Abstract

The identification of the dust characteristics in coal mine working faces is essential for preventing coal dust explosion and occupational diseases. In this paper, dust samples from the coal mines in southern Shanxi province and Henan province, central North China, were selected as the research objects. The results show that the dust contains primarily organic matter, as well as considerable amounts of minerals. The chemical composition of dust at the working faces is the most complex. According to the proportion of PM10, the dust composition can be divided into three types: symmetrical, fine-dominated, and coarse-dominated. The wettability of dust increases with the increase of the oxygen–carbon ratio on its surface, increase of ash content, decrease of fixed carbon content, and decrease of particle size. In addition, the great variety of harmful elements in dust, some with a high content, can harm the human body. An explosion index is proposed to evaluate the likeliness tendency of coal dust explosion based on several key affecting factors. The surfactant (0.05% AN solution) adopted in this paper can significantly increase the wettability of coal dust and inhibit the generation of dust greatly, showing good ability in preventing coal dust explosion and occupational diseases.
1. Introduction
Coal dust is the solid fine particle generated during coal mine production, of which dustfall settles to the ground and flying coal dust floats in the air. Coal dust plays a significant role in the gas explosion process.1−3 Meanwhile, coal dust can also cause explosion itself.4−6 Coal is an inherently combustible material, and when it is broken into dust, the contact area between coal and air significantly increases, leading to a higher explosion potential once an ignition source appears. The shockwaves generated by earlier explosions will raise secondary dust in the roadway, resulting in explosion propagation, which poses a great threat to miners and the mine. In addition, dust particles, containing harmful elements (e.g., silica), with an average diameter of less than 2.5 μm can directly enter the alveoli in the human body and combine with toxic host cells (especially macrophages) to cause permanent damage,7 leading to pneumoconiosis and other diseases.8−12 Moreover, dust can also accelerate the mechanical wear of equipment and reduce the visibility of the working face, thereby increasing the risk of safety hazards.13−15 In the past, much concerns have been concentrated on the prevention of sudden accidents rather than on the harmful influence of coal dust on the human body.16,17 In recent years, social and technological progress has led to enhanced health awareness and an ever-increasing emphasis on dust reduction in coal mines. The Chinese government issued the Technical Specifications of Comprehensive Dust Control Measures for Underground Coal Mines, which requires coal mines to adopt strict dust reduction measures. Therefore, the systematic study of dust characteristics is related to personal health, production safety, and environmental air quality, i.e., the HSE of the underground space of the mine. The working face generates about 60% of the total amount of dust in a coal mine,18 which deserves the focus of control efforts. The study of dust characteristics is an important step toward achieving the goal of dust control. Historical studies were conducted mainly by crushing coal in a laboratory to generate dust rather than collecting dust from the working face,19−22 which was unrealistic because the chemical composition, particle size, wettability, and harmful elements of real dust are different depending on individual coal types or the surrounding rock.
After a coal sample was crushed and screened, the particle size distribution of the sample was tested using a laser particle size analyzer. The analysis results generally showed only one single peak, and the distribution was in the shape of an “A” or occasionally in the shape of an “M”.23−26 The chemical composition of coal dust produced using the above method was identical to that of coal. Research on dust produced in the actual coal mining process was rare, and reports on its particle size characteristics and chemical composition were even rarer. The particle size distribution and surface morphology of dust generated by crushing varied substantially among different studies, which were determined by the different chemical compositions and mechanical properties of coal from different coal mines or coal beds.27
Coal dust wettability constitutes the theoretical basis for coal bed water injection and the water spraying method for dust reduction. It is also the main reference factor for the design and development of chemical inhibitors.28−31 Surfactant has an effect on the surface properties of different kinds of coal, and adding the appropriate concentration of surfactant can obviously reduce the contact angle of coal and improve the wetting effect.32−34 The pore structure of coal can be changed when the coal seam is injected with water to remove dust, and coal samples with different metamorphism have different pore structures.35 Opinions vary on the influence of coal properties on the wettability of dust. For example, some researchers have found that the wettability of coal dust has a negative correlation with the moisture content,36,37 while others believe that the moisture content does not have a decisive influence on the hydrophilicity of coal;38 some have found that the increase of its fixed carbon content will make coal dust more hydrophobic,37 while others have found that the fixed carbon content has no effect on dust wettability.36 Neither is there any consensus on the influence of ash content and volatiles on coal dust wettability.36−38 As early as in 1982, some scholars found that the wetting characteristics of dust are affected by particle size,39 showing that the smaller the particle size and the larger the specific surface area, the poorer the wettability of coal dust.6,40,41 Therefore, studying the particle size of coal dust can also provide useful information for dust reduction at the working faces of coal mines. Generally, infrared spectroscopy and X-ray photoelectron spectroscopy (XPS) have been used to study the wetting mechanism of coal,42,43 and the results show that benzene, aromatic hydrocarbons, methyl-containing aliphatic hydrocarbons, methylene, and other substances with macromolecular carbon structures are hydrophobic, while organic matters with oxygen-containing functional groups represented by hydroxyl and carboxyl, as well as silicate and carbonate minerals, are hydrophilic.23,28 XPS has been widely used in chemical structure analysis because it effectively enables the observation of the elemental composition and functional group information of the coal surface at a scale of 10 nm.44−46 Infrared spectroscopy is also a means of analyzing coal dust functional groups,47−49 but it is not as accurate as XPS. Notably, the controlling factors of dust wettability are very complex and related to the organic matter composition of coal, but also related to chemical composition and particle size.
Many trace elements can be enriched during the formation of coal, even to the extent that some elements (Ga, Ge, Li, U, etc.) become industrially recoverable.50−54 However, in the process of coal mining or utilization, some elements can enter the human body and cause harm.55−58 There are about 25 kinds of harmful trace elements in coal and coal dust,59−61 which can be roughly divided into five categories:59,62 toxic elements (B, Ba, Be, Cd, Hg, Pb, Sn, Tl, and V), carcinogenic elements (As, Be, Cd, Cr, Ni, and Pb), elements that pollute the atmosphere after combustion and cause further harm to the human body (As, Be, Cd, Co, Cr, f, Hg, Mn, Ni, Pb, Sb, Se, Th, and U), elements that are beneficial in small amounts but harmful when excessive (B, Co, Cu, Mn, Mo, Ni, and Zn), and radioactive elements (Th and U). Some scholars have systematically summarized the occurrence state of harmful trace elements in coal and studied the content,63,64 enrichment, and distribution of such elements in different regions,65−74 which is of great significance for protecting human living environments. The background values of trace elements in coal in China are generally comparable to those of the world, but some are higher.75 The main use of coal in China is to generate electricity and heat through combustion.76,77 In this process, trace elements, fly ash, and bottom ash are discharged into the atmosphere through different channels.78 Although China issued its Ambient Air Quality Standards (GB3095-2012), which provides the annual average standards for five heavy metal elements Hg, Pb, As, Cr (VI), and Cd, the pollution status of other airborne trace elements is so far unclear because there has been no routine monitoring or corresponding general survey of pollution sources.
