Abstract
Inherent and acquired factors determine the integrated autonomic response to cardiovascular stressors. Excessive sympathoexcitation to ischemic stress is a major contributor to the potential for sudden cardiac death. To define fundamental aspects of cardiac-related autonomic neural network interactions within the thoracic cord, specifically as related to modulating sympathetic preganglionic (SPN) neural activity. Adult, anesthetized Yorkshire pigs (n = 10) were implanted with penetrating high-density microarrays (64 electrodes) at the T2 level of the thoracic spinal cord to record extracellular potentials concurrently from left-sided dorsal horn (DH) and SPN neurons. Electrical stimulation of the T2 paravertebral chain allowed for antidromic identification of SPNs located in the intermediolateral cell column (57 of total 1,760 recorded neurons). Cardiac stressors included epicardial touch, occlusion of great vessels to transiently alter preload/afterload, and transient occlusion of the left anterior descending coronary artery (LAD). Spatial/temporal assessment of network interactions was characterized by cross-correlation analysis. While some DH neurons responded solely to changes in preload/afterload (8.5 ± 1.9%) or ischemic stress (10.5 ± 3.9%), the majority of cardiovascular-related DH neurons were multimodal (30.2 ± 4.7%) with ischemia sensitivity being one of the modalities (26.1 ± 4.7%). The sympathoexcitation associated with transient LAD occlusion was associated with increased correlations from baseline within DH neurons (2.43 ± 0.61 to 7.30 ± 1.84%, P = 0.04) and between SPN to DH neurons (1.32 ± 0.78 to 7.24 ± 1.84%, P = 0.02). DH to SPN network correlations were reduced during great vessel occlusion. In conclusion, increased intrasegmental network coherence within the thoracic spinal cord contributes to myocardial ischemia-induced sympathoexcitation.
NEW & NOTEWORTHY In an in vivo pig model, we demonstrate using novel high-resolution neural electrode arrays that increased intrasegmental network coherence within the thoracic spinal cord contributes to myocardial ischemia-induced sympathoexcitation.
Keywords: cardiopulmonary afferents, myocardial ischemia, spinal cord, sympathetic nervous system, sympathetic preganglionic neurons
INTRODUCTION
Cardiac control is achieved via a hierarchical network of interactions between and within central nervous system neurons (both medullary centers and the spinal cord), peripheral extracardiac-intrathoracic ganglia, and the intrinsic nervous system (ICN) (52). These interwoven neural networks for cardiac control are in constant communication with each other to maintain homeostasis of cardiac output and excitability in varying physiological conditions. Coordination of these neural networks involve descending (efferent), ascending (afferent), and intraganglionic interactions (11, 36, 52). As in all integrated neural systems, the elements within each network are recruited to optimize function, and as pathophysiological states develop, these neural networks reorganize in both compensatory and deleterious ways impacting function (4, 52, 57). Sudden cardiac death, the leading cause of mortality in the United States (59), reflects an abnormal cardiac electrophysiological substrate that is further amplified by aberrant remodeling of the cardiac nervous system (2, 28), involving both sympathetic and parasympathetic systems (52). To understand the adaptations of the cardiac neural networks in heart disease, it is critical to define the processing capabilities of these multilevel neural networks in response to cardiac stressors.
Peripheral ganglia for cardiac control, including those contained within the ICN and stellate/middle cervical ganglia, are capable of complex reflex processing even when disconnected from the central nervous system (8, 10, 50, 52). Rather than functioning as relay stations, their contained neural networks of postganglionic soma, local circuit neurons, and afferents are impacted by descending projections from higher centers that allow for beat-to-beat regulation of regional cardiac function (39, 52). The central projections to these peripheral ganglia arise from reflex networks arising in the brainstem, spinal cord, and from higher centers (13, 20, 21, 32).
For reflex control of sympathetic function, the spinal cord manifests integration of intraspinal reflexes and those involving higher centers (13, 21), with the sympathetic preganglionic neurons (SPNs) representing the final neural nexus point for central control of sympathetic activity. While recording of single unit activity has resolved much of the interconnectivity of spinal sympathetic neurons (21, 24, 26), the advent of high-density, penetrating microarrays has opened new avenues for dynamic assessments of local neuronal network functions that regulate sympathetic output to the heart. The current study aimed to use high-density microarray extracellular recording of the porcine thoracic spinal cord to characterize 1) neuronal discharge phenotypes of cardiac related dorsal horn (DH) neurons and 2) characterize neuronal connectivity within DH neurons and between DH and SPN neurons localized to the same spinal segment. We defined the neural interactions at baseline and in response to varying cardiac and extracardiac perturbations. Here we provide evidence for distinct populations of SPN and putative dorsal horn neurons that can be characterized as cardiac related, ischemia related, or multimodal. Further, we demonstrate functional interconnectivity within these neural networks, which can be enhanced (e.g., myocardial ischemia) or decreased (e.g., transient reductions in preload) depending on the characteristics of the cardiovascular stressor. Characterizing the neural network interactions is critical for defining the inherent and acquired factors that predispose to arrhythmia generation (28), progression of heart failure (23), and to identifying targets for neuromodulation (30, 57).
MATERIALS AND METHODS
Animal preparation.
All animal experiments were approved by the University of California, Los Angeles, Animal Research Committee, performed in accordance with guidelines set forth by the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (8th ed., 2011). No statistical differences in neural data was observed between male (n = 4) and female (n = 6) adult Yorkshire pigs, and thus all animals were included (n = 10). Animals weighing 42 ± 1.6 kg were sedated with intramuscular Telazol (4–6 mg/kg), intubated, and mechanically ventilated. General anesthesia was maintained with inhaled isoflurane (1.5–2.5%) and intravenous boluses of fentanyl (total, 10–30 µg/kg) during surgical preparation. Inhaled isoflurane was transitioned to intravenous α-chloralose (50 mg/kg bolus followed by a 20 mg·kg−1·h−1 continuous infusion) after surgical preparation was completed. Continuous intravenous saline was infused through the femoral vein throughout the experiments to maintain volume homeostasis. Arterial blood pressure was measured via a femoral arterial line. Heart rate was monitored by ECG using a surface 12-lead electrocardiogram. Left ventricular (LV) systolic pressure was measured using a pressure monitoring pigtail 5-Fr catheter inserted into the LV via the left carotid artery and connected to a MPVS Ultra Pressure Volume Loop System (Millar Instruments, Houston, TX). Arterial blood gas was tested hourly, and adjustment of ventilation and/or administration of sodium bicarbonate were made as necessary to maintain acid-base homeostasis. At the completion of the experiments, animals were humanely euthanized under anesthesia by inducing ventricular fibrillation via application of direct current to the heart.
Neuronal activity recording.
The activity generated by neuronal soma in the T2 level of the spinal cord was directly recorded in all 10 animals in situ. Thoracic laminectomy was performed to expose the spinal cord, and a small window was made in the spinal cord dura to access the T2 level of the spinal cord. A high-density two-dimensional (2D), penetrating microarray (64 electrodes distributed on 8 shanks; 5 mm in length; shanks, 200 µm apart; electrodes, 100 µm apart; NeuroNexus, Ann Arbor, MI), mounted on a micromanipulator, was inserted 2 mm into the spinal cord at T2 level, lateral to the midline (Fig. 1A). To be consistent across animals, we used anatomical landmarks and very precise micromanipulators to insert the electrodes to an exact depth each time. The animals remained stationary throughout the experiments. All reasonable precautions were taken to minimize any movement or degradation of the recording. This position allowed the recording sites to concurrently capture both dorsal horn (DH) and sympathetic preganglionic neuronal discharge from the intermediolateral (IML) cell column. The connecting wires of the multichannel electrode, along with ground and reference wires, were attached to a 64-channel headstage interface with 16-bit, 0.15-µV A/D resolution. For each channel, bandwidth filters were set to 500 Hz to 10 kHz and notch filter was set at 60 Hz. Extracellular potentials were recorded directly from the T2 region of the thoracic spinal cord, amplified, and digitized (SmartBox acquisition system, NeuroNexus, Ann Arbor, MI). The sampling frequency for neuronal data was 30 kHz.
