Abstract
Older adults are at increased risk of being bedridden and experiencing negative health outcomes including the loss of muscle tissue and functional capacity. We hypothesized that supplementing daily meals with a small quantity (3–4 g/meal) of leucine would partially preserve lean leg mass and function of older adults during bed rest. During a 7-day bed rest protocol, followed by 5 days of inpatient rehabilitation, healthy older men and women (67.8 ± 1.1 yr, 14 men; 6 women) were randomized to receive isoenergetic meals supplemented with leucine (LEU, 0.06 g/kg/meal; n = 10) or an alanine control (CON, 0.06 g/kg/meal; n = 10). Outcomes were assessed at baseline, following bed rest, and after rehabilitation. Body composition was measured by dual-energy X-ray absorptiometry. Functional capacity was assessed by knee extensor isokinetic and isometric dynamometry, peak aerobic capacity, and the short physical performance battery. Muscle fiber type, cross-sectional area, signaling protein expression levels, and single fiber characteristics were determined from biopsies of the vastus lateralis. Leucine supplementation reduced the loss of leg lean mass during bed rest (LEU vs. CON: −423 vs. −1035 ± 143 g; P = 0.008) but had limited impact on strength or endurance-based functional outcomes. Similarly, leucine had no effect on markers of anabolic signaling and protein degradation during bed rest or rehabilitation. In conclusion, providing older adults with supplemental leucine has minimal impact on total energy or protein consumption and has the potential to partially counter some, but not all, of the negative effects of inactivity on muscle health.
NEW & NOTEWORTHY Skeletal muscle morphology and function in older adults was significantly compromised by 7 days of disuse. Leucine supplementation partially countered the loss of lean leg mass but did not preserve muscle function or positively impact changes at the muscle fiber level associated with bed rest or rehabilitation. Of note, our data support a relationship between myonuclear content and adaptations to muscle atrophy at the whole limb and single fiber level.
Keywords: aging, bed rest, dietary supplementation, nutrition
INTRODUCTION
An accelerated loss of muscle mass and functional capacity are common features of skeletal muscle disuse (2, 14). In recent bed rest studies, we noted an approximate threefold greater loss of lean leg mass in middle-aged adults (~50 yr), compared with a separate earlier intervention focused on younger adults (15, 40). From a clinical perspective, older adults are more likely than younger adults to be physically incapacitated, hospitalized, or placed on bed rest for extended periods of time where they are at risk of experiencing a host of negative musculoskeletal and metabolic outcomes (16, 46).
Protein-energy supplementation is commonly prescribed to counter the loss of muscle mass and function in hospitalized or bed-ridden older adults (47, 51). Unfortunately, many dietary interventions fail because of obstacles including: prohibitive cost, poor palatability, satiety, dysphagia, or even a concomitant reduction in habitual food intake to accommodate the additional prescribed food/supplement (17, 38).
Leucine supplementation has the potential to simplify and positively impact current dietetic practice in some patient populations. Mechanistically, leucine has well-documented effects on translation initiation, activation of protein synthesis, and inhibition of protein breakdown (35). While the acute anabolic effects of supplementation do not always translate to overtly positive, chronic phenotypic changes during longer duration diet/exercise interventions (48), recent data from a cohort of middle-aged adults subjected to the catabolic stress of a 14-day bed rest protocol suggest that a small amount of supplemental leucine may partially protect several key markers of muscle health during relatively short periods of increased catabolism (15).
Bed rest studies in healthy volunteers are reductionist in design and provide an opportunity to isolate systemic and regional effects of muscular disuse and more fully characterize targeted interventions. The goal of this study was to determine if a relatively low quantity/volume leucine supplement could counter the catabolic influence of bed rest, protect indexes of muscle health, and facilitate recovery in a cohort of healthy older adults.
METHODS
Subjects.
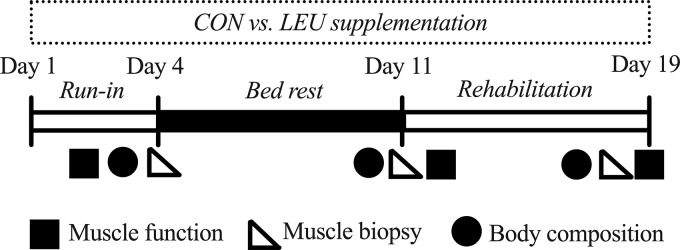
Twenty healthy, older adults aged 60–80 yr were recruited from the greater Galveston area, provided written informed consent, and were medically screened for this randomized, double-blind, placebo controlled clinical trial. Volunteers were randomized to a control cohort (CON; 7 men, 3 women) or a cohort receiving supplemental leucine (LEU; 7 men, 3 women). Volunteer demographic information is presented in Table 1. The inpatient phase consisted of a 4 day run-in period that included diet stabilization and pretesting of study variables. This was immediately followed by 7 days of horizontal bed rest and 5 days of inpatient rehabilitation. All study events and procedures took place in the University of Texas Medical Branch (UTMB) Institute for Translational Sciences, Clinical Research Center. The overall experimental design is depicted in Fig. 1. The study was conducted in accordance with the Declaration of Helsinki and approved by the UTMB Institutional Review Board. The CONSORT diagram is available in Supplemental Fig. S1 (https://doi.org/10.35092/yhjc.11320070.v1). This study was registered at clinicaltrials.gov (NCT01846130).
Table 1.
Baseline characteristics of the subjects randomized to the control and leucine-supplemented groups
| Age, yr | Sex, men/women | Height, cm | Body Mass, kg | BMI, kg/m2 | |
|---|---|---|---|---|---|
| CON | 68 ± 2 | 7/3 | 167 ± 0.3 | 73.6 ± 2.7 | 25.2 ± 0.7 |
| LEU | 68 ± 1 | 7/3 | 170 ± 0.4 | 82.1 ± 4.8 | 28.0 ± 1.0* |
Values are means ± SE. BMI, body mass index; CON, control; LEU, leucine supplement.
Significant difference from CON, P < 0.05.
Fig. 1.
Study timeline. Subjects admitted to the study underwent a 3-day inpatient run-in period to complete baseline testing of study outcomes and consumed a controlled diet. During the 7-day bed rest phase and 5-day inpatient rehabilitation phase, the subjects were supplemented with alanine (control; n = 10) or leucine (n = 10). Study outcomes were reassessed after bed rest and rehabilitation. CON, control; LEU, leucine.
Diet and supplementation.
The Harris-Benedict equation was used to calculate daily energy requirements with activity factors of 1.6 and 1.3 used for the ambulatory and bed-rest period, respectively (15, 41). Throughout the 19-day protocol (run-in, bed rest, and rehabilitation), subjects consumed individually prepared, isoenergetic diets (55% carbohydrate, 29% fat, and 16% protein) with protein and energy intake evenly distributed across three daily meals (0800, 1300, and 1800) (Table 2). Each meal was consumed within 20 min of presentation. Meals provided to the LEU group were supplemented with leucine (0.06 g/kg/meal), (Ajinomoto, Raleigh, NC), which resulted in an intake of 4.9 ± 0.3 g leucine/meal or 14.6 ± 0.8 g leucine/day. The CON group received the nonessential amino acid (NEAA) alanine (Ajinomoto, Raleigh, NC) as an isonitogenous, isoenergetic control (alanine: 0.06 g/kg body weight/meal; 4.4 ± 0.2 g/meal; 13.2 ± 0.5 g/day). Macronutrient intake and plate waste were analyzed by using Nutrition Data System for Research software (version 2013 NCC). Water was provided ad libitum.
