Abstract
Sepsis-induced cardiomyopathy (SIC) is associated with increased patient mortality. At present, there are no specific therapies for SIC. Previous studies have reported increased reactive oxygen species (ROS) and mitochondrial dysfunction during SIC. However, a unifying mechanism remains to be defined. We hypothesized that PKCδ is required for abnormal calcium handling and cardiac mitochondrial dysfunction during sepsis and that genetic deletion of PKCδ would be protective. Polymicrobial sepsis induced by cecal ligation and puncture (CLP) surgery decreased the ejection fraction of wild-type (WT) mice but not PKCδ knockout (KO) mice. Similarly, WT cardiomyocytes exposed to lipopolysaccharide (LPS) demonstrated decreases in contractility and calcium transient amplitude that were not observed in PKCδ KO cardiomyocytes. LPS treatment decreased sarcoplasmic reticulum calcium stores in WT cardiomyocytes, which correlated with increased ryanodine receptor-2 oxidation in WT hearts but not PKCδ KO hearts after sepsis. LPS exposure increased mitochondrial ROS and decreased mitochondrial inner membrane potential in WT cardiomyocytes. This corresponded to morphologic changes consistent with mitochondrial dysfunction such as decreased overall size and cristae disorganization. Increased cellular ROS and changes in mitochondrial morphology were not observed in PKCδ KO cardiomyocytes. These data show that PKCδ is required in the pathophysiology of SIC by generating ROS and promoting mitochondrial dysfunction. Thus, PKCδ is a potential target for cardiac protection during sepsis.
NEW & NOTEWORTHY Sepsis is often complicated by cardiac dysfunction, which is associated with a high mortality rate. Our work shows that the protein PKCδ is required for decreased cardiac contractility during sepsis. Mice with deletion of PKCδ are protected from cardiac dysfunction after sepsis. PKCδ causes mitochondrial dysfunction in cardiac myocytes, and reducing mitochondrial oxidative stress improves contractility in wild-type cardiomyocytes. Thus, PKCδ is a potential target for cardiac protection during sepsis.
Keywords: mitochondria, PKCδ, reactive oxygen species, sepsis
INTRODUCTION
Sepsis is a complex disease that causes multiple organ dysfunction. Sepsis-induced cardiomyopathy (SIC), defined as an acute decrease in systolic function during sepsis, is a common clinical problem that is associated with worse mortality (10, 16). There are no specific therapies for SIC. Previously, the generally accepted hypothesis was that systemic inflammation caused sepsis-induced cardiomyopathy (24). However, human studies show that the development of SIC does not correlate to circulating cytokine levels (18). Furthermore, anti-inflammatory therapies have not been effective in reducing mortality from sepsis in clinical trials. Anti-cytokine therapies have been at best mildly beneficial (13, 21). This indicates that there is a central component of the pathophysiology that is not being corrected by anti-inflammatory therapies (6).
If a patient recovers from sepsis, systolic function generally normalizes within a few weeks. The acute onset and potential for rapid recovery distinguish this form of cardiomyopathy from chronic systolic heart failure, suggesting that distinct mechanisms may be involved. Several molecular mechanisms have been proposed to cause SIC, including impaired cardiac energetics (4, 5, 19), autophagy (26), and increased reactive oxygen species (ROS) (3, 17, 30). Mitochondrial dysfunction is a consistent finding in sepsis models (15). Calcium handling abnormalities have also been described, but the relationships with mitochondrial dysfunction and oxidative stress are unclear (12).
Our prior work showed that NADPH oxidase-2 activation is upstream of cardiac calcium handling abnormalities and mitochondrial dysfunction during sepsis, but the specific downstream pathways involved remain unclear (15). We have also shown that PKCδ is activated in the heart during sepsis but the role of PKCδ in the pathophysiology of SIC is not well understood. We hypothesized that PKCδ causes cardiac mitochondrial dysfunction during sepsis, resulting in decreased contractility.
MATERIALS AND METHODS
Materials.
The OxyBlot Protein Oxidation Detection Kit and lipopolysaccharide (LPS) were purchased from Sigma. Other chemicals were purchased from Fisher Scientific. The anti-PKCδ antibody was purchased from Abcam (Cat. No. ab182126). The anti-SERCA antibody was purchased from Fisher Scientific (Cat. No. MA3–919). Phospholamban antibodies were purchased from Cell Signaling (Cat. Nos.14562 and 8496). Ryanodine receptor-2 (RyR2) antibodies were provided by the laboratory of Andrew Marks.
Animal care, echocardiograms, and cardiomyocyte isolation.
Animal protocols were approved by the Columbia University Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health’s “Guidelines for the Care and Use of Laboratory Animals.” Mice were housed under standard conditions and were fed standard chow (Picolab Rodent diet 20 from LabDiet) ad libitum. Isolation of cardiomyocytes was performed as previously described (25). Animals were randomly assigned to treatment groups; males and females were used in equal numbers. No animals were excluded from the analysis. Echocardiograms were performed and analyzed by blinded operators. The mice were sedated with isoflurane during echocardiogram recording. PKCδ KO mice were originally created by Dr. Keiichi Nakayama (Kyushu University), who gave us written permission to breed these mice.
Cardiomyocyte contractility and calcium transients.
Isolated cardiomyocyte contractility was measured using an Ionoptix system as previously described (15). Calcium transients were measured using Fura2 (Invitrogen). Sarcoplasmic reticulum (SR) calcium load was evoked with rapid application of caffeine (10 mM).
Measurements of ROS, mitochondrial calcium, and mitochondria inner membrane depolarization.
