Abstract
Real-world tasks, such as avoiding obstacles, require a sequence of interdependent choices to reach accurate motor actions. Yet, most studies on primate decision making involve simple one-step choices. Here we analyze motor actions to investigate how sensorimotor decisions develop over time. In a go/no-go interception task human observers (n = 42) judged whether a briefly presented moving target would pass (interceptive hand movement required) or miss (no hand movement required) a strike box while their eye and hand movements were recorded. Go/no-go decision formation had to occur within the first few hundred milliseconds to allow time-critical interception. We found that the earliest time point at which eye movements started to differentiate actions (go versus no-go) preceded hand movement onset. Moreover, eye movements were related to different stages of decision making. Whereas higher eye velocity during smooth pursuit initiation was related to more accurate interception decisions (whether or not to act), faster pursuit maintenance was associated with more accurate timing decisions (when to act). These results indicate that pursuit initiation and maintenance are continuously linked to ongoing sensorimotor decision formation.
NEW & NOTEWORTHY Here we show that eye movements are a continuous indicator of decision processes underlying go/no-go actions. We link different stages of decision formation to distinct oculomotor events during open- and closed-loop smooth pursuit. Critically, the earliest time point at which eye movements differentiate actions preceded hand movement onset, suggesting shared sensorimotor processing for eye and hand movements. These results emphasize the potential of studying eye movements as a readout of cognitive processes.
Keywords: eye movements, human action, manual interception, prediction, perceptual decision making, visual motion
INTRODUCTION
Sensorimotor decisions in real-world scenarios often require a sequence of interdependent actions. For example, when a pedestrian steps onto a bike lane, an approaching cyclist has to decide whether to stop or to veer around the obstacle. Depending on the initial decision outcome the cyclist then has to decide how hard to brake or in which direction to swerve. Dynamically evolving decision processes have been studied in ecologically inspired tasks, such as spatial navigation in rodents (Harvey et al. 2012; Krumin et al. 2018; Pfeiffer and Foster 2013) or during visual search and foraging in human observers (Diamond et al. 2017; Najemnik and Geisler 2005; Yoon et al. 2018). Yet, the time course of visually guided sequential decisions in simple movement tasks is relatively unexplored. This study probes decision-making processes using a speeded manual go/no-go interception task. We investigate continuous eye movements as a key signature of the dynamics of two-stage decision making, exhibited in the execution or withholding of a motor action (the interceptive hand movement). Given that our task involves a series of alternate action components in a go/no-go task—i.e., whether or not to move the hand, and when to move it—we consider this a sensorimotor decision formation process and refer to the individual action components as interception decision and timing decision. Considering human action as the manifestation of choice- or decision-making processes (von Mises 1998) is in line with how these terms are used in the sensorimotor literature (Gallivan et al. 2018).
Goal-directed hand, arm, and body movements, such as those during obstacle avoidance, are accompanied by eye movements. During many natural tasks the eyes fixate on target objects as the hand approaches and shift to the next target at around the time the hand arrives (e.g., Ballard et al. 1992; Johansson et al. 2001; Land et al. 1999). Past research has consistently found a behavioral interdependency between eye and hand movements, indicating common or coordinated control of eye and hand motor control. Moreover, eye movements are related to and affected by sensorimotor decision formation in simple motion direction-discrimination tasks (Joo et al. 2016; McSorley and McCloy 2009) and more complex go/no-go paradigms (Fooken and Spering 2019; Kim et al. 2005). Fooken and Spering (2019) showed that smooth pursuit and saccadic eye movement parameters provide reliable estimates of go/no-go manual interceptions in humans. However, it is possible that eye movement modulations simply reflect observers’ actions rather than the underlying perceptual decision. That is, the decision to act typically requires more accurate visual control than the decision not to act. Alternatively, eye movements may indicate an early readout of the decision formation itself, reflecting the observer’s response before it is executed. The current study examined the role of eye movements during the time course of a two-stage decision process: the decision whether and when to intercept a moving target.
MATERIALS AND METHODS
Overview.
This paper relates eye movements to the time course of decision formation during a rapid go/no-go track-intercept task. To investigate the relationship between eye movements and task outcome we performed new analyses on a previously published data set (Fooken and Spering 2019). Paradigm and procedure are identical to this published experiment and are reproduced here for the reader’s convenience. New analyses developed for the current paper are described in detail.
Observers.
We collected data from 45 male observers and excluded 3 participants who did not follow instructions and moved their hand in more than 80% of trials, regardless of stimulus conditions. The remaining 42 observers consisted of 25 members of the University of British Columbia (UBC) male varsity baseball (mean age 19.4 ± 1.2 yr) team and 17 age- and gender-matched nonathletes (mean age 22.1 ± 1.8 yr). Because this study was designed to investigate the relation between eye movements and hand motor action, but not examine accuracy of each measure, we do not differentiate between these two subgroups of observers (see Fooken and Spering 2019 for a report of differences between athletes and nonathletes in this task). All observers had normal or corrected-to-normal visual acuity confirmed by an Early Treatment Diabetic Retinopathy Study (ETDRS) acuity chart; 36 were right handed, six were left handed. All observers were unaware of the purpose of the experiment. The experimental protocol adheres to the Declaration of Helsinki and was approved by the Behavioral Research Ethics Board at the University of British Columbia; observers gave written informed consent before participation.
Visual display and apparatus.
The visual target was shown at a luminance of 5.4 candela per meter squared (cd/m2) on a uniform gray background (35.9 cd/m2). Stimuli were back-projected onto a translucent screen with a PROPixx video projector (VPixx Technologies, Saint-Bruno, QC, Canada; refresh rate 60 Hz, resolution 1,280 (horizontal) × 1,024 (vertical) pixels). The displayed window was 44.5 (horizontal) × 36 (vertical) cm or 55° × 45° in size. Stimulus display and data collection were controlled by a PC (NVIDIA GeForce GT 430 graphics card) and the experiment was programmed in MATLAB 7.1 using Psychtoolbox 3.0.8 (Brainard 1997; Kleiner et al. 2007; Pelli 1997). Observers were seated in a dimly lit room at 46 cm distance from the screen with their head supported by a combined chin and forehead rest.
Experimental paradigm.
