Abstract
Metabolic reprogramming is considered important in the pathogenesis of the occlusive vasculopathy observed in pulmonary hypertension (PH). However, the mechanisms that link reprogrammed metabolism to aberrant expression of genes, which modulate functional phenotypes of cells in PH, remain enigmatic. Herein, we demonstrate that, in mice, hypoxia-induced PH was prevented by glucose-6-phosphate dehydrogenase deficiency (G6PDDef), and further show that established severe PH in Cyp2c44−/− mice was attenuated by knockdown with G6PD shRNA or by G6PD inhibition with an inhibitor (N-ethyl-N′-[(3β,5α)-17-oxoandrostan-3-yl]urea, NEOU). Mechanistically, G6PDDef, knockdown and inhibition in lungs: 1) reduced hypoxia-induced changes in cytoplasmic and mitochondrial metabolism, 2) increased expression of Tet methylcytosine dioxygenase 2 (Tet2) gene, and 3) upregulated expression of the coding genes and long noncoding (lnc) RNA Pint, which inhibits cell growth, by hypomethylating the promoter flanking region downstream of the transcription start site. These results suggest functional TET2 is required for G6PD inhibition to increase gene expression and to reverse hypoxia-induced PH in mice. Furthermore, the inhibitor of G6PD activity (NEOU) decreased metabolic reprogramming, upregulated TET2 and lncPINT, and inhibited growth of control and diseased smooth muscle cells isolated from pulmonary arteries of normal individuals and idiopathic-PAH patients, respectively. Collectively, these findings demonstrate a previously unrecognized function for G6PD as a regulator of DNA methylation. These findings further suggest that G6PD acts as a link between reprogrammed metabolism and aberrant gene regulation and plays a crucial role in regulating the phenotype of cells implicated in the pathogenesis of PH, a debilitating disorder with a high mortality rate.
Keywords: metabolim, NADPH, PPP, pulmonary, smooth muscle cells
INTRODUCTION
Pulmonary hypertension (PH) is a multifactorial and debilitating disorder that contributes to the morbidity and mortality of several clinical conditions. Despite advances in our understanding of the underlying pathophysiology of PH, the processes that control remodeling of the pulmonary artery (PA) are still elusive, and treatment for most forms of the disease remains inadequate and challenging. Inflammation, sustained vasoconstriction, and PA remodeling all contribute to the pathogenesis of most types of PH. The PA remodeling is a consequence of endothelial cells, fibroblasts, and smooth muscle cells (SMCs), which acquire a hyperproliferative, antiapoptotic, and proinflammatory cancer-like phenotype in the PA wall (39). Concomitantly, they exhibit Warburg-like metabolism and upregulated activities of the enzymes in the glycolytic pathway and pentose phosphate pathway (PPP) (9).
Glucose oxidation, through either glycolysis or the PPP, produces energy or phosphoribose sugar moieties required for the de novo synthesis of nucleic acids (RNA and DNA) during cell replication and gene transcription. The PPP is also a major regulator of the cellular redox potential since it supplies the NADPH required for the recycling of oxidized glutathione, as well as for anabolic reactions, including fatty acid synthesis. Glucose-6-phosphate dehydrogenase (G6PD) is the rate-limiting enzyme in the PPP. Interestingly, G6PD deficiency is the most common enzymopathy in humans, with over 400 million people suffering from different degrees of enzymopathy worldwide. While G6PD deficiency increases the susceptibility of RBCs to oxidative hemolysis—a factor complicating antimalaria treatment with prooxidant quinone drugs—little is known regarding the role of G6PD deficiency in cardiovascular diseases. There are data to support a protective role of G6PD deficiency in decreasing the risk of heart disease and cardiovascular-associated deaths, perhaps through a decrease in cholesterol synthesis (16). However, limited clinical evidence indicates that G6PD deficiency may be associated with systemic hypertension (16). Studies in G6PD-deficient mice are mixed and provide evidence for both protective and deleterious effects (16). Moreover, the mechanisms through which G6PD mediates protective or deleterious effects on the cardiovascular system are not yet known. However, pyridine nucleotides (NADPH and NADH) are emerging as a potential link or important interface, above their coenzymatic activity, between cellular metabolism and various physiological and pathophysiological responses such as cardiovascular function and development of cancer and PH (40, 47).
Seminal studies from our and another laboratory suggested that inhibition of G6PD and pyruvate dehydrogenase kinase, a mitochondrial enzyme, respectively, decreases hypoxic pulmonary vasoconstriction (14) and hypoxia-induced PH (28). Subsequent studies, including ours, have shown that the expression and activity of G6PD and PPP are increased in 1) endothelin-1-treated pulmonary artery SMCs from idiopathic pulmonary arterial hypertension (iPAH) patients with a Bmpr2 exon1–8 deletion (48); 2) PA fibroblasts from iPAH patients and calves with hypoxia-induced PH (22); 3) hypoxic rat SMCs and CD133+ progenitor cells (6, 7); 4) SMCs derived from 4-wk-old lambs exposed to increased pulmonary blood flow (2); and 5) lungs from rat models of hypoxia- and monocrotaline-induced PH (6, 41). Interestingly, G6PD activity increases before PH develops (6, 35). In SMCs, hypoxia-induced H2O2 rapidly (within 2 h) increases G6PD expression by upregulating translation of G6pd mRNA (6). These studies collectively raise the possibility that G6PD and G6PD-dependent signaling pathways are etiological contributors to the pathogenesis of PH.
G6PD-derived NADPH regulates histone deacetylase 1 and 2 activity in C2 myoblasts (45), and expression of coding and noncoding genes in SMCs (8). Additionally, in preliminary studies, we found that G6PD interacts with and regulates the activity of histone and DNA methyltransferases and demethylases. In the present study, therefore, we tested the hypothesis that G6PD is a determinant of DNA methylation and PH pathology, and that genetic deficiency of G6PD caused by a mutation in G6pd promoter will prevent and lung-targeted G6pd gene ablation or pharmacological inhibition of G6PD will attenuate established PH and PH-associated heart failure. We further set out to determine the molecular mechanisms involved in these protective actions. Our results demonstrate a novel and previously unrecognized function for G6PD in modulating epigenetic modifications like DNA methylation, and that G6PD knockdown and inhibition alleviated PA remodeling and PH by regulating the expression of epigenetic modifier (Tet methylcytosine dioxygenase 2), long noncoding RNAs, and protein coding genes.
METHODS
Animal model of pulmonary hypertension.
All animal procedures were approved by the Institutional Animal Care and Use Committee. G6PD deficient (G6PDDef) mice (male and female, 25–30 g) (33) were provided by Dr. Jane Leopold, Department of Medicine at Brigham and Women’s Hospital, Harvard Medical School, Boston; and CYP2C44−/− mice (male and female, 25–30 g) (32) were provided by Dr. Michal Schwartzman, Department of Pharmacology at New York Medical College. Tet methylcytosine dioxygenase 2 global knockout (Tet2tm1.2Rao; male and female, 25–30 g) mice were purchased from Jackson Laboratories. G6PDDef, Cyp2c44−/−, Tet2tm1.2Rao, and eugenic wild-type mice were exposed to normobaric hypoxia (10% O2) for 5 wk to induce PH, as described previously (19). For reversal PH studies, Cyp2c44−/− mice were treated during weeks 3 and 4 with 25 μL of AdGFP-G6PD-shRNA (1012 p.f.u) or with a small inhibitor of G6PD activity, N-ethyl-N′-[(3β,5α)-17-oxoandrostan-3-yl]urea (NEOU). NEOU was injected (1.5 mg·kg−1·day−1; sc) daily during week 4. Tet2tm1.2Rao mice were treated with NEOU during week 4. After 5 wk of exposure to normobaric hypoxia (10% O2) with and without treatment, echocardiography and hemodynamic (by catherization of right and left ventricles) measurements were made, and mice were euthanized to harvest lungs, heart, and other organs. Blood was collected from left ventricle. Echocardiography was performed in 2% isoflurane-anesthetized mice using a Vevo 770 imaging system (VisualSonics, Toronto, ON, Canada). Catherization studies were performed in mice that were anesthetized using 4% isoflurane, after which 1–2% isoflurane was used to maintain anesthesia during the entire duration of the surgery and data acquisition. Detailed methods are given in online supplement (https://doi.org/10.6084/m9.figshare.11892846; all supplemental materials may be found at this site).
Statistical analysis.
Graphs and statistical analyses (ANOVA or t test) were prepared with GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA) and Metaboanalyst 3.0. Hierarchical clustering analyses and heat maps were plotted with GENE-E (Broad Institute, Boston, MA). Values are presented as the mean ± SD of the number of samples (n) from different animals and cell culture experiments. Kruskal-Wallis test followed by Dunn’s multiple-comparisons test, otherwise indicated, was used to compare multiple groups and unpaired Student’s t test was used to compare two groups. Values of P < 0.05 were considered significant.
RESULTS
G6PD deficiency reduced development of chronic hypoxia-induced pulmonary hypertension in mice.
