Abstract
A temporary discontinuation (drug holiday) of high-dose antiresorptive (AR) agents has been proposed to reduce the risk of medication-related osteonecrosis of the jaw (MRONJ). The aim of this systematic review was to answer the question: Is high-dose AR drug holiday, at the time of tooth extraction or dentoalveolar surgery, necessary to prevent the development of MRONJ in patients with cancer? This protocol was registered in the PROSPERO database. Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched for relevant studies up to and including April 2019. Randomized controlled trials (RCTs), cohort and cross-sectional studies, surveys, and case reports with more than five patients were included. Records were imported into www.covidence.org. Electronic searches were supplemented by manual searches and reference linkage. The Preferred Reporting Items for Systematic Reviews and Meta Analysis (PRISMA) were followed. Although only one study fitted the population, intervention, comparison, outcome (PICO) framework, valuable information on AR drug holiday could be extracted from 14 of 371 reviewed articles. Among these, 3 were prospective and 11 were retrospective studies. These studies described or evaluated high-dose AR drug holidays. In 2 studies, patients were being treated with denosumab, but neither showed that a drug holiday was effective. The remaining 12 studies evaluated bisphosphonate treatment and 2 of these studies found no reason to use AR drug holiday before surgery. Three studies recommended drug holidays, whereas most of the studies recommended assessing each patient separately. The only paper that fitted the PICO approach was a non-randomized, prospective study with a control group. This study concluded that drug holiday was not necessary. Thus, there are no evidence for using drug holiday, but it is also clear that caused by a limited numbers of eligible patients, and a great variation in between these patient, high-level evidence for using AR drug holiday is almost impossible to obtain.
Keywords: Cancer surgery, Clinical research, Dental surgery, Dentistry, Oral medicine, Pharmacology, Antiresorptive agents, Drug holiday, Holidays, MRONJ, Osteonecrosis of the jaw, Oral surgery, Tooth extraction
Cancer surgery; Clinical research; Dental surgery; Dentistry; Oral medicine; Pharmacology; Antiresorptive agents; Drug holiday; Holidays; MRONJ; Osteonecrosis of the jaw; Oral surgery; Tooth extraction.
1. Introduction
A considerable number of adults worldwide are treated using antiresorptive (AR) agents, including bisphosphonates (BPs) and denosumab. AR agents affect bone remodeling and are used to treat osteoporosis, metastatic bone cancer, and multiple myeloma [1, 2]. Treatment with high-dose denosumab or Zoledronic acid delays the onset of skeletal-related events (SREs) including fractures, lowers the risk of subsequent SREs and reduces pain in patients with cancer and bone metastases [2].
AR agents are used in low doses (e.g., Alendronate, Aclasta, Prolia) to treat osteoporosis, and in a high dose (e.g., Zometa, Pamifos, Xgeva) to manage cancer-related conditions. In recent years, AR agents have also been used as adjuvant therapies for patients with cancer [1].
A well-known serious adverse event of AR therapy is medication-related osteonecrosis of the jaw (MRONJ) [1]. The American Association of Oral and Maxillofacial Surgeons (AAOMS) defines MRONJ as an exposed area of bone, or bone that can be probed through an intra- or extra oral fistula that has persisted for more than eight weeks, in a non-irradiated jaw of a patient treated with AR or antiangiogenic agents. The AAOMS describes four stages of MRONJ: Stage 0 is the mildest variant with non-specific signs or symptoms. Stage I is defined as exposed bone or bone that can be probed through a fistula without subjective symptoms. Stage II is when patients feel pain, and stage III is when patients experience severe pain and have extensive necrotic bone development [1]. The risk of MRONJ developing depends on the frequency of administration of AR agents (osteoporosis versus cancer), the dose per administration (low versus high) and the duration of treatment (short versus long). Consequently, patients with cancer who are being treated with high doses of AR agents are at greater risk [2].
The etiology and pathogenesis of MRONJ is not completely understood, but several risk factors have been identified. Numerous studies have concluded that tooth extraction is the most important independent risk factor for the onset of MRONJ [1, 3, 4, 5, 6]. Therefore, patients being treated with high doses of AR agents are advised to avoid tooth extractions if possible [1]. If a tooth extraction is absolutely necessary, a temporary AR discontinuation in treatment, termed a ‘drug holiday’, is recommended or considered [1]. In some countries, a drug holiday is recommended by national guidelines or position papers based on expert opinions [7, 8, 9, 10, 11, 12, 13], but no international consensus regarding high-dose AR drug holidays has been reached and a systematic review evaluating the evidence for using AR drug holiday are missing. Current guidelines/position papers are summarized in Table 1 [1,7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18].
Table 1.
Guidelines/Position paper recommendations regarding high-dose antiresorptive drug holidays.
| Guideline/Position paper | Country | Year | Recommendations regarding high-dose antiresorptive drug holidays |
|---|---|---|---|
| Canadian Consensus of Practice Guidelines for Bisphosphonate Associated Osteonecrosis of the Jaw [7] | Canada | 2008 | Urgent invasive oral surgery: Discontinuation of BP therapy during healing period, if the medical conditions permits. Non-emergent procedure: BP drugs holiday for 3 to 6 months prior to oral surgery and until complete healing. |
| Osteonecrosis of the jaw complicating bisphosphonate treatment for bone disease in multiple myeloma: an overview with recommendations for prevention and treatment [8] | Australia | 2009 | If the patient's risk of skeletal-related events is low or intermediate: BP cessation for 2–3 months before extraction until complete healing. |
| The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network [16] | Europe | 2009 | Temporary suspension of BP treatment should be considered if invasive dental procedures are necessary, but any decision to suspend BP treatment should be considered on a case-by-case basis. |
| Management of patients at risk of bisphosphonate osteonecrosis in maxillofacial surgery units in the UK [17] | UK | 2009 | The use of BPs must be discussed with the prescribing physician. If continued BP use, any surgical treatment should be undertaken with at least a 2 weeks gap before the next treatment. |
| Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteonecrosis. Executive summary of recommendations from the American Dental Association Council on Scientific Affairs [9] | USA | 2011 | Drug holiday from AR drug therapy, or waiting periods before performing dental treatment, for prevention of MRONJ. |
| Guidelines for supportive care in multiple myeloma 2011 [10] | UK | 2011 | If the patient's fracture risks and disease status permits, it seems reasonable to stop the AR treatment and not recommence treatment until healing has occured. |
| Medication-Related Osteonecrosis of the Jaw - 2014 Update [1] | USA | 2014 | BP discontinuation prior to oral surgery is based on an evaluation of the individual patient's data. If MRONJ, the oncologist may consider a drug holiday until soft tissue closure. No studies support or refute the strategy of stopping Dmab in the prevention or treatment of MRONJ. |
| Diagnosis and Management of Osteonecrosis of the Jaw: A systematic review and International Consensus [11] | Canada | 2014 | Drug holiday after oral surgery and until complete soft tissue healing has occurred. |
| Medication Related Osteonecrosis of the Jaw: 2015 Position Statement of the Korean Society for Bone and Mineral Research and the Korean Association of Oral and Maxillofacial Surgeons [14] | Korea | 2015 | No definite conclusion is made regarding drug holiday. Only if MRONJ is present, the necessity of a drug holiday is clear. |
| "Positionspapier zur medikamentenassoziierten Osteonekrose des Kiefers (MRONJ)" [12] | Germany | 2016 | A 2 months drug holiday before oral surgery is recommended. If Dmab, the discontinuation can be shorter. Resumption when complete healing has occurred. |
| Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw [15] | Japan | 2016 | No consensus regarding drug holiday before invasive dental treatment. The decision on whether to implement a postoperative drug holiday should be made jointly by the physician and dentist based on fracture risk. Resumption of BP from 2 weeks to 2 months postoperatively. |
| Standard Operation Procedure, Medication-related Osteonecrosis of the Jaws (Not published) | Denmark | 2016 | The oncologist discontinues the ARs before referral to the oral surgeons. |
| Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the International Recommendations for Management From the International Task Force on ONJ [13] | Canada | 2017 | Interruption of BP or Dmab therapy is advised, if possible before oral surgery and until soft tissue healing has occurred. The treatment plan must be individualized for each patient. |
| Oral Health Management of Patients at Risk of Medication-related Osteonecrosis of the Jaw [18] | Scotland | 2017 | Drug holidays to avoid the risk of MRONJ associated with dental care are not recommended. |
AR: Antiresorptive; BP: Bisphosphonate; MRONJ: Medication-related osteonecrosis of the jaw; Dmab: Denosumab.
