Abstract
Patients with peripheral artery disease (PAD) have an accentuated exercise pressor reflex (EPR) during exercise of the affected limb. The underlying hemodynamic changes responsible for this, and its effect on blood flow to the exercising extremity, are unclear. We tested the hypothesis that the exaggerated EPR in PAD is mediated by an increase in total peripheral resistance (TPR), which augments redistribution of blood flow to the exercising limb. Twelve patients with PAD and 12 age- and sex-matched subjects without PAD performed dynamic plantar flexion (PF) using the most symptomatic leg at progressive workloads of 2–12 kg (increased by 1 kg/min until onset of fatigue). We measured heart rate, beat-by-beat blood pressure, femoral blood flow velocity (FBV), and muscle oxygen saturation () continuously during the exercise. Femoral blood flow (FBF) was calculated from FBV and baseline femoral artery diameter. Stroke volume (SV), cardiac output (CO), and TPR were derived from the blood pressure tracings. Mean arterial blood pressure and TPR were significantly augmented in PAD compared with control during PF. FBF increased during exercise to an equal extent in both groups. However, of the exercising limb remained significantly lower in PAD compared with control. We conclude that the exaggerated pressor response in PAD is mediated by an abnormal TPR response, which augments redistribution of blood flow to the exercising extremity, leading to an equal rise in FBF compared with controls. However, this increase in FBF is not sufficient to normalize the SmO2 response during exercise in patients with PAD.
NEW & NOTEWORTHY In this study, peripheral artery disease (PAD) patients and healthy control subjects performed graded, dynamic plantar flexion exercise. Data from this study suggest that previously reported exaggerated exercise pressor reflex in patients with PAD is driven by greater vasoconstriction in nonexercising vascular territories which also results in a redistribution of blood flow to the exercising extremity. However, this rise in femoral blood flow does not fully correct the oxygen deficit due to changes in other mechanisms that require further investigation.
Keywords: exercise pressor response, hemodynamic responses, peripheral artery disease
INTRODUCTION
Peripheral artery disease (PAD) is an atherosclerotic occlusive disease commonly affecting the arterial supply to the lower extremity, with an estimated prevalence of 8–12 million in the United States (45) and 202 million globally (51). The disease is progressive and portends a higher risk of cardiovascular morbidity and mortality to the affected individuals (9). A common symptomatic manifestation of the disease is exercise-induced pain known as intermittent claudication due to skeletal muscle demand ischemia. In addition to pain, the ischemic muscle during exercise is also responsible for triggering an exaggerated exercise pressor reflex (EPR) in patients with PAD (4, 29, 34, 38, 39, 46). This accentuated EPR in PAD occurs even at a very low intensity of exercise before the onset of pain, and the magnitude of response is positively correlated with the severity of disease (15, 38). Moreover, exaggerated blood pressure responses during exercise in PAD are not only negatively associated with functional limitation (16) but also predict mortality in this population (13, 14).
The EPR is a cardiovascular reflex with its afferent loop originating from thin afferent fibers in the exercising muscle. Mechanically sensitive group III afferents trigger the mechanoreflex, and group IV afferents sensitive to muscle metabolites trigger the metaboreflex, both of which are integral components to the EPR (1, 22, 26, 32, 48). The efferent loop modulates cardiac output, vascular resistance, and ventilation to raise blood pressure (8, 31, 35, 36) and ensure adequate delivery of oxygen to the exercising muscle (2, 42). Although pain can be a potent stimulator of cardiovascular reflexes (the “cardiovascular defense reaction”) (63), the exaggerated pressor response during exercise in PAD is seen before the onset of claudication (15, 38). Importantly, reductions in muscle oxygen saturation () before the onset of claudication are more pronounced in patients with PAD and closely associated with changes in blood pressure, suggesting that muscle ischemia plays a significant role in eliciting the exaggerated EPR in PAD (30, 54). It is thought that increased production and accumulation of muscle metabolites, such as hydrogen ion (57), potassium ion (24), diprotonated phosphate (58), lactic acid (23), and ATP (27), lead to increased activation of the EPR and augmented peripheral sympathetic outflow, and an exaggerated pressor response through its effect on cardiac output (CO) and total peripheral resistance (TPR). The effects of muscle mechanoreflex engagement on the augmented sympathetic response will be determined in future investigations. It is speculated that muscle metabolite accumulation in the ischemic muscle of patients with PAD sensitizes mechanically sensitive muscle afferent nerves, enhancing the sympathetic response during activation of muscle mechanoreflex. Whether the exaggerated pressor response in PAD is primarily flow mediated (i.e., predominantly via a rise in CO) or mediated by an increase in TPR is currently unclear. However, it has been shown that the hemodynamic “phenotype” of the EPR is dependent on the intensity of exercise (3), as well as underlying cardiovascular comorbidities, such as heart failure, which may limit the overall CO response during exercise (10, 19).
Activation of the EPR ensures that 1) there is adequate perfusion, thus delivery of O2, to the exercising muscle and 2) blood pressure is adequately maintained during exercise (2, 8, 31, 36, 42). The EPR maintains an adequate level of perfusion to the exercising muscle by increasing CO and, to a varying degree, mobilizing visceral blood flow to the extremities by increasing vascular resistance (7). However, excessive perfusion to the exercising muscle can lead to “outstripping” of the CO if not regulated (50). Thus the EPR can also negatively modulate skeletal muscle blood flow by increasing sympathetic vasoconstrictive tone to the exercising muscle, thus competing with local vasodilatory mechanisms (56). To what extent the EPR influences local blood flow in PAD, and how that might impact in the exercising muscle is currently unclear. Specifically, it is not clear whether the exaggerated EPR in PAD leads to an increase in blood flow or to further restriction of blood flow via sympathetic vasoconstriction in the exercising muscle of the diseased limb.
The purpose of this investigation was to characterize the systemic and local hemodynamic changes in response to activation of the EPR in patients with PAD. We sought to isolate and characterize the hemodynamic responses elicited by activation of the EPR by the affected lower extremity during dynamic exercise simulating normal ambulatory activity. This was performed using a previously described exercise paradigm using a foot pedal mounted to a platform that allowed the subjects to perform isolated plantar flexion in the supine position. Our prior studies have shown that this paradigm elicits an exaggerated EPR in patients with PAD compared with healthy subjects (34, 38, 39). We measured blood pressure, CO, TPR, and femoral blood flow (FBF), femoral conductance, and continuously to test the hypothesis that compared with healthy controls, an increase in TPR has a larger contribution to the exaggerated EPR in PAD, which augments blood flow to the exercising extremity.
