Abstract
In this paper, poly-α-olefins (PAO) containing quaternary carbon centers were prepared by two-step oligomerization using a metallocene catalyst followed by a Ziegler–Natta catalyst. First, the 1-decene dimer was oligomerized with [t-BuN(Me)2C(η5-C5H4)]ZrCl2, and the effects of the oligomerization temperature, Al/Zr molar ratio, and catalyst loading on the oligomerization were investigated. In the second step, the obtained 1-decene dimers were copolymerized with 1-decene with TiCl4/Et2AlCl, and the effects of the catalysts, monomer/dimer ratio, and α-olefin species on the copolymerization were investigated. The composition and structure of the dimers and copolymerization products were characterized by gas chromatography (GC) and 1H NMR and 13C NMR spectroscopy. The results of GC and 13C NMR analyses indicated that the metallocene catalyzed the formation of the 1-decene oligomerization product, resulting in the branched olefin dimer being the major product, and the existence of quaternary carbons in the 1-decene/1-decene dimer copolymerization product could also be found. The polymerization mechanism for the formation of the quaternary carbon centers is proposed. The 1-decene/1-decene dimer copolymerization product containing quaternary carbon centers has a kinematic viscosity of 10.8 mm2/s at 100 °C, a viscosity index of 165, and a pour point of −52 °C; thus, the product with quaternary carbon centers has a better viscosity–temperature performance and low-temperature fluidity than those of the 1-decene oligomerization product and typical PAO products, but the kinematic viscosity is similar.
Introduction
The synthetic lubricating base oil poly-α-olefin (PAO) has the advantages of high viscosity index, excellent low-temperature performance, good high-temperature oxidation stability, minimal environmental impact, low energy consumption, and long working lifetime, and it is widely used in the automobile industry, machinery industry, and aerospace industry.1,2 The monomers used in the synthesis of PAO are mainly linear C8–C12 α-olefins, and of these, PAO synthesized from 1-decene shows the best performance. PAOs synthesized from linear α-olefins usually have a wide product distribution, and in practice, the α-olefin dimer has problems such as high volatility; therefore, dimers of the α-olefin are usually removed after the completion of polymerization.3 This not only increases processing costs but also leads to a substantial waste of valuable materials. Therefore, developing a method to prepare PAOs with excellent performance using α-olefin dimers as reactants is of great importance. Kissin and Schwab4 separated dimers and trimers from an α-olefin oligomerization product mixture, and the separated vinylidene-containing dimers and trimers were polymerized by a cationic catalyst. The obtained polymerization product had excellent performance, and approximately 80% of the components in the product were C40 hydrocarbons. Nifant’ev et al.5 validated the results reported by Yury, and the oligomerization products from the oligomer have a “star-branched configuration”, meaning that the hydrocarbons have one or two alkyl groups of 6–10 carbons each attached near the center of normal paraffin, such as a trimer of 1-decene, and star-branched oligomers containing a quaternary carbon center have been shown to have the best lubricating properties.1,6−8 Nelson and Heckelsberg9 used a two-step method to obtain the product of this star-branched oligomer. In the first step, a mixture of 1-octene and 1-decene monomers was reacted using a ruthenium catalyst to produce C14 and C16 species as well as a C18 species with an internal olefin. In the second step, a C28–36 olefin product containing a star-branched oligomer was obtained by the BF3-catalyzed polymerization of the internal olefin obtained in the first step, but the ratio of the conversion of the internal olefin formed in the first step to the internal olefin selectivity was relatively low. The performance of the polymerization product obtained from the second step was affected, and its viscosity index could only range from 105 to 134. Evans et al.10 and Vautravers et al.11 used different catalysts to obtain dimers of olefins. Blewett and Turner12 provided a process whereby the dimer fraction obtained from a boron-trifluoride-catalyzed oligomerization process is reacted with an α-olefin in the presence of a phosphoric-acid-modified boron trifluoride catalyst to produce higher oligomeric products typically having viscosities (210 °F) in the 4–8 centiStoke (cSt) range. Their research has shown us the possibility to use α-olefin dimers as reactants for further polymerization. Dong et al.13 used a metallocene catalyst followed by a Ziegler–Natta catalyst to prepare a single-component polymer with an α-olefin tetramer content of 89.6% by the oligomerization of an α-olefin dimer. The product was shown to have a star-branched structure and contain α-olefins with quaternary carbon centers. Sadjadi et al.14 used an AlCl3/H2O catalytic system to oligomerize 1-hexene, 1-octene, and 1-decene, and the relationship between the monomer of the PAO homopolymer and CH + CH2/CH3 (S1/S2, the ratio of peak areas in the 1H NMR spectrum) was found by molecular weight analysis of the product, and this result provides a new platform for considering the relationships among the PAO molecular structure, the composition, and the performance. At present, there are few studies on α-olefin monomer/dimer copolymerization, and there are few studies on the relationships among the catalytic method, the PAO microstructure, and the properties of the product.