Coal dust explosion is mainly affected by coal rank,79−81 particle size,82−84 specific surface area,85−87 moisture content,88−90 volatile content,91−93 and the concentration.94−96 Given constant dust concentration and particle size, the explosion pressure increases with the increase of organic matter content.97 The higher the volatile content, the greater the explosion intensity of the dust, and the explosion pressure increases significantly faster with the decrease of particle size because the existence of fine particles greatly increases the total effective surface area and volatilization rate of dust, accelerating the dust explosion process.91 When the particle size is constant, the maximum explosion pressure and flame propagation speed increase with the dust concentration.82,98 In addition, the higher moisture content of coal dust can not only consume some of the reaction heat caused by dust explosion but also form capillary bridges among particles, reducing the interparticle distance, causing the fine dust particles to gradually agglomerate, which reduces the degree of dispersion.88
In this paper, the dustfall samples were collected directly from the working faces and roadways of coal mines. The surface elements and functional groups of the dust were quantitatively analyzed, and combined with the measured wetting contact angles, the wettability of the dust was evaluated. In addition, the harm of the coal dust to human body was determined by testing the harmful trace element contents. The above research aims to reduce dust and prevent coal dust explosion and occupational disease, thereby facilitating the construction of “green mines”.
2. Results and Discussion
2.1. Chemical and Mineral Compositions of Coal Dust
The proximate analysis results (Table 1) show that the dust in each mine is mainly composed of organic matter, along with different amounts of minerals. The ash content varies greatly among the mines within the range of 12.59–77.08% (avg. 28.85%). However, in the same mining area, the ash content of the dust from the working face dust is always higher than that of the dust from the air intake roadway and the return air roadway. In particular, the ash content of JLS-2 reaches 77.08%, while those of JLS-1 and JLS-3 are only 22.34 and 36.69%, respectively. The ash content of the dust from the different positions of Hebi no. 6 (HB6) shows low differences, with the ash content of HB6-2 of 17.83% and those of HB6-1 and HB6-3 being 17.30 and 14.7%, respectively. It can be seen that the dust composition is complex.
Table 1. Proximate Analysis of Dust.
| sample ID | dust source | sampling location | Mad (%) | Aad (%) | Vad (%) | FCad (%) |
|---|---|---|---|---|---|---|
| JLS-1 | Jiulishan coal mine | intake airway | 4.14 | 22.34 | 7.67 | 65.85 |
| JLS-2 | coalface | 4.20 | 77.08 | 7.23 | 11.49 | |
| JLS-3 | return airway | 1.5 | 36.69 | 6.24 | 55.57 | |
| HB6-1 | Hebi no. 6 coal mine | intake airway | 1.93 | 17.30 | 28.21 | 52.56 |
| HB6-2 | coalface | 1.17 | 17.83 | 25.12 | 55.88 | |
| HB6-3 | return airway | 0.67 | 14.7 | 13.18 | 71.45 | |
| DY | Daiyang coal mine | return airway | 12.43 | 12.59 | 8.03 | 66.95 |
| LTS | Lutaishan coal mine | return airway | 2.23 | 42.64 | 10.69 | 44.44 |
| PM2 | Pingmei no. 2 coal mine | return airway | 0.68 | 25.33 | 20.71 | 53.28 |
| SH | Sihe coal mine | return airway | 4.01 | 21.96 | 6.97 | 67.06 |
The X-ray diffraction (XRD) tests show that besides coal, the dust also contains certain amounts of clay minerals (illite, kaolinite, and montmorillonite), quartz, calcite, ankerite, halloysite, dickite, pyrite, galena, brookite, nacrite, and saponite. The mineral compositions at different sampling locations are different, but they invariably contain organic matter, quartz, and clay minerals (Figure 1). Calcite was found in all of the dust samples at 29° of 2θ except for Jiulishan (JLS) (Figure 1d–j). Ankerite, halloysite, and dickite were found in two sampling sites. Ankerite was found in HB6-2 and Lutaishan (LTS) at 31° of 2θ (Figure 1e,h), halloysite was found in JLS-1 and HB6-2 at 25° of 2θ (Figure 1a,e), and dickite was found in JLS-2 and HB6-1 at 12, 20, and 25° of 2θ (Figure 1b,d). Pyrite, galena, brookite, nacrite, and saponite were only present in one sampling site. Pyrite was found in many peaks where Pingmei no. 2 (PM2) ranges from 33 to 61° of 2θ (Figure 1i), galena was found in many peaks where Sihe (SH) ranges from 31 to 70° of 2θ (Figure 1j), brookite and nacrite were found in many peaks where HB6-3 ranges from 12 to 74° of 2θ (Figure 1f), and saponite was found in HB6-2 at 6° of 2θ (Figure 1e). The dust from the working face in JLS and HB6 has the highest mineral (ash) content and the most complex composition (Figure 1a–f) compared with that from the air intake and return air roadways. However, among the dust samples from the return air roadways of different mines, the JLS-3 and LTS samples have the highest mineral (ash) content but a relatively simple composition (Figure 1c,g); the Dayang (DY) samples have the lowest mineral (ash) content but the most complex composition (Figure 1h); and the mineral (ash) content and complexity of HB6-3, PM2, and SH are moderate.
Figure 1.
X-ray diffraction spectra of the dust from each mine. (a) JLS-1; (b) JLS-2; (c) JLS-3; (d) HB6-1; (e) HB6-2; (f) HB6-3; (g) LTS; (h) DY; (i) PM2; (j) SH.
The field emission scanning electron microscope (FE-SEM) observation results show that all samples contain coal and clay minerals, dominated by kaolinite and illite. The organic matter includes homogeneous vitrinite (Figure 2a,b), fusinite with a cell structure (Figure 2b,c), etc. Among the clay minerals, illite is generally leaf-shaped and has a silklike surface (Figure 2b,d), while kaolinite is accordion-shaped (Figure 2e). Among the other minerals, quartzite has a relatively intact hexagonal columnar shape (Figure 2f), calcite has an uneven surface and many intergranular pores (Figure 2g), and pyrite has a crystal size of about 5 μm and appears as strawberry-like aggregates (Figure 2h). In addition, richer clay minerals were also observed in the LTS and JLS-3 dust, which is consistent with the higher ash content.
Figure 2.
Field emission scanning electron microscope images of dust. (a, c) JLS-3; (b, d) HB6-3; (e) SH; (f) DY; (g, h) PM2.
The composition of dust from the working faces is more complicated than that from the air intake and return air roadways because the working face is the foremost source of dust generation, while the dust in the roadways has been sorted during its flight carried by wind flow. The composition of return air roadway dust also differs among the mining areas. The chemical composition is simpler when the ash content is higher because part of the dust with higher density and better wettability has settled after a short distance during its flight. However, the mineral composition is more complex when the ash content is lower because minerals and coal can easily form aggregates in dust with a low ash content, which is not conducive to be sorted during its flight.
