Abstract
Sensory processing deficits are increasingly recognized as core symptoms of autism spectrum disorders (ASDs). However the molecular and circuit mechanisms that lead to sensory deficits are unknown. We show that two molecularly disparate mouse models of autism display similar deficits in sensory-evoked responses in the mouse olfactory system. We find that both Cntnap2- and Shank3-deficient mice of both sexes exhibit reduced response amplitude and trial-to-trial reliability during repeated odor presentation. Mechanistically, we show that both mouse models have weaker and fewer synapses between olfactory sensory nerve (OSN) terminals and olfactory bulb tufted cells and weaker synapses between OSN terminals and inhibitory periglomerular cells. Consequently, deficits in sensory processing provide an excellent candidate phenotype for analysis in ASDs.
NEW & NOTEWORTHY The genetics of autism spectrum disorder (ASD) are complex. How the many risk genes generate the similar sets of symptoms that define the disorder is unknown. In particular, little is understood about the functional consequences of these genetic alterations. Sensory processing deficits are important aspects of the ASD diagnosis and may be due to unreliable neural circuits. We show that two mouse models of autism, Cntnap2- and Shank3-deficient mice, display reduced odor-evoked response amplitudes and reliability. These data suggest that altered sensory-evoked responses may constitute a circuit phenotype in ASDs.
Keywords: autism, calcium imaging, olfactory bulb, reliability, synaptic deficits
INTRODUCTION
The generation of appropriate behavior depends on neurons responding strongly and reliably to external stimuli. As sensory deficits have been recognized as an integral part of the diagnostic criteria for autism spectrum disorder (ASD) (DSM-5; American Psychiatric Association 2013), understanding the neural mechanisms that generate sensory defects will be critical to understanding autism. Moreover, sensory deficits provide an opportunity to use mouse models to study mechanisms that likely provide insight into common circuit alterations in ASD. In this study, we examined the amplitude and reliability of sensory-evoked responses in two different ASD mouse models, focusing on responses in the olfactory system, which is known to be a critical mediator of social behavior in mice (Brang and Ramachandran 2010; Dudova et al. 2011; Schilit Nitenson et al. 2015) and has been shown to be disrupted in autism (Koehler et al. 2018; Rozenkrantz et al. 2015; but see Larsson et al. 2017).
Two widely studied ASD risk genes, Cntnap2 and Shank3, encode cell adhesion and synaptic scaffolding proteins, respectively, and are thought to play roles in the structure and function of neuronal connections. Deletion of Cntnap2 and Shank3 causes similar behavioral phenotypes in humans and rodent models (Bozdagi et al. 2010; Durand et al. 2007; Peça et al. 2011; Peñagarikano et al. 2011; Selimbeyoglu et al. 2017; Wang et al. 2011), despite the fact that these genes differ in their subcellular locations, interactions with other proteins, and apparent functional roles (Anderson et al. 2012; Grabrucker et al. 2011; Kleijer et al. 2014; Poliak et al. 2003; Rodenas-Cuadrado et al. 2014; Varea et al. 2015). Additionally, Cntnap2- and Shank3-deficient mice show similar sensory abnormalities in pain processing (Dawes et al. 2018; Han et al. 2016). The data are less clear about olfactory deficits, as the few existing studies have contradictory findings (Gordon et al. 2016; Peñagarikano et al. 2011; Yang et al. 2012). However, indirect evidence suggests that the olfactory system is disrupted given that social communication is largely determined by the olfactory system (Bozdagi et al. 2010; Brang and Ramachandran 2010; Dudova et al. 2011; Schilit Nitenson et al. 2015) and is disrupted in both models (Durand et al. 2007; Peça et al. 2011; Peñagarikano et al. 2011; Selimbeyoglu et al. 2017; Wang et al. 2011). In this study, we sought to understand how such molecularly disparate genes could result in convergent functional deficits in the processing of olfactory sensory information.
Using both in vivo imaging and in vitro electrophysiology, we measured the stimulus-evoked responses and synaptic properties in two mouse models of autism, Cntnap2- and Shank3-deficient mice. We found that odor-evoked responses in both mouse models show reductions in response amplitude and reliability. Additionally, both mouse models displayed reductions in the number and strength of synapses between olfactory sensory neurons and olfactory bulb tufted cells, as well as a reduction in the strength of synapses onto inhibitory periglomerular cells.
MATERIALS AND METHODS
Ethical Approval
All experiments were completed in compliance with the guidelines established by the Institutional Animal Care and Use Committee of Carnegie Mellon University and the University of Pittsburgh.
Animals
For in vivo imaging experiments, adult mice (2–4 mo) of both sexes were used. For in vitro experiments, postnatal day 16–30 mice of both sexes were used. The experimental Cntnap2 and Shank3 mice were obtained by breeding heterozygotes with heterozygotes so that litters including wild-type (WT; +/+), heterozygote (HET; +/−), and homozygote (KO; −/−) mice could be obtained. The original breeder mice were obtained from the Jackson Laboratory (Bozdagi et al. 2010; Poliak et al. 2003). Cntnap2 mice were from stock no. 017482, and Shank3 mice were from stock no. 017890.
AAV-GCaMP6 Injection
AAV2/1.Syn.GCaMP6f.WPRE.SV40 (Penn Vector Core; titer ~2e13 GC/mL, volume ~0.1 µL) was injected into the dorsal olfactory bulb using a Picospritzer III, according to the method described by Kuhlman and Huang (2008). A glass pipette (tip size ~10 µm) was lowered through a hole in the skull to ~300 µm below the pial surface; 20 pulses (~10 ms long) were pressure injected at 0.3 Hz, and then the pipette was retracted 50 µm toward the surface and injection repeated as before at each site. This sequence was repeated until the pipette reached ~50 µm below the surface. This injection protocol resulted in a widespread and dense GCaMP6 expression in cell bodies and dendritic processes in mitral and tufted cells. Virus was allowed to express for 2–3 wk after injection before craniotomy and imaging were performed
In Vivo Two-Photon Calcium Imaging
Mice were anesthetized with ketamine (20 mg/mL)-xylazine (3.3 mg/mL) followed by supplemental ketamine. Reinjection of ketamine (20 mg/mL) was performed every 30 min to maintain a stable level of anesthesia throughout surgery and imaging (6–7 h). A window (1 mm ×1 mm) was created over the left dorsal olfactory bulb of mice and covered by no. 1.5 cover glasses (Zeiss) sealed with dental acrylic. A thin layer of low-melting-point agarose gel was placed in between the craniotomy and the cover glass to reduce motion during imaging. The craniotomy was performed on the same area of the dorsal surface across animals to ensure consistent placing of the craniotomy and thus sampling of glomeruli. Two-photon imaging of dendritic processes in the glomerular layer was performed on head-fixed mice using a Chameleon Ultra II laser tuned to a wavelength of 935 nm and an in vivo two-photon platform from Intelligent Imaging Innovations (3I), through a Zeiss W Plan-apochromatic ×20/1.0 objective. Data analysis of GCaMP6 activity was only performed within individual glomeruli. Because of the widespread expression of GCaMP6 across all layers of main olfactory bulb (MOB), GCaMP6 activity recorded in the superficial glomeruli is considered population activity and potentially attributed by a variety of cell types. Before each odor presentation, a 1-min-long video was captured to assess spontaneous event rate. For each odor presentation trial, a 20-s-long video capturing 5-s baseline and 15-s poststimulus fluorescence activity was collected in Slidebook5.5 (3I) at a frequency of 4.7 Hz. The imaging field had a resolution of 200 × 200 pixel2, corresponding to a 520 × 520-μm2 window. Positioning of the cranial window under the microscope was consistent so that similar subareas of the dorsal field were viewed across animals.
Odorant Stimulation
Brief pulses of isoamyl acetate (IAA; 100% saturated vapor, flow rate ~7.5 L/min) were delivered to the anesthetized mouse through a custom-built olfactometer to the animal’s left nostril. Odor pulses were controlled by valves opened via transistor-transistor logic pulses (Lee Valves). The distance of the tubing outlet to the nostril (~5 mm) was maintained throughout each experiment and across animals. Stimuli consisted of a single-pulse of IAA. In initial tests, we varied the stimulus duration between 5 and 1,000 ms. Single-trial responses of multiple glomeruli in the field were obtained for a range of stimulus durations to construct the relation between the stimulus duration and the fraction of activated glomeruli. Based on these initial results, we chose two stimulus durations, 200 and 20 ms, representing the high and intermediate stimulus levels to assess trial-to-trial response properties. Ten to fifteen trials for each of the two stimulus durations were delivered to the animal with an interval of 1.5–2 min. In a typical experiment, the 200-ms stimulus was presented first followed by the 20-ms stimulus. In a subset of experiments, we presented the 200-ms stimulus after the 20-ms stimulus and found no difference in the glomerular activity or trial-to-trial reliability, suggesting there is no systematic change in overall glomerular activity during our imaging sessions.