Observers were asked to track a small moving target (2° in diameter) and to predict whether it would pass (“go” response required) or miss (“no-go” required) a visible strike box (Fig. 1, A and B). We instructed observers to withhold a hand movement in miss trajectories and to intercept the ball while it was in the strike box in pass trajectories. Each interception started from a table-fixed position and was made with the index finger of the dominant hand.
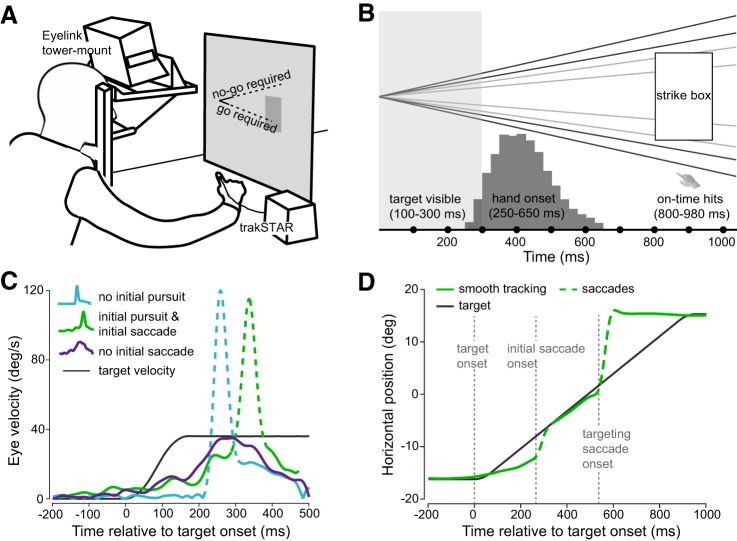
Fig. 1.
A: cartoon of the experimental setup. Observers had to judge whether a briefly presented target would pass (go required) or miss (no-go required) the strike box. Judgments were made by initiating or withholding an interceptive hand movement. B: illustration of task and interception events across time. The target was visible for 100, 200, or 300 ms, entered the strike box at ~800 ms, and was inside the box for ~180 ms. Observers initiated hand movements between 250 and 650 ms. The trial ended when the target reached the end of its trajectory (no-go), or when observers intercepted it (go). C: example initial eye velocity traces of single trials. Observers elicited three different types of eye movement patterns in response to target motion (black), either fixating until initiating a saccade (blue; 40.3% of trials), tracking the target before initiating a saccade (green; 53.9% of trials), or tracking it smoothly (purple; 5.8% of all trials). D: example eye position trace of a single trial in which a combination of smooth pursuit (solid green line) and saccades (dashed green line) was exhibited. Target motion onset, initial saccade onset, and targeting saccade onset are indicated by vertical dashed gray lines.
The stimulus followed a linear-diagonal path and either hit or missed a darker gray strike (31.5 cd/m2) box that was 6° × 10° in size and offset by 12° from the center to the side of interception (Fig. 1B). Stimulus velocity followed natural forces (gravity, drag force, Magnus force; Fooken et al. 2016). Launch angles were set to ± 5°, ± 7° (pass trajectories), ± 10°, or ± 12° (miss trajectories). Target speed was either 36 or 41°/s. Importantly, the target was only shown for 100, 200, or 300 ms and thus disappeared shortly after launch, making the task very challenging. All conditions were randomized and equally balanced. We instructed observers to track the target with their eyes and to follow its assumed trajectory even after it had disappeared. Each trial ended when observers either intercepted the target or when the target reached the edge of the screen (1–1.1 s). At the end of each trial observers received feedback about their performance; target end position was shown, and correct or incorrect actions were indicated. Each observer performed a familiarization session (16 trials; full trajectory visible) followed by 384 experimental trials in which the target viewing time was limited.
We defined four response types following conventions in the literature (Kim et al. 2005; Yang et al. 2010). Trials were classified as correct go if observers made an interception (i.e., touched the screen) in response to a pass trajectory and as incorrect go if observers moved their hands more than half way to the screen during a miss trajectory. Trials were classified as correct no-go or incorrect no-go if observers withheld a hand movement or moved their hand less than half way to the screen in response to a miss or pass trajectory, respectively. Interception decision accuracy (i.e., decision whether or not to move) was calculated as the percentage of all correct go and no-go responses.
Eye and hand movement recordings and preprocessing.
Eye position signals from the right eye were recorded with a video-based eye tracker (Eyelink 1000 tower mount; SR Research Ltd., Ottawa, ON, Canada) and sampled at 1,000 Hz. Eye movements were analyzed off-line using custom-made routines in MATLAB. Eye velocity profiles were filtered using a low-pass, second-order Butterworth filter with cutoff frequencies of 15 Hz (position) and 30 Hz (velocity). Saccades were detected based on a combined velocity and acceleration criterion: five consecutive frames had to exceed a fixed velocity criterion of 50°/s; saccade on- and offsets were then determined as acceleration minima and maxima, respectively, and saccades were excluded from pursuit analysis. Pursuit onset was detected in individual traces using a piecewise linear function that was fit to the filtered position trace (Fooken et al. 2016).
Finger position was recorded with a magnetic tracker (3D Guidance trakSTAR, Ascension Technology Corp., Shelburne, VT) at a sampling rate of 240 Hz; a lightweight sensor was attached to the observer’s dominant hand’s index fingertip with a small Velcro strap. The 2D finger interception position was recorded in x- and y-screen-centered coordinates. Each trial was manually inspected and a total of 345 trials (2%) were excluded across all observers due to eye or hand tracker signal loss.
Eye movement data analyses.
The stimulus characteristics in this paradigm triggered tracking behavior that most closely resembled short periods of smooth pursuit and catch-up saccades (Fig. 1, C and D). In some trials, observers tended to anticipate target motion (green and purple traces in Fig. 1C), whereas in other trials, observers fixated until initiating a catch-up saccade to match target speed (blue trace in Fig. 1C). To evaluate tracking behavior across time we analyzed eye movement quality during different time windows. In a previous study we found that observers typically made two to three saccades during the experimental paradigm. We defined the time interval from stimulus onset to the onset (or beginning) of the first saccade as our pursuit initiation time window. The time from the first saccade offset (or end of the first saccade) to the final saccade onset as the pursuit maintenance window (Fig. 1D). For trials, in which saccades occurred in between the first and last saccade we excluded the intermediate saccades from pursuit analysis. For the pursuit initiation and pursuit maintenance window we analyzed eye position and velocity relative to target position and velocity and extracted the following pursuit measures: mean 2D eye position and velocity error, relative eye velocity (gain), and absolute eye velocity. For each observer, we analyzed mean saccade rate across time as a temporal measure that is independent of spatial target position. For each trial we created a vector aligned to stimulus onset that contained an assigned value of 1 (eye in saccade state) or 0 (eye in fixation or pursuit state) at each time point. The mean saccade rate was then determined by calculating the mean probability of the eye being in saccade state at each time point.
