Abstract
Denosumab discontinuation has been associated with increased risk of rebound-associated multiple vertebral fractures. We report the cases of three patients, two females and one male, who had manifested rebound-associated vertebral fractures after denosumab discontinuation and sustained new vertebral fractures a few months later. Two of the patients had been previously treated with bisphosphonates. Patients discontinued denosumab after 2 to 8 years of treatment. One of the female patients was receiving prednisolone 7.5 mg daily for an unspecified connective tissue disorder and the male patient methylprednisolone 8 mg daily for dermatomyositis. We hypothesize that rebound-associated multiple vertebral fractures after denosumab discontinuation may occur, at least in some cases, sequentially instead of simultaneously. Our cases further underpin the need for prompt initiation of potent antiresorptives in patients who sustained rebound-associated vertebral fractures, in order to prevent not only bone loss but also a second round of fractures.
Keywords: Denosumab, Discontinuation, Fracture, Rebound, Osteoporosis
1. Introduction
Denosumab is the most potent antiresorptive agent available, causing substantial decrease of bone turnover which leads to continued increases of bone mineral density (BMD) and decreases of fracture rates at all sites for as long as treatment is administered (Bone et al., 2017; Anastasilakis et al., 2018). However, denosumab discontinuation results in a rapid, profound increase of bone resorption (Anastasilakis et al., 2017a) which has been associated with increased risk of rebound-associated multiple vertebral fractures (RAVFs) (Anastasilakis et al., 2017b; Cummings et al., 2018) occurring only a few months following the omission of the last denosumab injection. We report herein three patients from everyday clinical practice who suffered RAVFs in two or more different time points following denosumab discontinuation. Informed consent was obtained from all three patients for publication of their case reports and accompanying images.
2. Cases
2.1. Patient 1
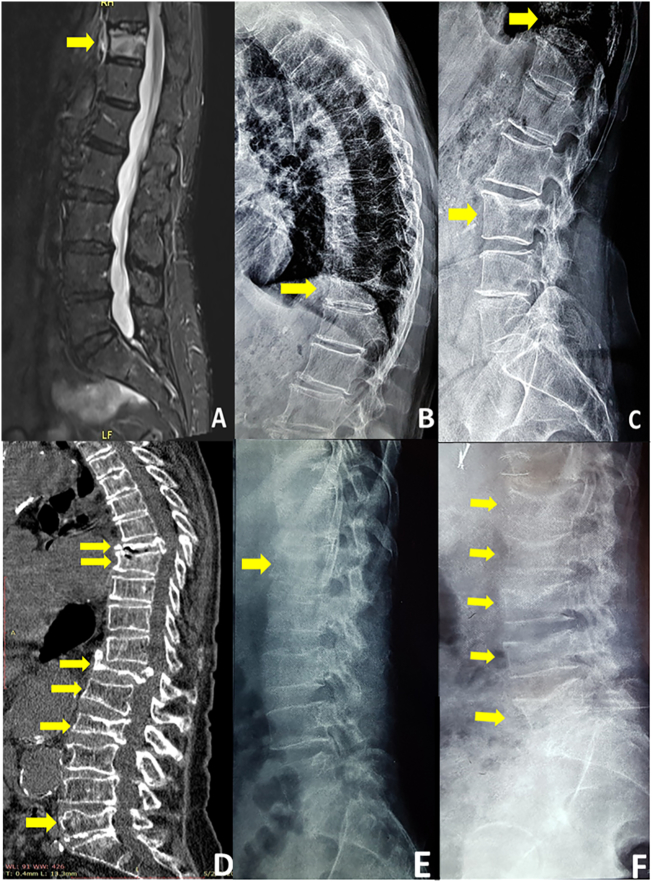
This patient is a postmenopausal woman, 71-years old at baseline, who received denosumab treatment on January 2012 following an allergic reaction to the first pill of alendronate, practically being treatment-naïve. At baseline she had a lumbar spine (LS) bone mineral density (BMD) T-score of −4.0, but was otherwise apparently healthy, with no clinical symptoms or evidence of a prevalent vertebral fracture in conventional X-rays. Her body mass index was 22.9 kg/m2, and she was not taking any concurrent medication. She received denosumab injections every 6 months for eight consecutive years (last injection on January 2019). Her LS BMD T-score at that time was −3.6. She discontinued treatment following her dentist's advice due to a pain in the lower jaw. In September 2019, eight months after the last denosumab injection, she experienced a sharp back pain and magnetic resonance imaging (MRI) revealed a Grade 2 fracture at T11 (Fig. 1, panels A and B). Patient remained off-treatment waiting for dental evaluation when, in November 2019, she experienced a new sharp back pain and subsequent X-rays showed a new Grade 1 fracture at L3 (Fig. 1, panel C). Secondary causes of fracture, such as multiple myeloma, bone metastases, celiac disease, and mastocytosis, were ruled out and the patient resumed denosumab treatment.
