Keywords: active stem cells, intestinal epithelium, plasticity model, reserve stem cells
Abstract
The gastrointestinal system is arguably one of the most complicated developmental systems in a multicellular organism, as it carries out at least four major functions: digestion of food, absorption of nutrients, excretion of hormones, and defense against pathogens. Anatomically, the fetal gut has a tubular structure with an outer layer of smooth muscle derived from lateral splanchnic mesoderm and an inner lining of epithelium derived from the definitive endoderm. During morphogenesis of the gut tube, the definitive endoderm transforms into a primitive gut tube with a foregut, midgut, and hindgut. During the course of further development, the midgut gives rise to the small and proximal large intestine and the hindgut gives rise to the distal large intestine and rectum. The small intestine is subdivided into three parts: duodenum, jejunum, and ileum, whereas the large intestine is subdivided into the cecum, colon, and rectum.
CELLULAR ARCHITECTURE WITHIN THE INTESTINAL EPITHELIUM
To maximize available absorptive surface area, during the transformation of the midgut to small intestine, the endoderm evaginates into the lumen and forms finger-like protrusions called villi. At the bottom of these villi lies the crypt of Lieberkühn, named after the German anatomist Jonathan Nathanael Lieberkühn (1711–1756) (14). Unlike the small intestine, the colon has a flat surface with crypts only and no villi.
It has been implied that a small number of stem cells reside at crypt bottoms. Daughter cells exit the stem cell compartment and become transit-amplifying (TA) cells that include secretory progenitors and absorptive progenitors. The secretory progenitor cells further differentiate into mucus-secreting goblet cells, hormone-secreting enteroendocrine cells, and bactericidal Paneth cells, while the absorptive progenitors differentiate into enterocytes, which are columnar in shape, with a luminal brush border (14). These differentiated cells continue to move upward towards the tip of the villus. Upon reaching the villus tip after 3–5 days, the differentiated cells undergo apoptosis and are shed into the gut lumen, except for the Paneth cells, which migrate toward the crypt bottom, where they reside for 6–8 wk before undergoing apoptosis (21). In a recent work, single-cell RNA-seq (scRNA-seq) analysis revealed the identity and characteristics of several previously unknown subtypes of intestinal epithelial cells and their gene signatures (21). The intestinal epithelium has one of the highest turnover rates, with 1011 epithelial cells (~200 g) being lost every day in humans (33). This indicates an immense proliferative process driven by the stem cell compartment located in the crypt.
INTESTINAL STEM CELL POSITIONS: A LONG, HISTORIC DEBATE
The concept of a common embryological origin was proposed by Joseph Paneth (1857–1890). It was further supported by Bizzozero in 1893, who noticed that mitosis occurs only in the crypts and thus concluded that the daughter cells must come out of the crypts to eventually give rise to the surface epithelium (4, 14). This controversial idea was further validated by injection of radioactive forms of the DNA precursors adenine and thymidine, followed by autoradiography (32, 44, 56). This approach provided a hypothesis that there was a pool of cells residing at the bottom of the crypt that fueled the proliferation and self-renewal. Almost 20 years later, Cheng and Leblond (13) provided further evidence to back up the hypothesis. Cheng and Leblond labeled phagosome-formed slender cells interspersed between Paneth cells by [3H]thymidine and then monitored them with electron microscopy. The cells with H3+-phagosome eventually ended up on different types of differentiated cells in the villi. This primitive tracing experiment formed the basis of the concept of common origin of differentiated cells.
Another idea emerged in the field in the 1980s known as the +4 stem cell model proposed by Potten and colleagues (40, 41). They reported that a certain group of cells at the +4 position retained tritiated thymidine as a label. Label-retaining cell (LRC) assays had been used in other studies as a means to identify mitotic quiescent cells, with the limitation that truly nondividing cells are unable to incorporate the label because the label can be incorporated only during S phase (62). Potten noted in his work that these +4 cells were cycling on a daily basis, and he reasoned that the label retention was not a consequence of lack of cell division but rather asymmetric segregation of the nascent and parental genomes during mitosis (41, 42). Before this, a concept of “immortal strand” had been suggested, which proposed that retaining the parental genome could be a protection mechanism against replication-induced mutation (10). However, it should be noted that later works showed evidence against this “immortal strand” theory (9, 19, 29, 47, 50).
