Abstract
Human immunodeficiency virus (HIV)-1 transactivator of transcription protein (Tat) is a viral protein that promotes transcription of the HIV genome and possesses cell-signaling properties. Long-term exposure of central nervous system (CNS) tissue to HIV-1 Tat is theorized to contribute to HIV-associated neurodegenerative disorder (HAND). In the current study, we sought to directly evaluate the effect of HIV-1 Tat expression on the intrinsic electrophysiological properties of pyramidal neurons located in layer 2/3 of the medial prefrontal cortex and in area CA1 of the hippocampus. Toward that end, we drove Tat expression with doxycycline (100 mg·kg−1·day−1 ip) in inducible Tat (iTat) transgenic mice for 7 days and then performed single-cell electrophysiological studies in acute tissue slices made through the prefrontal cortex and hippocampus. Control experiments were performed in doxycycline-treated G-tg mice, which retain the tetracycline-sensitive promoter but do not express Tat. Our results indicated that the predominant effects of HIV-1 Tat expression are excitatory in medial prefrontal cortical pyramidal neurons yet inhibitory in hippocampal pyramidal neurons. Notably, in these two populations, HIV-1 Tat expression produced differential effects on neuronal gain, membrane time constant, resting membrane potential, and rheobase. Similarly, we also observed distinct effects on action potential kinetics and afterhyperpolarization, as well as on the current-voltage relationship in subthreshold voltage ranges. Collectively, these data provide mechanistic evidence of complex and region-specific changes in neuronal physiology by which HIV-1 Tat protein may promote cognitive deficits associated with HAND.
NEW & NOTEWORTHY We drove expression of human immunodeficiency virus (HIV)-1 transactivator of transcription protein (Tat) protein in inducible Tat (iTat) transgenic mice for 7 days and then examined the effects on the intrinsic electrophysiological properties of pyramidal neurons located in the medial prefrontal cortex (mPFC) and in the hippocampus. Our results reveal a variety of specific changes that promote increased intrinsic excitability of layer II/III mPFC pyramidal neurons and decreased intrinsic excitability of hippocampal CA1 pyramidal neurons, highlighting both cell type and region-specific effects.
Keywords: electrophysiology, hippocampus, HIV, prefrontal cortex, Tat
INTRODUCTION
Approximately 36 million people are infected with the human immunodeficiency virus (HIV) globally, and the United States alone contributes 50,000 cases of new infections each year (UNAIDS 2018). Although viral load from HIV-1 infection can be effectively managed using current combinatorial anti-retroviral therapy (cART), the prevalence of various comorbid disorders associated with infection, such as HIV-associated neurocognitive disorder (HAND), remains unchanged at ~50% (Ellis et al. 2014; Heaton et al. 2010, 2011). While neurons are considered to remain uninfected by HIV-1, neighboring microglia and astrocytes are infected and release viral products into the extracellular milieu (Rojas-Celis et al. 2019). The HIV-1 transactivator of transcription protein (HIV-1 Tat) is one such viral product that, while necessary for viral transcription, is also released into the extracellular space. Clinical evidence suggests that a subset of virally suppressed patients continue to express HIV-1 Tat despite an undetectable viral load (Henderson et al. 2019; Johnson et al. 2013). Thus HIV-1 Tat may be a mediator of comorbid pathology in patients with a nonproductive viral state.
Substantial literature suggests viral proteins, including HIV-1 Tat, may induce neuronal dysregulation and alterations in neuronal synaptic function. For example, in vitro exposure to the HIV-1 Tat has been reported to enhance NMDA receptor function, L-type calcium channel function, and presynaptic glutamate release, while decreasing GABAergic function and shifting the brain’s excitatory/inhibitory balance toward hyperexcitability (Musante et al. 2010; Napier et al. 2014; Song et al. 2003; Wayman et al. 2015; Xu and Fitting 2016; Xu et al. 2016). Similarly, significant literature suggests a role for HIV-1 Tat in behavioral and cognitive deficits associated with HAND (Carey et al. 2012; Fitting et al. 2013; Jacobs et al. 2019). Across a wide range of studies focused on either cellular or behavioral effects of HIV-1 Tat, there is reason to speculate that the protein may have brain region-specific effects, particularly in the prefrontal cortex versus hippocampus (Jacobs et al. 2019; Kesby et al. 2016, 2018; Nookala et al. 2018).
Nevertheless, few studies have directly evaluated the effects of HIV-1 Tat or other viral proteins on detailed aspects of neuronal physiology and fewer yet have directly compared cellular and synaptic changes observed across key cortical and temporal areas within the same cohort of animals. This study was created to address this gap in the literature. Specifically, we use contemporary in vitro electrophysiological tools in combination with the Teton glial fibrillary acidic protein (GFAP)-inducible (iTat) mouse model to reveal detailed changes in both passive and active intrinsic electrophysiological properties of cortical and hippocampal pyramidal neurons following 7 days of HIV-1 Tat protein exposure in vivo. Overall, our results reveal complex and region-specific effects of HIV-1 Tat expression on neuronal physiology, with the weight of the observed changes favoring hyperexcitability in the prefrontal cortex (PFC) and hypoexcitability in the hippocampus.
METHODS
Animals.
The HIV-1 Tat inducible mouse model was generated as previously described (Kim et al. 2003). Briefly, the Teton-GFAP (G-tg) and the Tre-Tat86 (T-tg) lines were crossbred to produce a colony of mice that expressed both the tetracycline-sensitive promoter and the Tat gene (iTat mice). HIV-1 Tat expression was induced in these animals using doxycycline, solubilized in 0.9% saline, and delivered intraperitoneally once per day for 7 days at 100 mg/kg (injection volume was 250 µl/25 g of body weight). Doxycycline-treated iTat mice are referred to throughout this article as TatPos mice. Mice lacking the Tat gene, but retaining the tetracycline-sensitive GFAP promoter (G-tg mice), were used as controls. Control (G-tg) mice that received an identical 7 days of doxycycline treatment (100 mg/kg) intraperitoneally are referred to as TatNeg mice. All mice used were male, aged 7–12 wk, and all animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Florida.
Brain slice preparation.
Following 7 days of doxycycline treatment, animals were deeply anesthetized with a ketamine/xylazine mixture (0.1 ml of 10% ketamine and 0.05 ml of 2% xylazine) and then transcardially perfused with ice-cold sucrose laden artificial cerebrospinal fluid (aCSF) that contained the following (in mM): 206 sucrose, 10 d-glucose, 1 MgSO4 2 KCl, 1.25 NaH2PO4, 1 CaCl2, and 25 NaHCO3. Immediately following perfusion, brains were rapidly extracted and submerged in the same sucrose-laden aCSF, and coronal sections (300-μm thick) were made through both the dorsal hippocampus and through the medial prefrontal cortex using a VT1000s vibratome (Leica Microsystems). After sectioning, slices were maintained for 30 min at ~36°C in aCSF that contained the following (in mM): 124 NaCl, 2.5 KCl, 1.23 NaH2PO4, 3 MgSO4, 10 d-glucose, 1 CaCl2, and 25 NaHCO3. Following the incubation period, slices were equilibrated to room temperature for a minimum of 30 min before use in experiments. All solutions were saturated with 95% O2-5% CO2 and had a pH of 7.3.
In vitro whole cell recordings.