In this paper, vinylidene-containing 1-decene dimers were oligomerized by [t-BuN(Me)2C(η5-C5H4)]ZrCl2 and then the obtained 1-decene dimers were copolymerized with 1-decene with TiCl4/Et2AlCl, thus providing a method for synthesizing PAOs containing quaternary carbon centers using an α-olefin dimer as a reactant. The relationships among the product composition, structure, and properties were investigated. Further research results suggested that the good viscosity–temperature performance and the excellent low-temperature fluidity of the 1-decene/1-decene dimer copolymerization product can be attributed to the presence of quaternary carbon centers. The polymerization mechanism to form the quaternary carbon centers is proposed according to the gas chromatography (GC), 1H NMR, and 13C NMR characterization results.
Results and Discussion
Effect of Reaction Conditions on the Oligomerization of 1-Decene
The effects of the oligomerization conditions on the oligomerization of 1-decene were investigated. The results are presented in Table 1. Since the catalyst occlusion angle α is only 89.9°,15 it can be regarded as an open structure, indicating there is substantial space around the active center for polymerization and that the 1-decene monomer can freely coordinate in this large space. This leads to a relatively high conversion rate and dimerization selectivity.16
Table 1. Effect of Conditions on the Oligomerization of 1-Decenea.
| run | catalyst loading (μmol) | nAl/nZr | T (°C) | conversion rate (%) | dimer in products (wt %) |
|---|---|---|---|---|---|
| 1 | 10 | 300 | 70 | 84.3 | 68.6 |
| 2 | 30 | 300 | 70 | 84.4 | 70.5 |
| 3 | 50 | 300 | 70 | 89.4 | 72.6 |
| 4 | 90 | 300 | 70 | 88.5 | 82.3 |
| 5 | 90 | 200 | 70 | 80.3 | 81.9 |
| 6 | 90 | 100 | 70 | 77.2 | 81.6 |
| 7 | 90 | 50 | 70 | 76.6 | 80.6 |
| 8 | 90 | 50 | 80 | 83.4 | 82.9 |
| 9 | 90 | 50 | 90 | 56.8 | 87.0 |
| 10 | 90 | 50 | 100 | 36.9 | 89.2 |
Catalyst, [t-BuN(Me)2C(η5-C5H4)]ZrCl2. Reaction conditions: 1-decene, 50 mL; time, 3 h; and cocatalyst, methylaluminoxane (MAO).
As the catalyst loading was increased, the 1-decene monomer conversion rate and the dimer ratio increased gradually because the number of available catalytic active sites increased (Table 1, runs 1–4). As the number of catalytic active sites increased, a greater number of 1-decene monomers can initiate polymerization, resulting in shorter molecular chains and lower molecular weight. As the molar ratio of Al/Zr increases, the content of alkyl aluminum in the MAO increases, a great number of stable active sites can form in the catalytic system and the monomer conversion increases. MAO itself is also a chain-transfer agent, and excess MAO will hinder the formation of Zr active sites. For the above two reasons, the increase in the proportion of dimers formed was not significant when excess MAO was added (Table 1, runs 4–7). Increasing the temperature caused the chain-transfer rate to increase, which facilitates the diffusion and oligomerization of the monomer. When the temperature was further increased, the conversion rate decreased (Table 1, runs 7–10). The active site of the catalyst is gradually deactivated due to excessive temperature. Therefore, as long as the catalyst is thermally stable, suitable increases in temperature will facilitate the oligomerization of the monomer.
Structural Analysis of the α-Olefin Dimer
Under optimal reaction conditions, the metallocene ([t-BuN(Me)2C(η5-C5H4)]ZrCl2) catalyzed the oligomerization of α-olefins to afford a mixture of the corresponding oligomers, and after distillation under reduced pressure to remove the dimer, the content and structure of the dimer were determined. The results are shown in Figure 1–3.
Figure 1.
GC chromatogram of the 1-decene dimer.
Figure 3.
(a–c) Three different structures of 1-decene olefin dimers.
As seen from the analysis in Figure 1, distilling the oligomer mixture under reduced pressure afforded a small amount (3.6%) of 1-decene trimer (C30) in addition to the 1-decene dimer (C20). The unsaturated C20 olefins accounted for 91.3% of the material, and saturated alkanes (which were not affected by the second polymerization) accounted for 5.1% of the material. The 1-olefins and isomerized C20 olefins can be used for further polymerization.
Figure 2 shows the 13C NMR spectrum of the dimer (C20) obtained by the oligomerization of 1-decene. The 13C NMR spectrum shows that three distinct 1-decene dimers (Figure 3a–c) were formed by catalytic [t-BuN(Me)2C(η5-C5H4)]ZrCl2, and they each contain a double bond that can be used for the second step of the copolymerization with 1-decene, which is consistent with the results of GC and 1H NMR (Figure S1, Supporting Information). Combined with the GC results, the alkane accounts for a small amount of the dimer and does not affect the subsequent polymerization with TiCl4/Et2AlCl. Therefore, there will be no further discussion of the alkane dimers. According to the integration of the quantitative carbon spectrum, the a/b/c ratio is 1:0.05:0.04, and the 1-olefin dimer (dimer (a)) accounts for an extremely large proportion of the dimers obtained by oligomerization of 1-decene using [t-BuN(Me)2C(η5-C5H4)]ZrCl2. Based on the above analysis, the contents of the dimers are presented in Table 2. When a conversion rate of >83% is maintained, the dimerization selectivity is >82% and the 1-olefin dimer accounts for >88% of the product. This indicates that the metallocene catalyst ([t-BuN(Me)2C(η5-C5H4)]ZrCl2) is a highly selective dimerization catalyst under the developed conditions, the product uniformity is high, and the structure is quite regular, making the oligomerization dimers an ideal raw material for preparing lubricating base oil.
