Abstract
The adaptive immune response could play a major role in the resolution of lung injury. Although regulatory T cells (Tregs) have been implicated in promoting the resolution of lung injury, therapeutic strategies to enhance Treg quantity and activity at the site of injury need further exploration. In the current study, Akt inhibition using triciribine (TCBN), given 48 h after lipopolysaccharide (LPS) administration, increased Tregs-promoted resolution of acute lung injury (ALI). TCBN treatment enhanced the resolution of LPS-induced ALI on day 7 by reducing pulmonary edema and neutrophil activity associated with an increased number of CD4+/FoxP3+/CD103+ and CTLA4+ effector Tregs, specifically in the injured lungs and not in the spleen. Treatment of EL-4 T-lymphocytes with two Akt inhibitors (TCBN and MK-2206) for 72 h resulted in increased FoxP3 expression in vitro. On the other end, Treg-specific PTEN knockout (PTENTreg KO) mice that have a higher Akt activity in its Tregs exhibited a significant impairment in ALI resolution, increased edema, and neutrophil activity associated with a reduced number of CD4+/FoxP3+/CD103+ and CTLA4+ effector Tregs as compared with the control group. In conclusion, our study identifies a potential target for the treatment of late-stage ALI by promoting resolution through effector Treg-mediated suppression of inflammation.
Keywords: acute lung injury, Akt, PTEN, regulatory T cells, triciribine
INTRODUCTION
The initial response of the lung to injury caused by trauma, pneumonia, and sepsis, etc. is characterized by innate immune cell-mediated damage of alveolar epithelial and capillary endothelial barrier, leading to the exudation of protein-rich fluid into the alveoli (37). This results in reduced lung function and acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) in the long term. Unfortunately, despite the recent advances in supportive care, ARDS continues to have high mortality, as shown by a recent study where the hospital mortality was as high as 46.1% among those with severe ARDS (8).
An injury to the lung can lead to either irreversible fibroproliferation and scarring or resolution and healing. Controlling excessive inflammation can enhance the resolution of lung injury as opposed to fibroproliferation (36). Hence, the adaptive immune response could play a major role in the resolution of lung injury (10). Regulatory T cells (Tregs) are an anti-inflammatory subset of T cells that have been extensively studied to enhance the resolution of lung injury (10, 34, 35, 41). The quantity, as well as the activity, of Tregs is critical in determining the immune system response, as they have the capacity to prevent potentially damaging autoimmune and protective immune responses (26). The transcription factor FoxP3 directs the suppressive function of Tregs (31). The effector (active) Tregs, a minor fraction of the Treg population, are those that have encountered antigens and have a suppressive function (26). The effector Tregs are characterized by a higher expression of surface markers such as CD25, CD103, and CTLA4 along with high intracellular FoxP3 expression (26).
Several mechanisms by which Tregs resolve lung injury have been studied previously. Some of these include Treg-mediated proliferation of epithelial cells and repair of lung epithelium (12, 29), Treg-mediated reduction of fibrocyte recruitment along the CXCL12-CXCR4 axis (18), and Treg-induced macrophage polarization to anti-inflammatory M2-like macrophage (30). Most of these studies adopted the transfer of Tregs to enhance the resolution of lung injury. However, the adoptive transfer of foreign cells can result in complications such as graft versus host disease and immune intolerance (7, 24). Therefore, the clinically challenging question regarding Treg-mediated resolution of lung injury is the therapeutic strategy to enhance the quantity, stability, and activity of Tregs post-injury and during resolution. While this needs to be further explored, one potential solution lies in controlling molecular mechanisms that can enhance Treg differentiation from naïve T cells and/or those that can potentiate Treg’s immune-suppressive ability.
Results from two recent parallel studies have demonstrated that phosphatase and tensin homolog (PTEN)-mediated control of phosphoinositide 3-kinase (PI3K)-Akt activity is critical for the function and stability of mouse FoxP3+ Tregs (21, 33). Interestingly, silencing PTEN in Tregs resulted in reduced CD25 expression, leading to the accumulation of FoxP3+/CD25− cells that eventually lowered the expression of FoxP3 (21, 33). Loss of PTEN in Tregs exacerbated T-follicular helper and germinal center B cell responses, resulting in disrupted immune tolerance and homeostasis. PTEN controls the transcriptional program and metabolic balance in Tregs mainly via inhibition of mammalian target of rapamycin complex 2 (mTORC2)-mediated Akt activity. Mechanistically, PTEN signaling pathway is likely to orchestrate both metabolic and transcriptional programs like those regulated by FoxO1 and Blimp1 and thus regulate Treg stability as well as Treg-mediated suppression of TH1 and TFH cells (33). Another study in the meantime identified PTEN signaling in Tregs as an important driver of the immunosuppressive milieu in tumors (32). Ablation of PTEN in Tregs resulted in the loss of their suppressive phenotype and conversion into the proinflammatory helper cells (ex-Tregs) (32). Moreover, activation of the PI3K/Akt/FoxO1/3 signaling pathway in humans leads to the differentiation of Tregs to a Th1-Treg phenotype that has increased IFNγ secretion (23). These studies implied the importance of exploring PI3K/Akt signaling in modulating Treg function.
We previously showed that endothelial loss of Akt1 promotes LPS-induced ALI during the early stages (24–48 h) (6). Surprisingly, late-stage pharmacological Akt suppression (post 48 h) in mice promoted lung injury resolution on day 7 after LPS administration. Our study demonstrates that the pharmacological inhibition of Akt in the late-stage increases CD4+/FoxP3+/CTLA4+ & CD103+ effector Treg numbers in the lungs, thus enhancing injury resolution.
MATERIALS AND METHODS
Experimental protocol for LPS-induced ALI model in WT and PTENTreg KO mice.
Sex- and age-matched mice with similar body weights were randomly divided into two groups of 10 mice each, with an equal number of males and females. PTENTreg KO (FoxP3 Cre and PTEN floxed) mice were bred in-house, as previously published (32). Wild-type (WT) littermates were used as controls. LPS (5 mg/kg) was administered intratracheally (it). The experimental groups were as follows: saline injury with DMSO treatment, saline injury with LPS treatment, “control” group [it LPS injury with intraperitoneal DMSO from day 2 to day 6 after injury and lungs collected on day 7], and the “treatment” group [LPS injury with intraperitoneal triciribine (TCBN) (0.5 mg·kg−1·day−1) from days 2 to 6 after injury]. All of the mice were euthanized on day 7. Mouse weight was monitored daily over the course of the injury. The bronchoalveolar lavage (BAL), lung, and spleens were collected at the time of euthanasia. While the lower lung lobes were snap-frozen for further molecular analysis, the upper lobes of the right and left lungs were used for lung wet/dry (W/D) ratio analysis and sectioning and staining, respectively. A separate batch of animals was used for flow cytometry analysis of mouse lung cells. All animal experiments were performed as per the Charlie Norwood Veterans Affairs Medical Center Institutional Animal Care and Use Committee approved Animal Component of Research Protocol (ACORP) number 16-09-095.
