Abstract
Generally behavioral neuroscience studies of the common marmoset employ adaptations of well-established training methods used with macaque monkeys. However, in many cases these approaches do not readily generalize to marmosets indicating a need for alternatives. Here we present the development of one such alternate: a platform for semiautomated, voluntary in-home cage behavioral training that allows for the study of naturalistic behaviors. We describe the design and production of a modular behavioral training apparatus using CAD software and digital fabrication. We demonstrate that this apparatus permits voluntary behavioral training and data collection throughout the marmoset’s waking hours with little experimenter intervention. Furthermore, we demonstrate the use of this apparatus to reconstruct the kinematics of the marmoset’s upper limb movement during natural foraging behavior.
NEW & NOTEWORTHY The study of marmosets in neuroscience has grown rapidly and presents unique challenges. We address those challenges with an innovative platform for semiautomated, voluntary training that allows marmosets to train throughout their waking hours with minimal experimenter intervention. We describe the use of this platform to capture upper limb kinematics during foraging and to expand the opportunities for behavioral training beyond the limits of traditional training sessions. This flexible platform can easily incorporate other tasks.
Keywords: behavioral training, marmosets, motor control
INTRODUCTION
Neurophysiological recordings of isolated single neurons in awake, behaving macaques began in the late 1960s, whereas the first reports of single neuron recordings from awake marmosets did not occur until the early 2000s (Evarts 1968; Lu et al. 2001). There is growing interest in the use of marmosets as a model species for systems neuroscience, but the techniques for working with marmosets in this context are relatively new as compared with those used with more standard model primate species (e.g., rhesus macaques) in neuroscience research. Because of the success of the model, the approach to training a macaque to perform an experimental task has remained, with few exceptions, relatively unchanged for decades. In general, the monkey is restrained while engaging in a trained task for a few hours in exchange for water or juice. This method is popular because it generally yields hundreds to thousands of repetitions of a given behavior over the course of a training session. However, our experience and early behavioral work has indicated that this approach may be ill suited for working with marmosets. Marmosets seem much less tolerant to restraint as this approach yields far fewer trials and limits the expression of natural behavior (Eliades and Wang 2003; Johnston et al. 2018; Prins et al. 2017). To partly address these issues, Wang and colleagues developed a technique for wireless neural recordings which allowed for the study of sensorimotor processing in freely vocalizing marmosets (Roy and Wang 2012). However, there has not been a complimentary innovation in behavioral training paradigms to increase trial counts.
Marmoset ethology and its implications for experimental design.
Marmosets are obligate gum feeders and prey species. Field studies estimate that marmosets spend ~30% of their waking hours feeding on exudates (Maier et al. 1982 in Sussman and Kinzey 1984) and spend 25–30% of their waking time foraging for insects (Abreu et al. 2016; Stevenson and Rylands 1988). To feed on exudates, marmosets must gouge wounds into the trunks of trees to access the gum. They gouge new holes and revisit previously gouged holes to feed on newly accumulated gum (Stevenson and Rylands 1988). These visits only last a few seconds (Stevenson and Rylands 1988). Their daily behavioral repertoire generally does not involve them sitting in a single place engaging in repetitive behaviors for multiple hours. With this in mind, we designed an approach to training marmosets that would allow them to voluntarily engage in experimental behavior for short sessions throughout their waking hours. To do so we sought to modify an approach successful applied to rodents where rats voluntarily head-fixed themselves for in vivo calcium imaging (Scott et al. 2013). To implement this approach, researchers designed a set of custom elements to ensure stable imaging and slowly acclimated the rat to the apparatus, gradually extending the duration of head fixation. Once the animal was trained, the process of data collection could proceed with minimal experimenter involvement. This sort of voluntary setup, that allowed the animal to engage in the experiment throughout the day as an expression of its normal behavioral repertoire, has proven to be a promising approach to behavioral training of marmosets as described here.
MATERIALS AND METHODS
Subjects.
All work described were done with three common marmosets (Callithrix jacchus) (two females, and one male, 375–410 g). All methods were approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Design criteria.
Informed by field studies of the marmoset’s natural behavioral repertoire (Stevenson and Rylands 1988; Sussman and Kinzey 1984), early work with marmosets in neuroscience (Eliades and Wang 2003, 2005, 2008a), and the novel approach to training and in-vivo calcium imaging developed by Scott et al. (2013), we developed a behavioral training apparatus that attaches to the marmosets’ home cage. This apparatus allows multiple socially housed marmosets to voluntarily engage in behavioral training throughout their waking hours.
