Abstract
Ferrets are an attractive mammalian model for several diseases, especially those affecting the lungs, liver, brain, and kidneys. Many chronic human diseases have been difficult to model in rodents due to differences in size and cellular anatomy. This is particularly the case for the lung, where ferrets provide an attractive mammalian model of both acute and chronic lung diseases, such as influenza, cystic fibrosis, A1A emphysema, and obliterative bronchiolitis, closely recapitulating disease pathogenesis, as it occurs in humans. As such, ferrets have the potential to be a valuable preclinical model for the evaluation of cell-based therapies for lung regeneration and, likely, for other tissues. Induced pluripotent stem cells (iPSCs) provide a great option for provision of enough autologous cells to make patient-specific cell therapies a reality. Unfortunately, they have not been successfully created from ferrets. In this study, we demonstrate the generation of ferret iPSCs that reflect the primed pluripotent state of human iPSCs. Ferret fetal fibroblasts were reprogrammed and acquired core features of pluripotency, having the capacity for self-renewal, multilineage differentiation, and a high-level expression of the core pluripotency genes and pathways at both the transcriptional and protein level. In conclusion, we have generated ferret pluripotent stem cells that provide an opportunity for advancing our capacity to evaluate autologous cell engraftment in ferrets.
Keywords: animal models, ferret, induced pluripotent stem cells, multipotent differentiation, reprogramming
INTRODUCTION
Ferrets (Mustela putorius furo) are an attractive mammalian model for a number of diseases, especially those affecting the lungs (35, 44, 64, 68, 79, 82), pancreas (80), intestine (80), liver (2), brain (40), and kidney (39, 92). Use of currently available in vivo models for lung disease focus on rodents, which have a relatively short life span, limiting long-term investigation, and substantial differences in airway epithelial structure and function, has limited the translation of findings in the mouse into effective clinical therapies for humans. Ferrets, like humans, have submucosal glands throughout their proximal cartilaginous airways with the predominant secretory cell type being the mucus secreting goblet cell; in mice, this would be replaced by the club cell secreting more watery surfactants (26, 67). In addition to sharing similar lung physiology (57), ferrets also emulate many clinical features associated with human lung diseases (3, 57, 79, 80). For cystic fibrosis (CF), this includes, among other features: 1) a heterogeneous and severe lung phenotype, in which mucus plugging is observed in both the small and large airways, 2) deflated alveoli (atelectasis), 3) goblet cell hyperplasia, and 4) inflammatory cell infiltration and bacterial colonization (54). Coupled with their relatively small size and airways that are easily accessible by bronchoscopy, this makes them an indispensable model system for human lung diseases. The ferret also provides the opportunity to investigate disease pathophysiology from its inception before the onset of chronic disease: advanced disease stages may not reflect the initiating events of a given disease (77).
Information acquired from the ferret model of CF has significantly enhanced our understanding of CF pathology in multiple organs (80). Pancreatic and intestinal health impacts the quality of life of CF patients and can have a negative impact on lung health: both organs could benefit from gene or cell therapy (23, 56). The ferret, therefore, presents a unique opportunity to evaluate disease and therapeutic options at a variety of stages of CF organ disease (80). Avian influenza viruses (11), obliterative bronchiolitis following transplantation (79), and alpha-1A antitrypsin deficiency-induced emphysema (34) are also replicated well in the ferret model. In addition to the vast array of human lung-associated pathobiologies that the ferret recapitulates, there is also evidence for efficacy in modeling other diseases, including but not limited to tobacco carcinogen-associated hepatocellular carcinoma (2), age-dependent thrombocytopenia syndrome phlebovirus (62), and human primary microcephaly (40). In all of the above-mentioned diseases, ferrets have been shown to recapitulate many of the clinical manifestations of human infection and disease.
Since their discovery, induced pluripotent stem cells (iPSCs) have become a powerful cellular research tool and are being widely used to unravel disease-related molecular mechanisms, in particular, for genetically inherited diseases (33, 86). Furthermore, they hold great potential for regenerative therapy. In some organs, transplanted iPSC-derived cells have been shown to substantially improve tissue function; for example, iPSC-derived cardiomyocytes improve cardiac function in a porcine model of ischemic cardiomyopathy (37, 43, 95). Such patient-specific therapies using iPSCs have not been tested in the lung due to lack of a good animal model.
Given the advantages of using the ferret to model several diseases, it has the potential to be a valuable preclinical model for evaluating gene editing, gene therapy, and regenerative cell therapy. Toward testing cell therapy approaches, we need to generate ferret iPSCs (ftiPSCs) with the capacity for multilineage differentiation. To date, ferret pluripotent cells that can expand in vitro have not been generated: attempts to culture embryonic stem cells (ESCs) have failed and there have been no attempts to create iPSCs. iPSCs provide a self-renewing source of cells that could additionally be used to repair other organs affected by CF, such as the pancreas and intestine. Furthermore, iPSC progeny may be used to manipulate the stem cell niche to maintain the stem cell function of the reparative engrafted cells; for example, it may also be necessary to consider dual cell therapy, replacing hematopoietic cells to reduce the inflammatory environment (25). iPSCs are, therefore, a feasible option for the generation of enough autologous cells to achieve successful engraftment.
The capacity to fully evaluate the conditions essential to success of cellular therapy is not possible in humans; progression toward a cellular therapy for CF and other diseases will require a preclinical animal model system that closely resembles the physiology and function of the human organ and one that also recapitulates the pathogenesis of the human disease. The ferret is an ideal animal model to test the therapeutic potential of these cells, particularly within the lung as 1) ferrets develop pathology similar to that observed in human lungs; 2) the structure and histology of ferret lungs more closely resemble that of the human lung, as opposed to murine lungs; 3) knockout ferrets are now able to be generated; 4) ferret airways are easily accessible by bronchoscopy, making repeated interventions possible; and 5) the ferret genome is now annotated. This study demonstrates the generation of self-renewing, pluripotent cells from ferret somatic cells, providing the first step toward evaluation of autologous cell therapy in the ferret.
MATERIALS AND METHODS
Generation and culture of ferret iPSCs.
Transgene-free ferret iPSCs were generated from early passage ferret fetal fibroblasts (ftFB) isolated at the University of Iowa. All procedures involving live animals were approved by the Institutional Animal Care and Use Committee at the University of Iowa. All cells were screened for mycoplasma before reprogramming. 1×106 ferret fetal fibroblasts were nucleofected with the Epi5 Episomal iPSC reprogramming kit with episomal plasmids carrying human genes POU5F1, SOX2, KLF4, MYCL, LIN28A, mp53DD, and oriP/EBNA-1 (Epstein-Barr nuclear antigen-1) (24) (Thermo Fisher) using the Amaxa human stem cell nucleofector kit (Lonza) and Amaxa nucleofector program U-023 (Lonza). 500,000 cells were plated onto mitomycin-treated mouse embryonic fibroblasts (MEFs; Applied Stem Cell) on Geltrex (Thermo Fisher) coated 100-mm tissue culture dishes in fibroblast media containing 10% fetal bovine serum (FBS; Genesee Scientific), 1% GlutaMAX (Gibco), and 1% MEM nonessential amino acids solution (NEAA; Gibco) in DMEM/high glucose (Thermo Fisher). After 24 h, the media were replaced with custom stem cell media (see Supplemental Methods and Supplemental Figs. S1 to S8 and Supplemental Tables S1 to S7 are all available at https://doi.org/10.6084/m9.figshare.10248668 and Supplemental Table S7 at https://doi.org/10.6084/m9.figshare.10115864) supplemented with 100 ng/mL recombinant human basic FGF (hFGF2; Stem Cell Technologies), and media were changed every 48 h until iPSC colonies emerged. iPSC colonies were observed ~18 days after nucleofection, and single colonies were picked at 28 days. Ferret iPSC lines were maintained on mitomycin-treated MEFs in mTeSR (Stem Cell Technologies) with daily media changes and passaged by dissociation every 4–5 days using ReLeSR (Stem Cell Technologies). Cells were used for experiments between passages 5-15.
Alkaline phosphatase live staining.
At day 28 during reprogramming, cells were live stained for alkaline phosphatase using the alkaline phosphatase saining kit (Thermo Fisher).
Differentiation to three germ layers in vitro.
Embryoid bodies (EBs) were generated from single cell suspensions in ultra-low attachment plates in EB media containing DMEM/high glucose supplemented with, 1% MEM NEAA, 1% GlutaMAX, and 10% FBS. EBs were maintained for 10 days before plating on Geltrex-coated coverslips in EB media. Cells were fixed and stained 14 days later. Ferret iPSCs were differentiated to cells representative of the three germ layers using the Tri-Lineage differentiation kit as per the manufacturer’s guidelines (Stem Cell Technologies).
Definitive endoderm induction.
FtiPSCs were dissociated using TrypLE Express (Gibco), and 200,000 cells were plated per well of a 24-well Geltrex-coated-plate in mTeSR, and 10 μM Y-27632 (Stem Cell Technologies) for 24 h (day 0). On day 1, media were changed to complete serum free differentiation medium (cSFDM): 75% IMDM (Thermo Fisher), 25% Ham’s F12 (Gibco), 0.5× B-27 supplement (Thermo Fisher), 0.5× N-2 (Thermo Fisher), 0.75% albumin, bovine fraction V (RPI, Prospect, IL), 1× GlutaMAX, 50 μg/mL ascorbic acid (Sigma-Aldrich, Ventura, CA), 4.5 × 10−4 M 1-thioglycerol (Sigma-Aldrich), plus 20 ng/mL hFGF, 100 ng/mL recombinant human Wnt3a (R&D Systems, Minneapolis, MN), 1 μM CHIR99021 (Reagents Direct, Encinitas, CA), and 100 ng/mL activin A (Humanzyme, Chicago, IL). On day 2, media were changed to cSFDM containing 100 ng/mL activin A and 1 μM dorsomorphin homology 1 (DMH-1; Sigma-Aldrich), and media was changed daily. Definitive endoderm (DE) cells were evaluated at day 4 by quantitative PCR (qPCR) and immunofluorescence (IF).
Ectoderm induction.
The ectoderm differentiation protocol was adapted from a published protocol by Shi et al. (34). Briefly, ftiPSCs were dissociated by TrypLE Express and plated at 175,000 cells per well of a 24-well Geltrex-coated plate in mTeSR and 10 μM Y-27632. On day 2, half of the media was changed to neural induction media: DMEM/F12 (Thermo Fisher), 1% MEM NEAA, 1% GlutaMAX, 15% KnockOut Serum Replacement (Thermo Fisher), plus 5 μM SB431542 (Selleck Chemicals, Houston, TX), and 50 nM LDN-193189 (Cayman Chemical, Ann Arbor, MI). Media were changed after 48 h and 72 h. On day 6, media were changed to N2 media DMEM/F12, 1% MEM NEAA, 1% GlutaMAX, 1% N2 supplement, plus 10 μM SB431542 and 100 nM LDN-193189. Media were replaced after 24 h and then every 24 h to day 10 with N2 media only. Neural progenitors were evaluated at day 10 by IF and quantitative RT-PCR (qRT-PCR).