For the pursuit initiation interval we calculated a speed-accuracy score combining the latency of the initial saccade with initial pursuit velocity. We normalized eye velocity error ê (accuracy) and initial saccade latency l̂ (speed) across all observers and trials. Note that we accounted for the inverse relationship between velocity error and accuracy (i.e., a higher velocity error corresponds to lower tracking accuracy) by calculating 1−ê as speed score. We then added the normalized speed and accuracy score and calculated an average speed-accuracy score
Go/no-go separation time and statistical analyses.
To calculate the time at which the eye movement signature starts to differ between go and no-go actions, we calculated the saccade rate for each observer and split the data into go and no-go trials. We then compared the saccade rate between go and no-go actions across time. We calculated a moving average of the saccade rate across a 5-ms time interval and downsampled the data from 1,000 Hz to 500 Hz to decrease the risk of detecting false negatives. We then performed a Mann–Whitney test for each time interval. The separation time was determined as the first-time interval of at least three consecutive intervals for which a P value smaller than 0.01 was achieved. Differences between eye and hand movement measures were evaluated using Welch’s two-sample paired t tests. Correlations were assessed using the Pearson R test. All statistical analyses were performed in RStudio version 1.0.136 (RStudio, Boston, MA).
RESULTS
Relating eye movements to actions (go/no-go) and underlying sensorimotor decision formation (i.e., correct or incorrect interception and timing decisions) revealed three main findings. First, the earliest time point at which eye movements started to differentiate go/no-go actions preceded hand movement onset. This result indicates that differences in eye movements for go compared with no-go actions were not merely a consequence of interceptive hand movements, but they occurred before hand movement execution. Second, higher eye velocity during pursuit initiation was related to higher accuracy of interception decisions. Third, higher eye velocity during pursuit maintenance was related to higher accuracy of timing decisions (when to intercept), suggesting that different stages of decision formation were linked to continuously evolving eye movements. In the following we will first qualitatively describe observers’ eye and hand movement response over the time course of the go/no-go interception task and then present quantitative results to support our three main findings.
Eye movement separation coincides with hand movement onset.
The go/no-go task employed in this study triggered a combination of smooth pursuit and saccadic eye movements. To determine at which time point eye movements differentiated go and no-go actions (accuracy of interception decisions) we investigated the change in saccade rate—a temporal measure that is independent of the spatial target position—for go- compared with no-go decisions. Observers typically made two to three saccades in each trial (M = 2.6 ± 0.4). The initial catch-up saccade (i.e., the first saccade in each trial) was on average elicited 240 ms (SD = 41.6 ms) after target onset. This saccade was followed by a brief period of tracking before a final, targeting saccade was made on average 620 ms after target onset (SD = 58.5 ms; Fig. 1D).
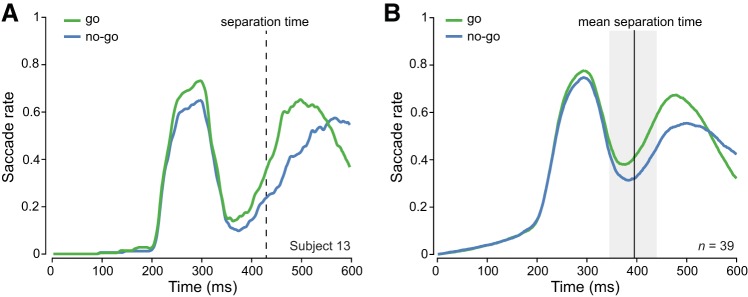
For each observer, we compared saccade rates between alternate action outcomes (go versus no-go). The time at which saccade rates first differed significantly (Mann–Whitney test, P < 0.01) was determined to be the go/no-go separation time (see materials and methods; Fig. 2A). For three observers we were unable to find a separation time until after the offset of the final saccade. Separation times for these observers differed by two or more standard deviations from the group mean and were therefore excluded from this analysis. For the remaining 39 observers the mean separation time was 395 ms (range 326–520 ms; Fig. 2B). In go trials, the same observers initiated a hand movement at 411 ms (range 320–536 ms), significantly later than the time point at which saccade rates started to differentiate [t(38) = 2.4, P = 0.02; Fig. 1B; Fig. 2]. These results indicate that the time at which eye movements started to reflect go/no-go actions preceded hand movement onset. Therefore, eye movement patterns that reflect go/no-go actions may not simply be a consequence of hand movement execution but could be an indicator of the ongoing decision formation underlying interception decisions.
Fig. 2.
Eye movement separation for go versus no-go actions. A: saccade rates for go versus no-go actions of a representative observer with a separation time of 428 ms. B: saccade rates for go versus no-go actions averaged across all observers that showed a differentiation (n = 39). Gray shaded area indicates standard deviation of separation time.
Pursuit initiation is related to interception decision accuracy.
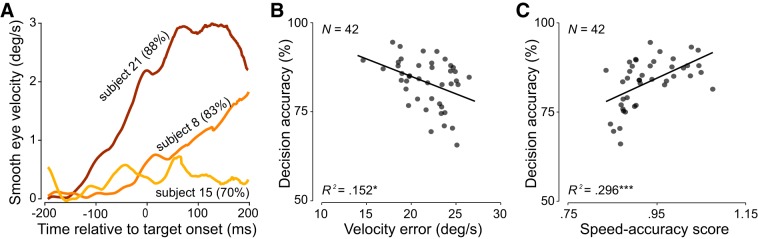
The time course of eye and hand movements suggests that decision formation underlying go/no-go actions must have occurred within the first few hundred milliseconds of each trial. The initial saccade offset was on average 350 ms (SD = 41.1 ms) after stimulus onset, only 50 ms before the average onset of interceptive hand movements. The brief delay between initial saccade offset and hand movement onset suggests that interception decision formation occurred before the initial saccade. We next investigated whether pursuit initiation (target onset to initial saccade onset) was related to the interception decision accuracy. Faster eye movements during the pursuit initiation period were associated with more accurate decisions whether or not to intercept (Fig. 3A), reflected in a significant positive correlation between pursuit initiation velocity and decision accuracy (r = 0.51, P < 0.001). Congruently, average eye velocity error (2D velocity difference between eye and target) during pursuit initiation was negatively correlated with interception decision accuracy (r = −0.39, P = 0.01; Fig. 3B). These results suggest that the initiation of smooth pursuit and the resulting decrease in velocity error might be related to target motion prediction and decision formation accuracy.