The three primary design criteria for the final apparatus were 1) that it mounts to the home cage to allow for voluntary engagement in training throughout the marmosets’ waking hours, 2) that it provides reliable positioning of marmosets and clear views of the upper limbs for capturing the kinematics of reaching movements, and 3) it provides a flexible way to present different experimental tasks. Additionally, to validate the effectiveness of the apparatus as a training instrument, it had to have a way to monitor and record the marmosets’ behavior within it. Finally, to facilitate training using operant conditioning, the apparatus also had to include a method for precisely timed reward delivery.
Hardware design and iteration.
Inspired by the gum feeding behavior in which marmosets naturally engage, the first version of the apparatus trained the marmosets to assume the appropriate posture to receive a small volume of yogurt (Fig. 1A). This posture placed them in front of a tray that contained foraging substrate. The next version of the apparatus removed the yogurt reward, and we found that marmosets would still engage in foraging behavior within the apparatus. After a series of iterations optimizing the form of the apparatus to multiple motion capture modalities (Fig. 1A), the current version of the apparatus (Fig. 1B) has allowed us to record the kinematics of this foraging behavior to study sensorimotor cortical responses related to upper limb movement.
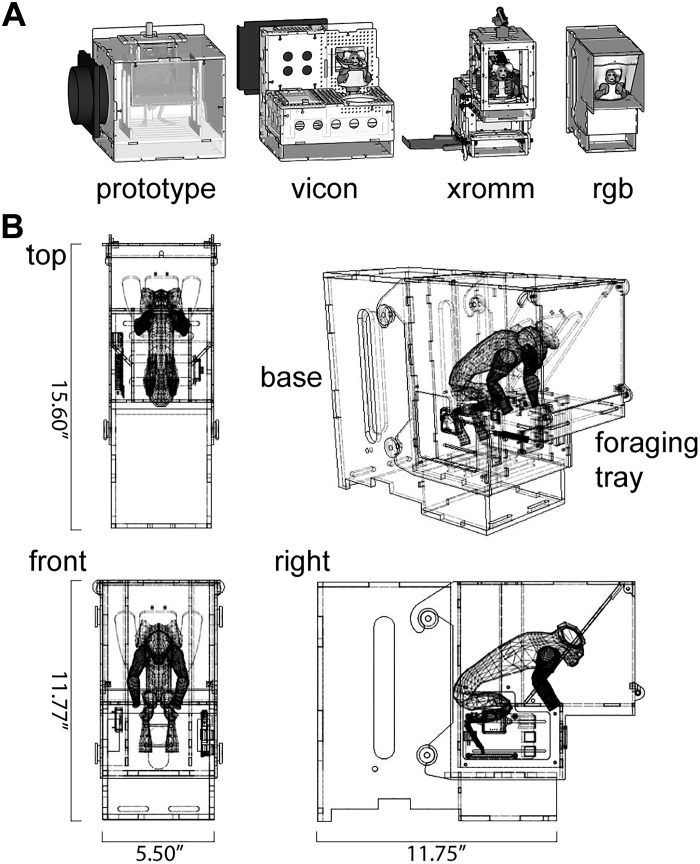
Fig. 1.
Developing a voluntary in-home cage approach to behavioral training with marmosets. A: iterations of apparatus design optimized for different motion capture modalities: the Vicon motion capture system, XROMM (X-ray reconstruction of moving morphology), and RGB video cameras. B: drawing of current version of the behavioral training apparatus.
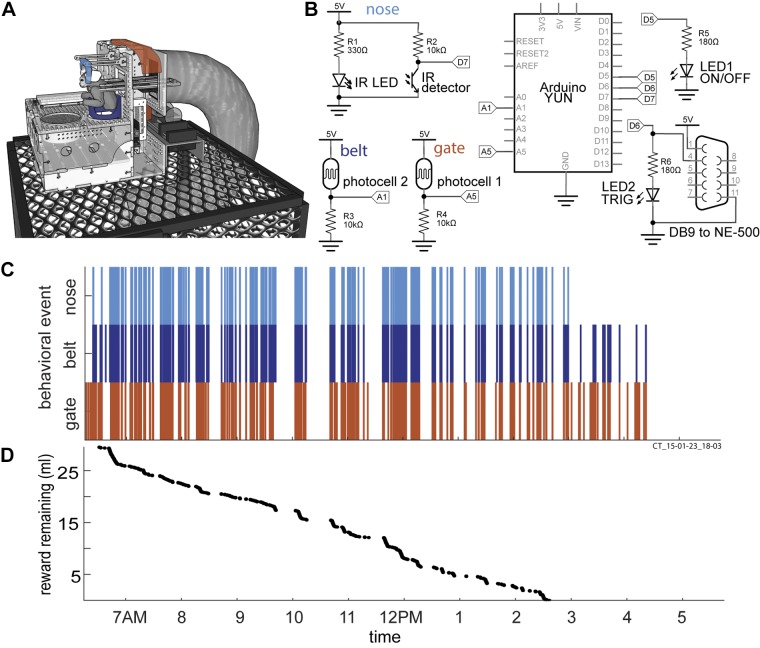
Designs of early versions of the behavioral training apparatus were done with CAD software called SketchUp, while later versions were designed using AutoDesk Fusion 360 (Fig. 1). The core of the apparatus was constructed using 1/8-in. or 1/4-in. thick clear acrylic sheets (continuous cast, McMaster Carr, Elmhurst, IL) that were cut into interlocking panels using a laser cutter (Universal Laser Systems VLS4.60). These panels were then assembled to achieve the form of the apparatus. To monitor the activity of the marmosets within the apparatus, we designed a simple circuit (Fig. 2, A and B) that included two photocells (CdS-photoresistor) and one infrared light based switch (IR switch comprising an IR phototransistor and IR LED pair), a syringe pump (syringepump.com, NE-500) and a network-connected microcontroller (Arduino YÚN). The sensors were recessed within the acrylic of the apparatus and acted as triggers to log the marmosets entering and leaving the gate, the belt, and the nosepiece of the apparatus. The sensor readings were logged to an SD card within the microcontroller, and the network connection of the microcontroller allowed remote operation of the apparatus. The apparatus sat on top of a round gate installed on the ceiling of the home cage (part no. 1822K314, McMaster-Carr).
Fig. 2.