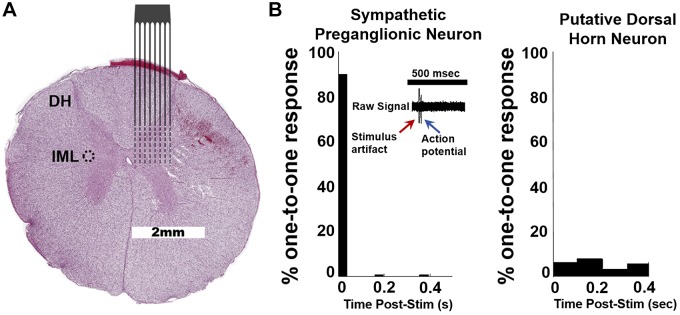
Fig. 1.
A: representative porcine spinal cord (T2; hematoxylin and eosin staining) with overlay of approximate location of recording electrode array. Intermediolateral nucleus (IML; circled). B: T2 paravertebral stimulation elicits spike firing in IML to identify sympathetic preganglionic neurons (SPNs). Stimulation should elicit a one-to-one response in SPNs (left) vs. other, putative dorsal horn (DH) neurons (1,703 neurons; 155 ± 48 per pig; n = 10). SPNs were identified as those that exhibited a one-to-one response to T2 paravertebral stimulation within <0.05 s at least 60% of the time. SPNs were only identified in 8 of 10 pigs (57 neurons; 6 ± 2 per pig; n = 8).
T2 paravertebral chain stimulation.
Sympathetic preganglionic neurons (SPNs) associated with cardiac control originate in the IML and project axons rostral in paravertebral chain to synapse with postganglionic contained with the stellate/middle cervical ganglia (18, 51). Left thoracotomy was performed to expose the sympathetic nerves of the posterior thorax, and bipolar needles were inserted into the left T2 paravertebral chain ganglia. To define threshold for activation of SPNs at the T2 ganglia, square wave stimulation pulses were delivered at 4 Hz, 4 ms duration by a Grass S88 Stimulator (Grass, Warwick, RI), functional threshold being defined as the current level that produced a 10% increase in systolic blood pressure. Thereafter, stimulation pulses were delivered to the T2 paravertebral ganglia 1 Hz at threshold intensity (7.0 ± 1.0 mA). The lower frequency was used to avoid evoking changes in neural activity secondary to changes in hemodynamics. SPNs were identified as those that fired with a constant latency of <0.05 s after T2 paravertebral chain stimulation pulses (Fig. 1B).
Cardiovascular stressors to perturb spinal cord neural networks.
To characterize interdependent neural network activities within and between the DH and SPN neurons, baseline activity was recorded and these networks were then sequentially and randomly challenged with cardiovascular stressors. To this purpose, following thoracotomy, umbilical tape was placed around the inferior vena cava (IVC) and descending aorta (Ao), and a 4-0 suture was place around the left anterior descending (LAD) coronary artery; each was led through polyethylene tubing. These allowed for transient and controlled occlusions of the great vessels (IVC, Ao; ~60-s duration) and the LAD coronary artery (~5-min duration). Activation of cardiac and somatic mechanosensitive receptive fields was accomplished by gentile touch of the ventricular epicardial surface using a moist Q-tip and gentle scratching of the left side of the chest and anterior forelimb respectively, each for ~60 s. Adequate time was given between perturbations for hemodynamic and electrophysiological measures to return to baseline between interventions (~15 min for great vessel occlusion and touch, and 30 min for LAD CAO).
Neuronal activity analysis.
Extracellular activity produced by individual neuronal somata located within the T2 level of the spinal cord was recorded. Neural activities with signal to noise ratios > 2:1 were identified. Based on impedance characteristics of the 2D microarrays, filter setting and high-impedance headstage, such activity reflects action potentials recorded from closely adjacent soma and not axons of passage since action potentials traveling along axons are difficult to record using this high-impedance technology because of relatively small ionic displacement as compared with the soma and thus extracellular potentials generated by the soma are orders of magnitude larger than that of axons (15, 52). Stratification of activity from individual neurons was completed off-line via available spike sorting tools (principal component analysis and cluster on measurements techniques) in Spike2 software (Cambridge Electronics Design, Cambridge, England). To determine significant neuronal activity changes and functionally delineate each identified neuron during cardiac and mechanical perturbations, a statistical test based on the Skellam distribution was performed using custom MatLab scripts (15, 56, 58). The Skellam distribution is the probability distribution of the difference of two random variables following a Poisson distribution. In this case, 1 min of neural firing rates before the perturbation (baseline) were compared with neural firing rates during the perturbation. Cardiac-related neurons were defined using Skellam analysis if their firing rate exhibited significant change (P ≤ 0.05; either increasing or decreasing) in response to at least one of the cardiac perturbations. Cardiac-related neurons were further classified as 1) ischemia sensitive if they elicited a significant response only to LAD occlusion, 2) cardiac load dependent if they responded significantly to aortic or IVC occlusion, or 3) multimodal if they responded significantly to two or more perturbations. Noncardiac neurons were classified as those that did not respond significantly to any cardiovascular perturbation; included in this group were neurons that only responded significantly to chest scratch.
Cross-correlation analysis.
With the use of a bin width of 0.2 ms, cross-correlation histograms were created with all possible pairs of simultaneously recorded neurons (48). A detectability index (DI) (1) was calculated for each correlogram as the peak relative to average activity (calculated over 20 ms before the trigger), divided by the SD. Features were considered significant if the DI was >10 (46), and only significant features in the positive direction were counted. Peaks are consistent with excitation between the initiating and target neurons (1, 40). Summary figures are displayed as the number of positive cross-correlogram features (CCs) of each putative cellular relationship, expressed as a percentage of the total number of possible positive features (i.e., based on the total number of recorded cells at each time point). Four potential correlative relationships were explored: 1) DH to SPN, 2) DH to DH, 3) SPN to DH, and 4) SPN to SPN. For statistical comparisons, the proportions of CCs were averaged for the periods before, during, and after each perturbation. With the use of these values, separate one-way, repeated-measures ANOVAs with all pairwise multiple comparisons analyzed via Tukey test as post hoc were run to analyze differences in the proportion of CCs between each period.
RESULTS
Classification of sympathetic preganglionic and putative dorsal horn neurons.
Principal component analysis of all neurons recorded within the second spinal thoracic segments (Fig. 1A) of all animals yielded a total of 1,760 identified spinal neurons. T2 paravertebral ganglion stimulation was used to antidromically activate SPNs as defined by a constant latency response to paravertebral chain stimulation (Fig. 1B, left). SPNs were identified in 8 of 10 animals (SPNs, 57 neurons; 6 ± 2 per pig; n = 8) and by default, all other recorded neurons were classified as putative dorsal horn neurons (DH, 1,703 neurons; 155 ± 48 per pig; n = 10). The putative DH neurons showed no phase locking to paravertebral stimulation (Fig. 1B, right)
Stratification of neural responses.
