Abstract
The objective of this study was to assess normative values for comprehensive forearm skin microcirculatory function: oxygen saturation, tissue fraction of red blood cells (RBCs), and speed-resolved perfusion. Furthermore, to examine the influence of age and sex on microcirculatory function. Measurements were performed using a noninvasive probe-based system, including diffuse reflectance spectroscopy and laser-Doppler flowmetry, yielding output data in absolute units. The study was conducted within the Swedish CArdioPulmonary BioImage Study (SCAPIS) and included 1,765 men and women aged 50–65 yr from the Linköping general population. Normative values are given at baseline, at the end of a 5-min occlusion of the brachial artery and during hyperemia after occlusion release. We found a consistent age distribution, in which the oldest individuals had the lowest peak oxygen saturation (P < 0.001) and the highest baseline low-speed perfusion (P < 0.001). Women had higher peak oxygen saturation (P < 0.001), lower RBC tissue fraction, in general (P < 0.001), lower baseline perfusion in all speed regions (P = 0.01), and lower peak high-speed perfusion at hyperemia (P < 0.001). The normative data can be used as reference values in future studies of disease-specific populations. The results show that age and sex are important aspects to consider in studies of microvascular function. Women and younger age were factors associated with higher peak oxygen saturation after ischemia. This is a novel parameter that reflects overall microcirculatory function associated with vascular dilation capacity.
NEW & NOTEWORTHY This study expands experimental microcirculatory research to clinical use by providing normative values on microcirculatory function in a large population-based cohort. Women and younger age were factors associated with higher peak oxygen saturation after ischemia, which implies that age and sex are important aspects to consider in studies of microvascular function. This study is the first step toward using microcirculatory assessment as a tool to improve diagnosis, prognosis, and treatment in disease-specific populations.
Keywords: ischemia, microvascular blood flow, oxygen saturation
INTRODUCTION
Cardiovascular disease (CVD) is a major cause of morbidity and mortality, which affects millions of people (21). Despite major efforts to reduce the impact of CVD, it remains a major cause of health loss for all regions of the world. The microcirculation consists of the smallest vessels (<150 µm) and is where oxygen and other essential substrates are delivered to tissue, and metabolic waste products are removed. Thus, microcirculatory function is the main prerequisite to maintain adequate tissue oxygenation and organ function. Changes in the microvascular structure and function are evident early in CVD as coronary artery disease and are related to the risk factors dyslipidemia, hypertension, and diabetes (7, 9, 13). Furthermore, microcirculatory function has significant impact on tissue blood perfusion and viability, and is, therefore, a potential indicator for cardiovascular health.
Forearm skin microcirculation has frequently been used for assessing microvascular reactivity. It is easily accessible with noninvasive optical methods such as laser-Doppler flowmetry (LDF). The postocclusive reactive hyperemia (PORH) test has proven to be a good model for assessing microvascular function (10, 23). PORH refers to the increase in skin blood flow following release from a vascular occlusion involving the endothelial vasodilator function. Peak skin perfusion and oxygen saturation after a 5-min arterial occlusion period may represent indices of overall microcirculatory function (10, 23) and the vasodilator capacity.
By integrating LDF and diffuse reflectance spectroscopy (DRS) in an individualized light transport skin model, it is possible to noninvasively simultaneously quantify skin microcirculatory oxygen saturation, speed-resolved perfusion and red blood cell (RBC) tissue fraction, in absolute units in real time (8, 14). Furthermore, speed-resolved perfusion has the capacity to separate the low-speed nutritive flow from the total blood flow, which has been shown to be reduced in type 2 diabetes (9, 13). This integrated technique was applied within the Swedish Cardiopulmonary bioimage Study (SCAPIS) (4). SCAPIS is a large population-based cohort study with the overall aim to study disease mechanisms and improve risk prediction of cardiovascular and pulmonary disease by extensively characterizing men and women aged 50 to 65 yr in Sweden.
By measuring microcirculatory parameters in absolute units, i.e., not merely in relative units, it is for the first time possible to compare data from different studies. There is now a possibility for true between-subject comparisons, since confounding factors, such as the effect of variations in tissue optical properties, can be reduced or eliminated (17). Therefore, there is a need for retrieving normative data.
There is a growing awareness of age and sex differences in microvascular physiology and function (3, 11, 20). Age has previously been shown to be inversely associated with skin microvascular dysfunction, measured as heat-induced skin hyperemia (26) and acetylcholine-induced vasodilation and capillary recruitment (12, 26). In the middle-aged population (59.7 ± 8.2 yr), men had lower skin hyperemia than women (26).
These studies show the importance of quantifying the influence of age and sex on microcirculatory function. Therefore, we performed post hoc analysis of the normative data to explore the age- and sex-related differences in clinical characteristics and profile.
The aim of this study was to assess normative values for comprehensive skin microcirculatory function at baseline and during a 5-min occlusion and release protocol in terms of oxygen saturation, tissue fraction of red blood cells, and speed-resolved perfusion in a large Swedish cohort. Furthermore, to examine the influence of age and sex on microcirculatory function.
METHODS
Study population and characterization.
This study on microcirculatory function was an add-on study at the Linköping site within the multicenter study, SCAPIS. The rationale and methodology of SCAPIS have been described previously (4). The study participants were randomly sampled from the population registry, including men and women between 50 and 65 yr living in Linköping, Sweden. During the time period January 1, 2016 to February 28, 2017, 1,765 subjects underwent the add-on study evaluating microcirculatory function.
SCAPIS has been approved as a multicenter trial by the Ethics Committee at Umeå University (Dnr 2010-228-31M with amendment, EPN Umeå) and adheres to the Declaration of Helsinki. The microcirculatory measurements and the analysis of data have been approved by the Ethics Committee in Linköping (Dnr 2018/156-31). Written informed consent was obtained from all subjects.
The subjects were characterized as described by Bergström et al. (4). Detailed information on self-reported health, family history, medication, occupational and environmental exposure, lifestyle, psychosocial well-being, socioeconomic status, and other social determinants were derived from a questionnaire.
Prevalent diabetes, hypertension, dyslipidemia, and smoking status were based on self-reported health in combination with laboratory results. Laboratory measurements were analyzed at Linköping University Hospital laboratory using a venous blood sample collected after an overnight fast. Obesity data were derived from direct measurements.