2.2. Particle Size Characteristics of Dust
2.2.1. Particle Size Distribution
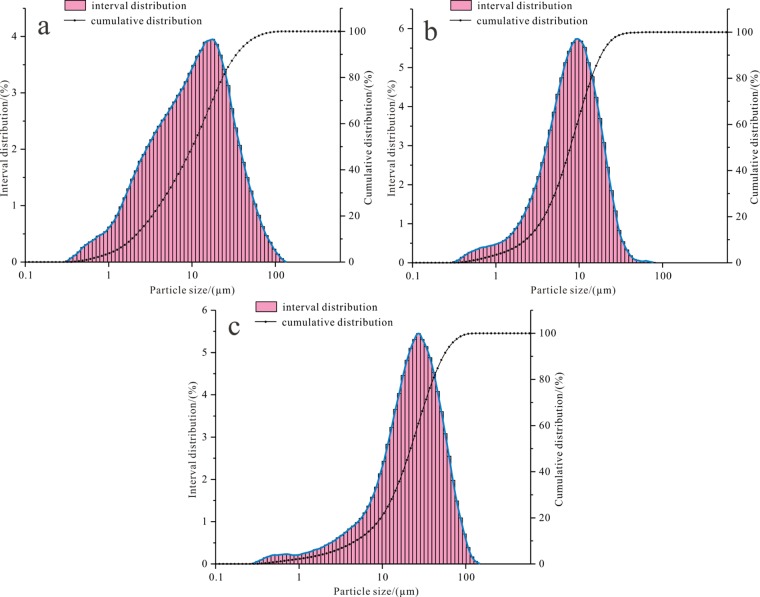
According to the particle size distribution characteristics, the dust is classified into three types, i.e., “fine type”, “symmetrical type”, and “coarse type”, referring to the conditions that particles smaller than 10 μm account for more than 55, 45–55%, and less than 45%, respectively. The test results of particle size with a laser (Table 2 and Figure 3) show that the particle size distribution of the JLS-2 and SH dust samples is the symmetrical type, in which the proportion of PM10 is 47.8 and 47.1%, respectively. The minimum and maximum sizes of JLS-2 are 0.319 and 132 μm, respectively, with the high peak appearing at 17.72 μm. The D50 size of it is 11.17 μm, and the specific surface area is 443 m2/kg (Figure 3a). There is a trough at 0.602 μm for SH. The particle size distribution of the JLS-3 and LTS dust samples is the fine type, in which the proportions of PM10 are 58.95 and 60.09%, respectively. The minimum and maximum sizes of LTS are 0.319 and 86.51 μm, respectively, with the high peak appearing at 9.404 μm, and there is a trough at 63 μm. The D50 size of it is 8.668 μm, and the specific surface area is 450.6 m2/kg (Figure 3b). The particle size distribution of the JLS-1, HB6-1, HB6-2, HB6-3, DY, and PM2 dust samples is the coarse type, in which the proportions of PM10 are 19.23, 20.57, 24.78, 36.81, 36.04, and 19.62%, respectively. The minimum and maximum sizes of PM2 are 0.287 and 146.7 μm, respectively, with the high peak appearing at 27.05 μm. The D50 size of it is 24.24 μm, and the specific surface area is 243.2 m2/kg (Figure 3c). There is a trough at 0.669, 0.542, 0.542, and 0.669 μm for JLS-1, HB6-3, DY, and PM2, respectively.
Table 2. Characteristics of Dust Particle Size.
| sample ID | distribution pattern | minimum size (μm) | low peak size (μm) | high peak size (μm) | maximum size (μm) | D10 (μm) | D50 (μm) | D90 (μm) | specific surface area (m2/kg) | proportion of PM10 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| JLS-2 | symmetrical type | 0.319 | 17.72 | 132 | 2.103 | 11.17 | 37.69 | 443 | 47.8 | |
| SH | 0.319 | 0.602 | 12.91 | 56.69 | 2.88 | 11.05 | 24.26 | 397.5 | 47.1 | |
| JLS-3 | fine type | 0.355 | 9.404 | 45.89 | 3.036 | 8.957 | 19.48 | 391.7 | 58.95 | |
| LTS | 0.319 | 63 | 9.404 | 86.51 | 2.545 | 8.668 | 20.23 | 450.6 | 60.09 | |
| JLS-1 | coarse type | 0.355 | 0.669 | 27.05 | 201.4 | 5.645 | 25.54 | 66.64 | 196.1 | 19.23 |
| HB6-1 | 0.355 | 27.05 | 146.7 | 5.628 | 22.27 | 53.26 | 207.2 | 20.57 | ||
| HB6-2 | 0.355 | 17.72 | 163 | 5.372 | 18.78 | 53.82 | 219.3 | 24.78 | ||
| HB6-3 | 0.258 | 0.542 | 15.95 | 118.7 | 3.213 | 13.99 | 34.27 | 353.7 | 36.81 | |
| DY | 0.319 | 0.542 | 14.35 | 106.8 | 4.389 | 13.43 | 29.97 | 287.6 | 36.04 | |
| PM2 | 0.287 | 0.669 | 27.05 | 146.7 | 5.297 | 24.24 | 59.61 | 243.2 | 19.62 |
Figure 3.
Particle size distribution of the dust. (a) symmetrical type (JLS-2); (b) fine type (LTS); (c) coarse type (PM2).
For samples collected from return air roadways, the FE-SEM observation results showed (Figure 4) that the proportions of coarse and fine particles are basically equal for the SH samples (Figure 4a); the JLS-3 and LTS samples contain more fine particles than coarse particles (Figure 4b,c); and the HB6-3, DY, and PM2 samples contain more coarse particles than fine particles (Figure 4d–f). These observations are consistent with the above XRD test results.
Figure 4.
Field emission scanning electron microscope images of dust. (a) SH; (b) JLS-3; (c) LTS; (d) HB6-3; (e) DY; (f) PM2.
2.2.2. Relationships between Dust Particles
According to the FE-SEM observation results, the relationships between the dust particles can be divided into the “independent type”, “agglomeration type”, and “adhesion type”. The dust particles of DY, HB6-3, and PM2 mainly exist in the form of agglomeration (Figure 2f,h), and those of LTS mainly exist in the form of adhesion (Figure 5a), while the proportion of agglomeration and adhesion is basically equal in the dust particles of JLS-3 and SH (Figure 5b). The independent type is rare as there are always more or less small particles attached to the surfaces of large particles (Figure 4e).
Figure 5.
Dust scanning electron microscope images. (a) LTS; (b) SH.
The above figure shows that the particle size distribution differs among the coal mines, but there is a common trend of decrease in particle sizes from the air intake roadway to the working face and then to the return air roadway, while the proportion of PM10 increases in that sequence. Such distribution is related to the sources and nature of coal dust particles, but the main cause is the flight distance and the sorting effect during flight. Particles with higher density and larger size settle easily, resulting in a larger amount of fine dust in the return air roadway, and the specific surface area of dust increases with the proportion of PM10.
2.3. Wettability of Dust
2.3.1. Contact Angle Test Results
The most straightforward characterization method of dust surface wettability is through measuring the contact angle.99 The smaller the contact angle, the better the hydrophilicity and wettability. The test results show that (Table 3, Figures 6 and7) the surface tension of the distilled water is 74.99 mN/m, and the contact angles between distilled water and the DY, HB6-3, HB6-1, PM2, and HB6-2 dust samples are 68.75, 66.51, 65.13, 64.25, and 63.2°, respectively, showing relatively poor wettability. The wettability of the SH, JLS-1, and JLS-3 dust samples is moderate, with the contact angles of 59, 55.37, and 48.4°, respectively, and the wettability of the LTS and JLS-2 dust samples is the greatest, with contact angles of 37.25 and 28.7°, respectively. The surface tension of the 0.05% AN solution is 26.134 mN/m, and the contact angle with the DY sample is the largest, although it is only 22.5°, while its contact angles with the dust from the other mines are all below 20°. In particular, the contact angles with the PM2, SH, HB6-2, LTS, and JLS-1 samples are 18.5, 17.25, 15.79, 14.25, and 12.31°, respectively, and the contact angles with JLS-3, HB6-1, and HB6-3 are merely 10, 9.8, and 7°, respectively.
Table 3. Contact Angle between Liquid and Dust.
| contact
angle (deg) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| liquid type | surface tension (mN/m) | JLS-2 | LTS | JLS-3 | JLS-1 | SH | HB6-2 | PM2 | HB6-1 | HB6-3 | DY |
| distilled water | 74.99 | 28.7 | 37.25 | 48.4 | 55.37 | 59 | 63.2 | 64.25 | 65.13 | 66.51 | 68.75 |
| 0.05% AN solution | 26.134 | 7.6 | 14.25 | 10 | 12.31 | 17.25 | 15.79 | 18.5 | 9.8 | 7 | 22.5 |
Figure 6.

