Abstract
In recent years, it has become increasingly clear that a number of learning processes are at play in visuomotor adaptation tasks. In addition to implicitly adapting to a perturbation, learners can develop explicit knowledge allowing them to select better actions in responding to it. Advances in visuomotor rotation experiments have underscored the important role of such “explicit learning” in shaping adaptation to kinematic perturbations. Yet, in adaptation to dynamic perturbations, its contribution has been largely overlooked. We therefore sought to approach the assessment of explicit learning in adaptation to dynamic perturbations, by developing two novel modifications of a force field experiment. First, we asked learners to abandon any cognitive strategy before selected force channel trials to expose consciously accessible parts of overall learning. Here, learners indeed reduced compensatory force compared with standard Catch channels. Second, we instructed a group of learners to mimic their right hand’s adaptation by moving with their naïve left hand. While a control group displayed negligible left hand force compensation, the mimicking group reported forces that approximated right hand adaptation but appeared to under-report the velocity component of the force field in favor of a more position-based component. Our results highlight the viability of explicit learning as a potential contributor to force field adaptation, though the fraction of learning under participants’ deliberate control on average remained considerably smaller than that of implicit learning, despite task conditions favoring explicit learning. The methods we employed provide a starting point for investigating the contribution of explicit strategies to force field adaptation.
NEW & NOTEWORTHY While the contribution of explicit learning has been increasingly studied in visuomotor adaptation, its contribution to force field adaptation has not been studied extensively. We employed two novel methods to assay explicit learning in a force field adaptation task and found that learners can voluntarily control aspects of compensatory force production and manually report it with their untrained limb. This supports the general viability of the contribution of explicit learning also in force field adaptation.
Keywords: action selection, declarative memory, internal model, sensorimotor, visuomotor adaptation
INTRODUCTION
Sensorimotor adaptation is considered important for maintaining skilled motor performance and has been studied extensively, with adaptation to kinematic (Helmholtz 1867; Cunningham 1989) and dynamic sensorimotor perturbations (Lackner and Dizio 1994; Shadmehr and Mussa-Ivaldi 1994) serving as model paradigms. When imposed perturbations are removed after training, adaptation is evidenced by characteristic aftereffects.
Frequently, adaptation has been treated as a property of implicit memory, outside participants’ deliberate cognitive control. The contribution of such an implicit process is supported by the observation that aftereffects occur despite learners being aware that a perturbation has been removed. Furthermore, aftereffects in amnesic patients, including patient HM, persisted over days, indicating that adaptation does not critically depend on declarative memory (Shadmehr et al. 1998).
Nevertheless, it has also been shown that learners can leverage declarative or propositional knowledge (Stanley and Krakauer 2013) to deliberately modify their actions in the face of a perturbation (Cohen 1967; Heuer and Hegele 2008; Jakobson and Goodale 1989; Redding and Wallace 2002; Taylor and Ivry 2011; Taylor et al. 2014), a capacity that we refer to as “explicit learning.” Recent advances in visuomotor rotation paradigms have quantified this component’s contribution to kinematic perturbations: Heuer and Hegele had learners provide perceptual judgements on the movement direction and/or amplitude required to compensate a visuomotor perturbation (Heuer and Hegele 2008, 2009). Benson and colleagues instructed learners about a cursor rotation, using a clock analogy, and tested the quality of their strategy after practice by a structured interview (Benson et al. 2011). Taylor and colleagues asked learners to verbally report their aiming strategy on individual trials based on a set of visual landmarks (Taylor et al. 2014). Results obtained by these methods suggest that explicit learning not only is a fundamental contributor to the learning outcome in such tasks but may underlie a range of behavioral phenomena that otherwise require extensions to standard models of implicit adaptation, such as savings (Morehead et al. 2015), structural learning (Bond and Taylor 2017), and context-dependent learning by abstract cues (Hegele and Heuer 2010; Schween et al. 2019) (see Krakauer et al. 2019 for a recent review).
Given the strong role of explicit learning in visuomotor adaptation, the question arises whether and how it contributes to adaptation to dynamic sensorimotor perturbations, most commonly investigated by perturbing movements through a robot-generated force field (Shadmehr and Mussa-Ivaldi 1994). A handful of studies have addressed potential explicit learning in this case (Hwang et al. 2006; Keisler and Shadmehr 2010; Kurtzer et al. 2003; McDougle et al. 2015; Thürer et al. 2016). Kurtzer and colleagues (2003) instructed a group of participants to “match the effort” of their baseline movements when confronted with a force field and found that it diminished learning compared with a group instructed to “match the kinematics.” This disengagement by instruction seems incompatible with purely implicit adaptation, which, in visual perturbation experiments, proceeds so stereotypically that it can sometimes harm task performance (Lee et al. 2018; Mazzoni and Krakauer 2006; Schween et al. 2014; Taylor and Ivry 2011). Hwang and colleagues (2006) assessed awareness as a marker of explicit memory by postexperimental questioning and found that awareness slightly improved learning of the force field and that more participants became aware when field direction was cued by a change of visual rather than proprioceptive workspace. However, the ability to verbalize awareness is limited by the learner’s capacity for communication and thus may not adequately assess explicit learning in all cases (Stadler 1997; Stanley and Krakauer 2013). Keisler and Shadmehr (2010), assuming a two-process model with a “fast” and a “slow” component of force field learning (Smith et al. 2006), found that a declarative memory task retroactively interfered with the fast component (Keisler and Shadmehr 2010), suggesting that this fast component, being susceptible to declarative memory load, might be explicit in nature (Morehead et al. 2011).
To our knowledge, the only study that attempted to quantify explicit contributions within participants comes from one of our laboratories, where we asked learners to report their off-target aim in response to a force field using a circular array of visual landmarks. Here, the time course of aiming reports overlapped with the putative fast process of adaptation (McDougle et al. 2015). However, whereas the circular array of landmarks appears adequate for reporting compensation for cursor rotations that can be offset by angular off-target aiming (Taylor et al. 2014), it is doubtful how adequate this reporting tool is for viscous force fields, where compensation is (ideally) velocity dependent and perpendicular to movement direction (Sing et al. 2009). In summary, these studies indicate a potential role of explicit learning in force adaptation, but we are currently lacking a suitable tool to assess them, leaving their precise role unclear.
To approach these issues, we here explored two novel methods for probing explicit contributions to adaptation in force fields. Our first approach (experiments 1 and 2) was rooted in “elimination” techniques frequently utilized in visuomotor rotation paradigms (Heuer and Hegele 2008; Werner et al. 2015): We instructed learners to refrain from using strategies on selected force channel trials and inferred explicit learning as the difference between these instructed and standard “Catch” channels (Scheidt et al. 2000). Our second approach (experiment 3) aimed to obtain reports in a way that is more suited to the viscous force field than circular landmarks (McDougle et al. 2015): We asked participants to mimic the force compensation learned with their right hand using their left hand, giving them the opportunity to express potential explicit force compensation strategies in the same velocity-dependent, perpendicular manner as with their right hand.
METHODS
We recruited 87 human volunteers from the participant pool maintained by Princeton University’s Psychology Department to participate in the experiments in exchange for payment or course credit. All participants provided written, informed consent. Experimental protocols were approved by Princeton University’s Institutional Review Board and complied with the relevant guidelines and regulations.
The apparatus was a Kinarm End-Point Laboratory (RRID: SCR_017060) run with commercial software (Dexterit-E) in a unimanual (experiments 1 and 2) or bimanual configuration (experiment 3). Participants sat in front of the robotic manipulandum, grasping its handle(s) at approximately lower chest level. They rested their head against the edge of a downward-facing, horizontal LCD monitor (LG47LD452C, LG Electronics, 47-in., 1,920×1,080 pixel resolution) and gazed into a horizontal mirror mounted below the monitor. This configuration creates the illusion that the visual display appears in the plane of the movement. By moving the robot handle, participants could move a screen cursor (blue disk, 10-mm diameter) that matched handle position.
The robot could generate field, null, and channel trials. On field trials, the robot generated a velocity-dependent force field (Shadmehr and Mussa-Ivaldi 1994):
| (1) |
where are directional velocities and a positive field constant b creates a clockwise force field. On null trials, b was zero, meaning that the robot did not actively influence the movement. On channel trials (Scheidt et al. 2000), the robot constrained the movement to a straight line through start and target by generating a spring of 12 kN/m with a damping coefficient of 50 N·m−1·s−1 (6 kN/m and 20 N·m−1·s−1 for experiment 3). The channels served as test trials, allowing us to measure the predictive forces participants learned to exert in response to the field in the absence of stiffness influences. With the above settings, the range of individual maximal, horizontal deviations from a straight line between start and target on channel trials was 1.9–3.7 mm for experiment 1, 1.2–3.0 mm for experiment 2, and 1.4–8.1 mm for experiment 3. Note that movements on experiment 3 differed from the other experiments in multiple ways and we do not intend to make direct comparisons across these experiments.
