Keywords: barrier, cytoskeleton, microRNA, translocation, tubulin
Abstract
Anemia is a frequent diagnosis in critically ill infants, but the clinical implications of severe anemia in these patients remain unclear. In this study, we examined preweaned mice to investigate the effects of severe anemia during early infancy on gut mucosal permeability. C57BL/6 mice were subjected to timed phlebotomy between postnatal days (P) 2–10 to induce severe anemia (hematocrits 20%–24%), and intestinal permeability was tracked longitudinally between P10 and P20 as intestine-to-plasma translocation of enteral macromolecules and bacterial translocation. Epithelial junctions were evaluated by electron microscopy, polymerase chain reactions, immunohistochemistry, and/or enzyme immunoassays on intestinal tissues, Caco-2 intestinal epithelial-like cells, and colonic organoids. Preweaned mouse pups showed an age-related susceptibility to severe anemia, with increased intestinal permeability to enteral macromolecules (dextran, ovalbumin, β-lactoglobulin) and luminal bacteria. Electron micrographs showed increased paracellular permeability and ultrastructural abnormalities of the adherens junctions. These findings were explained by the loss of E-cadherin in epithelial cells, which was caused by destabilization of the E-cadherin (Cdh1) mRNA because of microRNA let-7e-5p binding to the 3′-untranslated region. Severe anemia resulted in a disproportionate and persistent increase in intestinal permeability in preweaned mice because of the disruption of epithelial adherens junctions. These changes are mediated via microRNA let-7e-mediated depletion of Cdh1 mRNA.
NEW & NOTEWORTHY This research article shows that newborn infants with severe anemia show an age-related susceptibility to developing increased intestinal permeability to ingested macromolecules. This abnormal permeability develops because of abnormalities in intestinal epithelial junctions caused by a deficiency of the molecule E-cadherin in epithelial cells. The deficiency of E-cadherin is caused by destabilization of its mRNA precursor because of increased expression and binding of another molecule, the microRNA let-7e-5p, to the E-cadherin mRNA.
INTRODUCTION
Anemia is a frequently encountered diagnosis in premature and critically ill infants, who experience a rapid decline in hemoglobin levels within a few weeks after birth (6, 9). In these infants, severe anemia is also associated with increased risk of childhood morbidity and mortality (2, 33). The pathogenesis of anemia in these patients is complex and involves diverse factors, such as postnatal hypoactivity of the bone marrow, insufficient erythropoietin levels, phlebotomy losses, increased red blood cell (RBC) turnover, rapid somatic growth, and nutritional issues such as iron deficiency (2, 36). There is increasing recognition of the high frequency of RBC transfusions to correct anemia in premature and critically ill infants (5), but unfortunately, with most transfusions being administered to maintain hemoglobin/hematocrit levels above predetermined thresholds and not to replace actual blood loss, the transfusion practices continue to show a high level of inconsistency across centers. Practice improvement efforts with stringent transfusion guidelines (7, 16) can reduce the frequency of transfusions in these patients (37), but then these transfusion thresholds are not adequately supported in many situations by safety data and may even be too restrictive in some instances. Concerns remain that severe anemia during critical developmental epochs may cause harm (3, 28) and may place these infants at enhanced risk of altered neurodevelopment and necrotizing enterocolitis (NEC) (28, 36).
We have recently described a preclinical model of anemia and RBC transfusion-related NEC (26), in which severe anemia was associated with increased intestinal permeability. The present study was designed to investigate the effects and mechanisms by which anemia altered gut barrier function. C57BL/6 mouse pups were rendered anemic by timed phlebotomy between postnatal days (P) 2–10, and intestinal permeability was then tracked longitudinally between P10 and P20. Severe anemia was associated with increased intestinal permeability to dietary macromolecules and luminal bacteria. These changes occurred because of the disruption of the epithelial adherens junctions, which, in turn, was caused by microRNA let-7e-mediated depletion of E-cadherin.
MATERIALS AND METHODS
Animal model.
Animal studies were approved by the Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. C57BL/6 mice of both sexes (equal numbers) were subjected to facial vein phlebotomy to remove 20 μL blood/g body wt on P2, P4, P6, P8, and P10, and an equivalent amount of normal saline was administered subcutaneously. The definition of severe anemia with a hematocrit goal of ≤24% was based on levels utilized in the transfusion-restricted groups in the Premature Infants in Need of Transfusion (PINT) and Iowa trials (23, 24) and also based on consideration of normal postnatal changes in hematocrits in mice. Similar to human infants, mice were born relatively polycythemic and developed anemia during the postnatal period (8). Hematocrits, RBC indices, and reticulocyte hemoglobin were tracked at the time of phlebotomy (XT-2000iV veterinary hematology analyzer, Sysmex, Kobe, Japan). Nonphlebotomized control animals were handled similarly to phlebotomized animals with a daily nonphlebotomizing needle-stick.
Gut barrier function was measured on P10, P12, P14, P18, and P20 by administering fluorescein isothiocyanate (FITC)-conjugated dextran (10 kDa, 400 mg/kg; cat. no. D1820, ThermoFisher, Waltham, MA) by gavage and by measuring the fluorescence signal in the plasma 4 h later (31). In some experiments, we administered Escherichia coli (E. coli) expressing green-fluorescent protein (GFP) (108 colony-forming units by gavage; (cat. no. 25922GFP; American Type Culture Collection, Manassas, VA;) in P10 mice. The animals were euthanized 24 h later, and intestinal tissue was immunostained for GFP to identify these bacteria in the bowel wall. In other studies, we administered ovalbumin grade V or β-lactoglobulin (1 mg/g, gavage; cat. no. 41235 and L3908, Sigma, St. Louis, MO) and measured plasma levels by enzyme immunoassay (cat. no. MBS2087846 and MBS9426408, MyBioSource, San Diego, CA). For comparison, we also induced anemia in a few adult mice by removing 100 μL blood/phlebotomy on 5 consecutive days. Intestinal permeability was measured after the last phlebotomy.
Immunohistochemistry.
Formalin-fixed paraffin-embedded tissues (proximal and midcolon) were immunostained (25). Deparaffinized tissue sections were treated for antigen retrieval (EZ-AR Common solution, cat. no. HK545-XOK, Biogenex, San Remon, CA), digested with Proteinase K (20 μg/mL, 10 min; cat. no. AM2546, ThermoFisher), and blocked for 30 min (SuperBlock T20 blocking buffer, cat. no. 37536, ThermoFisher). These slides were incubated overnight at 4°C either with rabbit monoclonal anti-mouse E-cadherin (cat. no. 3195, Cell Signaling Technology, Danvers, MA) or with rabbit polyclonal anti-GFP antibodies (cat. no. ab6556, Abcam, Cambridge, MA). Secondary staining was performed with Alexa 488 or Alexa 568-conjugated antibodies for 30 min (Invitrogen, San Diego, CA). Nuclear staining was obtained with 4′,6-diamidino-2-phenylindole (DAPI) (cat no. D9542; Sigma), and the tissues were then imaged using a Nikon C2 confocal microscope. Images were analyzed using NIH Image J.
Transmission electron microscopy.
Tissue samples from proximal colon were fixed in 2% glutaraldehyde and then in 1% osmium tetroxide and processed for transmission electron microscopy (JEOL 1400 digital transmission electron microscope). The permeability of epithelial apical junctions was evaluated using the electron-dense dye ruthenium red (RR). Tissue samples were placed with the luminal side facing upward and fixed with 2.5% glutaraldehyde and 0.6% RR in 0.1 M sodium cacodylate buffer (pH 7.3) for 30 min and then placed in 2% osmium tetroxide and RR for 30 min. Finally, thin sections were photographed to analyze 10 apical junctions in tissue samples from each mouse.
RT quantitative PCR.
We used a standard SYBR green method to measure mRNA expression. Primers were designed using the Beacon Design software (Bio-Rad, Hercules, CA) and are listed in Table 1.
Table 1.