Contact angle between liquid and dust.
Figure 7.

Diagrams showing the measurement of contact angle between liquid and coal dust. (a) SH; (b) HB6-3.
2.3.2. XPS Test Results
2.3.2.1. Surface Element Test Results
The XPS test results (Table 4) show that the relative content of carbon elements on the dust surface varies from 53.76 to 81.5%, with the DY and HB6-3 samples possessing the highest content, both above 80%. The content of the LTS sample was the lowest at only 53.76%. The relative content of oxygen on the dust surface varies from 16.4 to 43.75%, with the LTS sample having the highest content, reaching 43.75%. Those of PM2, HB6-3, and DY are the lowest, all below 20%. The atomic ratios of oxygen and carbon (oxygen–carbon ratio) on the dust surfaces of the return air roadway samples from the six mines vary significantly, ranging from 20.12 to 81.38%, among which the oxygen–carbon ratios of the DY, HB6-3, and PM2 samples are 20.12, 20.76, and 24.49%, respectively; those of SH and JLS-3 are 45.21 and 49.18%, respectively; and that of LTS is 81.38%.
Table 4. Values of Oxygen and Carbon of Dust through the XPS Test.
| sample ID | relative content of carbon (%) | relative content of oxygen (%) | O/C (atom %) |
|---|---|---|---|
| LTS | 53.76 | 43.75 | 81.38 |
| JLS-3 | 65.66 | 32.29 | 49.18 |
| SH | 67.58 | 30.55 | 45.21 |
| PM2 | 77.68 | 19.02 | 24.49 |
| HB6-3 | 80.64 | 16.74 | 20.76 |
| DY | 81.5 | 16.4 | 20.12 |
2.3.2.2. Functional Group Test Results
The wettability of coal is mainly related to carbon and oxygen elements on the coal surface. Oxygen content can be analyzed based on C 1s and O 1s in the XPS test, but O 1s spectrograms are likely to have been interfered with by oxygen absorbed from the air or moisture on the coal surface.100 Therefore, only the C 1s spectrograms of the dust samples in the return air roadways of the mines were analyzed by peak fitting using XPSPEAK software. There are four forms of carbon in the surface structure of coal,101−103 among which the 284.8 eV peak is attributed to aromatic units and its substituted alkane (C–C, C–H), the 286.3 eV peak to phenolic carbon or ether carbon (C–O), the 287.5 eV peak to the carbonyl group (C=O, O–C–O), and the 289.0 eV peak to the carboxyl group (O=C–O). The content of each functional group was obtained through peak fitting (Table 5), which shows that the carbon elements on the dust surface mainly exist in the form of aromatic carbon C–C and fatty carbon C–H, with the relative content ranging from 78.78 to 94.47%. The oxygen-containing functional group mainly exists in the form of C–O, with the relative content ranging from 4.3 to 15.43%, and those of DY and LTS are the lowest and the highest, respectively. The relative content of carbonyl varies slightly within a range of 0.49–4.68%, showing that of PM2 and LTS are the lowest and the highest, respectively. The carboxyl content is low in all samples with a relative content ranging from 0 to 1.11%, and that of PM2, HB6-3, and DY samples is 0.
Table 5. XPS C 1s Analysis Results of Dust.
| relative
content (%) |
||||||
|---|---|---|---|---|---|---|
| carbon binding form | LTS | JLS-3 | SH | PM2 | HB6-3 | DY |
| C–C, C–H | 78.78 | 89.88 | 89.28 | 88.01 | 84.07 | 94.47 |
| C–O | 15.43 | 6.37 | 7.19 | 11.5 | 15.14 | 4.3 |
| C=O, O–C–O | 4.68 | 2.83 | 2.98 | 0.49 | 0.79 | 1.23 |
| O=C–O | 1.11 | 0.92 | 0.55 | 0 | 0 | 0 |
2.3.3. Analysis of Factors Affecting Dust Wettability
The wettability of the dust surface is mainly affected by the following four factors: dust surface elements, surface functional groups, ash and fixed carbon contents, and dust particle size.
2.3.3.1. Surface Elements
As shown in Figure 8, the dust wettability increases with the decrease of carbon content on the dust surface (Figure 8a), the increase of the surface oxygen content (Figure 8b), and the surface oxygen–carbon ratio (Figure 8c), indicating that the hydrophilicity of dust increases with the increase of oxygen elements.
Figure 8.
Relation between dust surface elements and contact angle. a, Relative content of carbon; b, relative content of oxygen; c, oxygen–carbon ratio.
2.3.3.2. Surface Functional Groups
Figure 9 shows that the dust wettability increases with the relative contents of C=O (O–C–O) and O=C–O on the dust surface (Figure 9a,b). This is because these polar oxygen-containing functional groups are associated with hydrogen in water molecules by hydrogen bonds under the action of dipole force, thereby exhibiting strong activity and promoting the wetting property of water to dust.
Figure 9.
Relation between functional group content and contact angle. (a) Relative contents of C=O and O–C–O; (b) relative content of O=C–O.
2.3.3.3. Proximate Analysis Parameters
Figure 10 indicates that the dust wettability increases with the ash content (Figure 10a) and decreases with the increase of fixed carbon content (Figure 10b). As ash is the residue obtained from the complete combustion of the minerals in dust under certain conditions, its content mainly depends on the amount of original minerals in the dust, mostly mudstone composed of clay minerals. As the hydrophilicity of mudstone is stronger than that of coal,104 the higher ash content and the lower fixed carbon content generally lead to better wettability.
Figure 10.
Relation of proximate analysis and the contact angle. (a) Ash; (b) fixed carbon.
2.3.3.4. Particle Size
As shown in Figure 11, the dust wettability increases with the PM10 content (Figure 11), and this is because the smaller the particle size, the smaller the contact area of the capillary bridges between particles, and the smaller the cohesive force (details to follow).
Figure 11.
Relation of particle size and contact angle.
2.3.4. Dust Reduction
2.3.4.1. Relationship between Wettability and Dust Occurrence Form
The wettability of dust determines its occurrence form. When wettability is poor, dust mostly exists in the form of agglomeration with the small-size particles filling in the pores of large-size particles. It is because, when the contact angle (θ) between the dust and the solution is large, and the particle size (r) is small, the area of capillary bridges between the particles and the cohesive force will be low (Figure 12) and the cohesive force will be small.105 As a result, capillary bridges with a sufficient contact area can only form between large-size particles, which encapsulate the small particles to settle as agglomerations, e.g., the case in the PM2, HB6-3, and DY samples. When the wettability is high, the contact angle between the dust and the solution is small, and capillary bridges with a large contact area can form even between smaller particles, which adhere to larger particles for settlement, e.g., the case in the LTS sample. With moderate wettability, both agglomerative and adherent types are possible, e.g., the case of JLS-3 and SH.
Figure 12.
Dust particle liquid bridge.
2.3.4.2. Dust Reduction Using Surfactant
Compared with distilled water, the 0.05% AN solution can significantly reduce the contact angle with dust (Table 3 and Figure 6), by 59.51° for HB6-3 and 21.1° for JLS-2, as well as moderate degrees for the samples from other mines. Although the reduction of the contact angle varies, the wettability of dust is significantly enhanced for all samples, increasing the areas of capillary bridges between the dust particles, which in turn increases the cohesion force, enables the dust to settle to the ground in the form of agglomeration or adhesion, and achieves the dust reduction effect.