Photoionization detector (PID) measurements of the odor plume were collected using a MiniRAE 3000 (RAE Systems, Inc., Sunnyvale, CA). The PID was placed roughly 5 mm from the end of the tubing. We chose this distance to mimic the distance from the tubing to the animal’s nostril. PID measurements were done using the same odor delivery system described above. PID measurements were digitized at 10 kHz using an ITC-18 (InstruTECH) controlled by custom software written in IgorPro (Wavemetrics).
Region of Interest Identification and Single-Trial Calcium Transients
Glomerular images were inspected and identified manually based on the shape and temporal correlation of pixels within a glomerulus. Selection of regions of interest (ROIs) was performed by examining responses to 200-ms pulses. ROIs (3 × 3 pixel2, pixel size: 1.04 µm/pixel) at the center of putative glomeruli were selected from single-trial movies to make sure the selected ROIs were fully contained in corresponding glomeruli. Normalized fluorescence changes expressed as ΔF/F for each trial in each ROI were extrapolated using the first 20 frames (4.2 s long) as the baseline activity. Only those ROIs that produced excitatory stimulus-evoked responses (i.e., generating positive changes in ΔF/F with non-zero trial-to-trial reliability) were included for further analysis. Methods for calculating trial-to-trial reliability are described in Data Analysis.
Respiration Monitoring
To assess if the alterations in neural responses by could be attributed to genotype differences in respiration rates, respiration was monitored simultaneously with imaging in a subset of Shank3 and Cntnap2 animals by wrapping a pressure-sensing cuff around the animal’s chest. The respiration rate was calculated as the average rate from the first 10 respiration cycles after the stimulus onset. The respiration rate and strength of inhalation of Shank3 HET and KO mice during odor-evoked activity did not differ from that of WT mice.
Data Analysis
All the code used for the analysis in this study was written in MATLAB.
Normalized single-trial response amplitude.
Distribution of single-trial response amplitudes in animals of each genotype (Cntnap2 or Shank3) was calculated by grouping the normalized single-trial peak amplitudes with the across-trial averages. For a given ROI, peak response amplitudes within the poststimulus window from single trials were defined by the normalized single-trial amplitude at a specific frame number corresponding to the maximum amplitude in the averaged across-trial response.
Definition of response reliability.
To assess if a single trial of a given ROI produces an evoked response (success trial), single-frame values of the first 20 frames from ΔF/F traces of all the trials (baseline activity) averaged at a given stimulus duration. The z score for a single-trial response was defined as the difference between the single-trial response and the mean baseline activity, normalized by the standard deviation (SD) of the baseline activity across the trials. A single trial is considered a success trial when peak activity above the threshold (z > 2) was detected within a poststimulus window of 20 frames (4.2 s) at the time corresponding to the maximum amplitude in the averaged response across all the trials. Response reliability for a given ROI was defined as the fraction of the success trials over all the trials.
Response latency.
Response latency was defined as the time difference between the stimulus onset and the response onset. We calculated the response latency of single ROIs from the averaged ΔF/F traces by taking the first frame where the averaged response intersects a horizontal line indicating 2 SD above the baseline activity within the evaluation window. The frame number was then converted to and reported in milliseconds (ms).
Area under calcium transients.
In addition to peak response amplitude, response magnitude was quantified using the area under the curve. Only the excitatory component of a response was considered in the calculation of area. First, the onset and offset times of success trials for each ROI were determined by finding the first and second intersection points (frames) with the 2 SD line of baseline activity (described in Response latency) within the poststimulus window of the averaged response. The area under the calcium transient between the onset and offset points was then calculated for individual success trials using the TRAPZ function in MATLAB, and a mean area was obtained for each ROI by averaging the areas from all the success trials (where number of success trials has to be >2). For ROIs whose responses did not decay to the baseline at the end of a trial, the offset times were set to be the end frame and the procedure remains the same.
Full width at half maximum.
The waveforms of responses were assessed by measuring the full width at half maximum (FWHM) of the peak amplitude in the averaged responses. The FWHM was determined by the difference between the onset and offset times when the averaged response trace intersects a horizontal line measuring half of the peak amplitude for a given ROI.
In Vitro Whole Cell Recording
Slice preparation.
Postnatal day 16–30 mice of both sexes were anaesthetized with isoflurane and decapitated into ice-cold oxygenated dissection solution containing (in mM) 125 NaCl, 25 glucose, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 3 MgCl2, and 0.5 CaCl2. Brains were rapidly isolated, and acute sagittal slices (300 µm thick) of the MOB were prepared using a vibratome (VT1200S; Leica). Slices were recovered for 15–30 min in ~37°C oxygenated Ringer solution that was identical to the dissection solution except for a lower Mg2+ concentration (1 mM MgCl2) and higher Ca2+ concentration (2 mM). Slices were then stored in room temperature oxygenated Ringer solution until recording.
Cell identification.
Quantification of excitatory postsynaptic current (EPSC) amplitude and paired-pulse ratio (PPR) were performed during recordings from tufted cells (TCs). These cells possess at least one lateral dendrite, reside in the external plexiform layer, and exhibit spontaneous long-lasting depolarizations (Burton and Urban 2014). Analysis of miniature EPSCs (mEPSCs) was performed from external tufted cells (ETCs), a cell type distinct from tufted cells (Antal et al. 2006), identified by lack of secondary dendrites and presence of spontaneous EPSCs (Hayar et al. 2004b; Tyler et al. 2007). Analysis of mEPSCs was also performed from periglomerular cells (PGCs), identified in the glomerular layer by high input resistance and small cell body diameter (<10 µm) and, when possible, biocytin-filled and further morphologically confirmed after tissue fixation (Hayar et al. 2004a).
Slice electrophysiology.
Cells were visualized using infrared differential interference contrast video microscopy. Whole cell current-clamp recordings of tufted cells were performed using a MultiClamp 700B amplifier (Molecular Devices). Data were low-pass filtered (4 kHz) and digitized at 10 kHz using an ITC-18 (InstruTECH) controlled by custom software written in IgorPro (Wavemetrics). Pipettes were pulled from borosilicate glass (1.5-mm outer diameter) on a Flaming/Brown micropipette puller (Sutter Instrument) to a resistance of 6–9 MΩ. The intracellular solution consisted of the following (in mM): 140 Cs-gluconate, 2 KCl, 10 HEPES, 10 sodium phosphocreatine, 4 Mg-ATP, 0.3 Na3GTP, 10 lidocaine N-ethyl bromide, and 0.25 Alexa Fluor 594 (Life Technologies). Series resistance was monitored throughout recordings and maintained below 50 MΩ. For extracellular stimulation, a monopolar glass electrode was filled with Ringer solution and connected to a stimulus isolation unit (World Precision Instruments) controlled by transistor-transistor logic pulses from the ITC-18 acquisition board.
For determining the excitatory drive onto TCs, we stimulated the olfactory nerve on the dorsal surface of OB slices consistently across all animals with extracellular electrical stimulation of increasing amplitudes (10, 20, 40, 60, 80, 100, 150, 200 µA) and recorded the amplitude of the fast component of the subsequent EPSC (Burton and Urban 2014). The mean evoked current amplitude was calculated from the single-trial responses obtained across 10 trials. Paired-pulse ratio (PPR) was determined by stimulating the olfactory nerve twice at four different interevent intervals (50, 100, 500, and 1,000 ms). The PPR was calculated by dividing the amplitude of the EPSC following the second pulse by the amplitude of the first EPSC. PPR was calculated as the mean of 10 trials using 80-µA stimulation intensity of the olfactory nerve. Miniature EPSCs (mEPSCs) were recorded in the presence of 0.5 µM tetrodotoxin (TTX) and were calculated during 2-min whole cell voltage-clamp recording (−70 mV holding potential) epochs. Events were detected and analyzed using AxoGraph X (Axograph Scientific).