METHODS
Study Subjects
Twelve patients with PAD and 12 healthy control subjects who were matched for sex, age, and body mass index (BMI) participated in the study. The study was designed to compare the EPR and the accompanying changes in systemic and local hemodynamics in subjects with and without PAD using noninvasive techniques. The study protocol, and all performed procedures were approved by the Institutional Review Board of the Penn State Health Milton S. Hershey Medical Center in agreement with the guidelines set forth by the Declaration of Helsinki. All subjects provided written informed consent. Subjects with PAD were recruited from the Penn State Heart and Vascular Institute Vascular Surgery Clinic, and those without PAD were recruited from the local community. Subjects with PAD were not excluded based on prior lower limb revascularization procedures; however, they were only considered if their most recent ankle-brachial index (ABI), the ratio of the blood pressure at the ankle and the arm, was <0.9, which is an indication of PAD in the lower limb. Subjects with critical limb ischemia (rest pain, ulcers, or gangrenes of the affected extremity) were excluded from the study. Subjects in the control group were selected so that each PAD subject had a corresponding match based on the same gender, similar age, and a BMI closely matched within 10%. All subjects underwent a thorough medical screening to perform eligibility. Exclusion criteria included 1) diabetic patients with poor glycemic control, or any evidence of peripheral neuropathy; 2) history of unstable angina or myocardial infarction within 6 mo of the study; and 3) chronic kidney disease (eGFR <59 mL/min). All subjects invited to the study underwent a screening medical examination before inclusion.
Study Protocol
The study protocol was designed with two primary aims: 1) measurement of systemic hemodynamic responses during lower extremity exercise; and 2) measurement of local hemodynamic responses and tissue oxygen saturation during lower extremity exercise. An isolated, single leg, graded, dynamic plantar flexion exercise in the supine position was chosen as the exercise modality for the lower extremity. This exercise modality (i.e., plantar flexion) showed good reliability and correlation with treadmill testing (66). Also, it has been used extensively by our group and our prior studies have shown that this exercise is well tolerated and elicits an exaggerated EPR in subjects with PAD (15, 30, 33, 38).
A total of 12 subjects with PAD and 12 control subjects (8 men and 4 women in each group) were deemed eligible to participate in the study. The average ages of the subjects were 64.4 ± 1.8 and 65.5 ± 1.9 in the control and PAD groups, respectively. The two groups differed mainly in their ABI (all P < 0.0001). The PAD cohort was Fontaine Stage IIb or less with an average ABI of 0.69 ± 0.04 for the right leg and 0.67 ± 0.04 for the left leg. The control cohort had minimum comorbidities compared with the PAD cohort, and had an average ABI of 1.12 ± 0.03 for the right leg and 1.07 ± 0.03 for the left leg. Further demographic and clinical characteristics of our study cohort are shown in Table 1. Ambulatory blood pressure and heart rate were similar in both groups (Table 2).
Table 1.
Subject characteristics
| Control | PAD | P Value | |
|---|---|---|---|
| n | 12 | 12 | |
| Sex, n | |||
| Men | 8 | 8 | |
| Women | 4 | 4 | |
| Age, yr | 65 ± 2 | 66 ± 2 | 0.6768 |
| Height, cm | 174.3 ± 2.9 | 169.4 ± 3.0 | 0.2547 |
| Weight, kg | 81.8 ± 4.8 | 77.8 ± 4.9 | 0.5725 |
| BMI, kg/m2 | 26.6 ± 1.0 | 26.6 ± 1.0 | 0.9899 |
| ABI | |||
| Right | 1.12 ± 0.03 | 0.69 ± 0.04* | <0.0001 |
| Left | 1.07 ± 0.03 | 0.67 ± 0.04* | <0.0001 |
| Prior revascularization | 4 | ||
| Disease location | |||
| Aortoiliac | 2 | ||
| Femoropopliteal | 8 | ||
| Infrapopliteal | 3 | ||
| Multiple | 3 | ||
| Medications | |||
| Anticoagulants | 2 | ||
| Plavix | 7 | ||
| Aspirin | 9 | ||
| Statins | 8 | ||
| Antihypertensives | 5 | ||
| β-Blockers | 4 | ||
| Drugs labeled for PAD | 3 |
Values are means ± SE. PAD, peripheral artery disease; BMI, body mass index; ABI, ankle-brachial index; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure.
P < 0.05, significant difference between control subjects and patients with PAD.
Table 2.
Baseline hemodynamics
| Control | PAD | P Value | |
|---|---|---|---|
| n | 12 | 12 | |
| SBP, mmHg | 117.4 ± 2.6 | 126.3 ± 4.4 | 0.0980 |
| DBP, mmHg | 75.8 ± 2.1 | 71.8 ± 3.0 | 0.2732 |
| MAP, mmHg | 89.7 ± 2.0 | 89.9 ± 2.7 | 0.9477 |
| HR, beats/min | 67.9 ± 2.0 | 66.3 ± 2.2 | 0.5750 |
| n | 11 | 11 | |
| SV, mL | 91.0 ± 5.6 | 78.5 ± 8.1 | 0.2190 |
| CO, L/min | 5.6 ± 0.3 | 4.9 ± 0.4 | 0.1782 |
| TPR, dyn·s·cm−5 | 1,367.8 ± 109.5 | 1,616.3 ± 133.7 | 0.1667 |
| n | 10 | 10 | |
| FBF, mL/min | 136.8 ± 37.4 | 184.0 ± 65.0 | 0.5396 |
| FVC, mL·min−1·mmHg−1 | 1.5 ± 0.4 | 2.0 ± 0.7 | 0.5166 |
Values are means ± SE. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; HR, heart rate; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance; FBF, femoral blood flow; FVC, femoral vascular conductance.
Of the 24 subjects enrolled in the study, one subject in the PAD group could not complete all three visits due to personal reasons. This subject, including the matched control subject, was not included in the analysis of systemic hemodynamic changes. Additionally, FBF could not be determined in one separate subject due to technical difficulties. This subject, including the matched control subject, was not included in the analysis of local hemodynamic changes. There were no significant changes to the average subject characteristics in the subset of subjects used for the final analysis.
All participants were asked to abstain from exercise, caffeine, and alcohol for 24 h and fast for at least 2 h before the study. Subjects were allowed to adhere to their normal medication schedule as prescribed. Whenever possible, subjects were asked to participate in three separate study visits to perform the measurements as described below. During the first visit, femoral blood flow velocity (FBV) and diameter of the exercising leg were collected and those of the nonexercising leg were collected during the second visit. The third visit was repeated to average systemic hemodynamic measurements obtained by finger plethysmography.
Graded, dynamic plantar flexion exercise protocol.
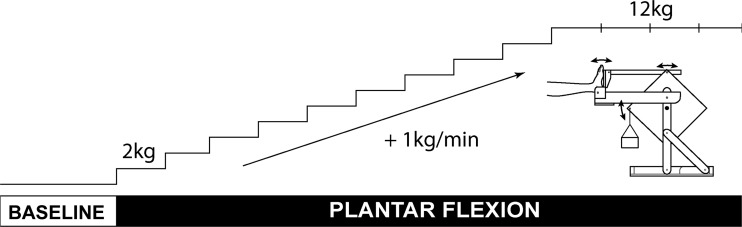
Subjects performed an isolated, graded plantar flexion exercise in the supine position similar to that described previously (38). Subjects had their most symptomatic foot (PAD) or left foot (control) strapped into a custom-made foot-pedal platform allowing rotation at the ankle. This foot pedal was connected via pushrods to a rotary device that lifted a weight pan during each plantar flexion followed by gravity-assisted return to neutral position. Weight was incrementally increased during each exercise paradigm. Subjects performed 30 plantar flexions/minute guided by a sound cue. The exercise was graded, starting at a workload of 2 kg, which was increased by 1 kg every minute up to 12 kg until subjects were unable to maintain the cadence of the protocol or reached the maximum exercise duration of 14 min (Fig. 1). After 12 kg, the workload remained constant for the remainder of the protocol. The level of fatigue at the end of exercise was assessed using the Borg scale, and the level of pain was assessed using a numeric pain intensity scale (0–10).