Table 2.
Mean energy and macronutrient intake in healthy adults during 7 days of bed rest and 5 days of rehabilitation
| Meal | Group | Energy, kcal | Protein, g | Protein, g/kg/day | Carbohydrate, g | Fat, g |
|---|---|---|---|---|---|---|
| Bed rest | ||||||
| Breakfast | CON | 578 ± 10.0 | 23 ± 0.38 | 0.32 ± 0.004 | 78 ± 1.7 | 20 ± 0.5 |
| LEU | 623 ± 13.8* | 25 ± 0.56* | 0.31 ± 0.003* | 85 ± 2.0* | 21 ± 0.6* | |
| Lunch | CON | 565 ± 12.0 | 23 ± 0.44 | 0.32 ± 0.004 | 78 ± 1.3 | 19 ± 0.8 |
| LEU | 625 ± 13.2* | 25 ± 0.53* | 0.31 ± 0.003 | 86 ± 1.7* | 22 ± 0.7* | |
| Dinner | CON | 577 ± 12.2 | 24 ± 0.45 | 0.32 ± 0.005 | 83 ± 2.4 | 18 ± 0.5 |
| LEU | 630 ± 13.1* | 25 ± 0.58* | 0.31 ± 0.005 | 90 ± 2.3 | 20 ± 0.5* | |
| Daily total | CON | 1722 ± 28.9 | 71 ± 1.2 | 0.97 ± 0.010 | 241 ± 4.1 | 56 ± 1.0 |
| LEU | 1883 ± 40.0* | 76 ± 1.8* | 0.94 ± 0.012 | 261 ± 5.6* | 63 ± 1.0* | |
| Rehabilitation | ||||||
| Breakfast | CON | 716 ± 17.1 | 29 ± 0.54 | 0.39 ± 0.004 | 99 ± 2.3 | 24 ± 0.9 |
| LEU | 774 ± 19.5 | 30 ± 0.82 | 0.37 ± 0.006* | 108 ± 2.7* | 26 ± 1.0 | |
| Lunch | CON | 688 ± 12.6 | 29 ± 0.50 | 0.39 ± 0.004 | 96 ± 1.4 | 23 ± 0.8 |
| LEU | 750 ± 18.0* | 30 ± 0.88 | 0.37 ± 0.006* | 105 ± 2.4* | 25 ± 0.8* | |
| Dinner | CON | 705 ± 12.5 | 29 ± 0.50 | 0.40 ± 0.005 | 101 ± 2.3 | 22 ± 0.5 |
| LEU | 743 ± 18.3 | 30 ± 0.89 | 0.36 ± 0.008* | 107 ± 2.7 | 23 ± 0.8 | |
| Daily total | CON | 2112 ± 37.1 | 86 ± 1.6 | 1.17 ± 0.012 | 296 ± 5.2 | 70 ± 1.4 |
| LEU | 2256 ± 53.8* | 90 ± 2.6 | 1.10 ± 0.017* | 319 ± 7.3* | 74 ± 2.1 | |
Values are means ± SE. The calculated values for protein (g) and protein (g/day) include the alanine (CON) and leucine (LEU) supplementation at each meal. The averages do not reflect the reduced caloric intake on the metabolic study days.
Significant difference from CON, P < 0.05.
Bed rest and rehabilitation.
Subject monitoring, safety, and comfort provisions were rigorous and consistent with our previous bed-rest studies (4, 15, 41). All bathing and toiletry activities were performed without weight bearing. Following bed rest, subjects completed daily 45 min sessions of structured rehabilitation that focused on stretching with some balance and strength-focused exercises. A complete description of the rehabilitation has been previously reported by Arentson-Lantz et al. (4).
Body composition.
Dual-energy X-ray absorptiometry (DEXA; Lunar iDXA; GE Medical Systems, Madison, WI) was used to assess whole body lean mass (WBLM), leg lean mass (LLM), and whole body fat mass (WBFM) on study days 3, 10, and 17 (Fig. 1). Subjects remained in a supine position for 10 min before each scan to standardize and minimize potential variation due to fluid shifts.
Muscle function.
Isokinetic dynamometry (Biodex System 4; Biodex Medical Systems, Shirley, NY) was used to measure unilateral knee extensor peak torque. Familiarization sessions were conducted before baseline testing. Peak isometric and isokinetic (60°/s) torque was assessed via three maximal repetitions at 60°/s.
Short physical performance battery.
All subjects completed the standardized short physical performance battery (SPPB) on days 2, 12, and 19 to provide a clinically focused assessment of functional capacity of the lower limbs. The standardized cut-offs of “0” (unable to complete task) and “4” (highest level of function) (19) were used to grade: 1) an 8-foot walk test, 2) standing five times from a seated position in a chair, and 3) standing balance. The score of each test was summed to calculate a composite SPPB score.
Peak aerobic capacity.
Peak oxygen uptake was assessed by a graded exercise test on a cycle ergometer (Monark Ergomedic 828E; Monark Exercise, Vansbro, Sweden) and metabolic cart (VMax Encore 29; CareFusion, Yorba Linda, CA). To provide perspective on changes in body composition during bed rest, data were expressed in absolute (L/min) and relative terms (mL·kg body mass−1·min−1 and mL·kg lean mass−1·min−1).
Muscle biopsies and protein extraction.
On the morning of days 4, 11, and 18, a muscle biopsy sample was obtained from the vastus lateralis muscle of fasted/rested subjects with a 5 mm Bergstrom biopsy needle and standard technique (6). We used 20 mg of tissue from each sample for protein analysis. Total protein was extracted with radioimmunoprecipitation assay (RIPA) buffer (Millipore, North Ryde, NSW, Australia) with 10 μL/mL Phosphatase and Protease Inhibitor Cocktail (Thermo Fisher Scientific, Waltham, MA). Total protein content was determined with the BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s instructions.
Western blotting.