In 1988, Winton published a work in which he used mutagen ethyl nitrosourea-based random mutagenesis to study the clonal organization of adult intestinal epithelium (58). This was the first visualization of clonal migration of cells from crypt bottom to the villi. Using a similar strategy, Bjerknes and Cheng (5, 7) showed that there were two kinds of marked clones: long lived and short lived. The long-lived clones were made up of different cell lineages but would invariably always incorporate crypt base columnar (CBC) cells. Bjerknes and Cheng proposed the stem cell zone hypothesis, arguing that CBCs and Paneth cells were located at the bottom of the crypt in a specific zone/niche that permitted the stem cell activity. Furthermore, daughter cells would leave this zone by reaching position 5, the common origin of differentiation. At this position, the daughter cells would start to commit to different lineages and migrate upward. Paneth cells, however, would migrate downward to the base of the crypt. This idea, though controversial, gained significant popularity and was eventually followed by many different studies on a quest to acquire a complete understanding.
ACTIVELY DIVIDING STEM CELLS
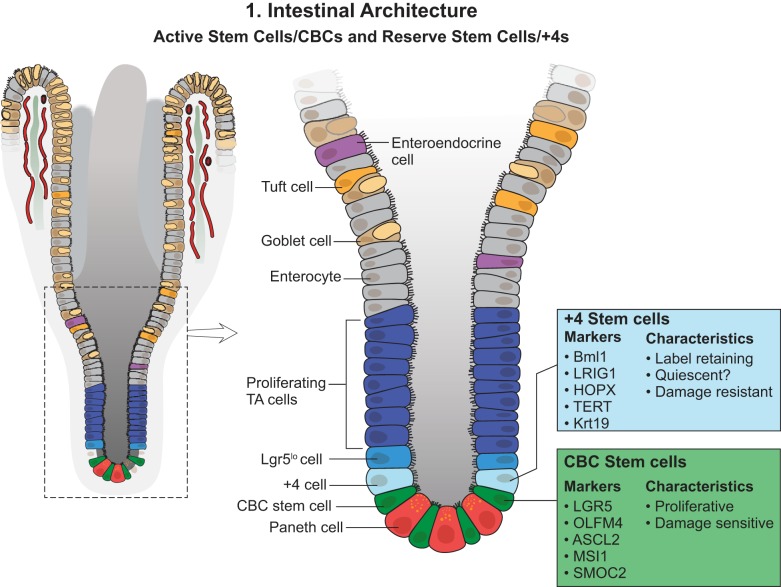
The Wnt signaling pathway is known to play a major role in the activity of stem cells in the crypt (9). This knowledge served as the basis for a microarray study on a human colorectal adenocarcinoma cell line, which facilitated the identification of ~80 Wnt target genes (9). Barker et al. (2) subjected the gene list to expression analysis using in situ hybridization and revealed that leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) was specifically expressed in CBCs but did not overlap with Paneth cells or TA cells. Using a Lgr5-CreER-driven lineage-tracing experiment, they further demonstrated that Lgr5+ CBCs gave rise to all epithelial lineages over a 60-day period. Further characterization revealed that there are ~15 Lgr5+ CBCs located at the bottom of every crypt between Paneth cells, and these CBCs divide every day. Lgr5 is a calcitonin gene-related peptide (GCRP) that acts as a receptor for a small family of Wnt pathway agonists called R-spondins (11). Subsequent work has shown that FACS-sorted Lgr5+ cells are capable of producing intestinal organoids in the in vitro culture system under specific culture conditions (1). Paneth cells have been shown to be necessary to the in vitro organoid formation by providing Wnt, epidermal growth factor, and Notch signaling (46). Wnt3a can replace Paneth cells to support growth of single intestinal stem cells (ISCs) into organoids, but only to a very limited extent; adding GSK3 inhibitor can improve the successful rate of organoid formation from single ISCs (57). Transcriptional and proteomics analyses have identified a gene signature for Lgr5+ CBCs. These include Ascl2, Olfm4, and Tnfrsf19, etc., and some remain to be studied (e.g., Aqp4, Cdca7, Cdk6, Clca4, Kcnq1, Nav1, Smoc2, and Soat1) (38) (Fig. 1). In this context, it is important to note that genetic ablation of Lgr5+ cells with diphtheria toxin showed that they are dispensable for intestinal homeostasis (53). This was further validated by Lgr5+ depletion by high-dose gamma irradiation (52, 60). In both cases, a subsequent regeneration event concomitant with reappearance of Lgr5+ cells suggested the existence of additional cells that are capable of compensating for CBC loss.
Fig. 1.