Statistics
The in vivo data across multiple animals are represented as means ± SE of the values for each animal. Comparisons of the response reliability, amplitude, and latency among WT, HET, and KO mice were performed on values of all the ROIs in each group using one-way ANOVA, followed by the Mann–Whitney U test. Differences in the cumulative distributions of groups of data were performed using the Kolmogorov–Smirnov (KS) test (*P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0001).
RESULTS
Adeno-associated virus vectors were used to drive diffuse expression of the calcium indicator GCAMP6f in cell bodies and dendrites throughout main olfactory bulb (MOB) lamina, consistent with the infection of most major cell types (Liu and Urban 2017; Wachowiak et al. 2013; Fig. 1A). Odor-evoked population activity was recorded by measuring changes in GCaMP6f fluorescence in the glomerular layer of MOB to the same odor stimulus (isoamyl acetate, IAA; Fig. 1B). Single-trial glomerular responses were stimulus-locked and had a high signal-to-noise ratio, enabling unambiguous detection of evoked response from a single trial. Glomerular responses were mostly excitatory and had a clearly identifiable peak across Cntnap2 and Shank3 wild-type (WT; +/+), heterozygote (HET; +/−), and homozygote (KO; −/−) mice (Fig. 1, B–E). Based on prior work (Carey et al. 2009) showing a logistic relationship between stimulus intensity and percentage of activated glomeruli, we chose two odor durations, 20 and 200 ms, to represent an intermediate and strong odor stimulus, with the hypothesis that any genotype differences in reliability may depend on the strength of the stimulus. We chose to vary odor pulse duration, and not other factors such as odor concentration, because changing concentration of odor delivery can result in trial-to-trial variation due to residual odor being present in the olfactometer. Additionally, because odor stimuli were highly reliable from trial to trial, any genotype-specific differences in neural variability are likely due to biological differences in circuit responses rather than differences in the stimuli themselves.
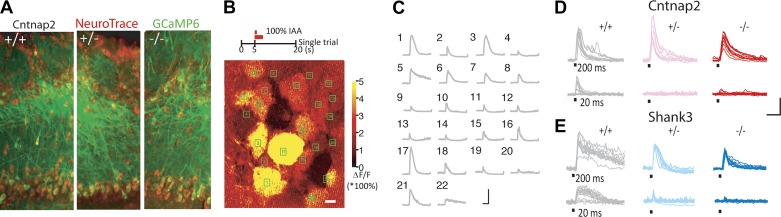
Fig. 1.
Calcium imaging in Cntnap2- and Shank3-deficient mice. A: gross anatomy of olfactory bulb (OB) does not differ among genotypes: +/+ (wild type; left), +/− (heterozygote; middle), and −/− (knockout; right). Red, NeuroTrace labeling cell bodies; green, GCaMP6. B: a representative experiment. A schematic of experimental design (top) and single-trial maximum normalized fluorescence change (ΔF/F) in response to 200-ms stimulus in dorsal OB of a representative animal (bottom) are shown. C: calcium transients in the identified regions of interest (ROIs) in the field of view as shown in B. Scale bars: 400% ΔF/F, 4 s. D and E: multiple single-trial responses to repeated odorant stimulation from representative ROIs in Cntnap2-deficient (D) and Shank3-deficient mice (E). Scale bars: 200% ΔF/F, 2 s. −2°.
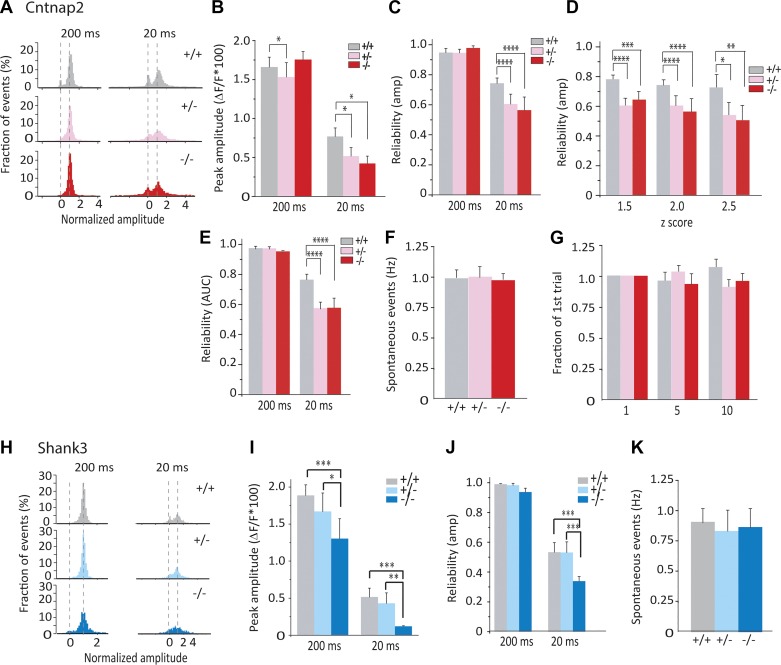
Response properties of olfactory bulb neurons in Cntnap2-deficient animals and littermates were investigated during repeated presentations of the same odor at two durations. Strong stimulation (200-ms presentation) resulted in highly consistent (greater than 90% reliable) activation of single glomeruli in all genotypes (Fig. 1D, top row). The probability of single glomeruli being activated dropped as stimulus duration decreased, resulting in more failure trials at the 20-ms stimulus (Fig. 1D, bottom row) and a clear bimodal response distribution indicating a binary response (Fig. 2A). While trial-average response amplitude at 200 ms was modestly reduced in HET mice (Fig. 2B), trial-average response amplitude at 20 ms was significantly smaller in Cntnap2 HET and KO mice (Fig. 2B). Moreover, at 20 ms, Cntnap2 HET and KO mice showed a decrease in reliability, as assessed by binarizing responses based on peak amplitude of the response (see materials and methods; Fig. 2C). Reliability was not biased by our method of binarizing responses, as genotype differences in reliability were unchanged regardless of z-score threshold (Fig. 2D). Additionally, reliability was not biased by the use of peak amplitude as our binarization metric, as similar genotype differences in reliability were found if total integrated response was used to binarize responses (Fig. 2E). Spontaneous event rates did not differ between Cntnap2 genotypes (Fig. 2F), indicating that changes in reliability are not likely due to differences in the signal-to-noise ratio. Finally, habituation across trials was minimal given our long (90 s) intertrial interval and did not vary across genotypes (Fig. 2G).
Fig. 2.
Cntnap2- and Shank3-deficient mice show reduced response amplitude and trial-to-trial reliability. A–G: data from Cntnap2-deficient mice. Data are from n = 95/6, 160/6, and 88/6 regions of interest (ROIs)/animals for wild type (WT; +/+), heterozygote (HET; +/−), and knockout (KO; −/−) mice, respectively. A: distribution of normalized single-trial peak amplitude at 200 and 20 ms from all animals. B: peak amplitude per ROI at 200 and 20 ms from all WT, HET, and KO animals. C: mean reliability in all conditions, using peak amplitude as binarization metric. D: mean reliability at 20 ms across z scores of 1.5, 2, and 2.5. E: mean reliability in all conditions, using integrated response as binarization metric. F: spontaneous event rate from all animals. G: habituation of odor-evoked response (ratio between 1st trial response and either 5th or 10th trial). H–K: data from Shank3-defient mice. Data are from n (ROIs/animals) = 105/7, 116/7, 77/5 for WT, HET, and KO mice, respectively. H: distribution of normalized single-trial peak amplitude (same as A). I: peak amplitude per ROI (same as B). J: mean reliability (same as C). K: spontaneous event rate (same as F). amp, Amplitude; AUC, area under the curve. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0001.
Next, we examined odor response properties in olfactory bulb neurons of Shank3 animals. Shank3 KO mice showed a reduction in average response amplitude across trials at both stimulus durations compared with WT and HET counterparts (Fig. 2, H and I). Although response reliability of Shank3 KO mice at 200 ms was not altered, reliability at 20 ms was greatly reduced (Fig. 2J). Additionally, spontaneous event rate did not differ between Shank3 genotypes (Fig. 2K). Therefore, we found that trial-to-trial response reliability was reduced in both Cntnap2 and Shank3 KO mice at 20 ms, and to a similar degree. We also note that while Cntnap2 HET mice showed reduction in reliability similar in magnitude to KO mice at 20 ms, Shank3 HET mice did not.