Proteins were separated with a 4–15% gradient Criterion tris-glycine extended (TGX) Stain Free gel (Bio-Rad, Hercules, CA) and transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked in 5% skim milk in TBST for 1 h at room temperature, after which they were incubated overnight at 4°C with a primary antibody against phospho-mTORSer2448, phospho-AKTSer473, phospho-4E-BP1Thr37∕46, phospho-p44/42MAPKThr202∕Tyr204, phospho-GSK-3βSer9, MurF1, FoxO1, and phospho-FoxO1Ser256 diluted in 5% bovine serum albumin (BSA) in Tris-buffered saline, 0.1% Tween 20 (TBST). The antibodies and conditions used have been previously reported (24, 45). Phospho-mTORSer2448, phospho-AKTSer473, phospho-4E-BP1Thr37∕46, phospho-p44/42MAPKThr202∕Tyr204, phospho-GSK-3βSer9, FoxO1, and phospho-FoxO1Ser256 antibodies were obtained from Cell Signaling Technology (Danvers, MA). These monoclonal antibodies constitute the gold standard in the field and have been validated against their recommended positive controls. MurF1 antibody was obtained from ECM Biosciences (MP3401; Versailles, KY) and was validated against the appropriate gain-of function and loss-of-function mutants [full validation data available at (44)]. Following overnight incubation, the membranes were washed in TBST and incubated for 1 h at room temperature with an anti-rabbit IgG antibody labeled with an infrared fluorescent 800 nm dye (Alexa Fluor 800; Thermo Fisher Scientific) diluted 1:5,000 in 5% BSA in TBST. After being washed, the proteins were exposed on an Odyssey CLx Infrared Imaging System, and individual protein band optical densities were determined by using the Odyssey Infrared Imaging System software. All blots were normalized against total protein load with the Bio-Rad Image Laboratory software (v6.0).
Immunohistochemistry.
By standard technique, a portion of muscle collected on days 4, 11, and 18 was oriented and mounted on foil-covered cork with Tissue Tek (Optimal Cutting Temperature; OCT Compound, Sakura Finetek, Torrance, CA) frozen in liquid-nitrogen cooled methylbutane and stored at −80 C until analysis. A small number of samples experienced freeze damage during the preservation process and were not included in the analysis (see results).
Muscle tissue used for cross-sectional area (CSA) analysis was cut into 7 um sections with a cryostat (HM525-NX, Thermo Fisher Scientific, Waltham, MA) and stained for fiber type as previously described (1). In brief, sections were incubated overnight in the following primary antibodies: BA.D5 (myosin heavy chain; MHC Type 1), SC.71 (MHC Type 2a), and 6H1 (Type 2x) all from Developmental Studies Hybridoma Bank (Iowa City, IA). The following day, species and isotype-specific fluorescent secondary antibodies were added, and the sections fixed in methanol and mounted with Vectashield fluorescence mounting medium (Vector Laboratories; Burlingame, CA). The number of fibers analyzed at each time point for CSA was 315 ± 53 fibers PreBR, 222 ± 44 fibers PostBR, and 332 ± 45 fibers PostRehab.
To assess single fiber width, volume, and myonuclear density, 15–20 mg of muscle from each biopsy was fixed in 4% paraformaldehyde for 48 h, then stored in phosphate-buffered saline (PBS) at room temperature until isolation. Isolation of the single fibers was completed by using a 40% NaOH digestion for 2 h followed by mechanical separation and washes in PBS as previously described (18, 22). To visualize myonuclei, suspended fibers were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen; Waltham, MA) and mounted on a slide with Vectashield fluorescence mounting medium (Vector Laboratories, Burlingame, CA). Single fiber analysis was only performed a subset of subjects (CON: n = 6; LEU: n = 7).
Immunohistochemical images of CSA and single fiber analysis were captured at ×100 and ×200 total magnification, respectively, at room temperature with a Zeiss upright microscope (AxioImager M1; Zeiss, Oberkochen, Germany). Analysis was performed with AxioVision Rel software (v4.9). Eight to ten fibers from each subject at each time point were analyzed for fiber width, volume, and myonuclear density. To determine volume, myofibers were analyzed with z-stack analysis by calculating fiber width (radius)2 × length of the measured fiber segment to give a fiber segment volume (μm)3 (22). Z-stack analysis was used to measure myonuclei per fiber.
Statistical analysis.
Analyses were performed with SPSS v24 software (IBM, Chicago, IL). Two-factor mixed analysis of variance (ANOVA) were used to analyze dependent variables with fixed effects of time (baseline/postbed rest/postrehab) as the within subject factor and supplement (CON/LEU) as the between subject factor. If the interaction of diet by time was significant (P < 0.05), individual post hoc tests with a Bonferroni adjustment were performed. Residual normality was tested by the Shapiro-Wilk test (P < 0.05) and Levene’s test of equality of error variance was used to check for equal variance. Data points greater than two standard deviations from the average were excluded from the analysis to better meet model assumptions. All data were expressed as means ± SE; significance was set at P < 0.05.
RESULTS
Protein intake relative to body weight was similar in LEU and CON groups (Table 2). The slightly taller and heavier LEU cohort consumed a greater total/absolute quantity of all macronutrients during bed rest. During rehabilitation, there were no differences in energy intake, although CON subjects consumed proportionally more protein (g/kg/day).
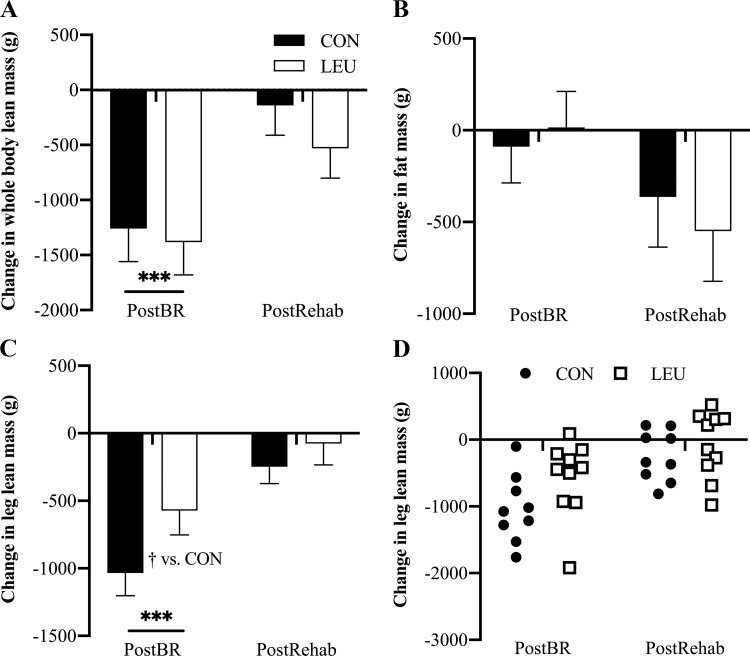
On a phenotypic level, there were no significant between-group differences in body weight change (CON vs. LEU; −0.54 vs. −0.93 ± 0.2 kg; P = 0.34) over the course of the study. Whole body and leg lean mass significantly decreased following bed rest (P < 0.001 for both) with no change in body fat (Fig. 2, A–C). Leucine supplementation partially protected leg lean mass (P = 0.01 vs. CON). Following rehabilitation, leg lean mass in both cohorts had returned to baseline (P = 0.74 vs. baseline).
Fig. 2.