To perform whole cell recordings, tissue sections were transferred to a slice chamber where they were visualized with infrared differential interference contrast microscopy (IR-DIC) using an Olympus BX51 WI upright microscope, an IR CCD camera (QImaging), and a ×40 water immersion objective. While in the slice chamber, tissue sections were continuously perfused at a rate of ~2 mL/min with aCSF that contained the following (in mM): 126 NaCl, 11 d-glucose, 1.5 MgSO4, 3 KCl, 1.2 NaH2PO4, 2.4 CaCl2, and 25 NaHCO3. This solution was saturated with 95% O2-5% CO2 and maintained at 30–32°C. Patch pipettes were made from borosilicate glass capillary tubes (BF150-86-10, Sutter Instruments) using a Flaming/Brown P-97 pipette puller (Sutter Instruments) and had an open tip resistance of 2–6 MΩ when filled with an internal solution that contained the following (in mM): 125 K-gluconate, 10 phosphocreatine, 1 MgCl2, 10 HEPES, 0.1 EGTA, 2 Na2ATP, and 0.25 Na3GTP. This solution was volume adjusted with KOH to a final pH of ~7.3 and final osmolarity of ~295 mOsm. In the hippocampus, CA1 pyramidal cells were readily identified by their large soma and presence in the CA1 pyramidal cell layer. A prominent apical dendrite extending toward stratum radiatum is often also discernable in IR-DIC. In the medial prefrontal cortex, layer 2/3 pyramidal neurons were identified (here almost exclusively in prelimbic cortex) by the location of their soma (in either layer 2 or 3) and by their pyramidal shape. The base of a prominent apical dendrite was also usually visible under IR-DIC. Throughout this study neurons in the both the medial PFC (mPFC) and dorsal hippocampus were examined in each animal, but no more than four cells were recorded in either brain region per mouse. Both current- and voltage-clamp recordings were made using a MultiClamp 700B amplifier (Molecular Devices), a Digidata 1440A digitizer (Molecular Devices), and Clampex 10.2 software (Molecular Devices). All data were sampled at 20 kHz. Voltage-clamp recordings were lowpass filtered at 2 kHz, and current-clamp recordings were lowpass filtered at 10 kHz. All data were analyzed using custom software written in OriginC (OriginLab, Northampton, MA) by C. J. Frazier. Additional details on specific protocols and analyses are provided below.
Neuronal gain.
To construct neuronal gain curves as presented in Fig. 1, neurons were held at 0 pA in current clamp, and then, a series of 17 half-second current injections (between −100 and 300 pA in 25-pA increments) were delivered through the recording pipette at 0.33 Hz. Action potentials produced by current injection were detected using parameter-based event detection software, and neuronal gain curves were constructed by plotting current injected versus frequency of firing observed.
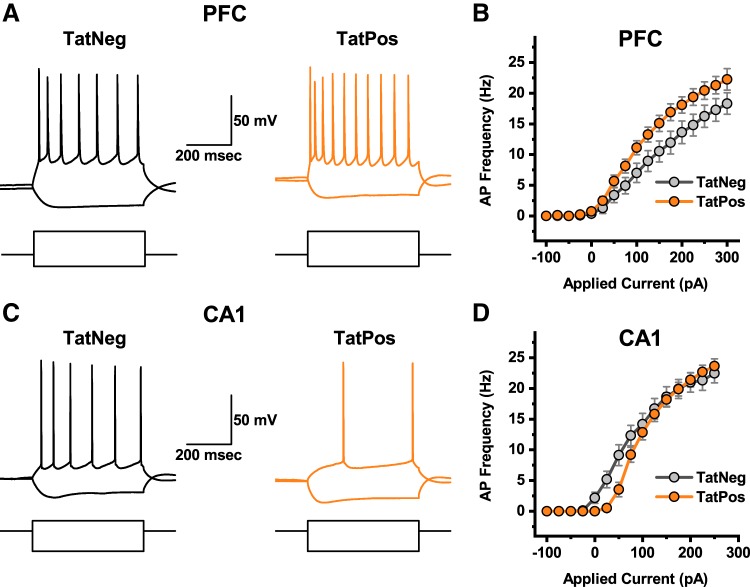
Fig. 1.
Region-specific effects of human immunodeficiency virus (HIV)-1 transactivator of transcription protein (Tat) on neuronal gain. A: response of a representative layer 2/3 medial prefrontal cortex (mPFC) pyramidal neuron obtained from a TatNeg animal (left, black traces) and TatPos animal (right, orange traces) to current steps of −100 pA and +200 pA (square pulses illustrated below the voltage traces). B: complete neuronal gain curves constructed using data from 14 TatNeg and 17 TatPos neurons indicate that HIV-1 Tat expression was associated with a clear increase in excitability of mPFC layer 2/3 pyramidal neurons (see results for additional details). C: response of a representative CA1 pyramidal neuron obtained from a TatNeg animal (left, black traces) and TatPos animal (right, orange traces) to current steps of −100 pA and +50 pA (square pulses illustrated below the voltage traces). D: complete neuronal gain curves constructed using data from 17 TatNeg and 17 TatPos neurons indicate that HIV-1 Tat expression was associated with a clear but selective decrease in excitability of CA1 pyramidal neurons, in response to current injections of <100 pA (see results for additional details).
Passive membrane properties.
To evaluate the effects of HIV-1 Tat expression on whole cell capacitance, input resistance, and membrane time constant, neurons were voltage clamped at −70 mV and the command voltage was transiently shifted to −80 mV first by delivery of a single 200-ms voltage step, and then 200 ms later, by two consecutive 50-ms voltage ramps (0.2 mV/ms) of opposing polarity. This series of voltage commands (both the step and ramp commands) was repeated 40 times over 23.4 s. For each square pulse, input resistance was calculated as Rm = [dV − (Ra × Iss)]/Iss, where dV is the change in voltage delivered during the step, Iss is the steady-state current observed at the end of the step, and Ra is the access resistance, calculated by dividing dV by the instantaneous current observed immediately after initiation of the voltage step. Whole cell capacitance was calculated from the current response to the voltage-clamp ramps as described in Golowasch et al. (2009). Membrane time constant was calculated as Rm × Cm and expressed in milliseconds.
Resting membrane potential, threshold, and rheobase.
Resting membrane potential was measured in current-clamp using techniques identical to those previously described for measuring tonic current in voltage clamp (Nahir et al. 2007). In brief, an amplitude histogram with 0.1-mV bins was constructed from 20,000 data points obtained during 1 s of recording at I = 0, and a Gaussian function was fit to the histogram using all bins that contained at least half the number of values as the largest bin. The resting membrane potential was then defined as the value observed at the center of that best-fit Gaussian curve. This technique was used rather than simply measuring the mean voltage observed at I = 0 because it is less sensitive to perturbation by spontaneous activity. To measure the action potential threshold and rheobase, cells were held briefly at I = 0 in current clamp and then exposed to a slow current ramp that increased current injection by 10 pA/s until action potentials were observed. Values reported for threshold and rheobase in Fig. 3 are those observed for the first action potential produced by the current ramp. Specifically, the threshold voltage was defined as the voltage at which dV/dt first exceeded 5% of the maximum rate observed during the rising phase of the spike. The rheobase was defined as the command current at the time of threshold. If a cell fired action potentials spontaneously at I = 0, then rheobase was defined as 0 pA, and threshold was still calculated as the voltage at which dV/dt first exceeded 5% of maximum.
