Abstract
Because of remarkable surgical and medical advances over the past several decades, there are growing numbers of infants and children living with single ventricle congenital heart disease (SV), where there is only one functional cardiac pumping chamber. Nevertheless, cardiac dysfunction (and ultimately heart failure) is a common complication in the SV population, and pharmacological heart failure therapies have largely been ineffective in mitigating the need for heart transplantation. Given that there are several inherent risk factors for ventricular dysfunction in the setting of SV in addition to probable differences in molecular adaptations to heart failure between children and adults, it is perhaps not surprising that extrapolated adult heart failure medications have had limited benefit in children with SV heart failure. Further investigations into the molecular mechanisms involved in pediatric SV heart failure may assist with risk stratification as well as development of targeted, efficacious therapies specific to this patient population. In this review, we present a brief overview of SV anatomy and physiology, with a focus on patients with a single morphological right ventricle requiring staged surgical palliation. Additionally, we discuss outcomes in the current era, risk factors associated with the progression to heart failure, present state of knowledge regarding molecular alterations in end-stage SV heart failure, and current therapeutic interventions. Potential avenues for improving SV outcomes, including identification of biomarkers of heart failure progression, implications of personalized medicine and stem cell-derived therapies, and applications of novel models of SV disease, are proposed as future directions.
Keywords: congenital heart disease, hypoplastic left heart syndrome, pediatric heart failure, single ventricle
INTRODUCTION
Congenital heart disease (CHD) with single ventricle physiology (SV) encompasses a heterogeneous group of severe abnormalities in cardiac structure where improper development of the fetal heart results in only one functional pumping chamber. Infants born with SV are critically ill in the newborn period, and SV is generally fatal without intervention. Nevertheless, in the past several decades, tremendous advances in surgical technique and postoperative care have resulted in an increasing number of SV patients living into adulthood. The incidence of infants born with SV physiology ranges from ~3.1–4.9 per 10,000 live births (39, 89, 129, 159), and beyond infancy, it is estimated that there are ~1.6 per 10,000 children living with SV physiology (39). Although patients with SV are uncommon, they require a substantial investment of health care resources over a lifetime, and their long-term prognosis remains guarded even after surviving the early neonatal period (129). In this high-risk population, medical therapies have been ineffective in preventing the development of ventricular dysfunction or limiting the progression of ventricular dysfunction to end-stage heart failure (HF). Specifically, CHD (including SV) is the most common cause of HF in children (86), and in the recent era, 57% of children were transplanted for CHD, with failed SV palliation as the predominant indication (199). Most clinicians view deterioration in ventricular function and shortened life expectancy as inevitable for patients with SV (169). Moreover, use of proven adult HF therapies has not achieved the same improvement in outcomes when applied to pediatric SV patients with ventricular dysfunction, which may be indicative of distinct etiologies and mechanisms of heart failure in these two populations.
In general, there is a spectrum of complex CHD types that result in SV physiology, usually arising from inadequate development of an atrioventricular valve and/or ventricular chamber. SV phenotypes can be further categorized by the morphology of the dominant ventricle: right ventricle (RV), left ventricle (LV), or indeterminate, with incidences of 50–57%, 42–43%, and 0–2%, respectively (3, 42). Additional variations in SV anatomy can further dictate need for subsequent surgical interventions. Although the anatomic variations of functional SV phenotypes are beyond the scope of this review, the most common CHD types with a dominant RV including hypoplastic left heart syndrome (HLHS, which is the most common type of SV disease, accounting for ~40% of SV cases) (3), double outlet right ventricle, and right-dominant atrioventricular septal defect will be the primary focus of this review. Several studies suggest that patients with SV of RV morphology are at higher risk for death, presumably because the geometry, fiber orientation, and metabolic adaptations of the RV are not tailored to support the high-pressure, systemic circulation long term (4, 5, 9, 42, 103, 128). Given the poor outcomes in this subgroup, we will focus this review on the unique physiological characteristics that drive the clinical management of patients with SV of RV morphology (with HLHS as an example), as well as highlight important gaps in knowledge regarding the pathogenesis of heart failure and the specific physiological and molecular adaptations in this vulnerable population.
COMPLEX GENETICS OF SV
Similar to other types of CHD, the underlying causes of SV remain relatively poorly understood (211). CHD, in general, is thought to be influenced by both genetic and environmental factors, yet the epidemiology of CHD suggests that genetics contributes to the majority of cases (211).
Aneuploidy, copy number variants, and environmental factors together account for less than half of all CHD cases, with the etiology for the majority of CHD cases being unknown or idiopathic (211). Interestingly, there is a relatively high level of heritability of SV heart disease, such that there is an 8% risk of sibling recurrence (85). First-degree probands of SV patients also have a higher frequency of cardiac defects than expected; in fact, siblings of SV patients display a 22% increased risk of having any cardiovascular malformation (21, 123). Together these data suggest that there is likely a genetic contribution to the development of SV heart disease. However, a large fraction of SV occurs in families with no other history of CHD; therefore, the possibility that a number of SV cases are also attributable to de novo genetic events, including chromosomal abnormalities, smaller copy number variants, and point mutations (211), also exists.
Although there are a few known mutations associated with SV anatomy, the etiology of SV disease in most patients appears to be multigenic and genetically heterogeneous in nature (85, 124, 150, 176, 207). For example, several cytogenic abnormalities (e.g., Turner and Jacobsen syndromes) and heterozygous mutations in genes (e.g., NKX2.5, GJA1, HAND1, TBX5, NOTCH1, MYH6, etc.) and a link to chromosome 2p15 have been reported in SV patients (48, 137, 215). Other studies have implicated a primary genetic basis for SV with autosomal recessive inheritance (176), and an overrepresentation of rare copy number variants and de novo copy number variants has been documented in patients with SV (72, 84, 87, 94, 201, 212). The SV phenotype is therefore generally thought to result from additive effects of multiple genes, interactions with other genes and environmental factors, epigenetic factors, and stochastic effects.
Overlapping Genetic Pathways Between Cardiac Function and Structure
Importantly, genetic contributions to CHD are likely to not only underlie structural CHD but also be a major contributor to CHD comorbidities, including HF, arrhythmia, and neurocognitive outcomes (211). As reviewed in Hebert et al. (82), an overlapping network of genes is implicated in both normal cardiac structure and normal cardiac function. Mutations in a single gene can result in multiple cardiac phenotypes ranging from congenital structural malformations to atrial fibrillation, dilated cardiomyopathy, hypertrophic cardiomyopathy, and LV noncompaction, among others (31, 162). Therefore, variability in genotype to phenotype correlation makes interpreting the relative contribution of genetic factors specific to SV etiology even more difficult. Critical biological pathways that may be impaired in the setting of CHD include chromatin remodeling, Notch signaling, cilia function, sarcomere structure and function, and renin angiotensin signaling (211), many of which have the potential to limit the compensatory ability of the myocardium in response to stress. Additionally, several genes (GATA4, GATA6) involved in embryonic cardiac development continue to be expressed in the adult heart, where they play a role in maintaining cardiomyocyte survival and adaptation (6, 34, 51). Specifically, evidence suggests that both the risk and course of HF itself may depend on genetic predisposition (31) and that combinatorial interactions between genes and hemodynamic stressors can result in HF in patients with CHD (52). There is also considerable overlap in mutations in sarcomeric genes associated with both CHD and cardiomyopathy (204). Furthermore, genetic abnormalities were identified as an independent risk factor for poor outcomes in SV patients undergoing staged surgical palliation, suggesting that genetic factors may not only influence abnormal cardiac structure, but also the development of HF in this population (86, 188).
FUNCTIONAL SV PHYSIOLOGY AND STAGED PALLIATION
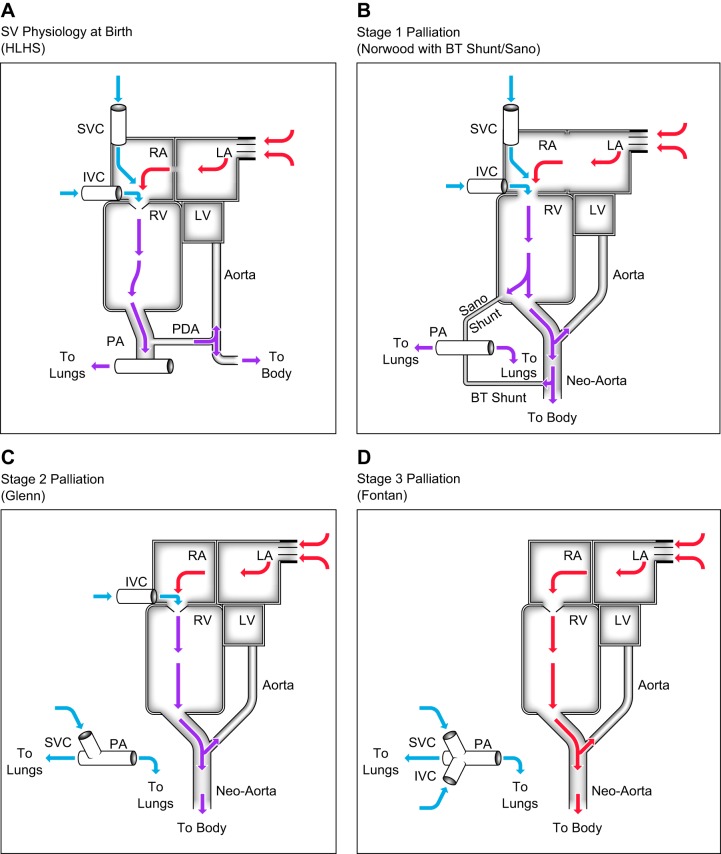
SV physiology is generally lethal without interventions to modify paths of blood flow. To this end, a series of three palliative surgical procedures may be required to accommodate for the single ventricle: ultimately, the functional ventricle is assigned to pump to the systemic circulation and blood flow to the lungs is passive (no subpulmonary pump). For example, in a newborn with HLHS (Fig. 1A), systemic blood flow arises from the pulmonary arteries via a patent ductus arteriosus (PDA); notably, this blood is relatively desaturated (oxygen saturation of ~75%). Within hours to weeks after birth, the PDA spontaneously closes and, in a neonate with SV physiology, blood flow to the body is compromised. Thus, pending surgical intervention, prostaglandin E1 is administered to preserve the patency of the ductus arteriosus to maintain systemic blood flow. In this setting, the pulmonary and systemic circulations are in parallel, with the total (single) ventricular output being twice the normal output in a biventricular (in series) circulation; ideally, the cardiac output is split equally between the lungs and the body, but this blood flow is ultimately controlled by the pulmonary and systemic vascular resistances. As pulmonary vascular resistance falls in the weeks after birth, the pulmonary bed receives more blood flow, which can compromise blood flow to the body. Nevertheless, it is important to note that SV physiology is complex, and surgical management will differ based on the underlying anatomy.
Fig. 1.
Path of blood flow in hypoplastic left heart syndrome (HLHS). Single ventricle (SV) physiology at birth (A), stage 1 palliation (B), stage 2 palliation (C), stage 3 palliation (D). Blue arrows indicate desaturated blood. Purple arrows indicate blood with intermediate oxygen saturation, typically 70–85%. Red arrows indicate fully oxygenated blood. BT, Blalock-Taussig; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; PA, pulmonary artery; PDA, patent ductus arteriosus; RA, right atrium; RV, right ventricle; SVC, superior vena cava.
Stage 1 Palliation (Norwood Procedure with Blalock-Taussig Shunt or Sano Shunt)
Most commonly, stage 1 palliation (Fig. 1B) is performed in the first week of life and consists of reconstruction of the aortic arch into the right ventricular outflow (Norwood), separation of branch pulmonary arteries from the RV, and creation of a stable, restrictive source of pulmonary blood flow from a systemic artery [Blalock-Taussig (BT) shunt] or directly from the single ventricle (right ventricle to pulmonary artery conduit, Sano shunt) (127, 144). While the pulmonary and systemic circulations remain in parallel, the primary goal of this stage of palliation is to establish a nonductal-dependent, permanent source of systemic blood flow in the form of a “neo-aorta” arising from the single ventricle. Pulmonary blood flow is provided by the BT or Sano shunt. Additionally, the atrial septum is largely removed to prevent pulmonary venous hypertension.
Stage 2 Palliation (Bidirectional, Superior Cavopulmonary Connection or Glenn/Hemi-Fontan Procedure)
In this operation, the BT or Sano shunts are taken down and the superior vena cava is anastomosed to the pulmonary artery to provide pulmonary blood flow (Fig. 1C), at around 3–5 mo of age. Performing the superior vena cava anastomosis before attaching the inferior vena cava has lower operative mortality and has been demonstrated to improve long-term survival of SV patients undergoing surgical palliation (58, 98).
Stage 3 Palliation (Total Cavopulmonary Connection or Fontan Procedure)
In the Fontan circulation (following completion of all 3 stages of surgical palliation, Fig. 1D), blood flow to the lungs is dependent on the pressure gradient from the systemic postcapillary vessels to the pulmonary postcapillary vessels. In contrast to a normal, biventricular circulation, there is no pump to add energy to the pulmonary circulation; thus, small changes in pulmonary resistance have a considerable impact on blood flow (68). Obstruction or abnormalities can occur at the level of the Fontan connection itself, pulmonary arteries, pulmonary capillary bed, or pulmonary veins. Additionally, nonpulsatile flow into the lungs and relaxation of the ventricle control the preload returning to the heart. The significantly diminished ability to augment cardiac output in a single ventricle system results in severe exercise limitations in patients with a Fontan circulation. Most centers proceed with Fontan completion when a single ventricle patient is between 2 and 4 yr of age.
Ultimately, attachment of both the superior (stage 2) and inferior (stage 3) vena cavae (total cavopulmonary connection or Fontan completion) decreases the volume load on the single ventricle, minimizing both wall stress and atrioventricular valve insufficiency. The goal of stages 2 and 3 of palliation is to allow for a circulation where the pulmonary and systemic systems are arranged in series, which improves efficiency as well as higher ventricular diastolic pressures and coronary artery perfusion (8, 40, 56).
SV OUTCOMES AND COMPLICATIONS IN THE CURRENT ERA
Early Time Period (First Year of Life)
Survival following the Norwood procedure has improved over the past several decades; however, the first year of life remains tenuous for SV patients, with high risk of mortality. Transplant-free survival in a cohort of 549 infants undergoing the Norwood procedure between 2005 and 2008 was only 69% at 1 yr of age (145). Heart transplant listing or death occurred in 12% of patients within 30 days of the Norwood procedure, 16% in the time period between the stage 1 and 2 operations (“interstage”), and 6% after the stage 2 palliation to 1 yr of age (145). Low birth weight (weight of less than 2.5 kg) and noncardiac abnormalities are independent risk factors for increased mortality after the Norwood procedure (182). The most common cause of death was low cardiac output in the immediate postoperative period, followed by unexpected cardiac arrest in patients seemingly making an uncomplicated recovery (182). Despite increased prevalence of SV disease in male subjects, a trend toward worse outcomes in female subjects with SV has been noted (182). Anatomic considerations, such as obstructed pulmonary venous return, also have a significant impact on the ultimate outcome of patients with SV. Chowdhury et al. (37) proposed a preoperative mortality risk score for neonates undergoing the Norwood procedure, taking into account patient birth weight, presence of obstructed pulmonary venous return, ascending aorta diameter, presence of a genetic syndromes, number of Norwood procedures performed by the surgeon each year, and anatomic diagnosis (HLHS vs. other SV anatomy). Hospital mortality was: ~5% for the low-risk score group (56–57% of patients), 22–30% for the medium-risk score group (32–38% of patients), and 55–75% for the high-risk score group (5–13% of patients) (37). Based on the outcomes of the Pediatric Heart Network study evaluating the effects of enalapril in 230 infants with SV physiology, the incidence of at least mild systemic ventricular dysfunction was 17–22% at 14 mo of age (92). Thus, the ability to risk stratify SV patients before the Norwood procedure may identify a subgroup of high-risk patients where alternatives to SV surgical palliation should be considered (such as the hybrid approach or primary cardiac transplant).