Hardware design and single day of behavior within the apparatus. A: illustration of behavioral training apparatus with sensors embedded into the gate (orange), belt (dark blue) and nosepiece (light blue) to log behavior throughout the day. The foraging tray is placed in front of the belt. A syringe pump is connected to deliver reward, and sits behind the apparatus. The whole assembly sits on top of the home cage. B: circuit diagram detailing the circuit logging behavior and delivery of reward. C and D: results for a single day of behavior within the apparatus. C: vertical ticks indicate the time of trigger events for sensors within the gate, belt and nosepiece. For instance, an orange tick indicates the marmoset crossed the gate of the apparatus, a dark blue tick indicates the marmoset is within the belt of the apparatus and a light blue tick indicates that the marmoset has its nose positioned within the nosepiece. When the marmoset stays within the nosepiece, 0.1 ml of yogurt is dispensed every 10 s as positive reinforcement for assuming the appropriate posture (body within the belt and nose in the nosepiece). D: reward remaining as a function of time of day. Apparatus shown is the version adapted for the Vicon motion capture system. Acrylic cover above the marmoset and foraging tray not shown.
Software for automating and monitoring training.
We wrote a library (C++) to coordinate logging activity within the apparatus, evaluate reward-conditions, deliver reward, and allow remote apparatus operation. The object-oriented design of this library is meant to facilitate integration of future experimental tasks.
XROMM.
Bi-planar X-ray sources and image intensifiers (90 kV, 25 mA at 200 fps) allowed us to reconstruct time varying joint angles by tracking the 3D position of radio-opaque tantalum beads (0.5–1 mm, Bal-tec) placed within the soft tissue of the arm, hand and torso. Briefly, marmosets were anesthetized with ketamine/dexmedatomidine (2–6 mg/kg, 75–125 μg/kg) in combination with Atipamezole (750–1250 μg/kg) for reversal and Isoflurane (1–3%) for maintenance during the procedure. Markers were placed subcutaneously using an angeocatheter (16G, Becton, Dickinson and Company) with the following steps: insert needle, withdraw needle leaving sheath in place, drop the marker into sheath and replace needle to move the marker into place under the skin. Once the marker is implanted, both the needle and sheath are removed carefully to ensure the marker remains in place. If necessary, veterinary adhesive is used to close the angiocatheter entry point.
Implanted marker positions were tracked and used to calculated rigid body translations and rotations of the torso, humerus, forearm and hand using XMA-laboratory (Knörlein et al. 2016). Three-dimensional movement of the upper limb was described using joint coordinate systems (JCS) to measure the relative movement of anatomical coordinate systems (ACS), located in the torso, humerus, forearm and hand. Anatomical and joint coordinate systems were created within Autodesk MAYA using 3D models of the marmoset upper limb and torso skeletal anatomy.
Precision thresholds for joint angles and marker position errors were estimated using a frozen marmoset cadaver with markers placed as in the in-vivo study (Brainerd et al. 2010; Menegaz et al. 2015). Trials were recorded as this frozen specimen was moved around within the XROMM capture volume, and those data were taken through the marker tracking and joint angle estimation pipeline. As the specimen is frozen, one would expect that the distance between markers, the intermarker distances, should remain fixed. Any deviations in intermarker distance would reflect measurement error. As such we use the standard deviation of intermarker distances as our measure of marker tracking error. Similarly, one would expect that, with a frozen specimen, there should be no changes in joint angles, and any change in joint angle observed in this precision study would be a reflection of error accumulated in the joint angle estimation pipeline. With this logic, we use the standard deviation of each joint angle observed in the precision study as the threshold for measurable motion at that joint angle. We did not account for soft-tissue artifacts.