This systematic review has the aim to investigate the evidence and efficacy of discontinuing high-dose AR therapy in relation to oral surgery. Thus, the hypothesis of the review is that high-dose AR drug holiday will reduce the risk of MRONJ.
2. Material and methods
In April 2018 a detailed protocol was written and registered online in the PROSPERO (International Prospective Register of Systematic Reviews) database; PROSPERO ID: 103124; registration number: CRD42018103124 [19]. The protocol and this systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20].
2.1. Focused question
At present, most patients undergoing high-dose AR therapy are advised to avoid dentoalveolar surgery or tooth extraction if possible. If an extraction is required, a temporary discontinuation of AR treatment prior to tooth extraction should be considered [1]. However, no evidence has been collected to evaluate the efficacy of high-dose AR drug holidays at the time of oral surgery. Therefore, we used the population, intervention, comparison, outcome (PICO) framework to develop the following focused question:
“Is a high-dose AR drug holiday at the time of tooth extraction, or other dentoalveolar surgery, necessary to prevent the development of MRONJ in patients with cancer?”
The PICO framework was defined as follows:
-
(P)
Population: Adults with malignant bone disease undergoing high-dose∗ AR therapy.
-
(I)
Intervention: Discontinuation (i.e., drug holiday) of high-dose AR therapy at the time of tooth extraction or dentoalveolar surgery.
-
(C)
Comparison: Continuation (i.e., no drug holiday) of high-dose AR therapy at the time of (prior to and/or after) tooth extraction or dentoalveolar surgery.
-
O)
Outcome: Primary outcome is development of MRONJ (+/-) and thereafter divided into the 4 stages of MRONJ defined by AAOMS.
∗High-dose is defined as monthly treatment with i.v. BP, daily treatment with oral ibandronic acid (Bondronate), or monthly treatment with subcutaneous denosumab (Xgeva) [1].
2.2. Inclusion and exclusion criteria
Randomized controlled trials (RCTs), cohort and cross-sectional studies were included. Surveys and case reports with more than five patients were also included if they included patients being treated with high doses of ARs (e.g., groups of patients, some being treated with low and some with high doses of ARs). All studies referring to the focused question (Is a high-dose AR drug holiday necessary to prevent the development of MRONJ in patients with cancer) were included.
In vitro studies, conference abstracts, and animal studies were excluded from the analysis.
2.3. Search strategy
The following online databases were searched:
-
•
Medline (PubMed)
-
•
Embase
-
•
Cochrane Central Register of Controlled Trials (CENTRAL)
We searched for clinical studies and manuscripts published from 1 January 1990 until and including April 2019. The search was limited to English language articles. An additional search was performed by screening the reference lists of all the relevant full-text articles obtained. The same search terms were used for all three databases.
The search strategy involved a combination of MeSH (Medical Subject Headings) terms and free text. MeSH terms and PubMed entry terms were examined to identify synonyms. A separate search was performed for each PICO element (population, intervention, comparison, and outcome). Finally, all searches were combined in one complete search:
Search (((((((malignant bone disease) OR (breast neoplasms OR breast tumor OR breast tumors OR breast cancer)) OR (prostate cancer OR metastatic prostate cancer OR prostatic neoplasms OR prostatic cancer)) OR (myelomatosis OR multiple myelomas OR multiple myeloma OR myelomatosis))) AND ((((((antiresorptive drug holiday) OR (antiresorptive agents OR antiresorptive agent OR antiresorptive drugs OR antiresorptive drug)) OR (diphosphonates OR bisphosphonates OR bisphosphonate)) OR bone density conservation agents) OR (alendronate OR zometa OR fosamax OR pamifos OR xgeva OR zoledronic acid OR denosumab[all])) OR (discontinue OR break OR suspension OR interruption OR cessation OR time out))) AND (tooth extraction OR tooth extractions OR extraction OR extractions OR oral surgical procedures OR alveolectomy)) AND (bisphosphonate-associated osteonecrosis of the jaw OR osteonecrosis OR jaw OR jaws OR ONJ OR medication related osteonecrosis of the jaw OR osteonecrosis of the jaw OR dead jaw bone OR bisphosphonate-related osteonecrosis of the jaw).
2.4. Filters: English
This search was conducted in April 2018 and all hits were imported into www.covidence.org for screening and reading. The complete search was performed once per week until 30 April 2019; all new hits were imported into Covidence for screening.
2.5. Study selection
Two review authors (C.O. and K.G.) independently assessed the studies for eligibility using Covidence. The studies were assessed first at the title and abstract level, and later at the full-text level. If no separate abstract was available, the full-text article including the abstract was used. The level of agreement between the reviewers evaluating abstracts for inclusion was assessed using Cohen's kappa coefficient [21].
All abstracts referring to an AR drug holiday (e.g., cessation or discontinuation) were included in the full-text screening to ensure important points of view regarding the intervention were not overlooked. The final decision on whether to include a study was always made at the full-text level. Any disagreements were resolved by discussion between the two review authors.
2.6. Data extraction
The following data items were collected from each literature source: Authors, year of publication, study design, study intervention, number of patients, primary diseases, type and duration of antiresorptive treatment, number of patients in drug holiday, duration of drug holiday, authors’ suggested drug holiday recommendations, development of medication-related osteonecrosis of the jaw, medication-related osteonecrosis of the jaw stages, reason for medication-related osteonecrosis of the jaw, treatment of medication-related osteonecrosis of the jaw and the follow-up period.
2.7. Risk of bias assessment
Bias within the included studies was assessed using the Newcastle–Ottawa Scale (NOS) [22]. The NOS is a quality assessment tool for non-randomized studies (cohort studies and case-control studies); it ranks studies by assigning 1 to 9 stars for each quality item defined in three domains: Selection, Comparability, Outcome/Exposure [22]. The number of stars are an expression of the quality of the study. The more stars, the lower the risk of bias. The NOS assessments can be converted into the more frequently used AHRQ standards (Agency for Healthcare Research and Quality standard assessments), which include good, fair, or poor quality depending on number of stars in each NOS domain [37].
3. Results
3.1. Study characteristics
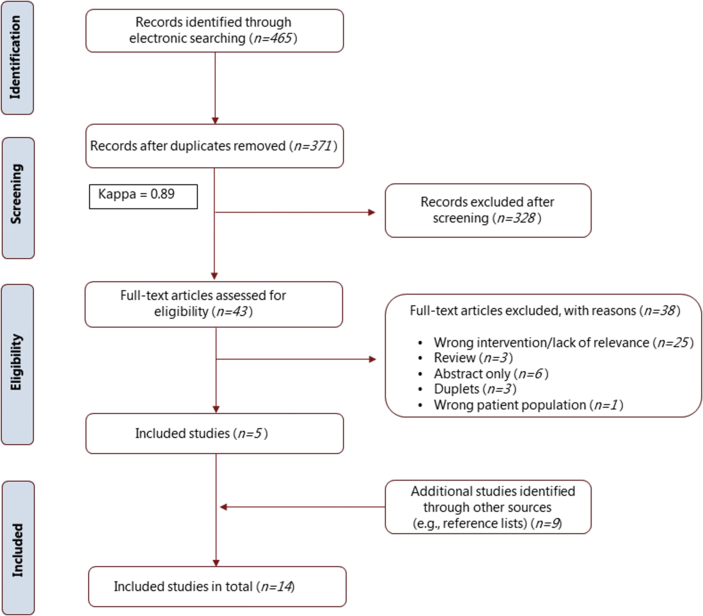
A total of 465 articles were identified from the preliminary electronic literature search. After duplicates were removed, 371 articles were screened on the basis of their titles and abstracts (kappa value = 0.89). Among these, 43 full-text articles were critically reviewed and 5 were identified as relevant. Nine additional studies, identified by hand-searching in the text and reference list of the identified literature, were also included (Figure 1). All 14 included studies were published in journals registered in either Medline (PubMed), Embase or Cochrane register.