Freshly isolated cardiomyocytes were divided into aliquots. To measure total cellular ROS, cells were loaded with the fluorescent dye 2′,7′-dichlorofluorescin diacetate (H2DCF-DA; 25 µM). Once it enters the cell, H2DCF-DA is converted by oxidation to DCF, which is fluorescent, and this is widely used as a measure of general oxidative stress (8). To measure mitochondrial ROS, MitoSOX Red (5 µM), was used to analyze mitochondrial superoxide generation. Cells were incubated with DCF or MitoSOX Red for 30 min in the dark. Excess DCF or Mitosox Red was removed with two washes of BSA solution. DCF fluorescence was recorded at excitation/emission wavelengths 488 and 532 nm, whereas MitoSOX Red was recorded at 525 and 620 nm, respectively. To assess the changes in mitochondrial membrane potential, cardiomyocytes were stained with 1 nM tetramethylrhodamine methyl (TMRM) ester for 30 min and recorded at 543 nm (excitation) and 590 nm (emission). TMRM preferentially accumulates in mitochondria due to its positive charge. As mitochondria are depolarized, they trap less TMRM, and so the signal is proportional to the inner membrane potential of the mitochondria. To determine the changes in mitochondrial calcium in living cells, cardiomyocytes were loaded with 10 µM Rhod 2-AM and incubated for 30 min, followed by a 1-h washout with cytosolic quenching using manganese, to produce specific mitochondrial calcium signal (2). Rhod 2-AM fluorescence was measured at 552 nm (excitation) and 581 nm (emission). Cells were loaded onto a 96-wells in triplicate and plated at 2,000 cells/ well, and fluorescence was measured with a Molecular Devices iD3 plate reader.
Electron microscopy.
Heart tissue was fixed with 2.5% glutaraldehyde and 2% paraformaldehyde in 100 mM sodium cacodylate buffer (pH 7.4) overnight at 4°C. Samples were then treated with 1% osmium tetroxide for 1 h, washed in distilled water four times, and then treated with 2% aqueous uranyl acetate overnight at 4°C in the dark. Samples were then washed and sequentially dehydrated with increasing concentrations of ethanol (20, 30, 50, 70, 90, and 100%) for 30 min each, followed by three additional treatments with 100% ethanol for 20 min each. Samples were then infiltrated with increasing concentrations of Spurr’s resin (25% for 1 h, 50% for 1 h, 75% for 1 h, 100% for 1 h, and 100% overnight at room temperature) and then incubated overnight at 70°C in a resin mold. Sections of 80 nm were cut on a Leica ultramicrotome with a diamond knife. Imaging then took place using a ThermoFisher Talos L120C transmission electron microscope operating at 120 kV.
Statistical analysis.
Results are given as means ± SE. The unpaired t-test was used for comparisons of two means. A two-tailed value of P < 0.05 was considered statistically significant. For groups of two or more, one-way analysis of variance (ANOVA) was used with Tukey post hoc testing or nonparametric methods with post hoc testing (Prism v5, GraphPad Software, La Jolla, CA).
RESULTS
Mitotempo prevents mitochondrial dysfunction and the decrease in contractility caused by low-dose of LPS in wild-type cardiomyocytes.
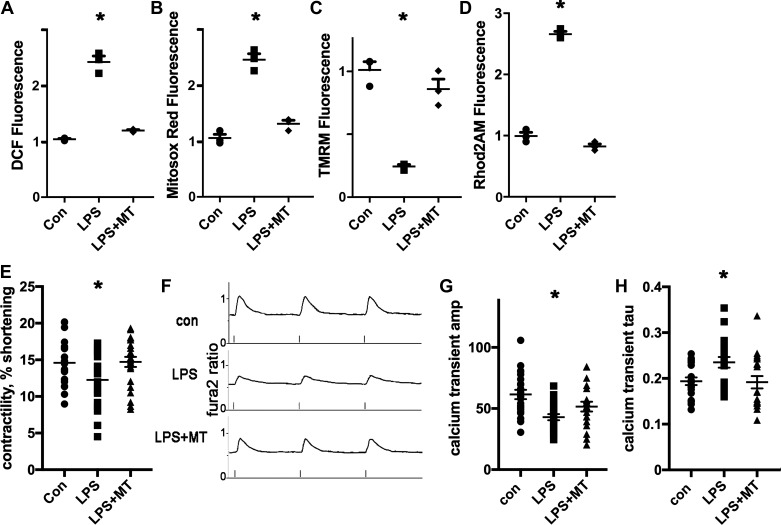
Since mitochondrial ROS appeared to be a part of the pathophysiology of SIC in prior work, we predicted that inhibiting mitochondrial ROS could protect cardiomyocytes from the abnormalities caused by LPS. For these experiments, we used isolated ventricular cardiomyocytes from wild-type (WT) mice and exposed cells to a low dose of LPS (20 ng/mL) for 1 h. Some cardiomyocytes were pretreated with the mitochondrial antioxidant mitotempo for 30 min. Mitotempo had a strong protective effect, normalizing ROS and preventing partial depolarization of the mitochondrial inner membrane (Fig. 1, A–D). Additional experiments demonstrate that control cardiomyocytes had a decrease in contractility and a corresponding decrease in calcium transient amplitude after exposure to LPS. Mitotempo preserved contractility and blunted the decrease in calcium transient amplitude caused by LPS (Fig. 1, E–G). The calcium transient decay time constant (τ), an estimate of sarco/endoplasmic reticulum calcium ATPase (SERCA) activity, was significantly increased by LPS in control cells, indicating slower reduction in cytosolic calcium. This was also prevented by pretreatment with mitotempo (Fig. 1F). Overall, these results indicate that mitochondrial ROS is responsible for the decrease in contractility and corresponding calcium-handling abnormalities in this cellular model of early sepsis.
Fig. 1.
Mitotempo (MT) prevents mitochondrial dysfunction and the decrease in contractility caused by lipopolysaccharide (LPS) in wild-type (WT) cardiomyocytes. A: isolated cardiomyocytes, height is 2',7'-dichlorofluorescein (DCF) fluorescence minus background, normalized arbitrary units, n = 3 wells, 2000 cells/well, bars represent means + SE. B: cardiomyocytes, MitoSOX red readout. C: cardiomyocytes, tetramethylrhodamine methyl (TMRM) signal to indicate mitochondrial inner membrane potential. D: cardiomyocytes, Rhod 2-AM signal, and mitochondrial calcium. E: WT cardiomyocyte contractility, sarcomere length (in μm), control solution, or LPS; bars represent means + SE; n = 21–23 cardiomyocytes from 3 isolations. F: representative calcium transients from WT cardiomyocytes; x-axis is fura2 ratio. G: WT cardiomyocyte calcium transient amplitude (Amp); means + SE; N = 19–21 cardiomyocytes from 3 isolations. H: WT cardiomyocyte calcium transient decay time constant (τ); means + SE. A–H: LPS = 20 ng/mL; 1-h exposure; MT = 20 μΜ. For all graphs, the means of the groups are different by ANOVA; *significantly different from control (Con) by post hoc test.