Primer sequences used for reverse transcriptase real-time polymerase chain reaction
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| HIF1a | GTTACGATTGTGAAGTTA | AAGGAATGAGATTAGGAA |
| Arnt1/HIF1b | GAGTGAGTGAGTGAGTGA | ATCTGTCTCTGTCTCTTACT |
| Epas1/HIF2a | TCATTCATATCCATACTAACAAG | CAACAAGAACAAGAACACT |
| HIF3a | TGCCACTACCACTACATT | CATACACAGAGACTCATACAC |
| Cldn1 | ATGCCTTCAACTGTTCTGTATCTC | AATCCAGGTCTACCAATGTCAATG |
| Cldn2 | AAGACCACAGCACCAGAG | CCACCTCAAGCACAATCC |
| Cldn3 | TGGAAGAGGACAGACAGG | ATTCGGCTTGGACAGTTC |
| Cldn4 | CGCTACTCTTGCCATTAC | CGTCCATCCACTCTACAG |
| Cldn5 | AGATGCTGCCTTAATGTCC | GGTTCAGTAGGTTCTTCTCC |
| Cldn7 | GCCTTAATGGTGGTGTCC | TCTTCGCTTTGTCATCTCC |
| Cldn8 | TCAGTATGTGTAGTTGTG | ATAATACCTCATTCTGCTAA |
| Cldn12 | TGGCTTCTTCATTGGTTTCAG | GACGCATTGTTAGTGTAATTGG |
| Cldn13 | GAGCCAACAACGATACCTTAG | TAGCCGCATCCAGAGTCC |
| Cldn15 | TATGCTGGAACCAAGTATG | GTATCACAACCGTAGAAGG |
| Jama | AAGCAGCAATTAGCAAGATAGG | ACAGCCGAGTTGGTTGAAG |
| Ocln | CTGCTGCTGATGAATATAATAG | CCTCTTGATGTGCGATAA |
| Tjp1 | CATAGTTCAACACAGCCTCCAG | CCATCCTCATCTTCATCTTCTTCC |
| Tjp2 | GCTGTGGACCTGCTCAACTAC | GGACTCAACCTCTGCCTTATGG |
| Tjp3 | CTCTTCACCGCCACCATCC | CGCCTGCTGTTGCTGTATTAC |
| Cgn | GCAAGAGGCTGAGAACAAG | CTTCATCTAACTCCGCTTCC |
| Marveld2 | TGTTGCTGCTGCTGCTATG | CCTTGCTCACTATGGACTGC |
| Cxadr | CCAAGACGCAGTATAACC | GGAATCATCACAGGAACC |
| Cdh1 | TTGTTCGGCTATGTGTCT | TGTGTACCTAAGAATCTGAGA |
| Cdh2 | ACAGAATATCAGATACACAA | AAGGAAGGTAGCATTATAG |
| Cdh3 | TATCACAACTCTGTCTCA | GGCAGGTTATCAATACTC |
| Cdh5 | CTCACAGACATAGAATAAGGT | TTACAGCAATGACTACAGAA |
| Ctnna1 | ACTTGATGAATGCTGTTGTG | TAATCTTGGTCTGCGTCTC |
| Ctnna2 | TCCTCCTGTGGTAGTTGC | CTGGTGTATGACGGTGTTC |
| Ctnna3 | TGACACAAGCAATGACATC | ATCTGGACACTGGTTAGC |
| Ctnnb1 | GGTCCTCTGTGAACTTGC | GTAATCCTGTGGCTTGTCC |
| Ctnnd1 | CTGCTCTGGTCCGATTGC | GTGGCTTACAGTCTTCATTAGG |
| Nectin | CTTATCTGTCAAGTGCTA | TCCGTCTCAATAATTCTG |
| Afdn | TATTCCTGTGATGTTGTC | CATAATCTGCTCCTTCAA |
| Jup | ACACTCAAGAAGACTACC | TTGTGGACATCTGGTATT |
| Ajap1 | CAACTTCCATCCGTCAGA | AACCACAGCATTCTTAATTCT |
Afdn, afadin/adherens junction formation factor; Ajap1, adherens junction-associated protein 1; Arnt, aryl hydrocarbon receptor nuclear translocator; Cdh, cadherin; Cgn; cingulin; Cldn, claudin family gene; Ctnna, catenin α; Ctnnb, catenin β; Ctnnd, catenin δ; Cxadr; coxsackie virus and adenovirus receptor; Epas, endothelial Per-Arnt-Sim domain-containing protein; HIF, hypoxia-inducible factor; Jama, junctional adhesion molecule A; Jup, junction plankoglobin; Marveld2, MARVEL (MAL and related proteins for vesicle trafficking and membrane link) domain-containing protein 2; Nectin, nectin cell adhesion molecule; Ocln, occludin; Tjp, tight junction protein.
Promoter methylation.
Pyrosequencing was performed at the Genetic Resources Core Facility at Johns Hopkins University. Genomic DNA was isolated using the DNEasy Blood and Tissue kit (cat. no. 69504, Qiagen, Germantown, MD) and bisulfite conversion was performed by the EpiTect bisulfite kit (cat. no. 59104, Qiagen). Mouse E-cadherin (Cdh1) promoter was investigated using Qiagen predesigned assays (PM00402794, Mm_Cdh1_01_PM; PM00402815, Mm_Cdh1_04_PM) in a Pyromark Q48 autoprep (Qiagen, Germany) per the manufacturer’s recommendations.
Enzyme immunoassay.
E-cadherin was measured in cell lysates by ELISA using a commercially available kit according to the manufacturer’s instructions (cat. no. ab197751, Abcam).
Caco-2 cells and colonic organoids.
Caco-2 cells (cat. no. HTB-37, American Type Culture Collection) were cultured in DMEM (cat. no. 10566016, ThermoFisher) with 10% fetal calf serum (cat. no. 16000044, ThermoFisher) in 6-well plates. In some experiments, we transfected Caco-2 with let-7e-5p inhibitor (cat. no. 4464084, ThermoFisher) and grew these cells under hypoxic conditions (1% oxygen).
Mouse colonic crypts were cultured using established methods (22). Briefly, the colon was rinsed with ice-cold phosphate-buffered saline (PBS) (cat. no. 10010023, ThermoFisher) with added penicillin (100 IU/mL) and streptomycin (100 μg/mL; cat. no. 11074440001, Sigma). Tissue pieces (2–4 mm) were rocked in PBS with 2 mM EDTA (cat. no. 324504, Sigma) for 30 min at 4°C and then vortexed for 10 s with 10 mL dissociation buffer (d-sorbitol 54.9 mM, sucrose 43.4 mM in PBS; cat. no. 240850 and S7903, respectively, Sigma). The solution was filtered (100 μm) and then centrifuged at 800 rpm for 5 min to harvest crypts. The pellet was resuspended in 100 μL PBS and then added to 250 μL matrigel (cat. no. 354234, Corning) [supplemented with 100 ng/mL mouse Wnt3a (cat. no. 1324-WN, R&D, Minneapolis, MN], 1 μg/mL R-spondin-1 (cat. no. 7150-RS, R&D), 100 ng/mL Noggin (cat. no. 6997-NG, R&D), and 50 ng/mL epidermal growth factor (cat. no. 4095-EG, R&D). Finally, 30 μL of this cell suspension was pipetted into each well of an 8-well chambered slide and incubated in 5% CO2 at 37°C. Media were replaced every 3–4 days.
In silico prediction of microRNA-binding sites.
We used STarMir (http://sfold.wadsworth.org/cgi-bin/starmirtest2.pl) and miRWalk2.0 (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) to predict microRNA-binding sites on Cdh1 mRNA. Functional conservation of these microRNA-binding sites between species was evaluated using TargetScan (http://www.targetscan.org/mmu_71/).
MicroRNA.
MicroRNAs were measured by poly(A) tailing and RT quantitative PCR (qPCR) (NCode Vilo miRNA cDNA synthesis and RT-qPCR kits; cat. no. 10461953, Invitrogen). Caco2 cells and colonic organoids were transfected with the miR-1 or let-7e-5p mimic (cat. no. AM17000, ThermoFisher) using the Lipofectamine RNAiMax reagent (cat. no. 13778030, ThermoFisher). To confirm microRNA-mRNA binding, Caco-2 cells were cotransfected with a luciferase construct containing the 3′-untranslated region (3′-UTR) sequence of Cdh1 mRNA (pMirTarget, cat. no. SC217485, OriGene, Rockville, MD) and the miR-1 or let-7e-5p mimic. Luciferase activity was measured using the Firefly Luciferase HS Assay Kit (cat. no. LF007, GeneCopoeia). In some experiments, Caco-2 cells transfected with miRNA mimics were treated with actinomycin D (10 μg/mL; cat. no. A1410, Sigma), and E-cadherin expression was measured by RT-qPCR at serial time points.
In vivo assay.
C57BL/6 mouse pups were treated intraperitoneally on P4, P6, and P8 with scrambled miR inhibitor or with specific miR let-7e-5p inhibitor (25 mg/kg body wt; miRCURY LNA let-7e-5p inhibitor, cat. no. 339203, Qiagen). There were four groups: 1) control mice treated with scrambled miR inhibitor, 2) control mice treated with let-7e-5p inhibitor, 3) anemic mice treated with scrambled miR inhibitor, and 4) anemic mice treated with let-7e-5p inhibitor. Intestinal permeability was measured by FITC-dextran measurements, and E-cadherin expression was measured by RT-qPCR in the intestines from each group on P10.