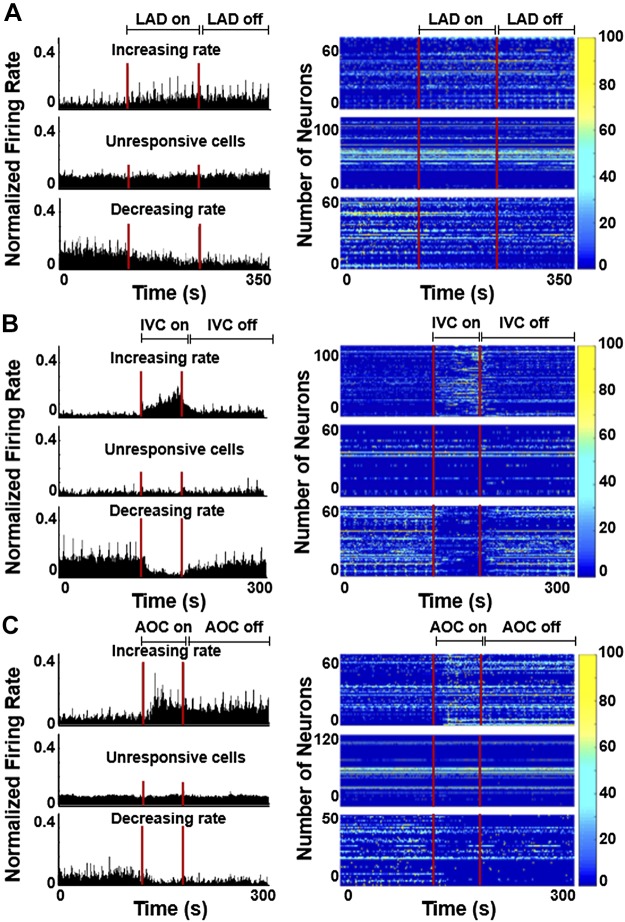
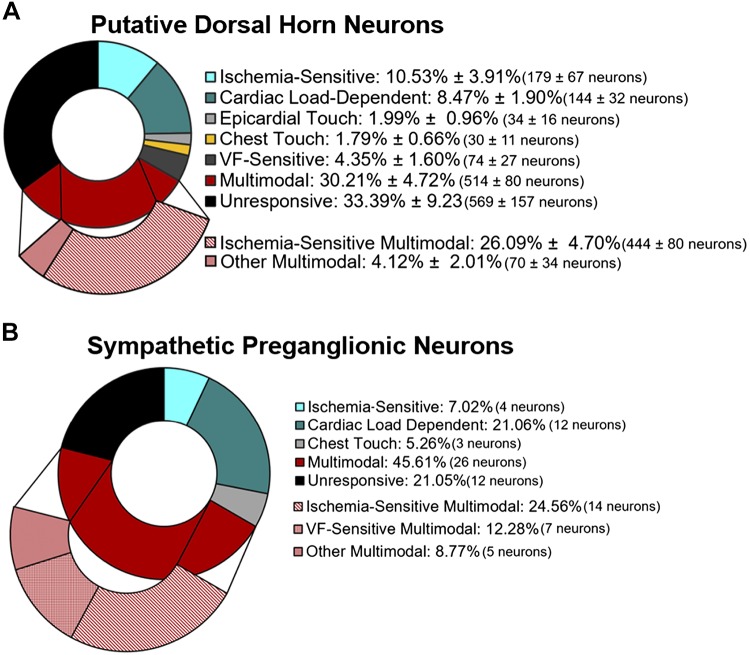
To determine if thoracic neurons were cardiac related, we used a post hoc Skellam analysis that compares the probability distribution of two variables with the assumption of a Poisson distribution (15, 56, 58). In this case, we compared the firing rates (pulse/s) of each 1-min baseline immediately preceding each intervention with the intervention and then again to the subsequent 1-min postperturbation (average responses shown in Tables 1 and 2). Neurons were initially classified as those that increased firing rate during a perturbation, those that were unresponsive, and those that decreased firing rate. In all animals, all three responses were seen during LAD ligation (Fig. 2A), IVC occlusion (Fig. 2B), and aortic occlusion (Fig. 2C). If a neuron’s firing rate increased or decreased, it was classified as responsive. In putative dorsal horn neurons, 10.53 ± 3.91% were singularly ischemia sensitive (e.g., LAD ligation), 8.47 ± 1.90% were singularly cardiac load dependent (e.g., IVC or aortic occlusion), 1.99 ± 0.96% were singularly sensitive to epicardial touch, 1.79 ± 0.66% were singularly sensitive to chest touch, 4.35 ± 1.60% were singularly responsive to ventricular fibrillation, 30.21 ± 4.72% were multimodal (i.e., responsive to 2 or more perturbations), and 33.39 ± 9.23% were unresponsive (Fig. 3A). Within the multimodal group, 26.09 ± 4.70% were ischemia sensitive. In identified sympathetic preganglionic neurons from all animals, 7.02% were singularly ischemia sensitive, 21.06% were singularly cardiac load dependent, 45.61% were multimodal, and 21.05% were unresponsive to any of the assigned perturbations (Fig. 3B). Within the multimodal group, 24.56% were ischemia sensitive and 12.28% were VF sensitive.
Table 1.
Dorsal horn neurons
| Chest | Heart | IVC | AOC | LAD | VF | |
|---|---|---|---|---|---|---|
| Increasing rate, pulses/s | ||||||
| Baseline | 4.1 ± 2.6 | 5.1 ± 3.8 | 2.0 ± 0.75 | 1.9 ± 0.58 | 2.2 ± 0.44 | 1.0 ± 0.56 |
| During | 6.6 ± 4.3* | 5.4 ± 3.9* | 3.6 ± 1.2* | 2.7 ± 0.76* | 3.6 ± 1.2* | 2.4 ± 0.94* |
| Unresponsive, pulses/s | ||||||
| Baseline/during | 0.0006 ± 0.0005 | 0.002 ± 0.002 | 0.002 ± 0.001 | 0.0008 ± 0.0005 | 0.004 ± 0.002 | 0.0008 ± 0.0006 |
| Decreasing rate, pulses/s | ||||||
| Baseline | 4.7 ± 2.4 | 2.8 ± 2.3 | 4.3 ± 0.85 | 4.7 ± 2.4 | 4.8 ± 2.5 | 3.2 ± 1.2 |
| During | 2.9 ± 1.5* | 2.5 ± 2.2* | 2.4 ± 0.54* | 3.2 ± 1.6* | 2.5 ± 0.63* | 1.7 ± 0.75* |
Values are means ± SE. Average pulses per second for dorsal horn neurons that increased, were unresponsive, or decreased firing rate during somatic (Chest, chest scratch), mechanical (Heart, heart touch), cardiac load (IVC, inferior vena cava occlusion; AOC, aortic occlusion), ischemic (LAD, left anterior descending coronary artery ligation), or ventricular fibrillation (VF) stimulation.
P < 0.03 vs. baseline.
Table 2.