Fig. 1.
Plantar flexion protocol schematics. After 3 min of baseline, subject performed plantar flexion exercise with rate of 30 contractions/min starting at workload of 2 kg. Workload was increased by 1 kg/min up to 12 kg until subjects were fatigued or reached the maximum exercise duration of 14 min.
Measurement of systemic hemodynamic responses during graded, dynamic plantar flexion.
All subjects underwent an echocardiographic evaluation (Vivid 7 dimension, GE Healthcare) on their first visit to ensure normal cardiac function and measure baseline CO. CO was calculated from the B-mode images using the EchoPAC (GE Healthcare). Baseline blood pressure was obtained using an automated pneumatic blood pressure cuff in the supine position, and used to calculate baseline TPR. Pulsatile blood pressure was measured continuously during the experiment using a finger plethysmograph (Finometer PRO, Finapress Medical Systems). Heart rate was monitored continuously using a three-lead ECG (Cardiocap/5, Datex-Ohmeda). CO and TPR were monitored continuously during the experiment using the Modelflow-based estimates from the Beatscope (Beatscope 1.1, Finapres Medical Systems) software attached to the Finometer. CO derived from finger plethysmographic recordings using the Modelflow technique has been validated against other standard techniques such as pulsed-Doppler cardiography, and inert gas rebreathing (6, 43, 60, 61, 64), and found to provide a reliable estimate of short-term changes in CO during an experiment. Baseline values from the finger plethysmograph measurements were calibrated based on baseline measurements using the pneumatic blood pressure cuff and echocardiography. Systemic hemodynamic measurements obtained by finger plethysmography during the three separate visits were averaged to obtain the mean hemodynamic responses to exercise for each individual subject. Before averaging, the data were assessed for outliers using robust estimates of the center and spread of the total peripheral resistance data for each group. The median and interquartile range for the total peripheral resistance data for each group across all exercise workloads and all three visits were used as the estimate for the center and spread. Values that were four times the spread away from the center were considered outliers. Visits containing data with outliers were excluded from the final average.
Measurement of local hemodynamic responses and during graded, dynamic plantar flexion.
Local hemodynamic responses to plantar flexion exercise were assessed using ultrasonographic (Philips iE33) evaluation of the common femoral artery. B-mode and pulsed-wave Doppler ultrasound were performed at baseline and during exercise to assess femoral artery diameter and FBV. FBV measurements were taken at a constant insonation angle of 60° with the sample volume adjusted to cover the width of the artery. High-resolution diameter measurements (6 MHz probe) were taken from 15-s recordings performed between measurements of flow velocity. The audio output containing the Doppler signal was converted to an analog signal proportional to blood flow velocity using a custom-made signal converter (Doppler Audio Translator) and sampled concurrently with other hemodynamic signals using PowerLab (ADInstruments). Measurements were separately performed in the exercising and nonexercising leg during two separate visits. Velocity measurements were sampled in real time (400 Hz) using a data-acquisition system (Powerlab, ADInstruments, Castle Hill, Australia). High-resolution diameter measurements were taken during baseline as 2D images and analyzed on the ultrasound device (Philips iE33). FBF at each workload was calculated by multiplying the baseline cross-sectional area of the femoral artery with mean blood velocity using the equation π(diameter/2)2 × velocity × 60. Femoral vascular conductance (FVC) was calculated by dividing FBF by mean arterial pressure (MAP). All vascular analyses were conducted by a single investigator.
In addition to blood flow velocity, a NIRS device (Moxy muscle oxygen monitor; Fortiori Design, Hutchinson, MN) was used to monitor local a measure of muscle oxygen saturation, at the medial gastrocnemius in both legs of every participant. The Moxy uses continuous-wave spectroscopy which uses light of constant intensity that travels through the tissue and is collected by the receiver a certain distance away from the light source (40). For its light source, the NIRS device uses four light emitting diodes (LEDs) at 680, 720, 760, and 800 nm. Also, Moxy has a unique data processing algorithm based on the Monte Carlo stimulation on a skin model with four different layers (epidermis, dermis, fat, and muscle). This allows the NIRS device to have improved accuracy of the level measure with more sensitivity to the muscle layer and less sensitivity to the fat layer. Moxy in particular has source and receiver distance of 12 and 25 mm, which means it can penetrate into the tissue down to 12.5 mm. To make sure the NIRS device was collecting reliable levels, the thickness of the skin and fat layers, at the site where the devices were placed, was measured using ultrasound imaging for every participant on their first visit. Three manually selected points were averaged and used to account for the length between the emitter optode’s furthest detector optode at 25 mm. Data from Moxy devices were recorded continuously at 2 Hz through software called PeriPedal (v2.4.8; Napoleon, IN). Local hemodynamic data were not replicated across the three visits, and therefore analysis was based on measurements obtained during a single visit.
Data Analysis
All signals collected through PowerLab were analyzed off-line using custom-written macro routines in PowerChart. Systemic hemodynamic parameters obtained in Beatscope was exported into PowerChart compatible format and analyzed in the same manner. data were exported into a text file and analyzed separately using spreadsheet software (Microsoft Excel). All continuously recorded values during the plantar flexion protocol were averaged over 1-min intervals. Baseline values were obtained by averaging the signal over the 3-min period preceding the exercise. Mean arterial pressures derived from the Finometer measurements were corrected using blood pressure measurements obtained using a brachial cuff during baseline. Baseline CO was calculated from the echocardiographic measurements obtained at baseline using EchoPAC (GE Healthcare). Baseline TPR was calculated using the CO from echo and blood pressure obtained using the brachial artery cuff during baseline. Changes in CO and TPR during exercise were calculated as percent changes from baseline.
Statistical Analysis
Between-group comparisons of demographic, clinical, and baseline hemodynamic characteristics were made using a Student’s t test. Between- and within-group changes in systemic and local hemodynamic parameters during graded plantar flexion exercise was analyzed using a two-way repeated measure analysis of variance (ANOVA). Post hoc comparisons for between- and within-group factors were performed when indicated using the Bonferroni or Dunnet’s method. Statistical analyses were performed using Prism 7 (GraphPad Software, La Jolla, CA) and JMP (SAS Institute). α-Levels were set at P < 0.05, with appropriate adjustments made for multiple comparisons. Final summary data are expressed as means ± SE.