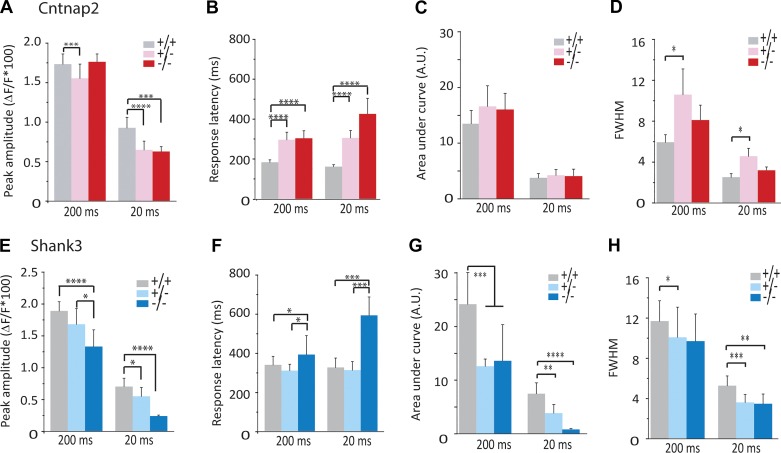
The smaller average response amplitude that we found in Cntnap2- and Shank3-deficient mice (Fig. 2, B and I) might be explained completely by a reduction in trial-to-trial reliability (Fig. 2, C and J), or it might reflect a reduction in the amplitude of successful responses. Consequently, we analyzed responses of the successful trials. Success-trial amplitude in Cntnap2 KO mice was reduced at 20 ms but not 200 ms (Fig. 3A), whereas in Cntnap2 HET mice, success-trial amplitude was reduced at both 20 and 200 ms (Fig. 3A). Similarly, success-trial amplitude of Shank3 KO mice was reduced at 200 and 20 ms (Fig. 3E). Shank3 HET mice displayed an intermediate reduction compared with KO mice (Fig. 3E). Taking these findings together, in addition to a reduction in response reliability, we observed that both Cntnap2- and Shank3-deficient mice (HET and KO) showed similar reductions in the response amplitudes of success trials elicited by 20-ms odor pulses. The reduction in success-trial amplitude may contribute to the reduction in trial-to-trial reliability if some very small amplitude responses are lower than our detection threshold and therefore may be categorized as failure trials. However, in some cases, these two effects were dissociated (Fig. 2I vs. Fig. 3E reliability vs. success-trial amplitude for Shank3 HET at 20 ms, where there is no change in the reliability but a reduction in the amplitude), suggesting that reduced response amplitude cannot completely account for the altered reliability.
Fig. 3.
Success-trial alterations in Cntnap2- and Shank3-deficient mice. A–D: data from Cntnap2-deficient mice. E–H: data from Shank3-deficient mice. Data are from n = 95/6, 160/6, and 88/6 regions of interest/animals for wild type (+/+), heterozygote (+/−), and knockout (−/−) mice, respectively. A and E: peak amplitude calculated from success trials. B and F: response latency. C and G: integrated response amplitude calculated using area under curve (A.U.). D and H: full width at half maximum (FWHM). *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0001.
Changes in response latency often accompany changes in response magnitude (Benedetti et al. 2009), and latency is an important variable in olfactory coding (Shusterman et al. 2011; Smear et al. 2011). We assessed the response latency in Cntnap2- and Shank3-deficient mice to the limits of our sampling rate (4.7 Hz) during success trials. The response latencies in Cntnap2 HET and KO mice were significantly longer compared with WT at 200 and 20 ms (Fig. 3B). Additionally, the response latency in Shank3 KO, but not HET, mice was significantly longer compared with that in WT mice at both 200 and 20 ms (Fig. 3F). Neither Cntnap2- or Shank3- deficient mice showed differences in their respiration rates (Cntnap2: WT: 2.41 ± 0.25 Hz, HET: 2.28 ± 0.14 Hz, KO: 2.35 ± 0.19 Hz, ANOVA: P = 0.81; Shank3: WT: 2.05 ± 0.14 Hz, HET: 1.96 ± 0.11 Hz, KO: 1.99 ± 0.11 Hz, ANOVA: P = 0.89) or strength of respiration (Cntnap2: WT: 42.01 ± 4.84 a.u., HET: 36.99 ± 5.45 a.u., KO: 43 ± 5.82 a.u., ANOVA: P = 0.65; Shank3: WT: 47 ± 5.98 a.u., HET: 44.48 ± 6.76 a.u., KO: 48.31 ± 6.35 a.u., ANOVA: P = 0.77). These findings show a shared increase in response latency in Cntnap2 and Shank3 KO mice.
Finally, we assessed the integrated response magnitude of successful trials. There were no differences in the integrated response of Cntanp2 HET and KO animals (Fig. 3C) despite longer response duration (Fig. 3D) in HET and KO mice, although not significantly so in KO mice. Increases in response duration in HET but not KO may be due to gene dosage effects. In contrast, integrated responses of Shank3 HET and KO mice were reduced at both 200 and 20 ms. Duration of responses was reduced in Shank3 HET mice at both 200 and 20 ms but was only reduced at 20 ms in Shank3 KO mice.
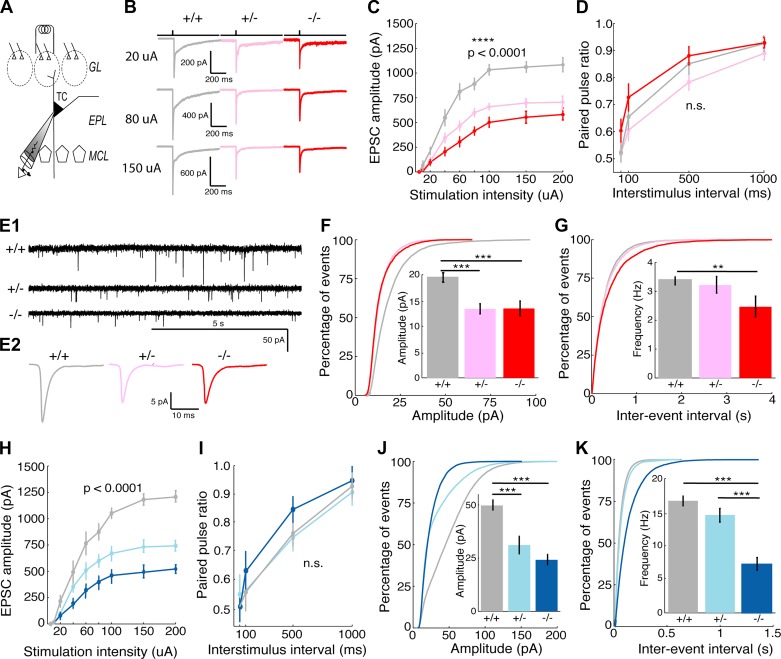
Next, we asked whether a common synaptic mechanism could be partially responsible for these network phenotypes. Using whole cell recordings from OB slices of these same strains of mice, we recorded excitatory postsynaptic currents (EPSCs) following electrical stimulation of olfactory sensory nerve inputs in tufted cells (TCs), one of the main projection neuron types of the MOB and one of the cell types that contributes to the measured dendritic calcium transients (Fig. 4A). We observed that stimulation-evoked EPSC amplitude in Cntnap2 HET and KO mice was reduced by ~50% compared with that in WT mice at the same stimulus intensity, indicating an overall reduction in excitatory drive onto TCs (Fig. 4, B and C; ANOVA: P < 1e-4). Paired-pulse ratio (PPR) was unaltered in these cells across genotypes (Fig. 4D; ANOVA: P = 0.52), suggesting that the reduction in excitatory input was not caused by changes in the probability of presynaptic glutamate release. Recordings of action potential-independent miniature EPSCs (mEPSCs) in external tufted cells (ETCs), a subset of TCs that receive strong direct input from olfactory sensory neurons (Hayar et al. 2004a), showed a significant reduction in mEPSC amplitude (Fig. 4, E and F; WT: 19.57 ± 0.70 pA, HET: 13.37 ± 0.51 pA, KO: 13.44 ± 0.89 pA, ANOVA: P < 1e-5, post hoc t test: WT vs. HET P < 1e-4, WT vs. KO P < 1e-4) and frequency (Fig. 4, F and G; WT: 3.40 ± 0.19 Hz, HET: 3.22 ± 0.40 Hz, KO: 2.47 ± 0.41 Hz, ANOVA: P < 1e-5, post hoc t test: WT vs. KO P < 1e-3) in the HET and KO mice, suggesting a decrease in synaptic strength and the number of synapses made onto these cells. Consistent with Cntnap2 mice, Shank3 mutants (HET and KO) showed strikingly similar changes in the synaptic properties (Fig. 4, H–K). We note, however, that there were large differences in mEPSC frequency between Cntnap2 and Shank3 WT animals, likely due to differences in genetic background between these strains. However, these background-specific effects will not alter our conclusions because WT, HET, and KO mice were born in the same litters. Collectively, in vitro results from Cntnap2- and Shank3-deficient mice (HET and KO) show a similar reduction in excitatory drive onto OB projection neurons due to a reduction in both the number and strength of these synapses.
