Abstract
Regular exercise enhances endothelial function in older men, but not consistently in estrogen-deficient postmenopausal women. Estradiol treatment improves basal endothelial function and restores improvements in endothelial function (flow-mediated dilation, FMD) to aerobic exercise training in postmenopausal women; however, estradiol treatment is controversial. Resveratrol, an estrogen receptor ligand, enhances exercise training effects on cardiovascular function and nitric oxide (NO) release in animal models, but impairs exercise training effects in men. We conducted a randomized cross-over, double-blinded, placebo-controlled pilot study to determine whether acute (single dose) resveratrol (250-mg tablet) or estradiol (0.05 mg/day transdermal patch) treatment enhances FMD at rest and after a single bout of moderate-intensity aerobic exercise in healthy estrogen-deficient postmenopausal women (n = 15, 58.1 ± 3.2 yr). FMD was measured before and after (30, 60, and 120 min) a 40-min bout of moderate-intensity treadmill exercise (60–75% peak heart rate) under the respective conditions (separated by 1-2 wk). FMD was higher (P < 0.05) before exercise and at all post-exercise time points in the resveratrol and estradiol conditions compared to placebo. FMD was increased from baseline by 120 min postexercise in the estradiol condition (P < 0.001), but not resveratrol or PL conditions. Consistent with our previous findings, estradiol also enhances endothelial function in response to acute endurance exercise. Although resveratrol improved basal FMD, there was no apparent enhancement of FMD to acute exercise and, therefore, may not act as an estradiol mimetic.
NEW & NOTEWORTHY The benefits of endurance exercise training on endothelial function are diminished in estrogen-deficient postmenopausal women, but estradiol treatment appears to restore improvements in endothelial function in this group. We show that basal endothelial function is enhanced with both acute estradiol and resveratrol treatments in estrogen-deficient postmenopausal women, but endothelial function is only enhanced following acute endurance exercise with estradiol treatment.
Keywords: aerobic exercise, endothelial function, nutraceutical, sex hormones, women
INTRODUCTION
Endothelial dysfunction, assessed via brachial artery flow-mediated dilation (FMD), is widely regarded as a biomarker of vascular aging and a predictor of future cardiovascular disease (CVD) morbidity and mortality (12, 21). Sex differences exist in the age-related decline in endothelial function, starting in the fourth decade of life for men, whereas an appreciably faster rate of decline is observed during the fifth decade of life in women (3). Accordingly, CVD prevalence is higher in men until 60 years of age, after which sex differences are no longer observed (38). The sex difference in the age-related decline in FMD and CVD prevalence has been attributed to the decline in estradiol with the menopause transition (3, 31, 33). Because of concerns about adverse effects of chronic estrogen-based hormone therapy in postmenopausal women, including estrogen-dependent cancers and CVD events, current guidelines reserve the prescription of estrogen-based hormone therapy for short-term treatment of menopausal symptoms (e.g., vasomotor), prevention of bone loss, and fractures in women at elevated risk, and hypoestrogenism caused by hypogonadism, surgical menopause, or primary ovarian insufficiency (54). Therefore, identification of alternative pharmacologic compounds and/or lifestyle prevention strategies (i.e., exercise) to enhance endothelial function in estrogen-deficient postmenopausal women are needed.
Resveratrol is a polyphenol (3,5,4′-trihydroxystilbene) present in many plant species and has been thought to contribute to the cardiovascular benefits of drinking red wine (i.e., “French Paradox”). Resveratrol has been identified as a vasoactive nutraceutical and has been shown to benefit vascular endothelial function in preclinical and human studies (30). Acute and chronic resveratrol treatment (dosed 30–270 mg) increased brachial artery FMD in healthy obese adults, obese adults with mild hypertension, and hypertensive postmenopausal women, with no differences between acute and chronic treatment (30, 61–64). However, whether resveratrol improves endothelial function in healthy postmenopausal women is unknown.
Lifestyle modifications, particularly endurance exercise, are strongly endorsed to confer multisystem health benefits and curb future risk of CVD-related events (13, 41). Although regular endurance exercise training preserves or restores endothelial function in middle-aged and older men, similar exercise training benefits are not consistently observed in sedentary estrogen-deficient postmenopausal women (8, 37, 44–46). Both the abrupt reductions in FMD coinciding with the menopause transition and the lack of apparent benefit on endothelial function with endurance exercise in estrogen-deficient postmenopausal women has spurred investigation into the possible modulatory influence of estradiol on vascular adaptations to exercise training. In this regard, we recently demonstrated that endothelial function is improved following 12 wk of moderate-intensity endurance exercise training in previously sedentary postmenopausal women who were treated with estradiol, but not in placebo-treated women (37).
The mechanisms by which estradiol modulates endothelial adaptations to exercise have not been completely elucidated (46). In our previous study, estradiol treatment restored the ability of exercise to induce an improvement in endothelial function in postmenopausal women by reducing oxidative stress (37). Estradiol and exercise also share common intracellular signaling pathways to phosphorylate and activate endothelial nitric oxide synthase (eNOS) and mediate nitric oxide (NO) release. Exercise increases blood flow and subsequent frictional forces (i.e., shear stress) along the surface of the endothelium, stimulating mechanosensors (e.g., integrins, G protein-coupled receptors) that transduce mechanical forces into biochemical signals to activate eNOS, and estradiol activates eNOS via estrogen receptor-α (ERα)-mediated genomic and nongenomic signaling of these same pathways (4, 24, 51, 57, 67). It is possible that estradiol modulates exercise-associated signal transduction to increase NO production and improve endothelial function. As such, it would be important to know whether prescribing endurance exercise training with pharmacological/nonpharmacological therapies that can attenuate oxidative stress and act via ERα can enhance exercise signaling and improve endothelial function in postmenopausal women not using estrogen-based hormone therapy.