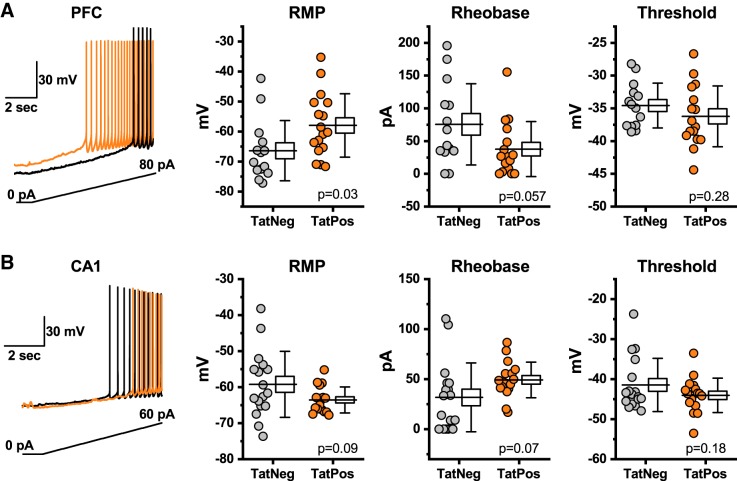
Fig. 3.
Region-specific effects of human immunodeficiency virus (HIV)-1 transactivator of transcription protein (Tat) on resting membrane potential (RMP), rheobase, and action potential threshold. A: HIV-1 Tat expression increases RMP and decreases rheobase in layer 2/3 pyramidal neurons without altering the threshold voltage. A, left: a slow current ramp (bottom trace) was delivered in current clamp until action potential threshold was reached. Black and orange current traces illustrate the response of representative Tat-negative (TatNeg) and Tat-positive (TatPos) layer 2/3 pyramidal neurons, respectively. Box plots illustrate the RMP, threshold, and rheobase for each cell tested. B: in contrast to effects observed in the medial prefrontal cortex (mPFC), HIV-1 Tat expression is associated with a trend toward decreased resting membrane potential and increased rheobase in CA1 pyramidal neurons. These trends are observed absent any apparent effects on the threshold voltage. B, left: illustrates the current protocol and representative data from a TatNeg and TatPos CA1 pyramidal cell (black trace and orange trace, respectively). Box plots are illustrated as described in the legend for Fig. 2 and in methods.
Fast action potential kinetics and the after hyperpolarization.
To examine fast action potential kinetics, we used custom software to extract the first action potential observed during a slow current ramp as above. Extracted action potentials were baseline subtracted to their threshold voltage, and aligned by peak amplitude. Average action potentials for each group were used to generate phase plots (dV/dt vs. dV) as presented in Fig. 4. For each individual action potential, half-width was defined as the width, in milliseconds, as observed at one-half amplitude, while amplitude was defined as the difference between the voltage at peak and the voltage at threshold. Afterhyperpolarization (AHP) amplitude is reported as the minimum value (relative to threshold) observed during the 20–130 ms after the action potential peak, and AHP area is defined as the area under the curve for the same time period.
Fig. 4.
Region-specific effects of human immunodeficiency virus (HIV)-1 transactivator of transcription protein (Tat) on action potential kinetics. A, left: illustrates first action potential (AP) observed in response to a slow current ramp in each medial prefrontal cortex (mPFC) neuron examined. Black traces are action potentials observed in neurons from TatNeg animals, while orange traces are from neurons obtained from TatPos animals. All traces are aligned by the action potential peak. A, middle: illustrates the average of the peak aligned action potentials from the TatNeg and TatPos groups. Shaded areas represent means ± SE. A, right: illustrates phase plots produced from those average traces. B: box plot illustrating the action potential half-width observed in each individual neuron from both the TatNeg and TatPos groups. Half-width was defined as the time (in ms) between the rising phase and the falling phase of the action potential, as observed at one-half amplitude (see methods for additional details). C, left: illustrates the first action potential observed in response to a slow current ramp in each CA1 pyramidal neuron examined. C, middle and right: illustrates the average of the peak aligned action potentials obtained from both TatNeg and TatPos neurons (shaded areas represent means ± SE) (middle) and phase plot constructed from those averages (right). D: box plot illustrating that HIV-1 Tat was associated with a significant increase in action potential half-width in CA1 pyramidal neurons.
Statistics.
For intrinsic properties represented by a single value measured from each neuron (input resistance, whole cell capacitance, resting membrane potential, threshold, rheobase, etc.) comparisons between TatPos and TatNeg groups of neurons were made using unpaired Student’s t tests. Welch’s correction was applied in cases where population variances were significantly different. For comparisons that involve multiple measurements per cell (e.g., neuronal gain and I–V curves), comparisons were made between TatPos and TatNeg groups using a repeated measures two-Way ANOVA with Holm-Sidak post hoc tests when appropriate. P ≤ alpha level of 0.05 was considered statistically significant, while P > 0.05 but ≤ 0.10 was considered representative of a trend. Error bars on all symbol + line plots (Figs. 1 and 6) illustrate the SE, while on box plots (Figs. 2–5) the line through the box center illustrates the population mean, box size illustrates means ± SE, and whiskers illustrate means ± SE.
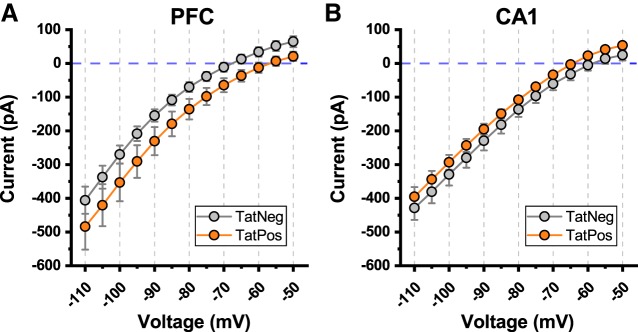
Fig. 6.
Current-voltage relationship at subthreshold voltages. A: illustrates the current voltage relationship observed in layer 2/3 medial prefrontal cortex (mPFC) pyramidal neurons obtained from both transactivator of transcription (Tat)Neg and TatPos animals. Holding current trended toward more negative values in TatPos versus TatNeg layer 2/3 pyramidal cells (see results for additional details). B: by contrast, human immunodeficiency virus (HIV)-1 Tat expression had no discernable effect on holding current when measured identically in CA1 pyramidal neurons (see results for additional details).
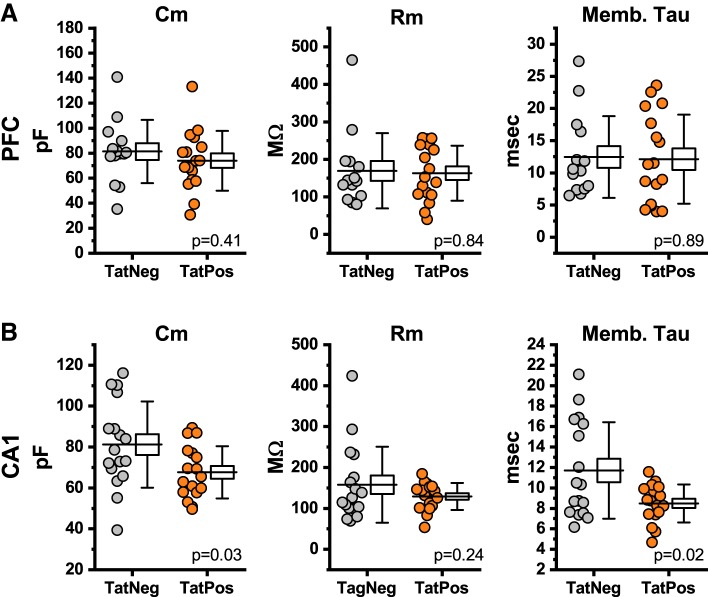
Fig. 2.
