Abstract
Adipose tissue is classically recognized as the primary site of lipid storage, but in recent years has garnered appreciation for its broad role as an endocrine organ comprising multiple cell types whose collective secretome, termed as adipokines, is highly interdependent on metabolic homeostasis and inflammatory state. Anatomical location (e.g. visceral, subcutaneous, epicardial etc) and cellular composition of adipose tissue (e.g. white, beige, and brown adipocytes, macrophages etc.) also plays a critical role in determining its response to metabolic state, the resulting secretome, and its potential impact on remote tissues. Compared with other tissues, the heart has an extremely high and constant demand for energy generation, of which most is derived from oxidation of fatty acids. Availability of this fatty acid fuel source is dependent on adipose tissue, but evidence is mounting that adipose tissue plays a much broader role in cardiovascular physiology. In this review, we discuss the impact of the brown, subcutaneous, and visceral white, perivascular (PVAT), and epicardial adipose tissue (EAT) secretome on the development and progression of cardiovascular disease (CVD), with a particular focus on cardiac hypertrophy and fibrosis.
Introduction
Adipose tissue biology is intricately linked to cardiovascular health, and the growing obesity epidemic increases the prevalence of cardiovascular disease (CVD) risk factors for hypertension, atherosclerosis, and myocardial infarction (MI). The heart has a constantly high demand for ATP generation, and the majority of this energy in healthy myocardium comes from oxidation of fatty acids, with adipose tissue providing a key source of free fatty acids (FFAs) [1]. Furthermore, it is well established that the metabolic fuel source and energy demands of the heart are altered in cardiac pathology, establishing a critical metabolic and physiological link between the heart as a primary source of FFA catabolism and adipose tissue as the primary source of FFA storage [1,2] Obesity comorbidities, including type 2 diabetes, have been linked to inflammation of the white adipose tissue (WAT) depots in both mice and men [3]. Adipose tissue is becoming increasingly recognized as an important source of paracrine signaling, through means such as adipocyte-derived exosomes and adipokines that influence CVD initiation and progression. In the setting of obesity, hypertrophic adipocytes are known to secrete numerous pro- and anti-inflammatory adipokines that have been shown to play a role in CVD. In addition to adipocytes, other cell types within adipose tissue, including smooth muscle, endothelial cells, fibroblasts, and macrophages, may also contribute to the paracrine signaling properties of adipose tissue [4,5]. Adipose tissue expansion in obesity is accompanied by an increase in total infiltrating immune cells and a shift in macrophage polarization toward a classical ‘M1’-like pro-inflammatory activation state [6,7] The relationship between obesity and CVD is indeed an interesting one and the ‘obesity paradox’, which postulates that while obesity may increase risk factors for CVD, mortality is actually reduced in the presence of obesity, continues to loom large in the field, and is yet to be satisfactorily explained on the mechanistic level [8–10]. The focus of this review is how adipose tissue-derived signaling, specifically associated with The pro-inflammatory milieu of obesity, impacts the development and progression of cardiac hypertrophy and fibrosis.
Heart failure (HF) is a leading cause of mortality in the United States with projections of affecting 8 million adults by 2030 [11]. HF is often preceded by pathological remodeling of cardiac structure and compliance in the forms of left ventricular (LV) hypertrophy (LVH) and fibrosis in response to injury (e.g. ischemia), increased peripheral resistance (e.g. chronic hypertension or obesity), or obstruction (e.g. valvular disease) [12–14]. The initial development of cardiac LVH is a beneficial and compensatory response to maintain cardiac output in the face of hemodynamic stress. Common etiologies for LVH can be physiological (e.g. normal cardiac muscle enlargement associated with athletes or pregnancy), pathological (e.g. in response to chronic hypertension, valvular disease, or MI, or congenital. The underlying physiology and differential molecular mechanism driving pathological and physiological LVH have been reviewed elsewhere [13], but our focus here is on the impact of adipose tissue on pathological cardiac remodeling.
A central theme of LVH that is distinctly specific to pathological hypertrophy is the activation of fibroblasts to myofibroblasts and subsequent accumulation of fibrosis within the myocardium. Fibroblasts in a healthy heart are quiescent, non-dividing cells responsible for homeostatic collagen turnover and continuous restructuring of the extracellular matrix (ECM) to optimize the contractile function of cardiomyocytes [14,15]. Myofibroblasts, on the other hand, have minimal contractile properties, acquire the ability to proliferate and migrate, and are marked by an excess deposition of ECM proteins [14,16]. Both compensated and decompensated hypertrophy and fibrosis are known clinically to be significant contributors and predictors of diastolic and systolic HF [17].
The potential for adipose tissue to impact cardiovascular physiology may seem quite obvious, but many of the mechanisms of how this tissue cross-talk occurs remain as the elusive topic of ongoing work. As this relatively new field grows and expands, so does the understanding that the varying adipose tissue depots have disparate impacts on CVD. First, adipose tissue itself can be broadly defined as either WAT or brown adipose tissue (BAT). WAT serves primarily as a fat (triglyceride; TG) droplet-rich energy store and can make up to 25% of body weight, whereas BAT is mitochondrial dense and metabolically active, accounting for as much as 20% of all energy expenditure [18]. More recently, beige adipocytes, or BAT-like cells within WAT depots, have been identified and shown to share many similarities with BAT, including expression of BAT marker genes, increased mitochondrial density, and heat induction in response to exercise, cold, or adrenergic stimulation [19,20]. However, due to the complexities of their inducible nature and interspersed location within larger WAT depots, the specific effects of beige adipocytes within the disease setting has been difficult to assess and their contribution remains unclear.
In addition to adipocyte composition (white, brown, beige), it has been known for decades that the specific anatomical location of adipose tissue has been shown to play a key role in cardiac influence [21,22]. For example, growing clinical evidence shows that excess visceral WAT is more strongly associated with CVD and associated comorbidities, potentially due to it being correlated with a more pro-inflammatory state compared with subcutaneous WAT [4,23,24]. Similarly, BAT is thought to be less inflammatory than WAT and have several cardioprotective properties [25]. Here, we will review the current state of the field with regard to the attributed role of visceral and subcutaneous white, brown, perivascular (PVAT), and epicardial adipose tissue (EAT) to cardiac hypertrophy and fibrosis.
Visceral and subcutaneous WAT
WAT acts as a major energy reservoir of the body. WAT also serves as an endocrine organ, where it can secrete hormones, lipids, and cytokines that regulate systemic energy homeostasis [26]. WAT is unique in that it is unilocular, possesses few mitochondria, and tends to be negative for uncoupling protein 1 (UCP1), which is vastly different than BAT [27]. The major anatomical WAT depots include intra-abdominal, upper-body subcutaneous, and lower body subcutaneous [28,29]. Further, intra-abdominal fat depots are made up of omental and mesenteric depots, otherwise termed as visceral fat. Lower body subcutaneous includes gluteal fat, subcutaneous leg fat, and intramuscular fat [29]. Subcutaneous fat is thought to be more heterogeneous, histologically, where it contains more mature unilocular adipocytes intercalated with small multilocular adipocytes. Visceral fat, however, consists of more large unilocular adipocytes and appears to be more uniform in nature [30–32].
In mice, subcutaneous WAT depots express higher levels of browning genes compared with visceral WAT [33]. This is consistent with the notion that the subcutaneous fat depot can undergo extensive browning after cold exposure and the appearance of multilocular beige adipocytes are mainly found in subcutaneous, but not visceral, fat depots [34]. On the contrary, human visceral WAT depots express higher levels of browning genes, suggesting a potential species-specific difference in the beiging of WAT [33]. Aside from these anatomical and genetic differences, it is well established that significant deposition of visceral fat is associated with metabolic syndrome, CVD, and several cancers [35,36]. On the contrary, subcutaneous fat depots are generally recognized to be protective in the context of cardiometabolic disease [35,37,38]. Therefore, the ratio of visceral fat to subcutaneous fat remains a predictor of metabolic syndrome and CVD [39].
Previous studies have reported that obese subjects have increased LV mass, LV wall thickness, and LV internal dimension compared with non-obese subjects [40–42]. Moreover, LV mass is significantly associated with waist-to-hip ratio and with waist-to-height ratio after adjusting for body mass index, suggesting that central obesity is an important risk factor for LV mass in older adults [43]. WAT, along with infiltrating immune cells such as monocytes and macrophages within the stromal vascular fraction (SVF), is known to express pro-inflammatory cytokines such as TNF-α and interleukin-6 (IL-6) in an obesity-dependent manner, though these cytokines are by no means specific to adipose tissue [44]. Here, we discuss the effects of WAT-selective adipokines on CVD, also highlighted in Table 1.
Table 1.
WAT-selective adipokines
| Adipokine | Tissue source | Cardiac function in animal models | Clinical correlation with CVD |
|---|---|---|---|
| Adiponectin | WAT, PVAT | Protective in acute I/R [47–49]; reduced ROS [48]; reduced Hypertrophy [50]; reduced fibrosis [51] | Uncertain/contradictory |
| Leptin | WAT, PVAT, EAT | Protective in acute I/R [62–67]; increased hypertrophy [68–75]; ECM remodeling [69,76–78] | Increased risk, specifically MI [56–60] |
| FABP4 | WAT, SVF | Unknown | Increased LV mass [82,83] |
| Resistin | WAT, EAT, SVF | Increased LV hypertrophy [89,90] | Increased CAD [91–93]; Increased HF [94] |
| Autotaxin | Increased hypertrophy and fibrosis [95] | Unknown | |
| Visfatin | SVF | Reduced apoptosis [97,98]; atherogenic plaque | Unknown |
| Destabilization [96,99] | |||
| Omentin | WAT, SVF, EAT | Protective in acute I/R [101,102]; reduced hypertrophy [103,104] | Decreased CAD [105–108] |
| Activin A, cTGF, MMPs | EAT | Increased fibrosis/ECM remodeling [184–188] | Increased atrial fibrillation [185,186,189–192] |
Central obesity and visceral adipose tissue accumulation are both inversely correlated with serum adiponectin [45], an anti-inflammatory adipokine that is traditionally viewed as an adipocyte-specific molecule but has also been found to be expressed locally in adult cardiomyocytes [46]. Adiponectin has been shown to play a protective role in acute ischemic injury [47], perhaps through an autophagy-dependent mechanism [48], and also plays a direct role in post-ischemic cardiac remodeling [49]. It has been demonstrated that mice lacking adiponectin display increased LV wall thickness with an accompanying ~74% decrease in cardiac output after transverse aortic constriction, compared with control animals [50]. Similarly, adiponectin-deficient mice exhibit severe angiotensin II (AngII)-induced cardiac fibrosis which was reversed with adiponectin supplementation [51]. In humans, however, the association between serum adiponectin and cardiac hypertrophy, and CVD risk in general, remains controversial [4]. A study in Japanese men demonstrated that serum adiponectin levels were inversely and independently associated with LV hypertrophy [52]. On the contrary, adiponectin secretion from WAT is dependent on systemic inflammation and stimulated by brain natriuretic peptide [53,54], which is increased in cardiac hypertrophy and HF, suggesting adiponectin to be predictive of adverse cardiac outcomes. Additionally, excessive visceral adiposity is associated with impaired diastolic parameters when assessing LV diastolic function, independently of decreased circulating adiponectin levels [55]. Nonetheless, it is evident that adiponectin is important in the underlying etiology of cardiac hypertrophy and fibrosis, but more work is needed to elucidate the precise mechanism(s) involved.