RESULTS
Foraging.
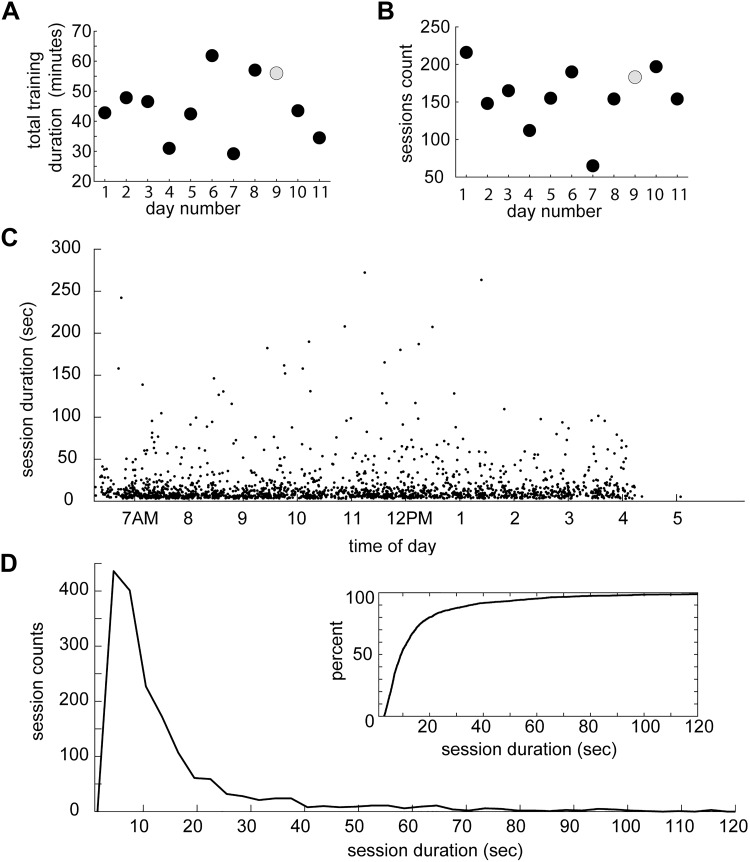
We began by studying foraging since this was a behavior in which the marmosets readily engaged. In early versions of the apparatus, foraging was coupled with the task of assuming an appropriate posture in exchange for yogurt reward. Marmosets engaged in behavior within the training apparatus throughout the day, and their engagement was sensitive to reward availability (Fig. 2, C and D). We measured each time a marmoset entered and exited the belt of the apparatus, i.e., the start and end of a session, to quantify the duration of these sessions. This measure allowed us to generate an estimate of how much time marmosets would spend engaging in behavior within the apparatus and how that behavior was distributed throughout their waking hours. Over the course of multiple days, we found that marmosets would spend up to an hour each day in the apparatus spanning 65–216 sessions (Fig. 3, A and B). Most of these sessions were not longer than 20 s, but some lasted almost five minutes (n = 1,739 sessions over 11 days, mean = 17.00 s, median = 9.36 s, Q1 = 6.99 s, Q3 = 16.67, max = 270 s) (Fig. 3, C and D).
Fig. 3.
Summary of sessions of behavior within the apparatus across days. A: total duration of all sessions within each day. B: number of sessions of behavior within each day. Gray circle indicates data point corresponding to day illustrated in Fig. 2. C: session durations as a function of time of day. Data were pooled across all days. Each point represents a single session. D: distribution of session durations. Inset: cumulative distribution of session durations.