PKCδ KO mice are protected from sepsis-induced cardiomyopathy.
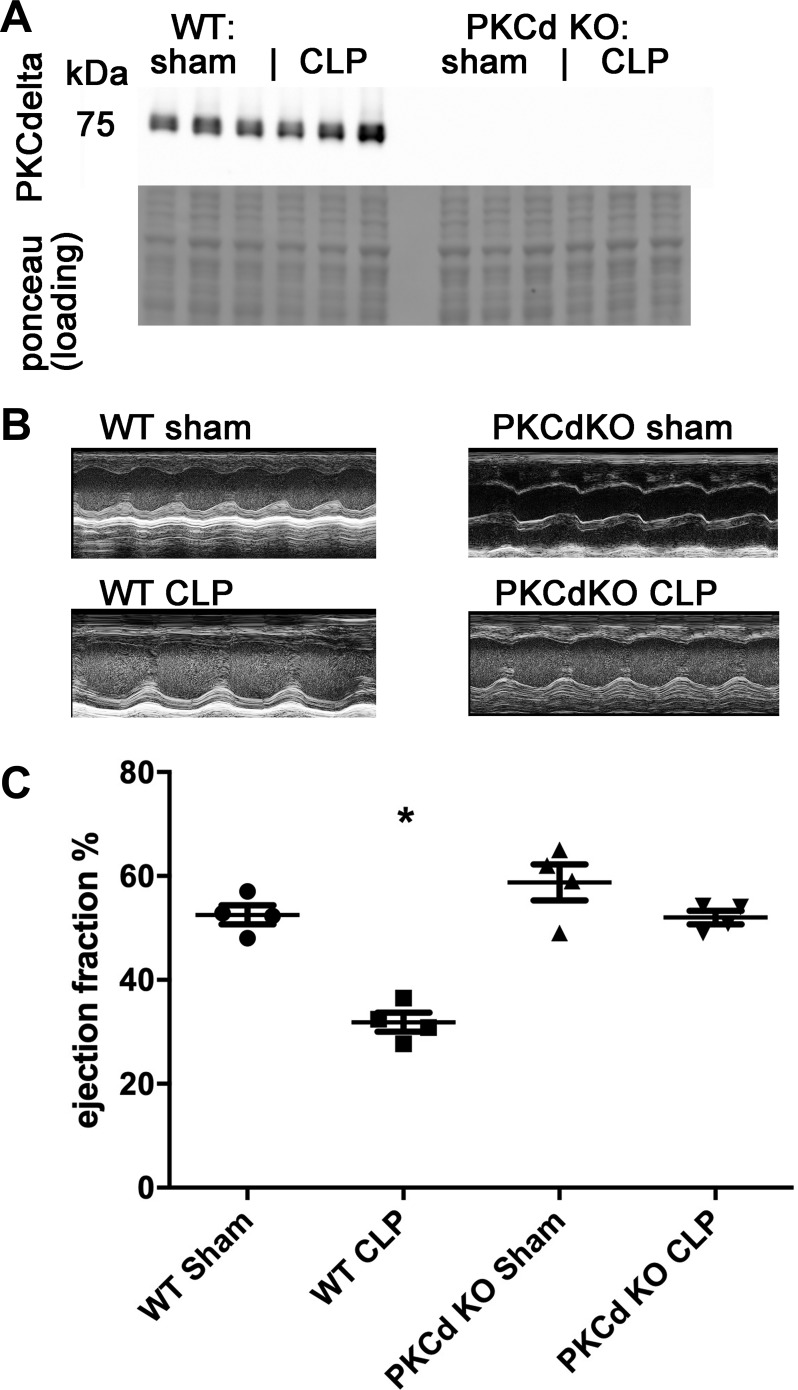
Our prior work showed that PKC isoforms are activated in the heart during SIC (15). To determine if PKCδ is required for mitochondrial dysfunction during sepsis and/or SIC, we obtained PKCδ KO mice. We confirmed genotypes with Western blot analysis using a primary antibody that is specific for PKCδ (Fig. 2A). We performed cecal ligation and puncture (CLP) surgery on 3-mo-old mice to induce polymicrobial sepsis and then performed echocardiograms 16–18 h later. The mice were euthanized and the heart was collected and divided into multiple samples. Sepsis consistently decreased the ejection fraction (EF) of WT mice, with a mean EF of 53% in WT sham-operated mice compared with a mean EF of 32% after CLP-induced sepsis. PKCδ KO mice were protected from the decrease in EF, with minimal differences comparing sham controls to CLP mice (Fig. 2, B and C, and Table 1). This indicates that PKCδ is necessary for systolic dysfunction in this animal model of polymicrobial sepsis.
Fig. 2.
PKCδ knockout (KO) mice are protected from sepsis induced cardiomyopathy. A: representative Western blot of PKCδ from heart lysates and loading control. B: representative echocardiography images. C: ejection fraction, n = 4 each, means are significantly different by ANOVA, *P < 0.05 by post hoc test. WT, wild-type; CLP, cecal ligation and puncture surgery.
Table 1.
Echocardiography measurements
| HR, beats/min | EF, % | LVEDd, mm | LVESd, mm | |
|---|---|---|---|---|
| WT | ||||
| Sham | 473.25 ± 70.45 | 52.7 ± 2 | 2.90 ± 0.39 | 1.63 ± 0.29 |
| CLP | 393.25 ± 79.13 | 31.8 ± 3* | 2.43 ± 0.39 | 1.98 ± 0.47 |
| PKCδ KO | ||||
| Sham | 451.25 ± 90.00 | 63.3 ± 6 | 3.03 ± 0.57 | 1.82 ± 0.24 |
| CLP | 323.25 ± 73.16 | 51.8 ± 1 | 3.05 ± 0.76 | 1.95 ± 0.58 |
Values are means + SE. WT, wild-type; CLP, cecal ligation and puncture surgery; KO, knockout; HR, heart rate; EF, ejection fraction; LVEDd, left ventricular end-diastolic diameter; LVESd, left ventricular end-systolic diameter.
P < 0.05, significantly different by ANOVA, by post hoc test.