Statistical methods.
Statistical analysis was performed using GraphPad Prism software, version 7.01 (GraphPad software, La Jolla, CA). Differences were considered significant at P < 0.05.
RESULTS
Severe neonatal anemia in murine pups.
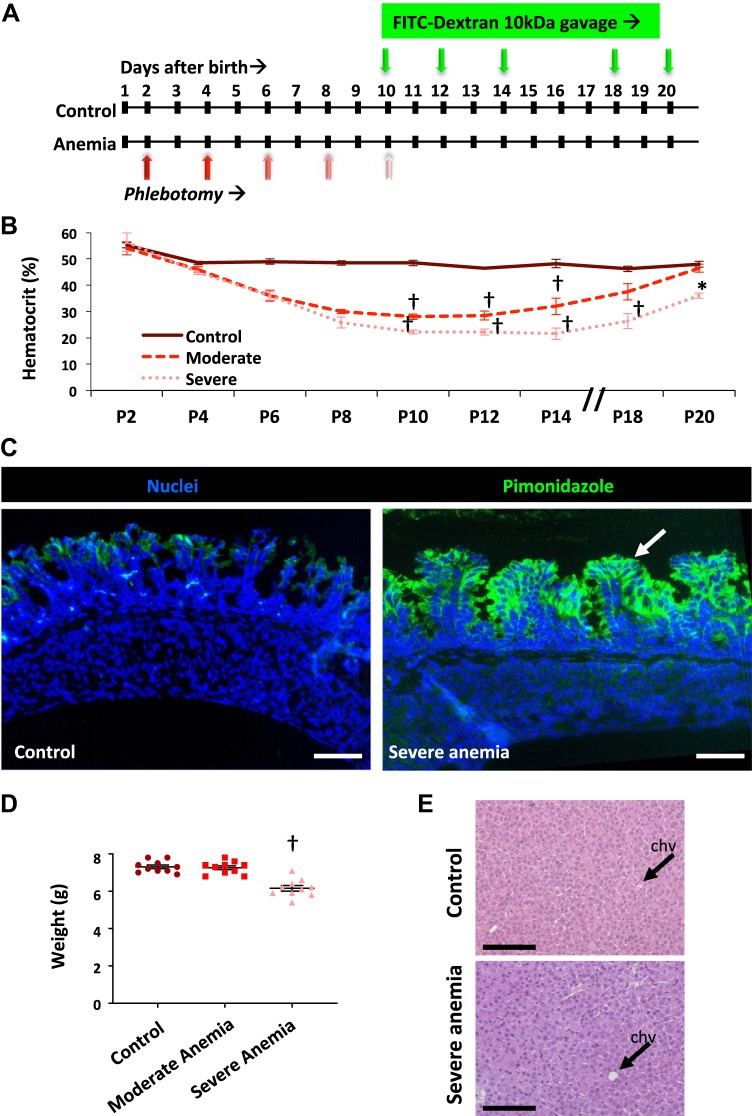
In neonatal mice, controlled phlebotomy on P2, P4, P6, P8, and P10 (Fig. 1A) resulted in a significant, predictable reduction in hematocrit. On P10, control mice showed a median hematocrit of 44% (range 42%–54%), which was higher than the hematocrit of 22.4% (range 18%–23%) in phlebotomized animals; P < 0.001. In phlebotomized animals, hematocrits recovered only partially over time and remained lower than those in controls on P20. Anemic pups showed lower mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and reticulocyte hemoglobin (Table 2).
Fig. 1.
Severe neonatal anemia in murine pups. A: schematic shows that C57BL/6 mouse pups were rendered anemic by repeated phlebotomy (red arrows) on postnatal day (P) 2, P4, P6, P8, and P10. Gut mucosal permeability was measured after enteral administration of fluorescein isothiocyanate (FITC)-dextran (green arrows) on P10, P12, P14, P18, and P20, and measurement of the plasma FITC signal 4 h later. B: line diagrams (means ± SE) show serial hematocrits in control (n = 4), moderately anemic (hematocrit 25%–30%; n = 6), and severely anemic mouse pups (hematocrit 20%–24%; n = 5); Kruskal-Wallis H test with Dunn’s posttest. †P < 0.001. C: fluorescence photomicrographs (×250 magnification) of control and severely anemic proximal colons showed increased pimonidazole immunoreactivity (green) in the epithelium in the anemic pups but not in the control pups; n = 6 pups/group. D: scatter plots (means ± SE) show body weights of mice in the three study groups. n = 10 mice/group; Kruskal-Wallis H test with Dunn’s posttest; †P < 0.001. E: photomicrographs (hematoxylin-eosin; ×200 magnification) of the liver showed no evidence of venous congestion in severe anemia [arrows indicate central hepatic veins (chvs)]. Scale bar = 100 μm; n = 6 mice/group.
Table 2.
Hematocrit and red cell indices in controls and anemic-transfused pups
| Parameter, median (range) | Naïve Control (n = 42) | Moderate Anemia (n = 21) | Severe Anemia (n = 40) |
|---|---|---|---|
| Hematocrit, % | 44 (42–54) | 28 (33–57) | 22.4 (18–23)† |
| Mean corpuscular volume, fL | 51.9 (45.1–75.3) | 53.5 (43.9–67.8) | 51.6 (41.5–62.3) |
| Mean corpuscular hemoglobin, pg | 15.8 (14.3–16.8) | 14 (12.8–15.8) | 13 (12–15.4)† |
| Mean corpuscular hemoglobin concentration, g/dL | 30.6 (26–32.8) | 29.2 (25.1–32.3) | 27.2 (24.7–32.1)** |
| Red cell distribution width, fL | 35.6 (26.9–48.5) | 35.9 (28.4–38.1)† | 36 (31.5–47.3) |
| Reticulocyte count, % | 1.98 (0–2.58) | 1.74 (1.1–3.44) | 3.24 (0–5.6)† |
| Reticulocyte hemoglobin, pg | 13.9 (12.4–16.8) | 12.6 (12.2–15.8) | 11.5 (10.6–16.7)† |
Kruskal-Wallis H test with Dunn’s posttest.
P < 0.01;
P < 0.001.
To evaluate for tissue hypoxia in the anemic intestine, we treated control and anemic animals with pimonidazole hydrochloride and then immunolocalized pimonidazole adducts in tissues harvested after euthanasia (1). Pimonidazole immunoreactivity was noted in distal ileal and proximal colonic epithelium in anemic animals but not in controls (Fig. 1C). Anemic pups gained less weight than controls (Fig. 1D), which attested to the physiological severity of their anemia. At autopsy, anemic animals did not show hepatic venous congestion (Fig. 1E), indicating that they were not likely to have experienced anemia-related cardiac failure.
We also included a few pups with “moderate” anemia (median hematocrit of 28%, range 26%–30%; P < 0.001). These animals gained weight similarly to controls and completely recovered their hematocrits by P20 (data incorporated in Fig. 1, B and C). We also studied a few adult mice that had been subjected to repeated phlebotomy to induce severe anemia with hematocrits of 20%–24%.
Severe anemia increased gut mucosal permeability in neonatal mice.