Sympathetic preganglionic neurons
| Chest | Heart | IVC | AOC | LAD | VF | |
|---|---|---|---|---|---|---|
| Increasing rate, pulses/s | ||||||
| Baseline | 4.6 ± 1.7 | 5.0 ± 2.6 | 4.3 ± 3.1 | 3.2 ± 1.6 | 1.4 ± 0.82 | 0.74 ± 0.584 |
| During | 5.3 ± 1.8* | 5.5 ± 3.0 | 7.4 ± 3.0* | 4.7 ± 2.2* | 2.1 ± 1.3* | 0.9 ± 1.0 |
| Unresponsive, pulses/s | ||||||
| Baseline/during | 0.0 ± 0.0 | 0.0002 ± 0.0002 | 0.07 ± 0.07 | 0.0 ± 0.0 | 0.003 ± 0.003 | 0.0 ± 0.0 |
| Decreasing rate, pulses/s | ||||||
| Baseline | 0.11 ± 0.10 | 2.5 ± 1.4 | 5.2 ± 1.8 | 1.7 ± 0.6 | 2.1 ± 0.8 | 2.1 ± 1.9 |
| During | 0.08 ± 0.08 | 2.2 ± 1.3 | 2.2 ± 1.2* | 0.7 ± 0.4* | 1.5 ± 0.5* | 0.4 ± 0.3 |
Values are means ± SE. Average pulses per second for sympathetic preganglionic neurons that increased, were unresponsive, or decreased firing rate during somatic (Chest, chest scratch), mechanical (Heart, heart touch), cardiac load (IVC, inferior vena cava occlusion; AOC, aortic occlusion), ischemic (LAD, left anterior descending coronary artery ligation), or ventricular fibrillation (VF) stimulation.
P < 0.03 vs. baseline.
Fig. 2.
Representative changes in normalized firing rate and heat maps of ischemia-sensitive neurons before, during, and after left anterior descending coronary artery (LAD) ligation (A), as well as cardiac load-dependent neurons before, during, and after inferior vena cava (IVC) occlusion (B) and aortic occlusion (AOC; C) of cells that increased (top), were unresponsive (middle), or decreased (bottom) firing during each perturbation. For all heat maps, each row represents the firing rate of a single neuron.
Fig. 3.
Responses to cardiac perturbations (from baseline) were analyzed using statistical approaches based on a Skellam distribution. A: in putative dorsal horn neurons, all perturbations elicited significant changes in neural firing rates (n = 10). Neurons were subsequently classified at being singularly responsive to left anterior descending coronary artery ligation (ischemia sensitive), inferior vena cava or aortic occlusion (cardiac load dependent), or ventricular fibrillation (VF) sensitive. Interestingly, the majority of neurons were either multimodal (responded to 2 or more perturbations) or unresponsive to the given stimuli. B: in animals with identified sympathetic preganglionic neurons, all perturbations elicited neuronal responses. No singularly responsive sympathetic preganglionic neurons (SPNs) were detected during VF. In the multimodal population of SPNs, the majority were ischemia sensitive or VF sensitive. Values are expressed as means ± SE.
Connectivity of putative dorsal horn and sympathetic preganglionic neurons during cardiac perturbations.
Cross-correlations were used to evaluate short-latency discharge synchrony among pairs of recorded neurons before, during, and after each perturbation. Significant peaks in each cross-correlogram (>10 SD above background) were defined as indicative of excitatory connections (40). Though troughs can effectively be seen as an inhibitory connection, this remains controversial (40), and thus we chose to focus only on excitation. From all animals, a total of 7,395 positive correlations was identified from 5,066,332 possible comparisons.
Examples of four cross-correlograms from one SPN to four DH neurons are shown in Fig. 4A, illustrating a recruitment of excitatory connections during LAD ligation. Indeed, the percentage of positive correlations (of all possible positive correlations of recorded neurons) from DH to SPN increased during LAD ligation and was significantly attenuated after the ligation was released (Fig. 4B). DH to DH and SPN to DH excitatory connections also significantly increased during LAD ligation versus before or after the perturbation (Fig. 4C and E), whereas there was no significant change in SPN to SPN connectivity.
Fig. 4.
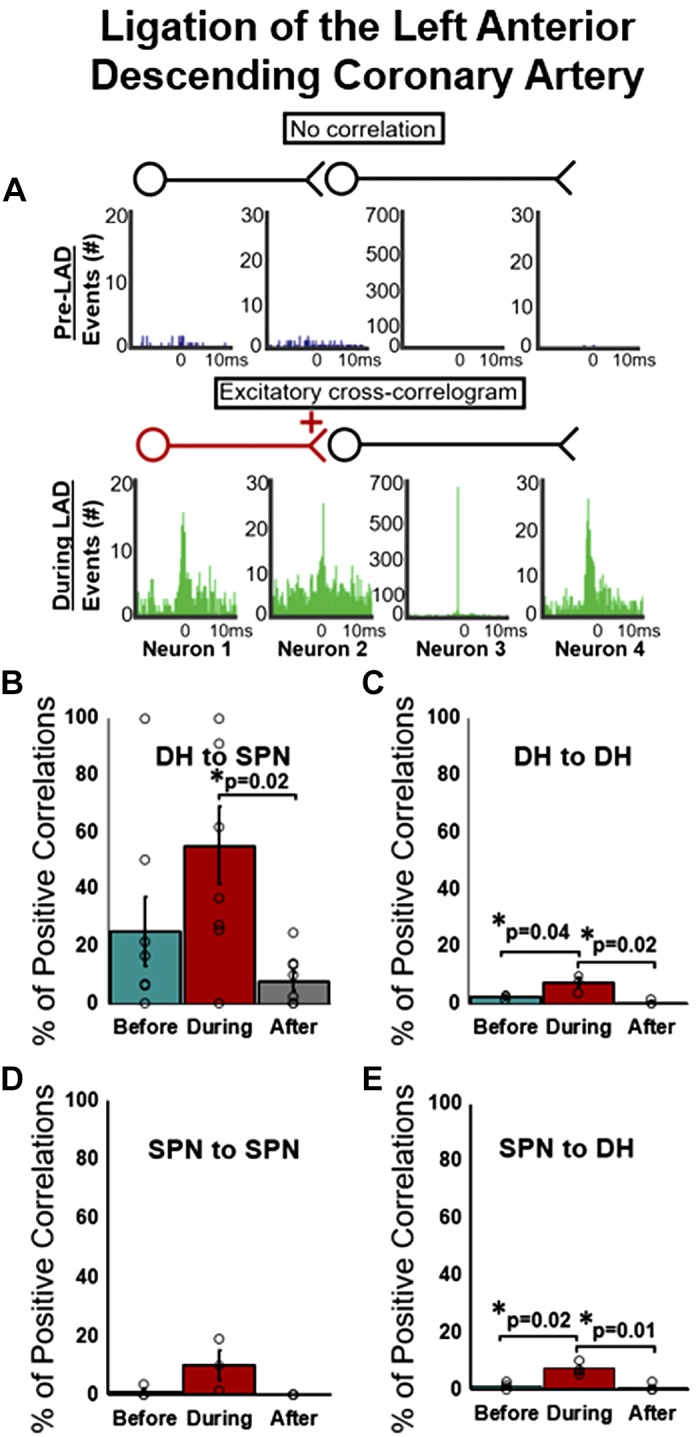
Excitatory connectivity of putative dorsal horn neurons (DH) and identified sympathetic preganglionic neurons (SPN) before, during, and after ligation of the left anterior descending coronary artery (LAD). A: representative cross-correlograms SPN and neurons demonstrating no correlative events (top) and excitatory relationships (green peaks; bottom) between 1 SPN neuron and 4 represenative DH neurons. Percentage of positive correlations from all possible positive correlations) between DH to SPN (B), DH to DH (C), SPN to SPN (D), and SPN to DH (E) are shown. Percentage of positive correlations significantly increased during LAD ligation (vs. baseline) between DH to DH neurons (C) and between SPN to DH neurons (E) and significantly decreased after ligation (B, C, and E). All values expressed as means ± SE. *Significantly different as indicated. One-way, repeated-measures ANOVA with multiple comparisons via Tukey post hoc test.