Figure 2.
13C NMR spectrum of C20.
Table 2. Oligomerization of α-Olefin and Proportions of Dimersa.
| α-olefin | conv. after 3 h, (%) | dimer in products (wt %)b | dimer in the separator (wt %)c | 1-olefin in the dimer (wt %)d | 1-olefin in the separator (wt %)e |
|---|---|---|---|---|---|
| 1-decene | 83.4 | 82.9 | 96.4 | 91.7 | 88.4 |
Reactionconditions: α-olefin, 50 mL; metallocene catalyst, 90 μmol; nAl/nZr= 50:1; T, 80 °C; and t, 3 h.
Determined by GC.
Determined by GC.
Determined by 13C NMR spectrocopy.
e = c × d.
α-Olefin Monomer/Dimer Copolymerization
The effects of the α-olefin species on the results of the TiCl4/Et2AlCl-catalyzed α-olefin monomer/dimer copolymerization were investigated, and the properties of the copolymerization products were compared with those of typical PAOs. When the reactants are 1-hexene, 1-octene, 1-decene, and their dimers, the copolymerization products all show relatively high viscosity index values and conversion rates (Table 3, runs 1–3). The kinematic viscosity of the copolymer of the 1-decene/1-decene dimer at 100 °C is significantly higher than those of the products obtained from 1-hexene and 1-octene. This is because the copolymer of 1-decene has longer branches. As the interactions between the molecules and the adhesion between the liquid and the solid wall increase, the difference between the moving speeds of the inner and outer layers increases and the frictional resistance between adjacent liquid layers increases, which leads to an increase in the viscosity.17 The product of the TiCl4/Et2AlCl-catalyzed monomer/dimer copolymerization of 1-hexene and 1-octene has a higher viscosity index than that of the typical PAO63 (Table 3, runs 1, 2, and 4). When the kinematic viscosity is similar, the low-temperature fluidity is significantly improved (Table 3, runs 2 and 4). Due to the presence of quaternary carbon centers, oligomers with a lower polymerization degree have a smaller radius of gyration than that of oligomers with a higher polymerization degree and will have a greater impact on the low-temperature fluidity of the PAOs. 1-Octene PAOs have more trimer and tetramer contents than those of 1-hexene PAOs, which can better improve the low-temperature fluidity and lead to a lower pour point for the same viscosity grade (Figure S2, Supporting Information). The 1-decene/1-decene dimer copolymerization product also showed a higher viscosity index and lower pour point than those of the typical PAO103 (Table 3, runs 3 and 5). In conclusion, the TiCl4/Et2AlCl-catalyzed α-olefin monomer/dimer copolymerization products have excellent performance, a high viscosity index, and a low pour point and can be used as a high-quality lubricant base oil.
Table 3. Effect of the α-Olefin Species on the α-Olefin Monomer/Dimer Copolymerizationa.
| run | catalyst | α-olefin | KV100 (cSt) | viscosity index | conversion rate (%) | PP (°C) |
|---|---|---|---|---|---|---|
| 1 | TiCl4/Et2AlCl | 1-hexene | 5.0 | 183 | 84.3 | –54 |
| 2 | TiCl4/Et2AlCl | 1-octene | 5.4 | 190 | 81.2 | –60 |
| 3 | TiCl4/Et2AlCl | 1-decene | 10.8 | 165 | 70.7 | –52 |
| 4 | PAO63 | 5.9 | 139 | –54 | ||
| 5 | PAO103 | 10.1 | 133 | –45 |
Runs 1–3: TiCl4 = 4 wt %; V (solvent)/V (α-olefin) = 1:4; mol (dimer)/mol (monomer) = 1:2; nAl/nTi = 0.5; reaction temperature, 60 °C; and reaction time, 4 h.
Table 4 shows the viscosity–temperature performance and pour point data of the products of the TiCl4/Et2AlCl-catalyzed copolymerization reactions with different ratios of the 1-decene/1-decene dimer. Also, the experiments were also carried out using AlCl3 as the catalyst for comparison. In general, the viscosity index of the 1-decene/1-decene dimer copolymer obtained with catalytic TiCl4/Et2AlCl is higher than that of the product obtained with AlCl3 and the low-temperature fluidity is better. This is because the coordination polymerization process with the Ziegler–Natta catalyst has stereotactic selectivity, resulting in a product with higher stereoregularity.
