Abstract
Cold environmental temperatures during exercise and recovery alter the acute response to cellular signaling and training adaptations. Approximately 3 wk is required for cold temperature acclimation to occur. To determine the impact of cold environmental temperature on training adaptations, fitness measurements, and aerobic performance, two groups of 12 untrained male subjects completed 1 h of cycling in 16 temperature acclimation sessions in either a 7°C or 20°C environmental temperature. Fitness assessments before and after acclimation occurred at standard room temperature. Muscle biopsies were taken from the vastus lateralis muscle before and after training to assess molecular markers related to mitochondrial development. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) mRNA was higher in 7°C than in 20°C in response to acute exercise before training (P = 0.012) but not after training (P = 0.813). PGC-1α mRNA was lower after training (P < 0.001). BNIP3 was lower after training in the 7°C than in the 20°C group (P = 0.017) but not before training (P = 0.549). No other differences occurred between temperature groups in VEGF, ERRα, NRF1, NRF2, TFAM, PINK1, Parkin, or BNIP3L mRNAs (P > 0.05). PGC-1α protein and mtDNA were not different before training, after training, or between temperatures (P > 0.05). Cycling power increased during the daily training (P < 0.001) but was not different between temperatures (P = 0.169). V̇o2peak increased with training (P < 0.001) but was not different between temperature groups (P = 0.460). These data indicate that a 3-wk period of acclimation/training in cold environmental temperatures alters PGC-1α gene expression acutely but this difference is not manifested in a greater increase in V̇o2peak and is dissipated as acclimation takes place.
NEW & NOTEWORTHY This study examines the adaptive response of cellular signaling during exercise in cold environmental temperatures. We demonstrate that peroxisome proliferator-activated receptor-γ coactivator 1α mRNA is different between cold and room temperature environments before training but after training this difference no longer exists. This initial difference in transcriptional response between temperatures does not lead to differences in performance measures or increases in protein or mitochondria.
Keywords: exercise, mitochondria, mRNA, PGC-1α, skeletal muscle
INTRODUCTION
Exercise training is an important way of mitigating disease and may provide an option for treatment. Some individuals may not be able to exercise at proper intensity for adaptive health benefits, necessitating alternative exercise models. Acute exercise in different environmental temperatures appears to elicit an altered physiological response. Specifically, oxygen consumption (V̇o2) at an absolute workload is reduced, and markers of mitochondrial development are reported to be enhanced in the skeletal muscle after acute aerobic exercise in the cold compared with room temperature (35, 39). Many aspects of acclimation to the cold are well documented (9, 33); however, there are limited data describing cold acclimation at the level of the skeletal muscle and how exercise training in the cold may affect whole body aerobic capacity. During acute exercise, peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) increases more at 7°C cold environmental temperature than at 20°C room temperature, leading to the speculation that mitochondrial and whole body aerobic capacity may be further enhanced after repeated exercise bouts in the cold compared with room temperature (39, 40). In this model, exercising in the cold may be an effective way to optimize the beneficial effects of exercise training.
Alternatively, the enhanced skeletal muscle PGC-1α response to the cold may become blunted with cold acclimation so that acute differences between temperatures diminish. Additionally, the effects of markers downstream of PGC-1α and other factors in mitochondrial biogenesis are not as well defined with temperature exposure, being that mRNA of nuclear respiratory factor 2 (NRF2), estrogen-related receptor-α (ERRα), and myocyte enhancer factor 2 (MEF2) are lower in the cold and vascular endothelial growth factor (VEGF) is higher in the cold (35, 39, 40). Markers related to the selective degradation of mitochondria (mitophagy) have also become of interest to researchers recently. The effect of temperature on mitophagy has yet to be determined in humans (15). Examination of mitophagy markers combined with biogenesis markers may provide insight into mitochondrial quality through increased turnover and dynamics.
Acclimation to environmental temperature occurs relatively quickly, i.e., within 10–14 days (6, 36) and involves an increase in metabolic heat production to raise body temperature due to both shivering and nonshivering thermogenesis (12). After acclimation occurs, the rate of shivering is decreased (14, 15, 26). Thus, cold acclimation during exercise training may reduce the initial differences that have been previously observed with acute exercise in the cold and ultimately lead to similar changes in whole body aerobic capacity as occurs from exercise in a room temperature environment. However, since past research has examined only acute exercise responses (39, 40), the purpose of this study was to determine the effects of exercise during a 3-wk acclimation and training period in 7°C compared with 20°C environmental temperatures. The information gained from this research will help determine the practicality of temperature-optimized training to increase the beneficial outcomes of exercise interventions and determine the influence of temperature acclimation on markers related to mitochondrial development.
METHODS
Study design.
Twenty-four untrained male subjects (age: 27 ± 2 yr, height 180 ± 3 cm) completed the procedures associated with this study after completing an Institutional Review Board-approved informed consent that conformed to the Declaration of Helsinki. Subjects were randomly assigned to cold (7°C) or room temperature (20°C) environmental temperatures. The determination of acclimation-induced influences on endurance training were the main interest of this study. Therefore, 7°C trials took place during warm months of the year (July–August) and 20°C trial took place during months of more moderate temperature (6 subjects in April–May, 6 subjects in September), so that subjects would not be preacclimated. All trials occurred in a temperature- and humidity-controlled environmental chamber with the relative humidity set at 40% (Darwin Chambers Co., St. Louis, MO). 7°C has been previously used to induce increased expression of PGC-1α mRNA and altered mRNA of transcription factors downstream of PGC-1α. Cycling shorts were provided for each subject, and all subjects wore short-sleeved T-shirts, so clothing was consistent between the two temperature groups. Subjects completed a total of 18 sessions for this study. Two fitness assessment trials were conducted in standard room temperature conditions 3 days before and 3 days after temperature intervention. The first and last training session were completed at an absolute intensity, the other 14 training sessions at a rating of perceived exertion (RPE) of 15 (hard). A self-selected intensity at an RPE of 15 was chosen during the training sessions as opposed to a fixed workload to allow a natural progressive overload during the 3 wk of training. All trials were completed Monday through Friday over a 22-day period, allowing subjects to recover during the weekend. Day 1 and day 22 represent the first and last training sessions at absolute intensity. Minimal exposure to environmental temperature, i.e., 1 h, was utilized, as this exposure has been sufficient to elicit differential response in gene expression acutely (39, 40). Cold acclimation occurring over a relatively short time period of 1–4 wk has also been observed with 1 to 2 h daily exposure and temperature above that used in the present study (4, 15, 25).
Fitness assessment trials.
During the first and last testing sessions, fitness assessments were completed for all subjects. The fitness assessments consisted of measuring height, weight, body composition, and maximal aerobic capacity (V̇o2peak). To make direct comparisons between temperature groups, these trials took place in standard room temperature conditions regardless of the temperature group that subjects were assigned to. Body density was measured through hydrodensitometry (Exertech, Dresbach, MN) with correction for estimated residual lung volume and gastrointestinal air volume (43). Body density was converted to percent fat using the Siri equation (38). Subjects performed a graded exercise test on an electronically braked cycle ergometer (Velotron; RacerMate, Seattle, WA) while breathing through a calibrated metabolic cart (ParvoMedics TrueOne Metabolic System, Sandy, UT) to determine V̇o2peak and to determine intensity for the absolute workload cycling bouts. Workload started at 95 W and increased by 35 W each stage. Workload associated with V̇o2peak (Wpeak) was calculated by multiplying the relative time spent in the final stage by 35 and adding to the workload of the previously completed stage. These measurements were repeated on the last trial (3 days after the last training session) to assess fitness alterations resulting from training in the 7°C and 20°C environmental conditions.