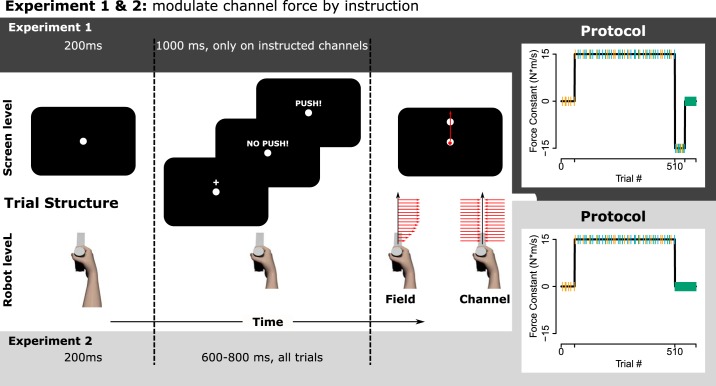
Experiment 1.
Each trial began with the robot passively moving the participant to a start location on the participant’s midline (white disk, 15-mm diameter), ~45 cm in front of their chest. After the participant held this position for 200 ms, a target appeared on the body midline (white disk, 15-mm diameter) 120 mm distal from the start position, and the participant’s task was to slice through the target in a rapid shooting movement. We chose these rather than more standard closed-loop movements (which require learners to successfully hit the target and thus emphasize feedback-based online corrections) as our channel instructions (see paragraph after next) would theoretically have prevented task success on field trials, and we did not want our participants to reason about the difference between field and channel trials. Target hits were reinforced by a pleasant “ding” sound. Movement speed was incentivized by a “too slow” message appearing if movement time exceeded 350 ms. If participants moved before the appearance of the target or if movement time exceeded 1,000 ms, the trial was aborted and repeated.
Twenty-five participants (mean age: 21, range: 18–26 yr, 16 female) practiced a force field with b=±15 N·m·s−1 and we tested their predictive force compensation on channel trials. Of these participants, three were not analyzed due to technical difficulties (two were tested with the calibration hardware still in place and one was subject to a software crash during testing). Two more participants were excluded because standardized postexperimental questioning indicated that they failed to follow instructions (one indicated that they interpreted the “Push” message to mean they should move to the target more quickly, the other responded to the question “What did you do on the trials with the ‘No Push!’ message?” in a way that spurred the experimenter to note “did not follow instructions,” though we unfortunately did not record the detailed answer). It was critical in this experiment for participants to follow instructions because we sought to test whether they could intentionally control part of the adaptive response to the force field. This required them to implement the correct intention on the respective channel trial.
We introduced two new types of channel trials in addition to the standard Catch channel: On these trials, participants saw a screen message before the reach (see Fig. 1), reminding them of an instruction given ~10 trials before the force field was introduced: One message read, “No Push.” For this message, participants were instructed to “act as in trials where the robot doesn’t push you off path and just move toward the target.” We thus aimed to probe their ability to voluntarily disengage a hypothetical explicit strategy. The other message read “Push,” reminding participants of an instruction to “expect the robot to push you off and act as on those trials.” We included this “Push” trial type as a control to ensure that any force modulation would be attributable to the associated instruction rather than other factors, such as delays introduced by the messages allowing a labile component of implicit memory to decay (Miyamoto et al. 2014; Zhou et al. 2017). Standard Catch channel trials were not preceded by a message. The full, scripted instructions (of experiment 2) are provided in the appendix.
Fig. 1.
Schematic methods for experiments 1 and 2. Left: after holding the start location for 200 ms, a fixation cross appeared. When the cross disappeared and the target appeared, the participant’s task was to “shoot through” the target in a single, fast movement, experiencing the force field (or null field in baseline). The red arrows schematically illustrate (potential) robot-generated forces. Note that the movement path on field trials could deviate substantially from the straight line illustrated. On channel trials (Scheidt et al. 2000), the robot constrained movements to the line connecting start and target. Catch channels were preceded by the fixation cross, making them indistinguishable, a priori, from field trials. On instructed channels, instead of the fixation cross, learners would see a keyword prompting them to either expect the robot not to push them off their path or to do so, and act accordingly. Note that for experiment 1 field and Catch trials, no fixation cross was shown and instead, the target appeared immediately. Right: perturbation schedules for the individual experiments, with channel trials highlighted by the colors used for results figures (green = No Push, yellow = Catch, Blue = Push.)
The protocol was an A-B-Clamp, or “rebound”-paradigm (McDougle et al. 2015; Smith et al. 2006), consisting of 40 trials familiarization, 60 trials baseline, 450 trials practice in a field A, 45 trials practice in an opposing field B, and 50 trials clamp. In the familiarization phase participants moved from start to target in a null field to get acquainted to the task and the time criterion. Baseline was also in a null field but contained six Catch channels to establish a baseline, with one channel per block of 10 trials and an additional constraint that channels could not be the first or last trial of a block, ensuring there were at least two nonchannel trials between any two channels. In field A practice, a force field was introduced, with the sign counterbalanced across participants. Each block of 10 trials contained one channel, as before (i.e., 10% channels), but now, metablocks of three blocks contained each of the three channel types (Catch, No Push, Push) once, in a random order. Field A practice thus contained 15 channels per type. Field B practice exposed participants to a force field of opposite polarity than field A. This phase contained one channel per block of five trials (i.e., 20% channels), with all other constraints as in field A. The final clamp phase consisted of only channel trials. This block no longer had screen messages, but participants were verbally instructed to consider all trials No Push trials from the beginning of the clamp phase until the end of the experiment to reveal behavior in the absence of potential explicit strategies (McDougle et al. 2015). We used the same randomization sequence for all of our participants in experiments 1 and 2.
Experiment 2.
Experiment 2 served as a replication of the main effect between channel types in experiment 1. Furthermore, we wanted to test whether behavior on No Push channels would match behavior on a more commonly used, longer error clamp period with the instruction that the perturbation was removed. We therefore omitted field B practice and instead had participants proceed directly into the final clamp phase, which now comprised 95 trials.
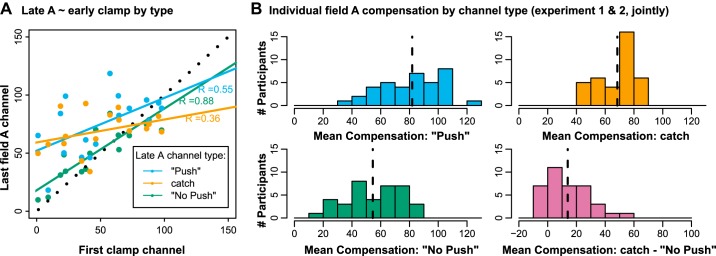
We tested 22 participants (mean age: 21, range: 18–28 yr, 16 female) on this experiment and excluded five. Of the exclusions, two participants indicated in postexperimental questioning that they “just went straight ahead” on the Push trials, one declared that they “still pushed” on the No Push trials, one reported (and according to data was actually) pushing in the direction of the force field rather than against it, and a final one was an influential outlier in correlations between last practice and first posttest channel (Fig. 5A; as in experiment 1, the potential success of the experiment was critically dependent on participants following the instructions.)
Fig. 5.
A: scatterplot with least squares regression lines: last channel of field A practice for the different types against the first clamp channel. Black dotted line is identity. B: histograms of individual means of force compensation index across field A practice (joint data from experiments 1 and 2). Dashed, vertical lines mark group means. Bottom right shows individual differences between No Push and Catch channels.
The procedures were the same as experiment 1 with the following exceptions: we suspected that, in experiment 1, the instructive message may have startled participants into moving prematurely (resulting in the trial being repeated), because it occurred around the time where they expected the target to appear. To avoid this problem, we now showed participants either the white text message or a white fixation cross (on null, field, and Catch trials). This was presented after the participants’ hand was in the start position for 200 ms. The target appeared 600–1,000 ms later (randomized, uniform distribution, Fig. 1) at which point the text or fixation cross was removed. We considered using another message instead of the fixation cross to match the channel messages in luminance and area, but decided against it, as we feared participants might become too accustomed to viewing a message and could potentially ignore it.
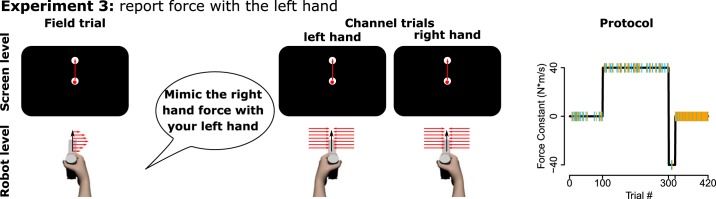
Experiment 3.
Experiment 3 aimed to test whether participants could mimic their right hand force compensation with their left hand and thereby express their explicit knowledge about the force field (Fig. 2). Participants adapted to a force field by closed-loop movements to a target 100 mm distal relative to the central start location. Field exposure was interspersed by right hand Catch channels and additional left hand channel trials. For these left hand channel trials, the Mimic group (N = 20, mean age: 21, range: 19–24 yr, 17 female, 1 excluded for technical issues) was instructed to “mimic the forces” they were applying with their right hand with the left hand, whereas the control group (N = 20, mean age: 22, range: 18–34 yr, 11 female, 3 excluded for technical issues) received no such instruction concerning left hand trials. To ensure that all participants could sense the force field, and to more closely match a previous experiment (McDougle et al. 2015), we increased the field constant to b=±40 N·m·s−1. Like experiment 1, the protocol was a field A-field B-Clamp paradigm, now consisting of 100 trials baseline (80 null, 10 right and 10 left hand channel), 200 trials field A practice (158 field, 21 right, 21 left hand channels), 20 trials practice of an opposing field B (18 field, 1 right, 1 left channel) and 100 clamp trials (90 right hand, 10 left hand channels). Field direction was counterbalanced across participants.
