Abstract
The greater muscular strength of long-term resistance-trained (LTT) individuals is often attributed to hypertrophy, but the role of other factors, notably maximum voluntary specific tension (ST), muscle architecture, and any differences in joint mechanics (moment arm), have not been documented. The aim of the present study was to examine the musculoskeletal factors that might explain the greater quadriceps strength and size of LTT vs. untrained (UT) individuals. LTT (n = 16, age 21.6 ± 2.0 yr) had 4.0 ± 0.8 yr of systematic knee extensor heavy-resistance training experience, whereas UT (n = 52; age 25.1 ± 2.3 yr) had no lower-body resistance training experience for >18 mo. Knee extension dynamometry, T1-weighted magnetic resonance images of the thigh and knee, and ultrasonography of the quadriceps muscle group at 10 locations were used to determine quadriceps: isometric maximal voluntary torque (MVT), muscle volume (QVOL), patella tendon moment arm (PTMA), pennation angle (QΘP) and fascicle length (QFL), physiological cross-sectional area (QPCSA), and ST. LTT had substantially greater MVT (+60% vs. UT, P < 0.001) and QVOL (+56%, P < 0.001) and QPCSA (+41%, P < 0.001) but smaller differences in ST (+9%, P < 0.05) and moment arm (+4%, P < 0.05), and thus muscle size was the primary explanation for the greater strength of LTT. The greater muscle size (volume) of LTT was primarily attributable to the greater QPCSA (+41%; indicating more sarcomeres in parallel) rather than the more modest difference in FL (+11%; indicating more sarcomeres in series). There was no evidence in the present study for regional hypertrophy after LTT.
NEW & NOTEWORTHY Here we demonstrate that the larger muscle strength (+60%) of a long-term (4+ yr) resistance-trained group compared with untrained controls was due to their similarly larger muscle volume (+56%), primarily due to a larger physiological cross-sectional area and modest differences in fascicle length, as well as modest differences in maximum voluntary specific tension and patella tendon moment arm. In addition, the present study refutes the possibility of regional hypertrophy, despite large differences in muscle volume.
Keywords: muscle architecture, regional hypertrophy, skeletal muscle, strength training
INTRODUCTION
Muscular strength is integral to athletic performance (21), helps to reduce injury risk (19) and the likelihood of developing musculoskeletal disorders such as osteoarthritis (84), and also facilitates independence and functional mobility (18, 53) with aging. Participation in resistance training (RT) is well known to increase strength and therefore is widely recommended on an on-going/continuous (i.e., long-term) basis for individuals of all ages as well as numerous patient groups (3, 49, 51, 72). Hence long-term RT individuals are known to be substantially stronger than untrained controls (UT) (9, 56), a functional difference that is often attributed to their larger muscle size [i.e., greater volume or cross-sectional area (CSA) due to hypertrophy]. However, the role of other morphological and mechanical differences that may also influence strength, notably specific tension (i.e., force per unit area), muscle architecture, and joint moment arm, have been poorly documented.
In fact, long-term systematic RT (i.e., multiple years) has been shown to result in substantially greater muscle size compared with untrained controls {+70–76% greater biceps brachii anatomical CSA [ACSA (9, 52)]; +85% greater quadriceps volume (38)}, but whether an increase in muscle size is accompanied by similar, smaller, or no changes in maximum voluntary specific tension (ST) remains unknown. Furthermore, the extent to which increases in overall muscle size (volume) after long-term RT are due to increases in either sarcomeres in parallel (i.e., increased physiological CSA; PCSA) and/or in series (i.e., fiber/fascicle length) has not been examined. Finally, the extent of region-specific hypertrophy, both between constituent muscles and along their length, after long-term RT remains to be elucidated. Therefore, a rigorous assessment of muscle size (ACSA, PCSA, and volume), ST, and architectural contributions to enhanced strength after long-term RT appears warranted.
ST during maximum voluntary contractions (MVCs) is a widely suggested adaptation to RT (24) that encompasses the functional consequences of any changes in neuromuscular activation of the agonist muscle, as well as any changes in intrinsic contractile ST (e.g., perhaps due to a shift in fiber type composition, decreases in antagonist activation, increase in lateral force transmission or reduced fat infiltration) (10). While ST has been quantified using a relatively crude calculation of external force/torque divided by ACSA, a more valid approach involves accounting for antagonist torque and moment arm to calculate agonist muscle force that can be expressed in proportion to PCSA to determine the ST of the agonist muscle. This more rigorous approach has only been used over 9 wk of RT (30) demonstrating an increase in ST of 17%; therefore the ST of individuals who have completed several years of regular systematic heavy RT, and thus the contribution of this variable to their greater strength, remains unknown.
Short-term RT (2–6 mo) appears to result in nonuniform hypertrophy both along and between muscles (25, 44, 61). For example, within the quadriceps numerous studies have found greater hypertrophy of the rectus femoris compared with the vastii (26, 43, 44, 58, 61, 69, 75, 81). Short-term RT studies have also reported the greatest increases in anatomical cross-sectional area (ACSA) to occur at surprisingly diverse points along the muscle: at maximal ACSA (ACSAmax; 24, 28, 30, 56), in the proximal (63) or distal (26, 35, 58), or even proximal and distal (4, 61) regions of the muscle. These diverse findings could potentially be due to the differences in the prescribed training task or be contraction mode dependent (33, 35, 68) or may in part reflect difficulties in accurately replicating measurement sites along the muscle/limb in studies that typically used a limited number of MRI slices [e.g., 3–7 slices (43, 44, 61)] or ultrasound measures (26, 58, 67), in which case careful description of ACSA along the whole muscle in relation to definitive anatomical landmarks (i.e., the ends of the underlying bone) are required. Moreover, if region-specific hypertrophy resulting from RT does exist it would be expected to be pronounced in long-term RT individuals that exhibit substantially larger muscles; however, this has not been examined.
The structural remodeling of muscle morphology in response to RT can be observed by examining muscle architecture, specifically pennation angle (ΘP) and fascicle length (FL). Numerous studies have found ΘP to increase after RT (1, 10, 57), and after RT interventions (12, 15, 16, 67); or be higher in resistance-trained versus untrained individuals on a cross-sectional basis (39, 47, 70). An increase in ΘP may facilitate an increase in the contractile material attaching to the tendon/aponeurosis, independent of any change in ACSA. However, the increase in ΘP also has a negative effect on force-generating capacity by reducing the transmission of force between the fibers and the tendon/aponeurosis (8). These contrary effects of ΘP on the force-generating capacity of the muscle are theoretically best reflected by effective PCSA (QEFFPCSA) that accounts for both the number of sarcomeres in parallel and force transmission to the aponeurosis/tendon.
The changes in FL after short-term RT remain controversial with reports of no change in FL [isometric RT (6); or conventional isoinertial RT (lifting and lowering) (14, 26, 29, 30, 80] and increased FL [isometric (65); isoinertial (7, 78)]. One study of long-term heavy RT individuals [RT history: 12.4 ± 5.4 yr (mean ± SD)] observed no difference in FL compared with controls (70). The controversy surrounding the architectural changes, especially FL, after RT, could in part be due to heterogeneous architectural changes throughout the muscle after RT (14, 59) in a similar manner, and potentially linked to region-specific hypertrophy. Therefore, comprehensive architectural measurements throughout the muscle may clarify whether FL changes after long-term RT.
Moment arm has been found to have a weak, but significant, association with maximal torque production (17, 77) in untrained controls (74, 79). For some muscles it has been suggested that muscle growth after RT may cause an advantageous increase in the moment arm by positioning the tendon further from the joint center (79). Although the anatomy of the patella and patella tendon wrapping around the distal femur mean that this may be unlikely for the quadriceps, the contribution of any differences in moment arm to the strength in long-term RT individuals compared with untrained individuals is unknown.
The aim of the present study was to determine the factors that explain the greater strength and larger muscle size (volume) of long-term RT individuals (LTT) vs. untrained (UT) individuals. This involved a comprehensive comparison of quadriceps (Q) morphology and mechanics, specifically: measures of muscle size (QVOL, QACSAMAX, QPCSA, QEFFPCSA) and regional hypertrophy/muscle mass distribution (between and along the quadriceps muscles) with MRI, agonist muscle ST (accounting for antagonist coactivation, moment arm and QEFFPCSA), muscle architecture (FL and ΘP) at 10 sites throughout the quadriceps with ultrasound imaging, and moment arm also assessed with MRI. It was hypothesized that: 1) the anticipated greater strength of LTT vs. UT would be due to both their greater muscle size (QVOL, QACSAMAX, QPCSA, QEFFPCSA) and higher ST; 2) the greater muscle volume of LTT would be due to higher PCSA rather than greater FL (i.e., sarcomeres in parallel not in series); and 3) there would be marked regional hypertrophy between and along constituent quadriceps muscles for LTT vs UT.
MATERIALS AND METHODS
Participants and ethical approval.