Karyotyping analysis.
Chromosomes from female and male ferret iPSCs and fibroblast cultures were harvested and analyzed using the standard GTW banding method (10, 71). Assignment of the chromosomes is based on the ideogram of the domestic ferret (16). Female lines 1, 2, and 4 and male lines 1, 2, and 3, reprogrammed using episomal plasmids, and two female lines, reprogrammed using the Sendai kit, were evaluated for karyotypic abnormalities.
Quantitative PCR.
Total RNA was extracted from cells using the Direct-zol RNA Miniprep kit (Zymo Research). RNA concentration was measured on the Nanodrop spectrophotometer, and 500 ng of RNA was reverse transcribed using high-capacity reverse transcription kit (Applied Biosystems). All primers used for qPCR are detailed in Supplemental Table S1 and PCR products validated in Supplemental Fig. S1B. Amplification was performed using SYBR-Green (Invitrogen) on the Applied Biosystems 7900VT fast real-time PCR system in 384 well format. The PCR conditions were 94°C for 2 min, 94°C for 30 s, 55°C for 30 s, and 72°C for 60 s, 30 cycles, and then 72°C for 10 min in a 5-μl volume.
Teratoma formation.
Ferret iPSCs were expanded onto 100-mm tissue culture plates in the presence of MEF feeder layers and collected by scraping after culturing for 5 days. The protocol was approved by the USC’s Institutional Animal Care and Use Committee (IACUC). Approximately 1 × 107 cells were injected in 100 µl DMEM/F12 containing 10% FBS above each shoulder into immunocompromised NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (Jackson). Mice were euthanized 5 wk later and growths were dissected for histological analysis. Samples were fixed in neutral buffered formalin for 48 h and then transferred to cassettes in 70% ethanol. Samples were embedded in paraffin, sectioned and stained with hematoxylin and eosin.
Immunocytochemistry.
Ferret lung tissues and teratomas were fixed in neutral buffered formalin for 48 h and then transferred to cassettes in 70% ethanol. Tissues were embedded in paraffin, and 5-µm sections placed on microslides in the USC Research Center for Liver Diseases Histology Core. First, tissues were deparaffinized and rehydrated in 100% Xylene (Sigma-Aldrich) and gradually decreased concentration of ethanol from 100%, 95%, 80%, and 75% for each 2–3 min. Antigen retrieval was achieved using citrate antigen retrieval solution (pH 6.0) comprising 10 mM sodium citrate (Abcam), 0.05% polysorbate 20 and samples were boiled for 20 min. Cells were fixed in 4% paraformaldehyde solution (Thermo Fisher) for 15 min at room temperature, washed three times with Dulbecco’s PBS (DPBS). All samples were permeabilized with 0.5% Triton X (Sigma-Aldrich) for 5 min at room temperature before blocking for 1–2 h at room temperature in blocking buffer: 2% albumin, bovine fraction V, and 5% normal donkey serum (NDS; Jackson ImmunoResearch) in DPBS. The cells were incubated in primary antibody diluted in blocking buffer for 4 h at room temperature or overnight at 4°C. Cells were washed three times in DPBS and incubated for 1 h in secondary antibody diluted in DPBS at room temperature. Cells were counterstained with DAPI for 5 min before mounting in Fluoromount-G (Southern Biotech). Epifluorescence images were taken on the Revolve microscope in its upright configuration (Echo Laboratories), and confocal images on a Zeiss LSM 800 confocal microscope in the USC Optical Imaging Core were processed using ImageJ. All antibodies are listed in Supplemental Table S2.
Western blot analysis.
Protein was extracted from cells in 2% SDS lysis buffer containing protease inhibitor cocktail (1:100, Thermo Scientific). Protein concentrations were measured using DC Protein Assay (Bio-Rad) and 10 μg of protein samples were equally loaded in a 12% Tris-glycine SDS-PAGE gel. Proteins were electrophoretically transferred onto Immuno-Blot PVDF membranes (Bio-Rad) and blocked in 5% nonfat milk. Primary antibodies included LIN28A (Cell Signaling), OCT4 (Cell Signaling), and SOX2 (Thermo Fisher). Blots were incubated with horseradish peroxidase-linked anti-IgG conjugates (Promega) for 1 h at room temperature. Samples were visualized by Pierce ECL Western blotting substrate (Thermo Scientific) or SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific) with an Azure Biosystems c300 Imaging System. Complete Western blots are included in Supplemental Fig. S1.
High-throughput RNA sequencing.
For RNA isolation and library preparation, RNA was prepared using the Direct-zol RNA Miniprep kit (Zymo Research) according to the manufacturer’s instructions. Strand-specific library preparation was carried out using a KAPA Stranded mRNA-Seq Kit, with KAPA mRNA Capture Beads (KAPA Biosystem). Sequencing libraries were validated using the Agilent Tape station 4200 (Agilent Technologies, Santa Clara, CA), and quantified by using Qubit 2.0 Fluorometer (Invitrogen), as well as by qPCR (Applied Biosystems). The sequencing libraries were multiplexed and clustered in one lane of a Flow Cell. The samples were sequenced using a 2 × 150 paired end (PE) configuration. Image analysis and base-calling were conducted by the HiSeq Control Software (HCS). Raw sequence data (.bcl files) generated from Illumina HiSeq were converted into Fastq files and demultiplexed using Illumina’s bcl2fastq 2.17 software. One mismatch was allowed for index sequence identification. RNA-seq was performed by GENEWIZ. For bioinformatic analyses of RNA-seq, the quality of Fastq files was checked with FastQC (Baraham Bioinformatics group, http://www.bioinformatics.babraham.ac.uk/). Supplemental Fig. S2 demonstrates our experimental workflow following quality validation of the sequence reads. Reads were trimmed for quality score and adaptor sequences were also removed. The high-quality reads (between 30 and 45 million) that passed quality filters were aligned to the human reference genome (GRCh38) using STAR aligner, in conjunction with the gene model from Ensemble 92. Reads were quantitated by counting the number of reads across exons. Differentially expressed genes were identified by using criteria false discovery rate (FDR)-adjusted P < 0.05 in DEseq (8). As detailed previously, DesEQ2 performs a likelihood ratio test that compares how well a gene count data fits a full model (with independent variable time) compared with a reduced model without those variables (51). Unless specified, hierarchical clustering, principal component analysis, and statistical analysis were performed in R (http://www.r-project.org). A Z-score algorithm was used to identify biological functions that are expected to be more active (positive Z-score) or repressed (negative Z-score) in deferentially expressed genes between iPSCs and fibroblasts. To enhance the stringency of our analysis, we considered functions with a Z-score ± 2. An overlapping P value was calculated with the Fisher’s exact test that reflects the likelihood of significant association between a set of genes in our data set and a biological function (FDRp < 0.05).
Statistical analysis.
All data are presented as means ± SE. Statistical analysis was performed using the paired Student’s t test with a value of P < 0.05 being considered statistically significant.
RESULTS
Generation of iPSCs from ferret fibroblasts.
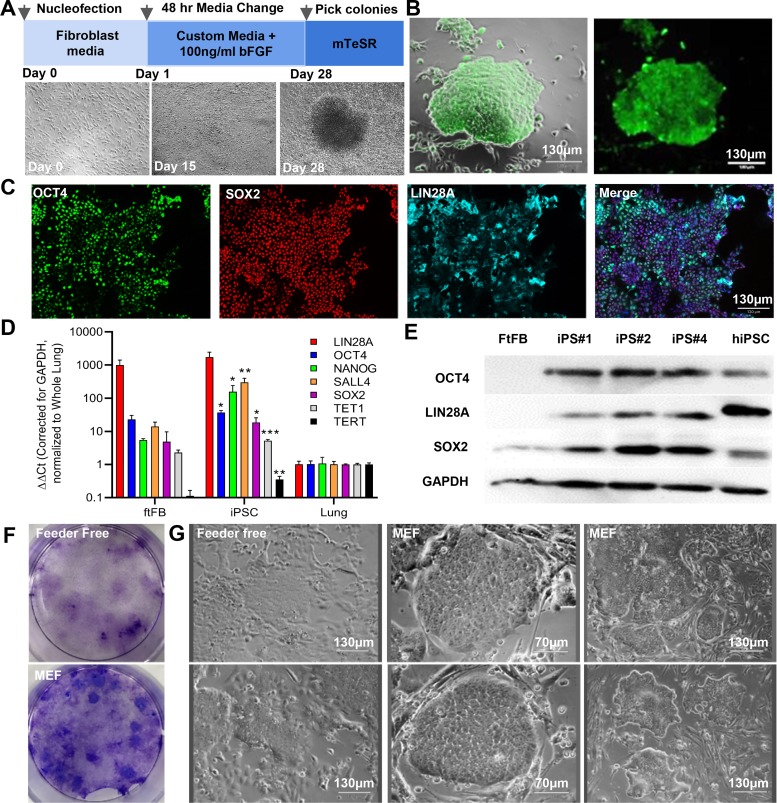
FtiPSCs were generated from fetal fibroblasts isolated from outbred ferrets (ftFBs) at passage 4 using episomal vectors carrying the human reprogramming genes, chosen because of the poor annotation of ferret pluripotency genes and their successful use in reprogramming cells from multiple species (12, 15). Over a 28-day reprogramming period (Fig. 1A), we observed the generation of compact, iPSC-like colonies, as indicated by cell morphology (Fig. 1B and Supplemental Fig. S3) and by live cell alkaline phosphatase (AP) staining (Fig. 1B). AP-positive colonies with iPSC morphology were picked on day 28 and expanded on mitomycin-treated MEFs in mTeSR. Using quantitative RT-PCR and primers specific to ferret genes, we evaluated the expression of endogenous pluripotency genes. Pluripotency-associated genes Octamer-binding transcription factor 4 (OCT3/4 or POU5F1), NANOG, sex-determining region Y-box (SOX2), Lin-28 homolog A (LIN28A), Reduced expression 1 (REX1), Sal-like protein 4 (SALL4), telomerase reverse transcriptase (TERT) and Ten-eleven translocation methylcytosine dioxygenase 1 (TET1) were all upregulated in ftiPSCs compared with the initial ftFBs (Fig. 1D). It should be noted that KLF4 and C-MYC are strongly expressed in both ftFBs and pluripotent cells, and thus no substantial increase was observed at the RNA level (data not shown). At the protein level, we observed expression of OCT3/4, LIN28A, and SOX2 by immunofluorescence (Fig. 1C) and increased expression by Western blot (Fig. 1E and Supplemental Fig. S1A). The initial fibroblast cultures did not stain with these antibodies (Supplemental Fig. S1D). Surprisingly, while the fibroblasts used for reprogramming were all karyotypically stable, there was a high tendency for female ftiPSCs to lose an X chromosome during reprogramming phase. Three of five female lines evaluated for karyotype all lost a single X chromosome by passage 1, whereas male ftiPSCs all retained normal karyotype (3/3 evaluated, Supplemental Fig. S3). The data in the current manuscript provide a summary of a minimum of three independent ftiPSC lines; with representative images shown for iPS#1, additional characterization of lines iPS#2 and iPS#4 are included in Supplemental Fig. S3. The expression of the episomal reprogramming vectors in the reprogrammed ferret iPSC colonies was evaluated at a variety of passages by PCR for EBNA-1, present in all five episomal plasmids. EBNA expression was not detected in ftiPSC lines after 10 passages (Supplemental Fig. S4).