Late Time Period (Post-Fontan and Beyond)
Long-term outcomes for SV are also poor, with a 10-yr transplant-free survival rate ranging from 39–50% (47, 66). In a single center retrospective review of 245 infants with SV, only one-third of children were alive and free of heart transplant at the age of 14 yr (129). Nevertheless, survival after the Fontan procedure has improved significantly since it was initially performed in 1968 (57), and it is estimated that patients surviving to the Fontan operation (excluding deaths in the early time period described above) today may have a 30-yr survival rate of 85% (171). Modifications to surgical technique, including the extracardiac conduit and incorporation of a fenestration between the systemic venous return and the pulmonary venous atrium, as well as improved patient selection and perioperative management, have contributed to improved outcomes. Multiple studies have established risk factors for death late after the Fontan, including male sex, presence of a common atrioventricular valve, older age at Fontan operation, elevated pulmonary artery pressures, and prolonged pleural effusions after Fontan completion (27, 41, 47, 65, 131, 154). However, it has been described that the Fontan circulation is characterized by chronically elevated systemic venous pressures and decreased cardiac output (168); thus, by definition, all patients with a Fontan have a physiology consistent with chronic HF. Yet only a subset of patients will have clinical symptoms of failure. In general, four distinct clinical phenotypes of failure exist in patients with a Fontan circulation: 1) type I Fontan failure with reduced ejection fraction, 2) type II Fontan failure with preserved ejection fraction, 3) type III Fontan failure with “normal heart,” and 4) type IV Fontan failure with abnormal lymphatics (17). In an analysis of a contemporary cohort of 500 Fontan patients, ventricular dysfunction (Type I Fontan failure) was the most common cause of Fontan failure (61%) (116).
Fontan Failure
Systolic dysfunction of the systemic ventricle is one clinical phenotype of Fontan failure (17). Although Fontan failure is similar to traditional HF in that the circulation can no longer meet the metabolic demands of the body, the clinical syndrome of Fontan failure can also occur with “normal” ventricular function and hemodynamics. The Fontan circulation impacts other organ systems by creating systemic venous hypertension resulting in hepatic congestion and potentially contributing to long-term abnormalities in the lymphatics, lungs, liver, kidneys, brain, and peripheral vessels. Complications inherent to the Fontan circulation are reviewed in a recent scientific statement from the American Heart Association (168). Because a significant proportion of Fontan patients develop HF with reduced ejection fraction (Type I Fontan failure), the remainder of this review will focus on the phenotype of ventricular dysfunction in the setting of SV disease of RV morphology.
RISK FACTORS FOR VENTRICULAR DYSFUNCTION
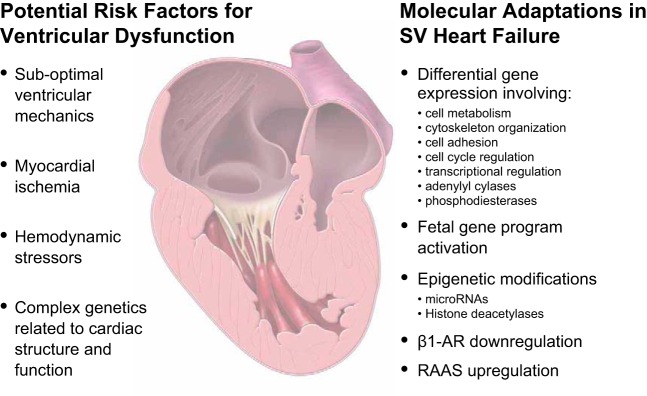
Although early survival following staged surgery for SV has dramatically improved, this surgical pathway is palliative in nature, and cardiac complications, including ventricular dysfunction and eventual failure, remain prominent (43, 86, 90, 91, 99, 183). Patients with SV have the highest incidence of ventricular dysfunction and HF at the time of diagnosis (81), and progressive ventricular failure is both a common cause of death and indication for heart transplantation in infants and children with SV (47, 80). In fact, increased survival of infants and children with SV translates into growing numbers of patients at risk for the development of this atypical form of HF. The failing single ventricle can become dilated and/or hypocontractile in addition to developing hypertrophy, which may partly be attributed to the large volume overload that occurs early in life, before the Fontan completion (67). Theoretically, systemic ventricular dysfunction may arise from residual anatomic obstructions, myocardial ischemia, hemodynamic consequences of different physiological states throughout surgical palliation, or genetic predispositions, as detailed above (31, 88, 162). Nevertheless, the true contribution of each of these factors remains ill-defined. For an individual SV patient, these components exert variable amounts of influence, resulting in unpredictable degrees of systolic or diastolic impairment. The addition of significant noncardiac morbidities arising from the chronic effects of the Fontan circulation, such as liver cirrhosis, hepatocellular carcinoma, protein-losing enteropathy, plastic bronchitis, and cognitive deficits, complicate the management of SV HF further (2, 30, 59, 153). Here we present a brief overview of select issues thought to impact ventricular function, with a focus primarily on systolic dysfunction of a systemic RV in SV disease (Fig. 2).
Fig. 2.
Summary figure. Potential risk factors associated with ventricular dysfunction and heart failure in patients with single ventricle (SV) physiology and known molecular adaptations in the SV failing heart. β1-AR, β1-adrenergic receptor; RAAS, renin-angiotensin-aldosterone system.
Suboptimal Ventricular Mechanics
The single ventricle is inherently predisposed to systolic and/or diastolic dysfunction (69). The geometry of a normal biventricular heart is the most efficient in terms of bioenergetics and coupling of ventricular and arterial structures. The normal systemic LV is ideally designed to generate significant torsion, which accomplishes a maximal volume change for a given amount of myocyte shortening during ventricular ejection and optimizes energetic efficiency. In contrast, these energy-conserving, torsion-related mechanisms do not occur to the same extent in the functional SV (either right or left ventricular morphology); thus, volume change during ejection is almost completely dependent on energy-consuming shortening of myocytes (54, 55, 148). Additionally, in a normally structured, biventricular heart, the right and left ventricles interact with each other to optimize cardiac function. As expected, these ventricular-ventricular interactions are suboptimal or absent in the patient with a functional single ventricle (54, 55, 148). Thus, although the hypoplastic, “secondary” ventricle is in many ways inadequate, the size of this rudimentary ventricle may provide a supporting role and influence exercise performance after Fontan (151).
In patients with SV disease of RV morphology, concerns arise regarding the ability of the RV to support the high-pressure systemic circulation. The development of the RV is controlled by a more distinct set of transcriptional pathways than those that give rise to the LV, and the morphology, physiology, and molecular adaptations of the RV are also different (12, 172). The RV, for instance, has a thinner wall and lacks the middle layer of circular fibers that normally make up a majority of the wall thickness of the LV. Moreover, it has been shown that the RV has a lower ability than the LV to adapt to hemodynamic load based on gene expression analysis comparing the two ventricles (106). Expression of genes related to adrenergic signaling, G proteins, and cytoskeletal and contractile components were lower, whereas maladaptive genes such as fibroblast growth factors, caspases, and ubiquitin were higher in the RV versus the LV. Additionally, although the RV is able to function appropriately under a wide variety of preload conditions, it is not well suited to adapt to changes in afterload (53). The RV also differs from the LV in its metabolic response to pressure loading, such that the RV has a decreased capacity to modify substrate oxidation to maintain energy balance in response to changes in workload (104, 143). These differences in RV versus LV may explain, at least in part, the varied molecular and histological response of the RV when subjected to pressure and volume overload.
Impaired Myocardial Perfusion
Abnormal myocardial perfusion is common in the setting of SV and may contribute varying amounts to ventricular dysfunction and ultimately HF. Similar to other cyanotic CHD, the myocardium of patients with SV is at risk for subendocardial ischemia due to severe systemic hypoxemia. At rest, the myocardium is relatively protected from ischemia, even in the setting of chronic hypoxemia, due to a high level of oxygen extraction (60–75%) by the myocardium from the circulation and a compensatory increase in hemoglobin to maintain oxygen carrying capacity. However, during periods of increased myocardial metabolic demand (i.e., higher heart rates related to stresses or exercise), the decreased oxygen content in severely hypoxemic patients becomes limiting and can result in ischemia. Although there is no immediate detectable impact of early ischemia, it remains to be determined whether this may preprogram the myocardium and contribute to maladaptive remodeling later in life (90). Additionally, SV often results in mismatched coronary flow versus demand, such that the typical coronary anatomy in SV is insufficient to supply an enlarged, hypertrophied RV over time (183). High wall stress from both increased afterload and decreased coronary flow reserve in SV physiology can be associated with myocardial hypoperfusion and a mismatch in supply and demand (167, 180). In particular, there are anatomic subgroups of SV patients (pulmonary atresia with intact ventricular septum and HLHS with mitral stenosis and aortic atresia) who have an increased incidence of ventricular-coronary fistulas, which are postulated to contribute to coronary steal and subsequent myocardial dysfunction (142). Furthermore, the BT shunt used in some patients for stage 1 palliation provides continuous forward flow into the pulmonary arteries, with resultant diastolic runoff and coronary steal (188). Thus, even in the absence of overt coronary artery abnormalities, tissue ischemia may still be present in SV physiology.
The SV myocardium is also subject to potential acquired damage by virtue of requiring multiple cardiopulmonary bypass runs as part of the staged palliation pathway. Additionally, the results of the Single Ventricle Reconstruction Trial demonstrated improved early outcomes (transplant-free survival to 1 yr of age) attributed to the Sano shunt over the BT shunt at the time of stage 1 palliation (188). However, the survival benefit in the Sano group was no longer significant after 1 yr of age, suggesting that any potential beneficial effects related to the Sano shunt are not sustained in the long term. Importantly, even after the Sano shunt is removed from the circulation at stage 2, there may be residual damage to the RV from the ventriculotomy, which is not present in patients who had BT shunts (188). A prior study demonstrated that by the time of Fontan completion, RV contractility was decreased in the group who underwent Sano shunt placement when compared with historical controls that received a BT shunt (189).
Hemodynamic Stressors
The hemodynamic consequences of SV physiology evolve as a patient proceeds through the various stages of surgical palliation. However, the effects of altered hemodynamics may accumulate in the years following surgery, resulting in development of early HF. Surgical repair and catheter-based interventions can ameliorate or mitigate some hemodynamic stressors, with subsequent improvement in ventricular function. Nevertheless, a number of abnormalities are inherent to SV disease and palliation, and it is unclear why some SV patients are able to tolerate these abnormalities while others progress to end-stage HF.
Increased Ventricular Volume Load
From birth until stage 2 palliation, the volume load of the single ventricle is as much as three times the normal cardiac output. The ability to tolerate this excess volume load may be influenced by ventricular morphology and inherent mechanisms of ventricular adaptation. In general, limiting the duration of increased volume load is thought to minimize pathological remodeling of the ventricle and associated adverse consequences. This volume-loaded state promotes ventricular dilatation and hypertrophy, leading to geometric distortion of the ventricle and atrioventricular valve regurgitation. A moderate amount of atrioventricular valve regurgitation, or greater, before stage 2 palliation is a risk factor for late adverse outcome (81). Although significant volume unloading of the ventricle occurs following stage 2 palliation, the degree of atrioventricular valve regurgitation does not improve in most SV patients; thus, if needed, valve repair at the time of stage 2 palliation is recommended to try to protect against development of ventricular failure (81). Atrioventricular valve failure occurs frequently in patients undergoing Fontan palliation, and those with valve failure are twice as likely to develop Fontan circulation failure than those without valve failure (110). The literature also supports attempting valve repair at the time of Fontan to try to prevent decline in ventricular function (135).
An additional source of ventricular volume load includes the formation of aortopulmonary collaterals, which are frequently observed in SV patients following stage 2 and stage 3 palliation (192). Theoretically, aortopulmonary collateral vessels may place an additional hemodynamic burden on the single ventricle due to significant left-to-right shunting, similar to high output HF of the structurally normal heart associated with arteriovenous malformations or anemia. Although increased end-diastolic ventricular volume has been shown in SV patients with high aortopulmonary collateral flow, function of the single ventricle was preserved (119), Although the precise role aortopulmonary collateral burden plays in the development of Fontan failure remains unclear, there does seem to be an association between aortopulmonary collateral burden and Fontan failure with preserved ejection fraction (177).
Lastly, as previously described, the incorporation of a Fontan fenestration has contributed to improved postoperative outcomes at the time of the Fontan procedure. Nevertheless, this communication between the systemic venous system and the right atrium can result in an additional volume load in the form of a right-to-left shunt. This right-to-left shunt may cause lower systemic oxygen saturations and cyanosis, with compensatory alterations in red blood cell indices and possible abnormalities in coagulation. The degree of volume load through the Fontan fenestration is variable based on the size of the fenestration and a patient’s hemodynamics, and closure of the Fontan fenestration may be considered in the years following the Fontan operation, although there is significant interinstitutional variability in this practice. Closure of the Fontan fenestration when hemodynamics are favorable is primarily performed to minimize stroke risk and cyanosis, as well as decrease ventricular volume load.
Systemic Outflow Obstruction and Elevated Systemic Vascular Resistance
Systemic ventricular outflow tract obstruction may be evident early on or may progress later after palliative surgeries are complete. In particular, aortic arch hypoplasia is a prominent feature in HLHS, which requires surgical augmentation at the time of stage 1 palliation. Residual or recurrent aortic arch obstruction is likely to exert an intolerable pressure load on the single RV, which is already limited in its ability to manage increased afterload, as discussed above. Systemic outflow obstruction often results in significant ventricular hypertrophy, which is an independent risk factor for death (112). Lastly, ongoing interactions between cardiac output and systemic vascular resistance have lifelong implications for SV patients. A recent study demonstrated that following stage 1 palliation, high systemic vascular resistance states were unstable, and these patients were at risk for developing low cardiac output (88). Elevated systemic vascular resistance is also a feature of Fontan failure with reduced or preserved ejection fraction, perhaps related to chronic low cardiac output (17).
Impairments in Systemic Ventricular Diastolic Function
Even in cases where ventricular systolic function is relatively well-preserved after the Fontan procedure, diastolic dysfunction of the systemic ventricle has been reported early after Fontan (1, 62, 149) and found to persist even in the decade following the Fontan (36). This has been classified as a distinct clinical phenotype (type II Fontan failure with preserved ejection fraction) with symptoms primarily resulting from pulmonary venous congestion, with secondary elevation of Fontan pressures and hepatic congestion. Hemodynamic assessment of these patients demonstrates normal or low cardiac output, elevated ventricular end-diastolic pressure, and elevated systemic vascular resistance (17). Development of restrictive physiology (end-diastolic pressure-volume relationship measured on the explanted heart and compared with an unused adult cardiac allograft) has been reported as a major contributing factor to Fontan failure requiring heart transplantation (197). The etiology of decreased ventricular compliance is hypothesized to be secondary to longstanding low preload of the systemic ventricle (36, 197), which is similar to what has been described in the chronically underfilled left ventricle in the setting of mitral stenosis in a biventricular heart (121). Notably, histological evaluation of the explanted myocardium in the aforementioned case report did not demonstrate signs of myocyte hypertrophy or fibrosis (197). Decreased ventricular compliance may contribute to a progressive increase in pulmonary venous pressure, leading to reductions in forward blood flow through the lungs and resultant low cardiac output. This may be one of the mechanisms underlying the functional deterioration late after the Fontan procedure (36).
