The rapid increase in popularity of “pod-mods” such as JUUL e-cigarettes, particularly in youth, has sparked many discussions on the possible harmful effects of JUUL. We spotlight key differences between JUUL, which contains 5% nicotine in benzoic salt form, and a conventional e-cigarette, Blu, which is claimed to contain 2.4% nicotine free base. We compared the measured pHs of JUUL and Blu E-liquids with pH values calculated based on chemical principles. The concentrations of protonated and unprotonated nicotine in these two kinds of e-cigarettes were also calculated. Theoretically, there is a clear distinction between the pH effects of the direct contacts of e-cigarette aerosol on the tissue in the inner surface of the respiratory tract and on other body systems via circulation after absorption. The concentration of protonated nicotine [the ligand of nicotinic acetylcholine receptors (nAChRs)] in JUUL (pH 6.0) is 7 times higher than in Blu that, hypothetically, excessively stimulates nAChRs that impact the epithelium inflammatory responses in the lungs and contribute to onset, progression, and proliferation of lung cancer. The concentration of unprotonated nicotine that readily diffuses across membranes (high absorption rate) in Blu (pH 8.26) is 26 times higher than that in JUUL. Based on pH and protonated versus unprotonated nicotine considerations, JUUL e-cigarettes potentially would lead to more detrimental effects on the lung, while conventional e-cigarettes such as Blu would lead to more systemic effects, such as on cardiovascular and nervous systems. Regulatory policies on the pH of E-liquid are implicated.
INTRODUCTION
A new type of electronic cigarettes, the “pod-mod” e-cigarette, has raised public health concerns in the press, the e-cigarette research community, and among regulatory agencies. A recent US FDA statement regarding safety issues of e-cigarette use, particularly in youth and young adults, states “we’re looking at the potential for direct effects of harm from e-cigarettes on the lungs as well as other health factors that these products could negatively impact. In particular, we have concerns about the direct effects of e-cigarettes on the airways. This includes the potential for the use of such products to cause changes to airways that could be a precursor to cancer” (11). In addition, a series of CDC and FDA announcements reported over 2,000 cases of respiratory illnesses associated with e-cigarette/vaping product use (11a). Patients develop shortness of breath, fatigue, chest pain, cough, anorexia, nausea, diarrhea, and weight loss, with symptoms worsening over days or weeks with some dying from this condition. More research is urgently needed to understand the causes and pathophysiology of the respiratory toxicity.
Traditional e-cigarette products use E-liquid with free-base nicotine while JUUL and other pod-mods use protonated nicotine formulations derived from the nicotine salts in loose-leaf tobacco. JUUL contains 0.7 ml E-liquid per pod with concentration of 50 mg/ mL (5%), which is 2 to 10 times those found in most free-base nicotine e-cigarette products—equivalent to ~20 combustible cigarettes (2). Goniewicz et al. (10) confirmed the concentration of nicotine in a JUUL pod to be 56.2 mg/mL. The JUUL website further states that the salt-based nicotine E-liquid formula is intended to help satisfy smokers when transitioning from cigarettes. Here we focus on discussions on the potential health effects of E-liquid pH, nicotine salt versus free-base nicotine, and protonated versus unprotonated nicotine, as well as an important distinction of pH effects on the lungs and other organ systems.
pH, PROTONATED VERSUS UNPROTONATED NICOTINE IN E-LIQUIDS
Nicotine in aqueous solution can exist in three forms: diprotonated, monoprotonated, and unprotonated. The diprotonated form is of low abundance and negligible importance in this context. We consider only the monoprotonated and unprotonated nicotine in the following discussion.