Sixty-eight young men provided written informed consent before completing this study, which was approved by the Loughborough University Ethical advisory committee and was conducted according to the principles expressed in the Declaration of Helsinki. All participants were healthy and free from musculoskeletal injury. Physical activity levels of all participants were assessed with the International Physical Activity Questionnaire [IPAQ, short format (22)]. The untrained control group (UT, n = 52, age 25 ± 2 yr; IPAQ: 2,286 ± 1312 metabolic equivalent min/wk) had no lower-body RT experience for >18 mo. The long-term resistance-trained group (LTT, n = 16, age 22 ± 2 yr; IPAQ: 5,383 ± 1495 metabolic equivalent min/wk) reported (via a detailed questionnaire and follow-up oral discussion) systematic, progressive, heavy RT of the quadriceps ~3 ×/wk for ≥3 yr (mean ± SD, 4 ± 1 yr; range, 3–5 yr), involving completion of several knee extensor exercises (e.g., squat, lunge, step-up, knee extension and leg press) within an individual session, and with the primary aim of developing maximum strength. The RT of this group had not been experimentally supervised although some of these participants had received variable coaching (technique and programming) support. Participation in weight classified or predominantly endurance sports was an exclusion criteria to avoid these potential confounders of morphological adaptation. Of the LTT group, resistance training was the only systematic physical activity of 50% (n = 8); 38% (n = 6) were national-level rugby union players, with the remaining 12% (n = 2) competing in powerlifting/body building. Use of androgenic-anabolic steroids was an exclusion criterion for all participants. Many individuals in the LTT group reported regular use of nutritional supplements (e.g., whey protein and creatine).
Experimental design.
Participants completed a familiarization session, involving practice of all voluntary contractions performed during subsequent measurement sessions, followed by two duplicate strength measurement sessions separated by 7–10 days. The duplicate strength measurement sessions were typically averaged (for 66 of 68 participants) to enhance the reliability of criterion measurements. Due to availability or injury occurring between sessions two participants completed only one measurement session (both in the LTT group).
Strength measurement sessions were performed at a consistent time of the day for each individual participant, and all sessions started between 1200 and 1900. Participants were instructed not to participate in strenuous physical activity or consume alcohol for 36 h, and to refrain from caffeine consumption for 6 h, before strength measurement sessions. These strength measurement sessions involved a series of incremental warm-up contractions followed by MVCs to establish maximum voluntary torque (MVT) for both the knee extensors and flexors of the dominant limb.
On a separate occasion, musculoskeletal imaging measurements (B-mode ultrasonography and MRI) were performed. Magnetic resonance T1-weighted axial plane images of the thigh were acquired to measure quadriceps muscle size (QVOL and QACSAMAX) with sagittal scans of the knee used to assess patella tendon moment arm (PTMA). Ultrasonographic images were captured at 10 locations throughout the four constituent muscles of the quadriceps (i.e., 2 or 3 locations per muscle) to comprehensively quantify FL and ΘP of the whole muscle group.
Torque and electromyographic measurements.
Participants were positioned in an isometric dynamometer with knee and hip angles of 115° and 125° (180° = full extension), respectively. Adjustable straps were tightly fastened across the pelvis and shoulders to prevent extraneous movement. An ankle strap (35 mm width reinforced canvas webbing) was placed ~15% of tibial length (distance from lateral malleolus to knee joint space) above the medial malleolus and positioned perpendicular to the tibia and in series with a calibrated S-Beam strain gauge (Force Logic UK, Berkshire, UK).
The analog force signal was amplified (×370; A50 amplifier, Force Logic UK, Berkshire, UK) and sampled at 2,000 Hz using an A/D converter (Micro 1401; CED, Cambridge, UK) and recorded with Spike 2 computer software (CED). In offline analysis, force signals were low-pass filtered at 500 Hz using a fourth-order zero-lag Butterworth filter (54), gravity corrected by subtracting baseline force, and multiplied by lever length, the distance from the knee joint space to the center of the ankle strap, to calculate torque.
Surface electromyography (EMG) of the hamstring muscles (biceps femoris long head and semitendinosus) was recorded using a wireless EMG system (Trigno; Delsys, Boston, MA). Skin preparation (shaving, abrading, and cleansing with 70% ethanol) was conducted before single differential Trigno Standard EMG sensors (Delsys; fixed 1-cm interelectrode distance) were placed on the biceps femoris long head and semitendinosus at 45% of thigh length above the popliteal fossa. Sensors were placed parallel to the presumed orientation of the underlying fibers. EMG signals were amplified at source (×300; 20- to 450-Hz bandwidth) before further amplification (overall effective gain, ×909), and sampled at 2,000 Hz via the same A/D converter and computer software as the force signal, to enable data synchronization. In offline analysis, EMG signals were corrected for the 48-ms delay inherent to the Trigno EMG system.
Knee extension and flexion maximum voluntary contractions.
Following a brief warm-up [3 s contractions at 50% (×3), 75% (×3), and 90% (×1) of perceived maximum], participants performed 3–4 MVCs of the knee extensors for 3–4 s duration interspersed with ≥30 s rest and were instructed to “push as hard as possible.” A horizontal cursor indicating the greatest torque obtained within the session was displayed for biofeedback, and verbal encouragement was provided during all MVCs. The highest instantaneous torque recorded during any MVC was defined as knee extension MVT. Tendon force was calculated as MVT divided by moment arm.
Using the same set-up and warm-up protocol as for the knee extensors, participants performed 3–4 knee flexion MVCs and were instructed to “pull as hard as possible” for 3–4 s and rest for ≥30 s between efforts. A torque-time curve with a horizontal cursor indicating the greatest torque obtained within that session was displayed for biofeedback, and verbal encouragement was provided during all MVCs. Knee flexion MVT was the greatest instantaneous torque achieved during any MVC during that measurement session.
Hamstrings EMG amplitude during knee flexor MVCs was calculated as the root mean square (RMS) of the filtered EMG signal of the biceps femoris long head and semitendinosus over a 500-ms epoch at knee flexion MVT (250 ms either side) and averaged across the two muscles to give HEMGMAX. Biceps femoris long head and semitendinosus (antagonist) EMG amplitude during a 500-ms window surrounding knee extension MVT (250 ms either side) was normalized to HEMGMAX from the corresponding EMG sensor. Normalized antagonist EMG amplitude was multiplied by the knee flexor MVT to estimate antagonist knee flexor torque during the knee extension MVCs (assuming a linear relationship between EMG amplitude and torque).
MRI measurements of quadriceps muscle size and patella tendon moment arm.
Participants reported to the MRI scanner (1.5-T Signa HDxt, GE) having not engaged in strenuous activity in the prior 36 h and were instructed to arrive in a relaxed state having eaten and drunk normally and sat quietly for 15 min before their MRI scans. T1-weighted MR images of the dominant leg (thigh and knee) were acquired in the supine position at a knee angle of 163° (180° = full extension; due to constraints in knee coil size) and analyzed using OsiriX software (Version 6.0, Pixmeo, Geneva, Switzerland). Using a receiver 8-channel whole body coil, axial images (image matrix 512 × 512, field of view 260 × 260 mm, pixel size 0.508 × 0.508 mm, slice thickness 5 mm, interslice gap 0 mm) were acquired from the anterior superior iliac spine to the knee joint space in two overlapping blocks. Oil-filled capsules placed on the lateral side of the thigh allowed alignment of the blocks during analysis.
The quadriceps muscles [vastus lateralis (VL), vastus intermedius (VI), vastus medialis (VM), and rectus femoris (RF)] were manually outlined to determine ACSA in every third image (i.e., every 15 mm; Fig. 1A) starting from the most proximal image in which each muscle appeared. This equated to the following number of slices being analyzed per muscle (VM, 23–26; VI, 24–27; VL, 24–27; and RF, 23–26 slices). The volume of each muscle was calculated using cubic spline interpolation of the measured ACSA values/slices (1,000 interpolated points/ACSA values per muscle; GraphPad Prism 6; GraphPad Software) and expressed relative to % femur length. Femur length was defined by the number of slices between the proximal greater trochanter and the knee joint space, multiplied by the slice thickness. For muscle mass distribution, interpolated ACSA for each individual muscle at 5% intervals of femur length were used and expressed relative to ACSAMAX. Total quadriceps volume (QVOL) was the sum of the individual muscle volumes. QACSAMAX was calculated by the summation of the maximal ACSA from each individual muscle. Previous data from our group has demonstrated a mean within-participant coefficient of variation for repeat quadriceps muscle volume measurements using the same protocol 12 wk apart with a control group to be 1.7% (11). Inter- and intrarater reliability for QVOL calculated from the repeated analysis of five MRI scans was 1.2 and 0.4%, respectively.
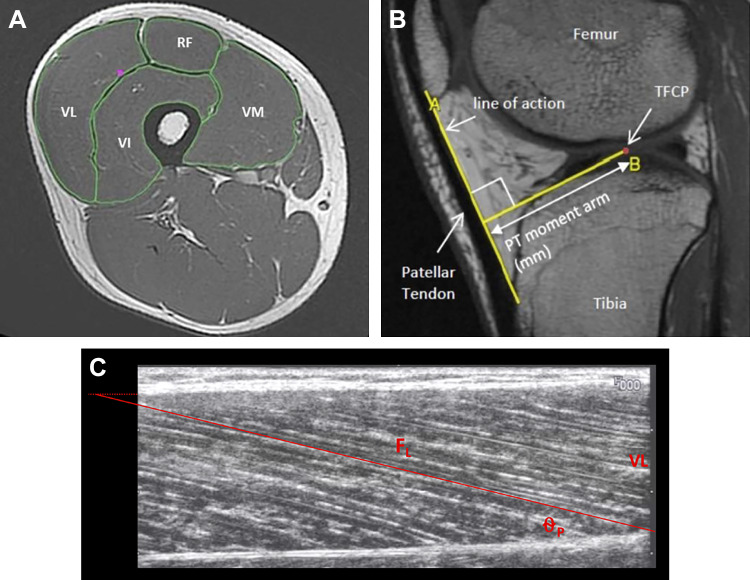
Fig. 1.
Representative axial MR image of the thigh (A); sagittal MRI image of the knee joint (B) and muscle architecture (C). Patellar tendon (PT) moment arm was defined as the perpendicular distance between the tendon line of action and the tibiofemoral contact point (TFCP). C demonstrates muscle architecture measurements of pennation angle (ΘP) and fascicle length (QFL) from the vastus lateralis.