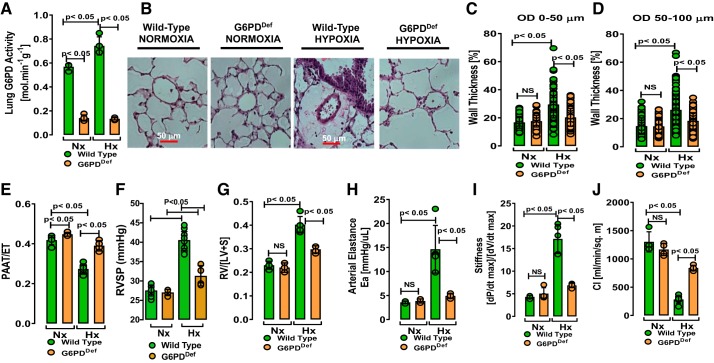
To unequivocally determine the role of increased G6PD in the pathogenesis of PH, we exposed G6PDDef and their eugenic wild-type (WT) mice to hypoxia (10% O2) for 5 wk. Whereas lung G6PD activity was significantly higher in WT-hypoxic than WT-normoxic mice, G6PD activity was significantly low in G6PDDef-normoxic or -hypoxic mice and hypoxia did not increase G6PD activity (Fig. 1A). Muscularization and increased wall thickness in small PAs, a hallmark of hypoxic PA remodeling, was significantly less in G6PDDef than in WT mice exposed to hypoxia (Fig. 1, B–D, and Supplemental Fig. S1). To assess PA and right ventricular (RV) systolic pressure, we measured pulmonary artery acceleration time-to-ejection time (PAAT-to-ET) ratios by echocardiography in normoxic and hypoxic mice. We observed a negative correlation between the PAAT-to-ET ratio and G6PD activity (Supplemental Fig. S2A). PAAT-to-ET ratios decreased less and remained higher in G6PDDef mice than in WT mice exposed to hypoxia (Fig. 1E). Similarly, RV systolic pressure increased to a greater extent in WT-hypoxic mice than in G6PDDef-hypoxic mice (Fig. 1F). RV hypertrophy [determined as RV-to-left ventricle plus septal wall (LV + S) ratio; Fulton’s Index] and increases in arterial elastance and LV stiffness were all attenuated in G6PDDef compared with WT hypoxic mice (Fig. 1, G–I). Conversely, the cardiac index was higher in G6PDDef-hypoxic than in WT-hypoxic mice (Fig. 1J).
Fig. 1.
Glucose-6-phosphate dehydrogenase deficiency (G6PDDef) prevented pulmonary artery remodeling and heart failure in a mouse hypoxia-induced pulmonary hypertension model. A: significantly reduced pulmonary G6PD activity in lungs of normoxic (Nx) and hypoxic (Hx) G6PDDef mice as compared with wild-type (WT) mice. B: representative H&E-stained sections showing increased wall thickness in WT, but not in G6PDDef, mice exposed to hypoxia. C–D: hypoxia-induced pulmonary artery (PA) remodeling (0–50 μm and 50–100 μm outer diameter) is prevented in G6PDDef mice. Note each circle in figures represents number of PAs on which morphometric analysis was performed in each mice group. E: the pulmonary artery acceleration time-to-ejection time (PAAT-to-ET) ratio is reduced in WT, but not in G6PDDef, mice exposed to hypoxia. F and G: hypoxia increased right ventricle systolic pressure (RVSP) and right ventricle (RV) hypertrophy [a ratio of RV-to-left ventricle plus septal wall (LV+S) mass] in WT, but not in G6PDDef, mice. H–J: alterations of systemic arterial elastance (Ea), left ventricular stiffness, and cardiac index, observed in hypoxic WT mice, are significantly attenuated in hypoxic G6PDDef mice. Values are means ± SD; n = 5 in Nx and n = 7 in Hx of WT group and n = 5 in Nx and Hx of G6PDDef group; males and females (3:2 ratio) were included in all groups.
Lung-targeted G6PD knockdown decreased established chronic hypoxia-induced pulmonary hypertension.
Since G6PD deficiency protected mice from hypoxic PH, we hypothesized that G6PD knockdown would stop progression of disease and even attenuate established PH. We first confirmed that we could successfully deliver a G6PD-shRNA (CGGAAACGUCGUACACUUtt) and a scrambled sequence (control), which is cloned by GeneScript into an adenoviral vector under the H1 promoter to drive short hairpin (sh) RNA expression, to the lungs through intratracheal aerosolization. To monitor transduction efficiency, the vector also carried a green fluorescent protein (GFP) marker (coral GPF, cGFP) under the control of the CMV promoter, hereafter referred to as AdGFP-G6PD-shRNA. As expected, G6PD-shRNA cloned in adenoviral vector (AdG6PD-shRNA) without a GFP-tag and packaged in adenovirus did not show green fluorescence in the lungs (Fig. 2A, top), while AdGFP-G6PD-shRNA showed evenly distributed green fluorescence in the lungs (Fig. 2A, bottom).
Fig. 2.
Established pulmonary hypertension is attenuated by glucose-6-phosphate dehydrogenase (G6PD) knockdown. A: representative micrograph showing lungs stained with green fluorescent protein and successful delivery of adenovirus (Ad)GFP-G6PD-shRNA (with GFP-tag; bottom) to the lungs after intratracheal aerosolization. AdG6PD-shRNA (without GFP-tag; top) delivered to the lungs after intratracheal aerosolization shows no GFP fluorescence. B: schematic showing the timeline for induction of hypoxia and therapeutic intervention. C: significantly reduced G6PD activity in lungs of hypoxic CYP2C44−/− mice treated with IT-shG6PD [25 μL of 1012 plaque-forming units (pfu); Hx+IT-shG6PD] as compared with IT-scrambled shRNA (25 μL of 1012 pfu; Hx). Nx, normoxia. D: representative H&E-stained sections showing reduced pulmonary artery remodeling in hypoxic CYP2C44−/− mice treated with IT-shG6PD as compared with IT-scrambled shRNA (Hx). E and F: hypoxia-induced pulmonary artery (PA) remodeling (0–50 μm and 50–100 μm outer diameter) is attenuated by IT-shG6PD (Hx-IT-shG6PD) as compared with IT-scrambled shRNA (Hx) treatment. Note that each circle in the figures represents the number of PAs on which morphometric analysis was performed in each mice in group. G–I: the pulmonary artery acceleration time-to-ejection time (PAAT-to-ET) ratio, right ventricle systolic pressure (RVSP), and hypertrophy [as the RV-to-left ventricle (LV)+ septal wall (S) mass ratio] is reduced in mice by IT-shG6PD as compared with IT-scrambled shRNA (Hx). I–L: systemic arterial elastance (Ea) and left ventricular stiffness is decreased, and cardiac index is increased in hypoxic mice by IT-shG6PD as compared with IT-scrambled shRNA (Hx) treatment. Values are means ± SD; n = 5 in normoxia; n = 10 in hypoxia; and n = 5 in hypoxia+TI-shG6PD group; males and females (3:2 ratio) were included in all groups.
We then tested whether AdGFP-G6PD-shRNA or AdGFP-scrambled shRNA, which was used as a control for AdGFP-GDPD-shRNA administration, would attenuate PA remodeling and PH in CYP2C44−/− mice. These mice develop severe PH, which is more severe in females than males, with formation of occlusive vascular lesions in PA under hypoxia (19) similar to that seen in the Sugen5416/hypoxia/normoxia rat model and in iPAH patients (1, 44). The CYP2C44−/− mice were exposed to hypoxia (10% O2) for 5 wk. They were also intratracheally administered aerosolized AdGFP-G6PD-shRNA (IT-shG6PD) or AdGFP-scrambled-shRNA (given once a week) during weeks 3 and 4 (Fig. 2B). We treated these mice once per week for 2 wk because shRNA took 3–5 days to decrease protein levels by 50%. Since hypoxia-induced PH was completely established by the end of 3 wk in CYP2C44−/− mice, we treated these mice in week 3 and then in week 4 to maintain decreased level of protein before the experiments were terminated at the end of week 5 for hemodynamic measurements. Application of IT-shG6PD, but not IT-Scrambled shRNA, for 2 wk reduced G6PD activity (Fig. 2C), which progressively increased in hypoxia (normoxia, Nx: 0.509 ± 0.089; 1.5 wk hypoxia, Hx: 0.680 ± 0.047; 5 wk Hx: 0.870 ± 0.079; in mol·min−1·g−1; *P < 0.05 vs. Nx), and reduced the PA wall thickening (Fig. 2, D–F) in the lungs of hypoxic mice. Further, we did not observe occluded PAs in lungs of treated mice. Correspondingly, PAAT-to-ET ratios (Fig. 2G), RV systolic pressure (Fig. 2H), and RV hypertrophy (RV-to-LV+S ratio; Fig. 2I) were reduced by IT-shG6PD therapy. Two weeks of treatment with IT-shG6PD also attenuated the increases in arterial elastance and LV stiffness (Fig. 2, J and K), and increased the cardiac index in hypoxic mice (Fig. 2L). CYP2C44−/− mice exposed to normoxia (21% O2) were used as normotensive controls.
G6PD inhibition with NEOU attenuated established chronic hypoxia-induced pulmonary hypertension.
