Abstract

Nowadays, green-chemistry principles offer an approach that fits to ensure chemical process sustainability by the use of low-cost renewable raw materials, waste prevention, inherent safer designs, among others. Based on this motivation, this study presents a novel methodology for sustainable process design that comprises the synthesis of a multifeedstock optimal biorefinery under simultaneous optimization of economic and environmental targets and further sustainability evaluation using the sustainability weighted return on investment metric (SWROIM). The first step of the proposed method is the formulation of an optimization model to generate the most suitable process alternatives. The model took into account various biomasses as available raw materials for production of ethanol, butanol, succinic acid, among others. Process technologies such as fermentation, anaerobic digestion, gasification, among others, were considered for biorefinery design. Once the model synthesizes the optimal biorefinery, we used environmental, safety, economic, and energy analyses to assess the process, which is a case study for north Colombia. Process simulation generated the data needed (extended mass and energy balances, property estimation, and modeling of downstream) to develop the process analysis stage via the Aspen Plus software. Results for the environmental and economic analyses showed that the assumption considered to solve the optimization problem was adequate, yielding promising environmental and economic outcomes. Finally, the overall sustainability evaluation showed a SWROIM of 27.29%, indicating that the case study showed higher weighted performance compared to the return on investment (ROI) metric of 14.33%.
Introduction
Over the recent decades, the interest in natural resource conservation has increased; thus, almost all industries and chemical plants have begun to apply sustainability criteria in process design. Along with an adequate disposition of resources, there are also concerns related to an increase in the world energy demand, which has raised greenhouse gas (GHG) emissions.1 In this regard, CO2 emissions to the atmosphere have increased since the last century, mainly due to the overuse of fossil fuels, along with the economic activities related to sectors such as chemical industries, agriculture, resource exploitation, among others.2 In this sense, biofuels have emerged as an essential alternative to complement energy generation and pollution mitigation. There is a positive projection regarding biofuels because these substances can mitigate fossil fuel dependence, along with the reduction of environmental impacts and emissions of chemicals derived from the decrease in the exploitation of such resources.3 For biofuel production, there are some key aspects closely related to the identification of biomass material and the definition of biorefinery technologies and processes. The above is crucial considering the relevance, suitability, and economics of the available alternatives.4
Consequently, the selection of processing routes for a biorefinery configuration becomes an essential task, which necessarily has to consider overall process sustainability. Therefore, there is a real motivation in the formulation of biorefinery synthesis and optimization models to support the decision-makers in the development of engineering projects. This goal comprises mathematical tools and methods for the selection of raw materials, processing pathways, and products.5 Likewise, this study tries to achieve this goal, presenting a way for the synthesis and evaluation of biorefinery configurations under sustainability parameters.
Due to the enormous number of possible alternatives and combinations of processing pathways that could be considered for adoption in biorefinery design, there is a growing number of studies addressing biorefinery synthesis and optimization and examining sustainability objectives.6 Bao et al.7 proposed a shortcut method for the preliminary synthesis of processing routes focusing on the optimization and application of the conceptual design of integrated biorefineries. Sengupta8 conducted a compilation of suitable raw materials, pathways, and biorefinery products related to sustainability-based processes using renewable biomasses. Pham and El-Halwagi9 addressed a process synthesis model for biorefinery configurations. González-Delgado et al.10 proposed a process synthesis model for a microalgae-based biorefinery based on combined forward–backward screening and superstructure approach. A mathematical programming method for bioprocess-based synthesis was introduced by Bonatsos et al.,11 which considers detailed design data and accurate cost estimation.
As described, some studies on biorefinery synthesis have addressed models under single- or multiobjective optimization, calculating profits, environmental impacts, risks, among others. Regarding the above, it is apparent that studies considering further process evaluation or sustainability assessment of synthesized processes in the same methodology are extensively absent. Therefore, this study presents a novel method that combines biorefinery synthesis and optimization along with process evaluation and sustainability measurement using technical analyses and a weighted metric. In this sense, the fundamental goal is to design more sustainable biorefinery pathways in terms of profits and environmental impacts and also consider the energy distribution and safety issues of the plant.
Furthermore, environmental impact evaluation has been incorporated in the process synthesis approach considering emissions and impact mitigation.12 Stefanis et al.13 introduced the life cycle analysis in the optimization framework showing the advantages of the application of multiobjective functions for the design of processes. Zondervan et al.14 synthesized optimal processing routes for ethanol, butanol, and succinic acid production through biorefinery optimization based on a mixer integer nonlinear programming (MINLP) model. Shabbir et al.15 developed a fuzzy optimization biorefinery synthesis model considering environmental and economic performance. Andiappan et al.16 proposed a multiobjective optimization approach considering economic, environmental, and energy parameters for synthesizing an integrated biorefinery. Meramo-Hurtado et al.17 introduced the evaluation of potential environmental impacts (PEI; along with exergy losses and profits) for biorefinery synthesis considering a three-dimensional (3D) multiobjective optimization.
The operation of biorefineries requires the use of high amounts of resources (such as freshwater, utilities, among others), so the evaluation and diagnosis of topologies is a crucial aspect regarding sustainable design. In this regard, the literature reports several studies addressing the role of process evaluation and analysis to measure sustainability performance. Ruiz-Mercado et al.18 reported several indicators to assess process sustainability considering economic, exergy/energy, environmental/safety, and technical parameters. Carvajal et al.19 used techno-economic and environmental analyses to compare lignin extraction processes. García Carlos et al.20 performed energetic and environmental assessments of thermochemical and biochemical routes for producing energy from agricultural residues. Exergy evaluation, along with techno-economic analysis, was used for assessing industrial production processes.21 Ojeda et al.22 developed an exergy analysis and process integration (heat integration) of different pathways for bioethanol production using acid pretreatment to identify the best route between separate hydrolysis and fermentation, simultaneous saccharification and fermentation, and simultaneous saccharification and co-fermentation technologies. Moreover, a multidimensional sustainability assessment was proposed to assess Fischer–Tropsch fuel processes considering economic, environmental, energy, and, also, social indicators.23
There is an increasing awareness to generate novel ways for producing biofuels and chemicals from renewable materials based on the development of process and biorefinery synthesis methods along with new tools for the evaluation of biomass transformation processes under sustainability parameters. Therefore, this study presents an optimization design approach for multifeedstock biorefinery configuration synthesis. Also, an optimal topology (synthesized by the solution of the model) is evaluated using technical analyses under environmental, economic, and safety parameters. The proposed model considered a case study according to the availability of agricultural wastes in the north Colombia region. For this purpose, production data and reports from government institutions were compiled for the most recent years, to establish real-approximate scenarios for the Colombian case.
Results and Discussion
The proposed case study took into account the reported production of agricultural residues generated in the northern zone of Colombia. The method required the selection of different processing routes for production of desired biorefinery products. In this sense, ethanol and succinic acid were chosen due to the high yield production rate of these substances (and the possibility of their simultaneous synthesis) from corn stover.24 The next step implied the selection of hydrogen, bio-oil, and biogas because these chemicals have great potential for energy generation or cogeneration in biorefinery processes.25 Bio-oil can also be considered as an essential intermediate in the production of petrochemical products.26 Levulinic acid is classified by the United States Department of Energy as one of the top 12 biorefinery products, and it is also considered as an essential intermediate for the production of several compounds with application in many industries.27 Finally, butanol was selected as a potential biorefinery product because it has become a substitute for fossil fuels, and this substance shows several advantages compared to other biofuels.28Table 1 shows the assignation for b, k, and p.
Table 1. Indexes for b, k, and p Parameters.
| b | biomass | p | pathways | k | products |
|---|---|---|---|---|---|
| 1 | corn stover | 1 | fermentation | 1 | ethanol |
| 2 | rice chaff | 2 | dark fermentation | 2 | hydrogen |
| 3 | banana rachis | 3 | acid dehydration | 3 | succinic acid |
| 4 | cassava waste | 4 | anaerobic digestion | 4 | biogas |
| 5 | cocoa husk | 5 | pyrolysis | 5 | bio-oil |
| 6 | ABE fermentation | 6 | butanol | ||
| 7 | gasification | 7 | levulinic acid |
The lignocellulosic raw material mainly contains cellulose, hemicellulose, and lignin; therefore, the mathematical expression for raw material mass flow, Fb, can be rewritten, as shown in eq 1.
| 1 |
where Fqb is the mass flow of a carbohydrate for each bioresource, while nqb represents the mass fraction of cellulose (q = 1), hemicellulose (q = 2), or lignin (q = 3) for each bioresource b. A linear programming problem (see the Methodologysection) is formulated, considering an objective function for simultaneous minimization of AI (Environmental Impacts) and maximization of E (gross profit), with 35 unknowns (xbp) subject to non-negativity restrictions (xb ≥ 0), market demand constraint (Jk ≤ Jmaxk), and the availability of raw materials (Fb ≤ Fbmax). Figure 1 shows the superstructure corresponding to the addressed case study, which represents the selection of bioresources, routes, and products. This arrangement comes from a generic superstructure (see the Methodology section), which allows establishing the mass balance equations. Finally, it considered the assignation of the parameters of each layer for this case study.
Figure 1.
Defined superstructure for the proposed case study.
The model uses hypothetical scenarios based on production and sales reports, among others, to determine market demand for biorefinery products, which include reports on bioethanol sales in Colombia, provided by the National Federation of Biofuels, and the natural gas demand data for 2015, reported by the Mining and Energy Planning Unit (UPME), among others.33Table 2 shows the reported prices and demand scenario for biorefinery products.
Table 2. Prices and Demand Scenario for Selected Biorefinery Products.
| product | Jkmax (t/y) | Ck (USD/kg) | scenario | refs |
|---|---|---|---|---|
| ethanol | 81 400 | 0.971 | 25% of sales for Colombia in 2016 | (29) |
| butanol | 30 000 | 2.742 | 1% of world demand | (30) |
| hydrogen | 16 175 | 2.453 | 25% of equivalent production for Colombia | (5) |
| biogas | 197 930 | 0.444 | 10% of the demand for natural gas in Colombia for 2015 | (31) |
| levulinic acid | 22 500 | 5.006 | 1% of world demand | (32) |
| succinic acid | 120 000 | 2.861 | 1% of world demand | (30) |
| bio-oil | 203 170 | 0.725 | 10% of crude oil production of Ecopetrola for 2017 | (7) |
Colombian Petroleum Company.
Also, the model evaluates the economic objective in terms of the annual profit from the sales, raw material costs, and production costs. Table 3 provides information about the costs of selected biorefinery routes, while Table 4 reports the prices for raw materials.