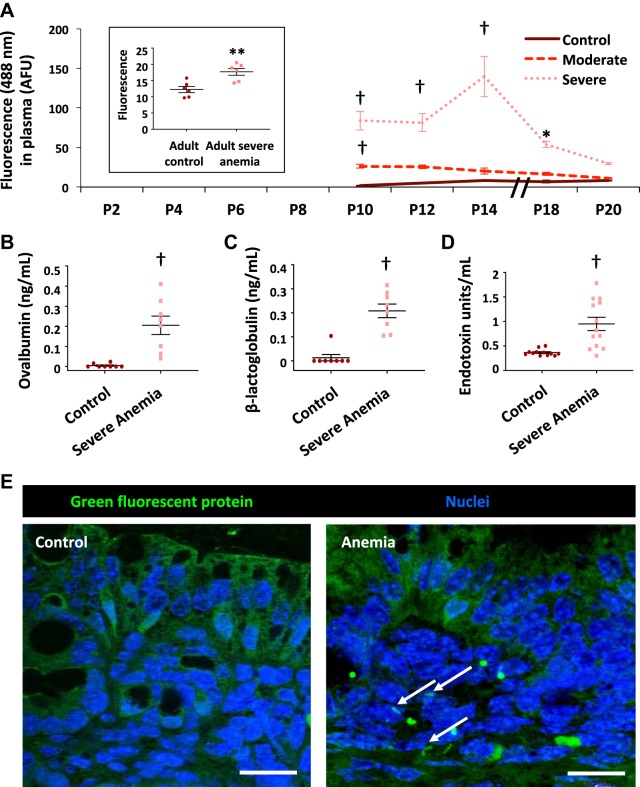
We measured gut mucosal permeability in control and anemic pups at serial time points between P10 and P20 by first administering FITC-dextran (molecular mass 10 kDa; 400 mg/kg; predetermined optimum) by gavage and then measuring the fluorescein signal in the plasma 4 h later (31). Experimentally induced severe anemia increased intestinal permeability; plasma FITC signals in control and severely anemic pups on P10 were 1.77 ± 0.68 and 83.79 ± 11.6 arbitrary fluorescence units (AFU), respectively; P < 0.001. The fluorescence signals continued to rise in the anemic pups and peaked on P14 (139.69 ± 25.3 AFU; P < 0.001 vs. control) even though there was no change in the hematocrits between P10–P14. There was some recovery of gut barrier function on P18 and beyond (Fig. 2A). These findings contrasted with those in pups with moderate anemia (lowest hematocrits between 25% and 30%), which showed increased intestinal permeability on P10 (26.36 ± 2.52 AFU) but recovered within 48 h (Fig. 2A). Compared with pups, adult mice with severe anemia showed a relatively modest increase in intestinal permeability; plasma fluorescein signals in control and severely anemic adult mice were 12.25 ± 0.95 AFU and 17.69 ± 1.06 AFU, respectively; P = 0.01 (Fig. 2A, inset).
Fig. 2.
Severe anemia increased gut mucosal permeability in neonatal mice. A: line diagrams (means ± SE) from control and anemic C57BL/6 mice show plasma fluorescein isothiocyanate (FITC) fluorescence [in artificial fluorescence units (AFU)] measured 4 h after enteral administration of FITC-dextran in control (n = 4), moderately anemic (hematocrit 25%–30%; n = 6), or severely anemic mouse pups (hematocrit 20%–24%; n = 5). Kruskal-Wallis H test with Dunn’s posttest. *P < 0.05; **P < 0.01; †P < 0.001. Left inset: Plasma FITC fluorescence in adult control mice vs. severely anemic adult mice. n = 6 mice/group. Mann-Whitney U test. B and C: scatter dot plots show plasma ovalbumin and β-lactoglobulin in control vs. anemic pups 4 h after gavage. n = 8 mice/group. Mann-Whitney U test. D: scatter dot plots show increased plasma LPS in control and severely anemic mouse pups (n = 12 mice/group); †P < 0.001. E: fluorescence photomicrographs show Escherichia coli expressing green fluorescence protein within the bowel wall in anemic mouse pups but not in control mouse pups. Scale bar = 25 μm; n = 5 pups/group.
To ascertain the translational relevance of these findings, we next evaluated anemic pups for intestinal permeability to dietary macromolecules and luminal bacteria. We first administered ovalbumin and β-lactoglobulin (1 mg/g body wt of each protein) by gavage (34) and measured plasma concentrations of these proteins after 4 h. Anemic mice showed elevated plasma concentrations of both proteins (Fig. 2, B and C). Severely anemic pups also showed elevated plasma lipopolysaccharide levels (Fig. 2D), suggesting bacterial translocation across the intestinal barrier. To confirm these findings, we administered genetically modified E. coli expressing green-fluorescent protein (GFP; 108 colony-forming units) by gavage; these bacteria were detectable in the bowel wall of anemic pups after 24 h, but were seen only in the lumen in control pups (Fig. 2E).
Taken together, these observations indicated a nonlinear and age-dependent effect of anemia on intestinal permeability, in which preweaned mouse pups showed greater susceptibility to the effects of anemia than adult mice. Because intestinal permeability increased disproportionately at hematocrits of ≤24%, subsequent experiments were focused on the comparison of control versus severely anemic pups (hematocrit of 20%–24%).
Severe anemia caused specific ultrastructural abnormalities in epithelial adherens junctions.
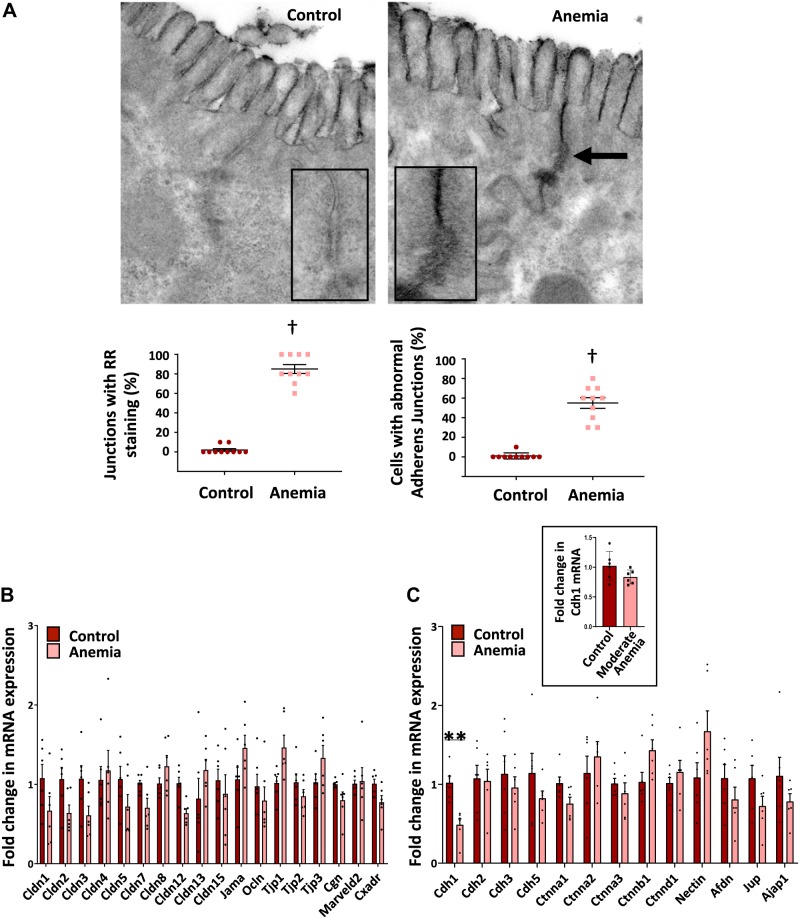
To elucidate the mechanism(s) underlying the abnormal gut permeability in anemic mice, we first used transmission electron microscopy to evaluate tissue specimens of proximal colon that had been fixed and processed after applying the electron-dense dye ruthenium red (RR) on the luminal side. These images confirmed increased paracellular permeability across the tight and adherens junctions. In addition, most adherens junctions showed ultrastructural abnormalities, such as longitudinal foreshortening, irregularity, and separation of membrane leaflets (Fig. 3A).
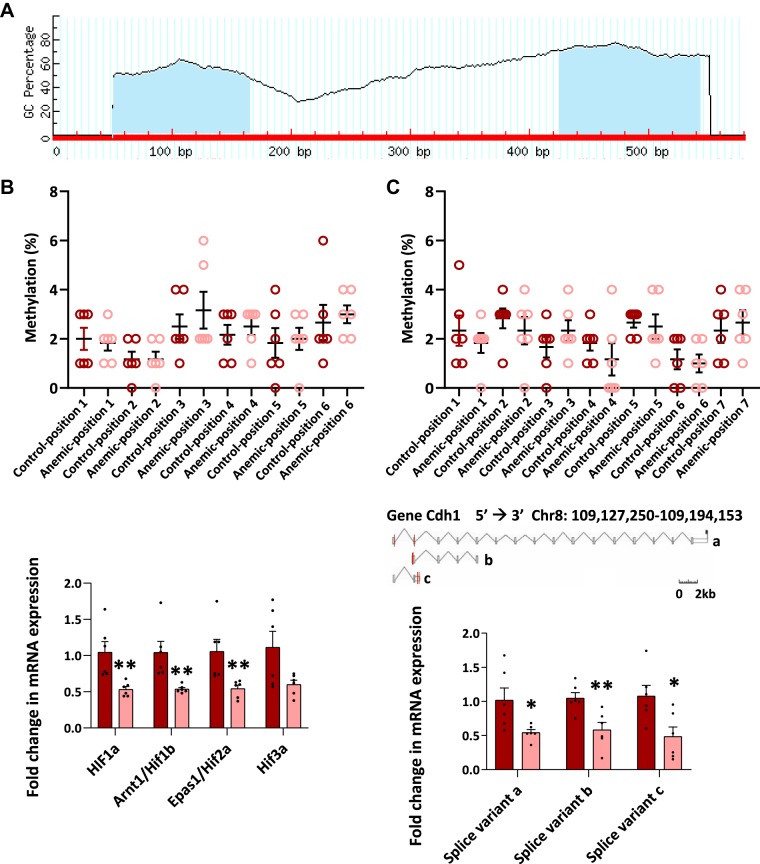
Fig. 3.