Table 4. Effect of the C20/C10 Ratio on the Copolymerization Resultsa,b,c.
| run | catalyst | m (dimer)/m (monomer) | conversion rate (%) | KV100 (cSt) | viscosity index | PP (°C) |
|---|---|---|---|---|---|---|
| 1 | TiCl4/Et2AlCl | 1:0 | 72.2 | 6.3 | 144 | –62 |
| 2 | TiCl4/Et2AlCl | 2:1 | 64.5 | 7.0 | 162 | –58 |
| 3 | TiCl4/Et2AlCl | 1:1 | 61.5 | 7.7 | 167 | –58 |
| 4 | TiCl4/Et2AlCl | 1:2 | 70.7 | 10.8 | 165 | –52 |
| 5 | TiCl4/Et2AlCl | 1:3 | 59.7 | 12.7 | 175 | –50 |
| 6 | TiCl4/Et2AlCl | 0:1 | 67.9 | 26.0 | 169 | –31 |
| 7 | TiCl4/Et2AlCl | 0:1 | 55.4 | 12.2 | 163 | –44 |
| 8 | AlCl3 | 1:0 | 88.2 | 8.9 | 141 | –46 |
| 9 | AlCl3 | 2:1 | 70.5 | 10.2 | 152 | –42 |
| 10 | AlCl3 | 1:1 | 78.4 | 10.0 | 136 | –42 |
| 11 | AlCl3 | 1:2 | 88.3 | 10.4 | 137 | –41 |
| 12 | AlCl3 | 1:3 | 76.7 | 10.2 | 132 | –41 |
| 13 | AlCl3 | 0:1 | 81.5 | 10.5 | 126 | –41 |
Runs 1–6: TiCl4 = 4 wt %; V (solvent)/V (1-decene) = 1:4; nAl/nTi = 0.5; reaction temperature, 60 °C; and reaction time, 4 h.
Runs 7: TiCl4 = 2.5 wt %; V (solvent)/V (1-decene) = 1:4; nAl/nTi = 0.5; reaction temperature, 80 °C; and reaction time, 4 h.
Runs 8–13: AlCl3 = 4 wt %; V (solvent)/V (1-decene) = 1:4; reaction temperature, 60 °C; and reaction time, 4 h.
The effects of the 1-decene/1-decene dimer ratio on the viscosity–temperature performance and pour point data of the products obtained with catalytic TiCl4/Et2AlCl are presented in Table 4, runs 1–7. When the kinematic viscosities of the products at 100 °C are similar, the viscosity index of the polymerization product obtained using the 1-olefin dimer as the reactant (Table 4, run 3) is higher than that of the 1-decene oligomerization product (Table 4, run 5), and the pour point of the product obtained in run 3 is lower than that of the oligomerization product. The improvements in the viscosity–temperature behavior and the low-temperature fluidity are related to the microstructure of the polymerization product.1,7−9,13 As the content of 1-olefin dimer in the reaction mixture decreases, the kinematic viscosity at 100 °C and the pour point of the product obtained with catalytic TiCl4/Et2AlCl increase (Table 4, runs 1–6). The steric hindrance causes 1-decene to polymerize to a higher degree than that seen with the 1-decene dimer, and it leads to an increase in the degree of polymerization and the molecular weight of the product, causing the kinematic viscosity to increase and the low-temperature fluidity to decrease. As the 1-olefin dimer content in the reaction mixture decreased, the viscosity index of the product tended to increase, which is caused by the increase in the viscosity index that occurs as the product’s kinematic viscosity increases18,19 (Table 4, runs 2–5).
The performance data of the AlCl3-catalyzed 1-decene/1-decene dimer copolymerization products are shown in Table 4, runs 8–13. The rapid chain transfer in the process of AlCl3-catalyzed polymerization and its resulting “light polymer” have a significant impact on the copolymerization products of the 1-decene/1-decene dimer.20,21 Due to the presence of the block dimer in the PAO, the copolymerization products with different 1-decene/1-decene dimer ratios have different viscosity indexes. These phenomena are worthy of more experimental and theoretical research in subsequent work. In conclusion, TiCl4/Et2AlCl is the more suitable catalyst to copolymerize the 1-decene/1-decene dimer than AlCl3 because of the TiCl4/Et2AlCl-catalyzed products’ better viscosity–temperature performance and low-temperature fluidity.
Structure and Properties of 1-Decene/1-Decene Dimer Copolymerization Products
Figure 4 shows the GC chromatograph of the TiCl4/Et2AlCl-catalyzed 1-decene/1-decene dimer copolymerization product. After distillation under reduced pressure, the obtained product contained almost no dimer. The peak at 19.23 min can be attributed to the 1-decene trimer, and the peak at 24.188 min can be attributed to the 1-decene tetramer. The pentamer and hexamer correspond to the peaks at 27.491 and 30.185 min, respectively. The main components in the TiCl4/Et2AlCl-catalyzed 1-decene/1-decene dimer copolymerization product are trimers and tetramers.
Figure 4.
GC chromatograph of the TiCl4/Et2AlCl-catalyzed C20/C10 copolymerization product. Polymerization conditions: TiCl4 = 4 wt %; V (solvent)/V (1-decene) = 1:4; m (C20)/m (C10) = 1:2; nAl/nTi = 0.5; reaction temperature, 60 °C; and reaction time, 4 h.