Table 3. Cost of Selected Biorefinery Processing Routes.
| product | route | Ckbp (USD/kg) | refs |
|---|---|---|---|
| ethanol | fermentation | 0.36 | (5) |
| succinic acid | fermentation | 0.38 | (24) |
| hydrogen | dark fermentation | 1.75 | (34) |
| butanol | ABE fermentation | 1.24 | (35) |
| biogas | anaerobic digestion | 0.36 | (7) |
| levulinic acid | acid dehydration | 0.50 | (32) |
| bio-oil | pyrolysis | 0.28 | (7) |
| hydrogen | gasification | 0.39 | (7) |
Table 4. Cost of Selected Bioresources.
| bioresource | price (USD/kg) | refs |
|---|---|---|
| corn stover | 0.033 | (24) |
| cassava waste | 0.023 | (5) |
| banana rachis | 0.020a | |
| cocoa husk | 0.020a | |
| rice straw | 0.005 | (36) |
For these residues, reported information about their prices was not found. We assumed an average from the other residues.
Otherwise, the method requires information about the potential environmental impacts of each biorefinery product and raw material. Table 5 shows the specific PEI for biorefinery products, and Table 6 reports specific PEI for selected bioresources.
Table 5. PEI of Biorefinery Products.
| products | Ak (PEI/kg) | products | Ak (PEI/kg) |
|---|---|---|---|
| ethanol | 0.51 | furfural | 5.80 |
| butanol | 1.37 | carbon dioxide | 0.0003 |
| succinic acid | 0.17 | hydrogen | 0.00 |
| acetic acid | 0.34 | bio-oil | 0.84 |
| formic acid | 0.39 | biogas | 0.01 |
| levulinic acid | 0.20 |
Table 6. PEI of Biorefinery Raw Materials.
| raw material | Ab (PEI/kg) |
|---|---|
| corn stover | 0.108 |
| rice chaff | 0.103 |
| banana rachis | 0.079 |
| cassava waste | 0.079 |
| cocoa peel | 0.106 |
Also, the environmental objective evaluates the generation of environmental effects from the operation of the biorefinery. These effects calculate the associated PEI using a basis of 1 kg/h of the produced product. Table 7 shows the rate of generated PEI for each screened biorefinery processing route.
Table 7. PEI of Biorefinery Processing Routes.
| process | Apk (PEI/(kg/h)) | basis |
|---|---|---|
| fermentation | 0.90 | 1 kg/h ethanol |
| fermentation | 1.06 | 1 kg/h succinic acid |
| dark fermentation | 9.05 | 1 kg/h H2 |
| acid dehydration | 6.14 | 1 kg/h levulinic acid |
| ABE fermentation | 0.67 | 1 kg/h butanol |
| anaerobic digestion | 0.74 | 1 kg/h biogas |
| pyrolysis | 6.22 | 1 kg/h bio-oil |
| gasification | 44.70 | 1 kg/h H2 |
According to the development of the optimization model, mathematical expressions were obtained for the multiobjective function and constraints established due to the availability of raw materials, conversion yields, costs, and potential environmental impact (PEI). The ε-constraint method is used to determine an optimal set of solutions that meets both environmental and economic objectives. Also, it provides the data for the construction of a Pareto curve that allows selecting an optimal design alternative.37 The open software LINGO 17.0 is used for the solution of the formulated optimization problem. Figure 2 shows the solution Pareto curve, indicating point A to reflect the minimum environmental impacts at the cost of the minimum economic benefits.
Figure 2.
Optimal Pareto solution curve.
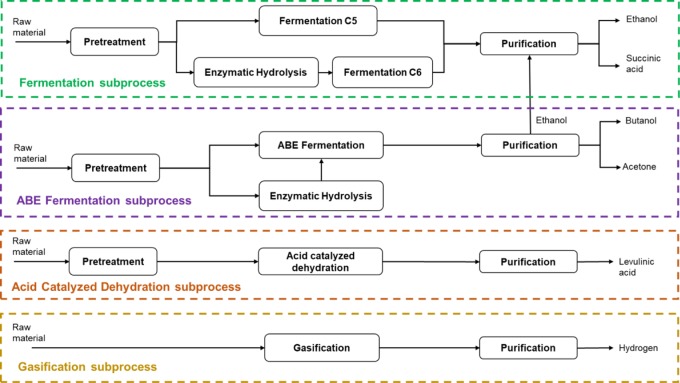
The economic performance at this point is 25.54 million USD/y, which represents approximately 9% of the maximum. Point C of the curve shows the maximum economic benefits (284.8 million USD/y) at the cost of maximum environmental impacts, with a value of 1.147 × 109 PEI/y, which would imply an increase of almost 12 times compared to the minimum. The outcomes for point C showed that the biorefinery with the highest economic potential would comprise (i) fermentation (345 763 t/y of cassava waste), (ii) acid dehydration (185 605 t/y of banana rachis), (iii) pyrolysis (236 117 t/y of corn stover, 178 612 t/y of rice chaff, 7842 48 t/y of banana rachis, and 2623 t/y of cocoa husk), and (iv) acetone–butanol–ethanol (ABE) fermentation (67 968 t/y of banana rachis and 51 665 t/y of cassava waste). Otherwise, point A (minimal environmental impacts) showed a biorefinery that would be composed of (i) anaerobic digestion (236 112 t/y of corn stover, 178 612 t/y of rice chaff, 55 640 t/y of cassava waste, and 2623 of cocoa husk), (ii) pyrolysis (7842.48 banana rachis), and (iii) ABE fermentation (253 573 t/y of banana rachis and 341 789 of cassava waste). It is essential to realize that the Pareto curve shows a set of optimal solutions that tradeoff both objectives simultaneously. The selection for the best solution can be made based on the requirements that best fit sustainability objectives. The development of the process synthesis model requires the choice of one scenario or solution to develop the sustainability assessment, represented by point B or case B. The optimization problem is solved using the extreme points of both objectives expressed at points A and C. The model constructs the curve ranging from the upper to lower values for the environmental target. The ε-constraint method converts the environmental goal into a constraint, which allows knowing how profits and PEI are changing within the evaluated range. This procedure generated data about the behavior of both objectives, which helped us to make the selection of case B, aiming at the maximum possible profits at a lower cost of outgoing environmental impacts of the process. The outcome offered by point B described better the aforementioned condition. Table 8 shows the raw material flows obtained for case B. It represents a solution that posts 30% of the maximum environmental impacts with a corresponding economic performance equal to 85.30% of the maximum. Case B shows the biorefinery pathways that comprise this scenario. These include the following subprocesses: (i) fermentation, (ii) acetone–butanol–ethanol (ABE) fermentation, (iii) acid dehydration, and (iv) gasification. This optimal biorefinery comprises three biochemical operations and one thermochemical technology. Figure 3 displays the simplified block diagram of biorefinery case B. Compared with the extreme points (A and C), only ABE fermentation technology remains as a subprocess for these biorefineries, with the highest total processing capacity (595 362 t/y) corresponding to the design with the minimum environmental impacts. This mass flow for case B represents a decrease of 79.51% with respect to this value for point A. Furthermore, the main difference between the technologies that comprise points C and B is that the first one considers the operation of a pyrolysis unit, while the second one changes that subprocess for a gasification stage (the mass flow of each feedstock also varies between these designs).
Table 8. Total and Specific Mass Flow of the Raw Material for Optimal Case B.
| subprocess | overall mass flow (t/y) | bioresource flow (t/y) | |
|---|---|---|---|
| gasification | 478 257 | 236 112 | corn stover |
| 178 612 | rice chaff | ||
| 60 910 | banana rachis | ||
| 2623 | cocoa husk | ||
| acid dehydration | 132 015 | 132 015 | banana rachis |
| ABE fermentation | 121 962 | 121 962 | cassava waste |
| fermentation | 343 958 | 68 491 | banana rachis |
| 275 467 | cassava waste | ||
Figure 3.
Simplified block diagram of biorefinery case B.
Fermentation
This subprocess was simulated based on the processing capacities (for each biomass) reported in Table 8. Due to the molecular structure of carbohydrates that comprise lignocellulosic biomass, a prehydrolysis stage enhances the formation of C6 and C5 sugars. In this sense, the process pathway starts with the simulation of the acid pretreatment unit, which operates under the following conditions: (i) the use of a dilute acid solution (H2SO4 at 0.5 wt %) and (ii) a solid concentration of 22 wt % in the reactor. The reaction mechanisms for this system occur at 190 °C and 13 atm.24 The outlet stream of the reactor is sent to a flash cooler to reach environmental temperature and pressure conditions. Large amounts of 5-hydroxymethylfurfural (HMF), furfural, and acetic acid are separated, so these substances are subsequently condensed and directed to wastewater treatment. A scrubber filter mixes and separates the hydrolysates for splitting the solids and liquids. Solids (mainly composed of nondegraded cellulose) are separated and further treated in the enzymatic hydrolysis unit for glucose production.38 Besides, the liquid stream continues to an ion-exchange unit for acetic and sulfuric acid removal; it requires the addition of an ammonia solution with a proportion of 1.1 N/N of removed ions. Subsequently, this mixture is overlimed with CaOH. For this purpose, an inlet flow of lime raises the pH to 10, and then, a heat exchanger heats the flow to 50 °C. This path forms gypsum as an undesired product. Finally, a hydrocyclone unit rejects about 99.5% of this substance.39 Besides, the other process line comprises the transport of the treated liquid stream for further pentose fermentation.
After pretreatment operations, a delignification unit separates the solubilized lignin from the pentose-rich mixture. The flow continues to the fermenter unit where the xylose and a small portion of glucose react to form ethanol, along with byproducts such as succinic acid, acetic acid, lactic acid, and carbon dioxide. There are different microorganisms used for the formation of ethanol, which allows the degradation of sugars of five and six carbons. This process simulation used the recombinant Zymomona mobilis for fermentation purposes. This microorganism can degrade carbohydrates with yields of up to 95% for ethanol production from glucose and 85% from xylose.24 The fermentation broth, with a high content of carbon dioxide and water, is sent to the beer column to remove CO2 and other gases.40 Due to the volatility of ethanol, the downstream processing requires the use of a scrubber unit. It separates the gas phase from the main flow to avoid product losses. The bottom flows of each tower are collected and mixed, obtaining a treated stream with a concentration of ethanol of about 8 wt %. The mixture reaches the azeotrope concentration formed between ethanol and water in the distillation tower.41 Then, incorporation of a specific separation system is needed to obtain the product with high purity. Kang et al.42 reported that through the use of molecular sieve units, a concentration of 99.7 wt % of ethanol could be achieved. Hence, the separation sequence of this topology ends considering the simulation of this type of unit.