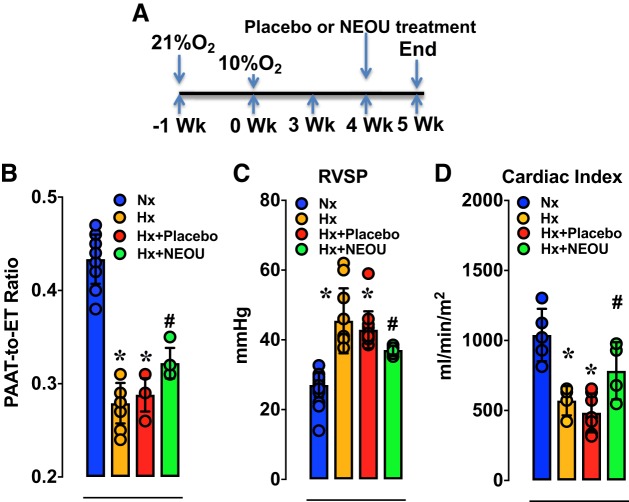
Given that PA remodeling and PH were prevented by G6PD deficiency and that established PH was reduced by G6PD knockdown, we next tested whether pharmacological inhibition of G6PD activity with a small molecule inhibitor (NEOU, N-ethyl-N’-[(3β,5α)-17-oxoandrostan-3-yl]urea) would attenuate established PH. Hypoxia progressively decreased PAAT-to-ET ratios (Nx: 0.429 ± 0.007; 1.5 wk Hx: 0.377 ± 0.007; 3 wk Hx: 0.318 ± 0.011; and 5 wk Hx: 0.292 ± 0.007; P < 0.05 vs. Nx), and increased RV systolic pressure (27.6 ± 0.4; 1.5 wk Hx: 32.5 ± 2.5; 3 wk Hx: 39.2 ± 0.8, and 5 wk Hx: 40.6 ± 0.6; in mmHg) and Fulton’s Index (Nx: 0.229 ± 0.008; 1.5 wk Hx: 0.240 ± 0.015; 3 wk Hx: 0.340 ± 0.007; and 5 wk Hx: 0.393 ± 0.015; P < 0.05 vs. Nx) in CYP2C44−/− mice. Small molecule inhibitor (NEOU) of G6PD activity is easily permeable into the cell and decreases G6PD activity within 12 h and has a half-life of 7–9 h. Therefore, as shown in Fig. 3A, we treated the mice with NEOU once per day in week 4 (for 7 days), and the experiments were terminated at the end of week 5 for hemodynamic measurements. Subcutaneous injections of NEOU (1.5 mg/kg/day sc), a selective and potent inhibitor of G6PD activity (IC50 = 1 μM) (15), for 1 wk to CYP2C44−/− mice with established PH effectively increased the PAAT-to-ET ratios (Fig. 3B), decreased RV systolic pressure (Fig. 3C), and increased the cardiac index (Fig. 3D). In addition to these observations, we also noticed that G6PD inhibition with NEOU dose-dependently relaxed PAs precontracted with KCl (Supplemental Fig. S2B). NEOU decreased G6PD activity (Nx: 0.704 ± 0.071, Hx: 0.917 ± 0.091, and Hx+NEOU: 0.435 ± 0.117 nmol·min−1·g protein−1; P < 0.05 vs. Hx). Consistently, we found that G6PD inhibition decreased Sugen+Hypoxia-induced PH in mice (19a). It is noteworthy that neither G6PD deficiency nor knockdown, nor inhibition caused hemolytic anemia. Hematocrit was increased in all Hx (46% to 65%) as compared with Nx (40% to 51%) groups. Kidney and liver function test parameters were within normal limits (Supplemental Tables S1 and S2). Furthermore, we did not observe neurotoxicity or loss of weight (not shown).
Fig. 3.
Established pulmonary hypertension is reduced by a novel glucose-6-phosphate dehydrogenase (G6PD) activity inhibitor. A: schematic showing the timeline for induction of hypoxia and therapeutic intervention in CYP2C44−/− mice. B–D: the pulmonary artery acceleration time-to-ejection time (PAAT-to-ET) ratio is increased, right ventricle systolic pressure (RVSP) is decreased, and cardiac indexes are increased by treating hypoxic mice with N-ethyl-N′-[(3β,5α)-17-oxoandrostan-3-yl]urea (NEOU; 1.5 mg·kg−1·day−1; sc) in week 4 (for 1 wk) as compared with placebo. Values are means ± SD; n = 20 (in A and B) and 5 (in C) Normoxia group; n = 11 (in A and B) and 4 (in C) in Hypoxia group; n = 6 in Hypoxia-Placebo group; and n = 5 (in A and B) and 4 (in C) Hypoxia+NEOU group; males and females (3:2 ratio) were included in all groups. *P < 0.05 vs. Nx and #P < 0.05 vs. Hx.
G6PD inhibition with NEOU normalized metabolism in lungs and right ventricles of chronically hypoxic mice.
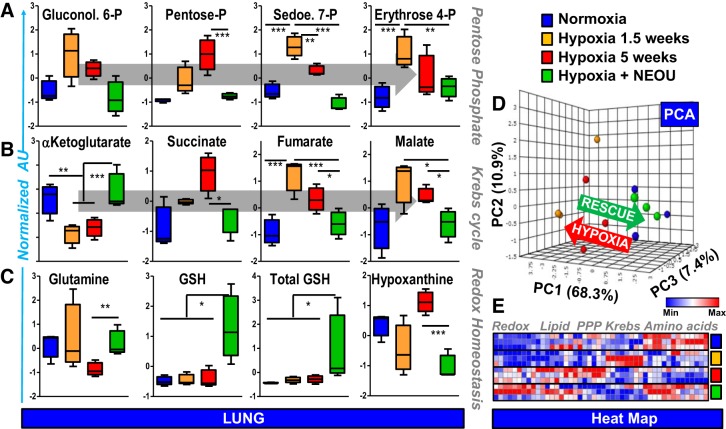
Next, we wanted to determine whether pharmacological inhibition of G6PD activity with NEOU in these CYP2C44−/− mice (see Fig. 3A) would reverse hypoxia-induced metabolic reprogramming in mouse lungs and right ventricles (RVs). NEOU-treated CYP2C44−/− mice exhibited normalization of the hypoxia-induced activation of the PPP, as shown by steady-state measurements of hexose monophosphate shunt, intermediates in lungs and RVs (Fig. 4A and Supplemental Fig. S3A). G6PD inhibition by NEOU also prevented the hypoxia-induced mitochondrial metabolic reprogramming and the accumulation, in both lungs and RVs, of late carboxylic acid intermediates of the Krebs cycle succinate, fumarate and malate (Fig. 4B and Supplemental Fig. S3B), all markers of mitochondrial metabolic derangement observed in PH (9). Furthermore, the levels of either α-ketoglutarate or 2-oxoglutarate were restored, suggestive of normalized hydroxylation capacity and thus promotion of degradation of the hypoxia-inducible factors. G6PD inhibition also decreased (P < 0.05) the ratios (control: 1.0 ± 0.1; hypoxia: 3.2 ± 0.7; and hypoxia+NEOU: 0.9 ± 0.1) of s-adenosylhomocysteine to s-adenosylmethionine (SAM). Although theoretically decreasing the oxidized glutathione-regeneration capacity, inhibition of G6PD did not negatively impact the total and reduced levels of glutathione, while it decreased glutaminolysis and accumulation of the hypoxic marker hypoxanthine (36), both in the lungs and RVs of treated mice vs. hypoxic mice (Fig. 4C, Supplemental Fig. S3D). Overall, exposure to hypoxia for 1.5 to 5 wk induced a progressive metabolic reprogramming, promoting amino acid catabolism, free fatty acid production (which synthesis is NADPH-dependent), lipid oxidation (eicosanoids in RV – Supplemental Fig. S3), activation of the PPP and accumulation of late Krebs cycle intermediates, a phenomenon rescued by inhibition of G6PD, as gleaned by unsupervised Principal Component Analysis (PCA; Fig. 4D and Supplemental Fig. S3E) and hierarchical clustering analysis of metabolomics data (heat maps shown in Fig. 4E and Supplemental Fig. S3F) in lungs and RV.
Fig. 4.
Pharmacological inhibition of glucose-6-phosphate dehydrogenase (G6PD) attenuated metabolic reprogramming in lungs of CYP2C44−/− hypoxic mice. A and B: exposure to hypoxia for 1.5 (orange) to 5 wk (red) of mouse lungs (blue) promotes the activation of the pentose phosphate pathway and accumulation of late Krebs cycle intermediates. This phenotype is rescued by inhibition of G6PD by NEOU (1.5 mg·kg−1·day−1; sc)-treated mice in week 4 (for 1 wk), which also promoted glutathione synthesis and increased the total glutathione pool (reduced + oxidized; C), while decreasing the levels of the hypoxic marker hypoxanthine (C). These pathways were highlighted by unsupervised principal component analysis (PCA; D), which showed a clear rescue by the G6PD inhibition treatment on the metabolic reprogramming induced by hypoxia in mouse lungs. An overview of the main pathways affected by the various treatments is provided in the heat map in E (blue to red = low to high levels of metabolites in that pathway across all replicates). n = 10 in the Normoxia Group; n = 5 in the Hypoxia Group at 1.5 wk; n = 5 in the Hypoxia Group at 5 wk; and n = 5 in the Hypoxia+NEOU Group; males and females (3:2 ratio) were included in all groups. *P < 0.05; **P < 0.01; and ***P < 0.005.
The aberrant expression of protein coding and noncoding genes is moderated in the lungs of hypoxic mice treated with a G6PD inhibitor NEOU.