Leptin, another well-studied adipokine, increases with obesity and has been clinically shown to be associated with increased cardiovascular risk [56–60]. Like adiponectin, leptin is predominately expressed by WAT; however, leptin can also be produced by epicardial and perivascular adipose tissue, as well as directly by cardiac myocytes, and is generally elevated in obese individuals [61]. Studies examining the role of leptin on acute ischemia/hypoxia have suggested that it reduces myocyte apoptosis [62–65] which gives it a cardioprotective effect of reducing myocardial infarct size [66,67]. However, the majority of studies examining the role of leptin in the setting of cardiac hypertrophy have determined it to have a detrimental role of promoting hypertrophic progression, potentially through a p38 MAPK-dependent signaling pathway [68–75]. Leptin has also been shown to modulate collagen synthesis and potentially mediate changes in ECM remodeling enzymes [69,76–78].
Adiponectin and leptin are possibly the most common, but certainly not the only WAT- derived adipokines with the potential to influence cardiac hypertrophy and fibrosis. Fatty acid binding protein 4 (FABP4) strongly correlates with adiposity and is released from adipocytes where it acts as an adipokine in several organs, including the heart [79–81]. Serum FABP4 concentrations have recently been identified to associate with LV mass in overweight and obese women [82], which is consistent with data in non-obese patients who were hospitalized for acute decompensated HF [83]. Interestingly though, FABP4 was once thought to be expressed primarily in adipocytes [84,85], but has since been shown to also be expressed in monocyte and macrophage lineages [86,87]. Resistin, which is similarly expressed by both mature adipocytes and monocytes/macrophages within the stromal–vascular fraction [88], has been shown to mediate cardiac dysfunction and LV hypertrophy [89,90] while also showing a strong clinical correlation with coronary artery disease (CAD) [91–93] and HF in human patients [94]. Additional adipokines have been suggested to play a cardioprotective role. Recent work demonstrated that specific inhibition of adipose tissue-derived autotaxin protected against HFD-induced LV hypertrophy and dysfunction [95]. Visfatin, secreted primarily by the SVF of WAT [96], has been suggested to reduce myocyte apoptosis [97,98], but has also been shown to play a pro-inflammatory role and mediate atherogenic plaque destabilization [96,99]. Omentin, also expressed primarily by the SVF of WAT [100], has also been suggested to be cardioprotective in the setting of acute ischemic injury, potentially through a reduction in apoptosis [101,102], as well as pathological hypertrophy [103,104]. Circulating omentin levels are also negatively correlated with CAD [105–108].
The specific cell signaling link between subcutaneous adiposity and its influence on cardiac hypertrophy and fibrosis remains poorly defined. In 2013, Ichikawa and colleagues [109] noted that the ratio of visceral adipose tissue to subcutaneous adipose tissue was an independent determinant of LV mass. Recent findings from the Dallas Heart Study demonstrate that lower body subcutaneous fat was associated with higher LV end-diastolic volume, reduced concentricity and wall thickness, and greater cardiac output. Visceral fat, however, remained associated with lower cardiac output and higher vascular resistance [110]. In accordance with mouse data, this information suggests that visceral adipose tissue remains positively correlated with CVD risk including increased LV mass and dysfunction, while subcutaneous adiposity remains protective in this context. Further work is needed to identify the underlying mechanisms, and potential adipose-specific adipokines, that may contribute to the etiology of LV hypertrophy and cardiac dysfunction.
BAT
The existence of functional BAT in adult humans was controversial until 2009 when it was demonstrated that adults indeed possess multiple depots of metabolically active BAT (see Figure 1 for anatomical distribution of BAT in humans) [111,112]. Importantly, it was shown that energy expenditure in BAT could be enhanced through cold or adrenergic stimulation similar to BAT in rodents [111–115]. The thermogenic uncoupling of mitochondrial ATP synthesis, which can account for up to 20% of total energy expenditure [18], is primarily achieved by uncoupling proteins, with UCP1 being expressed specifically in brown and beige adipocytes and the most well-studied mediator of thermogenesis [116].
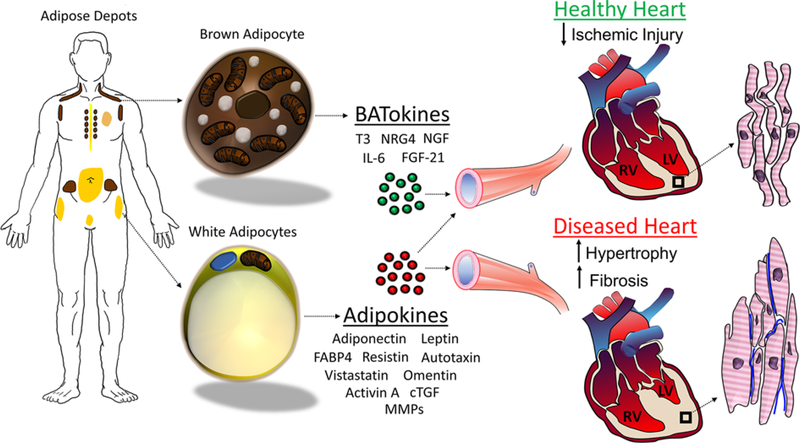
Figure 1. Adipose-mediated paracrine signaling effects on the heart.
WAT (yellow color), comprising PVAT, EAT, visceral, subcutaneous, and gonadal, is composed of droplet-rich and mitochondrial poor white adipocytes. BAT (brown color), comprising cervical, supraclavicular, auxillary, paravertebral, and suprarenal, is composed of multilocular and mitochondrial rich brown adipocytes. WAT releases several adipokines, which can result in cardioprotection, but is often associated with increased CVD, including hypertrophied cardiomyocytes and collagen deposition (blue). BAT releases BATokines that are most often associated with promoting healthy myocardium and cardioprotection from ischemic injury.
BAT has an important role in modulating various aspects of cardiovascular health. As other types of adipose tissue have shown to yield a negative cardiovascular effect, BAT has been shown to be cardioprotective in models of myocardial injury, myocardial fibrosis, and LV hypertrophy [117]. As such, transplantation of activated BAT has piqued interest as a novel therapeutic treatment of cardiac injury. Formerly, increasing the presence of adult BAT has served as a therapeutic target for obesity and associated diseases, such as type II diabetes, due to its thermogenic properties [118,119]. Activation of BAT as a therapeutic strategy for weight loss was suggested as early as the 1930s and has continued with agents such as glucocorticoids, capsinoids, and β3-adrenergic receptor (β3-AR) agonists, but all have been unsuccessful in achieving weight reduction without unwanted cardiovascular side effects [114,120–122]. Interestingly, normal cardiac function has been shown to be necessary for BAT-mediated thermogenesis [123]. Conversely, the molecular contributors to BAT development and function, such as norepinephrine, catecholamine, and natriuretic peptide release occurring along with sympathetic nervous system activation, also have clear cardiovascular functions and are correlated with cardiac injury and LV remodeling [117,124,125].
Utilizing a model of catecholamine-induced cardiomyopathy, Thoonen and colleagues [117] demonstrated that Ucp1–/– mice had observed increases in cardiomyocyte injury and fibrosis versus proficient controls, suggesting BAT has protective effects in the setting of myocardial injury and cardiac remodeling. They observed exacerbated hypertrophic remodeling of the LV in Ucp1–/– mice, accompanied by a decrease in LV systolic function in males, and an increase in hypertrophy in females [117]. The gender differences expressed in this model are consistent with the differences exhibited in models of humans [117,126] and other rodents [127,128]. Ucp1–/– mice subjected to isoproterenol treatment also displayed decreased survival and decreased phosphorylation of AKT compared with wild-type controls [117]. The AKT pathway is suggested to be cardioprotective in ischemia and reperfusion injury, and a decrease in phosphorylation is damaging to cardiomyocyte longevity [129–131]. However, upon transplantation of functional BAT to Ucp1–/– mice, improvements in myocardial injury and increased survival was observed, suggesting a cardioprotective function of BAT [117].
The complete endocrinological profile of BAT is largely unknown but is suggested to play a cardioprotective role. Compiling evidence suggests BAT yields autocrine and paracrine action through the release of ‘BATokines’, which often act to improve general metabolic function. Functional BAT may improve cardiovascular health just by virtue of an improved metabolic profile, including benefits on glucose metabolism, adipogenesis of BAT, increased energy expenditure, and insulin sensitivity, but is also suggested to mediate cardiac risk through the release of systemic cardioprotective factors [132]. A comprehensive list of BATokines and their roles in cardiometabolic disease can be found in Table 2.
Table 2.