IVC occlusion leads to a decrease in preload with resultant decreases in ventricular volume, cardiac output, and arterial pressure. Afferent signals from mechanosensitive cardiopulmonary and arterial baroreceptors would be expected to decrease during the active IVC occlusion phase. Figure 5 summarizes the short-term excitatory connections within and between DH and SPN neurons before, during, and in the 1 min following release of the IVC occlusion. The percentage of positive correlations from DH to SPN was significantly reduced during IVC occlusion (Fig. 5B); however, there was no significant change in DH-to-DH or SPN-to-DH connections (Fig. 5, C and D). Only one pair of SPNs in one animal was found to have a positive connection before IVC occlusion but that connection was lost during occlusion and subsequent recordings (data not shown).
Fig. 5.
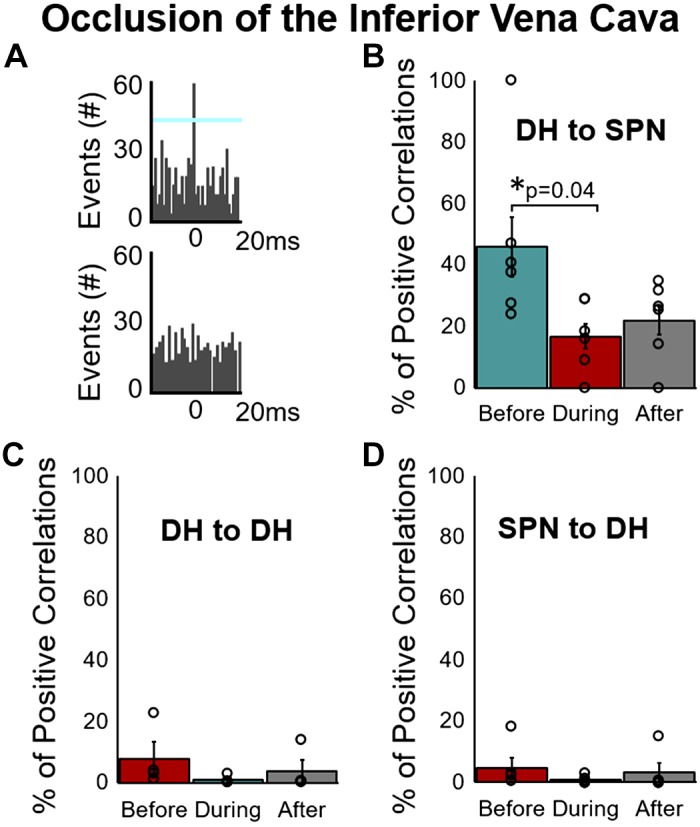
Excitatory connectivity of putative dorsal horn (DH) neurons and identified sympathetic preganglionic neurons (SPN) before, during, and after occlusion of the inferior vena cava (IVC). A: representative cross-correlograms of SPNs and DH neurons demonstrating an excitatory relationship (top) and an example of no correlative events (bottom). Percentage of positive correlations (from all possible positive correlations) between DH to SPN, DH to DH, SPN to SPN, and SPN to DH are shown in B–D. The percentage of positive correlations significantly decreased during occlusion of the IVC (vs. baseline) between DH and SPN (B). There were no significant changes from DH to DH (C) or SPN to DH (D), and there were 2 or fewer animals that demonstrated any positive correlations between SPN neurons (data not shown). All values expressed as means ± SE. *Significantly different as indicated. One-way, repeated-measures ANOVA with multiple comparisons via Tukey post hoc test.
Transient occlusion of the descending aorta leads to increased cardiac afterload (~50 mmHg in aortic blood pressure) and ventricular volume. Afferent signals from mechanosensitive cardiopulmonary and arterial baroreceptors would be expected to increase during the descending aortic occlusion. Excitatory connections before aortic occlusion (Fig. 6A) were diminished during occlusion and were significantly attenuated after the occlusion was released (Fig. 6B). No other significant connectivity relationships were found from DH to DH, SPN to DH, or SPN to SPN during the aortic occlusion stress. Additionally, no significant changes were seen in any neuronal pairing during the chest-anterior forelimb touch or epicardial touch of the ventricles (data not shown).
Fig. 6.
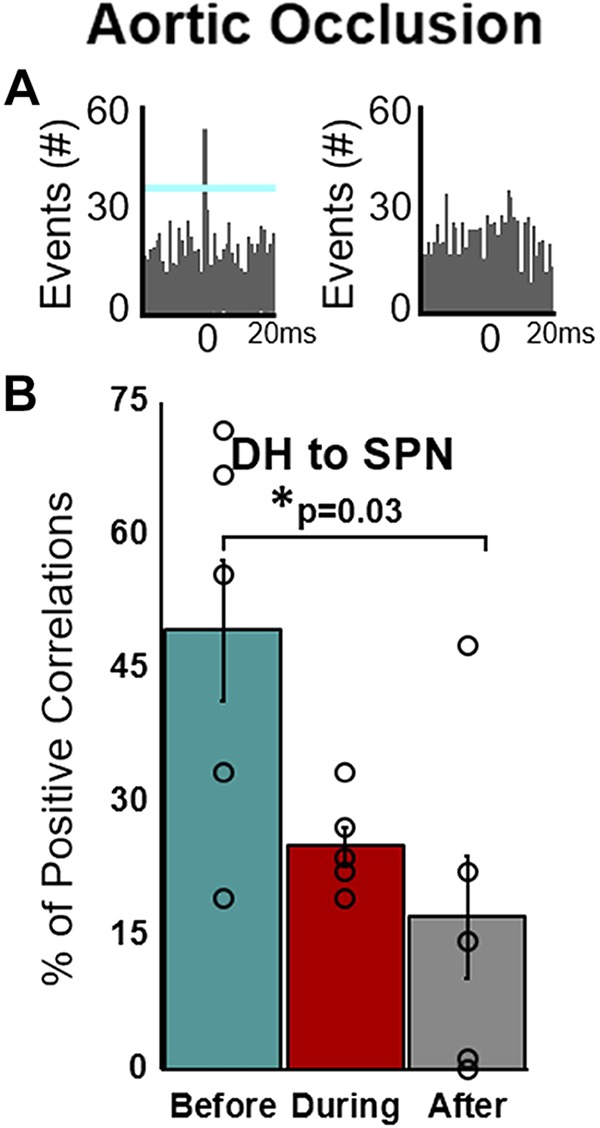
Excitatory connectivity of putative dorsal horn neurons (DH) and identified sympathetic preganglionic neurons (SPN) before, during, and after aortic occlusion. A: representative cross-correlograms of SPNs and DH neurons demonstrating an excitatory relationship (left) and an example of no correlative events (right). B: percentage of positive correlations from DH to SPN significantly decreases after aortic occlusion (vs. baseline). Two or fewer animals showed positive correlations from DH to DH, SPN to DH, and SPN to SPN before, during, or after aortic occlusion (data not shown). All values expressed as means ± SE. *Significantly different as indicated. One-way, repeated-measures ANOVA with multiple comparisons via Tukey post hoc test.
DISCUSSION
Data presented in this article defines fundamental aspects of cardiovascular-related sympathetic neural networks in the thoracic spinal cord. The 2D penetrating microarrays allow for spatial-temporal assessments of spinal cord neural networks. The major findings of this study are as follows. First, cardiac-related DH and SPN neurons show differential responses to activation of peripheral receptive fields. Second, while some DH neurons respond preferentially to changes in preload/afterload or ischemic stress, the majority of cardiovascular-related DH neurons are multimodal with ischemia sensitivity being one of the modalities. DH and SPN neurons, unaffected by CV stressors, have low-basal firing frequencies compared with those that are affected by CV stressors. Third, SPN activities are determined in part by intraspinal network interactions and supraspinal reflexes (21, 24). This convergence is evident in predominant multimodal response sensitivities of SPNs. Fourth, the sympathoexcitation associated with transient MI is associated with increased coordination within DH neurons and between DH to SPN neurons. Finally, DH to SPN network connectivity is reduced during great vessel occlusion. Together, these data are the benchmark against which future studies can define cardiac pathology-induced changes in central aspects of cardiac autonomic control and define potential targets for neuromodulation therapies for cardiac disease.