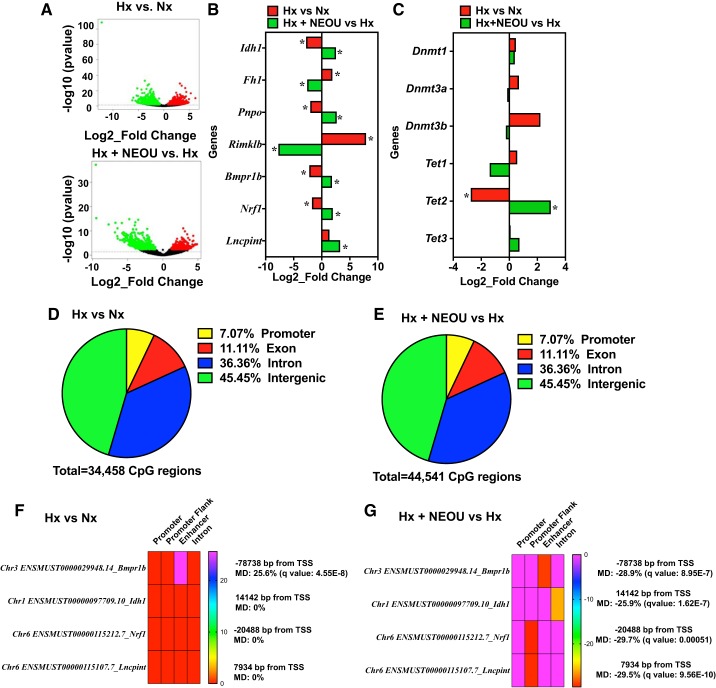
To begin to determine how G6PD deficiency/knockdown and inhibition of protected mice from chronic hypoxia-induced PH development, we took a genomic approach as PH is characterized by aberrant expression of genes involved in apoptosis resistance, proliferation, inflammation, and extracellular matrix remodeling. We performed whole-genome RNA-seq analyses in lungs from wild-type and CYP2C44−/− mice under normoxic or hypoxic conditions, treated with placebo or NEOU (see Fig. 3). While no changes (>1.5-fold) in gene expression were observed in lungs of CYP2C44−/− as compared with littermate WT controls, we found significant changes in the expression of several noncoding and protein coding genes with some being significantly upregulated and others downregulated in the lungs of hypoxic versus normoxic mice and in hypoxic + NEOU versus hypoxic mice (Fig. 5A). Specifically, a total of 1,016 and 952 genes were upregulated and downregulated, respectively, in lungs from hypoxic mice versus normoxic mice (≥2.5 fold). Also 1,535 and 1,362 genes were upregulated and downregulated, respectively, in hypoxia+NEOU versus hypoxia (≥2.5-fold). Top 100 upregulated and downregulated genes in hypoxia versus normoxia and hypoxia+NEOU versus hypoxia are given in Supplemental Tables S3 and S4, respectively. Gene Ontology analysis showed significant enrichment of pathways relevant for metabolic and biosynthetic processes, cell differentiation, muscle organ development, and EGFR and ERBB signaling pathways (Supplemental Fig. S4).
Fig. 5.
Pharmacological inhibition of glucose-6-phosphate dehydrogenase (G6PD) regulates the expression of noncoding and protein coding genes by controlling DNA methylation (epigenetic modification) in lungs of CYP2C44−/− hypoxic mice. Gene expression in lungs of normoxic (n = 3; male 2 and female 1), hypoxic (n = 3; male 2 and female 1), and hypoxic+NEOU (1.5 mg·kg−1·day−1; sc; n = 3; male 2 and female 1) was quantified by whole genome RNA-seq. A: volcano plots demonstrate several genes are upregulated and downregulated in the lungs of hypoxic vs. normoxic mice (top) and hypoxia+ N-ethyl-N′-[(3β,5α)-17-oxoandrostan-3-yl]urea (NEOU; 1.5·mg·kg−1·day−1; sc) vs. hypoxic mice (bottom). Red circles represent significantly upregulated genes, while green circles represent significantly downregulated genes between the groups. Black circles represent genes that were altered but not significantly. B: alterations in the expression of noncoding and protein coding [relevant to metabolic pathways and implicated in pulmonary hypertension (PH)] genes in lungs of hypoxia (Hx) vs. normoxia (Nx) mice, and hypoxia+NEOU (Hx+NEOU-treated for 1 wk) vs. hypoxia (Hx) mice is shown. C: expression of DNA (cytosine-5-)-methyltransferase 1, 3a, and 3b (Dnmt1, Dnmt3a, and Dnmt3b) and ten-eleven translocation methylcytosine dioxygenase 1, 2, and 3 (Tet1, Tet2, and Tet3) in lungs of hypoxic vs. normoxic mice and hypoxia+NEOU vs. hypoxic mice determined by RNAseq is shown. Change in gene expression is shown as Log2_fold change. *P < 0.05 (also see Table 1). DNA methylation in lungs of normoxic (n = 3; male 2 and female 1), hypoxic (n = 3; male 2 and female 1), and hypoxic+NEOU (1.5 mg·kg−1·day−1; sc; n = 3; male 2 and female 1) mice was determined by reduced representation bisulfite sequencing. D and E: pie graphs showing percentage of differentially methylated genes/regions (with a methylation difference threshold of 25% and a q value (SLIM) threshold of 0.05, calculated using the Chi-squared test) in lungs of hypoxia (Hx) vs. normoxia (Nx) and in hypoxia+NEOU (Hx+NEOU) vs. hypoxia (Hx), mice. A landscape map showing the DNA hypermethylation in same location on functional element of Bmpr1b in lung of hypoxia (Hx) vs. normoxia (Nx) (F), and DNA hypomethylation in functional elements of Bmpr1b, Idh1, Nrf1, and lncPint genes in lung of hypoxia+NEOU (Hx + NEOU) vs. Hx (G). Distance of functional elements from transcription start site (TSS), methylation difference (MD), and statistical significance are shown. Details of differential methylation analysis are given in the online supplement.
The effects of hypoxia and NEOU on a subset of genes that encode enzymes in metabolic pathways, such as the Krebs cycle and glutathione metabolism (Fig. 4, B and C), and that are relevant to PH are shown in Fig. 5B and Table 1. For example, expression of the genes that encode Krebs cycle enzymes were up (Idh1)- or down (Fh)-regulated, and the gene related to glutamate and glutathione metabolism (Rimklb) were downregulated, in the lungs of hypoxic mice treated with NEOU as compared with untreated hypoxic mice (Fig. 5B), and these results are consistent with the metabolomic data (Fig. 4, B and C). G6PD inhibition also augmented expression of Pnpo, a gene that encodes pyridoxine 5′-phosphate oxidase involved in the biosynthesis of vitamin B6, which regulates carbohydrate/mitochondrial/one carbon metabolism. Along with the genes encoding metabolic enzymes, expression of nuclear respiratory factor-1 [Nrf1, a transcription factor that activates expression of genes that encode ATP synthase and other enzymes of mitochondrial respiratory chain (4)] and long noncoding (lnc) RNA Pint [a p53-induced noncoding transcript that inhibits proliferation of lung tumor cells and correlates with the expression of gene in metabolic pathway (26)] was increased in lungs of hypoxic mice treated with NEOU as compared with untreated hypoxic mice (Fig. 5B). Expression of the genes relevant to PH (Bmpr1b) was upregulated in the lungs of hypoxic mice treated with NEOU as compared with untreated hypoxic mice (Fig. 5B), and in SMCs of G6PDDef mice as compared with WT mice (Supplemental Fig. S5, A–C). It is well established that the products of Idh1, Fh, and Bmpr1b genes are linked to various forms of PH.
Table 1.
Log2_fold change and statistical values of the genes in Fig. 5
| Hx vs. Nx |
Hx+NEOU vs. Hx |
|||||
|---|---|---|---|---|---|---|
| Gene Name | Log2_fold Change | P Value | Padj | Log2_fold Change | P Value | Padj |
| Idh1 | −2.45 | 0.021 | NA | 2.32 | 0.026 | 0.155 |
| Fh1 | 1.81 | 0.017 | NA | −2.37 | 0.002 | 0.032 |
| Rimklb | 7.89 | 2.92E-11 | 4.15E-08 | −7.72 | 2.27E-13 | 2.85E-10 |
| Pnpo | −2.04 | 0.026 | NA | 2.66 | 0.035 | 0.185 |
| Bmpr1b | −2.21 | 0.047 | NA | 1.82 | 0.049 | 0.366 |
| Nrf1 | −1.74 | 0.075 | NA | 1.97 | 0.052 | 0.226 |
| Lncpint | 1.36 | 0.284 | NA | 3.27 | 0.006 | 0.059 |
| Dnmt1 | 0.47 | 0.747 | 0.75 | 0.39 | 0.524 | 0.752 |
| Dnmt3a | 0.68 | 0.383 | 0.38 | −0.15 | 0.704 | 0.865 |
| Dnmt3b | 2.22 | 0.082 | NA | −0.24 | 0.814 | NA |
| Tet1 | 0.56 | 0.619 | NA | −1.40 | 0.253 | NA |
| Tet2 | −2.75 | 0.012 | NA | 2.96 | 0.002 | 0.038 |
| Tet3 | 0.09 | 0.856 | 0.949 | 0.72 | 0.141 | 0.383 |
Hx, hypoxia; Nx, normoxia.
Furthermore, while we found expression of DNA (cytosine-5)-methyltransferases (Dnmt1, Dnmt3a, and Dnmt3b) was not altered significantly, expression of TET methylcytosine dioxygenase 2 (Tet2), but not Tet1 and Tet3, was decreased (four-fold) in lungs of hypoxic versus normoxic mice (Fig. 5C and Table 1). Importantly, NEOU reversed expression of Tet2 (increased >7.6-fold) in lungs of hypoxic+NEOU versus hypoxic mice. These data indicated that NEOU reverses the alterations of gene expression, induced by hypoxia.
G6PD controls gene expression by regulating DNA methylation.
Association between G6PD and nuclear proteins has been found by coimmunoprecipitation and immunolocalization assays previously (37, 45). Therefore, to further shed light on the mechanisms by which G6PD regulates gene expression, we investigated whether G6PD complexes with proteins that regulate gene expression in SMCs. Using a proteomics approach, G6PD-interacting proteins were determined by pulldown with G6PD antibody or with IgG (control), followed by LC-MS/MS and MALDI-TOF/TOF analyses. We found evidence of a likely association between G6PD and numerous cellular proteins in various cellular compartments (Supplemental Fig. S6). We found that G6PD complexes with nuclear proteins (30% of total binding), including DNA (cytosine-5)-methyltransferase, which catalyzes the transfer of methyl groups to specific CpG elements in DNA during methylation of DNA; methyl CpG binding protein 2 (MeCP2), which binds to methylated DNA and modulates gene transcription; lysine-specific demethylase 3A and 5A, enzymes that demethylate Lys-9 and Lys-4 of histone3, respectively; HDAC5, a histone deacetylase involved in chromatin modification and gene transcription regulation; and RNA polymerases, enzymes involved in gene transcription (Supplemental Table S5).