In the hexose fermentation unit, C6 sugars are converted into succinic acid as the main product, while acetic acid is formed as a byproduct. Okino et al.43 reported the use of the recombinant Corynebacterium glutamicum microorganism, which can transform glucose into succinic acid with a yield of 0.7 kg/kg under anaerobic conditions and high CO2 concentration. The sodium succinate formation pathway allows the purification of succinic acid. In this regard, an evaporator separates water, oxygen, and carbon dioxide from the mainstream, which contains the produced succinic acid (and the other byproducts). The bottom contents of the evaporator are cooled to 30 °C and filtered. This results in the separation of the solid phase from the gas phase, along with a significant portion of the residual liquids.44 To recover succinic acid, H2SO4 reacts with sodium succinate, resulting in the precipitation of sodium sulfate, for further filtration. Finally, the product stream is brought to vacuum distillation for moisture removal, yielding succinic acid at a concentration of 98.5 wt %, and a crystallizer solidifies the product under 4 °C.14
ABE Fermentation
This section of the optimal biorefinery was simulated considering a processing capacity of 121 962 t/y of cassava waste, according to the data reported in Table 8. As performed for fermentation, this unit requires the operation of a pretreatment or prehydrolysis stage to increase substrate digestibility. Therefore, the simulation of this section implies the same conditions and operations used for alcoholic fermentation. Similarly, solids with high cellulose content pass to enzymatic hydrolysis to produce glucose. The ABE fermentation unit digests both glucose and xylose in the same reaction system, which requires a temperature of 35 °C and 1 atm.45 Qureshi et al.46 reported high yields in ABE production using Clostridium, with a production percentage of 50%. Due to the mixture formed between ABE products and residual gases (like CO2, H2, or O2), a gas-stripping unit is coupled at the end of the fermentation stage for dragging the desired products into the gas phase (it uses N2 or fermentation gases), which allows the separation of butyric acid and other unwanted byproducts that remain in the liquid phase.47 Otherwise, a condenser decreases the temperature to −10 °C (under 1 atm), which allows the separation of the gas stream in a further flash separator unit. The above operation allows isolating condensed gases such as H2, CO2, and N2 from the liquid phase, which is rich in ABE products and water.48 The process continues to a double-effect distillation stage, which comprises a series of distillation towers for the extraction of acetone, ethanol, and butanol. The first one is the beer column, which removes a high amount of water, increasing butanol concentration.
Given that a small portion of CO2 is solubilized in the mixture, the process uses a flash separator for rejecting this gas from the main flow. Otherwise, the bottom stream (liquid stream) is sent to the acetone column (which operates under 0.7 atm), so the acetone-rich distillate stream is compressed to bring the flow to atmospheric conditions.49 Finally, the distillate stream from the acetone column is sent to a flash separator that raises acetone from a concentration of 85 to 97 wt %. The bottom contents of the acetone tower are sent to the ethanol column to recover an amount of this product. In this part, the molecular sieve unit (in the alcoholic fermentation) also purifies the distillate rich in ethanol, taking advantage of its features. The process incorporates a decanter unit to overcome the heterogeneous azeotrope formed by water and butanol (the bottom contents of the ethanol tower).50 It divides the flow between the organic-rich mixture (77 wt % of butanol) and water-rich mixture. The butanol tower treats the organic mixture to purify this main product up to 96.7 wt %. The recirculation of the distillate stream allows the maximization of the product recovery.
Acid-Catalyzed Dehydration
This topology processes a feedstock flow of 132 015 t/y banana rachis (primary raw material). For this process, the lignocellulosic biomass was pretreated to increase the availability of sugars of five and six carbons (as described for the previous processes). Acid-catalyzed dehydration involves the degradation of reducing sugars through the use of an acid catalyst (H2SO4). Therefore, xylose and glucose streams before entering the reactor system are mixed with the catalyst and subsequently heated to 180 °C.51 It operates at 14.80 atm, forming levulinic acid from the dehydration of glucose. The reaction mechanism involves dehydration of C6 sugars to form HMF. This allows the formation of levulinic acid (along with formic acid) by rehydration of HMF. Otherwise, xylose is dehydrated to form furfural.52 The outlet stream is cooled to 30 °C and sent to the purification stage. This operation is carried out in an arrangement of two distillation towers (in series), obtaining levulinic acid from the bottom of the second column at a concentration of 98.5 wt %.32
Gasification
This design can process a total amount of 478 257 t/y in a multifeed system using a mixture of corn stover, rice chaff, banana rachis, and cocoa husk. The above comprises the solution of the model given in Table 8. Such feedstock flows are mixed and sent to a dryer unit to reduce moisture content.53 The dried stream is directed to the gasifier reactor under 750 °C and 1 atm. As a result of the gasification, solid and gas phases are generated. They are separated using a cyclone unit. The gas flow is flash-cooled to 15 °C, allowing the condensation of a high amount of water. This processing pathway incorporates an injector unit for rejecting CO2, which uses water as an absorbent medium. The injected flow is compressed to 6 bar and sent to a separator tower for the rejection of CO2.54 The water gas shift reaction increases H2 production. It involves the formation of H2 (and CO2) from the equilibrium reaction of CO and H2O. Product formation is favorable at conditions of 205 °C and 32 atm. The employed model to simulate the reactor in Aspen Plus is the Rstoic model, which uses the thermodynamic parameters of equilibrium reactions. Because of CO2 formation in the water gas shift system, the process uses an amine absorption unit. It allows the H2 purification, yielding this substance with a concentration of 97% in volume.55Table 9 summarizes the mass flows of raw material and products generated in case B.
Table 9. Mass Flows of Products and Raw Materials for the Simulation of Case B.
| raw materials (t/y) | |||||
|---|---|---|---|---|---|
| corn stover | rice chaff | banana rachis | cassava waste | cocoa husk | |
| 236 112 | 178 612 | 261 416 | 397 429 | 2623 | |
| products (t/y) | |||||
| ethanol | succinic acid | butanol | levulinic acid | hydrogen | acetonea |
| 22 036 | 119 225 | 11 428 | 34 804 | 14 260 | 4785 |
Co-product obtained by ABE fermentation.
Environmental Outcomes
The development of process simulation for the biorefinery configuration of case B allows the evaluation of the overall sustainability performance. Such examination requires information about the process performance, which is obtained from the application of technical analyses. Likewise, an environmental assessment was carried out using the WAste Reduction (WAR) algorithm methodology. Figure 4 shows the output rate of PEI/kg of the product for toxicological categories.
Figure 4.
PEI rates for toxicological impact categories.
Human toxicity potential by ingestion (HTPI) and terrestrial toxicity potential (TTP) are the most impacted categories among the toxicological ones, with a corresponding rate of 0.55 PEI/kg of the product. These values are associated with the handling or processing of organic substances like ethanol, acetic acid, hydrochloric acid, among others.56 Compared to the described categories, the output rate of environmental impacts for aquatic toxicity potential (ATP), with a rate of 0.12 PEI/kg of the product, is significantly lower, which means that the potential effects on aquatic systems could be moderately low. Finally, human toxicity potential by dermal exposure (HTPE) shows the best performance, posting a rate of 0.05 PEI/kg of the product, which represents that the possible emission of substances (in this process) is not highly harmful.
Figure 5 shows the environmental performance results for atmospheric categories. Acidification potential (AP) showed the highest output rate of environmental impacts with 0.30 PEI/kg of the product; this result is mainly related to the high amount of energy required for the operation of this biorefinery. WAR can evaluate a chemical process considering three different energy sources (natural gas, oil, and coal). We selected natural gas because this source has fewer environmental impacts compared with the other two options.57 This result might reveal that this topology has a high demand for utilities and energy supply. Photochemical oxidation potential (PCOP) posted a rate of 0.05 PEI/kg of the product, which, along with AP, is classified as a regional atmospheric impact category. Comparing their rates with those obtained for global warming potential (GWP) and ozone depletion potential (ODP), there are more concerns about the generation of local pollution (short time) than global contamination (long time). The above is stated, considering that GWP and ODP are metrics related to the measurement of global atmospheric impacts.
Figure 5.
PEI rates for toxicological impact categories.
Economic Results
For the calculation of process economics, Aspen Economic Analyzer is implemented, which allows the estimation of utility, equipment, piping, and electricity costs. The overall evaluation was performed based on the calculation of the net present value (NPV), the payback period (PBP), and the return on investment (ROI) metrics.58 Therefore, the calculation of the capital investment cost and the annualized operating cost is developed using the information provided by process simulation. The annual sales for the simulated biorefinery are calculated to be 581.52 million dollars, which correspond to an NPV of 268.77 million dollars, a PBP of 6.83 years, and an ROI of 32%. Luo et al.24 reported that the typical ROI for this type of project could vary between 25 and 35%, which means that the modeled optimal topology presents a moderate to high economic performance, showing performance parameters among the reported standards.
Process Safety Results
The sustainability evaluation of the optimal biorefinery assesses process safety as a third criterion for the overall valuation. This study uses the inherent safety index (ISI). This indicator evaluates the potential inherent risks associated with the industrial chemical process at the conceptual design stage.59 The heuristic basis of this method relates to the assumption that considers all situations as the worst possible case. Through this approach, it is possible to describe the riskiest state that may happen during process operation.60 The ISI comprises the chemical inherent safety (CIS) and process inherent safety (PIS) indexes. The first one requires information about the properties of the substances, corrosion, and reaction mechanism. This biorefinery develops several reactions and complex mechanisms; therefore, to establish a score for main and side reaction subindexes, the heat of reaction of all systems was calculated. As a result, ABE fermentation showed the highest heats of reaction for both chemical safety subindexes. In this sense, the conversion of xylose into butanol in the fermentation system is the most exothermic, showing a heat of reaction equal to −6536.16 J/g. In the case of the side reaction, the conversion mechanism of xylose into acetic acid is most exothermic, with an enthalpy equal to −6507.75 J/g. Chemical interaction between formic acid and hydroxymethylfurfural could represent a highly corrosive, toxic, and explosive mixture, which implies that the reactors and equipment require stainless steel or the best materials for their construction;61 this indicator obtains a score of 2. Also, it is worth mentioning that for acid pretreatment it is recommended to use special alloys to avoid corrosion of the construction materials.62 The dangerous substance subindex comprises the evaluation of toxicity, flammability, and explosivity of handled chemical compounds. Properties related to those aspects were checked in the reported safety datasheets, implying that formic acid is the most dangerous in this biorefinery configuration.