Fig. 1.
Magnetic resonance imaging of the spine (Panel A) and X-rays (Panel B) depicting the fracture at T11 in Patient 1. X-Rays showing the new fracture at L3 in Patient 1 (Panel C). Computed Tomography of the spine showing the multiple vertebral fractures in patient 2 (Panel D). X-rays depicting the first timepoint fracture at L1 (Panel E) and the additional fractures at L2, L3, L4, and L5 at the second timepoint in patient 3 (Panel F).
2.2. Patient 2
A postmenopausal woman initiated denosumab treatment on February 2017 at the age of 76, after a 5-year course with ibandronate. The patient had a prevalent fracture at L2 and was receiving prednisolone 7.5 mg daily for an unspecified connective tissue disorder. BMD T-score at baseline is not available. She received denosumab for 2 years (last injection in August 2018) and experienced a sudden sharp back pain after lifting weight in April 2019 (8 months after the last injection). A Grade 3 fracture at T12 was identified in MRI. She remained off-treatment by choice as she was grieving the loss of her husband, and on January 2020 (17 months after the last injection) she experienced back pain again, while standing, with no apparent cause. New fractures, Grade 2 at T8, L1, L5 and Grade 3 at T7, and a deterioration of the fracture at L2 were shown in CT imaging (Fig. 1, Panel D). The dose of prednisolone was invariable throughout the treatment with denosumab and its off-treatment period. Other secondary causes of fractures were ruled out. The patient was again set on denosumab.
2.3. Patient 3
The 3rd patient is a 53-year old male who had been previously treated with alendronate for three years. The patient had dermatomyositis treated with 8 mg of methylprednisolone daily. At baseline his body mass index was 27.8 kg/m2 and he had no clinical symptoms or evidence of a prevalent vertebral fracture in conventional X-rays. Having a BMD T-score of −3.2 at the left femoral neck despite treatment, he was switched to denosumab for three years from 2011 up to 2014. After that, patient decided to discontinue treatment and sustained a Grade 2 fracture at L1 (Fig. 1, panel E). Denosumab was reinitiated for another year, when a delay of three months in the next injection resulted in two new Grade 2 RAVFs at L3, and L5, and two Grade 1 RAVFs at L2, and L4 (Fig. 1, Panel F). As in patient 2, the dose of methylprednisolone was stable throughout the denosumab treatment and during the off-treatment period and other secondary conditions predisposing to fractures were ruled out. Denosumab was reinstated once more until November 2019 when a new two-month delay in the injection resulted in an additional Grade 2 fracture at T11.
3. Discussion
Herein we report three patients who discontinued denosumab after 2 to 8 years of treatment and sustained rebound-associated vertebral fractures sequentially in two or more different timepoints following discontinuation. Another similar case has very recently been reported (Niimi et al., 2020). Given the above incidences, we hypothesize that rebound-associated multiple vertebral fractures after denosumab discontinuation may occur, at least in some cases, sequentially and not at the same time, especially if osteoporosis treatment is not resumed. A second round of fractures had already been reported in such patients who had being subjected to vertebroplasty at the initially fractured vertebrae (Anastasilakis et al., 2017b). This had been attributed to the increased compressing forces exerted upon the previously normal vertebrae by their neighboring cemented vertebrae (Anastasilakis et al., 2017b). Taking all together, it seems that the suddenly and profoundly weakened skeleton, by the abrupt increase of bone turnover following denosumab discontinuation, remains prone to fractures for a long period of time and the event of an initial fracture does not necessarily mark the “peak” of fragility as even more fractures can be expected.