Elevation in Pulmonary Vascular Resistance
After the Fontan procedure, blood flow through the lungs is determined by the transpulmonary gradient (difference between the central venous pressure and pulmonary venous pressure) and pulmonary vascular resistance (PVR). PVR is defined as the resistance that blood must overcome to pass through the pulmonary vasculature, and the PVR index normalizes the patients PVR to their body surface area to account for the effect of body size on blood flow (11). Not surprisingly, increased PVR index leads to Fontan failure, which was noted by Fontan in his initial description of the operation (57). Mortality is increased in patients with a mean pre-Fontan pulmonary artery pressure >15 mmHg and a PVR index >2 Wood units/m2. Additionally, because decreased pulmonary blood flow results in decreased preload for the systemic ventricle, PVR strongly influences cardiac output at rest and with exercise (174). Increased pulmonary blood flow, increased pulmonary arterial pressure, and nonpulsatile pulmonary flow can all contribute to adverse pulmonary vascular remodeling and elevated PVR (163). Importantly, when coupled with low cardiac output, even mild elevations in PVR are associated with a high risk of Fontan failure (49). Nevertheless, the true PVR can be challenging to measure in SV patients for multiple reasons, one of which is the presence of aortopulmonary collateral vessels that form commonly in SV patients after superior cavopulmonary connection (70, 71, 205).
MOLECULAR ADAPTATIONS TO HEART FAILURE
Long-term survival and quality of life ultimately depend on preservation of systemic ventricular function in the SV population. However, from a molecular standpoint, it is not well understood how the SV myocardium adapts to the chronic altered hemodynamic conditions of palliated SV physiology. At the molecular level, HF is characterized by adaptive and compensatory alterations in transcriptional, translational, and epigenetic modifications in response to changes in functional myocardial demands. Persistence of SV dysfunction may reflect irreversible pathological remodeling that is presumably a precursor to eventual HF (108). Although extensive research efforts have aided in the identification of specific molecular mechanisms involved in adult biventricular HF, significantly less is known with regard to gene and protein expression changes in the pediatric SV population. A better understanding of the molecular pathways associated with SV adaptation and remodeling are necessary to gain insight into this unique physiological circumstance and would provide a rationale for disease-specific, efficacious pharmacological therapies. Below, we discuss the present state of knowledge regarding molecular alterations in SV HF.
Contributions of Fibrosis to SV Heart Failure
Fibrosis is defined as the excessive deposition of extracellular matrix components, resulting in tissue scarring and ultimately organ dysfunction. In the heart, fibrosis may be reparative (e.g., replacing areas of myocyte loss) or reactive (e.g., in response to chronic stress) (126, 191). Reactive fibrosis by histopathological evaluation and concomitant activation of the renin-angiotensin-aldosterone system (RAAS) is common in adults with end-stage HF, and the severity of cardiac fibrosis is independently associated with cardiovascular mortality (22, 78). However, the presence and role of myocardial fibrosis in the SV population is less clear. In young (10 yr of age or less) SV hearts, neither histological fibrosis nor a profibrotic gene expression profile was associated with HF (140). In contrast, quantification of fibrosis by cardiac MRI in adult congenital heart disease patients demonstrates increased fibrosis when compared with normal adult controls (25). These data suggest that SV HF can occur in the absence of fibrosis, particularly in younger children, whereas fibrotic contributions to SV HF are likely greater in adults.
Differential Gene Expression in SV Heart Disease
Previous studies have implicated both overlapping and unique gene expression changes associated with SV HF as compared with other HF etiologies. One common characteristic of the failing myocardium is recapitulation of the fetal gene program, where there is upregulation of a number of fetal genes [β-myosin heavy chain (β-MHC), brain natriuretic peptide (BNP), and atrial natriuretic factor (ANF)] coupled with downregulation of genes encoding proteins related to contractile function [α-myosin heavy chain (α-MHC), sarcoplasmic reticulum calcium-adenosine triphosphatase 2a (SERCA), and β1-adrenergic receptor (β1-AR)] (147, 178, 179, 184, 202). Pathological remodeling in SV myocardium is typified by increased expression of ANF and β-MHC and decreased expression of β1-AR, α-MHC, and SERCA (14, 132).
Cyclic nucleotide signaling plays a central role in the translation of primary signals to a cellular response. The three primary protein classes involved in cyclic nucleotide signaling include: 1) cyclases, which catalyze the formation of cyclic nucleotides, 2) phosphodiesterases (PDEs) that hydrolyze cyclic nucleotides, and 3) cyclic nucleotide receptor proteins. PDE enzymes regulate the intensity, duration, and compartmentalization of intracellular cyclic nucleotide signaling via hydrolysis of the potent second messenger molecules cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). At the level of the cardiomyocyte, modulation of cAMP and cGMP is critical for the regulation of a number of functions, including pacemaker function, contractility, cell growth, cell survival, and calcium handling. Therefore, alterations in cyclic nucleotide-mediated signaling via differential PDE or adenylyl cyclase (AC) expression, localization, and activity have been implicated in the progression of cardiovascular pathology. Global cAMP levels are decreased in the single right ventricular myocardium; however, phospholamban (PLN) phosphorylation appears preserved (139). Additionally, gene expression of a variety of AC and PDE isoforms is affected by both age and disease (141). Significantly higher AC5 and lower AC7 and upregulation of PDE isoforms PDE1C1 and PDE3B were seen in pediatric single right ventricular myocardium compared with the normal pediatric RV. Upregulation of PDE5A was also seen in single right ventricular myocardium, similar to what is seen in other HF etiologies. Correspondingly, PDE5 protein expression and activity are increased in the failing single right ventricular myocardium relative to the normal pediatric RV (64). Thus, modulation of intracellular cyclic nucleotides may represent a promising therapeutic target to support the failing SV.
Exon array analysis of infant, SV myocardium obtained at the time of the Norwood procedure demonstrated differentially expressed genes and alternative spliced transcripts relative to normal RV and LV controls (donor hearts) (161). Pathway analysis of these differentially expressed genes suggests changes in cell metabolism, amino acid degradation, cytoskeleton organization, and cell adherence in SV patients. A follow-up study identified a specific RNA binding protein, Rbfox2, as a major contributor to the transcriptome changes seen in infant single RV myocardium (Norwood samples). The subcellular localization of Rbfox2 is altered in pediatric SV patients, which adversely affects its role in RNA metabolism and likely contributes to the early transcriptome changes seen in these patients (198). Analysis of a similarly young SV patient cohort revealed differential gene expression related to chromatin remodeling (HDAC2, SET, and MYND domain containing 1), cell cycle regulation (CDK4 and p18), and transcriptional regulation (Foxp1) in SV patients compared with normal controls (donor hearts) (63).
Hypertrophy in SV Heart Disease
Cardiac hypertrophy is an initial, adaptive response to hemodynamic stressors; however, over time, hypertrophy becomes maladaptive and is associated with an increased risk of HF and arrhythmias in adults (60, 136). At the cellular level, cardiomyocyte hypertrophy is characterized by an increase in cell size, protein synthesis, and sarcomere disorganization. Whereas reinduction of the fetal gene program is present in the SV myocardium (14, 132), cardiomyocyte size in failing SV hearts has not been evaluated.
Epigenetic Modifications and Noncoding RNAs in SV Heart Disease
The discovery of epigenetic modifications and their functional relevance has revolutionized our understanding of the complexities associated with gene regulation. Epigenetic regulatory mechanisms play a central role in regulating a diverse range of biological and disease processes. microRNAs (miRNAs, miRs), for example, are small noncoding RNAs that can modulate gene expression via degradation or translational repression of target mRNAs, and it is estimated that more than 60% of the human genome is subject to their regulation (61). miRNAs can be present in cells or in the circulation, and recent studies have investigated the diagnostic and prognostic value of circulating miRNA levels in the CHD population (reviewed in 185). One such study showed an inverse correlation between the levels of miR-129-5p in circulating microvesicles of patients with SV physiology and the likelihood these patients will eventually develop heart failure (155). Another study demonstrated that differential expression of four miRNAs (miRNAs-19b, miR-22, miR29c, and miR-375) in the serum of pregnant women were highly predictive of the presence of fetal CHD (214). Moreover, specific MyomiRs (miR-499, miR208a, and miR-208b), named for their colocalization with muscle-specific genes, were highly upregulated in pediatric patients following open heart surgery, and their expression was associated with delayed recovery and known markers of cardiac injury (16). Another study demonstrated unique miRNA profiles in RV myocardium from SV patients relative to the normal control RV. Expressions of a number of miRNAs are correlated with stage of surgical palliation, suggesting volume load may modulate specific miRNA expression (187). Lastly, differential expressions of several miRNAs are associated with SV heart disease in general, independent of surgical stage, and the mRNA targets of these miRNAs are related to proper heart development and regulation of transcription.
Histone deacetylases (HDACs) are a class of epigenetic enzymes that remove acetyl groups from lysine residues of both histone and nonhistone proteins and have known roles in the modulation of gene expression and posttranslational modifications (173). Myocardial HDAC adaptations have been shown to occur in the SV heart. HDAC activity and expression is altered in the single right ventricular myocardium relative to the normal RV such that there is significantly increased activity of class I, IIa, and IIb HDACs. Correspondingly, the increased HDAC catalytic activity seen in class I and IIa HDACs correlates with increased levels of HDAC isoform protein expression, including significantly increased class I HDACs-1, -2, and -3 and class IIa HDAC5 (13). Whether HDAC inhibition represents a potential therapeutic target in SV patients with HF requires further investigation.
β-Adrenergic and Renin-Angiotensin-Aldosterone Systems in SV Heart Disease
The β-adrenergic system is an integral part of cardiac signaling, and β-receptor subtypes regulate cardiotoxicity and cardioprotection via crosstalk with other signaling pathways, alterations in intracellular calcium, and regulation of cell death. In a paradigm-shifting study, Bristow et al. (24) demonstrated that β1-receptors were downregulated by 60% in adult failing human hearts. It is now well established that chronic activation of β1-AR signaling and subsequent downregulation of β1-ARs is a major contributor to the development of adult HF. Similar radioligand binding assays demonstrated a significant decrease in β1-AR and preservation of β2-AR density in SV RV myocardium (132). Correspondingly, increased calcium/calmodulin-dependent protein kinase II (CaMKII) activity, a downstream effector of β1-AR stimulation, is also seen in SV RV myocardium (132).
The renin-angiotensin-aldosterone system (RAAS) also plays a significant role in the pathophysiology of HF. The RAAS system is an important mediator of the ventricular response to hemodynamic load, and it has been shown that persistent upregulation of RAAS and concomitant increased angiotensin II is detrimental in the setting of HF (109, 190). Previous studies have demonstrated increased renin and aldosterone consistent with RAAS activation in CHD patients, including those with SV physiology (15, 170). Additionally, pharmacogenomic trials investigating the effect of polymorphisms in RAAS-related genes determined that genotypes associated with RAAS upregulation negatively influence ventricular remodeling in patients with a systemic RV (26, 130).
HEART FAILURE THERAPIES
Although the significant amount of research conducted regarding the management of HF in adults has contributed to meaningful changes in the outcomes of adult HF patients, there are considerably fewer studies regarding HF in children. The limited number of pediatric studies that have been performed are often criticized for their small numbers, retrospective nature, and inconsistent outcome measures. Study design in pediatric HF has proved to be challenging, in part because of the comparatively small number of pediatric HF patients. Utilization of traditional clinical trial end points (e.g., death or hospitalization) leads to underpowered studies in this population. Additionally, given the phenotype of HF with preserved ejection fraction, ejection fraction may not be an appropriate end point. Tailored pediatric HF study design represents an area of potential innovation, with a need for identification and validation of relevant surrogate end points, application of novel statistical methods, and increased utilization of comparative effectiveness research, which may facilitate meaningful and significant results from pediatric HF studies (138). The current management of HF in children (including those with SV HF) is primarily based on expert consensus and the extrapolation of adult HF therapies (111). Unfortunately, well-established therapies for adult LV failure frequently fail to show significant benefit for SV failure, or studies have yielded conflicting results. Given the unique anatomic considerations and physiological adaptations of SV and age-related mechanisms influencing HF, perhaps it is not surprising that there are currently no proven medical therapies for the treatment or prevention of HF in the SV population. Here we present a brief overview of current therapeutic interventions empirically applied to the SV HF population, with a focus on their primary intended use.
Blocking Pathological Remodeling and Preventing Ventricular Dysfunction
Angiotensin-converting enzyme inhibitor (ACEi) therapy is commonly used in the SV population to decrease the afterload on the systemic ventricle. Additionally, given that early use of ACEi therapy in high-risk adult patients has been shown to significantly reduce the incidence of HF or death (113, 181), ACEi is also empirically used in SV patients in the hopes of similarly delaying pathological ventricular remodeling through RAAS blockade. However, although use of ACEi therapy in SV patients is common, the efficacy of ACEi use with regard to ventricular function and remodeling in SV is unclear (reviewed in 206). In infants with SV, administration of the ACEi enalapril did not demonstrate any benefit in terms of somatic growth, improvement of ventricular function, or prevention of HF in an unselected SV population without HF (92). Correspondingly, unfavorable cardiac remodeling and renal function were seen in SV patients with RAAS-upregulation genotypes, as was impaired somatic growth, particularly with ACEi administration (130). Similarly, in a single-center prospective study in post-Fontan adults, RAAS-upregulated genotypes were associated with diastolic dysfunction and higher serum brain natriuretic peptide (BNP) levels but were not associated with increased ventricular mass or impaired systolic function (26). However, a small single-center retrospective study demonstrated a significant reduction in end-diastolic, pulmonary, and atrial pressures following short-term treatment with ACEi in patients with SV physiology, suggesting short-term benefit while awaiting Fontan completion (210). Thus, although RAAS upregulation has been implicated in SV physiology, studies have not supported the prophylactic use of ACEi to prevent or delay pathological ventricular remodeling.
Support of the Failing SV
Support of the SV heart with systolic dysfunction.
Based on extrapolation from other etiologies of HF (32, 133) and evidence of RAAS activation in SV, ACEi may provide theoretical benefit in SV patients with systolic dysfunction. However, although the aforementioned Pediatric Heart Network study was underpowered to demonstrate the effect of enalapril on SV patients with ventricular dysfunction, no difference was seen in the small proportion of patients who died or underwent cardiac transplantation secondary to HF in this cohort (92). Additionally, a separate multicenter, double-blind, randomized controlled trial of longer-term valsartan (an ARB) administration in adult SV patients with single RV dysfunction found no benefit of 3-yr valsartan therapy on ejection fraction, maximum exercise capacity, quality of life, neurohormonal activation, or clinical outcomes (196). However, subgroup analysis suggested a significant difference in valsartan versus placebo in symptomatic HF patients such that RV ejection fraction remained unchanged in the valsartan group, whereas there was a significant decline in the placebo group after 3-yr follow up. Therefore, the use of ACEi or ARB therapy may be confounded by age, ventricular morphology, presence of ventricular dysfunction, duration of disease, and symptom presentation. In addition to targeting RAAS activation with an ARB, augmentation of natriuretic peptides via neprilysin inhibition (angiotensin receptor neprilysin inhibitor, ARNi) has shown promise in decreasing NH2-terminal pro-B-type natriuretic peptide in pediatric patients with biventricular, systemic LV systolic dysfunction and was recently approved by the Food and Drug Administration for this indication. Nevertheless, the use of ARNi in the single RV population has not yet been studied.
β-Adrenergic receptor antagonist (β-blocker) therapies such as carvedilol and metoprolol have long been associated with dose-related improvements in LV function, reverse-remodeling, and reductions in mortality in adult patients, particularly those with mild to severe systolic dysfunction. In fact, β-blockade therapies are an integral part of the standard care for adult HF patients (23). However, the efficacy of β-blockers in SV HF has not been proven. Specifically, a double-blind placebo controlled clinical trial on the use of carvedilol in children with systolic HF demonstrated no significant benefit of carvedilol for patients with SV, with a trend toward worse outcomes with carvedilol in patients with a systemic RV (175). In contrast, there are several small retrospective studies and case reports describing beneficial effects of β-blocker therapy for failure of the systemic RV (46, 95, 102, 120). Thus, the use of β-blocker therapy in SV patients is likely impacted by ventricular morphology, duration of disease, and presence of HF. Therefore, although it is reasonable to consider the use of adult-based therapies (e.g., ACEi, ARB, or β -blockers) for the treatment of SV HF, it is important to note that there is very little evidence, and no controlled clinical trials, to support their use. This represents a substantial knowledge gap in the field and underscores the importance of identifying therapeutic targets specific to the failing SV.