Region-specific effects of human immunodeficiency virus (HIV)-1 transactivator of transcription protein (Tat) on passive membrane properties. A: HIV-1 Tat expression had no significant effect on whole cell capacitance (Cm; left), input resistance (Rm; middle), or membrane time constant (Memb. Tau; right) in medial prefrontal cortex (mPFC) pyramidal neurons located in layer 2/3. B: in contrast, HIV-1 Tat expression was associated with a reduction in both whole cell capacitance and membrane time constant in hippocampal CA1 pyramidal neurons. In A and B, individual points represent data recorded from a single neuron (gray: TatNeg; orange: TatPos), and a box plot is provided next to the raw data points. The horizontal line that extends though the data points into the center of the box plot represents the population mean. The size of the box represents the means ± SE, and the whiskers on the box illustrate the means ± SD. P values provided in A and B are as produced by an unpaired Student’s t test performed on the illustrated data.
Fig. 5.
Region-specific effects of human immunodeficiency virus (HIV)-1 transactivator of transcription protein (Tat) on afterhyperpolarization (AHP) amplitude and area. A, left: illustrates the AHP as observed over the first 115 ms after the first action potential produced in response to a slow current ramp, as in Fig. 4, for each neuron evaluated. Traces from TatNeg neurons are illustrated in black, while traces from TatPos neurons are illustrated in orange. A, right: illustrates the average of all individual AHPs from TatNeg and TatPos neurons. Shaded areas represent the SE. B: box plots illustrating the AHP amplitude (left) and area (right) from each individual AHP in layer 2/3 medial prefrontal cortex (mPFC) pyramidal neurons obtained from both TatNeg and TatPos animals. C: illustrates individual (left) and average (right) AHPs observed in CA1 pyramidal cells obtained from both TatNeg and TatPos animals. D: box plots illustrating the AHP amplitude (left) and area (right) from each individual AHP in both TatNeg and TatPos CA1 pyramidal neurons are illustrated as described in Fig. 2 legend and methods.
RESULTS
Effects of HIV-1 Tat on neuronal gain.
To evaluate the effects of HIV-1 Tat expression on neuronal gain, we performed current clamp recordings from pyramidal neurons located either in layer 2/3 of the mPFC or in area CA1 of the hippocampus. Recordings were made in slices obtained from both TatNeg and TatPos mice (see methods). Neurons were held at I = 0 in current clamp, and a series of current steps were delivered between −100 and 300 pA (see methods). Figure 1, A and C, illustrates a subset of the raw data obtained from a representative cell in each group. Action potentials produced by current injection were detected using parameter-based event detection software, and neuronal gain curves were constructed by plotting current injected versus frequency of firing observed. Results indicated that HIV-1 Tat expression was associated with a significant increase in neuronal gain in layer 2/3 mPFC pyramidal neurons [Fig. 1B, main effect of HIV-1 Tat (genotype), P = 0.04, F1,29 = 4.64, TatNeg n = 14 neurons, 3 animals, TatPos n = 17 neurons, 5 animals]. In sharp contrast, in CA1 pyramidal cells, there was no main effect of HIV-1 Tat expression on neuronal gain (Fig. 1D, P = 0.40, F1,32 = 0.74, TatNeg n = 17 neurons, 5 animals, TatPos n = 17 neurons, 5 animals); however, we did note a significant HIV-1 Tat × Frequency interaction (P < 0.001, F1,14 = 3.29). Post hoc tests further revealed significantly less firing was observed in CA1 pyramidal neurons from TatPos neurons in response to both 25-pA and 50-pA current steps (P = 0.004 and P < 0.001, respectively). These results indicate HIV-1 Tat expression leads to a general increase in excitability in mPFC layer 2/3 pyramidal neurons yet, conversely, reduces excitability of hippocampal CA1 pyramidal cells in response to small/moderate current steps. In an effort to gain further insight on the specific changes underlying these divergent and complex phenotypes, we designed additional experiments to selectively and precisely evaluate the effects of HIV-1 Tat expression on a variety of additional passive and active electrophysiological properties of these neurons.
Effects of HIV-1 Tat on passive membrane properties.
Whole cell capacitance, input resistance, and membrane time constant were measured in both layer 2/3 pyramidal neurons and hippocampal CA1 pyramidal cells, from both TatNeg and TatPos mice, using voltage-clamp protocols as described in methods. HIV-1 Tat expression did not alter whole cell capacitance in mPFC pyramidal neurons (TatNeg: 81.3 ± 7.77 pF, TatPos: 73.9 ± 5.80 pF, n = 14, 17, P = 0.41, Fig. 2A, left), but was associated with a significant reduction in whole cell capacitance in hippocampal pyramidal cells (TatNeg: 81.2 ± 5.10 pF, TatPos: 67.6 ± 3.10 pF, n = 17, 17, P = 0.03, Fig. 2B, left). Consistent with this finding, the membrane time constant was also significantly reduced by HIV-1 Tat expression in hippocampal CA1 pyramidal cells (TatNeg: 11.7 ± 1.14 ms, TatPos: 8.49 ± 0.45 ms, n = 17, 17, P = 0.02, Fig. 2B, right) but not in mPFC pyramidal neurons (TatNeg: 12.5 ± 1.70 ms, TatPos: 12.1 ± 1.68 ms, n = 14, 17, P = 0.89, Fig. 2A, right). Finally, neuronal membrane resistance was not significantly altered by HIV-1 Tat expression in either mPFC pyramidal neurons (TatNeg: 169.5 ± 26.78 MΩ, TatPos: 163.2 ± 17.82 MΩ, n = 14, 17, P = 0.84, Fig. 2A, middle), or in hippocampal CA1 pyramidal cells (TatNeg: 157.8 ± 22.49 MΩ, TatPos: 129.2 ± 8.02 MΩ, n = 17, 17, P = 0.24, Fig. 2B, middle).
Effects of HIV-1 Tat on resting membrane potential, rheobase, and action potential threshold.