Fig. 1.
Generation of induced pluripotent stem cells from ferret fetal fibroblasts. A: schematic showing the timeline for reprogramming with representative phase-contrast images of ferret induced pluripotent stem cells (iPSC) colony formation. B: live cell alkaline phosphatase (green) staining of a ftiPSC colony. C: representative immunofluorescence images showing expression of pluripotency genes OCT4 (green), SOX2 (red), and LIN28A (cyan). Nuclei are counterstained with DAPI (blue). D: quantitative PCR analysis of ferret pluripotency genes in reprogrammed cells at passage 6–10 compared with ferret fetal fibroblasts (ftFB) and normalized to ferret fetal lung (ftLung). *P < 0.05, **P < 0.01, and ***P < 0.01; n = 4–6 biological replicates each with n = 3 technical replicates. E: Western blots for pluripotency genes OCT4, LIN28A, SOX2, and internal control GAPDH. FtFBs represent the negative control and human iPSCs (hiPSCs) a positive control. F: crystal violet staining of reprogramming day 28 in the presence and absence of MEF feeders. G: representative phase-contrast images of iPS#1 at P4 in the presence and absence of MEF feeders. Scale bars represent 70 and 130 μm, as indicated.
A variety of reprogramming and culture conditions were tested to determine the best methodology for supporting ferret reprogramming and pluripotency; the conditions are listed in Supplemental Table S3. iPSCs are known to exist in either 1) a primed FGF2-dependent state or 2) a naïve LIF-dependent state. Similar to the primed pluripotent state of human iPSCs, pluripotency of ftiPSCs was maintained in media containing FGF2, but not in other media formulations, including FGF2+ leukemia inhibitory factor (LIF), FGF2+2i (GSK 3β inhibitor, CHIR 99021 and MEK inhibitor PD0325901), LIF alone, 2i alone, or LIF+2i (Supplemental Fig. S5A). The only cells that survived and maintained colony formation after 72 h in the different culture conditions were those cultured in the presence of FGF2. FtiPSCs proliferate on Geltrex, or Matrigel (data not shown), in the presence and absence of feeder cells, but do not adhere, and grow on gelatin-coated dishes in any media (Supplemental Fig. S5B). It should also be noted that ftiPSCs were more efficiently generated, and maintained more consistent colony morphology, with the addition of a MEF feeder layer to the Geltrex (Fig. 1, F and G). Colonies grown on Geltrex alone, for example, tended to flatten and spread, losing their distinguishing boundaries (Fig. 1G). Reprogramming in LIF, 2i, or 3i (2i plus Y-27642, Rho-A kinase inhibitor) containing media did not generate any viable colonies; only in the presence of 100 ng/mL FGF2 were colonies derived and picked for expansion (conditions 1, 8, and 9 in Supplemental Table S3).
RNA-seq confirms an upregulation of a pluripotency signature in ftiPSCs.
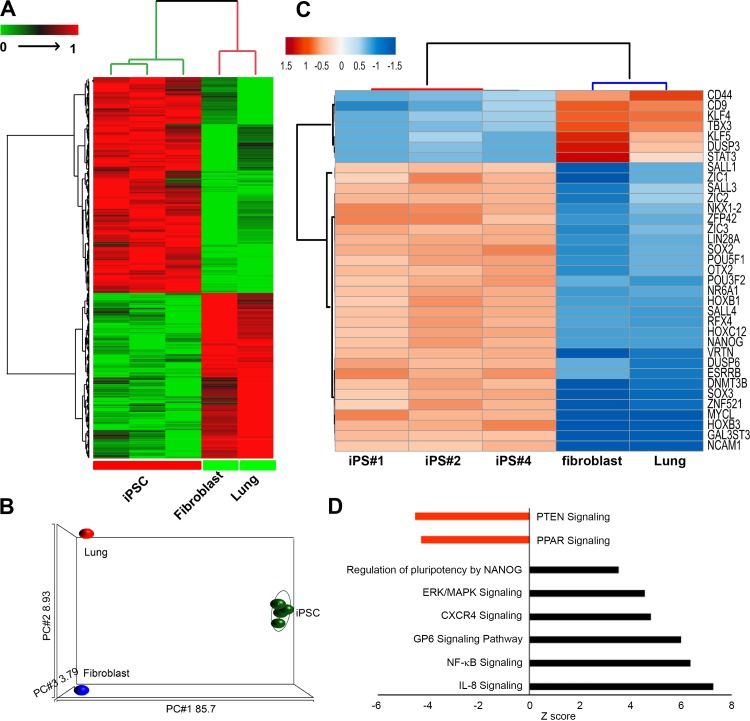
We generated a high-quality RNA-seq data, as summarized in Supplemental Tables S4 and S5 and Supplemental Fig. S2, from three independent ferret iPSC lines (iPS#1, #2, and #4) with an overall, mapping efficiency of ~90% (Supplemental Table S6). A two-way hierarchal clustering using Euclidean distance and average linkage suggests that the ftiPSC transcriptomes uniquely cluster together independent of the fibroblasts used for reprogramming and an unassociated control of ferret adult whole lung RNA (Fig. 2A) and the principle component analysis (Fig. 2B and Supplemental Fig. S2). A total of 2,754 genes are differentially expressed between the iPSCs, as compared with the control cells (fibroblasts) and tissues (lung). Of these 2,754 differentially expressed genes, 1,426 are upregulated and 1,328 downregulated in ftiPSCs (Supplemental Table S7). Evaluation of a subset of 35 genes, common genes associated with a pluripotent transcriptional signature, indicated an upregulation of 28 and downregulation of 7 genes in all three ftiPSC lines (Fig. 2C). We observe a significant upregulation of core pluripotency genes, including POU5F1 (OCT3/4), SOX2, LIN28A, L-MYC, and NANOG in the ftiPSCs. The heterochromatin-associated protein SALL1, a direct cofactor for NANOG signaling, SALL4, important for the regulation of the OCT3/4 and NANOG in regulating the pluripotency transcriptional network and ESRRB, a downstream target of Nanog, are also enriched in ftiPSCs (53, 55, 59, 78). This profile supported by the upregulation of genes in the transcriptional pathway associated with Nanog signaling in ftiPSCs (Fig. 2D). We additionally observed an enrichment of the genes corresponding to ERK/MAPK, CXCR4, GP6, NF-κB, and IL-8 signaling pathways and a downregulation of PTEN and PPAR signaling (Fig. 2D).
Fig. 2.
Differentially expressed genes between ferret induced pluripotent stem cells (ftiPSCs), fibroblasts, and lung tissue indicate a pluripotency signature in ftiPSCs. A two-dimensional hierarchical clustering (A) and principal component analysis (PCA) plot (B) of differentially expressed genes [false discovery rate-adjusted P value (FDRp) < 0.05] between ftiPSCs vs. ftFBs and whole lung showing a distinct expression pattern of iPSCs as compared with fibroblast and whole lung. C: a hierarchical clustering of a subset of differentially expressed [FDRp < 0.05; fold change (FC) ± 2] pluripotent genes showing a unique expression profile of ftiPSC cells. D: enrichment of various pathways in differentially expressed genes among ftiPSCs and fibroblasts identified in A.
