Keywords: endothelial heterogeneity, liver sinusoidal endothelial cell specification, transcription factors
Abstract
Liver sinusoidal endothelial cells (LSECs) are the first liver cells to encounter waste macromolecules, pathogens, and toxins in blood. LSECs are highly specialized to mediate the clearance of these substances via endocytic scavenger receptors and are equipped with fenestrae that mediate the passage of macromolecules toward hepatocytes. Although some transcription factors (TFs) are known to play a role in LSEC specialization, information about the specialized LSEC signature and its transcriptional determinants remains incomplete.
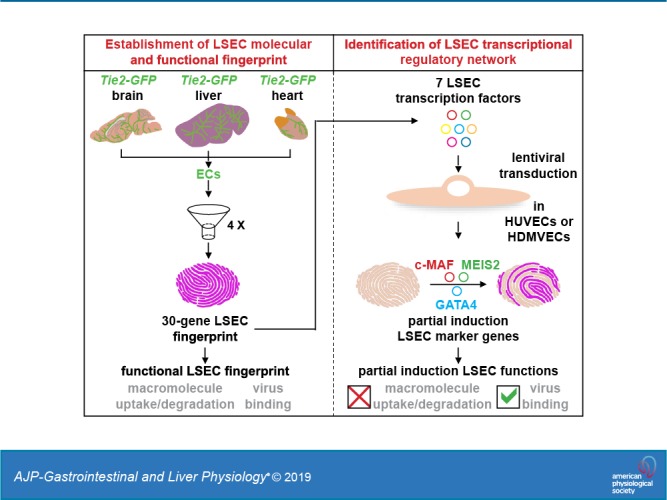
Based on a comparison of liver, heart, and brain endothelial cells (ECs), we established a 30-gene LSEC signature comprising both established and newly identified markers, including 7 genes encoding TFs. To evaluate the LSEC TF regulatory network, we artificially increased the expression of the 7 LSEC-specific TFs in human umbilical vein ECs. Although Zinc finger E-box-binding protein 2, homeobox B5, Cut-like homolog 2, and transcription factor EC (TCFEC) had limited contributions, musculoaponeurotic fibrosarcoma (C-MAF), GATA binding protein 4 (GATA4), and MEIS homeobox 2 (MEIS2) emerged as stronger inducers of LSEC marker expression. Furthermore, a combination of C-MAF, GATA4, and MEIS2 showed a synergistic effect on the increase of LSEC signature genes, including liver/lymph node-specific ICAM-3 grabbing non-integrin (L-SIGN) (or C-type lectin domain family member M (CLEC4M)), mannose receptor C-Type 1 (MRC1), legumain (LGMN), G protein-coupled receptor 182 (GPR182), Plexin C1 (PLXNC1), and solute carrier organic anion transporter family member 2A1 (SLCO2A1). Accordingly, L-SIGN, MRC1, pro-LGMN, GPR182, PLXNC1, and SLCO2A1 protein levels were elevated by this combined overexpression. Although receptor-mediated endocytosis was not significantly induced by the triple TF combination, it enhanced binding to E2, the hepatitis C virus host-binding protein. We conclude that C-MAF, GATA4, and MEIS2 are important transcriptional regulators of the unique LSEC fingerprint and LSEC interaction with viruses. Additional factors are however required to fully recapitulate the molecular, morphological, and functional LSEC fingerprint.
NEW & NOTEWORTHY Liver sinusoidal endothelial cells (LSECs) are the first liver cells to encounter waste macromolecules, pathogens, and toxins in the blood and are highly specialized. Although some transcription factors are known to play a role in LSEC specialization, information about the specialized LSEC signature and its transcriptional determinants remains incomplete. Here, we show that Musculoaponeurotic Fibrosarcoma (C-MAF), GATA binding protein 4 (GATA4), and Meis homeobox 2 (MEIS2) are important transcriptional regulators of the unique LSEC signature and that they affect the interaction of LSECs with viruses.
INTRODUCTION
The liver is the largest internal organ and has vital functions such as the elimination of toxins and waste products from the body, the production of factors important for food digestion and blood coagulation, and the storage of vitamins and carbohydrates. To optimally support these functions, the liver has a unique dual blood supply. Blood is drained from the intestines to the liver via the portal vein, and the liver receives oxygen-rich blood through the hepatic artery. Blood from both sources merges in the hepatic sinusoids and leaves the liver via the central veins. The hepatic sinusoids are lined with highly specialized liver sinusoidal endothelial cells (LSECs) to support the high demand for the transfer of substances, including nutrients, toxins, and waste products, from and to the hepatocytes. Therefore, they have fenestrae organized in sieve plates, lack an organized basement membrane, and express several scavenger receptors (2, 59, 60). LSECs have different expression profiles and functions according to their location in the liver lobule, and liver endothelial cells (ECs) are also implicated in determining metabolic zonation of liver lobules through Wingless-related integration site (Wnt)-mediated angiocrine signaling (6, 27, 38, 68). These specific characteristics make LSECs a prototypical example of endothelial heterogeneity.
LSECs, however, lose their specific characteristics early in liver fibrosis through a process called capillarization (18, 28). A similar process called pseudocapillarization occurs during aging (35). Although liver injury can be caused by different agents, including excess fat, toxins, alcohol, bacteria, or viruses, independent of the initial cause, liver injury invariably leads to collagen deposition and fibrosis. If the cause of liver injury is removed, the fibrotic liver can regenerate. LSECs play a central role in regeneration through the secretion of angiocrine signals, including Wingless-related integration site 2 (Wnt2) and angiopoietin-2 (20, 29, 31). However, if fibrosis progresses, it may become irreversible and lead to cirrhosis, which causes severe liver dysfunction and highly increases the risk of hepatocellular carcinoma. Currently, the only cure for cirrhosis is a liver transplantation (8). During liver disease, stellate cells become activated and deposit collagen in the liver. In the healthy liver, stellate cell activation is inhibited by LSECs through nitric oxide production; however, upon capillarization LSECs lose the ability to keep stellate cells quiescent, thereby further stimulating collagen deposition (19, 68). LSECs therefore play an essential role in liver function, protect against liver disease, and stimulate recovery.
Although many reports have documented the (unique) expression profile of LSECs by using different types of comparisons and techniques (17, 25, 41, 49, 50, 56), information still remains incomplete because novel LSEC markers, like GPR182, are still emerging from recent literature (56). Furthermore, although there is extensive literature on the rapid loss of LSEC hallmark characteristics in disease and culture, little is known about how LSECs actively acquire these specific characteristics. Upon culture, like many other EC types [including arterial ECs and cardiac capillary ECs (4, 16)], primary LSECs rapidly lose their specific properties, including the expression of LSEC markers and the presence of fenestrae (7, 21, 22, 24, 25), suggesting that environmental factors play an important role. Accordingly, coculture of LSECs with hepatocytes prevents loss of the LSEC marker Fc fragment of IgG receptor 2b (FcγRIIb) (7, 43). In a recent study, GATA4 has been put forward as a major transcriptional regulator of LSEC fate during development (24); however, it is unlikely that one single TF can fully determine the signature of an EC subtype. Like in arterial, lymphatic, or heart capillary ECs, a combination of TFs is most likely required to achieve an expression pattern that more closely approaches the specific EC molecular fingerprint (4, 5, 16). Canonical signaling pathways, such as VEGF signaling (9, 64), adrenomedullin-receptor activity modifying protein 2 signaling (3), and transforming growth factor β signaling (3), have been proposed to codetermine the acquisition of specific LSEC features during development, but their role in established LSECs remains to be determined.
Identifying what drives LSEC specialization could reveal therapeutic targets to prevent LSEC capillarization and thereby inhibit stellate cell activation and reverse fibrosis. Furthermore, because of species differences, ethical concerns, and costs, there is a high demand for reliable in vitro human models as alternatives to the use of animals in toxicity testing (63). Because LSECs not only control which compounds reach the hepatocytes but LSEC injury also plays a role in liver fibrosis (46, 68), the inclusion of LSEC-like cells in these in vitro liver models is crucial. Knowledge about the mechanisms driving LSEC specialization is therefore also important for developing LSECs for in vitro toxicity testing.
In this current study, we aimed to identify novel LSEC-enriched markers and TFs by comparing freshly isolated LSECs with ECs from the brain and heart. Furthermore, we intended to investigate how the specific LSEC fingerprint is transcriptionally regulated by testing the individual and combined effect of LSEC TFs on LSEC signature gene expression and on LSEC morphological and functional hallmarks. We identified a triple TF combination (C-MAF, GATA4, and MEIS2) that regulated a substantial part of the LSEC fingerprint in human umbilical vein ECs (HUVECs) and human dermal microvascular ECs (HDMVECs); however, this TF combination only partially induced LSEC-specific functions and did not give rise to fenestrae. Therefore, additional mechanisms remain to be identified for full induction of specific LSEC features.
MATERIALS AND METHODS
Isolation of cells and tissues.