Resting membrane potential, rheobase, and action potential threshold were measured in both layer 2/3 pyramidal neurons and hippocampal CA1 pyramidal cells, from both TatNeg and TatPos mice, using current-clamp protocols as described in methods. In the mPFC, HIV-1 Tat expression increased the resting membrane potential (to more positive/depolarized values) and decreased the rheobase observed in layer 2/3 pyramidal neurons, without altering the threshold voltage. Specifically, the resting membrane potential increased from −66.40 ± 2.7 mV in TatNeg neurons to −58.0 ± 5.6 mV in TatPos neurons (P = 0.03, n = 14, 17, Fig. 3A), while rheobase decreased from 75.7 ± 16.6 pA to 37.7 ± 10.5 pA (n = 14, 16, P = 0.057, Fig. 3A). Threshold was unaltered at −34.6 ± 0.9 mV versus −36.2 ± 1.2 mV in TatPos and TatNeg neurons, respectively (n = 14, 16, P = 0.3). Intriguingly, identical experiments performed in CA1 pyramidal neurons failed to reveal similar effects of HIV-1 Tat expression. Indeed, in TatNeg versus TatPos CA1 pyramidal neurons, the mean resting membrane potential tended to decrease to more negative/hyperpolarized values (rather than increase) from −59.2 ± 2.2 mV to −63.5 ± 0.9 mV (n = 17,17, P = 0.09, Fig. 3B), while rheobase tended to increase (rather than decrease) from 31.7 ± 8.34 pA to 49.2 ± 4.32 pA (n = 17, 17, P = 0.07, Fig. 3B). Threshold was again unaltered at −41.4 ± 1.61 mV versus −44.05 ± 1.04 mV (n = 17, 17, P = 0.18, Fig. 3B). Collectively, these data suggest that differential effects of HIV-1 Tat expression on neuronal gain observed in layer 2/3 pyramidal neurons versus hippocampal pyramidal cells (Fig. 1) are produced at least in part by differential effects on the resting membrane potential and rheobase.
Effects of HIV-1 Tat on action potential amplitude, kinetics, and afterhyperpolarization.
To evaluate the effect of HIV-1 Tat expression on action potential amplitude and kinetics, we extracted and analyzed the first action potential observed during the same slow current ramp used for experiments presented in Fig. 3. These individual action potentials are illustrated in Fig. 4A, left, and Fig. 4C, left (for mPFC pyramidal neurons and CA1 pyramidal neurons, respectively). Overall, HIV-1 Tat expression did not significantly impact action potential amplitude (TatNeg: 79.0 ± 3.4 mV, TatPos: 84.7 ± 3.0 mV, n = 14, 16, P = 0.22) or maximum rate of depolarization (TatNeg: 214 ± 17.6 mV/ms, TatPos: 250 ± 16.7 mV/ms, n = 14, 16, P = 0.15) in layer 2/3 mPFC pyramidal neurons; however, there was a trend toward increased rate of repolarization (47.3 ± 2.5 mv/ms, TatPos: 57.5 ± 5.1 mV/ms, n = 14, 16, P = 0.08). Phase plots constructed from the average of the peak aligned action potentials in each group also are indicative of somewhat faster kinetics in the TatPos group (Fig. 4A, left and middle). Further consistent with this observation, we also noted a trend toward decreased action potential half-width (Fig. 4B, TatNeg: 1.60 ± 0.06 ms, TatPos: 1.46 ± 0.06 ms, n = 14, 16, P = 0.10) in these neurons. In CA1 pyramidal neurons, there was no significant change in action potential amplitude (TatNeg: 92.3 ± 4.0 mV, TatPos: 98.3 ± 2.6 mV, n = 17, 17, P = 0.24), maximum rate of depolarization (TatNeg: 294 ± 22.9 mV/ms, TatPos: 300 ± 17.1 mV/ms, n = 17, 17, P = 0.84), or maximum rate of repolarization (TatNeg: 66.3 ± 2.81 mV/ms, TatPos: 62.5 ± 2.44 mV/ms, n = 17, 17, P = 0.31); however, in clear contrast to the mPFC, phase plots constructed from the average of the peak aligned action potentials in each group (Fig. 4B, left and middle) suggested slower overall action potential kinetics. Consistent with that observation, HIV-1 Tat was associated with a significant increase in action potential half-width in CA1 pyramidal neurons (Fig. 4D, TatNeg: 1.33 ± 0.04 ms, TatPos: 1.52 ± 0.05 ms, n = 17, 17, P = 0.006). Collectively these data indicate that HIV-1 Tat expression can very likely modulate a subset of voltage gated ionophores that influence fast action potential kinetics and also indicate that these effects are again unique and divergent when compared in cortical versus hippocampal pyramidal neurons.
To further evaluate potential effects of HIV-1 Tat expression on the AHP, we analyzed the 110 ms of data beginning 20 ms after the action potential observed in each neuron (see methods). Raw data including this time period from each individual neuron examined is illustrated in Fig. 5A, left, and Fig. 5C, left (for mPFC pyramidal neurons and CA1 pyramidal cells respectively), while average traces are shown in Fig. 5A, right, and Fig. 5C, right. Overall, we found HIV-1 Tat expression did not have any notable effect on AHP amplitude (Fig. 5B, left, TatNeg: −14.6 ± 1.18 mV, TatPos: −15.2 ± 0.62 mV, n = 14, 15, P = 0.63) or area (Fig. 5B, right, TatNeg: −1368 ± 124.9 mV × ms, TatPos: −1414 ± 75.6 mV × ms, n = 14, 15, P = 0.74) in layer 2/3 mPFC pyramidal neurons. In CA1 pyramidal cells the overall effects were again not statistically significant, although in this case there was a strong trend toward decreased AHP amplitude (TatNeg: −9.6 ± 1.20 mV, TatPos: −6.9 ± 0.76 mV, n = 17, 16, P = 0.06) and area (TatNeg: −886 ± 114 mV × ms, TatPos: −635 ± 73.6 mV × ms, n = 17, 16 in both cases, P = 0.08). A trend toward reduced AHP amplitude in CA1 pyramidal neurons might help maintain maximum firing rate during a large current injection as observed in Fig. 1, despite the trend toward a more negative resting membrane potential and increased rheobase, as reported in Fig. 3. In response to smaller current injections, AHP amplitude is expected to have minimal impact on observed firing rate.
Effects of HIV-1 Tat on the subthreshold current-voltage relationship.
To examine the effects of HIV-1 Tat expression on holding current in the subthreshold voltage range, neurons were voltage clamped for 1 s at each voltage between −110 mV and −50 mV in 5-mV steps. The I–V plots in Fig. 6 were constructed by plotting the mean current observed over the last 300 ms of each step versus the command voltage. Overall, the results of these experiments suggested a slight trend toward increased holding current in layer 2/3 mPFC pyramidal cells in this voltage range (Fig. 6A, main effect of HIV-1 Tat, P = 0.10, F1,29 = 2.9), and did not reveal an effect in CA1 pyramidal neurons (Fig. 6B, main effect of HIV-1 Tat, P = 0.22, F1,30 = 1.59). When the same data were analyzed exclusively between −95 and −50 mV, a stronger effect was apparent in mPFC pyramidal neurons (main effect of HIV-1 Tat, P = 0.05, F1,29 = 4.2), but there was still no apparent effect in CA1 pyramidal neurons (main effect of HIV-1 Tat, P = 0.16, F1,30 = 1.59). These findings suggest that HIV-1 Tat may produce a modest increase in holding current required at subthreshold voltages in mPFC pyramidal neurons, which is not apparent in CA1 pyramidal neurons.
DISCUSSION
HIV-1 Tat is a viral protein that promotes viral transcription, and it is also secreted into the extracellular space where it can have effects on neighboring cells. It is thought to contribute to cognitive deficits associated with HAND, even in cART patients with very low viral load. In the current study, we present the results of detailed in vitro electrophysiological experiments that demonstrate, for the first time, brain region-specific effects of in vivo HIV-1 Tat protein exposure on the intrinsic electrophysiological properties of pyramidal neurons located in the medial prefrontal cortex and hippocampus.