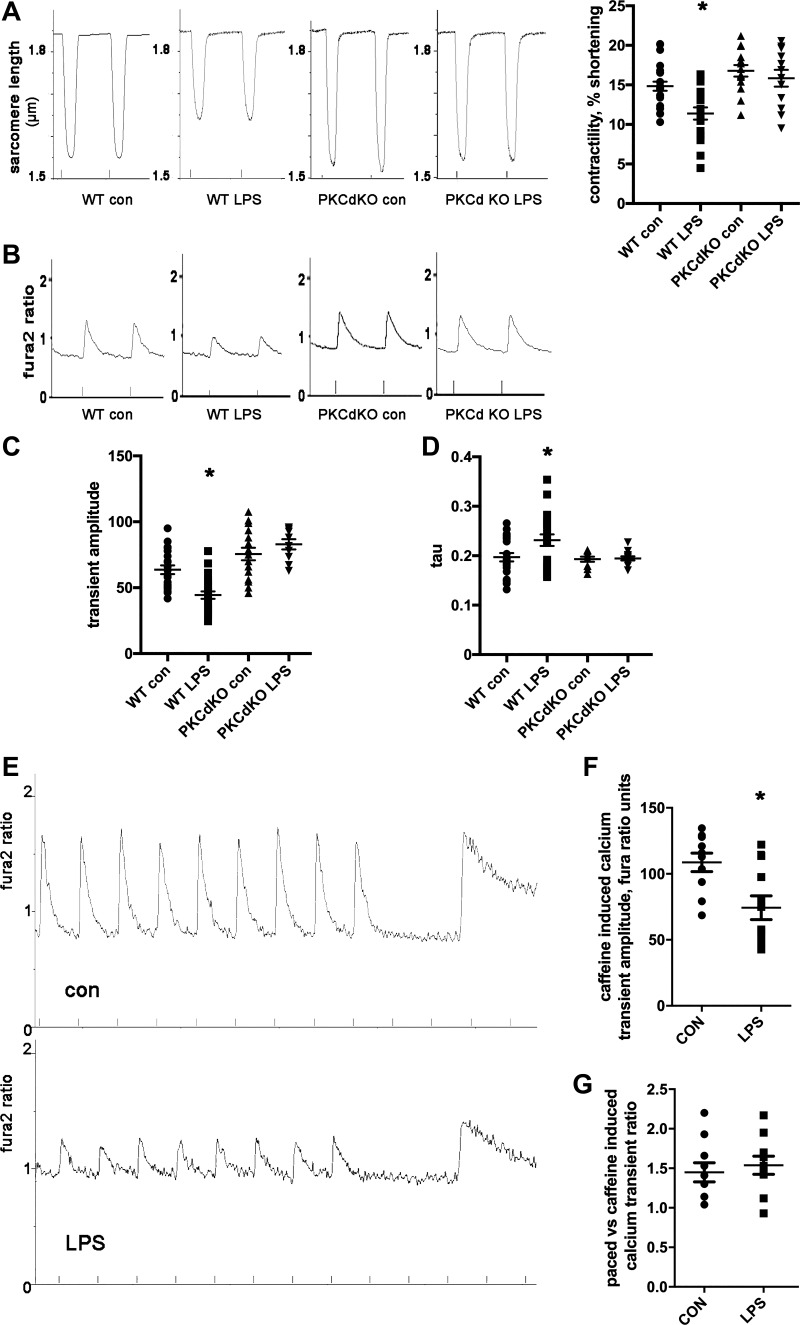
PKCδ KO cardiomyocytes are protected from decreased contractility.
To determine the mechanisms of sepsis pathophysiology at the cellular level, we isolated ventricular cardiomyocytes from PKCδ KO mice and WT littermates and exposed cells to LPS for 1 h. WT cardiomyocytes had a decrease in contractility and a corresponding decrease in calcium transient amplitude after exposure to LPS (Fig. 3A). In WT cardiomyocytes, the calcium transient τ was significantly increased by LPS, indicating slower return to baseline. In contrast, PKCδ KO cardiomyocytes did not have an abnormal contractility, calcium transient amplitudes, or calcium transient τ after LPS (Fig. 3, B–D). These results at the single-cell level are consistent with the prevention of systolic dysfunction in PKCδ KO mice in vivo and indicate that intracellular calcium handling abnormalities are involved.
Fig. 3.
PKCδ knockout (KO) cardiomyocytes are protected from decreased contractility and mitochondrial dysfunction after exposure to lipopolysaccharide (LPS). A: representative raw data of wild-type (WT) and PKCδ KO cardiomyocyte contractility, sarcomere length (in μm), control solution or LPS, and means + SE; N = 20–30 cells from 3 hearts each group. B: representative calcium transients from WT and PKCδ KO cardiomyocytes, control solution, or LPS. C: calcium transient amplitude; means + SE. D: calcium transient decay time constant (τ; in s), means + SE. For A–D, the means are significantly different by ANOVA; *statistically significant difference from WT control (Con) by post hoc test. LPS = 20 ng/mL, 1-h exposure. E: representative images of control cardiomyocyte and cardiomyocyte exposed to LPS, pacing-induced calcium transient, and then sarcoplasmic reticulum (SR) calcium release by caffeine. F: amplitude of sarcoplasmic reticulum (SR) calcium release by caffeine. *Statistically significant difference by t-test; N = 20–30 cells from 3 hearts. G: ratio of amplitude of paced calcium transient to amplitude of SR calcium release.
PKCδ KO cardiomyocytes are protected from SR calcium depletion.
To examine the cause of decreased calcium transient amplitude, we determined if LPS causes a reduction in SR calcium stores or fractional calcium release from the SR. After steady-state pacing was achieved, WT isolated ventricular cardiomyocytes were exposed to caffeine to release SR calcium. The caffeine-induced SR transient had lower amplitude in cardiomyocytes exposed to LPS, indicating that SR calcium stores were reduced (Fig. 3, E and F). The ratio of paced transient amplitude to caffeine-induced transient amplitude was similar in both groups of cardiomyocytes, indicating that fractional calcium release is not significantly different (Fig. 3G).