Absolute-intensity trials.
Subjects performed two absolute-intensity trials on the first day of training and the last day of training in their assigned temperature. Subjects were instructed to refrain from exercise a minimum of 48 h before these trials and to fast overnight. A dietary log of the previous day was self-recorded, and subjects were asked to replicate that diet the day before the final absolute-intensity trial. On the day of the trial, a muscle biopsy was taken from the vastus lateralis muscle before cycling for 60 min on an ergometer (Velotron) in an environmental chamber at an intensity of 50% Wpeak. This workload was chosen because it was anticipated to be an intensity that this group of untrained subjects could realistically exercise at for 60 min. Nude body weight was recorded before and immediately after exercise on a Befour PS-660 ST digital scale (Saukville, WI) to calculate sweat rate. Core temperature was monitored during exercise via RET-1 rectal probe (Physitemp Instruments, Clifton, NJ) inserted 12 cm past the anal sphincter. Skin temperature was recorded and averaged from the chest and back with an SST-1 skin probe (Physitemp Instruments Inc.). Both rectal and skin probes were logged and recorded via SDL200 four-channel thermometer SD logger (Extech, Nashua, NH). Heart rate was monitored continuously during exercise via Polar V800 watch and chest strap (Polar Electronic, Lake Success, NY). After exercise was completed, subjects recovered for 4 h in standard room temperature conditions (~22°C). After the 4-h recovery, a second muscle biopsy was taken from a separate incision ~2 cm proximal to the previous biopsy.
Daily training sessions.
Subjects arrived at the laboratory every weekday (Monday–Friday) for three straight weeks to complete a 60-min ride on a cycle ergometer (Phantom 3; Cycleops, Madison, WI) at the temperature previously assigned. Body weight was measured before and after each session. Subjects were instructed to cycle at an intensity of 15 (hard) on the RPE scale (5). Subjects were encouraged to increase their intensity progressively to maintain an RPE of 15 and create a progressive overload stimulus. Workload was self-adjusted by subjects and recorded using an iPad app (VirtualTraining s.r.o., Czech Republic). During the ride on the cycle ergometer, heart rate was monitored continuously (Polar Electronic, Lake Success, NY), and core body temperature was monitored before and after exercise via tympanic thermometer (Covidien Genius 2, Mansfield, MA). Water was given ad libitum, and the volume consumed was recorded so that daily sweat rate could be monitored.
Biopsies.
During the two absolute workload trials, two muscle biopsies (2 per trial × 2 trials) were obtained from the vastus lateralis using the percutaneous needle biopsy technique with suction (3). The biopsy sites were cleaned, and ~3–4 mL of 1% lidocaine was injected under the skin surface and around the muscle fascia before a small incision was made. The biopsy needle was then inserted into the muscle, and two cuts were made before the needle was extracted. The incision was closed using steri-strips and wrapped with a compression bandage. The biopsy samples were obtained before exercise and following the 4-h recovery. Biopsies for a given absolute-intensity trial were on the same leg, and subsequent biopsies of the same trial were performed ~2 cm proximal to the initial biopsy site. This procedure was repeated for the other absolute-intensity trial on the opposite leg. Samples were cleared of excess blood, separated from connective tissue, and portioned into All Protect Tissue Reagent (Qiagen, Valencia, CA). Samples were stored overnight at 4°C and then transferred to −30°C for later analysis.
Gene expression.
A 16.2 ± 0.2-mg piece of skeletal muscle was homogenized in 800 µL of TRIzol (Invitrogen, Carlsbad, CA) using an electric homogenizer (PowerGen 150; Fisher Scientific, Hampton, NH), and RNA was extracted as previously described (35). RNA concentration was quantified using a nanospectrophotometer (Nano-drop ND-2000; Thermo Scientific, Wilmington, DE). Average RNA yields were 182.8 ± 7.3 ng/µL. The average absorbance ratio at 260/280 was 1.9 indicating high purity of the RNA. The RNA integrity of the samples was assessed using an Agilent RNA 6000 kit and a 2100 Bioanalyzer (both from Agilent Technologies, Santa Clara, CA) according to the manufacturer’s instructions. The RNA integrity number (RIN) was 8.2 ± 0.1, indicating intact RNA. First-strand cDNA synthesis was achieved using the Superscript IV VILO Master Mix with ezDNase Enzyme (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The resulting cDNA was diluted with the appropriate amount of RNase-free water to achieve a final cDNA concentration of 0.5625 ng/µL in the PCR reaction. Each 10-µL qRT-PCR reaction volume contained 0.5 µL of probe and primer mix (PrimeTime qPCR assay; Integrated DNA Technologies, Coralville, IA), 5 µL of qPCR Master Mix (Integrated DNA Technologies), and 4.5 µL of sample cDNA. PCR was run in triplicate on a Stratagene mx3005p PCR system (Agilent Technologies) and a two-step protocol (1 cycle at 95°C for 5 s followed by 60°C for 20 s for 40 cycles). The probes and primers for our genes of interest and stable reference genes were designed by Integrated DNA Technologies (PrimeTime qPCR assay, Coralville, IA) and are presented in Table 1.
Table 1.
Probes and primers used for real-time reverse transcription quantitative PCR
| Genes | Primer 1 | Primer 2 | Probe |
|---|---|---|---|
| Housekeeping | |||
| ACTB |
AAGTCAGTGTA CAGGTAAGCC |
GTCCCCCAACT TGAGATGTATG |
CTGCCTCCAC CCACTCCCA |
| B2M |
ACCTCCATGA TGCTGCTTAC |
GGACTGGTCTTT CTATCTCTTGT |
CCTGCCGTGTGA ACCATGTGACT |
| CYC |
TCTTTCACTTT GCCAAACACC |
CATCCTAAAGC ATACGGGTCC |
TGCTTGCCATCCA ACCACTCAGTC |
| GAPDH |
TGTAGTTGAGG TCAATGAAGGG |
ACATCGCTCA GACACCATG |
AAGGTCGGAGTCA ACGGATTTGGTC |
| rpS18 |
GTCAATGTCTG CTTTCCTCAAC |
GTTCCAGCATA TTTTGCGAGT |
TCTTCGGCCCAC ACCCTTAATGG |
| Mitochondrial biogenesis | |||
| PGC-1α |
GCAATCCGTC TTCATCCACA |
CCAATCAGTACA ACAATGAGCCT |
AGCAGTCCTCACAG AGACACTAGACAG |
| NRF1 |
GTCATCTCACC TCCCTGTAAC |
GATGCTTCAGA ATTGCCAACC |
ATGGAGAGGTGGA ACAAAATTGGGC |
| GABPA |
TGTAGTCTTGGT TCTAGCAGTTTC |
TGGAACAGAGA AAGCAGAGTG |
TGGTTCATTGATG TCTATGGCCTGGC |
| TFAM |
GCCAAGACAGA TGAAAACCAC |
TGGGAAGGT CTGGAGCA |
CGCTCCCCCTTCA GTTTTGTGTATTT |
| ERRα |
TCTCCGCTTG GTGATCTCA |
CTATGGTGTG GCATCCTGTG |
TGGTCCTCTTGAA GAAGGCTTTGCA |
| VEGF |
GCGCTGATAG ACATCCATGA |
CCATGAACTTT CTGCTGTCTTG |
TGCTCTACCTCC ACCATGCCAAG |
| Mitophagy | |||
|
GTTGCTTGGG ACCTCTCTTG |
TGAACACAATG AGCCAGGAG |
TGTAAGTGACTGC TCCATACTCCCCA |
|
| Parkin |
GCTTGGTGGTT TTCTTGATGG |
TTGAAGCCTCA GGAACAACT |
CCTGCTCGGC GGCTCTTTCA |
| BNIP3 |
CCACTAACGAA CCAAGTCAGAC |
CATCTCTGCT GCTCTCTCAT |
AAAGGTGCTGGT GGAGGTTGTCA |
|
CAAACATGATC TGCCCATCTTC |
TCCTCATCCTC CATCCACAA |
TCTCACTGTGA CAGCCCTTCGC |
|
ACTB, β-actin; B2M, β2-microglobulin; CYC, cyclophilin; RPS18, ribosomal protein S18; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; NRF1, nuclear respiratory factor 1; GABPA, GA-binding protein-α; TFAM, mitochondrial transcription factor A; ERRα, estrogen-related receptor-α; VEGF, vascular endothelial growth factor; PINK1, PTEN-induced kinase-1; Parkin, an E3 ubiquitin ligase; BNIP3, Bcl2-interacting protein-3, BNIP3L, Bcl2-interacting protein 3-like.