PDE3, a cAMP-specific phosphodiesterase, is a known regulator of myocardial contractile function via cAMP and its regulation of PLN and SERCA activity. Therefore, PDE3 inhibition (PDE3i) is thought to have beneficial myocardial effects in the setting of systolic HF. In adult HF, short-term PDE3i inotropic therapy is variably efficacious and chronic PDE3i therapy is associated with arrhythmias and increased mortality (146, 186). Conversely, the PDE3i milrinone has been successfully used in pediatric SV HF as a bridge to transplant. In fact, milrinone therapy improves HF symptoms in SV patients such that they have a reduced frequency of HF-related emergency room visits and hospital admissions (11, 152). This differential response in therapy may be attributed to differences in cAMP compartmentalization and preservation of PLN activity seen in the SV myocardium (139). Therefore, although SV HF patients display suboptimal responses to many traditional HF medications, the increased efficacy and safety of chronic PDE3i use in SV HF again implies that both age and disease etiology may influence response to HF therapy.
The use of ventricular assist devices (VADs) have become an effective intervention for increasing survival of adult and pediatric patients with end-stage systolic LV HF. However, their application in SV HF introduces unique physiological and anatomic challenges. For example, use of VAD support as a bridge to heart transplant in older children and adolescents without CHD has been relatively successful, but options for VAD support in the infant and young child are more limited. The typical small size of pediatric SV patients and the often hypoplastic aortic arch impose severe anatomic obstacles given the size of the cannulas and VAD itself. Given the relatively limited demand for pediatric VAD support, the National Institutes of Health has had to incentivize development of novel circulatory support devices for pediatric patients with medically refractory HF (10). Additionally, the use of VADs in these patients is challenging not only due to issues related to size, but also to SV physiology itself. For example, although the pediatric VAD has been used to provide a bridge to transplant for children with SV, it is overall less successful for SV children than for biventricular patients (203). Moreover, given the unique circulation at each stage of SV palliation, VAD support is most successful in SV patients with a Fontan circulation and end-stage systolic HF compared with those SV patients with Glenn physiology, diastolic heart failure, or pulmonary vascular complications (35, 114, 168). Likewise, a small study suggested VAD use as a bridge to transplant or recovery is more successful in SV patients with superior or total cavopulmonary connections as opposed to those with shunted sources of pulmonary blood flow (203). Additionally, although a number of case reports exist regarding the efficacy of VAD use in the SV population, mixed clinical results suggest patient selection, timing, particular VAD type, and center experience are important considerations (reviewed in 33). However, it is important to note that experience with VADs to support the SV circulation is rapidly evolving, and continued innovations in this area will continue to improve the ability to support these patients.
Support of the SV heart with high pulmonary vascular resistance and/or diastolic dysfunction.
Patients with Fontan physiology are specifically at risk for ventricular failure, and although the pathophysiology of the failing Fontan is not completely understood, maintaining a low pulmonary vascular resistance is important for the optimal function of this circulation. Increased systemic vascular resistance, diastolic ventricular failure, and decreased exercise capacity are a growing problem in the post-Fontan SV population. Therefore, therapeutics aimed at reducing pulmonary artery pressures (pulmonary vasodilator therapies), such as endothelial-receptor antagonists, prostanoids, and phosphodiesterase type 5 inhibitors, are often used in this population of SV patients.
PDE5 is a cGMP-specific PDE with prominent expression throughout the pulmonary vasculature and in the coronary vessels. With regard to the cardiovascular system, myocardial and cardiomyocyte-specific PDE5 expression is increased under conditions of cardiac stress, including in the hypertrophied RV, and has been shown to be associated with maladaptive myocardial responses (64, 134, 141, 213). PDE5i therapy is often successfully used to treat pulmonary hypertension in adult and pediatric patients (reviewed in 194). The use of PDE5i therapy in the adult HF population, however, is confounded by inconsistencies in efficacy and lack of completion of large clinical trials (158, 200). Selective and competitive PDE5i therapy such as sildenafil and tadalafil are increasingly used in children and adults with SV physiology, with the intention of lowering pulmonary vascular resistance, increasing pulmonary venous return to the heart, and subsequently improving cardiac output (18). In fact, sildenafil has been associated with improved hemodynamics, exercise tolerance, and single right ventricular function by echocardiography in a small series of SV patients (29, 38, 73–76, 83, 97, 193). Additionally, in a recent Phase 3 clinical trial in Fontan patients, the PDE5i udenafil was associated with improvements in multiple measures of submaximal exercise performance at the ventilatory anaerobic threshold (77).
Circulating concentrations of the vasoconstrictor peptide endothelin-1 are increased in SV patients with Fontan physiology (208). Therefore, use of endothelin receptor antagonists is increasingly common in this population. Several small studies have suggested improved exercise capacity and cardiac performance in response to endothelin receptor antagonism (20, 44, 45). A larger randomized, placebo-controlled, double-blind study (TEMPO) was conducted in adolescents and adults after Fontan operation (82). This trial found that the endothelin receptor antagonist bosentan improved exercise capacity, exercise time, and functional class in Fontan patients, without serious adverse events or hepatotoxicity. Nevertheless, additional studies are required to assess the longer-term effects with regard to the potential hepatotoxicity of bosentan in the setting of Fontan-associated liver disease.
Although the use of prostanoids is relatively rare, two small studies evaluated the use of inhaled prostanoid therapy in Fontan patients. Inhaled iloprost was shown to improve exercise capacity, V̇o2max, and cardiac output in a small number of Fontan patients (93, 160).
Although there may be potential for the use of ACEi therapy in SV patients with diastolic dysfunction, the paucity of robust studies makes drawing major conclusions difficult. Given that ACEi improves exercise capacity in adult HF patients via decreased systemic vascular resistance and improved ventricular diastolic function, Kouatli et al. (117) assessed the effects of enalapril in post-Fontan adult SV patients. However, enalapril administration did not alter systemic vascular resistance, resting cardiac index, diastolic function, or exercise capacity in this cohort (117).
Heart Transplantation
Although palliative surgeries have dramatically improved survival of SV patients, SV failure is an increasing occurrence, and heart transplantation is often the only hope for long-term survival in this population. However, due to heterogeneous anatomy and surgical histories, high immunological exposure, and unique physiology, transplantation of SV patients poses unique technical difficulties (107). Although surgical palliation is the most common pathway for SV infants, heart transplantation without prior cardiac surgery (primary heart transplant) in infants with SV physiology was associated with better graft survival and lower probability of acute rejection (7). Nevertheless, a contemporary multicenter study determined that survival of cardiac transplantation in the SV population was comparable to that of adult HF recipient outcomes (19). However, CHD patients in general wait longer than non-CHD patients and carry a higher wait list mortality rate (105). Notably, patients with SV physiology were less likely to be listed for heart transplant due to increased risk for operative mortality (e.g., cirrhosis and synthetic liver dysfunction and irreversible pulmonary hypertension) (19). Moreover, given the complexities associated with SV physiology, cardiac transplantation is best performed at centers with substantial experience in transplantation of individuals with complex cardiac abnormalities. Although survival after cardiac transplantation of SV patients has improved, greater ability to determine risk, ideal timing, and role of transplantation in the lifelong management of SV are necessary.
AVENUES FOR IMPROVING OUTCOMES
Personalized Medicine for SV Patients
Biomarkers of heart failure progression and risk stratification.
Even though ventricular function is abnormal in the majority of SV patients, and all are subject to common risk factors for ventricular dysfunction to varying degrees, it remains a challenge to predict which patients will develop clinically significant HF. The use of standard HF prognostic tools may or may not apply to the SV population. BNP and NH2-terminal pro-brain natriuretic peptide (NT-proBNP) are well-established markers for heart failure in structurally normal hearts. A systematic review compiled data from 16 studies on BNP in patients with SV and concluded that BNP levels are elevated before completion of the Fontan or when patients have symptomatic HF. Interestingly, BNP levels in asymptomatic patients after the Fontan were comparable to healthy, age-matched controls; thus, sequential BNP measurements for each individual patient could represent a clinical marker of volume status and HF in SV (50). Nevertheless, not all SV patients with symptomatic HF have elevated BNP or NT-proBNP levels. Thus, although BNP and NT-proBNP can be useful tools to follow HF progression in the SV population, normal levels do not exclude clinically significant HF.
Additionally, the ability to accurately risk stratify SV patients would provide clinicians with the ability to customize therapies for an individual patient, potentially increasing the efficacy of therapies based on patient selection. Incorporation of circulating molecular biomarkers into SV risk stratification before proceeding toward surgical palliation may optimize the identification of patients at risk for poor outcomes with traditional surgical palliation, who may be candidates for other novel therapies. Furthermore, although complex, delineation of genetic contributors to the development of cardiac dysfunction in SV may assist with tailored management strategies (86).
Pharmacogenomics.
Pharmacogenomics, the combination of pharmacology and genomics, is defined as the association between genetic factors and response to therapy. The concept that an individual’s genetic makeup can impact their response, or lack thereof, to medications may be applied to the SV population to contribute to improved outcomes. Although a number of studies have already been conducted in the adult HF population (reviewed in 164), the nature of pediatric SV heart disease presents unique challenges. For example, the incomplete understanding of underlying genetic causes, the heterogeneity in ventricular dysfunction and HF progression, relatively small numbers of SV patients, and lack of established treatment and dosing guidelines make it difficult to conduct pharmacogenomics studies in the pediatric SV population. Ultimately, utilization of nontraditional clinical and translational research methodologies [such as application of Bayesian statistical methods (79) in clinical trial design or greater utilization of comparative effectiveness research] may assist in overcoming some of these challenges.
Stem cell therapies.
Stem cell-based therapies to restore ventricular function have been the subject of numerous recent investigations. Although it is thought that delivery of stem cells may elicit important effects to benefit myocardial function, evidence to date is small and inconsistent (reviewed in 125) even in the adult HF population. A phase 1 clinical trial in the pediatric SV population demonstrated that delivery of autologous stem cells directly into the RV myocardium during planned palliative surgery was safe and feasible; however, it was underpowered to assess efficacy (28). A follow-up phase 2 study then found that intracoronary infusion of cardiosphere-derived cells after staged palliation improved ventricular function and global strain (96). Other small studies and case reports have indicated the potential for stem cell-based therapies in SV patients, and the beneficial effects appear to be greater in children than in adults (165, 166). Two stem cell-based clinical trials are currently underway in the pediatric SV population, which will hopefully provide more information regarding the safety and efficacy of these stem cell-based interventions.
Novel models of SV heart disease.
Although animal models have been fundamental in providing a better understanding of disease progression, genotype-phenotype correlations, and therapeutic efficacy, the lack of a postnatal animal model of SV heart disease has hindered our ability to investigate the specific mechanisms involved in SV HF and the effects of pharmacological interventions. However, novel models of SV heart disease, together with modern research technology, will likely be key in providing a better understanding of the mechanisms associated with progression of ventricular dysfunction in this population.
Human induced pluripotent stem cell models.
The discovery and use of human induced pluripotent stem cells (iPSCs) has transformed translational research. iPSC-derived cardiomyocytes have emerged as a novel and robust platform to model specific cardiovascular disease processes and advance the science and clinical care in a wide range of diseases. A number of recent studies have demonstrated the use of patient-specific iPSCs to identify in vitro expression and functional correlates of SV. For example, SV patient iPSCs have a reduced ability to differentiate into mature cardiomyocytes and an enhanced ability to differentiate into smooth muscle cells. Additionally, once differentiated into cardiomyocytes, these cells are characterized by a lower beating rate, disorganized sarcomeres and sarcoplasmic reticulum, persistence of a fetal gene expression, alterations in calcium transient patterns, electrophysiological responses to β-AR antagonists, and alterations in genes involved in NOTCH signaling (101, 115, 209). Therefore, iPSC technology has the potential to better dissect the complex transcriptional and epigenetic mechanisms associated with the pathogenesis of SV physiology.
Systemic circulating factors.
Circulating factors present in patient serum or plasma can be important paracrine modulators of cardiac gene expression (100, 118). Correspondingly, in vitro treatment of primary cardiomyocytes with SV patient sera induces gene expression changes indicative of pathological myocardial remodeling (e.g., recapitulation of the fetal gene program), cardiac hypertrophy, and cardiac dysfunction. Importantly, confirmatory gene expression analysis in human SV RV myocardium revealed that gene expression changes induced by SV serum in primary cardiomyocytes are similar to those seen in human SV myocardium (64). These data suggest that this model may be a useful platform to enhance the mechanistic understanding of SV HF and aid in the identification of potential drug targets relevant to myocardial remodeling in SV.
Murine models of SV disease.
The complex anatomy and physiology of SV heart disease has hindered the development of animal models of this disease, which in turn has hampered progress in the development of new therapeutics and a more thorough understanding of the mechanisms associated with SV failure. Murine models of RV hypertrophy and chronic RV volume overload have increased our understanding of RV-specific adaptions (13, 156, 157, 195). However, to date there is no postnatal animal model of SV physiology. Nevertheless, a recent study using a mouse forward genetic screen approach was the first to isolate mutant mice with a hypoplastic LV and advance our understanding of the complex genetics associated with SV physiology (122). In this intricate study, Liu et al. (122) used mouse chemical mutagenesis coupled with ultrasound phenotyping of 100,000 fetal mice to identify a wide spectrum of CHD mutants, including recovery of 8 lines with a systemic RV. RNA-seq analysis of the hypoplastic LV from these mice found changes in gene expression related to metabolism and mitochondrial pathways, calcium signaling, cardiac muscle contraction, and dilated/hypertrophic cardiomyopathy, similar to what has been shown in human SV myocardium-based analysis. Studies such as this one will be important for improving understanding of the complex gene-to-phenotype associations and molecular mechanisms and evaluating novel therapeutics in SV heart disease.
CONCLUSIONS
SV heart disease requires unique consideration, given the complexity of interactions between numerous genetic, molecular, and pathophysiological processes. The relative heterogeneity in HF phenotypes, underlying molecular mechanisms, interactions with other comorbidities, and the genetic etiology of SV present unique challenges with regard to the management of this population. Building on the remarkable medical and surgical advances accomplished in the clinical care of SV patients in the past several decades, further improvement of clinical outcomes in the SV population is dependent on refining understanding of factors contributing to ventricular dysfunction and HF in this vulnerable population.
GRANTS
This work was supported by the National Institutes of Health (NIH) Grants K08-HL130592-01A1 (to S. J. Nakano), K12-HD-068372 (to S. J. Nakano), R01-HL-126928-03S1 (to A. M. Garcia), UL1-TR-001082 (to A. M. Garcia), and KL2-TR-002534 (to A. M. Garcia) and the Colorado Clinical and Translational Sciences Institute (supported in part by Colorado Clinical and Translational Science Award) Grants UL1-TR-002535, KL2-TR002534, and TL1-TR-002533 from NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.N. conceived and designed research; S.J.N. analyzed data; A.M.G. interpreted results of experiments; A.M.G., J.-T.B., and S.J.N. prepared figures; A.M.G. and S.J.N. drafted manuscript; A.M.G. and S.J.N. edited and revised manuscript; A.M.G., J.-T.B., and S.J.N. approved final version of manuscript.