Fig. 2.
Schematic methods for experiment 3. Left: participants practiced closed-loop target reaches in the field with their right hand and were tested on right hand (Catch) and left hand channels. On left hand channels, the Mimic group was instructed to mimic their right hand forces. The control group received no such instruction. Right: perturbation schedule with channel trials highlighted in color (yellow = right, blue = left hand channel.)
Data analysis.
We processed data in MATLAB (RRID: SCR_001622). We conducted statistical analysis and visualization in R (RRID: SCR_001905, using packages “car,” “psych,” “ez,” and “R.matlab”) and JASP (RRID: SCR_015823).
For experiments 1 and 2, we defined movement start as the instant when movement speed first exceeded 3 cm/s and movement end when the radial distance of the hand to the start first exceeded that of the target’s center. In experiment 3, we used a different start criterion of 5 cm/s for at least 100 ms, as we noticed the lower criterion included some premature movements whose occurrence we attribute to the different movement types (shooting versus closed loop). Movement end in experiment 3 was defined as the hand being within the target circle with a speed of less than 2.5 cm/s. We then truncated force and position data of each movement to the section between these events. We calculated axial velocities using a 4th order Savitsky-Golay filter and additional time frames at the start and end of the reach to avoid ramping effects. Data of participants experiencing the clockwise field in the A-phase were flipped about the y-axis before further processing.
We quantified percent force compensation on channel trials by calculating ideal compensatory forces based on velocity along the channel and regressing the actual forces exerted onto it. For this, we used zero-offset regression and assumed field A for ideal force calculation to give different signs to compensation for field A and field B. For shooting movements (experiments 1 and 2), we did this on the complete time series from start to end, whereas for closed-loop movements (experiment 3) we used a window of 400 ms prior and posterior to peak speed. If this window exceeded movement start or end, we clipped it at those events, respectively. To produce mean trajectories for plotting, we aligned force data to peak speed and padded shorter movements with NaNs at beginning and end.
Experiment 3 also measured the degree to which force expression was composed of position and velocity components for the left and right hands. To compute this, we fit perpendicular channel force profiles using a zero-offset linear model of position along the channel, velocity along the channel, or a linear combination of the two, respectively (Sing et al. 2009). We used adjusted R2 (as returned by R’s summary.lm function) to quantify variance accounted for, and we averaged across participants by transforming its square root to Fisher-Z-scores (Bortz and Schuster 2010), calculating their mean, and retransforming to R2. We did this for 1,000 bootstrap samples of individuals in this group and estimated 95% confidence intervals.
In all experiments, we computed reaction time (RT) on field trials as the time from target display to movement start, and removed RTs exceeding three standard deviations of participants’ individual means (experiment 1: 97 trials, 1%; experiment 2: 114 trials, 1%; experiment 3: 154 trials, 1.7%). We then calculated individual medians over the following blocks: late baseline, early field A practice, late field A practice, field B practice (where applicable) and early clamp, using 36 (experiments 1 and 2) or 18 (experiment 3) field trials per block, and averaged across participants. For experiments 1 and 2, late baseline excluded the last 10 trials of baseline as RTs there were distorted by instructions sometimes being given during the RT interval.
Statistical analysis.
We used mixed ANOVAs with group as between-subject factor, and channel type, hand, and/or block as within-subject factors. We checked for unequal variances between groups by Levene’s tests. Where Mauchly’s test indicated significant nonsphericity, we report Greenhouse–Geisser-corrected P values and degrees of freedom. For effect sizes, we report generalized eta-squared. To follow up on significant effects, we used one-way ANOVAs for simple main effects and t tests for post hoc comparisons and report Bonferroni–Holm corrected P values for the latter. For comparing dependent correlations, we used a test described in Bortz and Schuster (2010, p. 167–168).
RESULTS
Experiment 1.
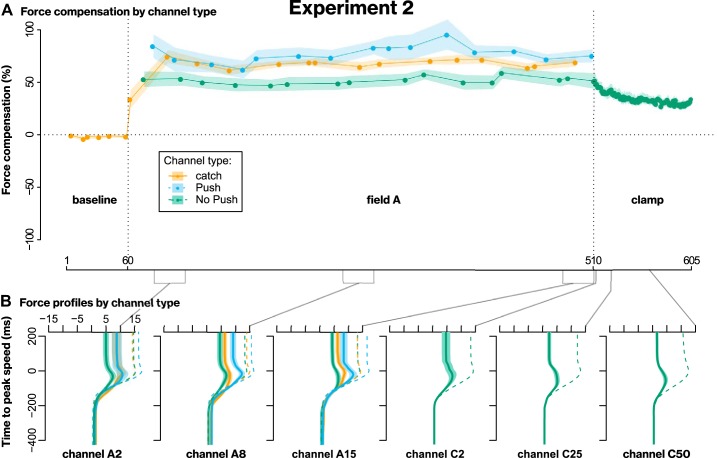
Experiment 1 tested whether predictive force compensation in channels is fully implicit, or whether it contains an explicit component, which learners can voluntarily modulate. Participants practiced shooting movements and were exposed to two velocity-dependent force fields, where they experienced an initial long block of field A, followed by a short block of an opposing field B, and a clamp block (McDougle et al. 2015; Smith et al. 2006). Channel trials tested participants’ predictive force compensation (Smith et al. 2006) on a fraction of trials. Whereas all baseline channels and one third of channels during practice were standard, unannounced Catch channels, the other two thirds were preceded by an on-screen message instructing participants about the upcoming trial (Fig. 1). One message type said “No Push!”, for which our instructions asked participants to “act as in trials where the robot doesn’t push you off path and just move toward the target.” We reasoned that if predictive compensation is fully implicit, participants would express the same amount of force on these trials as they did on Catch trials. On the other hand, if participants could spontaneously downregulate their force in response to the instruction, this would indicate that an explicit component was contributing to Catch channel behavior. The other message type said “Push!”, asking participants to expect the robot to push them off and act accordingly. The B-field was followed by a clamp block of all channel trials (McDougle et al. 2015; Smith et al. 2006), for which we instructed participants that they should treat all of them as “No Push!” trials, even in the absence of messages.
Figure 3A shows the force compensation index across trials for each type of instructed channel. During field A practice, participants expressed less force on No Push compared with Catch channels, revealing an ability to voluntarily eliminate a component of predictive force compensation in response to the instruction. Conversely, channels with the instruction to Push voluntarily show compensation that is not smaller, but even larger than on Catch channels, suggesting that behavior on No Push channels was under volitional control rather than just a general effect of the instruction. Figure 3B visualizes across participant average force profiles on selected channel trials.
Fig. 3.
Results of experiment 1. A: group mean force compensation (±standard error of mean; SE) across trials. B: group mean force profiles (±SE) obtained by averaging individual force profiles aligned to peak speed. Dashed lines are hypothetical, ideal field A compensatory forces calculated from velocity and used as reference. Labels on x-axis reference position in the protocol, with A2 being the 2nd trial in field A, C5 the 5th trial in the clamp phase, etc. Multiple lines per panel are from channels of the different types, respectively. Gray lines and brackets between panels indicate regions represented in this way.
To statistically analyze these data, we averaged the first, middle, and last three channels of each type from field A-practice into blocks and performed an ANOVA with the within-participant factors channel type and block. This revealed a significant main effect of channel type (F2,38 = 23.5, P < 0.001, = 0.25), no significant main effect of block (F1.3,24.0 = 0.27, P = 0.66, = 0.002), and a significant interaction (F2.1,39.0 = 5.2, P = 0.009, = 0.05). Simple main effects indicated that the effect of channel type was present at all three levels of block (early: F2,38 = 23.9, P < 0.001, = 0.32; middle: F1.4,26.9 = 14.3, P < 0.001, = 0.29; late: F1.3,25.1 = 8.7, P = 0.004, = 0.21) and post hoc t tests at these levels indicated significant differences between all pairs of channel types (all P < 0.03 after Bonferroni–Holm correction), except for the No Push versus Catch channels in the early block (P = 0.36) and for Push versus Catch channels in the late block (P = 0.13). Whereas we might have expected an effect of block, reflecting learning, the time courses of Catch channels (Fig. 3A) indicate that learning may have been too quick to be captured by the relatively infrequent channel trials. Such quick learning may be explained by the use of a single target, and by increased requirement for predictive compensation imposed by the shooting movements. Regardless, the main effect of channel type in combination with the simple main effects and post hoc tests suggests that the degree to which participants express learning is under volitional control, which would be consistent with the use of a strategy.