RESULTS
Changes in Systemic Hemodynamics During Graded, Dynamic Plantar Flexion
There were no group differences in baseline supine blood pressure or heart rate. Differences in baseline stroke volume, CO, and TPR determined using echocardiographic measurements during visit 1 were not statistically significant between the two groups. As expected, subjects with PAD could only perform plantar flexion up to an average peak load of 9.20 ± 0.7 kg vs. 12 kg in control subjects. This resulted in a significantly shorter exercise duration in subjects with PAD (528.6 ± 58.0 s) compared with control (832.8 ± 7.3 s). However, there were no group differences in the perceived exertion at completion of the plantar flexion routine (Table 3). Claudication was the primary limiting factor in subjects with PAD, with an average reported pain level of 5.3 ± 0.7 on a numerical rating scale. In contrast, control subjects completed the routine with minimal reported pain (Table 3).
Table 3.
Exercise performance
| Control | PAD | P Value | |
|---|---|---|---|
| n | 11 | 11 | |
| Duration, s | 832.8 ± 7.3 | 528.6 ± 58.0* | 0.0004 |
| Peak load, kg | 12.0 ± 0.03 | 9.2 ± 0.70* | 0.0026 |
| RPE | 15.4 ± 0.5 | 15.4 ± 0.7 | 1.0000 |
| Reported pain | 0.14 ± 0.14 | 5.3 ± 0.7* | <0.0001 |
Values are means ± SE. This table reports averages of 3 visits for exercise performance data. RPE, rated perceived exertion.
P < 0.05, significant difference between control subjects and patients with PAD.
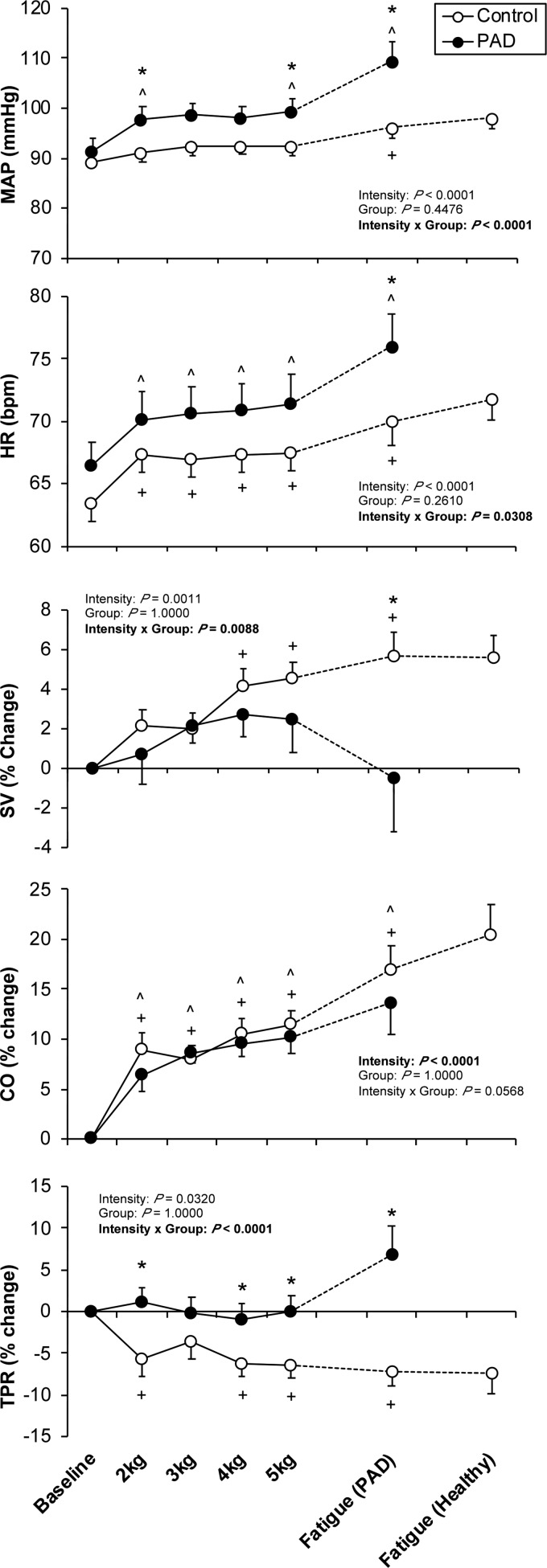
Subjects with PAD had a higher rise in blood pressure during plantar flexion compared with control. This exaggerated pressor response was evident during the early presymptomatic stage of the routine, and accentuated at fatigue (i.e., following the onset of symptoms). In contrast, the pressor response observed in the control subjects was not significantly above baseline until after a workload of 6 kg. The blood pressure response during the presymptomatic stage was accompanied by an increase in heart rate and CO in both groups. At the time of fatigue in the patients with PAD, the rise in heart rate was greater in subjects with PAD compared with control; however, there were no statistically significant group differences in CO at any stage of the plantar flexion routine. In contrast, there was a significant group difference in the changes in TPR during plantar flexion both during the presymptomatic stage of the routine and at fatigue. In control subjects, TPR dropped below baseline during plantar flexion. However, in subjects with PAD, this drop in TPR was absent during submaximal exercise and was significantly above baseline at the onset of fatigue (Fig. 2).
Fig. 2.
Systemic hemodynamic responses to plantar flexion exercise in peripheral artery diseases (PAD) patients and healthy controls. Data are expressed as means ± SE. Data are collected from finger plethysmography and derived by the ModelFlow technique in patients with PAD (n = 11, ●) and matched healthy controls (n = 11, ○) during plantar flexion exercise. HR, heart rate; MAP, mean arterial pressure; CO, cardiac output; TPR, total peripheral resistance; SV, stroke volume. *P < 0.05, significant difference between control and PAD. ^P < 0.05, significant difference from baseline in PAD patient. +P < 0.05, significant difference from baseline in healthy control.