REFERENCES
- 1.Akagi T, Benson LN, Gilday DL, Ash J, Green M, Williams WG, Freedom RM. Influence of ventricular morphology on diastolic filling performance in double-inlet ventricle after the Fontan procedure. J Am Coll Cardiol 22: 1948–1952, 1993. doi: 10.1016/0735-1097(93)90784-X. [DOI] [PubMed] [Google Scholar]
- 2.Alsaied T, Bokma JP, Engel ME, Kuijpers JM, Hanke SP, Zuhlke L, Zhang B, Veldtman GR. Factors associated with long-term mortality after Fontan procedures: a systematic review. Heart 103: 104–110, 2017. doi: 10.1136/heartjnl-2016-310108. [DOI] [PubMed] [Google Scholar]
- 3.Alsoufi B, Gillespie S, Kim D, Shashidharan S, Kanter K, Maher K, Kogon B. Impact of dominant ventricle morphology on palliation outcomes of single ventricle anomalies Ann Thorac Surg 102: 593–601, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Alsoufi B, Manlhiot C, Awan A, Alfadley F, Al-Ahmadi M, Al-Wadei A, McCrindle BW, Al-Halees Z. Current outcomes of the Glenn bidirectional cavopulmonary connection for single ventricle palliation. Eur J Cardiothorac Surg 42: 42–49, 2012. doi: 10.1093/ejcts/ezr280. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ 3rd; Pediatric Heart Network Investigators . Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol 52: 85–98, 2008. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci USA 101: 6975–6980, 2004. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auerbach SR, Smith JK, Gralla J, Mitchell MB, Campbell DN, Jaggers J, Pietra BA, Miyamoto SD. Graft survival is better without prior surgery in cardiac transplantation for functionally univentricular hearts. J Heart Lung Transplant 31: 987–995, 2012. doi: 10.1016/j.healun.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azakie T, Merklinger SL, McCrindle BW, Van Arsdell GS, Lee K-J, Benson LN, Coles JG, Williams WG. Evolving strategies and improving outcomes of the modified norwood procedure: a 10-year single-institution experience. Ann Thorac Surg 72: 1349–1353, 2001. doi: 10.1016/S0003-4975(01)02795-3. [DOI] [PubMed] [Google Scholar]
- 9.Backer CL. The functionally univentricular heart: which is better—right or left ventricle? J Am Coll Cardiol 59: 1186–1187, 2012. doi: 10.1016/j.jacc.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin JT, Borovetz HS, Duncan BW, Gartner MJ, Jarvik RK, Weiss WJ. The national heart, lung, and blood institute pediatric circulatory support program: a summary of the 5-year experience. Circulation 123: 1233–1240, 2011. doi: 10.1161/CIRCULATIONAHA.110.978023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg AM, Snell L, Mahle WT. Home inotropic therapy in children. J Heart Lung Transplant 26: 453–457, 2007. doi: 10.1016/j.healun.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol 18: 67–76, 2007. doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blakeslee WW, Demos-Davies KM, Lemon DD, Lutter KM, Cavasin MA, Payne S, Nunley K, Long CS, McKinsey TA, Miyamoto SD. Histone deacetylase adaptation in single ventricle heart disease and a young animal model of right ventricular hypertrophy. Pediatr Res 82: 642–649, 2017. doi: 10.1038/pr.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohlmeyer TJ, Helmke S, Ge S, Lynch J, Brodsky G, Sederberg JH 2nd, Robertson AD, Minobe W, Bristow MR, Perryman MB. Hypoplastic left heart syndrome myocytes are differentiated but possess a unique phenotype. Cardiovasc Pathol 12: 23–31, 2003. doi: 10.1016/S1054-8807(02)00127-8. [DOI] [PubMed] [Google Scholar]
- 15.Bolger AP, Sharma R, Li W, Leenarts M, Kalra PR, Kemp M, Coats AJS, Anker SD, Gatzoulis MA. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 106: 92–99, 2002. doi: 10.1161/01.CIR.0000020009.30736.3F. [DOI] [PubMed] [Google Scholar]
- 16.Bolkier Y, Nevo-Caspi Y, Salem Y, Vardi A, Mishali D, Paret G. Micro-RNA-208a, -208b, and -499 as biomarkers for myocardial damage after cardiac surgery in children. Pediatr Crit Care Med 17: e193–e197, 2016. doi: 10.1097/PCC.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 17.Book WM, Gerardin J, Saraf A, Marie Valente A, Rodriguez F 3rd. Clinical phenotypes of Fontan failure: implications for management. Congenit Heart Dis 11: 296–308, 2016. doi: 10.1111/chd.12368. [DOI] [PubMed] [Google Scholar]
- 18.Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res 8: 47–52, 1996. [PubMed] [Google Scholar]
- 19.Boucek D, Yetman AT, Yeung E, Miyamoto SD, Stehlik J, Kfoury AG, Kaza AK, Weng C, Everitt MD. Survival based on patient selection for heart transplant in adults with congenital heart disease: a multi-institutional study. Int J Cardiol 172: e89–e90, 2014. doi: 10.1016/j.ijcard.2013.12.133. [DOI] [PubMed] [Google Scholar]
- 20.Bowater SE, Weaver RA, Thorne SA, Clift PF. The safety and effects of bosentan in patients with a Fontan circulation. Congenit Heart Dis 7: 243–249, 2012. doi: 10.1111/j.1747-0803.2012.00635.x. [DOI] [PubMed] [Google Scholar]
- 21.Brenner JI, Berg KA, Schneider DS, Clark EB, Boughman JA. Cardiac malformations in relatives of infants with hypoplastic left-heart syndrome. Am J Dis Child 143: 1492–1494, 1989. doi: 10.1001/archpedi.1989.02150240114030. [DOI] [PubMed] [Google Scholar]
- 22.Brilla CG, Scheer C, Rupp H. Renin-angiotensin system and myocardial collagen matrix: modulation of cardiac fibroblast function by angiotensin II type 1 receptor antagonism. J Hypertens Suppl 15: S13–S19, 1997. doi: 10.1097/00004872-199715066-00004. [DOI] [PubMed] [Google Scholar]
- 23.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N; MOCHA Investigators . Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation 94: 2807–2816, 1996. doi: 10.1161/01.CIR.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 24.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res 59: 297–309, 1986. doi: 10.1161/01.RES.59.3.297. [DOI] [PubMed] [Google Scholar]
- 25.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging 3: 727–734, 2010. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burchill LJ, Redington AN, Silversides CK, Ross HJ, Jimenez-Juan L, Mital S, Oechslin EN, Dragulescu A, Slorach C, Mertens L, Wald RM. Renin-angiotensin-aldosterone system genotype and serum BNP in a contemporary cohort of adults late after Fontan palliation. Int J Cardiol 197: 209–215, 2015. doi: 10.1016/j.ijcard.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Burkhart HM, Dearani JA, Mair DD, Warnes CA, Rowland CC, Schaff HV, Puga FJ, Danielson GK. The modified Fontan procedure: early and late results in 132 adult patients. J Thorac Cardiovasc Surg 125: 1252–1258, 2003. doi: 10.1016/S0022-5223(03)00117-X. [DOI] [PubMed] [Google Scholar]
- 28.Burkhart HM, Qureshi MY, Rossano JW, Cantero Peral S, O’Leary PW, Hathcock M, Kremers W, Nelson TJ; Wanek HLHS Consortium Clinical Pipeline . Autologous stem cell therapy for hypoplastic left heart syndrome: safety and feasibility of intraoperative intramyocardial injections. J Thorac Cardiovasc Surg 158: 1614–1623, 2019. doi: 10.1016/j.jtcvs.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Butts RJ, Chowdhury SM, Baker GH, Bandisode V, Savage AJ, Atz AM. Effect of Sildenafil on pressure-volume loop measures of ventricular function in Fontan patients. Pediatr Cardiol 37: 184–191, 2016. doi: 10.1007/s00246-015-1262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byrne RD, Weingarten AJ, Clark DE, Huang S, Perri RE, Scanga AE, Menachem JN, Markham LW, Frischhertz BP. More than the heart: hepatic, renal, and cardiac dysfunction in adult Fontan patients. Congenit Heart Dis 14: 765–771, 2019. doi: 10.1111/chd.12820. [DOI] [PubMed] [Google Scholar]
- 31.Cahill TJ, Ashrafian H, Watkins H. Genetic cardiomyopathies causing heart failure. Circ Res 113: 660–675, 2013. doi: 10.1161/CIRCRESAHA.113.300282. [DOI] [PubMed] [Google Scholar]
- 32.Calabrò R, Pisacane C, Pacileo G, Russo MG. Hemodynamic effects of a single oral dose of enalapril among children with asymptomatic chronic mitral regurgitation. Am Heart J 138: 955–961, 1999. doi: 10.1016/S0002-8703(99)70023-2. [DOI] [PubMed] [Google Scholar]
- 33.Carlo WF, Villa CR, Lal AK, Morales DL. Ventricular assist device use in single ventricle congenital heart disease. Pediatr Transplant 21: e13031, 2017. doi: 10.1111/petr.13031. [DOI] [PubMed] [Google Scholar]
- 34.Charron F, Paradis P, Bronchain O, Nemer G, Nemer M. Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Mol Cell Biol 19: 4355–4365, 1999. doi: 10.1128/MCB.19.6.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Rosenthal DN, Murray J, Dykes JC, Almond CS, Yarlagadda VV, Wright G, Navaratnam M, Reinhartz O, Maeda K. Bridge to transplant with ventricular assist device support in pediatric patients with single ventricle heart disease. ASAIO J 66: 205–211, 2020. doi: 10.1097/MAT.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 36.Cheung YF, Penny DJ, Redington AN. Serial assessment of left ventricular diastolic function after Fontan procedure. Heart 83: 420–424, 2000. doi: 10.1136/heart.83.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury SM, Graham EM, Atz AM, Bradley SM, Kavarana MN, Butts RJ. Validation of a simple score to determine risk of hospital mortality after the Norwood Procedure. Semin Thorac Cardiovasc Surg 28: 425–433, 2016. doi: 10.1053/j.semtcvs.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciliberti P, Giardini A. Impact of oral chronic administration of sildenafil in children and young adults after the Fontan operation. Future Cardiol 7: 609–612, 2011. doi: 10.2217/fca.11.52. [DOI] [PubMed] [Google Scholar]
- 39.Coats L, O’Connor S, Wren C, O’Sullivan J. The single-ventricle patient population: a current and future concern a population-based study in the North of England. Heart 100: 1348–1353, 2014. doi: 10.1136/heartjnl-2013-305336. [DOI] [PubMed] [Google Scholar]
- 40.Cohen MS, Jacobs ML, Weinberg PM, Rychik J. Morphometric analysis of unbalanced common atrioventricular canal using two-dimensional echocardiography. J Am Coll Cardiol 28: 1017–1023, 1996. doi: 10.1016/S0735-1097(96)00262-8. [DOI] [PubMed] [Google Scholar]
- 41.d’Udekem Y, Iyengar AJ, Galati JC, Forsdick V, Weintraub RG, Wheaton GR, Bullock A, Justo RN, Grigg LE, Sholler GF, Hope S, Radford DJ, Gentles TL, Celermajer DS, Winlaw DS. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 130, Suppl 1: S32–S38, 2014. doi: 10.1161/CIRCULATIONAHA.113.007764. [DOI] [PubMed] [Google Scholar]
- 42.d’Udekem Y, Xu MY, Galati JC, Lu S, Iyengar AJ, Konstantinov IE, Wheaton GR, Ramsay JM, Grigg LE, Millar J, Cheung MM, Brizard CP. Predictors of survival after single-ventricle palliation: the impact of right ventricular dominance. J Am Coll Cardiol 59: 1178–1185, 2012. doi: 10.1016/j.jacc.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 43.Das BB. Current state of pediatric heart failure. Children (Basel) 5: 88, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demetriades P, Aziz A, Condliffe R, Bowater SE, Clift PF. The use of Macitentan in Fontan circulation: a case report. BMC Cardiovasc Disord 17: 131, 2017. doi: 10.1186/s12872-017-0567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derk G, Houser L, Miner P, Williams R, Moriarty J, Finn P, Alejos J, Aboulhosn J. Efficacy of endothelin blockade in adults with Fontan physiology. Congenit Heart Dis 10: E11–E16, 2015. doi: 10.1111/chd.12189. [DOI] [PubMed] [Google Scholar]
- 46.Doughan AR, McConnell ME, Book WM. Effect of beta blockers (carvedilol or metoprolol XL) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. Am J Cardiol 99: 704–706, 2007. doi: 10.1016/j.amjcard.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 47.Driscoll DJ, Offord KP, Feldt RH, Schaff HV, Puga FJ, Danielson GK. Five- to fifteen-year follow-up after Fontan operation. Circulation 85: 469–496, 1992. doi: 10.1161/01.CIR.85.2.469. [DOI] [PubMed] [Google Scholar]
- 48.Durbin MD, Cadar AG, Williams CH, Guo Y, Bichell DP, Su YR, Hong CC. Hypoplastic left heart syndrome sequencing reveals a novel NOTCH1 mutation in a family with single ventricle defects. Pediatr Cardiol 38: 1232–1240, 2017. doi: 10.1007/s00246-017-1650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egbe AC, Connolly HM, Miranda WR, Ammash NM, Hagler DJ, Veldtman GR, Borlaug BA. Hemodynamics of Fontan failure: the role of pulmonary vascular disease. Circ Heart Fail 10: e004515, 2017. doi: 10.1161/CIRCHEARTFAILURE.117.004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eindhoven JA, van den Bosch AE, Jansen PR, Boersma E, Roos-Hesselink JW. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol 60: 2140–2149, 2012. doi: 10.1016/j.jacc.2012.02.092. [DOI] [PubMed] [Google Scholar]
- 51.Fahed AC, Gelb BD, Seidman JG, Seidman CE. Genetics of congenital heart disease: the glass half empty. Circ Res 112: 707–720, 2013. doi: 10.1161/CIRCRESAHA.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fahed AC, Roberts AE, Mital S, Lakdawala NK. Heart failure in congenital heart disease: a confluence of acquired and congenital. Heart Fail Clin 10: 219–227, 2014. doi: 10.1016/j.hfc.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Files MD, Arya B. Pathophysiology, adaptation, and imaging of the right ventricle in Fontan circulation. Am J Physiol Heart Circ Physiol 315: H1779–H1788, 2018. doi: 10.1152/ajpheart.00336.2018. [DOI] [PubMed] [Google Scholar]
- 54.Fogel MA, Weinberg PM, Fellows KE, Hoffman EA. A study in ventricular-ventricular interaction. Single right ventricles compared with systemic right ventricles in a dual-chamber circulation. Circulation 92: 219–230, 1995. doi: 10.1161/01.CIR.92.2.219. [DOI] [PubMed] [Google Scholar]
- 55.Fogel MA, Weinberg PM, Gupta KB, Rychik J, Hubbard A, Hoffman EA, Haselgrove J. Mechanics of the single left ventricle: a study in ventricular-ventricular interaction II. Circulation 98: 330–338, 1998. doi: 10.1161/01.CIR.98.4.330. [DOI] [PubMed] [Google Scholar]
- 56.Fogel MA, Weinberg PM, Hoydu A, Hubbard A, Rychik J, Jacobs M, Fellows KE, Haselgrove J. The nature of flow in the systemic venous pathway measured by magnetic resonance blood tagging in patients having the Fontan operation. J Thorac Cardiovasc Surg 114: 1032–1041, 1997. doi: 10.1016/S0022-5223(97)70017-5. [DOI] [PubMed] [Google Scholar]
- 57.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 26: 240–248, 1971. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forbess JM, Cook N, Roth SJ, Serraf A, Mayer JE Jr, Jonas RA. Ten-year institutional experience with palliative surgery for hypoplastic left heart syndrome. Risk factors related to stage I mortality. Circulation 92, Suppl 9: 262–266, 1995. doi: 10.1161/01.CIR.92.9.262. [DOI] [PubMed] [Google Scholar]
- 59.Foulks MG, Meyer RML, Gold JI, Herrington CS, Kallin K, Menteer J. Postoperative heart failure after stage 1 palliative surgery for single ventricle cardiac disease. Pediatr Cardiol 40: 943–949, 2019. doi: 10.1007/s00246-019-02093-4. [DOI] [PubMed] [Google Scholar]