Lung wet/dry weight ratio.
The W/D ratio was assayed as previously described (6). Briefly, the upper lobe of the right lungs was excised, and the wet weights were determined immediately. These lobes were then placed on sterile nonenzymic paper and incubated at 80°C for 96 h to remove all moisture, and their dry weights were measured to calculate the W/D ratios.
Lung injury scoring.
Lung injury was scored based on predefined criteria (6) by three blinded reviewers. Averages of the three reviewer scores were considered for the analysis. Briefly, all lung fields at ×20 magnification were examined for each sample. Assessment of histological lung injury was performed by grading each sample as follows [scoring from 1 to 5, with 1 being the best (normal lung) and 5 being the worst (severe most lung injury)]: 1, normal; 2, focal (<50% lung section) interstitial congestion and inflammatory cell infiltration; 3, diffuse (>50% lung section) interstitial congestion and inflammatory cell infiltration; 4, focal (<50% lung section) consolidation (combining into a solid mass without the alveoli structure) and inflammatory cell infiltration; 5, diffuse (>50% lung section) consolidation and inflammatory cell infiltration.
BAL analysis.
At the time of euthanasia, animals were anesthetized using intraperitoneal ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively). When deep anesthesia was attained, the abdominal content and thorax were exposed through an abdominal midline incision, and a tracheostomy tube was subsequently inserted and secured in the trachea, the diaphragm was cut, and the chest wall was opened via a midline incision to expose the lungs. Lungs were then lavaged with 3 × 1 mL aliquots of saline, and each aliquot was instilled and withdrawn three times. The total volume of lavage fluid collected from each mouse was recorded. Lung lavage fluid was immediately centrifuged at 380 g for 10 min at 4°C to isolate the cell pellet; the supernatant was collected, and aliquots were frozen at −80°C for subsequent analyses. The supernatant-containing protein was used to measure BAL protein using the Bio-Rad protein assay (cat. no. 23208).
Mouse weight analysis.
Daily weights of mice were measured to assess the recovery from lung injury. The weights of the mice were normalized to day 0 weights before comparison.
Myeloperoxidase activity assay.
Myeloperoxidase (MPO) activity assay was performed as described previously (6) using the Mouse MPO ELISA kit from Hycult biotech (cat. no. HK210-02) according to the manufacturer’s protocol. Briefly, 10 mg of the lungs were homogenized in 200 μL of lysis buffer, which had the following composition: 200 mM NaCl, 5 mM EDTA, 10% glycerin, 1 mM PMSF, 1 μg/ml leupeptin, 28 μg/ml aprotinin, and Tris·HCl (pH 7.4). Sample aliquots were applied onto microtiter wells precoated with the capture antibody. After washing, a biotinylated tracer antibody was added to each well. After the incubation for binding of biotin to streptavidin-peroxidase conjugate, the color development with tetramethylbenzidine was performed. The color reaction was stopped by the addition of oxalic acid. The absorbance at 450 nm was measured with a spectrophotometer. The mouse MPO concentration of each sample was calculated from the standard curve with various concentrations of mouse MPO by serial dilution.
Western blot and flow cytometry analyses.
Western blot analysis was performed as described previously (6). Antibodies used included anti-p-Ser473Akt (cat. no. 9271L), anti-pFoxO1/3a (cat. no. 9464S), and anti-FoxO3a (cat. no. 2497S) from Cell Signaling Technology (Danvers MA). Anti-β-actin antibodies were obtained from Sigma (St. Louis, MO; cat. no. A5441), and mouse anti-FoxP3 antibodies were obtained from BioLegend (San Diego, CA; cat. no. 126402).
For flow cytometry, mouse lung lobes were digested with collagenase-dispase (Sigma-Aldrich, St. Louis, MO) at a 1 mg/mL concentration, and single cells were obtained by double filtration through sterile 100 μm and 40 μm gauges. Ack lysis buffer was used to disrupt red blood cells, followed by staining cells with surface markers. The lung sample was divided into two aliquots for staining with either anti-CTAL4 PE or anti-CD103 PE along with all other markers that were common for both aliquots. The following antibodies were used for surface staining: AlexFluor 700-conjugated anti-CD45 (cat. no. 103128; Biolegend), FITC-conjugated anti-CD4 (cat. no. 11-0043-85; eBioscience), PE-conjugated anti-CD103 (cat. no. 12-1031-82; eBioscience), and PE-conjugated anti-CTLA4 (cat. no. 61-1522-80; eBioscience). After the last wash, fixation and permeabilization with Fix/perm (cat. no. 00-5223-56 and cat. no. 00-5223-43) from eBioscience, Thermo Scientific (Hanover Park, IL) was performed. This was followed by intracellular staining for FoxP3 with APC-conjugated anti-FoxP3 (cat. no. 17-5773-82; eBioscience). This protocol was recommended by eBioscience (protocol B) for the FoxP3/Transcription Factor Staining Buffer Set (cat. no. 00-5523). Lymphocytes were gated with characteristic low forward scatter/side scatter, using BDFortessa or BDLSR II Flow cytometers. FlowJo was used for the analysis of the data. The gating strategy for CD45+/CD4+/FoxP3+/CD103+ and CTLA4+ is shown in Supplemental Fig. S1 (all Supplemental Material for this article can be found at https://doi.org/10.6084/m9.figshare.11791950.v1), and isotype controls for FITC, APC and PE are shown in Supplemental Fig. S2.
Statistical analysis.
All of the data are presented as means ± SD and were calculated from multiple independent experiments performed in triplicate. The n value for each figure implies the multiple independent experiments we performed. All of the data were analyzed by parametric testing using Student’s unpaired t test or one-way ANOVA, followed by the post hoc test using the GraphPad Prism 6.01 software. Data with P < 0.05 were considered significant.
RESULTS
Delayed Akt inhibition enhanced the resolution of LPS-induced mouse lung injury on day 7.