During field B, the time courses of the three channel types are largely consistent with previous findings in this paradigm (McDougle et al. 2015): when participants expect the robot to apply force (Push and Catch channels), predictive compensation quickly changes sign upon exposure to field B, while compensation when they do not expect the robot to apply force (No Push channels) decreases more gradually with practice, so that not only the sign of each individual channel but also the relation between the three channel types is inverted by the end of the B-phase. This was confirmed by a repeated-measures ANOVA on the individual differences between the last channel of field A and field B, respectively, which indicated a significant difference (F1.5,28.2 = 12.6, P < 0.001, = 0.40) with post hoc tests showing a significant difference between No Push and both Catch (t = −5.2, P < 0.001) and Push trials (t = −4.1, P = 0.001), but not between Catch and Push trials (t = 1.4, P = 0.18). This means that the change from field A to field B was significantly smaller on No Push than Push or Catch trials. Considering the short timeframe in focus, this is in line with the implicit No Push trials capturing a slow component of learning, while an additional fast explicit process is represented in Push and Catch trials (Keisler and Shadmehr 2010; McDougle et al. 2015).
In the final clamp phase consisting of only No Push channels, behavior rebounded. This observation would be difficult to explain by the No Push instruction directly eliciting volitional forces, and is more consistent with behavior seen in implicit learning of force fields and cursor rotations in this paradigm, where it has been explained by a fast, explicit component that is adapted to field B being quickly diminished by instruction or decay, revealing a slow component that is still adapted to field A (McDougle et al. 2015; Smith et al. 2006; also see discussion).
Experiment 2.
Experiment 1 supported our hypothesis that learners can voluntarily disengage a component of channel force compensation when instructed to do so right before the trial. Here, we wanted to replicate this finding and to verify that this is not just an idiosyncrasy of the single-trial instructions. In experiment 2, we therefore tested whether behavior on a more standard, postexperimental clamp phase with the No Push instruction administered only at phase onset, would indeed reflect similar behavior as the interspersed trials and further display the hallmark behavior of gradually decaying memory. We therefore replicated experiment 1 but removed field B practice.
Figure 4 shows that the instruction modulated force compensation on channel trials as before. Accordingly, like for experiment 1, an ANOVA on field A practice with factors block and channel type indicated a significant main effect of channel type (F2,32 = 17.0, P < 0.001, = 0.17) but not of block (F1.2,19.5 = 1.0, P = 0.34) and no interaction (F2.1,32.9 = 0.89, P = 0.42). Averaging over blocks and comparing channel types by post hoc t tests revealed differences between all pairs of channel types (all P < 0.02 after Bonferroni–Holm correction). Thus, experiment 2 replicates the main effect of channel type observed in experiment 1.
Fig. 4.
Results of experiment 2. A: group average force compensation measures across trials. B: group mean force profiles (±SE) on selected trials. Labels on x-axis reference position in the experiment, with A2 being the 2nd trial in field A, C25 the 25th trial in the clamp phase, etc.
When going directly from field A into the clamp phase, with the instruction not to push voluntarily, behavior on clamp appeared to pick up on the most recent interspersed No Push channel and to decay smoothly from there. When correlating individual participants’ performance on the first channel of the final phase and on the last interspersed channel of each instruction type, this correlation was significantly stronger for the No Push channel than for the Catch (z = 3.5, P < 0.001) or Push channel (z = 2.9, P = 0.003), and the regression line was close to unity (Fig. 5). While this result is merely correlational and not a strong indicator, it nevertheless fits with our assumption that the behavior in the final clamp phase reflects the same implicit processes exposed by the No Push channels interspersed during learning.
How is the difference between the channel types reflected in individual participants? Figure 5B shows the histograms of individual average force compensation during field A practice on Catch and No Push channels and the individual differences between the channel types for participants of experiments 1 and 2. It is clear that some participants responded strongly to the instruction whereas others show no force modulation. Besides actual differences in the extent of explicit learning, one explanation for this could be that participants differed in how they understood and followed our instructions. We excluded cases where standardized postexperimental questioning made it clear that participants misunderstood the instructions and either consciously compensated on No Push or did not compensate on “push” channels (see methods). However, some responses were ambiguous so we kept those participants in the analysis to avoid potential selection bias.
In summary, we take results of our manipulation in experiments 1 and 2 to show that predictive compensation of a force field expressed on standard Catch channels was not purely implicit. If learning is entirely implicit, we would expect participants to have no volitional control over its expression. Conversely, participants in our experiment were able to modulate predictive expression based on verbal instruction in line with an explicit component contributing to learning.
Experiment 3.
Experiments 1 and 2 show that force compensation performance can be modified based on verbal instruction, thus displaying a key characteristic of explicit learning. In experiment 3, we sought to complement this finding by testing whether participants can express their knowledge about the force field. In a previous experiment, we presented participants with a circular array of landmarks and asked where they would have to aim their movement to compensate for the perturbation (McDougle et al. 2015). Whereas this reporting method is well suited to cursor rotation experiments, where a participant can verbally express the task-relevant variable with some degree of precision in relation to a reference (i.e., the angle of the reach direction relative to the target), it is potentially not ideal for dynamic force field experiments. Here, adaptation requires counteracting a time-varying, velocity-dependent force field, which may be difficult to describe verbally or in relation to a spatial reference point. Instead, a better assay for a potential strategy would be one that would allow learners to express them in a similar way as the task-relevant variable—in this case force. To address this issue, we asked participants to express the movement strategies they were consciously using to compensate for the force field, by mimicking them with their left hand. Based on the finding that true, implicit intermanual transfer of force compensation is limited (Joiner et al. 2013; Malfait and Ostry 2004; Wang and Sainburg 2003), we reasoned that forces expressed with the left hand could thus serve as a proxy of explicit knowledge that participants had acquired about the force field with their right hand, and that the possibility to report this knowledge by movements of the contralateral effector would therefore enable more appropriate reports than visual aiming directions.
We tested two groups of participants on an experiment where the test channels interspersed between closed-loop reaches in a force field were alternatively conducted with the left or right hand by each participant. We asked participants in the Mimic group to “mimic the forces” they experienced on right hand trials with their left hand, whereas the control group was just told to move to the target.
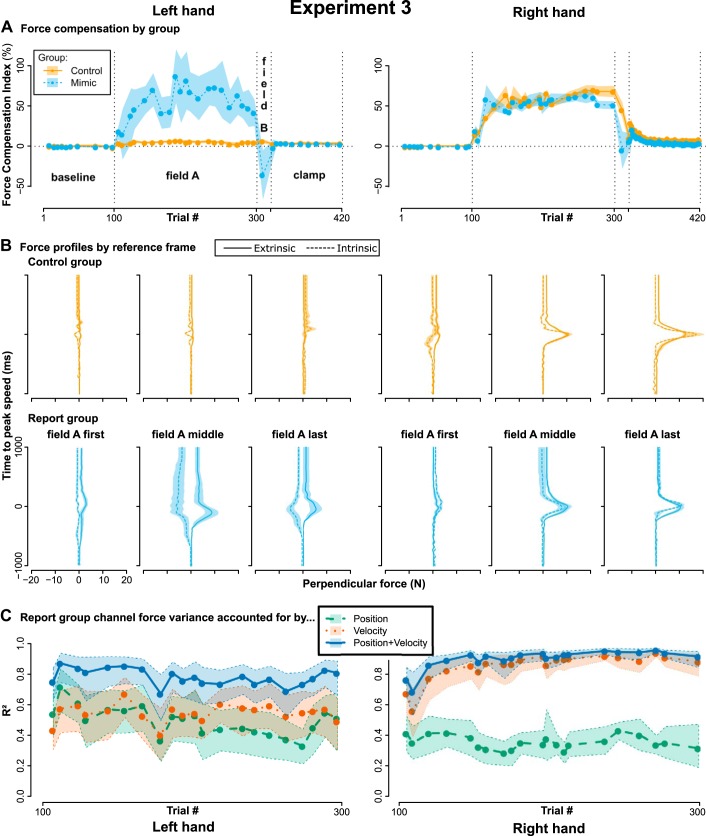
Figure 6, A and B show the force compensation index and force profiles of both groups by hand. Both groups compensated the force field about equally with their right hand. As for the left hand, the control group expressed very little force adaptation, confirming our expectation of limited intermanual transfer based on prior results (Joiner et al. 2013; Malfait and Ostry 2004; Wang and Sainburg 2003). For the Mimic group, we noted that different participants appeared to generate forces in different directions (see Fig. 6B). We interpret this as participants mimicking the force in different reference frames, i.e., in an extrinsic and an intrinsic reference frame. We therefore aligned left hand forces in the Mimic group to an extrinsic reference frame by sign-inverting left hand forces of participants whose median reports in field A matched an intrinsic reference frame (8 participants in Mimic group, 4 in control group). With this, left hand force compensation in the Mimic group on average trailed that of the right hand, albeit still with higher variability (Fig. 6A).
Fig. 6.
Results of experiment 3. A: across participant mean force compensation indices (±SE) of left and right hand force channel trials for the control group with no instruction and the Mimic group instructed to mimic the forces with their left hand. To average left hand performance, the signs of left hand forces were flipped for 8 of 19 participants in the Mimic group and 4 of 17 in the control group, as they appeared to report in an inverted reference frame. B: mean force profiles (±SE) at the beginning, middle and end of field A practice, with separate means for participants displaying a more external or more internal reference frame. C: variance in perpendicular channel force accounted for by along-channel position, velocity, or a linear combination of the two. Shaded areas are bootstrapped 95% confidence intervals.