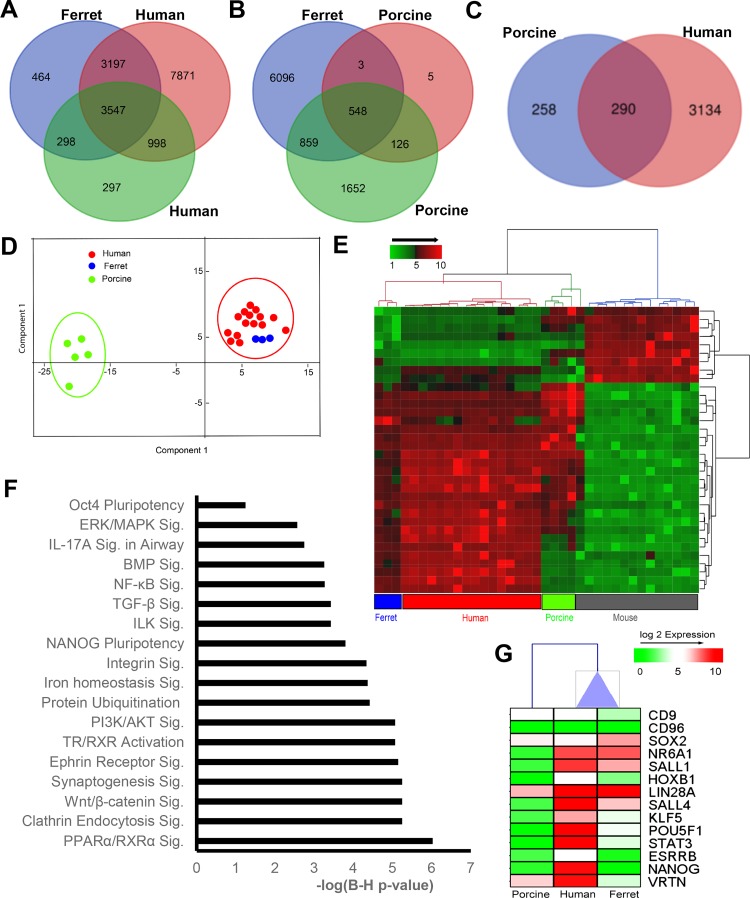
To compare the pluripotent signature of our ftiPSCs to that of existing and well-studied iPSCs from different species, we pooled our three ferret iPSC lines and compared with publicly available expression data sets from two human (GSE15148 and GSE67915), two mouse (GSE28262 and GSE127927), and two porcine iPSC lines (GSE92889 and GSE119664). As can be seen in Fig. 3, comparison of the significantly expressed genes in ferret iPSCs to those of human, porcine and mouse iPSCs indicates that the ferret iPSC transcriptomic profile is most similar to primed human iPSCs, sharing 3,547 common genes among all data sets (Fig. 3A). Ferret and porcine showed a set of 548 common genes (Fig. 3B), while the naïve murine iPSC shared a total of 7% genes with ferret iPSCs (data not shown). All of the 548 consensus genes shown in Fig. 3B were used to assign orthologs for both human and porcine. Since, both human and porcine iPSC show a significant level of overlap with ferret signatures, we further studied the relationship between FGF2-dependent human and porcine iPSCs with that of ftiPSCs. Any differences may reflect the challenges in maintaining both ferret and porcine iPSC over a high number of passages and will direct further studies to determine the differences in porcine, ferret, and human pluripotency. From a set of 290 consensus genes (Fig. 3C) between ferret and both human and porcine homologs principle component analysis confirms a clustering of the ferret and human iPSC away from the porcine iPSC (Fig. 3D). To extrapolate consensus genes among all data sets, all common genes were interconverted to the human orthologs, resulting in only 34 genes present in all the analyzed data sets (Supplemental Fig. S6A). Hierarchical clustering of these genes across data sets also supports clustering of the ferret and human iPSC distinct from the porcine and mouse iPSC (Fig. 3E). Ferret iPSCs have enrichment of several known pluripotency-mediated pathways, including that of OCT3/4- and NANOG-dependent stem cell pluripotency (83, 89) and NF-κB signaling (84). Additionally, BMP signaling (7) and Wnt/β-catenin signaling (21) have also been reported to be associated with human pluripotency (Fig. 3F). Furthermore, comparison of the relative expression of pluripotency genes across ferret, human, and porcine iPSCs (Fig. 3G) indicates a greater similarity of ferret iPSCs and human iPSCs than porcine iPSCs, which have a substantially lower expression level of key pluripotency genes POU5F1, SALL1, SALL4, KLF5, and STAT3. While SALL1, LIN28A, and SALL4 are similarly expressed in human and ferret iPSCs, other key pluripotency genes POU5F1, NANOG, ESRRB, and STAT3 are expressed but at a relatively lower expression level (Fig. 3G). Given the differential gene expression among individual iPSC transcriptomic data sets, we can conclude that our ferret iPSCs are most like human iPSCs and share common profiles with both human and porcine iPSCs. We will continue to utilize the information acquired in this study to improve the ferret iPSC, covert them to naïve iPSC, and evaluate true pluripotency through tetraploid complementation, these studies are extensive and beyond the scope of the current manuscript.
Fig. 3.
Comparative global induced pluripotent stem cells (iPSC) gene signature profiles generated from human and porcine. A Venn diagram showing overlap in the significantly expressed genes in ferret iPSCs with human (A) and porcine (B) iPSCs. C: 290 common genes expressed in ferret iPSCs overlapping with both human and porcine iPSCs. D: unsupervised principal component analysis (PCA) plot of the expression profile of 290 consensus genes among ferret, human, and porcine. Each circle represents an individual sample. E: hierarchical clustering, based on Euclidean distance and average linkage, of 34 consensus genes expressed in ferret, human, porcine, and mouse iPSC data sets. F: pathways enriched in common consensus genes among ferret versus porcine or human iPSCs (refer to C: 290 genes). G: one-way hierarchal clustering of the expression profile of key pluripotent genes in all three species: ferret, human, and porcine. Ferret showed an expression profile closer to humans.
Multilineage differentiation capacity of ftiPSCs.
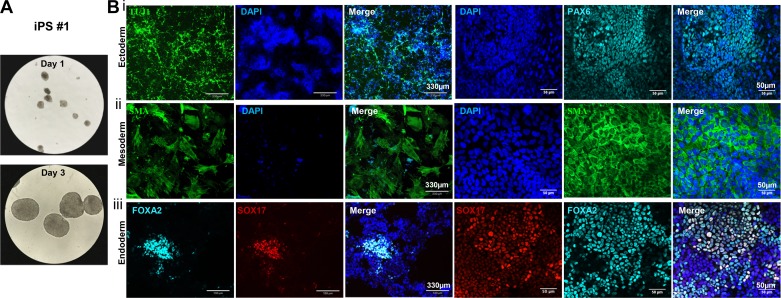
Multilineage differentiation capacity is a critical characteristic of pluripotent cells, both in vivo and in vitro. We evaluated the differentiation capability of ftiPSCs through both serum-stimulated spontaneous differentiation and lineage-directed differentiation in vitro and teratoma formation in vivo. For spontaneous differentiation, we generated EBs in ultra-low attachment plates (Fig. 4A) for 10 days and then transferred to Geltrex-coated plates for an additional 14 days before analysis for expression of markers of the three germ layers (38). Analysis of the final cultures using a panel of lineage-specific antibodies indicated differentiation to 1) ectoderm by expression of PAX6 and β-tubulin (TUJ1) (Fig. 4Bi), 2) mesoderm by expression of Brachyury and smooth muscle α-actin (SMA) (Fig. 4Bii), and 3) endoderm by expression of SRY-box containing gene 17 (SOX17) and Forkhead box A2 (FOXA2) (Fig. 4Biii).
Fig. 4.
Spontaneous differentiation capacity of ferret induced pluripotent stem cells (ftiPSCs). A: representative phase-contrast images of embryoid bodies (EBs) at days 1 and 3 of differentiation for iPS#1. B: representative immunofluorescence images of proteins expressed in each of the three germ layers for iPS#1: i) ectoderm: TUJ1 (green, left) and PAX6 (cyan, right); ii) mesoderm: smooth muscle α-actin (SMA, green), and iii) endoderm: FOXA2 (cyan), SOX17 (red). Scale bars represent 330 µm (left) and 50 µm (right) and all nuclei were counterstained with DAPI (blue).
FtiPSCs were also able to respond to cues to differentiate specifically into cells from all three germ layers. Neural progenitor cells were generated following an adapted protocol published by Shi et al. in 2012 (72). After driving ftiPSCs toward a neural lineage, we observed the formation of neural rosettes that expressed TUJ1 (neuron-specific Class III β-tubulin), SOX2, and PAX6, which are all associated with neural stem cells (74, 97) (Supplemental Fig. S7A). PAX6 plays a critical role in central nervous system (CNS) development and is an essential transcription factor for neural stem cell proliferation, multipotency, and neurogenesis in many regions of the CNS (61). Induction of neural progenitors was also stimulated using commercially available neural induction media from Stem Cell Technologies resulting in a similar expression of PAX6 and SOX2 after 7 days of differentiation (Supplemental Fig. S7B). TUJ1 expression was not present in our ferret iPSCs and only increased when iPSCs were cultured in differentiation conditions (Supplemental Fig. S7C).
Mesoderm arises during gastrulation and will eventually give rise to muscle, connective tissue, kidneys, and adrenal glands (45). After 5 days in mesoderm-induction media, ferret iPSCs expressed Brachyury, which has a direct role in the early events of mesoderm formation (27, 50) and smooth muscle α-actin (ACTA2 or SMA) (Supplemental Fig. S8A). Comparison by qRT-PCR shows upregulation of paraxial mesoderm marker, TBX6, and the lateral plate mesoderm transcription factor, FOXF1 (n = 4, experimental replicates). There are notable differences in the efficiency of differentiation across iPSC lines, indicated in the bar charts, resulting in a high variance in the data (17, 42, 52, 87, 90) (Supplemental Fig. S8A).
Definitive endoderm (DE) gives rise to lungs, thyroid, pancreas, liver, and intestines and is specified from the anterior primitive streak (APS). Initial DE induction, using Stem Diff (Stem Cell Technologies), was inefficient (<10%), as shown in the images showing FOXA2 and SOX17 expression after 5 days (Supplemental Fig. S8Ci). The addition of mycophenolic acid (MPA) to the media substantially increased the efficiency of generating FOXA2, SOX17 expressing DE (Supplemental Fig. S8Cii). A concomitant reduction in neuronal cells was observed in the presence of MPA, similar to the observations made by Nakashima and colleagues (2015) increasing pancreatic endoderm specification (58). The APS is induced through strong and synergistic activation of nodal and canonical Wnt signaling during gastrulation. This can be mimicked in culture using activin A and Wnt3a or Wnt agonist CHIR99021 (47). The APS can be pushed to DE through persistent activation of nodal signaling and inhibition of bone morphogenetic protein (BMP), using DMH-1, to suppress bias toward mesoderm differentiation. Further modification of the protocol replacing commercially available STEMDiff DE with activin A and DMH-1 resulted in an increased efficiency of DE specification to >70% of DAPI cells on day 5 from 20% in our original conditions (Supplemental Fig. S8Ciii). qRT-PCR supports the IF data indicating upregulation of FOXA2, SOX17 and CXCR4 mRNA (Supplemental Fig. S8D). Variability between iPSC lines is indicated in Supplemental Figs. S7 and S8, resulting in a high variance in the data (n = 4, experimental replicates). Importantly, SOX7, present in primitive, parietal, and visceral endoderm but not in DE, is not upregulated in ftiPSC-derived DE, supporting the specificity of induction (Supplemental Fig. S8D).