Severe anemia caused specific ultrastructural abnormalities in epithelial adherens junctions. A: transmission electron micrographs (×60,000) of proximal colon from control and anemic pups treated with ruthenium red (RR) immediately after euthanasia. Anemic colon showed electron-dense RR deposits in paracellular spaces in the anemic colon (closed arrow). n = 10 mice/group. Insets show high-magnification images highlight longitudinal foreshortening of the adherens junction and irregularity of membrane leaflets in the anemic intestine. Dot scatter plots (2 panels below A) show increased number (means ± SE) of adherens junctions with RR staining and ultrastructural abnormalities. †P < 0.001. Bar diagrams (means ± SE) show mRNA expression of key tight junction genes (B) and adherens junction genes (C) in control vs. anemic neonatal colon; Kruskal-Wallis H test with Dunn’s posttest. **P < 0.01; n = 8 mice/group. Bar diagram (means ± SE) of E-cadherin expression (inset in C) in control vs. moderately anemic neonatal colon. Kruskal-Wallis H test with Dunn’s posttest. Afdn, afadin/adherens junction formation factor; Ajap1, adherens junction-assoicated protein 1; Cdh, cadherin; Cgn, cingulin; Cldn, claudin family gene; Ctnna, catenin α; Ctnnb, catenin β; Ctnnd, catenin δ; cxadr, coxsackie virus and adenovirus receptor; Jama, junctional adhesion molecule A; Jup, junction plankoglobin; Marveld2, MARVEL (MAL and related proteins for vesicle trafficking and membrane link) domain-containing protein 2; Nectin, nectin cell adhesion molecule; Ocln, occludin; Tjp, tight junction protein.
We next used RT-qPCR to measure the expression of genes involved in the assembly of tight and adherens junctions (Fig. 3, B and C). Anemic intestine showed decreased E-cadherin (Cdh1), which is essential for the formation of adherens junctions (4). No significant changes were seen in other tight junction genes. There is no significant change in E-cadherin expression in the intestine during moderate anemia (Fig. 3C, inset).
Severe anemia was associated with decreased epithelial E-cadherin protein expression.
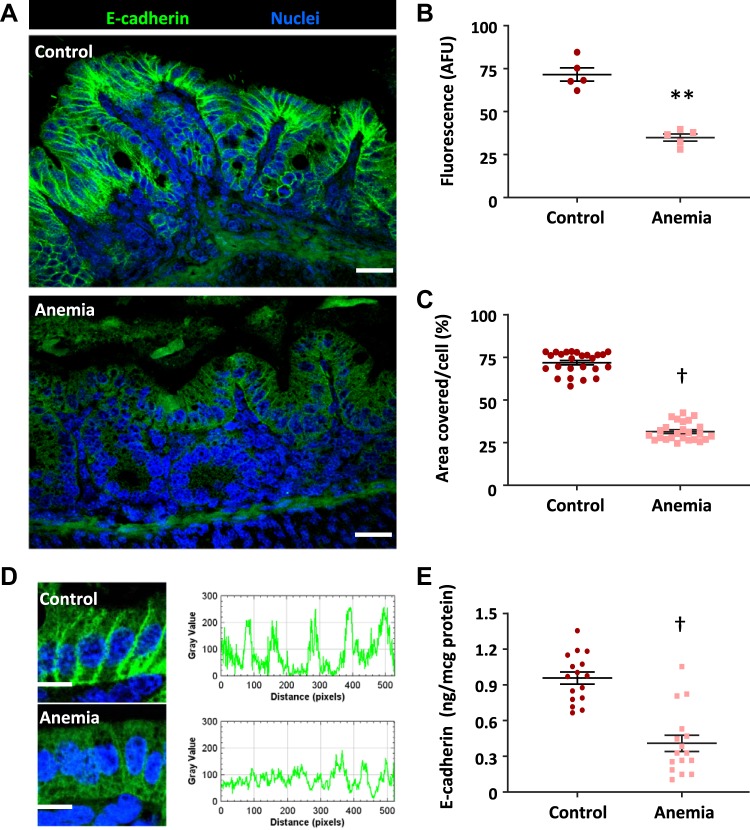
Immunohistochemistry showed decreased E-cadherin staining in the anemic intestine. There was a reduction in the fluorescence intensity of E-cadherin staining on the epithelial intercellular membranes (Fig. 4, A, B, and E) and the total cellular area covered by E-cadherin staining (Fig. 4C). Enzyme immunoassay confirmed decreased expression of E-cadherin protein in exfoliated epithelial cells (Fig. 4D).
Fig. 4.
Severe anemia was associated with decreased epithelial E-cadherin protein expression. Fluorescence photomicrographs (×240) showed decreased epithelial immunoreactivity for E-cadherin (green) in anemia (A). Scale bar = 25 μm. Scatter plots (means ± SE) right summarize fluorescence intensity (B) and area covered by E-cadherin staining (C), respectively; n = 5 mice/group. High-magnification images and fluorescence intensity measurements (Image J) showed loss of perimembrane E-cadherin immunoreactivity in anemic colonic epithelial cells (D). Direct measurements in exfoliated epithelial cells (E) showed decreased E-cadherin expression in the anemic colon (n = 16 animals/group); Mann-Whitney U test; **P < 0.01; †P < 0.001. AFU, artificial fluorescence units.
Anemia-related depletion of Cdh1 mRNA was not due to promoter methylation or transcriptional inhibitors.
To understand how severe anemia reduced E-cadherin mRNA expression in the intestine, we evaluated promoter methylation, tissue hypoxia-inducible transcriptional inhibitors of E-cadherin, and alternative splicing of E-cadherin transcripts as potential mechanisms. E-cadherin promoter area showed two sites that may undergo methylation, but comparison of these areas in control and severe anemia did not show a change in the frequency or site of methylation between the two groups (Fig. 5A).
Fig. 5.
Anemia-related depletion of E-cadherin (Cdh1) mRNA was not due to promoter methylation or transcriptional inhibitors. A: schematic shows two potential methylation sites in the E-cadherin promoter (means ± SE, percentage; shaded blue in schematic). GC, guanine-cytosine. B: dot plot (with means ± SE) from control and severely anemic mouse colons showed no difference in methylation of the E-cadherin promoter; n = 6 pups/group. Bar diagrams (means ± SE) summarize mRNA expression of the four major hypoxia-inducible factors (HIFs) in control vs. severely anemic neonatal colons: HIF-1α, HIF-1β/aryl hydrocarbon receptor nuclear translocator (Arnt), HIF-2α/endothelial Per-Arnt-Sim domain-containing protein-1 (Epas1), and HIF-3α. n = 6/group; each dot represents a different pup. C: bar diagram (means ± SE) with dots indicating readings from individual animals from control and severely anemic mice shows similar depletion of the three alternative mRNA transcripts of E-cadherin; Mann-Whitney U test. *P < 0.05; **P < 0.01. Chr8, chromosome 8.
Tissue hypoxia can inhibit epithelial E-cadherin gene expression by inducing/stabilizing one or more of the hypoxia-inducible factors (HIFs) (14, 20, 38). Therefore, we next used RT-qPCR to compare control versus anemic intestine for the expression of HIF-1α, HIF-1β/aryl hydrocarbon receptor nuclear translocator, HIF-2α/endothelial Per-Arnt-Sim domain-containing protein-1, and HIF-3α. Interestingly, HIF-1α, HIF-1β, and endothelial Per-Arnt-Sim domain-containing protein-1/HIF-2β were significantly decreased in anemia, and a trend was seen toward decreased Hif-3α (Fig. 5B). These findings did not support a HIF-mediated transcriptional mechanism for altered intestinal permeability and the loss of E-cadherin expression in severe anemia.
We also investigated alternative mRNA splicing as a potential mechanism for the observed loss of E-cadherin mRNA in severe anemia. RT-qPCR showed a similar depletion of all three major splice variants of the E-cadherin transcript and, therefore, did not support alternative splicing as a likely mechanism for decreased E-cadherin expression (Fig. 5C).