Fig. 3.
Relationship between eye movement initiation and interception decision accuracy. A: initial eye velocity from three observers averaged across 384 trials. Subject 15 (yellow) shows the lowest eye velocity during the pursuit initiation phase and had an overall lower decision accuracy (70%) than observers 8 (orange; 83%) and 21 (brown; 88%). B: decision accuracy is negatively related to eye velocity error in the interval from target onset to initial saccade onset. Asterisks denote significant regression results: *P < 0.05, ***P < 0.001. C: decision accuracy is positively related to modeled speed-accuracy score. Each data point represents the averaged value per observer.
However, we found that in ~40% of all trials observers fixated until initiating the first catch-up saccade (Fig. 1C). In these trials, observers might benefit from delaying the initial catch-up saccade to allow more time for evidence accumulation, resulting in a potential speed-accuracy tradeoff. To investigate whether initial saccade timing can account for some of the variability observed in the relationship between velocity error and decision accuracy (Fig. 3B), we calculated an average speed-accuracy score for each participant (see materials and methods) and related this score to each observer’s interception decision accuracy. The observed positive correlation between the speed-accuracy score and decision accuracy (r = 0.54, P < 0.001; Fig. 3C) indicates that the timing of the initial saccade plays an important role in decision formation accuracy. It would be preferable to confirm that the relation between eye velocity error and decision accuracy holds across individual observers by conducting trial-by-trial correlations. However, since go/no-go actions are binary, an underlying decision accuracy measure can only be calculated across trials.
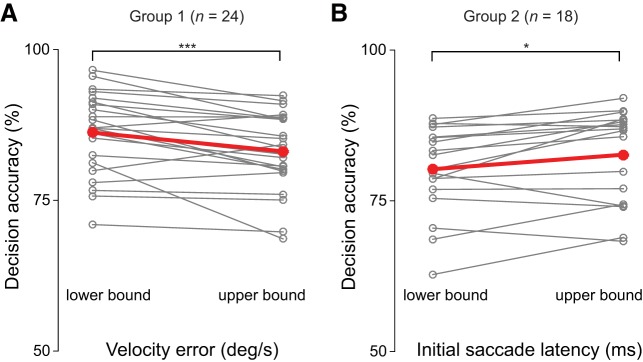
To further investigate the role of the accuracy and timing of pursuit initiation within observers we divided our sample into two groups—one that appeared to rely on reducing velocity error (group 1) and one that seemed to delay the initial saccade (group 2). Five observers did not reliably initiate smooth pursuit (<10% of the trials) and were automatically assigned to the saccade delay group (group 2). The remaining observers were assigned to groups as follows. We first performed a median split analysis on initial eye velocity error and initial saccade. For each observer we then calculated the mean interception decision accuracy below (lower bound) and above (upper bound) the median of eye velocity error and initial saccade latency. We then assigned observers to the group for which the change of interception decision accuracy between lower and upper bound was greater. For group 1 (n = 24), we found that decision accuracy was significantly higher in trials with a low compared with a high velocity error [t(23) = 4.1, P < 0.001; Fig. 4A]. For group 2 (n = 18) we found that decision accuracy was higher for late as compared with early initial saccade latencies [t(17) = 2.7, P = 0.01; Fig. 4B]. These results suggest that the timing and accuracy of pursuit initiation is related to go/no-go actions and underlying interception decision accuracy across as well as within observers.
Fig. 4.
Comparison of interception decision accuracy within each subject. A: difference in decision accuracy between trials in which eye velocity error was lower (lower bound) or higher (upper bound) than the median value for each observer (n = 24). B: difference in decision accuracy between trials in which initial saccade latency was earlier (lower bound) or later (upper bound) than the median value for each observer (n = 18). Gray lines show individual subject data; red thick line indicates group mean. Asterisks denote significant differences lower and upper bound: *P < 0.05, ***P < 0.001.
Pursuit maintenance is related to hitting accuracy.
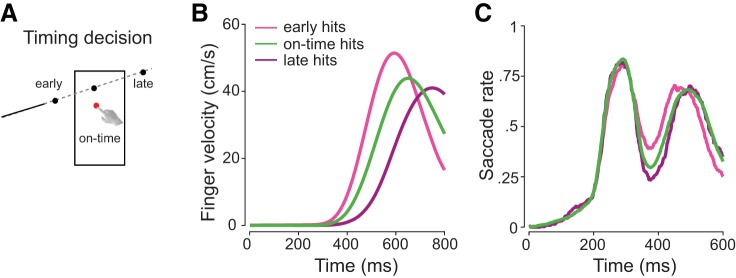
The previous results indicate that eye movement initiation is linked to the decision whether to intercept. In our task, a decision to intercept was always associated with a second decision: when to intercept (timing decision). Observers were instructed to hit the strike box while the target was inside. Whereas the spatial position of the target trajectory was restricted to the area of the strike box, observers had to time-critically judge horizontal target motion to successfully intercept the target. The timing of hitting the strike box was therefore crucial for accurate timing decisions (Fig. 5A). Across all interception (go-required) trials, observers’ interceptions were on time in 76 ± 6.6% of trials, too early in 18 ± 7.5% trials, and too late in 6 ± 4.5% trials. Incorrectly timed—early and late—interceptions were reflected in shifts in interceptive hand and eye movement onsets (Fig. 5, B and C; Table 1). In trials in which they intercepted too early as compared with trials in which they were on time, observers moved their hand earlier [t(41) = 15.3, P < 0.001] and faster [t(41) = 4.9, P < 0.001] and initiated the final targeting saccade earlier [t(41) = 5.8, P < 0.001]. Conversely, when interceptions were too late versus on time, observers initiated the interceptive hand movement later [t(41) = 11.9, P < 0.001] and made the final targeting saccade later [t(41) = 3.9, P < 0.001], whereas the finger velocity did not differ [t(41) = 0.1, P = 0.89]. We did not observe any difference in initial saccade latency when comparing on-time interceptions with timing errors that resulted in early [t(41) = 1.8, P = 0.08] or late interceptions [t(41) = 1.9, P = 0.06], suggesting that timing decision accuracy might not be related to the initial saccade.