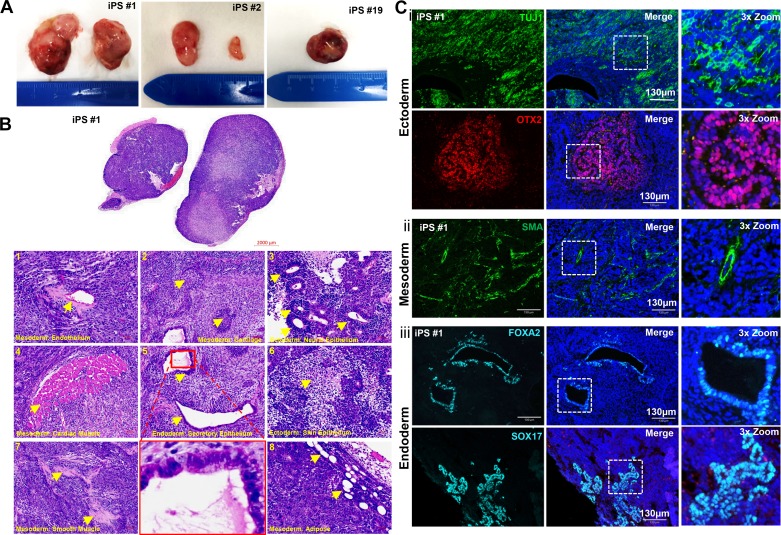
Teratoma formation in vivo remains a key index of pluripotency for iPSCs and ESCs. After subcutaneous administration of ftiPSC into NSG immunocompromised mice, tumors formed as early as 3 wk, and because of their rapid growth had to be harvested before 5 wk (Fig. 5A). The H&E staining of the teratomas, shown for iPS#1 and replicated in iPS#2 and #4 (data not shown), confirms the presence of cells differentiating toward all three germ layers and includes evidence for endothelial cells, cartilage, neural epithelium, cardiac muscle, secretory epithelium, skin epithelium, smooth muscle cells, and adipose tissue as shown in Fig. 5B, 1 through 8, respectively. We further confirmed multilineage differentiation by staining for markers specific to ectoderm, mesoderm, and endoderm (Fig. 5C). The teratomas contained a high percentage of ectodermal cells, indicated by the extensive TUJ1 and OTX2 staining (Fig. 5Ci). In addition, we detected SMA expressing smooth muscle surrounding artery-like structures, confirming mesoderm differentiation (Fig. 5Cii) and FOXA2 and SOX17 in airway-like structures, confirming definitive endoderm differentiation (Fig. 5Ciii). The relatively short growth period for the teratomas may account for a lack of more mature structures in the teratomas such as pigmented epithelium, cartilage, and keratin whorls.
Fig. 5.
Teratoma formation from ftiPSCs. A: images of teratomas formed from iPS lines #1, #2, and #19. B: representative H&E-stained teratoma sections from iPS#1 showing: 1, endothelial cells; 2, cartilage; 3, neural epithelium; 4, cardiac muscle; 5, secretory epithelium; 6, squamous epithelium; 7, smooth muscle; and 8, adipose cells. Representative cells are indicated by yellow arrowheads. Ci: representative immunofluorescence images for iPS#1 showing TUJ1 (green) and OTX2 (red)-expressing ectodermal cells. ii: smooth muscle α-actin (SMA; green)-expressing mesodermal cells. iii: FOXA2 (cyan) and SOX17 (cyan)-expressing endodermal cells. The white boxes indicate the regions featured in the 3× zoom (right panels). Scale bars represent 2,000 µm (B, whole teratoma), 50 µm (B, panels), and 130 µm (C) and all nuclei are counterstained with DAPI (blue).
DISCUSSION
With the advancing development of stem cell-derived therapeutics, the notion of using personalized therapies to replace injured or diseased tissues is progressing as an innovative therapeutic approach. In this study, we have generated iPSCs from ferret fibroblasts as the first step toward evaluating iPSC-derived cells as an autologous cell therapy for lung and other organ diseases, in which in vivo evaluation of cell engraftment is not plausible in humans. Therefore, we sought to select a large animal model system with a lung structure and function that closely resembles that of humans. Rodent models have several advantages, including cheaper housing requirements compared with large animals, and they are well characterized and readily genetically manipulated. Unfortunately, mouse models do not often recapitulate human lung disease, limiting their usefulness for evaluating cell therapy (54). Furthermore, the expression of lung disease-related genes is different between mice and humans. For example, in CFTR knockout mice, mutations in the CFTR gene do not result in the same CF symptoms seen in humans. Mice develop a milder lung disease phenotype compared with humans likely due to compensatory upregulation of alternative chloride transport systems (28). Both ferrets and pigs can serve as large animal models with lung pathophysiology closely reflecting that of humans, particularly in the case of CF (66, 76, 80–82, 85, 91). The ferret model has already been extensively used in the study of lung infections and influenza virus (30, 69, 75, 88, 96). Additionally, although lung transplantation is an effective therapy for end-stage lung disease, long-term viability of transplanted lung allografts is limited by chronic rejection. An orthotopic model of ferret lung transplant has been shown to accurately phenocopy human chronic rejection (79), and a study using this model and corroborated in humans recently discovered that airway stem cells are depleted in allografts as rejection sets in Ref. 82. Thus, the ftiPSCs described here may be used to explore the therapeutic potential of iPSC derivatives to treat chronic rejection following lung transplant, as well as other lung diseases. Finally, the ease of use of the ferret compared with the pig model system led to the selection of the ferret for this study.
As mentioned previously, ferret pluripotent stem cells, ESCs, or iPSCs have yet to be successfully derived or maintained in vitro. The aim of the current study was to directly reprogram ferret somatic cells to a pluripotent state. We initially reprogrammed these cells using Sendai virus as an efficient and nonintegrating delivery method. Although we were able to successfully reprogram the ferret fibroblasts, the cells maintained a high level of the Sendai virus, and thus expression of the exogenous pluripotency genes, for over 40 passages (data not shown). Continued overexpression of the exogenous genes is likely to confound further differentiation of the cells and increase the risk of tumor formation in vivo (29). It is worth noting that retention of Sendai virus has also been observed for porcine iPSC (19, 20). To avoid potential complications and to evaluate endogenous pluripotency in the ferret, we changed our reprogramming strategy to nonintegrating, episomal plasmids. After 28 days, ferret iPSC colonies exhibiting the characteristic hallmarks of pluripotency described in this manuscript could be picked and expanded. Cell surface markers were not detected in our ftiPSCs, a phenomenon also observed for bovine and ovine iPSCs, which do not express detectable SSEA-1, SSEA-3, SSEA-4, Tra-1-60, and Tra-1-81 (9). In the absence of ferret ESCs, we do not have a positive control to test the reactivity of antibodies against pluripotency genes in our ferret cells. For example, the antibodies available for Tra1-81 only share 44.6% homology with ferret and are, thus, not predicted to cross-react with ferret epitopes. Therefore, we are currently unable to determine whether the cells do or do not express these proteins/carbohydrates on their cell surface.
The transcriptomic analysis provided more in-depth characterization of the pluripotency signature for ftiPSCs. As expected, CD44 is highly expressed in ferret fetal fibroblast and absent in fully established ftiPSCs (65) and CD9, known to be dispensable for maintenance of mouse pluripotency, is not expressed by ftiPSCs (5). Krüppel-like factors (KLFs), including Klf4 and Klf5, have largely overlapping functions in maintaining pluripotency and can differentially regulate murine ESC differentiation to mesoderm and definitive endoderm (4). Surprisingly, the expression of both genes is decreased in ftiPSCs compared with the original fibroblasts; however, they are still robustly expressed.
The “naïve,” preimplantation pluripotent state of mouse iPSCs precedes the “primed,” epiblast pluripotent state that defines human iPSCs. This state is functionally and molecularly distinct from the early inner cell mass (13). “Naïve” pluripotent cells can transition to an epiblast state where expression dynamics resemble that observed in the late blastocyst, including decreased Nanog expression and upregulated OTX2 (1, 93). Other genes with augmented expression include SOX3, SALL2, and de novo methyltransferases DNMT3B and DNMT3B (14). Of these, the ftiPSCs express SOX3, DNMT3B, and OTX2, as shown in our transcriptomic profile, suggesting their existence in a state more reflective of the “primed” epiblast cells. NANOG, and associated genes such as ZIC3 and ESRRB, are significantly upregulated in ftiPSCs and are known to be important in maintaining pluripotency through inhibition of endodermal differentiation (22, 49). It should be noted, however, that these genes are expressed at a lower level than other pluripotency genes across the three ftiPSC lines evaluated. It is important to mention that there is substantial disconcordance in the NANOG transcripts between ferret and human, while POU5F1 and SOX2 retain significant homology across species, this may account for the differential expression of Nanog and related genes (Supplemental Fig. S6); indeed NANOG may not have the same role in ferret iPSCs, an avenue that we are continuing to investigate. Interestingly, one of the highest differentially expressed genes compared with the lung and fibroblast controls is Let-7 target gene, NR6A1, also known as germ cell nuclear factor (GCNF). NR6A1 is known to be a repressor of Nanog signaling (31, 60). Its high expression may be influencing pluripotency maintenance in ftiPSCs; its relative expression, however, is akin to that observed in human iPSCs (Fig. 3E). DUSP3, which our transcriptomic data indicate is not highly expressed in our ftiPSCs, does not appear to be as critical for maintenance of Nanog signaling and pluripotency as DUSP6 is, which is significantly upregulated (94). Interestingly, TBX3, a critical regulator of DPPA3, through repression of Wnt signaling, and of maintaining mouse pluripotency, is also absent in ftiPSCs (70, 98). In human iPSCs, TBX3 has been shown to increase the efficiency of reprogramming, although TBX3 knockout iPSCs still retain all the features of pluripotency, indicating that it is not a critical regulator of the “primed” state of pluripotency (46).
NF-κB signaling was among the most highly represented pathways that was differentially expressed between fibroblasts and ftiPSCs. The precise role of NF-κB in iPSC is not entirely clear. Augmentation of NF-κB activity has been observed in human pluripotent cells, and its expression decreases during differentiation. Repression of NF-κB signaling by p65 siRNA has been shown to reduce both OCT4 and NANOG expression, suggesting the pathway plays a role in the maintenance of pluripotency (84). ERK/MAPK signaling is also surrounded by conflicting reports regarding its precise role in regulating pluripotency. It is currently understood that FGF2 maintains a basal level of ERK activity that supports self-renewal and that can be upregulated when growth factors are deprived (73). Inhibition of PTEN can support the generation of “naïve” mouse pluripotent cells through inhibition of PI3K signaling (48). In human pluripotency, there is also evidence that PTEN is a key regulator of hESC fate; though PTEN knockdown was associated with upregulation of pluripotency network genes and increase in TRA-1-81-expressing cells (6). Together, the analysis of gene expression and pathway upregulation suggests our ftiPSCs are in a “primed” pluripotent state (32, 41), validating their dependence on FGF2.
Our Sendai virus-retaining ftiPSCs maintained a normal karyotype, while reprogramming with episomal vectors resulted in a loss of an X chromosome in all our female iPSC lines by Passage 2. It is known that pluripotent stem cells are associated with chromosomal loss or aneuploidy, in particular, at chromosomes 1, 12, 17, 20, and X, which are believed to assist in maintaining the pluripotent state (36, 99). The instability of XX karyotype has been documented in ESC (99) and may be epigenetically driven. DNA methylation has been reported to be directly related to the abnormal XX karyotype of ES cell lines (63). Hypomethylation may provide the selective pressure favoring deletion of one of the X chromosomes, and overall methylation takes place after the loss of one of the X chromosomes (XO state) (18, 99). Addressing the stability of the X chromosome in female ftiPSCs is ongoing in our laboratory and will be critical moving toward preclinical studies.