Otherwise, the PIS index evaluates the process using the operational conditions and the required equipment. The first subindex evaluated for PIS is the inventory. The calculation of this parameter assumes a retention time of 2 h. For this biorefinery, the stock is higher than 10 000 t, which corresponds to a score of 4. The gasification reaction showed the highest temperature, specifically in the gasification reaction, which operates at 750 °C and 32 atm. Also, this unit presents the most elevated pressure. The above might mean that the operation of the gasification reaction could be one of the riskiest units in the modeled biorefinery. The secure structure subindex reflects how a plant and its operational system work together in terms of inherent safety. This parameter shows a score of 3, considering that this process handles considerable amounts of mass and energy, along with the required equipment. Finally, the evaluated biorefinery design obtained a total ISI score of 36. This result reflects that this production process performs operations (and under conditions) in which this production process can be taken as moderate or highly risky. Furthermore, the above statement is confirmed considering the recommendation made by Heikkila,60 which mentioned that for chemical plants, a process that claims neutral safety performance commonly shows ISI scores equal to 24 or below. Table 10 summarizes the posted score for all ISI subindexes.
Table 10. Scores Assigned to ISI Subindexes for Case B.
| CIS indexes | score | PIS indexes | score | ISI |
|---|---|---|---|---|
| main reaction | 4 | inventory | 4 | 36 |
| side reactions | 4 | temperature | 4 | |
| interactions | 3 | pressure | 2 | |
| dangerous substances | 8 | equipment | 3 | |
| corrosivity | 2 | secure structure | 2 | |
| total | 21 | total | 15 |
Energy Analysis under Process Integration
The energy analysis was developed based on the application of energy integration to offer an arrangement of heat exchangers for reducing the demand for industrial utilities.63,64 The current requirements for industrial services correspond to 2476.95 GJ/h for cooling and 1002.67 GJ/h for heating. This result represents a total requirement of 3479.62 GJ/h. The pinch analysis methodology was applied to identify the potential savings for cooling and heating, considering the heat transfer capacity of process streams setting the heat recovery approximation temperature (see the Methodology section).65 The pinch analysis calculates the maximum potential savings from the data of the heat that a process stream is transporting within a process stage. As aforementioned, it classifies the flows as cold or hot, allowing to know the actual energy demand.66 After this, the temperature interval diagram was made, where the streams that provide their thermal loads for each temperature difference are verified. Tables 11 and 12 show the heat load of hot and cold streams of case B.
Table 11. Heat Load of Hot Streams of Case B.
| stream | Ts (°C) | Tt (°C) | mCp (GJ/h °C) | ΔH (GJ/h) |
|---|---|---|---|---|
| VAP | 167 | 28 | 0.161 | 22.322 |
| SOL | 167 | 30 | 0.041 | 5.659 |
| OUTEP | 100 | 30 | 0.342 | 23.945 |
| FOE | 115 | 30 | 0.053 | 4.487 |
| ET | 140 | 28 | 0.066 | 7.407 |
| SOLDS | 140 | 30 | 0.014 | 1.530 |
| ABEG | 88 | –10 | 0.025 | 2.496 |
| SOLD | 140 | 30 | 0.015 | 1.693 |
| LL | 180 | 30 | 0.022 | 3.300 |
| F2 | 257 | 28 | 0.010 | 2.414 |
| SOLDI7 | 750 | 28 | 78.2 | 56.467 |
| WAOU | 261 | 30 | 279.0 | 64.450 |
| GAS | 750 | 15 | 3103.1 | 2280.772 |
| total | 2476.947 |
Table 12. Heat Load of Cold Streams of Case B.
| stream | Ts (°C) | Tt (°C) | mCp (GJ/h °C) | ΔH (GJ/h) |
|---|---|---|---|---|
| M1 | 28 | 190 | 0.074 | –12.061 |
| AG1 | 28 | 190 | 0.294 | –47.681 |
| AGS | 28 | 190 | 0.125 | –20.328 |
| MY6 | 28 | 190 | 0.015 | –2.461 |
| CONDE | –10 | 25 | 0.042 | –1.481 |
| RT | 28 | 215 | 0.025 | –4.703 |
| KL | 30 | 180 | 0.008 | –1.310 |
| ING | 30 | 205 | 1.599 | –279.370 |
| UI | 144 | 450 | 2.070 | –633.276 |
| total | –1002.673 |
Likewise, the earlier step gives the current requirement for cooling and heating, which correspond to 2476.95 and 1002.67 GJ/h, respectively. Therefore, the total demand for energy is 3479.62 GJ/h. The pinch analysis showed that the maximum potential savings are 100% for heating and 55.34% for cooling. Also, it determines the pinch point at a temperature of 750 °C. The heat exchanger network (HEN) employs a total of 22 heat exchanger units, involving 13 hot streams and 9 cold streams. This HEN design uses eight heat exchangers to operate using hot and cold streams, while the remaining 14 use the external utility supply. Figure 6 displays the HEN designed for this biorefinery. The red color identifies the hot streams (GAS, SOLD7, WAOU, F2, LL, VAP, SOL, SOLDS, SOLD, ET, FOE, OUTSEP, and ABEG), while the blue color describes the cold streams (UI, RT, ING, M1, AG1, AGS, MY6, KL, and CONDE). The reuse of the heat load of the GAS stream was possible, reducing the heating utility of seven cold streams. The above means a total reduction of 999.883 GJ/h, which, along with the reuse of the F2 stream, was possible, decreasing the needed heating utility for KL, achieving integration of 1.31 GJ/h. This arrangement allows a total reduction of 40.52% for a cooling utility and 99.85% for a heating utility. The process can lead to energy savings very close to the maximum potentials determined through pinch analysis. Table 13 summarizes the results obtained from the heat integration of the process.
Figure 6.
Designed HEN for case B.
Table 13. Result of Pinch Analysis and Energy Integration.
| item | heating | cooling |
|---|---|---|
| utilities without integration (GJ/h) | 1002.67 | 2476.95 |
| maximum potential savings (%) | 100.00 | 55.34 |
| utilities with integration (GJ/h) | 1.48 | 1003.61 |
| integration saving (%) | 99.85 | 40.52 |
Sustainability Evaluation
Some assumptions were stated to evaluate the overall sustainability of the synthesized and modeled biorefinery. It is worth mentioning that the rural area of the Department of Bolivar is a suitable location for the biorefinery. In this zone, the raw material would be available from agricultural activities, so the corresponding weighting factor considers a medium relevance. In the case of the environmental aspect, the scenario is established based on the commitments that Colombia has acquired over the different international environmental agreements. Thus, this parameter gets maximum relevance. Otherwise, taking into account the integration developed (presented before) for the optimal biorefinery, the maximum degree of importance is assigned because of the high utility supplies required in the mainstream and downstream processing. Table 14 displays the corresponding parameters, indicators, and weighting factors associated with each technical aspect.
Table 14. Corresponding Parameters, Indicators, and Weighting Factors for Each Technical Parameter.
| aspect | index | indicatori | indicatortarget | wi |
|---|---|---|---|---|
| environmental | %RGWP | 67.40% | 100% | 0.60 |
| safety | %Sfn | 50% | 100% | 0.20 |
| energy | savings | 1.00 × 103 GJ/h | 1.00 × 103 GJ/h | 0.40 |
The application of SWROIM requires the definition of target indicators (Indicatortarget) for each technical parameter. Also, it needs the corresponding quantities of these parameters showing current process performance (indicatori). For process safety, indicatortarget is established considering that a process is neutral in terms of inherent risks when it reports an ISI score of 24 or below. As SWROIM evaluates projects in terms of improvements, we reformulated this indicator to show how close the process is to operate at a neutral safety point. So, eq 2 expresses this indicator as described.
| 2 |
%Sfn is the percentage of safety operation at a neutral point, ISIi is the inherent safety index of a project or alternative i, and ISIn is the neutral reference inherent safety index (ISIn = 24). Then, the target value for the safety indicator is equal to %Sfn = 100%. The current process safety performance of case B corresponds to an ISI = 36 (see the Process Safety Results section). Environmental and energy targets are set, taking into account the recommendation made by El-Halwagi,64 which suggested that process benchmarking techniques allow establishing the goals for sustainability indicators taking into account the best results for each parameter within the design alternatives. Therefore, such target indicators count the maximum potential energy savings from process integration and the maximum emissions in terms of CO2eq. Likewise, the improvement in the environmental performance counts the current reduction in the CO2 emissions (measured using GWPi) in terms of the theoretical maximum that can be generated from the case study of biorefinery production presented in this work. This value is the maximum environmental impact for the global warming potential category (GWPmax) shown by case C, which corresponds to 3.19 × 107 PEI/y. Furthermore, this design showed a current GPW = 1.04 × 107 PEI/y, according to the outcomes obtained from the environmental analysis. This indicator is calculated as shown in eq 3.
| 3 |
%RGWP is the reduction percentage of GWP. The aforementioned conditions imply that the evaluated targets should measure impacts in terms of better, equal, or worst performance for each aspect. The described approach helped us to set performance behaviors and target values for evaluating SWROIM. This was needed considering that the ISI and GWP evaluate and diagnose current performance for seeking opportunities of enhancements, but they do not reflect themselves an impact from a weighted estimation. Otherwise, integration savings come from the application of an improvement strategy through process integration; therefore, it has the ability to show improvement impacts, aiming at target and current performances. Likewise, process integration provided that through HEN this biorefinery can reach energy savings of 1.00 × 103 GJ/h. As the evaluation of optimal biorefinery comprises environmental, energy, and safety criteria, along with the baseline economic performance, the metric uses weighting factors (wi) for each indicator. It represents the relative importance of the sustainability indicator i compared to the economic baseline parameter (in this case, it is the ROI).67 Considering the performance data provided by the simulation and analysis of the optimal biorefinery, the overall sustainability of this process uses the SWROIM for estimating the performance of the whole process. Table 15 shows the results obtained for each used technical parameter and SWROIM for the modeled topology.
Table 15. Calculations for SWROIM and Evaluated Technical Parameters for Case B.
| scenario | AEP | TCI | GWP | ISI | energy savings | ROI | SWROIM |
|---|---|---|---|---|---|---|---|
| unit | $MM USD | $MM USD | PEI/y | GJ/h | % | % | |
| biorefinery case B | 41.98 | 293.01 | 1.04 × 107 | 36 | 1.00 × 103 | 14.33 | 27.29 |
These results reveal that this project (described as case B) presents a sustainability performance of 27.69%, which is a higher value than that obtained for ROI. This result might mean that the evaluated technical parameters had moderate positive effects that yield the economic performance of the plant. Notably, there is a positive contribution associated with energy savings through the application of process integration. Otherwise, the environmental impacts derived from this biorefinery could generate adverse effects on sustainability performance, but case B reaches a significant reduction in the GWP indicator with respect to the theoretical maximum. Such behavior indicates that the outcome shown by the proposed model fits with the results of this sustainability assessment. Finally, the developed inherent safety index suggests that there are real concerns regarding the risks associated with the operation of the plant. It shows 50% of a neutral operation, which seems to be a little bit far from an adequate safety performance. This outcome is mostly due to the operational conditions and equipment required for the gasification section; thus, process sustainability is moderately affected by this aspect.