Mice on an FVB background specifically expressing green fluorescent protein (GPF) in blood vascular ECs but not lymphatic ECs under the Tie2 promoter (47) were housed under standard conditions with 12-h light-dark cycles and ad libitum access to regular chow and water. Organs were dissected out from 8 to 12-wk-old mice, surrounding connective tissue and visible large vessels were removed, and tissues were enzymatically digested (using dispase for livers, collagenase type I for hearts, and crude collagenase for brains). Organs from several mice (with a 1:1 gender ratio) were pooled to obtain ECs for each sample. From each sample, ~106 GFP+ ECs per organ type were isolated by FACS directly in RLT lysis buffer (Qiagen) on an Aria I sorter (Beckton Dickinson) after exclusion of doublets based on forward-side scatter gating. To visualize GFP+ EC-coated vessels, frozen cross-sections were stained with Cy3-conjugated mouse anti-mouse α-smooth muscle actin (Sigma, C6198, 1:200). To visualize large liver vessels, paraffin cross-sections from Tie2-GFP livers were double-stained with phycoerythrin-conjugated rat anti-mouse CD34 (Beckton Dickinson, 551387) and chicken-anti-GFP (Abcam, ab13970). For liver ECs, to determine the size of the microvascular EC fraction, a subsample of the cells was stained with phycoerythrin-conjugated rat-anti-mouse CD34 or allophycocyanin-conjugated hamster-anti-mouse CD36 (Biolegend, 102612) to distinguish between CD34+ large vessel ECs and CD36+ microvascular ECs. To determine the expression of LSEC signature genes in hepatocytes and the remaining nonparenchymal fraction, livers from wild-type mice (FVB) were digested as described above, the hepatocytes were separated from the obtained monocellular suspensions by centrifugation, and the endothelial and nonendothelial nonparenchymal cell fractions were isolated from the remaining cell suspension by positive and negative selection for the panendothelial marker Meca32, using an Alexa700-conjugated rat-anti-Meca32 antibody (Novus). To evaluate the kinetics of TF expression loss upon culture, the liver EC fraction was isolated from liver monocellular suspensions of wild-type mice using magnetic beads conjugated with an anti-Meca32 antibody (Beckton Dickinson). Meca32+ cells were plated on gelatin-coated culture vessels in endothelial growth medium (EBM2 medium supplemented with EGM2-MV singlequots; Lonza), and cells were lysed for RNA isolation at 0 h (control), 12 h, or 24 h after plating. All mouse experiments were approved by the KU Leuven Animal Ethics Committee (Ethics Committee Dossier number 148/2010, 022/2011, 169/2014, and 208/2017) and performed in accordance with the Committee’s guidelines.
As a positive control for morphological and functional evaluation, LSECs were isolated from male Sprague-Dawley rats (Charles River, Sulzfeld, Germany), aged 6–11 wk. Rats were housed under controlled conditions (12-h light/12-h dark cycle) at the animal research facility at University of Tromsø – The Arctic University of Norway. The rats had free access to water and standard chow and were acclimatized for at least 1 wk before experiments. The experimental protocols were approved by the National Animal Research Authority at the Norwegian Food Safety Authority (Forsøksdyrforvatningen tilsyns- og søknadssystem , Mattilsynet, approval identification no. 8455), and experiments were performed in compliance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. Livers were perfused via the portal vein with collagenase. The resulting monocellular suspensions were subsequently subjected to Percoll density centrifugations as previously described (58). An initial selective adherence step in glutaraldehyde-treated human albumin-coated dishes was performed to remove Kupffer cells. Nonadherent cells were subsequently cultured in type I collagen-coated dishes in α-MEM supplemented with 10% serum and antibiotics.
Human liver biopsies used for immunostaining were collected in Gey’s buffer after obtaining informed consent from the donors (patients undergoing elective cholecystectomy). Biopsies were fixed overnight in 4% paraformaldehyde, embedded in paraffin, and sectioned for immunofluorescence staining. HUVECs were isolated from umbilical cords from babies delivered by Cesarean section after full-term pregnancy with informed consent from the mother. HDMVECs were purchased from Westburg (HMVECdNeo). The use of human biopsies, umbilical cords, and human cells was approved by the Ethics Committee of University Hospital Leuven (No. B32220152525871), and experiments were performed in accordance with the Committee’s guidelines.
Microarray and functional annotation analyses.
Microarrays on RNA from ECs from adult mouse livers, hearts, and brains (n = 5 per organ type) were performed by the Vlaams Instituut voor Biotechnologie Nucleomics core (KU Leuven, Leuven, Belgium) as previously described (16). Raw data were deposited at the National Center for Biotechnology Information Gene Expression Omnibus website (https://www.ncbi.nlm.nih.gov/geo/) (accession no. GSE48209). We obtained a (human) LSEC signature by a stepwise filtering process. First, genes enriched above a certain threshold in liver ECs (vs. other organ ECs and vs. the non-EC fractions from the liver) were retained, and genes without a human ortholog according to the Gene Cards human gene database (https://www.genecards.org) were removed. Finally, to complement the filtered gene list and obtain a 30-gene signature, we added 3 well-established (human) LSEC markers from the literature that were not meeting the criteria used or had no mouse ortholog that was represented on the mouse genome-wide Affymetrix Mogene 1 0ST array. Enriched biological themes (gene ontology terms) were identified using the database for annotation, visualization, and integrated discovery (DAVID) (https://david.ncifcrf.gov/summary.jsp).
Lentivirus production and lentiviral transduction.
Open reading frames of the LSEC TFs were cloned from cDNA-containing plasmids [purchased from Open Biosystems for human (h) TCFEC (clone ID no. 5179088), hMEIS2 (clone ID no. 6066728), and homeobox B5 (HOXB5) (clone ID no. 40125798) or from Thermo Scientific for human zinc finger E-box-binding 2 (ZEB2) (clone ID no. 40124124)] or by PCR from genomic DNA from mouse liver [mouse (m) cut-like homolog 2 (Cux2)], and were ligated into the pRRL2-PGK-Cherry lentiviral vector. If no successful overexpression was obtained with the in-house-made vectors, lentiviral vectors encoding GFP behind the TF of interest were purchased from Origene (hMEIS2, cat no. RC222807L; hC-MAF, RC209247L2). The lentiviral pLVX vector containing the hGATA4 sequence was generated in C. Verfaillie’s laboratory. Second generation lentiviruses were produced in human embryonic kidney 293 cells. Obtained viruses were titrated in HUVECs and HDMVECs, and the lowest concentration leading to 100% transduction efficiency, based on the presence of the coexpressed Cherry or GFP signal, was used in subsequent experiments. HUVECs and HDMVECs were grown in EBM2 medium supplemented with EGM2-MV singlequots (Lonza) on gelatin-coated plates. Cells were transduced with viruses (if multiple TFs were overexpressed, viruses were added simultaneously), medium was changed every other day, and 6 days after transduction, cells were lysed in TRIzol or radioimmunoprecipitation assay buffer with proteinase inhibitors for RNA or protein isolation, respectively, or were fixed in 4% paraformaldehyde for immunofluorescence.
RNA and protein preparation and quantification.
RNA was isolated with TRIzol (Invitrogen), and cDNA was made using the GoScript reverse transcription system (Promega) according to the manufacturer’s protocol. Quantitative real-time (RT) PCR was performed with the QuantStudio 3 system using SYBR green (Applied Biosystems). GAPDH was used as the housekeeping gene. Mouse and human gene primer sequences are provided in Supplemental Table S1 (https://doi.org/10.6084/m9.figshare.11830854.v1). Protein expression for a subset of LSEC markers was quantified by Western blotting or immunostaining using the rabbit-anti-L-SIGN, (Thermo Fisher, PA5–42164), goat-anti-LGMN (R&D Systems, AF2199), goat-anti-MRC1 (R&D Systems, AF2535), mouse-anti-human PLXNC1 (R&D Systems, MAB3887), rabbit-anti-human SLCO2A1 (Novus Biologicals, NBP2–13349), rabbit-anti-human GPR182 (Abcam, ab199177), and a secondary anti-goat, anti-mouse, or anti-rabbit horse radish peroxidase antibody (Santa Cruz for Western blotting or to Dako for immunofluorescence). GAPDH (Cell Signaling Technologies) was used as the loading control, and blots were developed using ECL (Bio-Rad). The immunofluorescence signal was enhanced through a Cy3-based amplification system (Perkin Elmer).
LSEC morphological and functional assays.
To evaluate TF-treated HUVECs/HDMVECs, control HUVECs/HDMVECs, or freshly isolated rat or mouse LSECs for the presence of fenestrae, scanning electron microscopy was performed on cells fixed with McDowell’s fixative. After washing, cells were treated with 1% tannic acid in 0.15 mol/L cacodylate buffer for 1 h, post-fixed with 1% osmium tetroxide in 0.1 mol/L cacodylate buffer for 30 min, dehydrated with graded alcohols, dried with hexamethyldisilazane, sputter-coated with 25 nm gold/palladium, and examined via a Zeiss Sigma Field emission scanning electron microscope (Carl Zeiss, Germany).
To test functional purity of ECs, Tie2-GFP mice were injected in the tail vein with goat-anti-rabbit Alexa660-conjugated IgG (Invitrogen, A-21073). Two hours after injection, mice were euthanized, and livers were dissected out and enzymatically digested, and the obtained monocellular suspensions were further processed for analysis by FACS, as previously described (61).
TF-treated HUVECs, control HUVECs, or freshly isolated rat LSECs were assessed for their endocytotic ability through the stabilins, mannose receptor, or FcγRIIb receptor, as described in Refs. 23 and 42). Briefly, cells were starved of serum for 4 h and incubated for 1 h with in-house-prepared radiolabeled (125I radiolabeling) formaldehyde-treated serum albumin (FSA) [a ligand for the stabilins (60)], RNAse B [a ligand for the mannose receptor (1)], or aggregated gamma globulin (AGG) [a ligand for FcγRIIb (60), AGG was prepared from human normal immunoglobulin (Baxter, Vienna, Austria) by diluting it 1:9 with PBS and incubating at 63°C for 1 h]. The incubations were terminated by transferring the media to tubes containing 20% trichloroacetic acid/0.5% phosphotungstic acid, and proteins were precipitated by centrifugation. Ligand degradation was determined by measuring the amount of free 125I in the supernatant. Cells were washed and lysed in 1% (wt/vol) SDS. Cell-associated ligands were quantified by measuring the radioactivity in the lysate. Total radioactivity was the sum of the cell-associated, degraded fraction and the protein-associated activity in the medium.