BATokines
| BATokine | Metabolic function | Cardiac function |
|---|---|---|
| Thriiodothyronine (T3) | Up-regulated in BAT post activation, effects areas such as growth, metabolism, and homeostasis [25,133,134] | Unclear, but thyroid hormones have shown cardioprotective effects in humans [25] |
| FGF-21 | Beneficial effects on lipid and glucose metabolism [135,138], acts on white adipose tissue and the liver [25,135] | Cardioprotective and antihypertrophic effects in animal models of ischemia and hypertrophy [25,139,140] |
| NRG4 | Adipogenesis and cold induced activation of BAT [25,141] | Cardioprotective effects in mice models of myocardial ischemia [25,141] |
| IL-6 | Mediates glucose metabolism and energy expenditure, promotes browning of WAT [25,142,143,144] | Diverse; cardioprotective effects in HF, contributing factor in cardiac remodeling [145] |
| NGF | Produced by BAT, but down-regulated upon BAT activation by cold or adrenergic stimulation [146–150] | Promotes cell survival and preserves function in ischemic and diabetic hearts [147–149] |
The first BATokine to be identified was the thyroid hormone Triiodothyronine (T3) which has an extensive network of action within the human body, affecting areas such as growth, metabolism, and heart rate [133]. The enzyme type II thyroxine 5′-deiodinase (DIO2) mediates the conversion of T3 from T4 and is shown to be highly up-regulated in brown fat upon activation [133]. Thus, increased levels of plasma T3 are also seen after BAT activation [25,133,134]. Thyroid hormones have shown cardioprotective effects in humans, however as T3 is not specifically secreted by BAT, the role of specific BAT-mediated production is still unclear [25].
Another BATokine, Fibroblast Growth Factor 21 (FGF21), is secreted by thermogenically stimulated BAT and acts to collectively improve metabolic and cardiac function [135,136]. Importantly, FGF21 also regulates the expression of UCP1 and is critical in the beiging of WAT [137]. FGF21 acts on WAT and the liver and is shown to yield beneficial effects on lipid and glucose metabolism [25,135], as well as having protective effects on type II diabetes [138]. Additionally, FGF21 has been shown to have antihypertrophic and cardioprotective effects in animal models of hypertrophy and ischemia [25,139,140].
Nrg4, another factor secreted by cold-induced BAT, is cardioprotective in mouse models of myocardial ischemia [25,141]. However, Nrg4 is also secreted by other organs such as the liver, so identification of Nrg4 as a product of BAT response to cardiac injury is uncertain.
IL-6, a pro-inflammatory cytokine released by BAT and other tissues, has been suggested to yield a variety of metabolic and cardiac effects. IL-6 has been shown to mediate glucose metabolism and energy expenditure [25]. When IL-6 production is inhibited, mice become highly susceptible to diet-induced obesity and glucose intolerance. However, when chronically activated, mice showed an increase in energy expenditure and the promotion of WAT beiging [142,143]. In studies of BAT transplantation, the metabolic improvements regarding glucose homeostasis and insulin sensitivity were eliminated when BAT from IL-6-null mice was used for transplant, indicating a necessary role for IL-6 in BAT-mediated metabolic function [144]. IL-6 plays a clear, but broad and diverse, role in cardiovascular physiology, making it an important paracrine signaling BATokine, but it is also produced by multiple tissue types, making it not specific to BAT [145].
Nerve Growth Factor (NGF) is a protein secreted by BAT and other tissues, which typically functions to promote sympathetic axon growth and the proliferation of neurons [146]. NGF has also been shown to promote cell survival in models of ischemic injury, as well as improving the general function of perfused diabetic hearts [147–149]. Paradoxically, however, NGF expression in mice is down-regulated upon BAT activation by either cold or adrenergic stimulation, leaving its role as a cardioprotective agent in question [150].
Certainly, there are many more BATokines and pathways of BAT-mediated cross-talk to the heart that are yet to be identified. In fact, while we have recently shown that activity of the RNA binding protein human antigen R (HuR) directly within cardiomyocytes mediates cardiac hypertrophy and fibrosis [151,152], new unpublished data from our lab show that deletion of HuR specifically in adipose tissue elicits the development of cardiac hypertrophy and fibrosis, which is potentially linked to the disruption of BAT-mediated thermogenic function. Interestingly, HuR has been shown to target IL-6 as well as many other inflammatory cytokines [153,154], but the underlying mechanism for the cardiac phenotype of adipocyte HuR-deletion remains unknown. In general, the pro-inflammatory profile of BAT is significantly lower than WAT, producing a phenotypically attenuated inflammatory tissue [132]. Overall, the endocrinological ability of BAT shows therapeutic avenues for cardiovascular health and CVD risk factors.
Epicardial, pericardial, and perivascular adipose tissue
The nomenclature and classification of epicardial (EAT), pericardial (PAT) and perivascular (PVAT) adipose tissue throughout the literature can be confusing [155–157]. EAT is the adipose depot that lies beneath the surface of the epicardium and shares the same microcirculation vasculature with the myocardium [155,158]. PAT, meanwhile, is contained on the outer surface of the parietal pericardium [159], and may also be referred to as intrathoracic, mediastinal, or paracardial adipose tissue [160]. PVAT is found almost ubiquitously on vasculature throughout the body, with the notable exception of the cerebral vasculature and, because it also comprises the adipose tissue on the coronary arteries, can be difficult to distinguish from EAT [161]. Coupled with the fact that mice have minimal EAT, this makes the nomenclature even more confusing [158].
EAT varies due to multiple different factors such as age, waist circumference, and heart weight which all suggest that aging, abdominal obesity, and myocardial hypertrophy are the main predictors of EAT volume [162–164]. EAT has been shown in adult humans to possess molecular features and a gene profile of beige adipocytes and may play a thermogenic role [165,166]. Evidence has shown that EAT is metabolically active, a source of several adipokines, and is an active endocrine organ [167,168].
Very little is known about the functional roles that epicardial fat play in cardiac hypertrophy or fibrosis. Given the proximity of EAT within the myocardium, it is not surprising that numerous studies have associated both EAT volume and thickness with risk of coronary events and HF (see Ansaldo and colleagues (2019) [164] for a thorough summary of clinical observations linking EAT to CVD). Echocardiography can be used to distinguish EAT from pericardial fat in humans and has been used to demonstrate a clinical correlation between EAT thickness and LV mass [169–171]. However, contrasting studies suggested a decrease in EAT volume in patients with HF [172–174]. Studies conducted on both obese and non-obese men show that men with CAD have more epicardial fat accumulation than those without CAD independent of obesity [162,175]. In fact, surgical removal of EAT has been shown to ameliorate atherogenesis in a porcine model suggesting a causal role for EAT in CAD [176,177].
EAT has been shown to express multiple adipokines, including adiponectin, in a secretome that changes with metabolic and inflammatory states, but the mechanisms by which EAT may contribute to CVD remain unclear [163,164,178–180]. Omentin, which we have previously mentioned as having potential cardioprotective effects [101,102], is increased in EAT in the setting of CAD [181] and may serve as a clinical predictor of adverse cardiovascular outcome [182,183]. Perhaps most interesting is that EAT has been shown to secrete direct mediators of fibrotic remodeling, such as activin A, connective tissue growth factor (cTGF), TGF-β1, and metalloproteinases [184–188]. Secreted factors from human EAT are sufficient to induce fibrosis in an organo-culture model of rat atria [184]. This pro-fibrotic effect of EAT may be enhanced by a pro-inflammatory state and play a strong predictive and mechanistic role in atrial fibrillation [185,186,189–192].
While these clinical observations are supportive of EAT playing a role in the development of CVD, mechanistic studies in animal models are scarce [163,164,180]. This is in part due to the fact that small animal models such as mice and rats often express little to no epicardial adipose, making them difficult laboratory models for the study of EAT [158]. Surgical resection of EAT in a rat model of MI suppressed inflammatory signaling and improved LV remodeling [193], though its mechanisms of action or direct role on the myocardium remain elusive. The secretome of EAT from guinea pig hearts has been shown to negatively influence myocyte contractility [194], and orosomucoid isolated from the human EAT secretome reduced hypoxia-induced apoptosis in H9c2 cells [195].
Because of the aforementioned nomenclature confusion, delineating the role of PAT can be difficult. Human PAT biopsies reveal elevated levels of the BAT-specific genes UCP1 and PPARγ as compared with subcutaneous adipose tissue, suggesting PAT may function similar to BAT, in a generally cardioprotective manner [196]. However, multiple clinical studies have found positive correlations between PAT volume and LV mass [197–200], systolic dysfunction [198], ejection fraction [199], and cardiac output [201]. Although PAT volume is not a better predictor of CVD than other measures of adiposity [197,200], it nonetheless appears to be associated with the development of cardiac hypertrophy and heart failure.
PVAT, depending on anatomical location, may closely resemble either WAT or BAT [161]. However, adipocytes composing PVAT arise from a different cell lineage than either classical WAT or BAT, sharing lineage markers with vascular smooth muscle cell precursors [202,203].
PVAT undoubtedly plays a critical role in cardiovascular health, with a classic role in mediating vascular tone being identified as early as 1991 when it was shown that intact PVAT blunted the ex vivo contractile effects of adrenaline [204]. It was subsequently shown that the same regulatory role for PVAT was also demonstrated for AngII and phenylephrine-mediated contractility in aortic rings [205]. However, obesity induces a shift to more pro-inflammatory state in PVAT that renders it as a vasoconstrictive mediator [206]. Thus, it is not surprising that inflammatory cell infiltration and resulting pro-inflammatory cytokine expression profile within PVAT has been noted to increase under conditions of obesity and high fat feeding [207,208]. The precise mechanisms of this inflammatory switch remain unknown, but are potentially downstream of hypoxia-induced HIF-1α signaling, which has been shown to mediate the up-regulation of pro-inflammatory cytokine gene expression concomitant with reduced expression of anti-inflammatory adiponectin [209–211]. Accordingly, HIF-1α has been shown to increase in obesity, and exposure of PVAT to hypoxia induces inflammatory gene expression and abrogates its vasorelaxive properties [212,213].
Given that PVAT has been shown to mediate both vasodilatory and vasoconstrictive roles, the precise role for PVAT in regulating vascular tone remains somewhat uncertain [25,214]. Since the presence of a direct-acting PVAT-derived relaxing factor (PVDF) was first hypothesized in 2002, numerous mediators of PVAT-mediated vasorelaxation, such as leptin, adiponectin, and inflammatory cytokines, have been identified [209]. Similarly, PVAT has also been shown to play antithetical roles with regard to atherosclerosis. However, it appears that the role of PVAT leans more protective in metabolically healthy subjects and injurious under conditions of obesity and diabetes, similar to what one might expect from an adipose depot that most closely resembles activatable beige adipocytes [203,215,216].