In this regard, similar to estradiol, resveratrol has antioxidant properties and increases NO release through eNOS activation (26, 40), effects that appear to be mediated, in part, through ER activation of eNOS (26). Additionally, resveratrol enhances exercise training effects on cardiovascular function and NO release in animal models (9, 39). Although, studies conducted in older men show that resveratrol may prevent exercise training adaptations on CVD risk factors and cardiovascular fitness (15), because of resveratrol’s estrogen-like properties, the effects of resveratrol on vascular responses to exercise could be sex-dependent and be more favorable in postmenopausal women (28). The potential of resveratrol to enhance endothelial function and NO in response to endurance exercise in postmenopausal women have not been determined. Acute exercise bouts have been readily used to provide insight into the mechanisms of longer-term adaptations to chronic exercise training (7). Accordingly, we conducted a pilot study to acquire preliminary insight as to whether pretreatment with acute resveratrol or estradiol enhances endothelial function at rest and in response to an acute single bout of moderate-intensity aerobic exercise in healthy estrogen-deficient postmenopausal women. We hypothesized that both resveratrol and estradiol treatment would equally improve FMD before and after an acute bout of aerobic exercise, whereas there would be no improvement in FMD with placebo treatment.
METHODS
Study population.
Fifteen healthy, estrogen-deficient postmenopausal women (52–63 years of age) were recruited from the community. Postmenopausal status was defined as an absence of menses for at least 1 year as defined by the Stages of Reproductive Aging Workshop, and as previously defined (33, 34, 37, 50). Participants were sedentary or recreationally active (<3 days/week of vigorous exercise), had not used hormone therapy for ≥6 months prior to enrollment, were nonsmokers, normotensive based on previous guidelines (resting blood pressure <140/90 mmHg), nondiabetic, had a fasted plasma glucose <110 mg/dL, not taking medications that influence cardiovascular function (i.e. antihypertensive, lipid-lowering medications), and were healthy, as determined by medical history, physical examination, standard blood chemistries, and resting and maximal exercise electrocardiogram. Participants taking vitamin supplements or nonsteroidal anti-inflammatory medications were asked to refrain from use for ≥4 wk prior to vascular testing. Women with a history of hysterectomy or oophorectomy were excluded. The study was approved by the Colorado Multiple Institutional Review Board, and all participants provided written informed consent.
Study design.
This was a randomized cross-over, double-blinded, placebo-controlled pilot study (Fig. 1). Participants were tested under three different conditions, in random order, at the same time of day, with at least 1 week between testing conditions. Treatment conditions consisted of placebo (inactive transdermal patch + inactive tablet); trans-resveratrol (250-mg resveratrol tablet + placebo transdermal patch); and estradiol (0.05 mg/day transdermal patch + placebo tablet). Swanson Ultra Resveratrol 250 is derived from the Chinese herb Polygonum cuspidatum. Both resveratrol and matching placebo tablets were provided by Belmar Pharmacy (Lakewood, CO). Placebo and estradiol transdermal patches were placed 2 days before the exercise visit, whereas placebo or resveratrol tablets were administered on the morning of the exercise visit. Preexercise (baseline) endothelial function measures and blood sampling were obtained 45 min after resveratrol/placebo administration. The dose and time after resveratrol supplementation were determined on the basis of prior studies that have demonstrated its efficacy in improving resting FMD (61, 63). Participants then completed a 5-min warm-up, followed by a 40-min bout of treadmill exercise at 60–75% of their peak heart rate (measured via Polar monitor) achieved during screening cardiopulmonary exercise testing. Women exercised at the same speed/grade for each of the three conditions. Endothelial function tests were performed 30, 60, and 120 min after completing the exercise bout; blood sampling (glucose and insulin) was performed before exercise and at 60 and 120 min after exercise.
Fig. 1.
Experimental design. BD, blood draw; FMD, flow-mediated dilation.
Measurements.
Participants were studied in the supine position following an overnight fast with proper hydration (water only). The study took place at the Colorado Clinical and Translational Sciences Institute (CCTSI) Clinical and Translational Research Center (CTRC).
Endothelial function.
Supine blood pressure was measured prior to measures of endothelial function. Ultrasound measurements of brachial artery flow-mediated dilation (FMD) were performed using a GE Vivid I with a multifrequency linear-array transducer, as previously described (33, 34). Briefly, a pediatric cuff was placed on the upper forearm, and brachial artery images were acquired ∼3–6 cm above the antecubital fossa. The ultrasound probe was clamped to prevent involuntary movement, and measurements were made to ensure the location of the same arterial segment for serial measurements. After obtaining concurrent measures of baseline brachial artery diameter and blood flow velocity, reactive hyperemia was produced by inflating the cuff to 250 mmHg for 5 min, followed by rapid deflation. After the release of the arterial occlusion, Doppler blood flow velocity was acquired, and B-mode ultrasound brachial artery diameter images were measured continuously for 2 min. Brachial artery diameter was analyzed using a commercially available software package (Vascular Analysis Tools 5.5.1; Medical Imaging Applications, Iowa City, IA). All images were coded by number, blinded to treatment condition, and analyzed by the same individual (K.L.M.). Images were considered valid when clear vascular boundaries could be identified; those without clear boundaries were not included in the analyses. Two participants had poor-quality preexercise brachial images during the placebo visit, and additional images that were removed from analyses included one participant’s resveratrol condition and one participant’s estradiol condition due to poor preexercise images, one participant’s placebo condition 30 min postexercise assessment, one participant’s estradiol condition 30 min postexercise assessment, one participant’s resveratrol condition 120 min postexercise assessment and estradiol condition 60 min postexercise assessment, and one participant’s resveratrol condition 30 min postexercise assessment and estradiol condition 60 min postexercise assessment. All procedures conformed strictly to published guidelines for assessing FMD in human participants (5).
Seated blood pressure, body composition, and peak aerobic capacity.