In the mPFC we report that 7 days of inducible HIV-1 Tat expression caused a clear increase in neuronal gain in layer 2/3 pyramidal neurons. This effect was not associated with changes in passive membrane properties of the neurons, yet it did appear to depend in part on a significant increase in resting membrane potential and a decrease in rheobase. We also noted a trend toward decreased action potential half-width. Collectively, these changes are indicative of hyperexcitability in mPFC layer 2/3 pyramidal neurons following exposure to HIV-1 Tat. This conclusion is consistent with a prior report indicating that bath-applied Tat can increase neuronal gain (and also modulate voltage-gated calcium channel function) in mPFC pyramidal neurons (Napier et al. 2014). Similarly, HIV-1 Tat protein may contribute to the observed increase in neuronal excitability reported in layer 5/6 pyramidal neurons in mPFC of the HIV-1 transgenic rat (Chen et al. 2019). Interestingly, a variety of prior cognitive and behavioral studies also provide some support for the hypothesis that hyperexcitability in the prefrontal cortex may contribute to HAND. For example, both optogenetic activation of pyramidal neurons in prefrontal cortex and exposure to HIV-1 Tat have been associated with increased anxiety-like behavior (Berg et al. 2019; Paris et al. 2014b, 2014c). Similarly, substantial work has demonstrated that HIV-1 Tat expression enhances the rewarding effects of various psychoactive compounds and reinstates drug seeking behavior (Gonek et al. 2018; McLaughlin et al. 2014; Paris et al. 2014a), while studies that rely on both chemogenetic and pharmacological modulation of activity in prefrontal cortex suggest such effects are associated with increased excitation and decreased inhibition (McFarland and Kalivas 2001; Warthen et al. 2016). Finally, it is worth noting that chronic pain is commonly reported in people living with HIV and is strongly correlated with psychiatric illness and intravenous drug use (Keltner et al. 2014; Merlin et al. 2012a, 2012b, 2014). The prefrontal cortex supports dynamic modulation of pain processing and is of particular interest in chronic pain (Ong et al. 2019). A recent report utilizing spared-nerve injury in C57BL/6 J mice (the parent strain of the transgenic mice used in this study) showed that peripheral injury resulting in chronic neuropathy increased the excitability of layer 2/3 pyramidal neurons in the prelimbic region of the mPFC (Mitrić et al. 2019). Overall, these types of studies further suggest that the medial prefrontal cortex is a potential hub for modifying not just cognition, but multiple varied comorbidities observed in HIV infection, and that underlying hyperexcitability may contribute to the observed phenotypes.
By contrast, in the hippocampus, we report a general shift toward hypoexcitability in CA1 pyramidal neurons after 7 days of exposure to HIV-1 Tat. Specifically, we found that neuronal gain was selectively reduced in response to low-to-moderate levels of current injection. Further data presented here indicate that this effect was associated with a trend toward a more negative resting membrane potential and an increase in rheobase. All of these effects are in the opposite direction of changes observed in layer 2/3 mPFC pyramidal neurons. Furthermore, we noted an increase in action potential half-width and a strong trend toward a reduced AHP amplitude that were unique to CA1 pyramidal neurons. Collectively, these results suggest that HIV-1 Tat exposure tends to promote hypoexcitability (rather than hyperexcitability) in CA1 pyramidal cells. Intriguingly, this conclusion is consistent with a recent report of reduced intrinsic excitability in CA1 pyramidal cells in HIV1-tg rats (Sokolova et al. 2019) and thus suggests that the HIV-1 Tat protein produced in those animals may contribute significantly to those prior observations. Exposure of cultured hippocampal neurons to HIV-1 Tat has also been reported to shift the balance of excitatory and inhibitory transmission in favor of inhibition (Hargus and Thayer 2013), while time dependent effects on NMDA receptor function in hippocampal neurons has been reported, with inhibitory effects predominating after 48 h of exposure (Krogh et al. 2014, 2015). It will be interesting for future studies to examine the relationship between HIV-1 Tat expression and NMDA receptor function in acute tissue preparations of both mPFC and hippocampus. Importantly, it is also worth highlighting that hypoexcitablity of CA1 neurons as reported here is also plausibly consistent with impairments in spatial learning and memory associated with HIV-1 Tat as reported in animal models and human patients (Fitting et al. 2008, 2013; Harricharan et al. 2015; Li et al. 2004; Nookala et al. 2018).
Additional changes we noted uniquely in CA1 pyramidal neurons exposed to HIV-1 Tat include a decrease in whole cell capacitance and a likely directly associated decrease in the membrane time constant, which were observed absent any significant change in membrane resistance. While these effects are less obviously related to the clear trend toward hypoexcitability, it is interesting to note that there is some prior evidence that HIV-1 Tat can alter cholesterol production and lipid metabolism in hippocampal astrocytes and neurons (Cotto et al. 2018; Mohseni Ahooyi et al. 2018). Similarly, it is worth noting that several lines of evidence suggest that HIV-1 Tat may alter synaptodendritic structure and function via mechanisms that depend on filamentous actin (e.g., see Bertrand et al. 2014; Festa et al. 2015; Krogh et al. 2015), a key cytoskeletal protein that may also be relevant to measurements of whole cell capacitance (Herring et al. 1999). Further study evaluating whether these types of metabolic and functional alterations in HIV-1 Tat-exposed brain also contribute to HAND may prove insightful.
In a broader sense, it is also worth considering how exposure to a single stressor, HIV-1 Tat protein, may ultimately lead to such divergent effects on the intrinsic excitability of pyramidal neurons in the prefrontal cortex compared with those in the hippocampus. In that regard, it is worth noting that prior studies have indicated the HIV-1 Tat is likely able to alter intracellular calcium homeostasis and mitochondrial function (Norman et al. 2008), promote inflammatory responses (Nookala and Kumar 2014; Nookala et al. 2013), increase oxidative stress (Agrawal et al. 2012; Kim et al. 2015), and even directly modulate gene transcription (Liu et al. 2018; Santerre et al. 2019). Given this wide range of mechanisms, it is in our view quite plausible that fundamentally similar expression of HIV-1 Tat in different brain regions could ultimately lead to modulation of unique downstream effectors, and thus divergent effects on intrinsic excitability, in distinct types of neurons. As such, detailed and reductionist functional analysis of HIV-1 Tat-induced changes in cellular and synaptic physiology will likely be necessary in multiple brain regions to develop a clear understanding of the full range of effects of HIV-1 Tat on CNS function. An additional challenging but fascinating goal in the future will be to develop a better understanding of how unique changes in distinct brain areas (such as hyperexcitability in mPFC and concurrent hypofunction in the hippocampus) can work synergistically to produce the complex behavioral and cognitive phenotypes associated with HAND.
GRANTS
This work was supported by National Institute on Drug Abuse Grant R01-DA-039044 (to J.P.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.J.C., S.W.H., J.P.M., and C.J.F. conceived and designed research; T.J.C. performed experiments; T.J.C. and C.J.F. analyzed data; S.W.H. and C.J.F. interpreted results of experiments; C.J.F. prepared figures; T.J.C. and C.J.F. drafted manuscript; T.J.C., S.W.H., J.P.M., and C.J.F. edited and revised manuscript; T.J.C., S.W.H., J.P.M., and C.J.F. approved final version of manuscript.