Body composition. Whole body lean mass (A), fat mass (B), and leg lean mass (C and D) were measured before bed rest (PreBR), after bed rest (PostBR), and after rehabilitation (PostRehab) by dual-energy X-ray absorptiometry (DEXA). In A, B, and C, data are shown as mean change from PreBR ± SE at PostBR and PostRehab. Data in D show the response of individual subjects to 7 days of bed rest and 5 days of rehabilitation with or without leucine supplementation. Whole body lean mass decreased significantly in both control (CON, black bars; n = 10) and leucine (LEU, white bars; n = 10) subjects during bed rest and was not different from baseline following rehabilitation. Fat mass did not significantly change during the study. Leg lean mass (LLM) was a primary outcome measure. Both CON (●, n = 9) and LEU (□, n = 10) lost a significant amount of LLM compared with baseline during 7 days of disuse; however, LEU subjects loss significantly less muscle than CON subjects. Leg lean mass was not significantly different from baseline following disuse. ***P < 0.001 vs. baseline. †P < 0.01 vs. CON.
Isometric knee extension strength did not change in response to bed rest or rehabilitation in either study cohort. Despite conferring a moderate protective effect on lean leg mass, leucine supplementation was associated with a greater reduction (P = 0.05 vs. CON) in peak knee extension torque during bed rest (Table 3). This reduction in isokinetic torque, in both groups, persisted through rehabilitation. Both cohorts also experienced a reduction in absolute and relative aerobic capacity following bed rest, which was restored during rehabilitation (Table 3). In both cohorts, bed rest significantly decreased/worsened (P = 0.02) chair rise and gait speed (P = 0.02) resulting in a significant decrease in composite SPPB scores of 0.95 ± 0.34 (Table 4). Following rehabilitation, SPPB scores returned to baseline.
Table 3.
Muscle strength and aerobic capacity (absolute and relative to body mass) for CON and LEU groups measured at baseline and the change following bed rest and rehabilitation
| Knee Extensor Torque: Isometric, Nm | Knee Extensor Torque: 60°/s, Nm | V̇o2peak, L/min | Relative V̇o2peak, ml/kg/min | |
|---|---|---|---|---|
| Baseline | ||||
| CON | 150.7 ± 12.5 | 133.0 ± 10.8 | 1.8 ± 0.2 | 24.5 ± 1.9 |
| LEU | 145.5 ± 12.5 | 130.0 ± 11.8 | 1.8 ± 0.2 | 23.4 ± 1.8 |
| Δ Post-BR | ||||
| CON | 0.41 ± 8.5 | −16.3 ± 5.5* | −0.1 ± 0.1* | −1.2 ± 0.9* |
| LEU | −7.1 ± 8.5 | −24.8 ± 4.5*† | −0.3 ± 0.1* | −3.4 ± 1.1* |
| Δ Post-Rehab | ||||
| CON | −1.1 ± 7.2 | −10.0 ± 4.1* | −0.1 ± 0.1 | −1.2 ± 0.9 |
| LEU | −2.1 ± 7.2 | −22.9 ± 4.8*† | −0.1 ± 0.1 | −1.1 ± 1.0 |
Values are means ± SE. Δ Post-BR, change from baseline following bed rest; Δ Post-Rehab, change from baseline following rehabilitation.
Significant change from baseline, P < 0.05;
significant difference from CON, P < 0.05.
Table 4.
Composite and categorical scores from short performance physical battery testing performed on days 2, 12, and 19
| SPPB | Balance | Chair Rise | Gait | |
|---|---|---|---|---|
| Baseline | ||||
| CON | 11.5 ± 0.2 | 3.8 ± 0.1 | 3.7 ± 0.5 | 4.0 ± 0.0 |
| LEU | 11.7 ± 0.2 | 3.8 ± 0.1 | 3.1 ± 0.4 | 4.0 ± 0.3 |
| Post-BR | ||||
| CON | 10.5 ± 0.4* | 3.8 ± 0.1 | 3.1 ± 0.4* | 3.5 ± 0.3* |
| LEU | 10.8 ± 0.6* | 3.8 ± 0.1 | 3.2 ± 0.4* | 3.8 ± 0.2* |
| Post-Rehab | ||||
| CON | 11.4 ± 0.3 | 3.9 ± 0.1 | 3.5 ± 0.3 | 4.0 ± 0.0 |
| LEU | 11.2 ± 0.4 | 3.8 ± 0.1 | 3.4 ± 0.3 | 4.0 ± 0.0 |
Values are means ± SE. SPPB, short performance physical battery testing; Baseline, day 2; Post-BR, day 12; Post-Rehab, day 19.
Significant change from baseline, P < 0.05.
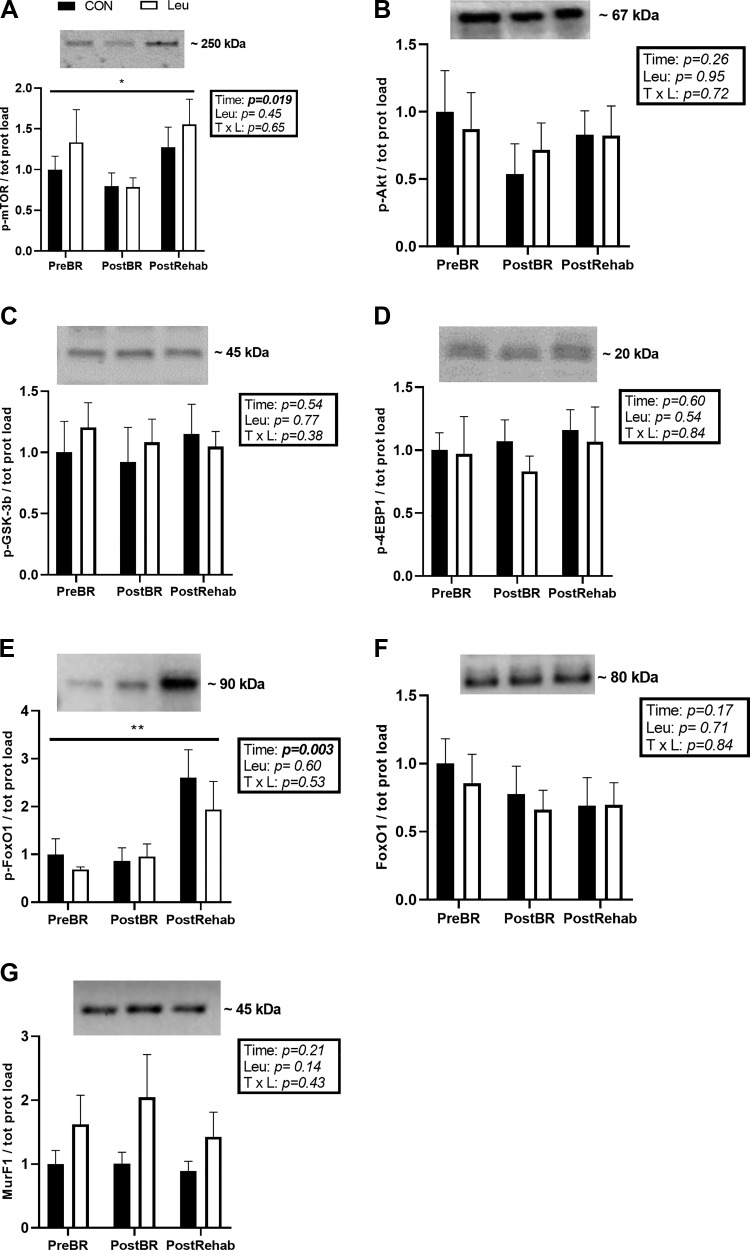
The expression of phosphorylated forms of a subset of proteins involved in anabolic signaling were measured by Western blot. There was a significant time effect on phospho-mTOR protein expression, which decreased by 0.68-fold after bed rest and recovered to be 1.21-fold higher than baseline postrehabilitation (P = 0.02) (Fig. 3A). In contrast, neither bed rest or rehabilitation changed the expression levels of the upstream proteins phospho-Akt and phospho-GSK-3β, or of the downstream effector phospho-4EBP1 (Fig. 3, B–D). Leucine supplementation was not provided on muscle biopsy days and had no residual effect on any of the proteins measured.