This could be of clinical importance, highlighting once more the need for prompt treatment re-initiation as soon as the first incident occurs to avoid the next “wave” of fractures. In this aspect, bisphosphonates are recommended by experts (Tsourdi et al., 2017) although the optimal regimen and treatment duration remain largely unknown. Up to now alendronate (Freemantle et al., 2012) and zoledronate (Anastasilakis et al., 2019) have shown to fully maintaining BMD gains achieved during denosumab treatment although a partial efficacy of zoledronate and lesser of risedronate have been reported in other studies (Everts-Graber et al., 2020; Reid et al., 2017; Lehmann and Aeberli, 2017; Horne et al., 2018). However, among the antiresorptive agents, denosumab achieves the most rapid reduction of bone turnover, being evident already within the first 12 h and reaching a nadir at about 1 month (Bekker et al., 2004). Therefore, from a pharmacokinetic point of view, denosumab re-initiation might be more appropriate in these cases of rebound-associated vertebral fractures, where an immediate reinstatement of an antiresorptive effect is urgently needed, although this cannot be universally expected in all cases as a recent case report has shown (Niimi et al., 2020). On the other hand and according to current limited evidence, zoledronate infusion may also prevent the rebound-vertebral fractures following denosumab discontinuation (Anastasilakis et al., 2019; Everts-Graber et al., 2020); however, these studies had BMD rather than fractures as primary end-point. Therefore, at this time it is difficult to draw conclusions about the potential of zoledronate to prevent RAVFs. In this regard, the use of bone turnover markers has been proposed as a valid approach of defining the most effective time-point to start treatment with zoledronate (Everts-Graber et al., 2020; Reid et al., 2017; Lehmann and Aeberli, 2017; Horne et al., 2018). However, a trial designed to test this hypothesis has shown that zoledronate did not fully prevent BMD loss in patients treated with denosumab for a mean of 4.6 years, regardless if it was given at 6 months or at 9 months after the last denosumab injection or when bone turnover markers increased above a prespecified limit (Sølling et al., 2019). Furthermore, zoledronate deterrent effect on RAVFs has not been tested yet in cases who already sustained an initial fracture event(s).
Unfortunately, we do not have available serum samples to measure bone turnover markers during the course of our patients. It is a fact that case reports provide the lowest level of evidence. Therefore, these may be simply cases of patients with severe osteoporosis who would have fractured anyway or we may be missing a yet unknown key factor acting together with the rebound effect on bone turnover and maintaining a high fracture risk in patients experiencing RAVFs. The fact that one vertebral fracture predisposes to the next is a well-established knowledge (Black et al., 1999) and could just indicate compromised bone strength irrespective of denosumab discontinuation. However, the short interval between the sequential fractures in our cases could be indicative of the abrupt and profound deterioration of bone status that usually characterizes patients who discontinue denosumab. Prospective cohort studies are needed to prove or reject whether resuming denosumab is the most favorable choice in patients that have sustained fractures following its discontinuation. In any case and for the time being prompt initiation of potent antiresorptives is of paramount importance in patients who sustained rebound-associated vertebral fractures after discontinuing denosumab.
CRediT author statement
Athanasios D. Anastasilakis: Conceptualization, Methodology, Validation, Writing Original Draft, Supervision; Gerasimos Evangelatos: Data curation, Resources, Visualization; Polyzois Makras: Writing-Review and Editing Visualization; Alexios Iliopoulos: Conceptualization, Resources, Writing- Reviewing and Editing.
Grant support
This work was not supported by any grant, fund, or institution.
Declaration of competing interest
A.D. Anastasilakis reports lecture fees from Amgen, Bianex, Eli-Lilly and ITF; G. Evangelatos has nothing to declare; Makras reports honoraria for lectures and research grants from Amgen; lecture fees from Glaxo, Lilly, Pfizer, Leo, Genesis, Elpen, and Vianex. A. Iliopoulos reports lecture fees from Amgen, MSD, and Novartis.