Sagittal plane images of the knee joint were acquired from the lateral to medial condyles of the femur using an 8-channel knee coil (image matrix 384 × 224, field of view 512 × 512 mm, slice thickness 2 mm, inter-slice gap 0 mm) to determine patella tendon moment arm (PTMA), defined as the perpendicular distance from the patellar tendon line of action to the tibiofemoral contact point (TFCP, the midpoint of the contact between the tibial and femoral condyles; Fig. 1B). For maximal voluntary specific tension measurements PTMA length for the MVT specific knee angle was estimated from previously published data fitted with a quadratic function (48) scaled to each participant’s measured moment arm length at 163° as previously (56).
Muscle architecture and calculation of PCSA/QEFFPCSA.
Architecture of all four quadriceps constituent muscles (VM, VL, VI, and RF) was examined in detail using B-mode ultrasonography (EUB-8500, Hitachi Medical Systems UK Ltd, Northamptonshire, UK) and a 92-mm, 5–10 MHz linear-array transducer (EUP-L53L). The participant sat in the same isometric dynamometer used for strength measurements while images were captured at rest at 2–3 sites per constituent muscle, for a total of 10 quadriceps architecture measurements sites. Specific sites were over the mid muscle belly (median longitudinal line, i.e., 50% of superficial mediolateral width) at the following percentages of thigh length proximal to the knee joint space: VM 20% (VMDIS) and 40% (VMPRX), VL and VI at 30% (VLDIS, VIDIS), 50% (VLMID, VIMID) and 70% (VLPRX, VIPRX), and RF 55% (RFMID) and 75% (RFPRX). The transducer (coated with water soluble transmission gel) was positioned parallel to the long axis of the thigh (femur), and perpendicular to the skin such that an image with the aponeuroses and the perimysium trajectory of several fascicles was clearly identifiable with no visible fascicle distortion at the edge of the image, and with minimal pressure applied on the dermal surface. Video output from the ultrasound machine was transferred to a computer (via an S-video to USB converter) and images recorded using ez-cap video capture software. Images were later imported into public domain software (Image J, v1.48, National Institutes of Health, Bethesda, MD) for analysis.
ΘP was measured as the angle of insertion of the muscle fascicles into the deep aponeurosis, taken as a mean of 3 individual fascicles per ultrasound site. Muscle fascicle length was used as an index of fiber length and sarcomeres in series, and was measured as the length of the fascicular path between the insertions into the superficial and deep aponeurosis; where the fascicular path extended beyond the acquired image the missing portion of the fascicle was estimated by extrapolating linearly the fascicular path and the aponeurosis (48). Due to the long 92 mm ultrasound probe, the extrapolation typically consisted of ≤10% of FL. ΘP and FL were averaged over each individual muscle, before calculating an overall quadriceps mean averaged over the four constituents (QΘP and QFL).
PCSA (PCSA) was calculated per constituent muscle as individual muscle volume divided by FL (mean of sites for that constituent), then summed to give quadriceps physiological cross-sectional area (QPCSA). Theoretically PCSA is the best index of contractile material (sarcomeres and cross-bridges) arranged in parallel. To correct for force transmission to the tendon, EFFPCSA was calculated as this theoretically best index of muscular force/torque production. Specifically, individual muscle EFFPCSA was calculated by multiplying PCSA by cosine of mean ΘP (28), before summing the four constituent muscles to give quadriceps effective PCSA (QEFFPCSA).
Calculation of ST.
ST was determined first by the calculation of maximal tendon force; this was done by correcting knee extension MVT for antagonist torque [HEMG at knee extensor MVT normalized to HEMGMAX (i.e., at KF MVT) multiplied by KF MVT] to provide torque from the knee extensors only (66). This knee extensor muscle torque was divided by corrected PTMA (see above) and the subsequent muscle force divided by QEFFPCSA to calculate ST.
Statistical analysis.
Muscle strength measured during the duplicate laboratory sessions was averaged to produce criterion values for statistical analysis. An a priori significance level of P < 0.05 was set for all statistical tests which were performed using SPSS Version 23.0 (IBM Corp., Armonk, NY). Descriptive data are presented as means ± standard deviation (SD) and percentage differences between groups calculated from group means. The influence of group (UT, LTT) on all muscle architecture and muscle size variables was examined by independent t-tests. To examine if the architectural differences between the groups varied with constituent muscle, a 4 × 2 ANOVA [constituent muscle (VL, VM, VI, RF) × group (LLT, UT)] was performed, and if interaction effects were found, then post hoc analysis (pairwise ANOVA contrasting only two muscles) was also performed. Effect size (ES) for absolute difference data was calculated as previously detailed for between-subject study designs (50) and classified as follows: <0.20 = “trivial,” 0.20–0.49 = “small,” 0.50–0.79 = “moderate,” or ≥0.80 = “large.” P values were corrected for multiple tests using the Benjamini–Hochberg procedure (13) with a false detection rate of 5%, and significance was defined as adjusted P < 0.05. For the whole cohort (i.e., data pooled from both LTT and UT groups, n = 68) the relationships between musculoskeletal variables and MVT were first assessed with independent Pearson's product- moment correlations, and then stepwise multiple regression analysis was performed, with only the significant predictors entered into the model.
RESULTS
Participant characteristics and strength.
LTT were taller and heavier than UT (183 ± 6 vs. 176 ± 2 cm; 91 ± 10 vs. 73 ± 10 kg; both P < 0.001). MVT was 60% greater in LTT than UT (388 ± 70 vs. 245 ± 43 N·m; P < 0.001, ES = 2.5).
Total quadriceps and constituent muscle size, and muscle mass distribution between and along the quadriceps muscles.
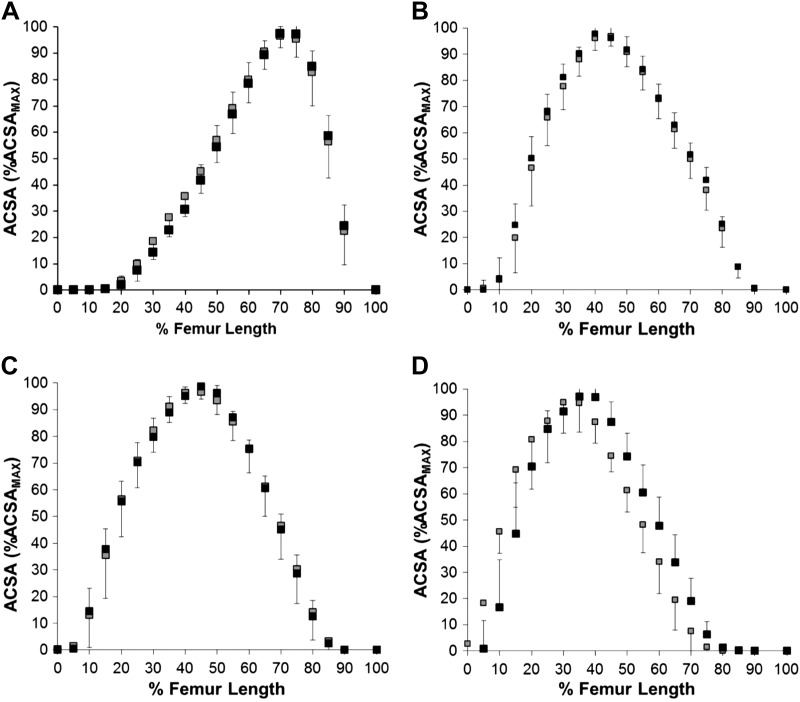
QVOL was 56% greater in LTT than UT (P < 0.001; ES = 3.7), QACSAMAX was 50% greater (P < 0.001, ES = 3.3) and QEFFPCSA 41% greater in LTT compared with UT (P < 0.001, ES = 4.1). LTT had greater volume of all the individual constituent muscles of the quadriceps (54–58%, P < 0.001, ES = 2.3–3.7; Table 1). Likewise, LTT had greater ACSAMAX, PCSA, and EFFPCSA of all the individual constituent muscles of the quadriceps (ACSAMAX, 46–52%, all P < 0.001, ES = 1.9–2.9; PCSA, +39–45%, all P < 0.001, ES = 1.9–2.6; EFFPCSA, +38–44%, all P < 0.001, ES = 2.2–2.7) than UT. However, the proportional volume, and ACSAMAX, of the individual constituent muscles (to total quadriceps muscle volume and QACSAMAX, respectively) were similar for LTT and UT (P = 0.56–0.94; volume data shown in Table 1) and the percentage of femur length where ACSAMAX of each constituent muscle occurred was also similar for both groups (VM: 28% vs. 29%; VI: 58% vs. 58%; VL: 57% vs. 56% and RF: 68% vs. 68% femur length for UT and LTT, respectively; P = 0.26–0.80; Fig. 2). To further assess regional hypertrophy, the relative distribution of muscle mass along the thigh was examined by plotting relative ACSA (%ACSAMAX) against femur length for each constituent muscle (Fig. 2). No differences in relative ACSA were observed between UT and LTT at any position along the femur for any of the constituent muscles (adjusted P > 0.21).
Table 1.