Figure 1.
Flowchart showing the electronic and manual search results. Abbreviation: n, number of studies.
3.2. Description of included studies
A total of 14 studies were included. Among these, three were prospective studies [23, 24, 25] and 11 were retrospective studies [26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]. Descriptive data, including detailed study characteristics such as patient population, ARs, AR treatment duration, MRONJ characteristics, drug holiday characteristics, and authors’ conclusions on drug holidays from the 14 studies fulfilling the inclusion criteria are presented in Tables 2, 3, and 4.
Table 2.
Descriptive characteristics of prospective studies.
| Author, Year | Design | Intervention | Patient population | Primary disease | Location | Type of AR | Duration of AR (months) | Drug holiday (patients) | Duration of drug holiday (months) | Development of MRONJ | Authors' conclusions on drug holidays | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saia et al., 2010 [23] | Prospective non-controlled cohort study | Surgical tooth extraction | 60 patients/total of 185 teeth | Metastatic bone disease, multiple myeloma or nonmalignant bone disease | 103 teeth (55.7%) in the mandible, 82 teeth (44.3%) in the maxilla | Zoledronate (63%), Pamidronate (40%), Neridronate (7%), Risedronate (3%)∗ | - | All 60 patients paused their BP therapy | 1 to >3 from the day of surgery | 5/60 (all cancer patients) | Resumption of BP treatment was not associated with BRONJ | 12 |
| Ferlito et al., 2011 [24] | Longitudinal observational non-controlled cohort study | Evaluate the time to bony sequestrum formation in patients with confirmed MRONJ | 94 | - | - | Zoledronate (77%), Alendronate (17%), Neridronate (4%), Ibandronate (1%), Clodronate (1%) | 1–24+ | 43 | <6–24 | 94 (All from study start) | Suspension of ARs was determined by the clinical condition of the patient. Bony sequestra were prolonged in patients continuing BP therapy. Discontinuation not recommended because the patient may develop systemic complications, such as a recurrence of pain or progression of the underlying disease | 6 |
| Bodem et al., 2015 [25] | Prospective cohort study | Surgical tooth extraction | 61 patients/102 extraction sites/total of 184 teeth | Breast cancer (38.9%), Multiple myeloma (17.6%), Prostatic cancer (9.25%), Other (19.4%) | 55 teeth (53.9%) in the maxilla, 47 teeth (46.1%) in the mandible | Zoledronic acid (62.4%), Ibandronate (28.3%), Pamidronate (9.3%) | 40.25 (Range 4–245) | 17 patients paused or completed their BP therapy at the time of surgery | 17.6 ± 15.9 (range, 1–63) before surgery | 1/17 developed MRONJ (+DH), 7/44 developed MRONJ (no DH) | Drug holidays should not be implemented for i.v. BP therapy | 3 |
AAOMS: American Association of Oral and Maxillofacial Surgeons; AR: Antiresorptive; BP: Bisphosphonate; BRONJ: Bisphosphonate induced osteonecrosis of the jaw; DH: Drug holiday; i.v.: Intravenous; MRONJ: Medication-related osteonecrosis of the jaw; -: Not described in the article.
percentages >100% as described in the publication by Saia et al.
Table 3.
Descriptive characteristics of retrospective studies.
| Study | Design | Aim | No. of patients | AR treatment | Duration of AR treatment (months) | Reasons for MRONJ | MRONJ stages | Treatment of MRONJ |
|---|---|---|---|---|---|---|---|---|
| Dimitrakopoulos et al., 2006 [36] | Case series >5 | Clinical evaluation of drug-induced avascular osteonecrosis | 11 | Zoledronate (6/11), Zoledronate + Pamidronate (4/11), Pamidronate + Ibandronate + Zoledronate (1/11) | 6–60 | Tooth extraction (7/11), Chronic denture trauma (1/11), Spontaneous onset (3/11) | - | Sequestrectomy (3/11), Debridement (6/11), No surgical treatment (2/11) |
| Wilde et al., 2011 [27] | Retrospective cohort study | Surgical treatment with bilayer mucosal closure | 24 (33 sites) | Zoledronate (14/24), Zoledronate + Bondronate (3/24), Zoledronate + Pamidronate (3/34), All three types of BP (4/24) | - | Misfitting dentures (6/24), Extraction (19/24), Incision after abscess (1/24), Periodontal disease (2/24), Other (1/24) | 1 (2/24), 2 (7/24), 3 (11/24), 4∗ (4/24) | All surgically treated |
| Jabbour et al., 2012 [29] | Retrospective cohort study | Investigating outcomes of conservative therapy alone or followed by surgical treatment | 14 | Alendronate (4/14), Pamidronate (2/14), Zoledronic acid (7/14), Pamidronate + Zoledronic acid (1/14) | 12–96 | Misfitting dentures (4/14), Extractions (7/14), Spontaneous onset (1/14), Other (1/14), Not available (1/14) | 2 (14/14) | Conservative treatment (8/14), Surgical treatment (6/14) |
| Voss et al., 2012 [30] | Retrospective cohort study | Surgical three-layered technique | 20 (manuscript describes 21, but only 20 in the summary table) | Ibandronate (2/20), Zoledronate (14/20), Alendronate (3/20), Pamidronate + Alendronate + Zoledronate (1/20) | 40.1 (mean) (range, 6–84) | Extractions (12/20), Other (8/20) | 2 (15/20), 3 (5/20) | All surgically treated |
| Wutzl et al., 2012 [26] | Retrospective analysis of a prospective cohort study | Surgery: therapeutic approach | 41 | Pamidronate (7/41), Zoledronic acid (25/41), Zoledonric acid + other bisphosphonate (8/41), Alendronate (1/41) | - | - | 0 (1/41), 1 (10/41), 2 (24/41), 3 (6/41) | All surgically treated |
| Kim et al., 2014 [31] | Retrospective cohort study | Investigating prognostic factors after surgical management of patients diagnosed with MRONJ | 54 | Alendronate (35/54), Risedronate (9/54), Ibandronate (3/54), Pamidronate (4/54), Zolendronate (4/54)† | Surgical treatment: 54 (mean); Conservative treatment: 86 (mean) | Extraction of teeth (33/54), Implant (4/54), Curettage (1/54), Partial dentures (2/54), Spontaneous onset (6/54), No data (8/54) | 0 (4/54), 1 (17/54), 2 (32/54), 3 (1/54) | Surgically treated with debridement or sequestrectomy (21/54), Conservatively treated (33/54) |
| Lopes et al., 2015 [32] | Retrospective observational cohort study | Evaluation of the efficacy of surgery | 33 (46 sites) | Zoledronate (22/33), Pamidronate (3/33), Zoledronate + Pamidronate (5/33), Alendronate (2/33), Zoledronate + Alendronate (1/33) | I.v. treatment: 26.3 (mean) (2 patients with Alendronate for 10 years) | Extractions (16/33), Implant treatment (3/33), Periodontal disease (9/33), Misfitting dentures (8/33), Palatal tori (2/33), Spontaneous onset (8/33) | 2 (37/46), 3 (9/46) | All surgically treated |
| Bodem et al., 2016 [33] | Monocentric retrospective cohort study | Analysis of surgical outcomes: i.e., drug holiday versus no drug holiday | 39 (47 sites) | Zoledronic acid (39/39) | 24 (range 2–120) | - | 2 (23/47), 3 (24/47) | All surgically treated |
| Hoefert et al., 2017 [34] | Retrospective review of medical records | Examination of clinical characteristics and operative and non-operative therapeutic outcomes | 17 | XGEVA (15), Prolia (2) | 19.7 ± 10.5 (range 4–48) | Misfitting dentures (7/17), Extractions (6/17), Peri-implantitis (1/17), Periodontitis (2/17), Spontaneous onset (1/17) | 1 (1/17), 2 (10/17), 3 (6/17) | Operative (7/17), Non-operative (10/17) |
| Aljohani et al., 2018 [35] | Retrospective multicenter case series | Analysis of AR characteristics, demographics, related comorbidities, local preceding events, treatment strategies, and treatment outcomes. | 63 | XGEVA (52/63), Prolia (11/63) (31/63 patients had a history of bisphosphonate use) | - | Extractions (28/63), Periodontitis (6/63), Misfitting dentures (4/63), Implant placement (2/63), Peri-implantitis (1/63), Other (9/63), Unknown (13/63) | 0 (2/63) 1 (6/63) 2 (41/63) 3 (8/63) Combined (6/63) | Surgical (60/63), Non-surgical (3/63) |
| Jung et al., 2018 [28] | Retrospective cross-sectional study (database) | Investigation of the gap between BP use and the occurrence of MRONJ. | 1569 | Alendronate, Clodronate, Etidronate, Ibandronate, Risedronate, Pamidronate, Zoledronic acid | 2.94 years (average) | Dental surgery, including extractions (915/1,569) | - | - |
AR: Antiresorptive; MRONJ: Medication-related osteonecrosis of the jaw; i.v.: Intravenous.