In conclusion, we have generated induced pluripotent stem cells from ferret somatic cells capable of directed differentiation to all three germ layers, providing an exciting first step toward evaluating the potential of iPSC-derived autologous cell therapy for human lung disease modeled in the ferret. While our data demonstrate the capacity for generating ftiPSCs for the first time, there are several issues that still need to be addressed to more effectively maintain these cells. First, although FGF2 was able to maintain a pluripotent-like state in our cells, it remains to be determined whether this condition is optimal for reprogramming and for the maintenance of ftiPSCs. The cells express genes characteristic of pluripotent cells; gradually, evaluated over 22 passages, pluripotent gene expression gradually decreases (data not shown). Second, female ftiPSCs are karyotypically unstable and readily lose an X chromosome. More favorable conditions are likely needed to maintain karyotypic stability in female ftiPSCs. Ultimately, the generation of germline-competent chimeras by injection of ftiPSCs into ferret postimplantation embryos will be the true test of pluripotency.
GRANTS
A.L.R. and K.P. are supported by Cystic Fibrosis Foundation Therapeutics FIRTH15XX0. A.L.R. is also supported by the Hastings Foundation. J.F.E. is supported by National Heart, Lung, and Blood Institute Grant R24 HL-123482 and National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-054759.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.G., S.P., X.S., L.A.B., J.F.E., K.R.P., and A.L.R. conceived and designed research; J.G., S.P., X.S., L.A.B., T.J.L., C.-L.H., R.E., Z.L., K.R.P., and A.L.R. performed experiments; J.G., S.P., X.S., L.A.B., T.J.L., C.-L.H., R.E., Z.L., V.P., K.R.P., and A.L.R. analyzed data; J.G., S.P., X.S., T.J.L., C.-L.H., V.P., K.R.P., and A.L.R. interpreted results of experiments; X.S. and A.L.R. prepared figures; J.G., S.P., J.F.E., and A.L.R. drafted manuscript; V.P., J.F.E., K.R.P., and A.L.R. edited and revised manuscript; J.F.E., K.R.P., and A.L.R. approved final version of manuscript.
ACKNOWLEDGMENTS
Microscopy was performed in the Optical Imaging Core, Keck School of Medicine of the University of Southern California (USC). Histology sample preparation was performed in the USC Research Center for Liver Diseases, Liver Histology Core.
REFERENCES
- 1.Acampora D, Omodei D, Petrosino G, Garofalo A, Savarese M, Nigro V, Di Giovannantonio LG, Mercadante V, Simeone A. Loss of the Otx2-binding site in the Nanog promoter affects the integrity of embryonic stem cell subtypes and specification of inner cell mass-derived epiblast. Cell Rep 15: 2651–2664, 2016. doi: 10.1016/j.celrep.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa K, Liu C, Tang S, Veeramachaneni S, Hu KQ, Smith DE, Wang XD. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int J Cancer 139: 1171–1181, 2016. doi: 10.1002/ijc.30161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aizawa K, Liu C, Veeramachaneni S, Hu KQ, Smith DE, Wang XD. Development of ferret as a human lung cancer model by injecting 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Lung Cancer 82: 390–396, 2013. doi: 10.1016/j.lungcan.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aksoy I, Giudice V, Delahaye E, Wianny F, Aubry M, Mure M, Chen J, Jauch R, Bogu GK, Nolden T, Himmelbauer H, Xavier Doss M, Sachinidis A, Schulz H, Hummel O, Martinelli P, Hübner N, Stanton LW, Real FX, Bourillot PY, Savatier P. Klf4 and Klf5 differentially inhibit mesoderm and endoderm differentiation in embryonic stem cells. Nat Commun 5: 3719, 2014. doi: 10.1038/ncomms4719. [DOI] [PubMed] [Google Scholar]
- 5.Akutsu H, Miura T, Machida M, Birumachi J, Hamada A, Yamada M, Sullivan S, Miyado K, Umezawa A. Maintenance of pluripotency and self-renewal ability of mouse embryonic stem cells in the absence of tetraspanin CD9. Differentiation 78: 137–142, 2009. doi: 10.1016/j.diff.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Alva JA, Lee GE, Escobar EE, Pyle AD. Phosphatase and tensin homolog regulates the pluripotent state and lineage fate choice in human embryonic stem cells. Stem Cells 29: 1952–1962, 2011. doi: 10.1002/stem.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amit M, Chebath J, Margulets V, Laevsky I, Miropolsky Y, Shariki K, Peri M, Blais I, Slutsky G, Revel M, Itskovitz-Eldor J. Suspension culture of undifferentiated human embryonic and induced pluripotent stem cells. Stem Cell Rev Rep 6: 248–259, 2010. doi: 10.1007/s12015-010-9149-y. [DOI] [PubMed] [Google Scholar]
- 8.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 11: R106, 2010. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao L, He L, Chen J, Wu Z, Liao J, Rao L, Ren J, Li H, Zhu H, Qian L, Gu Y, Dai H, Xu X, Zhou J, Wang W, Cui C, Xiao L. Reprogramming of ovine adult fibroblasts to pluripotency via drug-inducible expression of defined factors. Cell Res 21: 600–608, 2011. doi: 10.1038/cr.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayani J, Squire JA. Traditional banding of chromosomes for cytogenetic analysis. Curr Protoc Cell Biol Chap. 22: Unit 22.3, 2004. doi: 10.1002/0471143030.cb2203s23. [DOI] [PubMed] [Google Scholar]
- 11.Belser JA, Katz JM, Tumpey TM. The ferret as a model organism to study influenza A virus infection. Dis Model Mech 4: 575–579, 2011. doi: 10.1242/dmm.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Nun IF, Montague SC, Houck ML, Tran HT, Garitaonandia I, Leonardo TR, Wang YC, Charter SJ, Laurent LC, Ryder OA, Loring JF. Induced pluripotent stem cells from highly endangered species. Nat Methods 8: 829–831, 2011. doi: 10.1038/nmeth.1706. [DOI] [PubMed] [Google Scholar]
- 13.Bernardo AS, Jouneau A, Marks H, Kensche P, Kobolak J, Freude K, Hall V, Feher A, Polgar Z, Sartori C, Bock I, Louet C, Faial T, Kerstens HHD, Bouissou C, Parsonage G, Mashayekhi K, Smith JC, Lazzari G, Hyttel P, Stunnenberg HG, Huynen M, Pedersen RA, Dinnyes A. Mammalian embryo comparison identifies novel pluripotency genes associated with the naïve or primed state. Biol Open 7: bio033282, 2018. doi: 10.1242/bio.033282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boroviak T, Loos R, Lombard P, Okahara J, Behr R, Sasaki E, Nichols J, Smith A, Bertone P. Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev Cell 35: 366–382, 2015. doi: 10.1016/j.devcel.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canizo JR, Vazquez Echegaray C, Klisch D, Aller JF, Paz DA, Alberio RH, Alberio R, Guberman AS. Exogenous human OKSM factors maintain pluripotency gene expression of bovine and porcine iPS-like cells obtained with STEMCCA delivery system. BMC Res Notes 11: 509, 2018. doi: 10.1186/s13104-018-3627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavagna P, Menotti A, Stanyon R. Genomic homology of the domestic ferret with cats and humans. Mamm Genome 11: 866–870, 2000. doi: 10.1007/s003350010172. [DOI] [PubMed] [Google Scholar]
- 17.Chapman DL, Agulnik I, Hancock S, Silver LM, Papaioannou VE. Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol 180: 534–542, 1996. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- 18.Choi J, Clement K, Huebner AJ, Webster J, Rose CM, Brumbaugh J, Walsh RM, Lee S, Savol A, Etchegaray JP, Gu H, Boyle P, Elling U, Mostoslavsky R, Sadreyev R, Park PJ, Gygi SP, Meissner A, Hochedlinger K. DUSP9 modulates DNA hypomethylation in female mouse pluripotent stem cells. Cell Stem Cell 20: 706–719, 2017. doi: 10.1016/j.stem.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Congras A, Barasc H, Canale-Tabet K, Plisson-Petit F, Delcros C, Feraud O, Oudrhiri N, Hadadi E, Griscelli F, Bennaceur-Griscelli A, Turhan A, Afanassieff M, Ferchaud S, Pinton A, Yerle-Bouissou M, Acloque H. Corrigendum: Non integrative strategy decreases chromosome instability and improves endogenous pluripotency genes reactivation in porcine induced pluripotent-like stem cells. Sci Rep 8: 46931, 2018. doi: 10.1038/srep46931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Congras A, Barasc H, Canale-Tabet K, Plisson-Petit F, Delcros C, Feraud O, Oudrhiri N, Hadadi E, Griscelli F, Bennaceur-Griscelli A, Turhan A, Afanassieff M, Ferchaud S, Pinton A, Yerle-Bouissou M, Acloque H. Non integrative strategy decreases chromosome instability and improves endogenous pluripotency genes reactivation in porcine induced pluripotent-like stem cells. Sci Rep 6: 27059, 2016. doi: 10.1038/srep27059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci USA 109: 4485–4490, 2012. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Declercq J, Sheshadri P, Verfaillie CM, Kumar A. Zic3 enhances the generation of mouse induced pluripotent stem cells. Stem Cells Dev 22: 2017–2025, 2013. doi: 10.1089/scd.2012.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demeyer S, De Boeck K, Witters P, Cosaert K. Beyond pancreatic insufficiency and liver disease in cystic fibrosis. Eur J Pediatr 175: 881–894, 2016. doi: 10.1007/s00431-016-2719-5. [DOI] [PubMed] [Google Scholar]