Conclusions
In this study, an optimal biorefinery configuration was synthesized and assessed, considering a scenario for the North of Colombia. In this regard, computer-aided tools and sustainability analysis were useful to model and evaluate the developed alternative considering technical parameters. The model synthesized a multifeedstock biorefinery (case B) comprising fermentation, ABE fermentation, acid dehydration, and gasification to produce ethanol, levulinic acid, butanol, hydrogen, and succinic acid. The evaluation of case B allowed the estimation of the overall sustainability performance to diagnose and find improvement opportunities. The application of SWROIM required the simultaneous calculation of the technical parameters used to assess this biorefinery design. SWROIM showed a performance of 27.29%. The economic aspect was the most determinant feature in the result obtained with positive contributions associated with the energy integration based on the high savings that are reached by performing the HEN. This study also presented an optimization model for the selection of biorefinery processes and products under sustainability criteria. It comprises the maximization of economic profits and the minimization of environmental impacts. This work validated the model through a case study, which allows having a convenient and useful tool for the design of multifeedstock biorefineries. For future work, other studies can consider other essential parameters regarding process features and optimization model targets (such as exergy, resilience, or uncertainty parameters) and incorporate other technical indicators and metrics like exergy, process efficiency, business modeling, which allow a broader sustainability analysis. Also, the application of water recycle networks or process intensification techniques might positively contribute to the ongoing development of this field.
Methodology
According to the reported data about the annual production information of the agroindustry for the department of Bolivar (North of Colombia), crops of cassava, corn, rice, and cocoa are the most produced products in this region. Therefore, the generated wastes from such products are taken as raw materials for biorefinery design. The agroindustry activities of north Colombia are mostly developed from the center to the south of the Bolivar department.68Table 16 reports the data about the estimated generation of some of the most produced crops in the department of Bolivar.
Table 16. Corresponding Parameters, Indicators, and Weighting Factors for Each Technical Parameter.
| crop | production (t/y) | area (ha) | yield (t/ha) | waste generated (t/y) |
|---|---|---|---|---|
| corn | 167 455 | 108 476 | 1.60 | 236 112 |
| rice | 70 044 | 24 805 | 2.82 | |
| plantain | 42 514 | 5157 | 8.24 | |
| cassava | 397 270 | 41 445 | 9.60 | |
| cocoa | 2914 | 6433 | 0.45 | 2623 |
For a construction scenario, it is suggested that this plant should be located near agricultural production areas, which will allow better logistics management due to the availability of raw materials and their transportation.69 The approach presented in this study follows the following stages: (1) raw materials, routes, and product selection (hierarchy approach), (2) mathematical formulation of the optimization model based on the superstructure approach (showing the relationship of each stage in biorefinery processing units), (3) formulation of a solution strategy, (4) synthesis of optimal scenarios, and (5) evaluation of sustainability parameters of the optimal biorefinery configuration. Otherwise, the formulation of the constraints and mass balance equations of the model implies managing variables that involve the knowledge of the operating costs, raw material prices, conversion fractions, reaction yields, among others. This data is taken from reported studies and industrial production. Figure 7 shows the proposed step-wise methodology for the development of this study. The first step of the model allows generating process flowsheets considering well-defined conditions for a case study under economic and environmental objectives. Due to the simplicity and the assumptions made to develop the above point, the outcomes of that optimization cannot completely be used as a truthful diagnose of the synthesized process design. That is why we included a second stage of the methodology (sustainability assessment), which allows evaluating and obtaining more detailed data about the technical performance of the biorefinery.
Figure 7.
Proposed step-wise methodology for synthesis and evaluation of the multifeedstock optimal biorefinery.
Model Formulation
The optimization model presented in this work considers a set of available b bioresources (biomass feedstock), which can be converted into k different products through p process routes, where one option to obtain the desired products is not necessarily used. Figure 8 shows the generalized scheme of the superstructure for the selection of optimal pathways in a biorefinery configuration. The relationship between the available bioresources, process routes, and the desired product is given by a conversion factor (β) that refers to the efficiency of product formation from raw materials. The superstructure represents a variety of available options for process technologies, raw materials, and products and allows addressing the task to reach the best selection, taking into account economic and environmental criteria. Also, it considers the market demand for biorefinery products, which is a crucial constraint regarding the proposed optimization. This step corresponds to the biorefinery configuration synthesis stage, according to the methodology shown in Figure 7. It comprises the application of the green-chemistry principles through the use of renewable resources, the formulation of a mathematical structure for synthesizing biorefinery pathways, and the consideration of sustainability objectives.
Figure 8.
Generalized scheme of the superstructure.
On the other hand, the complexity of the model (and its solution) depends on the available options. Santibañez-Aguilar et al.5 mentioned that those formulations, which involve biomass transformation through biorefinery routes to biofuels and chemical derivatives, present nonlinearities that generate problems of nonconvergence. Thus, for this optimization formulation, a black-box model is considered, which allows linearizing the material balance equations. Therefore, these expressions can be set as a ratio between the mass flow of products, reactant flow, and a constant of proportionality that represents the overall efficiency or conversion. The scheme of Figure 7 permits to establish inlet and outlet variables for the mass balance according to eq 4.
| 4 |
where Jbpk represents the mass flow of product k, which is obtained through process p and from bioresource b, with a conversion yield βbp. This conversion factor refers to the amount of the product obtained from a specific raw material or substrate. Fbpk represents the mass flow of the raw material that enters the process path p to produce product k.
Market Demand for Biorefinery Products
A critical restriction in the optimization model comes from establishing or considering the needs of biorefinery products to avoid product flows higher than those required and optimize resources and guarantee sustainable use of these. Equation 5 shows the market demand restriction in mathematical terms.
| 5 |
where Jkmax represents the market demand for product k. The model needs to evaluate different scenarios for maximum production or market demand for each biorefinery product, considering the amount of waste available for this restriction to be significant in the model.
Objective Function
The objective function for the formulation of the optimization model simultaneously considers environmental and economic criteria and is expressed as follows in eq 6
| 6 |
where OF represents the multiobjective function of optimization, E represents gross profit, and IA the potential environmental impacts. The gross profits refer to the value of the total net income that is obtained from the sales of the products minus raw material and processing costs. Equation 7 shows the mathematical expression to evaluate economic performance.
| 7 |
where Ck represents the net price of product k, Cb the cost of the raw material b, and Ckbp the cost of the process of route p to produce product k from bioresource b. The parameter Ckbp includes total operating and annualized capital costs. Operational costs are related to the supply of chemical substances, energy, and expenses for supervisory work, operators, among others.
Regarding the measurement of environmental impacts, WAR GUI software, which was developed by the United States Environmental Protection Agency (USEPA), was used to perform the environmental impact assessment of the biorefinery configuration according to atmospheric and toxicological impact categories.70 This objective considers the potential impacts derived from the use of raw materials along with the effects associated with the selected processing routes and the generated biorefinery products. The environmental objective function is shown in eq 8
| 8 |
where Ak and Apk represent the potential impacts of the product k and the potential of the process path p to obtain k, respectively. Finally, Ab is the PEI of bioresource b. The measurement of potential environmental impacts of raw materials considers the concentration of carbohydrates in each biomass-based waste and the data reported by WAR GUI for cellulose, hemicellulose, and lignin.
Environmental Evaluation
For environmental assessment, the WAR methodology was selected. The WAR introduces the concept of PEI, which calculates the effects derived from a random discharge or emission of a substance on the environment.71 The above implies that a release would have quantizable effects on the surroundings, which count as potential environmental impacts. Potential environmental impact is a probabilistic and conceptual quantity that is not possible to be measured directly. Nevertheless, it can be estimated from measurable or estimable quantities.72 The main advantage of this method is associated with its capacity to quantify the PEI generated within a chemical process. Also, it allows the estimation of the rate at which environmental impacts enter or leave a chemical process.73 The WAR assesses chemical processes by the evaluation of eight impact categories, which include toxicological and atmospheric categories. In this sense, WAR uses the following parameters to evaluate a chemical process: Human toxicity potential by ingestion, Human toxicity potential by dermal exposure, terrestrial toxicity potential, aquatic toxicity potential (toxicological group), global warming potential, ozone depletion potential, photochemical oxidation potential, and acidification potential (atmospheric group).74 As aforementioned, the WAR evaluates the PEI emitted by a chemical process or system, which represents the rate of total output environmental impacts. It can be expressed in PEI/h or PEI/kg (of the product). Equation 9 shows the expression to calculate the total output rate of PEI on a time basis, while eq 10 displays the total output rate of PEI on the mass of the product basis.
Here, Iout(t) and Iout(m) are the total output rate of PEI/h and the total rate of PEI/kg of the product due to chemical interactions within the system, respectively. Iin(cp) and Igen(cp) are the total inlet rate of PEI and the total generation of PEI, respectively. αi is the weighting factor of the impact category i; Mv(in) and Mv(out) are the input and output mass flows of stream v, respectively; Xa is the mass fraction of a component a in the stream v; ψa is the overall potential environmental impact of substance a; and Kk is the mass flow rate of product k.75 The WAR algorithm uses both eqs 8 and 9 to calculate the PEI balance for a well-bounded or defined process.
| 9 |
| 10 |
Economic Evaluation
This criterion is evaluated considering the estimation of the net present value (NPV), payback period (PBP), and return on investment (ROI) metrics. In this sense, the calculation of these parameters requires the knowledge of economic indicators such as total capital investment (TPI), annualized operational cost, among others.76 These costs are estimated, taking into account the information provided by process simulation.77 The approach assumes a construction period of 3 years to establish a baseline for economic evaluation. In the first year, 10% of the capital cost related to engineering and construction is spent, 80% for the second year, and the total capital is completely used until the third year.24 The biorefinery operates at 75% of its maximum for the third year (actually, it is its first year of operation). It produces at its maximum capacity for the rest of the plant life.