Stage 4 is defined in this study; -: Not described in the article.
This may be an error in the original article because n = 55, not 54 as described.
Table 4.
Drug holiday recommendations from the retrospective studies.
| Study | Primary disease | Drug holiday (patients)∗ | Duration of drug holiday (months) | Healing of MRONJ (patients with drug holidays) | Authors' conclusions on drug holidays |
|---|---|---|---|---|---|
| Dimitrakopoulos et al., 2006 [36] | Breast cancer (1/11), Prostate cancer (2/11), Multiple myeloma (5/11), Neuroendocrine cancer (1/11), Lung cancer (1/11), Fibrous dysplasia (1/11) | 10/11 | 2–8 | 5/10 | Discontinuation of BP, combined with surgical debridement, is the treatment of choice. More than 3 months of cessation appears to be necessary |
| Wilde et al., 2011 [27] | Breast cancer (6/24), Prostate cancer (7/24), Multiple myeloma (7/24), Thyroid cancer (1/24), Hodgkin's lymphoma (1/24), Non-Hodgkin's lymphoma (1/24), Kidney cancer (1/24) | 10/24 | - | 10/10 | Treatment results were not significantly affected, whether BP therapy was continued or discontinued. The results of this study indicate that there is no reason to interrupt AR therapy for surgery |
| Jabbour et al., 2012 [29] | Osteoporosis (4/14), Breast cancer (5/14), Prostate cancer (2/14), Multiple myeloma (1/14), Kidney cancer (2/14), | 9/14 (7 with cancer) | - | 10/14 | There was no standard protocol for a drug holiday in this study. None of the patients died or had their health status changed due to discontinuation of BP therapy |
| Voss et al., 2012 [30] | Osteoporosis (4/20), Breast cancer (9/20), Prostate cancer (1/20), Thyroid cancer (1/20), Plasmacytoma (3/20), Vulva cancer (1/20), Kidney cancer (1/20) | 20/20 | 1–1.5 (4 weeks before, 6 weeks after) | 19/20 | An individual approach in consultation with the prescribing oncologist is recommended |
| Wutzl et al., 2012 [26] | Osteoporosis (5/41), Breast cancer (9/41), Prostate cancer (3/41), Multiple myeloma (20/41), Histiocytosis X (1/41), Lung cancer (2/41), Anal cancer (1/41) | 28/41 | ?–6 (6 post-operatively) | - | Discontinuation of BPs before surgery favored significantly better treatment outcomes |
| Kim et al., 2014 [31] | Osteoporosis (47/54), Breast cancer (1/54), Multiple myeloma (5/54), Malignant lymphoma (1/54) | 54/54 | Surgical treatment group: 6.9 Conservative treatment group: 7.2 | 11/20 | A correlation was found between drug holidays and prognoses in the surgical treatment group. Drug holiday durations should be at least 4 months to prevent a poor prognosis after surgical management |
| Lopes et al., 2015 [32] | Osteoporosis (2/33), Breast cancer (18/33), Prostate cancer (4/33), Multiple myeloma (4/33), Lung cancer (4/33), Kidney cancer (1/33) | 31/33 | 6.8 ± 9.2 | - | A total of 40/46 sites (87%) healed. No conclusions can be drawn from this study |
| Bodem et al., 2016 [33] | Malignant disease (39/39) | 15/39 | - | 9/15 showed complete healing, 4/15 showed relative healing 2/15 showed no healing | No statistically significant differences were observed between patients who were still receiving their i.v. BPs at the time of surgery and those on a drug holiday |
| Hoefert et al., 2017 [34] | Osteoporosis (1/17), Breast cancer (9/17), Prostate cancer (6/17), Lung cancer (1/17) | 10/17 | - | 5/10 | Cessation of denosumab treatment had no apparent effect on healing outcomes |
| Aljohani et al., 2018 [35] | Osteoporosis (9/63), Breast cancer (27/63), Prostate cancer (17/63), Multiple myeloma (2/63), Lung cancer (1/63), Melanoma (1/63), Thyroid cancer (2/63), Kidney cancer (4/63) | 42/63 | 6 ± 3.4 | Healing (5/42), Partial healing (3/42), No healing (5/42), Missing data (9/42) | No associations were observed between denosumab drug holidays and healing outcomes |
| Jung et al., 2018 [28] | Cancer (317/1569), Other (1252/1569) | 836/1569 | 1 day to >4 years | - | Among all the cases of ONJ that occurred during the study, 53.3% occurred after BP therapy was suspended. Most ONJ cases occurred within 2–3 years of BP therapy being discontinued. Different approaches are needed to determine whether drug holidays are likely to be beneficial. The benefits of continuing therapy may outweight the risks of suspending it |
AR: Antiresorptive; BP: Bisphosphonate; MRONJ: Medication-related osteonecrosis of the jaw; i.v.: Intravenous.
If no information is provided, then it was unclear whether the patients with drug holidays had a cancer diagnosis in the original studies; -: Not described in the article.
3.3. Methodological quality assessment
The included studies were quality assessed using the NOS (Table 5). Only one study was awarded 9/9 stars (range: 3–9) [25]. When converting the NOS to the AHRQ standards [37], 4 studies were of good quality [23, 24, 25, 26], 8 studies were of fair quality [27, 28, 29, 30, 31, 32, 33, 34] and 2 studies were of poor quality [35, 36].
Table 5.