Afferent signaling.
In the heart, a dense network of both myelinated and nonmyelinated sensory fibers project to somata contained within the DRG of the thoracic spinal cord with projections on second-order DH neurons via their bipolar morphology (52). Cardiac-related DRG neurons represent a small population of the individual DRG ganglia (34). While there are small subsets of cardiac-related DRG neurons that demonstrate sole mechanosensitivity or chemosensitivity, the majority are multimodal (12, 17, 43, 44). Modality stratification within the DH neurons can be reflective of the primary sensory input or convergence of DRG input onto individual neurons. As demonstrated in Fig. 3, while some DH neurons respond preferentially to changes in preload/afterload or ischemic stress, the majority of responsive cardiovascular related DH neurons are multimodal with ischemia sensitivity being one of the modalities. As demonstrated by Fig. 4, ischemic stress augmented DH to DH coordination, a finding that may be reflective of recruitment of nociceptive afferents inputs to DH neurons by the ischemic stress and by convergent/divergent projections onto DH neurons.
Myocardial ischemia and spinal networks.
There are inherent and acquired factors that determine the integrated autonomic response to cardiovascular stressors (28). Reflex control of cardiac function involves the dynamic interplay between peripheral and central aspects of the cardiac nervous system (6, 11, 12, 63). Imbalances within these multitier reflex control systems can lead to lethal cardiac disease (28, 38, 64) with excessive sympathoexcitation to the heart amplifying diseased-induced cardiac substrate abnormalities (3, 28). The spinal cord serves as a nexus point for neural regulation of central aspects of sympathetic control (21, 25, 26). Acute occlusion of coronary arteries elicits local myocardial ischemia and activates afferent inputs to the thoracic spinal cord (42, 45). Figure 4 defines a critical aspect of this reflex response where the short-term coordination between DH to SPN more than doubles, indicating that local afferent-efferent neuronal network interaction in the spinal cord significantly contributes to increase in cardiac sympathetic activity. Previous reports have also suggested the role of complex spinal circuits in the integration of afferent information and reflex sympathoexcitation (22, 47, 61). In contrast, we show that SPN-to-SPN, short-term coordination was not enhanced during transient myocardial ischemia, suggesting that neural network interactions within the IML are not central to the sympathoexcitatory response.
Cardiac pathology can induce both short- and long-term effects on neural networks involved in cardiac control, some giving rise to exaggerated reflex responses in some while others are blunted (23, 28, 31, 55, 64). Gene expression changes in thoracic spinal cord indicative of neuronal stress response, including inflammation and apoptosis, and also potentially contribute to following both acute and chronic MI (53). Such neuronal remodeling has the potential to develop conflicts between central and peripheral reflexes of the cardiac nervous system (30, 38), a condition predisposing to arrhythmia formation and/or deterioration of contractile function (11, 23, 28). It remains to be determined how chronic myocardial ischemia/infarction remodels these spinal cord neural networks as defined in the current study.
Spinal processing of mechano-sensitive afferents inputs.
Spinal cord neural networks are modified by DRG afferent transduction from cardiopulmonary sensory neurites and via supraspinal neural networks responding to arterial baroreceptor inputs with resultant changes in reticulospinal projection to SPNs in the spinal cord (13, 52). For the spinal cord neural network evaluated herein, we defined the DH-to-DH and DH-to-SPN interactions in response to transient decreases in preload (IVC occlusion) versus transient increases in afterload (occlusion of descending aorta). IVC occlusion, by decreasing venous return, leads to a decrease in ventricular volume, cardiac output, and arterial pressure. Afferent signals from mechanosensitive cardiopulmonary and arterial baroreceptors would be expected to decrease during the active IVC occlusion phase. While DH-to-DH coordination was unaffected by IVC occlusion, DH-to-SPN coordination was reduced, both during and in the 1 min following this cardiovascular stressor. This may reflect a general reduction in DH activity as cardiopulmonary DRG afferents are unloaded by the decreased cardiac volume. In contrast, transient occlusion of the descending aorta leads to increased cardiac afterload (~50 mmHg in aortic blood pressure) and ventricular volume. Afferent signals from mechanosensitive cardiopulmonary and arterial baroreceptors would be expected to increase during pressure increase associated with descending aortic occlusion phase (27, 33) (41). The decrease in DH to SPN short-term interactions may reflect higher center reflex functions (e.g., brainstem) responding to baroreceptor input and with resultant decreased activity on the reticulospinal projections to SPNs in thoracic and lumbar segments of the spinal cord.
Translational perspectives.
Heart disease remains the primary cause of morbidity and mortality in the world with its impact cutting across all segments of the population (49). The fundamental premise of neurocardiology is that it is the dynamic interactions between autonomic control and the heart that determines how cardiac disease progresses (11, 52, 57). Through a mechanistic understanding of neurohumoral-cardiac adaptations and maladaptations, a rationale for therapies designed to maintain cardiovascular homeostasis can be defined (30, 57). Hyperdynamic reflexes, especially sympathoexcitatory ones, are a major contributor in the potential for sudden cardiac death (28) and progression into heart failure (23). While pharmacological options remain the mainstay of treatments for arrhythmias and heart failure (5, 37, 62), targeted neuromodulation therapies are an emerging field in the treatment of heart disease. This ranges from short-term interventions, such as spinal epidural anesthesia (16, 35), surgical interventions directed at stellate ganglia (60), and device-based bioelectronic therapies targeted at spinal cord (6, 25, 54) and peripheral paravertebral ganglia (19). Defining specific targets and refining stimulation protocols as impacting central and or peripheral neural networks for control of regional cardiac function are the cornerstone for future advances in neuromodulation-based therapeutics.
Study limitations.
Experiments were done in anesthetized animals that can alter background activity and autonomic reflexes. This was mitigated in part by the use of α-chloralose, which is the preferred anesthetic of choice for acute studies for evaluation of autonomic function in normal and controlled cardiac pathology states. It maintains a high level of basal sympathetic and parasympathetic tone that can be reflexly modified by afferent inputs and responds in robust ways to direct activation of autonomic inputs (7, 14, 15, 29, 52). Spinal cord recordings were limited to one segment and one side. While neural activities arising from the same spinal segment have similar characteristics (13), future studies should consider deploying microarrays bilaterally and to at least two adjacent spinal cord segments. This would allow for concurrent assessments of intra- and intersegmental interactions, as well as define functional maps of afferent inputs to different spinal segments. While defining critical aspects of neural network function, the current studies provide no new information on the neurotransmitters involved in network interactions. Finally, sudden cardiac death can result from pump failure. Herein we focused on sympathetic amplification leading to ventricular tachycardia/ventricular fibrillation VT/VF in the acute setting. Abnormalities in cardiac substrate, as amplified by heterogeneous reflex activation of sympathetic efferents, are central to the induction of arrhythmias (9, 28).