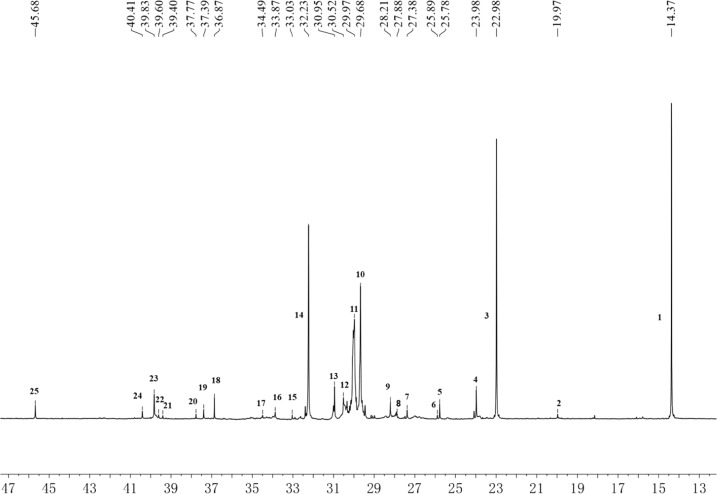
The 13C NMR spectrum of the product (Figure 5) showed that the trimer and tetramer were obtained in the TiCl4/Et2AlCl-catalyzed 1-decene/1-decene dimer copolymerization (Figure 6). There are four main trimer and tetramer structures: olefins and alkanes with a head-to-tail structure and olefins and alkanes with a star-branched structures containing a quaternary carbon. The chemical shift of the quaternary carbon in the 1-decene trimer alkane is 30.95 ppm, the chemical shift of the methyl branched-CH3 connected to the quaternary carbon is 25.78 ppm, and the chemical shifts of the α-CH2 and β-CH2 groups connected to the quaternary carbon are 39.40 and 23.98 ppm, respectively. The quaternary carbon in the star-branched 1-decene trimer olefin has a chemical shift of 27.88 ppm due to the presence of the double bond. The chemical shifts of the remaining carbons are similar to those in the corresponding alkane structures. The chemical shifts of α-CH2 connected to CH groups in 1-decene tetramer alkanes are 45.68 and 36.87 ppm. The chemical shifts of the quaternary carbon and −CH3 group carbon in the 1-decene star-branched tetramer olefin are 27.38 and 25.89 ppm, respectively, due to the presence of double bonds, and the chemical shifts of the remaining carbons are also similar to those of the corresponding alkane.
Figure 5.
13C NMR spectrum of the TiCl4/-Et2AlCl-catalyzed copolymerization products.
Figure 6.
(a) Trimer in the C20/C10 copolymerization product mixture obtained with catalytic TiCl4/Et2AlCl. (b) Tetramer in the C20/C10 copolymerization product mixture obtained with catalytic TiCl4/Et2AlCl. Polymerization conditions: TiCl4 = 4 wt %; V (solvent)/V (1-decene) = 1:4; m (C20)/m (C10) = 1:2; nAl/nTi = 0.5; reaction temperature, 60 °C; and reaction time, 4 h.
The 13C NMR spectrum shows that one of the products of the TiCl4/Et2AlCl-catalyzed 1-decene/1-decene dimer copolymerization contains a quaternary carbon center. This quaternary carbon will cause the product to have excellent viscosity–temperature performance and a low pour point1,7−9,13 (Table 4). This is because products containing quaternary carbons have more carbon atoms distributed on the branch chains, the product is structurally more compact, and the product has a higher degree of linearity with a linear polymer backbone and other branches. The polymers with quaternary carbon centers have a smaller rotation radius than polymers without such centers9 and have weaker interactions with adjacent molecular segments and a smaller sweep volume, which gives products with quaternary carbon centers excellent low-temperature flow performance. Besides, further research shows that products containing quaternary carbons have better oxidation stability compared to that of the commercial PAO base oil because of the saturation of quaternary carbons (Table S1, Supporting Information).
Mechanism of the Polymerization to form Quaternary Carbon Centers
Due to the high content of the 1-olefin dimer in the reactant, it can be speculated that not only do the trimeric and tetrameric products from the TiCl4/Et2AlCl-catalyzed 1-decene/1-decene dimer copolymerization contain a quaternary carbon center but also there must be a quaternary carbon structure in the pentameric and higher oligomers. Therefore, the reaction mechanism of quaternary carbon formation in the 1-decene/1-decene dimer copolymerization catalyzed by TiCl4/Et2AlCl was speculated based on the product structure (Figure 7). TiCl4 is activated by Et2AlCl to afford catalytically active Ti complex II.22−25 The catalytically active Ti complex coordinates to the C=C bond of monomeric 1-decene (II → III), and then the C=C bond breaks and inserts into the Ti–C bond to form a new Ti–C bond (III → IV).26 The new Ti–C bond then coordinates with the C=C bond to complete the chain growth process (IV → V). During the chain growth process, the 1-decene dimer also inserts into the Ti–C bond (V → VI), the β-H elimination occurs in the polymer chain, and the product with a quaternary carbon structure is obtained (Figure 8).