Quantification of mRNA for genes of interest were completed using the 2−∆∆CT method (24). For each subject, the geometric mean of five housekeeping genes: β-actin (ACTB), β2-microglobulin (B2M), cyclophilin (CYC), and ribosomal protein S18 (rpS-18), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the stable reference point. This combination of genes was determined to be stable using NormFinder software (22, 37). To determine the difference in acute exercise response before and after training, postexercise mRNA data were normalized relative to the preexercise resting sample of that training state. To determine the effect of training, posttraining resting mRNA data was normalized relative to pretraining resting mRNA. For average values below 1.00 (downregulation of gene expression), the negative inverse was taken for equal graphic representation for upregulated and downregulated gene expression.
DNA extraction and quantification.
DNA extraction was obtained during phase separation of the aqueous and organic phase of the TRIzol extraction concurrently with RNA extraction. The interphase containing DNA was extracted, and the remaining aqueous phase overlying the interphase was removed. Next, 100% EtOH was added to precipitate the DNA pellet. The phenol-ethanol supernatant containing protein was transferred to a separate tube from the resulting DNA pellet. The DNA pellet was washed in 0.1 M sodium citrate in 10% ethanol followed by 30-min incubation on a tube rotator. The supernatant was discarded and resuspended in 1,200 μL of 75% EtOH, and the pellet was resuspended in 400 µL of 8 mM NaOH and incubated overnight at 50°C. The DNA was centrifuged and transferred to a new tube removing insoluble materials, and the pH was adjusted with 0.1 M HEPES and quantified on the Nanodrop. The concentration of this DNA was then adjusted to contain the same amount of DNA within a given subject for qRT-PCR analysis (19.7 ng/µL). PCR was then performed on the DNA to measure mitochondrial (mt)DNA relative copy number (mtMinArc). The mtMinArc copies were determined using the 2×2ΔCT equation to determine the number of mtDNA copies per nuclear DNA copy (30). Values were expressed relative to pretraining.
Protein quantification.
Protein extraction was obtained during phase separation of the aqueous and organic phase of the TRIzol extraction. The interphase containing DNA was extracted for separate analysis, and 1.2 mL of isopropanol was added to the remaining phenol-ethanol supernatant liquid. Samples were centrifuged to pellet the proteins and the supernatant was discarded. The protein pellet was resuspended in 1.6 mL of 0.3 M guanidine hydrochloride in 95% ethanol and incubated for 20 min. Samples were then centrifuged at 7,500 g for 5 min at 4°C, and the supernatant was discarded. This step was repeated twice more, with 1.5 mL of 100% EtOH. After a 20-min incubation in EtOH, samples were centrifuged at 7,500 g for 5 min at 4°C. The EtOH was then removed, and the pellets were then resuspended in 150 mL of 1% SDS and incubated overnight at 50°C. The following day, samples were centrifuged at 10,000 g for 10 min at 4°C, and the supernatant was transferred to a new tube to remove insoluble materials for protein quantification.
Protein quantity of each fraction was measured using a commercially available BCA protein assay kit (Pierce Biotechnologies) according to the manufacturer’s instructions. Absorbance of samples was read at 562 nm using a spectrophotometer (Nano-drop ND 2000, Thermo Scientific). Protein concentrations were then calculated using a standard curve according to kit instructions. Sample concentrations were 3.5 ± 0.2 µg/µL.
Gel electrophoresis and Western blotting.
Proteins were separated by SDS-PAGE as previously described, with modifications (18). Briefly, gels were run at 250 V for 45 min in a Mini Trans-Blot tank (Bio-Rad, Hercules, CA). Gels were then transferred to a nitrocellulose membrane using a Trans-Blot Turbo transfer system (Bio-Rad). Total protein was imaged using Revert Total Protein Stain (Li-Cor). The membrane was then blocked for 85 min at room temperature with Li-Cor blocking buffer (Li-Cor), and incubated overnight at 4°C in rabbit anti-human primary antibody at a ratio of 1:1,000 for PGC-1α (Novus Biologicals, catalog no. NBP1-04676, Centennial, CO) in a plate rocker. This antibody has been previously validated and used for Western blot analysis (16, 17, 20). The next day, the membrane was washed and then incubated in secondary antibody using 1 µL of Li-Cor goat anti-rabbit 800 CW antibody (Li-Cor, catalog no. 926-32211) in 20 mL of blocking buffer for 65 min. The membrane was imaged by infrared fluorescence using an Odyssey Fc imaging system (Li-Cor). Bands at 92 kDa were quantified using Image Studio 5.2 software (Li-Cor).
Statistical analysis.
A two-way mixed-design ANOVA (time × temperature) was used for all dependent variables, with time being the repeated measure and temperature being the between-subject factor. If the F ratio values of the ANOVA were found to be significant, a Fisher’s protected least significant difference post hoc test was performed to evaluate where significance occurred. A probability of type I error of less than 5% was considered significant (P < 0.05). All statistical data were analyzed using the Statistical Package for Social Sciences software (SPSS 24.0).
RESULTS
Daily training.
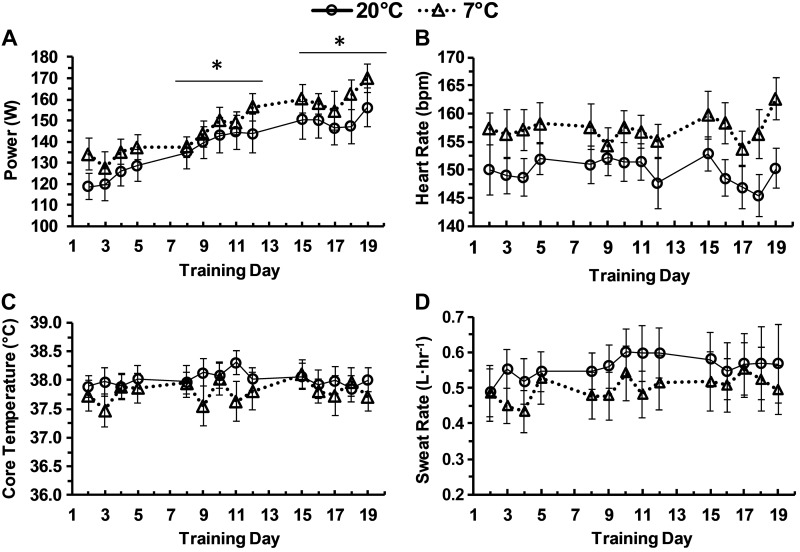
Power increased during each week of training (P < 0.001) but was not different between temperatures (P = 0.331). Heart rate did not change during the 3 wk of training (P = 0.988) but had a trend towards being higher in the 7°C group compared with the 20°C group (P = 0.095). Sweat rate had a trend toward increasing during the 3 wk of training (P = 0.100) but was not different between the 7°C and 20°C group. Core temperature was not different during training (P = 0.431) or between temperature groups (P = 0.233). Data for the daily training trials are presented in Fig. 1.