The group difference was supported by a mixed ANOVA with factors group (control versus Mimic), block (early, middle, late field A practice) and hand (right, left). This yielded a significant main effect of group (F1,34 = 6.6, P = 0.015, = 0.16) and block (F1.4,48.0 = 6.3, P = 0.009, = 0.15) and a significant interaction between group and hand (F1,34 = 5.6, P = 0.023, = 0.13), but no significant effect of hand (F1,34 = 4.1, P = 0.051, = 0.09) and no other significant two- or threefold interactions (all P > 0.29). Following up on the group-by-hand interaction, simple main effects indicated a significant difference between left and right hand in the control (F = 103.8, P < 0.001), but not in the Mimic group (F = 0.04, P = 0.85).
The brevity of the field B phase only allowed us to test one channel per hand in its center. These data were highly variable; for example, the Mimic group had right hand mean: −5%, SE: 101%, median: 21%, and left hand mean: −36%, SE: 134%, median: 5%). Closer inspection revealed that this was not due to few outliers but indeed reflected the group being spread out between maintaining compensation, switching the sign of their compensation, or even increasing. Accordingly, neither t test (t18 = 0.6, P = 0.55), nor sign test (S = 7, P = 0.18) indicated a significant difference between left and right hand for differences between the last field A channel and the field B channel. We speculate that this is reflective of some learners adopting a new strategy that is appropriate to the sign-switched force field, while others did not, potentially because they chose to mimic in a single reference frame. We therefore refrain from overinterpreting the field B or clamp phase in this experiment and note that the method would need to be improved to allow inferences on field switches or rebound.
Representation of force compensation.
Field A practice data afforded us the opportunity to determine whether force compensation reflected separable components of learning. A hallmark of adaptation to velocity-dependent force fields is that compensatory forces are also velocity dependent, suggesting that the brain builds an internal model of the velocity-dependent perturbation (Conditt and Mussa-Ivaldi 1999). Later studies found that the compensatory response to a force field is determined by hand velocity and to a lesser extent by hand position (Sing et al. 2009) and the position-dependent component has subsequently been attributed to a different, model-free learning mechanism (Haith et al. 2011). Considering that recent studies have found proposed model-free reinforcement learning to depend on explicit strategies (Codol et al. 2018; Holland et al. 2018; Shmuelof et al. 2012), this can be taken to suggest that the strategic component of learning should depend on position and, in this respect, resemble off-target aiming typical of cursor rotation experiments (Benson et al. 2011; Bond and Taylor 2017; Taylor et al. 2014). Alternatively, strategies could also depend on velocity. Different from previous approaches (McDougle et al. 2015), experiment 3 offered learners a way to express a strategy in exactly the dimension of the field, allowing us to infer the nature of strategy representation. Figure 5D displays the proportion of variance accounted for on all of the field A channels in experiment 3 when fitting channel-perpendicular force by position, velocity, or a linear combination of the two. For the right hand, the proportion of variance accounted for by velocity is larger than that accounted for by position and increases with practice, in line with learning an internal model of the velocity-dependent force field (Haith et al. 2011; Sing et al. 2009). For the left hand, position and velocity accounted for more equal portions of variance throughout practice. Thus, the putative explicit strategy did not appear to capture the velocity-dependent nature of the force field as adequately as right hand learning, even when the two were assessed by the same method. This supports our interpretation that the explicit component expressed by the left hand is qualitatively different from adaptation of an internal model and suggests that the complexity of explicit learning may be limited to simple, position-dependent strategies, as are typically observed in cursor rotation experiments.
Experiments 1 to 3: reaction times.
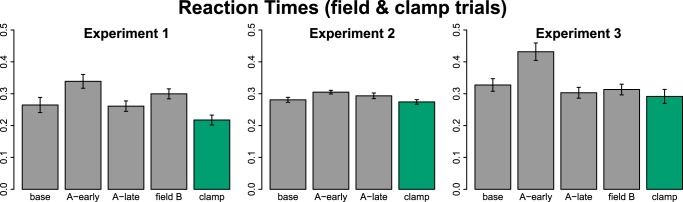
Reaction time (RT) has been shown to sharply increase at the onset of learning in visuomotor rotation tasks (Hinder et al. 2008; McDougle and Taylor 2019; Shabbott et al. 2010), and if RT is constrained, performance is significantly impaired (Fernandez-Ruiz et al. 2011; Haith et al. 2015), suggestive of strategy use. Figure 7 shows RTs from all experiments, summarized over 36 (18 for experiment 3) consecutive field trials (or channel trials for clamp) for selected blocks. In all experiments, RTs increased with the introduction of the force field: Across-block ANOVAs indicated significant differences for experiment 1 (F2.5,47.1 = 13.9, P < 0.001, = 0.42), and experiment 2 (F2.2,35.4 = 5.1, P = 0.009, = 0.24) and post hoc t tests confirmed significant differences from baseline to early field A (experiment 1: t = −4.1, P = 0.004; experiment 2: t = −3.1, P = 0.03). For experiment 3, ANOVA indicated a significant effect of block (F3.3,110.5 = 18.6, P < 0.001, = 0.35), but not of group (F1,34 = 0.04, P = 0.85, = 0.001), and post hoc t tests showed a significant increase from baseline to early field A (t = −4.6, P < 0.001). By the end of field A practice, RTs were decreased relative to early field A in experiment 1 (t = 5.5, P < 0.001), and 3 (t = 7.2, P < 0.001), though this difference was not significant in experiment 2 (t = 1.1, P = 0.6). RTs no longer differed significantly from baseline at the end of field A training (experiment 1: t = 0.2, P = 0.86; experiment 2: t = −1.4, P = 0.6; experiment 3: t = 1.3, P = 1.0). The introduction of field B caused another increase in RT in experiment 1 (compared with late field A: t = −3.3, P = 0.02), but not experiment 3 (t = 0.2, P = 1.0). The latter fact may indicate that the highly variable behavior in experiment 3’s B-phase was truly reflective of learners not adopting a new strategy in response to field B, though this remains speculative.
Fig. 7.
Across participant mean reaction times (±SE) from selected blocks of 36 (experiments 1 and 2) or 18 (experiment 3) trials representing late baseline, early and late field A practice, complete field B practice, and early clamp, respectively. Gray bars are field trials; green bars are No Push trials for experiments 1 and 2 and right hand channel trials for experiment 3.
Critically, a purely implicit learning mechanism would not predict any measurable modulation of RTs upon the imposition of a perturbation. RT, however, is a reliable reflection of action selection/decision-making processes; thus, the observed RT increases likely reflect planning behaviors. After many trials of training, RTs are reduced, perhaps reflecting the “caching” of reinforced actions (McDougle and Taylor 2019), or habituation (Hardwick et al. 2019; Huberdeau et al. 2019). Importantly, whereas our interventions only tested for an explicit component on channel trials, the RT results suggest that this component also contributed on the standard force field learning trials.
DISCUSSION
We designed two novel approaches to probe explicit contributions to force field learning similar to the ways this has been done for aiming strategies in visuomotor adaptation (Heuer and Hegele 2008; Taylor et al. 2014). Under the conditions we studied, our data support an explicit contribution in three ways. First, in experiments 1 and 2, instructing participants to not expect the force field on an upcoming (channel) trial reduced force expression compared with unannounced Catch channels. Conversely, instructing them to expect the force field caused no such reduction, showing that the former was not just a stereotyped response to changes in trial conditions but truly reflects the semantic content of the instruction. Despite the problems surrounding verbalization as a necessary criterion, responsiveness to the content of verbal instruction is a hallmark of explicit learning, whereas implicit adaptation is believed to be immune to this content (Heuer and Hegele 2008; Mazzoni and Krakauer 2006; Schween et al. 2014; Taylor and Ivry 2011). Second, in experiment 3, participants were able to express a learned pattern of compensatory forces with their left hand in response to a field learned with their right hand. This shows that they were both explicitly aware of the force field experienced with their right hand and were able to intentionally utilize that knowledge to shape the motor response of their left hand. During this intentional expression, participants appeared to report in different reference frames. Whereas in principle both implicit and explicit learning could differ in their reference frames across participants, individual interpretations of the instruction to “mimic the force” arise naturally from explicit learning being responsive to instructions. Thus, individual differences in the implicit component would require further explanation, making it more parsimonious to explain them by explicit learning. Furthermore, right hand channel force displayed velocity dependence that increased with practice, whereas left hand force depended equally on position and velocity components. Velocity dependence is a hallmark of adapting putative internal models to viscous force fields, and its reduced presence in the left hand reports suggests that these reflect a qualitatively different component of learning. Third, across all experiments, introduction of the force field led to a transient increase in RT, in line with predictive compensation on normal field trials initially requiring some degree of cognitive processing and subsequently becoming more proceduralized over time (Hardwick et al. 2019; Huberdeau et al. 2019; Leow et al. 2020; McDougle and Taylor 2019). Conversely, the introduction of the final clamp phase led to a reduction in RT, consistent with strategies being abandoned.