GRANTS
This work was supported by National Institutes of Health Grants U18-EB-021799 (to J. L. Ardell), UO1-EB-025138 (to J. L. Ardell), and HL-136836 (to A. Mahajan and J. L. Ardell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.A.D., J.L.A., and A.M. conceived and designed research; E.A.D., J.K., Y.K., P.A.C., J.L.A., and A.M. performed experiments; E.A.D., J.K., Y.K., M.D.S., P.A.C., J.L.A., and A.M. analyzed data; E.A.D., J.K., Y.K., M.D.S., P.A.C., J.L.A., and A.M. interpreted results of experiments; E.A.D., J.K., Y.K., M.D.S., J.L.A., and A.M. prepared figures; E.A.D., J.K., J.L.A., and A.M. drafted manuscript; E.A.D., J.K., Y.K., M.D.S., P.A.C., J.L.A., and A.M. edited and revised manuscript; E.A.D., J.K., Y.K., M.D.S., P.A.C., J.L.A., and A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank S. Salavatian for software support.
Present address of E. A Dale: Dept. of Physiology and Functional Genomics, College of Medicine, University of Florida.
Present address of A. Mahajan: University of Pittsburgh School of Medicine.
REFERENCES
- 1.Aertsen AM, Gerstein GL. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res 340: 341–354, 1985. doi: 10.1016/0006-8993(85)90931-X. [DOI] [PubMed] [Google Scholar]
- 2.Ajijola OA, Hoover DB, Simerly TM, Brown TC, Yanagawa J, Biniwale RM, Lee JM, Sadeghi A, Khanlou N, Ardell JL, Shivkumar K. Inflammation, oxidative stress, and glial cell activation characterize stellate ganglia from humans with electrical storm. JCI Insight 2: 94715, 2017. doi: 10.1172/jci.insight.94715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol 305: H1031–H1040, 2013. doi: 10.1152/ajpheart.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL, Shivkumar K. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: neuropeptide and morphologic changes. Heart Rhythm 12: 1027–1035, 2015. doi: 10.1016/j.hrthm.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 15: e190–e252, 2018. doi: 10.1016/j.hrthm.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Ardell JL. Heart failure: Mechanisms of spinal cord neuromodulation for heart disease. Nat Rev Cardiol 13: 127–128, 2016. doi: 10.1038/nrcardio.2016.8. [DOI] [PubMed] [Google Scholar]
- 7.Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am J Physiol 260: H713–H721, 1991. doi: 10.1152/ajpheart.1991.260.3.H713. [DOI] [PubMed] [Google Scholar]
- 8.Ardell JL, Cardinal R, Vermeulen M, Armour JA. Dorsal spinal cord stimulation obtunds the capacity of intrathoracic extracardiac neurons to transduce myocardial ischemia. Am J Physiol Regul Integr Comp Physiol 297: R470–R477, 2009. doi: 10.1152/ajpregu.90821.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardell JL, Foreman RD, Armour JA, Shivkumar K. Cardiac sympathectomy and spinal cord stimulation attenuate reflex-mediated norepinephrine release during ischemia preventing ventricular fibrillation. JCI Insight 4: 131648, 2019. doi: 10.1172/jci.insight.131648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armour JA. Neuronal activity recorded extracellularly in chronically decentralized in situ canine middle cervical ganglia. Can J Physiol Pharmacol 64: 1038–1046, 1986. doi: 10.1139/y86-177. [DOI] [PubMed] [Google Scholar]
- 11.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93: 165–176, 2008. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 12.Armour JA, Kember G. Cardiac sensory neurons. In: Basic and Clinical Neurocardiology. New York: Oxford University Press, 2004, p. 79–117. [Google Scholar]
- 13.Barman SM, Yates BJ. Deciphering the neural control of sympathetic nerve activity: status report and directions for future research. Front Neurosci 11: 730, 2017. doi: 10.3389/fnins.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beam DM, Neto-Neves EM, Stubblefield WB, Alves NJ, Tune JD, Kline JA. Comparison of isoflurane and α-chloralose in an anesthetized swine model of acute pulmonary embolism producing right ventricular dysfunction. Comp Med 65: 54–61, 2015. [PMC free article] [PubMed] [Google Scholar]
- 15.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. doi: 10.1113/jphysiol.2013.259382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, Boyle NG, Mahajan A, Narasimhan C, Lokhandwala Y, Shivkumar K. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 121: 2255–2262, 2010. doi: 10.1161/CIRCULATIONAHA.109.929703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown AM. Cardiac reflexes. In: The Cardiovascular System. Handbook of Physiology Bethesda, MD: American Physiological Society, 1979, p. 677–689. [Google Scholar]
- 18.Buckley U, Yamakawa K, Takamiya T, Andrew Armour J, Shivkumar K, Ardell JL. Targeted stellate decentralization: implications for sympathetic control of ventricular electrophysiology. Heart Rhythm 13: 282–288, 2016. doi: 10.1016/j.hrthm.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chui RW, Buckley U, Rajendran PS, Vrabec T, Shivkumar K, Ardell JL. Bioelectronic block of paravertebral sympathetic nerves mitigates post-myocardial infarction ventricular arrhythmias. Heart Rhythm 14: 1665–1672, 2017. doi: 10.1016/j.hrthm.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Coote JH. Myths and realities of the cardiac vagus. J Physiol 591: 4073–4085, 2013. doi: 10.1113/jphysiol.2013.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deuchars SA, Lall VK. Sympathetic preganglionic neurons: properties and inputs. Compr Physiol 5: 829–869, 2015. doi: 10.1002/cphy.c140020. [DOI] [PubMed] [Google Scholar]
- 22.Felder RB, Thames MD. The cardiocardiac sympathetic reflex during coronary occlusion in anesthetized dogs. Circ Res 48: 685–692, 1981. doi: 10.1161/01.RES.48.5.685. [DOI] [PubMed] [Google Scholar]
- 23.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 114: 1815–1826, 2014. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 24.Foreman RD, Garrett KM, Blair RW. Mechanisms of cardiac pain. Compr Physiol 5: 929–960, 2015. doi: 10.1002/cphy.c140032. [DOI] [PubMed] [Google Scholar]
- 25.Foreman RD, Linderoth B. Neural mechanisms of spinal cord stimulation. Int Rev Neurobiol 107: 87–119, 2012. doi: 10.1016/B978-0-12-404706-8.00006-1. [DOI] [PubMed] [Google Scholar]
- 26.Foreman RD, DeJongste MJ, Linderoth B. Integrative control of cardiac function by cervical and thoracic spinal neurons. In: Basic and Clinical Neurocardiology. New York: Oxford University Press, 2004, p. 153–186. [Google Scholar]
- 27.Fu Q, Shook RP, Okazaki K, Hastings JL, Shibata S, Conner CL, Palmer MD, Levine BD. Vasomotor sympathetic neural control is maintained during sustained upright posture in humans. J Physiol 577: 679–687, 2006. doi: 10.1113/jphysiol.2006.118158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 116: 2005–2019, 2015. doi: 10.1161/CIRCRESAHA.116.304679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibbons DD, Southerland EM, Hoover DB, Beaumont E, Armour JA, Ardell JL. Neuromodulation targets intrinsic cardiac neurons to attenuate neuronally mediated atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 302: R357–R364, 2012. doi: 10.1152/ajpregu.00535.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanna P, Shivkumar K, Ardell JL. Calming the nervous heart: autonomic therapies in heart failure. Card Fail Rev 4: 92–98, 2018. doi: 10.15420/cfr.2018.20.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardwick JC, Ryan SE, Beaumont E, Ardell JL, Southerland EM. Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Autonomic Neurosci 181: 4–12, 2014. doi: 10.1016/j.autneu.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harper RM, Kumar R, Macey PM, Ogren JA, Richardson HL. Functional neuroanatomy and sleep-disordered breathing: implications for autonomic regulation. Anat Rec (Hoboken) 295: 1385–1395, 2012. doi: 10.1002/ar.22514. [DOI] [PubMed] [Google Scholar]