Fig. 3.
Representative immunoblots of phospho-mTORSer2448 (A), phospho-AKTSer473 (B), phospho-GSK-3βSer9 (C), phospho-4E-BP1Thr37∕46 (D), phospho-FoxO1Ser256 (E), total FoxO1 (F), and MuRF1 (G), and corresponding quantification. Data are shown as mean change from before bed rest (PreBR) ± SE at after bed rest (PostBR) and after rehabilitation (PostRehab) with the control (CON) group represented by the black bars (n = 10) and the leucine (LEU) group represented by the white bars (n = 10). There was a main effect of time for phospho-mTOR and phospsho-FOXO1 protein expression. *P < 0.05; **P < 0.01. All samples were derived at the same time and processed in parallel; no adjustment to digital images do alter the information contained therein. Since there was no effect of LEU for any of the proteins or phospho-proteins measured at any time point, a single representative band is displayed for each time point.
Expression of the members of the muscle protein degradation pathway FoxO1, phospho-FoxO1 and MuRF1, were measured at all time points (Fig. 3, E and F). The inactive form of the FoxO1 protein, phospho-FoxO1, did not change with bed rest but increased by 2.65-fold after rehabilitation, compared with before bed rest (P = 0.002) (Fig. 3E). There was no effect of leucine. Total FoxO1 (Fig. 3F) and MurF1 (Fig. 3G) protein expression did not change in response to bed rest or supplementation.
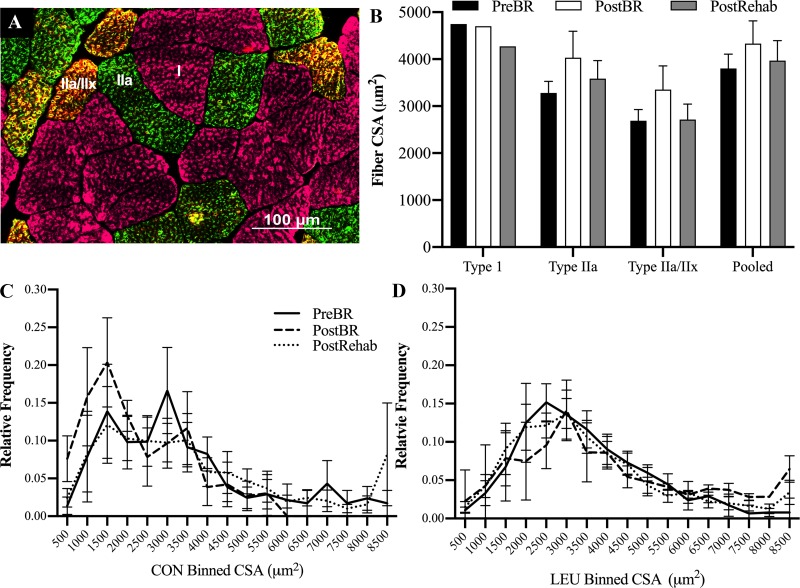
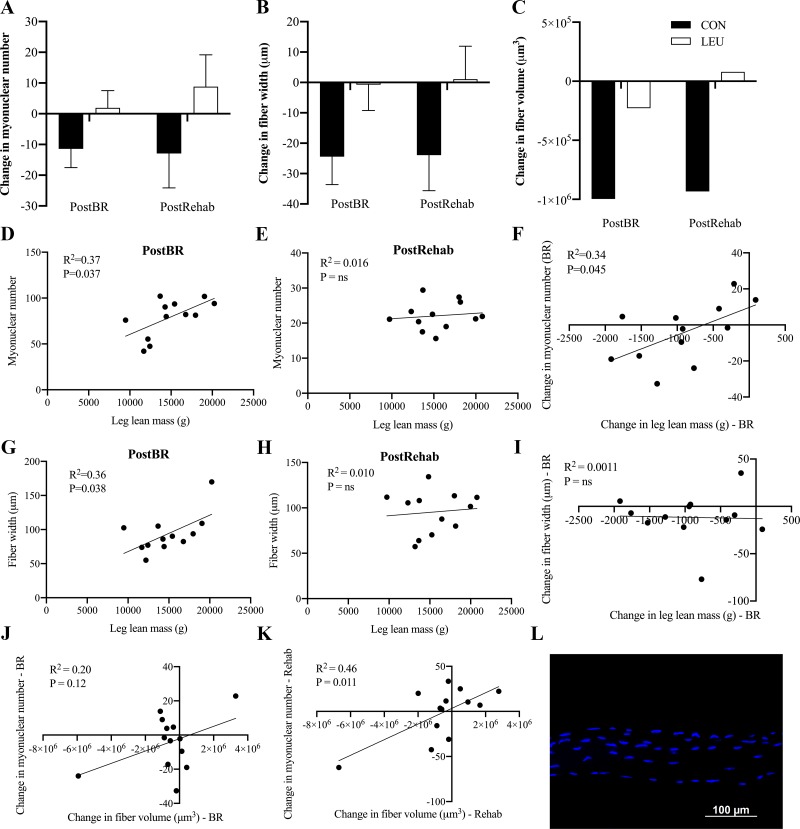
There were no significant changes in fiber type-specific or pooled CSA of myofibers following bed rest or rehabilitation in either cohort (Fig. 4). However, CON subjects exhibited a shift toward an increase the frequency of smaller fibers (< 2,000 µm2) and decrease in the frequency of larger fibers (> 6,000 µm2) following bed rest (Fig. 4C) that was not seen in the LEU subjects (Fig. 4, C and D). There was a tendency for leucine to reduce the loss of fiber width and fiber volume during bed rest (Fig. 5B, P = 0.09 and Fig. 5C, P = 0.08). However, there were no significant time, treatment, or time by treatment effects on myonuclear number, fiber width, or volume (Fig. 5, A–C). Myonuclear number was significantly associated with lean leg mass following bed rest (Fig. 5D, P = 0.037), but not postrehabilitation (Fig. 5E). The change in myonuclear number during disuse was also significantly associated with the change in lean leg mass (Fig. 5F, P = 0.045). Fiber width was significantly associated with leg lean mass following bed rest (Fig. 5G, P = 0.036) but not postrehabilitation (Fig. 5H). The change in fiber width also was not associated with the change in leg lean mass during bed rest (Fig. 5I). The change in myonuclear number approached but was not positively associated with the change in fiber volume during bed rest (Fig. 5J, P = 0.12). During rehabilitation, the change in myonuclear number was positively associated with the change in fiber volume (Fig. 5K, P = 0.011).