Expression of genes can be regulated by methylation of nucleosomal histones and/or DNA. DNA methylation and demethylation is mediated by epigenetic modifers: DNA (cytosine-5)-methyltransferases and demethylases/Tet methylcytosine dioxygenases, respectively. Chromatin structure alterations, including alterations of histone modifications, have been suggested to play an important role in the persistently activated phenotypes of cells in the wall of PAs (18). Since our screening identified a potential interaction between G6PD with cytosine-5-methyltransferase (Supplemental Table S5) and because G6PD inhibition decreased one-carbon metabolism, which produces substrate (SAM) for DNA methylation and increased Tet2 expression (Fig. 5C), we determined whether G6PD inhibition regulates expression of protein noncoding and coding gene levels through mechanisms of DNA methylation. Using reduced-representation bisulfite sequencing (RRBS), we found a total of 34,458 (16,298 hypermethylated and 18,159 hypomethylated) CpG regions in lungs of hypoxic as compared with normoxic mice (Fig. 5D), and 44,541 (21,522 hyper-methylated and 22,998 hypo-methylated) CpG regions in lungs of hypoxic mice treated with NEOU as compared with untreated hypoxic mice (Fig. 5E) were differentially methylated (with a methylation diff >25% and q value < 0.05). Of all the changed CpG in Hx vs. Nx or Hx+NEOU vs. Hx, only 7% of CpG regions were located near the promoter regions (including promoter, promoter flank, and enhancer), which could be particularly important for altering gene expression. The differential methylation patterns of the top 150 genes between hypoxia versus normoxia and hypoxia+NEOU versus hypoxia are shown in Supplemental Tables S6 and S7, respectively. Although not all CpG regions that were differentially methylated in Hx versus Nx were normalized by NEOU (~14.6% genes were not normalized by NEOU), NEOU did reverse the methylation pattern of most genes. For example, for the genes listed in Fig. 5B, hypoxia increased CpG methylation in enhancer of Bmpr1b (Fig. 5E), which was reversed by NEOU treatment (Fig. 5F). Moreover, additional 22.4% new CpG regions were differentially methylated in lungs of hypoxia+NEOU versus hypoxia mice as compared with hypoxia versus normoxia mice. For example, for the genes listed in Fig. 5B, Idh1, Nrf1, and lncPint were not differentially methylated in lungs of Hx versus Nx mice (Fig. 5E), but these genes were hypomethylated in specific regulatory regions in lungs of Hx+NEOU versus Hx mice (Fig. 5F), and concomitantly expression of most genes were increased in lungs of Hx+NEOU versus Hx mice (Fig. 5B). Thus, RRBS findings corroborating with the gene expression results suggest that methylation pattern of genes is modified by G6PD inhibition in lungs of hypoxic mice.
G6PD inhibition neither increased gene expression nor reduced pulmonary hypertension in Tet methylcytosine dioxygenase 2 knockout mice.
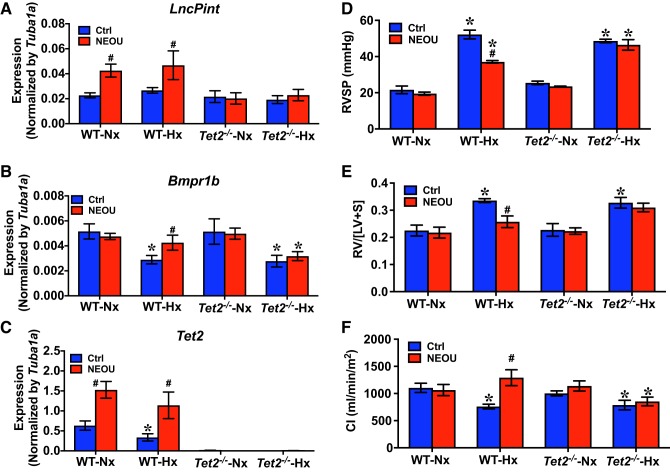
Since NEOU increased Tet2 expression and hypomethylated genes in lungs of hypoxic mice (Fig. 5), we speculated that functional TET2 might be required for G6PD inhibition-elicited upregulation of gene expression and reduction of established PH. Therefore, we determined lncPint, Bmpr1b, and Tet2 expression levels in lungs of normoxic, normoxic+NEOU (1.5 mg·kg−1·day−1; sc), hypoxic, and hypoxic+NEOU (1.5 mg·kg−1·day−1; sc) Tet2tm1.2Rao and WT mice. Mice were kept in normoxia and normoxia+NEOU or exposed to hypoxia and hypoxia+NEOU, as described in Fig. 3A. While levels of lncPint did not decrease in lungs of both WT and Tet2tm1.2Rao mice under hypoxia, compared with the mice under normoxia (Fig. 6A); Bpmr1b and Tet2, levels were similarliy reduced (P < 0.05) in lungs of both WT and Tet2tm1.2Rao mice under hypoxia (Fig. 6, B and C). In the NEOU-treated mice, NEOU treatment increased the levels of lncPint, Bmpr1b, and Tet2 in lungs of WT mice under hypoxia, but not in Tet2tm1.2Rao mice under hypoxia. Alongside NEOU-induced changes of gene expression in a TET2-dependent manner, we also observed that NEOU: 1) reduced hypoxia-induced increases in RV systolic pressure (Fig. 6D), RV hypertrophy (Fig. 6E), and 2) restored decreased cardiac index (CI; Fig. 6F), in WT mice, but not in Tet2tm1.2Rao mice, under hypoxia.
Fig. 6.
Ten-eleven translocation methylcytosine dioxygenase 2 (TET2)-mediated glucose-6-phosphate dehydrogenase (G6PD) inhibition-induced upregulation of molecular footprints and reversal of hypoxia-induced pulmonary hypertension (PH). G6PD inhibitor N-ethyl-N′-[(3β,5α)-17-oxoandrostan-3-yl]urea (NEOU; 1.5 mg·kg−1·day−1; sc): increased expression of lncPint, Bmpr1b, and Tet2 genes in lungs of wild-type (WT) mice, but not in TET2 global knockout (Tet2tm1.2Rao; Tet2−/−) mice (A–C); significantly reduced hypoxia-induced increase of right ventricle systolic pressure (RVSP) and right ventricle hypertrophy (RVH) (D and E); and increased cardiac index (CI) in WT mice, but not in Tet2tm1.2Rao (Tet2−/−) mice (F). Values are means ± SD; n = 5 in WT group (male 3 and female 2) and n = 4 in Tet2tm1.2Rao (male 2 and female 2). *P < 0.05 vs. normoxia and #P < 0.05 vs. control (ctrl).
G6PD inhibition with NEOU normalized metabolism, increased TET2 and lncPINT, and reduced growth of human iPAH-SMC.
To determine whether G6PD regulates metabolic reprogramming in the human SMCs and to establish relevance of findings in mice to human, we applied NEOU to SMCs isolated from PAs of iPAH patients and performed metabolomic analysis. Although metabolic reprogramming is observed in endothelial cells, fibroblasts, and SMCs, we decided to study metabolic phenotype and gene expression in SMCs because SMCs are the most studied PA vascular cells. Furthermore, SMCs acquire a dedifferentiated phenotype and unique metabolic state in vivo and remain in this phenotypically activated state ex vivo in cell culture (22, 49). Our results suggest that application of NEOU effectively inhibited the PPP, as shown by steady-state measurements of hexose monophosphate shunt intermediates in iPAH-SMCs (Fig. 7A, top). G6PD inhibition with NEOU also decreased accumulation of late carboxylic acid (the Krebs cycle) intermediates (Fig. 7A, middle), markers of mitochondrial metabolic derangement observed in PH (9), and one carbon metabolism intermediates (Fig. 7A, bottom), which are substrates for the methylation reactions (25); and normalized the metabolism, as gleaned by unsupervised PCA (Fig. 7B) and hierarchical clustering analysis of metabolomics data (heat maps shown for highlighted metabolites as group averages in Fig. 7C, raw measurements provided as Supplemental Table S8) in iPAH-SMCs.
Fig. 7.
Pharmacological inhibition of glucose-6-phosphate dehydrogenase (G6PD) attenuated metabolic reprogramming in idiopathic pulmonary arterial hypertension (iPAH) smooth muscle cells (SMCs). A: metabolomic analysis indicates activation of the pentose phosphate pathway, accumulation of late Krebs cycle intermediates, and increase of one carbon metabolism in SMCs isolated and cultured from pulmonary arteries (PAs) of iPAH patients (red) as compared with non-iPAH individuals (control; blue). This phenotype is rescued by inhibition of G6PD with NEOU (1 μM; green) treatment for 48 h. These pathways were highlighted by unsupervised principal component analysis (PCA; B), which showed a clear rescue by the G6PD-inhibition treatment on the metabolic reprogramming in iPAH-SMCs. An overview of the main pathways affected by the various treatments is provided in the heat map in C (blue to red = low to high levels of metabolites in that pathway across all replicates). *P < 0.05 and ***P < 0.005.