Small intestinal structure. The small intestine consists of monolayer epithelial cells covering invaginated gland structures (crypts) and finger-like protrusions (villi). Intestinal stem cells (ISCs) reside in the bottom of the crypts, composed of crypt base columnar cells (CBCs) (active stem cells) and +4 stem cells (reserve stem cells), which could be distinguished by their certain markers but mainly are based on cell cycle status and functional roles. Absorptive and secretory progenitor cells are located above the ISC zone, and more differentiated cells are at the villi. TA, transit amplifying; Lgr5, leucine-rich repeat-containing G protein-coupled receptor 5; BMI1, polycomb group RING finger protein-4; LRIG1, leucine-rich repeats and immunoglobulin-like domains protein-1; HOPX, homeodomain-only protein homeobox; TERT, telomerase reverse transcriptase; Krt19, keratin, type I cytoskeletal 19; OLFM4, olfactomedin 4; ASCL2, achaete-scute complex homolog 2; MSI1, Musashi RNA-binding protein-1; SMOC2, SPARC-related modular calcium-binding protein-2.
RESERVE STEM CELLS
Speculation around the concept of a reserve (or back-up) intestinal stem cell (rISC) population capable of rescuing CBC loss in the intestine has led to many different studies over the years (36, 49). A number of markers for rISCs have been investigated during the past decade (Fig. 1). Bmi1 (polycomb group RING finger protein-4), a gene that is a part of the polycomb group gene family, was shown to be involved in the self-renewal of neuronal, hematopoietic, and leukemic cells (34, 35). An investigation based on this knowledge led to the finding that Bmi1 was expressed in rare cells at the +4 cell position in the proximal small intestine (45). They found that in vivo lineage tracing utilizing a Bmi1-ires-CreER mouse yielded differentiated cell lineages under homeostatic conditions that resembled lineage tracing from Lgr5+ cells. Moreover, diphtheria toxin-mediated ablation of Lgr5+ cells did not perturb intestinal homeostasis, whereas Bmi1+ cell ablation led to crypt loss, which further supported the notion that mammals might use more than one molecularly distinguishable adult stem cell subpopulation to maintain organ homeostasis and regeneration under stress. It was suggested that the Bmi1+ population is distinct from the Lgr5+ population in being radiation resistant and quiescent and capable of regeneration upon damage-induced CBC loss (52, 60). However, there has been some controversy about the specificity of Bmi1 expression, stemming from the observation that Bmi1 mRNA was also expressed in Lgr5+ stem cells. Both staining for Bmi1 protein and single mRNA molecule FISH approaches indicated that Bmi1 was broadly expressed at roughly equal levels by all proliferative crypt cells, including the Lgr5+ CBC cells (26, 38).
Hopx (homeodomain-only protein homeobox) encodes an atypical homeodomain-containing protein (HOP) that has previously been studied in the heart and neural stem cells (12, 16). Using Hopx-lacZ reporter mice, Takeda et al. (51) noted that Hopx was expressed throughout the entire intestine, with strongest expression in the +4 region. Moreover, lineage tracing experiments revealed that Hopx+ cells were found to be relatively quiescent and radiation resistant and to rapidly proliferate in response to irradiation-induced injury. Comparative expression profiling and ex vivo organoid culture supported the idea that Hopx+ cells give rise to CBCs and all mature intestinal epithelial lineages. Conversely, CBCs can give rise to Hopx+ cells. Based on these findings, a model was suggested in which proliferating Lgr5+ stem cells and quiescent Hopx+ stem cells are located at distinct locations within the crypt and efficiently interconvert during epithelial homeostasis or under stress. However, it should be noted that several other studies have indicated that Hopx is expressed rather broadly, including in Lgr5+ stem cells (37, 38).
Telomerase is a ribonucleoprotein complex that catalyzes the extension of chromosome ends, and its expression is associated with cells avoiding cellular senescence (66). Stimulated by the idea that such resistance to senescence is a characteristic of stem cells, Breault et al. (8) conducted an investigation and reported the occurrence of mouse telomerase reverse transcriptase (mTert)-GFP+ cells in 1 per 150 crypts. A fraction of these mTert-GFP+ cells were found to be LRCs. A subsequent study from the same group showed that mTert expression marks a radiation-resistant pool of stem cells distinct from Lgr5+ cells (8). Lineage-tracing studies demonstrated that mTert+ cells give rise to all differentiated intestinal cell types, persist long term, and contribute to the regenerative response following injury. Similar to the previously mentioned reserve stem cell markers, mTert was also found to be expressed in Lgr5+ stem cells (38, 58).
To identify and characterize colonic SC markers with known functions, Powell et al. (43) performed gene expression profiling of CD24-purified mouse colonic epithelial progenitor cells and identified the leucine-rich repeats and immunoglobulin-like domains 1 (Lrig1) gene. The Lrig1 gene encodes a single-pass transmembrane receptor that functions as a conditional inhibitor of ERB proteins in several adult tissues (31). Powell et al. used Lrig1-CreERT2 reporter mice and showed that Lrig1+ cells are predominately noncycling, long-lived stem cells that are located at the crypt base and that, upon injury, proliferate and divide to replenish damaged crypts. Transcriptome profiling showed Lrig1+ stem cells were significantly different from Lgr5+ stem cells; genes upregulated in the Lrig1+ population included those involved in cell cycle repression and oxidative damage response, among others. In a separate study, Jensen et al. (59) reported that approximately one-third of all small intestinal crypt cells express Lrig1, with the highest levels in Lgr5+ stem cells.