Fig. 4.
There were no significant changes in mean fiber type-specific or pooled cross-sectional area (CSA) of myofibers following 7 days of disuse and 7 days of rehabilitation in older adults. Due to issues during the freezing process, samples from 7 control (CON) subjects and 8 leucine (LEU) subjects were of sufficient quality for cross-sectional area analysis. A: representative immunohistochemical image of MHC type 1 (pink), type 2a (green), and type 2a/2x (orange) fiber identification. B: there were no significant differences between CON and LEU CSA, so the groups were pooled and reported as the mean fiber type-specific and pooled cross-sectional area of myofibers at pre bed rest (black bars), following 7 days of bed rest (white bars) and 5 days of rehabilitation (gray bars). The CSA of MHC type 1 fibers was not normally distributed, so was square root transformed and reported with a 95% confidence interval (2366, 7478). C, D: histogram distributions of fiber CSA for the CON and LEU group, respectively, at PreBR (solid line), PostBR (dashed line), and PostRehab (dotted line).
Fig. 5.
Myonuclear number is associated with the loss of muscle mass and single fiber volume experienced during 7 days of disuse in older adults. Muscle from a subset of subjects was analyzed for changes in single fiber volume, width, and myonuclear number [control (CON), black bars, n = 6; leucine (LEU), white bars, n = 7]. A: change in myonuclear number from baseline following 7 days of bed rest and 7 days of rehabilitation. B: change in myofiber width from baseline following 7 days of bed rest and 7 days of rehabilitation. C: change in myofiber volume from baseline following 7 days of bed rest and 7 days of rehabilitation. Myonuclear number and myofiber width are reported as means SE. Myofiber volume was not normally distributed so was log transformed and reported with back-transformed physiologically meaningful values with a 95% confidence interval (1.04 × 108, 1.40 × 108). D: association of myonuclear number and lean leg mass (LLM) following 7 days of bed rest. E: association of myonuclear number and LLM following 5 days of rehabilitation. F: association of the change in in myonuclear number and the change in LLM during 7 days of bed rest. G: association of fiber width and LLM following 7 days of bed rest. H: association of fiber width and LLM following 7 days of rehabilitation. I: association of change of fiber width and change of LLM during 7 days of bed rest. J: association of change in myonuclear number and change in fiber volume following 7 days of disuse. K: association of change in myonuclear number and change in fiber volume following 7 days of rehabilitation. L: representative image of single fiber staining. Myonuclei are stained in blue.
DISCUSSION
Successful dietary approaches to counter the catabolic effect of short-term physical inactivity must overcome obstacles related to cost, effectiveness, efficiency, and practicality. In this paper, we demonstrated that adding a small quantity of supplemental leucine (∼5 g/meal) to standardized meals containing ∼75 g of whole food-based protein (0.95 g protein/kg/day), partially preserved lean leg mass in older adults during 7 days of bed rest. However, leucine supplementation did not affect muscle fiber cross-sectional area or other common indexes of muscle strength and function. The partial protective effect of leucine on lean leg mass broadly mirrored recent data from a middle-aged cohort (50 vs. 68 yr) who received leucine during a similarly structured 14-day bed rest period (15).
Leucine supplementation is a pragmatic, mechanistically attractive strategy to reduce the loss of muscle mass and function during brief periods of physical inactivity. Specifically, a relatively small quantity of leucine (>3 g), added to a whole food, mixed nutrient diet, can efficiently trigger a robust anabolic stimulus (12, 50). In comparison, supplementation with traditional whole protein sources such as whey, soy, or collagen typically involve servings of ≥20 g (37). This quantity of leucine has minimal impact on total protein or energy consumption and may also offer practical advantages that extend to the cost and ease of incorporation into meal plans. However, in its isolated form, the palatability of leucine remains an obstacle and is likely inversely proportional to the quantity consumed (38).
Historically, dietary approaches to counter catabolism and disuse atrophy focused on enhancing overall protein intake. At the most basic level, this manifests as the blunt provision of potentially large quantities of supplemental protein or amino acids and, in many cases, increased energy intake (33, 39, 42). To this end, most clinical societies (e.g., the American Society for Parenteral and Enteral Nutrition, and the European Society for Clinical Nutrition and Metabolism) highlight the inherent benefits of consuming sufficient protein, within the context of an energy-controlled or even hypocaloric/trophic diet (30, 34). As a baseline, we provided our healthy volunteer cohort with standardized diets containing ~0.95 g protein/kg/day, evenly distributed between each of the three daily meals (Table 2). It is possible that a more traditional inpatient diet, containing less protein and energy and perhaps skewing food intake toward the evening meal, may have increased catabolic stress, resulting in a greater loss of muscle mass and function in both groups during bed rest (28). Consequently, it is important to view these data through the lens of a study that represents a “best case” model to test the efficacy of the leucine intervention.
Recently, Deutz et al. (13) demonstrated that supplementation with a small quantity of the leucine metabolite β-hydroxy-β-methylbutyrate (HMB ~3 g/day) preserved muscle mass in a cohort of older adults (60–76 yr) following 10 days of bed rest. Similarly, we demonstrated that small amounts of supplemental leucine (4.5 g/meal) not only partially protected lean mass but also preserved indexes of muscle strength and endurance in middle-aged adults (52 ± 1 yr) following 14 days of bed rest. Nonetheless, in the present study, the beneficial effect of leucine on lean mass is at odds with its failure to preserve other regulators and markers of skeletal muscle mass and muscle function. While it is possible this discrepancy reflects an inherent failure of leucine to influence these outcome measures in our study population, more mundane factors were also likely to play a role. This includes intersubject variability. Despite rigorous study screening/inclusion criteria and unavoidable “healthy subject bias,” which likely increased homogeneity, we observed considerable variation in many outcomes, particularly those requiring maximal effort from participants (e.g., strength and endurance tests). A second potential source of intersubject variability, and also an opportunity for more targeted investigations moving forward, was the inclusion of both sexes in our study cohorts (7 men, 3 women in each group). Recent data from our group suggest that the transition from middle to older age may disproportionally increase the risk of disuse atrophy in women (3, 4, 15). The loss of ovarian follicular activity and altered hormonal status [e.g., decreased estrogen, inhibin A, increased follicle-stimulating hormone (11, 20)] following menopause has been associated with increased risk of sarcopenia and functional impairment (31). However, there is limited information on potential sex-specific responses to an acute catabolic insult, such as disuse.