Quality assessment of studies (Newcastle-Ottawa Scale).
| Reference | Selection |
Comparability |
Outcome |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Was the exposed cohort representative? | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | Overall quality assessment NOS score (0–9) | |
| J.P Bodem et al., 2015 [25] | ∗ Truly representative of typical patients being treated with high-dose ARs in the community. | ∗ Drawn from the same community as the exposed cohort | ∗ Secure record | ∗ Yes |
∗ Study controls for ongoing vs. completed/paused therapy. ∗Study controls for additional factors |
∗ Independent assessment | ∗ Yes | ∗ Complete follow up - all subjects accounted for | 9 |
| G. Saia et al., 2010 [23] | ∗ Truly representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | ∗ Yes | ∗ Study controls for additional factors | ∗ Independent assessment | ∗ Yes | ∗ Complete follow up - all subjects accounted for | 7 |
| Ferlito et al., 2011 [24] | ∗ Somewhat representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | ∗ Yes | ∗ Study controls for type of bisphosphonates | ∗ Independent assessment | ∗ Yes | ∗ Complete follow up - all subjects accounted for | 7 |
| Dimitrakopoulos et al., 2006 [36] | Selected group of users. | No description | ∗ Secure record | No | - | ∗ Independent assessment | ∗ Yes | ∗ Complete follow up - all subjects accounted for | 3 |
| Wilde et al., 2011 [27] | ∗ Somewhat representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | No |
∗ Study controls for patients' generel health status. ∗Study controls for additional factors |
∗ Independent assessment | ∗ Yes | ∗ 37.5% lost to follow-up, description provided of those lost | 4 |
| Jabbour et al., 2012 [29] | ∗ Somewhat representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | No | - | ∗ Independent assessment | ∗ Yes | ∗ Complete follow up - all subjects accounted for | 5 |
| Voss et al., 2012 [30] | ∗ Somewhat representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | No |
∗ Study controls for duration of bisphosphonates. ∗Study controls for additional factors |
∗ Independent assessment | ∗ Yes | ∗ Complete follow up - all subjects accounted for | 7 |
| Wutzl et al., 2012 [26] | ∗ Truly representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | ∗ Yes |
∗ Study controls for the effect of continuing therapy with bisphosphonates. ∗Study controls for additional factors. |
∗ Independent assessment | ∗ Yes | ∗ 15% lost to follow-up, but unlikely to produce bias | 8 |
| Kim et al., 2014 [31] | ∗ Somewhat representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | No |
∗ Study controls for drug holiday. ∗Study controls for additional factors. |
∗ Independent assessment | ∗ Yes | ∗ Complete follow up - all subjects accounted for | 7 |
| Lopes et al., 2015 [32] | ∗ Somewhat representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | No | - | ∗ Independent assessment | ∗ Yes | ∗ 61% lost to follow up. Descriptions provided for the subjects who could not be followed up. | 5 |
| Bodem et al., 2016 [33] | ∗ Truly representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | No | ∗ Study controls for drug holidays. ∗Study controls for additional factors. | ∗ Independent assessment | ∗ Yes | ∗ Complete follow up - all subjects accounted for | 7 |
| Hoefert et al., 2017 [34] | ∗ Somewhat representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | No | ∗ Study controls for the duration of therapy. ∗Study controls for additional factors. | ∗ Independent assessment | ∗ Yes | ∗ Complete follow up - all subjects accounted for | 7 |
| Aljohani et al., 2018 [35] | ∗ Truly representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | No |
∗ Study controls for denosumab dose. ∗Study controls for additional factors. |
∗ Independent assessment | ∗ Yes | 17% lost to follow-up. No descriptions of the subjects who could not be followed up. | 6 |
| Jung et al., 2018 [28] | ∗ Somewhat representative of typical patients being treated with high-dose ARs in the community. | No description | ∗ Secure record | No |
∗ Study controls for drug holidays. ∗Study controls for additional factors. |
∗ Independent assessment | No statement | No statement | 4 |
AR: Antiresorptive.
One star is awarded for each item, with a maximum of two stars being awarded for comparability; -: Not described in the article.
3.4. Outcomes: prospective studies
Of all the included studies, only the one performed by Bodem et al. fitted the PICO framework (25). This was the only study with surgical tooth extraction as the intervention and a related BP drug holiday, which included a control group.
Bodem et al. investigated whether patients undergoing i.v. BP treatment during surgery had a higher risk of BP-related osteonecrosis of the jaw (BRONJ) than patients who had a drug holiday, i.e., patients who had completed their BP treatment or had it temporarily suspended. A total of 17 of 61 patients had their high-dose AR therapy temporarily suspended prior to tooth extraction. Only 1 of the 17 patients developed MRONJ (5.9%). In total, 7 of the 44 patients in the control group developed MRONJ (16%) No significant differences were found (p = 0.4232) [25].
In the study by Saia et al., BP therapy was discontinued at the time of surgical tooth extractions, and all patients discontinued the AR treatment. The majority of the patients restarted BP treatment 1 month after the extractions. The objective was to reduce BP accumulation in the alveolar sockets. Six patients discontinued the AR therapy for a longer period due to the presence of osteomyelitis in bone biopsies, and 2 of these 6 patients restarted the therapy 3 months later due to metastatic progression. In this study, resumption of BP treatment was not associated with BRONJ 12 months after tooth extraction. The incidence of MRONJ in this study, which included a drug holiday, was 5 of the 60 patients (8.3%) [23].
The final prospective study that was included described a protocol for treating BRONJ [24]. In this study by Ferlito et al., all patients had BRONJ diagnosed from the start of the study. In total, 43 of 94 patients had drug holidays of differing durations. The authors found that prolonged bone sequestration occurred in patients continuing BP therapy, because the last patient to develop a sequestrum had continued treatment with zoledronate. However, these authors did not recommend drug holidays [24].
None of the prospective studies involved a denosumab drug holiday.
3.5. Outcomes: retrospective studies
None of the 11 retrospective studies that were included fitted the PICO framework, but all of them drew conclusions on drug holidays. Patients were receiving denosumab therapy in 2 of the 11 studies [34, 35]. Drug holidays were recommended in 3 studies [26, 30, 35], whereas 3 studies found no reason to discontinue treatment [27, 33, 34] and 5 studies suggested drug holidays should be considered on a case-by-case basis (Table 4) [28, 29, 31, 32, 36]. Only in the study by Voss et al. a detailed drug holiday description was given, high-dose AR was paused 4 weeks before operation and restarted 6 weeks postoperatively [30].
Most of the retrospective studies were mixed studies, meaning that the patient population was a mixture of patients in high-dose AR treatment (cancer patients) and low-dose AR treatment (osteoporotic patients). The one retrospective study that included only patients diagnosed with a malignant disease was that performed by Bodem et al. [32].This study analyzed the surgical outcomes for 39 patients with malignant diseases who received high-dose zoledronic acid treatment and had established BRONJ. At the time of surgical resection, 15 patients were registered as having a drug holiday. No statistically significant differences for healing after surgery were observed between patients undergoing ongoing BP therapy and those with temporarily suspended/completed BP therapy.
4. Discussion
In this systematic review, we investigated the evidence and efficacy of a high-dose AR drug holiday. Only one prospective study with a control group fitted the PICO framework, and this study concluded that drug holidays should not be implemented for i.v. BP therapy [25]. Thus, based on this study the hypothesis should be rejected. Drug holiday of high-dose AR will not reduce the risk of MRONJ. However, it would be wrong only to make this conclusion based on one underpowered, prospective controlled study. Although the other included studies did not directly compare discontinuation with continuation of high-dose AR therapy, they draw conclusions on implementing drug holidays based on different observations. It is clear that the level of evidence was low, but represents the best available information on this subject. None of the studies were randomized and most of them had a relatively small sample size, resulting in low-grade scientific evidence. Although identifying an important research question is relatively straightforward, the low incidence of MRONJ and high variation among patients and AR therapies mean it is difficult to complete RCTs or controlled prospective studies with sufficient patient numbers to answer that question. Even relevant low-level evidence studies are difficult to perform and should be respected for the accumulation of knowledge within this difficult to reach subject area. Consequently, some of these studies were mixed (e.g., including patients undergoing both high-dose and low-dose AR therapy). This is clearly one of the limitations of this review, because most of the included studies did not exclude patients undergoing low-dose AR treatment (e.g., patients with osteoporosis). Nevertheless, these studies are important and must be analyzed to understand the reasons for implementing high-dose AR drug holidays. Another limitation is related to the search strategy with MeSH terms and free text, where only 5 studies were identified. Thus, 9 studies were added through other sources. This was mainly due to the limited size of the topic and the great variation in used text words.