Figure 7.
Mechanism of quaternary carbon formation.
Figure 8.

Metallocene catalyst [t-BuN(Me)2C(η5-C5H4)]ZrCl2.
Experimental Section
Main Raw Materials
Toluene and hexane (analytically pure, Shanghai Lingfeng Chemical Reagent Co., Ltd.) were refluxed over sodium metal for 24 h using benzophenone as an indicator. A-olefin (polymerization grade, 95%, Beijing Bailingwei Technology Co., Ltd.) was purified by precolumn chromatography, dried for 48 h, and then distilled from sodium hydride. MAO (1.5 mol/L toluene solution) was obtained from Lanzhou Chemical Research Center, China National Petroleum Corporation. TiCl4 (AR, 99%) was obtained from Shanghai Aladdin Biotechnology Co., Ltd. Et2AlCl (1 mol/L toluene solution) was obtained from Sinopharm Chemical Reagent Co., Ltd. The metallocene catalyst ([t-BuN(Me)2C(η5-C5H4)]ZrCl2) was synthesized according to a previously reported procedure.27
Preparation of the Dimer
All synthetic experiments were conducted under an argon atmosphere. A three-necked 250 mL flask was charged with 50 mL of freshly distilled α-olefin using a hypodermic syringe. Next, 1.5 M MAO solution was added. The reaction was initiated by the addition of toluene solution of the metallocene catalyst after heating to the desired reaction temperature. After reacting for 3 h, the reaction was terminated by the addition of ethanolic hydrochloric acid, and the product was separated by filtration; washed sequentially with base and water; and the ethanol, toluene, and unreacted monomer were removed by distillation under atmospheric pressure. The dimer was obtained by distillation under reduced pressure. The basic properties (boiling point and GC retention time) of the polymers of the oligomers are shown in Table 5. The distillations were carried out based on the boiling point, and the content of each product was confirmed by GC.
Table 5. Boiling Points and GC Retention Times of the Oligomers.
| α-olefin | characteristic | dimer | trimer | tetramer | pentamer | hexamer |
|---|---|---|---|---|---|---|
| 1-hexene | bp (°C/mm Hg) | 80/7 | 120/0.5 | 145/0.2 | 190/0.2 | |
| GC retention time, min | 6.4 | 8.7 | 13.7 | 16.8 | 19.6 | |
| 1-octene | bp (°C/mm Hg) | 105/0.8 | 166/0.8 | 212/0.8 | 242/0.2 | |
| GC retention time, min | 6.4 | 14.5 | 19.5 | 22.7 | 25.3 | |
| 1-decene | bp (°C/mm Hg) | 125/0.5 | 182/0.3 | 235/0.3 | 290/0.2 | |
| GC retention time, min | 13.6 | 19.3 | 24.3 | 27.5 | 30.0 |
Copolymerization of the α-Olefin Monomer/Dimer
All synthetic experiments were conducted under an argon atmosphere. A three-necked 250 mL flask was charged with freshly distilled α-olefin using a hypodermic syringe, and then a hexane solution of the obtained α-olefin dimer was added. Next, the cocatalyst was slowly added to the flask with sufficient agitation. TiCl4 was added after the desired reaction temperature was reached. After reacting for 4 h, the reaction was quenched by the addition of ethanolic hydrochloric acid. The product was separated by filtration; sequentially washed with a base and water; and the ethanol, hexane, and unreacted monomer were removed by distillation under atmospheric pressure. A small amount of unreacted dimer was removed by distillation under reduced pressure.
Oligomer Analysis Method
Determination of the Kinematic Viscosity (KV), Viscosity Index (VI), and Pour Point (PP) of the Oligomers
The kinematic viscosity (unit, cSt) of the oligomers was measured at 40 and 100 °C with a GB/T-265 kinematic viscometer, the VI was measured based on the kinematic viscosity according to ASTM D2270-10 (2016), and the PP was measured according to the ASTM D97 method.
Determination of the Composition of the Oligomer
The compositions of the oligomers were measured by GC–mass spectrometry (MS), and the contents and ratios of the different components were obtained by an internal standard method. GC–MS analyses were performed on a PY-7890A-5975C color-mass spectrometer, model Agilent 7890A GC/5975C MSD, using a hydrogen flame ionization detector. The carrier gas was nitrogen, the column front pressure was 0.07 MPa, the hydrogen flow rate was 30 mL/min, the carrier gas flow rate was 250 mL/min, the inlet temperature was 250 °C, the detector temperature was 400 °C, the vaporization chamber temperature was 450 °C, and the split ratio was 100:1. The temperature was programmed as follows: the initial temperature was 50 °C, this temperature was maintained for 10 min, the temperature was increased at 9 °C/min to 380 °C, and this temperature was held for 10 min.
Nuclear Magnetic Resonance Analysis of the Oligomer (13C NMR and 1H NMR Spectroscopy)
A Bruker AVANCE-6 nuclear magnetic resonance spectrometer (manufactured by Bruker) was used with CDCl3 as the solvent, tetramethylsilane (TMS) as the internal standard, and a pulse angle of 90°. The NMR spectra of samples with a mass fraction of 15% were acquired at room temperature.