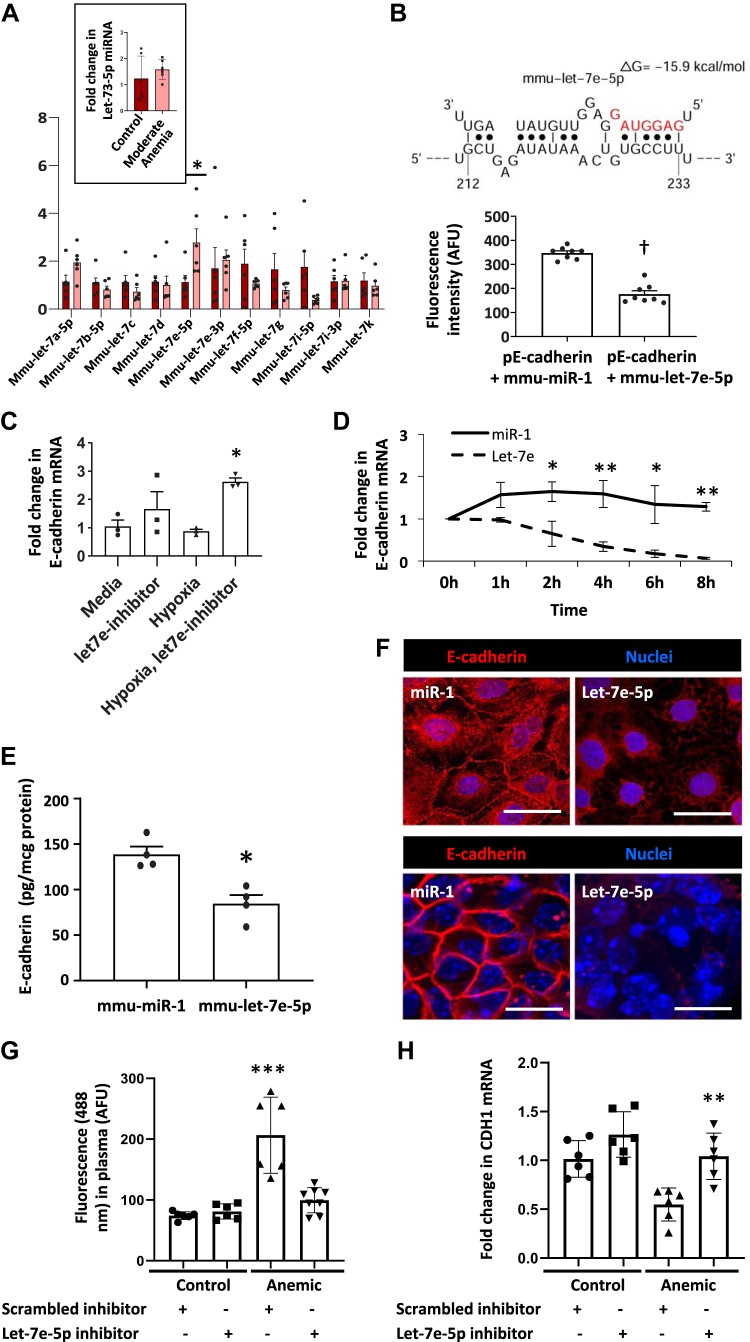
MicroRNA let-7e destabilized Cdh1 mRNA in the anemic intestine.
Cdh1 mRNA displays a substantial 3′-untranslated region (3′-UTR), and therefore, we next asked whether this UTR could be a microRNA target. To predict potential microRNA-binding sites, we analyzed the 3′UTR sequences in silico, using online tools STarMir and miRWalk2.0. Sorting by statistical significance, lowest predicted binding energy, and highest accessibility, we identified several members of the let-7 microRNA family as likely to bind the 3′-UTR region of E-cadherin. Quantitative PCR measurements showed increased microRNA let-7e-5p in the anemic intestine (Fig. 6A). To confirm let-7e-5p binding to the 3′-UTR of Cdh1 mRNA, we transfected Caco-2 cells with luciferase reporters containing 3′-UTR sequences and miR let-7e-5p (or control miR-1). Cotransfection with let-7e-5p reduced the luciferase activity of the modified Cdh1 mRNA (Fig. 6B). Identification of let-7e-5p as a potential mechanism for decreased Cdh1 mRNA was interesting because it is a known hypoxia-responsive microRNA (21). To determine how hypoxia induced let-7e expression in anemia, Caco-2 cells were grown under normal and hypoxic conditions (17% or 1% oxygen, respectively), and cells in some plates were transfected with a let-7e inhibitor. Hypoxia increased let-7e-5p and suppressed E-cadherin expression, which was reversed by the let-7e inhibitor (Fig. 6C).
Fig. 6.
MicroRNA let-7e destabilized E-cadherin (Cdh1) mRNA in the anemic intestine. A: quantitative PCR showed expression of various let-7 microRNAs in the severely anemic intestine. Inset: bar diagram (means ± SE) shows the expression of miR let-7e-5p in control vs. moderately anemic neonatal intestine. Mann-Whitney U test; *P < 0.001. B: schematic shows predicted binding configuration of miR let-7e-5p with the 3′-untranslated region (3′UTR) of E-cadherin. Bar diagram (means ± SE) below summarizes direct fluorescence measurements from where a luciferase construct containing the 3′-UTR sequence of E-cadherin mRNA binds miR let-7e-5p. Mann-Whitney U test; †P < 0.001. C: hypoxia effects on let-7e-5p expression in Caco-2 cells are reversed by a miR let-7e inhibitor. Caco-2 cells grown under normal (defined as 17% oxygen) and hypoxic (1% oxygen) conditions. Kruskal-Wallis H test with Dunn’s posttest; *P < 0.05. D: line diagram (means ± SE) shows that miR let-7e-5p, but not control (miR-1), suppressed E-cadherin expression in Caco-2. n = 8 (4 replicates/group in 2 runs). Repeated measures test. **P < 0.01; *P < 0.05. E: bar diagram (means ± SE) shows decreased E-cadherin protein expression in Caco-2 cells transfected with miR let-7e-5p but not in those transfected with control miRNA (miR-1). Mann-Whitney U test; *P < 0.05. F: fluorescence photomicrographs (×240) showed decreased perimembrane immunoreactivity for E-cadherin (red) intestinal epithelial cells. Overexpression of miR let-7e-5p, but not of miR1, disrupted E-cadherin expression in Caco-2 monolayers (scale bar = 25 μm; top) and colonic organoids (scale bar = 15 μm; bottom). G: bar diagrams (means ± SE) show plasma fluorescein isothiocyanate (FITC) fluorescence [in artificial fluorescence units (AFU)] measured 4 h after enteral administration of FITC-dextran in 1) postnatal day (P) 10 control mice treated with scrambled miR inhibitor (n = 6), 2) control mice treated with miR let-7e-5p inhibitor (n = 6), 3) anemic mice treated with scrambled miR inhibitor (n = 6), and 4) anemic mice treated with miR let-7e-5p inhibitor (n = 8). All pups received these reagents intraperitoneally on P4, P6, and P8. Kruskal-Wallis H test with Dunn’s posttest; ***P < 0.001. H: quantitative PCR showed decreased E-cadherin expression in 1) P10 control mice treated with scrambled miR inhibitor (n = 6), 2) control mice treated with miR let-7e-5p inhibitor (n = 6), 3) anemic mice treated with scrambled miR inhibitor (n = 6), and 4) anemic mice treated with miR let-7e-5p inhibitor (n = 6). E-cadherin expression was restored in anemic mice treated with miR let-7e-5p inhibitor. Mann-Whitney U test; **P < 0.01.
In mice with moderate anemia, tissue expression of let-7e-5p resembled that found in controls and was significantly lower than in mice with severe anemia (Fig. 6A, inset). Adult mice with severe anemia did not upregulate let-7e-5p. These findings are consistent with the effects of moderate and severe anemia on intestinal permeability shown in Fig. 1. We looked for, but did not find, evidence of age-related epigenetic regulation of let-7e expression. The let-7e gene promoter carries a 5′-C-phosphate-G-3′ island, but there was no evidence of methylation in either pups or adults.
To confirm miR let-7e effects on mRNA stability, we transfected Caco-2 cells with miR let-7e-5p (or control miR-1) and added actinomycin D to block ongoing transcription. RT-qPCR measurements at serial time points showed decreased stability of miR let-7e-5p compared with the control (Fig. 6D). In further experiments, Caco-2 cells transfected with miR let-7e-5p showed lower E-cadherin expression at the protein level by ELISA than cells transfected with control microRNA did (Fig. 6E). Consistently, miR let-7e-5p overexpression also reduced E-cadherin localization at intercellular junctions in Caco-2 cells (Fig. 6F, top) and in colonic organoids derived from neonatal mouse crypts (Fig. 6F, bottom). Thus, let-7e expression recapitulated the entire set of findings seen in severe neonatal anemia.