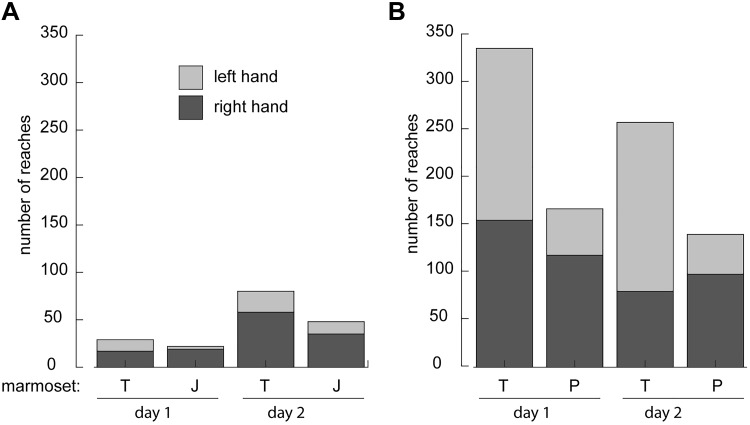
After validating that marmosets engage in voluntary behavioral training and gaining a sense of their attention span, we optimized the design of the apparatus to provide unobscured views of the upper limb (Fig. 1), removed the yogurt reward and set out to characterize foraging behavior within the apparatus. Using a custom-written algorithm (MATLAB) to define the video frame when the animal started foraging, reaches were subsequently counted manually. Reaches with both hands were counted over the course of a day (12 h). When only the foraging mix (some assortment of dried and fresh fruit pieces, mealworms and beetles, mushrooms and miniature marshmallows in a substrate of either dry oatmeal or aspen shavings) was provided in the apparatus, marmosets performed between 20 and 80 reaches while foraging each day (Fig. 4A). In contrast, if their entire daily diet (i.e., foraging mix and 1–2 oz ZuPreem Marmoset Diet) was provided within the behavioral training apparatus, the marmosets performed 100–300 reaches per day (Fig. 4B), and they would spend up to 1.5 h in the apparatus. Both of these conditions stand in contrast to our efforts to employ the traditional restraint based approach, similar to that described by Wang and colleagues (Remington et al. 2012) but with arms free to reach, which we found eliminated all foraging behavior regardless of the contents of the foraging mix. While restrained, the marmoset would reach for a desirable food item when an experimenter presented one, but would not voluntarily engage in foraging.
Fig. 4.
Reaches counted during foraging behavior within the apparatus. A: counts of reaches for two marmosets (T and J) across 2 days when only foraging mix was provided in the apparatus. B: counts of reaches for two marmosets (T and P) across 2 days when their entire daily diet was provided within the training apparatus. Extensions of the hand away from the torso to make contact with foraging substrate were manually counted as reaches.
Recording upper limb kinematics during foraging with XROMM.
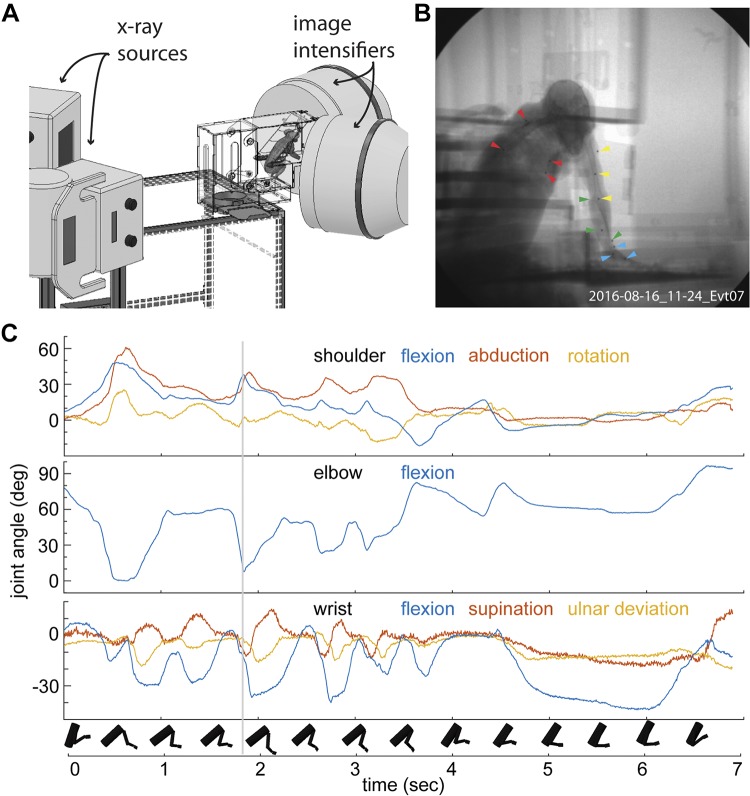
We next sought to record the kinematics of the upper limb during foraging. After confirming that marmosets do not tolerate retro-reflective markers placed on their skin needed for traditional near infrared based motion capture systems (e.g., VICON) (Takemi et al. 2014; Young et al. 2016) we moved to using an X-ray based system called XROMM or X-Ray Reconstruction of Moving Morphology (Brainerd et al. 2010) (Fig. 5A). Using a set of tools developed at Brown University (Brainerd et al. 2010; Knörlein et al. 2016; Miranda et al. 2011) and adaptations of joint coordinate systems for the upper limb (Baier and Gatesy 2013; Wu et al. 2005), we could translate the positions of these markers, with sub-millimeter precision, into joint kinematics (Fig. 5, B and C). Based on intermarker distance standard deviations for 13 markers, the mean marker tracking precision was 0.06 mm over 1,930 frames (n = 78 intermarker distances). The marker set allowed reconstruction of the seven degrees of freedom of the shoulder, elbow and wrist (Fig. 5C). A typical foraging sequence (Fig. 5C) consisted of multiple extensions of the arm toward food items (e.g., mealworm) within the foraging substrate followed by one to a few instances of coupled supination and flexion of the wrist as the marmoset attempted to grasp the food item. We report flexion, abduction and rotation of the shoulder, flexion of the elbow, and flexion, supination, and ulnar deviation of the wrist. We did not attempt to record the motion of individual digits. We estimate that we are able to reconstruct all reported joint angles to precisions within 1 degree, except shoulder rotation, for which the precision was within 2 degrees.