Conclusion
Collectively, adipose-derived signaling molecules have been established as important contributors to CVD, further strengthening the link between central obesity and CVD risk. It is clear that the secretome from adipose tissue, collectively termed as adipokines, have the potential to have a profound effect on cardiac physiology. Additionally, the secretome profile of adipokines changes with the metabolic state (e.g. lean vs. obese), anatomical location (visceral, subcutaneous, EAT etc.), and cell identity (BAT vs. WAT) of the adipose depot (summarized in Figure 1). The use of animal models has facilitated the experimental investigation of mechanistic and causal relationships between adipose tissue and CVD (see Table 3 for a summary of animal models discussed herein). Thus, while there is tremendous untapped potential in harnessing the effects of adipose tissue on cardiac hypertrophy and fibrosis for the clinical application of diagnostic biomarkers and therapeutic interventions, much work remains to be done to fill the gaps in understanding of how the dynamic changes in adipokines in response to obesity/inflammation effect cardiovascular health.
Table 3.
Animal models demonstrating direct adipokine/batokine-mediated cardiac effects
| Species | Animal Model | Adipokine/BATokine | Observed effect |
|---|---|---|---|
| Mouse | TAC | Adiponectin Omentin |
Decreased hypertrophy in wild-type and diabetic (db/db) mice [50] Decreased hypertrohy and fibrosis [104] |
| AngII infusion | Adiponectin | Decreased ROS [48]; decreased fibrosis [51] | |
| Permanent coronary ligation | Adiponectin Leptin |
Improved LV function [49] Decreased apoptosis [64]; improved LV function and compensated hypertrophy [75] |
|
| Coronary ischemia/reperfusion | Adiponectin Leptin Visfatin Omentin |
Reduced infarct size [47] Reduced infarct size [66]Reduced infarct size [98] Reduced infarct size [101] |
|
| Diet-induced obesity | Autotaxin | Secretion in obesity increased hypertrophy and fibrosis [95] | |
| Isoproterenol infusion; BAT transplant | BAT (general) | Functional BAT reduced hypertrophy and fibrosis [117] | |
| Rat | Langendorff isolated heart | Leptin | Reduced infarct size [67] |
| Permanent coronary ligation | Leptin | Increased hypertrophy and fibrosis [76] | |
| AAV overexpression | Resistin | Increased hypertrophy, fibrosis, and apoptosis with reduced LV function [90] | |
| Surgical excision of EAT | EAT (general) | Removal of EAT reduced infarct size and preserved cardiac function [193] | |
| Pig | Surgical excision of EAT | EAT (general) | Removal of EAT reduced CAD [176,177] |
Abbreviations: AAV, adeno-associated virus; ROS, reactive oxygen species; TAC, transverse aortic constriction.
Acknowledgments
Funding
This work is supported by the NHLBI R01 [grant number HL132111 (to M.T.)]; the American Heart Association Transformational Project Award [grant number 19TPA34910086 (to M.T. and A.P.O.III)]; and the American Heart Association Innovative Project Award [grant number 19IPLOI34770131 (to M.T.)].
Abbreviations
- AngII
angiotensin II
- BAT
brown adipose tissue
- CAD
coronary artery disease
- CVD
cardiovascular disease
- EAT
epicardial adipose tissue
- ECM
extracellular matrix
- FABP4
fatty acid binding protein 4
- FFA
free fatty acid
- FGF21
fibroblast growth factor 21
- HF
heart failure
- HFD
high fat diet
- HIF-1α
hypoxia inducible factor 1 alpha
- HuR
human antigen R
- IL-6
interleukin-6
- LV
left ventricular
- LVH
LV hypertrophy
- MI
myocardial infarction
- NGF
nerve growth factor
- Nrg1
neuregulin 1
- PAT
pericardial adipose tissue
- PVAT
perivascular adipose tissue
- SVF
stromal vascular fraction
- T3
triiodothyronine
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS and Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol. Rev 90, 207–258, 10.1152/physrev.00015.2009 [DOI] [PubMed] [Google Scholar]
- 2.Fillmore N, Mori J and Lopaschuk GD (2014) Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol 171, 2080–2090, 10.1111/bph.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancello R. and Clément K. (2006) Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 113, 1141–1147, 10.1111/j.1471-0528.2006.01004.x [DOI] [PubMed] [Google Scholar]
- 4.Fuster JJ, Ouchi N, Gokce N and Walsh K (2016) Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res 118, 1786–1807, 10.1161/CIRCRESAHA.115.306885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynes MD and Tseng Y-H (2018) Deciphering adipose tissue heterogeneity. Ann. N.Y. Acad. Sci 1411, 5–20, 10.1111/nyas.13398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cildir G, Aknclar SC and Tergaonkar V (2013) Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol. Med 19, 487–500, 10.1016/j.molmed.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 7.Lumeng CN., Bodzin JL. and Saltiel AR. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest 117, 175–184, 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB and Kumar SA (2014) Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl. Res 164, 345–356, 10.1016/j.trsl.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 9.Lavi CJ., Milan RV. and Ventur HO. (2014) Disparate effects of metabolically healthy obesity in coronary heart disease and heart failure. J. Am. Coll. Cardiol 63, 1079–1081, 10.1016/j.jacc.2013.10.080 [DOI] [PubMed] [Google Scholar]
- 10.Carbone S, Lavie CJ and Arena R (2017) Obesity and heart failure: focus on the obesity paradox. Mayo Clin. Proc 92, 266–279, 10.1016/j.mayocp.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R et al. (2017) Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135, e146–e603, 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braunwald E. (2013) Heart failure. JACC Heart Fail. 1, 1–20, 10.1016/j.jchf.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Shimizu I and Minamino T (2016) Physiological and pathological cardiac hypertrophy. J. Mol. Cell Cardiol 97, 245–262, 10.1016/j.yjmcc.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 14.Travers JG, Kamal FA, Robbins J, Yutzey KE and Blaxall BC (2016) Cardiac fibrosis: the fibroblast awakens. Circ. Res 118, 1021–1040, 10.1161/CIRCRESAHA.115.306565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadoshima J and Weiss JN (2014) Cardiac fibroblasts: the good, the bad, the ugly, the beautiful. J. Mol. Cell Cardiol 70, 1, 10.1016/j.yjmcc.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong P, Christia P and Frangogiannis NG (2014) The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci 71, 549–574, 10.1007/s00018-013-1349-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Negishi K, Otahal P and Marwick TH (2015) Clinical prediction of incident heart failure risk: a systematic review and meta-analysis. Open Heart 2, e000222, 10.1136/openhrt-2014-000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolfe DF and Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev 77, 731–758, 10.1152/physrev.1997.77.3.731 [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A-H et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376, 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L et al. (2012) Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 7, e49452, 10.1371/journal.pone.0049452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK et al. (1982) Relation of body fat distribution to metabolic complications of obesity. J. Clin. Endocrinol. Metab 54, 254–260, 10.1210/jcem-54-2-254 [DOI] [PubMed] [Google Scholar]
- 22.Krotkiewski M, Björntorp P, Sjöström L and Smith U (1983) Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Invest 72, 1150–1162, 10.1172/JCI111040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neeland IJ, Poirier P and Després J-P (2018) Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation 137, 1391–1406, 10.1161/CIRCULATIONAHA.117.029617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin T, Lamendola C, Liu A and Abbasi F (2011) Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J. Clin. Endocrinol. Metab 96, E1756–E1760, 10.1210/jc.2011-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thoonen R, Hindle AG and Scherrer-Crosbie M (2016) Brown adipose tissue: The heat is on the heart. Am. J. Physiol. Heart Circ. Physiol 310, H1592–H1605, 10.1152/ajpheart.00698.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng Y and Scherer PE (2010) Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci 1212, E1–E19, 10.1111/j.1749-6632.2010.05875.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Gurmaches J, Hung C-M and Guertin DA (2016) Emerging complexities in adipocyte origins and identity. Trends Cell Biol 26, 313–326, 10.1016/j.tcb.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chusyd DE, Wang D, Huffman DM and Nagy TR (2016) Relationships between rodent white adipose fat pads and human white adipose fat depots. Front. Nutr 3, 10, 10.3389/fnut.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD et al. (2013) Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 17, 644–656, 10.1016/j.cmet.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry DC, Stenesen D, Zeve D and Graff JM (2013) The developmental origins of adipose tissue. Development 140, 3939–3949, 10.1242/dev.080549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchernof A, Bélanger C, Morisset A-S, Richard C, Mailloux J, Laberge P et al. (2006) Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes 55, 1353–1360, 10.2337/db05-1439 [DOI] [PubMed] [Google Scholar]
- 32.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T et al. (2007) Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am. J. Physiol. Endocrinol. Metab 292, E298–E307, 10.1152/ajpendo.00202.2006 [DOI] [PubMed] [Google Scholar]
- 33.Zuriaga MA, Fuster JJ, Gokce N and Walsh K (2017) Humans and mice display opposing patterns of “Browning” gene expression in visceral and subcutaneous white adipose tissue depots. Front. Cardiovasc. Med 4, 27, 10.3389/fcvm.2017.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang QA, Tao C, Gupta RK and Scherer PE (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med 19, 1338–1344, 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meisinger C, Döring A, Thorand B, Heier M and Löwel H (2006) Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am. J. Clin. Nutr 84, 483–489, 10.1093/ajcn/84.3.483 [DOI] [PubMed] [Google Scholar]