It is somewhat perplexing that all of the +4 markers described above are expressed rather broadly and are most abundant in the Lgr5 stem cells, leading to inconclusive interpretation (38, 57). One possible explanation could be that disrupted native 3′/5′-untranslated region (UTR) sequence in transgenic reporter mouse might affect transcription in a way that influences the expression of the gene of interest and thereby inaccurately reflects the endogenous expression. In fact, a recent work confirmed this discordant expression pattern using several different transgenic mice, such as Lgr5-EGFP, Lgr5-CreERT2, Bmi1-EGFP, Bmi1-CreERT2, Hopx-EGFP, and Hopx-CreERT2 (37). In addition to that, it was reported that there was significant discrepancy between the molecular profile of the different +4 populations FACS sorted based on the different putative +4 markers. These observations clearly underlie the importance of mindful interpretation of molecular profiles taken from bulk populations, as population heterogeneity might complicate the picture. Finally, one should be careful of antibody specificity as well, which has been shown to give rise to conflicting observations in the past by cross-reacting with different epitopes (6).
THE PLASTICITY MODEL: INTESTINAL REGENERATION WITHOUT ISCs
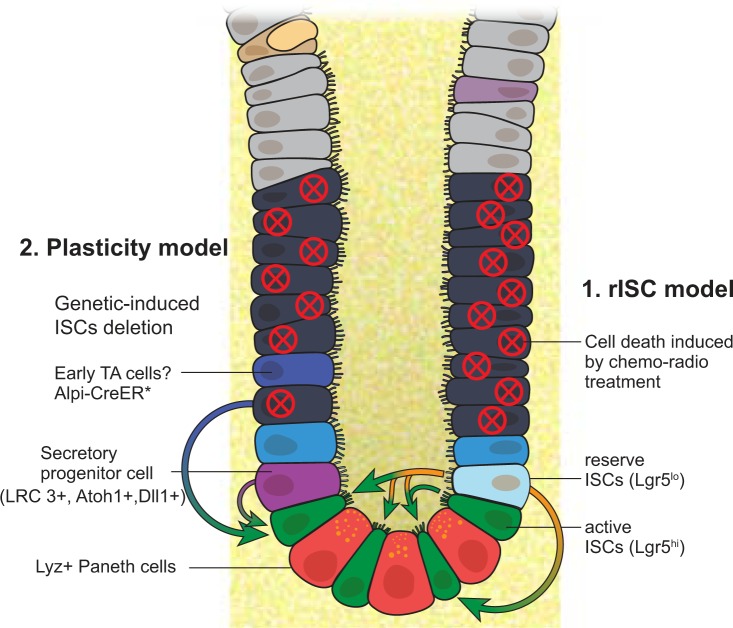
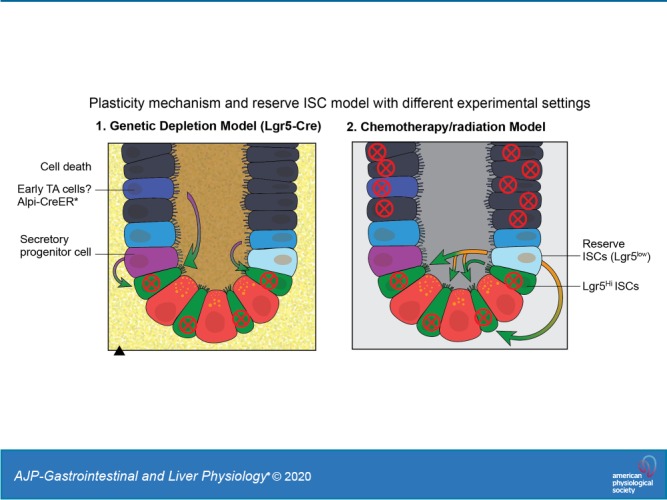
A large body of work shows that, when ISCs are depleted, such as from using Lgr5-DTR (Diphtheria Toxin Receptor) system or severe damage from using dextran sulfate sodium (DSS), a variety of intestinal progenitor cells, and even more differentiated cells can reprogram back to ISC-like status and are responsible for regeneration (Fig. 2). Transcriptomic profiling analyses identified that Prox1+ cells, which have common features between ISCs and enteroendocrine cells, contribute to crypt homeostasis and regeneration, suggesting that plasticity of the differentiated secretory lineage supports epithelial reconstitution following ISC loss (17, 18, 22, 39, 61). Atoh1 (protein atonal homolog 1) is a master transcription factor for secretory lineage determination. Recent studies have shown that Atoh1+ cell could exhibit its stem cell properties as evidenced by the detection of Atoh1+ cell-derived ribbons in both homeostatic and pathological conditions (25). Moreover, inhibition of phosphorylation of Atoh1 could promote cells differentiated into EE cells and prevent progenitors from gaining self-renewal ability (54). Another study has elucidated that Dll1+ crypt cells are short-lived secretory progenitors that express the EE marker neurogenin 3 (Neurog3) and regain stemness and establish long-lived lineage traces upon radiation (55). And the mechanism is that these secretory progenitors have adopted a unique epigenetic signature and chromatin accessibility that lead to reprograming into ISCs (27). It also has been demonstrated that other differentiated intestinal epithelial cells could acquire stem cell properties under normal or injury condition. For example, Paneth cells could participate in epithelial replenishment following stem cell loss (48, 63). It has been well demonstrated that various signaling pathways derived from niche cells are essential for regulation of intestinal homeostasis and regeneration, which further promote plasticity of intestine. All these observations seem against the idea