Fig. 5.
Capturing the kinematics of upper limb during foraging with XROMM. A: an illustration of the biplanar X-ray motion capture system, XROMM, together with the behavioral training apparatus and marmoset in the capture volume. B: a single frame of X-ray video of a marmoset foraging within the apparatus. Note radio-opaque markers placed within the marmoset’s torso (red), upper arm (yellow), forearm (green), and hand (blue). C: seven degrees of freedom of upper limb movement reconstructed by tracking the movement of the radio-opaque markers seen in B. Gray line indicates timestamp of the frame in B. Rigid bodies represent kinematics of the torso, upper arm, forearm and hand over the course of the foraging sequence. Precision thresholds estimated for joint angles are as follows: shoulder abduction: 0.304°; flexion: 0.208°; rotation: 1.824°; elbow flexion: 0.203°; wrist flexion: 0.424°; ulnar deviation: 0.636°; and supination: 1.107°.
DISCUSSION
Here we present a method for in home-cage, semiautomated, and voluntary behavioral training of marmosets that has in our experience been more prolific than adaptations of the more traditional approaches. The method presented also allows for the training of multiple marmosets in parallel. It provides a flexible platform for a variety of experimental tasks and liberates the animals from excessive restraints and provides a platform for marmosets to self-initiate natural behavior and engage in more traditional operant paradigms. It allows for behavioral engagement in short sessions throughout the marmosets waking hours rather than extended sessions, which are limited by marmoset cooperation and satiation. This flexible approach should allow us to contextualize results from relatively constrained and overtrained experimental tasks within the space of the marmoset’s natural behavioral repertoire. Toward this end, we are in the process of implementing an additional motor learning task and we are pairing this training approach with wireless neural recordings.
While we were able to successfully reconstruct foraging kinematics with XROMM, and have found that the XROMM does not interfere with our neural recordings (data not shown), XROMM is not the ideal complement to the in-home cage behavioral training we developed. First, the marmosets cannot have unrestricted 24-h access to the XROMM as it is a shared facility and requires a human operator to be present for X-ray generation. While the marmosets are able to voluntarily engage in behavioral training throughout their waking hours, we are only able to use XROMM to record the kinematics of that behavior for a brief portion of that time. Additionally, X-ray generation is limited to durations of ten seconds at a time to prevent overheating of the X-ray sources. As a result, the recording of foraging sequences often gets truncated, missing both the beginning and the end of a given sequence. To address these shortcomings, we are currently using XROMM to validate DeepLabCut, a markerless approach to capturing the kinematics of marmoset behavior that is becoming a widely used solution for quantifying kinematics due in part to its accessibility (Mathis et al. 2018). We plan to present a more detailed analysis of marmoset upper limb kinematics in a future paper with the validation of this approach.
