Abstract
Aging induces physiological decline in human skeletal muscle function and morphology, including type II fiber atrophy and an increase in type I fiber frequency. Resistance exercise training (RET) is an effective strategy to overcome muscle mass loss and improve strength, with a stronger effect on type II fibers. In the present study, we sought to determine the effect of a 12-wk progressive RET program on the fiber type-specific skeletal muscle hypertrophic response in older adults. Nineteen subjects [10 men and 9 women (71.1 ± 4.3 yr)] were studied before and after the 12-wk program. Immunohistochemical analysis was used to quantify myosin heavy chain (MyHC) isoform expression, cross-sectional area (CSA), satellite cell abundance, myonuclear content, and lipid droplet density. RET induced an increase in MyHC type II fiber frequency and a concomitant decrease in MyHC type I fiber frequency. Mean CSA increased significantly only in MyHC type II fibers (+23.3%, P < 0.05), but myonuclear content increased only in MyHC type I fibers (P < 0.05), with no change in MyHC type II fibers. Satellite cell content increased ~40% in both fiber types (P > 0.05). RET induced adaptations to the capillary supply to satellite cells, with the distance between satellite cells and the nearest capillary increasing in type I fibers and decreasing in type II fibers. Both fiber types showed similar decrements in intramuscular lipid density with training (P < 0.05). Our data provide intriguing evidence for a fiber type-specific response to RET in older adults and suggest flexibility in the myonuclear domain of type II fibers during a hypertrophic stimulus.
NEW & NOTEWORTHY In older adults, progressive resistance exercise training (RET) increased skeletal muscle fiber volume and cross-sectional area independently of myonuclear accretion, leading to an expansion of the myonuclear domain. Fiber type-specific analyses illuminated differential adaptation; type II fibers underwent hypertrophy and exhibited myonuclear domain plasticity, whereas myonuclear accretion occurred in type I fibers in the absence of a robust hypertrophic response. RET also augmented satellite cell-capillary interaction and reduced intramyocellular lipid density to improve muscle quality.
Keywords: aging, hypertrophy, myonuclear domain, skeletal muscle
INTRODUCTION
Aging induces a decline in human skeletal muscle function and mass, with age-associated maladaptations to muscle morphology accompanying sarcopenia. On a myocellular level, advanced age results in smaller fiber size, specific to type II fibers (46), in addition to reduced capillary content (42) and an overall change in fiber type distribution with an increase in type I fiber frequency (29, 52). From a functional perspective, type II fibers produce greater relative and absolute force with a relatively shorter contraction time (4, 21). An emerging body of literature has drawn attention to the fiber type-specific effect(s) of aging on muscle deterioration. Aging has been shown to promote type II fiber denervation (9, 31, 35), atrophy, and reduced satellite cell (SC) content (60). The preferential atrophy of type II fibers reduces older adults’ muscle strength and power compared with younger subjects. Preserving type II fiber size with age is a key target to optimize, or at least maintain, muscle function in older adults to improve quality of life. With a better understanding of the processes that promote type II fiber hypertrophy, it would be possible to develop targeted interventions to mitigate age-related functional decline.
Resistance exercise training (RET) is an effective strategy to overcome age-related atrophy and weakness. Previous studies have shown that older adults can increase the relative frequency and cross-sectional area (CSA) of type II fibers following RET (30, 41, 59). Skeletal muscle fiber hypertrophy is accompanied by the addition of new myonuclei within existing fibers. SCs are the primary muscle stem cells that contribute new myonuclei. During resistance training, SCs are stimulated to proliferate, differentiate, and ultimately fuse into fibers, adding new myonuclei that support enhanced ribosomal function and thus myofiber hypertrophy (46, 47, 64). The addition of new myonuclei from SCs during hypertrophy maintains a constant myonuclear domain (MND). The ‘MND theory’ is defined as the sarcoplasmic volume that is transcriptionally governed by an individual myonucleus and an expansion of sarcoplasmic volume during hypertrophy that is accompanied by concurrent myonuclear accretion to maintain a relatively constant MND (44, 51). However, more recent studies have challenged this theory and suggested that the MND can be flexible and adaptations to exercise may occur in a fiber type-specific manner (10, 12, 57).
It has been observed that SC proximity to capillaries facilitates their activation in response to exercise (8). This finding suggests that local perfusion supports SC activity and subsequent muscle growth. Capillaries are responsible for the delivery of nutrients, oxygen, and hormones to skeletal muscle to help govern its adaptation during resistance training. We and others have shown that a reduction in capillary density and/or a greater distance between SCs and their nearest capillary may impair skeletal muscle adaptation in older adults (40, 53). However, controversy exists surrounding the ability of RET to improve capillary density in older adults, with some studies showing positive adaptation (17, 61) and others showing an equivocal effect of RET on capillary density (15, 40, 53).
Additionally, recent studies have advanced the hypothesis that the accumulation of intramyocellular lipids (IMCLs) may also promote muscle dysfunction during aging (7, 16). Because of their oxidative capacity, type I fibers have greater lipid droplet density (14), and perturbations in the accumulation of IMCLs may influence the fast-to-slow fiber transition and muscle fiber atrophy (36).
Physiological and metabolic attributes of glycolytic and oxidative characteristics underpin their sensitivity to specific anabolic/catabolic stimuli. Resistance exercise training is known to promote muscle growth, but our understanding of the molecular mechanisms governing muscle hypertrophy continues to be refined. Recent research underscores a greater gap in knowledge regarding the fiber type-specific response to exercise. Additionally, as compared with type I fibers, type II fibers are more vulnerable to age-related atrophy. Thus, a greater comprehension of the mechanisms directing fiber type adaptation to resistance training is of clinical relevance to support evidence-based strategies to overcome sarcopenia. In light of these considerations, the aim of this study was to investigate fiber type-specific adaptations to myonuclear content, SC abundance, and muscle fiber structure and quality in sedentary older adults after 12 wk of progressive resistance exercise.
METHODS
Subjects
The present study is an analysis of secondary data from a recently published study from our group (39). Nineteen older adults (10 male, 9 female) participated in the study [age 71.1 ± 4.4 yr; body mass index (BMI) 29.7 ± 3.1 kg/m2]. Volunteers were considered eligible with an age between 65 and 80 yr and BMI < 30 kg/m2. All subjects were healthy; BMI > 35 kg/m2, frailty and any significant chronic disease, active infection, or cancer served as exclusion criteria for subjects in our study based on a physical examination and laboratory tests. Additionally, subjects were classified as nondiabetic by an oral glucose tolerance test and classified as nonactive based on their daily steps (<10,000 steps/day) and not engaging in any form of structured exercise training (<2 weekly sessions of moderate intensity exercise). Before enrollment, all subjects read and signed a written informed consent form approved by the Institutional Review Board of the University of Texas Medical Branch (UTMB, Galveston, TX), which is in accordance with the standards set by the latest revision of the Declaration of Helsinki.