Microcirculatory function assessment.
The measurement was performed with a PeriFlux 6000 EPOS system (Enhanced Perfusion and Oxygen Saturation; Perimed AB, Järfälla, Stockholm, Sweden). The system consisted of a PF 6010 laser-Doppler unit (including a laser light source at 785 nm and an optical passband filter at 785 ± 40 nm), a PF 6060 spectroscopy unit, a broadband white light source (Avalight-HAL-S, Avantes BV, The Netherlands) and a fiber-optic probe.
In PeriFlux 6000 EPOS, the microcircular parameters oxygen saturation, RBC tissue fraction and speed-resolved perfusion are assessed using a model-based approach that has previously been described thoroughly (8). The principle is that a multiparameter tissue model has been adopted to measure diffuse reflectance and laser-Doppler power spectra in each measurement point. The same types of spectra can be calculated from the model, and the parameters are iteratively updated, until calculated spectra match the measured spectra. The parameters cover all relevant aspects affecting the spectra in skin, such as epidermis thickness, melanin content, and light scattering, as well as the microcircular parameters themselves, i.e., the fraction of oxygenized and hemoglobin and RBC flow speed distribution. When the optimal parameter set is found, i.e., the set of parameters that match the measured diffuse reflectance and Doppler power spectra simultaneously, the microcircular parameters are derived directly from the model. In that manner, the parameters can be expressed in absolute units, and the perfusion can be expressed in a speed-resolved manner, contrary to the perfusion estimate from conventional laser-Doppler flowmetry.
Resolving the perfusion in different speed regions facilitates the study of the microvasculature. In general, the flow with speeds below 1 mm/s are associated with capillary flow, although especially some venular flow may also fall into that category. The speeds 1–10 mm/s are foremost constituted by flow in venules, small arterioles and, under some circumstances, veins. The flow speeds above 10 mm/s are primarily related to flow in larger arterioles and other larger vessels situated in the sampling volume. Although this general relation between flow speed and vessel type should be interpreted with some caution, especially during an occlusion release provocation, it is likely to provide new insights about the microvascular function.
The subjects were asked to refrain from large meals and coffee for 3 h, nicotine for 4 h, and alcohol for 12 h before the measurements. Medications were omitted in the morning of the study, except for anticoagulants, contraceptives, or medications for Parkinson’s disease, diabetes, epilepsy, chronic pain, and spasticity.
The subjects were acclimatized in a temperature-controlled room (23.7 ± 0.7°C, mean ± SD) for at least 15 min before start of measurement. The subjects were resting in a supine position both before and during the measurement. A fiber-optic probe was attached to the volar surface of the lower right arm using double-sided adhesive tape, avoiding visible veins, moles, and hair. The microcirculatory protocol included a 5-min baseline, 5-min arterial occlusion of the forearm using a blood pressure cuff rapidly inflated to 250 mmHg, and a 10-min reperfusion phase.
Baseline values of oxygen saturation, tissue fraction of red blood cells, and speed-resolved perfusion were calculated as the median over the first 3 min of the 5-min baseline recording. Occlusion values were calculated as the median over 10 s, starting 30 s before release of the cuff. PORH peak values were defined as the highest value reached after release of the cuff. Before calculating peak values, the perfusion estimates were low-pass filtered using a Butterworth filter and a median filter.
Data exclusion.
If the occlusion period was less than 5 min or more than 5.5 min, the subject was excluded. The occlusion was defined to be incomplete if the median of the total perfusion during the first minute of occlusion was above the median baseline perfusion value. Motion artifacts during the occlusion were detected by quantifying the total perfusion standard deviation during occlusion. On the basis of empirical experience, the subject was excluded if the standard deviation was above 0.04% RBC × mm/s. If the hemoglobin signature in DRS spectra was too weak, the RBC oxygen saturation estimate became unstable. Hence, spectra with a small hemoglobin signature or an estimated RBC tissue fraction below 0.1% were discarded (14). Also, DRS or LDF spectra with model-to-measurement fitting error above 10% were omitted from the analysis.
Statistical analysis.
Data are presented as means ± SD for continuous variables and percentages for categorial variables. To study age effects, the subjects were divided into three groups based on age (50–55, 55–60, and 60–65 yr). Statistical differences between men and women or age groups were assessed by using unpaired t-tests or one-way ANOVA, respectively. A P value of ≤0.05 was regarded as significant. All statistical analyses were performed using IBM SPSS, version 23 (SPSS, Chicago, IL).
RESULTS
Four subjects chose to end the measurement before the end of the protocol and were, therefore, omitted from the data analysis. Twenty subjects were excluded due to data acquisition failure. Thirty subjects had been smoking or drinking coffee less than 2 h before the measurement occasion and were excluded. Nine subjects were excluded due to a too short or too long occlusion time. The occlusion was incomplete in 36 subjects, and motion artifacts were present in 14 subjects. Subjects were also excluded when data were missing on blood pressure (n = 1), waist circumference (n = 1), Hb (n = 2), LDL cholesterol (n = 30) or from questionnaire (diagnosis, smoking, and previous cardiovascular events) (n = 61). If there existed a possible circulatory dysfunction in the right arm (e.g., due to surgery, nerve damage, or illness), the measurement was performed on the left arm (n = 12).
Clinical characteristics.
After exclusion, 1,557 subjects remained for further analyses. The clinical characteristics of the study population are shown in Table 1. Among the 1,557 subjects, 114 had diabetes, 173 had dyslipidemia, and 346 had hypertension. There were 315 subjects that had used medications for hypertension and 128 subjects for dyslipidemia at some point during the last 2 wk before the measurement occasion.
Table 1.