Therefore, the key to dust prevention and control is to reduce the contact angle between dust and water to induce agglomeration or adhesion-type settlements.
2.4. Harmful Elements in Dust
According to the results of the inductively coupled plasma mass spectrometry (ICP-MS) test (Table 6), the content of harmful trace elements in the dust is generally higher than that of the coal. In particular, the contents of As, Cr, Mn, Ba, V, Zn, and P in the dust are much higher than in the coal in all mining areas.
Table 6. Contents of Harmful Trace Elements in Coal and Dust.
| content (μg/g) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTS |
JLS-3 |
SH |
PM2 |
HB6-3 |
DY |
||||||||
| hazard | element | dust | coal | dust | coal | dust | coal | dust | coal | dust | coal | dust | coal |
| three | Be | 2.0 | 0.7 | 1.9 | 6.5 | 1.1 | 0.7 | 1.5 | 2.6 | 0.7 | 0.9 | 0.8 | 0.6 |
| Cd | 0.15 | 0.0 | 0.4 | 0.05 | 0.08 | 0.1 | 0.59 | 0.1 | 0.23 | 0.1 | 0.09 | 0.1 | |
| Pb | 30.7 | 3.6 | 23.1 | 14.2 | 23.5 | 16.2 | 11.4 | 7.2 | 11.4 | 8.5 | 13.0 | 24.8 | |
| Ni | 12.3 | 12.1 | 20.0 | 21.0 | 6.6 | 1.9 | 25.8 | 26.5 | 2.5 | 23.9 | 4.1 | 1.7 | |
| two | As | 47.93 | 0.4 | 0.6 | 3.16 | 2.79 | 2.9 | 10.44 | 0.9 | 10.31 | 0.9 | 8.41 | 1.4 |
| Cr | 60.8 | 7.7 | 48.2 | 24.2 | 42.7 | 14.1 | 61.3 | 12.6 | 33.3 | 9.1 | 32.9 | 8.0 | |
| Co | 7.2 | 2.3 | 11.2 | 32.9 | 6.6 | 0.1 | 4.0 | 6.7 | 2.8 | 9.8 | 3.0 | 0.5 | |
| Mn | 153.6 | 3.0 | 98.9 | 39.9 | 118.6 | 28.0 | 91.9 | 29.7 | 100.8 | 5.0 | 38.6 | 19.5 | |
| Th | 2.80 | 0.4 | 1.2 | 5.62 | 2.72 | 0.3 | 2.77 | 0.5 | 2.56 | 0.6 | 2.92 | 0.3 | |
| U | 1.71 | 1.5 | 0.3 | 1.63 | 0.78 | 1.2 | 1.70 | 1.1 | 0.57 | 0.3 | 0.76 | 0.3 | |
| B | 5.5 | 2.1 | 59.3 | 1.9 | 6.3 | 49.5 | 7.4 | 1.6 | 8.9 | 50.0 | 8.7 | 29.9 | |
| Hg | 0.14 | 1.5 | 0.9 | 0.07 | 0.24 | 0.8 | 0.11 | 0.5 | 0.17 | 1.0 | 0.12 | 1.6 | |
| one | Ba | 225.3 | 32.8 | 535.7 | 43.6 | 147.7 | 86.9 | 43.6 | 10.6 | 136.4 | 86.3 | 77.8 | 48.7 |
| Sn | 9.2 | 9.6 | 0.5 | 5.0 | 8.6 | 4.8 | 9.7 | 7.2 | 7.0 | 4.7 | 10.4 | 4.6 | |
| Tl | 5.8 | 0.1 | 0.9 | 2.7 | 6.3 | 0.6 | 3.0 | 0.4 | 2.7 | 0.4 | 3.1 | 0.6 | |
| V | 54.0 | 5.0 | 65.6 | 23.0 | 16.4 | 8.1 | 42.8 | 7.9 | 14.4 | 9.5 | 17.1 | 10.1 | |
| Sb | 3.4 | 2.9 | 4.3 | 0.2 | 5.1 | 1.4 | 1.4 | 0.0 | 1.7 | 0.0 | 3.5 | 1.9 | |
| Se | 0.34 | 0.0 | 1.5 | 4.68 | 1.89 | 6.2 | 1.30 | 5.9 | 0.56 | 5.9 | 5.69 | 6.3 | |
| Cu | 21.0 | 8.5 | 21.5 | 11.3 | 15.4 | 9.8 | 17.6 | 15.8 | 14.6 | 12.5 | 11.7 | 7.7 | |
| Mo | 1.7 | 4.0 | 2.6 | 1.9 | 1.8 | 2.3 | 16.7 | 2.7 | 1.8 | 2.4 | 3.6 | 3.5 | |
| Zn | 642.7 | 2.4 | 53.2 | 6.7 | 60.7 | 2.9 | 50.5 | 11.4 | 443.6 | 3.9 | 61.1 | 2.9 | |
| Ag | 1.8 | 0.0 | 0.3 | 0.8 | 0.3 | 0.7 | 1.8 | 0.2 | 0.9 | 1.3 | 2.1 | 0.7 | |
| P | 316.2 | 127.4 | 134.4 | 29.5 | 706.4 | 62.3 | 82.3 | 39.9 | 362.8 | 19.5 | 327.3 | 51.3 | |
Be, Cd, Pb, and Ni elements belong to three hazardous categories. Specifically, Be, Cd, and Pb are toxic and carcinogenic elements harmful to the human body after combustion; their average contents are 1.3, 0.25, and 18.8 μg/g in dust, respectively, and 2, 0.1, and 12.4 μg/g in coal, respectively. The content of Be and Cd is less than that of Pb. The content of Pb in the dust is higher than that of the coal for all samples except for DY. The content of Pb in the dust has the highest value of 30.7 μg/g in LTS and the lowest value of 11.4 μg/g in PM2 and HB6-3. The content of Pb in the coal has the highest value of 24.8 μg/g in DY and the lowest value of 3.6 μg/g in LTS. Ni is a carcinogenic element, harmful to the human body after combustion, and beneficial in small amounts but harmful when excessive. Its contents in the dust and coal in LTS, JLS-3, and PM2 show little difference. The Ni content in the dust is higher than that of the coal in SH and DY, while that in the coal is much higher than that of the dust for HB6-3. The average contents of Ni in the dust and coal are 11.9 and 14.5 μg/g, respectively.
As, Cr, Co, Mn, Th, U, B, and Hg elements belong to two hazardous categories. As and Cr are both carcinogenic and harmful to the human body after combustion; their average contents are 13.41 and 46.5 μg/g in the dust, respectively, and 1.6 and 12.6 μg/g in the coal, respectively. Co and Mn are elements that are both harmful to the human body after combustion and beneficial in small amounts but harmful when excessive; their average contents are 5.8 and 100.4 μg/g in the dust, respectively, and 8.7 and 20.8 μg/g in the coal, respectively. The average content of Mn in the dust is five times that in the coal, and the content of Mn in the dust has the highest value of 153.6 μg/g in LTS and the lowest value of 38.6 μg/g in DY. However, the content of Mn in the coal has the highest value of only 39.9 μg/g in JLS-3. Th and U are elements that are both harmful to the human body after combustion and radioactive; their average contents are 2.49 and 0.98 μg/g in the dust, respectively, and 1.3 and 1 μg/g in the coal, respectively. B is both a toxic element and an element beneficial in small amounts but harmful when excessive, and its average contents in the dust and coal are 16 and 22.5 μg/g, respectively. Hg is both a toxic element and an element harmful to the human body after combustion, and its average contents in the dust and coal are 0.28 and 0.9 μg/g, respectively.