Finally, to determine whether G6PD regulates gene expression in human-SMCs and to establish relevance of our findings regarding gene expression in mice to humans, we applied NEOU to iPAH- and control-SMCs and measured gene expression, as well as cell growth. NEOU increased (P < 0.05) expression of TET2 in human control-SMCs cultured in hypoxia and in normoxia (Fig. 8A), and in iPAH-SMCs as compared with untreated iPAH-SMCs, which had significantly less TET2 than in control SMCs (Fig. 8B). Concomitantly, NEOU increased (P < 0.05) expression of lncPINT in iPAH-SMCs (Fig. 8C) and in control-SMCs (Ctrl: 0.000270 ± 0.000009 and NEOU: 0.000462 ± 0.000116; P = 0.053). Since lncPINT overexpression inhibits proliferation of lung tumor-derived cells (26), we postulated that upregulation of TET2 and lncPINT in iPAH-SMCs by G6PD inhibition suppresses their growth. Therefore, we determined cell proliferation with a Cyquant assay. As expected, human control-SMC (placebo: 2418 ± 97 versus NEOU: 1741 ± 265; P < 0.05) and iPAH-SMC (Fig. 8D) numbers were reduced by NEOU as compared with placebo control. Conversely, lncPINT knockdown increased growth of human control-SMCs cultured in normoxia and hypoxia (Fig. 8E). If lncPINT is required for G6PD inhibition to reduce SMC growth then NEOU should be ineffective in reducing growth of SMCs lacking lncPINT. Therefore, we tested whether NEOU decreases growth of human control-SMCs, in which lncPINT was knocked out by siRNA. As anticipated, inhibition of G6PD activity with NEOU was unable to reduce growth of human control-SMCs, lacking lncPINT, cultured in normoxia. However, NEOU partially decreased growth of human control-SMCs, lacking lncPINT, cultured in hypoxia (Fig. 8C). These results suggested that G6PD inhibition-elicited decreased growth of human control-SMCs in hypoxia was mediated via lncPINT and additional pathways/mechanisms.
Fig. 8.
Pharmacological inhibition of glucose-6-phosphate dehydrogenase (G6PD) modulated molecular footprints, such as the expression of lncPINT and attenuated growth of idiopathic pulmonary arterial hypertension (iPAH) smooth muscle cells (SMCs). A and B: TET2 expression increased significantly in hypoxic human control-SMCs-NEOU and in iPAH+NEOU-SMCs as compared with iPAH-SMCs. C: LncPINT expression increased significantly in iPAH+NEOU-SMCs as compared with iPAH- and control-SMCs. D: growth of iPAH-SMCs decreased by application of NEOU (1 μM; n = 4–6 different experiments) as compared with iPAH-SMCs. E: hypoxia and knockdown of lncPINT increased growth of human control-SMCs. Data are means ± SD. F: schematic illustrating potential mechanisms by which G6PD knockdown or inhibition attenuated hypoxia-induced PA remodeling and PH. Chronic hypoxia increased G6PD activity and elicited metabolic reprogramming (shown by green arrows and plus sign). Deficiency (Def), knockdown (KD), and inhibition (Inh) of G6PD stopped metabolic reprogramming (shown by red arrow and a minus sign), normalized metabolism, and increased α-ketoglutarate (α-KG)—a cosubstrate of TET methylcytosine dioxygenase—levels. Additionally, it increased TET2 gene expression, which elicited DNA hypomethylation of noncoding and coding genes, including lncPINT. Increased lncPINT decreased growth of control and iPAH-SMCs and prompted normalization of hyperproliferative SMCs. *P < 0.01 vs. Ctrl-siRNA-Normoxia Group and #P < 0.05 vs. LncPINT-SiRNA-Hypoxia Group.
DISCUSSION
Metabolic reprogramming is emerging as a significant mediator of PA inflammation and remodeling in most forms of PH (9). However, the cellular mechanisms that link reprogrammed metabolism to aberrant expression of genes, which modulate functional phenotypes of cells composing PA wall and remodeling of arteries, remain incompletely understood. Recent studies suggest that G6PD expression and activity are increased in several cell types (endothelial cells, SMCs, and fibroblasts) in PH animal models and in patients with idiopathic and heritable PH (2, 6, 22, 41, 48, 49), raising the possibility that G6PD could have a pivotal role in the pathogenesis of PH. Herein, we demonstrated that 1) hypoxia-induced PH is reduced in G6PDDef mice; 2) occlusive PA remodeling and established PH were reduced by G6PD knockdown with lung-directed delivery of G6PD-shRNA in CYP2C44−/− mice; and 3) treatment with the G6PD inhibitor (NEOU) for 1 wk reverted mitochondrial metabolic derangements, reduced remodeling, attenuated established PH, and improved heart function as compared with placebo in PH CYP2C44−/− mice; 4) G6PD knockdown and inhibition induced DNA hypomethylation and increased transcription of lncPint and suppressed growth of iPAH-SMCs; and 5) G6PD inhibition neither increased lncPint in lungs of Tet2tm1.2Rao mice nor reduced hypoxia-induced PH in Tet2tm1.2Rao mice. Together, these findings suggest that G6PD plays a crucial role in mediating the growth of SMCs, which contribute to PA remodeling (23), partly by regulating methylation of DNA, an epigenetic modification. While inhibitors of HDACs, the NAD(P)H-sensitive transcriptional co-regulator CtBP1, PKM2, and PDH kinase have been shown to individually attenuate metabolic derangement, cell proliferation, and PA inflammation (22, 27, 49), G6PD knockdown or inhibition singularly and efficaciously normalized gene transcription, restored glucose and mitochondrial metabolism, reduced PA remodeling, and attenuated PH. Collectively, these data support our hypothesis that G6PD plays a key role in PH pathology (Fig. 8D).
Glucose and fatty acid metabolism is required for cell proliferation and survival. Fatty acid catabolism promotes energy generation in the mitochondria, while fatty acid anabolism supports lipid biosynthesis for organelles and cell membrane expansion. We found in the lungs of hypoxic mice as compared with normoxic mice, that 1) metabolism of l-glutamine, which contributes to l-alanine and succinate generation (9), is decreased; 2) reduced and total glutathione pools, which confers protection from oxidative stress in the tissue, are increased [suggestive of increased synthesis in the face of decreased capacity to reduce oxidized glutathione back to its reduced form (a NADPH-dependent reaction)] are elevated; and 3) glucose metabolism, through the Krebs cycle and the PPP, is increased in a time-dependent manner. Moreover, one-carbon metabolism, the PPP, and late Krebs cycle intermediates are increased in iPAH-SMCs—consistent with our previous observations in iPAH-fibroblasts (49)—as compared with control SMCs. These reversible metabolic changes observed upon G6PD inhibition are attributable to a direct effect, probably driven by law of mass action, and decreased levels of NADPH and ribose phosphate. Additionally, G6PD inhibition-induced reductions in accumulation of glycolytic pathway and the Krebs cycle intermediates in the lungs of hypoxic mice and in iPAH-SMCs are potentially attributable to 1) normalization of Idh1 and Fh1 expression and 2) increased Nrf1 expression, which activates expression of respiratory chain enzymes and increases mitochondrial metabolism (4). Metabolic reprogramming or the Warburg effect, as in cancer cells, is an adaptive response in PH (3, 9, 17, 22, 49). Since reprogramming of metabolism occurred 1.5 wk after hypoxia, and metabolism remained altered thereafter, we suggest that metabolic reprogramming began as an adaptive change and progressively became maladaptive during the development of PH (3–5 wk after hypoxia). Consistently, activity of aerobic glycolysis and the PPP increases before monocrotaline- and hypoxia-induced PH develops (6, 35). Moreover, accumulation of glycolytic and the Krebs cycle metabolites, lactate, and succinate, promotes a proinflammatory phenotype of fibroblasts and macrophages in the PA wall (22) and remodeling of the PA (9). Reprogrammed metabolism in the various cell types of the PA wall now appears to be associated with remodeled large and small PA in most forms of PH (2, 6, 22, 41, 48, 49). As α-ketoglutarate, a coactivator of TET methylcytosine dioxygenases (42), is increased and SAM, a substrate for DNA methylation (9), is decreased in hypoxic lungs by G6PD inhibition, we, therefore, suggest that increased G6PD activity is critical in reprogramming metabolism and interconnecting deranged metabolism to aberrant gene transcription by regulating metabolic substrates required for the methylation of DNA in the cells of hypoxic PAs and lungs.
In this study, we determined the methylation status of the CpG islands in the whole genome by RRBS. After scanning the genome using 500-bp tiling windows, only 7% of differentially methylated regions were located in the promoter regions, and a very few were near the transcription start site and enhancers. Our results demonstrated that G6PD inhibition hypomethylated promoter flanking region in Nrf1 and lncPint; and enhancer in Bmpr1b, to increase their transcripts in the lungs of hypoxic mice. Nrf1 product, NRF1, and BMPR1b-related signaling is implicated in regulating mitochondrial metabolism and in the pathogenesis of PH (4, 11). Although the relevance of the other genes that were significantly hyper- or hypo-methylated in the lungs of hypoxic mice to the pathogenesis of PH remains to be investigated, these data suggest that modifications of DNA methylation in the functional elements dictated gene expression programs in lungs of hypoxic mice. A recent RRBS study has reported similar distinct differentially regulated patterns of DNA methylation in different functional elements across the genome of cancer and normal B cells (31). While the mechanism(s) of how epigenetic modifers like methyltranferases and demethylases, enzymes responsible for the differential methylation of genes, are recruited to specific genomic sites remains a mystery; the unprecedented methylation landscape map taken together with increased Tet2 expression and α-ketoglutarate levels, the essential cosubtrate for TET activity (42), in lungs of hypoxic+NEOU mice and human-SMCs indicate that TET2-mediated demethylation and/or hypomethylation (epigenetic modification) potentially contributed to increase noncoding and coding genes in lungs of hypoxic+NEOU mice and human SMCs. This notion was further confirmed by our results demonstrating NEOU neither increased gene expression nor reduced the hypoxia-induced PH in Tet2 global knockout, Tet2tm1.2Rao, mice. TET2 is decreased in iPAH-SMCs (Fig. 8B), and TET2 deficiency and/or somatic TET2 mutation in hematopoietic cells is associated with overproduction of proinflammatory cytokines and pathogenesis of atherosclerosis (13), and SMC dedifferentiation (24)—both inflammation and SMC dedifferentiation are incriminated in the pathogenesis of PH (9). Based on these studies and our findings that Tet2 is downregulated in lungs of hypoxic mice, we anticipated that Tet2tm1.2Rao mice would either spontaneously develop PH or worsen hypoxia-induced PH. Although Tet2tm1.2Rao mice neither spontaneously developed PH nor worsened hypoxia-induced PH for reasons yet unknown, we suggest that TET2 is a critical contributor to G6PD inhibition-induced DNA hypomethylation of noncoding and coding genes. In addition, decrease of ancillary metabolism pathways like polyamine pathway and one carbon metabolism, which produces substrate for methylation of DNA (9, 34), suggest that decreased substrates for DNA methylation in lungs of hypoxic+NEOU, at least partly, contributed to the G6PD inhibition-induced hypomethylation of genes mice observed in this study.