- 36.Shuster A, Patlas M, Pinthus JH and Mourtzakis M (2012) The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br. J. Radiol 85, 1–10, 10.1259/bjr/38447238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ and Fox CS (2009) Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 32, 1068–1075, 10.2337/dc08-2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB et al. (2005) Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 48, 301–308, 10.1007/s00125-004-1637-7 [DOI] [PubMed] [Google Scholar]
- 39.Bouchi R, Takeuchi T, Akihisa M, Ohara N, Nakano Y, Nishitani R et al. (2015) High visceral fat with low subcutaneous fat accumulation as a determinant of atherosclerosis in patients with type 2 diabetes. Cardiovasc. Diabetol 14, 136–137, 10.1186/s12933-015-0302-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammond IW, Devereux RB, Alderman MH and Laragh JH (1988) Relation of blood pressure and body build to left ventricular mass in normotensive and hypertensive employed adults. J. Am. Coll. Cardiol 12, 996–1004, 10.1016/0735-1097(88)90467-6 [DOI] [PubMed] [Google Scholar]
- 41.Lauer MS, Anderson KM, Kannel WB and Levy D (1991) The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 266, 231–236, 10.1001/jama.1991.03470020057032 [DOI] [PubMed] [Google Scholar]
- 42.Messerli FH, Sundgaard-Riise K, Reisin ED, Dreslinski GR, Ventura HO, Oigman W et al. (1983) Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann. Intern. Med 99, 757–761, 10.7326/0003-4819-99-6-757 [DOI] [PubMed] [Google Scholar]
- 43.Lee TC, Jin Z, Homma S, Nakanishi K, Elkind MSV, Rundek T et al. (2019) Changes in left ventricular mass and geometry in the older adults: role of body mass and central obesity. J. Am. Soc. Echocardiogr 32, 1318–1325, 10.1016/j.echo.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wellen KE and Hotamisligil GS (2003) Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest 112, 1785–1788, 10.1172/JCI20514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M et al. (2004) Adiponectin as a biomarker of the metabolic syndrome. Circ.J 68, 975–981, 10.1253/circj.68.975 [DOI] [PubMed] [Google Scholar]
- 46.Lau WB, Ohashi K, Wang Y, Ogawa H, Murohara T, Ma X-L et al. (2017) Role of adipokines in cardiovascular disease. Circ. J 81, 920–928, 10.1253/circj.CJ-17-0458 [DOI] [PubMed] [Google Scholar]
- 47.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K et al. (2005) Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med 11, 1096–1103, 10.1038/nm1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Essick EE., Wilson RM., Pimentel DR., Shimano M., Baid S., Ouchi N. et al. (2013) Adiponectin modulates oxidative stress-induced autophagy in cardiomyocytes. PLoS ONE 8, e68697, 10.1371/journal.pone.0068697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joki Y, Ohashi K, Yuasa D, Shibata R, Ito M, Matsuo K et al. (2015) FGF21 attenuates pathological myocardial remodeling following myocardial infarction through the adiponectin-dependent mechanism. Biochem. Biophys. Res. Commun 459, 124–130, 10.1016/j.bbrc.2015.02.081 [DOI] [PubMed] [Google Scholar]
- 50.Shibata R., Ouchi N., Ito M., Kihara S., Shiojima I., Pimente DR. et al. (2004) Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med 10, 1384–1389, 10.1038/nm1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H et al. (2008) Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler. Thromb. Vasc. Biol 28, 863–870, 10.1161/ATVBAHA.107.156687 [DOI] [PubMed] [Google Scholar]
- 52.Mitsuhashi H, Yatsuya H, Tamakoshi K, Matsushita K, Otsuka R, Wada K et al. (2007) Adiponectin level and left ventricular hypertrophy in Japanese men. Hypertension 49, 1448–1454, 10.1161/HYPERTENSIONAHA.106.079509 [DOI] [PubMed] [Google Scholar]
- 53.Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L et al. (2016) Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR- γ/adiponectin signalling. Circ. Res 118, 842–855, 10.1161/CIRCRESAHA.115.307856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonopoulos AS, Margaritis M, Coutinho P, Digby J, Patel R, Psarros C et al. (2014) Reciprocal effects of systemic inflammation and brain natriuretic peptide on adiponectin biosynthesis in adipose tissue of patients with ischemic heart disease. Arterioscler. Thromb. Vasc. Biol 34, 2151–2159, 10.1161/ATVBAHA.114.303828 [DOI] [PubMed] [Google Scholar]
- 55.Sawada N, Daimon M, Kawata T, Nakao T, Kimura K, Nakanishi K et al. (2019) The significance of the effect of visceral adiposity on left ventricular diastolic function in the general population. Sci. Rep 9, 4435–4438, 10.1038/s41598-018-37137-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Söderberg S, Ahrén B, Jansson JH, Johnson O, Hallmans G, Asplund K et al. (1999) Leptin is associated with increased risk of myocardial infarction. J. Intern. Med 246, 409–418, 10.1046/j.1365-2796.1999.00571.x [DOI] [PubMed] [Google Scholar]
- 57.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, Gami AS, Sert Kuniyoshi FH, Wolk R et al. (2007) Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am. J. Cardiol 100, 234–239, 10.1016/j.amjcard.2007.02.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallerstedt SM., Eriksson A-L., Niklason A., Ohlsson C. and Hedner T. (2004) Serum leptin and myocardial infarction in hypertension. Blood Press. 13, 243–246, 10.1080/08037050410021405 [DOI] [PubMed] [Google Scholar]
- 59.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A et al. (2001) Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 104, 3052–3056, 10.1161/hc5001.101061 [DOI] [PubMed] [Google Scholar]
- 60.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD and Somers VK (2004) Plasma leptin and prognosis in patients with established coronary atherosclerosis. J. Am. Coll. Cardiol 44, 1819–1824, 10.1016/j.jacc.2004.07.050 [DOI] [PubMed] [Google Scholar]
- 61.Sweeney G (2010) Cardiovascular effects of leptin. Nat. Rev. Cardiol 7, 22–29, 10.1038/nrcardio.2009.224 [DOI] [PubMed] [Google Scholar]
- 62.Shin E-J, Schram K, Zheng X-L and Sweeney G (2009) Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J. Cell. Physiol 221, 490–497, 10.1002/jcp.21883 [DOI] [PubMed] [Google Scholar]
- 63.Eguchi M, Liu Y, Shin E-J and Sweeney G (2008) Leptin protects H9c2 rat cardiomyocytes from H2O2-induced apoptosis. FEBS J. 275, 3136–3144, 10.1111/j.1742-4658.2008.06465.x [DOI] [PubMed] [Google Scholar]
- 64.McGaffin KR, Zou B, McTiernan CF and O’Donnell CP (2009) Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc. Res 83, 313–324, 10.1093/cvr/cvp071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trivedi P, Yang R and Barouch LA (2008) Decreased p110alpha catalytic activity accompanies increased myocyte apoptosis and cardiac hypertrophy in leptin deficient ob/ob mice. Cell Cycle 7, 560–565, 10.4161/cc.7.5.5529 [DOI] [PubMed] [Google Scholar]
- 66.Smith CCT., Mocanu MM., Davidson SM., Wynne AM., Simpkin JC. and Yellon DM. (2006) Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br. J. Pharmacol 149, 5–13, 10.1038/sj.bjp.0706834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixon RA, Davidson SM, Wynne AM, Yellon DM and Smith CCT (2009) The cardioprotective actions of leptin are lost in the Zucker obese (fa/fa) rat. J. Cardiovasc. Pharmacol 53, 311–317, 10.1097/FJC.0b013e31819d6152 [DOI] [PubMed] [Google Scholar]
- 68.Rajapurohitam V, Javadov S, Purdham DM, Kirshenbaum LA and Karmazyn M (2006) An autocrine role for leptin in mediating the cardiomyocyte hypertrophic effects of angiotensin II and endothelin-1. J. Mol. Cell Cardiol 41, 265–274, 10.1016/j.yjmcc.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 69.Madani S, De Girolamo S, Muñoz DM, Li R-K and Sweeney G (2006) Direct effects of leptin on size and extracellular matrix components of human pediatric ventricular myocytes. Cardiovasc. Res 69, 716–725, 10.1016/j.cardiores.2005.11.022 [DOI] [PubMed] [Google Scholar]
- 70.Rajapurohitam V, Gan XT, Kirshenbaum LA and Karmazyn M (2003) The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ. Res 93, 277–279, 10.1161/01.RES.0000089255.37804.72 [DOI] [PubMed] [Google Scholar]
- 71.Xu F-P, Chen M-S, Wang Y-Z, Yi Q, Lin S-B, Chen AF et al. (2004) Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation 110, 1269–1275, 10.1161/01.CIR.0000140766.52771.6D [DOI] [PubMed] [Google Scholar]
- 72.Majumdar P, Chen S, George B, Sen S, Karmazyn M and Chakrabarti S (2009) Leptin and endothelin-1 mediated increased extracellular matrix protein production and cardiomyocyte hypertrophy in diabetic heart disease. Diabetes Metab. Res. Rev 25, 452–463, 10.1002/dmrr.964 [DOI] [PubMed] [Google Scholar]
- 73.Zeidan A, Javadov S and Karmazyn M (2006) Essential role of Rho/ROCK-dependent processes and actin dynamics in mediating leptin-induced hypertrophy in rat neonatal ventricular myocytes. Cardiovasc. Res 72, 101–111, 10.1016/j.cardiores.2006.06.024 [DOI] [PubMed] [Google Scholar]
- 74.Zeidan A, Javadov S, Chakrabarti S and Karmazyn M (2008) Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc. Res 77, 64–72, 10.1093/cvr/cvm020 [DOI] [PubMed] [Google Scholar]
- 75.Abe Y, Ono K, Kawamura T, Wada H, Kita T, Shimatsu A et al. (2007) Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. Am. J. Physiol. Heart Circ. Physiol 292, H2387–96 [DOI] [PubMed] [Google Scholar]
- 76.Purdham DM, Rajapurohitam V, Zeidan A, Huang C, Gross GJ and Karmazyn M (2008) A neutralizing leptin receptor antibody mitigates hypertrophy and hemodynamic dysfunction in the postinfarcted rat heart. Am. J. Physiol. Heart Circ. Physiol 295, H441–6 [DOI] [PubMed] [Google Scholar]
- 77.Schram K, Wong MMC, Palanivel R, No EK, Dixon IMC and Sweeney G (2008) Increased expression and cell surface localization of MT1-MMP plays a role in stimulation of MMP-2 activity by leptin in neonatal rat cardiac myofibroblasts. J. Mol. Cell Cardiol 44, 874–881, 10.1016/j.yjmcc.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 78.Xia Q-G, Na T, Guo Y-M, Bi Y-T, Zhang H-Y and Dai D-Z (2007) Improvement of chronic heart failure by dexamethasone is not associated with downregulation of leptin in rats. Acta Pharmacol. Sin 28, 202–210, 10.1111/j.1745-7254.2007.00503.x [DOI] [PubMed] [Google Scholar]
- 79.Coe NR, Simpson MA and Bernlohr DA (1999) Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J. Lipid Res 40, 967–972 [PubMed] [Google Scholar]
- 80.Rodríguez-Calvo R, Girona J, Alegret JM, Bosquet A, Ibarretxe D and Masana L (2017) Role of the fatty acid-binding protein 4 in heart failure and cardiovascular disease. J. Endocrinol 233, R173–R184, 10.1530/JOE-17-0031 [DOI] [PubMed] [Google Scholar]