REFERENCES
- Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 Tat neurotoxicity: a model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis 45: 657–670, 2012. doi: 10.1016/j.nbd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Berg L, Eckardt J, Masseck OA. Enhanced activity of pyramidal neurons in the infralimbic cortex drives anxiety behavior. PLoS One 14: e0210949, 2019. doi: 10.1371/journal.pone.0210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Mactutus CF, Aksenova MV, Espensen-Sturges TD, Booze RM. Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens. J Neurochem 128: 140–151, 2014. doi: 10.1111/jnc.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 229: 48–56, 2012. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Khodr CE, Al-Harthi L, Hu XT. Aging and HIV-1 alter the function of specific K+ channels in prefrontal cortex pyramidal neurons. Neurosci Lett 708: 134341, 2019. doi: 10.1016/j.neulet.2019.134341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotto B, Natarajaseenivasan K, Ferrero K, Wesley L, Sayre M, Langford D. Cocaine and HIV-1 Tat disrupt cholesterol homeostasis in astrocytes: implications for HIV-associated neurocognitive disorders in cocaine user patients. Glia 66: 889–902, 2018. doi: 10.1002/glia.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Letendre S, Vaida F, Haubrich R, Heaton RK, Sacktor N, Clifford DB, Best BM, May S, Umlauf A, Cherner M, Sanders C, Ballard C, Simpson DM, Jay C, McCutchan JA. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis 58: 1015–1022, 2014. doi: 10.1093/cid/cit921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa L, Gutoskey CJ, Graziano A, Waterhouse BD, Meucci O. Induction of interleukin-1β by human immunodeficiency virus-1 viral proteins leads to increased levels of neuronal ferritin heavy chain, synaptic injury, and deficits in flexible attention. J Neurosci 35: 10550–10561, 2015. doi: 10.1523/JNEUROSCI.4403-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal injection of the HIV-1 proteins gp120 and Tat: differential effects on behavior and the relationship to stereological hippocampal measures. Brain Res 1232: 139–154, 2008. doi: 10.1016/j.brainres.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry 73: 443–453, 2013. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Thomas G, Taylor AL, Patel A, Pineda A, Khalil C, Nadim F. Membrane capacitance measurements revisited: dependence of capacitance value on measurement method in nonisopotential neurons. J Neurophysiol 102: 2161–2175, 2009. doi: 10.1152/jn.00160.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonek M, McLane VD, Stevens DL, Lippold K, Akbarali HI, Knapp PE, Dewey WL, Hauser KF, Paris JJ. CCR5 mediates HIV-1 Tat-induced neuroinflammation and influences morphine tolerance, dependence, and reward. Brain Behav Immun 69: 124–138, 2018. doi: 10.1016/j.bbi.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargus NJ, Thayer SA. Human immunodeficiency virus-1 Tat protein increases the number of inhibitory synapses between hippocampal neurons in culture. J Neurosci 33: 17908–17920, 2013. doi: 10.1523/JNEUROSCI.1312-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harricharan R, Thaver V, Russell VA, Daniels WM. Tat-induced histopathological alterations mediate hippocampus-associated behavioural impairments in rats. Behav Brain Funct 11: 3, 2015. doi: 10.1186/s12993-014-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I; CHARTER Group . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75: 2087–2096, 2010. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I; CHARTER Group; HNRC Group . HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17: 3–16, 2011. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LJ, Johnson TP, Smith BR, Reoma LB, Santamaria UA, Bachani M, Demarino C, Barclay RA, Snow J, Sacktor N, Mcarthur J, Letendre S, Steiner J, Kashanchi F, Nath A. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 33, Suppl 2: S145–S157, 2019. doi: 10.1097/QAD.0000000000002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring TL, Cohan CS, Welnhofer EA, Mills LR, Morris CE. F-actin at newly invaginated membrane in neurons: implications for surface area regulation. J Membr Biol 171: 151–169, 1999. doi: 10.1007/s002329900567. [DOI] [PubMed] [Google Scholar]
- Jacobs IR, Xu C, Hermes DJ, League AF, Xu C, Nath B, Jiang W, Niphakis MJ, Cravatt BF, Mackie K, Mukhopadhyay S, Lichtman AH, Ignatowska-Jankowska BM, Fitting S. Inhibitory control deficits associated with upregulation of CB1R in the HIV-1 Tat transgenic mouse model of hand. J Neuroimmune Pharmacol 14: 661–678, 2019. doi: 10.1007/s11481-019-09867-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci USA 110: 13588–13593, 2013. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JR, Fennema-Notestine C, Vaida F, Wang D, Franklin DR, Dworkin RH, Sanders C, McCutchan JA, Archibald SL, Miller DJ, Kesidis G, Cushman C, Kim SM, Abramson I, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Corkran S, Cherner M, Duarte NA, Alexander T, Robinson-Papp J, Gelman BB, Simpson DM, Collier AC, Marra CM, Morgello S, Brown G, Grant I, Atkinson JH, Jernigan TL, Ellis RJ; CHARTER Group . HIV-associated distal neuropathic pain is associated with smaller total cerebral cortical gray matter. J Neurovirol 20: 209–218, 2014. doi: 10.1007/s13365-014-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Fields JA, Chang A, Coban H, Achim CL, Semenova S; TMARC Group . Effects of HIV-1 TAT protein and methamphetamine exposure on visual discrimination and executive function in mice. Behav Brain Res 349: 73–79, 2018. doi: 10.1016/j.bbr.2018.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S; TMARC Group . Effects of HIV/TAT protein expression and chronic selegiline treatment on spatial memory, reversal learning and neurotransmitter levels in mice. Behav Brain Res 311: 131–140, 2016. doi: 10.1016/j.bbr.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol 162: 1693–1707, 2003. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Smith AJ, Tan J, Shytle RD, Giunta B. MSM ameliorates HIV-1 Tat induced neuronal oxidative stress via rebalance of the glutathione cycle. Am J Transl Res 7: 328–338, 2015. [PMC free article] [PubMed] [Google Scholar]
- Krogh KA, Lyddon E, Thayer SA. HIV-1 Tat activates a RhoA signaling pathway to reduce NMDA-evoked calcium responses in hippocampal neurons via an actin-dependent mechanism. J Neurochem 132: 354–366, 2015. doi: 10.1111/jnc.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh KA, Wydeven N, Wickman K, Thayer SA. HIV-1 protein Tat produces biphasic changes in NMDA-evoked increases in intracellular Ca2+ concentration via activation of Src kinase and nitric oxide signaling pathways. J Neurochem 130: 642–656, 2014. doi: 10.1111/jnc.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ST, Matsushita M, Moriwaki A, Saheki Y, Lu YF, Tomizawa K, Wu HY, Terada H, Matsui H. HIV-1 Tat inhibits long-term potentiation and attenuates spatial learning [corrected]. Ann Neurol 55: 362–371, 2004. doi: 10.1002/ana.10844. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou D, Feng J, Liu Z, Hu Y, Liu C, Kong X. HIV-1 protein Tat1-72 impairs neuronal dendrites via activation of PP1 and regulation of the CREB/BDNF pathway. Virol Sin 33: 261–269, 2018. doi: 10.1007/s12250-018-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21: 8655–8663, 2001. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Ganno ML, Eans SO, Mizrachi E, Paris JJ. HIV-1 Tat protein exposure potentiates ethanol reward and reinstates extinguished ethanol-conditioned place preference. Curr HIV Res 12: 415–423, 2014. doi: 10.2174/1570162X1206150311160133. [DOI] [PubMed] [Google Scholar]