Inherent Safety Evaluation
The third evaluated criterion in the sustainability assessment is the inherent safety of the optimal biorefinery configuration. The safety assessment is applied through the inherent safety index (ISI) method, which assesses the inherent risks associated with the operation of a chemical process. The ISI method has the advantage that it can be used at the conceptual design stage. It is an essential feature compared with other methodologies that require more detailed data, which is not always available in early engineering design stages. The inherent safety index takes into account the contribution of the CIS and PIS. Equation 11 calculates the score of both subindexes as follows.59
| 11 |
The ISI considers chemical factors and properties that could affect the inherent safety of the process. Therefore, it evaluates parameters like chemical reactivity, flammability, explosivity, toxicity, corrosivity, among others. Otherwise, the ISI estimates the inherent risk about process operations considering pressure, temperature, equipment safety, inventory, and secure structure. Equations 12 and13 show the parameters referring to the inherent chemical safety and process safety indicators, respectively.
| 12 |
| 13 |
where IRMMAX and IRSMAX are the indexes for the main and side reactions, respectively. IINTMAX is the chemical interaction index, (IFL + IEX + ITOX)MAX is the total dangerous substance index, and ICORMAX is the corrosivity index. In the case of PIS, II is the inventory index; ITMAX and IPMAX are the maximum process temperature and pressure indexes, respectively; IEQMAX is the equipment safety index; and ISTMAX is the secure structure index. The safety assessment through the ISI methodology is developed assuming the worst possible scenario or situation, and this approach is likely to describe the riskiest situations that might occur during process operation.78
Energy Integration
Energy performance is a crucial aspect that has to be taken into account for the evaluation of process sustainability. The energy assessment is developed by energy integration along with the application of pinch analysis and the design of a HEN.79 This approach allows the reduction of requirements for external heating and cooling utilities through the principles of the first and second laws of thermodynamics and heat transference. The task is reached, taking advantage of the energy integration potential of hot and cold streams of the process. Otherwise, pinch analysis determines these saving potentials. It uses the thermal information of the process to determine the maximum savings that can be reached by a network of heat exchangers.80 The pinch analysis comes from an initial condition related to the transference between a hot stream and a cold stream. The system reaches equilibrium when the temperature of these streams is equal (see eq 14).
| 14 |
T is the temperature of the hot stream, and t is the temperature of the cold stream. As it is well known, to reach this thermal equilibrium, the heat transference area tends to infinite. Thus, the system has to consider a differential of temperature potential (considered as ΔTmin), which involves the cold and hot temperatures as follows in eq 15.
| 15 |
Equation 15 ensures that the process integration (and the heat transference) takes into account the restriction of the second law of thermodynamics. Otherwise, ΔTmin can be used to tradeoff the operating and capital costs. This study uses a ΔTmin = 10 °C, which has worked with reliable and positive outcomes for developed heat integration in previous works.65,81 The method follows with the construction of the cascade diagram, which gives a list of exchangeable loads for each temperature interval and the information about the minimum heating and cooling requirements.
The following step is the design of the heat exchange network that satisfies the identified minimum energy requirements. The method splits the problem between the heat exchange below the pinch and above it. The target value for the number of heat exchanger units to achieve potential savings is the sum of the number of units below and above the pinch point. The integration takes into account some matching rules considering the pinch point, the number of units below and above the pinch, the flow rates per specific heat (also called FCp) of each available stream, and the ΔTmin.
Sustainability Assessment Methodology
To determine the sustainability performance of the synthesized optimal biorefinery, the approach proposed in this study implies the evaluation of environmental, economic, safety, and energy parameters. As aforementioned, these technical indicators are evaluated using computer-aided process engineering (CAPE) tools. For accomplishing this goal, the SWROIMT is used to provide a single value that shows the overall sustainability performance. The calculation of this parameter follows the expression shown in eq 16.
| 16 |
wi is the weighting factors of sustainability indicator i; indicatori and indicatortarget are the current and target values of sustainability indicator i, respectively; AEP is the annual net profit; and TCI is the total capital investment. The assignation of values for wi mainly depends on the priority of the decision-makers.82 This study proposes some directions for establishing such weighted factors or assignations. It is crucial to understand how this weighting factor works; a value of wi = 1 means that the technical parameter has the same relevance for the designer as the annual net profit of the project (AEP), then values below this number are related to a lower importance, while a value above 1 implies a higher significance. The assignation for process safety parameters can be established considering the potential hazards derived from biorefinery operation, which can affect the engineers, operators, and staff. Also, depending on the size and the type of processes, the population surrounding the location of the plant can be affected, which would imply developing important safety and contingency measures. If the plant location is near to an urban area of a city with high population density, the importance of safety performance becomes maximum, so a project characterized by being high risk would negatively affect the economy and sustainability of the plant. This implies that the ISI indicator is set as negative within the SWROIM calculation. For urban areas, a wi = 0.5 is recommended, while for industrial and rural zones, the weighting factor takes values of 0.35 and 0.20, respectively. The above consideration is supported because process safety has been used as a tool for measuring the societal component83 in the triple dimension of sustainable development.18
The energy parameter related to the evaluation of process sustainability is established under the results of the energy integration developed for case B. The method applied allowed the optimization of heat streams of the process, achieving a significant reduction in the requirements of industrial utilities so that the savings of the energy integration positively affect the economic performance of the project, and the relative importance of energy criteria is based on the heat savings of the integration. If a design has a high demand for industrial utilities, the application of heat exchange networks becomes crucial. In this sense, the energy integration of those processes with lower requirements would be less relevant in the economy and performance of the sustainability of an engineering project. Taking into consideration the above, Table 17 reports the recommended values for wi in the case of energy integration.
Table 17. Recommended Weighting Factors for the Energy Parameter.
| energy requirements | relevance | wi |
|---|---|---|
| high | maximum | 0.40 |
| mid | moderate | 0.27 |
| low | moderate | 0.18 |
| very low | minimal | 0.10 |
The evaluation of sustainability contemplates the efforts for the reduction of GHG emissions in engineering projects. The carbon dioxide equivalent (CO2eq) is the unit used for estimating GHG emissions and is also a measure of GWP. This implies that the weighting factor for this parameter takes negative values, since high amounts of CO2eq have more significant impacts on the environment.84 Therefore, the assignation of wi for this criterion indicates the degree of compliance with the commitments entered in the framework of international environmental agreements, and for Colombia, the government regulates the economic activities and their impacts on the environment. Such normativity establishes sanctions for those companies that generate emissions above the permissible limits set by the law. Table 18 shows the recommended weighting factor for the environmental aspect.
Table 18. Recommended Weighting Factors for the Safety Parameter.
| compliance with environmental regulations | relevance | wi |
|---|---|---|
| high | maximum | 0.60 |
| mid | moderate | 0.45 |
| none | minimal | 0.25 |
Acknowledgments
The authors thank the Universidad de Cartagena and Fundación Universitaria Colombo Internacional for their support in the development of this research.
Glossary
Abbreviations
- TPA
tones per annum
- LA
luvulinic acid
- HMF
hydroxymethylfurfural
- ACD
acid-catalyzed dehydration
- NRTL
nonrandom two liquid
- PEI
potential environmental impact
- EPA
Environmental Protection Agency
- HTPI
human toxicity potential by ingestion
- HTPE
human toxicity potential by dermal exposure
- ATP
aquatic toxicity potential
- TTP
terrestrial toxicity potential
- GWP
global warming potential
- ODP
ozone depletion potential
- PCOP
photochemical oxidation potential
- AP
acidification potential
- CIS
chemical inherent safety
- PIS
process inherent safety
- ISI
inherent safety index
- FYP
fraction yield to product
- WAR
waste reduction algorithm
- TLV
threshold limit values
- ISBL
inside battery limits
- OSBL
outside battery limits
The Universidad de Cartagena supported this paper through the strengthening plan of the IDAB Group Act 069-2018.
The authors declare no competing financial interest.