of the existence of reserve ISCs. While the functional robustness and contribution of these lineage-committed cells to intestinal homeostasis and restoration upon tissue damage remain unclear, it will be worth future investigation; however, the disagreement whether reserve stem cells exist may also arise from a presumption that Lgr5 marks a homogenous cycling stem cell population. In reality, it was reported that Lgr5lo cells with expression of Mex3a represent slow-cycling ISC population that can survive the stress and support crypt regeneration including giving rise to Lgr5hi ISCs (3). However, in some studies using the Lgr5-DTR system, the Lgr5lo ISCs mostly likely were also eliminated.
Fig. 2.
Reserve intestinal stem cell (rISC) model vs. plasticity mechanism. Exposure to irradiation or chemotherapeutic agents eliminates actively proliferating cells, including crypt base columnar cells (CBCs) and transit-amplifying (TA) cells. In response to DNA damage, rISCs (Lgr5lo) enter the cell cycle to replenish active ISCs (Lgr5hi) and epithelial cells. In addition, some reports demonstrate that Lgr5lo cells above the crypt, potentially derived from the Lgr5hi CBCs, can resist DNA damage-induced cell death and contribute to regeneration. Furthermore, in genetic-induced ISCs, depletion settings in which ISCs, including both Lgr5hi and Lgr5lo subsets, are eliminated with diphtheria toxin (*), progenitors of either enterocyte or secretory lineages, and even transit-amplifying cells can reprogram to ISCs and support regeneration. This is termed the plasticity model. Lgr5, leucine-rich repeat-containing G protein-coupled receptor 5; LRC, label-retaining cell; Alpi-CreER, alkaline phosphatase, intestinal-Cre recombinase; Atoh1, protein atonal homolog 1; Dil1, dynein intermediate light chain 1.
In other stem cell systems, including hematopoietic and hair follicle stem cells, the distinction between quiescent and active stem cells is based on cycle state and metabolic activity. Both quiescent and active stem cells coexpress multiple stem cell markers, such as c-Kit and Sca-1 in hematopoietic stem cells and CD34 in hair follicle stem cells (20, 24). A recent study revealed that the difference between reserve and primed HSCs is based on the level of CD49b expression, with the latter expression higher and the former lower (64). In line with this point, evidence has emerged showing that there are Lgr5hi and Lgr5lo subsets of ISCs, in which the former enriches cycling ISCs and the latter enriches slow-cycling ISCs (3). As described earlier, the intestinal crypts are thought to harbor two principal pools of stem cells: actively cycling CBCs marked by Lgr5 and slowly cycling cells located around the +4 position. Unlike hematopoietic and hair follicle stem cells, in which quiescent stem cells give rise to active stem cells with a so-called hierarchical model (24, 64), Lgr5hi ISCs normally give rise to the Lgr5lo ISCs under homeostasis (30), but Lgr5lo ISCs conversely can give rise to Lgr5hi ISCs under stress, a mutual conversion relationship (51, 53, 60). This might be due to the small intestine being the fastest turnover tissue in the body. Lgr5lo cells sitting at the +4 position, maintained as slow-cycling cells being exposed to Bmp4 signaling from the adjacent stromal cell niche (23), can monitor whether or not loss of active ISCs occurs at the crypt bottom.