For virus association studies, HUVECs were trypsinized and starved in nonadherent tubes for 4 h. HUVECs were subsequently incubated with the hepatitis C virus (HCV) E2 protein for 2 h, followed by incubation with anti-E2 antibody (AP33, a kind gift from A.H. Patel at the Centre for Virus Research, Medical Research Council University of Glasgow in Glasgow, Scotland, UK) (52), followed by incubation with a Alexa660-conjugated secondary antibody (Invitrogen). E2 binding was visualized by flow cytometry (on a Canto II; Beckton Dickinson), successfully transduced cells were identified by the presence of the GFP signal, and the fraction of E2-binding cells within the successfully transduced cell population was determined.
Statistics.
Data are expressed as means ± SE. The Student’s t test was used to compare two groups. One-way ANOVA was used to compare three groups, and the Wilcoxon signed-rank test was used to analyze fold increase. P < 0.05 was considered statistically significant, and 0.05 < P < 0.1 was considered borderline significant. Graphpad Prism 7 was used for statistical analysis.
RESULTS
Establishment of a 30-gene (human) LSEC fingerprint.
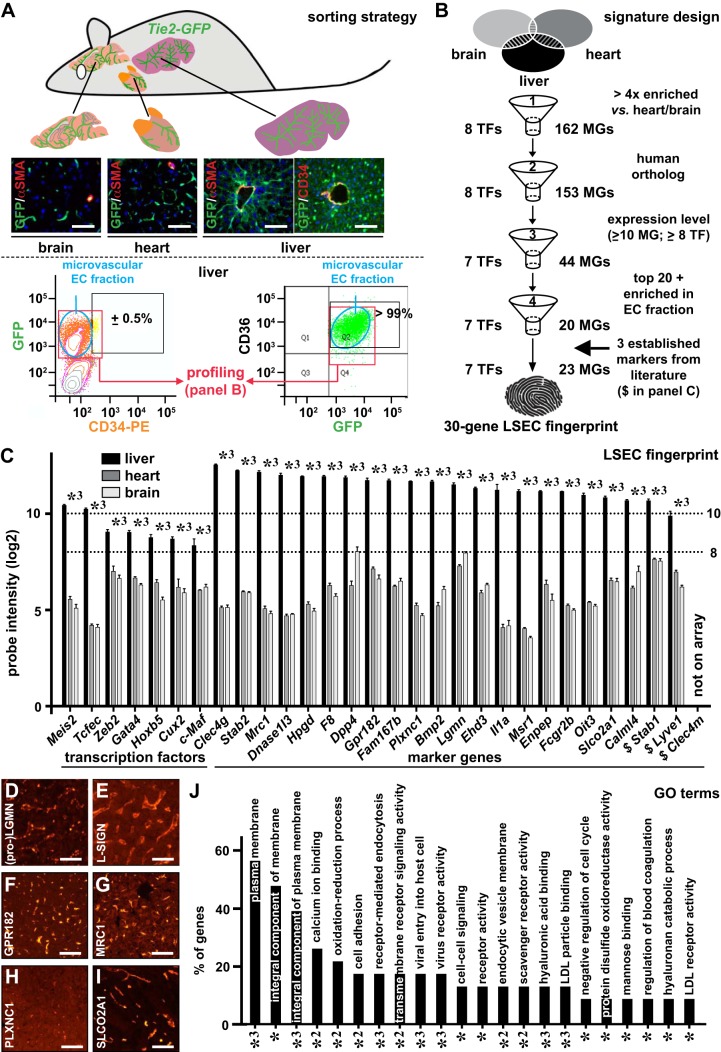
For expression profiling, GFP+ ECs were isolated from the livers, brains, and hearts of Tie2-GFP mice (Fig. 1A). Previously, we showed that the majority of heart ECs are microvascular by staining sorted GFP+ ECs from Tie2-GFP mice with the microvascular marker CD36 (16). Similarly, FACS analysis revealed that the majority (>99%) of GFP+ liver ECs were positive for the microvascular marker CD36, and, complementarily, costaining of GFP+ liver ECs with the macrovascular marker CD34 revealed that <1% of the GFP+ liver ECs were CD34+, indicating that >99% of the isolated ECs were microvascular (Fig. 1A). The obtained expression profile of GFP+ liver ECs therefore reflects that of microvascular ECs. Comparative profiling revealed remarkably different gene signatures for ECs from the 3 organs, as evidenced by the Volcano plots showing 1,596 (858 up/738 down) and 1,858 (920 up/938 down) differentially expressed probe sets between liver ECs and heart or brain ECs, respectively (Supplemental Fig. S1, A–C). Purity of the endothelial fraction was confirmed by the very low abundance of lymphatic EC, hepatocyte, stellate cell, Kupffer cell, and biliary epithelial cell markers compared with general EC markers (Supplemental Fig. S1, D and E), by the ubiquitous presence of sieve plates, and by the observation that >97.5% of GFP+ cells had the ability to take up fluorescently labeled IgGs (Supplemental Fig. S1, F and G). To obtain a (human) LSEC gene signature, a four-step filtering process was designed (Fig. 1B). In the first step, after retaining the best probe set for each gene and ranking the genes according to the fold upregulation in the liver versus in the heart, 170 genes (162 encoding an LSEC marker gene; 8 encoding a TF) that were at least fourfold enriched in liver ECs were retained. In the second step, we only selected genes for which a human ortholog is described [this resulted in the dismissal of 9 LSEC marker genes: alcohol dehydrogenase 6B, aldo-keto reductase family 1 member B8, KH RNA binding domain containing signal transduction associated 3, major urinary protein 1 (Mup1), Mup2, Mup3, Mup7, Mup20, and testis protein 18 (Tex18) (Supplemental Table S2)]. In the third step, LSEC marker genes with a log2 probe intensity of less than 10 and TFs with a log2 probe intensity of less than 8 were discarded (the latter threshold was set lower given the generally lower expression levels of TFs). In the last step, we selected the top 20 ranked genes that were significantly enriched in ECs versus hepatocytes and nonparenchymal cells other than ECs. By using quantitative RT-PCR on ECs from an independent group of mice, we confirmed that the remaining 20 marker genes were significantly more highly expressed in the EC fraction (Supplemental Fig. S2). Finally, we added three well-established human LSEC markers, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), stabilin1 (STAB1) and L-SIGN, to complement the filtered gene list and to obtain a 30-gene fingerprint. LYVE1 was filtered out after the third step because it had a log2 probe intensity of <10 (Supplemental Table S2); STAB1 was not retained after the fourth step, as it did not belong to the top 20 ranked genes (Supplemental Table S2). L-SIGN (or CLEC4M) was not represented on the mouse microarray. This finally gave us a 30-gene signature comprising 7 genes encoding a TF and 23 genes encoding an LSEC marker protein (Fig. 1C). This signature contained both previously established markers (e.g., STAB2, CLEC4G, FCGR2B) and known TFs (GATA4, TCFEC, and C-MAF) as well as new markers (LGMN, glutamyl aminopeptidase (ENPEP), Family with Sequence Similarity 167 Member B (FAM167B)) and new TFs (ZEB2, MEIS2, HOXB5, and CUX2). Expression of some markers was confirmed at the protein level on human liver sections (Fig. 1, D–I). DAVID analysis showed that, among the LSEC markers, there were many membrane proteins and that they were involved in several LSEC-specific functions, including virus binding and macromolecule uptake (Fig. 1J; Supplemental Table S3).
Fig. 1.
Establishment of a 30-gene (human) liver sinusoidal endothelial cell (LSEC) fingerprint. Brains, hearts, and livers were isolated from Tie2-green fluorescent protein (GFP) mice (A), which express GFP specifically in endothelial cells (ECs). Frozen cross-sections of these organs stained for α-smooth muscle actin (αSMA; red) and DAPI (as nuclear counterstain) confirmed GFP expression (green) in all ECs, and costaining for CD34 (red) and GFP (green) on liver cross-sections confirmed CD34 as a macrovascular EC marker. FACS analysis after colabeling of GFP+ ECs from Tie2-GFP livers with CD34 (left) or CD36 (right) revealed that <1% and >99% of the ECs represented macrovascular and microvascular ECs, respectively, such that the profiles of GFP+ cells reflected that of (sinusoidal) capillaries. A (human) LSEC-specific 30-gene signature consisting of genes encoding transcription factors (TFs) (left) and marker genes (right) arranged according liver EC probe set intensity (C) was established from comparative gene expression profiling using a four-step filtering process and by adding three established markers not retained by the filtering process (B). In C, the expression of the 30 genes in mouse livers (closed bars), hearts (dark shaded bars), and brains (light shaded bars) are shown as mean log2 probe intensities ± SE (n = 5 for each organ; *3P < 0.001 vs. heart or brain by ANOVA). Dotted horizontal lines in C indicate the expression threshold levels used in filter step three. Human liver cross-sections (female donor) immunostained (red) for Legumain (LGMN) (D), liver/lymph node-specific ICAM-3 grabbing non-integrin (L-SIGN) (E), G protein-coupled receptor 182 (GPR182) (F), mannose receptor C-type 1 (MRC1) (G), PlexinC1 (PLXNC1) (H), and solute carrier organic anion transporter family member 2A1 (SLCO2A1) (I). Diagram (J) displays annotations [gene ontology (GO) terms] identified by database for annotation, visualization, and integrated discovery analysis significantly (adjusted P-values: *P < 0.05, *2P < 0.01, or *3P < 0.001) associated with the identified LSEC markers, expressed as the % of genes from the 30-gene signature allocated to the corresponding terms. Scale bars = 100 μm in A and D–I. $Established markers.
Identification of transcriptional determinants of the LSEC fingerprint.