Ba, Sn, Tl, V, Sb, Se, Cu, Mo, Zn, Ag, and P elements belong to a single hazardous category. Ba, Sn, Tl, and V are all toxic elements; their average contents are 194.4, 7.5, 3.6, and 35 μg/g in the dust, respectively, and 51.5, 6, 0.8, and 10.6 μg/g in the coal, respectively. The average content of Ba in the dust is nearly four times than in the coal, and the content of Ba in the dust has the highest value of 535.7 μg/g in JLS-3 and the lowest value of 43.6 μg/g in PM2. However, the content of Ba in the coal has the highest value of only 86.9 μg/g in SH. Sb, Se, and Cu are all elements harmful to the human body after combustion; their average contents are 3.2, 1.88, and 17 μg/g in the dust, respectively, and 1.1, 4.8, and 10.9 μg/g in the coal, respectively. Mo and Zn are elements that are both beneficial in small amounts and harmful when excessive; their average contents are 4.7 and 218.6 μg/g in the dust, respectively, and 2.8 and 5 μg/g in the coal, respectively. The average content of Zn in the dust differs greatly from that in the coal, and the content of Zn in the dust has the highest value of 642.7 μg/g in LTS and the lowest value of 50.5 μg/g in PM2. However, the content of Zn in the coal has the highest value of only 11.4 μg/g in SH. Although Ag and P are harmful elements, they rarely affect the human body; their average contents are 1.2 and 321.6 μg/g in the dust, respectively, and 0.6 and 55 μg/g in the coal, respectively. The average content of P in the dust is nearly six times than in the coal, and the content of P in the dust has the highest value of 706.4 μg/g in SH and the lowest value of 82.3 μg/g in PM2. However, the content of P in the coal has the highest value of 127.4 μg/g in LTS.
The above findings indicate that the sources of harmful elements in dust are complex and not all originated from coal. Some come from the surrounding rocks. These elements may have been enriched in the process of flight, and the content of the dust might have changed in the process of migration with wind flow.
3. Research Significance
3.1. Coal Dust Explosion Evaluation
Coal dust explosion is affected by multiple factors. Generally, the higher the organic matter content, the lower the ash content, the finer the particle size, the larger the specific surface area of the particles, and the worse the wettability, the greater the explosion risk.
The material composition of coal is one of the key indicators for determining the explosion possibility. Volatile matter, fixed carbon, and ash contents reflect the material composition of dust, among which the volatile matter and fixed carbon reflect the organic quality. The organic content of HB6-3, HB6-2, and HB6-1 samples is above 80%, indicating the highest explosion tendency among all of the samples; the DY, SH, PM2, and JLS-1 samples contain 70–80% organic matter, indicating a moderate explosion tendency; and the content of organic matter in JLS-3, LTS, and JLS-2 is less than 70%, indicating a low explosion tendency. The ash content mostly comes from minerals. The ash content of DY, HB6-3, HB6-1, and HB6-2 is below 20%, indicating a high explosion tendency; the ash content in SH, JLS-1, and PM2 ranges from 20 to 30%, indicating a moderate explosion tendency; and the ash content in JLS-3, LTS, and JLS-2 is above 30%, indicating a low explosion tendency. Particle size is another key indicator for determining the dust explosion possibility. The proportion of PM10 in JLS-3 and LTS is above 55%, indicating a high explosion tendency; the proportion of PM10 in JLS-2 and SH ranges from 45 to 55%, indicating a moderate explosion tendency; and the proportion of PM10 in JLS-1, HB6-1, HB6-2, HB6-3, DY, and PM2 is below 45%, indicating the lowest explosion tendency. The specific surface area of the LTS and JLS-2 coal dust samples was above 400 m2/kg, indicating a high explosion tendency; the specific surface areas of the SH, JLS-3, and HB6-3 coal dust samples are between 300 and 400 m2/kg, indicating a moderate explosion tendency; and the DY, PM2, HB6-2, HB6-1, and JLS-1 coal dust samples have specific surface areas below 300 m2/kg, indicating a relatively low explosion tendency. The better the wettability, the more easily capillary bridges can form between the coal dust particles, and the easier it is for the dust to agglomerate and settle. The dust in DY, HB6-3, HB6-1, PM2, and HB6-2 has the poorest wettability and the highest explosion tendency, followed by SH, JLS-1, and JLS-3; LTS and JLS-2 have the best wettability and so the lowest explosion tendency.
In this paper, the organic matter content (or ash content) and particle size (or specific surface area) of dust are taken as the highest weight indicators, with a weight of 40% for each, followed by wettability with a weight of 20%. The reason for this solution is that the organic matter content and ash content are opposite to each other, as are particle size and specific surface area. Particle size is more accurately reflected by the dust content below PM10. Accordingly, the explosion index is calculated by the following equation:
| 1 |
where E is the explosion index (dimensionless), O is the organic matter content (%), P is the particle size (PM10 content) (%), and W is the contact angle, degrees (°).
The results (Table 7 and Figure 13) show that the explosion indexes of HB6-3 and SH are above 60, indicating a high explosion tendency; those of DY, JLS-3, HB6-2, HB6-1, LTS, and PM2 range from 50 to 60, indicating a moderate explosion tendency; and those of JLS-1 and JLS-2 are below 50, indicating the lowest explosion tendency.
Table 7. Explosion Index of Dust.
| sample ID | HB6-3 | SH | DY | JLS-3 | HB6-2 | HB6-1 | LTS | PM2 | JLS-1 | JLS-2 |
|---|---|---|---|---|---|---|---|---|---|---|
| explosion index | 61.878 | 60.252 | 58.158 | 57.984 | 54.952 | 53.562 | 53.538 | 50.294 | 48.174 | 32.348 |
Figure 13.
Coal dust explosion evaluation.
It should be pointed out that due to the differences in the geological background of the mining area where the dust is located and the complexity of the explosion, the application of the explosion index is limited and still in the exploratory stage. Applying the index to other mining areas requires further research.
3.2. Evaluation of Occupational Disease Risks
The research in this paper shows that organic matter is the dominant content in coal mine dust, but the dust generally contains a certain amount of silica (quartzite). However, the inhalation of silica can directly lead to silicosis. Coal dust particles with size lower than 10 μm can directly enter the respiratory tract and alveoli, causing permanent injury to the human body. The proportions of PM10 in the LTS and JLS-3 samples are the highest, both above 55%, indicating the most harm to the human body through long-term inhalation. The proportions of PM10 in the JLS-2, SH, HB6-3, and DY dust samples range between 35 and 50%, indicating moderate harm to the human body. The proportions of PM10 in HB6-2, HB6-1, PM2, and JLS-1 are below 25%, indicating relatively lower harm to the human body. In addition, the amount of fine dust is the highest in the return air roadways, followed by the working faces, and the air intake roadways, correlating to the long-term injury degree to the human body, which indicates that underground workers should try to avoid staying in return air roadways for long periods of time.
During long-term underground work, miners will inhale a large amount of dust, which generally contains higher harmful contents than that of coal. Be, Cd, Pb, and Ni are the elements with three types of hazardous effects at the same time. The content of Be and Cd in the dust is relatively low, with the content lower than 2 and 0.59 μg/g, respectively. The dust in LTS has the highest content of Pb at 30.7 μg/g, making it the most harmful to the human body, and long-term inhalation would cause poisoning and cancer. Meanwhile, the PM2 and HB6-3 samples have the lowest Pb content at 11.4 μg/g, indicating the least harmful to the human body. The PM2 sample has the highest content of Ni at 25.8 μg/g, and long-term inhalation of excessive Ni would cause cancer and other hazards to the human body. The HB6-3 sample has the lowest content of Ni at only 2.5 μg/g. The results of the other mines fall within this range, indicating different degrees of harm to the human body through long-term inhalation.