Study Design
After enrollment, participants reported to the UTMB Clinical Research Center for an initial biopsy (pretraining). Pre- and post-biopsies were collected after an overnight fast and 72 h following the last training session. Percutaneous muscle biopsies were obtained from the vastus lateralis using a Bergström 5-mm muscle biopsy needle with suction. The following week, subjects underwent a familiarization week of training before starting a 12-wk progressive RET program. Training was performed at the exercise training facility located in the UTMB Center for Recovery, Physical Activity and Nutrition. The RET protocol consisted of three sessions per week of a total body workout at a progressive intensity. Exercises involving the vastus lateralis were the leg press and leg extension. Training volume and intensity adapted over the course of the 12 wk; initially, subjects completed 3 sets of 15 reps at 60% of 1 repetition maximum (RM), and at the end of the training program subjects were completing 3 sets of 10 reps at 70% of 1 RM. The relative load was adjusted to ensure that all exercises during every session were performed to failure. A 6 RM test was performed monthly before increasing the intensity of the training (from 60 to 65 to 70% of 1 RM). For complete details of the RET protocol, please see Moro et al. (39). To minimize any possible acute effect of training on our outcomes (i.e., satellite cell activation), a second muscle biopsy (posttraining) was collected 72 h following the last acute exercise bout.
Muscle Processing
Approximately 20 mg of well-oriented muscle tissue was placed on a cork with Tissue Tek optimal cutting temperature (OCT; Thermo Fisher Scientific, Rockford, IL) and frozen in liquid nitrogen-cooled isopentane. Samples were then stored at −80°C until analysis. Sections (7-μm thick) were cut in a cryostat and allowed to air dry for 1 h. Approximately 10 mg of muscle tissue was fixed immediately in 4% paraformaldehyde for single fiber isolation.
Immunohistochemistry
Fiber typing and grouping.
Unfixed slides were incubated overnight at room temperature with specific antibodies against anti-myosin heavy chain (MyHC) isoforms type I (DSHB; BA.D5, IgG2b) and laminin (no. L9393; Sigma Aldrich, St. Louis, MO). The following day, slides were incubated with immunoglobulin-specific secondary antibodies: goat anti-mouse IgG2b AF647 (Invitrogen; A21242, type I) and goat anti-rabbit AF350 (Invitrogen; A21068, laminin). After being postfixed in methanol for 5 min, slides were mounted with fluorescent mounting media (Vectashield, no. H-1000; Vector Laboratories, Burlingame, CA). Whole cross-sectional images of the muscle biopsies were captured at ×100 total magnification using the mosaic and stitching functions on an Axioimager MI upright microscope (Zeiss). We quantified fiber type distribution as MyHC type I+ and MyHC type I− (type II fibers) as relative frequency by quantifying the number of MyHC+ relative to the total number of fibers. The prevalence of type I and II fiber grouping was then assessed as two contiguous fibers of the same type surrounded by only fibers of the same type to constitute a grouping of that fiber type, which is similar to analysis reported by Messi et al. (37). We also assessed CSA (µm2) of type I and II fibers. Type I fiber CSA has been previously published (39).
Satellite cell, myonuclei, and capillary assessment.
After 10-min fixation in ice-cold acetone, slides had endogenous peroxidases blocked with 3% H2O2 and were then blocked for 1 h in 2.5% normal horse serum (no. S-2012; Vector Laboratories). Slides were then incubated overnight with the following antibodies and reagents: anti-MyHC type I IgG2b BA.D5 (DHSB Iowa), anti-laminin (no. L9393; Sigma Aldrich, St. Louis, MO), anti-Pax7 (DHSB), and rhodamine-labeled Ulex Europaeus agglutinin I (no. RL- 1062; Vector Laboratories), a human endothelial cell marker (18). The following day, slides were incubated in secondary antibodies: goat anti-rabbit IgG AF647 (no. A21245; Invitrogen, laminin), goat anti- mouse IgG2b, AF647 (no. A21242; Invitrogen, type I), and goat anti-mouse IgG biotinylated antibody (no. 115-065-205; Jackson ImmunoResearch, West Grove, PA) for 1 h. After 1 h of incubation in streptavidin-horseradish peroxidase and a reaction with AF488 tyramide included with the TSA kit (no. T20935; Invitrogen), slides were stained with DAPI (4′,6-diamidino-2-phenylindole, no. D35471; Invitrogen) before being mounted with fluorescent mounting media. Whole cross-sectional images of the muscle biopsies were captured at ×100 total magnification using the mosaic and stitching functions on an Axioimager MI upright microscope (Zeiss). SC abundance was analyzed by quantifying Pax7+/DAPI+ nuclei residing within the laminin border. SC abundance was subdivided by fiber type: MyHC type I+ and MyHC type I− (type II fibers), as we have previously published (2, 12). Myonuclear number was analyzed by quantifying Pax7−/DAPI+ nuclei residing within the laminin border and subdivided by fiber type. Additionally, capillary-to-fiber ratio and the distance between SC and their nearest capillary was measured using Fiji software (https://fiji.sc/), as reported by Nederveen et al. (42).
Intramuscular lipid droplet analysis.
Fresh-cut (without air drying) slides were fixed in 4% paraformaldehyde for 7 min at room temperature. Sections were then permeabilizated in 0.5% Triton-X100 in PBS (T8787; Sigma Aldrich, St. Louis, MO) for 10 min. Slides were then incubated for 2 h with primary antibody for anti-MyHC type I (M8421, Sigma Aldrich, St. Louis, MO), followed by 1 h in secondary antibody (goat anti-mouse IgG1 AF555; no. A21127; Invitrogen). Slides were then stained with Bodipy (D3922; ThermoFisher) and Wheat Germ Agglutinin AF350 conjugate (W11263; ThermoFisher) before being mounted with fluorescent mounting media. Slides were kept in a dark container and protected from light exposure until imaging, and the time between mounting and imaging was kept consistent between samples. It is worth noting that the use of Triton-X100 may have led to some lipid extraction, impacting our IMCL quantification. This is a limitation of our lipid droplet analysis; however, all samples underwent the same procedure, allowing for qualitative IMCL analysis before and after RET in our subjects. Whole cross-sectional images of the muscle biopsies were captured at ×100 total magnification using the mosaic and stitching functions on an Axioimager MI upright microscope (Zeiss). Quantification of intramyocellular lipid droplets (IMCL) was performed using Fiji software. Individual muscle fibers were manually delineated as individual regions of interest, and an intensity threshold was uniformly selected and applied to represent a positive signal for IMCL droplets. At least 50 fibers of each type from each biopsy were analyzed for IMCL droplet density. The density of IMCL droplets was expressed as the positively stained area fraction relative to the single fiber area of each muscle fiber, and results were subdivided by fiber type: MyHC type I+ and MyHC type I− (type II fibers).