In the prospective studies by Bodem et al. and Saia et al., the number of patients and the surgical techniques were similar. Most patients in the two studies were undergoing high-dose BP treatment, but the health status of the patients varied, which also is a limitation difficult to avoid. In the study by Bodem et al., all the patients had been diagnosed with metastatic bone cancer, which means that the health of these patients had been compromised compared to patients who did not have cancer (e.g., patients diagnosed with osteoporosis) [25]. A compromised immune response may increase susceptibility to infections and possibly MRONJ onset [16]. In the study by Saia et al., only 72% of the patients were diagnosed with cancer [23].
The specific ARs used may be important for any relationship between drug holidays and MRONJ development. Although all AR agents modify bone remodeling by inducing apoptosis and inhibiting osteoclast mediated bone resorption, BPs and denosumab behave differently.
There are different types of BPs; all have a high affinity for bone, but their binding strengths are different. BPs have half-lives of approximately 10–12 years and continue to be recycled when bone is remodeled [38, 39].
Denosumab is a more recently developed AR agent, which is also used frequently. Denosumab is a human monoclonal antibody directed against the receptor activator of nuclear factor kappa B ligand (RANKL) [38]. RANKL is a membrane protein expressed in several tissues and organs. Denosumab targets osteoclast precursor cells in the bone marrow and also functions in bone tissue, where it stops osteoclast precursor cells from differentiating into mature osteoclast cells, as well as inhibiting the function and survival of existing osteoclasts. Denosumab has a half-life of 25.4 days, which means a single dose is reduced to an insignificant level after approximately 4–6 months [38].
The effects of BP and denosumab drug holidays may be very different, due to the different pharmacokinetic properties of the two agents. A BP drug holiday may be ineffective, due to the long half-life of the drugs. BPs will remain in the bone for years after patients have stopped receiving them. A temporary discontinuation of denosumab could be favorable due to denosumab's short half-life [40] – but an AR drug holiday may also increase the risk of SREs including recurrence of bone pain and possibly progression of bone metastases in the patients [41, 42, 43, 44]. When compared with zoledronic acid in a phase 3 trial, denosumab was associated with an increased rate of progression-free survival [43].
Patients were receiving denosumab therapy in only 2 of the 14 studies that were included [33, 34]. Hoefert et al. described data from 17 patients who underwent denosumab therapy. In total, 15 of these patients had high-doses of denosumab (Xgeva), but only 6 of the 17 patients had tooth extractions prior to MRONJ onset. However, in this study, the authors concluded that a denosumab drug holiday seemed to have no effect on healing outcomes and MRONJ onset [33]. Aljohani et al. also found no association between a denosumab holiday and MRONJ healing among 63 patients. In total, 49 of the 63 patients were treated using high-doses of Xgeva, and tooth extraction was the reason for MRONJ onset in 28 patients [34]. Thus, neither of the denosumab studies found that a drug holiday had any effect.
The remaining 12 studies, including the 3 prospective studies, involved BPs and only 2 of these studies clearly indicated that AR therapy should not be interrupted [25, 27]. Wilde et al. reported a retrospective study of 24 patients with a surgical aspect, which included resection of all necrotic bone, smoothing sharp bone edges, and primary wound closure. In total, 10 patients discontinued their BP therapy after being diagnosed with MRONJ. The authors observed no treatment failures within this group of patients, but among the 14 patients that continued their BP therapy, treatment was unsuccessful in four cases. However, the authors found no significant relationship between continued BP therapy and treatment failure, and they concluded that the results of the investigation did not support discontinuing BP therapy to perform surgery [27].
The remaining 10 studies recommended discontinuing BP therapy, suggested making decisions on a case-by-case basis with no standard protocol for drug holidays, or reported no conclusions regarding drug holidays at the time of tooth extraction.
Three retrospective studies found that a drug holiday for oral surgery was beneficial [26, 30, 35]. All patients in these retrospective studies had a MRONJ diagnosis at the time of the drug holidays. All 3 studies concluded that discontinuing BP therapy for more than 3 months had a positive effect on surgical outcomes. However, in the study performed by Kim et al., only 7 of the 54 patients had a malignant disease and these patients were not described as a group [30]. Dimitrakopoulos et al. found that the oncologist's opinion of the patient's disease status was a crucial factor in the decision to stop BP therapy for MRONJ treatment [35]. Wutzl et al. stated that the effectiveness of drug holidays, either for elective oral surgery or therapeutic reasons, should be assessed by future controlled studies [26]. These findings are consistent with some existing position papers and guidelines [7, 8, 9, 10, 11, 12, 13], whereas others suggest continuing drug therapy or making decisions on a case-by-case basis [1, 14, 15, 16, 17, 18].
Most of the studies included in this systematic review did not give any detailed description of the use of drug holidays (e.g. how long preoperatively, how long postoperatively) and did not draw specific conclusions on drug holidays either, but recommended assessing each case separately – most often in close collaboration with the physician [24, 28, 29, 31, 32, 36]. In the study performed by Voss et al., all patients discontinued their BP medication 4 weeks before and 6 weeks after surgery. This is the most details description of the use of drug holiday. These authors recommended early surgery because many oncologists discontinue BP-treatment once the patient develops exposed bone, to promote wound healing. However, there is a risk of pathological fractures and/or progression of the underlying disease due to the drug holiday [29]. In the study described by Jabbour et al., temporary discontinuation of BP therapy was discussed with the specialist responsible for treatment. However, the most recent follow-up to this study found that drug holidays had not apparently changed the health status of any of the patients [28]. Bodem et al. investigated whether patients who were undergoing i.v. BP therapy at the time of surgery had a higher risk of treatment failure compared with patients who had completed their BP therapy or had it temporarily suspended. This retrospective cohort study included a total of 39 patients with cancer. At the time of surgery, 15 patients (31.9%) were registered as having a drug holiday. In total, 9 of these 15 patients (60%) showed complete healing, but these findings were not statistically significant [32]. These results contrast with those reported by Wutzl et al. [26]; although, this may be because Wutzl et al. did not have a homogeneous study population. Jung et al. investigated the relationship between BP treatment and the occurrence of ONJ using a national database. A total of 1,569 patients were included based on 4-years retrospective periods, and only 317 patients were being treated with high-dose BP therapy. The authors found that 53.3% of all incidences of ONJ during the study period occurred after a drug holiday and that the frequency of ONJ occurrence declined steadily as the length of the drug holidays increased [36].
As with many similar studies, a limitation of the research performed by Jung et al. was that the study population was not homogeneous. In addition, many of these studies do not include patients who do not have MRONJ, and few studies compare drug holidays against no drug holidays. For example, Saia et al. reported that all patients included in their prospective cohort study had a drug holiday, meaning that no comparison was made between a drug holiday and “no drug holiday”. In the study by Bodem et al., only 17 patients had their BP treatment temporarily suspended; one of these patients developed MRONJ [25]. Saia et al. reported that 5 of their 44 cancer patients developed MRONJ. However, the AR agent treatment durations were not described [23]. Given the strong correlation between AR treatment duration and the risk of MRONJ, this is a limitation of the study performed by Saia et al.