Fig. 1.
Daily training values. Power (W), heart rate, core temperature, and sweat rate during training each day at an rating of perceived exertion (RPE) of 15 in 20°C or 7°C. Data are means ± SE. *P < 0.05 from previous week.
Fitness.
Subject descriptive data before and after training in the 7°C and 20°C groups are presented in Table 2. Fat free mass (P = 0.028) increased after training in the 20°C group (P < 0.001) but not in the 7°C group (P = 0.200). V̇o2peak increased after training (P < 0.001) but was not different between temperatures (P = 0.460). Total body mass did not change from training (P = 0.762) or between temperature groups (P = 0.903). Percent body fat and fat mass decreased from training (P < 0.001, P < 0.001, respectively) but were not different between temperature groups (P = 0.996, P = 0.894, respectively).
Table 2.
Pretraining and posttraining physiological variables in 20°C and 7°C environments
| Pre |
Post |
|||||
|---|---|---|---|---|---|---|
| 20°C | 7°C | Combined | 20°C | 7°C | Combined | |
| Body mass, kg | 85.3 ± 7.5 | 87.0 ± 6.0 | 86.1 ± 4.7 | 85.9 ± 7.7 | 86.5 ± 5.8 | 86.2 ± 4.7 |
| Body fat, % | 21.9 ± 3.2 | 21.7 ± 2.1 | 21.7 ± 1.8 | 20.4 ± 3.2 | 20.7 ± 2.1 | 20.5 ± 1.8* |
| Fat mass, kg | 20.7 ± 4.9 | 19.9 ± 2.9 | 20.2 ± 2.7 | 19.6 ± 4.8 | 19.0 ± 2.9 | 19.2 ± 2.7* |
| Fat free mass, kg | 64.5 ± 3.7 | 67.1 ± 3.4 | 65.8 ± 2.4 | 66.2 ± 3.8* | 67.6 ± 3.2 | 66.9 ± 2.4 |
| V̇o2peak, L/min | 3.21 ± 0.21 | 3.47 ± 0.16 | 3.34 ± 0.13 | 3.65 ± 0.20 | 3.80 ± 0.10 | 3.72 ± 0.11* |
| V̇o2peak, mL·kg−1·min−1 | 39.4 ± 2.8 | 40.8 ± 1.8 | 40.1 ± 1.6 | 44.2 ± 2.5 | 45.5 ± 2.3 | 44.8 ± 1.6* |
| Core temperature, °C | 37.4 ± 0.3 | 38.0 ± 0.1 | 37.7 ± 0.2 | 37.5 ± 0.2 | 37.7 ± 0.1 | 37.6 ± 0.1 |
| Skin temperature, °C | 33.6 ± 0.4 | 30.1 ± 0.8† | 31.8 ± 0.5 | 32.9 ± 0.4 | 31.2 ± 0.5*† | 32.0 ± 0.3 |
| Heart rate, beats/min | 141 ± 5 | 147 ± 4 | 144 ± 3 | 127 ± 3 | 129 ± 3 | 128 ± 2.0* |
| Sweat rate, mL/h | 505 ± 83 | 456 ± 55 | 481 ± 49 | 687 ± 90 | 537 ± 88 | 612 ± 63* |
Data are means ± SE.
P < 0.05 from pretraining;
P < 0.05 from 20°C.
Absolute-intensity trials.
The absolute-intensity trials allowed for assessment of temperature acclimation and the effect of training during the 3-wk temperature and exercise intervention. Skin temperature was higher in the 20°C group than in the 20°C group both before training (P = 0.001) and after training (P = 0.013). Additionally, skin temperature of the 20°C group did not change from training (P = 0.186) but increased after training in the 7°C group (P = 0.031). Heart rate decreased and sweat rate increased from before to after training (P < 0.001, P = 0.050, respectively) but neither were different between temperatures (P = 0.424, P = 0.307, respectively). No differences in core temperature occurred from pretraining to posttraining (P = 0.251) or between the 7°C and 20°C groups (P = 0.643). Data for the temperature tolerance trials are presented in Table 2.
mRNA response to acute exercise before and after training.
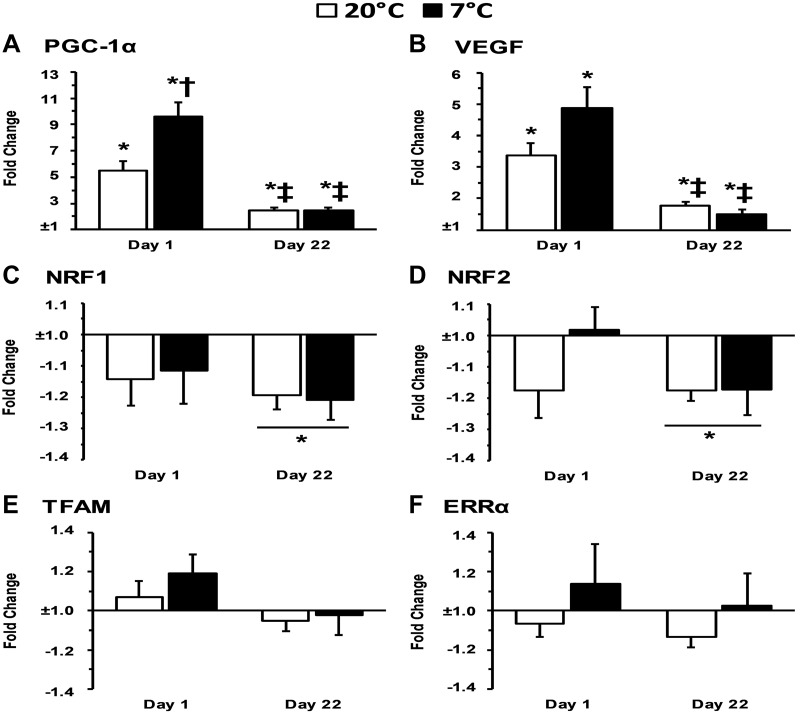
PGC-1α mRNA increased with acute exercise before training and after training in both 7°C and 20°C training groups (P < 0.001). PGC-1α mRNA was higher in 7°C than in 20°C after acute exercise before training (P = 0.012), but this difference between 7°C and 20°C was not observed after training (P = 0.813). Furthermore, PGC-1α mRNA was lower after training in response to acute exercise for both 7°C and 20°C than before training (P < 0.001). VEGF mRNA increased with acute exercise before training and after training in both 7°C and 20°C training groups (P < 0.001). There was a trend for VEGF mRNA to be higher in 7°C than in 20°C after acute exercise before training (P = 0.072), but this trend for a difference between 7°C and 20°C was not observed after training (P = 0.206). Furthermore, VEGF mRNA was lower after training in response to acute exercise for both 7°C (P = 0.002) and 20°C (P < 0.001) than before training. NRF1 and NRF2 mRNA decreased with acute exercise after training regardless of temperature (P = 0.001 and P = 0.001, respectively) and showed a trend to decrease with acute exercise only before training (P = 0.055 and P = 0.080, respectively). TFAM and ERRα mRNA were not altered with acute exercise, training, or temperature (P > 0.05). The mitochondrial biogenesis-related mRNA response to acute exercise before and after training in 7°C and 20°C data are presented in Fig. 2, A–F.