According to the manufacturer, JUUL E-liquid contains 5% nicotine (308 mM) as a salt of benzoic acid. For comparison, a conventional tank e-cigarette (Blu e-cigarettes) contains 2.4% nicotine (148 mM). The logarithmic acid dissociation constant (pKa) of nicotine is 7.89 at 25°C (5), pKb = 14 − 7.89 = 6.11. The pH can be calculated (1) using:
| (1) |
where Nic denotes unprotonated nicotine and NicH+ denotes protonated nicotine. We assume 148 mM of free-base nicotine is present in Blu E-liquid. We assume that equal molar concentrations (308 mM) of nicotine and benzoate are present in JUUL E-liquid. With the pKa of benzoic acid being 4.2, the calculated pHs of Blu and JUUL are listed in Table 1.
Table 1.
pH, protonated and unprotonated nicotine in Blu and JUUL e-cigarettes
| Nicotine Concentration (mM) | Calculated pH | Measured pH | Protonated Nicotine (mM) | Unprotonated Nicotine (mM) | |
|---|---|---|---|---|---|
| Blu | 148 (free base) | 10.53 | 8.26 (SD 0.01) | 44.3 | 103.7 |
| JUUL | 308 (benzoic salt) | 6.05 | 6.0 (SD 0.03) | 304 | 4 |
We then measured the pH of commercial JUUL and Blu E-liquids (purchased from https://www.JUUL.com and https://www.Blu.com; both classic tobacco flavor). E-liquid samples were diluted 1:1 with deionized H2O and measured with a well-calibrated pH meter (AB15, Accumet). The samples were analyzed in triplicate, and the results are listed in Table 1.
The Blu E-liquid is basic and ~2 pH units lower than what was expected from the calculation assuming 148 mM free-base nicotine is in the E-liquid. In contrast, JUUL is acidic, close to our calculated value. Our measured pH of JUUL is consistent with that of Talih et al. (17), and pH of Blu is consistent with Stepanov and Fujioka (15), although this is not a direct comparison, as the nicotine concentration of the Blu E-liquid we used is higher (24 mg/mL). We suggest that the pH of conventional e-cigarette, such as Blu may have been buffered with acids and other acidic components during the manufacturing process.
Based on the Henderson-Hasselbalch equation, the ratio of the protonated versus unprotonated nicotine is a function of pH:
| (2) |
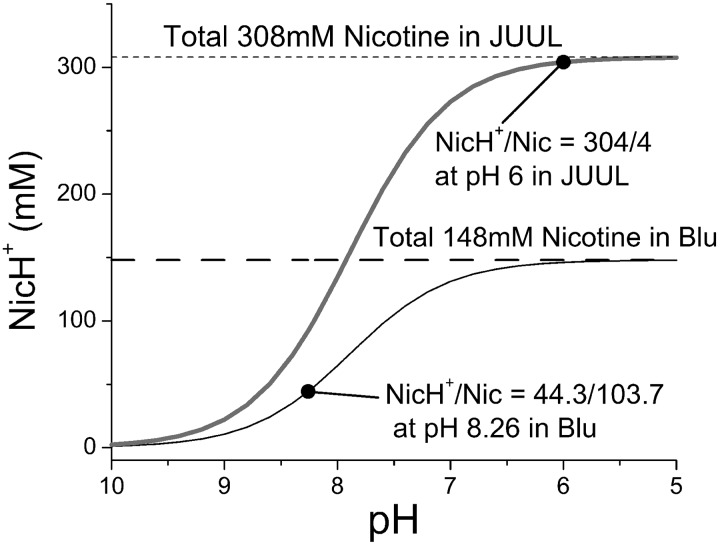
Therefore, in Blu E-liquid (where the pH = 8.26 and initial [Nic] = 148 mM), the protonated and unprotonated nicotine would be [NicH+] = 44.3 mM and [Nic] = 103.7 mM. In JUUL E-liquid (where the pH = 6.0 and initial [Nic] = 308 mM), the [NicH+] = 304 mM, while [Nic] = 4 mM (Table 1 and Fig. 1).
Fig. 1.