Fig. 5.
A: timing decision accuracy depended on the timing of the interception. If the target had not yet reached the strike box at the time of interception the observer was too early; if it had already left the strike at the time of interception the observer was too late. Finger velocity (B) and saccade rate (C) separated by early (light pink), on-time (green), and late (purple) hits reflect timing errors.
Table 1.
Hand and eye movement differences for early, on-time, and late hits
| Hand Latency | Hand Peak Velocity | Initial Saccade Latency | Targeting Saccade Latency | |
|---|---|---|---|---|
| Early hit | 372.4 ± 38.2 ms | 59.7 ± 6.2 cm/s | 236.7 ± 43.1 ms | 506.5 ± 65.6 ms |
| On-time | 426.8 ± 44.4 ms | 57.3 ± 5.5 cm/s | 232.4 ± 38.4 ms | 542.8 ± 60.4 ms |
| Late hit | 513.1 ± 60.8 ms | 57.4 ± 6.9 cm/s | 219.1 ± 52.5 ms | 581.6 ± 81.7 ms |
Values indicate group averages ± standard deviation. Hand and targeting saccade latencies are relative to stimulus onset. The initial saccade was defined as the first saccade and the targeting saccade as the final saccade of each trial.
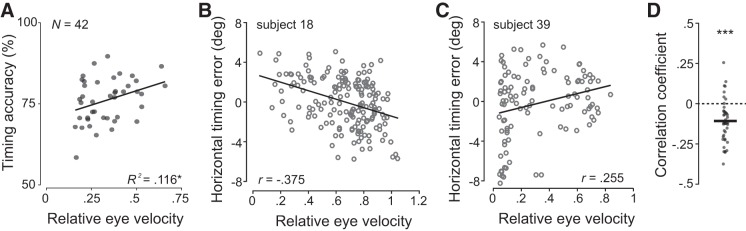
To investigate the relationship between eye movements and timing decision accuracy we analyzed eye movement velocity relative to target velocity during the pursuit maintenance phase (initial saccade offset to targeting saccade onset; Fig. 1C). Across observers, we observed a positive correlation between relative eye velocity and timing decision accuracy (r = 0.34, P = 0.03; Fig. 6A). Within observers we found that faster tracking of the target (higher relative eye velocity) was associated with earlier interceptions (negative horizontal timing error, Fig. 6B). This negative relationship was seen in the majority of our observers (correlation coefficients smaller than zero, Fig. 6D). For some observers, we found that poor tracking of the target (relative eye velocity around zero) was associated with overall poor timing decisions (see example in Fig. 6C). Overall correlation coefficients from within-subject correlations differed significantly from zero [one-sample t test: t(41) = 5.2, P < 0.001; Fig. 6D].
Fig. 6.
A: relationship between timing decision accuracy and relative eye velocity during maintenance phase. Each circle represents an observer. Asterisk denotes significant regression results: *P < 0.05. B and C: relationship between horizontal timing error and relative eye velocity for two representative observers. Each circle represents a single trial. D: correlation coefficient r between horizontal timing error and relative eye velocity of each observer. Mean correlation coefficient is significantly different from zero denoted by asterisks. ***P < 0.001.
Positional measures of tracking quality (2D or horizontal eye position error, saccade amplitudes) were not related to timing accuracy. These results indicate that observers benefit from matching eye and target velocity during pursuit maintenance when tasked to accurately judge target speed and successfully time an interception.
DISCUSSION
In this study we related continuously evolving eye movements to two-stage perceptual decisions in a go/no-go interception task. We showed that eye movements distinguished go/no-go actions early in the decision process, before the hand first started to move, and are therefore unlikely to merely be a consequence of motor execution. We also revealed that accurate smooth pursuit initiation was related to interception decision accuracy (reflecting the decision whether or not to act) and that accurate smooth pursuit maintenance was linked to timing decision accuracy (reflecting the decision when to act). These findings suggest that smooth pursuit eye movements continuously contribute to dynamic decision formation.
Eye movements as an early indicator of go/no-go actions.
Eye movements are closely related to cognitive goals in a variety of everyday tasks that require an interaction with objects, such as brick stacking or sandwich making (Hayhoe 2017; Hayhoe and Ballard 2005; Land et al. 1999). A particularly strong link between eye movements and action is seen in the context of goal-directed hand movements. It is commonly observed that the eye leads the hand when tasks require pointing, hitting, or catching (Bekkering et al. 1994; Belardinelli et al. 2016; Brenner and Smeets 2011; Land and McLeod 2000; Mrotek and Soechting 2007). We recently showed that eye movements reliably decoded go/no actions; go compared with no-go actions were associated with earlier targeting saccades to guide the interceptive hand movement (Fooken and Spering 2019).
The current study goes beyond previous work by addressing the question whether eye movements are the consequence of a perceptual decision, manifested in a hand movement, or whether they might instead reflect decision formation over time. Our results reveal that eye movements differentiated between later decisions whether or not to intercept at an early point in time, before the onset of the interceptive hand movement. This finding emphasizes that eye movements may indicate go/no-go actions before hand movements are executed. The concurrence of eye movement separation time and hand movement onset is further evidence for common neural processing of action goals (e.g., Andersen and Cui 2009; Crawford et al. 2004; Hwang et al. 2014). Moreover, our findings are closely related to the observation that eye and hand movements are interdependent during movement planning (Leclercq et al. 2013) and execution (Chen et al. 2016; Danion and Flanagan 2018; Fooken et al. 2016, 2018; Maiello et al. 2018). The interdependence and timing of eye and goal-directed hand movement preparation and execution could further be studied by combining eye tracking with measurements of the electromyographic activity of arm muscles during reach or point movements (e.g., Gribble et al. 2002).