Characteristics of the analyzed subjects
| Parameter | Value |
|---|---|
| Age, yr | 57.7 ± 4.4 |
| Women | 779 (50) |
| Systolic blood pressure, mmHg | 132 ± 18 |
| Diastolic blood pressure, mmHg | 84 ± 11 |
| Height, cm | 172.4 ± 9.5 |
| Weight, kg | 80.2 ± 15.2 |
| BMI, kg/m2 | 26.9 ± 4.2 |
| Waist circumference, cm | |
| Men | 98.4 ± 10.6 |
| Women | 88.4 ± 12.1 |
| Smoking status | |
| No, never | 871 (55.9) |
| Yes | 93 (6.0) |
| Yes, occasionally | 64 (4.1) |
| No, have quit | 506 (32.5) |
| Don’t want to answer/cannot answer | 23 (1.5) |
| Medical history | |
| Diabetes | 114 (7.3) |
| Dyslipidemia | 173 (11.1) |
| Hypertension | 346 (22.2) |
| Blood sample analyses | |
| Glucose, mmol/L | 5.7 ± 1.1 |
| Hb, g/L | 141.7 ± 11.3 |
| HbA1c, mmol/mol | 36.4 ± 6.3 |
| Creatinine, µmol/L | 80.9 ± 14.8 |
| Total cholesterol, mmol/L | 5.6 ± 1.1 |
| Triglycerides, mmol/L | 1.2 ± 0.6 |
| HDL cholesterol, mmol/L | 1.7 ± 0.5 |
| LDL cholesterol, mmol/L | 3.4 ± 1.0 |
| C-reactive protein, mmol/L | 1.7 ± 2.7 |
Continuous values are means ± SD, and categorical values are n (%); N = 1557. BMI, body mass index; Hb, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Microcirculatory parameters.
An example of an individual recording of the microcirculatory parameters is given in Fig. 1. The marked time intervals representing baseline and occlusion, respectively are marked in gray and the peak after release of occlusion is marked with an asterisk.
Fig. 1.
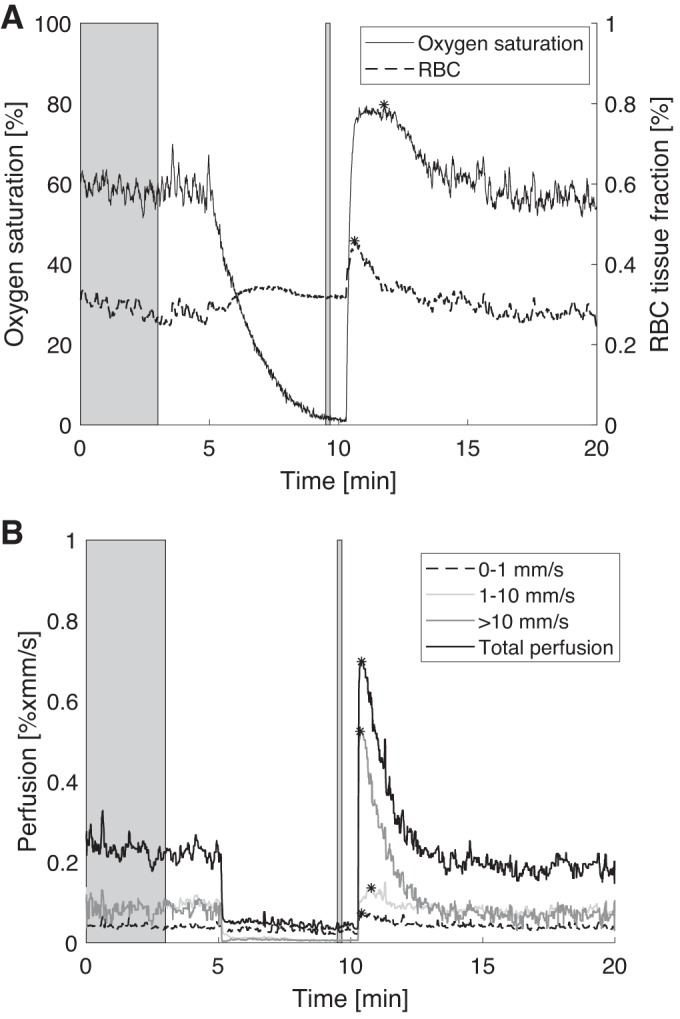
An individual recording of the microcirculatory parameters. A: oxygen saturation and red blood cell (RBC) tissue fraction. B: total perfusion and speed resolved perfusion for speeds <1, 1–10, and >10 mm/s. The marked time intervals in gray represent calculated baseline and occlusion values, and peak values after release are marked with asterisks.
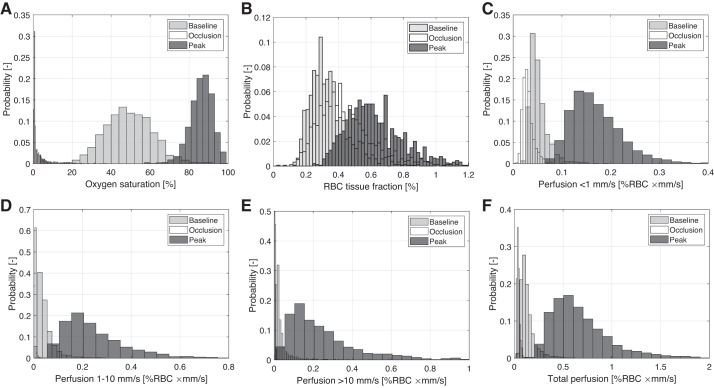
Mean values ± SD for all microcirculatory parameters during baseline, occlusion and PORH peak are given in Table 2. Additional descriptive data are given in the appendix, for the group of subjects without diabetes, dyslipidemia, or hypertension (n = 1,117), and separately for the groups of subjects with diabetes (n = 114), dyslipidemia (n = 173), and hypertension (n = 346). Data from the whole population (Table 2) are used for further analyses. Peak values were determined for each of the parameters. Histograms for all parameters during baseline, occlusion, and PORH peak are given in Fig. 2.
Table 2.