CONCLUSIONS AND PERSPECTIVE
Recent literature seems to question whether rISCs exist, given that there is a robust plasticity mechanism in response to loss of ISCs. However, keeping in mind that “reserve” ISCs is a functional definition; the existence of a plasticity mechanism does not have to exclude the existence of rISCs, especially the Lgr5lo subset of ISCs as discussed above. From an evolutionary viewpoint, the rISC mechanism has the advantage of long-term sustainability as the genome of ISC has less accumulated damage. The plasticity mechanism can compensate severe tissue damage, including loss of both active and slow-cycling stem cell pools, but at the expense of genomic integrity, and thus is not optimal for long-term sustainability. The existence of plasticity also suggests that the niche might be a crucial factor affecting this dynamic behavior. Following injury, once the stem cell pool is lost, the change in the microenvironment, particularly inflammation cells with the requisite signals, stimulates reversion of progenitors or even differentiated cells to ISCs, thereby initiating a regeneration event.
GRANTS
This work was supported by Stowers Institute for Medical Research and the Intestinal Stem Cell Consortium (U01 DK-085507 to L. Li.), a collaborative research project funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
L. Li and several laboratory members are inventors on the following patents/patent applications: “Methods, kits and compositions for stem cell self-renewal,” “PTEN/Akt methods and compositions relating to BMP,” “Methods for treating chemoresistant cancer-initiating cells,” “Methods and systems useful in culturing hematopoietic stem cells,” and “Methods of identifying stem cells in normal and cancerous tissues and related progeny.” The patents and patent applications are all assigned to the Stowers Institute for Medical Research. The inventors are entitled to share in any proceeds generated by the intellectual property. Currently, none of the above is actively licensed. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
S.K., L.D., and X.C.H. prepared figures; S.K., L.D., X.C.H., and L.L. drafted manuscript; S.K., L.D., X.C.H., and L.L. edited and revised manuscript; S.K. and L.L. approved final version of manuscript.
REFERENCES
- 1.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611, 2009. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 2.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 3.Barriga FM, Montagni E, Mana M, Mendez-Lago M, Hernando-Momblona X, Sevillano M, Guillaumet-Adkins A, Rodriguez-Esteban G, Buczacki SJA, Gut M, Heyn H, Winton DJ, Yilmaz OH, Attolini CS, Gut I, Batlle E. Mex3a marks a slowly dividing subpopulation of Lgr5+ intestinal stem cells. Cell Stem Cell 20: 801–816.e7, 2017. doi: 10.1016/j.stem.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bizzozero G. Ueber die schlauchförmigen Drüsen des Magendarmkanals und die Beziehungen ihres Epithels zu dem Oberflächenepithel der Schleimhaut Dritte Mittheilung. Arch mikrosk Anat 42: 82–152, 1893. doi: 10.1007/BF02975307. [DOI] [Google Scholar]
- 5.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116: 7–14, 1999. doi: 10.1016/S0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 6.Bjerknes M, Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 289: G381–G387, 2005. doi: 10.1152/ajpgi.00160.2005. [DOI] [PubMed] [Google Scholar]
- 7.Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol 283: G767–G777, 2002. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 8.Breault DT, Min IM, Carlone DL, Farilla LG, Ambruzs DM, Henderson DE, Algra S, Montgomery RK, Wagers AJ, Hole N. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci USA 105: 10420–10425, 2008. doi: 10.1073/pnas.0804800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495: 65–69, 2013. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 10.Cairns J. Mutation selection and the natural history of cancer. Nature 255: 197–200, 1975. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 11.Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci USA 108: 11452–11457, 2011. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, Nazarian R, Schnepp R, Jen K, Biben C, Runke G, Mackay JP, Novotny J, Schwartz RJ, Harvey RP, Mullins MC, Epstein JA. Hop is an unusual homeobox gene that modulates cardiac development. Cell 110: 713–723, 2002. doi: 10.1016/S0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- 13.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141: 537–561, 1974. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 14.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell 154: 274–284, 2013. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 16.De Toni A, Zbinden M, Epstein JA, Ruiz i Altaba A, Prochiantz A, Caillé I. Regulation of survival in adult hippocampal and glioblastoma stem cell lineages by the homeodomain-only protein HOP. Neural Dev 3: 13, 2008. doi: 10.1186/1749-8104-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer E-M, Olsen J, Sundler F, Christensen JP, Wierup N, Olsen JV, Holst JJ, Zigman JM, Poulsen SS, Schwartz TW. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153: 5782–5795, 2012. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelstoft MS, Egerod KL, Lund ML, Schwartz TW. Enteroendocrine cell types revisited. Curr Opin Pharmacol 13: 912–921, 2013. doi: 10.1016/j.coph.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Escobar M, Nicolas P, Sangar F, Laurent-Chabalier S, Clair P, Joubert D, Jay P, Legraverend C. Intestinal epithelial stem cells do not protect their genome by asymmetric chromosome segregation. Nat Commun 2: 258, 2011. doi: 10.1038/ncomms1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming WH, Alpern EJ, Uchida N, Ikuta K, Spangrude GJ, Weissman IL. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J Cell Biol 122: 897–902, 1993. doi: 10.1083/jcb.122.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grün D, Lyubimova A, Kester L, Wiebrands K, Basak O, Sasaki N, Clevers H, van Oudenaarden A. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 525: 251–255, 2015. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- 22.Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CA, Parker HE, Morley TC, Yeo GS, Reimann F, Gribble FM. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153: 3054–3065, 2012. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He XC, Zhang J, Tong W-G, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signaling. Nat Genet 36: 1117–1121, 2004. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 24.Hsu Y-C, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144: 92–105, 2011. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishibashi F, Shimizu H, Nakata T, Fujii S, Suzuki K, Kawamoto A, Anzai S, Kuno R, Nagata S, Ito G, Murano T, Mizutani T, Oshima S, Tsuchiya K, Nakamura T, Watanabe M, Okamoto R. Contribution of ATOH1+ cells to the homeostasis, repair, and tumorigenesis of the colonic epithelium. Stem Cell Reports 10: 27–42, 2018. doi: 10.1016/j.stemcr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, van Oudenaarden A. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol 14: 106–114, 2012. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadhav U, Saxena M, O’Neill NK, Saadatpour A, Yuan G-C, Herbert Z, Murata K, Shivdasani RA. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell 21: 65–77.e5, 2017. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature 449: 238–242, 2007. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim T-H, Saadatpour A, Guo G, Saxena M, Cavazza A, Desai N, Jadhav U, Jiang L, Rivera MN, Orkin SH, Yuan GC, Shivdasani RA. Single-cell transcript profiles reveal multilineage priming in early progenitors derived from Lgr5+ intestinal stem cells. Cell Reports 16: 2053–2060, 2016. doi: 10.1016/j.celrep.2016.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laederich MB, Funes-Duran M, Yen L, Ingalla E, Wu X, Carraway KL III, Sweeney C. The leucine-rich repeat protein LRIG1 is a negative regulator of ErbB family receptor tyrosine kinases. J Biol Chem 279: 47050–47056, 2004. doi: 10.1074/jbc.M409703200. [DOI] [PubMed] [Google Scholar]
- 32.Leblond CP, Messier B. Renewal of chief cells and goblet cells in the small intestine as shown by radioautography after injection of thymidine-H3 into mice. Anat Rec 132: 247–259, 1958. doi: 10.1002/ar.1091320303. [DOI] [PubMed] [Google Scholar]
- 33.Leblond CP, Walker BE. Renewal of cell populations. Physiol Rev 36: 255–276, 1956. doi: 10.1152/physrev.1956.36.2.255. [DOI] [PubMed] [Google Scholar]
- 34.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423: 255–260, 2003. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 35.Leung C, Lingbeek M, Shakhova O, Liu J, Tanger E, Saremaslani P, Van Lohuizen M, Marino S. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 428: 337–341, 2004. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 327: 542–545, 2010. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Yousefi M, Nakauka-Ddamba A, Jain R, Tobias J, Epstein JA, Jensen ST, Lengner CJ. Single-cell analysis of proxy reporter allele-marked epithelial cells establishes intestinal stem cell hierarchy. Stem Cell Reports 3: 876–891, 2014. doi: 10.1016/j.stemcr.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJ, Clevers H. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4′ cell markers. EMBO J 31: 3079–3091, 2012. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrova TV, Nykänen A, Norrmén C, Ivanov KI, Andersson LC, Haglund C, Puolakkainen P, Wempe F, von Melchner H, Gradwohl G, Vanharanta S, Aaltonen LA, Saharinen J, Gentile M, Clarke A, Taipale J, Oliver G, Alitalo K. Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 13: 407–419, 2008. doi: 10.1016/j.ccr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Potten CS, Al-Barwari SE, Searle J. Differential radiation response amongst proliferating epithelial cells. Cell Tissue Kinet 11: 149–160, 1978. doi: 10.1111/j.1365-2184.1978.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 41.Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell 15: 899–906, 1978. doi: 10.1016/0092-8674(78)90274-X. [DOI] [PubMed] [Google Scholar]