- 81.She WJ., Sridhar K., Bernlohr DA. and Kraemer FB. (1999) Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proc. Natl Acad. Sci. U.S.A 96, 5528–5532, 10.1073/pnas.96.10.5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Engeli S, Utz W, Haufe S, Lamounier-Zepter V, Pofahl M, Traber J et al. (2013) Fatty acid binding protein 4 predicts left ventricular mass and longitudinal function in overweight and obese women. Heart 99, 944–948, 10.1136/heartjnl-2013-303735 [DOI] [PubMed] [Google Scholar]
- 83.Liu M, Zhou M, Bao Y, Xu Z, Li H, Zhang H et al. (2013) Circulating adipocyte fatty acid-binding protein levels are independently associated with heart failure. Clin. Sci 124, 115–122, 10.1042/CS20120004 [DOI] [PubMed] [Google Scholar]
- 84.Bernlohr DA, Bolanowski MA, Kelly TJ and Lane MD (1985) Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J. Biol. Chem 260, 5563–5567 [PubMed] [Google Scholar]
- 85.Yang VW, Christy RJ, Cook JS, Kelly TJ and Lane MD (1989) Mechanism of regulation of the 422(aP2) gene by cAMP during preadipocyte differentiation. Proc. Natl Acad. Sci. U.S.A 86, 3629–3633, 10.1073/pnas.86.10.3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu Y, Luo N and Lopes-Virella MF (2000) Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J. Lipid Res 41, 2017–2023 [PubMed] [Google Scholar]
- 87.Pelton PD, Zhou L, Demarest KT and Burris TP (1999) PPARgamma activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem. Biophys. Res. Commun 261, 456–458, 10.1006/bbrc.1999.1071 [DOI] [PubMed] [Google Scholar]
- 88.Lee P., Linderman JD., Smith S., Brychta RJ., Wang J., Idelson C. et al. (2014) Irisin and FGF21 are cold- induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309, 10.1016/j.cmet.2013.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang S, Chemaly ER, Hajjar RJ and Lebeche D (2011) Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J. Biol. Chem 286, 18465–18473, 10.1074/jbc.M110.200022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chemaly ER, Hadri L, Zhang S, Kim M, Kohlbrenner E, Sheng J et al. (2011) Long-term in vivo resistin overexpression induces myocardial dysfunction and remodeling in rats. J. Mol. Cell Cardiol 51, 144–155, 10.1016/j.yjmcc.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muse ED, Feldman DI, Blaha MJ, Dardari ZA, Blumenthal RS, Budoff MJ et al. (2015) The association of resistin with cardiovascular disease in the multi-ethnic study of atherosclerosis. Atherosclerosis 239, 101–108, 10.1016/j.atherosclerosis.2014.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohmori R, Momiyama Y, Kato R, Taniguchi H, Ogura M, Ayaori M et al. (2005) Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J. Am. Coll. Cardiol 46, 379–380, 10.1016/j.jacc.2005.04.022 [DOI] [PubMed] [Google Scholar]
- 93.Pischon T, Bamberger CM, Kratzsch J, Zyriax B-C, Algenstaedt P, Boeing H et al. (2005) Association of plasma resistin levels with coronary heart disease in women. Obes. Res 13, 1764–1771, 10.1038/oby.2005.215 [DOI] [PubMed] [Google Scholar]
- 94.Takeishi Y, Niizeki T, Arimoto T, Nozaki N, Hirono O, Nitobe J et al. (2007) Serum resistin is associated with high risk in patients with congestive heart failure–a novel link between metabolic signals and heart failure. Circ. J 71, 460–464, 10.1253/circj.71.460 [DOI] [PubMed] [Google Scholar]
- 95.Xu Y, Wang Y, Liu J, Cao W, Li L, Du H et al. (2019) Adipose tissue-derived autotaxin causes cardiomyopathy in obese mice. J. Mol. Endocrinol 63, 113–121, 10.1530/JME-18-0242 [DOI] [PubMed] [Google Scholar]
- 96.Romacho T., Sánchez-Ferrer CF. and Peiró C. (2013) Visfatin/Nampt: an adipokine with cardiovascular impact. Mediators Inflamm. 2013, 946427–15, 10.1155/2013/946427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao J, Sun B, Li M, Wu Y and Sun X-B (2013) A novel adipocytokine visfatin protects against H(2)O(2) -induced myocardial apoptosis: a missing link between obesity and cardiovascular disease. J. Cell. Physiol 228, 495–501, 10.1002/jcp.24257 [DOI] [PubMed] [Google Scholar]
- 98.Lim SY., Davidson SM., Paramanathan AJ., Smith CCT., Yellon DM. and Hausenlo DJ. (2008) The novel adipocytokine visfatin exerts direct cardioprotective effects. J. Cell. Mol. Med 12, 1395–1403, 10.1111/j.1582-4934.2008.00332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li B, Zhao Y, Liu H, Meng B, Wang J, Qi T et al. (2016) Visfatin destabilizes atherosclerotic plaques in apolipoprotein E-deficient mice. PLoS ONE 11, e0148273, 10.1371/journal.pone.0148273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tan BK, Adya R and Randeva HS (2010) Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc. Med 20, 143–148, 10.1016/j.tcm.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 101.Kataoka Y, Shibata R, Ohashi K, Kambara T, Enomoto T, Uemura Y et al. (2014) Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. J. Am. Coll. Cardiol 63, 2722–2733, 10.1016/j.jacc.2014.03.032 [DOI] [PubMed] [Google Scholar]
- 102.Kazama K, Okada M and Yamawaki H (2015) Adipocytokine, omentin inhibits doxorubicin-induced H9c2 cardiomyoblasts apoptosis through the inhibition of mitochondrial reactive oxygen species. Biochem. Biophys. Res. Commun 457, 602–607, 10.1016/j.bbrc.2015.01.032 [DOI] [PubMed] [Google Scholar]
- 103.Greulich S, Chen WJY, Maxhera B, Rijzewijk LJ, van der Meer RW, Jonker JT et al. (2013) Cardioprotective properties of omentin-1 in type 2 diabetes: evidence from clinical and in vitro studies. PLoS ONE 8, e59697, 10.1371/journal.pone.0059697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matsuo K, Shibata R, Ohashi K, Kambara T, Uemura Y, Hiramatsu-Ito M et al. (2015) Omentin functions to attenuate cardiac hypertrophic response. J. Mol. Cell Cardiol 79, 195–202, 10.1016/j.yjmcc.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 105.Shibata R, Ouchi N, Kikuchi R, Takahashi R, Takeshita K, Kataoka Y et al. (2011) Circulating omentin is associated with coronary artery disease in men. Atherosclerosis 219, 811–814, 10.1016/j.atherosclerosis.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 106.Onur I, Oz F, Yildiz S, Oflaz H, Sigirci S, Elitok A et al. (2014) Serum omentin 1 level is associated with coronary artery disease and its severity in postmenopausal women. Angiology 65, 896–900, 10.1177/0003319713511322 [DOI] [PubMed] [Google Scholar]
- 107.Zhong X, Zhang H-Y, Tan H, Zhou Y, Liu F-L, Chen F-Q et al. (2011) Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol. Sin 32, 873–878, 10.1038/aps.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shang F-J, Wang J-P, Liu X-T, Zheng Q-S, Xue Y-S, Wang B et al. (2011) Serum omentin-1 levels are inversely associated with the presence and severity of coronary artery disease in patients with metabolic syndrome. Biomarkers 16, 657–662, 10.3109/1354750X.2011.622789 [DOI] [PubMed] [Google Scholar]
- 109.Ichikawa R, Daimon M, Miyazaki T, Kawata T, Miyazaki S, Maruyama M et al. (2013) Influencing factors on cardiac structure and function beyond glycemic control in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol 12, 38–39, 10.1186/1475-2840-12-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR et al. (2013) Relation of regional fat distribution to left ventricular structure and function. Circ. Cardiovasc. Imaging 6, 800–807, 10.1161/CIRCIMAGING.113.000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND et al. (2009) Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med 360, 1500–1508, 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- 112.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med 360, 1509–1517, 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med 360, 1518–1525, 10.1056/NEJMoa0808949 [DOI] [PubMed] [Google Scholar]
- 114.Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA et al. (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38, 10.1016/j.cmet.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thyagarajan B and Foster MT (2017) Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Horm. Mol. Biol. Clin. Investig 31, 1500. [DOI] [PubMed] [Google Scholar]
- 116.Harper M-E, Green K and Brand MD (2008) The efficiency of cellular energy transduction and its implications for obesity. Annu. Rev. Nutr 28, 13–33, 10.1146/annurev.nutr.28.061807.155357 [DOI] [PubMed] [Google Scholar]
- 117.Thoonen R, Ernande L, Cheng J, Nagasaka Y, Yao V, Miranda-Bezerra A et al. (2015) Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J. Mol. Cell Cardiol 84, 202–211, 10.1016/j.yjmcc.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tseng Y-H, Cypess AM and Kahn CR (2010) Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov 9, 465–482, 10.1038/nrd3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Townsend K and Tseng Y-H (2012) Brown adipose tissue: recent insights into development, metabolic function and therapeutic potential. Adipocyte 1, 13–24, 10.4161/adip.18951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosen ED and Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156, 20–44, 10.1016/j.cell.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y et al. (2013) Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest 123, 3404–3408, 10.1172/JCI67803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramage LE, Akyol M, Fletcher AM, Forsythe J, Nixon M, Carter RN et al. (2016) Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 nbegulation. Cell Metab. 24, 130–141, 10.1016/j.cmet.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schreiber R, Diwoky C, Schoiswohl G, Feiler U, Wongsiriroj N, Abdellatif M et al. (2017) Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab. 26, 753–763.e7, 10.1016/j.cmet.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Manfrini O, Morgagni G, Pizzi C, Fontana F and Bugiardini R (2004) Changes in autonomic nervous system activity: spontaneous versus balloon-induced myocardial ischaemia. Eur. Heart J 25, 1502–1508, 10.1016/j.ehj.2004.03.019 [DOI] [PubMed] [Google Scholar]
- 125.Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M et al. (2004) Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J. Am. Coll. Cardiol 44, 1988–1995, 10.1016/j.jacc.2004.07.057 [DOI] [PubMed] [Google Scholar]
- 126.Villar AV, Llano M, Cobo M, Expósito V, Merino R, Martín-Durán R et al. (2009) Gender differences of echocardiographic and gene expression patterns in human pressure overload left ventricular hypertrophy. J. Mol. Cell Cardiol 46, 526–535, 10.1016/j.yjmcc.2008.12.024 [DOI] [PubMed] [Google Scholar]