Seated brachial arterial blood pressure was measured in triplicate with a semiautomated device in the morning following an overnight fast, as previously described (53). Total (percent of total mass) and regional (percent of mass in trunk region) body fat were determined using dual-energy X-ray absorptiometry (Hologic Discovery, version 12.6, Bedford, MA). Peak oxygen consumption was determined using an individualized incremental treadmill exercise protocol (52). A warm-up period was used to determine the walking speed that elicited a heart rate roughly 70% of age-predicted maximum. This speed was maintained during the test while the treadmill grade was increased by 2% every 2 min until volitional fatigue. A 12-lead electrocardiogram (Quinton Q4500; Quinton Instruments, Seattle, WA) was used to monitor heart rhythm and rate continuously, while blood pressure was measured during each exercise stage. Cardiorespiratory data were collected at 30-s intervals using a ParvoMedics TrueOne 2400 automated metabolic gas analysis system. The highest oxygen uptake (V̇o2) value achieved was recorded as V̇o2peak.
Blood sampling.
Fasted plasma concentrations of glucose, insulin (RIA immunoassay), estradiol (chemiluminescence methodology, Beckman Coulter), total- (Roche Diagnostic Systems, Indianapolis, IN) and high-density lipoprotein cholesterol (HDL; Diagnostic Chemicals, Oxford, CT) were determined using enzymatic/colorimetric methods. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation (14). Because exercise can influence circulating glucose and insulin levels, and both can modulate endothelial function (60), blood sampling for glucose, insulin, and estradiol occurred on the day of vascular testing. All assays were performed by the CCTSI CTRC core laboratory.
Statistical analysis.
Descriptive statistics were used to examine all data elements. The repeated measurements of FMD were modeled using a general linear mixed model. This approach is conceptually identical to repeated-measures analysis of variance but has the advantage of using all available data for all 15 women, including those whose FMD images were considered missing due to image quality; estimates are unbiased under the assumption that missing data are missing at random. As this was a cross-over study with repeated measures, a direct product compound symmetric covariance structure was used to model the variance/covariance between the repeated measures within each treatment and within each subject. Linear contrasts were used to estimate preexercise hemodynamic, blood, and vascular measures to postexercise measures within condition (i.e., the within-group effects) as well as to compare measures at the same time points among conditions (i.e., the between-group effects); maximum likelihood was used to estimate the covariance structure. Standard statistical conventions of performing two-sided tests and P values < 0.05 designating statistically significant results were used. All analyses were conducted using SAS software version 9.4 M5 (SAS Institute, Cary, NC).
RESULTS
Participant characteristics.
Participant characteristics are presented in Table 1. On average, participants were 7.1 ± 4.2 (means ± SD) years past menopause; 4 (27%) had used hormone therapy in the past for an average of 6.7 ± 5.0 months. Clinical characteristics in past hormone therapy users did not significantly differ from nonusers. All 15 women completed all three treatments and acute exercise bouts. One participant was provided the incorrect treatment; therefore, an additional study visit was performed with the correct treatment.
Table 1.
Participant characteristics
| Parameter | Value |
|---|---|
| Age, yr | 58.1 ± 3.1 |
| Body mass, kg | 60.6 ± 12.0 |
| BMI, kg/m2 | 23.2 ± 34.9 |
| Total body fat, % | 31.5 ± 5.8 |
| Seated systolic BP, mmHg | 110 ± 12 |
| Seated diastolic BP, mmHg | 67 ± 9 |
| Total cholesterol, mg/dL | 203 ± 32 |
| LDL cholesterol, mg/dL | 113 ± 15 |
| HDL cholesterol, mg/dL | 71 ± 25 |
| Glucose, mg/dl | 87 ± 9 |
| Peak systolic BP, mmHg | 161 ± 15 |
| Peak diastolic BP, mmHg | 77 ± 7 |
| Peak HR, bpm | 164 ± 11 |
| Respiratory exchange ratio | 1.06 ± 0.07 |
| V̇o2peak, ml·kg−1·min−1 | 32.6 ± 5.7 |
Data are expressed as means ± SD; n = 15. BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; HR, heart rate; LDL, low-density lipoprotein; V̇o2, oxygen consumption.
Hemodynamic, glucose, and insulin at baseline and after a single bout of exercise.
Preexercise and postexercise hemodynamic responses to the three acute exercise bouts are outlined in Table 2. Preexercise systolic blood pressure was lower in the estradiol condition compared to placebo; systolic, diastolic and mean arterial blood pressures (MAP) were lower between estradiol and resveratrol conditions. No hemodynamic differences existed between resveratrol and placebo conditions. There were no differences in preexercise heart rate (HR) among estradiol or resveratrol and placebo conditions.
Table 2.