Since inhibition of G6PD activity modulated DNA methylation, it is reasonable to envision this could regulate the binding activity of metabolic- and redox-sensitive transcription factors and coregulators to the DNA and alter the gene expression profile in various cell types in the lungs, including SMCs, endothelial cells, and fibroblasts. Additionally, as G6PD is associated with nuclear enzymes that modify histones (acetylation and methylation) and DNA/RNA polymerases (Supplemental Table S5), we suspect that G6PD potentially regulates gene expression through chromatin modification, which has been suggested to persistently activate cells in the wall of PAs (18), followed by polymerase-dependent transcription. G6PD is present in the nucleus of hepatic cells (37), and its nuclear localization was recently confirmed by CRISPR-Cas9 editing (38). These studies support our idea that G6PD contributes to the epigenetic modification and regulation of the expression of genes that orchestrate the accumulation of persistently activated cell types involved in remodeling of hypertensive PAs.
G6PD inhibition increased expression of TET2 and lncPINT, and reduced growth of control- and iPAH-SMCs, which exhibit a hyperproliferative phenotype ex vivo (12, 23). LncPINT, a polycomb repressive complex 2-interacting lncRNA, is a tumor suppressor that reduces proliferation of colon primary tumors by regulating expression of genes of the TGF-β, MAPK, and p53 pathways (26). Silencing of lncPINT increased growth of control-SMCs cultured in normoxia and hypoxia. Inhibiting G6PD activity partially decreased cell numbers of hypoxic, but not normoxic, control-SMCs lacking (knockdown) lncPINT. This suggests that lncPINT-mediated the antiproliferative effects of G6PD inhibition in normoxia but in hypoxia the anti-proliferative effects of G6PD inhibition were mediated by lncPINT and additional mechanism(s). Since G6PD inhibition increased TET2 expression in iPAH-SMCs and control-SMCs, we propose that TET2-dependent mechanisms, which suppresses leukemia and mast cell proliferation (29, 46), also contributed to G6PD inhibition-induced decreased growth of SMCs. Previous studies have implicated increased G6PD activity in growth factor- and hypoxia-induced proliferation of SMCs, endothelial cells, Balb/c 3T3 fibroblasts, and macrophages (6, 21, 30, 43). Our new findings demonstrate that TET2 and lncPINT potentially contributed to decrease iPAH-SMCs growth and moderated PA remodeling within the lungs of hypoxic mice observed upon G6PD inhibition.
Collectively then, these findings demonstrate a new function for G6PD as a controller of DNA methylation. Furthermore, we suggest that G6PD serves as a link between metabolic reprogramming and gene expression programs that regulate SMC phenotype. Therefore, a moderate decrease of G6PD activity would have beneficial effects in PH (Fig. 8F).
Limitation.
Although our results clearly suggest that G6PD deficiency, knockdown, and inhibition were beneficial in reducing hypoxia-induced PH, these findings must be interpreted cautiously, as there are contradicting reports in the literature regarding association of loss-of-function mutation in G6PD, with hemolysis and PH. Studies suggest that loss-of-function mutation in G6PD and a moderate loss of G6PD activity with hemolysis is associated with idiopathic pulmonary arterial hypertension (5, 20). In contrast another study suggests that PH is associated with increased hemolysis/sickle cell disease but not with G6PD deficiency (10). Our preliminary studies suggest that G6PDDef mice without or with hemolysis are not predisposed to PH. However, more studies are needed to confirm these findings, and this topic should be addressed elaborately in the future. Nonetheless, G6PD deficiency and effects on SMC signaling are perhaps a balancing and protective influence on PH.
GRANTS
This study was supported by National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) Grant RO1 HL132574 and the American Heart Association Grant 17GRNT33670454 (to S. A. Gupte); NHLBI Grant P01 HL014985, NHLBI Grant R01 HL114887, and Department of Defense Grant W81XWH-15-1-0280 (to K. R. Stenmark). I. Waddell and A. Jordon were wholly funded by Cancer Research UK (Grants C480/A1141 and C5759/A17098).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.G. conceived and designed research; S.R.J., A.K., C.J., R.H., V.D., A.R., C.Z., H.Z., and A.D. performed experiments; S.R.J., V.D., A.D., and S.A.G. analyzed data; A.J., I.W., J.A.L., I.F.M., A.D., K.R.S., and S.A.G. interpreted results of experiments; S.R.J., V.D., C.Z., A.D., and S.A.G. prepared figures; ; S.A.G. drafted manuscript; S.R.J., R.H., V.D., J.A.L., C.-J.H., I.F.M., A.D., K.R.S., and S.A.G. edited and revised manuscript; S.R.J., A.K., C.J., R.H., V.D., A.R., H.Z., A.J., I.W., J.A.L., C.-J.H., I.F.M., A.D., K.R.S., and S.A.G. approved final version of manuscript.
REFERENCES
- 1.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 2.Boehme J, Sun X, Tormos KV, Gong W, Kellner M, Datar SA, Kameny RJ, Yuan JX, Raff GW, Fineman JR, Black SM, Maltepe E. Pulmonary artery smooth muscle cell hyperproliferation and metabolic shift triggered by pulmonary overcirculation. Am J Physiol Heart Circ Physiol 311: H944–H957, 2016. doi: 10.1152/ajpheart.00040.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caruso P, Dunmore BJ, Schlosser K, Schoors S, Dos Santos CC, Perez-Iratxeta C, Lavoie JR, Zhang H, Long L, Flockton AR, Frid MG, Upton PD, D’Alessandro A, Hadinnapola C, Kiskin FN, Taha M, Hurst LA, Ormiston ML, Hata A, Stenmark KR, Carmeliet P, Stewart DJ, Morrell NW. Identification of MicroRNA-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary arterial hypertension via PTBP1 (polypyrimidine tract binding protein) and pyruvate kinase M2. Circulation, 136: 2451–2467, 2017. doi: 10.1161/CIRCULATIONAHA.117.028034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau CM, Evans MJ, Scarpulla RC. Nuclear respiratory factor 1 activation sites in genes encoding the gamma-subunit of ATP synthase, eukaryotic initiation factor 2α, and tyrosine aminotransferase. Specific interaction of purified NRF-1 with multiple target genes. J Biol Chem 267: 6999–7006, 1992. [PubMed] [Google Scholar]
- 5.Chen LY, Lai EJ, Collins DR, Ostrow DN, Sreenivasan GM. A young woman with episodic angioedema, papilledema, and eosinophilia. Am J Hematol 85: 124–127, 2010. doi: 10.1002/ajh.21584. [DOI] [PubMed] [Google Scholar]
- 6.Chettimada S, Gupte R, Rawat D, Gebb SA, McMurtry IF, Gupte SA. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: implication in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L287–L300, 2015. doi: 10.1152/ajplung.00229.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chettimada S, Joshi SR, Alzoubi A, Gebb SA, McMurtry IF, Gupte R, Gupte SA. Glucose-6-phosphate dehydrogenase plays a critical role in hypoxia-induced CD133+ progenitor cells self-renewal and stimulates their accumulation in the lungs of pulmonary hypertensive rats. Am J Physiol Lung Cell Mol Physiol 307: L545–L556, 2014. doi: 10.1152/ajplung.00303.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chettimada S, Joshi SR, Dhagia V, Aiezza A II, Lincoln TM, Gupte R, Miano JM, Gupte SA. Vascular smooth muscle cell contractile protein expression is increased through protein kinase G-dependent and -independent pathways by glucose-6-phosphate dehydrogenase inhibition and deficiency. Am J Physiol Heart Circ Physiol 311: H904–H912, 2016. doi: 10.1152/ajpheart.00335.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Alessandro A, El Kasmi KC, Plecitá-Hlavatá L, Ježek P, Li M, Zhang H, Gupte SA, Stenmark KR. Hallmarks of pulmonary hypertension: mesenchymal and inflammatory cell metabolic reprogramming. Antioxid Redox Signal 28: 230–250, 2018. doi: 10.1089/ars.2017.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahoui HA, Hayek MN, Nietert PJ, Arabi MT, Muwakkit SA, Saab RH, Bissar AN, Jumaa NM, Farhat FS, Dabbous IA, Bitar FF, Abboud MR. Pulmonary hypertension in children and young adults with sickle cell disease: evidence for familial clustering. Pediatr Blood Cancer 54: 398–402, 2010. doi: 10.1002/pbc.22306. [DOI] [PubMed] [Google Scholar]
- 11.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, Cao A, Wang L, Reddy S, Chen PI, Nakahira K, Alcazar MA, Hopper RK, Ji L, Feldman BJ, Rabinovitch M. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab 21: 596–608, 2015. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, Fini MA, Graham BB, Tuder RM, Friedman JE, Eltzschig HK, Sokol RJ, Stenmark KR. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 193: 597–609, 2014. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andrés V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355: 842–847, 2017. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupte SA, Li KX, Okada T, Sato K, Oka M. Inhibitors of pentose phosphate pathway cause vasodilation: involvement of voltage-gated potassium channels. J Pharmacol Exp Ther 301: 299–305, 2002. doi: 10.1124/jpet.301.1.299. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton NM, Dawson M, Fairweather EE, Hamilton NS, Hitchin JR, James DI, Jones SD, Jordan AM, Lyons AJ, Small HF, Thomson GJ, Waddell ID, Ogilvie DJ. Novel steroid inhibitors of glucose 6-phosphate dehydrogenase. J Med Chem 55: 4431–4445, 2012. doi: 10.1021/jm300317k. [DOI] [PubMed] [Google Scholar]