Although bed rest provides a useful model to examine the mechanistic impact of disuse on muscle health, its invasive nature and the need to limit enrollment to healthy volunteers cannot provide direct insight into a more compromised, clinical population. Specifically, it is notable that older adults can lose upwards of 1 kg of lean leg mass with variable and often minimal impact on traditional strength outcomes, perhaps reflecting a “reserve” in muscle function in healthy adults. In contrast, older hospitalized adults can experience an accelerated loss of both muscle mass and function driven by additional catabolic stressors (16, 18).
Even in the absence of overt stressors such as injury, inflammation, or hypercortisolemia, simple disuse exerts profound catabolic effects on skeletal muscle, culminating in atrophy that can be observed at both the whole limb (2, 14) and muscle fiber level. Concomitant decrements in myonuclear content are less well defined, but were recently observed in our middle-aged bed rest cohort (1). In the current study we detected nonsignificant decrements in single fiber width, volume, and myonuclear content in the control group that appeared to be mitigated in the leucine-supplement group. However, myonuclear content was positively correlated with changes in leg lean mass and single fiber volume following bed rest and rehabilitation. These regressions were driven by subjects exhibiting the greatest degree of atrophy (with concomitant reductions in myonuclear content), offering intriguing insight into the permanence of myonuclei during periods of significant atrophy. In rodent models of disuse atrophy, the effect of inactivity on myonuclear content remains controversial with data supporting both myonuclear loss (21, 26) and equivocal changes in myonuclear content (9, 10). Myonuclear adaptations in human studies of disuse are less well reported, but there is evidence supporting disuse-mediated adaptations to myonuclear density. For example, 28 days of bed rest in middle-aged adults induced a nonsignificant ∼11% decline in myonuclear content (8). Our data in older adults support a relationship between myonuclear content and adaptations to muscle atrophy at both the whole limb and single fiber level.
Protein kinase B (Akt/PKB) is a positive regulator of skeletal muscle mass that acts via mTORC1 to activate protein synthesis in response to exercise or feeding (5, 27). In response to bed rest, we observed a decrease in (postabsorptive) mTORC1 phosphorylation. Phosphorylation returned to baseline following rehabilitation. While this reflects a repression of the Akt/mTORC1 pathway during disuse, others have demonstrated that research models involving disuse of a single lower limb may not be sufficient to affect mTORC1 phosphorylation levels (32, 49). In addition, Akt phosphorylates the Forkhead box FOXO proteins and inactivates their catabolic action of by sequestering them to the cytosol (43). This prevents the activation of their downstream targets involved in the atrophy process, including FBXO32 and MuRF1 (7). In this study, rehabilitation triggered an increase in the inactive form of the FOXO1 protein, phopho-FOXO1, suggesting a compensatory inhibition of the muscle protein degradation pathways following a period of disuse.
In our experimental model, leucine was consumed three times/day with meals during the bed rest and rehabilitation periods, but not immediately before (within ~15 h) the collection of the muscle tissue samples. The quantity of leucine provided (~5 g/meal) was likely greater than the amount generally thought to “trigger” translation initiation and muscle protein synthesis (35). The fact that we did not observe any effect of leucine on the activation of the anabolic or catabolic signaling pathways following bed rest or rehabilitation suggests that its anabolic effect is transient and perhaps temporally linked to each supplemented meal and may not be readily observed when analyzing tissue obtained following an overnight fast. Conversely, there is a growing body of evidence to suggest that leucine supplementation may only be effective in certain clinical situations. Specifically, leucine appears to have the greatest potential for benefit in situations where study participants are physically inactive and have a habitual protein intake less than ~1.0 g protein/kg/day (23, 25, 29). In situations where participants are physical active and/or consume >1.0 g protein/kg/day, the benefits of leucine appear to be absent or blunted (25, 48). For example, a study employing a 7-day, single leg disuse model in a healthy, young, male cohort consuming 1.2 g protein/kg/day failed to demonstrate positive outcomes following leucine supplementation.
In conclusion, 7 days of bed rest has a profoundly negative effect on skeletal muscle morphology and function in older adults. Adding a small quantity of supplemental leucine to standardized, mixed nutrient meals had minimal impact on total energy or protein consumption but partially countered the loss of lean leg mass. However, leucine supplementation did not preserve muscle function or positively impact changes at the muscle fiber level associated with bed rest or rehabilitation.
GRANTS
The study was supported by Nursing Research at the National Institutes of Health (R01 NR012973 to D. Paddon-Jones) and in part by the Claude D. Pepper Older Americans Independence Center grant (P30 AG-024832); and the National Center for Research Resources (1UL1RR-029876). This study was conducted with the support of UTMB’s Institute for Translational Sciences, supported by Clinical and Translational Science Awards (UL1TR000071 and UL1TR001439) from the National Center for Advancing Translational Sciences.
DISCLOSURES
D.P.-J. has participated on scientific advisory panels, provided educational seminars and received travel reimbursements and honoraria from Leprino Foods, National Cattlemens Beef Association, National Dairy Council, Sabra Wellness and Nutrition and the US Dairy Export Council. E.J.A.-L. K.N.F., K.J.A.-C., R.R.D., A.W., C.S.F., and S.L. report no conflicts of interest.
AUTHOR CONTRIBUTIONS
E.J.A.-L. and D.P.-J. conceived and designed research; E.J.A.-L., K.N.F., K.J.A.-C., R.R.D., A.W., C.S.F., S.L., and D.P.-J. performed experiments; E.J.A.-L., C.S.F., and S.L. analyzed data; E.J.A.-L., C.S.F., S.L., and D.P.-J. interpreted results of experiments; E.J.A.-L. and S.L. prepared figures; E.J.A.-L. and D.P.-J. drafted manuscript; E.J.A.-L., C.S.F., S.L., and D.P.-J. edited and revised manuscript; E.J.A.-L., K.N.F., K.J.A.-C., R.R.D., A.W., C.S.F., S.L., and D.P.-J. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank Glenda Blaskey, Syed Husaini, Adetutu Odejimi, Sneha Nagamma, Jessica Spahn, Elena Volpi, and staff of the Institute for Translational Sciences-Clinical Research Center for assistance.