The time course effect of LPS-induced ALI on mouse lung histopathology showing the alveolar inflammatory cell infiltration and injury scoring revealed significant lung injury until day 7 after it LPS instillation in C57BL6 mice (Supplemental Fig. S3). As reported previously, the lung injury was completely resolved in mice by day 10 after a single it LPS instillation on day 0 (10). To test the hypothesis that late pharmacological inhibition of Akt enhances the resolution of lung injury on day 7 after injury, C57BL6 mice were administered with LPS and then treated with Akt inhibitor, TCBN, or vehicle (DMSO) starting 48 h after the injury until euthanasia on day 7 (Fig. 1A). TCBN has been demonstrated to inhibit Akt activity in cells (1, 17) and animal models of pulmonary fibrosis (2) and cancer (14). Control animals administered it saline did not exhibit any signs of injury, as assessed by histological examinations of mice lungs (Supplemental Fig. S4). Assessments of LPS-induced lung injury on day 7 on lung histology (Fig. 1 B and C), lung edema (Fig. 1D), BAL protein levels (Fig. 1E), mouse weights (Fig. 1F), and lung neutrophil (MPO) activity (Fig. 1G) were performed. A significant reduction in lung injury score was observed with Akt inhibition accompanied by an improvement in body weights as well as reduced lung edema and neutrophil activity. Furthermore, TCBN treatment significantly reduced BAL protein levels. These results indicate the enhanced resolution of lung injury with Akt inhibition in C57BL6 mice on day 7 post-LPS-induced lung injury.
Fig. 1.
Delayed Akt inhibition enhances the resolution of lung injury. A: a depiction of the experimental design. B: representative images of mouse lung sections (hematoxylin and eosin staining) from control (DMSO) and treatment group showing enhanced resolution of lung injury on day 7 with the triciribine (TCBN) treatment starting from day 2. C: scatterplot showing a significant increase in lung injury score (blindly scored by 3 reviewers) on day 7 after LPS administration that was significantly reduced with TCBN treatment compared with DMSO treated control (n = 6). D: scatterplot showing lung wet/dry weight ratio as a measure of lung edema, demonstrating a significant increase in lung edema with LPS injury that was rescued with TCBN treatment (n = 6–10). E: scatterplot showing significantly decreased bronchoalveolar lavage (BAL) protein content in TCBN-treated lungs compared with DMSO-treated control lungs from LPS-injured mice (n = 6). F: graph showing mouse body weight measured over a period of 7 days after LPS injury, with significant improvement (increase) in body weight with TCBN treatment on day 7 (n = 6). G: bar graph showing significant reduction of myeloperoxidase (MPO) activity in TCBN-treated mice lungs compared with control (n = 6). Data are presented as means ± SD. *P < 0.05; #P < 0.01.
Akt inhibition in LPS-treated lungs significantly increased CD4+/FoxP3+/CD103−/CTLA4− resting Tregs as well as CD4+/FoxP3+/CD103+ and CTLA4+ effector Tregs.
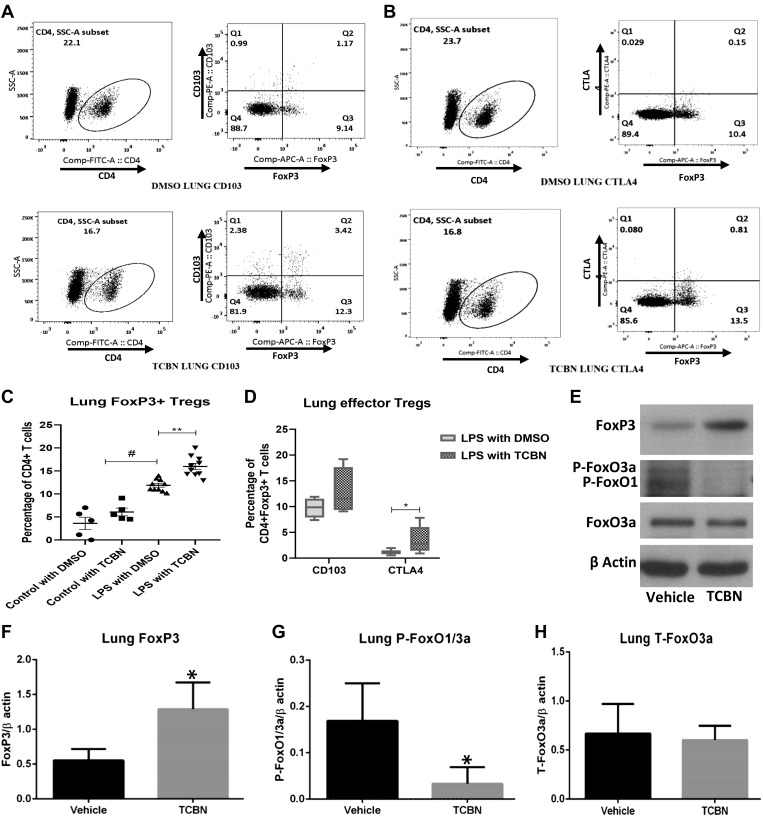
Because Akt inhibition from day 2 post-it LPS was associated with reduced inflammatory cell infiltration and enhanced resolution of lung injury on day 7, we sought to determine whether this was accompanied by changes in the number of anti-inflammatory Tregs. Single-cell suspension of mouse lungs subjected to flow cytometry analysis for effector Treg markers revealed increased numbers of CD4+/FoxP3+/CD103−/CTLA4− resting Tregs (Fig. 2, A–C) as well as the effector Tregs expressing either CD103+ (Fig. 2, A and D) or CTLA4+ (Fig. 2, B and D and Supplemental Fig. S5) on day 7 with TCBN treatment. Note that there were no significant changes in lung Tregs with the treatment for control mice that were injured with it saline (Supplemental Fig. S6).
Fig. 2.
Akt inhibition increases lung CD4+/FoxP3+/CD103+/cytotoxic T lymphocyte-associated protein 4 (CTLA4)+ effector regulatory T cells (Tregs). A and B: representative images of mouse lung single-cell suspension showing increased FoxP3+ Tregs as well as FoxP3+/CD103+ and FoxP3+/CTLA4+ effector Tregs with treatment (bottom) as compared with control (top). C: scatterplot showing the analysis of mouse lung cell analysis of Tregs with an increase in the percentage of FoxP3+ Tregs in LPS injured lungs that were further enhanced with triciribine (TCBN) treatment compared with control (n = 5–10). D: graph showing the analysis of effector Tregs in the lungs with a significant increase in CD4+/FoxP3+/CTLA4+ and a modest increase in CD4+/FoxP3+/CD103+ effector Tregs with TCBN treatment compared with control (n = 7). E: representative Western blot images showing lung FoxP3, phospho-FoxO1/3a, and total FoxO3a expression in LPS-instilled lung lysates in the presence of either vehicle (control) or TCBN treatment. F–H: bar graphs showing ImageJ-based band densitometry analysis of lung FoxP3, phospho-FoxO1/3a, and total FoxO3a expression in LPS-instilled lung lysates, with significantly higher expression of FoxP3 associated with significantly lower expression of phospho-FoxO1/3a and no change in the expression of total FoxO3a in TCBN group compared with vehicle (n = 6). Data are presented as means ± SD. *P < 0.05; #P < 0.01; **P < 0.001. SSC-A, side scatter area.