Hemodynamic, brachial artery and metabolic parameters before acute treadmill exercise, and at 30, 60, and 120 min after treadmill exercise, during placebo, resveratrol, and estradiol treatments
| Placebo |
Resveratrol |
Estradiol |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preexercise | 30 min | 60 min | 120 min | Preexercise | 30 min | 60 min | 120 min | Preexercise | 30 min | 60 min | 120 min | |
| FMD | ||||||||||||
| Brachial diameter, mm | 3.85 ± 0.12 | 3.90 ± 0.11 | 3.87 ± 0.11 | 3.79 ± 0.11 | 3.69 ± 0.10 | 3.71 ± 0.10 | 3.74 ± 0.10 | 3.67 ± 0.10 | 3.68 ± 0.11 | 3.73 ± 0.11 | 3.77 ± 0.11 | 3.74 ± 0.11 |
| Brachial FMD, mm∆ | 0.16 ± 0.02 | 0.14 ± 0.02 | 0.13 ± 0.02 | 0.16 ± 0.02 | 0.25 ± 0.03* | 0.22 ± 0.03* | 0.26 ± 0.03* | 0.27 ± 0.03* | 0.22 ± 0.02* | 0.21 ± 0.03* | 0.27 ± 0.03*† | 0.31 ± 0.02*† |
| Blood markers | ||||||||||||
| Glucose, mg/dL | 93 ± 2 | 93 ± 2 | 92 ± 2 | 94 ± 2 | 94 ± 2 | 92 ± 2 | 90 ± 2*§ | 91 ± 2§ | 90 ± 2 | |||
| Insulin, mIU/L | 6.8 ± 0.8 | 6.6 ± 0.8 | 6.2 ± 0.8 | 6.4 ± 1.0 | 6.1 ± 1.0 | 6.3 ± 1.0 | 5.9 ± 0.7 | 5.9 ± 0.7 | 5.6 ± 0.7 | |||
| Estradiol, pg/mL | 10.6 ± 3.2 | 10.5 ± 3.6 | 64.6 ± 26.6*§ | |||||||||
| HR and BP FMD | ||||||||||||
| HR, bpm | 58 ± 1 | 68 ± 1† | 64±1† | 59 ± 1 | 59±1 | 57 ± 2† | 66 ± 2† | 58 ± 2 | 58 ± 1 | 68 ± 1† | 64 ± 1† | 60 ± 1† |
| SBP, mmHg | 114 ± 2 | 108 ± 2† | 110±2† | 113 ± 2 | 115±2 | 107 ± 2† | 108 ± 2† | 115 ± 2 | 109 ± 1*§ | 104 ± 1† | 104 ± 1†* | 108 ± 1*§ |
| DBP, mmHg | 69 ± 2 | 66 ± 2† | 67±2† | 69 ± 2 | 71±2 | 66 ± 2† | 66 ± 2† | 70 ± 2 | 66 ± 2§ | 63 ± 2† | 64 ± 2* | 67 ± 2* |
| MAP, mmHg | 84 ± 2 | 81 ± 2† | 82±2 | 85 ± 2 | 88±2 | 81 ± 2† | 82 ± 2† | 86 ± 2 | 81 ± 1§ | 77 ± 1†§ | 78 ± 1†§ | 82 ± 1§ |
Values presented for the 15 subjects are expressed as means ± SE. BP, blood pressure; bpm, beats per minute; HR, heart rate; MAP, mean arterial pressure.
Significantly different (P < 0.05) from placebo condition at same time point.
Significantly different (P < 0.05) from pre-exercise within condition.
Significantly different (P < 0.05) from resveratrol at the same time point.
Average exercise intensities did not differ among the three conditions (placebo: 67.4 ± 2.1% of peak HR; resveratrol: 67.0 ± 2.6% of peak HR; estradiol 67.0 ± 2.6% of peak HR; P > 0.05), with all participants exercising in the prescribed exercise dose each visit. During all three conditions, the heart rate and blood pressure responses postexercise were biphasic, such that HR increased (P < 0.05) and blood pressure decreased (P < 0.05) compared to preexercise values, then normalized to preexercise levels by 120 min postexercise (P > 0.05) with the exception of the estradiol condition. Postexercise systolic, diastolic, and MAP did not differ between the placebo and resveratrol conditions at the respective time points (30, 60, and 120 minutes postexercise). Small, statistically significant differences were noted between the placebo and estradiol visits. Postexercise 60- and 120-min systolic and diastolic blood pressure were significantly lower (P < 0.05) in the estradiol treatment condition compared to placebo. Systolic blood pressure at 120 min after exercise was significantly lower in the estradiol treatment condition compared to resveratrol. MAP was significantly lower 30, 60, and 120 min after exercise in the estradiol compared to the resveratrol conditions.
There were small significant differences in preexercise glucose levels among conditions between the placebo and estradiol conditions, as well as between resveratrol and estradiol conditions. Glucose 60 min after exercise was lower in the estradiol compared to resveratrol condition. Moreover, there were no changes from preexercise values in glucose or insulin levels following the acute exercise bout in any condition.
Preexercise brachial artery FMD and estradiol.
Brachial artery baseline diameters did not significantly differ among conditions (Table 2). Preexercise brachial artery FMD, both percent (Fig. 2) and absolute (Table 2), were greater in the resveratrol and estradiol conditions than the placebo condition (both P < 0.05). As expected, estradiol concentration was higher in the estradiol condition than the placebo and resveratrol conditions (P < 0.001). There were no differences in estradiol concentrations between resveratrol and placebo.
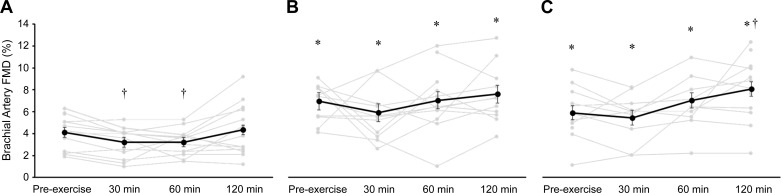
Fig. 2.
Brachial artery flow-mediated dilation (FMD) within placebo condition (A; n = 13), resveratrol condition (B; n = 12 before and 60 min after exercise, n = 11 30 and 120 min after exercise), estradiol condition (C; n = 12 before exercise and 120 min after exercise, n = 10 30 and 60 min after exercise). Individual responses are presented in gray, and values are expressed as group means ± SE, presented by the black circles and lines. The repeated measurements of FMD were modeled using a general linear mixed model to account for missing data due to image quality. †P < 0.05 vs. preexercise value within condition. *P < 0.05 different from placebo condition within the same time point.
Acute exercise effects on brachial artery FMD.
Brachial artery FMD, both percent (Fig. 2) and absolute (Table 2), was greater in resveratrol and estradiol conditions compared with placebo at all postexercise time points (Fig. 2; all P < 0.001). Within the placebo condition, FMD decreased at 30 min and 60 min after exercise (both P < 0.05; Cohen’s d: both 0.6), but normalized at 120 min after exercise and was no longer different (P = 0.54; Cohen’s d: 0.2) from preexercise FMD (Fig. 2). Within the resveratrol condition, there were no differences between postexercise FMD at any time point compared to preexercise FMD (Fig. 2; Cohen’s d: 0.4, 0.03, and 0.3, respectively). Within the estradiol condition, FMD was greater at 120 min after exercise compared to preexercise FMD (8.0 ± 0.7% and 5.9 ± 0.7%, respectively, P < 0.001; Cohen’s d: 1.1), but 30- and 60-min postexercise FMD values were not different from preexercise (P = 0.46 and P = 0.08, respectively; Cohen’s d: 0.5 and 1.1, respectively).