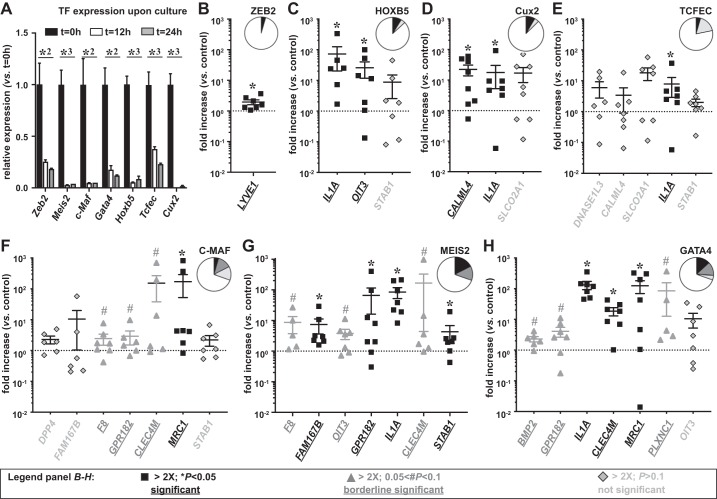
To establish the LSEC signature, we used freshly isolated ECs. However, many, if not all, EC types lose their specific expression pattern in culture when deprived of their natural environment. In arterial and heart ECs, we have shown that this expression loss occurs very rapidly and also affects TFs (4, 16). Likewise, we performed a kinetic expression analysis of the 7 LSEC TFs in ECs isolated from mouse livers and found that TF expression was significantly downregulated after 12 h in culture and was almost completely absent after 24 h (Fig. 2A). Because long-term cultivation (up to 6 days required for lentiviral overexpression) of primary LSECs was not achievable, we used HUVECs to study whether we could induce the expression of the 23 LSEC marker genes by artificially overexpressing the TFs. Therefore, we generated lentiviruses encoding each of the seven TFs and verified that all TFs were successfully overexpressed in HUVECs (Supplemental Table S4).
Fig. 2.
Identification of transcription factors (TFs) regulating liver sinusoidal endothelial cell (LSEC) marker gene expression (A). Expression of LSEC-specific TFs in freshly isolated (t = 0 h) primary mouse LSECs (closed bars), primary mouse LSECs cultured for 12 h (open bars), or primary mouse LSECs cultured for 24 h (shaded bars). Data were analyzed by ANOVA and are expressed as mean ± SE and represent expression levels relative to t = 0 h (n = 3; *P < 0.05; *2P < 0.01; *3P < 0.001). LSEC signature genes induced after 6 days of lentiviral overexpression in human umbilical vein endothelial cells of Zinc finger E-box-binding protein 2 (ZEB2) (B), homeobox B5 (HOXB5) (C), Cut-like homolog 2 (Cux2) (D), transcription factor EC (TCFEC) (E), musculoaponeurotic fibrosarcoma (C-MAF) (F), MEIS homeobox 2 (MEIS2) (G), or GATA binding protein 4 (GATA4) (H). Genes with significant (*P < 0.05) induction, borderline significant (#0.05 < P < 0.1) induction or at least twofold not significant (P > 0.1) induction are indicated in closed squares, shaded triangles or lightly shaded diamonds, respectively. Insets: pie charts of corresponding distributions of these four categories of B–H. Data are expressed as means ± SE and represent fold increase relative to control virus (n = 6–8 from 2 independent experiments; Wilcoxon signed ranked test).
In a first experiment using four independent HUVEC clones, we identified the LSEC markers for each TF that showed at least a twofold induction upon TF overexpression, and we repeated the experiment for these markers on an additional number of samples. We determined which markers were increased by at least twofold by using three significance levels: significant, borderline significant, and not significant (Fig. 2, B–H). Overall, ZEB2, HOXB5, TCFEC, and Cux2 only increased few LSEC markers (Fig. 2, B–E), whereas GATA4, MEIS2, and C-MAF increased a broader set of LSEC markers (Fig. 2, F–H). Among the statistically significantly (P < 0.05) regulated markers were MRC1 for C-MAF (Fig. 2F); FAM167B, GPR182, interleukin 1A (IL1A), and STAB1 for MEIS2 (Fig. 2G); and IL1A, CLEC4M, and MRC1 for GATA4 (Fig. 2H). Multiple genes (7 out of 23 or 30%, including some established markers like STAB2, CLEC4G, and FCGR2B) were not responsive (“no induction”) to any individual TF (Fig. 2, B–H, and Fig. 3B).
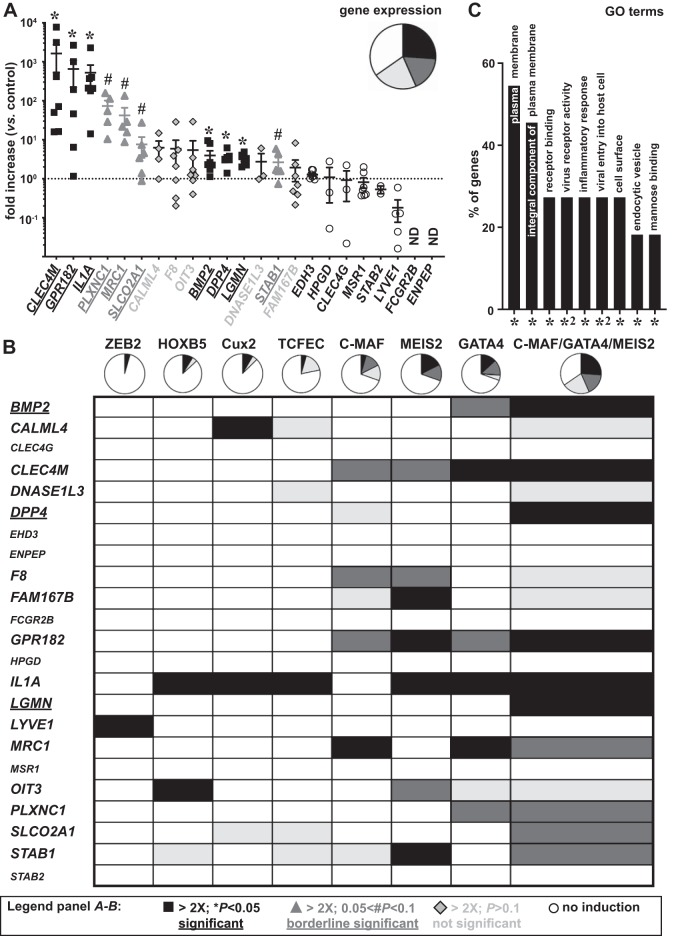
Fig. 3.
Identification of a transcription factor (TF) combination regulating liver sinusoidal endothelial cell (LSEC) marker gene expression. A: expression of LSEC signature genes (arranged according to the fold increase) after 6 days of combined lentiviral overexpression of musculoaponeurotic fibrosarcoma (C-MAF), GATA binding protein 4 (GATA4) and MEIS homeobox 2 (MEIS2) in human umbilical vein endothelial cells (HUVECs). Genes with at least twofold significant (*P < 0.05) induction, borderline significant (#0.05 < P < 0.1) induction, at least twofold not significant (P > 0.1) induction, or no induction are indicated in closed squares, shaded triangles, lightly shaded diamonds, or open circles, respectively. Corresponding distributions of these four categories are shown as a pie chart (upper right inset). Data are expressed as means ± SE and represent fold increase relative to control virus-transduced HUVECs from the same donor (n = 6–8 from 2 independent experiments; Wilcoxon signed ranked test). B: table schematically representing the effect of each TF or the triple combination on expression of the signature genes and corresponding pie charts (color codes same as those in A). C: annotations [gene ontology (GO) terms] identified by database for annotation, visualization, and integrated discovery analysis significantly (adjusted P values: *P < 0.05; *2P < 0.01) associated with the identified LSEC markers, expressed as the % of genes from the 30-gene signature allocated to the corresponding terms. Genes only statistically significantly induced by the three TF combination or still not induced by the three TF combination are underlined or shown in smaller font size, respectively. ND, not detectable.
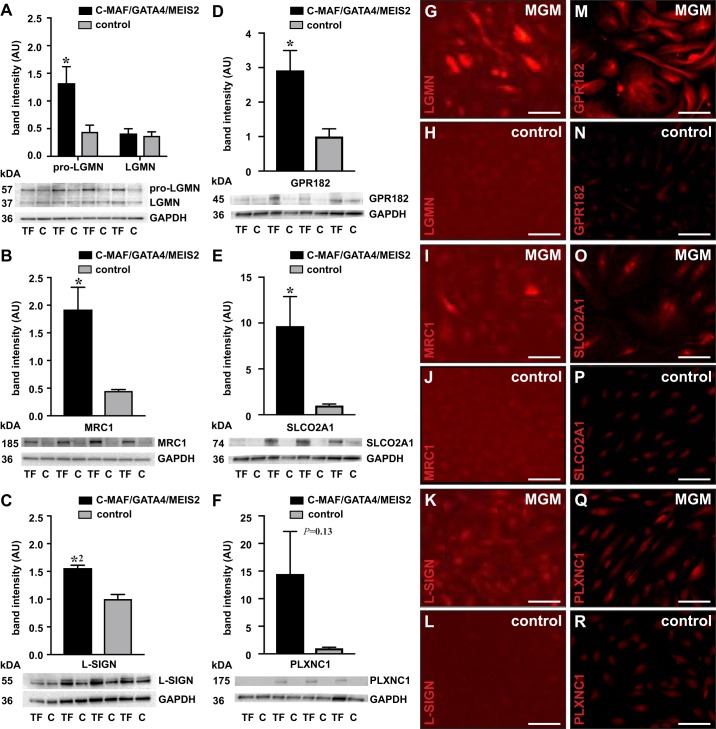
Because none of the identified TFs were able to regulate the majority of LSEC markers, and some markers were not induced at all, we hypothesized that a combination of TFs would be required to regulate the complete LSEC fingerprint. To test this hypothesis, we combined the three most successful inducers of LSEC marker expression. The level of overexpression that the TFs achieved in this combined setting was similar to that in the individual setting (Supplemental Table S4). In line with a synergistic effect, C-MAF, GATA4, and MEIS2 together were able to increase 43% of the LSEC markers by at least twofold when considering the (borderline) significant levels (Fig. 3, A and B). Some markers (bone morphogenetic protein 2 (BMP2), dipeptidyl peptidase 4 (DDP4), and LGMN) that were not significantly responsive to any individual TF were now significantly induced by the three TF combination, further supporting the synergistic effect (Fig. 3B). However, the expression of some LSEC markers, including 15-hydroxyprostaglandin dehydrogenase (HPGD), EH domain containing 3 (EHD3), ENPEP, STAB2, LYVE1, CLEC4G, MSR1, and FCGR2B, was still not increased by the triple combination of TFs (Fig. 3B), suggesting that these are regulated by other factors. DAVID analysis showed that the regulated genes were mainly related to receptor binding (BMP2 and IL1A), inflammation (BMP2, IL1A, and STAB1), virus binding and entry into the cell (DPP4, CLEC4M, MRC1, and PLXNC1), and endocytosis/mannose binding (CLEC4M, MRC1, and DPP4) (Fig. 3C). Overexpression of a subset of regulated genes [L-SIGN, MRC1, pro-LGMN (which is the precursor encoded by the LGMN gene), PLXNC1, GPR182, and SLCO2A1] was confirmed at the protein level by Western blotting on cell lysates (Fig. 4, A–F) and by immunofluorescence staining on confluent cell monolayers (Fig. 4, G–R). Because HUVECs are macrovascular ECs, we wondered whether combined overexpression of C-MAF, GATA4, and MEIS2 in a microvascular EC type, i.e., HDMVECs, would offer a better inductive effect. We found that the overall effect was quite comparable (both in terms of fold induction and type/number of responsive genes) to that found in HUVECs (Supplemental Fig. S3A and Supplemental Table S4).