- 33.Head GA, Saigusa T, Mayorov DN. Angiotensin and baroreflex control of the circulation. Braz J Med Biol Res 35: 1047–1059, 2002. doi: 10.1590/S0100-879X2002000900005. [DOI] [PubMed] [Google Scholar]
- 34.Hoover DB, Shepherd AV, Southerland EM, Armour JA, Ardell JL. Neurochemical diversity of afferent neurons that transduce sensory signals from dog ventricular myocardium. Autonomic Neurosci 141: 38–45, 2008. doi: 10.1016/j.autneu.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard-Quijano K, Takamiya T, Dale EA, Yamakawa K, Zhou W, Buckley U, Mahajan A. Effect of thoracic epidural anesthesia on ventricular excitability in a porcine model. Anesthesiology 126: 1096–1106, 2017. doi: 10.1097/ALN.0000000000001613. [DOI] [PubMed] [Google Scholar]
- 36.Jänig W. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis. New York: Cambridge University Press, 2006. [Google Scholar]
- 37.January CT, Wann LS, Calkins H, Field ME, Chen LY, Furie KL, Cigarroa JE, Heidenreich PA, Cleveland JC Jr, Murray KT, Ellinor PT, Shea JB, Ezekowitz MD, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 16: e66–e93, 2019. doi: 10.1016/j.hrthm.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Kember G, Armour JA, Zamir M. Neural control hierarchy of the heart has not evolved to deal with myocardial ischemia. Physiol Genomics 45: 638–644, 2013. doi: 10.1152/physiolgenomics.00027.2013. [DOI] [PubMed] [Google Scholar]
- 39.Kember G, Armour JA, Zamir M. Neural control of heart rate: the role of neuronal networking. J Theor Biol 277: 41–47, 2011. doi: 10.1016/j.jtbi.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Kirkwood PA. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods 1: 107–132, 1979. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- 41.Lohmeier TE. The sympathetic nervous system and long-term blood pressure regulation. Am J Hypertens 14: 147S–154S, 2001. doi: 10.1016/S0895-7061(01)02082-9. [DOI] [PubMed] [Google Scholar]
- 42.Malliani A, Lombardi F, Pagani M. Functions of afferents in cardiovascular sympathetic nerves. J Auton Nerv Syst 3: 231–236, 1981. doi: 10.1016/0165-1838(81)90065-5. [DOI] [PubMed] [Google Scholar]
- 43.Malliani A, Lombardi F, Pagani M, Recordati G, Schwartz PJ. Spinal cardiovascular reflexes. Brain Res 87: 239–246, 1975. doi: 10.1016/0006-8993(75)90421-7. [DOI] [PubMed] [Google Scholar]
- 44.Malliani A, Pagani M, Pizzinelli P, Furlan R, Guzzetti S. Cardiovascular reflexes mediated by sympathetic afferent fibers. J Auton Nerv Syst 7: 295–301, 1983. doi: 10.1016/0165-1838(83)90082-6. [DOI] [PubMed] [Google Scholar]
- 45.Malliani A, Schwartz PJ, Zanchetti A. A sympathetic reflex elicited by experimental coronary occlusion. Am J Physiol 217: 703–709, 1969. doi: 10.1152/ajplegacy.1969.217.3.703. [DOI] [PubMed] [Google Scholar]
- 46.Melssen WJ, Epping WJ. Detection and estimation of neural connectivity based on crosscorrelation analysis. Biol Cybern 57: 403–414, 1987. doi: 10.1007/BF00354985. [DOI] [PubMed] [Google Scholar]
- 47.Minisi AJ, Thames MD. Activation of cardiac sympathetic afferents during coronary occlusion. Evidence for reflex activation of sympathetic nervous system during transmural myocardial ischemia in the dog. Circulation 84: 357–367, 1991. doi: 10.1161/01.CIR.84.1.357. [DOI] [PubMed] [Google Scholar]
- 48.Moore GP, Segundo JP, Perkel DH, Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J 10: 876–900, 1970. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131: e29–e322, 2015. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 50.Murphy DA, Thompson GW, Ardell JL, McCraty R, Stevenson RS, Sangalang VE, Cardinal R, Wilkinson M, Craig S, Smith FM, Kingma JG, Armour JA. The heart reinnervates after transplantation. Ann Thorac Surg 69: 1769–1781, 2000. doi: 10.1016/S0003-4975(00)01240-6. [DOI] [PubMed] [Google Scholar]
- 51.Norris JE, Foreman RD, Wurster RK. Responses of the canine heart to stimulation of the first five ventral thoracic roots. Am J Physiol 227: 9–12, 1974. doi: 10.1152/ajplegacy.1974.227.1.9. [DOI] [PubMed] [Google Scholar]
- 52.Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol 594: 321–341, 2016. doi: 10.1113/JP271165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saddic LA, Howard-Quijano K, Kipke J, Kubo Y, Dale EA, Hoover D, Shivkumar K, Eghbali M, Mahajan A. Progression of myocardial ischemia leads to unique changes in immediate-early gene expression in the spinal cord dorsal horn. Am J Physiol Heart Circ Physiol 315: H1592–H1601, 2018. doi: 10.1152/ajpheart.00337.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salavatian S, Ardell SM, Hammer M, Gibbons D, Armour JA, Ardell JL. Thoracic spinal cord neuromodulation obtunds dorsal root ganglion afferent neuronal transduction of the ischemic ventricle. Am J Physiol Heart Circ Physiol 317: H1134–H1141, 2019. doi: 10.1152/ajpheart.00257.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schultz HD, Marcus NJ, Del Rio R. Role of the carotid body in the pathophysiology of heart failure. Curr Hypertens Rep 15: 356–362, 2013. doi: 10.1007/s11906-013-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin HC, Aggarwal V, Acharya S, Schieber MH, Thakor NV. Neural decoding of finger movements using Skellam-based maximum-likelihood decoding. IEEE Trans Biomed Eng 57: 754–760, 2010. doi: 10.1109/TBME.2009.2020791. [DOI] [PubMed] [Google Scholar]
- 57.Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, Esler M, De Ferrari GM, Fishbein MC, Goldberger JJ, Harper RM, Joyner MJ, Khalsa SS, Kumar R, Lane R, Mahajan A, Po S, Schwartz PJ, Somers VK, Valderrabano M, Vaseghi M, Zipes DP. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol 594: 3911–3954, 2016. doi: 10.1113/JP271870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skellam JG. The frequency distribution of the difference between two Poisson variates belonging to different populations. J R Stat Soc [Ser A] 109: 296, 1946. doi: 10.2307/2981372. [DOI] [PubMed] [Google Scholar]
- 59.Steinberg C, Laksman ZW, Krahn AD. Sudden cardiac death: A reappraisal. Trends Cardiovasc Med 26: 709–719, 2016. doi: 10.1016/j.tcm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Vaseghi M, Barwad P, Malavassi Corrales FJ, Tandri H, Mathuria N, Shah R, Sorg JM, Gima J, Mandal K, Sàenz Morales LC, Lokhandwala Y, Shivkumar K. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol 69: 3070–3080, 2017. doi: 10.1016/j.jacc.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webb SW, Adgey AA, Pantridge JF. Autonomic disturbance at onset of acute myocardial infarction. Br Med J 3: 89–92, 1972. doi: 10.1136/bmj.3.5818.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136: e137–e161, 2017. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 63.Yuan BX, Ren HM, Yang GD, Yang YJ, Qi L. Cardiac responses activated by nicotine in canine ganglial plexus between aorta and pulmonary artery. Zhongguo Yao Li Xue Bao 15: 331-335, 1994. [PubMed] [Google Scholar]
- 64.Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart Fail Clin 8: 87–99, 2012. doi: 10.1016/j.hfc.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]