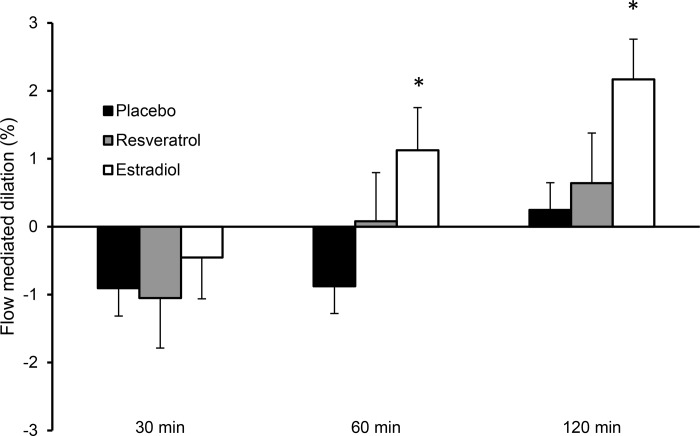
When comparing the change scores (FMD at postexercise time points – preexercise FMD) between conditions, only the 60- and 120-min change scores in the estradiol condition were significantly different (both P < 0.01; Cohen’s d: 1.5 and 1.4, respectively) from the placebo change scores at the same time period (Fig. 3). There were no significant differences in change scores between resveratrol and placebo at any postexercise time point (30 min after exercise, P = 0.86; 60 min after exercise, P = 0.24; 120 min after exercise, P = 0.63), and no difference between resveratrol and estradiol (30 min after exercise, P = 0.52, Cohen’s d: 0.1; 60 min after exercise, P = 0.26, Cohen’s d: 0.3; 120 min after exercise, P = 0.10, Cohen’s d: 0.1).
Fig. 3.
Brachial artery flow-mediated dilation (FMD) change scores within placebo condition (A; n = 13), resveratrol condition (B; n = 12 before and 60 min after exercise, n = 11, 30 and 120 min after exercise), estradiol condition (C; n = 12 before exercise and 120 min after exercise, n = 10, 30, and 60 min after exercise). Individual responses are presented in gray, and values are expressed as group means ± SE, presented by the black circles and lines. The repeated measurements of FMD were modeled using a general linear mixed model to account for missing data due to image quality. *P < 0.05 different from placebo condition within the same time point.
DISCUSSION
In the present study, we extended our previous observations demonstrating the essential role of estradiol in basal endothelial function and endothelial adaptations to chronic endurance exercise training in postmenopausal women by examining whether a potential “estrogen mimetic” (i.e., resveratrol) could modulate basal endothelial function and responses to a single bout of exercise in postmenopausal women. We first show that a single dose of resveratrol or estradiol increased brachial artery FMD to a similar extent compared to placebo. However, only estradiol treatment led to an enhanced brachial artery FMD response to exercise. These data provide further support for the important role of estradiol in the endothelial response to endurance exercise in postmenopausal women and suggest that estrogen mimetics like resveratrol may improve basal endothelial function, but whether it enhances endothelial function in response to exercise warrants further exploration.
Modulatory action of resveratrol on basal endothelial function in estrogen-deficient postmenopausal women.
Resveratrol is a vasoactive nutraceutical, with both acute and chronic administration improving vascular endothelial function to a similar degree (61, 64). Analogous to estradiol, the improvement in endothelial function is mediated by increased NO release through eNOS activation (25, 40). The increase in eNOS activity with resveratrol is attenuated with estrogen receptor blockade, indicating that the effects of resveratrol on endothelial function are mediated, in part, through estrogen receptor activation of eNOS (26), and thus, resveratrol may be a nutraceutical substitute for estradiol in women.
To our knowledge, the present study was the first to examine the effects of resveratrol on endothelial function in healthy postmenopausal women. The robust increase in resting brachial artery FMD with acute resveratrol dosed at 250 mg was similar in magnitude to that observed by Marques et al. (from 4.2 to 7.1%) with acute administration of a 300-mg dose of trans-resveratrol in hypertensive women aged 45–65 years (30). Interestingly, Marques et al. (30) showed no improvement in FMD with resveratrol in age-matched hypertensive men (from 4.4 to 4.9%). Our findings extend these previous observations by incorporating an estradiol arm. As expected, acute estradiol treatment led to a significant increase in baseline FMD compared to placebo, and to a similar degree reported previously (37).
Effects of resveratrol or estradiol treatment on the postexercise FMD response.
Acute exercise has been suggested to present the cardiovascular system with a hormetic challenge that, with repetition, leads to physiological adaptations (7). Previous investigations, primarily in young men, have characterized the time course of brachial artery FMD responses to an acute bout of endurance exercise (6, 7). Generally, a biphasic FMD response occurs after a bout of moderate- or vigorous-intensity exercise, with a decline in FMD immediately after exercise and a subsequent normalization or increase >60 min after exercise (1, 22, 23). The decrease in FMD immediately after exercise may be related to elevated retrograde shear, shear-induced substrate depletion, and/or a reduced shear stimulus during the FMD test (7). Additionally, a decrease in blood pressure from peak to postexercise may be associated with a reduction in NO endothelium-dependent dilation due to arterial baroreflex-induced increase in sympathetic nervous system activity (20, 42).