References
- Anastasilakis A.D., Yavropoulou M.P., Makras P., Sakellariou G.T., Papadopoulou F., Gerou S., Papapoulos S.E. Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur. J. Endocrinol. 2017;176:677–683. doi: 10.1530/EJE-16-1027. [DOI] [PubMed] [Google Scholar]
- Anastasilakis A.D., Polyzos S.A., Makras P., Aubry-Rozier B., Kaouri S., Lamy O. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J. Bone Miner. Res. 2017;32:1291–1296. doi: 10.1002/jbmr.3110. [DOI] [PubMed] [Google Scholar]
- Anastasilakis A.D., Polyzos S.A., Makras P. THERAPY OF ENDOCRINE DISEASE: Denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur. J. Endocrinol. 2018;179:R31–R45. doi: 10.1530/EJE-18-0056. [DOI] [PubMed] [Google Scholar]
- Anastasilakis A.D., Papapoulos S.E., Polyzos S.A., Appelman-Dijkstra N.M., Makras P. Zoledronate for the prevention of Bone loss in women discontinuing denosumab treatment. A prospective 2-year clinical trial. J. Bone Miner. Res. 2019;34:2220–2228. doi: 10.1002/jbmr.3853. [DOI] [PubMed] [Google Scholar]
- Bekker P.J., Holloway D.L., Rasmussen A.S., Murphy R., Martin S.W., Leese P.T., Holmes G.B., Dunstan C.R., DePaoli A.M. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J. Bone Miner. Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- Black D.M., Arden N.K., Palermo L., Pearson J., Cummings S.R. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of osteoporotic fractures research group. J. Bone Miner. Res. 1999;14:821–828. doi: 10.1359/jbmr.1999.14.5.821. [DOI] [PubMed] [Google Scholar]
- Bone H.G., Wagman R.B., Brandi M.L. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- Cummings S.R., Ferrari S., Eastell R. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J. Bone Miner. Res. 2018;33:190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- Everts-Graber J., Reichenbach S., Ziswiler H.R., Studer U., Lehmann T. A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of Bone mass gains. J. Bone Miner. Res. 2020 doi: 10.1002/jbmr.3962. [DOI] [PubMed] [Google Scholar]
- Freemantle N., Satram-Hoang S., Tang E.T., Kaur P., Macarios D., Siddhanti S., Borenstein J., Kendler D.L., Investigators D Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos. Int. 2012;23:317–326. doi: 10.1007/s00198-011-1780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne A.M., Mihov B., Reid I.R. Bone loss after romosozumab/denosumab: effects of bisphosphonates. Calcif. Tissue Int. 2018;103:55–61. doi: 10.1007/s00223-018-0404-6. [DOI] [PubMed] [Google Scholar]
- Lehmann T., Aeberli D. Possible protective effect of switching from denosumab to zoledronic acid on vertebral fractures. Osteoporos. Int. 2017;28:3067–3068. doi: 10.1007/s00198-017-4108-y. [DOI] [PubMed] [Google Scholar]
- Niimi R., Kono T., Nishihara A., Hasegawa M., Kono T., Sudo A. Second rebound-associated vertebral fractures after denosumab discontinuation. Arch. Osteoporos. 2020;15:7. doi: 10.1007/s11657-019-0676-0. [DOI] [PubMed] [Google Scholar]
- Reid I.R., Horne A.M., Mihov B., Gamble G.D. Bone loss after denosumab: only partial protection with zoledronate. Calcif. Tissue Int. 2017;101:371–374. doi: 10.1007/s00223-017-0288-x. [DOI] [PubMed] [Google Scholar]
- Sølling A.N., Harsløf T., Langdahl B.L. Treatment with zoledronic acid subsequent to treatment with denosumab. J. Bone Miner. Res. 2019;34:60. [Google Scholar]
- Tsourdi E., Langdahl B., Cohen-Solal M., Aubry-Rozier B., Eriksen E.F., Guanabens N., Obermayer-Pietsch B., Ralston S.H., Eastell R., Zillikens M.C. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]