Quadriceps muscle size indices, individual constituent muscle volumes and proportional volumes of untrained (UT) and long-term resistance-trained (LTT) men
| Muscle and Size Variable | UT (n = 52) | LTT (n = 16) | %Difference | Effect Size |
|---|---|---|---|---|
| Quadriceps | ||||
| QVOL (cm3) | 1,838.2 ± 262.9 | 2,881.9 ± 308.1* | 56 | 3.7 |
| QACSAMAX (cm2) | 86.2 ± 11.2 | 135.0 ± 15.0* | 50 | 3.3 |
| QPCSA (cm2) | 174.4 ± 19.8 | 245.7 ± 16.8* | 41 | 3.9 |
| QEFFPCSA (cm2) | 167.7 ± 18.8 | 236.8 ± 15.1* | 41 | 4.1 |
| Individual muscle volume (cm3) | ||||
| VM | 441.4 ± 67.8 | 691.2 ± 87.0* | 57 | 3.2 |
| VI | 546.9 ± 104 | 846.4 ± 124.0* | 55 | 2.6 |
| VL | 609.8 ± 98.4 | 964.3 ± 90.6* | 58 | 3.8 |
| RF | 240.2 ± 46.7 | 374.6 ± 72.0* | 56 | 2.3 |
| Proportional muscle volume (%QVOL) | ||||
| VM | 24.0 ± 1.7 | 24.1 ± 1.9 | 0 | 0.0 |
| VI | 29.7 ± 2.8 | 29.3 ± 1.6 | 1 | −0.2 |
| VL | 33.2 ± 2.6 | 33.6 ± 2.3 | 1 | 0.2 |
| RF | 13.1 ± 1.8 | 13.0 ± 1.8 | 1 | −0.1 |
Data are means ± SD, QVOL = quadriceps volume; QACSAMAX = sum of maximal anatomical cross-sectional areas from individual muscles; QPCSA = quadriceps physiological cross-sectional area; QEFFPCSA = effective physiological cross-sectional area; VM = vastus medialis; VI = vastus intermedius; VL = vastus lateralis; RF = rectus femoris;
indicates adjusted P < 0.01
Fig. 2.
Muscle mass distribution (% of ACSAmax) along the femur (at 5% increments from proximal (0%) to distal (100%)) in untrained men (UT, gray squares; n = 52) and long-term resistance-trained men (LTT, black squares ; n = 16) for the constituent quadriceps muscles: vastus medialis (A), vastus intermedius (B), vastus lateralis (C), and rectus femoris (D). Data are means ± SD. There were no differences between groups for muscle mass distribution (% of ACSAmax) for any muscle or 5% increment along the femur (all adjusted P ≥ 0.21). ACSA, anatomical cross-sectional area.
Muscle architecture.
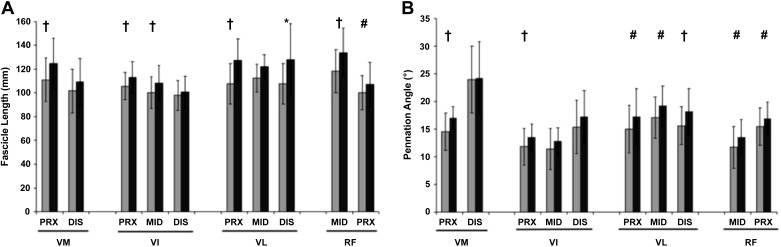
QFL, based on the mean of 10 sites, was 11% greater in LTT than UT (P < 0.001, ES = 1.2; Table 2), and mean FL of each individual muscle was longer (VM: +12%, ES = 0.7; VL: +13%, ES = 1.0; and RF: +12%, ES = 0.8; all P < 0.05) or showed a tendency to be longer (VI: +7%; P = 0.06, ES = 0.8) for LTT than UT. The outcome of the ANOVA revealed a constituent muscle (VL, VM, VI, RF) × group (LTT, UT) interaction effect (i.e., bigger differences between groups for some muscles than others; P = 0.03), and post hoc analysis showed larger differences between UT and LTT in the VM, VL, and RF compared with VI (pairwise ANOVA with only two muscles; group × muscle interaction; all P ≤ 0.008). Considering the specific measurement sites, 6 of 10 sites showed greater FL of LTT vs. UT (VMPRX, VIPRX, VIMID, RFMID, VLDIS, and VLPRX sites; all P < 0.001), with a tendency to be longer for RFPRX (P = 0.06) and no differences at the remaining three measurement sites (all P > 0.15; Fig. 3A).
Table 2.
Muscle architecture variables, fascicle length (FL) and angle of pennation (ΘP), for untrained (UT) and long-term resistance-trained (LTT) men
| Variable | Muscle | Sites Measured | UT (n = 52) | LTT (n = 16) | %Difference | Effect Size |
|---|---|---|---|---|---|---|
| FL (mm) | ||||||
| Q | 10 | 106.4 ± 9.0 | 118.0 ± 10.0* | 11 | 1.2 | |
| VM | 2 | 104.6 ± 16.4 | 117.1 ± 17.4† | 12 | 0.7 | |
| VI | 3 | 100.9 ± 8.1 | 107.5 ± 7.8# | 7 | 0.8 | |
| VL | 3 | 111.1 ± 11.5 | 125.7 ± 16.8† | 13 | 1.0 | |
| RF | 2 | 109.0 ± 14.8 | 121.6 ± 17.8† | 12 | 0.8 | |
| ΘP (mm) | ||||||
| Q | 10 | 15.4 ± 2.9 | 17.3 ± 2.0* | 13 | 0.7 | |
| VM | 2 | 19.2 ± 3.9 | 20.8 ± 3.4 | 8 | 0.4 | |
| VI | 3 | 12.9 ± 2.6 | 14.5 ± 2.2# | 13 | 0.7 | |
| VL | 3 | 15.9 ± 2.6 | 18.2 ± 3.3† | 15 | 0.8 | |
| RF | 2 | 13.5 ± 2.5 | 15.6 ± 2.4† | 16 | 0.9 |
Data are means ± SD. Quadriceps and individual constituent muscle values are based on the mean of ten or two/three sites, respectively. Q = mean quadriceps, VM = vastus medialis, VI = vastus intermedius, VL = vastus lateralis, RF = rectus femoris, ΘP = angle of pennation, FL = fascicle length. Adjusted P values are indicated by:
P < 0.01,
P < 0.05;
tendency P = 0.05–0.07.
Fig. 3.
Differences in fascicle length (A) and pennation angle (B) between untrained (UT) men (gray bars; n = 52) and long-term resistance-trained men (LTT, black bars; n = 16) at two or three sites of each of the constituent quadriceps muscle. VM, vastus medialis; VI, vastus intermedius; VL, vastus lateralis; RF, rectus femoris; PRX, proximal; MID, middle; DIS, distal. Data are means ± SD. Symbols indicate adjusted P values: *P < 0.01, †P < 0.05; #tendency for a difference P = 0.05–0.07.
QΘP was 13% greater in LTT than UT (P < 0.001, ES = 0.7; Table 2), and reflected a greater mean ΘP in the VL (15%, P = 0.02, ES = 0.8) and RF (15.5%, P = 0.01, ES = 0.9) but not the VM (9%, P = 0.21, ES = 0.4) or VI (13%, P = 0.07, ES = 0.7). There were no group × constituent muscle interactions (P = 0.826). LTT had greater ΘP than UT at 3 of 10 sites (VMPRX, VIPRX, VLDIS; P < 0.05), with a tendency to be greater observed at four further sites (VLPRX, VLMID and both RF sites; adjusted 0.05 ≤ P ≤ 0.07; Fig. 3B).
Patella tendon moment arm (PTMA) and maximum voluntary specific tension (ST).
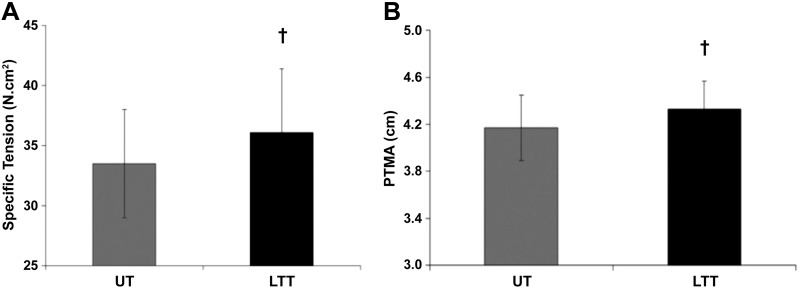
LTT had a 4% greater PTMA than UT (4.17 ± 0.28 cm vs. 4.33 ± 0.24 cm; P = 0.03; ES = 0.6: see Fig. 4B). However, when normalized to participant’s height, there was no difference in PTMA between groups (PTMA/Height ratio: UT, 0.0237 ± 0.0017 vs. LTT, 0.0236 ± 0.0009; P = 0.92; ES = 0.2). Tendon force was 54% greater in LTT than UT (5,576 ± 905 N vs. 8,564 ± 1,410 N; P < 0.001, ES = 2.6). There was 8% greater ST of the quadriceps in LTT than UT (33.3 ± 4.5 N·cm2 vs 36.1 ± 5.3 N·cm2; P = 0.04, ES = 0.6; Fig. 4A) when accounting for antagonist coactivation, corrected PTMA, and QEFFPCSA.
Fig. 4.
Maximal voluntary specific tension (ST) (A) and patella tendon moment arm (PTMA) (B) in untrained (UT; gray bars, n = 52) and long-term resistance-trained (LTT; black bars, n = 16) individuals. †Adjusted P < 0.05.
Factors that explain the greater strength and muscle mass (volume) of long-term RT individuals.