The observation that compensation on the Push channels in experiments 1 and 2 not only matched but exceeded Catch channel behavior was unexpected. We assume that the instruction prompted participants to more voluntarily compensate for the force than when just expecting a standard field trial, and this could explain the excess force applied. If such a direct effect of the manipulation occurs on Push trials, could it also explain performance on No Push trials? That is, could decreased expression be explained by participants voluntarily pushing in the other direction—which, notably, would still imply them consciously applying force—rather than just stopping to apply an explicit strategy? The time course of behavior when switching to field B does not support this possibility. Specifically, if participants were applying counterforces in direct response to the instruction, we would expect these to remain of approximately the same magnitude in the B-phase, albeit potentially with a sign switch. Whereas this may be seen in the Push channels, behavior on No Push channels appears to follow a time course that is much more independent from that on Catch channels (Fig. 3A).
More generally, the B-phase data of, experiment 1 are broadly in line with our previous interpretation that “the fast timescale” of force field adaptation may arise from an explicit learning component (McDougle et al. 2015): Push and Catch channels, which include the explicit component, showed rapid adaptation to field B upon its introduction, whereas No Push channels, which exclude it, adapted considerably more slowly. Nevertheless, we note that not all aspects of the behavior we observed can be explained by a two-state model with one fast and one slow component. Under such a model, behavior on No Push channels representing the implicit component should blend directly into clamp phase channels behavior. We suggest two explanations for this: first, it is likely the case that more than two processes contribute to motor adaptation (Forano and Franklin 2019; Inoue et al. 2015; Kim et al. 2015; Miyamoto et al. 2014). For example, Miyamoto and colleagues (2014) identified a single explicit process and two implicit processes. Of the two implicit processes, one was temporally stable (i.e., “slow”), whereas the other was temporally labile (i.e., “fast”). As such, we may suspect that No Push channels reflect both implicit components and that the labile component is responsible for their unexpectedly extensive compensation of field B. Notably, this labile implicit component learns from performance errors rather than sensory prediction errors and is therefore qualitatively different from standard implicit learning of internal models. We may suspect that this component reflects implicit changes in action selection that complement explicit ones (Morehead et al. 2015) and may be driven by reinforcement learning (Codol et al. 2018). Second, if we therefore assume that No Push channels include an implicit component that alters the movement plan, different performance on these channels across phases may additionally be strengthened by another effect: recent studies have shown that implicit memory is specific to the movement plan (Day et al. 2016; Hirashima and Nozaki 2012; McDougle et al. 2017; Schween et al. 2018; Sheahan et al. 2016) and changed movement plans in the different phases should therefore probe and adapt different parts of implicit memory. In summary, we take our results to show that explicit compensation strategies can contribute to force field adaptation, at least for some learners under specific conditions. We find signs of this component contributing to predictive compensation on Catch channels, as well as to compensation on field trials.
A question raised by this is under which specific circumstances does explicit compensation contribute to force field learning. We note that our experimental conditions were deliberately designed to facilitate strategy formation and expression. We consider three choices particularly relevant in this respect: First, we instructed participants about the potential use of strategies. We considered this necessary as we suspected that reducing clarity in our instructions to test more “natural” behavior would have come at the risk of masking an explicit component through misinterpretation of instructions. In contrast, some adaptation studies deliberately instruct their participants to “not think about the task,” which should lower explicit contributions if followed (Morehead et al. 2017). Second, for similar reasons, all of our experiments used only a single target direction, as this reduces the participants’ experience of the force field to mainly the horizontal component. Practicing compensation to different target directions is arguably more complex and may reduce learners’ capacity to extract useful explicit strategies. As such, the frequently used circular multitarget practice protocol (Heald et al. 2018a; Howard et al. 2012, 2013, 2015; Osu et al. 2004; Shadmehr and Mussa-Ivaldi 1994; Sheahan et al. 2016; Stockinger et al. 2014) may be less prone to the contribution of explicit strategies. However, we also note that more simple target arrangements are not uncommon (Brennan and Smith 2015; Heald et al. 2018b; Hirashima and Nozaki 2012; Howard and Franklin 2016; Keisler and Shadmehr 2010; Nozaki et al. 2016; Sarwary et al. 2015; Smith et al. 2006; Stockinger et al. 2015; Vaswani and Shadmehr 2013). Third, we used rather large field constants in experiment 3; lower field constants would likely spur less explicit learning, similar to the way smaller or gradually introduced cursor rotations do (Gaffin-Cahn et al. 2019; Kagerer et al. 2006; Malfait and Ostry 2004; Werner et al. 2014). Overall, it seems likely that the explicit component plays less of a role in force field paradigms that use small field constants, multiple targets, and incentives to reduce strategy use. Whereas our current results are thus not yet suited to make strong claims about force field learning in general, we take them to emphasize that explicit learning can play a role and to provide directions toward ways of better assessing them.
Where would we suspect a relevant role of explicit learning in force adaptation studies? We note that there was considerable individual variation in force modulation on experiments 1 and 2 (Fig. 5B), which parallels findings of high across-participant variability of explicit contribution in cursor rotation studies (Schween and Hegele 2017). This suggests that specific participant groups may be more prone to learn by explicit processes. For example, it has previously been shown that “motor experts” utilize more explicit strategies in a gross-motor prism adaptation task (Leukel et al. 2015) and it is conceivable they could also do so in force adaptation. On the other hand, older age has been identified as a factor apparently limiting strategy use (Hegele and Heuer 2013; Heuer and Hegele 2008; Vandevoorde and Orban de Xivry 2019).
Furthermore, a remaining role for explicit strategies may be suspected in specific scenarios, where the effectiveness of implicit compensation is impaired and explicit strategies could be reasonably assumed to compensate for it. A scenario where standard force field learning is known to be impaired is when participants have to repeatedly switch between different force environments (“dual adaptation”). When exposure alternates between two opposing force fields there is interference, leading to no learning on average (Gandolfo et al. 1996). Studies have identified contextual cues that allow learners to overcome this interference (Heald et al. 2018b; Hirashima and Nozaki 2012; Howard et al. 2012, 2013, 2015; Nozaki et al. 2016; Osu et al. 2004; Proud et al. 2019; Sheahan et al. 2016). Whereas most of these designs seem likely to test more implicit learning based on, e.g., their use of multiple targets, our results suggest that, given the right conditions, explicit strategies could jump in to learn otherwise interfering contexts, and this may explain some divergent findings. For example, results have differed regarding the effectiveness of abstract color cues in supporting dual adaptation (Gandolfo et al. 1996; Howard et al. 2013; Osu et al. 2004). A potential explanation is that the effect of color cues may be restricted to a strategic component whose contribution to solving the task depends on specific experimental conditions. An interesting case is the separation of visual workspaces, which has been a very effective cue in force field experiments (Howard et al. 2013) but appears effective only for explicit learning in cursor rotation studies (Hegele and Heuer 2010; Schween et al. 2018, 2019). Besides explicit learning, possible explanations include separate visual workspaces constituting separate states in the state space relevant for force field, but not for cursor rotation learning. The methods we present here provide a possibility to investigate these questions in more detail.
In conclusion, our two novel methods provide converging evidence that an explicit component can contribute to force field learning that shares important properties with explicit aiming strategies in visual perturbation paradigms. It seems likely that the size of this contribution depends on specific experimental conditions, and the extent to which it is relevant in more standard experiments remains to be determined. The methods we used here provide a starting point for doing this.
GRANTS
J.A.T. received funding from the United States’ National Institute of Neurological Disorders and Stroke, grant R01NS084948 and S.D.M. from the National Institute of Mental Health, grant F32MH119797. M.H. and R.S. received funding from a grant within the Priority Program, SPP 1772, from the German Research Foundation (Deutsche Forschungsgemeinschaft), grant He7105/1.1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.S., S.D.M., and J.A.T. conceived and designed research; R.S., S.D.M., and J.A.T. performed experiments; R.S., S.D.M., and J.A.T. analyzed data; R.S., S.D.M., M.H., and J.A.T. interpreted results of experiments; R.S. prepared figures; R.S. drafted manuscript; R.S., S.D.M., M.H., and J.A.T. edited and revised manuscript; R.S., S.D.M., M.H., and J.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Chandra Greenberg and Krista Bond for support with data collection, as well as Peter Butcher, Carlo Campagnoli, and Eugene Poh for support with experiment setup. We also thank Aaron Wong for help with the design of experiment 3 and Marion Forano for providing feedback on a previous manuscript version.
APPENDIX
Scripted instructions of experiment 2 (experiment 1 was similar). As a general rule, we use this script as a guideline but administer instructions in free speech with individual variations and answer questions asked by the participants within certain limits. While just reading the scripted instructions out loud or having participants read them may seem like better standardization, we consider our method more suited to achieve similar understanding on the side of participants rather than just giving them the same input.
GENERAL TASK INSTRUCTIONS (INSTRUCT AND DEMONSTRATE)
Welcome to our experiment. Today, we investigate how you learn to reach toward a target.
We will ask you to sit down in front of this robotic arm, grasp its handle, and look into the screen below your eye level.
On each trial, the robot will guide your hand to a start location. Once your hand is in the start location, you will see a Plus sign which I ask you to fixate with your view. After a short interval, the sign will disappear and a white target dot will appear. You don’t need to fixate on the Plus after it has disappeared.