- 24.Drozd AM, Walczak MP, Piaskowski S, Stoczynska-Fidelus E, Rieske P, Grzela DP. Generation of human iPSCs from cells of fibroblastic and epithelial origin by means of the oriP/EBNA-1 episomal reprogramming system. Stem Cell Res Ther 6: 122, 2015. doi: 10.1186/s13287-015-0112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duchesneau P, Besla R, Derouet MF, Guo L, Karoubi G, Silberberg A, Wong AP, Waddell TK. Partial restoration of CFTR function in cftr-null mice following targeted cell replacement therapy. Mol Ther 25: 654–665, 2017. doi: 10.1016/j.ymthe.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 2: 240–248, 1992. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 27.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-β signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA 103: 16806–16811, 2006. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gianotti A, Ferrera L, Philp AR, Caci E, Zegarra-Moran O, Galietta LJ, Flores CA. Pharmacological analysis of epithelial chloride secretion mechanisms in adult murine airways. Eur J Pharmacol 781: 100–108, 2016. doi: 10.1016/j.ejphar.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 29.González F, Boué S, Izpisúa Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming à la carte. Nat Rev Genet 12: 231–242, 2011. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 30.Gooch KE, Marriott AC, Ryan KA, Yeates P, Slack GS, Brown PJ, Fothergill R, Whittaker CJ, Carroll MW. Heterosubtypic cross-protection correlates with cross-reactive interferon-γ-secreting lymphocytes in the ferret model of influenza. Sci Rep 9: 2617, 2019. doi: 10.1038/s41598-019-38885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurtan AM, Ravi A, Rahl PB, Bosson AD, JnBaptiste CK, Bhutkar A, Whittaker CA, Young RA, Sharp PA. Let-7 represses Nr6a1 and a mid-gestation developmental program in adult fibroblasts. Genes Dev 27: 941–954, 2013. doi: 10.1101/gad.215376.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haghighi F, Dahlmann J, Nakhaei-Rad S, Lang A, Kutschka I, Zenker M, Kensah G, Piekorz RP, Ahmadian MR. bFGF-mediated pluripotency maintenance in human induced pluripotent stem cells is associated with NRAS-MAPK signaling. Cell Commun Signal 16: 96, 2018. doi: 10.1186/s12964-018-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halevy T, Urbach A. Comparing ESC and iPSC-based models for human genetic disorders. J Clin Med 3: 1146–1162, 2014. doi: 10.3390/jcm3041146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He N, Rosen BH, Gray JS, Evans IA, Zieger M, Yan Z, Borel F, Liang B, Sun X, Moll SR, Brodsky MH, Mueller C, Engelhardt JF. Generation of alpha-1 antitrypsin knockout and PI*ZZ ferrets using Crispr/Cas9. A genetic model of emphysema (Abstract) Ann Am Thorac Soc 15, Suppl 4: S292–S293, 2018. doi: 10.1513/AnnalsATS.201806-429MG [DOI] [Google Scholar]
- 35.Huo C, Xiao K, Zhang S, Tang Y, Wang M, Qi P, Xiao J, Tian H, Hu Y. H5N1 influenza a virus replicates productively in pancreatic cells and induces apoptosis and pro-inflammatory cytokine response. Front Cell Infect Microbiol 8: 386, 2018. doi: 10.3389/fcimb.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.International Stem Cell Initiative; Amps K, Andrews PW, Anyfantis G, Armstrong L, Avery S, Baharvand H, Baker J, Baker D, Munoz MB, Beil S, Benvenisty N, Ben-Yosef D, Biancotti JC, Bosman A, Brena RM, Brison D, Caisander G, Camarasa MV, Chen J, Chiao E, Choi YM, Choo AB, Collins D, Colman A, Crook JM, Daley GQ, Dalton A, De Sousa PA, Denning C, Downie J, Dvorak P, Montgomery KD, Feki A, Ford A, Fox V, Fraga AM, Frumkin T, Ge L, Gokhale PJ, Golan-Lev T, Gourabi H, Gropp M, Lu G, Hampl A, Harron K, Healy L, Herath W, Holm F, Hovatta O, Hyllner J, Inamdar MS, Irwanto AK, Ishii T, Jaconi M, Jin Y, Kimber S, Kiselev S, Knowles BB, Kopper O, Kukharenko V, Kuliev A, Lagarkova MA, Laird PW, Lako M, Laslett AL, Lavon N, Lee DR, Lee JE, Li C, Lim LS, Ludwig TE, Ma Y, Maltby E, Mateizel I, Mayshar Y, Mileikovsky M, Minger SL, Miyazaki T, Moon SY, Moore H, Mummery C, Nagy A, Nakatsuji N, Narwani K, Oh SK, Oh SK, Olson C, Otonkoski T, Pan F, Park IH, Pells S, Pera MF, Pereira LV, Qi O, Raj GS, Reubinoff B, Robins A, Robson P, Rossant J, Salekdeh GH, Schulz TC, Sermon K, Sheik Mohamed J, Shen H, Sherrer E, Sidhu K, Sivarajah S, Skottman H, Spits C, Stacey GN, Strehl R, Strelchenko N, Suemori H, Sun B, Suuronen R, Takahashi K, Tuuri T, Venu P, Verlinsky Y, Ward-van Oostwaard D, Weisenberger DJ, Wu Y, Yamanaka S, Young L, Zhou Q. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol 29: 1132–1144, 2011. doi: 10.1038/nbt.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishigami M, Masumoto H, Ikuno T, Aoki T, Kawatou M, Minakata K, Ikeda T, Sakata R, Yamashita JK, Minatoya K. Human iPS cell-derived cardiac tissue sheets for functional restoration of infarcted porcine hearts. PLoS One 13: e0201650, 2018. doi: 10.1371/journal.pone.0201650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med 6: 88–95, 2000. doi: 10.1007/BF03401776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson CN, Rogers AB, Maurer KJ, Lofgren JL, Fox JG, Marini RP. Cystic renal disease in the domestic ferret. Comp Med 58: 161–167, 2008. [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson MB, Sun X, Kodani A, Borges-Monroy R, Girskis KM, Ryu SC, Wang PP, Patel K, Gonzalez DM, Woo YM, Yan Z, Liang B, Smith RS, Chatterjee M, Coman D, Papademetris X, Staib LH, Hyder F, Mandeville JB, Grant PE, Im K, Kwak H, Engelhardt JF, Walsh CA, Bae BI. Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size. Nature 556: 370–375, 2018. doi: 10.1038/s41586-018-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung JH, Lee SJ, Kim J, Lee S, Sung HJ, An J, Park Y, Kim BS. CXCR2 and its related ligands play a novel role in supporting the pluripotency and proliferation of human pluripotent stem cells. Stem Cells Dev 24: 948–961, 2015. doi: 10.1089/scd.2014.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalinichenko VV, Gusarova GA, Shin B, Costa RH. The Forkhead box F1 transcription factor is expressed in brain and head mesenchyme during mouse embryonic development. Gene Expr Patterns 3: 153–158, 2003. doi: 10.1016/S1567-133X(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 43.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126, Suppl 1: S29–S37, 2012. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 44.Keiser NW, Birket SE, Evans IA, Tyler SR, Crooke AK, Sun X, Zhou W, Nellis JR, Stroebele EK, Chu KK, Tearney GJ, Stevens MJ, Harris JK, Rowe SM, Engelhardt JF. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am J Respir Cell Mol Biol 52: 683–694, 2015. doi: 10.1165/rcmb.2014-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiecker C, Bates T, Bell E. Molecular specification of germ layers in vertebrate embryos. Cell Mol Life Sci 73: 923–947, 2016. doi: 10.1007/s00018-015-2092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klingenstein M, Raab S, Achberger K, Kleger A, Liebau S, Linta L. TBX3 knockdown decreases reprogramming efficiency of human cells. Stem Cells Int 2016: 6759343, 2016. doi: 10.1155/2016/6759343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development 131: 1651–1662, 2004. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 48.Liao J, Marumoto T, Yamaguchi S, Okano S, Takeda N, Sakamoto C, Kawano H, Nii T, Miyamato S, Nagai Y, Okada M, Inoue H, Kawahara K, Suzuki A, Miura Y, Tani K. Inhibition of PTEN tumor suppressor promotes the generation of induced pluripotent stem cells. Mol Ther 21: 1242–1250, 2013. doi: 10.1038/mt.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim LS, Hong FH, Kunarso G, Stanton LW. The pluripotency regulator Zic3 is a direct activator of the Nanog promoter in ESCs. Stem Cells 28: 1961–1969, 2010. doi: 10.1002/stem.527. [DOI] [PubMed] [Google Scholar]
- 50.Lolas M, Valenzuela PD, Tjian R, Liu Z. Charting Brachyury-mediated developmental pathways during early mouse embryogenesis. Proc Natl Acad Sci USA 111: 4478–4483, 2014. doi: 10.1073/pnas.1402612111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahlapuu M, Ormestad M, Enerbäck S, Carlsson P. The Forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 128: 155–166, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Martello G, Sugimoto T, Diamanti E, Joshi A, Hannah R, Ohtsuka S, Göttgens B, Niwa H, Smith A. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 11: 491–504, 2012. [Erratum in Cell Stem Cell 12: 630, 2013.] doi: 10.1016/j.stem.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarron A, Donnelley M, Parsons D. Airway disease phenotypes in animal models of cystic fibrosis. Respir Res 19: 54, 2018. doi: 10.1186/s12931-018-0750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller A, Ralser M, Kloet SL, Loos R, Nishinakamura R, Bertone P, Vermeulen M, Hendrich B. Sall4 controls differentiation of pluripotent cells independently of the nucleosome remodelling and deacetylation (NuRD) complex. Development 143: 3074–3084, 2016. doi: 10.1242/dev.139113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moheet A, Moran A. CF-related diabetes: containing the metabolic miscreant of cystic fibrosis. Pediatr Pulmonol 52, S48: S37–S43, 2017. doi: 10.1002/ppul.23762. [DOI] [PubMed] [Google Scholar]
- 57.Munder A, Tümmler B. Origins of cystic fibrosis lung disease. N Engl J Med 372: 1574–1575, 2015. doi: 10.1056/NEJMc1502191. [DOI] [PubMed] [Google Scholar]
- 58.Nakashima R, Morooka M, Shiraki N, Sakano D, Ogaki S, Kume K, Kume S. Neural cells play an inhibitory role in pancreatic differentiation of pluripotent stem cells. Genes Cells 20: 1028–1045, 2015. doi: 10.1111/gtc.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novo CL, Tang C, Ahmed K, Djuric U, Fussner E, Mullin NP, Morgan NP, Hayre J, Sienerth AR, Elderkin S, Nishinakamura R, Chambers I, Ellis J, Bazett-Jones DP, Rugg-Gunn PJ. The pluripotency factor Nanog regulates pericentromeric heterochromatin organization in mouse embryonic stem cells. Genes Dev 30: 1101–1115, 2016. doi: 10.1101/gad.275685.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okumura LM, Lesch BJ, Page DC. The ligand binding domain of GCNF is not required for repression of pluripotency genes in mouse fetal ovarian germ cells. PLoS One 8: e66062, 2013. doi: 10.1371/journal.pone.0066062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells 26: 1663–1672, 2008. doi: 10.1634/stemcells.2007-0884. [DOI] [PubMed] [Google Scholar]