It is clear that marmosets are well poised to contribute to our understanding of the operating principles of neocortex as attested by their increasing prevalence in published systems neuroscience reports (Miller 2017). Moreover the structure of marmoset neocortex provides a strong potential for targeted circuit manipulations (Izpisua Belmonte et al. 2015; Sasaki et al. 2009). Marmosets have been trained to perform experimental tasks such as eye fixation and smooth pursuit (Mitchell et al. 2014, 2015) and basic reaching and neuroprosthetic tasks (Ebina et al. 2018; Pohlmeyer et al. 2012, 2014, 2014) using training procedures common in macaque studies. But the quantity of behavior marmosets produce using these procedures is generally limited in comparison to that of macaques. In contrast, the techniques we designed dramatically increased the time available for behavioral training by eliminating the use of restraint and making the experimental training apparatus available to the marmosets throughout their waking hours. With this paradigm, our initial estimates suggest that we can minimally double and can often quadruple the quantity of experimentally useful behavioral trials with the added benefit that this behavior is self-initiated rather than generated through restriction. While we have demonstrated the utility of this approach for capturing foraging behavior, we believe it can be adapted to many other tasks where a subject needs to be positioned for stimulus presentation or interaction with some manipulandum. In addition, this approach creates a situation in which training time could be drastically reduced. We are beginning to use this approach to train marmosets on a visually cued reaching task on a tablet. Two of the marmosets exposed to the basic version of the task were able to learn the task within a few days of exposure, in contrast to the multiple months common with restraint based approaches. In the literature as well as in our experience, there is significant variability in task engagement across marmosets. Our in-home-cage training approach could also be used to support high throughput screening of individual marmosets for behavioral aptitude with relatively little experimenter involvement, before restraint acclimation in paradigms where restraint is deemed necessary. Finally, as we have argued (Walker et al. 2017), natural behaviors in of themselves warrant study and this particular experimental behavioral paradigm facilitates this class of study by providing an opportunity for marmosets to participate in more traditional experimental tasks within their natural behavioral repertoire.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01-NS-104898, National Science Foundation (NSF) Grants NSF-MRI-1338066 and NSF-IGERT, UChicago Big Vision Fund, and the Tarrson Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.W., J.N.M., and N.G.H. conceived and designed research; J.D.W., F.P., and N.G. performed experiments; J.D.W. and F.P. analyzed data; J.D.W. and F.P. interpreted results of experiments; J.D.W. and F.P. prepared figures; J.D.W. drafted manuscript; J.D.W., F.P., N.G., J.N.M., and N.G.H. edited and revised manuscript; J.D.W., F.P., N.G., J.N.M., and N.G.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the veterinary staff at the University of Chicago for assistance with marmoset care.
REFERENCES
- Abreu F, De la Fuente MFC, Schiel N, Souto A. Feeding ecology and behavioral adjustments: flexibility of a small neotropical primate (Callithrix jacchus) to survive in a semiarid environment. Mammal Res 61: 221–229, 2016. doi: 10.1007/s13364-016-0262-4. [DOI] [Google Scholar]
- Baier DB, Gatesy SM. Three-dimensional skeletal kinematics of the shoulder girdle and forelimb in walking Alligator. J Anat 223: 462–473, 2013. doi: 10.1111/joa.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd EL, Baier DB, Gatesy SM, Hedrick TL, Metzger KA, Gilbert SL, Crisco JJ. X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J Exp Zool A Ecol Genet Physiol 313: 262–279, 2010. doi: 10.1002/jez.589. [DOI] [PubMed] [Google Scholar]
- Ebina T, Masamizu Y, Tanaka YR, Watakabe A, Hirakawa R, Hirayama Y, Hira R, Terada SI, Koketsu D, Hikosaka K, Mizukami H, Nambu A, Sasaki E, Yamamori T, Matsuzaki M. Two-photon imaging of neuronal activity in motor cortex of marmosets during upper-limb movement tasks. Nat Commun 9: 1879, 2018. doi: 10.1038/s41467-018-04286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol 89: 2194–2207, 2003. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Dynamics of auditory-vocal interaction in monkey auditory cortex. Cereb Cortex 15: 1510–1523, 2005. doi: 10.1093/cercor/bhi030. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Chronic multi-electrode neural recording in free-roaming monkeys. J Neurosci Methods 172: 201–214, 2008a. doi: 10.1016/j.jneumeth.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol 31: 14–27, 1968. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Izpisua Belmonte JC, Callaway EM, Caddick SJ, Churchland P, Feng G, Homanics GE, Lee K-F, Leopold DA, Miller CT, Mitchell JF, Mitalipov S, Moutri AR, Movshon JA, Okano H, Reynolds JH, Ringach D, Sejnowski TJ, Silva AC, Strick PL, Wu J, Zhang F. Brains, genes, and primates. Neuron 86: 617–631, 2015. doi: 10.1016/j.neuron.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston KD, Barker K, Schaeffer L, Schaeffer D, Everling S. Methods for chair restraint and training of the common marmoset on oculomotor tasks. J Neurophysiol 119: 1636–1646, 2018. doi: 10.1152/jn.00866.2017. [DOI] [PubMed] [Google Scholar]