FoxO1/3a are transcription factors known to regulate the expression of FoxP3 in Tregs (20). Because Akt inhibition enhances FoxP3-expressing Treg cell infiltration in the injured lung, we determined the phosphorylation changes in FoxO1/3a in mouse lung lysates after treatment with TCBN. Western blot analysis showed a significant increase in FoxP3 expression in TCBN-treated mouse lungs, which was associated with reduced phosphorylation of FoxO3a and FoxO1, two major substrates of Akt, without altering the total expression of FoxO3a (Fig. 2, E–H).
On the other hand, Akt inhibition in LPS- and TCBN-cotreated mouse spleen resulted in increased CD4+/FoxP3+/CD103−/CTLA4− resting Treg numbers compared with DMSO-treated controls (Fig. 3, A–C). This, however, did not increase effector Treg numbers expressing either CD103 (Fig. 3, A and D; for control group refer to Supplemental Fig. S7, A and C) or CTLA4 (Fig. 3, B and D; for control group refer to Supplemental Fig. S7, B and C) surface markers. These results indicate that Akt inhibition enhances the resolution of lung injury, and this was associated with a significant increase in lung effector Tregs that are either CD4+/FoxP3+/CD103+ or CD4+/FoxP3+/CTLA4+ at the site of injury and not in the spleen, which had enhanced FoxP3+ Tregs but not those that expressed these effector surface markers. Note that control mice administered it saline did not have any changes in spleen Tregs (Supplemental Fig. S7).
Fig. 3.
Triciribine (TCBN) treatment increases spleen CD4+/FoxP3+ regulatory T cells (Tregs) but not CD4+/FoxP3+/CD103+/cytotoxic T lymphocyte-associated protein 4 (CTLA4)+ effector Tregs. A and B: representative images of mouse spleen single-cell suspension showing increased FoxP3+ Tregs but not CD4+/FoxP3+/CD103+ and CD4+/FoxP3+/CTLA4+ effector Tregs with treatment (bottom) as compared with control (top). C: scatterplot showing the analysis of mouse spleen Tregs with an increase in the percentage of CD4+/FoxP3+ Tregs with TCBN treatment compared with control (n = 7). D: graph showing the analysis of effector Tregs in spleen, with no significant changes in CD4+/FoxP3+/CTLA4+ or CD4+/FoxP3+/CD103+ effector Tregs with TCBN treatment compared with control (n = 7). Data are presented as means ± SD. *P < 0.05. SSC-A, side scatter area.
Akt inhibition in murine T lymphocyte cell line enhances FoxP3 expression in vitro.
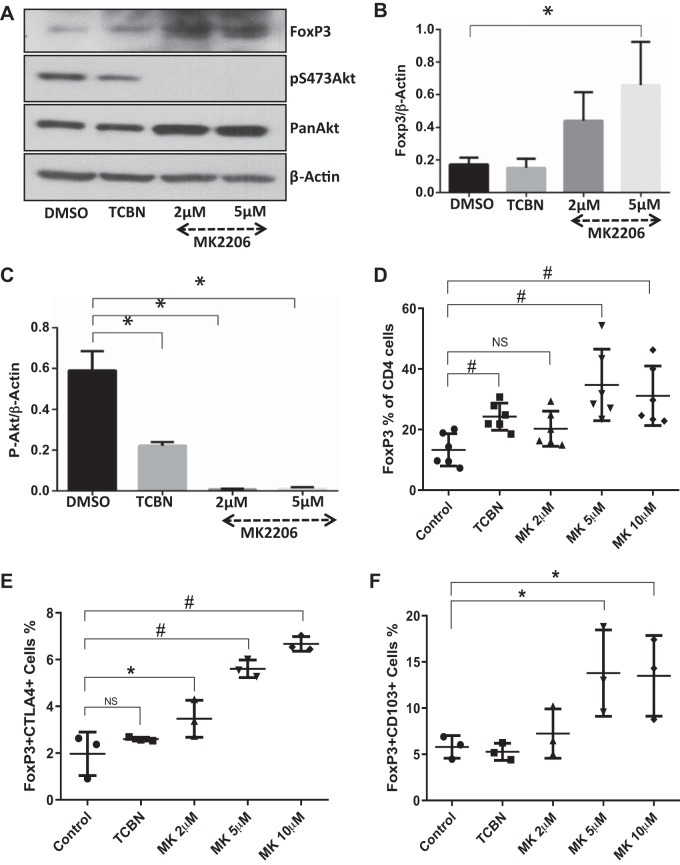
To confirm in vivo results, mouse T lymphocyte cell line (EL-4) cells were treated with two Akt inhibitors: TCBN, a compound that helps to normalize the Akt activity without cytotoxicity; and MK2206, a compound that is a potent inhibitor of Akt with high cytotoxicity; or vehicle (DMSO) for 72 h. These cells were then subjected to Western blot and flow cytometry analysis for various marker expressions. Both the Akt inhibitors reduced Akt phosphorylation and also enhanced FoxP3 expression after 72 h of treatment (Fig. 4, A–C). Notably, 24 h of inhibitor treatment was not sufficient to produce this effect (data not shown), indicating that this cellular transformation is a long-term process. Flow cytometry analysis of these cells showed a significant increase in FoxP3-expressing Tregs with TCBN treatment and MK (2, 5, and 10 µM) treatment (Fig. 4D). Interestingly, despite the very low population of CD4 cells in the EL-4 cell culture, the expression of effector Treg markers CTLA4 or CD103 were significantly increased with the higher dose of MK compound (Fig. 4, E and F).
Fig. 4.
Akt inhibition in vitro differentiated a subpopulation of EL4 T lymphocytes to CD4+/FoxP3+/CD103+/cytotoxic T lymphocyte-associated protein 4 (CTLA4)+ effector Tregs. A–C: representative Western blot images and band densitometry analysis showing increased FoxP3 expression in EL4 T lymphocyte cell lysates associated with reduced Akt phosphorylation after 72 h of treatment with Akt inhibitors triciribine (TCBN; 10 µM) and MK-2206 (2 and 5 µM) (n = 3). D: scatterplot of flow cytometry data showing an increased percentage of CD4+/FoxP3+ EL-4 T lymphocyte population by treatment with either TCBN (10 µM) or MK-2206 (MK; 2, 5, and 10 µM) (n = 6). E and F: scatterplots of flow cytometry data showing increased percentage of CD4+/FoxP3+/CTLA4+ and CD4+/FoxP3+/CD103+ EL-4 T lymphocyte cell population by treatment with TCBN (10 µM) or MK (2, 5, and 10 µM) (n = 3). Data are presented as means ± SD, *P < 0.05; #P < 0.01. NS, not significant.
FoxP3 promoter-driven Treg-specific PTEN-knockout mice had increased lung edema and less FoxP3+ Treg infiltration to the site of lung injury.