Mean values and standard deviation for all microcirculatory parameters during baseline, occlusion, and PORH peak
| Baseline | Occlusion | Peak | |
|---|---|---|---|
| Oxygen saturation, % | 49 ± 12 | 3 ± 5 | 86 ± 6 |
| RBC tissue fraction, % | 0.34 ± 0.12 | 0.44 ± 0.18 | 0.63 ± 0.19 |
| Perfusion (0–1 mm/s), %RBC × mm/s | 0.051 ± 0.019 | 0.030 ± 0.012 | 0.16 ± 0.051 |
| Perfusion (1–10 mm/s), %RBC × mm/s | 0.047 ± 0.035 | 0.006 ± 0.004 | 0.25 ± 0.16 |
| Perfusion (>10 mm/s), %RBC × mm/s | 0.036 ± 0.047 | 0.004 ± 0.003 | 0.25 ± 0.20 |
| Total perfusion, %RBC × mm/s | 0.13 ± 0.08 | 0.040 ± 0.014 | 0.63 ± 0.30 |
Values are means ± SD. PORH, postocclusive reactive hyperemia; RBC, red blood cells.
Fig. 2.
Histogram of oxygen saturation (A), red blood cell (RBC) tissue fraction (B) and speed-resolved perfusion, below 1 mm/s (C), 1–10 mm/s (D), above 10 mm/s (E), and total perfusion (F). Note that the widths of the bars differ to improve visibility.
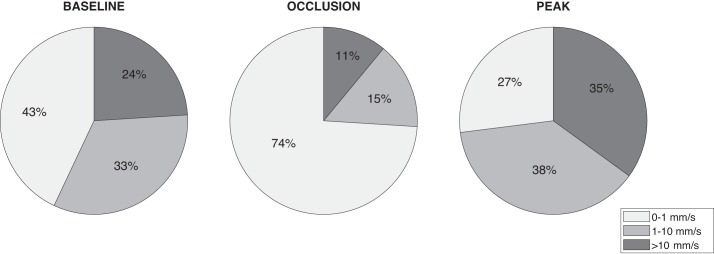
Mean percentage of total perfusion for the three perfusion components during baseline, occlusion, and total perfusion peak are given in Fig. 3.
Fig. 3.
Mean percentage of total perfusion for the three perfusion components during baseline, occlusion, and peak.
Since the perfusion estimates are close to zero during the occlusion phase, these values are not included in the further analysis of age and sex effects on the microcirculatory function.
Age and microcirculatory parameters.
For each age group, mean values ± SD for all microcirculatory parameters during baseline, occlusion, and peak are given in Table 3. There were significant differences in peak oxygen saturation (P < 0.001), baseline RBC tissue fraction (P = 0.029), perfusion 0–1 mm/s (P < 0.001), perfusion 1–10 mm/s (P = 0.049), and total perfusion (P = 0.018).
Table 3.
Age-specific microcirculatory parameters
| 50–55 yr | 55–60 yr | 60–65 yr | P | |
|---|---|---|---|---|
| n | 519 | 489 | 547 | |
| Oxygen saturation, % | ||||
| Baseline | 49 ± 12 | 49 ± 12 | 49 ± 12 | 0.573 |
| Occlusion | 3 ± 4 | 3 ± 5 | 3 ± 5 | 0.279 |
| Peak | 87 ± 6 | 87 ± 6 | 85 ± 6 | <0.001 |
| RBC tissue fraction, % | ||||
| Baseline | 0.33 ± 0.11 | 0.33 ± 0.13 | 0.35 ± 0.12 | 0.023 |
| Occlusion | 0.43 ± 0.17 | 0.44 ± 0.18 | 0.45 ± 0.18 | 0.136 |
| Peak | 0.62 ± 0.18 | 0.62 ± 0.19 | 0.64 ± 0.19 | 0.170 |
| Perfusion (0–1 mm/s), %RBC × mm/s | ||||
| Baseline | 0.049 ± 0.017 | 0.051 ± 0.021 | 0.053 ± 0.019 | <0.001 |
| Peak | 0.16 ± 0.05 | 0.16 ± 0.05 | 0.16 ± 0.05 | 0.755 |
| Perfusion (1–10 mm/s), %RBC × mm/s | ||||
| Baseline | 0.046 ± 0.035 | 0.045 ± 0.032 | 0.050 ± 0.038 | 0.049 |
| Peak | 0.25 ± 0.14 | 0.26 ± 0.18 | 0.25 ± 0.15 | 0.182 |
| Perfusion (>10 mm/s), %RBC × mm/s | ||||
| Baseline | 0.034 ± 0.041 | 0.037 ± 0.050 | 0.038 ± 0.051 | 0.399 |
| Peak | 0.26 ± 0.21 | 0.24 ± 0.18 | 0.25 ± 0.20 | 0.347 |
| Total perfusion, %RBC × mm/s | ||||
| Baseline | 0.13 ± 0.07 | 0.13 ± 0.08 | 0.14 ± 0.08 | 0.018 |
| Peak | 0.64 ± 0.30 | 0.64 ± 0.31 | 0.63 ± 0.29 | 0.872 |
Values are means ± SD. P values in bold indicate significant difference. RBC, red blood cells.
Average time traces for the two age groups 50–55 and 60–65 yr are presented in Fig. 4 (the group 55–60 yr is excluded for clarity). When calculating these average curves, a few additional measurements were excluded due to timing differences for start and end of occlusion, giving n = 511 for the group aged 50–55 yr and n = 538 for the group aged 60–65 yr.
Fig. 4.
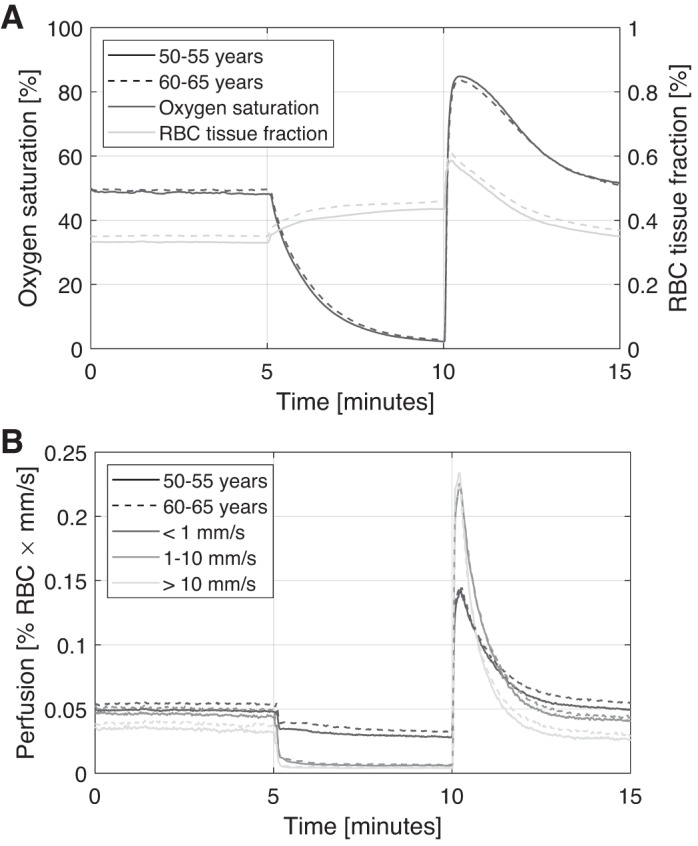
Average time traces of oxygen saturation and red blood cell (RBC) tissue fraction (A) and speed-resolved perfusion (B) for two age groups.