- 16.Hecker PA, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am J Physiol Heart Circ Physiol 304: H491–H500, 2013. doi: 10.1152/ajpheart.00721.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2: ra73, 2009. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu CJ, Zhang H, Laux A, Pullamsetti SS, Stenmark KR. Mechanisms contributing to persistently activated cell phenotypes in pulmonary hypertension. J Physiol 597: 1103–1119, 2019. doi: 10.1113/JP275857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi SR, Lakhkar A, Dhagia V, Zias AL, Soldatos V, Oshima K, Jiang H, Gotlinger K, Capdevila JH, Schwartzman ML, McMurtry IF, Gupte SA. Cyp2c44 gene disruption exacerbated pulmonary hypertension and heart failure in female but not male mice. Pulm Circ 6: 360–368, 2016. doi: 10.1086/688060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Kitagawa A, Gupte S. Hemodynamic impact of glucose-6-phosphate dehydrogenage inhibitor in pulmonary arterial hypertension model mice (Abstract). Circulation 140: A9544, 2019. [Google Scholar]
- 20.Kurdyukov S, Eccles CA, Desai AA, Gonzalez-Garay M, Yuan JX, Garcia JGN, Rafikova O, Rafikov R. New cases of glucose-6-phosphate dehydrogenase deficiency in pulmonary arterial hypertension. PLoS One 13: e0203493, 2018. doi: 10.1371/journal.pone.0203493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leopold JA, Walker J, Scribner AW, Voetsch B, Zhang YY, Loscalzo AJ, Stanton RC, Loscalzo J. Glucose-6-phosphate dehydrogenase modulates vascular endothelial growth factor-mediated angiogenesis. J Biol Chem 278: 32100–32106, 2003. doi: 10.1074/jbc.M301293200. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Riddle S, Zhang H, D’Alessandro A, Flockton A, Serkova NJ, Hansen KC, Moldovan R, McKeon BA, Frid M, Kumar S, Li H, Liu H, Caánovas A, Medrano JF, Thomas MG, Iloska D, Plecitá-Hlavatá L, Ježek P, Pullamsetti S, Fini MA, El Kasmi KC, Zhang Q, Stenmark KR. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation 134: 1105–1121, 2016. doi: 10.1161/CIRCULATIONAHA.116.023171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol 187: 2711–2722, 2011. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 128: 2047–2057, 2013. doi: 10.1161/CIRCULATIONAHA.113.002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13: 572–583, 2013. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marín-Béjar O, Marchese FP, Athie A, Sánchez Y, González J, Segura V, Huang L, Moreno I, Navarro A, Monzó M, García-Foncillas J, Rinn JL, Guo S, Huarte M. Pint lincRNA connects the p53 pathway with epigenetic silencing by the Polycomb repressive complex 2. Genome Biol 14: R104, 2013. doi: 10.1186/gb-2013-14-9-r104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, Cupitt J, Paterson I, Thompson RB, Chow K, O’Regan DP, Zhao L, Wharton J, Kiely DG, Kinnaird A, Boukouris AE, White C, Nagendran J, Freed DH, Wort SJ, Gibbs JSR, Wilkins MR. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med 9: eaao4583, 2017. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 28.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 105: 244–250, 2002. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 29.Montagner S, Leoni C, Emming S, Della Chiara G, Balestrieri C, Barozzi I, Piccolo V, Togher S, Ko M, Rao A, Natoli G, Monticelli S. TET2 regulates mast cell differentiation and proliferation through catalytic and non-catalytic activities. Cell Reports 15: 1566–1579, 2016. [Erratum in Cell Rep 20: 1744, 2017.] doi: 10.1016/j.celrep.2016.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J, Choe SS, Choi AH, Kim KH, Yoon MJ, Suganami T, Ogawa Y, Kim JB. Increase in glucose-6-phosphate dehydrogenase in adipocytes stimulates oxidative stress and inflammatory signals. Diabetes 55: 2939–2949, 2006. doi: 10.2337/db05-1570. [DOI] [PubMed] [Google Scholar]
- 31.Pei L, Choi JH, Liu J, Lee EJ, McCarthy B, Wilson JM, Speir E, Awan F, Tae H, Arthur G, Schnabel JL, Taylor KH, Wang X, Xu D, Ding HF, Munn DH, Caldwell C, Shi H. Genome-wide DNA methylation analysis reveals novel epigenetic changes in chronic lymphocytic leukemia. Epigenetics 7: 567–578, 2012. doi: 10.4161/epi.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pozzi A, Popescu V, Yang S, Mei S, Shi M, Puolitaival SM, Caprioli RM, Capdevila JH. The anti-tumorigenic properties of peroxisomal proliferator-activated receptor α are arachidonic acid epoxygenase-mediated. J Biol Chem 285: 12,840–12,850, 2010. doi: 10.1074/jbc.M109.081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pretsch W, Charles DJ, Merkle S. X-linked glucose-6-phosphate dehydrogenase deficiency in Mus musculus. Biochem Genet 26: 89–103, 1988. doi: 10.1007/BF00555491. [DOI] [PubMed] [Google Scholar]
- 34.Puleston DJ, Villa M, Pearce EL. Ancillary activity: beyond core metabolism in immune cells. Cell Metab 26: 131–141, 2017. doi: 10.1016/j.cmet.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rafikova O, Meadows ML, Kinchen JM, Mohney RP, Maltepe E, Desai AA, Yuan JX, Garcia JG, Fineman JR, Rafikov R, Black SM. Metabolic changes precede the development of pulmonary hypertension in the monocrotaline exposed rat lung. PLoS One 11: e0150480, 2016. doi: 10.1371/journal.pone.0150480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell GA, Jeffers G, Cooke RW. Plasma hypoxanthine: a marker for hypoxic-ischaemic induced periventricular leucomalacia? Arch Dis Child 67: 388–392, 1992. doi: 10.1136/adc.67.4_Spec_No.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer NY, Yan Z, Boudreau RL, Zhang Y, Luo M, Li Q, Tian X, Shah AM, Davisson RL, Davidson B, Banfi B, Engelhardt JF. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J Biol Chem 286: 8977–8987, 2011. doi: 10.1074/jbc.M110.193821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer NY, Yan Z, Cong L, Zhang Y, Engelhardt JF, Stanton RC. Definitive localization of intracellular proteins: novel approach using CRISPR-Cas9 genome editing, with glucose 6-phosphate dehydrogenase as a model. Anal Biochem 494: 55–67, 2016. doi: 10.1016/j.ab.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenmark KR, Tuder RM, El Kasmi KC. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J Appl Physiol (1985) 119: 1164–1172, 2015. doi: 10.1152/japplphysiol.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, Olin-Sandoval V, Grüning NM, Krüger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc 90: 927–963, 2015. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X, Kumar S, Sharma S, Aggarwal S, Lu Q, Gross C, Rafikova O, Lee SG, Dasarathy S, Hou Y, Meadows ML, Han W, Su Y, Fineman JR, Black SM. Endothelin-1 induces a glycolytic switch in pulmonary arterial endothelial cells via the mitochondrial translocation of endothelial nitric oxide synthase. Am J Respir Cell Mol Biol 50: 1084–1095, 2014. doi: 10.1165/rcmb.2013-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324: 930–935, 2009. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian WN, Braunstein LD, Pang J, Stuhlmeier KM, Xi QC, Tian X, Stanton RC. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem 273: 10609–10617, 1998. doi: 10.1074/jbc.273.17.10609. [DOI] [PubMed] [Google Scholar]
- 44.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 28: 23–42, 2007. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogelauer M, Krall AS, McBrian MA, Li JY, Kurdistani SK. Stimulation of histone deacetylase activity by metabolites of intermediary metabolism. J Biol Chem 287: 32006–32016, 2012. doi: 10.1074/jbc.M112.362467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Xiao M, Chen X, Chen L, Xu Y, Lv L, Wang P, Yang H, Ma S, Lin H, Jiao B, Ren R, Ye D, Guan KL, Xiong Y. WT1 recruits TET2 to regulate its target gene expression and suppress leukemia cell proliferation. Mol Cell 57: 662–673, 2015. doi: 10.1016/j.molcel.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao W, Wang RS, Handy DE, Loscalzo J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid Redox Signal 28: 251–272, 2018. doi: 10.1089/ars.2017.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao C, Yu J, Taylor L, Polgar P, McComb ME, Costello CE. Protein expression by human pulmonary artery smooth muscle cells containing a BMPR2 mutation and the action of ET-1 as determined by proteomic mass spectrometry. Int J Mass Spectrom 378: 347–359, 2015. doi: 10.1016/j.ijms.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, Riddle S, Kumar S, Flockton A, McKeon BA, Frid MG, Reisz JA, Caruso P, El Kasmi KC, Ježek P, Morrell NW, Hu CJ, Stenmark KR. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a MicroRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation 136: 2468–2485, 2017. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]