Consistent with previous observations in men (1, 23, 66), we showed a significant decline (~1%) in FMD 30 min after the acute bout of exercise with placebo treatment that persisted 60 min after exercise, but normalized by 120 minutes after exercise. However, our data are in contrast to previous observations of Serviente et al. (47) and Yoo et al. (66), who reported no significant change in FMD 20–30 min after acute, moderate-intensity, and high intensity interval training treadmill exercise in postmenopausal women. These findings may be explained, in part, by the relatively short bout of exercise (~30 min), as previous studies have determined that longer bouts are associated with an initial decline in FMD adults (7). Our data are also in contrast to Harvey et al. (18), who showed an increase in FMD ~60 min after 45 min of moderate intensity (60% V̇o2max) in postmenopausal women. The conflicting results could be due to methodological differences; Harvey et al. (18) fed their participants a light meal post-treadmill exercise and assessed FMD following upper arm occlusion, whereas participants in the present study were fasted, and FMD was assessed following forearm occlusion. Previous work that established the existence of macronutrient-specific effects on FMD (2, 56), as well as different FMD responses between upper and forearm cuff occlusions (10) has contributed to guidelines recommending FMD to be performed in the fasted stated (≥12 h) and using a forearm occlusion model (55).
Although the decrease in FMD at 30 min after exercise in the resveratrol condition approached ~1%, it was not statistically significant (P = 0.16). This may be explained by the relatively large standard deviation (±2.4%), perhaps reflecting the exclusion (n = 4) of poor-quality images at this time point. In contrast to the placebo condition, estradiol treatment prevented the transient decrease in FMD at 30- and 60-min after exercise and led to an increase in FMD 120 min after exercise. These data are disparate to Harvey et al. (19), who did not show additional improvements in FMD following a single bout of treadmill exercise in postmenopausal women treated with oral estradiol (2 mg/day). In this study, both acute exercise and estradiol treatment with no exercise improved FMD, but their effects were not additive. The conflicting results could be due to differences in assessment of FMD (i.e., upper arm occlusion in Harvey et al. vs. forearm occlusion in the present study). Serviente et al. (47) determined whether the brachial artery FMD response following acute moderate-intensity treadmill exercise would differ in women with varying ovarian hormone exposure (perimenopausal who have higher estrogen exposure than postmenopausal women, who have low estrogen exposure). Brachial artery FMD increased 30 min after exercise in the perimenopausal, but did not change in the postmenopausal women, despite having similar FMD before exercise. In a subsequent study, fitness appeared to modulate the response to acute exercise, with only low-fit perimenopausal women demonstrating an increase in FMD 30 min after exercise (48). These data are consistent with the present study’s findings that higher levels of circulating estradiol may prevent the transient decrease in FMD following acute exercise and provides support for the importance of estradiol in permitting endothelial adaptations to exercise in women.
Although resveratrol did not enhance the brachial artery response to a single bout of exercise, the present study’s findings that FMD was higher at all postexercise time points in the resveratrol condition compared with placebo is in contrast to a study conducted in older men that reported attenuation of improvements in cardiovascular function (MAP and maximal oxygen uptake) with chronic exercise training in resveratrol-treated compared to placebo-treated men (16). Results from the present study indicate that resveratrol does not negatively impact the endothelial response to exercise in estrogen-deficient postmenopausal women; whether resveratrol has a permissive effect on endothelial adaptations to chronic exercise training in older women warrants further investigation.
Potential mechanisms underlying the modulatory influence of resveratrol and estradiol on basal endothelial function and responses to acute exercise.
We can only speculate on the mechanisms that explain the favorable effects of resveratrol and estradiol on basal FMD and FMD responses following an acute exercise bout with estradiol treatment. Oxidative stress is a key modulator of NO and basal endothelial function in estrogen-deficient postmenopausal women (58). Reactive oxygen species (ROS), such as superoxide, scavenge NO, reducing its overall bioavailability and associated endothelial vasodilatory response (27). Additionally, it has been suggested that oxidative stress from increased ROS production during exercise may contribute to the transient decrease in FMD with acute exercise (7, 49). Because both resveratrol and estradiol can increase NO bioavailability through antioxidant effects (58, 59, 65), the higher basal FMD (i.e., preexercise) in both conditions compared to placebo may reflect reduced oxidative stress in these conditions. Moreover, the decrease in FMD 30 and 60 min after exercise in the placebo condition could be due to increased exercise induced ROS production, whereas the higher FMD at all time-points after exercise in both resveratrol and estradiol conditions compared to placebo may reflect prevention or attenuation of exercise-induced ROS and decreased FMD after exercise. Consistent with this, resveratrol supplementation prevented the increase in ROS (i.e., xanthine oxidase and hydrogen peroxide) and increased the activity of the antioxidants catalase and manganese superoxide dismutase in skeletal muscles following isometric contractions in aged mice (43). Additionally, we previously reported that brachial artery FMD increased during a systemic infusion of ascorbic acid (a well-described experimental model to acutely suppress oxidative stress) (11) following 12 wk of endurance exercise training in placebo-treated postmenopausal women, whereas no improvement was seen in estradiol-treated women (36). These results indicate that estrogen may play a key permissive role in the adaptive response of the endothelium to endurance exercise in women through antioxidant actions.
Other potential mechanisms that may contribute to the initial decline in endothelial function postexercise include increases in inflammation (17) and vasoconstrictors (e.g., endothelin-1) (29). For a detailed review of this topic, readers are directed to the following articles (32, 35, 46).
Experimental considerations and limitations.
This pilot study had several limitations. The objective was to characterize the basal and postexercise endothelial vasodilatory responses with resveratrol or estradiol treatment in healthy estrogen-deficient postmenopausal women. Findings may not extend to other populations, including men, and those with CVD risk factors. Without robust markers of NO and oxidative stress (i.e., both ROS and endogenous antioxidants), we can only speculate on the mechanistic role that NO and ROS may play in response to the treatment conditions. Future studies, including more robust and sensitive measures of NO and oxidative stress, are needed to begin to elucidate the mechanistic effects of acute and chronic resveratrol or estradiol treatment on vascular function in women. Although the FMD response at rest with resveratrol treatment was generally favorable, the implications of chronic resveratrol supplementation in conjunction with endurance exercise training in postmenopausal women are unknown. Additionally, because we did not measure endothelial-independent vasodilation, we cannot state for certain that the effects of the interventions and acute exercise on brachial artery FMD were mediated entirely by the vascular endothelium; it is plausible that the effects were also mediated by vascular smooth muscle cell function. Lastly, although blood glucose and insulin did not change after exercise from preexercise during any treatment condition, glucose levels during the estradiol treatment condition were lower at preexercise compared to the placebo condition. The small difference (3 mg/dL) was not considered clinically meaningful.