- Merlin JS, Cen L, Praestgaard A, Turner M, Obando A, Alpert C, Woolston S, Casarett D, Kostman J, Gross R, Frank I. Pain and physical and psychological symptoms in ambulatory HIV patients in the current treatment era. J Pain Symptom Manage 43: 638–645, 2012a. doi: 10.1016/j.jpainsymman.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Westfall AO, Raper JL, Zinski A, Norton WE, Willig JH, Gross R, Ritchie CS, Saag MS, Mugavero MJ. Pain, mood, and substance abuse in HIV: implications for clinic visit utilization, antiretroviral therapy adherence, and virologic failure. J Acquir Immune Defic Syndr 61: 164–170, 2012b. doi: 10.1097/QAI.0b013e3182662215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Zinski A, Norton WE, Ritchie CS, Saag MS, Mugavero MJ, Treisman G, Hooten WM. A conceptual framework for understanding chronic pain in patients with HIV. Pain Pract 14: 207–216, 2014. doi: 10.1111/papr.12052. [DOI] [PubMed] [Google Scholar]
- Mitrić M, Seewald A, Moschetti G, Sacerdote P, Ferraguti F, Kummer KK, Kress M. Layer- and subregion-specific electrophysiological and morphological changes of the medial prefrontal cortex in a mouse model of neuropathic pain. Sci Rep 9: 9479, 2019. doi: 10.1038/s41598-019-45677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni Ahooyi T, Shekarabi M, Torkzaban B, Langford TD, Burdo TH, Gordon J, Datta PK, Amini S, Khalili K. Dysregulation of neuronal cholesterol homeostasis upon exposure to HIV-1 Tat and cocaine revealed by RNA-sequencing. Sci Rep 8: 16300, 2018. doi: 10.1038/s41598-018-34539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musante V, Summa M, Neri E, Puliti A, Godowicz TT, Severi P, Battaglia G, Raiteri M, Pittaluga A. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cereb Cortex 20: 1974–1984, 2010. doi: 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- Nahir B, Bhatia C, Frazier CJ. Presynaptic inhibition of excitatory afferents to hilar mossy cells. J Neurophysiol 97: 4036–4047, 2007. doi: 10.1152/jn.00069.2007. [DOI] [PubMed] [Google Scholar]
- Napier TC, Chen L, Kashanchi F, Hu XT. Repeated cocaine treatment enhances HIV-1 Tat-induced cortical excitability via over-activation of L-type calcium channels. J Neuroimmune Pharmacol 9: 354–368, 2014. doi: 10.1007/s11481-014-9524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nookala AR, Kumar A. Molecular mechanisms involved in HIV-1 Tat-mediated induction of IL-6 and IL-8 in astrocytes. J Neuroinflammation 11: 214, 2014. doi: 10.1186/s12974-014-0214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nookala AR, Schwartz DC, Chaudhari NS, Glazyrin A, Stephens EB, Berman NEJ, Kumar A. Methamphetamine augment HIV-1 Tat mediated memory deficits by altering the expression of synaptic proteins and neurotrophic factors. Brain Behav Immun 71: 37–51, 2018. doi: 10.1016/j.bbi.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nookala AR, Shah A, Noel RJ, Kumar A. HIV-1 Tat-mediated induction of CCL5 in astrocytes involves NF-κB, AP-1, C/EBPα and C/EBPγ transcription factors and JAK, PI3K/Akt and p38 MAPK signaling pathways. PLoS One 8: e78855, 2013. doi: 10.1371/journal.pone.0078855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Reynolds HM, Kiebala M, De Mesy Bentley KL, Trejo M, Volsky DJ, Maggirwar SB, Dewhurst S, Masliah E, Gelbard HA. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS One 3: e3731, 2008. doi: 10.1371/journal.pone.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Stohler CS, Herr DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol 56: 1137–1166, 2019. doi: 10.1007/s12035-018-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP. Effects of conditional central expression of HIV-1 tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology 39: 380–388, 2014a. doi: 10.1038/npp.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Fenwick J, McLaughlin JP. Progesterone protects normative anxiety-like responding among ovariectomized female mice that conditionally express the HIV-1 regulatory protein, Tat, in the CNS. Horm Behav 65: 445–453, 2014b. doi: 10.1016/j.yhbeh.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl) 231: 2349–2360, 2014c. doi: 10.1007/s00213-013-3385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Celis V, Valiente-Echeverría F, Soto-Rifo R, Toro-Ascuy D. New challenges of HIV-1 infection: how HIV-1 attacks and resides in the central nervous system. Cells 8: E1245, 2019. doi: 10.3390/cells8101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santerre M, Bagashev A, Gorecki L, Lysek KZ, Wang Y, Shrestha J, Del Carpio-Cano F, Mukerjee R, Sawaya BE. HIV-1 Tat protein promotes neuronal dysregulation by inhibiting E2F transcription factor 3 (E2F3). J Biol Chem 294: 3618–3633, 2019. doi: 10.1074/jbc.RA118.003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova IV, Szucs A, Sanna PP. Reduced intrinsic excitability of CA1 pyramidal neurons in human immunodeficiency virus (HIV) transgenic rats. Brain Res 1724: 146431, 2019. doi: 10.1016/j.brainres.2019.146431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Nath A, Geiger JD, Moore A, Hochman S. Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-D-aspartate receptors at an allosteric zinc-sensitive site. J Neurovirol 9: 399–403, 2003. doi: 10.1080/13550280390201704. [DOI] [PubMed] [Google Scholar]
- UNAIDS UNAIDS Data 2018. [2018]. https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf.
- Warthen DM, Lambeth PS, Ottolini M, Shi Y, Barker BS, Gaykema RP, Newmyer BA, Joy-Gaba J, Ohmura Y, Perez-Reyes E, Güler AD, Patel MK, Scott MM. Activation of pyramidal neurons in mouse medial prefrontal cortex enhances food-seeking behavior while reducing impulsivity in the absence of an effect on food intake. Front Behav Neurosci 10: 63, 2016. doi: 10.3389/fnbeh.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman WN, Chen L, Persons AL, Napier TC. Cortical consequences of HIV-1 Tat exposure in rats are enhanced by chronic cocaine. Curr HIV Res 13: 80–87, 2015. doi: 10.2174/0929867322666150311164504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Fitting S. Inhibition of GABAergic neurotransmission by HIV-1 Tat and opioid treatment in the striatum involves μ-opioid receptors. Front Neurosci 10: 497, 2016. doi: 10.3389/fnins.2016.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Hermes DJ, Mackie K, Lichtman AH, Ignatowska-Jankowska BM, Fitting S. Cannabinoids occlude the HIV-1 Tat-induced decrease in GABAergic neurotransmission in prefrontal cortex slices. J Neuroimmune Pharmacol 11: 316–331, 2016. doi: 10.1007/s11481-016-9664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]