Changes in Local Hemodynamics During Graded, Dynamic Plantar Flexion
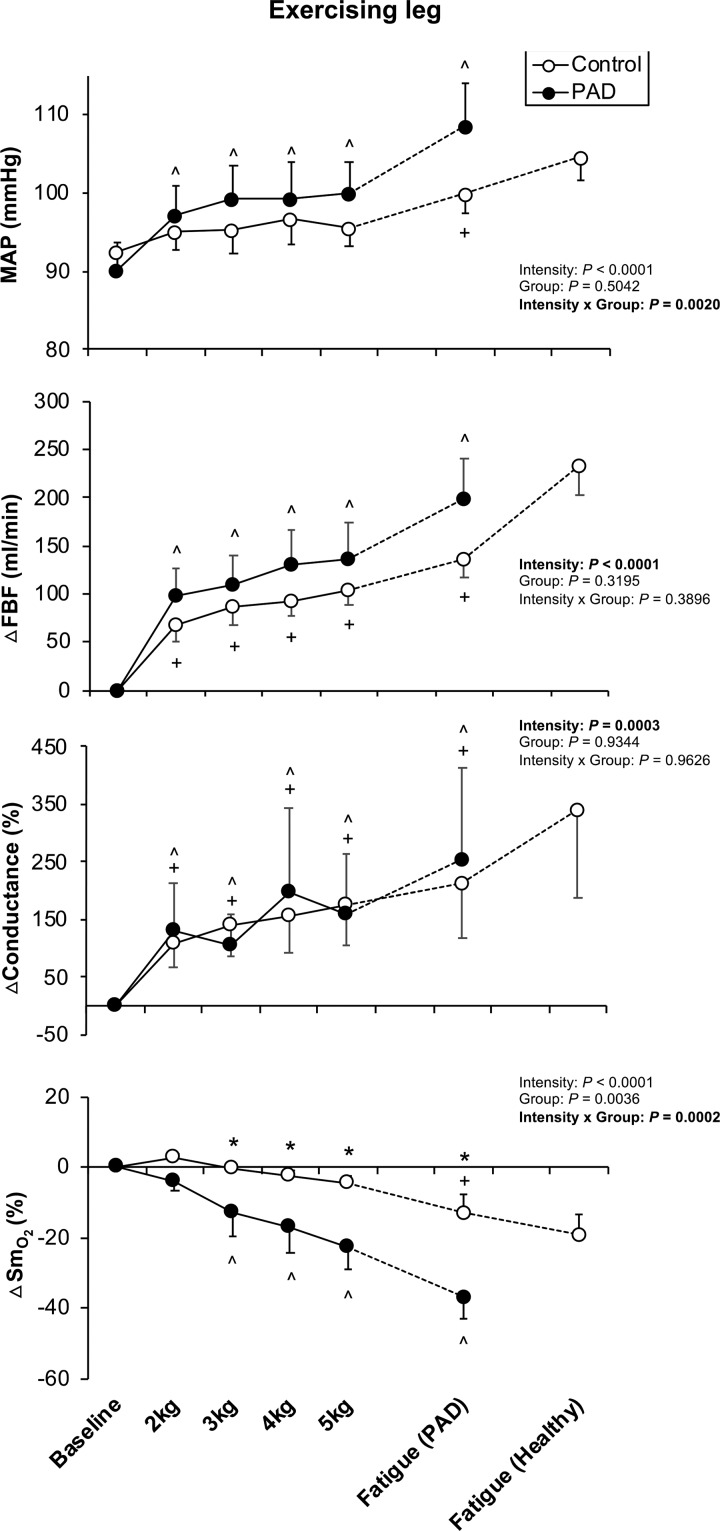
During plantar flexion exercise, a significant interaction was observed for MAP (interaction P = 0.0020; Fig. 2). Throughout the plantar flexion exercise, patients with PAD had a higher MAP response compared with the control group (MAP at peak: 108.7 ± 5.2 mmHg in PAD vs. 100.0 ± 2.6 mmHg in control). FBV was also significantly greater in PAD compared with control (interaction P = 0.0002), starting from 4 kg significant augmentation in FBV was observed in PAD. Despite the changes in FBV, FBF showed no significant interaction for group and intensity (interaction P = 0.3896). Moreover, FVC calculated from FBF and MAP also revealed no significant interaction (interaction P = 0.9626). On the other hand, patients with PAD had a significantly greater reduction in in the more affected leg during plantar flexion exercise (interaction P = 0.0002) compared with healthy control (Δ at peak: −37.0 ± 5.7% in PAD vs. −12.8 ± 5.3% in control). This exaggeration of reduction in was observed at early stages of exercise (i.e., 3 kg) and continued until the fatigue (Fig. 3). For comparison, the less affected leg was tested on a separate day (visit 2) and showed no significant differences in FBF, FVC, and between control subjects and patients with PAD (all P > 0.05).
Fig. 3.
Local hemodynamic and muscle oxygenation responses to plantar flexion exercise in peripheral artery disease (PAD) patients and healthy controls. Data are expressed as means ± SE. Data are collected from the leg of patients with PAD (n = 10, ●) and same leg of the matched healthy controls (n = 10, ○). Data are from exercising leg (most symptomatic leg) on visit 1. MAP, mean arterial pressure; FBF, femoral blood flow; , skeletal muscle oxygen saturation. *P < 0.05, significant difference between control and PAD. ^P < 0.05, significant difference from baseline in patients with PAD. +P < 0.05, significant difference from baseline in healthy control.
DISCUSSION
The objective of this study was to 1) examine the systemic hemodynamic mechanisms contributing to the exaggerated EPR; and 2) determine the effect of the EPR on blood flow to the exercising extremity in patients with PAD. We found that activation of the EPR during graded dynamic plantar flexion exercise causes a similar rise in CO in both healthy subjects and patients with PAD. There was no change in TPR until the onset of claudication in patients with PAD; however, this response differed significantly compared with healthy subjects where a decrease in TPR was noted during plantar flexion. FBF increased, and vascular conductance in the exercising extremity increased equally during exercise in healthy controls and patients with PAD. These findings suggest that the exaggerated EPR during dynamic plantar flexion exercise in patients with PAD is driven by an altered TPR response compared with healthy individuals. The exaggerated EPR raises FBF to a similar extent to that seen in healthy individuals; however, the increase in FBF is not sufficient to normalize in the lower limb in patients with PAD.
Exaggerated EPR During Dynamic Plantar Flexion is Mediated by an Altered TPR Response in PAD
The systemic hemodynamic mechanism mediating the EPR is dependent on several factors including the modality of exercise (static vs. rhythmic), intensity of exercise, and the limb evoking the response (arm vs. leg) (7). During submaximal, rhythmic exercise of the lower extremity, however, studies in canine models (25, 59) and humans (2) suggest that increases in CO is the primary mediator of the pressor response in this reflex. Our results in healthy controls (an EPR accompanied by rise in CO with a concomitant drop in TPR) is consistent with the primary role of CO as a mediator of the EPR. In control subjects, TPR decreased likely as a result of increased vascular conductance to the exercising leg as supported by the increase in FVC measured during exercise. On the other hand, TPR in patients with PAD did not decrease during the presymptomatic stages of the exercise as seen in controls, and subsequently rose above baseline at fatigue. Although patients with PAD have been shown to have impaired endothelial-dependent and -independent mechanisms of vasodilation (28, 52), the observation that FVC rose equally suggests that local impairment in vasodilatory function did not have a significant impact on the absence of a reduction in TPR during exercise. Additionally, there was no difference in the CO response between the two groups, suggesting that a limitation in the CO response was not responsible for the larger contribution of TPR as has been reported in canine models and human patients with heart failure (10, 21, 41, 55)
Our results suggest that the altered TPR response in patients with PAD is secondary to an increase in sympathetic vasoconstrictor tone during exercise, which then mediates the incrementally larger EPR. In healthy subjects, sympathetic discharge during exercise is related to the intensity, mode, and duration of exercise, and related to the degree of metabolite accumulation in the exercising muscle (53). It is possible that the more ischemic environment within the exercising muscle of patients with PAD leads to an incrementally higher level of sympathetic outflow following activation of the EPR, leading to the observed response in TPR. This could be due to an increased level of metabolite accumulation for any given amount of exercise, increased sensitivity of group III/IV muscle afferents to mechanical and metabolic stimuli, or a combination of both (12, 47, 49). Increased sensitivity of group III/IV muscle afferents is supported by a human study performing muscle stretch with metabolite accumulation (12) and by animal studies which have shown that exposure to skeletal muscle ischemia-reperfusion leads to sensitization of both group III/IV muscle afferents, an increase in renal sympathetic nerve activity, and an exaggerated EPR (47). Alternatively, changes in vasomotor responsiveness to adrenergic stimuli may be altered in PAD, which could produce the observed TPR response in the absence of an increase in sympathetic discharge. Enhanced vascular reactivity to an adrenergic stimulus has been shown in animal models of atherosclerosis (20). A desensitized baroreflex in patients with PAD can also contribute to the altered TPR response during exercise as this patient population has attenuated baroreflex sensitivity and vagal tone compared with healthy controls (11).