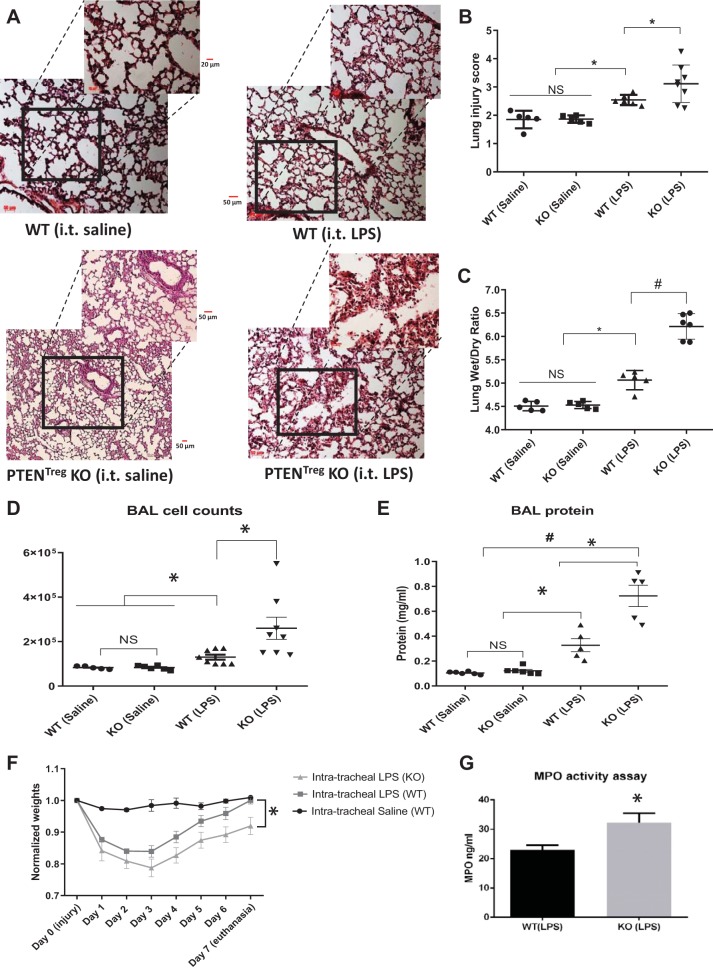
Treg-specific deletion of PTEN (a negative regulator of Akt) results in enhanced Akt activity in these Tregs (32). To confirm whether Akt signaling is directly responsible for Treg activation and promotion of lung injury resolution, we researched on Treg-specific PTEN knockout (PTENTreg KO) mice that have a high level of Akt activity in Tregs. PTENTreg KO mice were subjected to it LPS treatment and compared for injury resolution on day 7 with respective WT littermate controls. While saline administration did not show any morphological changes in these mice, indicating healthy pulmonary morphology, LPS administration caused a significant injury (Fig. 5A). Lung injury resolution on day 7 was significantly impaired in PTENTreg KO mice compared with WT (Fig. 5, A and B). Pulmonary edema as measured by the Lung wet/dry ratio was enhanced in PTENTreg KO mice (Fig. 5C). Impaired resolution in PTENTreg KO was further confirmed by decreased body weights on day 7 (Fig. 5F) as well as enhanced BAL cell counts and protein in PTENTreg KO mice (Fig. 5, D and E). Neutrophil activity is the hallmark of lung injury, which is usually reduced during injury resolution. However, PTENTreg KO mice had higher MPO activity on day 7 compared with WT (Fig. 5G).
Fig. 5.
Regulatory T cell (Treg)-specific phosphatase and tensin homolog (PTEN) knockout (PTENTreg KO) mice exhibited severe lung injury and edema as well as the loss of body weight compared with wild-type (WT) mice. A: representative images of mouse lung sections (hematoxylin and eosin staining) from saline-injured WT and PTENTreg KO controls as well as LPS-treated WT and PTENTreg KO group showing impaired resolution of lung injury on day 7 in PTENTreg KO lungs compared with WT. B and C: scatterplots showing blinded analysis of acute lung injury (ALI) score and lung wet/dry weight ratio indicating a significant increase in lung injury and lung edema in LPS-instilled PTENTreg KO mice compared with WT (n = 5–7). D and E: scatterplot showing elevated bronchoalveolar lavage fluid (BAL) total cell counts and BAL total protein content in LPS-instilled WT mouse lungs compared with the saline-instilled control lungs, which was further exacerbated in LPS-instilled PTENTreg KO mice (n = 5–8). F: body weights of mice measured over a period of 7 days after LPS administration showing a significant decrease in body weights of PTENTreg KO mice compared with WT control (n = 7). G: bar graph showing a significant increase in neutrophil myeloperoxidase (MPO) activity in LPS-instilled PTENTreg KO mouse lungs compared with respective WT control lungs (n = 7). Data are presented as means ± SD. *P < 0.05; #P < 0.01. NS, not significant.
PTENTreg KO mouse lungs exhibited reduced lung infiltration of CD4+/FoxP3+/CD103−/CTLA4− resting as well as CD4+/FoxP3+/CD103+ or CTLA4+ effector Tregs.