Sex and microcirculatory function.
There was no significant age difference between men and women. Microcirculatory mean values ± SD for men and women are given in Table 4. Women had a significantly higher oxygen saturation peak (P < 0.001), lower RBC tissue fraction during all three phases in the occlusion protocol (P < 0.001). There were also differences in perfusion both in baseline (0–1 mm/s, P < 0.001; 1–10 mm/s, P = 0.01; >10 mm/s, P < 0.001; and total perfusion, P < 0.001) and peak (1–10 mm/s, P = 0.04; >10 mm/s, P < 0.001; and total perfusion, P < 0.001).
Table 4.
Microcirculatory parameters for men and women
| Men | Women | P | |
|---|---|---|---|
| n | 778 | 779 | |
| Oxygen saturation, % | |||
| Baseline | 49 ± 11 | 49 ± 13 | 0.42 |
| Occlusion | 3 ± 5 | 3 ± 4 | 0.42 |
| Peak | 85 ± 6 | 88 ± 6 | <0.001 |
| RBC tissue fraction, % | |||
| Baseline | 0.37 ± 0.13 | 0.31 ± 0.10 | <0.001 |
| Occlusion | 0.50 ± 0.18 | 0.40 ± 0.15 | <0.001 |
| Peak | 0.67 ± 0.20 | 0.58 ± 0.17 | <0.001 |
| Perfusion (0–1 mm/s) | |||
| Baseline, %RBC × mm/s | 0.054 ± 0.020 | 0.048 ± 0.017 | <0.001 |
| Peak, %RBC × mm/s | 0.16 ± 0.05 | 0.16 ± 0.05 | 0.16 |
| Increase from baseline, % | 221 ± 93 | 250 ± 96 | <0.001 |
| Perfusion (1–10 mm/s) | |||
| Baseline, %RBC × mm/s | 0.049 ± 0.039 | 0.045 ± 0.032 | 0.01 |
| Peak, %RBC × mm/s | 0.26 ± 0.17 | 0.24 ± 0.15 | 0.04 |
| Increase from baseline, % | 593 ± 473 | 596 ± 478 | 0.90 |
| Perfusion (>10 mm/s) | |||
| Baseline, %RBC × mm/s | 0.041 ± 0.057 | 0.032 ± 0.036 | <0.001 |
| Peak, %RBC × mm/s | 0.28 ± 0.22 | 0.21 ± 0.16 | <0.001 |
| Increase from baseline, % | 1,295 ± 3,319 | 864 ± 1,060 | 0.001 |
| Total perfusion | |||
| Baseline, %RBC × mm/s | 0.14 ± 0.09 | 0.12 ± 0.06 | <0.001 |
| Peak, %RBC × mm/s | 0.68 ± 0.32 | 0.59 ± 0.27 | <0.001 |
| Increase from baseline, % | 412 ± 175 | 401 ± 171 | 0.22 |
Values are means ± SD. Values in bold indicate significant difference. RBC, red blood cells.
Average time traces for men and women are presented in Fig. 5. When calculating these average curves, a few additional measurements were excluded because of timing differences for start and end of occlusion, giving n = 767 for men and n = 756 for women.
Fig. 5.
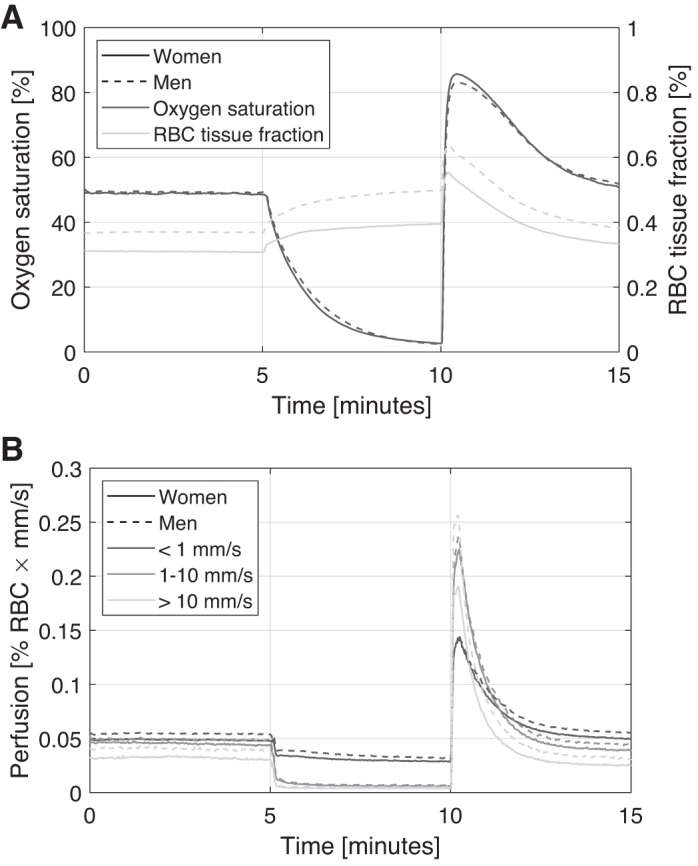
Average time traces of oxygen saturation and red blood cell (RBC) tissue fraction (A) and speed-resolved perfusion (B) for men and women.