Each of the elements As, Cr, Co, Mn, Th, U, B, and Hg has two types of hazardous effects. The contents of Co, Th, U, and Hg are relatively low with the contents lower than 11.2, 2.92, 1.71, and 0.9 μg/g, respectively, in all mines, showing little differences among different mines. The content of As in LTS dust is the highest at 47.93 μg/g, making it the most likely to cause cancer, followed by that in PM2, which is only 10.44 μg/g. The content of As in JLS-3 is the lowest at only 0.6 μg/g, indicating relatively little harm to the human body. The content of Cr in the dust of each mine is above 30 μg/g, with the highest values of 60.8 and 61.3 μg/g found in LTS and PM2, respectively. The lowest values are found in HB6-3 and DY but are still as high as 33.3 and 32.9 μg/g, indicating a high risk of causing cancer. As an essential element of the human body, Mn is beneficial to the human body in small amounts but harmful when excessive. The Mn content in the dust of all mines is higher than 90 μg/g in LTS, SH, HB6-3, JLS-3, and PM2, which is very harmful to the human body after long-term inhalation. The content of Mn in the dust of DY is the lowest at only 38.6 μg/g, which is the least harmful to the human body. The content of B in JLS-3 is as high as 59.3 μg/g, indicating that long-term inhalation is very likely to cause poisoning and other hazards. However, the content of B in the other mines ranges between 5.5 and 8.9 μg/g.
Ba, Sn, Tl, V, Sb, Se, Cu, Mo, Zn, Ag, and P are elements with only one type of hazardous effect. The contents of Sn, Tl, Sb, Se, Mo, and Ag are relatively low, with the contents lower than 10.4, 6.3, 5.1, 5.69, 16.7, and 2.1 μg/g, respectively, and would cause relatively low harm to the human body. The content of Ba in JLS-3 reaches 535.7 μg/g, which is the most likely to cause poisoning through long-term inhalation. The content of Ba in LTS, SH, and HB6-3 ranges between 100 and 250 μg/g and that of DY and PM2 is below 100 μg/g, indicating relatively low harm. The content of V in LTS, JLS-3, and PM2 is above 40 μg/g, while that in DY, SH, and HB6-3 is below 20 μg/g. However, V is a toxic element that may cause poisoning through long-term inhalation. The content of Cu as a heavy metal element in the dust shows low differences among all mines, ranging from 11.7 to 21.5 μg/g. Zn is an essential element for the human body. The content of Zn in LTS and HB6-3 is above 400 μg/g, indicating harm to the human body if inhaled excessively, while that in JLS-3, SH, PM2, and DY is below 61.1 μg/g. Although P rarely causes harm to the human body, the content of P in each mine ranges between 82.3 and 706.4 μg/g, which is relatively high and would cause harm to the human body in the case of long-term inhalation.
To sum up, special attention should be paid to the elements that are more harmful to the human body and possess relatively high contents, such as As Pb, Ni, As, Cr, Mn, B, Hg, Ba, V, Cu, Zn, P, etc. In addition, most of the harmful elements in LTS and JLS-3 have the highest content, indicating the highest harm to the human body, followed by SH, PM2, and HB6-3. DY has a relatively small content of harmful elements, posing relatively light harm to the human body.
4. Conclusions
The investigation of dust characteristics is the basis for dust removal and the prevention of coal dust explosions and occupational diseases. In this paper, the dustfall collected from the working faces and roadways of six coal mines was systematically studied. The main conclusions are as follows.
-
(1)
The mineral composition differs at different sampling locations. The material composition of dust at working faces is the most complex and that in air intake and return air roadways is relatively simple. In addition, the higher the ash content in the return airway, the simpler the mineral composition.
-
(2)
The interval distribution of dust particle size generally exhibits a partial normal pattern, and there is a trough in the fine or coarse section in some cases. According to the proportion of PM10, the dust can be divided into symmetrical, fine, and coarse types. There is a decreasing trend of PM10 proportion from the air intake roadway to the working face, to the return air roadway. The specific surface area and proportion of PM10 are positively correlated to each other. Moreover, dust mainly exists in the form of particle agglomeration and small particles adhering to large particles.
-
(3)
Dust wettability is mainly affected by the carbon and oxygen contents on the dust surface, the oxygen–carbon ratio, oxygen-containing functional groups, ash content, fixed carbon, and particle size of the dust. The surfactant (0.05% AN solution) used in this paper can enhance the wetting effect on dust and achieve the dust removal effect.
-
(4)
According to the influence degree of various factors on coal dust explosion, this paper explored the use of an explosion index to evaluate the coal dust explosion tendency, but further research is needed for its wide application. The harmful elements such as Cr, Mn, Ba, V, Zn, and P in the dust are far higher than those in the coal. The long-term inhalation of dust containing a higher content of harmful elements would undoubtedly cause harm to the human body, but the degree of such harm is still unknown. To protect the health of millions of coal mine workers, it is imperative to carry out the research and evaluation of air quality in coal mines.
5. Experimental Section
5.1. Experimental Materials
Coal dust samples were collected from the coal mines in southern Shanxi Province and Henan Province in central North China where coal mining activities are concentrated, including Dayang Mine (DY), Lutaishan Mine (LTS), and Sihe Mine (SH) in Shanxi and Jiulishan Mine (JLS), Pingmei No. 2 Mine (PM2), and Hebi No. 6 Mine (HB6) in Henan (Figure 14). Detailed sampling was carried out in JLS and HB6, where dustfall was collected from the air intake roadways, working faces, and the return air roadways. For the other four mines, dust was collected in the return air roadways near the mined-out line. When collecting, using a clean brush gently sweeps the dustfall on the pipelines and supports in the roadways and working faces into the sealed bags, and using a hammer knocks down about 20 g of unpolluted fresh coal samples in the coal wall of the return airway to seal it into bags for later use. All of the coal beds in the above mines are located in the Lower Permian Shanxi Formation. The surfactant used in this paper is 0.05% AN solution.106
Figure 14.
Location of samples.
5.2. Experimental Methods
The collected dust samples were subjected to proximate analysis according to the Chinese national standard GB/T212-2001. A D8 series X-ray diffractometer (XRD) produced by the German company Bruker AXS was used for qualitative analyzing of the mineral composition of the coal dust. Particle size distribution was tested using a BT-9300S laser particle size analyzer according to the international standard ISO13320-2009 and the Chinese national standard GB/T19077.1-2008. FE-SEM was then used to observe the composition, particle size, and occurrence mode of dust, followed by the XPS quantitative analysis of elements and functional groups on the coal surface using a Thermo Fisher Scientific-Escalab 250Xi photoelectron spectrometer. The contents of trace elements in the dust and coal were determined by means of ICP-MS. Furthermore, the dust was pressed into tablets with a diameter of 10 mm and a thickness of 2 mm using a powder tableting machine, and its contact angle with distilled water and surfactant (0.05% AN solution) and the surface tension of distilled water and surfactant were measured using a JC2000D contact angle measuring instrument.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41872176).
Glossary
Abbreviations
- XRD
X-ray diffraction
- FE-SEM
field emission scanning electron microscope
- PM10
particulate matter with diameter <10 μm
- XPS
X-ray photoelectron spectroscopy
- ICP-MS
inductively coupled plasma mass spectrometry
The authors declare no competing financial interest.