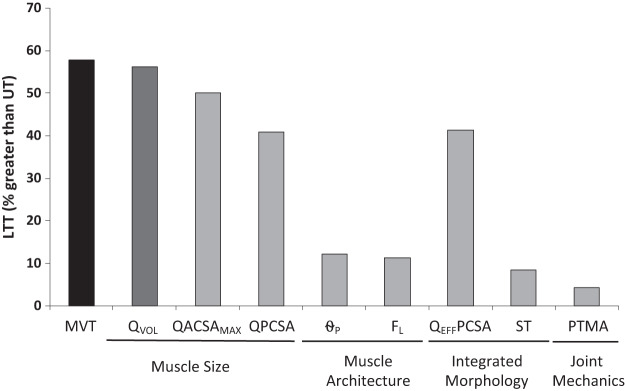
The difference in strength between LTT and UT (+60%) in comparison to the differences between the groups in a range of underpinning musculoskeletal variables, specifically those variables that were each significantly greater in LTT than UT, are shown in Fig. 5. Of the musculoskeletal variables, the largest differences were in the muscle size indices (QVOL +56%; QACSAMAX +50%) which therefore provide the primary explanation for the greater strength of LTT. This greater muscle size of LTT in combination with a more modest difference in QΘP (+12%) resulted in a difference in QEFFPCSA (+40%), which alongside other smaller contributions from ST (+8%) and moment arm (+4%) appears to explain the strength difference. The greater muscle volume of LTT vs. UT (QVOL +56%) appeared to be primarily due to increased QPCSA (+41%) with a much smaller contribution of QFL (+11%; Fig. 5). Bivariate correlations for the whole cohort (i.e., both groups, n = 68) were found between all musculoskeletal variables and MVT [QVOL r = 0.90 (Fig. 6); QACSAMAX r = 0.87; QEFFPCSA r = 0.87; QΘP r = 0.47; QFL r = 0.61; ST r = 0.56; PTMA r = 0.41; all P < 0.01]. Stepwise multiple regression analysis revealed that the only variable to contribute to the explained variance in MVT was QVOL (R2 = 0.81; P < 0.001).
Fig. 5.
Musculoskeletal variables that appear to contribute to the greater strength and larger muscle volume of long-term resistance-trained (LTT) compared with untrained (UT) men. Data are percentage differences in group mean values for maximal voluntary torque (MVT), quadriceps volume (QVOL), sum of maximal anatomical cross-sectional area (QACSAMAX), quadriceps physiological cross-sectional area (QPCSA); quadriceps effective physiological cross-sectional area (QEFFPCSA), mean quadriceps angle of pennation (QΘP), mean quadriceps fascicle length (QFL), maximum voluntary specific tension (ST), and patella tendon moment arm (PTMA) between untrained and long-term resistance-trained participants.
Fig. 6.
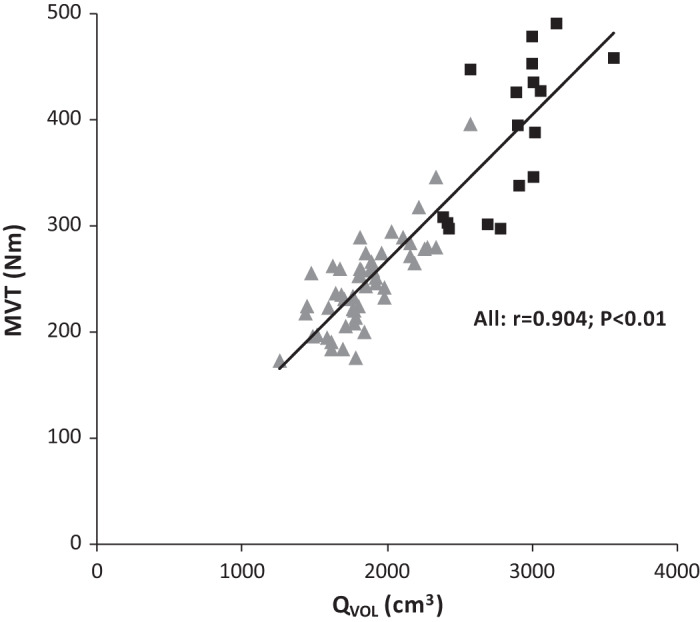
Scatterplot of the relationship between maximal voluntary torque (MVT; N·m) and quadriceps volume (QVOL; cm3) in untrained (UT; n = 52: triangles) and long-term resistance-trained (LTT; n = 16: squares) individuals. Regression line is for all participants(r = 0.90; P < 0.01).
DISCUSSION
The aim of the present study was to determine the musculoskeletal factors that explain the greater strength and larger muscle size (volume) of long-term RT individuals vs. untrained individuals. Previous RT studies have typically been short-duration interventions or examined a limited range of musculoskeletal factors, and thus our knowledge of the adaptations to prolonged RT have been limited. In accordance with our first hypothesis the greater muscle strength of LTT (+60%) was accompanied by both a greater quantity of skeletal muscle and higher ST. However, the differences between LTT vs. UT for the indices of muscle size (e.g., ranging from volume +56% to QEFFPCSA 41%) were substantially larger than was the case for ST (+8%), or in fact PTMA (+4%), and thus muscle size was the primary explanation for the greater strength of LTT. For the second hypothesis, the greater QVOL (+56%) of LTT was due primarily to enhanced QPCSA (41%), indicating more sarcomeres in parallel, although we also found convincing evidence for greater QFL (+11%), indicating a modest difference in sarcomeres in series. Finally, despite the large differences in QVOL, and contrary to our third hypothesis, we found no evidence for regional hypertrophy/muscle mass distribution between or along the constituent quadriceps muscles.
The difference in MVT of LTT vs. UT in the current study was substantial (+60%), but somewhat lower than observed in one previous study [+77% (70)]. The greater MVT of LTT was accompanied by both a greater quantity of skeletal muscle and higher specific tension, although it was clear from the magnitude of the differences that the indices of muscle size (e.g., volume +56%, QEFFPCSA +41%) were substantially larger than was the case for ST (+8%), or in fact PTMA (+4%), and thus muscle size was the primary explanation for the greater strength of LTT. The importance of muscle volume for strength was reinforced by our regression analysis of the whole cohort that found muscle volume was the only determinant of MVT, alone explaining 81% of the variance in strength. Several other studies have found substantially greater muscle size of long-term resistance-trained participants [70% to 86% (9, 37, 46, 49)], but none have previously examined maximum voluntary specific tension to investigate the contribution of force per unit area to the enhanced strength of LTT.
We found modest differences in specific tension (+8%), even after the average 4 yr of regular, heavy RT of LTT. While no previous studies have examined the specific tension of LTT individuals, after short-term (9 wk) RT maximum voluntary specific tension has been reported to increase by 20% (30), which is clearly somewhat contrary to the more modest 8% difference we have found for LTT vs. UT. However, it is notable that Erskine et al. (30), reported average isometric strength gains ~2-fold greater than we have found [31% vs. 11.5–18.2% (31, 32)], with almost identical training regimes and the same number of training sessions, and this discrepancy likely explains the large increase in specific tension they have reported. Nonetheless, numerous short-term RT studies have shown greater increases in strength/force than cross-sectional area, indicating an increase in the specific tension (23, 27, 29, 42, 45, 61, 69, 83). Increased specific tension could be attributable to changes in neuromuscular activation [e.g., increased agonist activation (10, 60)] or an increase in the intrinsic contractile specific tension, perhaps due to a shift in muscle fiber phenotype (20) or alterations in muscle architecture (24). Moreover, the modest difference we have found in specific tension after LTT suggests that increases in specific tension that occur with RT may be relatively limited, and thus the underpinning mechanisms for increased maximum voluntary specific tension (i.e., increased agonist neuromuscular activation or intrinsic contractile specific tension) are also relatively small.
The larger volume of muscle of LTT was primarily due to their greater PCSA (+41%; i.e., sarcomeres in parallel) rather than QFL (+11%; i.e., sarcomeres in series). To our knowledge this is the first report to quantify the contribution of these different aspects of muscle morphology to the enhanced muscle mass of substantially hypertrophied human muscle, and it is clear that muscle growth primarily occurs due to an increase in the contractile material arranged in parallel with a smaller contribution from increased sarcomeres in series. To provide a comprehensive assessment of quadriceps muscle architecture we measured ΘP and FL at 10 sites within the quadriceps, which revealed LTT to have a greater QΘP (+13%) and QFL (11%) than UT. A greater QΘP facilitates the attachment of more contractile material, and thus the application of more force, to the tendon/aponeurosis [i.e., as reflected by PCSA (40, 45, 47, 61)], independently from any increase in muscle ACSA or volume, although force transmission to the tendon is increasingly compromised (according to the cosine of ΘP). Overall a greater QΘP is thought to be beneficial for isometric force production up to an optimum angle of 45° (8). Resistance-trained individuals/bodybuilders have previously been found to have much higher ΘP in both the triceps brachii [33° vs. 15°; +120% (47)], mid-point vastus lateralis (20.4° vs. 15.5°; +31% (39)], and medial gastrocnemius [24.6° vs. 18.4°; +34% (39)], which are clearly a larger difference than we found in the present study (QΘP: +11%). This contrast may indicate an anatomical specificity to muscle architectural changes after RT or site-specific differences. Furthermore, the findings of the present study are surprisingly similar to the increases in ΘP observed following short-term lower body RT (2, 10, 26, 35), perhaps suggesting that changes in lower body ΘP may not continue to adapt with prolonged RT and could predominantly occur in the early phase of a training program (i.e., first 3 mo).
The possibility of FL increases after RT, largely based on short-term RT studies, has been controversial (7, 16, 26, 29, 30, 64, 78, 82). Using architecture measurements at 10 sites throughout the quadriceps we found the LTT group to have an 11% greater QFL compared with UT. One previous study of LTT vs. UT reported no differences between their groups (39); however, they assessed FL at only one site, equivalent to the VLMID site of our experiment, where we also observed no differences between LTT and UT (Fig. 3A). In contrast, we found a clear difference for 3 of 4 of the individual muscles (VM, VL, and RF), a tendency for a difference in the fourth (VI), and over the whole muscle group QFL showed a highly significant difference with a large effect size (+11%, P < 0.01 ES 1.2). We also found quantitative evidence for a training group (LTT vs. UT) by constituent muscle interaction for FL, demonstrating inhomogeneous adaptations to LTT. Thus it seems likely that the regional variability in FL changes, the error associated with a single measurement site, the differences in the mode of resistance training used and the short duration of previous reports contribute to the equivocal findings in the literature (34). The current study using a comprehensive assessment at 10 sites throughout the quadriceps muscle group indicates that QFL does increase with prolonged RT. Interestingly, based on geometric modeling it has recently been argued that relatively modest changes in FL can have disproportionately large effects on ACSA and muscle volume (46). In essence, longer (extended) fascicles due to the addition of sarcomeres in parallel appears to result in a disproportionately larger increase of sarcomeres in parallel and therefore could be a key explanation for the differences in muscle size (ACSA, PCSA, and volume) we have observed.