Fig. 4.
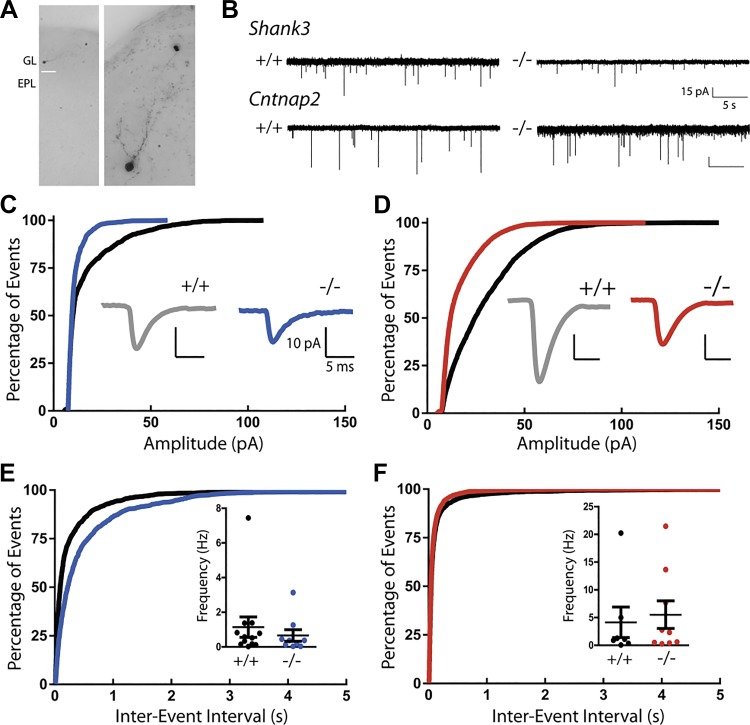
Miniature excitatory postsynaptic current (mEPSC) frequency and amplitude are reduced at olfactory sensory nerve (OSN)-tufted cell (TC) synapses in Cntnap2- and Shank3-deficient mice. A: schematic of experimental setup and olfactory bulb anatomy. TCs reside in the external plexiform layer (EPL) between the mitral cell layer (MCL) and glomerular layer (GL). B: example traces of monosynaptic EPSCs following olfactory nerve stimulation in Cntnap2 mice. C and H: TCs from heterozygote (HET) and knockout (KO) mice in both Cntnap2 (C) and Shank3 mice (H) show reductions in excitatory drive compared with TCs from wild-type (WT) animals [n (cells/animals) = 12/4 per genotype]. Repeated measures ANOVA: ****P < 0.0001. D and I: reductions in excitatory drive are not due to reductions in release probability at primary olfactory synapses in TCs from either Cntnap2 (D) or Shank3 (I) mice (12 cells from 4 animals per genotype. Repeated measures ANOVA: P > 0.5 (n.s.). Paired-pulse ratio experiments were conducted at interstimulus intervals of 50, 100, 500, and 1,000 ms. E1: example trances of mEPSCs (0.5 µM TTX, 2 min) measured in external tufted cells (ETCs), a subset of TCs, from Cntnap2 mice. E2: average mEPSC traces from ETCs in Cntnap2 mice. F, G, J, and K: average amplitude (F and J) and frequency (G and K) of mEPSCs is decreased in ETCs in both Cntnap2 (F and G) and Shank3 (J and K) HET and KO mice. Data are the cumulative frequency distribution from all events. Insets: average mEPSC amplitude and frequency of each cell. Data in F and G are taken from 6,070 events in 15 WT cells from 4 animals, 4,977 events in 13 HET cells from 4 animals, and 3,716 events in 13 KO cells from 4 animals. Data in J and K are taken from 20,707 events in 12 WT cells from 4 animals, 18,292 events in 12 HET cells from 4 animals, and 9,945 events in 12 KO cells from 4 animals. ANOVA followed by post hoc Tukey’s test: **P < 0.001; ***P < 0.0001.
Finally, we determined whether changes in inhibition may also contribute to the in vivo alterations reported by recording mEPSCs onto inhibitory periglomerular cells (PGCs; Fig. 5B). The amplitude of mEPSCs is smaller in Shank3 KO mice compared with WT mice, as shown in the shift toward smaller values in the cumulative histogram of event amplitudes (Fig. 5C). A similar difference was observed in Cntnap2 KO compared with WT control mice (Fig. 5D). No change was found in the frequency of mEPSCs onto PGCs from either Shank3 KO (Fig. 5E; WT: 1.1 ± 0.59 Hz, n = 12; KO: 0.66 ± 0.33 Hz, n = 9; t test with Welch’s correction, P = 0.49) or Cntnap2 KO mice (Fig. 5F; WT: 4.1 ± 2.8 Hz, n = 7; KO: 5.5 ± 2.5 Hz, n = 9; t test with Welch’s correction, P = 0.72) compared with WT mice. Consequently, both mouse models of autism show weaker excitatory synapses onto PGCs. These findings provide a window into the potential synaptic mechanisms that may be responsible for the in vivo alterations of circuit-level activity observed in the mice.
Fig. 5.
Miniature excitatory postsynaptic current (mEPSC) amplitude at synapses onto periglomerular cells (PGCs) is reduced in Cntnap2- and Shank3-deficient mice. A: images of a Neurobiotin-filled PGC in the glomerular layer (GL) of the olfactory bulb. EPL, external plexiform layer. B: example traces of mEPSCs (0.5 µM TTX) measured from PGCs. C: cumulative frequency distribution of amplitude from all events. Inset: average mEPSC trace from PGCs in Shank3 +/+ and −/− mice. D: cumulative frequency distribution of amplitude. Inset: average trace from PGCs in Cntnap2 +/+ and −/− mice. mEPSC amplitude is decreased in PGCs in both Shank3 (C) and Cntnap2 (D) KO mice (Shank3 KO vs. WT, P < 0.0001; Cntnap2 KO vs. WT, P < 0.0001 by Kolmogorov–Smirnov test). E: cumulative frequency distribution of interevent interval from all events. Inset: average mEPSC frequency from each cell in Shank3 +/+ and −/− mice showing no difference between groups (KO vs. WT, P = 0.49 by t test with Welch’s correction). F: cumulative frequency distribution of interevent interval. Inset: average frequency from PGCs in Cntnap2 +/+ and −/− mice, also showing no difference between conditions (KO vs. WT, P = 0.72 by t test with Welch’s correction). Data in C and E are taken from 1,655 events in 12 WT cells from 5 animals and 720 events in 9 KO cells from 4 animals. Data in D and F are taken from 3,480 events in 7 WT cells from 3 animals and 5,966 events in 9 KO cells from 5 animals.
DISCUSSION
Sensory deficits have been recognized as an integral part of the diagnostic criteria for ASD (DSM-5), and understanding the neural mechanisms that cause these sensory defects may provide an opportunity for gaining insight into common circuit alterations in ASD. In this study, we observed consistent reductions in the success-trial amplitude and trial-to-trial reliability in vivo and similar changes in excitatory synapses onto both excitatory and inhibitory neurons in vitro in the olfactory bulbs of Cntnap2- and Shank3-deficient mice.
A large number of molecularly disparate genes have been identified that increase risk for developing ASDs. Determining circuit-level phenotypes that are shared across a variety of genetic variants may lead to identification of common mechanisms and suggest possible pharmacological interventions. Many factors influence amplitude and reliability of neuronal responses, providing multiple mechanisms by which many different molecular alterations may affect behavioral features linked to autism. The recent discovery that individuals with autism display unreliable sensory-evoked responses in multiple sensory areas (Dinstein et al. 2012; Haigh et al. 2015) suggests that altered neural reliability may be an interesting phenotype to explore, especially given the increasing recognition that sensory deficits are core symptoms of ASDs.