Single Fiber Analyses
Muscle samples in 4% paraformaldehyde were allowed to fix for 48 h, and they were then stored at room temperature in PBS. Individual fiber bundles were isolated, and the bundles were digested in 40% NaOH solution for 2 h. Single fibers were then mechanically separated through pipette trituration and washed with PBS before being stained with DAPI for nuclear visualization. Suspended fibers were mounted with Vectashield fluorescent mounting media (Vector Laboratories). Images were captured at ×200 magnification using Z-stacking to combine multiple images taken at focal distances separated by 1 µm to provide a composite image with a greater depth of field on an Axioimager MI upright microscope (Zeiss). All fiber and nuclear measurements were made using AxioVision Rel software (version 4.8). A minimum of ten fibers from each subject was measured for fiber width (µm), and Z-stack analysis was used to count total myonuclei. Myonuclei per fiber volume (myonuclear domain, MND) were then calculated by dividing the fiber segment volume (µm3), which was calculated by multiplying one-half the fiber width (radius)2 × π × length of the measured fiber segment, and then dividing by the total number of myonuclei to obtain MND (µm3 per myonucleus). Fiber type-specific MND was estimated in two dimensions by expressing fiber CSA relative to myonuclear content (µm2 per myonucleus) as previously described (2, 38).
Statistical Analysis
After normality of the data distribution was assessed with a Kolmogorov–Smirnov test, a two-way ANOVA with repeated measures was used to identify the relationship between training and muscle fiber type (training × fiber type); in instances where significant main effects or interactions occurred, Bonferroni post hoc testing was performed. Dependent variables were compared pre- versus posttraining by performing a Student’s paired t test with significance set at P < 0.05. Sex differences at baseline and posttraining were tested with fiber type × sex ANOVA. All analyses were done with GraphPad Prism 7.0 (GraphPad Software, La Jolla CA, https://www.graphpad.com/).
RESULTS
Fiber Typing and Grouping
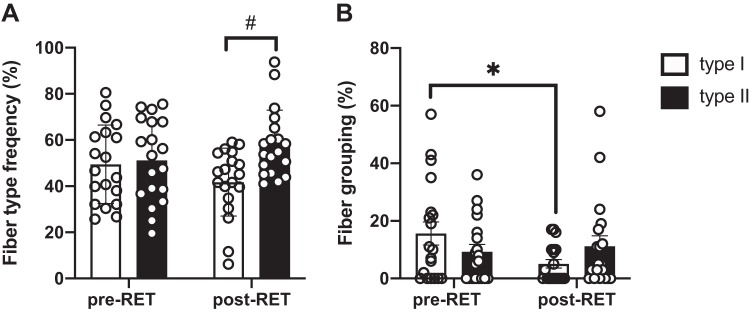
After 12 wk of RET, we observed a shift in fiber type distribution. There was a main effect of RET on fiber type distribution (P = 0.02), with a decrease in type I fiber frequency (from 49 ± 17% to 42 ± 15%) and a concomitant increase in type II fiber frequency (from 51 ± 18% to 58 ± 15%) (Fig. 1A). The percentage of type I fibers that were grouped significantly decreased after RET (P = 0.03), whereas type II fiber grouping did not change (Fig. 1B). No significant sex difference was observed in fiber type distribution at baseline or in response to training.
Fig. 1.
Resistance exercise training (RET) induces a shift in fiber type distribution and reduced grouping of type I fibers in older adults. A: quantification of fiber type from immunohistochemical analysis; data are presented as mean fiber frequency ± SE. B: quantification of spatial distribution of myofiber type grouping, presented as relative percentage ± SE. #Significantly different from type I fiber frequency (P < 0.05). *Significantly different from pretraining (pre-RET) value (P < 0.05).
Myonuclear Domain and Cross-Sectional Area
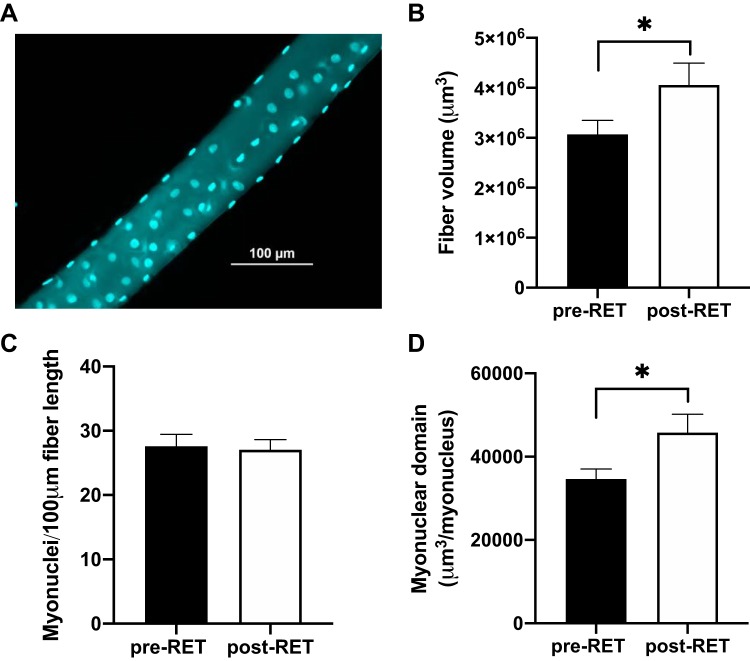
Isolated, single fibers from each subject were analyzed to determine fiber volume and MND, without distinction for fiber type. After 12 wk of RET, fiber volume increased by 42% (Fig. 2B, P = 0.05). Total myonuclear content per 100 µm of fiber length did not change (Fig. 2C, P = 0.76). As a result, MND was significantly higher (45%, P = 0.04) in response to training (Fig. 2D).
Fig. 2.
Resistance exercise training (RET) increases single fiber volume in the absence of myonuclear accretion in older adults. A: representative immunohistochemical image demonstrating a single fiber with associated myonuclei (turquoise). B: mean fiber volume (μm3). C: myonuclear density presented as mean number of myonuclei per 100 μm. D: quantification of myonuclear domain, calculated as mean fiber segment volume (µm3) relative to the number of myonuclei per fiber. Data are expressed as mean ± SE. *Significantly different from pretraining (pre-RET) value (P < 0.05).