While ΘP did not show such strong evidence for inhomogeneous adaptations to LTT (no training group × muscle interaction effect) there were a range of differences when comparing the four constituent muscles (ΘP 8–15%; FL 6–13%). Therefore, this study further highlights the need for multiple sites to comprehensively quantify architectural differences or changes after training as single sites may be difficult to replicate (36) and as seen in the present study and others, a single site measurement similar to VLMID is not reflective of overall architecture differences across the quadriceps muscle group following RT (26, 35).
Despite the 56% greater muscle volume of LTT vs. UT we found no evidence for regional hypertrophy either between the constituent quadriceps muscles or along their length. Previous short-term RT studies, documenting relatively limited hypertrophy, have, however, repeatedly reported nonuniform regional hypertrophy, both between and along the individual quadriceps muscles, although curiously the pattern of regional hypertrophy has been surprisingly diverse [i.e., which muscles and locations had the greatest hypertrophy (26, 35, 37, 43, 44, 57, 58, 61, 69, 75, 76)]. In the current study, we scanned the entire length of the thigh to accurately identify the ends of the bone and subsequently define the precise position of each of a large number of axial images (slices per muscle: VM, 23–26; VI, 24–27; VL, 24–27; RF, 23–26) relative to those absolute landmarks to carefully quantify regional differences in muscle size. In addition, we recently found a mean within-participant coefficient of variation for repeat quadriceps muscle volume measurements using the same protocol 12 wk apart with a control group to be 1.7%, indicating the reliability of our measurements (11). In contrast, previous studies typically used a small number of slices and positioned slices based on relatively imprecise surface anatomical measurements. Therefore, previous reports of regional hypertrophy may have been confounded by the inconsistent location of the images. Alternatively, as the LTT individuals in the current study had been doing a range of different training practices it is conceivable that this may have resulted in diverse individual hypertrophic responses that cumulatively canceled out and led to no overall regional hypertrophy. However, inspection of the variability (between participant standard deviation) indicates that the proportional size of the individual quadriceps’ muscles (Table 1) and distribution of muscle mass along the femur (Fig. 3) were no more variable for LTT than UT groups. In summary, given the careful methods and large difference in muscle volume in the current study without any evidence for regional hypertrophy it seems likely that this phenomenon may have been overestimated by previous studies.
In addition to morphological changes in the muscle, joint mechanical properties such as PTMA may make a small contribution to maximal torque production (17, 77). In the present study, PTMA was 5% greater in LTT compared with UT. In other muscle groups it has been suggested that muscle hypertrophy may result in biomechanically advantageous increases in leverage of muscular force application (5, 73, 74, 79). However, for the quadriceps the anatomy of the patella and patella tendon wrapping around the distal femur mean that this is unlikely to be the case. In addition, when PTMA was normalized to height there was no difference between the groups indicating that the 4% greater height of LTT group was in large part responsible for their greater PTMA.
There are a number of limitations within the current study that should be recognized. Although the current cross-sectional study design provided a pragmatic approach to examining the substantial adaptations that occur after LTT, due to the cross-sectional nature of the current study and the extensive, retrospective RT background (mean 4 yr RT) of these participants we have relatively limited information regarding their exact training (e.g., precise loads, types of contractions, periodization). Nonetheless these participants all had the primary goal of increasing maximum strength, and were demonstrably stronger than controls (+60%), and we excluded participants involved in activities (e.g., weight category and endurance sports) that might compromise morphological adaptations to RT. A repeated-measurement design on the same participants before, potentially during, and after a prolonged period of RT is clearly a stronger design. Although this approach would be practically challenging, there are very few supervised RT studies of ≥6 mo duration; it would facilitate an in-depth examination of the time course of adaptations to prolonged RT and could be informative for a number of the measures investigated in the current experiment (e.g., specific tension, architecture, regional hypertrophy). The acquisition of clear T1 MR images along the whole thigh (~25 min) is not compatible with measurements during contraction, and in our experience, it is also challenging to record clear ultrasound images of all the constituent muscles during MVCs (55). Thus, the imaging measurements of muscle size, architecture, and moment arm within the current experiment were made at rest to facilitate precise measurements. In addition, due to the constraints of the bore within the MRI scanner, muscle size and moment arm measurements were also taken at a different knee joint angle to the strength measurements. These discrepancies could potentially confound the comparison of strength and morphological variables. For example, quadriceps femoris CSAs and architecture are known to change substantially between rest and maximum contraction (55). Although we have recently found LTT to have a stiffer patella tendon compared with UT, the greater strength of this group appears to produce similar muscle shortening, and thus presumably architectural changes, at MVC (56). Therefore, we are not aware of any systematic effects that might interact with these potential confounders and influence the comparison of LTT and UT groups within the current study.
Finally, the use of B-mode ultrasound presents a number of methodological issues when quantifying muscle architecture in vivo (for a review, see Ref. 36). In the present study by using a relatively long probe (92 mm vs. commonly used 40–60 mm) we were able to minimize the need for extrapolation of fascicle trajectory beyond the recorded image (typically <10% of the measured FL was extrapolated). Architecture measurements were also performed in the knee isometric dynamometer with a knee angle of 115° (i.e., the same knee joint angle as the strength measurements), and this longer muscle length relative to rest explains why FL was longer in the present study than in some previous reports (35, 70). However, we are conscious that ultrasound images are a 2-D representation of a complex 3-D structure and recommend that future work utilize more sophisticated 3-D techniques (e.g., diffusion tensor MRI).
In conclusion, the present study demonstrates that the larger quadriceps strength of LTT individuals was primarily due to greater muscle size with smaller differences in specific tension and moment arm, and thus muscle size was the primary explanation for the greater strength of LTT. The greater muscle volume (+56%) of LTT was due primarily to enhanced PCSA (41%), indicating more sarcomeres in parallel, although we also found convincing evidence for greater QFL (+11%), indicating a modest difference in sarcomeres in series. Finally, there was no evidence for regional hypertrophy either between or along the quadriceps muscles after long-term RT.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.M.M-W, T.G.B., G.M., and J.P.F. conceived and designed research; T.M.M-W, T.G.B., and G.M. performed experiments; T.M.M-W, T.G.B., and G.M. analyzed data; T.M.M-W, T.G.B., G.M., and J.P.F. interpreted results of experiments; T.M.M-W, T.G.B., G.M., and J.P.F. prepared figures; T.M.M-W, T.G.B., G.M., and J.P.F. drafted manuscript; T.M.M-W, T.G.B., G.M., and J.P.F. edited and revised manuscript; T.M.M-W, T.G.B., G.M., and J.P.F. approved final version of manuscript.
GLOSSARY
- ΘP
Pennation angle
- ACSA
Anatomical cross-sectional area
- QACSAMAX
Sum of maximal anatomical cross-sectional areas
- CSA
Cross-sectional area
- EMG
Electromyography
- FL
Fascicle length
- HEMGMAX
Hamstrings EMG amplitude
- IPAQ
International Physical Activity Questionnaire
- KF MVT
Knee flexor maximal voluntary torque
- LTT
Long term resistance trained
- MRI
Magnetic resonance imaging
- MVC
Maximal voluntary contraction
- MVT
Quadriceps maximal isometric voluntary torque
- PTMA
Patella tendon moment arm
- PCSA
Physiological cross-sectional area
- EFFPCSA
Effective physiological cross-sectional area
- QEFFPCSA
Sum of effective physiological cross-sectional area
- QVOL
Quadriceps volume
- QFL
Mean quadriceps fascicle length
- QΘP
Mean quadriceps pennation angle
- RT
Resistance training
- RF
Rectus femoris
- ST
Maximal voluntary specific tension
- UT
Untrained
- VI
Vastus intermedius
- VL
Vastus lateralis
- VM
Vastus medialis
ACKNOWLEDGMENTS
We thank all participants for their time and efforts in completing the study. In addition, the we extend thanks to Matthew Casey and Barnaby Davies for technical assistance during data collection and Karen Voigt and the team at Alliance Medical for their MRI expertise.