While we report that both Cntnap2 and Shank3 mice show reductions in neural reliability, we cannot rule out the possibility that our method of calculating reliability by thresholding data could bias our results. For instance, if the amplitudes of the original calcium signals were continuous, then the binarized data would strongly depend on which z-score threshold is used. However, this is unlikely in our case because our ΔF/F data as displayed in Fig. 2, A and H, show a clear bimodal distribution indicating that glomerular responses are binary. Additionally, Fig. 2D shows that reliability differences across genotypes do not change whether the threshold to binarize responses is set to z = 1.5, 2, or 2.5. However, given these limitations, future work exploring variability with threshold-free methods such as odor-evoked spike trains in individual projection neurons in these animal models may better determine the extent of unreliable odor-evoked responses.
Many deficits at multiple levels of a neural circuit, whether alone or in combination, are theoretically capable of generating unreliable neural circuits. Prior modeling and in vitro work has shown that excitation/inhibition balance has the ability to influence the reliability of network activity (Carvalho and Buonomano 2009; Galán et al. 2006; Marder and Buonomano 2004; Pouille and Scanziani 2001; Schoppa and Urban 2003; Tiesinga et al. 2008). Additionally, disruptions in the excitation/inhibition balance have been thought to play an important role in the pathogenesis of ASDs (Lee et al. 2017; Nelson and Valakh 2015). Prior work suggests that deletion of either Cntnap2 or Shank3 effects this excitation/inhibition balance. Shank3 is known to be important in the formation of glutamatergic synapses onto GABAergic interneurons (Mei et al. 2016; Peça et al. 2011). Additionally, multiple studies have indicated that Cntnap2 is important for glutamatergic transmission (Anderson et al. 2012), whereas others have highlighted Cntnap2’s importance in the migration, development, and function of inhibitory interneurons (Jurgensen and Castillo 2015; Peñagarikano et al. 2011: Pinatel et al. 2015). Because glomerular responses are less reliable and have smaller amplitudes, excitation/inhibition balance appears to be altered in the direction of inhibition. Indeed, this is supported by our in vitro results showing reductions in excitatory drive onto TCs and PGCs. We note, however, that we do not provide direct evidence of changes in the amount of inhibition onto excitatory interneurons. However, if inhibition were reduced to a similar extent as excitation and excitation/inhibition remained balanced, glomerular responses would likely show no overall change in amplitude or reliability. Together, these findings suggest that alterations in the excitation/inhibition balance lead to unreliable neural circuits, which, in turn, may contribute to the pathogenesis of sensory deficits in ASDs. Additionally, these findings highlight the idea that the mechanism of circuit unreliability may manifest as similar cellular or synaptic alterations despite differences in genetic susceptibility and environmental risk factors between individuals with ASD. Future work should determine whether the intrinsic excitability of mitral or tufted cells or other sources of inhibition such as granule cells (Arevian et al. 2008; Geramita and Urban 2016; Geramita et al. 2016) differ in each mouse model and contribute to circuit unreliability.
While multiple circuit-level changes are capable of producing unreliable neural circuits, we cannot rule out the possibility of external sources of unreliability. For instance, anesthesia may differentially affect Cntnap2 and Shank3 deletion animals, although we found no evidence of such differences in these experiments and did not observe differences in the dose or time required to anesthetize these mice. Future experiments in awake-behaving mice can both rule out any potential effect of anesthesia and begin to address whether these differences in circuit reliability lead to differences in olfactory-guided behaviors.
Additionally, we report calcium signals that are not from a specific cell type. However, the main results presented in this article, showing that multiple mouse models of autism display higher unreliability to olfactory stimuli than control mice, are unlikely to be affected by a lack of cell-type specificity. Because of a variety of circuit mechanisms, both excitatory and inhibitory neurons of a single glomerulus are activated in a highly nonlinear fashion to low-intensity stimuli (Chen and Shepherd 2005; Koulakov et al. 2007). This nearly binary response occurs because most cells types receive little or no direct excitation from OSNs. Only ~30% of inhibitory periglomerular cells (PGCs) receive direct excitation from OSNs (Shao et al. 2009), and mitral cells only receive weak direct excitation (Gire and Schoppa 2009; Gire et al. 2012, Najac et al. 2011). External tufted cells (ETCs), an intrinsically bursting excitatory interneuron, provide the majority of excitation onto mitral and tufted cells (MTCs) and inhibitory PGCs (Hayar et al. 2004a, 2004b, 2005). ETC-mediated excitation is long lasting and synchronized across all cells within a glomerulus via gap junctions (Gire and Schoppa 2009; Hayar et al. 2005) and glutamate spillover (Urban and Sakmann 2002). Consequently, as the strength of OSN input increases, all neurons associated with a glomerulus become active in a stepwise fashion (Gire et al. 2012). In vivo calcium imaging from PGCs and MTCs shows highly correlated activity maps indicating that responses across both inhibitory and excitatory cell types within a glomerulus are highly synchronized (Wachowiak et al. 2013). Consequently, we designed our initial analysis in Figs. 2 and 3 to take advantage of these nonlinear responses by using binarized glomerular responses to calculate reliability. Therefore, this measure of reliability does not depend on which population of cells is being imaged. Furthermore, the similar decreases in success-trial amplitude across mouse models (Fig. 3) are likely due to decreases in the responsiveness of both PGCs and MTCs given the reduction in the strength of OSN inputs to both tufted cells and PGCs (Figs. 4 and 5). However, future work should definitively determine which cell type drives this decrease in success-trial amplitude by performing cell-type-specific calcium imaging.
Do the deficits described here depend on the number of mutant alleles? Cntnap2 HET mice, in general, show the same pattern of reduced odor-evoked amplitude and reliability as KO mice. This differs from prior work showing that Cntnap2 HET mice more closely resemble WT mice. This discrepancy likely arises due to the differences in brain area and experimental paradigm. The original description of Cntnap2 mice focused on cortical dysfunction. HET mice showed no differences in EEG signal, neuronal migration, number of interneurons, or synchrony compared with WT mice (Peñagarikano et al. 2011). In this study, we explored sensory processing in the olfactory bulb. Additionally, Cntnap2 HET mice may have less extensive synaptic deficits compared with KO mice, and consequently, alterations may not emerge during global measures such as EEG signals. As for Shank3 HET mice, our data are more in line with previously published data. Prior work using slice electrophysiology showed reduced synaptic amplitude, long-term potentiation, and prepulse inhibition in CA1 neurons from Shank3 HET mice compared with WT mice (Bozdagi et al. 2010). Similarly, we find weaker synaptic drive onto TCs in Shank3 HET mice compared with WT mice in vitro. However, Shank3 HET mice more closely resemble WT mice in vivo in that they do not show decreased amplitude or reliability. This discrepancy between in vivo and in vitro results indicates that one or more compensatory mechanisms, such as increased cellular excitability, produce normal in vivo glomerular responses.
How could the increased neural variability that we observe and that is characteristic of individuals with ASDs (Dinstein et al. 2012; Haigh et al. 2015) relate to the sensory symptoms now included as a diagnostic criteria of ASDs in the DSM5? Decades of work have shown that the responses of single neurons and populations are predictive of an animal’s choice in perceptual decision-making tasks. These trial-to-trial correlations between the activity of individual neurons and behavior have been found in a variety of brain areas that span several sensory modalities and occur in a variety of tasks in which difficult perceptual decisions are made (Britten et al. 1996; Celebrini and Newsome 1994; Cook and Maunsell 2002; Grunewald et al. 2002; Law and Gold 2008; Liu and Newsome 2005; Logothetis and Schall 1989; Nienborg and Cumming 2006; Uka and DeAngelis 2004). Consequently, deficits in intrinsic excitability or synaptic connectivity that alter the reliability by which a neuron’s activity reflects sensory input are likely to alter an animal’s behavior, especially in difficult tasks. Indeed, increased neural variability has been proposed as an explanation for deficits in visual perception (Dakin and Frith 2005; Simmons et al. 2009) and in predicting the local environment (Lawson et al. 2014; Pellicano and Burr 2012; Sinha et al. 2014; Van de Cruys et al. 2014) in individuals with ASD. Others have proposed that stereotyped motor behaviors in ASD may, in part, be a form of repeated resampling of sensory stimuli and may reflect a compensatory mechanism for unreliable perception in individuals with ASD (Dinstein et al. 2012; Haigh et al. 2015). Additionally, if similar deficits occur in motor systems, unreliability may explain why individuals with ASDs exhibit increased variability in eye saccade (Schmitt et al. 2014) and arm movement accuracy (Izawa et al. 2012), reaction times (Adamo et al. 2014; Karalunas et al. 2014), and the pitch of speech (Bonneh et al. 2011).