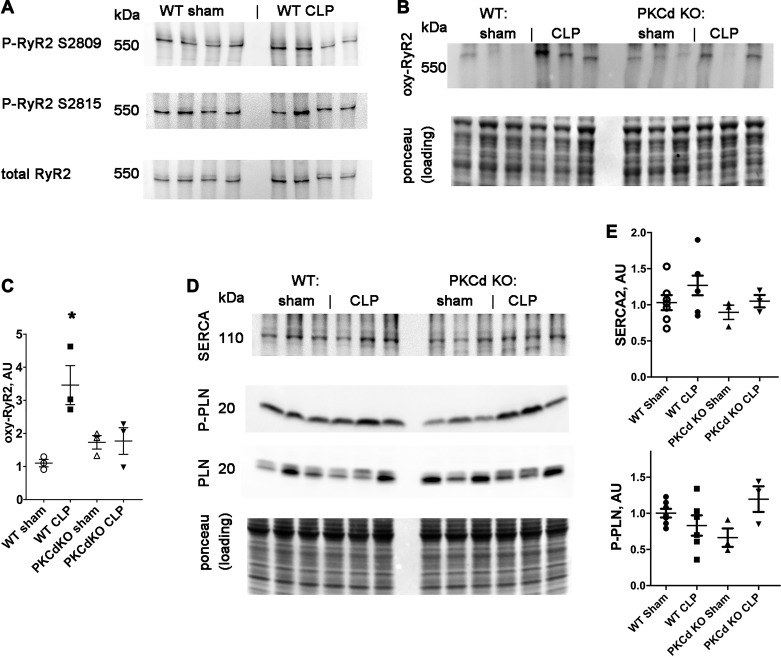
Our group and other laboratories have previously shown that sepsis causes increased SR calcium leak in the form of calcium sparks (15, 31). This is the most likely explanation for the decrease in SR calcium. To determine the molecular mechanism, we examined RyR2 calcium channels, since posttranslational modification of RyR2 can cause SR calcium leak (22). Phosphorylation of RyR2 was not significantly increased after CLP (Fig. 4A). In contrast, we found that WT mice have increased oxidation of cardiac RyR2 after CLP, compared with sham controls (Fig. 4, B and C). Oxidation of RyR is known to increase calcium leak (28). Furthermore, PKCδ KO mice did not have an increase in oxidation of cardiac RyR2 after CLP (Fig. 4, B and C). Calcium uptake into the SR is controlled by SERCA and the small regulatory protein phospholamban, with binding determined by phosphorylation of phospholamban. Western blot analysis of SERCA and phospho-phospholamban did not show any significant difference between groups (Fig. 4, E and F). Thus, any change in SERCA function, indicated by calcium transient τ, is not due to a decrease in protein level or change in phosphorylation of phospholamban.
Fig. 4.
Sepsis causes oxidation of ryanodine receptor 2 (RyR2). A: representative Western blots of RyR2. B: blot of oxidized (oxy) RyR2 with loading control. C: quantification of oxidized RyR2. The means of the groups are different by ANOVA, *statistically significant difference from control by post hoc test. D: Western blots of sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA), phospholamban, and phosphorylated phospholamban, with loading control. E: quantification of SERCA and phosphorylated (P) phospholamban (PLN) in arbitrary units (AU). Phosphorylated phospholamban is normalized to total phospholamban. The results of ANOVA for these Western blots are not significantly different. WT, wild-type: CLP, cecal ligation and puncture surgery; KO, knockout.
PKCδ KO cardiomyocytes are protected from mitochondrial dysfunction.
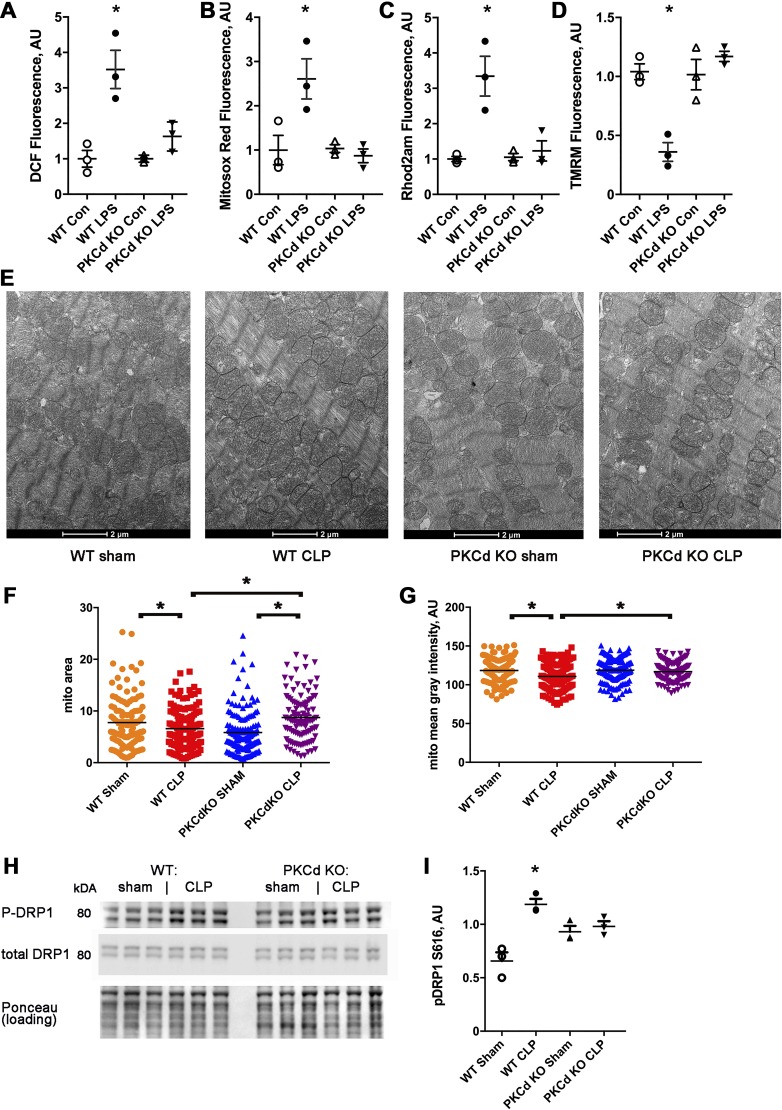
To elucidate the molecular mechanisms causing abnormalities in WT cardiomyocytes exposed to LPS, we examined oxidative stress and mitochondrial function. WT cardiomyocytes had a significant increase in total and mitochondrial ROS (Fig. 5, A and B). Unlike WT cardiomyocytes, PKCδ KO cardiomyocytes did not have an increase in total or mitochondrial ROS after LPS exposure (Fig. 5, A and B). WT cardiomyocytes had a significant increase in mitochondrial calcium and a significant decrease in mitochondrial inner membrane potential, whereas PKCδ KO cardiomyocytes did not have these abnormalities. (Fig. 5, C and D). Thus, the prevention of ROS and mitochondrial dysfunction correlated with preservation of single-cell contractility and calcium transient amplitude for PKCδ KO cardiomyocytes.