Protonated nicotine (NicH+) concentration as a function of pH in E-liquids of Blu and JUUL based on Henderson-Hasselbalch equation. Unprotonated nicotine (Nic) concentration is the difference between total nicotine and NicH+ concentrations at each pH. Note that at pH = pKa of nicotine = 7.89, [NicH+] = [Nic].
JUUL E-liquid has a protonated nicotine concentration that is 7 times higher than that in Blu. The unprotonated nicotine concentration is 4% of that in Blu E-liquid, whereas in JUUL E-liquid, the unprotonated nicotine is 1.3% of its protonated form.
BIOLOGICAL CONSEQUENCES AND CLINICAL RELEVANCE OF PH AND SALTS IN INHALED NICOTINE
Nicotine aerosol with appropriate particle size distribution, such as tobacco smoke, e-cigarette aerosol, and aerosolized nicotine solution, deposits in the alveolar regions of the lungs where it is quickly absorbed. In the 1990s to 2000s, there were many discussions on whether the tobacco industry manipulated the pH of tobacco cigarettes to increase the addiction potential (7). Now, JUUL is using low-pH salt E-liquid to produce a “smoother taste” such that users can take a higher dose of nicotine. An article in the Los Angeles Times uncovered that JUUL took the idea of adding acid to nicotine to develop nicotine salt liquid to make the product more palpable and appealing to youths (3). Those discussions on tobacco cigarettes have been controversial since it is hard to define pH in tobacco smoke which fails to match the conventional definition of pH. Since we can define pH in nicotine aerosol generated from nicotine solution (13) or E-liquids, and the ratio of protonated vs unprotonated nicotine is a function of pH (Fig. 1), we have a methodological framework for further studies to understand how pH and the protonated versus unprotonated nicotine contribute to nicotine pulmonary toxicity, absorption/rate of transfer in the lungs, and the bioavailability. The biological consequences of the differences in pHs and concentrations of protonated versus unprotonated nicotine between JUUL and Blu are as follows:
1) Nicotine binds to nicotinic acetylcholine receptors (nAChRs) that mediate its actions. It has been identified that it is the protonated, not unprotonated, nicotine that is the ligand of nAChRs (19). Bronchial epithelial cells in the lungs express functional nAChRs (12). Nicotine modulates multiple inflammatory responses in the lung through the nAChR subtype α7 (9). nAChRs are also expressed on lung cancer cells (14). These nAChRs readily interact with inhaled nicotine aerosol. With JUUL, the concentrations of ligand (protonated nicotine) binding to nAChRs are 7 times higher than Blu; we propose that high concentrations of protonated nicotine excessively stimulate (activate and desensitize) nAChRs that impact the epithelium responses in the lungs to the bacterial inflammogen (9) as well as contribute to onset, progression, and proliferation of lung cancer (8, 16). Thus, JUUL e-cigarettes could potentially produce more pronounced toxic effects in the lungs, including lung cancer promotion, than conventional e-cigarettes such as Blu.
2) The unprotonated free-base form of nicotine is lipophilic and thus readily diffuses across membranes (18) of the respiratory tract into the blood, whereas the protonated form of nicotine is hydrophilic and does not as readily diffuse across the membranes. Higher pH (increasing the ratio of unprotonated nicotine) in aerosolized nicotine produces a higher peak plasma nicotine concentration in humans (4). As drug delivery rate contributes to addiction potential, increased nicotine free-base levels lead to an increase in the delivery rate, enhancing the addiction potential. In contrast, the lower pH in JUUL E-liquid and aerosol decreases the concentrations of unprotonated nicotine (4 mM in JUUL versus 103.7 mM in Blu-cig calculated above) that reduces the amount absorbed in the lungs, as a consequence, reducing bioavailability of nicotine and potentially reducing its systemic detrimental effects (13) in organ systems including its addiction potential.