In addition to exploring the causes of circuit unreliability in the olfactory bulb, future work should look to determine whether the sensory-evoked reliability is reduced in other sensory systems in these mouse models. Human neuroimaging studies have identified reductions in sensory-evoked reliability across the visual, somatosensory, and auditory cortices of individuals with autism. Furthermore, given that Cntnap2 and Shank3 are differentially expressed across many brain areas (Lein et al. 2007), determining whether the mechanisms that generate circuit reliability are similar across brain areas within individual mouse models will be critical to the development of novel pharmacological targets. If the mechanisms of circuit unreliability are shared across brain areas in specific genetic mouse models or subsets of individuals with similar genetic susceptibilities, then identifying these mechanisms and creating novel drugs to reverse these alterations are warranted. However, if mechanisms of unreliability vary across brain areas within an individual, then methods to improve network reliability that are effective across a variety of pathological mechanisms are needed.
GRANTS
This work was supported by Simons Foundation Grant 274741 (to N. N. Urban) and National Institute of Deafness and Other Communications Disorders Grants R56DC015978 and R01DC016560 (to N. N. Urban).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.G., J.A.W., and N.N.U. conceived and designed research; M.A.G., J.A.W., and M.D.R. performed experiments; M.A.G., J.A.W., M.D.R., and N.N.U. analyzed data; M.A.G., J.A.W., M.D.R., and N.N.U. interpreted results of experiments; M.A.G., J.A.W., and M.D.R. prepared figures; J.A.W. drafted manuscript; M.A.G., J.A.W., M.D.R., and N.N.U. edited and revised manuscript; M.A.G., M.D.R., and N.N.U. approved final version of manuscript.
REFERENCES
- Adamo N, Huo L, Adelsberg S, Petkova E, Castellanos FX, Di Martino A. Response time intra-subject variability: commonalities between children with autism spectrum disorders and children with ADHD. Eur Child Adolesc Psychiatry 23: 69–79, 2014. doi: 10.1007/s00787-013-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (5th ed). Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- Anderson GR, Galfin T, Xu W, Aoto J, Malenka RC, Südhof TC. Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development. Proc Natl Acad Sci USA 109: 18120–18125, 2012. doi: 10.1073/pnas.1216398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal M, Eyre M, Finklea B, Nusser Z. External tufted cells in the main olfactory bulb form two distinct subpopulations. Eur J Neurosci 24: 1124–1136, 2006. doi: 10.1111/j.1460-9568.2006.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevian AC, Kapoor V, Urban NN. Activity-dependent gating of lateral inhibition in the mouse olfactory bulb. Nat Neurosci 11: 80–87, 2008. doi: 10.1038/nn2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti BL, Glazewski S, Barth AL. Reliable and precise neuronal firing during sensory plasticity in superficial layers of primary somatosensory cortex. J Neurosci 29: 11817–11827, 2009. doi: 10.1523/JNEUROSCI.3431-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh YS, Levanon Y, Dean-Pardo O, Lossos L, Adini Y. Abnormal speech spectrum and increased pitch variability in young autistic children. Front Hum Neurosci 4: 237, 2011. doi: 10.3389/fnhum.2010.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism 1: 15, 2010. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brang D, Ramachandran VS. Olfactory bulb dysgenesis, mirror neuron system dysfunction, and autonomic dysregulation as the neural basis for autism. Med Hypotheses 74: 919–921, 2010. doi: 10.1016/j.mehy.2008.11.048. [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996. doi: 10.1017/S095252380000715X. [DOI] [PubMed] [Google Scholar]
- Burton SD, Urban NN. Greater excitability and firing irregularity of tufted cells underlies distinct afferent-evoked activity of olfactory bulb mitral and tufted cells. J Physiol 592: 2097–2118, 2014. doi: 10.1113/jphysiol.2013.269886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Verhagen JV, Wesson DW, Pírez N, Wachowiak M. Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. J Neurophysiol 101: 1073–1088, 2009. doi: 10.1152/jn.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho TP, Buonomano DV. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron 61: 774–785, 2009. doi: 10.1016/j.neuron.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J Neurosci 14: 4109–4124, 1994. doi: 10.1523/JNEUROSCI.14-07-04109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR, Shepherd GM. The olfactory glomerulus: a cortical module with specific functions. J Neurocytol 34: 353–360, 2005. doi: 10.1007/s11068-005-8362-0. [DOI] [PubMed] [Google Scholar]
- Cook EP, Maunsell JH. Dynamics of neuronal responses in macaque MT and VIP during motion detection. Nat Neurosci 5: 985–994, 2002. doi: 10.1038/nn924. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron 48: 497–507, 2005. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dawes JM, Weir GA, Middleton SJ, Patel R, Chisholm KI, Pettingill P, Peck LJ, Sheridan J, Shakir A, Jacobson L, Gutierrez-Mecinas M, Galino J, Walcher J, Kühnemund J, Kuehn H, Sanna MD, Lang B, Clark AJ, Themistocleous AC, Iwagaki N, West SJ, Werynska K, Carroll L, Trendafilova T, Menassa DA, Giannoccaro MP, Coutinho E, Cervellini I, Tewari D, Buckley C, Leite MI, Wildner H, Zeilhofer HU, Peles E, Todd AJ, McMahon SB, Dickenson AH, Lewin GR, Vincent A, Bennett DL. Immune or genetic-mediated disruption of CASPR2 causes pain hypersensitivity due to enhanced primary afferent excitability. Neuron 97: 806–822.e10, 2018. doi: 10.1016/j.neuron.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable evoked responses in autism. Neuron 75: 981–991, 2012. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudova I, Vodicka J, Havlovicova M, Sedlacek Z, Urbanek T, Hrdlicka M. Odor detection threshold, but not odor identification, is impaired in children with autism. Eur Child Adolesc Psychiatry 20: 333–340, 2011. doi: 10.1007/s00787-011-0177-1. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsäter H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Rogé B, Héron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39: 25–27, 2007. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán RF, Fourcaud-Trocmé N, Ermentrout GB, Urban NN. Correlation-induced synchronization of oscillations in olfactory bulb neurons. J Neurosci 26: 3646–3655, 2006. doi: 10.1523/JNEUROSCI.4605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geramita M, Urban NN. Postnatal odor exposure increases the strength of interglomerular lateral inhibition onto olfactory bulb tufted cells. J Neurosci 36: 12321–12327, 2016. doi: 10.1523/JNEUROSCI.1991-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geramita MA, Burton SD, Urban NN. Distinct lateral inhibitory circuits drive parallel processing of sensory information in the mammalian olfactory bulb. eLife 5: e16039, 2016. doi: 10.7554/eLife.16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Franks KM, Zak JD, Tanaka KF, Whitesell JD, Mulligan AA, Hen R, Schoppa NE. Mitral cells in the olfactory bulb are mainly excited through a multistep signaling path. J Neurosci 32: 2964–2975, 2012. doi: 10.1523/JNEUROSCI.5580-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci 29: 13454–13464, 2009. doi: 10.1523/JNEUROSCI.2368-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A, Salomon D, Barak N, Pen Y, Tsoory M, Kimchi T, Peles E. Expression of Cntnap2 (Caspr2) in multiple levels of sensory systems. Mol Cell Neurosci 70: 42–53, 2016. doi: 10.1016/j.mcn.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Grabrucker AM, Schmeisser MJ, Schoen M, Boeckers TM. Postsynaptic ProSAP/Shank scaffolds in the cross-hair of synaptopathies. Trends Cell Biol 21: 594–603, 2011. doi: 10.1016/j.tcb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Grunewald A, Bradley DC, Andersen RA. Neural correlates of structure-from-motion perception in macaque V1 and MT. J Neurosci 22: 6195–6207, 2002. doi: 10.1523/JNEUROSCI.22-14-06195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh SM, Heeger DJ, Dinstein I, Minshew N, Behrmann M. Cortical variability in the sensory-evoked response in autism. J Autism Dev Disord 45: 1176–1190, 2015. doi: 10.1007/s10803-014-2276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Kim YH, Wang X, Liu D, Zhang ZJ, Bey AL, Lay M, Chang W, Berta T, Zhang Y, Jiang YH, Ji RR. SHANK3 deficiency impairs heat hyperalgesia and TRPV1 signaling in primary sensory neurons. Neuron 92: 1279–1293, 2016. doi: 10.1016/j.neuron.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci 24: 6676–6685, 2004a. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci 24: 1190–1199, 2004b. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci 25: 8197–8208, 2005. doi: 10.1523/JNEUROSCI.2374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Pekny SE, Marko MK, Haswell CC, Shadmehr R, Mostofsky SH. Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Res 5: 124–136, 2012. doi: 10.1002/aur.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgensen S, Castillo PE. Selective dysregulation of hippocampal inhibition in the mouse lacking autism candidate gene CNTNAP2. J Neurosci 35: 14681–14687, 2015. doi: 10.1523/JNEUROSCI.1666-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT. Annual research review: reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. J Child Psychol Psychiatry 55: 685–710, 2014. doi: 10.1111/jcpp.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijer KT, Schmeisser MJ, Krueger DD, Boeckers TM, Scheiffele P, Bourgeron T, Brose N, Burbach JP. Neurobiology of autism gene products: towards pathogenesis and drug targets. Psychopharmacology (Berl) 231: 1037–1062, 2014. doi: 10.1007/s00213-013-3403-3. [DOI] [PubMed] [Google Scholar]