- 127.Montalvo C, Villar AV, Merino D, García R, Ares M, Llano M et al. (2012) Androgens contribute to sex differences in myocardial remodeling under pressure overload by a mechanism involving TGF-β. PLoS ONE 7, e35635, 10.1371/journal.pone.0035635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu JC, Nasseri BA, Bloch KD, Picard MH and Scherrer-Crosbie M (2003) Influence of sex on ventricular remodeling after myocardial infarction in mice. J. Am. Soc. Echocardiogr 16, 1158–1162, 10.1067/S0894-7317(03)00648-5 [DOI] [PubMed] [Google Scholar]
- 129.Murphy E and Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev 88, 581–609, 10.1152/physrev.00024.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Berlo JH, Maillet M and Molkentin JD (2013) Signaling effectors underlying pathologic growth and remodeling of the heart. J. Clin. Invest 123, 37–45, 10.1172/JCI62839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sussman MA, Völkers M, Fischer K, Bailey B, Cottage CT, Din S et al. (2011) Myocardial AKT: the omnipresent nexus. Physiol. Rev 91, 1023–1070, 10.1152/physrev.00024.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Villarroya J, Cereijo R and Villarroya F (2013) An endocrine role for brown adipose tissue? Am. J. Physiol. Endocrinol. Metab 305, E567–72, 10.1152/ajpendo.00250.2013 [DOI] [PubMed] [Google Scholar]
- 133.Silva JE and Larsen PR (1983) Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305, 712–713, 10.1038/305712a0 [DOI] [PubMed] [Google Scholar]
- 134.Fernandez JA, Mampel T, Villarroya F and Iglesias R (1987) Direct assessment of brown adipose tissue as a site of systemic tri-iodothyronine production in the rat. Biochem. J 243, 281–284, 10.1042/bj2430281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gimeno RE and Moller DE (2014) FGF21-based pharmacotherapy–potential utility for metabolic disorders. Trends Endocrinol. Metab 25, 303–311, 10.1016/j.tem.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 136.Domouzoglou EM, Naka KK, Vlahos AP, Papafaklis MI, Michalis LK, Tsatsoulis A et al. (2015) Fibroblast growth factors in cardiovascular disease: The emerging role of FGF21. Am. J. Physiol. Heart Circ. Physiol 309, H1029–H1038, 10.1152/ajpheart.00527.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cuevas-Ramos D, Mehta R and Aguilar-Salinas CA (2019) Fibroblast growth factor 21 and browning of white adipose tissue. Front. Physiol 10, 37, 10.3389/fphys.2019.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K et al. (2010) Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59, 1817–1824, 10.2337/db09-1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC et al. (2013) Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci. Rep 3, 2767, 10.1038/srep02767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R et al. (2013) Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat. Commun 4, 2019, 10.1038/ncomms3019 [DOI] [PubMed] [Google Scholar]
- 141.Liu SQ, Tefft BJ, Roberts DT, Zhang L-Q, Ren Y, Li YC et al. (2012) Cardioprotective proteins upregulated in the liver in response to experimental myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol 303, H1446–58, 10.1152/ajpheart.00362.2012 [DOI] [PubMed] [Google Scholar]
- 142.Pal M, Febbraio MA and Whitham M (2014) From cytokine to myokine: the emerging role of interleukin-6 in metabolic regulation. Immunol. Cell Biol 92, 331–339, 10.1038/icb.2014.16 [DOI] [PubMed] [Google Scholar]
- 143.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J et al. (2014) A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447, 10.1016/j.cmet.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 144.Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM et al. (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest 123, 215–223, 10.1172/JCI62308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fontes JA, Rose NR and Čiháková D (2015) The varying faces of IL-6: from cardiac protection to cardiac failure. Cytokine 74, 62–68, 10.1016/j.cyto.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Néchad M, Ruka E and Thibault J (1994) Production of nerve growth factor by brown fat in culture: relation with the in vivo developmental stage of the tissue. Comp. Biochem. Physiol. Comp. Physiol 107, 381–388, 10.1016/0300-9629(94)90396-4 [DOI] [PubMed] [Google Scholar]
- 147.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B et al. (2008) Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 15, 299–311, 10.1038/sj.cdd.4402263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wei K, Liu L, Xie F, Hao X, Luo J and Min S (2015) Nerve growth factor protects the ischemic heart via attenuation of the endoplasmic reticulum stress induced apoptosis by activation of phosphatidylinositol 3-kinase. Int. J. Med. Sci 12, 83–91, 10.7150/ijms.10101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zheng L-R, Zhang Y-Y, Han J, Sun Z-W, Zhou S-X, Zhao W-T et al. (2015) Nerve growth factor rescues diabetic mice heart after ischemia/reperfusion injury via up-regulation of the TRPV1 receptor. J. Diabetes Complications 29, 323–328, 10.1016/j.jdiacomp.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 150.Nisoli E, Tonello C, Benarese M, Liberini P and Carruba MO (1996) Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology 137, 495–503, 10.1210/endo.137.2.8593794 [DOI] [PubMed] [Google Scholar]
- 151.Slone S, Anthony SR, Wu X, Benoit JB, Aubé J, Xu L et al. (2016) Activation of HuR downstream of p38 MAPK promotes cardiomyocyte hypertrophy. Cell. Signal 28, 1735–1741, http://linkinghub.elsevier.com/retrieve/pii/S0898656816302030, 10.1016/j.cellsig.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Green LC, Anthony SR, Slone S, Lanzillotta L, Nieman ML, Wu X et al. (2019) Human antigen R as a therapeutic target in pathological cardiac hypertrophy. JCI Insight 4, 47, 10.1172/jci.insight.121541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gurgis FMS, Yeung YT, Tang MXM, Heng B, Buckland M, Ammit AJ et al. (2015) The p38-MK2-HuR pathway potentiates EGFRvIII-IL-1β-driven IL-6 secretion in glioblastoma cells. Oncogene 34, 2934–2942, 10.1038/onc.2014.225 [DOI] [PubMed] [Google Scholar]
- 154.Chen J, Cascio J, Magee JD, Techasintana P, Gubin MM, Dahm GM et al. (2013) Posttranscriptional gene regulation of IL-17 by the RNA-binding protein HuR is required for initiation of experimental autoimmune encephalomyelitis. J. Immunol 191, 5441–5450, 10.4049/jimmunol.1301188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iacobellis G, Corradi D and Sharma AM (2005) Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med 2, 536–543, 10.1038/ncpcardio0319 [DOI] [PubMed] [Google Scholar]
- 156.Iacobellis G (2009) Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 17, 625, 10.1038/oby.2008.575 [DOI] [PubMed] [Google Scholar]
- 157.Sacks HS and Fain JN (2007) Human epicardial adipose tissue: a review. Am. Heart J 153, 907–917, 10.1016/j.ahj.2007.03.019 [DOI] [PubMed] [Google Scholar]
- 158.Yamaguchi Y, Cavallero S, Patterson M, Shen H, Xu J, Kumar SR et al. (2015) Adipogenesis and epicardial adipose tissue: a novel fate of the epicardium induced by mesenchymal transformation and PPARγ activation. Proc. Natl Acad. Sci. U.S.A 112, 2070–2075, 10.1073/pnas.1417232112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nelson AJ, Worthley MI, Psaltis PJ, Carbone A, Dundon BK, Duncan RF et al. (2009) Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J. Cardiovasc. Magn. Reson 11, 15–18, 10.1186/1532-429X-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yamada H and Sata M (2015) Role of pericardial fat: the good, the bad and the ugly. J. Cardiol 65, 2–4, 10.1016/j.jjcc.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 161.Horimatsu T, Kim HW and Weintraub NL (2017) The role of perivascular adipose tissue in non- atherosclerotic vascular disease. Front. Physiol 8, 969, 10.3389/fphys.2017.00969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Silaghi A, Piercecchi-Marti M-D, Grino M, Leonetti G, Alessi MC, Clement K et al. (2008) Epicardial adipose tissue extent: relationship with age, body fat distribution, and coronaropathy. Obesity (Silver Spring) 16, 2424–2430, 10.1038/oby.2008.379 [DOI] [PubMed] [Google Scholar]
- 163.Antonopoulos AS and Antoniades C (2017) The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. J. Physiol. (Lond.) 595, 3907–3917, 10.1113/JP273049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ansaldo AM, Montecucco F, Sahebkar A, Dallegri F and Carbone F (2019) Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol 278, 254–260, 10.1016/j.ijcard.2018.09.089 [DOI] [PubMed] [Google Scholar]
- 165.Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H et al. (2013) Adult epicardial fat exhibits beige features. J. Clin. Endocrinol. Metab 98, E1448–55, 10.1210/jc.2013-1265 [DOI] [PubMed] [Google Scholar]
- 166.Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F et al. (2009) Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab 94, 3611–3615, 10.1210/jc.2009-0571 [DOI] [PubMed] [Google Scholar]
- 167.Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK and Wong DTL (2014) Epicardial adipose tissue: far more than a fat depot. Cardiovasc. Diagn. Ther 4, 416–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lima-Martínez MM, Blandenier C and Iacobellis G (2013) Epicardial adipose tissue: more than a simple fat deposit? Endocrinol. Nutr 60, 320–328, 10.1016/j.endonu.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 169.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U et al. (2003) Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes. Res 11, 304–310, 10.1038/oby.2003.45 [DOI] [PubMed] [Google Scholar]
- 170.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV and Leonetti F (2004) Relation between epicardial adipose tissue and left ventricular mass. Am. J. Cardiol 94, 1084–1087, 10.1016/j.amjcard.2004.06.075 [DOI] [PubMed] [Google Scholar]
- 171.Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV et al. (2004) The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol 13, 313–316, 10.1016/j.carpath.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 172.Khawaja T, Greer C, Chokshi A, Chavarria N, Thadani S, Jones M et al. (2011) Epicardial fat volume in patients with left ventricular systolic dysfunction. Am. J. Cardiol 108, 397–401, 10.1016/j.amjcard.2011.03.058 [DOI] [PubMed] [Google Scholar]