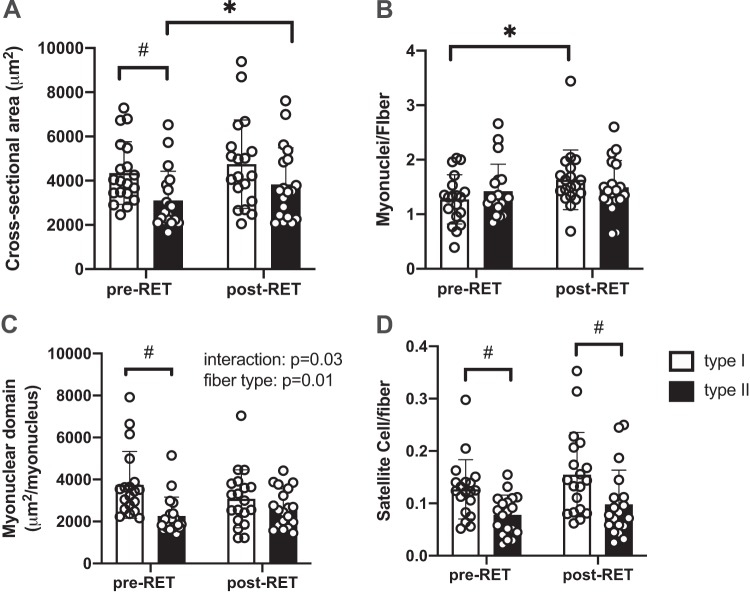
To assess the fiber type-specific response to training, we estimated the MND in 2 dimensions by expressing fiber CSA relative to myonuclear content. Pooled muscle fiber CSA (irrespective of fiber type) showed a statistical tendency toward hypertrophy (20.6% increase, P = 0.09, Student’s t test). When segregated into type I and type II fibers, there was a significant main effect for both fiber type and training (Fig. 3A). Post hoc analyses revealed that pretraining type I fiber CSA was greater than type II fiber CSA (P = 0.04) and that with training, only MyHC type II fiber CSA significantly increased (23.3% increase, P = 0.03). As shown in Fig. 3B, the number of myonuclei per fiber significantly increased after 12 wk of RET in type I fibers (38.1% increase, P = 0.01) but not in type II fibers (9.5% % increase, P > 0.05 fiber × training interaction, P = 0.08). As a result (Fig. 3C), the two-dimensional MND decreased in type I fibers (−8.5%) and increased in type II fibers (24.1%, interaction: training × fiber type, P = 0.03; main effect for fiber type, P = 0.006). These data provide evidence for fiber type-specific myonuclear accrual following RET, with type II fibers showing greater flexibility in their two-dimensional MND.
Fig. 3.
Resistance exercise training (RET) induces hypertrophy of type II fibers in the absence of myonuclear accretion in older adults. A: mean fiber cross-sectional area (CSA) of type I and II fibers are presented as mean µm2 ± SE. B: quantification of myonuclei in type I and II fibers; data are expressed as mean number of myonuclei per fiber ± SE. C: quantification of myonuclear domain, calculated as the mean fiber CSA (µm2) relative to the number of myonuclei per fiber ± SE. D: quantification of satellite cell (SC) content in type I and II fibers: type II fiber SC abundance was significantly lower in both pre- and posttraining conditions. Data are expressed as mean SC number per fiber ± SE. *Significantly different from pretraining (pre-RET) value (P < 0.05); #Significantly different from type I fiber (P < 0.05).
Before training, a main effect for sex was found in myonuclei associated with type II fibers and in both type I and type II fiber CSA. In particular, women were noted to have fewer myonuclei than men in type II fibers (1.69 ± 0.18 vs. 1.13 ± 0.05, P = 0.01); moreover, type I and II fibers were ~30–50% smaller in women compared with men (P < 0.05). As a result, MND was smaller in women compared with men, but this difference was not statistically significant (P = 0.35). Additionally, the effect of training on the fiber type-specific response to myonuclear density, fiber size, and MND was similar between sexes (Table 1). It is worth noting that type I fiber myonuclear accretion post-RET was driven by men, as post hoc tests revealed that men, and not women, increased their myonuclear density.
Table 1.
Sex differences
| Male |
Female |
|||
|---|---|---|---|---|
| Pre-RET | Post-RET | Pre-RET | Post-RET | |
| n | 10 | 10 | 9 | 9 |
| Fiber cross-sectional area, μm2 | ||||
| Fiber type I | 5,173.85 ± 516.19 | 5,083.99 ± 596.32 | 3,614.85 ± 208.72# | 4,318.74 ± 716.19 |
| Fiber type II | 4,042.67 ± 427.67 | 4,517.13 ± 540.38 | 2,180.97 ± 86.07# | 2,945.45 ± 370.56# |
| Myonuclei/fiber | ||||
| Fiber type I | 1.30 ± 0.14 | 1.78 ± 0.20* | 1.24 ± 0.16 | 1.46 ± 0.13 |
| Fiber type II | 1.69 ± 0.18 | 1.69 ± 0.14 | 1.13 ± 0.05# | 1.23 ± 0.13# |
| Myonuclear domain, μm2/myonucleus | ||||
| Fiber type I | 4,212.55 ± 447.51 | 3,003.93 ± 325.21 | 3,408.49 ± 595.26 | 3,169.74 ± 584.21 |
| Fiber type II | 2,590.19 ± 362.15 | 2,710.33 ± 263.79 | 1,976.30 ± 138.56 | 2,566.17 ± 345.38 |
Values are means ± SE. RET, resistance exercise training.
Significantly different from pretraining value (P < 0.05);
Significantly different from male subjects (P < 0.05).
Satellite Cells and Capillaries
Quantification of SC abundance demonstrated a similar but nonstatistically significant (47.9% type I, P = 0.25; 56.2% type II, P = 0.25) increase after RET in both fiber types (Fig. 3D). The number of capillaries per fiber increased only in type II fibers, but the difference was not statistically significant after 12 wk of RET (type I: 1.43 ± 0.1 pre-RET to 1.44 ± 0.1 post-RET; type II: 1.03 ± 0.1 pre-RET to 1.21 ± 0.1 post-RET, P > 0.05). Additionally, at baseline, the distance between an SC and its nearest capillary showed a trend to be greater in type II fibers compared with type I fibers (P = 0.06). We did observe a training by fiber type interaction (P = 0.04), with the distance increasing between capillaries and type I SC and decreasing between capillaries and type II SC following RET (Fig. 4A). We did not find any significant sex difference in our outcome measures at baseline or in response to training.