DISCUSSION
The results of this study provide normative data for comprehensive skin microcirculatory function in a middle-aged Swedish population-based cohort. This study is the first to report normative data on oxygen saturation, tissue fraction of red blood cells, and speed-resolved perfusion in a large cohort (1,557 individuals). This population-based normative data can be used as reference values in future studies of disease-specific populations. The method provides data in absolute units in contrast to many previous assessments of microcirculation that only provide data in arbitrary units. Additional descriptive data for subjects without diabetes, hypertension, and hyperlipidemia, as well as for these groups separately, are given in the appendix.
The PORH is a commonly used provocation to study cutaneous microcirculatory function (6, 22). The PORH has been shown to be a good model for overall microcirculatory function, anchored in relevant pathophysiological pathways (24). These pathways are of specific interest when studying relationships between microvascular and macrovascular function. The increase in blood flow after release of an arterial occlusion is considered to be locally mediated by many factors: sensory nerves, endothelium-derived hyperpolarizing factor (EDHF), prostaglandins and nitric oxide (NO), and local potassium channels (18, 23). The applied cuff pressure and the occlusion length varies between studies, in which there is a linear correlation between laser-Doppler perfusion amplitude during hyperemia and the ischemic period (27). The lowest recommendation of cuff pressure is 50 mmHg above the systolic blood pressure. Few previous studies have addressed, however, the feasibility and patient acceptability of the method, which are important factors to gain clinical acceptance for the method. In this study, only 0.2% of the subjects made a choice to end the measurement before the end of the protocol, even though the applied cuff pressure of 250 mmHg was higher than the lowest cuff pressure recommendation. This shows that postocclusive reactive hyperemia is a feasible method to use in clinical settings.
Previous investigations have found large spatial and temporal variability in the perfusion, both during baseline and at PORH peak (22). Our previous work found that the spatiotemporal repeatability was better for oxygen saturation and red blood cell tissue fraction than for perfusion (25). Because we found differences in oxygen saturation peak at PORH and not in the perfusion estimates, it is possible that the spatial heterogeneity of skin blood flow affects the perfusion estimate more than the oxygen saturation, which could be more homogenous over the microvascular bed.
The results support that sex and age are important aspects to consider in studies of microvascular function. There was a consistent age distribution, where the oldest individuals (60 to 65 yr) had the lowest oxygen saturation peak and the highest baseline perfusion in speed region 0–1 mm/s. Women had higher oxygen saturation peak at PORH, lower RBC tissue fraction, in general, and lower baseline perfusion in all speed regions. Peak perfusion at PORH showed more variance in the different speed regions, where women had lower values in total perfusion and perfusion in speed region >10 mm/s. However, we also found differences in baseline measures, where there was an age-related increase in baseline perfusion in the two lower speed regions (<10 mm/s). Previous investigations have found similar associations between age and perfusion, where the increased perfusion at baseline conditions was proposed to originate from a decreased basal vasoconstrictive tone in older individuals (19). However, other studies have shown both a decreased perfusion in older individuals (29) and no relationship between age and basal skin perfusion (15, 28). The divergence in the results points out the importance of quantitative measure that enables comparison.
An important finding in this study was the differences in oxygen saturation at PORH peak with both age and sex, both being risk factors for cardiovascular disease. Similarly, for type 2 diabetes, Adingupu et al. (1) found that oxygen saturation in resting state and at PORH were reduced. They suggested that this was caused by structural changes in the microvasculature, which could impair the microvessel response to a period of ischemia. This may also apply to the findings of this study. Furthermore, we found that mean oxygen saturation in baseline was 49% and in peak 86%. This is in line with several previous findings. Oxygen saturation in skin has been reported in smaller studies (n < 100) in healthy individuals of ages from 25 to 65 yr, ranging from 40% to 47% at rest (2, 5, 13, 14, 16). Similarly, peak oxygen saturation in healthy individuals was reported to range from 81.4 to 88% (14, 16, 25).
Limitations and considerations.
Our data on cutaneous microcirculation originate from measurements on Caucasian skin. How darker skin possibly affects estimations of perfusion, RBC tissue fraction, or oxygen saturation from the EPOS system has not been evaluated. The three-layered model can account for different amounts of melanin in the epidermis, but with a larger amount of melanin, less light will reach lower blood-containing layers and hence change the signal-to-noise ratio. Whether that alters the ability of the method to estimate the parameters remains unknown.
The participants in our study are a mixture of apparently healthy individuals and patients with various diseases, including diseases affecting the vascular system, like diabetes and cardiovascular diseases. Future work will address microcirculation in relation to different vascular diseases and cardiovascular risk factors.
GRANTS
This study was supported by Sweden’s innovation agency VINNOVA via the program MedTech4Health (no. 2016-02211). The main funding body of The Swedish CArdioPulmonary bioImage Study (SCAPIS) is the Swedish Heart-Lung Foundation. The study is also funded by the Knut and Alice Wallenberg Foundation, the Swedish Research Council, and VINNOVA (Sweden’s Innovation agency), the University of Gothenburg and Sahlgrenska University Hospital, Karolinska Institutet and Stockholm County Council, Linköping University and University Hospital, Lund University and Skåne University Hospital, Umeå University and University Hospital, and Uppsala University and University Hospital.
DISCLOSURES
I. Fredriksson is part-time employed by Perimed, AB, which is developing products related to research described in this publication. None of the other authors have disclosable conflicts of interests.
AUTHOR CONTRIBUTIONS
H.J., S.B., M.L., C.J.Ö., and T.S. conceived and designed research; H.J. and S.B. performed experiments; H.J. and I.F. analyzed data; H.J., S.B., and T.S. interpreted results of experiments; H.J. and I.F. prepared figures; H.J., S.B., M.L., and T.S. drafted manuscript; H.J., S.B., I.F., M.L., C.J.Ö., and T.S. edited and revised manuscript; H.J., S.B., C.J.Ö., and T.S. approved final version of manuscript.
APPENDIX
Additional descriptive data are shown below for the groups of subjects without diabetes, dyslipidemia, or hypertension (n = 1,117; Table A1) and for the groups of subjects with diabetes (n = 114; Table A2), hypertension (n = 346; Table A3), or dyslipidemia (n = 173; Table A4).