Fig. 2.
A–F: gene expression associated with mitochondrial biogenesis 4-h postexercise relative to preexercise. PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; NRF, nuclear respiratory factor; TFAM, mitochondrial transcription factor A; ERRα, estrogen-related receptor-α; VEGF, vascular endothelial growth factor. *P < 0.05 from preexercise; †P < 0.05 from 20°C; ‡P < 0.05 from day 1 4-h time point.
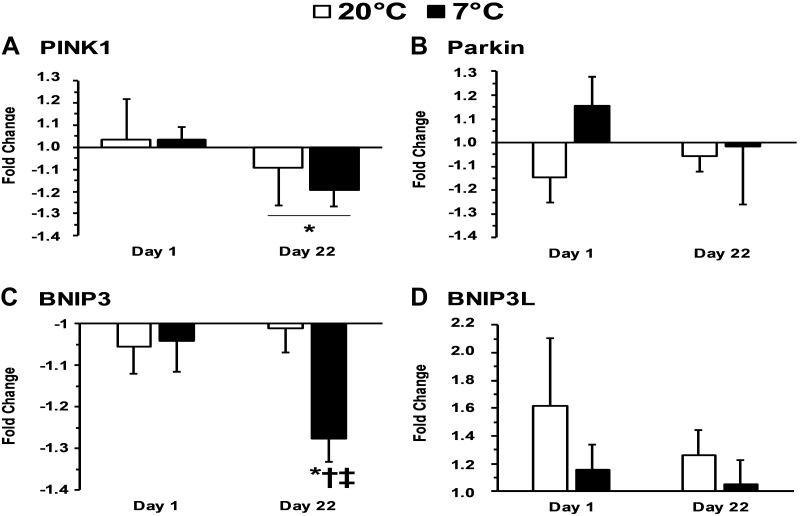
PINK1 (PTEN-induced kinase-1) mRNA did not change with acute exercise before training (P = 0.571) but PINK1 decreased with acute exercise after 3 wk of training in both the 7°C and 20°C group (P = 0.036). There was not a difference in PINK1 mRNA between the 7°C and 20°C groups before or after training (P = 0.796). BNIP3 (Bcl2 interacting protein-3) decreased with acute exercise after training (P < 0.001) and was lower after training compared with before training (P = 0.014). BNIP3 was also lower in the 7°C group than in the 20°C group after training (P < 0.017) but not before training (P = 0.549). Parkin (an E3 ubiquitin ligase) and BNIP3L (BNIP3-like) were not altered with acute exercise, training, or temperature (P > 0.05). The mitophagy-related mRNA response to acute exercise before and after training in both 7°C and 20°C groups are presented in Fig. 3, A–D.
Fig. 3.
A–D: gene expression associated with mitophagy 4-h after exercise relative to preexercise. PINK1, PTEN-induced kinase-1; BNIP3, Bcl2-interacting protein-3, BNIP3L, Bcl2-interacting protein 3-like; Parkin, an E3 ubiquitin ligase. *P < 0.05 from preexercise; †P < 0.05 from 20°C; ‡P < 0.05 from day 1 4-h time point.
Resting mRNA response to training in 7°C and 20°C.
The effect of the 3-wk training in the different environments on mRNA was determined by expressing the resting biopsy after the 3 wk relative to the resting biopsy before the 3-wk training period. PGC-1α (P = 0.731), VEGF (P = 0.129), and NRF1 (P = 0.731) mRNA did not change with training and were not different between 7°C and 20°C training groups (P > 0.05). NRF2 (P = 0.010), TFAM (P = 0.003), and ERRα (P = 0.014) mRNA decreased with training but were not different between 7°C and 20°C training groups (P > 0.05). PINK1 increased (P = 0.025), whereas Parkin (P = 0.001) and BNIP3L (0.008) decreased with training. BNIP3 was trending toward a decrease (P = 0.064) with training. No difference occurred in mitophagy-related genes between temperatures (P > 0.05). The resting mRNA response to training in 7°C and 20°C data are presented in Table 3.
Table 3.
Preexercise gene expression on day 22 relative to day 1
| Biogenesis | 20°C | 7°C | Combined |
|---|---|---|---|
| PGC-1α | −1.043 ± 0.104 | 1.124 ± 0.117 | 1.041 ± 0.078 |
| NRF1 | −1.248 ± 0.075 | −1.050 ± 0.184 | −1.136 ± 0.102 |
| GABPA | −1.162 ± 0.079 | −1.130 ± 0.063 | −1.145 ± 0.049* |
| TFAM | −1.204 ± 0.050 | −1.168 ± 0.106 | −1.185 ± 0.059* |
| ERRα | 1.005 ± 0.111 | −1.253 ± 0.120 | −1.057 ± 0.086* |
| VEGF | −1.215 ± 0.101 | 1.079 ± 0.116 | −1.045 ± 0.080 |
| Mitophagy | 20°C | 7°C | Combined |
|---|---|---|---|
| PINK1 | 1.228 ± 0.186 | 1.412 ± 0.124 | 1.324 ± 0.109* |
| Parkin | −1.353 ± 0.094 | −1.224 ± 0.101 | −1.283 ± 0.068* |
| BNIP3 | −1.149 ± 0.061 | −1.031 ± 0.099 | −1.084 ± 0.059 |
| BNIP3L3 | −1.305 ± 0.095 | −1.117 ± 0.148 | −1.120 ± 0.089* |
Data are means ± SE. PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; NRF1, nuclear respiratory factor 1; GABPA, GA-binding protein-α; TFAM, mitochondrial transcription factor A; ERRα, estrogen-related receptor-α; VEGF, vascular endothelial growth factor; PINK1, PTEN-induced kinase-1; ; Parkin, an E3 ubiquitin ligase; BNIP3, Bcl2-interacting protein-3, BNIP3L3, Bcl2-interacting protein 3-like 3.
P < 0.05 from day 1 Pre value of ±1.
mtDNA relative Amount.
Mitochondrial quantity was determined by comparing the relative amount of mtDNA to nuclear DNA. The relative amount of mtDNA did not change from pre- to posttraining (pre: 1.00 ± 0.00, post: 1.08 ± 0.10, P = 0.445) and was not different between temperature groups (7°C: 1.09 ± 0.08, 20°C: 0.99 ± 0.05, P = 0.271).
PGC-1α protein.
PGC-1α protein did not change from pre- to posttraining (pre: 0.048 ± 0.014 AU, post: 0.059 ± 0.014 AU, P = 0.529) and was not different between temperature groups (7°C: 0.049 ± 0.015 AU, 20°C: 0.058 ± 0.012 AU, P = 0.674).
DISCUSSION
Developing techniques to stimulate key genes related to mitochondrial growth and development is a desirable model for improving therapies and athletic performance. The results from this investigation provide insight into cold exposure during several weeks of exercise. The main finding from this study is that PGC-1α mRNA exhibits an adaptive response after a 3-wk temperature acclimation and exercise-training period. Specifically, the 7°C temperature group had higher PGC-1α mRNA before 3 wk of training than the 20°C temperature groups but was not different after 3 wk of training between the two temperatures. The mRNA of transcription factors downstream of PGC-1α were analyzed as well as mitophagy mRNA to provide a comprehensive view of mitochondrial dynamics. V̇o2peak increased after the 3 wk of training regardless of temperature exposure. Thus, it appears that the enhanced PGC-1α mRNA expression in the cold before training and acclimation does not manifest itself into enhanced mitochondrial function. Both temperature groups were matched for relative fitness, body composition, and age before the training protocol.