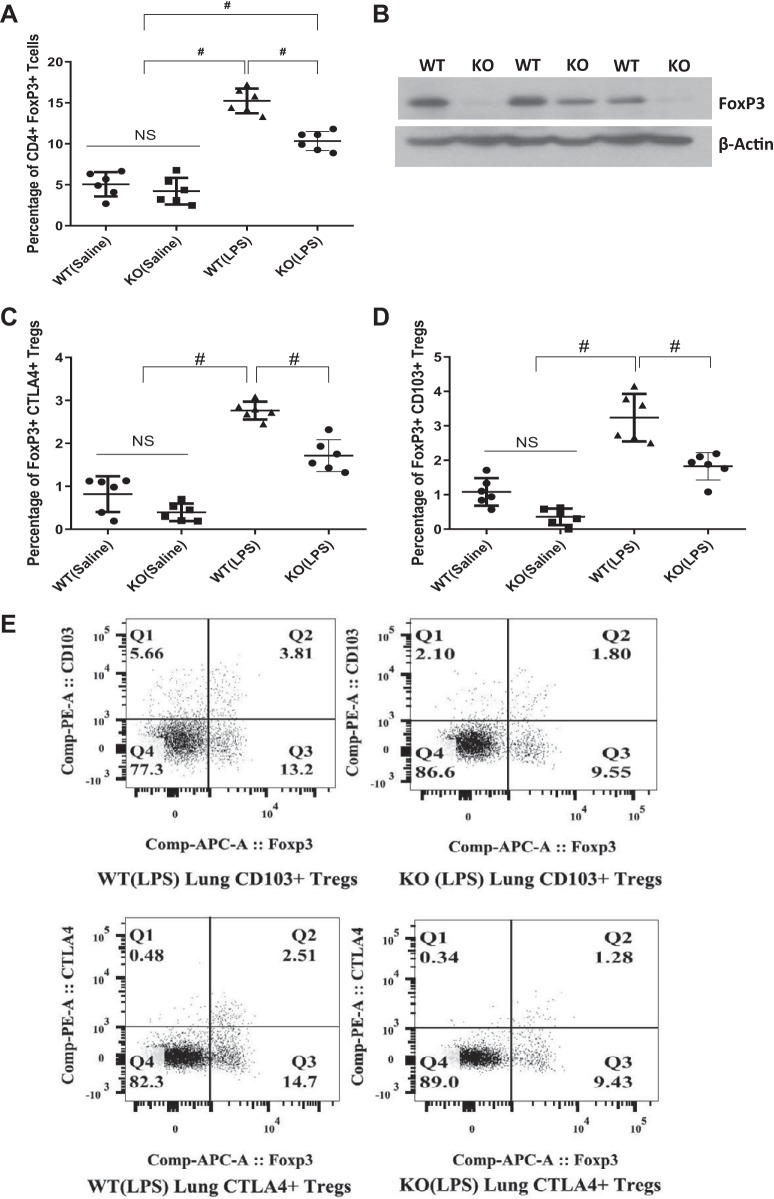
Because PTENTreg KO mice had impaired resolution of LPS-induced lung injury, we next investigated the presence of anti-inflammatory Tregs in the injured lungs. The number of both the resting (CD4+/FoxP3+/CD103−/CTLA4−) Tregs (Fig. 6, A and B and Supplemental Fig. S8A) and effector (CD4+/FoxP3+/CD103+ or CTLA4+) Tregs (Fig. 6, C–E and Supplemental Fig. S8, B and C) were significantly reduced in LPS-injured PTENTreg KO mouse lungs as compared with the LPS-treated WT littermate mice. The importance of Tregs in the resolution of lung injury has been reported, which explains the reason for impaired injury resolution in PTENTreg KO mouse lungs. The saline-administered PTENTreg KO mice did not exhibit lung injury or changes in lung Tregs. In summary, enhanced Akt activity in Tregs, as observed in PTENTreg KO mice, impairs the injury resolution capacity of Tregs and thus promotes LPS-induced lung injury.
Fig. 6.
Regulatory T cell (Treg)-specific phosphatase and tensin homolog (PTEN) knockout (PTENTreg KO) mice exhibited severe lung edema and fewer nos. of resting and effector Tregs at the site of injury. A: scatterplot showing flow cytometry analysis of LPS-injured mouse lung single-cell suspension, demonstrating a significant decrease in the total no. of infiltrated CD4+/FoxP3+ Tregs in PTENTreg KO mice compared with wild-type (WT) on day 7 after injury (n = 6). B: representative Western blot images showing decreased lung FoxP3 expression in PTENTreg KO mice lung lysates compared with WT, indicating a reduced number of Tregs in PTENTreg KO lungs. C and D: scatterplot showing flow cytometry analysis of effector Tregs in LPS-instilled mouse lung single-cell suspension, indicating a significant decrease in the no. of total CD4+/FoxP3+/CD103+ as well as CD4+/FoxP3+/CTLA4+ effector Tregs in PTENTreg KO mice compared with WT lungs on day 7 after injury (n = 6). E: representative images from flow cytometry analysis of mouse lung single-cell suspension showing significantly reduced effector Tregs in LPS-injured PTENTreg KO mice compared with WT littermates. Data are presented as means ± SD. #P < 0.01. NS, not significant.
DISCUSSION
Although remarkable progress has been made in understanding the pathophysiology of ARDS in recent years, we still lack a therapy to completely prevent mortality in ARDS patients. Supportive care, including ventilation and prone positioning, is the current management for ARDS, as specific pharmacological therapies have not yet been successful. Since 2010, the overall in-hospital mortality rate is 45% (38% in the intensive care unit), with a 30% 28- to 30-day mortality and 32% 60-day mortality (28). The cause of death in patients with ARDS is not lung pathology per se but mostly concurrent extrapulmonary organ dysfunction. However, early mortality is mostly associated with lung injury alone, and the late mortality corresponds to complications such as multiple organ failure (28, 38). Irreversible damage to the lung associated with multiple organ failure is due to uncontrolled injury, inflammation, and fibroproliferation in ARDS. Therefore, it is important to prevent persistent inflammation and enhance the resolution of acute lung injury to reduce mortality. Because of the pathological complexity, stage-specific therapy might be beneficial for ALI/ARDS patients.
Regulatory T cells expressing FoxP3 mediate the resolution of lung injury by modulating innate immune responses (10). Several mechanisms could be contributing simultaneously to Treg-mediated resolution of lung injury (5), but it is now well established from recent studies that Tregs are very important players in the resolution of lung injury (3, 10, 11, 29). Intriguingly, while Tregs do not play a role in the pathogenesis of lung injury, they play a prominent role in the injury resolution (10). However, LPS-induced ALI and inflammation correlate with a decrease in the percentage of alveolar FoxP3+ Tregs (10). Molecular mechanisms that enhance FoxP3+ Tregs at the site of injury (induced Tregs or iTregs) are not clear. In the current study, we show the efficacy of inhibiting Akt activity and activating downstream FoxO1/3a in enhancing the number of Tregs at the site of injury. Our data (Supplemental Fig. S3), along with previous studies (10), indicate that lung injury resolution in mice that are injured with single-dose intratracheal LPS instillation, starts after day 4. To mimic the pathological changes that occur in humans with lung injury, LPS injured mice were left for 48 h after the injury before the treatment began. Akt inhibitor TCBN enhanced the resolution of lung injury 7 days after it LPS instillation. Treatment with TCBN reduced lung injury score, decreased lung wet/dry ratio (pulmonary edema), improved mouse body weight, and decreased neutrophil MPO activity in mouse lungs on day 7 compared with the vehicle (DMSO) treatment. Interestingly, while TCBN treatment increased the number of CD4+/FoxP3+/CD103− or CD4+/FoxP3+/CTLA4− resting Tregs in both mouse lungs and spleen, the CD4+/FoxP3+/CTLA4+ or CD4+/FoxP3+/CD103+ activated/effector Tregs were enhanced only in the mouse lungs (site of injury) and not in the spleen. A simultaneous decrease in the phosphorylated FoxO1/3a along with increased FoxP3 expression was observed in the TCBN-treated mouse lungs. TCBN treatment to suppress Akt activity systemically could have several other effects on different cells but had a clear beneficial effect on lung injury resolution in LPS-injured mice. Overall, our study shows that pharmacological Akt suppression increases the percentage of effector Tregs at the site of injury, which is associated with enhanced recovery from LPS-induced ALI in mice.