- 173.Doesch C, Haghi D, Flüchter S, Suselbeck T, Schoenberg SO, Michaely H et al. (2010) Epicardial adipose tissue in patients with heart failure. J. Cardiovasc. Magn. Reson 12, 40–49, 10.1186/1532-429X-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schejbal V (1989) Epicardial fatty tissue of the right ventricle–morphology, morphometry and functional significance. Pneumologie 43, 490–499 [PubMed] [Google Scholar]
- 175.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S et al. (2001) Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis 157, 203–209, 10.1016/S0021-9150(00)00709-7 [DOI] [PubMed] [Google Scholar]
- 176.McKenney-Drake ML, Rodenbeck SD, Bruning RS, Kole A, Yancey KW, Alloosh M et al. (2017) Epicardial adipose tissue removal potentiates outward remodeling and arrests coronary atherogenesis. Ann. Thorac. Surg 103, 1622–1630, 10.1016/j.athoracsur.2016.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD et al. (2014) Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J. Cardiothorac. Surg 9, 2–11, 10.1186/1749-8090-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G et al. (2005) Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 29, 251–255 [DOI] [PubMed] [Google Scholar]
- 179.Zapardiel I, Blancafort C, Cibula D, Jaunarena I, Gorostidi M, Gil-Moreno A et al. (2017) Utility and actual use of European and Spanish Guidelines on the management of endometrial cancer among gynecologic oncologists in Spain. Int. J. Gynecol. Cancer 27, 1293–1297, 10.1097/IGC.0000000000001017 [DOI] [PubMed] [Google Scholar]
- 180.Packer M (2018) Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J. Am. Coll. Cardiol 71, 2360–2372, 10.1016/j.jacc.2018.03.509 [DOI] [PubMed] [Google Scholar]
- 181.Harada K, Shibata R, Ouchi N, Tokuda Y, Funakubo H, Suzuki M et al. (2016) Increased expression of the adipocytokine omentin in the epicardial adipose tissue of coronary artery disease patients. Atherosclerosis 251, 299–304, 10.1016/j.atherosclerosis.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 182.Saely CH, Leiherer A, Muendlein A, Vonbank A, Rein P, Geiger K et al. (2016) High plasma omentin predicts cardiovascular events independently from the presence and extent of angiographically determined atherosclerosis. Atherosclerosis 244, 38–43, 10.1016/j.atherosclerosis.2015.10.100 [DOI] [PubMed] [Google Scholar]
- 183.Narumi T, Watanabe T, Kadowaki S, Kinoshita D, Yokoyama M, Honda Y et al. (2014) Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failure. Cardiovasc. Diabetol 13, 84–87, 10.1186/1475-2840-13-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F et al. (2015) Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J 36, 795a–805a, 10.1093/eurheartj/eht099 [DOI] [PubMed] [Google Scholar]
- 185.Hatem SN and Sanders P (2014) Epicardial adipose tissue and atrial fibrillation. Cardiovasc. Res 102, 205–213, 10.1093/cvr/cvu045 [DOI] [PubMed] [Google Scholar]
- 186.Wang Q, Xi W, Yin L, Wang J, Shen H, Gao Y et al. (2018) Human epicardial adipose tissue cTGF expression is an independent risk factor for atrial fibrillation and highly associated with atrial fibrosis. Sci. Rep 8, 3585–3589, 10.1038/s41598-018-21911-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miksztowicz V, Morales C, Barchuk M, López G, Póveda R, Gelpi R et al. (2017) Metalloproteinase 2 and 9 activity increase in epicardial adipose tissue of patients with coronary artery disease. Curr. Vasc. Pharmacol 15, 135–143, 10.2174/1570161114666161024124244 [DOI] [PubMed] [Google Scholar]
- 188.Zeller J, Krüger C, Lamounier-Zepter V, Sag S, Strack C, Mohr M et al. (2019) The adipo-fibrokine activin A is associated with metabolic abnormalities and left ventricular diastolic dysfunction in obese patients. ESC Heart Fail. 6, 362–370, 10.1002/ehf2.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mazurek T, Kiliszek M, Kobylecka M, Skubisz-Gluchowska J, Kochman J, Filipiak K et al. (2014) Relation of proinflammatory activity of epicardial adipose tissue to the occurrence of atrial fibrillation. Am. J. Cardiol 113, 1505–1508, 10.1016/j.amjcard.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 190.Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT et al. (2010) Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ. Arrhythm. Electrophysiol 3, 345–350, 10.1161/CIRCEP.109.912055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muhib S, Fujino T, Sato N and Hasebe N (2013) Epicardial adipose tissue is associated with prevalent atrial fibrillation in patients with hypertrophic cardiomyopathy. Int. Heart J 54, 297–303, 10.1536/ihj.54.297 [DOI] [PubMed] [Google Scholar]
- 192.Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N et al. (2017) Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J 38, 53–61, 10.1093/eurheartj/ehv625 [DOI] [PubMed] [Google Scholar]
- 193.Chang H-X, Zhao X-J, Zhu Q-L, Hou Q and Li Y (2018) Removal of epicardial adipose tissue after myocardial infarction improves cardiac function. Herz 43, 258–264, 10.1007/s00059-017-4555-4 [DOI] [PubMed] [Google Scholar]
- 194.Greulich S, de Wiza DH, Preilowski S, Ding Z, Mueller H, Langin D et al. (2011) Secretory products of guinea pig epicardial fat induce insulin resistance and impair primary adult rat cardiomyocyte function. J. Cell. Mol. Med 15, 2399–2410, 10.1111/j.1582-4934.2010.01232.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lage R, Moscoso I, Fernández-Trasancos Á, Cebro M, Couselo M, Fandiño-Vaquero R et al. (2015) Differential behaviour of epicardial adipose tissue-secretomes with high and low orosomucoid levels from patients with cardiovascular disease in H9C2 cells. Mol. Cell. Endocrinol. 416, 77–87, 10.1016/j.mce.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 196.Cheung L, Gertow J, Werngren O, Folkersen L, Petrovic N, Nedergaard J et al. (2013) Human mediastinal adipose tissue displays certain characteristics of brown fat. Nutr. Diabetes 3, e66, 10.1038/nutd.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM et al. (2009) Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation 119, 1586–1591, 10.1161/CIRCULATIONAHA.108.828970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pucci G, Battista F, de Vuono S, Boni M, Scavizzi M, Ricci MA et al. (2014) Pericardial fat, insulin resistance, and left ventricular structure and function in morbid obesity. Nutr. Metab. Cardiovasc. Dis 24, 440–446, 10.1016/j.numecd.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 199.Shah RV, Anderson A, Ding J, Budoff M, Rider O, Petersen SE et al. (2017) Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: the multi-ethnic study of atherosclerosis. JACC Cardiovasc. Imaging 10, 1016–1027, 10.1016/j.jcmg.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu J, Fox CS, Hickson D, Sarpong D, Ekunwe L, May WD et al. (2010) Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes Care 33, 1635–1639, 10.2337/dc10-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hua N, Chen Z, Phinikaridou A, Pham T, Qiao Y, LaValley MP et al. (2014) The influence of pericardial fat upon left ventricular function in obese females: evidence of a site-specific effect. J. Cardiovasc. Magn. Reson 16, 37–38, 10.1186/1532-429X-16-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cai X, Lin Y, Hauschka PV and Grottkau BE (2011) Adipose stem cells originate from perivascular cells. Biol. Cell 103, 435–447, 10.1042/BC20110033 [DOI] [PubMed] [Google Scholar]
- 203.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C et al. (2012) Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126, 1067–1078, 10.1161/CIRCULATIONAHA.112.104489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Soltis EE and Cassis LA (1991) Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin. Exp. Hypertens. A 13, 277–296 [DOI] [PubMed] [Google Scholar]
- 205.Löhn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M and Sharma AM (2002) Periadventitial fat releases a vascular relaxing factor. FASEB J. 16, 1057–1063, 10.1096/fj.02-0024com [DOI] [PubMed] [Google Scholar]
- 206.Xia N and Li H (2017) The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol 174, 3425–3442, 10.1111/bph.13650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fitzgibbons TP and Czech MP (2014) Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J. Am. Heart Assoc 3, e000582, 10.1161/JAHA.113.000582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G et al. (2009) Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ. Res 104, 541–549, 10.1161/CIRCRESAHA.108.182998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Agabiti-Rosei C, Paini A, De Ciuceis C, Withers S, Greenstein A, Heagerty AM et al. (2018) Modulation of vascular reactivity by perivascular adipose tissue (PVAT). Curr. Hypertens. Rep 20, 44, 10.1007/s11906-018-0835-5 [DOI] [PubMed] [Google Scholar]
- 210.Huang Cao ZF, Stoffel E and Cohen P (2017) Role of perivascular adipose tissue in vascular physiology and pathology. Hypertension 69, 770–777, 10.1161/HYPERTENSIONAHA.116.08451 [DOI] [PubMed] [Google Scholar]
- 211.Chen B, Lam KSL, Wang Y, Wu D, Lam MC, Shen J et al. (2006) Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem. Biophys. Res. Commun 341, 549–556, 10.1016/j.bbrc.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 212.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C et al. (2005) Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54, 2277–2286, 10.2337/diabetes.54.8.2277 [DOI] [PubMed] [Google Scholar]
- 213.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M et al. (2009) Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119, 1661–1670, 10.1161/CIRCULATIONAHA.108.821181 [DOI] [PubMed] [Google Scholar]
- 214.Houben AJ, Eringa EC, Jonk AM, Serne EH, Smulders YM and Stehouwer CD (2012) Perivascular fat and the microcirculation: relevance to insulin resistance, diabetes, and cardiovascular disease. Curr. Cardiovasc. Risk Rep 6, 80–90, 10.1007/s12170-011-0214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Verhagen SN, Vink A, van der Graaf Y and Visseren FLJ (2012) Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study. Atherosclerosis 225, 99–104, 10.1016/j.atherosclerosis.2012.08.031 [DOI] [PubMed] [Google Scholar]
- 216.Lastra G and Manrique C (2015) Perivascular adipose tissue, inflammation and insulin resistance: link to vascular dysfunction and cardiovascular disease. Horm. Mol. Biol. Clin. Investig 22, 19–26 [DOI] [PubMed] [Google Scholar]