Conclusions
-
(1)
Dimeric 1-decene was prepared with a metallocene catalyst ([t-BuN(Me)2C(η5-C5H4)]ZrCl2) with high dimerization selectivity. The dimerization ratio reached 83.1%. GC and 13C NMR analyses confirmed that 1-olefin dimers accounted for the vast majority of the dimer species. This dimer mixture is an ideal raw material for further polymerization to prepare PAO.
-
(2)
Compared with PAO prepared with catalytic AlCl3 and typical PAO, the PAO product obtained by 1-decene/1-decene dimer copolymerization using TiCl4/Et2AlCl shows excellent viscosity–temperature performance and good low-temperature fluidity. PAOs with different kinematic viscosities can be prepared by changing the ratio of the 1-decene/1-decene dimer.
-
(3)
The GC and 13C NMR results show that the products of the TiCl4/Et2AlCl-catalyzed 1-decene/1-decene dimer copolymerization possess quaternary carbon, making these products more consistent with the ideal structure of lubricating base oils, namely, star-branched structures. The quaternary structure is the molecular-level reason for the products’ excellent viscosity–temperature performance and good low-temperature fluidity.
In this paper, a two-step polymerization method was used to prepare PAO lubricating base oils. The dimers synthesized with a metallocene catalyst with high dimerization selectivity were polymerized with TiCl4/Et2AlCl to afford PAO lubricating base oils with good viscosity–temperature properties and low-temperature fluidity. The quaternary carbon center in the polymerization product greatly improved product performance. This two-step polymerization method for preparing PAO lubricating base oils has great value for further research.
Acknowledgments
We are grateful to the National Key R&D Program of China (2017YFB0306701) and Project of Shanghai Key Discipline and Key Laboratory, China (Nos. B502, 08DZ2230500).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04361.
1H NMR spectrum of 1-decene dimers catalyzed by [t-BuN(Me)2C(η5-C5H4)]ZrCl2, GC chromatograms of 1-octene PAOs and 1-hexene PAOs, and oxidation stability test data of the PAOs prepared (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kioupis L. I.; Maginn E. J. Molecular Simulation of Poly-α-olefin Synthetic Lubricants: Impact of Molecular Architecture on Performance Properties. J. Phys. Chem. B 1999, 103, 10781–10790. 10.1021/jp992399n. [DOI] [Google Scholar]
- Ray S.; Rao P. V. C.; Choudary N. V. Poly-α-Olefin-Based Synthetic Lubricants: A Short Review on Various Synthetic Routes. Lubr. Sci. 2012, 24, 23–44. 10.1002/ls.166. [DOI] [Google Scholar]
- Zhang J.-T.; Hou X.-Y.; Li K.-W.; Liang S.-R.; Zhang J.-H. Use Situation and Processing Technology of PAO (group IV) Base Stocks. J. Xi’an Shiyou Univ., Nat. Sci. Ed. 2007, 22, 52–57. [Google Scholar]
- Kissin Y. V.; Schwab F. C. Post-Oligomerization of α-Olefin Oligomers: A Route to Single-Component and Multicomponent Synthetic Lubricating Oils. J. Appl. Polym. Sci. 2009, 111, 273–280. 10.1002/app.29030. [DOI] [Google Scholar]
- Nifant’ev I. E.; Vinogradov A. A.; Vinogradov A. A.; Sedov I. V.; Dorokhov V. G.; Lyadov A. S.; Ivchenko P. V. Structurally Uniform 1-Hexene, 1-Octene, and 1-Decene Oligomers: Zirconocene/Mao-Catalyzed Preparation, Characterization, and Prospects of their Use as Low-Viscosity Low-Temperature Oil Base Stocks. Appl. Catal., A 2018, 549, 40–50. 10.1016/j.apcata.2017.09.016. [DOI] [Google Scholar]
- Nifant’ev I.; Ivchenko P.; Tavtorkin A.; Vinogradov A.; Vinogradov A. Non-Traditional Ziegler-Natta Catalysis in A-Olefin Transformations: Reaction Mechanisms and Product Design. Pure Appl. Chem. 2017, 89, 1017–1032. 10.1515/pac-2016-1131. [DOI] [Google Scholar]
- Graessley W. W. Effect of Long Branches on the Flow Properties of Polymers. Acc. Chem. Res. 1977, 10, 332–339. 10.1021/ar50117a004. [DOI] [Google Scholar]
- Miller S. J.; O’Rear D. J.; Rosenbaum J.. Processes for Producing Lubricant Base Oils with Optimized Branching. U.S. Patent US7018525B2, 2010.
- Nelson W. T.; Heckelsberg L. F. Synthetic Lubricants: Star-Branched Oligomers via Metathesis/Dimerization of 1-Octene and or 1-Decene. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 178–181. 10.1021/i300010a005. [DOI] [Google Scholar]
- Evans M. E.; Atienza C. C. H.; Canich J. A. M.; Hagadorn J. R.; Cano D. A.; Day G. S.; Chen P. C.. Production of Olefin Dimers. WO Patent WO20190052592019.