Fig. 4.
Liver sinusoidal endothelial cell (LSEC) marker protein expression induced by combined musculoaponeurotic fibrosarcoma (C-MAF), GATA binding protein 4 (GATA4), and MEIS homeobox 2 (MEIS2) overexpression. Western blots showing protein expression in human umbilical vein endothelial cells (HUVECs) transduced with a combination of C-MAF, GATA4, and MEIS2 coding lentiviruses [transcription factors (TFs)] (closed bars) or with control virus (C) (shaded bars) for (pro)-legumain (LGMN) (A), mannose receptor C-Type 1 (MRC1) (B), liver/lymph node-specific ICAM-3 grabbing non-integrin (L-SIGN) (C), G-Protein-Coupled Receptor 182 (GPR182) (D), Solute Carrier Organic Anion Transporter Family Member 2A1 (SLCO2A1) (E), and PlexinC1 (PLXNC1) (F). GAPDH is used as loading control (the same GAPDH loading control was used for GPR182 and SLCO2A1 because both proteins were detected on the same blot). Data represent mean band intensity ± SE, expressed as arbitrary units (AUs) (n = 4, representative of 2–3 independent experiments) (*P < 0.05; *2P < 0.01 vs. control virus by Student’s t test). Representative pictures of HUVECs treated with the triple TF combination (MGM) or with control virus and stained (red) for pro-LGMN (G and H), MRC1 (I and J), L-SIGN (K and L), GPR182 (M and N), SLCO2A1 (O and P), and PLXNC1 (Q and R). Scale bars = 250 μm.
Identification of transcriptional regulators of LSEC morphology and functions.
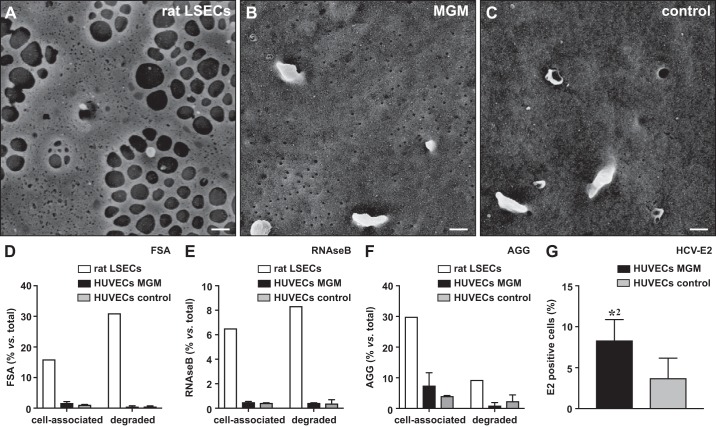
To investigate whether the triple TF combination also induces the morphological hallmark feature of LSECs, i.e., the formation of fenestrae, we performed SE. Unlike in freshly isolated rat or mouse LSECs, no fenestrae were observed in control HUVECs/HDMVECs or in HUVECs/HDMVECs overexpressing C-MAF, GATA4, and MEIS2 (Fig. 5, A–C, and Supplemental Fig. S3, B–D).
Fig. 5.
Molecular and functional specialization on combined musculoaponeurotic fibrosarcoma (C-MAF), GATA binding protein 4 (GATA4), and MEIS homeobox 2 (MEIS2) overexpression. Scanning electron micrographs of freshly isolated rat liver sinusoidal endothelial cells (LSECs) (A), human umbilical vein endothelial cells (HUVECs) transduced with a combination of C-MAF, GATA4, and MEIS2 coding lentiviruses (MGM) (B), or HUVECs transduced with control virus (C). Diagrams representing cell-associated and degraded (endocytosed) formaldehyde-treated serum albumin (FSA) (D), RNAseB (E), or aggregated gamma globulin (AGG) (F) after 1 h incubation with freshly isolated rat LSECs (open bars), HUVECs transduced with a combination of C-MAF, GATA4, and MEIS2 coding lentiviruses (closed bars), or HUVECs transduced with control virus (shaded bars). Data represent means ± SE (n = 1 for rat LSECs; n = 4 for HUVECs). Diagram (G) representing quantification of the fraction of transduced [green fluorescent protein positive (GFP+)] cells (quadrant 1 + 2) that was bound to the E2 protein of the hepatitis C virus (HCV-E2) in HUVECs transduced with a combination of C-MAF, GATA4, and MEIS2 coding lentiviruses (closed bar) or control virus (shaded bar). Data represent mean fraction of E2+ cells ± SE, expressed relative to the total amount of GFP+ cells (n = 3 from 3 independent experiments; *P < 0.01 vs. control virus by Student’s t test). Scale bars in A–C = 200 nm.
Because viral entry/receptor activity and endocytosis/mannose binding were among the functions emerging from the DAVID analyses (Fig. 3C), we next assessed whether these functions were enabled by the triple TF combination. To assess endocytosis, we measured the binding and degradation of radioactively labeled substrates for STAB1 and STAB2 (FSA), MRC1 (RNAseB), or FcγRIIB (AGG). However, we did not observe differences between control HUVECs and HUVECs expressing C-MAF, GATA4, and MEIS2, whereas endocytosis was seen with freshly isolated rat LSECs (Fig. 5, D–F). As the DAVID analysis also showed enrichment of genes involved in virus binding and entry to the cell, we tested the binding of the HCV-E2 protein. Interestingly, we observed a higher association of the E2 protein with cells expressing C-MAF, GATA4, and MEIS2 compared with that found in HUVECs transduced with control virus (Fig. 5G; Supplemental Fig. S4). Altogether, this suggests that the currently tested TFs do not regulate the scavenger function of LSECs or fenestrae formation, but they do affect the interaction of LSECs with viruses.
DISCUSSION
Although LSECs are highly specialized cells, little is known about how they acquire their specific expression pattern. Gaining insight into how LSECs obtain their specific properties may help to develop ways to preserve LSEC function not only during liver disease and aging but also for in vitro usage. Because of ethical concerns, species differences, and high costs, there is a high demand for in vitro models with human cells to test the liver toxicity of drugs as alternative to animal models (63). Unfortunately, because there is currently no commercial source of bona fide LSECs and because there is insufficient knowledge of how to maintain primary LSECs in vitro or generate them de novo from other sources (e.g., from stem cells or other EC types) (22, 25, 32), available in vitro liver models usually do not include LSECs. Yet, LSECs are important determinants of drug toxicity for several reasons: they are the first liver cells that come into contact with toxins from the blood, they control what macromolecules can reach the hepatocytes, in vitro coculture with them maintains the phenotype of hepatocytes (51), and their damage is associated with the onset of liver disease.
In this study, we identified a 30-gene fingerprint of 23 LSEC markers and 7 LSEC-enriched TFs by comparing ECs from the liver, heart, and brain. We limited the signature to 30 genes mainly for practical reasons, as we aimed to design a signature that could routinely be used in large-scale experiments involving multiple conditions. By applying a stringent multistep filtering process, we eliminated some of the marker genes previously known to be enriched in LSECs. We therefore decided to add those genes that have been elaborately documented in the field as being of high functional importance back to the signature. Among the 23 marker genes, 16 had been previously found to be part of the mouse or human LSEC gene signature (Supplemental Table S5), supporting the accuracy of our LSEC fingerprint. We also found calmodulin-like 4 (CALML4), a gene previously not linked to LSECs or even to ECs of any kind (Supplemental Table S5). Likewise, three of the TFs have previously been described to be enriched in LSECs (C-MAF, GATA4, and TCFEC) (24, 25), but the other four have not been associated with LSECs before (Supplemental Table S5). When considering the list of 170 genes that were at least fourfold enriched in liver ECs versus in heart or brain ECs, we found pericentral (e.g., R-Spondin 3, oncoprotein-induced transcript 3 (OIT3), DNASE1L3), periportal [e.g., Il1a, factor 8 (F8); defined according to the recent papers by Halpern et al. (27) or MacParland et al. (41)], and midzonal [e.g., Gpr182 (56)] landmark genes, suggesting that we captured the entire zonal spectrum. A new LSEC marker gene that was not previously associated with any kind of endothelium is CALML4. CALML4 encodes a Ca2+-binding protein putatively involved in calcification of hypertrophic cartilage (11). The other six new LSEC marker genes had been previously described to be expressed in certain EC types. FAM167B (or Diora-2) belongs to the group of intrinsically disordered proteins with highest expression in the adrenal glands, kidney, and liver and with little or no expression in other organs (44). It was recently described to be expressed in liver ECs, although it was uncertain whether it was expressed in LSECs (41). ENPEP, an aminopeptidase involved in cleavage of angiotensin II, is expressed in several EC types, including glomerular ECs (30, 33, 39). HPGD, an enzyme belonging to the nonmetalloenzyme alcohol dehydrogenase family involved in the metabolism and inactivation of prostaglandins, is highly expressed in the lungs and liver and in coronary ECs (14, 36); LGMN, an endopeptidase inducing chemotaxis of ECs is expressed in ECs of atherosclerotic lesions (13, 53); PLXNC1, a semaphorin 7A receptor, is expressed in lung microvascular ECs (71); and SLCO2A1, a prostaglandin transporter, is expressed in arterial ECs and in capillary ECs of several well-perfused organs, including the heart, brain, and kidney (10, 48, 62). Hpgd-deficient mice and mice lacking Slco2a1 show impaired remodeling of the ductus arteriosus, indicating a role of these proteins in the vasculature (10, 14).