Fig. 5.
PKCδ knockout (KO) cardiomyocytes are protected from mitochondrial dysfunction induced by lipopolysaccharide (LPS) and mitochondrial morphologic changes after cecal ligation and puncture surgery (CLP). A: isolated cardiomyocytes, 2',7'-dichlorofluorescein (DCF) fluorescence minus background, n = 3 wells, 2,000 cells/well, bars represent means + SE. AU, arbitrary units. The means of the groups are different by ANOVA, *significantly different from control (Con) by post hoc test. B: cardiomyocytes, MitoSOX red readout. C: cardiomyocytes, Rhod 2-AM signal, and mitochondrial calcium. D: cardiomyocytes, tetramethylrhodamine methyl (TMRM) signal to indicate mitochondrial inner membrane potential. E: representative electron microscopy from ventricular samples of wild-type (WT) sham, WT CLP, and PKCδ KO. F: mean mitochondrial area. G: mean mitochondrial pixel density. B and C: N = 100–140 mitochondrial from each group, n = 4 hearts each group, with 3 images from each heart sample. The means of the groups are different by ANOVA, *significantly different by post hoc test. H: representative Western blots of phospho-dynamin-related protein 1 (Drp1) and total Drp1. I: quantification of phospho-Drp1 normalized to total Drp1. The means of the groups are different by ANOVA, *significantly different by post hoc test.
PKCδ KO mice are protected from sepsis-induced changes in mitochondrial morphology.
To determine if sepsis caused structural changes in cardiac mitochondria, ventricular samples were prepared for electron microscopy. Electron microscopy images, quantified by a blinded reader, showed that mitochondrial structure had mild abnormalities, comparing WT CLP ventricular samples with WT sham samples. There was a mild but statistically significant decrease in mitochondrial size in the WT CLP group and a mild but significant reduction in mean pixel density that indicates a decrease in cristae density, consistent with mitochondrial damage (Fig. 5, E–G) (20). When the PKCδ KO groups are compared, there was a small increase in size after CLP with no significant difference in mean pixel density (Fig. 5, F and G). Thus, PKCδ KO hearts do not exhibit the morphologic changes in cardiac mitochondria that are found in WT hearts after sepsis.
Dynamin-related protein 1 (DRP1) is a regulator of mitochondrial fission/fusion. Previous studies have shown that phosphorylation of DRP1 at serine-616 increases mitochondrial fission, resulting in smaller average mitochondrial size. Although baseline levels of fission are necessary for normal mitochondrial physiology, excessive fission can lead to mitochondrial dysfunction and increased oxidative stress. We measured the level of DRP1 phosphorylation in heart lysates following CLP surgery. DRP1 phosphorylation is significantly increased after sepsis in WT mice (Fig. 5, H and I). DRP1 phosphorylation was not significantly increased in PKCδ KO hearts after sepsis. This result corresponds to the mild decrease in mitochondrial size seen in septic WT hearts but not in PKCδ KO hearts. Thus, DRP1 phosphorylation may contribute to the alterations in cardiac mitochondria after sepsis.
DISCUSSION
Sepsis is a common cause of hospitalization and death in developed countries (1). Cardiac damage frequently complicates sepsis (16). Studies using human tissue samples have shown that cardiomyocyte cell death is rare during sepsis and does not explain sepsis-induced cardiomyopathy (SIC) (27). Cardiac mitochondrial dysfunction is a consistent finding in sepsis animal models (15, 29), but the molecular mechanisms are obscure. The results of this investigation show that PKCδ is upstream of mitochondrial dysfunction and decreased contractility during sepsis.
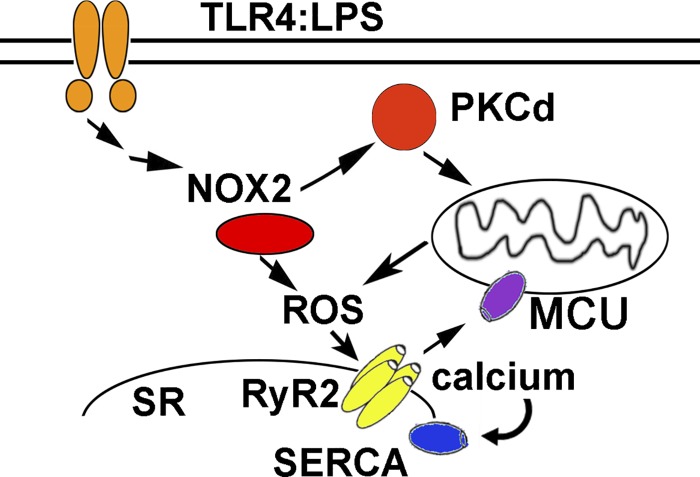
Prior work has shown that PKC isoforms are activated in the heart during sepsis (15). Although PKCδ is known to be involved in some forms of heart disease (7), to the best of our knowledge, this is the first time that PKCδ has been demonstrated to play a critical role in SIC. Our work indicates that genetic deletion of PKCδ has significant beneficial effects on cardiac mitochondrial function during sepsis. Our findings also show that PKCδ is necessary for the decrease in contractility during sepsis. In our working model, the initial increase in ROS caused by PKCδ-induced mitochondrial dysfunction causes oxidation of RyR2, increasing SR calcium leak. This results in mitochondrial calcium overload and worse mitochondrial dysfunction, causing a pathological feed-forward cycle (Fig. 6). Our data suggest that a decrease in SERCA activity is also involved. Regulation of SERCA is complex, with posttranslational modifications, phospholamban, and other small peptide regulators. Prior work had demonstrated that posttranslational modification of SERCA, due to oxidative stress, can decrease SERCA activity in the hearts of mice injected with LPS (11). Our data are consistent with this prior work.
Fig. 6.