Fig. 4.
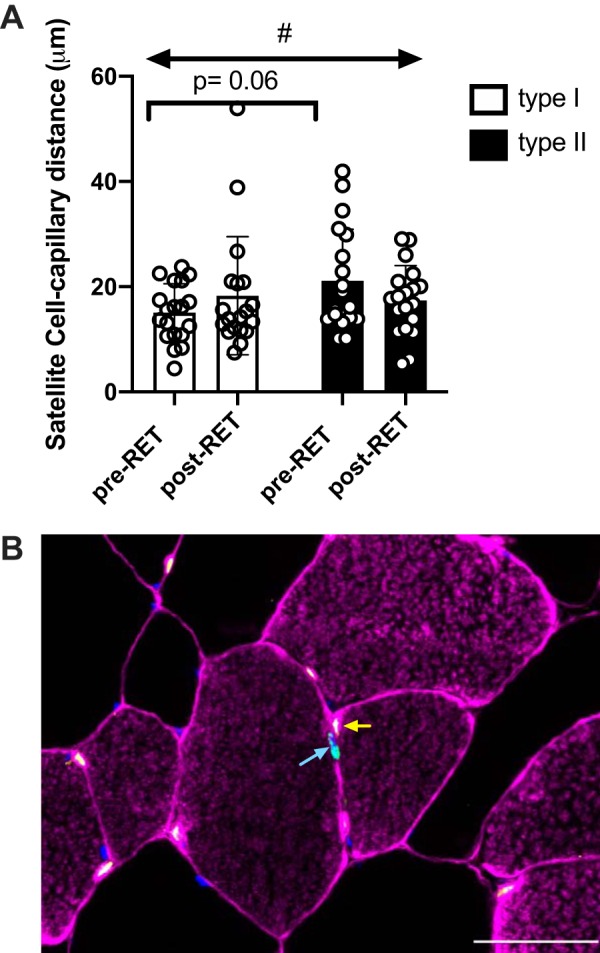
Resistance exercise training (RET) alters the distance between satellite cells and their nearest capillary. A: satellite cell distance to nearest capillary in type I and type II fibers; data are expressed as mean µm ± SE. At baseline, the difference between the distance of satellite cells and their capillaries in each fiber type trended toward statistical significance (P = 0.06). B: representative immunohistochemical image demonstrating Laminin and type I fibers (pink), capillaries (yellow), myonuclei (blue), and satellite cells (green). A blue arrow denotes a Pax7+ satellite cell and a yellow arrow points to its closet capillary. #Significant interaction type × training (P < 0.05). Scale bar = 50 µm.
Intramuscular Lipid Droplet
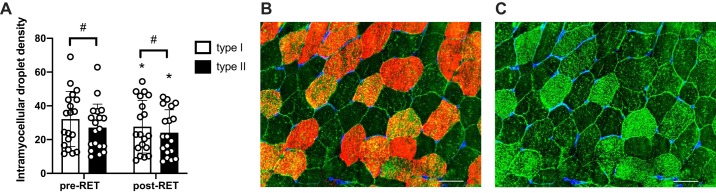
At baseline, quantification of IMCL droplets showed that type I fibers had a higher IMCL density in the subsarcolemmal region compared with type II fibers (P < 0.001). After RET, both fiber types showed a similar decrease in IMCL density (−7.6% type I; −5.4% type II; P < 0.001), which maintained fiber type-specific differences in IMCL density post-RET (Fig. 5A). We did not find any significant sex difference in our outcome measures at baseline or in response to training.
Fig. 5.
Resistance exercise training (RET) induces a decrease in intramyocellular lipid droplet density in type I and II fibers. A: quantification of lipid droplet density in type I and II fibers: individual muscle fibers were manually delineated and lipid droplet abundance assessed within each fiber. B: representative immunohistochemical image demonstrating lipid droplets (green), type I fibers (red), and Wheat Germ Agglutinin (blue). C: representative immunohistochemical image demonstrating lipid droplet (green) and Wheat Germ Agglutinin (blue): fibers with a greater lipid droplet density (brighter green) correspond to type I fibers in B. Data are expressed as mean droplet density ± SE. Scale bar = 50 µm. *Significantly different from pretraining (pre-RET) value (P < 0.05); #Significantly different from type I fiber (P < 0.05).
DISCUSSION
Our findings of the differential adaptation of oxidative and glycolytic skeletal muscle fibers following 12 wk of progressive RET support fiber type-specific flexibility of the MND in older adults. In addition to the restorative effects of RET on type II fiber size, we observed that the hypertrophic response of type II fibers was accompanied by remodeling of satellite cells and capillaries but no discernible myonuclear accretion. By comparison, type I fibers exhibited less dramatic hypertrophy that was sufficient to promote myonuclear accrual, leading to a reduction in their respective MND. These findings expand upon observations made in younger adults (12) and aged mice (32) in which relatively limited type I fiber hypertrophy occurred concurrently with an increase in myonuclear content, leading to a reduction in MND.
Aging induces a progressive loss of muscle mass and quality, which resolves on a myocellular level with decreased type II fiber frequency and CSA. In addition to well-defined atrophy, older adults show a greater incidence of grouped type I fibers (27, 34, 58). This phenomenon is likely related to progressive denervation of type II fibers seen during aging. To overcome reduced neural input to type II fibers, the nearby axons of type I fibers reinnervate the fiber, promoting a transition in phenotype from type II to type I (27). As a result, the number of type I fibers increases, and the type I fibers appear ‘grouped’ together with minimal infiltration of type II fibers. RET has been shown to increase type II fiber CSA and frequency both in young (23, 45, 46) and older adults (46, 59). Prior to RET, subjects in our study had similar frequencies of type I and type II fibers. After 12 wk of RET, we show a difference in fiber type distribution with greater frequency of type II fibers in conjunction with a significant decrease in the frequency of grouped type I fibers. It has been shown that RET increases the relative proportion of type II fibers (in particular, MyHC IIa) in young adults (1, 11) due to metabolic demands and neuronal activity required during weight lifting. The presence of fiber type grouping in older skeletal muscle is a well-established marker of motor unit remodeling (26, 27), as higher type I grouping is associated with greater neuromuscular junction deterioration and motor unit activation, which has been shown to precede functional decline (26). The reduction we observed in type I fiber frequency and grouping may suggest an attenuation or reversal of age-related neuromuscular degeneration. It may be possible that some of the myofibers were in an ambiguous denervation/reinnervation state and exercise training stimulated the transition of grouped type I fibers into type II (26). Moreover, mean fiber CSA increased after RET, with a greater hypertrophic response in type II fibers (+23% type II, +8% type I). However, it is worth noting that type I fiber CSA remained larger than type II fiber CSA even after training.