Usually, the decision on whether to implement a drug holiday is taken by the oncologist and maxillofacial surgeon in close collaboration. The oncologist's decision is based on how healthy each patient is, and this is considered the internationally accepted procedure.
4.1. Expert opinion
The question whether to pause high-dose antiresorptive therapy in cancer patients when tooth extraction is needed is a worldwide concern. To date, there do not exist any high-evidence trials that give us information about this question. To fill out the gap of knowledge about high-dose drug holiday, we have designed a single-blinded randomized controlled trial, which aim is to evaluate high-dose antiresorptive drug holiday related to tooth extraction with primary mucosal closure in cancer patients, including how a drug holiday affects the health related quality of life. A feasibility study of the designed trail is right now ongoing at the department of Oral & Maxillofacial Surgery, Copenhagen University Hospital, Denmark. If this study indicate that the study setup is useful and that there are no clear adverse effects of not using a drug holiday, additional studies with necessary statistical power should be initiated.
As previously told, it is gold standard to pause the high-dose antiresorptive treatment in relation to tooth extraction in Denmark, like in many other countries worldwide, even though the lack of evidence. If our clinical trial reveals that a drug holiday is not necessary to avoid the development of MRONJ, it will have high impact on how to treat the oncological patients in the future. If cancer patients can continue their antiresorptive therapy, and thus the potential good effect on controlling metastatic progression and skeletal related pain, it will certainly benefit the patients. We hope that our randomized trial will contribute to answer the question whether to use or not to use high-dose drug holiday in relation to tooth extraction.
5. Conclusions
The efficacy of a high-dose AR drug holiday remains uncertain. Applying the PICO approach suggests that the focused question cannot be answered using high-level evidence, because no RCTs and only one controlled prospective study were identified. This study indicated that drug holiday of high-dose AR will not reduce the risk of MRONJ and must therefore be seen as unnecessary. Retrospective studies and case-series without controls suggested that high-dose AR drug holidays could produce different results. Good quality, large prospective studies may lead to firmer conclusions, although the unusual outcome and limited numbers of eligible patients make such studies difficult to perform and complete.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Not applicable.
References
- 1.Ruggiero S.L., Dodson T.B., Fantasia J., Goodday R., Aghaloo T., Mehrotra B., O’Ryan F. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw - 2014 update. J. Oral Maxillofac. Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Otto S., Pautke C., Van den Wyngaert T., Niepel D., Schiødt M. Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Canc. Treat Rev. 2018;69:177–187. doi: 10.1016/j.ctrv.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Ruggiero S.L., Mehrotra B., Rosenberg T.J., Engroff S.L. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J. Oral Maxillofac. Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Yazdi P.M., Schiodt M. Dentoalveolar trauma and minor trauma as precipitating factors for medication-related osteonecrosis of the jaw (ONJ): a retrospective study of 149 consecutive patients from the Copenhagen ONJ Cohort. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015;119:416–422. doi: 10.1016/j.oooo.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Schiodt M., Reibel J., Oturai P., Kofod T. Comparison of nonexposed and exposed bisphosphonate-induced osteonecrosis of the jaws: a retrospective analysis from the Copenhagen cohort and a proposal for an updated classification system. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014;117:204–213. doi: 10.1016/j.oooo.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Filleul O., Crompot E., Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J. Canc. Res. Clin. Oncol. 2010;136:1117–1124. doi: 10.1007/s00432-010-0907-7. [DOI] [PubMed] [Google Scholar]
- 7.Khan A.A., Sándor G.K., Dore E. Canadian consensus practice guidelines for bisphosphonate associated osteonecrosis of the jaw. J. Rheumatol. 2008;35:1391–1397. [PubMed] [Google Scholar]
- 8.Dickinson M., Prince H.M., Kirsa S., Zannettino A., Gibbs S.D.J., Mileshkin L., O’Grady J., Seymour J.F., Szer J., Horvath N., Joshua D.E. Osteonecrosis of the jaw complicating bisphosphonate treatment for bone disease in multiple myeloma: an overview with recommendations for prevention and treatment. Intern. Med. J. 2009;39:304–316. doi: 10.1111/j.1445-5994.2008.01824.x. [DOI] [PubMed] [Google Scholar]
- 9.Hellstein J.W., Adler R.A., Edwards B., Jacobsen P.L., Kalmar J.R., Koka S., Migliorati C.A., Ristic H. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2011;142:1243–1251. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 10.Snowden J.A., Ahmedzai S.H., Ashcroft J., D’Sa S., Littlewood T., Low E., Lucraft H., Maclean R., Feyler S., Pratt G., Bird J.M. Guidelines for supportive care in multiple myeloma 2011. Br. J. Haematol. 2011;154:76–103. doi: 10.1111/j.1365-2141.2011.08574.x. [DOI] [PubMed] [Google Scholar]
- 11.Khan A.A., Morrison A., Hanley D.A., Felsenberg D., McCauley L.K., O’Ryan F., Reid I.R., Ruggiero S.L., Taguchi A., Tetradis S., Watts N.B., Brandi M.L., Peters E., Guise T., Eastell R., Cheung A.M., Morin S.N., Masri B., Cooper C., Morgan S.L., Obermayer-Pietsch B., Langdahl B.L., Al Dabagh R., Davison K.S., Kendler D.L., Sandor G.K., Josse R.G., Bhandari M., El Rabbany M., Pierroz D.D., Sulimani R., Saunders D.P., Brown J.P., Compston J., Sándor G.K., Josse R.G., Bhandari M., El Rabbany M., Pierroz D.D., Sulimani R., Saunders D.P., Brown J.P., Compston J. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J. Bone Miner. Res. 2015;30:3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 12.Svejda B., Muschitz C., Gruber R., Brandtner C., Svejda C., Gasser R.W., Santler G., Dimai H.P. [Position paper on medication-related osteonecrosis of the jaw (MRONJ)] Wien Med. Wochenschr. 2016;166:68–74. doi: 10.1007/s10354-016-0437-2. [DOI] [PubMed] [Google Scholar]
- 13.Khan A.A., Morrison A., Kendler D.L., Rizzoli R., Hanley D.A., Felsenberg D., McCauley L.K., O’Ryan F., Reid I.R., Ruggiero S.L., Taguchi A., Tetradis S., Watts N.B., Brandi M.L., Peters E., Guise T., Eastell R., Cheung A.M., Morin S.N., Masri B., Cooper C., Morgan S.L., Obermayer-Pietsch B., Langdahl B.L., Al Dabagh R., Davison K.S., Sándor G.K., Josse R.G., Bhandari M., El Rabbany M., Pierroz D.D., Sulimani R., Saunders D.P., Brown J.P., Compston J. Case-based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the international task force on ONJ. J. Clin. Densitom. 2017;20:8–24. doi: 10.1016/j.jocd.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Kim K.M., Rhee Y., Kwon Y.-D., Kwon T.-G., Lee J.K., Kim D.-Y. Medication related osteonecrosis of the jaw: 2015 position statement of the Korean society for bone and mineral research and the Korean association of oral and maxillofacial surgeons. J. Bone Metab. 2015;22:151. doi: 10.11005/jbm.2015.22.4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoneda T., Hagino H., Sugimoto T., Ohta H., Takahashi S., Soen S., Taguchi A., Nagata T., Urade M., Shibahara T., Toyosawa S. Antiresorptive agent-related osteonecrosis of the jaw: position paper 2017 of the Japanese allied committee on osteonecrosis of the jaw. J. Bone Miner. Metabol. 2017;35:6–19. doi: 10.1007/s00774-016-0810-7. [DOI] [PubMed] [Google Scholar]