3) Human blood is a huge buffering system so that after absorption into the blood, the pH of the inhaled nicotine aerosol would not affect the pH of the arterial blood. The concentration of nicotine in the blood depends on the absorption in the lungs while the pH is constant in the blood. Therefore, the ratio of protonated versus unprotonated nicotine would be constant and not be a factor in the binding of nAChRs in organ systems such as the central nervous system (CNS), cardiovascular system, and fetal development in pregnancy.
4) There have been reports that nicotine salts in pod-mods such as JUUL reduce harshness and result in a satisfying experience even at high nicotine concentrations (2). Slightly acidic JUUL may be less likely to have the harsh taste. High (basic) pH in Blu may make nicotine appear harsh and the pHs of some other brands of e-cigarettes are even higher (6, 15). A satisfying experience as promoted on the JUUL website is a complex phenomenon where pH, the rate of nicotine absorption, pharmacokinetics, flavor, and the conjugated base of the relevant acid, e.g., benzoic acid in JUUL, may play a role. How lower pH and less unprotonated nicotine contribute to satisfying experience needs more research.
CONCLUSION
Theoretically there is a clear distinction between the pH effects of the direct contacts of e-cigarette aerosol on the inner surface of the respiratory tract and those on other body systems via circulation. The effects of pH of inhaled e-cigarette aerosol, which determines the ratio of protonated versus unprotonated nicotine, are 2-fold. 1) Lower pH in JUUL e-cigarettes increases the concentrations of the protonated nicotine activating/desensitizing nAChRs on the epithelial and lung cancer cells in the inner surface of the respiratory tract before entering the circulation. These high concentrations of nicotine potentially have a substantial impact on the immune responses and on lung cancers. 2) The higher acidity of JUUL reduces the concentrations of unprotonated nicotine that reduce the bioavailability and toxicity to all body systems including the CNS (and addiction potential) to which nicotine distributes via circulation after absorption in the lungs. More investigation on nicotine pharmacokinetics and inhalation toxicity on the lungs of vapers or animal models is necessary for public health and for regulatory policies on the pH of E-liquids.
GRANTS
This study was supported by California Tobacco-Related Disease Research Program (TRDRP) Grant 251P003 (to T. C. Friedman); and by National Heart, Lung, and Blood Institute Grant 1R01-HL-135623-01 and National Institute on Drug Abuse Grant 2R42-DA-044788-02 (to X. M. Shao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.M.S. and T.C.F. conceived and designed research; X.M.S. performed experiments; X.M.S. analyzed data; X.M.S. and T.C.F. interpreted results of experiments; X.M.S. drafted manuscript; X.M.S. and T.C.F. edited and revised manuscript; X.M.S. and T.C.F. approved final version of manuscript.
REFERENCES
- 1.Atkins P, Jones L, Laverman L. The pH of Aqueous Solutions. In: Chemical Principles: The Quest for Insight. New York: W. H. Freeman, 2016, p. 472–482. [Google Scholar]
- 2.Barrington-Trimis JL, Leventhal AM. Adolescents’ use of “pod mod” e-cigarettes - urgent concerns. N Engl J Med 379: 1099–1102, 2018. doi: 10.1056/NEJMp1805758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgaertner E. Juul wanted to revolutionize vaping. It took a page from Big Tobacco’s chemical formulas. Los Angeles Times. November 19, 2019. https://www.latimes.com/politics/story/2019-11-19/juul-vaping-chemical-formulas-based-in-big-tobacco.