Acute exercise effects.
The present study is the first to couple acute physiological measurements with functional performance measurements after a period of cold temperature acclimation. We had previously observed increased PGC-1α mRNA during exercise and subsequent recovery in a 7°C environmental condition (39) and when a 7°C environmental temperature was used for recovery after 20°C exercise (40). In the current study, PGC-1α was higher in 7°C than in 20°C before the 3-wk training intervention. After 3 wk of training, PGC-1α mRNA was increased from acute exercise but to a smaller magnitude compared with before training and was not different between temperatures. Collectively, this disparity of acute response before and after training may be a result of the change in training state of the subjects and/or the acclimation to the cold. Sedentary subjects were chosen in the present study to obtain a robust response in acute gene expression (29), whereas previously we had used recreationally trained subjects (35, 40). The overall attenuated response of PGC-1α at 4 h postexercise after the 3-wk training intervention was expected, due to the increased fitness level of the subjects without an increase in intensity during this trial (29, 41, 44). Absolute intensity was equal from the pretraining trial to the posttraining trial in the current study. The 7°C trial occurred during the summer months to ensure subjects were not preacclimated to the cold temperature. Our present data indicate that cold stimulus during exercise is sufficient to elicit an increase in PGC-1α mRNA when the subject is not acclimated to the cold. After the 3-wk intervention, the difference in PGC-1α mRNA was not present, which may be have been due to an adaptive mRNA response. However, gene expression after multiple weeks of temperature exposure remains poorly characterized. Mice housed below their thermoneutral zone, i.e., room temperature, have higher exercise-induced markers of mitochondrial biogenesis in adipose tissue compared with mice housed in their thermoneutral zone at 29°C (27). Systemic effects of core and skin temperature may have also been responsible for the PGC-1α mRNA alterations before and after training. Skin temperature afferent signaling inhibits activation of the preoptic area, causing an increase in efferent signaling for thermogenesis to tissues such as brown adipose tissue and skeletal muscle (10, 28). Core temperature did not change in either group, whereas skin temperature increased in the 7°C group from pretraining to posttraining but not in the 20°C group. This increased skin temperature after training in the cold is indicative of habituation and acclimation to the cold (23). More research is needed to determine whether skin cooling with exercise is responsible for the additive effect of increased PGC-1α and alteration of other transcription factors.
VEGF is an angiogenic factor that has an additional role in stimulating nuclear encoded mitochondrial genes (46). VEGF trended toward being higher in the 7°C group before training and increased from the exercise response in both temperatures. VEGF was also blunted posttraining compared with before training, similarly to the response of PGC-1α. The trend in VEGF being increased was likely due to its role as the master regulator of angiogenesis. As cold temperature increases, blood is shunted toward skeletal muscle, which requires an increased demand for skeletal muscle vasculature (1). Several studies indicate that VEGF increases with environmental cold exposure and cold-water immersion (11, 20, 35). This putative cold-induced increase in skeletal muscle vasculature likely requires the coordination of VEGF via the angiogenic pathway. VEGF is also involved in pathways related to mitochondrial biogenesis though the induction of ERRα. No changes in ERRα or TFAM were observed before or after training. Some have reported ERRα to peak around 2 h postexercise (7). The 4-h postexercise time point used in the current study may have missed this peak expression time. The TFAM expression may be explained by the nutritional status of the subjects. TFAM mRNA appears to be increased when one is fed but not when fasted (34). The subjects in the present study were required to fast overnight before the absolute-intensity trials, which might have influenced these results. TFAM was of interest despite this knowledge to determine the effects of temperature acclimation on gene expression.
NRF1 and NRF2 are transcription factors downstream of PGC-1α and are responsible for promoting the transcription of TFAM. No acute changes were present in NRF1 and NRF2 before the 3-wk training period, but both decreased after acute exercise after the 3-wk period. A lower NRF2 mRNA response has been observed previously in 7°C compared with 20°C after acute exercise (35, 40) but was not present in the current study. This may have been due to differences in fitness status between subjects, as in the current study sedentary subjects were recruited whereas recreationally trained subjects were used in previous studies. Others have reported NRF2 to peak at 24 h postexercise (7). The time point taken in the current study may not be representative of peak transcription of NRF2, but an additional time point of 24 h was not included, being that peak gene expression occurs at 2–6 h postexercise in most of the transcription factors measured and to reduce the total number of biopsies taken. No difference occurred between temperatures in the present study, but it is interesting to note the discrepancy of these transcription factors before and after training. To our knowledge, this is the first study to demonstrate an adaptive response in gene expression of NRF1 and NRF2 after temperature acclimation.
Before the training and temperature intervention occurred, markers of mitophagy did not change from acute exercise and were not different between temperature groups. After the 3-wk training protocol, PINK1 and BNIP3 were decreased from acute exercise, whereas Parkin and BNIP3L did not change. These findings may also be attributed to the use of healthy, young subjects without the presence of disease. Although the subjects were relatively unfit, they were young and healthy, and increasing mitochondria may have precedence over mitochondrial remodeling due to absence of damaged mitochondria. Changes in markers of mitophagy in the current study may be affected by the fasted state of the subjects. BNIP mRNA is elevated in the fasted compared with the fed state in mice, although Parkin remains unchanged (19). The basal mRNA levels of some of these mitophagy markers may have increased because of the overnight fast leaving little room for increases beyond the basal level. The time course of mRNA markers of mitophagy is not characterized in humans as it is for markers of mitochondrial biogenesis. Markers of mitochondrial biogenesis peak around the 2- to 6-h time-point, and it is expected that markers of mitophagy would peak around the same time but may occur on a different time-course.
Three-week training temperature effects.
Comparing basal day 22 to day 1 mRNA allowed for assessment of the 3-wk temperature and exercise intervention. Participants did not exercise for 3 days before these assessments to ensure a chronic effect and not an effect of the previous acute-exercise bout. Most basal markers of mitochondrial biogenesis and mitophagy either did not change or decreased. PINK1 mRNA increased basally after the 3 wk of training. This may suggest the beginning stages of mitochondrial remodeling due to the stress from the 3 wk of training. However, the other markers of mitophagy were not indicative of this.
Total PGC-1α protein and mtDNA:gDNA were measured to examine the cumulative effect of exercise and temperature from the daily exercise. Both PGC-1α protein and the relative amount of mtDNA did not change during the 3-wk-training protocol. Similarly, cold-acclimated fish contain similar mtDNA copy number to unacclimated control despite greater mitochondrial enzyme activity in the cold-acclimated fish (2). The lack of change in mtDNA and PGC-1α protein may have been due to the relatively short training period. In rats, noticeable increases in PGC-1α protein occur in as little as 4 days of training, with greater increases occurring for up to 53 days (42). This time course is not well characterized in humans, as 10 days of training was reported to not increase PGC-1α protein beyond the initial training shift (41). Discrepancies occur as to the time course of when protein and mtDNA increases can be observed (29, 45). A longer training period of at least 6 wk may be needed to discern an appreciable accumulation in protein (32) and mtDNA (2). A longer training period was not used in the current study, since the goal was to assess the influence of temperature acclimation as opposed to training. Future research may focus further on the exercise training and mitochondrial responses. However, with the normalization of PGC-1α mRNA response with training/acclimation, little evidence in humans suggest a cold potentiation of the exercise response.