Previous studies have indicated that co-stimulation through CD28, although required for the generation of nTregs (natural Tregs), is not necessary for the development of iTregs (4, 25, 40). Moreover, a recently published study used EL4 T lymphocytes to induce Tregs in the absence of co-stimulation (30). In our study, we used two Akt inhibitors, TCBN (10 µM) and MK-2206 (2, 5, and 10 µM) on EL4 cells to test our hypothesis that pharmacological Akt inhibition in vitro alone is enough to induce FoxP3 expression. After 72 h of Akt inhibition (24 h did not have any effect) in mouse EL-4 T-lymphocytes, enhanced FoxP3 expression was observed using flow cytometry. Treatment with a potent Akt inhibitor, MK-2206, also increased the number of CD4+/FoxP3+/CTLA4+ or CD103+ effector Tregs at higher doses. One concern was that the partial inhibition of Akt phosphorylation was not sufficient to enhance effector Treg markers as compared with the significant effect achieved with a higher dose of potent Akt inhibitor MK-2206. This is probably because of the very low number of CD4+ T cells in the mixed population of EL-4 T-lymphocytes. Nevertheless, these results confirm the role of Akt inhibition in enhancing the number of effector iTregs.
Loss of PTEN, a negative regulator of Akt, in Tregs results in uncontrolled Akt activity, leading to loss of FoxP3 expression and disrupted immune tolerance (21, 32, 33). Because the injury resolution is dependent on Treg activity and not just the number, we determined whether the damage caused by LPS instillation in PTENTreg KO mice, which have hyperactive Akt in Tregs, affecting their stability and activity, would be more severe and associated with impaired resolution than the WT. This model will also inform us of the effect of Treg-specific Akt activity as opposed to global Akt activity in the resolution of ALI. The loss of PTEN in Tregs has been reported to reduce CD25 expression and eventual loss of FoxP3 expression and promote lymphoproliferative disease in mice at the age of 15 wk or later (32). Our mice, using a different Cre system and different PTEN-floxed mice, had a somewhat milder phenotype and did not develop autoantibodies until late in life (32). For this study, we used young mice (age 8–10 wk), and at this age the mice are healthy and do not have autoimmune problems. We also showed that the lung morphology of these mice that are not injured is healthy and comparable with WT mice. However, the resolution of lung injury was impaired in PTENTreg KO mice in comparison with WT mice, as evidenced by the increased pulmonary edema, enhanced lung MPO activity, enhanced lung injury score, increased BAL cell counts, increased BAL protein, and reduced body weight. Lack of activated/effector CD4+/FoxP3+/CTLA4+ Tregs was associated with impaired injury resolution in these mice. Because one of the main differences between the two groups was enhanced Akt activity in Tregs (associated with PTENTreg KO mice), our results demonstrate the importance of pharmacologically targeting Akt to prevent excessive inflammation and enhance injury resolution in ALI. However, previous studies have associated the absence of PTEN with the absence of p53 that is independent of enhanced Akt activity; moreover, Akt-independent pathways are also involved in PTEN-induced cell cycle arrest and apoptosis (13, 39). Thus, loss of PTEN in Tregs could also have an Akt-independent effect on Treg activity.
We have previously shown that Akt, Akt1 in particular, is essential for the endothelial-barrier integrity, that systemic or endothelial-specific suppression of Akt1 activity leads to vascular leakage (9, 16, 17), and that inhibition of Akt activity during the early stages of lung injury is deleterious (6). Intriguingly, a previous study from our group has also demonstrated that the suppression of Akt activity by TCBN halts the progression of TGFβ-induced pulmonary fibrosis (2), suggesting that Akt activity modulation in the early and late stages of ALI can have contrasting effects. The short- and long-term effects of Akt activity regulation has also been demonstrated by our laboratory in ischemic heart disease (22, 27) as well as cancer (14, 15, 19). This indicates the complexity of therapeutically targeting Akt for ALI and other diseases without considering disease severity and progression. Furthermore, the current study demonstrates the specific role of Akt in the resolution of lung injury in the recovery phase of ALI. In conclusion, our study provides a potential therapeutic target to pharmacologically enhance Treg-mediated resolution of lung injury and prevent irreversible damage to the lung that occurs during the fibroproliferative phase. Clinically, we speculate that Akt inhibitor could be used in the post-exudative stage of ARDS to enhance the resolution of lung injury and restore lung function.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant R01 HL103952, National Center for Advancing Translational Sciences Grant UL1 TR002378, and Wilson Pharmacy Foundation (intramural) and Translational Research Initiative grant (intramural). This work has been accomplished using the resources and facilities at the Veterans Affairs Medical Center in Augusta, GA. The funders had no role in the study design, data collection, analysis, or decision to publish the data. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A., A.V., S.M., D.H.M., and P.R.S. conceived and designed research; S.A., A.V., A.A., M.S.A., and P.R.S. performed experiments; S.A., A.V., A.A., M.S.A., and P.R.S. analyzed data; S.A., A.V., and P.R.S. interpreted results of experiments; S.A., A.V., and P.R.S. prepared figures; S.A. and P.R.S. drafted manuscript; S.A., A.V., A.A., M.S.A., S.M., D.H.M., and P.R.S. edited and revised manuscript; S.A., A.V., A.A., M.S.A., S.M., D.H.M., and P.R.S. approved final version of manuscript.