Table A1.
Mean values and standard deviation for all microcirculatory parameters during baseline, occlusion and PORH peak for subjects without diabetes, hypertension, or dyslipidemia
| Baseline | Occlusion | Peak | |
|---|---|---|---|
| Oxygen saturation, % | 50 ± 12 | 3 ± 5 | 87 ± 6 |
| RBC tissue fraction, % | 0.34 ± 0.12 | 0.44 ± 0.18 | 0.62 ± 0.19 |
| Perfusion (0–1 mm/s), %RBC × mm/s | 0.051 ± 0.019 | 0.030 ± 0.011 | 0.16 ± 0.049 |
| Perfusion (1–10 mm/s), %RBC × mm/s | 0.047 ± 0.035 | 0.006 ± 0.004 | 0.25 ± 0.15 |
| Perfusion (>10 mm/s), %RBC × mm/s | 0.036 ± 0.045 | 0.004 ± 0.004 | 0.25 ± 0.20 |
| Total perfusion, %RBC × mm/s | 0.14 ± 0.07 | 0.040 ± 0.014 | 0.64 ± 0.30 |
Values are means ± SD; n = 1,117 subjects. PORH, postocclusive reactive hyperemia; RBC, red blood cells.
Table A2.
Mean values and standard deviation for all microcirculatory parameters during baseline, occlusion, and PORH peak for subjects with diabetes
| Baseline | Occlusion | Peak | |
|---|---|---|---|
| Oxygen saturation, % | 46 ± 11 | 3 ± 5 | 84 ± 6 |
| RBC tissue fraction, % | 0.34 ± 0.13 | 0.49 ± 0.19 | 0.66 ± 0.20 |
| Perfusion (0–1 mm/s), %RBC × mm/s | 0.051 ± 0.021 | 0.032 ± 0.014 | 0.17 ± 0.06 |
| Perfusion (1–10 mm/s), %RBC × mm/s | 0.040 ± 0.033 | 0.006 ± 0.002 | 0.26 ± 0.20 |
| Perfusion (>10 mm/s), %RBC × mm/s | 0.034 ± 0.045 | 0.004 ± 0.004 | 0.23 ± 0.19 |
| Total perfusion, %RBC × mm/s | 0.13 ± 0.07 | 0.042 ± 0.015 | 0.62 ± 0.32 |
Values are means ± SD; n = 114. PORH, postocclusive reactive hyperemia; RBC, red blood cells.
Table A3.
Mean values and standard deviation for all microcirculatory parameters during baseline, occlusion, and PORH peak for subjects with hypertension
| Baseline | Occlusion | Peak | |
|---|---|---|---|
| Oxygen saturation, % | 48 ± 11 | 3 ± 4 | 85 ± 6 |
| RBC tissue fraction, % | 0.34 ± 0.12 | 0.46 ± 0.18 | 0.64 ± 0.18 |
| Perfusion (0–1 mm/s), %RBC × mm/s | 0.052 ± 0.019 | 0.032 ± 0.013 | 0.16 ± 0.06 |
| Perfusion (1–10 mm/s), %RBC × mm/s | 0.047 ± 0.039 | 0.006 ± 0.003 | 0.25 ± 0.17 |
| Perfusion (>10 mm/s), %RBC × mm/s | 0.037 ± 0.056 | 0.004 ± 0.003 | 0.23 ± 0.19 |
| Total perfusion, %RBC × mm/s | 0.14 ± 0.09 | 0.042 ± 0.015 | 0.62 ± 0.30 |
Values are means ± SD; n = 346. PORH, postocclusive reactive hyperemia; RBC, red blood cells.
Table A4.
Mean values and standard deviation for all microcirculatory parameters during baseline, occlusion, and PORH peak for subjects with dyslipidemia
| Baseline | Occlusion | Peak | |
|---|---|---|---|
| Oxygen saturation, % | 47 ± 11 | 3 ± 4 | 85 ± 7 |
| RBC tissue fraction, % | 0.34 ± 0.12 | 0.47 ± 0.18 | 0.64 ± 0.19 |
| Perfusion (0–1 mm/s), %RBC × mm/s | 0.051 ± 0.020 | 0.032 ± 0.013 | 0.16 ± 0.06 |
| Perfusion (1–10 mm/s), %RBC × mm/s | 0.045 ± 0.037 | 0.006 ± 0.003 | 0.24 ± 0.18 |
| Perfusion (>10 mm/s), %RBC × mm/s | 0.039 ± 0.052 | 0.005 ± 0.003 | 0.23 ± 0.19 |
| Total perfusion, %RBC × mm/s | 0.14 ± 0.08 | 0.042 ± 0.014 | 0.60 ± 0.30 |
Values are means ± SD; n = 173. PORH, postocclusive reactive hyperemia; RBC, red blood cells.