Schematic of proposed pathways. Prior work indicates that sepsis activates NADPH oxidase 2 (NOX2) in cardiomyocytes, increasing reactive oxygen species (ROS) and stimulating PKCδ, which causes mitochondrial dysfunction. Increased ROS due to NOX2 and mitochondrial dysfunction results in increased calcium leak from the sarcoplasmic reticulum (SR). Calcium leak causes mitochondrial calcium overload, promoting mitochondrial dysfunction and increasing the generation of mitochondrial ROS in a vicious cycle. In addition to SR calcium leak, impaired SR calcium uptake by sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) contributes to decreased calcium transient amplitude. LPS, lipopolysaccharide; MCU, mitochondrial calcium uniporter; RyR2, ryanodine receptor 2; TLR4, Toll-like receptor 4.
Pharmacological inhibition of mitochondrial ROS is protective for cardiomyocytes, supporting mitochondrial ROS as a part of the causal pathway and not merely an epiphenomenon. Although the mitochondrial morphologic changes detected in WT hearts by electron microscopy are not severe, they are not present in the PKCδ KO hearts. This supports the hypothesis that PKCδ causes SIC by inducing mitochondrial abnormalities.
We choose an early time point for our studies to identify the initial steps of the pathophysiology of SIC. It is likely that later stages of sepsis could involve additional pathways. For example, prior work examining gene expression in human heart samples taken from patients who died from sepsis showed downregulation of gene expression for sarcomere and mitochondrial genes (23). The fact that we can detect significant mitochondrial abnormalities in WT cardiomyocytes after a 1-h exposure to LPS is consistent with a rapid process, such as kinase activation and posttranslational modification of proteins. These in vitro experiments with freshly isolated cardiomyocytes also demonstrate that contractile dysfunction can happen in the absence of the immune system. Inflammation is certainly a prominent feature of sepsis, but the present work and our prior work (15, 17) show that cardiac dysfunction is not necessarily due to systemic inflammation. Inflammation may have a role in the cardiac pathology at later time points.
Our data indicate that inhibiting mitochondrial calcium uptake could be protective. Future work could focus on the role of mitochondrial calcium uniporter and its regulatory subunits during SIC. Other pathways may also play a role in mitochondrial dysfunction during sepsis. There is a prior report that LPS activates Drp1 in the H9c2 cell line, which promotes mitochondrial dysfunction (9). Our work demonstrates that a similar pathway exists in vivo. However, we detected relatively modest changes in mitochondrial size, indicating that alterations in fission/fusion dynamics are probably not a major part of the pathophysiology in the early stages of sepsis. The increase in mitochondrial ROS and decrease in inner membrane potential detected in isolated cardiomyocytes does not necessarily involve morphologic changes in mitochondria.
A limitation of our work is that we used whole body PKCδ KO mice. Thus, some of the in vivo benefits may not be a direct function of the genetic deletion in cardiomyocytes. However, the isolated cardiac myocyte experiments indicate that the benefits occur without the presence of other cells types. In conclusion, we have shown that genetic deletion of PKCδ prevents SIC in vivo, and we have determined that PKCδ cardiomyocytes do not have mitochondrial abnormalities after exposure to LPS. Inhibiting mitochondrial ROS prevents the decrease in contractility in cardiomyocytes. We conclude that PKCδ-induced mitochondrial dysfunction is a critical component of the pathophysiology of SIC. Specific PKCδ inhibitors have been developed for others diseases (14). Our work indicates that inhibition of PKCδ might be a treatment for sepsis-induced cardiomyopathy.
GRANTS
This work was supported in part by National Institutes of Health Grants T32-HL-007343 (to L. C. Joseph), R01-HL-136758 (to J. P. Morrow), R01-GM-066189 (to G. Hasko), and R01-HL-130218 (to K. Drosatos).
DISCLOSURES
G. Hasko owns stock in Purine Pharmaceuticals, Inc. and has patents related to sepsis but not specifically related to sepsis-induced cardiomyopathy. The other authors declare that they have no financial conflicts of interest.
AUTHOR CONTRIBUTIONS
L.C.J. and J.P.M. conceived and designed research; L.C.J., M.V.R., K.R.L., B.H.G., and J.P.M. performed experiments; L.C.J., M.V.R., K.R.L., B.H.G., K.D., and J.P.M. analyzed data; L.C.J., M.V.R., K.R.L., G.H., K.D., and J.P.M. interpreted results of experiments; L.C.J. and J.P.M. prepared figures; J.P.M. drafted manuscript; L.C.J., M.V.R., G.H., K.D., and J.P.M. edited and revised manuscript; L.C.J., M.V.R., G.H., K.D., and J.P.M. approved final version of manuscript.