Your task is to “shoot” through the target with the cursor in a swift, accurate movement. If you hit the target, it will turn green and you will hear a tone. If you miss it, neither of the two will happen. Independent of hit or miss, you may see a “Too Slow!” warning after the movement indicating that you should move faster. Your goal is to achieve the hit (i.e., target turning green and tone) without Too Slow warning as often as possible.
Note that the interval of target appearance is randomized—we do that so that you really have to react to the visual stimuli and cannot just fall into a rhythm. If you move before the target has appeared, the robot will resist your movement and bring you back toward the start location.
We will find you a comfortable position now. Please keep this position and take particular care not to move the chair.
INSTRUCT AT TRIAL 90 (CLOSE TO END OF BASELINE)
After a few trials, the robot is going to push you off your path while you are moving toward the target. Please try to successfully hit the target in time as often as possible despite being pushed off!
Before some trials you will see a message instead of the Plus sign. If the message says “Push!” please expect the robot to push you off and act as on those trials. Note that you will not feel the robot push you on the trials with the “Push!” message but we nevertheless ask you to act as if it was going to! If the message says “No Push!” we would like you to act as in trials where the robot doesn’t push you off path and just move toward the target.
To sum up, please try to hit the target in time and stay alert to the messages. The important things for you to remember are that you treat “Push!” message trials as if the robot was going to push you even though you will not feel it, and that on “No push!” trials, act as if it was not going to push you.
Note that it is a hard task, so don’t be frustrated if you don’t immediately manage to hit. Try your best and see if you can get better.
AFTER TRAIL 550 (BEFORE ERROR CLAMP)
Starting now, for the remainder of the experiment, we would like you to not push deliberately or apply any aiming strategies you may have developed. Just move toward the target. This is the same as on “No push!” trials, but you won’t be seeing any more messages.
REFERENCES
- Benson BL, Anguera JA, Seidler RDD. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J Neurophysiol 105: 2843–2851, 2011. doi: 10.1152/jn.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond KM, Taylor JA. Structural learning in a visuomotor adaptation task is explicitly accessible. eNeuro 4: ENEURO.0122-17.2017, 2017. doi: 10.1523/ENEURO.0122-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz J, Schuster C. Statistik für Human- und Sozialwissenschaftler (7th ed.). Heidelberg, Germany: Springer, 2010. [Google Scholar]
- Brennan AE, Smith MA. The decay of motor memories is independent of context change detection. PLOS Comput Biol 11: e1004278, 2015. doi: 10.1371/journal.pcbi.1004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codol O, Holland PJ, Galea JM. The relationship between reinforcement and explicit control during visuomotor adaptation. Sci Rep 8: 9121, 2018. doi: 10.1038/s41598-018-27378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM. Continuous versus terminal visual feedback in prism aftereffects. Percept Mot Skills 24: 1295–1302, 1967. doi: 10.2466/pms.1967.24.3c.1295. [DOI] [PubMed] [Google Scholar]
- Conditt MA, Mussa-Ivaldi FA. Central representation of time during motor learning. Proc Natl Acad Sci USA 96: 11625–11630, 1999. doi: 10.1073/pnas.96.20.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham HA. Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol Hum Percept Perform 15: 493–506, 1989. doi: 10.1037/0096-1523.15.3.493. [DOI] [PubMed] [Google Scholar]
- Day KA, Roemmich RT, Taylor JA, Bastian AJ. Visuomotor learning generalizes around the intended movement. eNeuro 3: e0005–e0016, 2016. doi: 10.1523/ENEURO.0005-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wong W, Armstrong IT, Flanagan JR. Relation between reaction time and reach errors during visuomotor adaptation. Behav Brain Res 219: 8–14, 2011. doi: 10.1016/j.bbr.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Forano M, Franklin DW. Timescales of motor memory formation in dual-adaptation (Preprint). bioRxiv 698167, 2019. doi: 10.1101/698167. [DOI] [PMC free article] [PubMed]
- Gaffin-Cahn E, Hudson TE, Landy MS. Did I do that? Detecting a perturbation to visual feedback in a reaching task. J Vis 19: 5, 2019. doi: 10.1167/19.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo F, Mussa-Ivaldi FA, Bizzi E. Motor learning by field approximation. Proc Natl Acad Sci USA 93: 3843–3846, 1996. doi: 10.1073/pnas.93.9.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith A, Pekny SE, Shadmehr R, Krakauer JW. Evidence for model free learning during force field adaptation. Translational and Computational Motor Control (TCMC) Washington, DC, November 12, 2011. [Google Scholar]
- Haith AM, Huberdeau DM, Krakauer JW. The influence of movement preparation time on the expression of visuomotor learning and savings. J Neurosci 35: 5109–5117, 2015. doi: 10.1523/JNEUROSCI.3869-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Forrence AD, Krakauer JW, Haith AM. Time-dependent competition between goal-directed and habitual response preparation. Nat Hum Behav 3: 1252–1262, 2019. doi: 10.1038/s41562-019-0725-0. [DOI] [PubMed] [Google Scholar]
- Heald JB, Franklin DW, Wolpert DM. Increasing muscle co-contraction speeds up internal model acquisition during dynamic motor learning. Sci Rep 8: 16355, 2018a. doi: 10.1038/s41598-018-34737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald JB, Ingram JN, Flanagan JR, Wolpert DM. Multiple motor memories are learned to control different points on a tool. Nat Hum Behav 2: 300–311, 2018b. doi: 10.1038/s41562-018-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele M, Heuer H. Implicit and explicit components of dual adaptation to visuomotor rotations. Conscious Cogn 19: 906–917, 2010. doi: 10.1016/j.concog.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Hegele M, Heuer H. Age-related variations of visuomotor adaptation result from both the acquisition and the application of explicit knowledge. Psychol Aging 28: 333–339, 2013. doi: 10.1037/a0031914. [DOI] [PubMed] [Google Scholar]
- Helmholtz H. Handbuch der physiologischen Optik. Leipzig, Germany: Leopold Voss, 1867. [Google Scholar]
- Heuer H, Hegele M. Adaptation to visuomotor rotations in younger and older adults. Psychol Aging 23: 190–202, 2008. doi: 10.1037/0882-7974.23.1.190. [DOI] [PubMed] [Google Scholar]
- Heuer H, Hegele M. Adjustment to a complex visuo-motor transformation at early and late working age. Ergonomics 52: 1039–1054, 2009. doi: 10.1080/00140130902912795. [DOI] [PubMed] [Google Scholar]
- Hinder MR, Tresilian JR, Riek S, Carson RG. The contribution of visual feedback to visuomotor adaptation: how much and when? Brain Res 1197: 123–134, 2008. doi: 10.1016/j.brainres.2007.12.067. [DOI] [PubMed] [Google Scholar]
- Hirashima M, Nozaki D. Distinct motor plans form and retrieve distinct motor memories for physically identical movements. Curr Biol 22: 432–436, 2012. doi: 10.1016/j.cub.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Holland P, Codol O, Galea JM. Contribution of explicit processes to reinforcement-based motor learning. J Neurophysiol 119: 2241–2255, 2018. doi: 10.1152/jn.00901.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Franklin DW. Adaptive tuning functions arise from visual observation of past movement. Sci Rep 6: 28416, 2016. doi: 10.1038/srep28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Franklin DW, Wolpert DM. Gone in 0.6 seconds: the encoding of motor memories depends on recent sensorimotor states. J Neurosci 32: 12756–12768, 2012. doi: 10.1523/JNEUROSCI.5909-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Wolpert DM, Franklin DW. The effect of contextual cues on the encoding of motor memories. J Neurophysiol 109: 2632–2644, 2013. doi: 10.1152/jn.00773.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Wolpert DM, Franklin DW. The value of the follow-through derives from motor learning depending on future actions. Curr Biol 25: 397–401, 2015. doi: 10.1016/j.cub.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberdeau DM, Krakauer JW, Haith AM. Practice induces a qualitative change in the memory representation for visuomotor learning. J Neurophysiol 122: 1050–1059, 2019. doi: 10.1152/jn.00830.2018. [DOI] [PubMed] [Google Scholar]
- Hwang EJ, Smith MA, Shadmehr R. Dissociable effects of the implicit and explicit memory systems on learning control of reaching. Exp Brain Res 173: 425–437, 2006. doi: 10.1007/s00221-006-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Uchimura M, Karibe A, O’Shea J, Rossetti Y, Kitazawa S. Three timescales in prism adaptation. J Neurophysiol 113: 328–338, 2015. doi: 10.1152/jn.00803.2013. [DOI] [PubMed] [Google Scholar]
- Jakobson LS, Goodale MA. Trajectories of reaches to prismatically-displaced targets: evidence for “automatic” visuomotor recalibration. Exp Brain Res 78: 575–587, 1989. doi: 10.1007/BF00230245. [DOI] [PubMed] [Google Scholar]