Conclusions.
The importance of estradiol for basal endothelial function and endothelial adaptations to exercise in women has been demonstrated in our previous work and others and was confirmed in the present study (46). However, alternatives to estradiol are needed given concerns about the potential harms of estrogen-based hormone therapy in postmenopausal women (46). We showed here for the first time that 1) a single dose of resveratrol improved endothelial function at rest in healthy estrogen-deficient postmenopausal women to the same extent as transdermal estradiol; and 2) only estradiol may interact with endurance exercise to enhance endothelial function further, consistent with our previous observations (37). Accordingly, these findings do not support resveratrol as an estrogen mimetic for enhancing endothelial function in response to acute aerobic exercise in older women.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01AG-028746, R56HL-114073, T32ST63009794; Colorado Clinical and Translational Sciences Institute No. UL1-TR001082 and Colorado Nutrition and Obesity Research Center No. P30 DK-048520; the University of Colorado Center for Women’s Health Research, and the Veterans Affairs Eastern Colorado Geriatric Research, Education and Clinical Center (GRECC), Denver. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the U.S. Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.L.M. and D.R.S. conceived and designed research; C.O., K.J.H., R.B. and K.L.M performed experiments; C.O., P.J.B., and K.L.M analyzed data; C.O., K.L.H., P.J.B., D.R.S., W.M.K., and K.L.M interpreted results of experiments; C.O. and K.L.M prepared figures, C.O., P.J.B., and K.L.M. drafted manuscript; C.O., K.L.H., P.J.B., K.J.H., R.B., D.R.S., W.M.K., and K.L.M. edited and revised manuscript, and approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Teresa Witten and Lila Sisbarro for their assistance.
REFERENCES
- 1.Birk GK, Dawson EA, Batterham AM, Atkinson G, Cable T, Thijssen DH, Green DJ. Effects of exercise intensity on flow mediated dilation in healthy humans. Int J Sports Med 34: 409–414, 2013. doi: 10.1055/s-0032-1323829. [DOI] [PubMed] [Google Scholar]
- 2.Brock DW, Davis CK, Irving BA, Rodriguez J, Barrett EJ, Weltman A, Taylor AG, Gaesser GA. A high-carbohydrate, high-fiber meal improves endothelial function in adults with the metabolic syndrome. Diabetes Care 29: 2313–2315, 2006. doi: 10.2337/dc06-0917. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 4.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 23: 665–686, 2002. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 5.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 6.Dawson EA, Cable NT, Green DJ, Thijssen DHJ. Do acute effects of exercise on vascular function predict adaptation to training? Eur J Appl Physiol 118: 523–530, 2018. doi: 10.1007/s00421-017-3724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson EA, Green DJ, Cable NT, Thijssen DH. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol (1985) 115: 1589–1598, 2013. doi: 10.1152/japplphysiol.00450.2013. [DOI] [PubMed] [Google Scholar]
- 8.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 9.Dolinsky VW, Jones KE, Sidhu RS, Haykowsky M, Czubryt MP, Gordon T, Dyck JRB. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol 590: 2783–2799, 2012. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001. doi: 10.1042/cs1010629. [DOI] [PubMed] [Google Scholar]
- 11.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J 26: 65–69, 2005. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol 72: 1622–1639, 2018. doi: 10.1016/j.jacc.2018.08.2141. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 15.Gliemann L, Schmidt JF, Olesen J, Biensø RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol 591: 5047–5059, 2013. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gliemann L, Schmidt JF, Olesen J, Biensø RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol 591: 5047–5059, 2013. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzales JU, Thompson BC, Thistlethwaite JR, Scheuermann BW. Association between exercise hemodynamics and changes in local vascular function following acute exercise. Appl Physiol Nutr Metab 36: 137–144, 2011. doi: 10.1139/H10-097. [DOI] [PubMed] [Google Scholar]
- 18.Harvey PJ, Morris BL, Kubo T, Picton PE, Su WS, Notarius CF, Floras JS. Hemodynamic after-effects of acute dynamic exercise in sedentary normotensive postmenopausal women. J Hypertens 23: 285–292, 2005. doi: 10.1097/00004872-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Harvey PJ, Picton PE, Su WS, Morris BL, Notarius CF, Floras JS. Exercise as an alternative to oral estrogen for amelioration of endothelial dysfunction in postmenopausal women. Am Heart J 149: 291–297, 2005. doi: 10.1016/j.ahj.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Hijmering ML, Stroes ESG, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39: 683–688, 2002. doi: 10.1016/S0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 21.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 22.Johnson BD, Padilla J, Wallace JP. The exercise dose affects oxidative stress and brachial artery flow-mediated dilation in trained men. Eur J Appl Physiol 112: 33–42, 2012. doi: 10.1007/s00421-011-1946-8. [DOI] [PubMed] [Google Scholar]
- 23.Katayama K, Fujita O, Iemitsu M, Kawano H, Iwamoto E, Saito M, Ishida K. The effect of acute exercise in hypoxia on flow-mediated vasodilation. Eur J Appl Physiol 113: 349–357, 2013. doi: 10.1007/s00421-012-2442-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids 73: 864–869, 2008. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem 280: 7460–7468, 2005. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 26.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem 280: 7460–7468, 2005. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 27.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 43: 562–571, 1999. doi: 10.1016/S0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 28.Louis XL, Raj P, Chan L, Zieroth S, Netticadan T, Wigle JT. Are the cardioprotective effects of the phytoestrogen resveratrol sex-dependent? 1. Can J Physiol Pharmacol 97: 503–514, 2019. doi: 10.1139/cjpp-2018-0544. [DOI] [PubMed] [Google Scholar]
- 29.Maeda S, Miyauchi T, Sakane M, Saito M, Maki S, Goto K, Matsuda M. Does endothelin-1 participate in the exercise-induced changes of blood flow distribution of muscles in humans? J Appl Physiol (1985) 82: 1107–1111, 1997. doi: 10.1152/jappl.1997.82.4.1107. [DOI] [PubMed] [Google Scholar]