- Koehler L, Fournel A, Albertowski K, Roessner V, Gerber J, Hummel C, Hummel T, Bensafi M. Impaired odor perception in autism spectrum disorder is associated with decreased activity in olfactory cortex. Chem Senses 43: 627–634, 2018. doi: 10.1093/chemse/bjy051. [DOI] [PubMed] [Google Scholar]
- Koulakov A, Gelperin A, Rinberg D. Olfactory coding with all-or-nothing glomeruli. J Neurophysiol 98: 3134–3142, 2007. doi: 10.1152/jn.00560.2007. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Huang ZJ. High-resolution labeling and functional manipulation of specific neuron types in mouse brain by Cre-activated viral gene expression. PLoS One 3: e2005, 2008. doi: 10.1371/journal.pone.0002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Tirado C, Wiens S. A meta-analysis of odor thresholds and odor identification in autism spectrum disorders. Front Psychol 8: 679, 2017. doi: 10.3389/fpsyg.2017.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat Neurosci 11: 505–513, 2008. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RP, Rees G, Friston KJ. An aberrant precision account of autism. Front Hum Neurosci 8: 302, 2014. doi: 10.3389/fnhum.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Lee J, Kim E. Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol Psychiatry 81: 838–847, 2017. doi: 10.1016/j.biopsych.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Liu A, Urban NN. Prenatal and early postnatal odorant exposure heightens odor-evoked mitral cell responses in the mouse olfactory bulb. eNeuro 4: ENEURO.0129-17.2017, 2017. doi: 10.1523/ENEURO.0129-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Correlation between speed perception and neural activity in the middle temporal visual area. J Neurosci 25: 711–722, 2005. doi: 10.1523/JNEUROSCI.4034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Schall JD. Neuronal correlates of subjective visual perception. Science 245: 761–763, 1989. doi: 10.1126/science.2772635. [DOI] [PubMed] [Google Scholar]
- Marder CP, Buonomano DV. Timing and balance of inhibition enhance the effect of long-term potentiation on cell firing. J Neurosci 24: 8873–8884, 2004. doi: 10.1523/JNEUROSCI.2661-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Monteiro P, Zhou Y, Kim JA, Gao X, Fu Z, Feng G. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature 530: 481–484, 2016. doi: 10.1038/nature16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najac M, De Saint Jan D, Reguero L, Grandes P, Charpak S. Monosynaptic and polysynaptic feed-forward inputs to mitral cells from olfactory sensory neurons. J Neurosci 31: 8722–8729, 2011. doi: 10.1523/JNEUROSCI.0527-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87: 684–698, 2015. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienborg H, Cumming BG. Macaque V2 neurons, but not V1 neurons, show choice-related activity. J Neurosci 26: 9567–9578, 2006. doi: 10.1523/JNEUROSCI.2256-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472: 437–442, 2011. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E, Burr D. When the world becomes ‘too real’: a Bayesian explanation of autistic perception. Trends Cogn Sci 16: 504–510, 2012. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147: 235–246, 2011. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinatel D, Hivert B, Boucraut J, Saint-Martin M, Rogemond V, Zoupi L, Karagogeos D, Honnorat J, Faivre-Sarrailh C. Inhibitory axons are targeted in hippocampal cell culture by anti-Caspr2 autoantibodies associated with limbic encephalitis. Front Cell Neurosci 9: 265, 2015. doi: 10.3389/fncel.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol 162: 1149–1160, 2003. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293: 1159–1163, 2001. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Rodenas-Cuadrado P, Ho J, Vernes SC. Shining a light on CNTNAP2: complex functions to complex disorders. Eur J Hum Genet 22: 171–178, 2014. doi: 10.1038/ejhg.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenkrantz L, Zachor D, Heller I, Plotkin A, Weissbrod A, Snitz K, Secundo L, Sobel N. A mechanistic link between olfaction and autism spectrum disorder. Curr Biol 25: 1904–1910, 2015. doi: 10.1016/j.cub.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilit Nitenson A, Stackpole EE, Truszkowski TL, Midroit M, Fallon JR, Bath KG. Fragile X mental retardation protein regulates olfactory sensitivity but not odorant discrimination. Chem Senses 40: 345–350, 2015. doi: 10.1093/chemse/bjv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LM, Cook EH, Sweeney JA, Mosconi MW. Saccadic eye movement abnormalities in autism spectrum disorder indicate dysfunctions in cerebellum and brainstem. Mol Autism 5: 47, 2014. doi: 10.1186/2040-2392-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci 26: 501–506, 2003. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- Selimbeyoglu A, Kim CK, Inoue M, Lee SY, Hong ASO, Kauvar I, Ramakrishnan C, Fenno LE, Davidson TJ, Wright M, Deisseroth K. Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci Transl Med 9: eaah6733, 2017. doi: 10.1126/scitranslmed.aah6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT. Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 101: 1988–2001, 2009. doi: 10.1152/jn.91116.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nat Neurosci 14: 1039–1044, 2011. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res 49: 2705–2739, 2009. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, Diamond SP, Held RM. Autism as a disorder of prediction. Proc Natl Acad Sci USA 111: 15220–15225, 2014. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O’Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature 479: 397–400, 2011. doi: 10.1038/nature10521. [DOI] [PubMed] [Google Scholar]
- Tiesinga P, Fellous JM, Sejnowski TJ. Regulation of spike timing in visual cortical circuits. Nat Rev Neurosci 9: 97–107, 2008. doi: 10.1038/nrn2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Petzold GC, Pal SK, Murthy VN. Experience-dependent modification of primary sensory synapses in the mammalian olfactory bulb. J Neurosci 27: 9427–9438, 2007. doi: 10.1523/JNEUROSCI.0664-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uka T, DeAngelis GC. Contribution of area MT to stereoscopic depth perception: choice-related response modulations reflect task strategy. Neuron 42: 297–310, 2004. doi: 10.1016/S0896-6273(04)00186-2. [DOI] [PubMed] [Google Scholar]
- Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol 542: 355–367, 2002. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, Wagemans J. Precise minds in uncertain worlds: predictive coding in autism. Psychol Rev 121: 649–675, 2014. doi: 10.1037/a0037665. [DOI] [PubMed] [Google Scholar]
- Varea O, Martin-de-Saavedra MD, Kopeikina KJ, Schürmann B, Fleming HJ, Fawcett-Patel JM, Bach A, Jang S, Peles E, Kim E, Penzes P. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc Natl Acad Sci USA 112: 6176–6181, 2015. doi: 10.1073/pnas.1423205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Economo MN, Díaz-Quesada M, Brunert D, Wesson DW, White JA, Rothermel M. Optical dissection of odor information processing in vivo using GCaMPs expressed in specified cell types of the olfactory bulb. J Neurosci 33: 5285–5300, 2013. doi: 10.1523/JNEUROSCI.4824-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, Lorenzo I, Wu G, Weinberg RJ, Ehlers MD, Philpot BD, Beaudet AL, Wetsel WC, Jiang YH. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet 20: 3093–3108, 2011. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wöhr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci 32: 6525–6541, 2012. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]