REFERENCES
- 1.Abdalla M, Goc A, Segar L, Somanath PR. Akt1 mediates α-smooth muscle actin expression and myofibroblast differentiation via myocardin and serum response factor. J Biol Chem 288: 33483–33493, 2013. doi: 10.1074/jbc.M113.504290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdalla M, Sabbineni H, Prakash R, Ergul A, Fagan SC, Somanath PR. The Akt inhibitor, triciribine, ameliorates chronic hypoxia-induced vascular pruning and TGFβ-induced pulmonary fibrosis. Br J Pharmacol 172: 4173–4188, 2015. doi: 10.1111/bph.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal NR, Tsushima K, Eto Y, Tripathi A, Mandke P, Mock JR, Garibaldi BT, Singer BD, Sidhaye VK, Horton MR, King LS, D’Alessio FR. Immunological priming requires regulatory T cells and IL-10-producing macrophages to accelerate resolution from severe lung inflammation. J Immunol 192: 4453–4464, 2014. doi: 10.4049/jimmunol.1400146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol 3: 756–763, 2002. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 5.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 162: 1078–1089, 2015. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artham S, Gao F, Verma A, Alwhaibi A, Sabbineni H, Hafez S, Ergul A, Somanath PR. Endothelial stromelysin1 regulation by the forkhead box-O transcription factors is crucial in the exudative phase of acute lung injury. Pharmacol Res 141: 249–263, 2019. doi: 10.1016/j.phrs.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baruch EN, Berg AL, Besser MJ, Schachter J, Markel G. Adoptive T cell therapy: An overview of obstacles and opportunities. Cancer 123: 2154–2162, 2017. doi: 10.1002/cncr.30491. [DOI] [PubMed] [Google Scholar]
- 8.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A; LUNG SAFE Investigators; ESICM Trials Group . Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315: 788–800, 2016. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med 11: 1188–1196, 2005. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 119: 2898–2913, 2009. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Alessio FR, Zhong Q, Jenkins J, Moldobaeva A, Wagner EM. Lung angiogenesis requires CD4(+) forkhead homeobox protein-3(+) regulatory T cells. Am J Respir Cell Mol Biol 52: 603–610, 2015. doi: 10.1165/rcmb.2014-0278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dial CF, Tune MK, Doerschuk CM, Mock JR. Foxp3+ regulatory T cell expression of keratinocyte growth factor enhances lung epithelial proliferation. Am J Respir Cell Mol Biol 57: 162–173, 2017. doi: 10.1165/rcmb.2017-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3: 117–130, 2003. doi: 10.1016/S1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 14.Gao F, Alwhaibi A, Artham S, Verma A, Somanath PR. Endothelial Akt1 loss promotes prostate cancer metastasis via β-catenin-regulated tight-junction protein turnover. Br J Cancer 118: 1464–1475, 2018. doi: 10.1038/s41416-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao F, Alwhaibi A, Sabbineni H, Verma A, Eldahshan W, Somanath PR. Suppression of Akt1-β-catenin pathway in advanced prostate cancer promotes TGFβ1-mediated epithelial to mesenchymal transition and metastasis. Cancer Lett 402: 177–189, 2017. doi: 10.1016/j.canlet.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao F, Artham S, Sabbineni H, Al-Azayzih A, Peng XD, Hay N, Adams RH, Byzova TV, Somanath PR. Akt1 promotes stimuli-induced endothelial-barrier protection through FoxO-mediated tight-junction protein turnover. Cell Mol Life Sci 73: 3917–3933, 2016. doi: 10.1007/s00018-016-2232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao F, Sabbineni H, Artham S, Somanath PR. Modulation of long-term endothelial-barrier integrity is conditional to the cross-talk between Akt and Src signaling. J Cell Physiol 232: 2599–2609, 2017. doi: 10.1002/jcp.25791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garibaldi BT, D’Alessio FR, Mock JR, Files DC, Chau E, Eto Y, Drummond MB, Aggarwal NR, Sidhaye V, King LS. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol 48: 35–43, 2013. doi: 10.1165/rcmb.2012-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goc A, Liu J, Byzova TV, Somanath PR. Akt1 mediates prostate cancer cell microinvasion and chemotaxis to metastatic stimuli via integrin β3 affinity modulation. Br J Cancer 107: 713–723, 2012. doi: 10.1038/bjc.2012.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu Y-C. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med 207: 1381–1391, 2010. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, Bluestone JA, Turka LA. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol 16: 188–196, 2015. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr BA, Ma L, West XZ, Ding L, Malinin NL, Weber ME, Tischenko M, Goc A, Somanath PR, Penn MS, Podrez EA, Byzova TV. Interference with akt signaling protects against myocardial infarction and death by limiting the consequences of oxidative stress. Sci Signal 6: ra67, 2013. doi: 10.1126/scisignal.2003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitz A, de Marcken M, Gautron AS, Mitrovic M, Hafler DA, Dominguez-Villar M. AKT isoforms modulate Th1-like Treg generation and function in human autoimmune disease. EMBO Rep 17: 1169–1183, 2016. doi: 10.15252/embr.201541905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klaver Y, Kunert A, Sleijfer S, Debets R, Lamers CH. Adoptive T-cell therapy: a need for standard immune monitoring. Immunotherapy 7: 513–533, 2015. doi: 10.2217/imt.15.23. [DOI] [PubMed] [Google Scholar]
- 25.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol 6: 1219–1227, 2005. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 26.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol 14: 154–165, 2014. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 27.Ma L, Kerr BA, Naga Prasad SV, Byzova TV, Somanath PR. Differential effects of Akt1 signaling on short- versus long-term consequences of myocardial infarction and reperfusion injury. Lab Invest 94: 1083–1091, 2014. doi: 10.1038/labinvest.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Máca J, Jor O, Holub M, Sklienka P, Burša F, Burda M, Janout V, Ševčík P. Past and present ARDS mortality rates: a systematic review. Respir Care 62: 113–122, 2017. doi: 10.4187/respcare.04716. [DOI] [PubMed] [Google Scholar]
- 29.Mock JR, Garibaldi BT, Aggarwal NR, Jenkins J, Limjunyawong N, Singer BD, Chau E, Rabold R, Files DC, Sidhaye V, Mitzner W, Wagner EM, King LS, D’Alessio FR. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol 7: 1440–1451, 2014. doi: 10.1038/mi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao X, Rao P, Zhang Y, Liu L, Pang M, Wang H, Hu M, Tian X, Zhang J, Zhao Y, Wang XM, Wang C, Yu H, Guo F, Cao Q, Wang Y, Wang YM, Zhang GY, Lee VW, Alexander SI, Zheng G, Harris DCH. Redirecting TGF-β signaling through the β-catenin/Foxo complex prevents kidney fibrosis. J Am Soc Nephrol 29: 557–570, 2018. doi: 10.1681/ASN.2016121362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10: 490–500, 2010. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 32.Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, Wolchok JD, Mautino MR, Celis E, Sharpe AH, Francisco LM, Powell JD, Yagita H, Mellor AL, Blazar BR, Munn DH. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv 1: e1500845, 2015. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol 16: 178–187, 2015. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer BD, King LS, D’Alessio FR. Regulatory T cells as immunotherapy. Front Immunol 5: 46, 2014. doi: 10.3389/fimmu.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer BD, Mock JR, Aggarwal NR, Garibaldi BT, Sidhaye VK, Florez MA, Chau E, Gibbs KW, Mandke P, Tripathi A, Yegnasubramanian S, King LS, D’Alessio FR. Regulatory T cell DNA methyltransferase inhibition accelerates resolution of lung inflammation. Am J Respir Cell Mol Biol 52: 641–652, 2015. doi: 10.1165/rcmb.2014-0327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 377: 562–572, 2017. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 37.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 377: 1904–1905, 2017. doi: 10.1056/NEJMc1711824. [DOI] [PubMed] [Google Scholar]
- 38.Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa S, Fernández RL, Kacmarek RM; ALIEN Network . The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 37: 1932–1941, 2011. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 39.Weng L, Brown J, Eng C. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Hum Mol Genet 10: 237–242, 2001. doi: 10.1093/hmg/10.3.237. [DOI] [PubMed] [Google Scholar]
- 40.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci 66: 2603–2622, 2009. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang F, Li MY, Lan YT, Wang CB. Imbalance of Th17/Tregs in rats with smoke inhalation-induced acute lung injury. Sci Rep 6: 21348, 2016. doi: 10.1038/srep21348. [DOI] [PMC free article] [PubMed] [Google Scholar]