- 4.Burch SG, Gann LP, Olsen KM, Anderson PJ, Hiller FC, Erbland ML. Effect of pH on nicotine absorption and side-effects produced by aerosolized nicotine. J Aerosol Med 6: 45–52, 1993. [Google Scholar]
- 5.Clayton PM, Vas CA, Bui TT, Drake AF, McAdam K. Spectroscopic investigations into the acid–base properties of nicotine at different temperatures. Anal Methods 5: 81–88, 2013. doi: 10.1039/C2AY25678A. [DOI] [Google Scholar]
- 6.Etter JF, Bugey A. E-cigarette liquids: constancy of content across batches and accuracy of labeling. Addict Behav 73: 137–143, 2017. doi: 10.1016/j.addbeh.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Ferris Wayne G, Connolly GN, Henningfield JE. Brand differences of free-base nicotine delivery in cigarette smoke: the view of the tobacco industry documents. Tob Control 15: 189–198, 2006. doi: 10.1136/tc.2005.013805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman JR, Richbart SD, Merritt JC, Brown KC, Nolan NA, Akers AT, Lau JK, Robateau ZR, Miles SL, Dasgupta P. Acetylcholine signaling system in progression of lung cancers. Pharmacol Ther 194: 222–254, 2019. doi: 10.1016/j.pharmthera.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gahring LC, Myers EJ, Dunn DM, Weiss RB, Rogers SW. Nicotinic alpha 7 receptor expression and modulation of the lung epithelial response to lipopolysaccharide. PLoS One 12: e0175367, 2017. doi: 10.1371/journal.pone.0175367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goniewicz ML, Boykan R, Messina CR, Eliscu A, Tolentino J. High exposure to nicotine among adolescents who use Juul and other vape pod systems (‘pods’). Tob Control 28: 676–677, 2019. doi: 10.1136/tobaccocontrol-2018-054565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottlieb S, Abernethy A. Statement from FDA Commissioner Scott Gottlieb, M.D., and Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D., on FDA’s Ongoing Scientific Investigation of Potential Safety Issue Related to Seizures Reported Following E-Cigarette Use, Particularly in Youth and Young Adults. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm635157.htm. [16 June, 2019].
- 11a.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, Navon L, Hoots B, Salvatore PP, Elderbrook M, Haupt T, Kanne J, Patel MT, Saathoff-Huber L, King BA, Schier JG, Mikosz CA, Meiman J. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin-Final Report. N Engl J Med 382: 903–916, 2020. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 12.Maus AD, Pereira EF, Karachunski PI, Horton RM, Navaneetham D, Macklin K, Cortes WS, Albuquerque EX, Conti-Fine BM. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol 54: 779–788, 1998. doi: 10.1124/mol.54.5.779. [DOI] [PubMed] [Google Scholar]
- 13.Shao XM, Xu B, Liang J, Xie XS, Zhu Y, Feldman JL. Nicotine delivery to rats via lung alveolar region-targeted aerosol technology produces blood pharmacokinetics resembling human smoking. Nicotine Tob Res 15: 1248–1258, 2013. doi: 10.1093/ntr/nts261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song P, Spindel ER. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in lung cancer provides a new target for cancer therapy. J Pharmacol Sci 106: 180–185, 2008. doi: 10.1254/jphs.FM0070091. [DOI] [PubMed] [Google Scholar]
- 15.Stepanov I, Fujioka N. Bringing attention to e-cigarette pH as an important element for research and regulation. Tob Control 24: 413–414, 2015. doi: 10.1136/tobaccocontrol-2014-051540. [DOI] [PubMed] [Google Scholar]
- 16.Sun HJ, Jia YF, Ma XL. Alpha5 nicotinic acetylcholine receptor contributes to nicotine-induced lung cancer development and progression. Front Pharmacol 8: 573, 2017. doi: 10.3389/fphar.2017.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talih S, Salman R, El-Hage R, Karam E, Karaoghlanian N, El-Hellani A, Saliba N, Shihadeh A. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob Control 28: 678–680, 2019. doi: 10.1136/tobaccocontrol-2018-054616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Surgeon General Chemistry and toxicology of cigarette smoke and biomarkers of exposure and harm, In: How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General, 2010, chapt 3, p. 27–102. [Google Scholar]
- 19.Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA. Nicotine binding to brain receptors requires a strong cation-pi interaction. Nature 458: 534–537, 2009. doi: 10.1038/nature07768. [DOI] [PMC free article] [PubMed] [Google Scholar]