REFERENCES
- 1.Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol 534: 613–623, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol 534: 613–623, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41: 687–708, 2009. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 4.Ahtiainen JP, Pakarinen A, Alen M, Kraemer WJ, Häkkinen K. Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur J Appl Physiol 89: 555–563, 2003. doi: 10.1007/s00421-003-0833-3. [DOI] [PubMed] [Google Scholar]
- 5.Akagi R, Iwanuma S, Hashizume S, Kanehisa H, Yanai T, Kawakami Y. In vivo measurements of moment arm lengths of three elbow flexors at rest and during isometric contractions. J Appl Biomech 28: 63–69, 2012. doi: 10.1123/jab.28.1.63. [DOI] [PubMed] [Google Scholar]
- 6.Alegre LM, Ferri-Morales A, Rodriguez-Casares R, Aguado X. Effects of isometric training on the knee extensor moment-angle relationship and vastus lateralis muscle architecture. Eur J Appl Physiol 114: 2437–2446, 2014. doi: 10.1007/s00421-014-2967-x. [DOI] [PubMed] [Google Scholar]
- 7.Alegre LM, Jiménez F, Gonzalo-Orden JM, Martín-Acero R, Aguado X. Effects of dynamic resistance training on fascicle length and isometric strength. J Sports Sci 24: 501–508, 2006. doi: 10.1080/02640410500189322. [DOI] [PubMed] [Google Scholar]
- 8.Alexander R, Vernon A. The dimensions of knee and ankle muscles and the forces they exert. J Hum Mov Stud 1: 115–123, 1975. [Google Scholar]
- 9.Alway SE, Stray-Gundersen J, Grumbt WH, Gonyea WJ. Muscle cross-sectional area and torque in resistance-trained subjects. Eur J Appl Physiol Occup Physiol 60: 86–90, 1990. doi: 10.1007/BF00846026. [DOI] [PubMed] [Google Scholar]
- 10.Balshaw TG, Massey GJ, Maden-Wilkinson TM, Morales-Artacho AJ, McKeown A, Appleby CL, Folland JP. Changes in agonist neural drive, hypertrophy and pre-training strength all contribute to the individual strength gains after resistance training. Eur J Appl Physiol 117: 631–640, 2017. doi: 10.1007/s00421-017-3560-x. [DOI] [PubMed] [Google Scholar]
- 11.Balshaw TG, Massey GJ, Maden-Wilkinson TM, Tillin NA, Folland JP. Training-specific functional, neural, and hypertrophic adaptations to explosive- vs. sustained-contraction strength training. J Appl Physiol (1985) 120: 1364–1373, 2016. doi: 10.1152/japplphysiol.00091.2016. [DOI] [PubMed] [Google Scholar]
- 12.Baroni BM, Geremia JM, Rodrigues R, De Azevedo Franke R, Karamanidis K, Vaz MA. Muscle architecture adaptations to knee extensor eccentric training: rectus femoris vs. vastus lateralis. Muscle Nerve 48: 498–506, 2013. doi: 10.1002/mus.23785. [DOI] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 14.Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol (1985) 103: 1565–1575, 2007. doi: 10.1152/japplphysiol.00578.2007. [DOI] [PubMed] [Google Scholar]
- 15.Blazevich AJ, Gill ND, Bronks R, Newton RU. Training-specific muscle architecture adaptation after 5-wk training in athletes. Med Sci Sports Exerc 35: 2013–2022, 2003. doi: 10.1249/01.MSS.0000099092.83611.20. [DOI] [PubMed] [Google Scholar]
- 16.Blazevich AJ, Gill ND, Deans N, Zhou S. Lack of human muscle architectural adaptation after short-term strength training. Muscle Nerve 35: 78–86, 2007. doi: 10.1002/mus.20666. [DOI] [PubMed] [Google Scholar]
- 17.Blazevich AJ, Coleman DR, Horne S, Cannavan D. Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur J Appl Physiol 105: 869–878, 2009. doi: 10.1007/s00421-008-0972-7. [DOI] [PubMed] [Google Scholar]
- 18.Brandon LJ, Gaasch DA, Boyette LW, Lloyd AM. Effects of long-term resistive training on mobility and strength in older adults with diabetes. J Gerontol A Biol Sci Med Sci 58: M740–M745, 2003. doi: 10.1093/gerona/58.8.M740. [DOI] [PubMed] [Google Scholar]
- 19.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol 67: 428–433, 2010. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 88: 50–60, 2002. doi: 10.1007/s00421-002-0681-6. [DOI] [PubMed] [Google Scholar]
- 21.Comfort P, Haigh A, Matthews MJ. Are changes in maximal squat strength during preseason training reflected in changes in sprint performance in rugby league players? J Strength Cond Res 26: 772–776, 2012. doi: 10.1519/JSC.0b013e31822a5cbf. [DOI] [PubMed] [Google Scholar]
- 22.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35: 1381–1395, 2003. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 23.Davies J, Parker DF, Rutherford OM, Jones DA. Changes in strength and cross sectional area of the elbow flexors as a result of isometric strength training. Eur J Appl Physiol Occup Physiol 57: 667–670, 1988. doi: 10.1007/BF01075986. [DOI] [PubMed] [Google Scholar]
- 24.Degens H, Erskine RM, Morse CI. Disproportionate changes in skeletal muscle strength and size with resistance training and ageing. J Musculoskelet Neuronal Interact 9: 123–129, 2009. [PubMed] [Google Scholar]
- 25.Earp JE, Newton RU, Cormie P, Blazevich AJ. Inhomogeneous quadriceps femoris hypertrophy in response to strength and power training. Med Sci Sports Exerc 47: 2389–2397, 2015. doi: 10.1249/MSS.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 26.Ema R, Wakahara T, Miyamoto N, Kanehisa H, Kawakami Y. Inhomogeneous architectural changes of the quadriceps femoris induced by resistance training. Eur J Appl Physiol 113: 2691–2703, 2013. doi: 10.1007/s00421-013-2700-1. [DOI] [PubMed] [Google Scholar]
- 27.Erskine RM, Degens H, Jones DA. Factors contributing to an increase in quadriceps specific tension following resistance training in young men (Abstract). Proc Phys Soc 11: C89, 2008. [Google Scholar]
- 28.Erskine RM, Jones DA, Maganaris CN, Degens H. In vivo specific tension of the human quadriceps femoris muscle. Eur J Appl Physiol 106: 827–838, 2009. doi: 10.1007/s00421-009-1085-7. [DOI] [PubMed] [Google Scholar]
- 29.Erskine RM, Jones DA, Maffulli N, Williams AG, Stewart CE, Degens H. What causes in vivo muscle specific tension to increase following resistance training? Exp Physiol 96: 145–155, 2011. doi: 10.1113/expphysiol.2010.053975. [DOI] [PubMed] [Google Scholar]
- 30.Erskine RM, Jones DA, Williams AG, Stewart CE, Degens H. Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur J Appl Physiol 110: 1117–1125, 2010. doi: 10.1007/s00421-010-1601-9. [DOI] [PubMed] [Google Scholar]
- 31.Folland JP, Irish CS, Roberts JC, Tarr JE, Jones DA. Fatigue is not a necessary stimulus for strength gains during resistance training. Br J Sports Med 36: 370–373, 2002. doi: 10.1136/bjsm.36.5.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folland J, Leach B, Little T, Hawker K, Myerson S, Montgomery H, Jones D. Angiotensin-converting enzyme genotype affects the response of human skeletal muscle to functional overload. Exp Physiol 85: 575–579, 2000. doi: 10.1111/j.1469-445X.2000.02057.x. [DOI] [PubMed] [Google Scholar]
- 33.Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 37: 145–168, 2007. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 34.Franchi MV, Atherton PJ, Maganaris CN, Narici MV. Fascicle length does increase in response to longitudinal resistance training and in a contraction-mode specific manner. Springerplus 5: 94, 2016. doi: 10.1186/s40064-015-1548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franchi MV, Atherton PJ, Reeves ND, Flück M, Williams J, Mitchell WK, Selby A, Beltran Valls RM, Narici MV. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol (Oxf) 210: 642–654, 2014. doi: 10.1111/apha.12225. [DOI] [PubMed] [Google Scholar]
- 36.Franchi MV, Raiteri BJ, Longo S, Sinha S, Narici MV, Csapo R. Muscle architecture assessment: strengths, shortcomings and new frontiers of in vivo imaging techniques. Ultrasound Med Biol 44: 2492–2504, 2018. doi: 10.1016/j.ultrasmedbio.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Franchi MV, Ruoss S, Valdivieso P, Mitchell KW, Smith K, Atherton PJ, Narici MV, Flück M. Regional regulation of focal adhesion kinase after concentric and eccentric loading is related to remodelling of human skeletal muscle. Acta Physiol (Oxf) 223: e13056, 2018. doi: 10.1111/apha.13056. [DOI] [PubMed] [Google Scholar]
- 38.Fukutani A, Kurihara T. Tendon cross-sectional area is not associated with muscle volume. J Appl Biomech 31: 176–180, 2015. doi: 10.1123/jab.2014-0183. [DOI] [PubMed] [Google Scholar]
- 39.Fukutani A, Kurihara T. Comparison of the muscle fascicle length between resistance-trained and untrained individuals: cross-sectional observation. Springerplus 4: 341, 2015. doi: 10.1186/s40064-015-1133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gans C, Bock WJ. The functional significance of muscle architecture–a theoretical analysis. Ergeb Anat Entwicklungsgesch 38: 115–142, 1965. [PubMed] [Google Scholar]
- 42.Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, Kraemer WJ, Newton RU, Alen M. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol (1985) 84: 1341–1349, 1998. doi: 10.1152/jappl.1998.84.4.1341. [DOI] [PubMed] [Google Scholar]
- 43.Häkkinen K, Pakarinen A, Kraemer WJ, Häkkinen A, Valkeinen H, Alen M. Selective muscle hypertrophy, changes in EMG and force, and serum hormones during strength training in older women. J Appl Physiol (1985) 91: 569–580, 2001. doi: 10.1152/jappl.2001.91.2.569. [DOI] [PubMed] [Google Scholar]