- 62.Park SJ, Kim YI, Park A, Kwon HI, Kim EH, Si YJ, Song MS, Lee CH, Jung K, Shin WJ, Zeng J, Choi Y, Jung JU, Choi YK. Ferret animal model of severe fever with thrombocytopenia syndrome phlebovirus for human lethal infection and pathogenesis. Nat Microbiol 4: 438–446, 2019. doi: 10.1038/s41564-018-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasque V, Karnik R, Chronis C, Petrella P, Langerman J, Bonora G, Song J, Vanheer L, Sadhu Dimashkie A, Meissner A, Plath K. X chromosome dosage influences DNA methylation dynamics during reprogramming to mouse iPSCs. Stem Cell Reports 10: 1537–1550, 2018. doi: 10.1016/j.stemcr.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng X, Alföldi J, Gori K, Eisfeld AJ, Tyler SR, Tisoncik-Go J, Brawand D, Law GL, Skunca N, Hatta M, Gasper DJ, Kelly SM, Chang J, Thomas MJ, Johnson J, Berlin AM, Lara M, Russell P, Swofford R, Turner-Maier J, Young S, Hourlier T, Aken B, Searle S, Sun X, Yi Y, Suresh M, Tumpey TM, Siepel A, Wisely SM, Dessimoz C, Kawaoka Y, Birren BW, Lindblad-Toh K, Di Palma F, Engelhardt JF, Palermo RE, Katze MG. The draft genome sequence of the ferret (Mustela putorius furo) facilitates study of human respiratory disease. Nat Biotechnol 32: 1250–1255, 2014. doi: 10.1038/nbt.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quintanilla RH Jr, Asprer JS, Vaz C, Tanavde V, Lakshmipathy U. CD44 is a negative cell surface marker for pluripotent stem cell identification during human fibroblast reprogramming. PLoS One 9: e85419, 2014. doi: 10.1371/journal.pone.0085419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raju SV, Kim H, Byzek SA, Tang LP, Trombley JE, Jackson P, Rasmussen L, Wells JM, Libby EF, Dohm E, Winter L, Samuel SL, Zinn KR, Blalock JE, Schoeb TR, Dransfield MT, Rowe SM. A ferret model of COPD-related chronic bronchitis. JCI Insight 1: e87536, 2016. doi: 10.1172/jci.insight.87536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson NP, Venning L, Kyle H, Widdicombe JG. Quantitation of the secretory cells of the ferret tracheobronchial tree. J Anat 145: 173–188, 1986. [PMC free article] [PubMed] [Google Scholar]
- 68.Rosen BH, Evans TIA, Moll SR, Gray JS, Liang B, Sun X, Zhang Y, Jensen-Cody CW, Swatek AM, Zhou W, He N, Rotti PG, Tyler SR, Keiser NW, Anderson PJ, Brooks L, Li Y, Pope RM, Rajput M, Hoffman EA, Wang K, Harris JK, Parekh KR, Gibson-Corley KN, Engelhardt JF. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am J Respir Crit Care Med 197: 1308–1318, 2018. doi: 10.1164/rccm.201708-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rudenko L, Kiseleva I, Krutikova E, Stepanova E, Rekstin A, Donina S, Pisareva M, Grigorieva E, Kryshen K, Muzhikyan A, Makarova M, Sparrow EG, Torelli G, Kieny MP. Rationale for vaccination with trivalent or quadrivalent live attenuated influenza vaccines: Protective vaccine efficacy in the ferret model. PLoS One 13: e0208028, 2018. doi: 10.1371/journal.pone.0208028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell R, Ilg M, Lin Q, Wu G, Lechel A, Bergmann W, Eiseler T, Linta L, Kumar P P, Klingenstein M, Adachi K, Hohwieler M, Sakk O, Raab S, Moon A, Zenke M, Seufferlein T, Schöler HR, Illing A, Liebau S, Kleger A. A dynamic role of TBX3 in the pluripotency circuitry. Stem Cell Reports 5: 1155–1170, 2015. doi: 10.1016/j.stemcr.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitt H, Sasiadek M, Jagielski J, Blin N. Classical and molecular cytogenetic methods in diagnosis of a rare translocation t(3;21). Int J Mol Med 1: 569–571, 1998. doi: 10.3892/ijmm.1.3.569. [DOI] [PubMed] [Google Scholar]
- 72.Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc 7: 1836–1846, 2012. doi: 10.1038/nprot.2012.116. [DOI] [PubMed] [Google Scholar]
- 73.Singh AM, Reynolds D, Cliff T, Ohtsuka S, Mattheyses AL, Sun Y, Menendez L, Kulik M, Dalton S. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell 10: 312–326, 2012. doi: 10.1016/j.stem.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci 5: 438–445, 2002. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 75.Starbæk SMR, Brogaard L, Dawson HD, Smith AD, Heegaard PMH, Larsen LE, Jungersen G, Skovgaard K. Animal models for influenza A virus infection incorporating the involvement of innate host defenses: enhanced translational value of the porcine model. ILAR J 59: 323–337, 2018. doi: 10.1093/ilar/ily009. [DOI] [PubMed] [Google Scholar]
- 76.Sterner-Kock A, Kock M, Braun R, Hyde DM. Ozone-induced epithelial injury in the ferret is similar to nonhuman primates. Am J Respir Crit Care Med 162: 1152–1156, 2000. doi: 10.1164/ajrccm.162.3.9812153. [DOI] [PubMed] [Google Scholar]
- 77.Stripp BR. Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell? Proc Am Thorac Soc 5: 695–698, 2008. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stuart HT, van Oosten AL, Radzisheuskaya A, Martello G, Miller A, Dietmann S, Nichols J, Silva JC. NANOG amplifies STAT3 activation and they synergistically induce the naive pluripotent program. Curr Biol 24: 340–346, 2014. doi: 10.1016/j.cub.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sui H, Olivier AK, Klesney-Tait JA, Brooks L, Tyler SR, Sun X, Skopec A, Kline J, Sanchez PG, Meyerholz DK, Zavazava N, Iannettoni M, Engelhardt JF, Parekh KR. Ferret lung transplant: an orthotopic model of obliterative bronchiolitis. Am J Transplant 13: 467–473, 2013. doi: 10.1111/j.1600-6143.2012.04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, Kinyon JM, Lei-Butters DC, Griffin MA, Naumann P, Luo M, Ascher J, Wang K, Frana T, Wine JJ, Meyerholz DK, Engelhardt JF. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 120: 3149–3160, 2010. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun X, Yan Z, Yi Y, Li Z, Lei D, Rogers CS, Chen J, Zhang Y, Welsh MJ, Leno GH, Engelhardt JF. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest 118: 1578–1583, 2008. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swatek AM, Lynch TJ, Crooke AK, Anderson PJ, Tyler SR, Brooks L, Ivanovic M, Klesney-Tait JA, Eberlein M, Pena T, Meyerholz DK, Engelhardt JF, Parekh KR. Depletion of airway submucosal glands and TP63+KRT5+ basal cells in obliterative bronchiolitis. Am J Respir Crit Care Med 197: 1045–1057, 2018. doi: 10.1164/rccm.201707-1368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 84.Takase O, Yoshikawa M, Idei M, Hirahashi J, Fujita T, Takato T, Isagawa T, Nagae G, Suemori H, Aburatani H, Hishikawa K. The role of NF-κB signaling in the maintenance of pluripotency of human induced pluripotent stem cells. PLoS One 8: e56399, 2013. doi: 10.1371/journal.pone.0056399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.ter Meulen J, Bakker AB, van den Brink EN, Weverling GJ, Martina BE, Haagmans BL, Kuiken T, de Kruif J, Preiser W, Spaan W, Gelderblom HR, Goudsmit J, Osterhaus AD. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet 363: 2139–2141, 2004. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tiscornia G, Vivas EL, Izpisúa Belmonte JC. Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nat Med 17: 1570–1576, 2011. doi: 10.1038/nm.2504. [DOI] [PubMed] [Google Scholar]
- 87.Ustiyan V, Bolte C, Zhang Y, Han L, Xu Y, Yutzey KE, Zorn AM, Kalin TV, Shannon JM, Kalinichenko VV. FOXF1 transcription factor promotes lung morphogenesis by inducing cellular proliferation in fetal lung mesenchyme. Dev Biol 443: 50–63, 2018. doi: 10.1016/j.ydbio.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Zhao J, Tang S, Ye Z, Hewlett I. Viremia associated with fatal outcomes in ferrets infected with avian H5N1 influenza virus. PLoS One 5: e12099, 2010. doi: 10.1371/journal.pone.0012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell 10: 440–454, 2012. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 90.Wardle FC, Papaioannou VE. Teasing out T-box targets in early mesoderm. Curr Opin Genet Dev 18: 418–425, 2008. doi: 10.1016/j.gde.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe T, Watanabe S, Shinya K, Kim JH, Hatta M, Kawaoka Y. Viral RNA polymerase complex promotes optimal growth of 1918 virus in the lower respiratory tract of ferrets. Proc Natl Acad Sci USA 106: 588–592, 2009. doi: 10.1073/pnas.0806959106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Woods JB, Schmitt CK, Darnell SC, Meysick KC, O’Brien AD. Ferrets as a model system for renal disease secondary to intestinal infection with Escherichia coli O157:H7 and other Shiga toxin-producing E. coli. J Infect Dis 185: 550–554, 2002. doi: 10.1086/338633. [DOI] [PubMed] [Google Scholar]
- 93.Yang SH, Kalkan T, Morissroe C, Marks H, Stunnenberg H, Smith A, Sharrocks AD. Otx2 and Oct4 drive early enhancer activation during embryonic stem cell transition from naive pluripotency. Cell Reports 7: 1968–1981, 2014. doi: 10.1016/j.celrep.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang SH, Kalkan T, Morrisroe C, Smith A, Sharrocks AD. A genome-wide RNAi screen reveals MAP kinase phosphatases as key ERK pathway regulators during embryonic stem cell differentiation. PLoS Genet 8: e1003112, 2012. doi: 10.1371/journal.pgen.1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15: 750–761, 2014. [Erratum in Cell Stem Cell 16: 102, 2015.] doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yum J, Ku KB, Kim HS, Seo SH. H7N9 influenza virus is more virulent in ferrets than 2009 pandemic H1N1 influenza virus. Viral Immunol 28: 590–599, 2015. doi: 10.1089/vim.2015.0052. [DOI] [PubMed] [Google Scholar]
- 97.Zhang J, Jiao J. Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. BioMed Res Int 2015: 727542, 2015. doi: 10.1155/2015/727542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao D, Wu Y, Chen K. Tbx3 isoforms are involved in pluripotency maintaining through distinct regulation of Nanog transcriptional activity. Biochem Biophys Res Commun 444: 411–414, 2014. doi: 10.1016/j.bbrc.2014.01.093. [DOI] [PubMed] [Google Scholar]
- 99.Zvetkova I, Apedaile A, Ramsahoye B, Mermoud JE, Crompton LA, John R, Feil R, Brockdorff N. Global hypomethylation of the genome in XX embryonic stem cells. Nat Genet 37: 1274–1279, 2005. doi: 10.1038/ng1663. [DOI] [PubMed] [Google Scholar]