- 16.Terpos E., Sezer O., Croucher P.I., García-Sanz R., Boccadoro M., San Miguel J., Ashcroft J., Bladé J., Cavo M., Delforge M., Dimopoulos M.A., Facon T., Macro M., Waage A., Sonneveld P. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann. Oncol. 2009;20:1303–1317. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]
- 17.McLeod N.M.H., Davies B.J.B., Brennan P.A. Management of patients at risk of bisphosphonate osteonecrosis in maxillofacial surgery units in the UK. Surgeon. 2009;7:18–23. doi: 10.1016/s1479-666x(09)80062-0. [DOI] [PubMed] [Google Scholar]
- 18.Scottish Dental Clinical Effectiveness Programme Oral health management of patients at risk of medication-related osteonecrosis of the jaw. Br. Dent. J. 2017;222:930. doi: 10.1038/sj.bdj.2017.539. [DOI] [PubMed] [Google Scholar]
- 19.PROSPERO . 2019. International Prospective Register of Systematic Reviews.https://www.crd.york.ac.uk/prospero/ accessed April 2019. [Google Scholar]
- 20.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P.A., Straus S., Thorlund K., Jansen J.P., Mulrow C., Catala-Lopez F., Gotzsche P.C., Dickersin K., Boutron I., Altman D.G., Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 21.McHugh M.L. Interrater reliability: the kappa statistic. Biochem. Med. 2012;22(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 22.G.S. Wells, D. O´Connel, J. Peterson, The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses, (2019). http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 23.Saia G., Blandamura S., Bettini G., Tronchet A., Totola A., Bedogni G., Ferronato G., Nocini P.F., Bedogni A. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J. Oral Maxillofac. Surg. 2010;68:797–804. doi: 10.1016/j.joms.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Ferlito S., Puzzo S., Palermo F., Verzì P. Treatment of bisphosphonate-related osteonecrosis of the jaws: presentation of a protocol and an observational longitudinal study of an Italian series of cases. Br. J. Oral Maxillofac. Surg. 2012;50:425–429. doi: 10.1016/j.bjoms.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Bodem J.P., Kargus S., Eckstein S., Saure D., Engel M., Hoffmann J., Freudlsperger C. Incidence of bisphosphonate-related osteonecrosis of the jaw in high-risk patients undergoing surgical tooth extraction. J. Cranio-Maxillofacial Surg. 2015;43:510–514. doi: 10.1016/j.jcms.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Wutzl A., Pohl S., Sulzbacher I., Seemann R., Lauer G., Ewers R., Drach J., Klug C. Factors influencing surgical treatment of bisphosphonate-related osteonecrosis of the jaws. Head Neck. 2012;36:194–200. doi: 10.1002/hed.21708. [DOI] [PubMed] [Google Scholar]
- 27.Wilde F., Heufelder M., Winter K., Hendricks J., Frerich B., Schramm A., Hemprich A. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw, Oral Surgery. Oral Med. Oral Pathol. Oral Radiol. Endodontology. 2011;111:153–163. doi: 10.1016/j.tripleo.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Jabbour Z., El-Hakim M., Mesbah-Ardakani P., Henderson J.E., Albuquerque R. The outcomes of conservative and surgical treatment of stage 2 bisphosphonate-related osteonecrosis of the jaws: a case series. Int. J. Oral Maxillofac. Surg. 2012;41:1404–1409. doi: 10.1016/j.ijom.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Voss P.J., Joshi Oshero J., Kovalova-Müller A., Veigel Merino E.A., Sauerbier S., Al-Jamali J., Lemound J., Metzger M.C., Schmelzeisen R. Surgical treatment of bisphosphonate-associated osteonecrosis of the jaw: technical report and follow up of 21 patients. J. Cranio-Maxillofacial Surg. 2012;40:719–725. doi: 10.1016/j.jcms.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y.H., Lee H.K., Il Song S., Lee J.K. Drug holiday as a prognostic factor of medication-related osteonecrosis of the jaw. J. Korean Assoc. Oral Maxillofac. Surg. 2014;40:206. doi: 10.5125/jkaoms.2014.40.5.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes R.N., Rabelo G.D., Rocha A.C., Carvalho P.A.G., Alves F.A. Surgical therapy for bisphosphonate-related osteonecrosis of the jaw: six-year experience of a single institution. J. Oral Maxillofac. Surg. 2015;73:1288–1295. doi: 10.1016/j.joms.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Bodem J.P., Schaal C., Kargus S., Saure D., Mertens C., Engel M., Hoffmann J., Freudlsperger C. Surgical management of bisphosphonate-related osteonecrosis of the jaw stages II and III, Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016;121:367–372. doi: 10.1016/j.oooo.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 33.Hoefert S., Yuan A., Munz A., Grimm M., Elayouti A., Reinert S. Clinical course and therapeutic outcomes of operatively and non-operatively managed patients with denosumab-related osteonecrosis of the jaw (DRONJ) J. Cranio-Maxillofacial Surg. 2017;45:570–578. doi: 10.1016/j.jcms.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Aljohani S., Gaudin R., Weiser J., Tröltzsch M., Ehrenfeld M., Kaeppler G., Smeets R., Otto S. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J. Cranio-Maxillofacial Surg. 2018;46:1515–1525. doi: 10.1016/j.jcms.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 35.Dimitrakopoulos I., Magopoulos C., Karakasis D. Bisphosphonate-induced avascular osteonecrosis of the jaws: a clinical report of 11 cases. Int. J. Oral Maxillofac. Surg. 2006;35:588–593. doi: 10.1016/j.ijom.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Jung S.Y., Suh H.S., Park J.W., Kwon J.W. Drug holiday patterns and bisphosphonate-related osteonecrosis of the jaw. Oral Dis. 2018:1–10. doi: 10.1111/odi.12966. [DOI] [PubMed] [Google Scholar]
- 37.AHRQ Standards. 2019. https://www.ncbi.nlm.nih.gov/books/NBK115843/.../appe-fm3.pdf [Google Scholar]
- 38.Damm D.D., Jones D.M. Bisphosphonate-related osteonecrosis of the jaws: a potential alternative to drug holidays. Gen. Dent. 2013;61:33–38. [PubMed] [Google Scholar]
- 39.Ruggiero S.L., Fantasia J., Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management, Oral Surgery. Oral Med. Oral Pathol. Oral Radiol. Endodontology. 2006;102:433–441. doi: 10.1016/j.tripleo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Stopeck A.T., Fizazi K., Body J.-J., Brown J.E., Carducci M., Diel I., Fujiwara Y., Martín M., Paterson A., Tonkin K., Shore N., Sieber P., Kueppers F., Karsh L., Yardley D., Wang H., Maniar T., Arellano J., Braun A. Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer, Support. Care Cancer. 2016;24:447–455. doi: 10.1007/s00520-015-2904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallego L., Junquera L. Consequence of therapy discontinuation in bisphosphonate-associated osteonecrosis of the jaws. Br. J. Oral Maxillofac. Surg. 2009;47:67–68. doi: 10.1016/j.bjoms.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Morgan G.J., Davies F.E., Gregory W.M., Cocks K., Bell S.E., Szubert A.J., Navarro-Coy N., Drayson M.T., Owen R.G., Feyler S., Ashcroft A.J., Ross F., Byrne J., Roddie H., Rudin C., Cook G., Jackson G.H., Child J.A. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raje N., Terpos E., Willenbacher W., Shimizu K., García-Sanz R., Durie B., Legieć W., Krejčí M., Laribi K., Zhu L., Cheng P., Warner D., Roodman G.D. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19:370–381. doi: 10.1016/S1470-2045(18)30072-X. [DOI] [PubMed] [Google Scholar]
- 44.Van Poznak C.H., Temin S., Yee G.C., Janjan N.A., Barlow W.E., Biermann J.S., Bosserman L.D., Geoghegan C., Hillner B.E., Theriault R.L., Zuckerman D.S., Von Roenn J.H. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J. Clin. Oncol. 2011;29:1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]