- 60.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation 109: 1580–1589, 2004. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 61.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frommelt PC, Snider AR, Meliones JN, Vermilion RP. Doppler assessment of pulmonary artery flow patterns and ventricular function after the Fontan operation. Am J Cardiol 68: 1211–1215, 1991. doi: 10.1016/0002-9149(91)90195-Q. [DOI] [PubMed] [Google Scholar]
- 63.Gambetta K, Al-Ahdab MK, Ilbawi MN, Hassaniya N, Gupta M. Transcription repression and blocks in cell cycle progression in hypoplastic left heart syndrome. Am J Physiol Heart Circ Physiol 294: H2268–H2275, 2008. doi: 10.1152/ajpheart.91494.2007. [DOI] [PubMed] [Google Scholar]
- 64.Garcia AM, Nakano SJ, Karimpour-Fard A, Nunley K, Blain-Nelson P, Stafford NM, Stauffer BL, Sucharov CC, Miyamoto SD. Phosphodiesterase-5 is elevated in failing single ventricle myocardium and affects cardiomyocyte remodeling in vitro. Circ Heart Fail 11: e004571, 2018. doi: 10.1161/CIRCHEARTFAILURE.117.004571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaynor JW, Bridges ND, Cohen MI, Mahle WT, Decampli WM, Steven JM, Nicolson SC, Spray TL. Predictors of outcome after the Fontan operation: is hypoplastic left heart syndrome still a risk factor? J Thorac Cardiovasc Surg 123: 237–245, 2002. doi: 10.1067/mtc.2002.119337. https://www.ncbi.nlm.nih.gov/pubmed/11828282 [DOI] [PubMed] [Google Scholar]
- 66.Gentles TL, Gauvreau K, Mayer JE Jr, Fishberger SB, Burnett J, Colan SD, Newburger JW, Wernovsky G. Functional outcome after the Fontan operation: factors influencing late morbidity. J Thorac Cardiovasc Surg 114: 392–403, 1997. doi: 10.1016/S0022-5223(97)70184-3. [DOI] [PubMed] [Google Scholar]
- 67.Gewillig M. The Fontan circulation. Heart 91: 839–846, 2005. doi: 10.1136/hrt.2004.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gewillig M, Goldberg DJ. Failure of the Fontan circulation. Heart Fail Clin. 10: 105–116, 2014. [DOI] [PubMed] [Google Scholar]
- 69.Gewillig MH, Lundström UR, Deanfield JE, Bull C, Franklin RC, Graham TP Jr, Wyse RK. Impact of Fontan operation on left ventricular size and contractility in tricuspid atresia. Circulation 81: 118–127, 1990. doi: 10.1161/01.CIR.81.1.118. [DOI] [PubMed] [Google Scholar]
- 70.Glatz AC, Harrison N, Small AJ, Dori Y, Gillespie MJ, Harris MA, Fogel MA, Rome JJ, Whitehead KK. Factors associated with systemic to pulmonary arterial collateral flow in single ventricle patients with superior cavopulmonary connections. Heart 101: 1813–1818, 2015. doi: 10.1136/heartjnl-2015-307703. [DOI] [PubMed] [Google Scholar]
- 71.Glatz AC, Rome JJ, Small AJ, Gillespie MJ, Dori Y, Harris MA, Keller MS, Fogel MA, Whitehead KK. Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes. Circ Cardiovasc Imaging 5: 218–225, 2012. doi: 10.1161/CIRCIMAGING.111.966986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glidewell SC, Miyamoto SD, Grossfeld PD, Clouthier DE, Coldren CD, Stearman RS, Geraci MW. Transcriptional impact of rare and private copy number variants in hypoplastic left heart syndrome. Clin Transl Sci 8: 682–689, 2015. doi: 10.1111/cts.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldberg DJ, French B, McBride MG, Marino BS, Mirarchi N, Hanna BD, Wernovsky G, Paridon SM, Rychik J. Impact of oral sildenafil on exercise performance in children and young adults after the Fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation 123: 1185–1193, 2011. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldberg DJ, French B, Szwast AL, McBride MG, Marino BS, Mirarchi N, Hanna BD, Wernovsky G, Paridon SM, Rychik J. Impact of sildenafil on echocardiographic indices of myocardial performance after the Fontan operation. Pediatr Cardiol 33: 689–696, 2012. doi: 10.1007/s00246-012-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldberg DJ, Zak V, Goldstein BH, Chen S, Hamstra MS, Radojewski EA, Maunsell E, Mital S, Menon SC, Schumacher KR, Payne RM, Stylianou M, Kaltman JR, deVries TM, Yeager JL, Paridon SM; Pediatric Heart Network Investigators . Results of a phase I/II multi-center investigation of udenafil in adolescents after Fontan palliation. Am Heart J 188: 42–52, 2017. doi: 10.1016/j.ahj.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldberg DJ, Zak V, Goldstein BH, McCrindle BW, Menon SC, Schumacher KR, Payne RM, Rhodes J, McHugh KE, Penny DJ, Trachtenberg F, Hamstra MS, Richmond ME, Frommelt PC, Files MD, Yeager JL, Pemberton VL, Stylianou MP, Pearson GD, Paridon SM; Pediatric Heart Network Investigators . Design and rationale of the Fontan Udenafil Exercise Longitudinal (FUEL) trial. Am Heart J 201: 1–8, 2018. doi: 10.1016/j.ahj.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldberg DJ, Zak V, Goldstein BH, Schumacher KR, Rhodes J, Penny DJ, Petit CJ, Ginde S, Menon SC, Kim SH, Kim GB, Nowlen TT, DiMaria MV, Frischhertz BP, Wagner JB, McHugh KE, McCrindle BW, Shillingford AJ, Sabati AA, Yetman AT, John AS, Richmond ME, Files MD, Payne RM, Mackie AS, Davis CK, Shahanavaz S, Hill KD, Garg R, Jacobs JP, Hamstra MS, Woyciechowski S, Rathge KA, McBride MG, Frommelt PC; Pediatric Heart Network Investigators . Results of the Fontan Udenafil Exercise Longitudinal (FUEL) trial. Circulation, 2019. doi: 10.1161/CIRCULATIONAHA.119.044352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TDH, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O’Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 309: 896–908, 2013. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 79.Gupta SK. Use of Bayesian statistics in drug development: advantages and challenges. Int J Appl Basic Med Res 2: 3–6, 2012. doi: 10.4103/2229-516X.96789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutgesell HP, Massaro TA. Management of hypoplastic left heart syndrome in a consortium of university hospitals. Am J Cardiol 76: 809–811, 1995. doi: 10.1016/S0002-9149(99)80232-X. [DOI] [PubMed] [Google Scholar]
- 81.Hansen JH, Uebing A, Furck AK, Scheewe J, Jung O, Fischer G, Kramer HH. Risk factors for adverse outcome after superior cavopulmonary anastomosis for hypoplastic left heart syndrome. Eur J Cardiothorac Surg 40: e43–e49, 2011. doi: 10.1016/j.ejcts.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 82.Hebert A, Mikkelsen UR, Thilen U, Idorn L, Jensen AS, Nagy E, Hanseus K, Sørensen KE, Søndergaard L. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) study. Circulation 130: 2021–2030, 2014. doi: 10.1161/CIRCULATIONAHA.113.008441. [DOI] [PubMed] [Google Scholar]
- 83.Hill KD, Tunks RD, Barker PCA, Benjamin DK Jr, Cohen-Wolkowiez M, Fleming GA, Laughon M, Li JS. Sildenafil exposure and hemodynamic effect after stage II single-ventricle surgery. Pediatr Crit Care Med 14: 593–600, 2013. doi: 10.1097/PCC.0b013e31828aa5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hinton RB, Martin LJ, Rame-Gowda S, Tabangin ME, Cripe LH, Benson DW. Hypoplastic left heart syndrome links to chromosomes 10q and 6q and is genetically related to bicuspid aortic valve. J Am Coll Cardiol 53: 1065–1071, 2009. doi: 10.1016/j.jacc.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinton RB Jr, Martin LJ, Tabangin ME, Mazwi ML, Cripe LH, Benson DW. Hypoplastic left heart syndrome is heritable. J Am Coll Cardiol 50: 1590–1595, 2007. doi: 10.1016/j.jacc.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 86.Hinton RB, Ware SM. Heart failure in pediatric patients with congenital heart disease. Circ Res 120: 978–994, 2017. doi: 10.1161/CIRCRESAHA.116.308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hitz MP, Lemieux-Perreault LP, Marshall C, Feroz-Zada Y, Davies R, Yang SW, Lionel AC, D’Amours G, Lemyre E, Cullum R, Bigras JL, Thibeault M, Chetaille P, Montpetit A, Khairy P, Overduin B, Klaassen S, Hoodless P, Awadalla P, Hussin J, Idaghdour Y, Nemer M, Stewart AF, Boerkoel C, Scherer SW, Richter A, Dubé MP, Andelfinger G. Rare copy number variants contribute to congenital left-sided heart disease. PLoS Genet 8: e1002903, 2012. [Erratum in PLoS Genet 9: 2013] doi: 10.1371/journal.pgen.1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoffman GM, Scott JP, Ghanayem NS, Stuth EA, Mitchell ME, Woods RK, Hraska V, Niebler RA, Bertrandt RA, Mussatto KA, Tweddell JS. Identification of time-dependent risks of hemodynamic states after Stage 1 Norwood palliation. Ann Thorac Surg, 2020. doi: 10.1016/j.athoracsur.2019.06.063. [DOI] [PubMed] [Google Scholar]
- 89.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 39: 1890–1900, 2002. doi: 10.1016/S0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 90.Hsu DT, Pearson GD. Heart failure in children: part I: history, etiology, and pathophysiology. Circ Heart Fail 2: 63–70, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.820217. [DOI] [PubMed] [Google Scholar]
- 91.Hsu DT, Pearson GD. Heart failure in children: part II: diagnosis, treatment, and future directions. Circ Heart Fail 2: 490–498, 2009. doi: 10.1161/CIRCHEARTFAILURE.109.856229. [DOI] [PubMed] [Google Scholar]
- 92.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, Barker PC, Ravishankar C, McCrindle BW, Williams RV, Altmann K, Ghanayem NS, Margossian R, Chung WK, Border WL, Pearson GD, Stylianou MP, Mital S; Pediatric Heart Network Investigators . Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation 122: 333–340, 2010. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim YH, Chae MH, Choi DY. Inhaled iloprost for the treatment of patient with Fontan circulation. Korean J Pediatr 57: 461–463, 2014. doi: 10.3345/kjp.2014.57.10.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iascone M, Ciccone R, Galletti L, Marchetti D, Seddio F, Lincesso AR, Pezzoli L, Vetro A, Barachetti D, Boni L, Federici D, Soto AM, Comas JV, Ferrazzi P, Zuffardi O. Identification of de novo mutations and rare variants in hypoplastic left heart syndrome. Clin Genet 81: 542–554, 2012. doi: 10.1111/j.1399-0004.2011.01674.x. [DOI] [PubMed] [Google Scholar]
- 95.Ishibashi N, Park I-S, Waragai T, Yoshikawa T, Murakami Y, Mori K, Mimori S, Ando M, Takahashi Y, Doi S, Mizutani S, Nakanishi T. Effect of carvedilol on heart failure in patients with a functionally univentricular heart. Circ J 75: 1394–1399, 2011. doi: 10.1253/circj.CJ-10-0845. [DOI] [PubMed] [Google Scholar]
- 96.Ishigami S, Ohtsuki S, Eitoku T, Ousaka D, Kondo M, Kurita Y, Hirai K, Fukushima Y, Baba K, Goto T, Horio N, Kobayashi J, Kuroko Y, Kotani Y, Arai S, Iwasaki T, Sato S, Kasahara S, Sano S, Oh H. Intracoronary cardiac progenitor cells in single ventricle physiology: the PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) Randomized Phase 2 Trial. Circ Res 120: 1162–1173, 2017. doi: 10.1161/CIRCRESAHA.116.310253. [DOI] [PubMed] [Google Scholar]
- 97.Jackson KW, Butts RJ, Svenson AJ, McQuinn TC, Atz AM. Response to a single dose of sildenafil in single-ventricle patients: an echocardiographic evaluation. Pediatr Cardiol 34: 1739–1742, 2013. doi: 10.1007/s00246-012-0415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jacobs ML, Norwood WI Jr. Fontan operation: influence of modifications on morbidity and mortality. Ann Thorac Surg 58: 945–952, 1994. doi: 10.1016/0003-4975(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 99.Jayaprasad N. Heart failure in children. Heart Views 17: 92–99, 2016. doi: 10.4103/1995-705X.192556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang X, Sucharov J, Stauffer BL, Miyamoto SD, Sucharov CC. Exosomes from pediatric dilated cardiomyopathy patients modulate a pathological response in cardiomyocytes. Am J Physiol Heart Circ Physiol 312: H818–H826, 2017. doi: 10.1152/ajpheart.00673.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang Y, Habibollah S, Tilgner K, Collin J, Barta T, Al-Aama JY, Tesarov L, Hussain R, Trafford AW, Kirkwood G, Sernagor E, Eleftheriou CG, Przyborski S, Stojković M, Lako M, Keavney B, Armstrong L. An induced pluripotent stem cell model of hypoplastic left heart syndrome (HLHS) reveals multiple expression and functional differences in HLHS-derived cardiac myocytes. Stem Cells Transl Med 3: 416–423, 2014. doi: 10.5966/sctm.2013-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Josephson CB, Howlett JG, Jackson SD, Finley J, Kells CM. A case series of systemic right ventricular dysfunction post atrial switch for simple D-transposition of the great arteries: the impact of beta-blockade. Can J Cardiol 22: 769–772, 2006. doi: 10.1016/S0828-282X(06)70293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Julsrud PR, Weigel TJ, Van Son JA, Edwards WD, Mair DD, Driscoll DJ, Danielson GK, Puga FJ, Offord KP. Influence of ventricular morphology on outcome after the Fontan procedure. Am J Cardiol 86: 319–323, 2000. doi: 10.1016/S0002-9149(00)00922-X. [DOI] [PubMed] [Google Scholar]
- 104.Kajimoto M, Nuri M, Isern NG, Robillard-Frayne I, Des Rosiers C, Portman MA. Metabolic response of the immature right ventricle to acute pressure overloading. J Am Heart Assoc 7: e008570, 2018. doi: 10.1161/JAHA.118.008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karamlou T, Hirsch J, Welke K, Ohye RG, Bove EL, Devaney EJ, Gajarski RJ. A United Network for Organ Sharing analysis of heart transplantation in adults with congenital heart disease: outcomes and factors associated with mortality and retransplantation. J Thorac Cardiovasc Surg 140: 161–168, 2010. doi: 10.1016/j.jtcvs.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 106.Kaufman BD, Desai M, Reddy S, Osorio JC, Chen JM, Mosca RS, Ferrante AW, Mital S. Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. J Card Fail 14: 760–767, 2008. doi: 10.1016/j.cardfail.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 107.Kenny LA, DeRita F, Nassar M, Dark J, Coats L, Hasan A. Transplantation in the single ventricle population. Ann Cardiothorac Surg 7: 152–159, 2018. doi: 10.21037/acs.2018.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khairy P, Fernandes SM, Mayer JE Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 117: 85–92, 2008. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 109.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev 52: 11–34, 2000. [PubMed] [Google Scholar]
- 110.King G, Ayer J, Celermajer D, Zentner D, Justo R, Disney P, Zannino D, d’Udekem Y. Atrioventricular valve failure in Fontan palliation. J Am Coll Cardiol 73: 810–822, 2019. doi: 10.1016/j.jacc.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 111.Kirk R, Dipchand AI, Rosenthal DN, Addonizio L, Burch M, Chrisant M, Dubin A, Everitt M, Gajarski R, Mertens L, Miyamoto S, Morales D, Pahl E, Shaddy R, Towbin J, Weintraub R. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: Executive summary. J Heart Lung Transplant 33: 888–909, 2014. [Erratum in J Heart Lung Transplant 42: 1104, 2014.] doi: 10.1016/j.healun.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 112.Kirklin JK, Blackstone EH, Kirklin JW, Pacifico AD, Bargeron LM Jr. The Fontan operation. Ventricular hypertrophy, age, and date of operation as risk factors. J Thorac Cardiovasc Surg 92: 1049–1064, 1986. doi: 10.1016/S0022-5223(19)35821-0. [DOI] [PubMed] [Google Scholar]