Finally, we investigated whether inhibition of miR let-7e-5p in vivo decreases intestinal permeability. We administered miR let-7e-5p inhibitor (scrambled miR inhibitor as control; 25 mg/kg body wt) to control and anemic pups intraperitoneally on P4, P6, and P8. Intestinal permeability in anemic pups treated with the miR let-7e-5p inhibitor decreased to levels similar to those in control pups (Fig. 6G). RT-qPCR in the miR let-7e-5p inhibitor-treated anemic mouse intestine showed E-cadherin expression restored to control levels (Fig. 6H).
DISCUSSION
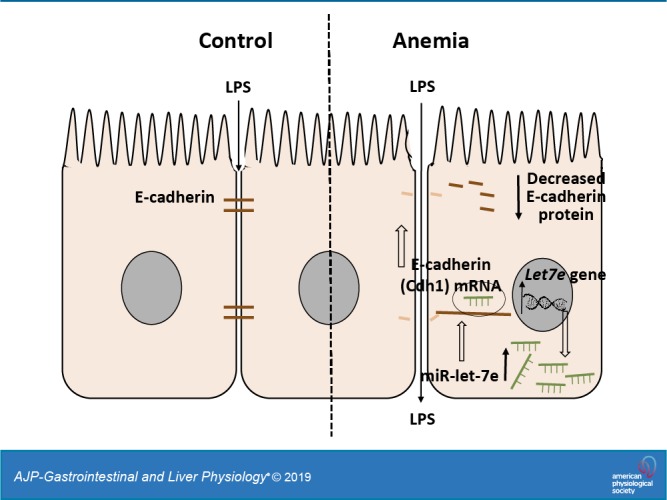
We present a detailed investigation of the effects of anemia on intestinal permeability in preweaned mice. In our model, severe anemia (lowest hematocrits of 20%–24%) increased gut mucosal permeability to foodborne macromolecules and enteral bacteria. These effects were specific to the neonate; adult mice with hematocrits in the 20%–24% range showed only minimal changes. At an ultrastructural level, we found abnormalities in the adherens junctions. These findings were explained by decreased E-cadherin expression, which in turn, was caused by hypoxia-mediated upregulation of microRNA let-7e-5p and the consequent destabilization of the E-cadherin 3′-UTR. Our findings call for the evaluation of immediate and long-term effects of severe anemia in young infants to allow informed decision-making and to balance the risks of RBC transfusions with those of untreated and prolonged severe anemia.
We detected a nonlinear relationship between falling hematocrits and intestinal permeability. In pups with lowest hematocrits between 20% and 24%, intestinal permeability increased between P10 and P14, although the hematocrits did not change during this period. After P14, there was gradual recovery of the barrier function by P20. In contrast, pups with moderate anemia (lowest hematocrit 25%–30%) showed increased gut permeability on P10 but recovered within 48 h. Increased intestinal permeability to larger foodborne macromolecules, such as ovalbumin and β-lactoglobulin, in severe anemia was also intriguing. The current epidemiological evidence about the impact of early exposure to foodborne macromolecules on the immune system is conflicting; anemia and iron deficiency in childhood have been linked with atopic disease and food allergies (11), but there is also evidence for the tolerogenic effect of early exposure to some foodborne macromolecules, such as peanut allergens (12). Similarly, bacterial translocation in severe anemia may also have important short-term and long-term implications. In the last decade, several retrospective case-control studies and the meta-analysis of these data suggest that a third of all cases of necrotizing enterocolitis (NEC) follow RBC transfusions. Although the pathogenesis of NEC is complex and not well understood, the importance of bacterial translocation is widely accepted (18). Singh et al. (32) reported that the risk of NEC following transfusion progressively increased with decreasing hematocrit. In a recent large prospective clinical study, Patel et al. (28) also showed NEC to be associated in their cohort with anemia and not RBC transfusions.
In our model, we phlebotomized mouse pups between P2 and P10 and measured intestinal permeability at serial time points between P10 and P20. Our justification for using mice in these age ranges was based on the strong resemblance of the murine intestine at birth to the preterm human intestine; the murine intestine attains the structural/functional maturity of the term human neonate (mucosal histoarchitecture, leukocyte populations, digestive enzymes, and transporters) only by P18–P21 (35). The baseline hematocrits of 40%–50% in mouse pups are similar to those seen in human neonates, and with repeated phlebotomy, the hematocrits dropped to 20%–24% to resemble those of preterm infants with anemia of prematurity. The use of phlebotomy to induce anemia was based on existing modeling and clinical data that emphasize blood draws for laboratory testing as a leading contributor to anemia in premature and critically ill infants (30). Anemic pups showed normocytic, hypochromic anemia and low reticulocyte hemoglobin, indicating that incipient iron deficiency (from chronic blood loss) may also have contributed (17). These hematological changes are also similar to this patient population (30).
Neonatal anemia was associated with decreased epithelial expression of E-cadherin. Ultrastructural abnormalities, such as irregularity of the apposed membrane leaflets and longitudinal foreshortening of the adherens junctions, were consistent with the localization of E-cadherin within the adherens junctions and in the paracellular spaces (27). The role of E-cadherin in epithelial barrier function is well established during development, in diverse inflammatory states, and in colon cancer (4, 10, 24). In most situations, E-cadherin expression is mediated via hypoxic signaling involving the HIFs, which may promote promoter hypermethylation, transregulation of E-cadherin transcription, or posttranscriptional modifications such as tyrosine phosphorylation (19, 39). In our study, anemia did not induce HIFs, despite evidence of tissue hypoxia, which could possibly be due to autocrine inhibitory loops that limit HIF expression in chronic hypoxia (29).
MicroRNA let-7e-5p expression has been previously documented in the neonatal intestinal epithelium (15). Our studies have now shown that the induction of microRNA let-7e during severe anemia disrupts E-cadherin function and increases intestinal permeability in neonates. The effects of specific inhibition of microRNA let-7e in vitro and in vivo add specific evidence to these observations. Developmentally regulated let-7 microRNAs can promote gut epithelial differentiation, and, therefore, the expression of these microRNAs in the neonatal intestine is not surprising. During fetal development, hypoxic stress may simulate altitude-related adaptation, vascular development, wound healing, cell survival or cell proliferation (which may be regulated by gene expression), mRNA stability, internal ribosomal entry site-mediated translation, hypoxia effects on protein synthesis, or even to effects independent of hypoxia, such as altered calcium flux and chromatin/histone remodeling (23). Further work is needed to elucidate the mechanisms for let-7e induction in neonatal anemia. Existing information also shows hypoxia to induce let-7e in the endothelium and in cancer, in which these microRNAs can derepress vascular endothelial growth factor expression and promote angiogenesis (8). Recent findings on the role of let-7 on intestinal healing during inflammatory conditions are also interesting (13).
In conclusion, we have shown that severe neonatal anemia can alter intestinal permeability to enteral macromolecules and to the luminal bacterial flora. The strength of this study is the development of a robust preclinical model of neonatal anemia and the mechanistic insights it has generated. There are some obvious limitations: animal models may not always capture the complexity of a natural, multifactorial process such as anemia. Studies in small animals may also overlook physiological covariates such as feeding experience, comorbidities, and microbial flora. Nevertheless, our findings indicate a need for careful evaluation of the implications of severe anemia in premature and critically ill infants. We also need to revisit the transfusion thresholds for these patients and determine if, in our expectant approach, we might be allowing these infants to become far too anemic without adequate information on safety.
GRANTS
This work is supported by the National Heart, Lung, and Blood Institute Grant HL-124078 (to A. Maheshwari).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.M. and A.M. conceived and designed research; K.M., K.N., N.S., N.G.A., V.S., J.K.D., Y.C., and J.W.B. performed experiments; K.M., K.N., N.S., N.G.A., V.K.S., J.K.D., Y.C., J.W.B., and A.M. analyzed data; K.M., K.N., V.K.S., and A.M. interpreted results of experiments; K.M., N.G.A., and A.M. prepared figures; K.M. and A.M. drafted manuscript; K.M., K.N., V.K.S., J.K.D., Y.C., and A.M. edited and revised manuscript; K.M., V.K.S., J.K.D., J.W.B., and A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Sysmex America, Scientific Marketing Department, for the instrument loan (Sysmex XT-2000iV) and technical support. They also thank Dr. Jack Widness for the introduction to the Sysmex team and to automated veterinary hematology analyzers. A part of this work has been presented at the Pediatric Academic Societies meetings and published in abstract form.