- 44.Housh DJ, Housh TJ, Johnson GO, Chu WK. Hypertrophic response to unilateral concentric isokinetic resistance training. J Appl Physiol (1985) 73: 65–70, 1992. doi: 10.1152/jappl.1992.73.1.65. [DOI] [PubMed] [Google Scholar]
- 45.Jones DA, Rutherford OM. Human muscle strength training: the effects of three different regimens and the nature of the resultant changes. J Physiol 391: 1–11, 1987. doi: 10.1113/jphysiol.1987.sp016721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jorgenson KW, Hornberger TA. The overlooked role of fiber length in mechanical load-induced growth of skeletal muscle. Exerc Sport Sci Rev 47: 258–259, 2019. doi: 10.1249/JES.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol (1985) 74: 2740–2744, 1993. doi: 10.1152/jappl.1993.74.6.2740. [DOI] [PubMed] [Google Scholar]
- 48.Kellis E, Baltzopoulos V. In vivo determination of the patella tendon and hamstrings moment arms in adult males using videofluoroscopy during submaximal knee extension and flexion. Clin Biomech (Bristol, Avon) 14: 118–124, 1999. doi: 10.1016/S0268-0033(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 49.de Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millán-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr 15: 154, 2015. doi: 10.1186/s12877-015-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4: 863, 2013. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev (3): CD002759, 2009. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacDougall JD, Sale DG, Alway SE, Sutton JR. Muscle fiber number in biceps brachii in bodybuilders and control subjects. J Appl Physiol 57: 1399–1403, 1984. doi: 10.1152/jappl.1984.57.5.1399. [DOI] [PubMed] [Google Scholar]
- 53.Maden-Wilkinson TM, McPhee JS, Jones DA, Degens H. Age-related loss of muscle mass, strength, and power and their association with mobility in recreationally-active older adults in the United Kingdom. J Aging Phys Act 23: 352–360, 2015. doi: 10.1123/japa.2013-0219. [DOI] [PubMed] [Google Scholar]
- 54.Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J. Rate of force development: physiological and methodological considerations. Eur J Appl Physiol 116: 1091–1116, 2016. doi: 10.1007/s00421-016-3346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Massey G, Evangelidis P, Folland J. Influence of contractile force on the architecture and morphology of the quadriceps femoris. Exp Physiol 100: 1342–1351, 2015. doi: 10.1113/EP085360. [DOI] [PubMed] [Google Scholar]
- 56.Massey GJ, Balshaw TG, Maden-Wilkinson TM, Folland JP. Tendinous tissue properties after short- and long-term functional overload: Differences between controls, 12 weeks and 4 years of resistance training. Acta Physiol (Oxf) 222: e13019, 2018. doi: 10.1111/apha.13019. [DOI] [PubMed] [Google Scholar]
- 57.Matta TT, Nascimento FX, Fernandes IA, Oliveira LF. Heterogeneity of rectus femoris muscle architectural adaptations after two different 14-week resistance training programmes. Clin Physiol Funct Imaging 35: 210–215, 2015. doi: 10.1111/cpf.12151. [DOI] [PubMed] [Google Scholar]
- 58.Matta TT, Nascimento FX, Trajano GS, Simão R, Willardson JM, Oliveira LF. Selective hypertrophy of the quadriceps musculature after 14 weeks of isokinetic and conventional resistance training. Clin Physiol Funct Imaging 37: 137–142, 2017. doi: 10.1111/cpf.12277. [DOI] [PubMed] [Google Scholar]
- 59.McMahon GE, Morse CI, Burden A, Winwood K, Onambélé GL. Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J Strength Cond Res 28: 245–255, 2014. doi: 10.1519/JSC.0b013e318297143a. [DOI] [PubMed] [Google Scholar]
- 60.Moritani T, DeVries H. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58: 115–130, 1979. [PubMed] [Google Scholar]
- 61.Narici MV, Hoppeler H, Kayser B, Landoni L, Claassen H, Gavardi C, Conti M, Cerretelli P. Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiol Scand 157: 175–186, 1996. doi: 10.1046/j.1365-201X.1996.483230000.x. [DOI] [PubMed] [Google Scholar]
- 63.Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol 59: 310–319, 1989. doi: 10.1007/BF02388334. [DOI] [PubMed] [Google Scholar]
- 64.Noorkõiv M, Nosaka K, Blazevich AJ. Effects of isometric quadriceps strength training at different muscle lengths on dynamic torque production. J Sports Sci 33: 1952–1961, 2015. doi: 10.1080/02640414.2015.1020843. [DOI] [PubMed] [Google Scholar]
- 65.Noorkoiv M, Stavnsbo A, Aagaard P, Blazevich AJ. In vivo assessment of muscle fascicle length by extended field-of-view ultrasonography. J Appl Physiol (1985) 109: 1974–1979, 2010. doi: 10.1152/japplphysiol.00657.2010. [DOI] [PubMed] [Google Scholar]
- 66.Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol 91: 116–118, 2004. doi: 10.1007/s00421-003-0961-9. [DOI] [PubMed] [Google Scholar]
- 67.Scanlon TC, Fragala MS, Stout JR, Emerson NS, Beyer KS, Oliveira LP, Hoffman JR. Muscle architecture and strength: adaptations to short-term resistance training in older adults. Muscle Nerve 49: 584–592, 2014. doi: 10.1002/mus.23969. [DOI] [PubMed] [Google Scholar]
- 68.Seger JY, Arvidsson B, Thorstensson A, Seger JY. Specific effects of eccentric and concentric training on muscle strength and morphology in humans. Eur J Appl Physiol Occup Physiol 79: 49–57, 1998. doi: 10.1007/s004210050472. [DOI] [PubMed] [Google Scholar]
- 69.Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol (1985) 102: 368–373, 2007. doi: 10.1152/japplphysiol.00789.2006. [DOI] [PubMed] [Google Scholar]
- 70.Seynnes OR, Kamandulis S, Kairaitis R, Helland C, Campbell E-L, Brazaitis M, Skurvydas A, Narici MV. Effect of androgenic-anabolic steroids and heavy strength training on patellar tendon morphological and mechanical properties. J Appl Physiol (1985) 115: 84–89, 2013. doi: 10.1152/japplphysiol.01417.2012. [DOI] [PubMed] [Google Scholar]
- 72.Stamatakis E, Lee IM, Bennie J, Freeston J, Hamer M, O’Donovan G, Ding D, Bauman A, Mavros Y. Does strength-promoting exercise confer unique health benefits? a pooled analysis of data on 11 population cohorts with all-cause, cancer, and cardiovascular mortality endpoints. Am J Epidemiol 187: 1102–1112, 2018. doi: 10.1093/aje/kwx345. [DOI] [PubMed] [Google Scholar]
- 73.Sugisaki N, Wakahara T, Miyamoto N, Murata K, Kanehisa H, Kawakami Y, Fukunaga T. Influence of muscle anatomical cross-sectional area on the moment arm length of the triceps brachii muscle at the elbow joint. J Biomech 43: 2844–2847, 2010. doi: 10.1016/j.jbiomech.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 74.Sugisaki N, Wakahara T, Murata K, Miyamoto N, Kawakami Y, Kanehisa H, Fukunaga T. Influence of muscle hypertrophy on the moment arm of the triceps brachii muscle. J Appl Biomech 31: 111–116, 2015. doi: 10.1123/JAB.2014-0126. [DOI] [PubMed] [Google Scholar]
- 75.Tesch PA, Trieschmann JT, Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. J Appl Physiol (1985) 96: 1451–1458, 2004. doi: 10.1152/japplphysiol.01051.2003. [DOI] [PubMed] [Google Scholar]
- 76.Tracy BL, Ivey FM, Hurlbut D, Martel GF, Lemmer JT, Siegel EL, Metter EJ, Fozard JL, Fleg JL, Hurley BF. Muscle quality. II. Effects Of strength training in 65- to 75-yr-old men and women. J Appl Physiol (1985) 86: 195–201, 1999. doi: 10.1152/jappl.1999.86.1.195. [DOI] [PubMed] [Google Scholar]
- 77.Trezise J, Collier N, Blazevich AJ. Anatomical and neuromuscular variables strongly predict maximum knee extension torque in healthy men. Eur J Appl Physiol 116: 1159–1177, 2016. doi: 10.1007/s00421-016-3352-8. [DOI] [PubMed] [Google Scholar]
- 78.Ullrich B, Holzinger S, Soleimani M, Pelzer T, Stening J, Pfeiffer M. Neuromuscular Responses to 14 Weeks of Traditional and Daily Undulating Resistance Training. Int J Sports Med 36: 554–562, 2015. doi: 10.1055/s-0034-1398529. [DOI] [PubMed] [Google Scholar]
- 79.Vigotsky AD, Contreras B, Beardsley C. Biomechanical implications of skeletal muscle hypertrophy and atrophy: a musculoskeletal model. PeerJ 3: e1462, 2015. doi: 10.7717/peerj.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wakahara T, Ema R, Miyamoto N, Kawakami Y. Increase in vastus lateralis aponeurosis width induced by resistance training: implications for a hypertrophic model of pennate muscle. Eur J Appl Physiol 115: 309–316, 2015. doi: 10.1007/s00421-014-3012-9. [DOI] [PubMed] [Google Scholar]
- 81.Wakahara T, Ema R, Miyamoto N, Kawakami Y. Inter- and intramuscular differences in training-induced hypertrophy of the quadriceps femoris: association with muscle activation during the first training session. Clin Physiol Funct Imaging 37: 405–412, 2017. doi: 10.1111/cpf.12318. [DOI] [PubMed] [Google Scholar]
- 82.Wakahara T, Fukutani A, Kawakami Y, Yanai T. Nonuniform muscle hypertrophy: its relation to muscle activation in training session. Med Sci Sports Exerc 45: 2158–2165, 2013. doi: 10.1249/MSS.0b013e3182995349. [DOI] [PubMed] [Google Scholar]
- 83.Young A, Stokes M, Round JM, Edwards RH. The effect of high-resistance training on the strength and cross-sectional area of the human quadriceps. Eur J Clin Invest 13: 411–417, 1983. doi: 10.1111/j.1365-2362.1983.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 26: 355–369, 2010. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]