Pursuit eye movements are related to interception and timing decision accuracy.
In the current study we show that the timing and accuracy of pursuit initiation was related to go/no-go actions; that is, higher pursuit initiation velocity was associated with higher interception decision accuracy. It should be noted, however, that we do not directly show a causal link between accurate eye movement initiation and interception decision formation. An alternative interpretation could be that observers initiated faster smooth pursuit eye movements because they perceived and extrapolated motion signals more accurately. Further studies are needed to identify the causality of the underlying mechanisms.
We further show that eye velocity during pursuit maintenance is linked to accurate interception timing. Previous research has shown that engaging in smooth pursuit aids accurate motion prediction, a benefit that is thought to arise from additional motion information provided through efference copy signals during pursuit maintenance (Bennett et al. 2010; Spering et al. 2011). Moreover, observers’ speed perception critically depends on the rate and direction of corrective saccades during tracking. Compared with trials in which observers tracked the target with pure smooth pursuit, observers overestimated target speed when tracking was accompanied by forward saccades and underestimated target speed when backward saccades were elicited (Goettker et al. 2018). Corrective saccades during smooth pursuit also affected manual interception accuracy: observers intercepted ahead or behind of the target when eliciting forward or backward saccades, respectively (Goettker et al. 2019). In our task, target motion was predictable and only forward saccades were elicited. We did not find any relationship between saccade rate or amplitude and accurate interception timing. Instead, we found that relative eye velocity with respect to the target velocity was linked to timing accuracy. These results complement previous findings showing that more accurate smooth pursuit eye movements (lower 2D position error) were linked to spatially more accurate manual interceptions (Fooken et al. 2016). Taken together these results suggest that velocity and positional error signals during smooth pursuit eye movements may contribute to different aspects of motion perception.
We further found that both saccades and smooth pursuit measures were related to interception and timing decision accuracy. These results highlight the interdependence of the two eye movement systems and indicate that smooth pursuit and saccades work in synchrony to enable accurate motion prediction (Barborica and Ferrera 2004; Blohm et al. 2003; de Brouwer et al. 2002; Orban de Xivry et al. 2006; Orban de Xivry and Lefèvre 2007; Schreiber et al. 2006). Our findings show that an increase in eye velocity as well as timing of the initial saccade are both beneficial for the accuracy of go/no-go actions, a novel finding that needs to be investigated further to identify the underlying mechanisms and speed-accuracy tradeoffs.
Perceptual decision making and hand motor responses are interdependent.
Perceptual decisions can be biased by motor actions. For example, when participants indicated their choice in a motion discrimination task by left- or right-handed reaches that were associated with different mechanical loads, their motion perception was biased toward the side that had lower resistance. Interestingly, this perceptual bias occurred even though participants were not aware of the difference in motor cost between the two hands (Hagura et al. 2017). Notwithstanding these biases, eye and hand movements are modulated by prior perceptual decisions. When observers made visually guided (Joo et al. 2016) or choice-indicating (McSorley and McCloy 2009) saccades just after a perceptual judgement, saccades in the decision-congruent direction were initiated earlier and faster. When observers’ hand movements were perturbed while making manual choice responses in a motion discrimination task, arm muscular reflex gains scaled with stimulus motion strength (Selen et al. 2012). This finding suggests that sensorimotor control is linked to ongoing perceptual decision making. Taken together, these findings indicate that there is a continuous cross talk between perceptual decision processes and evolving motor plans.
Further evidence for the close relationship between perceptual and motor processing during decision making comes from studies of neural activity in motor cortex in human and nonhuman primates. Neural population activity measured by magnetoencephalography in human observers were predictive of decision outcome in a motion detection task before observers indicated their choice (Donner et al. 2009; Pape and Siegel 2016). Furthermore, electrophysiological recordings of the dorsal premotor and primary motor cortex of macaque monkeys revealed that neural activity reflects changes of mind during reach target selection when the position of correct targets had to be updated dynamically (Kaufman et al. 2015; Thura and Cisek 2014). These results suggest that the readout of sensory information is continuously coupled to motor preparation and execution.
Cortical decision correlates.
Neural and behavioral correlates of perceptual decision making have classically been studied using random-dot motion stimuli gradually adding to our understanding of decision networks in human observers (Gold and Shadlen 2007; Heekeren et al. 2008; Schall 2013). Yet, real-world scenarios require more complex perceptual decisions than judging net motion. In a sequential decision task nonhuman primates were trained to select a target that was associated with a certain rule (pick the smaller or darker target). Monkeys then had to discriminate two visual targets based on their initial choice and responded by making a saccade to the chosen target (Abzug and Sommer 2018). Rule selection and sequential decision monitoring were related to neural activity in the supplementary eye field, an area also associated with the predictive control of eye movements (Fukushima et al. 2006).
Similarly, a series of seminal studies investigating go/no-go actions in human (Heinen et al. 2006) and nonhuman primates (Kim et al. 2005; Yang and Heinen 2014; Yang et al. 2010) revealed neural decision correlates in the supplementary and frontal eye fields. Speed-accuracy tradeoff of saccadic eye movements in a visual search task is also encoded in the frontal eye fields (Heitz and Schall 2012). Taken together, these findings indicate that neural activity in the supplementary and frontal eye fields governs timing and performance monitoring of visual decision making and may play a key role in our paradigm.
Limitations.
One limitation of using a go/no-go paradigm is that interception decision accuracy is a binary variable; that is, the decision to go (or not to go) is either correct or incorrect. Designing a task with a continuous measure of decision accuracy would allow us to carry out a more detailed trial-by-trial analysis than the median split analysis presented here (Fig. 4). Yet, go/no-go actions are interesting to study because the motor response is all-or-none and decisions cannot be corrected online. Another consideration is that manual interceptions had to occur within a specific time window. Hand movement onset or interception time can therefore not be interpreted as a classic measure of reaction time. The effect of decision timing on hand movement reaction time could be investigated in a future study. Notwithstanding these limitations, our results provide evidence for an interdependency of eye and hand movements with sensorimotor decision processes in human observers.
Conclusion.