References
- Tilak P.; El-Halwagi M. M. Process Integration of Calcium Looping with Industrial Plants for Monetizing CO2 into Value-Added Products. Carbon Resour. Convers. 2018, 1, 191–199. 10.1016/j.crcon.2018.07.004. [DOI] [Google Scholar]
- Rüstemoğlu H.; Rodriguez A. Determinants of CO2 Emissions in Brazil and Russia between 1992 and 2011: A Decomposition Analysis. Environ. Sci. Policy 2016, 58, 95–106. 10.1016/j.envsci.2016.01.012. [DOI] [Google Scholar]
- Costa C. B. B.; Potrich E.; Cruz A. J. G. Multiobjective Optimization of a Sugarcane Biorefinery Involving Process and Environmental Aspects. Renewable Energy 2016, 96, 1142–1152. 10.1016/j.renene.2015.10.043. [DOI] [Google Scholar]
- Hellsmark H.; Mossberg J.; Söderholm P.; Frishammar J. Innovation System Strengths and Weaknesses in Progressing Sustainable Technology: The Case of Swedish Biorefinery Development. J. Cleaner Prod. 2016, 131, 702–715. 10.1016/j.jclepro.2016.04.109. [DOI] [Google Scholar]
- Santibañez-Aguilar J. E.; González-Campos J. B.; Ponce-Ortega J. M.; Serna-González M.; El-Halwagi M. M. Optimal Planning of a Biomass Conversion System Considering Economic and Environmental Aspects. Ind. Eng. Chem. Res. 2011, 50, 8558–8570. 10.1021/ie102195g. [DOI] [Google Scholar]
- Cardona-Alzate C. A.; Solarte-Toro J. C.; Peña Á. Fermentation, Thermochemical and Catalytic Processes in the Transformation of Biomass through Efficient Biorefineries. Catal. Today 2018, 302, 61–72. 10.1016/j.cattod.2017.09.034. [DOI] [Google Scholar]
- Bao B.; Ng D. K. S.; Tay D. H. S.; Jimenez A.; El-Halwagi M. M. A Shortcut Method for the Preliminary Synthesis of Process-Technology Pathways: An Optimization Approach and Application for the Conceptual Design of Integrated Biorefineries. Comput. Chem. Eng. 2011, 35, 1374–1383. 10.1016/j.compchemeng.2011.04.013. [DOI] [Google Scholar]
- Sengupta D. Green.Chemistry and Chemical Engineering: Chemicals from Biomass: Integrating Bioprocesses into Chemical Production Complexes for Sustainable Development; CRC Press: London, GBR, 2012, ProQuest Ebrary. Web, 12 August 2015. Copyright 2015, August. [Google Scholar]
- Pham V.; El-Halwagi M. M. Process Synthesis and Optimization of Biorefinery Configuration. AIChE J. 2012, 58, 1212–1221. 10.1002/aic.12640. [DOI] [Google Scholar]
- González-Delgado Á.-D.; Kafarov V.; El-Halwagi M. Development of a Topology of Microalgae-Based Biorefinery: Process Synthesis and Optimization Using a Combined Forward-Backward Screening and Superstructure Approach. Clean Technol. Environ. Policy 2015, 17, 2213–2228. 10.1007/s10098-015-0946-5. [DOI] [Google Scholar]
- Bonatsos N.; Dheskali E.; Freire D. M. G.; de Castro A. M.; Koutinas A. A.; Kookos I. K. A Mathematical Programming Formulation for Biorefineries Technology Selection. Biochem. Eng. J. 2016, 116, 135–145. 10.1016/j.bej.2016.05.001. [DOI] [Google Scholar]
- Cambero C.; Sowlati T.; Pavel M. Economic and Life Cycle Environmental Optimization of Forest-Based Biorefinery Supply Chains for Bioenergy and Biofuel Production. Chem. Eng. Res. Des. 2016, 107, 218–235. 10.1016/j.cherd.2015.10.040. [DOI] [Google Scholar]
- Stefanis S. K.; Livingston A. G.; Pistikopoulos E. N. Minimizing the Environmental Impact of Process Plants: A Process Systems Methodology. Comput. Chem. Eng. 1995, 19, 39–44. 10.1016/0098-1354(95)87012-1. [DOI] [Google Scholar]
- Zondervan E.; Nawaz M.; de Haan A. B.; Woodley J. M.; Gani R. Optimal Design of a Multi-Product Biorefinery System. Comput. Chem. Eng. 2011, 35, 1752–1766. 10.1016/j.compchemeng.2011.01.042. [DOI] [Google Scholar]
- Shabbir Z.; Tay D. H. S.; Ng D. K. S. A Hybrid Optimisation Model for the Synthesis of Sustainable Gasification-Based Integrated Biorefinery. Chem. Eng. Res. Des. 2012, 90, 1568–1581. 10.1016/j.cherd.2012.02.015. [DOI] [Google Scholar]
- Andiappan V.; Ko A.; Lau V.; Ng L.; Ng R.; Chemmangattuvalappil N.; Ng D. Synthesis of Sustainable Integrated Biorefinery via Reaction Pathway. AIChE J. 2015, 61, 132–146. 10.1002/aic.14616. [DOI] [Google Scholar]
- Meramo-Hurtado S.-I.; González-Delgado Á.-D. Biorefinery Synthesis and Design Using Sustainability Parameters and Hierarchical/3D Multi-Objective Optimization. J. Cleaner Prod. 2019, 240, 118134 10.1016/j.jclepro.2019.118134. [DOI] [Google Scholar]
- Ruiz-Mercado G. J.; Smith R. L.; Gonzalez M. A. Sustainability Indicators for Chemical Processes: I. Taxonomy. Ind. Eng. Chem. Res. 2012, 51, 2309–2328. 10.1021/ie102116e. [DOI] [Google Scholar]
- Carvajal J. C.; Gómez Á.; Cardona C. A. Comparison of Lignin Extraction Processes: Economic and Environmental Assessment. Bioresour. Technol. 2016, 214, 468–476. 10.1016/j.biortech.2016.04.103. [DOI] [PubMed] [Google Scholar]
- García Carlos A.; Peña A.; Betancourt R.; Cardona C. A. Energetic and Environmental Assessment of Thermochemical and Biochemical Ways for Producing Energy from Agricultural Solid Residues: Coffee Cut-Stems Case. J. Environ. Manage. 2018, 216, 160–168. 10.1016/j.jenvman.2017.04.029. [DOI] [PubMed] [Google Scholar]
- Abusoglu A.; Kanoglu M. Exergetic and Thermoeconomic Analyses of Diesel Engine Powered Cogeneration: Part 1 - Formulations. Appl. Therm. Eng. 2009, 29, 234–241. 10.1016/j.applthermaleng.2008.02.025. [DOI] [Google Scholar]
- Ojeda K.; Sánchez E.; El-Halwagi M.; Kafarov V. Exergy Analysis and Process Integration of Bioethanol Production from Acid Pre-Treated Biomass: Comparison of SHF, SSF and SSCF Pathways. Chem. Eng. J. 2011, 176–177, 195–201. 10.1016/j.cej.2011.06.083. [DOI] [Google Scholar]
- Yang S.; Xiao L.; Yang S.; Kraslawski A.; Man Y.; Qian Y. Sustainability Assessment of the Coal/Biomass to Fischer-Tropsch Fuel Processes. ACS Sustainable Chem. Eng. 2014, 2, 80–87. 10.1021/sc400336e. [DOI] [Google Scholar]
- Luo L.; van der Voet E.; Huppes G. Biorefining of Lignocellulosic Feedstock - Technical, Economic and Environmental Considerations. Bioresour. Technol. 2010, 101, 5023–5032. 10.1016/j.biortech.2009.12.109. [DOI] [PubMed] [Google Scholar]
- Cheng J.; Lin R.; Ding L.; Song W.; Li Y.; Zhou J.; Cen K. Fermentative Hydrogen and Methane Cogeneration from Cassava Residues: Effect of Pretreatment on Structural Characterization and Fermentation Performance. Bioresour. Technol. 2015, 179, 407–413. 10.1016/j.biortech.2014.12.050. [DOI] [PubMed] [Google Scholar]
- Xiu S.; Shahbazi A. Bio-Oil Production and Upgrading Research: A Review. Renewable Sustainable Energy Rev. 2012, 16, 4406–4414. 10.1016/j.rser.2012.04.028. [DOI] [Google Scholar]
- Antonetti C.; Licursi D.; Fulignati S.; Valentini G.; Raspolli Galletti A. New Frontiers in the Catalytic Synthesis of Levulinic Acid: From Sugars to Raw and Waste Biomass as Starting Feedstock. Catalysts 2016, 6, 196 10.3390/catal6120196. [DOI] [Google Scholar]
- Lodi G.; De Guido G.; Pellegrini L. A. Simulation and Energy Analysis of the ABE Fermentation Integrated with Gas Stripping. Biomass Bioenergy 2018, 116, 227–235. 10.1016/j.biombioe.2018.06.012. [DOI] [Google Scholar]
- Colombia F. N. deB.de Indicatores. http://www.fedebiocombustibles.com. (accessed Dec 19, 2019).
- Ahlkvist J.Catalytic Conversion of Biomass to Biofuels, Umea University, 2014. [Google Scholar]
- SIMEC. Sistemade Informacion de Petroleo y Gas Colombiano. http://www.sipg.gov.co/?TabId=53&ctl=Privacy (accessed Dec 19, 2019).
- Hayes D. J.; Ross J.; Hayes M. H. B.; Fitzpatrick S. The Biofine Process: Production of Levulinic Acid, Furfural and Formic Acid from Lignocellulosic Feedstocks. Biorefineries: Ind. Processes Prod. 2005, 1, 54053349 10.1002/9783527619849.ch7. [DOI] [Google Scholar]
- UPME. Balance de Gas Natural En Colombia 2016–2025. Unidad de Planeación Minero Energética, 2016, No. 69, 33.
- Mejía J. G.; Acevedo C. A. Proyección Al Año 2025 Para El Uso Del Hidrógeno En El Sector Transporte Del Valle de Aburrá. Sci. Tech. 2013, 18, 327–334. 10.22517/23447214.8743. [DOI] [Google Scholar]
- Sengupta D.Green Chemistry and Chemical Engineering: Chemicals from Biomass: Integrating Bioprocesses into Chemical Production Complexes for Sustainable Development; CRC Press: London, GBR, 2012, ProQuest Ebrary. Web, 12 August 2015. Copyright 2015. [Google Scholar]
- Quintero J A.; Moncada J.; Cardona C. A. Techno-Economic Analysis of Bioethanol Production from Lignocellulosic Residues in Colombia: A Process Simulation Approach. Bioresour. Technol. 2013, 139, 300–307. 10.1016/j.biortech.2013.04.048. [DOI] [PubMed] [Google Scholar]
- Caramia M.; Dell’Olmo P.. Multi-Objective Management in Freight Logistics Increasing Capacity, Service Level and Safety with Optimization Algorithms; Springer, 2008; pp 11–36. [Google Scholar]
- Giarola S.; Bezzo F.; Shah N. A Risk Management Approach to the Economic and Environmental Strategic Design of Ethanol Supply Chains. Biomass Bioenergy 2013, 58, 31–51. 10.1016/j.biombioe.2013.08.005. [DOI] [Google Scholar]
- Wooley R.; Ruth M.; Sheehan J.; Majdeski H.; Galvez A.. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis Current and Futuristic Scenarios; Technical Report NREL/TP-510-32438, 1999; p 132.
- Kumar D.; Murthy G. S. Impact of Pretreatment and Downstream Processing Technologies on Economics and Energy in Cellulosic Ethanol Production. Biotechnol. Biofuels 2011, 4, 27 10.1186/1754-6834-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirbas A.Biorefineries: For Biomass Upgrading Facilities; Springer: Trabzon, 2010. [Google Scholar]
- Kang Q.; Huybrechts J.; Van Der Bruggen B.; Baeyens J.; Tan T.; Dewil R. Hydrophilic Membranes to Replace Molecular Sieves in Dewatering the Bio-Ethanol/Water Azeotropic Mixture. Sep. Purif. Technol. 2014, 136, 144–149. 10.1016/j.seppur.2014.09.009. [DOI] [Google Scholar]
- Okino S.; Noburyu R.; Suda M.; Jojima T.; Inui M.; Yukawa H. An Efficient Succinic Acid Production Process in a Metabolically Engineered Corynebacterium glutamicum Strain. Appl. Microbiol. Biotechnol. 2008, 81, 459–464. 10.1007/s00253-008-1668-y. [DOI] [PubMed] [Google Scholar]
- Olajuyin A. M.; Yang M.; Liu Y.; Mu T.; Tian J.; Adaramoye O. A.; Xing J. Efficient Production of Succinic Acid from Palmaria Palmata Hydrolysate by Metabolically Engineered Escherichia coli. Bioresour. Technol. 2016, 214, 653–659. 10.1016/j.biortech.2016.04.117. [DOI] [PubMed] [Google Scholar]
- Xin F.; Wu Y. R.; He J. Simultaneous Fermentation of Glucose and Xylose to Butanol by Clostridium Sp. Strain BOH3. Appl. Environ. Microbiol. 2014, 80, 4771–4778. 10.1128/AEM.00337-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N.; Singh V.; Liu S.; Ezeji T. C.; Saha B. C.; Cotta M. A. Process Integration for Simultaneous Saccharification, Fermentation, and Recovery (SSFR): Production of Butanol from Corn Stover Using Clostridium beijerinckii P260. Bioresour. Technol. 2014, 154, 222–228. 10.1016/j.biortech.2013.11.080. [DOI] [PubMed] [Google Scholar]
- Haigh K. F.; Petersen A. M.; Gottumukkala L.; Mandegari M.; Naleli K.; Görgens J. F. Simulation and Comparison of Processes for Biobutanol Production from Lignocellulose via ABE Fermentation. Biofuels, Bioprod. Biorefin. 2018, 12, 1023–1036. 10.1002/bbb.1917. [DOI] [Google Scholar]
- Naleli K.Process Modelling In Production of Biobutanol from Lignocellulosic Biomass via ABE Fermentation. Master’s Thesis, 2016. [Google Scholar]
- Colyer L.Fermentation 2015: The ABE Process. Spec. Chem. Mag. 2015. [Google Scholar]
- Kaymak D. B. Design and Control of an Alternative Process for Biobutanol Purification from ABE Fermentation. Ind. Eng. Chem. Res. 2019, 58, 1957–1965. 10.1021/acs.iecr.8b03818. [DOI] [Google Scholar]
- Maria A.; Galletti R.; Antonetti C.; De Luise V.; Licursi D.; Nassi N.; Nasso D. Levulinic Acid Production From Waste Biomass. BioResources 2012, 7, 1824–1835. 10.15376/biores.7.2.1824-1835. [DOI] [Google Scholar]
- Frankiewicz A. Overview of 4-Oxopentanoic (Levulinic) Acid Production Methods - an Intermediate in the Biorefinery Process. Chemik 2016, 70, 206–208. [Google Scholar]
- Levine J.; Lakshmanan A.; Peers Z.. Jump Start: Modeling Convective Dryers 2013, pp 13.