Our study cannot differentiate between these possible mechanisms, which would be an important subject for future studies. We have previously shown that renal vasoconstriction is enhanced during exercise in patients with PAD (15), suggesting that muscle afferent activation during dynamic plantar flexion leads to increased visceral vasoconstriction that may contribute to the overall TPR response seen in the present study.
Exaggerated EPR Raises Blood Flow to the Exercising Extremity During Dynamic Plantar Flexion Exercise in Patients with PAD
As we have previously reported, the early drop in during plantar flexion exercise plays an essential role in triggering the exaggerated EPR seen in patients with PAD (30). Without measurements of local blood flow, however, it was unclear whether engagement of the EPR was acting to restore flow to the ischemic leg in patients with PAD or whether it had a negative impact on local perfusion. The finding in this report showing a similar increase in FBF and FVC between patients with PAD and controls suggests that early engagement of the EPR during dynamic plantar flexion in patients with PAD raises blood flow to levels that match those seen in healthy controls performing the same intensity of exercise. However, despite this matched perfusion at the level of the femoral artery, is persistently lower in patients with PAD compared with controls. Several factors could contribute to this observation, including 1) blood flow at the level of the femoral artery is not indicative of calf perfusion; and 2) the response in calf muscle is influenced by changes in skeletal muscle efficiency specific to patients with PAD.
Since the local blood flow was measured at the level of the common femoral artery, the regional blood flow distribution downstream from the site of measurements, especially in the presence of downstream occlusive lesions, may not be represented by FBF. In fact, the majority of patients in our PAD study cohort had femoropopliteal disease (66%), 25% had infrapopliteal disease, and another 25% had multiple lesions spanning lower extremity circulation. Therefore, the disparity between matched FBF and attenuated calf in patients with PAD may be a result of impaired calf muscle perfusion due to stenosis or occlusion distal to the measurement site during exercise. This could be addressed in future studies comparing the local distribution of blood flow distal to the common femoral artery using more sophisticated imaging modalities (17).
The observed discrepancy in FBF and the calf response between healthy subjects and patients with PAD is consistent with a prior report showing a larger drop in during a static plantar flexion exercise at a low workload (10% of MVC) despite having similar levels of blood flow compared with healthy controls (5). This may be explained by consistently reported impairments in muscle quality in patients with PAD. The patient population is shown to have an increased level of apoptosis (37), skeletal muscle degeneration (62), and fibrosis in their gastrocnemius muscles (18). Moreover, a prior investigation reported higher oxygen uptake levels for pain-free ambulation in patients of PAD compared with age-matched healthy controls. This suggests that patients with PAD have impaired walking economy, a functional measure of muscle efficiency (65). Collectively, the observation of a greater reduction in calf despite matched FBF in patients with PAD may be explained by an increased O2 cost due to the skeletal muscle inefficiency to perform the same absolute workload as the healthy control group. To elucidate the relationship between skeletal muscle efficiency and the hemodynamic response in patients with PAD, further investigations are warranted.
Experimental Considerations and Limitations
First of all, for FBF calculation, an average of three resting femoral artery diameters measured in between resting FBV measurements was used due to the difficulty in data acquisition. A second consideration is the use of finger blood pressure waveform analysis (Modelflow) to estimate stroke volume, CO, and TPR. While also limited with respect to absolute values, this is a widely used method in the EPR literature that acceptably tracks changes in these parameters. Thus considerable efforts were undertaken to calibrate Modelflow values to the resting echo and to calculate the percent change from baseline. Another limitation of this study is the potential effects of the medications used in patients with PAD on the EPR. Medications could have altered the mechanisms of blood pressure control from a CO-mediated to a vasoconstriction-mediated rise in arterial pressure, leading to an exaggerated TPR response to exercise (55). However, in the present study, the doses of the β-blockades used were relatively low (25–50 mg of metoprolol and atenolol), and the HR response to plantar flexion was not altered by them (data not shown). Moreover, the other medications used would have tended to reduce TPR responses. Thus we are confident that the greater TPR responses to plantar flexion in patients with PAD were not caused by medications taken.
In this study, patients with PAD with prior lower limb revascularization procedures were included if they had ABI values <0.9. All prior surgical revascularization procedures were done more than 2 yr before the study visit. One patient was studied 8 mo after an angioplasty had been performed for an occluded graft. At the time of the study, ABI was 0.66 in the exercising leg. Three of these four patients had recent duplex scans showing reocclusion or occlusion at new distal or proximal sites. The exaggerated pressor response seen at the time of fatigue in the patients with PAD could have been due to greater activation of nociceptors in the patients with PAD since these subjects had significantly greater levels of pain (5.3 ± 0.7) compared with those seen in the healthy controls (0.14 ± 0.14) (Table 2). Nociceptor activation during small muscle exercise can influence the blood pressure response (44). Thus the significantly greater EPR seen in our patients with PAD could have been due, at least in part, to greater activation of muscle nociceptors. We should note however, that we saw increases in TPR at workloads before the time when limb pain was sensed.
It also needs to be noted that the patients with PAD compared with the control subjects were working at a higher relative workload when the reflex effects were observed. This could have increased central command activation, resulting in an augmented increase in the blood pressure response to exercise. Finally, in this study, we do not report data on microvascular perfusion of the exercising calf muscles.
Conclusion and Clinical Significance
In conclusion, despite the matched FBF, patients with PAD exhibit a greater than healthy controls during the same absolute workload of plantar flexion. Collectively, these results suggest that patients with PAD have an exaggerated EPR via vasoconstriction in vascular territories other than the exercising extremity, resulting in redistribution of blood flow to the exercising extremity. However, this rise in FBF does not fully correct the oxygen deficit due to changes in other mechanisms that require further investigation. Future work will be necessary to determine whether interventions such as rehabilitation reduce TPR and alter the distribution of flow to the ischemic lower limb musculature. Our data suggest that during exercise the exaggerated pressor response improves conduit flow but does not restore perfusion of the ischemic muscle.
GRANTS
This project was funded by National Heart, Lung, and Blood Institute Grant P01-HL-134609 (to L. I. Sinoway) and Penn State Clinical and Translational Science Institute Grant UL1-TR002014 (to L I. Sinoway).