- 113.Kleber FX, Sabin GV, Winter UJ, Reindl I, Beil S, Wenzel M, Fischer M, Doering W. Angiotensin-converting enzyme inhibitors in preventing remodeling and development of heart failure after acute myocardial infarction: results of the German multicenter study of the effects of captopril on cardiopulmonary exercise parameters (ECCE). Am J Cardiol 80: 162A–167A, 1997. doi: 10.1016/S0002-9149(97)00474-8. [DOI] [PubMed] [Google Scholar]
- 114.Knoll C, Chen S, Murray JM, Dykes JC, Yarlagadda VV, Rosenthal DN, Almond CS, Maeda K, Shin AY. A quality bundle to support high-risk pediatric ventricular assist device implantation. Pediatr Cardiol 40: 1159–1164, 2019. doi: 10.1007/s00246-019-02123-1. [DOI] [PubMed] [Google Scholar]
- 115.Kobayashi J, Yoshida M, Tarui S, Hirata M, Nagai Y, Kasahara S, Naruse K, Ito H, Sano S, Oh H. Directed differentiation of patient-specific induced pluripotent stem cells identifies the transcriptional repression and epigenetic modification of NKX2-5, HAND1, and NOTCH1 in hypoplastic left heart syndrome. PLoS One 9: e102796, 2014. doi: 10.1371/journal.pone.0102796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kotani Y, Chetan D, Zhu J, Saedi A, Zhao L, Mertens L, Redington AN, Coles J, Caldarone CA, Van Arsdell GS, Honjo O. Fontan failure and death in contemporary Fontan circulation: analysis from the last two decades. Ann Thorac Surg 105: 1240–1247, 2018. doi: 10.1016/j.athoracsur.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 117.Kouatli AA, Garcia JA, Zellers TM, Weinstein EM, Mahony L. Enalapril does not enhance exercise capacity in patients after Fontan procedure. Circulation 96: 1507–1512, 1997. doi: 10.1161/01.CIR.96.5.1507. [DOI] [PubMed] [Google Scholar]
- 118.Kumar A, Kumar A, Michael P, Brabant D, Parissenti AM, Ramana CV, Xu X, Parrillo JE. Human serum from patients with septic shock activates transcription factors STAT1, IRF1, and NF-kappaB and induces apoptosis in human cardiac myocytes. J Biol Chem 280: 42619–42626, 2005. doi: 10.1074/jbc.M508416200. [DOI] [PubMed] [Google Scholar]
- 119.Latus H, Gummel K, Diederichs T, Bauer A, Rupp S, Kerst G, Jux C, Akintuerk H, Schranz D, Apitz C. Aortopulmonary collateral flow is related to pulmonary artery size and affects ventricular dimensions in patients after the Fontan procedure. PLoS One 8: e81684, 2013. doi: 10.1371/journal.pone.0081684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lindenfeld J, Keller K, Campbell DN, Wolfe RR, Quaife RA. Improved systemic ventricular function after carvedilol administration in a patient with congenitally corrected transposition of the great arteries. J Heart Lung Transplant 22: 198–201, 2003. doi: 10.1016/S1053-2498(02)00656-3. [DOI] [PubMed] [Google Scholar]
- 121.Liu CP, Ting CT, Yang TM, Chen JW, Chang MS, Maughan WL, Lawrence W, Kass DA. Reduced left ventricular compliance in human mitral stenosis. Role of reversible internal constraint. Circulation 85: 1447–1456, 1992. doi: 10.1161/01.CIR.85.4.1447. [DOI] [PubMed] [Google Scholar]
- 122.Liu X, Yagi H, Saeed S, Bais AS, Gabriel GC, Chen Z, Peterson KA, Li Y, Schwartz MC, Reynolds WT, Saydmohammed M, Gibbs B, Wu Y, Devine W, Chatterjee B, Klena NT, Kostka D, de Mesy Bentley KL, Ganapathiraju MK, Dexheimer P, Leatherbury L, Khalifa O, Bhagat A, Zahid M, Pu W, Watkins S, Grossfeld P, Murray SA, Porter GA Jr, Tsang M, Martin LJ, Benson DW, Aronow BJ, Lo CW. The complex genetics of hypoplastic left heart syndrome. Nat Genet 49: 1152–1159, 2017. doi: 10.1038/ng.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Loffredo CA, Chokkalingam A, Sill AM, Boughman JA, Clark EB, Scheel J, Brenner JI. Prevalence of congenital cardiovascular malformations among relatives of infants with hypoplastic left heart, coarctation of the aorta, and d-transposition of the great arteries. Am J Med Genet A 124A: 225–230, 2004. doi: 10.1002/ajmg.a.20366. [DOI] [PubMed] [Google Scholar]
- 124.MacRae CA. Mendelian forms of structural cardiovascular disease. Curr Cardiol Rep 15: 399, 2013. doi: 10.1007/s11886-013-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madonna R, Van Laake LW, Davidson SM, Engel FB, Hausenloy DJ, Lecour S, Leor J, Perrino C, Schulz R, Ytrehus K, Landmesser U, Mummery CL, Janssens S, Willerson J, Eschenhagen T, Ferdinandy P, Sluijter JPG. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J 37: 1789–1798, 2016. doi: 10.1093/eurheartj/ehw113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res 91: 1103–1113, 2002. doi: 10.1161/01.RES.0000046452.67724.B8. [DOI] [PubMed] [Google Scholar]
- 127.Pigott JD, Murphy JD, Barber G, Norwood WI. Palliative reconstructive surgery for hypoplastic left heart syndrome. Ann Thorac Surg 45: 122–128, 1988. doi: 10.1016/S0003-4975(10)62420-4. [DOI] [PubMed] [Google Scholar]
- 128.McGuirk SP, Winlaw DS, Langley SM, Stumper OF, de Giovanni JV, Wright JG, Brawn WJ, Barron DJ. The impact of ventricular morphology on midterm outcome following completion total cavopulmonary connection. Eur J Cardiothorac Surg 24: 37–46, 2003. doi: 10.1016/S1010-7940(03)00186-6. [DOI] [PubMed] [Google Scholar]
- 129.Menon SC, Erickson LK, McFadden M, Miller DV. Effect of ventriculotomy on right-ventricular remodeling in hypoplastic left heart syndrome: a histopathological and echocardiography correlation study. Pediatr Cardiol 34: 354–363, 2013. doi: 10.1007/s00246-012-0462-x. [DOI] [PubMed] [Google Scholar]
- 130.Mital S, Chung WK, Colan SD, Sleeper LA, Manlhiot C, Arrington CB, Cnota JF, Graham EM, Mitchell ME, Goldmuntz E, Li JS, Levine JC, Lee TM, Margossian R, Hsu DT; Pediatric Heart Network Investigators . Renin-angiotensin-aldosterone genotype influences ventricular remodeling in infants with single ventricle. Circulation 123: 2353–2362, 2011. doi: 10.1161/CIRCULATIONAHA.110.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mitchell ME, Ittenbach RF, Gaynor JW, Wernovsky G, Nicolson S, Spray TL. Intermediate outcomes after the Fontan procedure in the current era. J Thorac Cardiovasc Surg 131: 172–180, 2006. doi: 10.1016/j.jtcvs.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 132.Miyamoto SD, Stauffer BL, Polk J, Medway A, Friedrich M, Haubold K, Peterson V, Nunley K, Nelson P, Sobus R, Stenmark KR, Sucharov CC. Gene expression and β-adrenergic signaling are altered in hypoplastic left heart syndrome. J Heart Lung Transplant 33: 785–793, 2014. doi: 10.1016/j.healun.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mori Y, Nakazawa M, Tomimatsu H, Momma K. Long-term effect of angiotensin-converting enzyme inhibitor in volume overloaded heart during growth: a controlled pilot study. J Am Coll Cardiol 36: 270–275, 2000. doi: 10.1016/S0735-1097(00)00673-2. [DOI] [PubMed] [Google Scholar]
- 134.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St. Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 116: 238–248, 2007. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 135.Naito Y, Hiramatsu T, Kurosawa H, Agematsu K, Sasoh M, Nakanishi T, Imai Y, Yamazaki K. Long-term results of modified Fontan operation for single-ventricle patients associated with atrioventricular valve regurgitation. Ann Thorac Surg 96: 211–218, 2013. doi: 10.1016/j.athoracsur.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 136.Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15: 387–407, 2018. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 137.Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H (). Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology. Tokyo, Japan: Springer, 2016. doi: 10.1007/978-4-431-54628-3 [DOI] [PubMed] [Google Scholar]
- 138.Nakano SJ, Miyamoto SD, Price JF, Rossano JW, Cabrera AG. Pediatric heart failure: an evolving public health concern. J Pediatr, 2019. doi: 10.1016/j.jpeds.2019.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nakano SJ, Nelson P, Sucharov CC, Miyamoto SD. Myocardial response to milrinone in single right ventricle heart disease. J Pediatr 174: 199–203.e5, 2016. doi: 10.1016/j.jpeds.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nakano SJ, Siomos AK, Garcia AM, Nguyen H, SooHoo M, Galambos C, Nunley K, Stauffer BL, Sucharov CC, Miyamoto SD. Fibrosis-related gene expression in single ventricle heart disease. J Pediatr 191: 82–90.e2, 2017. doi: 10.1016/j.jpeds.2017.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakano SJ, Sucharov J, Van R, Cecil M, Nunley K, Wickers S, Karimpur-Fard A, Stauffer BL, Miyamoto SD, Sucharov CC. Cardiac adenylyl cyclase and phosphodiesterase expression profiles vary by age, disease, and chronic phosphodiesterase inhibitor treatment. J Card Fail, 2017. doi: 10.1016/j.cardfail.2016.07.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nathan M, Williamson AK, Mayer JE, Bacha EA, Juraszek AL. Mortality in hypoplastic left heart syndrome: review of 216 autopsy cases of aortic atresia with attention to coronary artery disease. J Thorac Cardiovasc Surg 144: 1301–1306, 2012. doi: 10.1016/j.jtcvs.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 143.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L’Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 293: H3270–H3278, 2007. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- 144.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med 308: 23–26, 1983. doi: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 145.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW; Pediatric Heart Network Investigators . Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med 362: 1980–1992, 2010. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL; The PROMISE Study Research Group . Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med 325: 1468–1475, 1991. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 147.Patten M, Hartogensis WE, Long CS. Interleukin-1β is a negative transcriptional regulator of alpha1-adrenergic induced gene expression in cultured cardiac myocytes. J Biol Chem 271: 21134–21141, 1996. doi: 10.1074/jbc.271.35.21134. [DOI] [PubMed] [Google Scholar]
- 148.Penny DJ, Krishnamurthy R. Clinical-physiological considerations in patients undergoing staged palliation for a functionally single ventricle. Pediatr Crit Care Med 17, Suppl 1: S347–S355, 2016. doi: 10.1097/PCC.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 149.Penny DJ, Rigby ML, Redington AN. Abnormal patterns of intraventricular flow and diastolic filling after the Fontan operation: evidence for incoordinate ventricular wall motion. Br Heart J 66: 375–378, 1991. doi: 10.1136/hrt.66.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pierpont ME, Brueckner M, Chung WK, Garg V, Lacro RV, McGuire AL, Mital S, Priest JR, Pu WT, Roberts A, Ware SM, Gelb BD, Russell MW; American Heart Association Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine . Genetic basis for congenital heart disease: revisited: a scientific statement from the American Heart Association. Circulation 138: e653–e711, 2018. [Erratum in Circulation 138: e73, 2018]. doi: 10.1161/CIR.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Prakash A, Travison TG, Fogel MA, Hurwitz LM, Powell AJ, Printz BF, Puchalski MD, Shirali GS, Yoo SJ, Geva T; Pediatric Heart Network Investigators . Relation of size of secondary ventricles to exercise performance in children after fontan operation. Am J Cardiol 106: 1652–1656, 2010. doi: 10.1016/j.amjcard.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Price JF, Towbin JA, Dreyer WJ, Moffett BS, Kertesz NJ, Clunie SK, Denfield SW. Outpatient continuous parenteral inotropic therapy as bridge to transplantation in children with advanced heart failure. J Card Fail 12: 139–143, 2006. doi: 10.1016/j.cardfail.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 153.Pundi K, Pundi KN, Kamath PS, Cetta F, Li Z, Poterucha JT, Driscoll DJ, Johnson JN. Liver disease in patients after the Fontan operation. Am J Cardiol 117: 456–460, 2016. doi: 10.1016/j.amjcard.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 154.Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O’Leary PW, Driscoll DJ, Cetta F. 40-year follow-up after the Fontan operation: long-term outcomes of 1,052 patients. J Am Coll Cardiol 66: 1700–1710, 2015. doi: 10.1016/j.jacc.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 155.Ramachandran S, Lowenthal A, Ritner C, Lowenthal S, Bernstein HS. Plasma microvesicle analysis identifies microRNA 129-5p as a biomarker of heart failure in univentricular heart disease. PLoS One 12: e0183624, 2017. doi: 10.1371/journal.pone.0183624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reddy S, Zhao M, Hu DQ, Fajardo G, Hu S, Ghosh Z, Rajagopalan V, Wu JC, Bernstein D. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics 44: 562–575, 2012. doi: 10.1152/physiolgenomics.00163.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reddy S, Zhao M, Hu DQ, Fajardo G, Katznelson E, Punn R, Spin JM, Chan FP, Bernstein D. Physiologic and molecular characterization of a murine model of right ventricular volume overload. Am J Physiol Heart Circ Physiol 304: H1314–H1327, 2013. doi: 10.1152/ajpheart.00776.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E; RELAX Trial . Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309: 1268–1277, 2013. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr 153: 807–813, 2008. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rhodes J, Ubeda-Tikkanen A, Clair M, Fernandes SM, Graham DA, Milliren CE, Daly KP, Mullen MP, Landzberg MJ. Effect of inhaled iloprost on the exercise function of Fontan patients: a demonstration of concept. Int J Cardiol 168: 2435–2440, 2013. doi: 10.1016/j.ijcard.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ricci M, Xu Y, Hammond HL, Willoughby DA, Nathanson L, Rodriguez MM, Vatta M, Lipshultz SE, Lincoln J. Myocardial alternative RNA splicing and gene expression profiling in early stage hypoplastic left heart syndrome. PLoS One 7: e29784, 2012. doi: 10.1371/journal.pone.0029784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Richards AA, Garg V. Genetics of congenital heart disease. Curr Cardiol Rev 6: 91–97, 2010. doi: 10.2174/157340310791162703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ridderbos FJ, Wolff D, Timmer A, van Melle JP, Ebels T, Dickinson MG, Timens W, Berger RM. Adverse pulmonary vascular remodeling in the Fontan circulation. J Heart Lung Transplant 34: 404–413, 2015. doi: 10.1016/j.healun.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 164.Roden DM, McLeod HL, Relling MV, Williams MS, Mensah GA, Peterson JF, Van Driest SL. Pharmacogenomics. Lancet 394: 521–532, 2019. doi: 10.1016/S0140-6736(19)31276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rupp S, Jux C, Bönig H, Bauer J, Tonn T, Seifried E, Dimmeler S, Zeiher AM, Schranz D. Intracoronary bone marrow cell application for terminal heart failure in children. Cardiol Young 22: 558–563, 2012. doi: 10.1017/S1047951112000066. [DOI] [PubMed] [Google Scholar]