REFERENCES
- 1.Adingupu DD, Thorn CE, Casanova F, Elyas S, Gooding K, Gilchrist M, Aizawa K, Gates PE, Shore AC, Strain DW. Blood oxygen saturation after ischemia is altered with abnormal microvascular reperfusion. Microcirculation 22: 294–305, 2015. doi: 10.1111/micc.12198. [DOI] [PubMed] [Google Scholar]
- 2.Beckert S, Witte MB, Königsrainer A, Coerper S. The impact of the Micro-Lightguide O2C for the quantification of tissue ischemia in diabetic foot ulcers. Diabetes Care 27: 2863–2867, 2004. doi: 10.2337/diacare.27.12.2863. [DOI] [PubMed] [Google Scholar]
- 3.Bentov I, Reed MJ. The effect of aging on the cutaneous microvasculature. Microvasc Res 100: 25–31, 2015. doi: 10.1016/j.mvr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergström G, Berglund G, Blomberg A, Brandberg J, Engström G, Engvall J, Eriksson M, de Faire U, Flinck A, Hansson MG, Hedblad B, Hjelmgren O, Janson C, Jernberg T, Johnsson Å, Johansson L, Lind L, Löfdahl CG, Melander O, Östgren CJ, Persson A, Persson M, Sandström A, Schmidt C, Söderberg S, Sundström J, Toren K, Waldenström A, Wedel H, Vikgren J, Fagerberg B, Rosengren A. The Swedish CArdioPulmonary BioImage Study: objectives and design. J Intern Med 278: 645–659, 2015. doi: 10.1111/joim.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernjak A, Stefanovska A, McClintock PV, Owen-Lynch PJ, Clarkson PB. Coherence between fluctuations in blood flow and oxygen saturation. Fluct Noise Lett 11: 1240013, 2012. doi: 10.1142/S0219477512400135. [DOI] [Google Scholar]
- 6.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 27: 503–508, 2006. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Debbabi H, Bonnin P, Ducluzeau PH, Lefthériotis G, Levy BI. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens 23: 541–546, 2010. doi: 10.1038/ajh.2010.10. [DOI] [PubMed] [Google Scholar]
- 8.Fredriksson I, Burdakov O, Larsson M, Strömberg T. Inverse Monte Carlo in a multilayered tissue model: merging diffuse reflectance spectroscopy and laser Doppler flowmetry. J Biomed Opt 18: 127004, 2013. doi: 10.1117/1.JBO.18.12.127004. [DOI] [PubMed] [Google Scholar]
- 9.Fredriksson I, Larsson M, Nyström FH, Länne T, Östgren CJ, Strömberg T. Reduced arteriovenous shunting capacity after local heating and redistribution of baseline skin blood flow in type 2 diabetes assessed with velocity-resolved quantitative laser Doppler flowmetry. Diabetes 59: 1578–1584, 2010. doi: 10.2337/db10-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 11.Huxley VH, Kemp SS. Sex-specific characteristics of the microcirculation. Adv Exp Med Biol 1065: 307–328, 2018. doi: 10.1007/978-3-319-77932-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IJzerman RG, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serné EH, Stehouwer CDA. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest 33: 536–542, 2003. doi: 10.1046/j.1365-2362.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 13.Jonasson H, Bergstrand S, Nystrom FH, Länne T, Östgren CJ, Bjarnegård N, Fredriksson I, Larsson M, Strömberg T. Skin microvascular endothelial dysfunction is associated with type 2 diabetes independently of microalbuminuria and arterial stiffness. Diab Vasc Dis Res 14: 363–371, 2017. doi: 10.1177/1479164117707706. [DOI] [PubMed] [Google Scholar]
- 14.Jonasson H, Fredriksson I, Pettersson A, Larsson M, Strömberg T. Oxygen saturation, red blood cell tissue fraction and speed resolved perfusion. A new optical method for microcirculatory assessment. Microvasc Res 102: 70–77, 2015. doi: 10.1016/j.mvr.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Kelly RI, Pearse R, Bull RH, Leveque JL, de Rigal J, Mortimer PS. The effects of aging on the cutaneous microvasculature. J Am Acad Dermatol 33: 749–756, 1995. doi: 10.1016/0190-9622(95)91812-4. [DOI] [PubMed] [Google Scholar]
- 16.Kuliga KZ, McDonald EF, Gush R, Michel C, Chipperfield AJ, Clough GF. Dynamics of microvascular blood flow and oxygenation measured simultaneously in human skin. Microcirculation 21: 562–573, 2014. doi: 10.1111/micc.12136. [DOI] [PubMed] [Google Scholar]
- 17.Larsson M, Steenbergen W, Strömberg T. Influence of optical properties and fiber separation on laser doppler flowmetry. J Biomed Opt 7: 236–243, 2002. doi: 10.1117/1.1463049. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585: 295–303, 2007. doi: 10.1113/jphysiol.2007.143867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogrin R, Darzins P, Khalil Z. Age-related changes in microvascular blood flow and transcutaneous oxygen tension under basal and stimulated conditions. J Gerontol A Biol Sci Med Sci 60: 200–206, 2005. doi: 10.1093/gerona/60.2.200. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberry R, Munson M, Chung S, Samuel TJ, Patik J, Tucker WJ, Haykowsky MJ, Nelson MD. Age-related microvascular dysfunction: novel insight from near-infrared spectroscopy. Exp Physiol 103: 190–200, 2018. doi: 10.1113/EP086639. [DOI] [PubMed] [Google Scholar]
- 21.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi J-Y, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang Y-H, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin M-J, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70: 1–25, 2017. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roustit M, Blaise S, Millet C, Cracowski JL. Reproducibility and methodological issues of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetry. Microvasc Res 79: 102–108, 2010. doi: 10.1016/j.mvr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Roustit M, Cracowski JL. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation 19: 47–64, 2012. doi: 10.1111/j.1549-8719.2011.00129.x. [DOI] [PubMed] [Google Scholar]
- 24.Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci 34: 373–384, 2013. doi: 10.1016/j.tips.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Strömberg T, Sjöberg F, Bergstrand S. Temporal and spatiotemporal variability in comprehensive forearm skin microcirculation assessment during occlusion protocols. Microvasc Res 113: 50–55, 2017. doi: 10.1016/j.mvr.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Sörensen BM, Houben AJHM, Berendschot TTJM, Schouten JSAG, Kroon AA, van der Kallen CJH, Henry RMA, Koster A, Dagnelie PC, Schaper NC, Schram MT, Stehouwer CDA. Cardiovascular risk factors as determinants of retinal and skin microvascular function: The Maastricht Study. PLoS One 12: e0187324, 2017. doi: 10.1371/journal.pone.0187324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tee GBY, Rasool AHG, Halim AS, Rahman ARA. Dependence of human forearm skin postocclusive reactive hyperemia on occlusion time. J Pharmacol Toxicol Methods 50: 73–78, 2004. doi: 10.1016/j.vascn.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Tikhonova IV, Tankanag AV, Chemeris NK. Age-related changes of skin blood flow during postocclusive reactive hyperemia in human. Skin Res Technol 19: e174–e181, 2013. doi: 10.1111/j.1600-0846.2012.00624.x. [DOI] [PubMed] [Google Scholar]
- 29.Van den Brande P, von Kemp K, De Coninck A, Debing E. Laser Doppler flux characteristics at the skin of the dorsum of the foot in young and in elderly healthy human subjects. Microvasc Res 53: 156–162, 1997. doi: 10.1006/mvre.1996.1995. [DOI] [PubMed] [Google Scholar]