Our findings emphasize commonalities in the timing and accuracy of oculomotor and hand movement control during sensorimotor decision making that underlies such actions. Eye movements provide a continuous readout of cognitive processes during two-stage decision formation. Because eye movements occur naturally and spontaneously, this may open new avenues for studying decision making processes in real-world scenarios.
GRANTS
This work was supported by Natinoal Sciences and Engineering Research Council Discovery and Accelerator Grants (RGPIN 418493) and a Canada Foundation for Innovation John R. Evans Leaders Fund equipment grant to M.S.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.F. and M.S. conceived and designed research; J.F. performed experiments; J.F. analyzed data; J.F. and M.S. interpreted results of experiments; J.F. prepared figures; J.F. drafted manuscript; J.F. and M.S. edited and revised manuscript; J.F. and M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Rose Shannon for help with data collection and preprocessing and Terry McCaig and the UBC Varsity Baseball team for their collaboration. Data were presented in preliminary form at the 2019 Vision Sciences Society meeting in St. Pete Beach, FL (Fooken and Spering, Program No.: 21.12) and the 2019 Gordon Research Seminar on Eye Movements in Lewiston, ME.
REFERENCES
- Abzug ZM, Sommer MA. Neuronal correlates of serial decision-making in the supplementary eye field. J Neurosci 38: 7280–7292, 2018. doi: 10.1523/JNEUROSCI.3643-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron 63: 568–583, 2009. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Ballard DH, Hayhoe MM, Li F, Whitehead SD. Hand-eye coordination during sequential tasks. Philos T R Soc B 337: 331–339, 1992. doi: 10.1098/rstb.1992.0111. [DOI] [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Modification of saccades evoked by stimulation of frontal eye field during invisible target tracking. J Neurosci 24: 3260–3267, 2004. doi: 10.1523/JNEUROSCI.4702-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkering H, Adam JJ, Kingma H, Huson A, Whiting HT. Reaction time latencies of eye and hand movements in single- and dual-task conditions. Exp Brain Res 97: 471–476, 1994. doi: 10.1007/BF00241541. [DOI] [PubMed] [Google Scholar]
- Belardinelli A, Stepper MY, Butz MV. It’s in the eyes: planning precise manual actions before execution. J Vis 16: 18, 2016. doi: 10.1167/16.1.18. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Baures R, Hecht H, Benguigui N. Eye movements influence estimation of time-to-contact in prediction motion. Exp Brain Res 206: 399–407, 2010. doi: 10.1007/s00221-010-2416-y. [DOI] [PubMed] [Google Scholar]
- Blohm G, Missal M, Lefèvre P. Interaction between smooth anticipation and saccades during ocular orientation in darkness. J Neurophysiol 89: 1423–1433, 2003. doi: 10.1152/jn.00675.2002. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Brenner E, Smeets JBJ. Continuous visual control of interception. Hum Mov Sci 30: 475–494, 2011. doi: 10.1016/j.humov.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Chen J, Valsecchi M, Gegenfurtner KR. Role of motor execution in the ocular tracking of self-generated movements. J Neurophysiol 116: 2586–2593, 2016. doi: 10.1152/jn.00574.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JD, Medendorp WP, Marotta JJ. Spatial transformations for eye-hand coordination. J Neurophysiol 92: 10–19, 2004. doi: 10.1152/jn.00117.2004. [DOI] [PubMed] [Google Scholar]
- Danion FR, Flanagan JR. Different gaze strategies during eye versus hand tracking of a moving target. Sci Rep 8: 10059, 2018. doi: 10.1038/s41598-018-28434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brouwer S, Yuksel D, Blohm G, Missal M, Lefèvre P. What triggers catch-up saccades during visual tracking? J Neurophysiol 87: 1646–1650, 2002. doi: 10.1152/jn.00432.2001. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Wolpert DM, Flanagan JR. Rapid target foraging with reach or gaze: the hand looks further ahead than the eye. PLOS Comput Biol 13: e1005504, 2017. doi: 10.1371/journal.pcbi.1005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Siegel M, Fries P, Engel AK. Buildup of choice-predictive activity in human motor cortex during perceptual decision making. Curr Biol 19: 1581–1585, 2009. doi: 10.1016/j.cub.2009.07.066. [DOI] [PubMed] [Google Scholar]
- Fooken J, Lalonde KM, Mann GK, Spering M. Eye movement training is most effective when it involves a task-relevant sensorimotor decision. J Vis 18: 18, 2018. doi: 10.1167/18.4.18. [DOI] [PubMed] [Google Scholar]
- Fooken J, Spering M. Decoding go/no-go decisions from eye movements. J Vis 19: 5, 2019. doi: 10.1167/19.2.5. [DOI] [PubMed] [Google Scholar]
- Fooken J, Yeo S-H, Pai DK, Spering M. Eye movement accuracy determines natural interception strategies. J Vis 16: 1–15, 2016. doi: 10.1167/16.14.1. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Kurkin S, Kaneko CR, Fukushima K. The vestibular-related frontal cortex and its role in smooth-pursuit eye movements and vestibular-pursuit interactions. J Vestib Res 16: 1–22, 2006. [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Chapman CS, Wolpert DM, Flanagan JR. Decision-making in sensorimotor control. Nat Rev Neurosci 19: 519–534, 2018. doi: 10.1038/s41583-018-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettker A, Braun DI, Schütz AC, Gegenfurtner KR. Execution of saccadic eye movements affects speed perception. Proc Natl Acad Sci USA 115: 2240–2245, 2018. doi: 10.1073/pnas.1704799115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettker A, Brenner E, Gegenfurtner KR, de la Malla C. Corrective saccades influence velocity judgments and interception. Sci Rep 9: 5395, 2019. doi: 10.1038/s41598-019-41857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci 30: 535–574, 2007. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Gribble PL, Everling S, Ford K, Mattar A. Hand-eye coordination for rapid pointing movements. Arm movement direction and distance are specified prior to saccade onset. Exp Brain Res 145: 372–382, 2002. doi: 10.1007/s00221-002-1122-9. [DOI] [PubMed] [Google Scholar]