REFERENCES
- 1.Arentson-Lantz E, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol 120: 965–975, 2016. doi: 10.1152/japplphysiol.00799.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arentson-Lantz E, Galvan E, Wacher A, Fry CS, Paddon-Jones D. 2000 Steps/Day Does Not Fully Protect Skeletal Muscle Health in Older Adults during Bed Rest. J Aging Phys Act 27: 191–197, 2018. doi: 10.1123/japa.2018-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol (1985) 120: 965–975, 2016. doi: 10.1152/japplphysiol.00799.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arentson-Lantz EJ, Galvan E, Ellison J, Wacher A, Paddon-Jones D. Improving Dietary Protein Quality Reduces the Negative Effects of Physical Inactivity on Body Composition and Muscle Function. J Gerontol A Biol Sci Med Sci 74: 1605–1611, 2019. doi: 10.1093/gerona/glz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J 19: 786–788, 2005. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- 6.Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35: 609–616, 1975. doi: 10.3109/00365517509095787. [DOI] [PubMed] [Google Scholar]
- 7.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 8.Brooks NE, Cadena SM, Vannier E, Cloutier G, Carambula S, Myburgh KH, Roubenoff R, Castaneda-Sceppa C. Effects of resistance exercise combined with essential amino acid supplementation and energy deficit on markers of skeletal muscle atrophy and regeneration during bed rest and active recovery. Muscle Nerve 42: 927–935, 2010. doi: 10.1002/mus.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruusgaard JC, Egner IM, Larsen TK, Dupre-Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J Appl Physiol (1985) 113: 290–296, 2012. doi: 10.1152/japplphysiol.00436.2012. [DOI] [PubMed] [Google Scholar]
- 10.Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest 118: 1450–1457, 2008. doi: 10.1172/JCI34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update 13: 559–565, 2007. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- 12.Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr 31: 512–519, 2012. doi: 10.1016/j.clnu.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr 32: 704–712, 2013. doi: 10.1016/j.clnu.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, Volpi E. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 302: E1113–E1122, 2012. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr 103: 465–473, 2016. doi: 10.3945/ajcn.115.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr 91: 1123S–1127S, 2010. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 17.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330: 1769–1775, 1994. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 18.Finnerty CC, McKenna CF, Cambias LA, Brightwell CR, Prasai A, Wang Y, El Ayadi A, Herndon DN, Suman OE, Fry CS. Inducible satellite cell depletion attenuates skeletal muscle regrowth following a scald-burn injury. J Physiol 595: 6687–6701, 2017. doi: 10.1113/JP274841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49: M85–M94, 1994. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 20.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab 92: 3060–3067, 2007. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 21.Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol 301: R701–R715, 2011. doi: 10.1152/ajpregu.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol 303: C854–C861, 2012. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob KJ, Chevalier S, Lamarche M, Morais JA. Leucine Supplementation Does Not Alter Insulin Sensitivity in Prefrail and Frail Older Women following a Resistance Training Protocol. J Nutr 149: 959–967, 2019. doi: 10.1093/jn/nxz038. [DOI] [PubMed] [Google Scholar]
- 24.Lamon S, Zacharewicz E, Arentson-Lantz E, Gatta PA, Ghobrial L, Gerlinger-Romero F, Garnham A, Paddon-Jones D, Russell AP. Erythropoietin Does Not Enhance Skeletal Muscle Protein Synthesis Following Exercise in Young and Older Adults. Front Physiol 7: 292, 2016. doi: 10.3389/fphys.2016.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, Saris WH, van Loon LJ. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr 141: 1070–1076, 2011. doi: 10.3945/jn.111.138495. [DOI] [PubMed] [Google Scholar]
- 26.Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 288: R1288–R1296, 2005. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- 27.Léger B, Cartoni R, Praz M, Lamon S, Dériaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576: 923–933, 2006. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 144: 876–880, 2014. doi: 10.3945/jn.113.185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Arnau FM, Fonfría-Vivas R, Cauli O. Beneficial Effects of Leucine Supplementation on Criteria for Sarcopenia: A Systematic Review. Nutrients 11: E2504, 2019. doi: 10.3390/nu11102504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta NM, Bechard LJ, Zurakowski D, Duggan CP, Heyland DK. Adequate enteral protein intake is inversely associated with 60-d mortality in critically ill children: a multicenter, prospective, cohort study. Am J Clin Nutr 102: 199–206, 2015. doi: 10.3945/ajcn.114.104893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messier V, Rabasa-Lhoret R, Barbat-Artigas S, Elisha B, Karelis AD, Aubertin-Leheudre M. Menopause and sarcopenia: A potential role for sex hormones. Maturitas 68: 331–336, 2011. doi: 10.1016/j.maturitas.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Møller AB, Vendelbo MH, Schjerling P, Couppé C, Møller N, Kjær M, Hansen M, Jessen N. Immobilization Decreases FOXO3a Phosphorylation and Increases Autophagy-Related Gene and Protein Expression in Human Skeletal Muscle. Front Physiol 10: 736, 2019. doi: 10.3389/fphys.2019.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J Gerontol A Biol Sci Med Sci 70: 57–62, 2015. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 34.Nicolo M, Heyland DK, Chittams J, Sammarco T, Compher C. Clinical Outcomes Related to Protein Delivery in a Critically Ill Population: A Multicenter, Multinational Observation Study. JPEN J Parenter Enteral Nutr 40: 45–51, 2016. doi: 10.1177/0148607115583675. [DOI] [PubMed] [Google Scholar]
- 35.Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J Nutr 136: 533S–537S, 2006. doi: 10.1093/jn/136.2.533S. [DOI] [PubMed] [Google Scholar]
- 37.Oikawa SY, Kamal MJ, Webb EK, McGlory C, Baker SK, Phillips SM. Whey protein but not collagen peptides stimulate acute and longer-term muscle protein synthesis with and without resistance exercise in healthy older women: a randomized controlled trial. Am J Clin Nutr 111: 708–718, 2020. doi: 10.1093/ajcn/nqz332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paddon-Jones D, Leidy H. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care 17: 5–11, 2014. doi: 10.1097/MCO.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Aarsland A, Wolfe RR, Ferrando AA. The catabolic effects of prolonged inactivity and acute hypercortisolemia are offset by dietary supplementation. J Clin Endocrinol Metab 90: 1453–1459, 2005. doi: 10.1210/jc.2004-1702. [DOI] [PubMed] [Google Scholar]
- 40.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab 89: 4351–4358, 2004. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- 41.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286: E321–E328, 2004. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 42.Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, Faliva MA, Solerte BS, Fioravanti M, Lukaski H, Perna S. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr 103: 830–840, 2016. doi: 10.3945/ajcn.115.113357. [DOI] [PubMed] [Google Scholar]
- 43.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stefanetti R. Atrogin-1 and MURF-1 signalling in human skeletal muscle (Ph.D thesis). School of Exercise and Nutrition Sciences, Deakin University, 2014, p. 166. [Google Scholar]
- 45.Stefanetti RJ, Zacharewicz E, Della Gatta P, Garnham A, Russell AP, Lamon S. Ageing has no effect on the regulation of the ubiquitin proteasome-related genes and proteins following resistance exercise. Front Physiol 5: 30, 2014. doi: 10.3389/fphys.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985) 107: 1172–1180, 2009. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 47.Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, de Groot LC. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 13: 720–726, 2012. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr 89: 1468–1475, 2009. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- 49.Wall BT, Dirks ML, Snijders T, van Dijk JW, Fritsch M, Verdijk LB, van Loon LJ. Short-term muscle disuse lowers myofibrillar protein synthesis rates and induces anabolic resistance to protein ingestion. Am J Physiol Endocrinol Metab 310: E137–E147, 2016. doi: 10.1152/ajpendo.00227.2015. [DOI] [PubMed] [Google Scholar]
- 50.Wall BT, Hamer HM, de Lange A, Kiskini A, Groen BB, Senden JM, Gijsen AP, Verdijk LB, van Loon LJ. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr 32: 412–419, 2013. doi: 10.1016/j.clnu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev 71: 195–208, 2013. doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]