- Vautravers N.; Teles J. H.; Berkessel A.; Paul M.; Yatham V. R.. Process for the Dimerization of Activated Olefins. U.S. Patent US9796654B2, 2017.
- Blewett C. W.; Turner S. W.. Converting α-Olefin Dimers to Higher More Useful Oligomers. U.S. Patent US4469912A, 1984.
- Dong S. Q.; Mi P. K.; Xu S.; Zhang J.; Zhao R. D. Preparation and Characterization of Single-Component Poly-α-Olefin Oil Base Stocks. Energy Fuels 2019, 33, 9796–9804. 10.1021/acs.energyfuels.9b02938. [DOI] [Google Scholar]
- Sadjadi S.; Bahri-Laleh N.; Nekoomanesh-Haghighi M.; Ziaee F.; Dehghani S.; Shirbakht S.; Rahbar A.; Barough M. S.; Mirmohammadi S. A. Rationalizing Chain Microstructure in the Polyα-Olefins Synthesized by Cationic AlCl3/H2O Catalytic System. Int. J. Polym. Anal. Charact. 2019, 24, 556–570. 10.1080/1023666X.2019.1627027. [DOI] [Google Scholar]
- Xu S.; Huang J. Syntheses of Bulkily Substituted Zirconocene Dichloride Complexes and Study on their Intramolecular Elimination Reaction. Acta Chim. Sin. 2005, 63, 1318–1322. [Google Scholar]
- Gao Z.; Gu J.; Mi P.; Zhang Y. Oligomerization of 1-Decene with Different Structure Metallocene Catalysts. Pet. Process. Petrochem. 2016, 47, 14–19. [Google Scholar]
- Guo Q.-P.; Yao T.; Fei Y.-W.; Yang H.-W. Property Analysis of Poly-α-Olefin Synthetic Aviation Lubricating Base Oil. Contemp. Chem. Ind. 2014, 43, 1790–1792. [Google Scholar]
- Verdier S.; Coutinho J. A. P.; Silva A. M. S.; Alkilde O. F.; Hansen J. A. A Critical Approach to Viscosity Index. Fuel 2009, 88, 2199–2206. 10.1016/j.fuel.2009.05.016. [DOI] [Google Scholar]
- Xiang W.; Xiong C.; Dong J.; Jin K. Preparation of Low Viscosity Synthetic Oil from Cracked Olefins of Slack Wax. Pet. Process. Petrochem. 2000, 31, 13–16. [Google Scholar]
- Dimitrov P.; Emert J.; Hua J.; Keki S.; Faust R. Mechanism of Isomerization in the Cationic Polymerization of Isobutylene. Macromolecules 2011, 44, 1831–1840. 10.1021/ma102645w. [DOI] [Google Scholar]
- Jiang Y.; Sun E.; Liu T.; Yan Y.; Cao Y.; Zhao T. Research on Viscosity Temperature Characteristics and Low Temperature Performance of Oligomer from 1- Decene Catalyzed by AlCl3. Adv. Fine Petrochem. 2016, 17, 41–44. [Google Scholar]
- Asanuma T.; Nishimori Y.; Ito M.; Uchikawa N.; Shiomura T. Preparation of Syndiotactic Polyolefins by Using Metallocene Catalysts. Polym. Bull. 1991, 25, 567–570. 10.1007/BF00293515. [DOI] [Google Scholar]
- Yang H.; Zhang L.; Zang D.; Fu Z.; Fan Z. Effects of Alkylaluminum as Cocatalyst on the Active Center Distribution of 1-Hexene Polymerization with MgCl2-Supported Ziegler–Natta Catalysts. Catal. Commun. 2015, 62, 104–106. 10.1016/j.catcom.2015.01.023. [DOI] [Google Scholar]
- Kaminsky W.Polyolefins: 50 Years after Ziegler and Natta I[M]//Polyolefins: 50 years after Ziegler and Natta II; Springer: Berlin Heidelberg, 2013. [Google Scholar]
- Nayeri H. H.; Taromi F. A.; Hemmati M.; Rekabdar F. Preparation Method of Superactive Ziegler–Natta Catalysts to Produce Ultra-High Molecular Weight Amorphous Poly(1-Octene), Poly(1-Decene), and their Copolymers. J. Coord. Chem. 2014, 67, 3270–3278. 10.1080/00958972.2014.958475. [DOI] [Google Scholar]
- Wang S.; Li L.; Sun E.; Jiang Y.; Cao Y.; Wang J. Ziegler-Natta Catalyst System with Two Co-Catalysts for Synthesis of Lube Base Oil from Mixed Decene. Petrochem. Technol. 2017, 46, 433–438. [Google Scholar]
- Mi P. K.; Kong X. J.; Xu S.; Zhu Y. L.; Cheng L. Synthesis of sp3C1-Bridged Constrained Geometry Complexes and their Application for Copolymerization of Ethylene and 1-Octene. Adv. Mater. Res. 2013, 683, 280–288. 10.4028/www.scientific.net/AMR.683.280. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.