The results presented in this study suggest differential growth mechanisms between different fiber types. Although we also confirmed that resistance training is an effective strategy to address aging-related muscle atrophy, our findings show some inconsistencies to those obtained after longer training protocols in older adults (3, 46) or in younger subjects (24, 49). Interestingly, recent work from Verdijk et al. (59) shows a greater effect of 12-wk resistance training on the magnitude of muscle hypertrophy in older adults compared with our results. If we compare pre-RET fiber size, however, our subjects have a smaller pre-RET CSA (59). The discrepancies in the hypertrophic capacity of older adults may suggest that older adults with smaller fiber size require a longer period of RET to induce robust hypertrophy.
Muscle hypertrophy is accompanied by the addition of new myonuclei into preexisting fibers from the activation and fusion of satellite cells. Previous studies have shown that RET induces an expansion in SC content resulting in an increase in myonuclei (22). Fry et al. (12) show that this relationship holds true in type I fibers, but hypertrophy of type II fibers was not associated with myonuclear accrual after aerobic training in young and middle-aged adults. Our current data confirm this fiber type-specific response to training in older adults. In the present study, SC density increased nonsignificantly by ∼40% in both fiber types, but myonuclear content was only increased in type I fibers. The degree of hypertrophy in type II fibers was also far greater than type I (23% vs. 8%, respectively), highlighting the discordant relationship between hypertrophy and myonuclear accrual. Myonuclei have been shown to be crucial in supporting ribosome activity during muscle hypertrophy: in a recent study published by Stec et al. (55), myonuclear addition correlated with muscle hypertrophy and rRNA accumulation in older adults. Similarly, in a rodent model of overload-induced hypertrophy, increased myonuclear transcription rates accompanied greater RNA synthesis during hypertrophy (28). Given these data, our findings would suggest that type II fiber myonuclei are capable of supporting increased transcriptional activity to sustain RET-induced hypertrophy. Support for this idea was observed during mechanical overload of the plantaris (enriched for type II fiber content), where hypertrophy in the absence of myonuclear fusion resulted in existing myonuclei upregulating transcriptional activity to support muscle growth (28). Type II fibers may have a greater ‘ceiling’ for their MND than type I fibers, allowing for hypertrophy in the absence of SC fusion/myonuclear accrual. The MND is commonly presented as the sarcoplasmic volume under transcriptional control by a single myonucleus (6). Current research indicates that the MND can be flexible until existing fibers undergo hypertrophy of ~25%, at which point SC-mediated myonuclear addition occurs (55). Growth beyond this point would then necessitate SC activation and fusion to continue supporting further hypertrophy. In our study, type II fiber CSA increased by 23% without a concomitant increase in myonuclear content, approaching the ‘ceiling theory’ and providing support for a flexible MND within type II fibers (46, 47, 59). Intriguingly, type I fibers showed a relatively limited hypertrophic response associated with a surprisingly robust addition of myonuclei. These data provide further support for a fiber type-specific response of SC to training-induced hypertrophy (12). Additional evidence is seen in isolated single fibers, which show increased volume following RET with no increase in myonuclear content, leading to an elevated MND after training. Although our single fiber data do not provide information on fiber type, it can be assumed that the majority of isolated fibers expressed type II MyHC, as our single fiber data mirror the adaptation observed in type II fiber cross-sectional analyses.
Recent studies have also underscored the importance of capillaries on SC proliferation and muscle hypertrophy (42). After acute exercise, SCs become activated in part due to local cytokines present in the interstitial muscle space (5, 54). Cytokines and other anabolic hormones are delivered to fibers and SCs through capillaries and microvascular flow. Recent work in aging muscle has shown that type II fiber SCs are located at a greater distance from the nearest capillary compared with younger adults (42). Similar studies have also shown that when activated in young adults, the distance between SCs and the nearest capillary decreases (42, 43); suggesting that the distance between SCs and the capillary likely plays an important role in governing the muscles’ response to exercise. Snijders and colleagues (53) report that in older adults the distance between type II fiber-associated SC and their nearest capillary was greater than that associated with type I fibers, and this relationship was maintained after 24 wk of training with no significant alteration over time. Consistently with Nederveen and others (42), in our subjects the pre-RET distance between type I fiber SCs and their nearest capillary was smaller than type II fiber-associated SC; however, our results show that the gap between type I and II SC was reduced by 12 wk of RET. Moreover, Leenders et al. (33) [using the same subjects as those reported by Snijders et al. (53)] observed an increase in myonuclear content in type II fibers but not type I fibers with training. On the contrary, we observed that the distance between type I fiber SCs and capillaries exhibited a nonsignificant increase concomitantly with an increase in SC content and significant myonuclear addition, which would challenge the necessity of this relationship to facilitate myonuclear accretion (53). On the other hand, type II fiber SC-capillary distance decreased after RET, which may have supported type II fiber remodeling and hypertrophy apart from the traditional addition of a new myonucleus. Indeed, this phenomena has been reported before, where the contribution of satellite cells is thought to perhaps aid in fiber type transition during high-intensity interval training (20). The discrepancy between our study and the others may have several explanations. First, the intensity of exercise used in our study was lower compared with the one used by Snijders et al. (53) (from 60% to 75% 1 RM vs. from 60% to 80% 1 RM, respectively). Higher-intensity exercise may have caused greater muscle damage, promoting an increase in SC activation and proliferation (50). Additionally, although not significant, we observed an increase in type II fiber capillarization (~30%) whereas Snijders et al. (53) reported no change in capillary density. The emergence of new capillaries may have resulted in a reduced distance between them and type II fiber SCs, which may help explain differences between our two studies. Moreover, as recently confirmed by Kargl et al. (25), the cross-talk between endothelial cells and satellite cells seems to be crucial for the mitogenesis of both cells. It may be possible that in older adults, capillary expansion has to occur first to attract SCs and stimulate their activity. Another consideration is the influence of habitual physical activity on myonuclear adaptation to training. Our subjects were less active (as assessed by daily step count) compared with those in the study by Leenders et al. (33); thus, the initial aerobic demands after training may be greater than those in more active subjects. This may have resulted in a nonhypertrophic remodeling of type I fibers, with greater fusion of SC and increased myonuclear content, which may support additional nonmyofibrillar transcriptional demands (19). Conversely, in more active older adults, this early metabolic adaptation may not be required, and resistance training may directly increase SC activation and myonuclear accrual preferentially in type II fibers (33, 53).