- Hagura N, Haggard P, Diedrichsen J. Perceptual decisions are biased by the cost to act. eLife 6: e18422, 2017. [Erratum in eLife 6: e26902, 2017]. doi: 10.7554/eLife.18422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484: 62–68, 2012. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhoe M, Ballard D. Eye movements in natural behavior. Trends Cogn Sci 9: 188–194, 2005. doi: 10.1016/j.tics.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Hayhoe MM. Vision and Action. Annu Rev Vis Sci 3: 389–413, 2017. doi: 10.1146/annurev-vision-102016-061437. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nat Rev Neurosci 9: 467–479, 2008. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Rowland J, Lee B-T, Wade AR. An oculomotor decision process revealed by functional magnetic resonance imaging. J Neurosci 26: 13515–13522, 2006. doi: 10.1523/JNEUROSCI.4243-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz RP, Schall JD. Neural mechanisms of speed-accuracy tradeoff. Neuron 76: 616–628, 2012. doi: 10.1016/j.neuron.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang EJ, Hauschild M, Wilke M, Andersen RA. Spatial and temporal eye-hand coordination relies on the parietal reach region. J Neurosci 34: 12884–12892, 2014. doi: 10.1523/JNEUROSCI.3719-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Westling G, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci 21: 6917–6932, 2001. doi: 10.1523/JNEUROSCI.21-17-06917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo SJ, Katz LN, Huk AC. Decision-related perturbations of decision-irrelevant eye movements. Proc Natl Acad Sci USA 113: 1925–1930, 2016. doi: 10.1073/pnas.1520309113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Ryu SI, Shenoy KV. Vacillation, indecision and hesitation in moment-by-moment decoding of monkey motor cortex. eLife 4: e04677, 2015. doi: 10.7554/eLife.04677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-G, Badler JB, Heinen SJ. Trajectory interpretation by supplementary eye field neurons during ocular baseball. J Neurophysiol 94: 1385–1391, 2005. doi: 10.1152/jn.00109.2005. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s new in Psychtoolbox-3. Perception 36: 1–16, 2007. doi: 10.1068/v070821. [DOI] [Google Scholar]
- Krumin M, Lee JJ, Harris KD, Carandini M. Decision and navigation in mouse parietal cortex. eLife 7: e42583, 2018. doi: 10.7554/eLife.42583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M, Mennie N, Rusted J. The roles of vision and eye movements in the control of activities of daily living. Perception 28: 1311–1328, 1999. doi: 10.1068/p2935. [DOI] [PubMed] [Google Scholar]
- Land MF, McLeod P. From eye movements to actions: how batsmen hit the ball. Nat Neurosci 3: 1340–1345, 2000. doi: 10.1038/81887. [DOI] [PubMed] [Google Scholar]
- Leclercq G, Blohm G, Lefèvre P. Accounting for direction and speed of eye motion in planning visually guided manual tracking. J Neurophysiol 110: 1945–1957, 2013. doi: 10.1152/jn.00130.2013. [DOI] [PubMed] [Google Scholar]
- Maiello G, Kwon M, Bex PJ. Three-dimensional binocular eye-hand coordination in normal vision and with simulated visual impairment. Exp Brain Res 236: 691–709, 2018. doi: 10.1007/s00221-017-5160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley E, McCloy R. Saccadic eye movements as an index of perceptual decision-making. Exp Brain Res 198: 513–520, 2009. doi: 10.1007/s00221-009-1952-9. [DOI] [PubMed] [Google Scholar]
- Mrotek LA, Soechting JF. Target interception: hand-eye coordination and strategies. J Neurosci 27: 7297–7309, 2007. doi: 10.1523/JNEUROSCI.2046-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najemnik J, Geisler WS. Optimal eye movement strategies in visual search. Nature 434: 387–391, 2005. doi: 10.1038/nature03390. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Bennett SJ, Lefèvre P, Barnes GR. Evidence for synergy between saccades and smooth pursuit during transient target disappearance. J Neurophysiol 95: 418–427, 2006. doi: 10.1152/jn.00596.2005. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Lefèvre P. Saccades and pursuit: two outcomes of a single sensorimotor process. J Physiol 584: 11–23, 2007. doi: 10.1113/jphysiol.2007.139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape A-A, Siegel M. Motor cortex activity predicts response alternation during sensorimotor decisions. Nat Commun 7: 13098, 2016. doi: 10.1038/ncomms13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497: 74–79, 2013. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. Macrocircuits: decision networks. Curr Opin Neurobiol 23: 269–274, 2013. doi: 10.1016/j.conb.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber C, Missal M, Lefèvre P. Asynchrony between position and motion signals in the saccadic system. J Neurophysiol 95: 960–969, 2006. doi: 10.1152/jn.00315.2005. [DOI] [PubMed] [Google Scholar]
- Selen LPJ, Shadlen MN, Wolpert DM. Deliberation in the motor system: reflex gains track evolving evidence leading to a decision. J Neurosci 32: 2276–2286, 2012. doi: 10.1523/JNEUROSCI.5273-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering M, Schütz AC, Braun DI, Gegenfurtner KR. Keep your eyes on the ball: smooth pursuit eye movements enhance prediction of visual motion. J Neurophysiol 105: 1756–1767, 2011. doi: 10.1152/jn.00344.2010. [DOI] [PubMed] [Google Scholar]
- Thura D, Cisek P. Deliberation and commitment in the premotor and primary motor cortex during dynamic decision making. Neuron 81: 1401–1416, 2014. doi: 10.1016/j.neuron.2014.01.031. [DOI] [PubMed] [Google Scholar]
- von Mises L. Human Action: A Treatise on Economics. The Scholar’s Edition. Auburn, AL: Ludwig von Mises Institute, 1998. [Google Scholar]
- Yang SN, Heinen S. Contrasting the roles of the supplementary and frontal eye fields in ocular decision making. J Neurophysiol 111: 2644–2655, 2014. doi: 10.1152/jn.00543.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Hwang H, Ford J, Heinen S. Supplementary eye field activity reflects a decision rule governing smooth pursuit but not the decision. J Neurophysiol 103: 2458–2469, 2010. doi: 10.1152/jn.00215.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Geary RB, Ahmed AA, Shadmehr R. Control of movement vigor and decision making during foraging. Proc Natl Acad Sci USA 115: E10476 –E10485, 2018. [Erratum in Proc Natl Acad Sci USA 115: E11884, 2018]. doi: 10.1073/pnas.1812979115. [DOI] [PMC free article] [PubMed] [Google Scholar]