Two of the newly identified TFs have known roles in ECs other than liver ECs. HOXB5 has been shown to play a role in EC differentiation, sprouting, and intussusceptive angiogenesis (66, 67). ZEB2 may be involved in angiogenesis via regulation of mesenchyme homeobox 2 (MEOX2) and affects EC proliferation, migration, and apoptosis in hyperglycemic HUVECs (12, 65). Although expression of ZEB2 in LSECs was not previously studied, it is expressed in other nonparenchymal liver cells, including stellate cells (70) and Kupffer cells (57). CUX2 is expressed in liver to a higher extent in female rodents (34), in which it regulates genes enriched in female mice (15), but it has no documented role in ECs. No studies are published about MEIS2 in the liver or vasculature (45).
Overexpression of individual TFs in HUVECs, a macrovascular EC type, revealed that some TFs had more impact on LSEC marker gene expression than others. Among the more influential TFs was GATA4, which has recently been shown to be involved in LSEC specification during mouse development (24). Interestingly, Géraud et al. showed that although GATA4 positively affected a subset of LSEC marker genes, it also had a role in repressing genes associated with nonfenestrated continuous ECs. A similar role for other TFs from our list may be true, e.g., for CUX2, which has been shown to exclusively act as a transcriptional repressor in NIH3T3 fibroblasts (26). Full specification most likely requires a cooperative effort of several TFs. Accordingly, combining the overexpression of the 3 most influential TFs in HUVECs resulted in an at least 2-fold upregulation of 43% of the LSEC signature markers at the (borderline) significant level and 65% overall. Comparable results were obtained when transducing a microvascular EC type, i.e., HDMVECs. To fully exploit this synergistic effect, we also tried the combined overexpression of all seven TFs. Although this 7 TF combination indeed had an even broader effect on the signature [48% and 78% of the markers being increased at the (borderline) significant level and overall, respectively] (Supplemental Fig. S5), combined overexpression of the 7 TFs had several limitations. First, for many genes the variation was higher, which was probably due to the presence of subpopulations of cells that were not transduced with all seven viral vectors. In addition, we could not obtain many cells using this combination, most likely because of cell death caused by the high virus exposure. Ways of regulating all seven TFs other than viral transduction would therefore be desirable.
Some LSEC markers, including well-established ones, such as STAB2, CLEC4G, and FCGR2B, were not regulated by any of the TFs or by the triple TF combination in either macrovascular or microvascular ECs. This suggests that other factors may be required to induce the expression of these marker genes. Adrenomedullin, together with transforming growth factor β inhibition, was shown to boost the expression of LYVE1, STAB2, and FCGR2B in embryonic ECs, but it remains unclear whether these compounds also have a role in the induction/maintenance of the established adult LSEC signature (3). Another possibility is that to fully achieve the adult LSEC signature, LSEC-specific TFs have to cooperate with more general TFs, as has been previously shown for lymphatic EC (LEC) specification, which requires synergy between the LEC-specific TF prospero homeobox 1 (PROX1) and COUP transcription factor 2 (COUP-TFII), the latter of which is commonly expressed in LECs and venous ECs (5, 37, 69). Furthermore, to test the effects of the seven TFs, we used HUVECs that may lack certain LSEC-specific cofactors needed for the optimal function of these TFs. Finally, in addition to the intrinsic regulation by TFs, environmental cues, such as contact with neighboring cells, may have an important contribution in LSEC specification (7, 43). Similarly, because we were not able to induce fenestrae in HUVECs nor in HDMVECs, the induction of this morphological LSEC feature may require a combination of specific and nonspecific TFs and environmental cues. Providing these environmental cues will likely require sophisticated coculture platforms, such as three-dimensional systems or organoids.
In addition to the role of the identified TFs in inducing LSEC marker expression, we also studied LSEC-specific functions. Functional annotation of our gene signature revealed functional terms that had been previously associated with LSECs, including binding of macromolecules, endocytosis, and virus binding. Because MRC1 was among the genes that were upregulated by the triple TF combination, we expected an increased ability to bind, endocytose, and degrade RNAseB. This was, however, not the case. This may in part be due to the nonuniform expression of MRC1 induced by lentiviral transduction, or the level of MRC1 protein may not be sufficient. The lack of increased endocytosis and degradation of FSA and AGG was in accordance with the lack of upregulation of the corresponding receptors, STAB1, STAB2, and FCγRIIB, respectively. As the function of LGMN in LSECs is currently unknown, we could not evaluate the functional consequences of LGMN upregulation by the triple TF combination. Functional analysis showed that genes that were regulated by C-MAF, MEIS2, and GATA4 included not only those involved in inflammation and receptor binding but also those involved in virus binding and entry, which is an important but often underappreciated functional property of LSECs (22). In this study, we showed that HCV-E2 binding was enhanced in HUVECs transduced with lentiviruses overexpressing C-MAF, GATA4, and MEIS2, likely through the upregulation of L-SIGN, which has been previously shown to be involved in HCV-E2 binding to LSECs (40, 55), DPP4, and MRC1, which are also involved in virus binding according to our annotation analysis (Supplementary Table S3). However, CLEC4G, another known mediator of virus uptake (Supplementary Table S3), was not upregulated. Finally, dendritic cell-specific ICAM-3-grabbing nonintegrin 1 (DC-SGN), which was also shown to be involved in HCV binding by LSECs (40, 55), was not part of our LSEC signature. The combination of TFs we identified could be potentially used to study mechanisms of virus interaction with the endothelium in an in vitro liver model (54).
In conclusion, we identified a new 30-gene LSEC signature, including 7 TF genes and 23 marker genes. We identified a triple TF combination that regulates a substantial part of the specific LSEC signature. However, additional regulatory mechanisms remain to be identified to explain full molecular, morphological, and functional specification of LSECs, which can then be implemented in models of in vitro drug toxicity testing.
GRANTS
This work was supported by a European Research Council grant (FP7-StG-IMAGINED203291) to A. Luttun; a Cosmetics Europe/European Commission FP7 grant (FP7-Health-HemiBio266777) to B. Smedsrød, C. Verfaillie, and A. Luttun; a Program Financing grant (PF/10/014) to A. Luttun; an Interuniversity Attraction Poles grant (IUAP/P7/07) to A. Luttun; the C1 KU Leuven research grants (C14/19/095 to A. Luttun and C12/16/023 to W. de Haan); a Marie Sklodowska-Curie Actions postdoctoral fellowship to W. de Haan (H2020-MSCA-IF-REZONABLE658666); a Fonds voor Wetenschappelijk Onderzoek (FWO) predoctoral fellowship to W. Dheedene (1157318N); and an Agentschap voor Innovatie door Wetenschap en Technologie predoctoral fellowship to M. Beerens (SB/0881071).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.d.H., C.Ø., M. Benkheil, G.C., X.L.A., B.S., and A.L. conceived and designed research; W.d.H., C.Ø., M. Benkheil, W.D., S.V., G.C., X.L.A., M. Beerens, J.J., and B.T. performed experiments; W.d.H., C.Ø., M. Benkheil, W.D., G.C., and X.L.A. analyzed data; W.d.H., C.Ø., W.D., S.V., G.C., X.L.A., B.S., and A.L. interpreted results of experiments; W.d.H. and A.L. prepared figures; W.d.H. drafted manuscript; W.d.H., C.Ø., M. Benkheil, W.D., S.V., G.C., X.L.A., M. Beerens, J.J., B.T., C.V., B.S., and A.L. approved final version of manuscript; C.Ø., M. Benkheil, W.D., G.C., X.L.A., M. Beerens, C.V., B.S., and A.L. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Petra Vandervoort and Dr. Ruomei Li for technical support and Dr. Arvind H. Patel for providing the anti-E2 antibody. Current addresses of coauthors: G. Coppiello and X. L. Aranguren: Hematology and Cell Therapy Area, Clinica Universidad de Navarra, and Division of Oncology, Center for Applied Medical Research, University of Navarra, Pamplona, Spain; M. Beerens: Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
REFERENCES