Additionally, recent work has proposed that greater intramuscular accumulation of lipid with aging negatively affects the contractile capacity of skeletal muscle (48) and may predict mobility limitations in older adults (62). Computed tomography studies have clearly shown an association between fat infiltration and attenuated muscle strength and quality (13, 62, 63). Because of differences in oxidative capacity, lipid accumulation is not homogenous across different muscles (56) or fiber types (7, 14), with type I fibers having greater IMCL density. With aging-associated type II fiber atrophy and greater fat infiltration within muscle, it has been proposed that aging-related lipid accumulation may contribute to the transition of type II fibers to type I (36). In our study, type I fibers had greater IMCL density in the subsarcolemmal region compared with type II fibers (~20%). However, the lipid density decreased equally for both fiber types following 12 wk of RET, delineating a non-fiber type-specific response to training. These results confirm the positive effect of exercise to restore muscle quality, and the reduced IMCL density may enhance contractile capacity of both type I and type II fibers in older adults following RET.
In conclusion, after 12 wk of RET, older adults exhibited type II fiber hypertrophy that was not associated with myonuclear accretion. Conversely, progressive resistance training induced a significant increase in myonuclear content in the absence of a robust hypertrophic response in type I fibers. Our findings support plasticity of the MND in aging muscle as well as fiber type-specificity in the hypertrophic response to training.
GRANTS
This work was supported by the National Institute on Aging at the NIH (Grant Nos. R56 AG051267, P30 AG024832, and T32 AG000270) and the National Center for Advancing Translational Sciences at the NIH (Grant No. UL1 TR001439).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.M., B.B.R., and C.S.F. conceived and designed research; T.M., C.R.B., and C.S.F. performed experiments; T.M. analyzed data; T.M. and C.S.F. interpreted results of experiments; T.M. prepared figures; T.M. drafted manuscript; T.M., C.R.B., E.V., B.B.R., and C.S.F. edited and revised manuscript; T.M., C.R.B., E.V., B.B.R., and C.S.F. approved final version of manuscript.
REFERENCES
- 1.Andersen JL, Klitgaard H, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of sprinters: influence of training. Acta Physiol Scand 151: 135–142, 1994. doi: 10.1111/j.1748-1716.1994.tb09730.x. [DOI] [PubMed] [Google Scholar]
- 2.Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol (1985) 120: 965–975, 2016. doi: 10.1152/japplphysiol.00799.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamman MM, Hill VJ, Adams GR, Haddad F, Wetzstein CJ, Gower BA, Ahmed A, Hunter GR. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci 58: B108–B116, 2003. doi: 10.1093/gerona/58.2.B108. [DOI] [PubMed] [Google Scholar]
- 4.Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495: 573–586, 1996. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantini M, Massimino ML, Rapizzi E, Rossini K, Catani C, Dalla Libera L, Carraro U. Human satellite cell proliferation in vitro is regulated by autocrine secretion of IL-6 stimulated by a soluble factor(s) released by activated monocytes. Biochem Biophys Res Commun 216: 49–53, 1995. doi: 10.1006/bbrc.1995.2590. [DOI] [PubMed] [Google Scholar]
- 6.Cheek DB. The control of cell mass and replication. The DNA unit—a personal 20-year study. Early Hum Dev 12: 211–239, 1985. doi: 10.1016/0378-3782(85)90144-6. [DOI] [PubMed] [Google Scholar]
- 7.Choi SJ, Files DC, Zhang T, Wang ZM, Messi ML, Gregory H, Stone J, Lyles MF, Dhar S, Marsh AP, Nicklas BJ, Delbono O. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci 71: 557–564, 2016. doi: 10.1093/gerona/glv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christov C, Chrétien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18: 1397–1409, 2007. doi: 10.1091/mbc.e06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupont-Versteegden EE, Murphy RJ, Houlé JD, Gurley CM, Peterson CA. Mechanisms leading to restoration of muscle size with exercise and transplantation after spinal cord injury. Am J Physiol Cell Physiol 279: C1677–C1684, 2000. doi: 10.1152/ajpcell.2000.279.6.C1677. [DOI] [PubMed] [Google Scholar]
- 11.Flück M, Hoppeler H. Molecular basis of skeletal muscle plasticity—from gene to form and function. Rev Physiol Biochem Pharmacol 146: 159–216, 2003. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- 12.Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA, Peterson CA. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol 592: 2625–2635, 2014. doi: 10.1113/jphysiol.2014.271288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 90: 2157–2165, 2001. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 14.Gueugneau M, Coudy-Gandilhon C, Théron L, Meunier B, Barboiron C, Combaret L, Taillandier D, Polge C, Attaix D, Picard B, Verney J, Roche F, Féasson L, Barthélémy JC, Béchet D. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J Gerontol A Biol Sci Med Sci 70: 566–576, 2015. doi: 10.1093/gerona/glu086. [DOI] [PubMed] [Google Scholar]
- 15.Hagerman FC, Walsh SJ, Staron RS, Hikida RS, Gilders RM, Murray TF, Toma K, Ragg KE. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci 55: B336–B346, 2000. doi: 10.1093/gerona/55.7.B336. [DOI] [PubMed] [Google Scholar]
- 16.Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol (Lausanne) 7: 69, 2016. doi: 10.3389/fendo.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on V̇o2peak and the capillary supply to skeletal muscle. J Appl Physiol (1985) 82: 1305–1310, 1997. doi: 10.1152/jappl.1997.82.4.1305. [DOI] [PubMed] [Google Scholar]
- 18.Holthöfer H, Virtanen I, Kariniemi AL, Hormia M, Linder E, Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest 47: 60–66, 1982. [PubMed] [Google Scholar]
- 19.Joanisse S, Gillen JB, Bellamy LM, McKay BR, Tarnopolsky MA, Gibala MJ, Parise G. Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J 27: 4596–4605, 2013. doi: 10.1096/fj.13-229799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joanisse S, McKay BR, Nederveen JP, Scribbans TD, Gurd BJ, Gillen JB, Gibala MJ, Tarnopolsky M, Parise G. Satellite cell activity, without expansion, after nonhypertrophic stimuli. Am J Physiol Regul Integr Comp Physiol 309: R1101–R1111, 2015. doi: 10.1152/ajpregu.00249.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joumaa V, Power GA, Hisey B, Caicedo A, Stutz J, Herzog W. Effects of fiber type on force depression after active shortening in skeletal muscle. J Biomech 48: 1687–1692, 2015. doi: 10.1016/j.jbiomech.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, Olsen S, Kjaer M. The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch 451: 319–327, 2005. doi: 10.1007/s00424-005-1406-6. [DOI] [PubMed] [Google Scholar]
- 23.Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558: 1005–1012, 2004. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol 113: 99–103, 2000. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- 25.Kargl CK, Nie Y, Evans S, Stout J, Shannahan JH, Kuang S, Gavin TP. Factors secreted from high glucose treated endothelial cells impair expansion and differentiation of human skeletal muscle satellite cells. J Physiol 597: 5109–5124, 2019. doi: 10.1113/JP278165. [DOI] [PubMed] [Google Scholar]