- 42.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115: 2381–2388, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149: 146–158, 2012. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quastler H, Sherman FG. Cell population kinetics in the intestinal epithelium of the mouse. Exp Cell Res 17: 420–438, 1959. doi: 10.1016/0014-4827(59)90063-1. [DOI] [PubMed] [Google Scholar]
- 45.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920, 2008. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J 30: 1104–1109, 2011. doi: 10.1038/emboj.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitt M, Schewe M, Sacchetti A, Feijtel D, van de Geer WS, Teeuwssen M, Sleddens HF, Joosten R, van Royen ME, van de Werken HJG, van Es J, Clevers H, Fodde R. Paneth cells respond to inflammation and contribute to tissue regeneration by acquiring stem-like features through SCF/c-Kit signaling. Cell Reports 24: 2312–2328.e7, 2018. doi: 10.1016/j.celrep.2018.07.085. [DOI] [PubMed] [Google Scholar]
- 49.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology 134: 849–864, 2008. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 50.Steinhauser ML, Bailey AP, Senyo SE, Guillermier C, Perlstein TS, Gould AP, Lee RT, Lechene CP. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature 481: 516–519, 2012. doi: 10.1038/nature10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science 334: 1420–1424, 2011. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao S, Tang D, Morita Y, Sperka T, Omrani O, Lechel A, Sakk V, Kraus J, Kestler HA, Kühl M, Rudolph KL. Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. EMBO J 34: 624–640, 2015. doi: 10.15252/embj.201490700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011. [Erratum in Nature 482: 120, 2012.] doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomic G, Morrissey E, Kozar S, Ben-Moshe S, Hoyle A, Azzarelli R, Kemp R, Chilamakuri CSR, Itzkovitz S, Philpott A, Winton DJ. Phospho-regulation of ATOH1 is required for plasticity of secretory progenitors and tissue regeneration. Cell Stem Cell 23: 436–443.e7, 2018. doi: 10.1016/j.stem.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens ACM, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14: 1099–1104, 2012. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker BE, Leblond CP. Sites of nucleic acid synthesis in the mouse visualized by radioautography after administration of C14-labelled adenine and thymidine. Exp Cell Res 14: 510–531, 1958. doi: 10.1016/0014-4827(58)90158-7. [DOI] [PubMed] [Google Scholar]
- 57.Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, Smith NR, Lei NY, Davies PS, Fuller MK, Haug JS, McClain M, Gracz AD, Ding S, Stelzner M, Dunn JC, Magness ST, Wong MH, Martin MG, Helmrath M, Li L. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 145: 383–395.e21, 2013. doi: 10.1053/j.gastro.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winton DJ, Blount MA, Ponder BA. A clonal marker induced by mutation in mouse intestinal epithelium. Nature 333: 463–466, 1988. doi: 10.1038/333463a0. [DOI] [PubMed] [Google Scholar]
- 59.Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, Watt FM, Winton DJ, Clevers H, Jensen KB. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol 14: 401–408, 2012. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109: 466–471, 2012. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, Roelf K, Calderon RI, Cynn E, Hu X, Mandleywala K, Wilhelmy J, Grimes SM, Corney DC, Boutet SC, Terry JM, Belgrader P, Ziraldo SB, Mikkelsen TS, Wang F, von Furstenberg RJ, Smith NR, Chandrakesan P, May R, Chrissy MAS, Jain R, Cartwright CA, Niland JC, Hong YK, Carrington J, Breault DT, Epstein J, Houchen CW, Lynch JP, Martin MG, Plevritis SK, Curtis C, Ji HP, Li L, Henning SJ, Wong MH, Kuo CJ. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 21: 78–90.e6, 2017. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yousefi M, Li L, Lengner CJ. Hierarchy and plasticity in the intestinal stem cell compartment. Trends Cell Biol 27: 753–764, 2017. doi: 10.1016/j.tcb.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu S, Tong K, Zhao Y, Balasubramanian I, Yap GS, Ferraris RP, Bonder EM, Verzi MP, Gao N. Paneth cell multipotency induced by notch activation following injury. Cell Stem Cell 23: 46–59.e5, 2018. doi: 10.1016/j.stem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao M, Tao F, Venkatraman A, Li Z, Smith SE, Unruh J, Chen S, Ward C, Qian P, Perry JM, Marshall H, Wang J, He XC, Li L. N-cadherin-expressing bone and marrow stromal progenitor cells maintain reserve hematopoietic stem cells. Cell Reports 26: 652–669.e6, 2019. doi: 10.1016/j.celrep.2018.12.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zvereva MI, Shcherbakova DM, Dontsova OA. Telomerase: structure, functions, and activity regulation. Biochemistry (Mosc) 75: 1563–1583, 2010. doi: 10.1134/S0006297910130055. [DOI] [PubMed] [Google Scholar]