The molecular signaling events of no difference in PGC-1α between temperature groups are in correspondence with the functional performance improvements of V̇o2peak and body composition. The 3-wk training protocol for 1 h/day increased V̇o2peak, decreased fat mass, and decreased percent body fat regardless of temperature. Fat-free mass increased in the 20°C group from pre- to posttraining trial but not in the 7°C group. During resistance exercise, myogenic markers such as myogenin, myogenic regulatory factor 4, and myogenic factor 5 have a higher response in warmer compared with colder local temperature application (47). Cold stimuli such as cold-water immersion during recovery may also attenuate skeletal muscle hypertrophy in resistance training (31). The combined use of endurance exercise and cold temperature may have prohibited increases in skeletal muscle hypertrophy in the current study, whereas the 20°C may have not had the same inhibition, thus increasing fat-free mass posttraining. This may explain the increase in fat-free mass in the 20°C group in the present study, although further examination of this increase may be warranted. A disconnect appears with the mtDNA molecular events and V̇o2peak. Despite no change in mtDNA after training, V̇o2peak increased after 3 wk of training. This may have been due to improvements in skeletal muscle markers not measured such as increased mitochondrial efficiency and muscle oxidative capacity (13, 48). This may also be due to improvements to whole body metabolism such as increased muscle fiber recruitment, capillary density, arteriovenous differences, and cardiac output (13).
Conclusion.
Understanding the characteristics related to the process of temperature acclimation are of importance due to the potential for novel therapeutic treatments and health benefits and possible effects on performance. The findings from this study demonstrate that the initial acute difference of PGC-1α is changed after several weeks of acclimation and training in environmental cold. PGC-1α normalized between temperatures, and this may explain the lack of differences in VO2peak performance after training between the groups. Differences between 7°C and 20°C environments may not exist after an acclimation period, as skeletal muscle acclimation may be the adaptation responsible. This is likely due to the acclimation process and not the fitness status of the subjects, as more recreationally trained individuals have been used previously. These data indicate that, despite initial differences in PGC-1α gene expression, temperature-acclimated skeletal muscle does not elicit the same response of increased signaling in the cold related to mitochondrial development. Clinicians and athletes looking to rehabilitate and improve performance, respectively, may not find additional benefits in further improving aerobic capacity in cold environmental temperatures. Future work may compare the long-term aspects of training in cold environmental temperatures to determine the influence of training adaptations after acclimation has occurred. Different forms of cold therapy such as local cooling or cold-water immersion should also be considered in attempting to improve training outcomes.
GRANTS
The research was funded by the Department of Defense United States Army Medical Research and Materiel Command (DOD USAMRMC: W81XWH-15-2-0075), the National Institute of General Medical Sciences Nebraska IDeA Networks of Biomedical Research Excellence (NIGMS-P20GM103427), and a University of Nebraska at Omaha Graduate Research and Creative Activity (GRACA) grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.S., B.R., and D.S. conceived and designed research; R.S., K.M., M.O., H.S., B.R., and D.S. performed experiments; R.S., K.M., M.O., H.S., B.R., and D.S. analyzed data; R.S., K.M., M.O., H.S., B.R., and D.S. interpreted results of experiments; R.S., K.M., H.S., and D.S. prepared figures; R.S., K.M., and D.S. drafted manuscript; R.S., K.M., M.O., H.S., B.R., and D.S. edited and revised manuscript; R.S., K.M., M.O., H.S., B.R., and D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Walter Hailes, Caleb Ross, Roksana Zak, Zohal Alizai, and Chris Collins for their various contributions in data collection and analysis.
REFERENCES
- 1.Banchero N, Kayar SR, Lechner AJ. Increased capillarity in skeletal muscle of growing guinea pigs acclimated to cold and hypoxia. Respir Physiol 62: 245–255, 1985. doi: 10.1016/0034-5687(85)90118-5. [DOI] [PubMed] [Google Scholar]
- 2.Battersby BJ, Moyes CD. Influence of acclimation temperature on mitochondrial DNA, RNA, and enzymes in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 275: R905–R912, 1998. doi: 10.1152/ajpregu.1998.275.3.R905. [DOI] [PubMed] [Google Scholar]
- 3.Bergström J. Muscle Electrolytes in Man: Determined by Neutron Activation Analysis on Needle Biopsy Specimens: a Study on Normal Subjects, Kidney Patients, Patients with Chronic Diarrhoea; from the Clinical Laboratory, St. Erik’s Sjukhus. Stockholm, Sweden: Scandinavian University Press, 1962. [Google Scholar]
- 4.Blondin DP, Daoud A, Taylor T, Tingelstad HC, Bézaire V, Richard D, Carpentier AC, Taylor AW, Harper ME, Aguer C, Haman F. Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue. J Physiol 595: 2099–2113, 2017. doi: 10.1113/JP273395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports 5: 90–93, 1973. [PubMed] [Google Scholar]
- 6.Brück K, Baum E, Schwennicke HP. Cold-adaptive modifications in man induced by repeated short-term cold-exposures and during a 10-day and-night cold-exposure. Pflugers Arch 363: 125–133, 1976. doi: 10.1007/BF01062280. [DOI] [PubMed] [Google Scholar]
- 7.Cartoni R, Léger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Dériaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 567: 349–358, 2005. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellani JW, Young AJ. Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton Neurosci 196: 63–74, 2016. doi: 10.1016/j.autneu.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Cui J, Durand S, Crandall CG. Baroreflex control of muscle sympathetic nerve activity during skin surface cooling. J Appl Physiol (1985) 103: 1284–1289, 2007. doi: 10.1152/japplphysiol.00115.2007. [DOI] [PubMed] [Google Scholar]
- 11.D’Souza RF, Zeng N, Markworth JF, Figueiredo VC, Roberts LA, Raastad T, Coombes JS, Peake JM, Cameron-Smith D, Mitchell CJ. Divergent effects of cold water immersion versus active recovery on skeletal muscle fiber type and angiogenesis in young men. Am J Physiol Regul Integr Comp Physiol 314: R824–R833, 2018. doi: 10.1152/ajpregu.00421.2017. [DOI] [PubMed] [Google Scholar]
- 12.Daanen HA, Van Marken Lichtenbelt WD. Human whole body cold adaptation. Temperature (Austin) 3: 104–118, 2016. doi: 10.1080/23328940.2015.1135688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daussin FN, Zoll J, Dufour SP, Ponsot E, Lonsdorfer-Wolf E, Doutreleau S, Mettauer B, Piquard F, Geny B, Richard R. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: relationship to aerobic performance improvements in sedentary subjects. Am J Physiol Regul Integr Comp Physiol 295: R264–R272, 2008. doi: 10.1152/ajpregu.00875.2007. [DOI] [PubMed] [Google Scholar]
- 14.Davis TR. Chamber cold acclimatization in man. J Appl Physiol 16: 1011–1015, 1961. doi: 10.1152/jappl.1961.16.6.1011. [DOI] [PubMed] [Google Scholar]