DISCLAIMERS
The grants specifically disclaim responsibility for any data collection, analyses, interpretations, or conclusions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.J.-K.K., M.T.K., J.C., Z.G., A.J.M., and L.I.S. conceived and designed research; D.J.-K.K., M.T.K., J.C., Z.G., J.C.L., S.P., and A.J.M. performed experiments; D.J.-K.K., M.T.K., J.C.L., and S.P. analyzed data; D.J.-K.K., M.T.K., J.C., Z.G., J.C.L., and L.I.S. interpreted results of experiments; D.J.-K.K. and M.T.K. prepared figures; D.J.-K.K., M.T.K., and L.I.S. drafted manuscript; D.J.-K.K., M.T.K., J.C., Z.G., J.C.L., S.P., A.J.M., and L.I.S. edited and revised manuscript; D.J.-K.K., M.T.K., J.C., Z.G., J.C.L., S.P., A.J.M., and L.I.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Cheryl Blaha and Aimee Cauffman for nursing assistance and Jen Stoner and Kris Gray for administrative support, and the Heart and Vascular Institute at the Pennsylvania State University College of Medicine for support.
REFERENCES
- 1.Abrahams VC. Group III and IV receptors of skeletal muscle. Can J Physiol Pharmacol 64: 509–514, 1986. doi: 10.1139/y86-083. [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O’Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. doi: 10.1152/ajpheart.2001.280.4.H1645. [DOI] [PubMed] [Google Scholar]
- 4.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- 5.Bauer TA, Brass EP, Barstow TJ, Hiatt WR. Skeletal muscle StO2 kinetics are slowed during low work rate calf exercise in peripheral arterial disease. Eur J Appl Physiol 100: 143–151, 2007. doi: 10.1007/s00421-007-0412-0. [DOI] [PubMed] [Google Scholar]
- 6.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 7.Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf) 199: 367–383, 2010. doi: 10.1111/j.1748-1716.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- 8.Coote JH, Pérez-González JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol 208: 261–278, 1970. doi: 10.1113/jphysiol.1970.sp009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 116: 1509–1526, 2015. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 10.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 11.Cui J, Kuroki MT, Leiter T, Gao Z, Kim DJ-K, Blaha C, Pai S, Sinoway LI. The baroreflex control of heart rate is impaired in patients with peripheral arterial disease. The FASEB Journal 33: 746.3, 2019. [Google Scholar]
- 12.Cui J, Mascarenhas V, Moradkhan R, Blaha C, Sinoway LI. Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am J Physiol Regul Integr Comp Physiol 294: R458–R466, 2008. doi: 10.1152/ajpregu.00475.2007. [DOI] [PubMed] [Google Scholar]
- 13.de Liefde II, Hoeks SE, van Gestel YR, Bax JJ, Klein J, van Domburg RT, Poldermans D. Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. Am J Cardiol 102: 921–926, 2008. doi: 10.1016/j.amjcard.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 14.de Liefde II, Verhagen HJ, Stolker RJ, van Domburg RT, Poldermans D . The value of treadmill exercise test parameters together in patients with known or suspected peripheral arterial disease. Eur J Prev Cardiol 19: 192–198, 2012. doi: 10.1177/1741826711399986. [DOI] [PubMed] [Google Scholar]
- 15.Drew RC, Muller MD, Blaha CA, Mast JL, Heffernan MJ, Estep LE, Cui J, Reed AB, Sinoway LI. Renal vasoconstriction is augmented during exercise in patients with peripheral arterial disease. Physiol Rep 1: e00154, 2013. doi: 10.1002/phy2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner AW, Montgomery PS, Wang M, Chen C, Kuroki M, Kim DJ. Greater exercise pressor response is associated with impaired claudication outcomes in symptomatic peripheral artery disease. Angiology 70: 220–228, 2019. doi: 10.1177/0003319718790876. [DOI] [PubMed] [Google Scholar]
- 17.Gliemann L, Mortensen SP, Hellsten Y. Methods for the determination of skeletal muscle blood flow: development, strengths and limitations. Eur J Appl Physiol 118: 1081–1094, 2018. doi: 10.1007/s00421-018-3880-5. [DOI] [PubMed] [Google Scholar]
- 18.Ha DM, Carpenter LC, Koutakis P, Swanson SA, Zhu Z, Hanna M, DeSpiegelaere HK, Pipinos II, Casale GP. Transforming growth factor-beta 1 produced by vascular smooth muscle cells predicts fibrosis in the gastrocnemius of patients with peripheral artery disease. J Transl Med 14: 39, 2016. doi: 10.1186/s12967-016-0790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O’Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- 20.Hof RP, Hof A. Vasoconstrictor and vasodilator effects in normal and atherosclerotic conscious rabbits. Br J Pharmacol 95: 1075–1080, 1988. doi: 10.1111/j.1476-5381.1988.tb11741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichinose MJ, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, O’Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol 298: H245–H250, 2010. doi: 10.1152/ajpheart.00909.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman MP, Rotto DM, Rybicki KJ. Pressor reflex response to static muscular contraction: its afferent arm and possible neurotransmitters. Am J Cardiol 62: 58E–62E, 1988. doi: 10.1016/S0002-9149(88)80013-4. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61: I60–I65, 1987. [PubMed] [Google Scholar]
- 25.Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O’Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. doi: 10.1152/ajpheart.00648.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenagy J, VanCleave J, Pazdernik L, Orr JA. Stimulation of group III and IV afferent nerves from the hindlimb by thromboxane A2. Brain Res 744: 175–178, 1997. doi: 10.1016/S0006-8993(96)01211-5. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Gao Z, Kehoe V, Xing J, King N, Sinoway L. Interstitial adenosine triphosphate modulates muscle afferent nerve-mediated pressor reflex. Muscle Nerve 38: 972–977, 2008. doi: 10.1002/mus.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao JK, Bettmann MA, Sandor T, Tucker JI, Coleman SM, Creager MA. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ Res 68: 1027–1034, 1991. doi: 10.1161/01.RES.68.4.1027. [DOI] [PubMed] [Google Scholar]
- 29.Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation 46: 257–263, 1972. doi: 10.1161/01.CIR.46.2.257. [DOI] [PubMed] [Google Scholar]
- 30.Luck JC, Miller AJ, Aziz F, Radtka JF III, Proctor DN, Leuenberger UA, Sinoway LI, Muller MD. Blood pressure and calf muscle oxygen extraction during plantar flexion exercise in peripheral artery disease. J Appl Physiol (1985) 123: 2–10, 2017. doi: 10.1152/japplphysiol.01110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol 342: 383–397, 1983. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller AJ, Luck JC, Kim DJ, Leuenberger UA, Aziz F, Radtka JF III, Sinoway LI, Muller MD. Peripheral revascularization attenuates the exercise pressor reflex and increases coronary exercise hyperemia in peripheral arterial disease. J Appl Physiol (1985) 125: 58–63, 2018. doi: 10.1152/japplphysiol.01046.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller AJ, Luck JC, Kim DJ, Leuenberger UA, Proctor DN, Sinoway LI, Muller MD. Blood pressure and leg deoxygenation are exaggerated during treadmill walking in patients with peripheral artery disease. J Appl Physiol (1985) 123: 1160–1165, 2017. doi: 10.1152/japplphysiol.00431.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell J, Schmidt R. Cardiovascular reflex control by afferent fibers from skeletal muscle receptors. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow, edited by Shepherd JT, Abboud FM.. Bethesda, MD: American Physiological Society, 1983, vol. III, p. 623–658. [Google Scholar]