- Joiner WM, Brayanov JB, Smith MA. The training schedule affects the stability, not the magnitude, of the interlimb transfer of learned dynamics. J Neurophysiol 110: 984–998, 2013. doi: 10.1152/jn.01072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Bo J, Clark JE. Abrupt, but not gradual visuomotor distortion facilitates adaptation in children with developmental coordination disorder. Hum Mov Sci 25: 622–633, 2006. doi: 10.1016/j.humov.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Keisler A, Shadmehr R. A shared resource between declarative memory and motor memory. J Neurosci 30: 14817–14823, 2010. doi: 10.1523/JNEUROSCI.4160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ogawa K, Lv J, Schweighofer N, Imamizu H. Neural substrates related to motor memory with multiple timescales in sensorimotor adaptation. PLoS Biol 13: e1002312, 2015. doi: 10.1371/journal.pbio.1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Hadjiosif AM, Xu J, Wong AL, Haith AM. Motor learning. Compr Physiol 9: 613–663, 2019. doi: 10.1002/cphy.c170043. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, DiZio P, Lackner J. Task-dependent motor learning. Exp Brain Res 153: 128–132, 2003. doi: 10.1007/s00221-003-1632-0. [DOI] [PubMed] [Google Scholar]
- Lackner JR, Dizio P. Rapid adaptation to Coriolis force perturbations of arm trajectory. J Neurophysiol 72: 299–313, 1994. doi: 10.1152/jn.1994.72.1.299. [DOI] [PubMed] [Google Scholar]
- Lee K, Oh Y, Izawa J, Schweighofer N. Sensory prediction errors, not performance errors, update memories in visuomotor adaptation. Sci Rep 8: 16483, 2018. doi: 10.1038/s41598-018-34598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow L-A, Marinovic W, de Rugy A, Carroll TJ. Task errors drive memories that improve sensorimotor adaptation. J Neurosci 1506-19, 2020. doi: 10.1523/JNEUROSCI.1506-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leukel C, Gollhofer A, Taube W. In Experts, underlying processes that drive visuomotor adaptation are different than in Novices. Front Hum Neurosci 9: 50, 2015. doi: 10.3389/fnhum.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait N, Ostry DJ. Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci 24: 8084–8089, 2004. doi: 10.1523/JNEUROSCI.1742-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle SD, Bond KM, Taylor JA. Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. J Neurosci 35: 9568–9579, 2015. doi: 10.1523/JNEUROSCI.5061-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle SD, Bond KM, Taylor JA. Implications of plan-based generalization in sensorimotor adaptation. J Neurophysiol 118: 383–393, 2017. doi: 10.1152/jn.00974.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle SD, Taylor JA. Dissociable cognitive strategies for sensorimotor learning. Nat Commun 10: 40, 2019. doi: 10.1038/s41467-018-07941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto YR, Wang S, Brennan AE, Smith MA. Distinct forms of implicit learning that respond differentially to performance errors and sensory prediction errors. Translational and Computational Motor Control (TCMC) Washington, DC, November 14, 2014. [Google Scholar]
- Morehead JR, Butcher PA, Taylor JA. Does fast learning depend on declarative mechanisms? J Neurosci 31: 5184–5185, 2011. doi: 10.1523/JNEUROSCI.0040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead JR, Qasim SE, Crossley MJ, Ivry R. Savings upon re-aiming in visuomotor adaptation. J Neurosci 35: 14386–14396, 2015. doi: 10.1523/JNEUROSCI.1046-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead JR, Taylor JA, Parvin DE, Ivry RB. Characteristics of implicit sensorimotor adaptation revealed by task-irrelevant clamped feedback. J Cogn Neurosci 29: 1061–1074, 2017. doi: 10.1162/jocn_a_01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki D, Yokoi A, Kimura T, Hirashima M, Orban de Xivry JJ. Tagging motor memories with transcranial direct current stimulation allows later artificially-controlled retrieval. eLife 5: e15378, 2016. doi: 10.7554/eLife.15378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osu R, Hirai S, Yoshioka T, Kawato M. Random presentation enables subjects to adapt to two opposing forces on the hand. Nat Neurosci 7: 111–112, 2004. [Erratum in Nat Neurosci 7: 314, 2004]. doi: 10.1038/nn1184. [DOI] [PubMed] [Google Scholar]
- Proud K, Heald JB, Ingram JN, Gallivan JP, Wolpert DM, Flanagan JR. Separate motor memories are formed when controlling different implicitly specified locations on a tool. J Neurophysiol 121: 1342–1351, 2019. doi: 10.1152/jn.00526.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Strategic calibration and spatial alignment: a model from prism adaptation. J Mot Behav 34: 126–138, 2002. doi: 10.1080/00222890209601935. [DOI] [PubMed] [Google Scholar]
- Sarwary AME, Stegeman DF, Selen LPJ, Medendorp WP. Generalization and transfer of contextual cues in motor learning. J Neurophysiol 114: 1565–1576, 2015. doi: 10.1152/jn.00217.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol 84: 853–862, 2000. doi: 10.1152/jn.2000.84.2.853. [DOI] [PubMed] [Google Scholar]
- Schween R, Hegele M. Feedback delay attenuates implicit but facilitates explicit adjustments to a visuomotor rotation. Neurobiol Learn Mem 140: 124–133, 2017. doi: 10.1016/j.nlm.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Schween R, Langsdorf L, Taylor JA, Hegele M. How different effectors and action effects modulate the formation of separate motor memories. Sci Rep 9: 17040, 2019. doi: 10.1038/s41598-019-53543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schween R, Taube W, Gollhofer A, Leukel C. Online and post-trial feedback differentially affect implicit adaptation to a visuomotor rotation. Exp Brain Res 232: 3007–3013, 2014. doi: 10.1007/s00221-014-3992-z. [DOI] [PubMed] [Google Scholar]
- Schween R, Taylor JA, Hegele M. Plan-based generalization shapes local implicit adaptation to opposing visuomotor transformations. J Neurophysiol 120: 2775–2787, 2018. doi: 10.1152/jn.00451.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbott BA, Sainburg RL. Learning a visuomotor rotation: simultaneous visual and proprioceptive information is crucial for visuomotor remapping. Exp Brain Res 203: 75–87, 2010. doi: 10.1007/s00221-010-2209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Brandt J, Corkin S. Time-dependent motor memory processes in amnesic subjects. J Neurophysiol 80: 1590–1597, 1998. doi: 10.1152/jn.1998.80.3.1590. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan HR, Franklin DW, Wolpert DM. Motor planning, not execution, separates motor memories. Neuron 92: 773–779, 2016. doi: 10.1016/j.neuron.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Huang VS, Haith AM, Delnicki RJ, Mazzoni P, Krakauer JW. Overcoming motor “forgetting” through reinforcement of learned actions. J Neurosci 32: 14617–14621a, 2012. doi: 10.1523/JNEUROSCI.2184-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing GC, Joiner WM, Nanayakkara T, Brayanov JB, Smith MA. Primitives for motor adaptation reflect correlated neural tuning to position and velocity. Neuron 64: 575–589, 2009. doi: 10.1016/j.neuron.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4: e179, 2006. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler MA. Distinguishing implicit and explicit learning. Psychon Bull Rev 4: 56–62, 1997. doi: 10.3758/BF03210774. [DOI] [Google Scholar]
- Stanley J, Krakauer JW. Motor skill depends on knowledge of facts. Front Hum Neurosci 7: 503, 2013. doi: 10.3389/fnhum.2013.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger C, Focke A, Stein T. Catch trials in force field learning influence adaptation and consolidation of human motor memory. Front Hum Neurosci 8: 231, 2014. doi: 10.3389/fnhum.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger C, Thürer B, Focke A, Stein T. Intermanual transfer characteristics of dynamic learning: direction, coordinate frame, and consolidation of interlimb generalization. J Neurophysiol 114: 3166–3176, 2015. doi: 10.1152/jn.00727.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Flexible cognitive strategies during motor learning. PLOS Comput Biol 7: e1001096, 2011. doi: 10.1371/journal.pcbi.1001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34: 3023–3032, 2014. doi: 10.1523/JNEUROSCI.3619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thürer B, Stockinger C, Focke A, Putze F, Schultz T, Stein T. Increased gamma band power during movement planning coincides with motor memory retrieval. Neuroimage 125: 172–181, 2016. doi: 10.1016/j.neuroimage.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Vandevoorde K, Orban de Xivry JJ. Internal model recalibration does not deteriorate with age while motor adaptation does. Neurobiol Aging 80: 138–153, 2019. doi: 10.1016/j.neurobiolaging.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Vaswani PA, Shadmehr R. Decay of motor memories in the absence of error. J Neurosci 33: 7700–7709, 2013. doi: 10.1523/JNEUROSCI.0124-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149: 520–526, 2003. doi: 10.1007/s00221-003-1392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Schorn CF, Bock O, Theysohn N, Timmann D. Neural correlates of adaptation to gradual and to sudden visuomotor distortions in humans. Exp Brain Res 232: 1145–1156, 2014. doi: 10.1007/s00221-014-3824-1. [DOI] [PubMed] [Google Scholar]
- Werner S, van Aken BC, Hulst T, Frens MA, van der Geest JN, Strüder HK, Donchin O. Awareness of sensorimotor adaptation to visual rotations of different size. PLoS One 10: e0123321, 2015. doi: 10.1371/journal.pone.0123321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Fitzgerald J, Colucci-Chang K, Murthy KG, Joiner WM. The temporal stability of visuomotor adaptation generalization. J Neurophysiol 118: 2435–2447, 2017. doi: 10.1152/jn.00822.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]