- 1.Aarnoudse CA, Bax M, Sánchez-Hernández M, García-Vallejo JJ, van Kooyk Y. Glycan modification of the tumor antigen gp100 targets DC-SIGN to enhance dendritic cell induced antigen presentation to T cells. Int J Cancer 122: 839–846, 2008. doi: 10.1002/ijc.23101. [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Phenotypic heterogeneity of the endothelium. II. Representative vascular beds. Circ Res 100: 174–190, 2007. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 3.Arai T, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Iinuma N, Iesato Y, Koyama T, Yoshizawa T, Uetake R, Yamauchi A, Yang L, Kawate H, Ogawa S, Kobayashi A, Miyagawa S, Shindo T. Induction of LYVE-1/stabilin-2-positive liver sinusoidal endothelial-like cells from embryoid bodies by modulation of adrenomedullin-RAMP2 signaling. Peptides 32: 1855–1865, 2011. doi: 10.1016/j.peptides.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Aranguren XL, Agirre X, Beerens M, Coppiello G, Uriz M, Vandersmissen I, Benkheil M, Panadero J, Aguado N, Pascual-Montano A, Segura V, Prósper F, Luttun A. Unraveling a novel transcription factor code determining the human arterial-specific endothelial cell signature. Blood 122: 3982–3992, 2013. doi: 10.1182/blood-2013-02-483255. [DOI] [PubMed] [Google Scholar]
- 5.Aranguren XL, Beerens M, Coppiello G, Wiese C, Vandersmissen I, Lo Nigro A, Verfaillie CM, Gessler M, Luttun A. COUP-TFII orchestrates venous and lymphatic endothelial identity by homo- or hetero-dimerisation with PROX1. J Cell Sci 126: 1164–1175, 2013. doi: 10.1242/jcs.116293. [DOI] [PubMed] [Google Scholar]
- 6.Asumendi A, Alvarez A, Martinez I, Smedsrød B, Vidal-Vanaclocha F. Hepatic sinusoidal endothelium heterogeneity with respect to mannose receptor activity is interleukin-1 dependent. Hepatology 23: 1521–1529, 1996. doi: 10.1002/hep.510230632. [DOI] [PubMed] [Google Scholar]
- 7.Bale SS, Golberg I, Jindal R, McCarty WJ, Luitje M, Hegde M, Bhushan A, Usta OB, Yarmush ML. Long-term coculture strategies for primary hepatocytes and liver sinusoidal endothelial cells. Tissue Eng Part C Methods 21: 413–422, 2015. doi: 10.1089/ten.tec.2014.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 58: 593–608, 2013. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter B, Lin Y, Stoll S, Raffai RL, McCuskey R, Wang R. VEGF is crucial for the hepatic vascular development required for lipoprotein uptake. Development 132: 3293–3303, 2005. doi: 10.1242/dev.01902. [DOI] [PubMed] [Google Scholar]
- 10.Chang HY, Locker J, Lu R, Schuster VL. Failure of postnatal ductus arteriosus closure in prostaglandin transporter-deficient mice. Circulation 121: 529–536, 2010. doi: 10.1161/CIRCULATIONAHA.109.862946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Fink T, Zhang XY, Ebbesen P, Zachar V. Quantitative transcriptional profiling of ATDC5 mouse progenitor cells during chondrogenesis. Differentiation 73: 350–363, 2005. doi: 10.1111/j.1432-0436.2005.00038.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Banda M, Speyer CL, Smith JS, Rabson AB, Gorski DH. Regulation of the expression and activity of the antiangiogenic homeobox gene GAX/MEOX2 by ZEB2 and microRNA-221. Mol Cell Biol 30: 3902–3913, 2010. doi: 10.1128/MCB.01237-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerin V, Shih HH, Deng N, Hebert G, Resmini C, Shields KM, Feldman JL, Winkler A, Albert L, Maganti V, Wong A, Paulsen JE, Keith JC Jr, Vlasuk GP, Pittman DD. Expression of the cysteine protease legumain in vascular lesions and functional implications in atherogenesis. Atherosclerosis 201: 53–66, 2008. doi: 10.1016/j.atherosclerosis.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Coggins KG, Latour A, Nguyen MS, Audoly L, Coffman TM, Koller BH. Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat Med 8: 91–92, 2002. doi: 10.1038/nm0202-91. [DOI] [PubMed] [Google Scholar]
- 15.Conforto TL, Zhang Y, Sherman J, Waxman DJ. Impact of CUX2 on the female mouse liver transcriptome: activation of female-biased genes and repression of male-biased genes. Mol Cell Biol 32: 4611–4627, 2012. doi: 10.1128/MCB.00886-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppiello G, Collantes M, Sirerol-Piquer MS, Vandenwijngaert S, Schoors S, Swinnen M, Vandersmissen I, Herijgers P, Topal B, van Loon J, Goffin J, Prósper F, Carmeliet P, García-Verdugo JM, Janssens S, Peñuelas I, Aranguren XL, Luttun A. Meox2/Tcf15 heterodimers program the heart capillary endothelium for cardiac fatty acid uptake. Circulation 131: 815–826, 2015. doi: 10.1161/CIRCULATIONAHA.114.013721. [DOI] [PubMed] [Google Scholar]
- 17.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One 5: e13741, 2010. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 61: 1740–1746, 2015. doi: 10.1002/hep.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLeve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 48: 920–930, 2008. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505: 97–102, 2014. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elvevold K, Nedredal GI, Revhaug A, Bertheussen K, Smedsrød B. Long-term preservation of high endocytic activity in primary cultures of pig liver sinusoidal endothelial cells. Eur J Cell Biol 84: 749–764, 2005. doi: 10.1016/j.ejcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Elvevold K, Smedsrød B, Martinez I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol 294: G391–G400, 2008. doi: 10.1152/ajpgi.00167.2007. [DOI] [PubMed] [Google Scholar]
- 23.Elvevold KH, Nedredal GI, Revhaug A, Smedsrød B. Scavenger properties of cultivated pig liver endothelial cells. Comp Hepatol 3: 4, 2004. doi: 10.1186/1476-5926-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Géraud C, Koch PS, Zierow J, Klapproth K, Busch K, Olsavszky V, Leibing T, Demory A, Ulbrich F, Diett M, Singh S, Sticht C, Breitkopf-Heinlein K, Richter K, Karppinen SM, Pihlajaniemi T, Arnold B, Rodewald HR, Augustin HG, Schledzewski K, Goerdt S. GATA4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopoiesis. J Clin Invest 127: 1099–1114, 2017. doi: 10.1172/JCI90086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Géraud C, Schledzewski K, Demory A, Klein D, Kaus M, Peyre F, Sticht C, Evdokimov K, Lu S, Schmieder A, Goerdt S. Liver sinusoidal endothelium: a microenvironment-dependent differentiation program in rat including the novel junctional protein liver endothelial differentiation-associated protein-1. Hepatology 52: 313–326, 2010. doi: 10.1002/hep.23618. [DOI] [PubMed] [Google Scholar]
- 26.Gingras H, Cases O, Krasilnikova M, Bérubé G, Nepveu A. Biochemical characterization of the mammalian Cux2 protein. Gene 344: 273–285, 2005. doi: 10.1016/j.gene.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Halpern KB, Shenhav R, Massalha H, Toth B, Egozi A, Massasa EE, Medgalia C, David E, Giladi A, Moor AE, Porat Z, Amit I, Itzkovitz S. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat Biotechnol 36: 962–970, 2018. doi: 10.1038/nbt.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn T, Christoffersen P, Henriksen JH. Alcoholic liver injury: defenestration in noncirrhotic livers--a scanning electron microscopic study. Hepatology 7: 77–82, 1987. doi: 10.1002/hep.1840070117. [DOI] [PubMed] [Google Scholar]
- 29.Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, Terhardt D, Vogel MJ, Cao L, Korn C, Bartels S, Thomas M, Augustin HG. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science 343: 416–419, 2014. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 30.Johnson AR. Human pulmonary endothelial cells in culture. Activities of cells from arteries and cells from veins. J Clin Invest 65: 841–850, 1980. doi: 10.1172/JCI109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein D, Demory A, Peyre F, Kroll J, Augustin HG, Helfrich W, Kzhyshkowska J, Schledzewski K, Arnold B, Goerdt S. Wnt2 acts as a cell type-specific, autocrine growth factor in rat hepatic sinusoidal endothelial cells cross-stimulating the VEGF pathway. Hepatology 47: 1018–1031, 2008. doi: 10.1002/hep.22084. [DOI] [PubMed] [Google Scholar]
- 32.Krause P, Markus PM, Schwartz P, Unthan-Fechner K, Pestel S, Fandrey J, Probst I. Hepatocyte-supported serum-free culture of rat liver sinusoidal endothelial cells. J Hepatol 32: 718–726, 2000. doi: 10.1016/S0168-8278(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 33.Kugler P. Ultracytochemistry of aminopeptidase A (angiotensinase A) in the kidney glomerulus and juxtaglomerular apparatus. Histochemistry 74: 199–212, 1982. doi: 10.1007/BF00495830. [DOI] [PubMed] [Google Scholar]
- 34.Laz EV, Holloway MG, Chen CS, Waxman DJ. Characterization of three growth hormone-responsive transcription factors preferentially expressed in adult female liver. Endocrinology 148: 3327–3337, 2007. doi: 10.1210/en.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Couteur DG, Warren A, Cogger VC, Smedsrød B, Sørensen KK, De Cabo R, Fraser R, McCuskey RS. Old age and the hepatic sinusoid. Anat Rec (Hoboken) 291: 672–683, 2008. doi: 10.1002/ar.20661. [DOI] [PubMed] [Google Scholar]
- 36.Lee MY, Tse HF, Siu CW, Zhu SG, Man RY, Vanhoutte PM. Genomic changes in regenerated porcine coronary arterial endothelial cells. Arterioscler Thromb Vasc Biol 27: 2443–2449, 2007. doi: 10.1161/ATVBAHA.107.141705. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, Ramu S, Lee J, Hong YK. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood 113: 1856–1859, 2009. doi: 10.1182/blood-2008-03-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leibing T, Géraud C, Augustin I, Boutros M, Augustin HG, Okun JG, Langhans CD, Zierow J, Wohlfeil SA, Olsavszky V, Schledzewski K, Goerdt S, Koch PS. Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice. Hepatology 68: 707–722, 2018. doi: 10.1002/hep.29613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Wu Q, Wang J, Bucy RP, Cooper MD. Widespread tissue distribution of aminopeptidase A, an evolutionarily conserved ectoenzyme recognized by the BP-1 antibody. Tissue Antigens 42: 488–496, 1993. doi: 10.1111/j.1399-0039.1993.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 40.Lozach PY, Lortat-Jacob H, de Lacroix de Lavalette A, Staropoli I, Foung S, Amara A, Houles C, Fieschi F, Schwartz O, Virelizier JL, Arenzana-Seisdedos F, Altmeyer R. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J Biol Chem 278: 20358–20366, 2003. doi: 10.1074/jbc.M301284200. [DOI] [PubMed] [Google Scholar]