- 26.Kelly NA, Hammond KG, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Effects of aging and Parkinson’s disease on motor unit remodeling: influence of resistance exercise training. J Appl Physiol (1985) 124: 888–898, 2018. doi: 10.1152/japplphysiol.00563.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve 57: E52–E59, 2018. doi: 10.1002/mus.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirby TJ, Patel RM, McClintock TS, Dupont-Versteegden EE, Peterson CA, McCarthy JJ. Myonuclear transcription is responsive to mechanical load and DNA content but uncoupled from cell size during hypertrophy. Mol Biol Cell 27: 788–798, 2016. doi: 10.1091/mbc.E15-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 140: 55–62, 1990. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- 30.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101: 531–544, 2006. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 31.Kramer IF, Snijders T, Smeets JSJ, Leenders M, van Kranenburg J, den Hoed M, Verdijk LB, Poeze M, van Loon LJC. Extensive type II muscle fiber atrophy in elderly female hip fracture patients. J Gerontol A Biol Sci Med Sci 72: 1369–1375, 2017. doi: 10.1093/gerona/glw253. [DOI] [PubMed] [Google Scholar]
- 32.Lee JD, Fry CS, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Aged muscle demonstrates fiber-type adaptations in response to mechanical overload, in the absence of myofiber hypertrophy, independent of satellite cell abundance. J Gerontol A Biol Sci Med Sci 71: 461–467, 2016. doi: 10.1093/gerona/glv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci 68: 769–779, 2013. doi: 10.1093/gerona/gls241. [DOI] [PubMed] [Google Scholar]
- 34.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. doi: 10.1007/BF00293457. [DOI] [PubMed] [Google Scholar]
- 35.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 36.Mastrocola R, Collino M, Nigro D, Chiazza F, D’Antona G, Aragno M, Minetto MA. Accumulation of advanced glycation end-products and activation of the SCAP/SREBP Lipogenetic pathway occur in diet-induced obese mouse skeletal muscle. PLoS One 10: e0119587, 2015. doi: 10.1371/journal.pone.0119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messi ML, Li T, Wang ZM, Marsh AP, Nicklas B, Delbono O. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J Gerontol A Biol Sci Med Sci 71: 1273–1280, 2016. doi: 10.1093/gerona/glv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore DR, Kelly RP, Devries MC, Churchward-Venne TA, Phillips SM, Parise G, Johnston AP. Low-load resistance exercise during inactivity is associated with greater fibre area and satellite cell expression in older skeletal muscle. J Cachexia Sarcopenia Muscle 9: 747–754, 2018. doi: 10.1002/jcsm.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moro T, Brightwell CR, Deer RR, Graber TG, Galvan E, Fry CS, Volpi E, Rasmussen BB. Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J Nutr 148: 900–909, 2018. doi: 10.1093/jn/nxy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moro T, Brightwell CR, Phalen DE, McKenna CF, Lane SJ, Porter C, Volpi E, Rasmussen BB, Fry CS. Low skeletal muscle capillarization limits muscle adaptation to resistance exercise training in older adults. Exp Gerontol 127: 110723, 2019. doi: 10.1016/j.exger.2019.110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moro T, Ebert SM, Adams CM, Rasmussen BB. Amino acid sensing in skeletal muscle. Trends Endocrinol Metab 27: 796–806, 2016. doi: 10.1016/j.tem.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM, Parise G. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 7: 547–554, 2016. doi: 10.1002/jcsm.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nederveen JP, Snijders T, Joanisse S, Wavell CG, Mitchell CJ, Johnston LM, Baker SK, Phillips SM, Parise G. Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am J Physiol Regul Integr Comp Physiol 312: R85–R92, 2017. doi: 10.1152/ajpregu.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connor RS, Pavlath GK. Point:Counterpoint: satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol (1985) 103: 1099–1100, 2007. doi: 10.1152/japplphysiol.00101.2007. [DOI] [PubMed] [Google Scholar]
- 45.Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol 573: 525–534, 2006. doi: 10.1113/jphysiol.2006.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291: E937–E946, 2006. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 47.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985) 104: 1736–1742, 2008. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 48.Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, Frontera WR, Fielding RA. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol 114: 29–39, 2014. doi: 10.1007/s00421-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reidy PT, Fry CS, Igbinigie S, Deer RR, Jennings K, Cope MB, Mukherjea R, Volpi E, Rasmussen BB. Protein supplementation does not affect myogenic adaptations to resistance training. Med Sci Sports Exerc 49: 1197–1208, 2017. doi: 10.1249/MSS.0000000000001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol A Biol Sci Med Sci 56: B240–B247, 2001. doi: 10.1093/gerona/56.6.B240. [DOI] [PubMed] [Google Scholar]
- 51.Schiaffino S, Bormioli SP, Aloisi M. The fate of newly formed satellite cells during compensatory muscle hypertrophy. Virchows Arch B Cell Pathol 21: 113–118, 1976. [DOI] [PubMed] [Google Scholar]
- 52.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 53.Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJ, Parise G. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle 8: 267–276, 2017. doi: 10.1002/jcsm.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJ, Parise G. Satellite cells in human skeletal muscle plasticity. Front Physiol 6: 283, 2015. doi: 10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310: E652–E661, 2016. doi: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res 2012: 172957, 2012. doi: 10.1155/2012/172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van der Meer SF, Jaspers RT, Degens H. Is the myonuclear domain size fixed? J Musculoskelet Neuronal Interact 11: 286–297, 2011. [PubMed] [Google Scholar]
- 58.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve 25: 17–25, 2002. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 59.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 64: 332–339, 2009. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292: E151–E157, 2007. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- 61.Verdijk LB, Snijders T, Holloway TM, van Kranenburg J, van Loon LJ. Resistance training increases skeletal muscle capillarization in healthy older men. Med Sci Sports Exerc 48: 2157–2164, 2016. doi: 10.1249/MSS.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 62.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60: 324–333, 2005. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 63.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 50: 897–904, 2002. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 64.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67, 2013. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]