- 166.Rupp S, Zeiher AM, Dimmeler S, Tonn T, Bauer J, Jux C, Akintuerk H, Schranz D. A regenerative strategy for heart failure in hypoplastic left heart syndrome: intracoronary administration of autologous bone marrow-derived progenitor cells. J Heart Lung Transplant 29: 574–577, 2010. doi: 10.1016/j.healun.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 167.Rutz T, de Marchi SF, Schwerzmann M, Vogel R, Seiler C. Right ventricular absolute myocardial blood flow in complex congenital heart disease. Heart 96: 1056–1062, 2010. doi: 10.1136/hrt.2009.191718. [DOI] [PubMed] [Google Scholar]
- 168.Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, Hsia T-YY, Hsu DT, Kovacs AH, McCrindle BW, Newburger JW, Pike NA, Rodefeld M, Rosenthal DN, Schumacher KR, Marino BS, Stout K, Veldtman G, Younoszai AK, D’Udekem Y; American Heart Association Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing . Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation 140: e234–e284, 2019. doi: 10.1161/CIR.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 169.Sable C, Foster E, Uzark K, Bjornsen K, Canobbio MM, Connolly HM, Graham TP, Gurvitz MZ, Kovacs A, Meadows AK, Reid GJ, Reiss JG, Rosenbaum KN, Sagerman PJ, Saidi A, Schonberg R, Shah S, Tong E, Williams RG. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation 123: 1454–1485, 2011. doi: 10.1161/CIR.0b013e3182107c56. [DOI] [PubMed] [Google Scholar]
- 170.Scammell AM, Diver MJ. Plasma renin activity in infants with congenital heart disease. Arch Dis Child 62: 1136–1138, 1987. doi: 10.1136/adc.62.11.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schilling C, Dalziel K, Nunn R, Du Plessis K, Shi WY, Celermajer D, Winlaw D, Weintraub RG, Grigg LE, Radford DJ, Bullock A, Gentles TL, Wheaton GR, Hornung T, Justo RN, d’Udekem Y. The Fontan epidemic: population projections from the Australia and New Zealand Fontan registry. Int J Cardiol 219: 14–19, 2016. doi: 10.1016/j.ijcard.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 172.Sedmera D. Form follows function: developmental and physiological view on ventricular myocardial architecture. Eur J Cardiothorac Surg 28: 526–528, 2005. doi: 10.1016/j.ejcts.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol 6: a018713, 2014. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shachar GB, Fuhrman BP, Wang Y, Lucas RV Jr, Lock JE. Rest and exercise hemodynamics after the Fontan procedure. Circulation 65: 1043–1048, 1982. doi: 10.1161/01.CIR.65.6.1043. [DOI] [PubMed] [Google Scholar]
- 175.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY, Pediatric Carvedilol Study Group F; Pediatric Carvedilol Study Group . Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA 298: 1171–1179, 2007. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 176.Shokeir MH. Hypoplastic left heart. Evidence for possible autosomal recessive inheritance. Birth Defects Orig Artic Ser 10: 223–227, 1974. [PubMed] [Google Scholar]
- 177.Simpson KE, Cibulka N, Lee CK, Huddleston CH, Canter CE. Failed Fontan heart transplant candidates with preserved vs impaired ventricular ejection: 2 distinct patient populations. J Heart Lung Transplant 31: 545–547, 2012. doi: 10.1016/j.healun.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 178.Simpson P, McGrath A, Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res 51: 787–801, 1982. doi: 10.1161/01.RES.51.6.787. [DOI] [PubMed] [Google Scholar]
- 179.Singh K, Communal C, Colucci WS. Inhibition of protein phosphatase 1 induces apoptosis in neonatal rat cardiac myocytes: role of adrenergic receptor stimulation. Basic Res Cardiol 95: 389–396, 2000. doi: 10.1007/s003950070038. [DOI] [PubMed] [Google Scholar]
- 180.Singh TP, Humes RA, Muzik O, Kottamasu S, Karpawich PP, Di Carli MF. Myocardial flow reserve in patients with a systemic right ventricle after atrial switch repair. J Am Coll Cardiol 37: 2120–2125, 2001. doi: 10.1016/S0735-1097(01)01283-9. [DOI] [PubMed] [Google Scholar]
- 181.Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 1: 18–20, 2000. doi: 10.3317/jraas.2000.002. [DOI] [PubMed] [Google Scholar]
- 182.Stasik CN, Gelehrter S, Goldberg CS, Bove EL, Devaney EJ, Ohye RG. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg 131: 412–417, 2006. [Erratum in J Thorac Cardiovasc Surg 133: 602, 2007]. doi: 10.1016/j.jtcvs.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 183.Stout KK, Broberg CS, Book WM, Cecchin F, Chen JM, Dimopoulos K, Everitt MD, Gatzoulis M, Harris L, Hsu DT, Kuvin JT, Law Y, Martin CM, Murphy AM, Ross HJ, Singh G, Spray TL; American Heart Association Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, and Council on Cardiovascular Radiology and Imaging . Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation 133: 770–801, 2016. doi: 10.1161/CIR.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 184.Sucharov CC, Dockstader K, Nunley K, McKinsey TA, Bristow M. β-Adrenergic receptor stimulation and activation of protein kinase A protect against α1-adrenergic-mediated phosphorylation of protein kinase D and histone deacetylase 5. J Card Fail 17: 592–600, 2011. doi: 10.1016/j.cardfail.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sucharov CC, Miyamoto SD, Garcia AM. Circulating microRNAs as biomarkers in pediatric heart diseases. (2018). doi: 10.1016/j.ppedcard.2018.02.008. [DOI] [Google Scholar]
- 186.Sucharov CC, Nakano SJ, Slavov D, Schwisow JA, Rodriguez E, Nunley K, Medway A, Stafford N, Nelson P, McKinsey TA, Movsesian M, Minobe W, Carroll IA, Taylor MRG, Bristow MR. A PDE3A Promoter Polymorphism Regulates cAMP-Induced Transcriptional Activity in Failing Human Myocardium. J Am Coll Cardiol 73: 1173–1184, 2019. doi: 10.1016/j.jacc.2018.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sucharov CC, Sucharov J, Karimpour-Fard A, Nunley K, Stauffer BL, Miyamoto SD. Micro-RNA expression in hypoplastic left heart syndrome. J Card Fail 21: 83–88, 2015. doi: 10.1016/j.cardfail.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tabbutt S, Ghanayem N, Ravishankar C, Sleeper LA, Cooper DS, Frank DU, Lu M, Pizarro C, Frommelt P, Goldberg CS, Graham EM, Krawczeski CD, Lai WW, Lewis A, Kirsh JA, Mahony L, Ohye RG, Simsic J, Lodge AJ, Spurrier E, Stylianou M, Laussen P; Pediatric Heart Network Investigators . Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg 144: 882–895, 2012. doi: 10.1016/j.jtcvs.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tanoue Y, Kado H, Shiokawa Y, Fusazaki N, Ishikawa S. Midterm ventricular performance after Norwood procedure with right ventricular-pulmonary artery conduit. Ann Thorac Surg 78: 1965–1971, 2004. doi: 10.1016/j.athoracsur.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 190.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 45: 205–251, 1993. [PubMed] [Google Scholar]
- 191.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res 118: 1021–1040, 2016. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Triedman JK, Bridges ND, Mayer JE Jr, Lock JE. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol 22: 207–215, 1993. doi: 10.1016/0735-1097(93)90836-P. [DOI] [PubMed] [Google Scholar]
- 193.Tunks RD, Barker PCA, Benjamin DK Jr, Cohen-Wolkowiez M, Fleming GA, Laughon M, Li JS, Hill KD. Sildenafil exposure and hemodynamic effect after Fontan surgery. Pediatr Crit Care Med 15: 28–34, 2014. doi: 10.1097/PCC.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Unegbu C, Noje C, Coulson JD, Segal JB, Romer L. Pulmonary hypertension therapy and a systematic review of efficacy and safety of PDE-5 inhibitors. Pediatrics 139: e20161450, 2017. doi: 10.1542/peds.2016-1450. [DOI] [PubMed] [Google Scholar]
- 195.Urashima T, Zhao M, Wagner R, Fajardo G, Farahani S, Quertermous T, Bernstein D. Molecular and physiological characterization of RV remodeling in a murine model of pulmonary stenosis. Am J Physiol Heart Circ Physiol 295: H1351–H1368, 2008. doi: 10.1152/ajpheart.91526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, van Dijk APJ, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJM. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation 127: 322–330, 2013. doi: 10.1161/CIRCULATIONAHA.112.135392. [DOI] [PubMed] [Google Scholar]
- 197.Van Puyvelde J, Verbeken E, Gewillig M, Meyns B. Fontan failure associated with a restrictive systemic ventricle. J Thorac Cardiovasc Surg 154: e7–e8, 2017. doi: 10.1016/j.jtcvs.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 198.Verma SK, Deshmukh V, Nutter CA, Jaworski E, Jin W, Wadhwa L, Abata J, Ricci M, Lincoln J, Martin JF, Yeo GW, Kuyumcu-Martinez MN. Rbfox2 function in RNA metabolism is impaired in hypoplastic left heart syndrome patient hearts. Sci Rep 6: 30896, 2016. doi: 10.1038/srep30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Voeller RK, Epstein DJ, Guthrie TJ, Gandhi SK, Canter CE, Huddleston CB. Trends in the indications and survival in pediatric heart transplants: a 24-year single-center experience in 307 patients. Ann Thorac Surg 94: 807–816, 2012. doi: 10.1016/j.athoracsur.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 200.Wang H, Anstrom K, Ilkayeva O, Muehlbauer MJ, Bain JR, McNulty S, Newgard CB, Kraus WE, Hernandez A, Felker GM, Redfield M, Shah SH. Sildenafil treatment in heart failure with preserved ejection fraction: targeted metabolomic profiling in the RELAX trial. JAMA Cardiol 2: 896–901, 2017. doi: 10.1001/jamacardio.2017.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Warburton D, Ronemus M, Kline J, Jobanputra V, Williams I, Anyane-Yeboa K, Chung W, Yu L, Wong N, Awad D, Yu CY, Leotta A, Kendall J, Yamrom B, Lee YH, Wigler M, Levy D. The contribution of de novo and rare inherited copy number changes to congenital heart disease in an unselected sample of children with conotruncal defects or hypoplastic left heart disease. Hum Genet 133: 11–27, 2014. doi: 10.1007/s00439-013-1353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Waspe LE, Ordahl CP, Simpson PC. The cardiac beta-myosin heavy chain isogene is induced selectively in alpha 1-adrenergic receptor-stimulated hypertrophy of cultured rat heart myocytes. J Clin Invest 85: 1206–1214, 1990. doi: 10.1172/JCI114554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Weinstein S, Bello R, Pizarro C, Fynn-Thompson F, Kirklin J, Guleserian K, Woods R, Tjossem C, Kroslowitz R, Friedmann P, Jaquiss R. The use of the Berlin Heart EXCOR in patients with functional single ventricle. J Thorac Cardiovasc Surg 147: 697–705, 2014. doi: 10.1016/j.jtcvs.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 204.Wessels MW, Willems PJ. Mutations in sarcomeric protein genes not only lead to cardiomyopathy but also to congenital cardiovascular malformations. Clin Genet 74: 16–19, 2008. doi: 10.1111/j.1399-0004.2008.00985.x. [DOI] [PubMed] [Google Scholar]
- 205.Whitehead KK, Harris MA, Glatz AC, Gillespie MJ, DiMaria MV, Harrison NE, Dori Y, Keller MS, Rome JJ, Fogel MA. Status of systemic to pulmonary arterial collateral flow after the Fontan procedure. Am J Cardiol 115: 1739–1745, 2015. doi: 10.1016/j.amjcard.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wilson TG, Iyengar AJ, d’Udekem Y. The use and misuse of ace inhibitors in patients with single ventricle physiology. Heart Lung Circ 25: 229–236, 2016. doi: 10.1016/j.hlc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 207.Xia C, Bao Z, Yue C, Sanborn BM, Liu M. Phosphorylation and regulation of G-protein-activated phospholipase C-β3 by cGMP-dependent protein kinases. J Biol Chem 276: 19770–19777, 2001. doi: 10.1074/jbc.M006266200. [DOI] [PubMed] [Google Scholar]
- 208.Yamagishi M, Kurosawa H, Hashimoto K, Nomura K, Kitamura N. The role of plasma endothelin in the Fontan circulation. J Cardiovasc Surg (Torino) 43: 793–797, 2002. [PubMed] [Google Scholar]
- 209.Yang C, Xu Y, Yu M, Lee D, Alharti S, Hellen N, Ahmad Shaik N, Banaganapalli B, Sheikh Ali Mohamoud H, Elango R, Przyborski S, Tenin G, Williams S, O’Sullivan J, Al-Radi OO, Atta J, Harding SE, Keavney B, Lako M, Armstrong L. Induced pluripotent stem cell modelling of HLHS underlines the contribution of dysfunctional NOTCH signalling to impaired cardiogenesis. Hum Mol Genet 26: 3031–3045, 2017. doi: 10.1093/hmg/ddx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yim DL, Jones BO, Alexander PM, d’Udekem Y, Cheung MM. Effect of anti-heart failure therapy on diastolic function in children with single-ventricle circulations. Cardiol Young 25: 1293–1299, 2015. doi: 10.1017/S1047951114002376. [DOI] [PubMed] [Google Scholar]
- 211.Zaidi S, Brueckner M. Genetics and genomics of congenital heart disease. Circ Res 120: 923–940, 2017. doi: 10.1161/CIRCRESAHA.116.309140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, Romano-Adesman A, Bjornson RD, Breitbart RE, Brown KK, Carriero NJ, Cheung YH, Deanfield J, DePalma S, Fakhro KA, Glessner J, Hakonarson H, Italia MJ, Kaltman JR, Kaski J, Kim R, Kline JK, Lee T, Leipzig J, Lopez A, Mane SM, Mitchell LE, Newburger JW, Parfenov M, Pe’er I, Porter G, Roberts AE, Sachidanandam R, Sanders SJ, Seiden HS, , et al. . De novo mutations in histone-modifying genes in congenital heart disease. Nature 498: 220–223, 2013. doi: 10.1038/nature12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang M, Takimoto E, Hsu S, Lee DI, Nagayama T, Danner T, Koitabashi N, Barth AS, Bedja D, Gabrielson KL, Wang Y, Kass DA. Myocardial remodeling is controlled by myocyte-targeted gene regulation of phosphodiesterase type 5. J Am Coll Cardiol 56: 2021–2030, 2010. doi: 10.1016/j.jacc.2010.08.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhu S, Cao L, Zhu J, Kong L, Jin J, Qian L, Zhu C, Hu X, Li M, Guo X, Han S, Yu Z. Identification of maternal serum microRNAs as novel non-invasive biomarkers for prenatal detection of fetal congenital heart defects. Clin Chim Acta 424: 66–72, 2013. doi: 10.1016/j.cca.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 215.Zhu Y, Gramolini AO, Walsh MA, Zhou YQ, Slorach C, Friedberg MK, Takeuchi JK, Sun H, Henkelman RM, Backx PH, Redington AN, Maclennan DH, Bruneau BG. Tbx5-dependent pathway regulating diastolic function in congenital heart disease. Proc Natl Acad Sci USA 105: 5519–5524, 2008. doi: 10.1073/pnas.0801779105. [DOI] [PMC free article] [PubMed] [Google Scholar]