- 30.Marques BCAA, Trindade M, Aquino JCF, Cunha AR, Gismondi RO, Neves MF, Oigman W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin Exp Hypertens 40: 218–223, 2018. doi: 10.1080/10641963.2017.1288741. [DOI] [PubMed] [Google Scholar]
- 31.Moreau KL. Intersection between gonadal function and vascular aging in women. J Appl Physiol (1985) 125: 1881–1887, 2018. doi: 10.1152/japplphysiol.00117.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau KL, Hildreth KL. Vascular aging across the menopause transition in healthy women. Adv Vasc Med 2014: 204390, 2014. doi: 10.1155/2014/204390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreau KL, Ozemek C. Vascular adaptations to habitual exercise in older adults: time for the sex talk. Exerc Sport Sci Rev 45: 116–123, 2017. doi: 10.1249/JES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131: e29–e322, 2015. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 39.Murase T, Haramizu S, Ota N, Hase T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology 10: 423–434, 2009. doi: 10.1007/s10522-008-9177-z. [DOI] [PubMed] [Google Scholar]
- 40.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal 11: 2851–2897, 2009. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 41.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The Physical activity guidelines for Americans. JAMA 320: 2020–2028, 2018. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raine NM, Cable NT, George KP, Campbell IG. The influence of recovery posture on post-exercise hypotension in normotensive men. Med Sci Sports Exerc 33: 404–412, 2001. doi: 10.1097/00005768-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Ryan MJ, Jackson JR, Hao Y, Williamson CL, Dabkowski ER, Hollander JM, Alway SE. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci 65: 815–831, 2010. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos-Parker JR, Strahler TR, Vorwald VM, Pierce GL, Seals DR. Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J Appl Physiol (1985) 122: 11–19, 2017. doi: 10.1152/japplphysiol.00732.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985) 105: 1323–1332, 2008. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seals DR, Nagy EE, Moreau KL. Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol 597: 4901–4914, 2019. doi: 10.1113/JP277764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serviente C, Troy LM, de Jonge M, Shill DD, Jenkins NT, Witkowski S. Endothelial and inflammatory responses to acute exercise in perimenopausal and late postmenopausal women. Am J Physiol Regul Integr Comp Physiol 311: R841–R850, 2016. doi: 10.1152/ajpregu.00189.2016. [DOI] [PubMed] [Google Scholar]
- 48.Serviente C, Witkowski S. Follicle-stimulating hormone, but not cardiorespiratory fitness, is associated with flow-mediated dilation with advancing menopausal stage. Menopause 26: 531–539, 2019. doi: 10.1097/GME.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silvestro A, Scopacasa F, Oliva G, de Cristofaro T, Iuliano L, Brevetti G. Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis 165: 277–283, 2002. doi: 10.1016/S0021-9150(02)00235-6. [DOI] [PubMed] [Google Scholar]
- 50.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Stages of Reproductive Aging Workshop (STRAW). J Womens Health Gend Based Med 10: 843–848, 2001. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 51.Tan E, Gurjar MV, Sharma RV, Bhalla RC. Estrogen receptor-α gene transfer into bovine aortic endothelial cells induces eNOS gene expression and inhibits cell migration. Cardiovasc Res 43: 788–797, 1999. doi: 10.1016/S0008-6363(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka H, Desouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. J Appl Physiol (1985) 83: 1947–1953, 1997. doi: 10.1152/jappl.1997.83.6.1947. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 54.The NAMS 2017 Hormone Therapy Position Statement Advisory Panel The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 24: 728–753, 2017. doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 55.Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ, Ghiadoni L. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40: 2534–2547, 2019. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 56.Thom NJ, Early AR, Hunt BE, Harris RA, Herring MP. Eating and arterial endothelial function: a meta-analysis of the acute effects of meal consumption on flow-mediated dilation. Obes Rev 17: 1080–1090, 2016. doi: 10.1111/obr.12454. [DOI] [PubMed] [Google Scholar]
- 57.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 18: 677–685, 1998. doi: 10.1161/01.ATV.18.5.677. [DOI] [PubMed] [Google Scholar]
- 58.Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 101: 2258–2263, 2000. doi: 10.1161/01.CIR.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 59.Wagner AH, Schroeter MR, Hecker M. 17β-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J 15: 2121–2130, 2001. doi: 10.1096/fj.01-0123com. [DOI] [PubMed] [Google Scholar]
- 60.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 97: 1695–1701, 1998. doi: 10.1161/01.CIR.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 61.Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I, Howe PR. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens 31: 1819–1827, 2013. doi: 10.1097/HJH.0b013e328362b9d6. [DOI] [PubMed] [Google Scholar]
- 62.Wong RH, Coates AM, Buckley JD, Howe PR. Evidence for circulatory benefits of resveratrol in humans. Ann N Y Acad Sci 1290: 52–58, 2013. doi: 10.1111/nyas.12155. [DOI] [PubMed] [Google Scholar]
- 63.Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis 21: 851–856, 2011. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Wong RHX, Howe PRC, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis 21: 851–856, 2011. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Xia N, Daiber A, Förstermann U, Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol 174: 1633–1646, 2017. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoo JK, Pinto MM, Kim HK, Hwang CL, Lim J, Handberg EM, Christou DD. Sex impacts the flow-mediated dilation response to acute aerobic exercise in older adults. Exp Gerontol 91: 57–63, 2017. doi: 10.1016/j.exger.2017.02.069. [DOI] [PubMed] [Google Scholar]
- 67.Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signaling kinases. J Physiol 587: 3911–3920, 2009. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]