REFERENCES
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 369: 840–851, 2013. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Bowser DN, Minamikawa T, Nagley P, Williams DA. Role of mitochondria in calcium regulation of spontaneously contracting cardiac muscle cells. Biophys J 75: 2004–2014, 1998. doi: 10.1016/S0006-3495(98)77642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceylan-Isik AF, Zhao P, Zhang B, Xiao X, Su G, Ren J. Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis. J Mol Cell Cardiol 48: 367–378, 2010. doi: 10.1016/j.yjmcc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drosatos K, Drosatos-Tampakaki Z, Khan R, Homma S, Schulze PC, Zannis VI, Goldberg IJ. Inhibition of c-Jun-N-terminal kinase increases cardiac peroxisome proliferator-activated receptor alpha expression and fatty acid oxidation and prevents lipopolysaccharide-induced heart dysfunction. J Biol Chem 286: 36331–36339, 2011. doi: 10.1074/jbc.M111.272146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drosatos K, Khan RS, Trent CM, Jiang H, Son NH, Blaner WS, Homma S, Schulze PC, Goldberg IJ. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ Heart Fail 6: 550–562, 2013. doi: 10.1161/CIRCHEARTFAILURE.112.000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep 12: 130–140, 2015. doi: 10.1007/s11897-014-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duquesnes N, Lezoualc’h F, Crozatier B. PKC-delta and PKC-epsilon: foes of the same family or strangers? J Mol Cell Cardiol 51: 665–673, 2011. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 594: 57–72, 2010. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 9.Haileselassie B, Mukherjee R, Joshi AU, Napier BA, Massis LM, Ostberg NP, Queliconi BB, Monack D, Bernstein D, Mochly-Rosen D. Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy. J Mol Cell Cardiol 130: 160–169, 2019. doi: 10.1016/j.yjmcc.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havaldar AA. Evaluation of sepsis induced cardiac dysfunction as a predictor of mortality. Cardiovasc Ultrasound 16: 31, 2018. doi: 10.1186/s12947-018-0149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobai IA, Buys ES, Morse JC, Edgecomb J, Weiss EH, Armoundas AA, Hou X, Khandelwal AR, Siwik DA, Brouckaert P, Cohen RA, Colucci WS. SERCA Cys674 sulphonylation and inhibition of L-type Ca2+ influx contribute to cardiac dysfunction in endotoxemic mice, independent of cGMP synthesis. Am J Physiol Heart Circ Physiol 305: H1189–H1200, 2013. doi: 10.1152/ajpheart.00392.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobai IA, Edgecomb J, LaBarge K, Colucci WS. Dysregulation of intracellular calcium transporters in animal models of sepsis-induced cardiomyopathy. Shock 43: 3–15, 2015. doi: 10.1097/SHK.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13: 862–874, 2013. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeno F, Inagaki K, Rezaee M, Mochly-Rosen D. Impaired perfusion after myocardial infarction is due to reperfusion-induced deltaPKC-mediated myocardial damage. Cardiovasc Res 73: 699–709, 2007. doi: 10.1016/j.cardiores.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph LC, Kokkinaki D, Valenti MC, Kim GJ, Barca E, Tomar D, Hoffman NE, Subramanyam P, Colecraft HM, Hirano M, Ratner AJ, Madesh M, Drosatos K, Morrow JP. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI Insight 2: 94248, 2017. doi: 10.1172/jci.insight.94248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalla C, Raveh D, Algur N, Rudensky B, Yinnon AM, Balkin J. Incidence and significance of a positive troponin test in bacteremic patients without acute coronary syndrome. Am J Med 121: 909–915, 2008. doi: 10.1016/j.amjmed.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 17.Kokkinaki D, Hoffman M, Kalliora C, Kyriazis ID, Maning J, Lucchese AM, Shanmughapriya S, Tomar D, Park JY, Wang H, Yang XF, Madesh M, Lymperopoulos A, Koch WJ, Christofidou-Solomidou M, Drosatos K. Chemically synthesized Secoisolariciresinol diglucoside (LGM2605) improves mitochondrial function in cardiac myocytes and alleviates septic cardiomyopathy. J Mol Cell Cardiol 127: 232–245, 2019. doi: 10.1016/j.yjmcc.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landesberg G, Levin PD, Gilon D, Goodman S, Georgieva M, Weissman C, Jaffe AS, Sprung CL, Barak V. Myocardial dysfunction in severe sepsis and septic shock: no correlation with inflammatory cytokines in real-life clinical setting. Chest 148: 93–102, 2015. doi: 10.1378/chest.14-2259. [DOI] [PubMed] [Google Scholar]
- 19.Langley RJ, Tsalik EL, van Velkinburgh JC, Glickman SW, Rice BJ, Wang C, Chen B, Carin L, Suarez A, Mohney RP, Freeman DH, Wang M, You J, Wulff J, Thompson JW, Moseley MA, Reisinger S, Edmonds BT, Grinnell B, Nelson DR, Dinwiddie DL, Miller NA, Saunders CJ, Soden SS, Rogers AJ, Gazourian L, Fredenburgh LE, Massaro AF, Baron RM, Choi AM, Corey GR, Ginsburg GS, Cairns CB, Otero RM, Fowler VG Jr, Rivers EP, Woods CW, Kingsmore SF. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med 5: 195ra95, 2013. doi: 10.1126/scitranslmed.3005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Jeong EM, Liu H, Xie A, So EY, Shi G, Jeong GE, Zhou A, Dudley SC Jr. Magnesium supplementation improves diabetic mitochondrial and cardiac diastolic function. JCI Insight 4: 123182, 2019. doi: 10.1172/jci.insight.123182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med 20: 195–203, 2014. doi: 10.1016/j.molmed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Marx SO, Marks AR. Dysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseases. J Mol Cell Cardiol 58: 225–231, 2013. doi: 10.1016/j.yjmcc.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matkovich SJ, Al Khiami B, Efimov IR, Evans S, Vader J, Jain A, Brownstein BH, Hotchkiss RS, Mann DL. Widespread down-regulation of cardiac mitochondrial and sarcomeric genes in patients with sepsis. Crit Care Med 45: 407–414, 2017. doi: 10.1097/CCM.0000000000002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merx MW, Weber C. Sepsis and the heart. Circulation 116: 793–802, 2007. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 25.Morrow JP, Katchman A, Son NH, Trent CM, Khan R, Shiomi T, Huang H, Amin V, Lader JM, Vasquez C, Morley GE, D’Armiento J, Homma S, Goldberg IJ, Marx SO. Mice with cardiac overexpression of peroxisome proliferator-activated receptor γ have impaired repolarization and spontaneous fatal ventricular arrhythmias. Circulation 124: 2812–2821, 2011. doi: 10.1161/CIRCULATIONAHA.111.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, Xie Y, Carlson D, Rothermel BA, Sun Y, Levine B, Hill JA, Wolf SE, Minei JP, Zang QS. Beclin-1-Dependent Autophagy Protects the Heart During Sepsis. Circulation 138: 2247–2262, 2018. doi: 10.1161/CIRCULATIONAHA.117.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187: 509–517, 2013. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terentyev D, Györke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Györke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103: 1466–1472, 2008. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Wyngene L, Vandewalle J, Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol Med 10: 8712, 2018. doi: 10.15252/emmm.201708712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C, Yi C, Wang H, Bruce IC, Xia Q. Mitochondrial nitric oxide synthase participates in septic shock myocardial depression by nitric oxide overproduction and mitochondrial permeability transition pore opening. Shock 37: 110–115, 2012. doi: 10.1097/SHK.0b013e3182391831. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Bernecker OY, Manohar NS, Hajjar RJ, Hellman J, Ichinose F, Valdivia HH, Schmidt U. Increased leakage of sarcoplasmic reticulum Ca2+ contributes to abnormal myocyte Ca2+ handling and shortening in sepsis. Crit Care Med 33: 598–604, 2005. doi: 10.1097/01.CCM.0000152223.27176.A6. [DOI] [PubMed] [Google Scholar]