- 41.MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, Manuel J, Khuu N, Echeverri J, Linares I, Gupta R, Cheng ML, Liu LY, Camat D, Chung SW, Seliga RK, Shao Z, Lee E, Ogawa S, Ogawa M, Wilson MD, Fish JE, Selzner M, Ghanekar A, Grant D, Greig P, Sapisochin G, Selzner N, Winegarden N, Adeyi O, Keller G, Bader GD, McGilvray ID. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun 9: 4383, 2018. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malovic I, Sørensen KK, Elvevold KH, Nedredal GI, Paulsen S, Erofeev AV, Smedsrød BH, McCourt PA. The mannose receptor on murine liver sinusoidal endothelial cells is the main denatured collagen clearance receptor. Hepatology 45: 1454–1461, 2007. doi: 10.1002/hep.21639. [DOI] [PubMed] [Google Scholar]
- 43.March S, Hui EE, Underhill GH, Khetani S, Bhatia SN. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology 50: 920–928, 2009. doi: 10.1002/hep.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mentlein L, Thorlacius GE, Meneghel L, Aqrawi LA, Ramírez Sepúlveda JI, Grunewald J, Espinosa A, Wahren-Herlenius M. The rheumatic disease-associated FAM167A-BLK locus encodes DIORA-1, a novel disordered protein expressed highly in bronchial epithelium and alveolar macrophages. Clin Exp Immunol 193: 167–177, 2018. doi: 10.1111/cei.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minehata K, Kawahara A, Suzuki T. meis1 regulates the development of endothelial cells in zebrafish. Biochem Biophys Res Commun 374: 647–652, 2008. doi: 10.1016/j.bbrc.2008.07.075. [DOI] [PubMed] [Google Scholar]
- 46.Miyao M, Kotani H, Ishida T, Kawai C, Manabe S, Abiru H, Tamaki K. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Lab Invest 95: 1130–1144, 2015. doi: 10.1038/labinvest.2015.95. [DOI] [PubMed] [Google Scholar]
- 47.Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, Richardson CD, Kawate T, Kuno J, Weinstein BM, Stainier DY, Sato TN. Universal GFP reporter for the study of vascular development. Genesis 28: 75–81, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura Y, Nakanishi T, Shimada H, Shimizu J, Aotani R, Maruyama S, Higuchi K, Okura T, Deguchi Y, Tamai I. Prostaglandin transporter OATP2A1/SLCO2A1 is essential for body temperature regulation during fever. J Neurosci 38: 5584–5595, 2018. doi: 10.1523/JNEUROSCI.3276-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26: 204–219, 2013. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nonaka H, Sugano S, Miyajima A. Serial analysis of gene expression in sinusoidal endothelial cells from normal and injured mouse liver. Biochem Biophys Res Commun 324: 15–24, 2004. doi: 10.1016/j.bbrc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Ortega-Ribera M, Fernández-Iglesias A, Illa X, Moya A, Molina V, Maeso-Díaz R, Fondevila C, Peralta C, Bosch J, Villa R, Gracia-Sancho J. Resemblance of the human liver sinusoid in a fluidic device with biomedical and pharmaceutical applications. Biotechnol Bioeng 115: 2585–2594, 2018. doi: 10.1002/bit.26776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owsianka A, Tarr AW, Juttla VS, Lavillette D, Bartosch B, Cosset FL, Ball JK, Patel AH. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol 79: 11095–11104, 2005. doi: 10.1128/JVI.79.17.11095-11104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papaspyridonos M, Smith A, Burnand KG, Taylor P, Padayachee S, Suckling KE, James CH, Greaves DR, Patel L. Novel candidate genes in unstable areas of human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 26: 1837–1844, 2006. doi: 10.1161/01.ATV.0000229695.68416.76. [DOI] [PubMed] [Google Scholar]
- 54.Petropolis DB, Faust DM, Tolle M, Rivière L, Valentin T, Neuveut C, Hernandez-Cuevas N, Dufour A, Olivo-Marin JC, Guillen N. Human liver infection in a dish: easy-to-build 3D liver models for studying microbial infection. PLoS One 11: e0148667, 2016. doi: 10.1371/journal.pone.0148667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pöhlmann S, Soilleux EJ, Baribaud F, Leslie GJ, Morris LS, Trowsdale J, Lee B, Coleman N, Doms RW. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci USA 98: 2670–2675, 2001. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmid CD, Schledzewski K, Mogler C, Waldburger N, Kalna V, Marx A, Randi AM, Géraud C, Goerdt S, Koch PS. GPR182 is a novel marker for sinusoidal endothelial differentiation with distinct GPCR signaling activity in vitro. Biochem Biophys Res Commun 497: 32–38, 2018. doi: 10.1016/j.bbrc.2018.01.185. [DOI] [PubMed] [Google Scholar]
- 57.Scott CL, T’Jonck W, Martens L, Todorov H, Sichien D, Soen B, Bonnardel J, De Prijck S, Vandamme N, Cannoodt R, Saelens W, Vanneste B, Toussaint W, De Bleser P, Takahashi N, Vandenabeele P, Henri S, Pridans C, Hume DA, Lambrecht BN, De Baetselier P, Milling SWF, Van Ginderachter JA, Malissen B, Berx G, Beschin A, Saeys Y, Guilliams M. The transcription factor ZEB2 is required to maintain the tissue-specific identities of macrophages. Immunity 49: 312–325.e5, 2018. doi: 10.1016/j.immuni.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smedsrød B, Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol 38: 213–230, 1985. doi: 10.1002/jlb.38.2.213. [DOI] [PubMed] [Google Scholar]
- 59.Sørensen KK, McCourt P, Berg T, Crossley C, Le Couteur D, Wake K, Smedsrød B. The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol 303: R1217–R1230, 2012. doi: 10.1152/ajpregu.00686.2011. [DOI] [PubMed] [Google Scholar]
- 60.Sørensen KK, Simon-Santamaria J, McCuskey RS, Smedsrød B. Liver sinusoidal endothelial cells. Compr Physiol 5: 1751–1774, 2015. doi: 10.1002/cphy.c140078. [DOI] [PubMed] [Google Scholar]
- 61.Stradiot L, Verhulst S, Roosens T, Øie CI, Moya IM, Halder G, Mannaerts I, van Grunsven LA. Functionality based method for simultaneous isolation of rodent hepatic sinusoidal cells. Biomaterials 139: 91–101, 2017. doi: 10.1016/j.biomaterials.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 62.Topper JN, Cai J, Stavrakis G, Anderson KR, Woolf EA, Sampson BA, Schoen FJ, Falb D, Gimbrone MA Jr. Human prostaglandin transporter gene (hPGT) is regulated by fluid mechanical stimuli in cultured endothelial cells and expressed in vascular endothelium in vivo. Circulation 98: 2396–2403, 1998. doi: 10.1161/01.CIR.98.22.2396. [DOI] [PubMed] [Google Scholar]
- 63.Underhill GH, Khetani SR. Bioengineered liver models for drug testing and cell differentiation studies. Cell Mol Gastroenterol Hepatol 5: 426–439.e1, 2018. doi: 10.1016/j.jcmgh.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walter TJ, Cast AE, Huppert KA, Huppert SS. Epithelial VEGF signaling is required in the mouse liver for proper sinusoid endothelial cell identity and hepatocyte zonation in vivo. Am J Physiol Gastrointest Liver Physiol 306: G849–G862, 2014. doi: 10.1152/ajpgi.00426.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang LJ, Wu ZH, Zheng XT, Long JY, Dong YM, Fang X. Zinc finger E-box binding protein 2 (ZEB2) suppress apoptosis of vascular endothelial cells induced by high glucose through mitogen-activated protein kinases (MAPK) pathway activation. Med Sci Monit 23: 2590–2598, 2017. doi: 10.12659/MSM.904678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winnik S, Klinkert M, Kurz H, Zoeller C, Heinke J, Wu Y, Bode C, Patterson C, Moser M. HoxB5 induces endothelial sprouting in vitro and modifies intussusceptive angiogenesis in vivo involving angiopoietin-2. Cardiovasc Res 83: 558–565, 2009. doi: 10.1093/cvr/cvp133. [DOI] [PubMed] [Google Scholar]
- 67.Wu Y, Moser M, Bautch VL, Patterson C. HoxB5 is an upstream transcriptional switch for differentiation of the vascular endothelium from precursor cells. Mol Cell Biol 23: 5680–5691, 2003. doi: 10.1128/MCB.23.16.5680-5691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, Gaarde WA, Deleve LD. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 142: 918–927.e6, 2012. doi: 10.1053/j.gastro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells 14: 425–434, 2009. doi: 10.1111/j.1365-2443.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 70.Yang J, Lu Y, Yang P, Chen Q, Wang Y, Ding Q, Xu T, Li X, Li C, Huang C, Meng X, Li J, Zhang L, Wang X. MicroRNA-145 induces the senescence of activated hepatic stellate cells through the activation of p53 pathway by ZEB2. J Cell Physiol 234: 7587–7599, 2019. doi: 10.1002/jcp.27521. [DOI] [PubMed] [Google Scholar]
- 71.Zhang M, Yan X, Liu W, Sun R, Xie Y, Jin F. Endothelial semaphorin 7A promotes seawater aspiration-induced acute lung injury through plexin C1 and β1 integrin. Mol Med Rep 16: 4215–4221, 2017. doi: 10.3892/mmr.2017.7097. [DOI] [PubMed] [Google Scholar]