- 15.Gordon K, Blondin DP, Friesen BJ, Tingelstad HC, Kenny GP, Haman F. Seven days of cold acclimation substantially reduces shivering intensity and increases nonshivering thermogenesis in adult humans. J Appl Physiol (1985) 126: 1598–1606, 2019. doi: 10.1152/japplphysiol.01133.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasan-Olive MM, Lauritzen KH, Ali M, Rasmussen LJ, Storm-Mathisen J, Bergersen LH. A ketogenic diet improves mitochondrial biogenesis and bioenergetics via the PGC1α-SIRT3–UCP2 axis. Neurochem Res 44: 22–37, 2019. doi: 10.1007/s11064-018-2588-6. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto Y, Shiina M, Kato T, Yamamura S, Tanaka Y, Majid S, Saini S, Shahryari V, Kulkarni P, Dasgupta P, Mitsui Y, Sumida M, Deng G, Tabatabai L, Kumar D, Dahiya R. The role of miR-24 as a race related genetic factor in prostate cancer. Oncotarget 8: 16581–16593, 2017. doi: 10.18632/oncotarget.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heesch MW, Shute RJ, Kreiling JL, Slivka DR. Transcriptional control, but not subcellular location, of PGC-1α is altered following exercise in a hot environment. J Appl Physiol (1985) 121: 741–749, 2016. doi: 10.1152/japplphysiol.01065.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamart C, Naslain D, Gilson H, Francaux M. Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. Am J Physiol Endocrinol Metab 305: E964–E974, 2013. doi: 10.1152/ajpendo.00270.2013. [DOI] [PubMed] [Google Scholar]
- 20.Joo CH, Allan R, Drust B, Close GL, Jeong TS, Bartlett JD, Mawhinney C, Louhelainen J, Morton JP, Gregson W. Passive and post-exercise cold-water immersion augments PGC-1α and VEGF expression in human skeletal muscle. Eur J Appl Physiol 116: 2315–2326, 2016. doi: 10.1007/s00421-016-3480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julian GS, Oliveira RW, Tufik S, Chagas JR. Analysis of the stability of housekeeping gene expression in the left cardiac ventricle of rats submitted to chronic intermittent hypoxia. J Bras Pneumol 42: 211–214, 2016. doi: 10.1590/S1806-37562015000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leppäluoto J, Korhonen I, Hassi J. Habituation of thermal sensations, skin temperatures, and norepinephrine in men exposed to cold air. J Appl Physiol (1985) 90: 1211–1218, 2001. doi: 10.1152/jappl.2001.90.4.1211. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Mäkinen TM, Mäntysaari M, Pääkkönen T, Jokelainen J, Palinkas LA, Hassi J, Leppäluoto J, Tahvanainen K, Rintamäki H. Autonomic nervous function during whole-body cold exposure before and after cold acclimation. Aviat Space Environ Med 79: 875–882, 2008. doi: 10.3357/ASEM.2235.2008. [DOI] [PubMed] [Google Scholar]
- 26.Mathew L, Purkayastha SS, Jayashankar A, Nayar HS. Physiological characteristics of cold acclimatization in man. Int J Biometeorol 25: 191–198, 1981. doi: 10.1007/BF02184518. [DOI] [PubMed] [Google Scholar]
- 27.McKie GL, Medak KD, Knuth CM, Shamshoum H, Townsend LK, Peppler WT, Wright DC. Housing temperature affects the acute and chronic metabolic adaptations to exercise in mice. J Physiol 597: 4581–4600, 2019. doi: 10.1113/JP278221. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiros PM, Goyal A, Jha P, Auwerx J. Analysis of mtDNA/nDNA Ratio in Mice. Curr Protoc Mouse Biol 7: 47–54, 2017. doi: 10.1002/cpmo.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts LA, Raastad T, Markworth JF, Figueiredo VC, Egner IM, Shield A, Cameron-Smith D, Coombes JS, Peake JM. Post-exercise cold water immersion attenuates acute anabolic signalling and long-term adaptations in muscle to strength training. J Physiol 593: 4285–4301, 2015. doi: 10.1113/JP270570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Dériaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-γ coactivator-1 and peroxisome proliferator-activated receptor-α in skeletal muscle. Diabetes 52: 2874–2881, 2003. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 33.Sawka MN, Young AJ. Physiological systems and their responses to conditions of heat and cold. In: ACSM’s Advance Exercise Physiology, edited by Sawka MN, Tipton CM. Hagerstown, MD: Lippincott Williams and Wilkins, 2006, p. 536–563. [Google Scholar]
- 34.Schwalm C, Deldicque L, Francaux M. Lack of activation of mitophagy during endurance exercise in human. Med Sci Sports Exerc 49: 1552–1561, 2017. doi: 10.1249/MSS.0000000000001256. [DOI] [PubMed] [Google Scholar]
- 35.Shute RJ, Heesch MW, Zak RB, Kreiling JL, Slivka DR. Effects of exercise in a cold environment on transcriptional control of PGC-1α. Am J Physiol Regul Integr Comp Physiol 314: R850–R857, 2018. doi: 10.1152/ajpregu.00425.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silami-Garcia E, Haymes EM. Effects of repeated short-term cold exposures on cold induced thermogenesis of women. Int J Biometeorol 33: 222–226, 1989. doi: 10.1007/BF01051081. [DOI] [PubMed] [Google Scholar]
- 37.Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol 7: 33, 2006. doi: 10.1186/1471-2199-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siri WE. Body composition from fluid spaces and density: analysis of methods. Nutrition 61: 223–244, 1961. [PubMed] [Google Scholar]
- 39.Slivka DR, Dumke CL, Tucker TJ, Cuddy JS, Ruby B. Human mRNA response to exercise and temperature. Int J Sports Med 33: 94–100, 2012. doi: 10.1055/s-0031-1287799. [DOI] [PubMed] [Google Scholar]
- 40.Slivka D, Heesch M, Dumke C, Cuddy J, Hailes W, Ruby B. Effects of post-exercise recovery in a cold environment on muscle glycogen, PGC-1α, and downstream transcription factors. Cryobiology 66: 250–255, 2013. doi: 10.1016/j.cryobiol.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Stepto NK, Benziane B, Wadley GD, Chibalin AV, Canny BJ, Eynon N, McConell GK. Short-term intensified cycle training alters acute and chronic responses of PGC1α and cytochrome C oxidase IV to exercise in human skeletal muscle. PLoS One 7: e53080, 2012. doi: 10.1371/journal.pone.0053080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor EB, Lamb JD, Hurst RW, Chesser DG, Ellingson WJ, Greenwood LJ, Porter BB, Herway ST, Winder WW. Endurance training increases skeletal muscle LKB1 and PGC-1α protein abundance: effects of time and intensity. Am J Physiol Endocrinol Metab 289: E960–E968, 2005. doi: 10.1152/ajpendo.00237.2005. [DOI] [PubMed] [Google Scholar]
- 43.Thomas TR, Etheridge GL. Hydrostatic weighing at residual volume and functional residual capacity. J Appl Physiol 49: 157–159, 1980. doi: 10.1152/jappl.1980.49.1.157. [DOI] [PubMed] [Google Scholar]
- 44.Tunstall RJ, Mehan KA, Wadley GD, Collier GR, Bonen A, Hargreaves M, Cameron-Smith D. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab 283: E66–E72, 2002. doi: 10.1152/ajpendo.00475.2001. [DOI] [PubMed] [Google Scholar]
- 45.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem 282: 194–199, 2007. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 46.Wright GL, Maroulakou IG, Eldridge J, Liby TL, Sridharan V, Tsichlis PN, Muise-Helmericks RC. VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J 22: 3264–3275, 2008. doi: 10.1096/fj.08-106468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zak RB, Hassenstab BM, Zuehlke LK, Heesch MWS, Shute RJ, Laursen TL, LaSalle DT, Slivka DR. Impact of local heating and cooling on skeletal muscle transcriptional response related to myogenesis and proteolysis. Eur J Appl Physiol 118: 101–109, 2018. doi: 10.1007/s00421-017-3749-z. [DOI] [PubMed] [Google Scholar]
- 48.Zoll J, Sanchez H, N’Guessan B, Ribera F, Lampert E, Bigard X, Serrurier B, Fortin D, Geny B, Veksler V, Ventura-Clapier R, Mettauer B. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 543: 191–200, 2002. doi: 10.1113/jphysiol.2002.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]