- 36.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell RG, Duscha BD, Robbins JL, Redfern SI, Chung J, Bensimhon DR, Kraus WE, Hiatt WR, Regensteiner JG, Annex BH. Increased levels of apoptosis in gastrocnemius skeletal muscle in patients with peripheral arterial disease. Vasc Med 12: 285–290, 2007. doi: 10.1177/1358863X07084858. [DOI] [PubMed] [Google Scholar]
- 38.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller MD, Drew RC, Ross AJ, Blaha CA, Cauffman AE, Kaufman MP, Sinoway LI. Inhibition of cyclooxygenase attenuates the blood pressure response to plantar flexion exercise in peripheral arterial disease. Am J Physiol Heart Circ Physiol 309: H523–H528, 2015. doi: 10.1152/ajpheart.00267.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishiyasu T, Tan N, Kondo N, Nishiyasu M, Ikegami H. Near-infrared monitoring of tissue oxygenation during application of lower body pressure at rest and during dynamical exercise in humans. Acta Physiol Scand 166: 123–130, 1999. doi: 10.1046/j.1365-201x.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 41.O’Leary DS. Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp Physiol 91: 73–77, 2006. doi: 10.1113/expphysiol.2005.031179. [DOI] [PubMed] [Google Scholar]
- 42.O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Ogoh S, Fadel PJ, Nissen P, Jans Ø, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peikert D, Smolander J. The combined effect of the cold pressor test and isometric exercise on heart rate and blood pressure. Eur J Appl Physiol Occup Physiol 62: 445–449, 1991. doi: 10.1007/BF00626618. [DOI] [PubMed] [Google Scholar]
- 45.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125: e2–e220, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross AJ, Gao Z, Luck JC, Blaha CA, Cauffman AE, Aziz F, Radtka JF III, Proctor DN, Leuenberger UA, Sinoway LI, Muller MD. Coronary exercise hyperemia is impaired in patients with peripheral arterial disease. Ann Vasc Surg 38: 260–267, 2017. doi: 10.1016/j.avsg.2016.05.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross JL, Queme LF, Shank AT, Hudgins RC, Jankowski MP. Sensitization of group III and IV muscle afferents in the mouse after ischemia and reperfusion injury. J Pain 15: 1257–1270, 2014. doi: 10.1016/j.jpain.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985) 64: 2306–2313, 1988. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- 49.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol (1985) 68: 861–867, 1990. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- 50.Rowell LB. Muscle blood flow in humans: how high can it go? Med Sci Sports Exerc 20, Suppl: S97–S103, 1988. doi: 10.1249/00005768-198810001-00001. [DOI] [PubMed] [Google Scholar]
- 51.Sampson UK, Fowkes FG, McDermott MM, Criqui MH, Aboyans V, Norman PE, Forouzanfar MH, Naghavi M, Song Y, Harrell FE Jr, Denenberg JO, Mensah GA, Ezzati M, Murray C. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart 9: 145–158.e21, 2014. doi: 10.1016/j.gheart.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Schellong SM, Böger RH, Burchert W, Bode-Böger SM, Galland A, Frölich JC, Hundeshagen H, Alexander K. Dose-related effect of intravenous L-arginine on muscular blood flow of the calf in patients with peripheral vascular disease: a H215O positron emission tomography study. Clin Sci (Lond) 93: 159–165, 1997. doi: 10.1042/cs0930159. [DOI] [PubMed] [Google Scholar]
- 53.Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. In: Exercise and Sport Sciences Reviews, edited by Holloszy JO. Baltimore, MD: Williams and Wilkins, chapt. 9, vol. 19, 1991, p. 313–350. [PubMed] [Google Scholar]
- 54.Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987. doi: 10.1152/ajpheart.1987.253.5.H1199. [DOI] [PubMed] [Google Scholar]
- 55.Sheriff DD, Augustyniak RA, O’Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998. doi: 10.1152/ajpheart.1998.275.3.H767. [DOI] [PubMed] [Google Scholar]
- 56.Sinoway L, Prophet S. Skeletal muscle metaboreceptor stimulation opposes peak metabolic vasodilation in humans. Circ Res 66: 1576–1584, 1990. doi: 10.1161/01.RES.66.6.1576. [DOI] [PubMed] [Google Scholar]
- 57.Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol (1985) 66: 429–436, 1989. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- 58.Sinoway LI, Smith MB, Enders B, Leuenberger U, Dzwonczyk T, Gray K, Whisler S, Moore RL. Role of diprotonated phosphate in evoking muscle reflex responses in cats and humans. Am J Physiol Heart Circ Physiol 267: H770–H778, 1994. doi: 10.1152/ajpheart.1994.267.2.H770. [DOI] [PubMed] [Google Scholar]
- 59.Spranger MD, Sala-Mercado JA, Coutsos M, Kaur J, Stayer D, Augustyniak RA, O’Leary DS. Role of cardiac output versus peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise versus postexercise muscle ischemia. Am J Physiol Regul Integr Comp Physiol 304: R657–R663, 2013. doi: 10.1152/ajpregu.00601.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stok WJ, Baisch F, Hillebrecht A, Schulz H, Meyer M, Karemaker JM. Noninvasive cardiac output measurement by arterial pulse analysis compared with inert gas rebreathing. J Appl Physiol (1985) 74: 2687–2693, 1993. doi: 10.1152/jappl.1993.74.6.2687. [DOI] [PubMed] [Google Scholar]
- 61.Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179: 361–366, 2003. doi: 10.1046/j.0001-6772.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 62.Teräväinen H, Mäkitie J. Striated muscle ultrastructure in intermittent claudication. Arch Pathol Lab Med 101: 230–235, 1977. [PubMed] [Google Scholar]
- 63.Watkins L, Sherwood A, Goldstein DS, Maixner W. Mechanisms underlying cardiovascular defense reaction evoked by dorsal periaqueductal gray stimulation. Am J Physiol Regul Integr Com Physiol 265: R1155–R1161, 1993. doi: 10.1152/ajpregu.1993.265.5.R1155. [DOI] [PubMed] [Google Scholar]
- 64.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol (1985) 74: 2566–2573, 1993. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 65.Womack CJ, Sieminski DJ, Katzel LI, Yataco A, Gardner AW. Oxygen uptake during constant-intensity exercise in patients with peripheral arterial occlusive disease. Vasc Med 2: 174–178, 1997. doi: 10.1177/1358863X9700200303. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto K, Miyata T, Onozuka A, Koyama H, Ohtsu H, Nagawa H. Plantar flexion as an alternative to treadmill exercise for evaluating patients with intermittent claudication. Eur J Vasc Endovasc Surg 33: 325–329, 2007. doi: 10.1016/j.ejvs.2006.10.012. [DOI] [PubMed] [Google Scholar]