- Knörlein BJ, Baier DB, Gatesy SM, Laurence-Chasen JD, Brainerd EL. Validation of XMALab software for marker-based XROMM. J Exp Biol 219: 3701–3711, 2016. doi: 10.1242/jeb.145383. [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Neural representations of temporally asymmetric stimuli in the auditory cortex of awake primates. J Neurophysiol 85: 2364–2380, 2001. doi: 10.1152/jn.2001.85.6.2364. [DOI] [PubMed] [Google Scholar]
- Maier W, Alonso C, Langguth A. Field observations on Callithrix jacchus jacchus. Z Saugetier-Kunde 47: 334–346, 1982. [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21: 1281–1289, 2018. doi: 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
- Menegaz RA, Baier DB, Metzger KA, Herring SW, Brainerd EL. XROMM analysis of tooth occlusion and temporomandibular joint kinematics during feeding in juvenile miniature pigs. J Exp Biol 218: 2573–2584, 2015. doi: 10.1242/jeb.119438. [DOI] [PubMed] [Google Scholar]
- Miller CT. Why marmosets? Dev Neurobiol 77: 237–243, 2017. doi: 10.1002/dneu.22483. [DOI] [PubMed] [Google Scholar]
- Miranda DL, Schwartz JB, Loomis AC, Brainerd EL, Fleming BC, Crisco JJ. Static and dynamic error of a biplanar videoradiography system using marker-based and markerless tracking techniques. J Biomech Eng 133: 121002, 2011. doi: 10.1115/1.4005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Priebe NJ, Miller CT. Motion dependence of smooth pursuit eye movements in the marmoset. J Neurophysiol 113: 3954–3960, 2015. doi: 10.1152/jn.00197.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Reynolds JH, Miller CT. Active vision in marmosets: a model system for visual neuroscience. J Neurosci 34: 1183–1194, 2014. doi: 10.1523/JNEUROSCI.3899-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmeyer EA, Mahmoudi B, Geng S, Prins N, Sanchez JC. Brain-machine interface control of a robot arm using actor-critic rainforcement learning. Conf Proc IEEE Eng Med Biol Soc 2012: 4108–4111, 2012. doi: 10.1109/EMBC.2012.6346870. 23366831 [DOI] [PubMed] [Google Scholar]
- Pohlmeyer EA, Mahmoudi B, Geng S, Prins NW, Sanchez JC. Using reinforcement learning to provide stable brain-machine interface control despite neural input reorganization. PLoS One 9: e87253, 2014. doi: 10.1371/journal.pone.0087253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins NW, Pohlmeyer EA, Debnath S, Mylavarapu R, Geng S, Sanchez JC, Rothen D, Prasad A. Common marmoset (Callithrix jacchus) as a primate model for behavioral neuroscience studies. J Neurosci Methods 284: 35–46, 2017. doi: 10.1016/j.jneumeth.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington ED, Osmanski MS, Wang X. An operant conditioning method for studying auditory behaviors in marmoset monkeys. PLoS One 7: e47895, 2012. doi: 10.1371/journal.pone.0047895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Wang X. Wireless multi-channel single unit recording in freely moving and vocalizing primates. J Neurosci Methods 203: 28–40, 2012. doi: 10.1016/j.jneumeth.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature 459: 523–527, 2009. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Scott BB, Brody CD, Tank DW. Cellular resolution functional imaging in behaving rats using voluntary head restraint. Neuron 80: 371–384, 2013. doi: 10.1016/j.neuron.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson MF, Rylands AB. The marmosets, genus callithrix. In: Ecology and Behavior of Neotropical Primates, edited by Mittermeier RA, Rylands AB, Coimbra-Filho AF, da Fonseca GAB. Washington, DC: World Wildlife Fund, 1988, vol. 2, p. 131–222. [Google Scholar]
- Sussman RW, Kinzey WG. The ecological role of the callitrichidae: a review. Am J Phys Anthropol 64: 419–449, 1984. doi: 10.1002/ajpa.1330640407. [DOI] [PubMed] [Google Scholar]
- Takemi M, Kondo T, Yoshino-Saito K, Sekiguchi T, Kosugi A, Kasuga S, Okano HJ, Okano H, Ushiba J. Three-dimensional motion analysis of arm-reaching movements in healthy and hemispinalized common marmosets. Behav Brain Res 275: 259–268, 2014. doi: 10.1016/j.bbr.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Walker J, MacLean J, Hatsopoulos NG. The marmoset as a model system for studying voluntary motor control. Dev Neurobiol 77: 273–285, 2017. doi: 10.1002/dneu.22461. [DOI] [PubMed] [Google Scholar]
- Wu G, van der Helm FCT, Veeger HE, Makhsous M, Van Roy P, Anglin C, Nagels J, Karduna AR, McQuade K, Wang X, Werner FW, Buchholz B; International Society of Biomechanics . ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—Part II: shoulder, elbow, wrist and hand. J Biomech 38: 981–992, 2005. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Young JW, Stricklen BM, Chadwell BA. Effects of support diameter and compliance on common marmoset (Callithrix jacchus) gait kinematics. J Exp Biol 219: 2659–2672, 2016. doi: 10.1242/jeb.140939. [DOI] [PubMed] [Google Scholar]