REFERENCES
- 1.Aguilera KY, Brekken RA. Hypoxia studies with pimonidazole in vivo. Bio Protoc 4: 1254, 2014. doi: 10.21769/BioProtoc.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman ST, Lacroix J, Boven K, Forbes P, Barton R, Thomas NJ, Jacobs B, Markovitz B, Goldstein B, Hanson JH, Li HA, Randolph AG; Pediatric Acute Lung Injury and Sepsis Investigators Network . Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med 178: 26–33, 2008. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- 3.Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, Cress GA, Johnson KJ, Kromer IJ, Zimmerman MB. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics 115: 1685–1691, 2005. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondow BJ, Faber ML, Wojta KJ, Walker EM, Battle MA. E-cadherin is required for intestinal morphogenesis in the mouse. Dev Biol 371: 1–12, 2012. doi: 10.1016/j.ydbio.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen JR, Patterson JA, Roberts CL, Isbister JP, Irving DO, Ford JB. Red cell and platelet transfusions in neonates: a population-based study. Arch Dis Child Fetal Neonatal Ed 100: F411–F415, 2015. doi: 10.1136/archdischild-2014-307716. [DOI] [PubMed] [Google Scholar]
- 6.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr 131, 2S-2: 636S–648S, 2001. doi: 10.1093/jn/131.2.636S. [DOI] [PubMed] [Google Scholar]
- 7.Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, Peterson N, Ramsey G, Rao SV, Roback JD, Shander A, Tobian AA. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 316: 2025–2035, 2016. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, Wang YT, Sun W, Cui X, Li YS, Fang T, Zhao H, Padmanabhan C, Sun R, Wang DL, Jin H, Chau GY, Huang HD, Hsiao M, Shyy JY. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest 123: 1057–1067, 2013. doi: 10.1172/JCI65344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q 38: 302–316, 1985. [PubMed] [Google Scholar]
- 10.Dorudi S, Hanby AM, Poulsom R, Northover J, Hart IR. Level of expression of E-cadherin mRNA in colorectal cancer correlates with clinical outcome. Br J Cancer 71: 614–616, 1995. doi: 10.1038/bjc.1995.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drury KE, Schaeffer M, Silverberg JI. Association between atopic disease and anemia in US children. JAMA Pediatr 170: 29–34, 2016. doi: 10.1001/jamapediatrics.2015.3065. [DOI] [PubMed] [Google Scholar]
- 12.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G; LEAP Study Team . Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 372: 803–813, 2015. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng H, Bu HF, Liu F, Wu L, Pfeifer K, Chou PM, Wang X, Sun J, Lu L, Pandey A, Bartolomei MS, De Plaen IG, Wang P, Yu J, Qian J, Tan XD. In inflamed intestinal tissues and epithelial cells, interleukin 22signaling increases expression of H19 Long Noncoding RNA, which promotes mucosal regeneration. Gastroenterology 155: 144–155, 2018. doi: 10.1053/j.gastro.2018.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gort EH, Groot AJ, van der Wall E, van Diest PJ, Vooijs MA. Hypoxic regulation of metastasis via hypoxia-inducible factors. Curr Mol Med 8: 60–67, 2008. doi: 10.2174/156652408783565568. [DOI] [PubMed] [Google Scholar]
- 15.Grosshans H, Johnson T, Reinert KL, Gerstein M, Slack FJ. The temporal patterning microRNA let-7 regulates several transcription factors at the larval to adult transition in C. elegans. Dev Cell 8: 321–330, 2005. doi: 10.1016/j.devcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Guillén U, Cummings JJ, Bell EF, Hosono S, Frantz AR, Maier RF, Whyte RK, Boyle E, Vento M, Widness JA, Kirpalani H. International survey of transfusion practices for extremely premature infants. Semin Perinatol 36: 244–247, 2012. doi: 10.1053/j.semperi.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hempel EV, Bollard ER. The evidence-based evaluation of iron deficiency anemia. Med Clin North Am 100: 1065–1075, 2016. doi: 10.1016/j.mcna.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol 6: 6–23, 2003. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, Konishi I. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol 163: 1437–1447, 2003. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jing SW, Wang YD, Chen LQ, Sang MX, Zheng MM, Sun GG, Liu Q, Cheng YJ, Yang CR. Hypoxia suppresses E-cadherin and enhances matrix metalloproteinase-2 expression favoring esophageal carcinoma migration and invasion via hypoxia inducible factor-1 alpha activation. Dis Esophagus 26: 75–83, 2013. doi: 10.1111/j.1442-2050.2011.01321.x. [DOI] [PubMed] [Google Scholar]
- 21.Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ 15: 667–671, 2008. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Chatterjee I, Gujral T, Alakkam A, Coffing H, Anbazhagan AN, Borthakur A, Saksena S, Gill RK, Alrefai WA, Dudeja PK. Activation of nuclear factor-kappaB by tumor necrosis factor in intestinal epithelial cells and mouse intestinal epithelia reduces expression of the chloride transporter SLC26A3. Gastroenterology 153: 1338–1350.e3, 2017. doi: 10.1053/j.gastro.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Lee J. Hypoxia-inducible factor-1 (HIF-1)-independent hypoxia response of the small heat shock protein hsp-16.1 gene regulated by chromatin-remodeling factors in the nematode Caenorhabditis elegans. J Biol Chem 288: 1582–1589, 2013. doi: 10.1074/jbc.M112.401554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta S, Nijhuis A, Kumagai T, Lindsay J, Silver A. Defects in the adherens junction complex (E-cadherin/ β-catenin) in inflammatory bowel disease. Cell Tissue Res 360: 749–760, 2015. doi: 10.1007/s00441-014-1994-6. [DOI] [PubMed] [Google Scholar]
- 25.MohanKumar K, Namachivayam K, Chapalamadugu KC, Garzon SA, Premkumar MH, Tipparaju SM, Maheshwari A. Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr Res 79: 951–961, 2016. doi: 10.1038/pr.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MohanKumar K, Namachivayam K, Song T, Jake Cha B, Slate A, Hendrickson JE, Pan H, Wickline SA, Oh JY, Patel RP, He L, Torres BA, Maheshwari A. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun 10: 3494, 2019. doi: 10.1038/s41467-019-11199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta 1778: 562–571, 2008. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, Easley KA, Josephson CD. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA 315: 889–897, 2016. doi: 10.1001/jama.2016.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qutub AA, Popel AS. Three autocrine feedback loops determine HIF1 alpha expression in chronic hypoxia. Biochim Biophys Acta 1773: 1511–1525, 2007. doi: 10.1016/j.bbamcr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosebraugh MR, Widness JA, Nalbant D, Veng-Pedersen P. A mathematical modeling approach to quantify the role of phlebotomy losses and need for transfusions in neonatal anemia. Transfusion 53: 1353–1360, 2013. doi: 10.1111/j.1537-2995.2012.03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiou SR, Yu Y, Chen S, Ciancio MJ, Petrof EO, Sun J, Claud EC. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem 286: 12123–12132, 2011. doi: 10.1074/jbc.M110.154625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R, Visintainer PF, Frantz ID III, Shah BL, Meyer KM, Favila SA, Thomas MS, Kent DM. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol 31: 176–182, 2011. doi: 10.1038/jp.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strauss RG. Anaemia of prematurity: pathophysiology and treatment. Blood Rev 24: 221–225, 2010. doi: 10.1016/j.blre.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strobel S, Ferguson A. Modulation of intestinal and systemic immune responses to a fed protein antigen, in mice. Gut 27: 829–837, 1986. doi: 10.1136/gut.27.7.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walthall K, Cappon GD, Hurtt ME, Zoetis T. Postnatal development of the gastrointestinal system: a species comparison. Birth Defects Res B Dev Reprod Toxicol 74: 132–156, 2005. doi: 10.1002/bdrb.20040. [DOI] [PubMed] [Google Scholar]
- 36.Widness JA. Pathophysiology of anemia during the neonatal period, including anemia of prematurity. Neoreviews 9: e520–e525, 2008. doi: 10.1542/neo.9-11-e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. J Pediatr 129: 680–687, 1996. doi: 10.1016/S0022-3476(96)70150-6. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Zhang X, Zhang Y, Zhu D, Zhang L, Li Y, Zhu Y, Li D, Zhou J. HIF-2α promotes epithelial-mesenchymal transition through regulating Twist2 binding to the promoter of E-cadherin in pancreatic cancer. J Exp Clin Cancer Res 35: 26, 2016. doi: 10.1186/s13046-016-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Shi X, Peng Y, Wu M, Zhang P, Xie R, Wu Y, Yan Q, Liu S, Wang J. HIF-1α promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLoS One 10: e0129603, 2015. doi: 10.1371/journal.pone.0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]