- Alamia A.; Lind F.; Thunman H.. Hydrogen from Biomass Gasification for Utilization in Oil Refineries 2012, no (1), , pp 2–3.
- Meramo-Hurtado S.; Ojeda-Delgado K.; Sanchez-Tuiran E. Environmental Assessment of a Biorefinery: Case Study of a Purification Stage in Biomass Gasification. Contemp. Eng. Sci. 2018, 11, 113–120. 10.12988/ces.2018.813. [DOI] [Google Scholar]
- Meramo-Hurtado S.; Alarcon C.; Gonzalez A. Exergetic Sensibility Analysis and Environmental Evaluation of Chitosan Production from Shrimp Exoskeleton in Colombia. J. Cleaner Prod. 2019, 248, 119285 10.1016/j.jclepro.2019.119285. [DOI] [Google Scholar]
- Herrera-Aristizábal R.; Salgado-Dueñas J. S.; Peralta-Ruiz Y. Y.; González-Delgado ÁD. Environmental Evaluation of a Palm-Based Biorefinery under North-Colombian Conditions. Chem. Eng. Trans. 2017, 57, 193–198. 10.3303/CET1757033. [DOI] [Google Scholar]
- Hernández V.; Romero-García J. M.; Dávila J. A.; Castro E.; Cardona C. A. Techno-Economic and Environmental Assessment of an Olive Stone Based Biorefinery. Resour., Conserv. Recycl. 2014, 92, 145–150. 10.1016/j.resconrec.2014.09.008. [DOI] [Google Scholar]
- Palaniappan C.; Srinivasan R.; Tan R. Selection of Inherently Safer Process Routes: A Case Study. Chem. Eng. Process. Process Intensif. 2004, 43, 647–653. 10.1016/j.cep.2002.12.001. [DOI] [Google Scholar]
- Heikkila A.-M.Inherent Safety in Process Plant Design; VTT Publications, 1999; pp 1–132. [Google Scholar]
- de Jong E.; Gosselink R. J. A.. Lignocellulose-Based Chemical Products; Elsevier, 2014. [Google Scholar]
- Haynes International. HASTELLOY C-2000 Alloy, 2017.
- Oh S. Y.; Yun S.; Kim J. K. Process Integration and Design for Maximizing Energy Efficiency of a Coal-Fired Power Plant Integrated with Amine-Based CO2 capture Process. Appl. Energy 2018, 216, 311–322. 10.1016/j.apenergy.2018.02.100. [DOI] [Google Scholar]
- El-Halwagi M. M.Sustainable Design Through Process Integration: Fundamentals and Applications to Industrial Pollution Prevention, Resource Conservation, and Profitability Enhancement; Elsevier, 2012. [Google Scholar]
- Lopez-Castrillon C.; Leon J. A.; Palacios-Bereche M. C.; Palacios-Bereche R.; Nebra S. A. Improvements in Fermentation and Cogeneration System in the Ethanol Production Process: Hybrid Membrane Fermentation and Heat Integration of the Overall Process through Pinch Analysis. Energy 2018, 156, 468–480. 10.1016/j.energy.2018.05.092. [DOI] [Google Scholar]
- Mangili P. V.; Prata D. M. Improvement of the Butyl Acetate Process through Heat Integration: A Sustainability-Based Assessment. Chem. Eng. Process. Process Intensif. 2019, 135, 93–107. 10.1016/j.cep.2018.11.020. [DOI] [Google Scholar]
- El-Halwagi M. M. A Return on Investment Metric for Incorporating Sustainability in Process Integration and Improvement Projects. Clean Technol. Environ. Policy 2017, 19, 611–617. 10.1007/s10098-016-1280-2. [DOI] [Google Scholar]
- Meramo S.; Ojeda K. A.; Sánchez E. L. Integrated Biorefinery from Corn Waste Biomass: A Case Study in the North of Colombia. Int. J. ChemTech Res. 2018, 11, 33–40. [Google Scholar]
- Igbinadolor R. O.; Onilude A. A. Bioprocess Systems Applied for the Production of Bio-Ethanol from Lignocellulosic Biomass of Cocoa Pod Husk (Theobroma cacao L.) and Other Agricultural Residues: A Review. Afr. J. Biotechnol. 2016, 12, 5375–5388. 10.5897/AJB2013.12890. [DOI] [Google Scholar]
- Meramo-Hurtado S.; Urbina-Suarez N.; González-Delgado Á. Computer-Aided Environmental and Exergy Analyses of a Large-Scale Production of Chitosan Microbeads Modified with TiO2 Nanoparticles. J. Cleaner Prod. 2019, 237, 117804 10.1016/j.jclepro.2019.117804. [DOI] [Google Scholar]
- Young D.; Scharp R.; Cabezas H. The Waste Reduction (WAR) Algorithm: Environmental Impacts, Energy Consumption, and Engineering Economics. Waste Manag. 2000, 20, 605–615. 10.1016/S0956-053X(00)00047-7. [DOI] [Google Scholar]
- Young D. M.; Cabezas H. Designing Sustainable Processes with Simulation: The Waste Reduction (WAR) Algorithm. Comput. Chem. Eng. 1999, 23, 1477–1491. 10.1016/S0098-1354(99)00306-3. [DOI] [Google Scholar]
- Moreno-Sader K.; Meramo-Hurtado S. I.; González-Delgado A. D. Computer-Aided Environmental and Exergy Analysis as Decision-Making Tools for Selecting Bio-Oil Feedstocks. Renewable Sustainable Energy Rev. 2019, 112, 42–57. 10.1016/j.rser.2019.05.044. [DOI] [Google Scholar]
- Petrescu L.; Cormos C. C. Waste Reduction Algorithm Applied for Environmental Impact Assessment of Coal Gasification with Carbon Capture and Storage. J. Cleaner Prod. 2015, 104, 220–235. 10.1016/j.jclepro.2014.08.064. [DOI] [Google Scholar]
- Arteaga-Díaz S. J.; Meramo-Hurtado S. I.; León-Pulido J.; Zuorro A.; González-Delgado A. D. Environmental Assessment of Large Scale Production of Magnetite (Fe3O4) Nanoparticles via Coprecipitation. Appl. Sci. 2019, 9, 1682 10.1016/j.jclepro.2019.117804. [DOI] [Google Scholar]
- González-Delgado Á.; Peralta-Ruiz Y. A Hierarchical Techno-Economic Sensitivity Approach for Evaluation of Agroindustrial Production Chains. Int. J. ChemTech Res. 2017, 10, 921–929. [Google Scholar]
- Do T. X.; Lim Y.-i.; Yeo H. Techno-Economic Analysis of Biooil Production Process from Palm Empty Fruit Bunches. Energy Convers. Manag. 2014, 80, 525–534. 10.1016/j.enconman.2014.01.024. [DOI] [Google Scholar]
- Gangadharan P.; Singh R.; Cheng F.; Lou H. H. Novel Methodology for Inherent Safety Assessment in the Process Design Stage. Ind. Eng. Chem. Res. 2013, 52, 5921–5933. 10.1021/ie303163y. [DOI] [Google Scholar]
- Li J.; Demirel S. E.; Hasan M. M. F. Process Integration Using Block Superstructure. Ind. Eng. Chem. Res. 2018, 57, 4377–4398. 10.1021/acs.iecr.7b05180. [DOI] [Google Scholar]
- Diban P.; El-Halwagi M. M.; Foo D. C. Y. A Decomposition-Based Approach for the Optimum Integration of Heating Utility and Phase Separation Systems in Oil and Gas Platform. Ind. Eng. Chem. Res. 2019, 58, 21584–21601. 10.1021/acs.iecr.9b03205. [DOI] [Google Scholar]
- Sánchez E.; Ojeda K.; El-Halwagi M.; Kafarov V. Biodiesel from Microalgae Oil Production in Two Sequential Esterification/Transesterification Reactors: Pinch Analysis of Heat Integration. Chem. Eng. J. 2011, 176–177, 211–216. 10.1016/j.cej.2011.07.001. [DOI] [Google Scholar]
- Sikdar S. K.; Sengupta D.; Mukherjee R.. Measuring Progress towards Sustainability: A Treatise for Engineers; Springer-Verlag, 2016. [Google Scholar]
- Jafari M. J.; Mohammadi H.; Reniers G.; Pouyakian M.; Nourai F.; Torabi S. A.; Rafiee Miandashti M. Exploring Inherent Process Safety Indicators and Approaches for Their Estimation: A Systematic Review. J. Loss Prev. Process Ind. 2018, 52, 66–80. 10.1016/j.jlp.2018.01.013. [DOI] [Google Scholar]
- Rodrigues Gurgel da Silva A.; Giuliano A.; Errico M.; Rong B. G.; Barletta D. Economic Value and Environmental Impact Analysis of Lignocellulosic Ethanol Production: Assessment of Different Pretreatment Processes. Clean Technol. Environ. Policy 2019, 21, 637–654. 10.1007/s10098-018-01663-z. [DOI] [Google Scholar]