References
- Wang Y. J.; Jiang S. G.; Wu Z. Y.; Shao H.; Wang K.; Wang L. Study on the inhibition influence on gas explosions by metal foam based on its density and coal dust. J. Loss Prev. Process Ind. 2018, 56, 451–457. 10.1016/j.jlp.2018.09.009. [DOI] [Google Scholar]
- Si R.; Li R.; Huang Z. Material Evidence Analysis upon Accident Investigation of Gas and Coal Dust Explosion. Procedia Eng. 2012, 45, 458–463. 10.1016/j.proeng.2012.08.186. [DOI] [Google Scholar]
- Yueze L.; Akhtar S.; Sasmito A. P.; Kurnia J. C. Prediction of air flow, methane, and coal dust dispersion in a room and pillar mining face. J. Min. Sci. Technol. 2017, 27, 657–662. 10.1016/j.ijmst.2017.05.019. [DOI] [Google Scholar]
- Wu D.; Schmidt M.; Berghmans J. Spontaneous ignition behaviour of coal dust accumulations: A comparison of extrapolation methods from lab-scale to industrial-scale. Proc. Combust. Inst. 2019, 37, 4181–4191. 10.1016/j.proci.2018.05.140. [DOI] [Google Scholar]
- Zheng Y. P.; Feng C. G.; Jing G. X.; Qian X. M.; Li X. J.; Liu Z. Y.; Huang P. A statistical analysis of coal mine accidents caused by coal dust explosions in China. J. Loss Prev. Process Ind. 2009, 22, 528–532. 10.1016/j.jlp.2009.02.010. [DOI] [Google Scholar]
- Lin S.; Liu Z.; Qian J.; Li X. Comparison on the explosivity of coal dust and of its explosion solid residues to assess the severity of re-explosion. Fuel 2019, 251, 438–446. 10.1016/j.fuel.2019.04.080. [DOI] [Google Scholar]
- Heppleston A. G. Silica, pneumoconiosis, and carcinoma of the lung. Am. J. Ind. Med. 1985, 7, 285–294. 10.1002/ajim.4700070404. [DOI] [PubMed] [Google Scholar]
- Jin Y.; Fan J. G.; Pang J.; Wen K.; Zhang P. Y.; Wang H. Q.; Li T. Risk of Active Pulmonary Tuberculosis among Patients with Coal Workers’Pneumoconiosis: A Case-control Study in China. Biomed. Environ. Sci. 2018, 31, 448–453. 10.3967/bes2018.058. [DOI] [PubMed] [Google Scholar]
- Erol I.; Aydin H.; Didari V.; Ural S. Pneumoconiosis and quartz content of respirable dusts in the coal mines in Zonguldak, Turkey. Int. J. Coal Geol. 2013, 116–117, 26–35. 10.1016/j.coal.2013.05.008. [DOI] [Google Scholar]
- Dai S.; Li W.; Tang Y.; Zhang Y.; Feng P. The sources, pathway, and preventive measures for fluorosis in Zhijin County, Guizhou, China. Appl. Geochem. 2007, 22, 1017–1024. 10.1016/j.apgeochem.2007.02.011. [DOI] [Google Scholar]
- Zhao C.; Luo K. Household consumption of coal and related sulfur, arsenic, fluorine and mercury emissions in China. Energy Policy 2018, 112, 221–232. 10.1016/j.enpol.2017.10.021. [DOI] [Google Scholar]
- Huang X.; Chen H.; Long R.; Li S. Development and validation of the quality of life scale for Chinese coal miners with pneumoconiosis (QOL-CMP): Measurement method and empirical study. J. Cleaner Prod. 2019, 232, 1062–1075. 10.1016/j.jclepro.2019.05.398. [DOI] [Google Scholar]
- Ni W. Y.; Liu B.; Gai W. The Research on Integrated Visual Information Management System of the Mine Ventilation and Safety. Procedia Eng. 2011, 26, 2070–2074. 10.1016/j.proeng.2011.11.2407. [DOI] [Google Scholar]
- Wang D.; Lu X.; Wang H.; Chen M. A new design of foaming agent mixing device for a pneumatic foaming system used for mine dust suppression. Int. J. Min. Sci. Technol. 2016, 26, 187–192. 10.1016/j.ijmst.2015.12.002. [DOI] [Google Scholar]
- Wang H.; Wang D.; Yan T.; Wang Q. Foaming agent self-suction properties of a jet-type foam preparation device used in mine dust suppression. Process Saf. Environ. Prot. 2015, 98, 231–238. 10.1016/j.psep.2015.08.001. [DOI] [Google Scholar]
- Azam S.; Mishra D. P. Effects of particle size, dust concentration and dust-dispersion-air pressure on rock dust inertant requirement for coal dust explosion suppression in underground coal mines. Process Saf. Environ. Prot. 2019, 126, 35–43. 10.1016/j.psep.2019.03.030. [DOI] [Google Scholar]
- Haas E. J. Using self-determination theory to identify organizational interventions to support coal mineworkers’ dust-reducing practices. Int. J. Min. Sci. Technol. 2019, 29, 371–378. 10.1016/j.ijmst.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Study on Regularity of on-way Dust Distribution and Effect of Shearer’s External Spray on Coal Face. M.S. Thesis, Liaoning University of Engineering Technology, November 2012. [Google Scholar]
- Wang X.; Yuan S.; Jiang B. Experimental investigation of the wetting ability of surfactants to coals dust based on physical chemistry characteristics of the different coal samples. Adv. Powder Technol. 2019, 30, 1696–1708. 10.1016/j.apt.2019.05.021. [DOI] [Google Scholar]
- Rybak W.; Moron W.; Ferens W. Dust ignition characteristics of different coal ranks, biomass and solid waste. Fuel 2019, 237, 606–618. 10.1016/j.fuel.2018.10.022. [DOI] [Google Scholar]
- Xu C.; Wang D.; Wang H.; Ma L.; Zhu X.; Zhu Y.; Zhang Y.; Liu F. Experimental investigation of coal dust wetting ability of anionic surfactants with different structures. Process Saf. Environ. Prot. 2019, 121, 69–76. 10.1016/j.psep.2018.10.010. [DOI] [Google Scholar]
- Sarver E.; Keles C.; Rezaee M. Characteristics of respirable dust in eight appalachian coal mines: A dataset including particle size and mineralogy distributions, and metal and trace element mass concentrations. Data in Brief 2019, 25, 104032. 10.1016/j.dib.2019.104032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Zhang L.; Wang D.; He X. Experimental investigation on the wettability of respirable coal dust based on infrared spectroscopy and contact angle analysis. Adv. Powder Technol. 2017, 28, 3130–3139. 10.1016/j.apt.2017.09.018. [DOI] [Google Scholar]
- Li Q.; Lin B.; Zhao S.; Dai H. Surface physical properties and its effects on the wetting behaviors of respirable coal mine dust. Powder Technol. 2013, 233, 137–145. 10.1016/j.powtec.2012.08.023. [DOI] [Google Scholar]
- Ren W.; Shi J.; Guo Q.; Zhao Q.; Bai L. The influence of dust particles on the stability of foam used as dust control in underground coal mines. Process Saf. Environ. Prot. 2017, 111, 740–746. 10.1016/j.psep.2017.08.043. [DOI] [Google Scholar]
- Wang X.; Huang X.; Zhang X.; Zhang Y.; Zhang Y. Numerical simulation of coal dust explosion suppression by inert particles in spherical confined storage space. Fuel 2019, 253, 1342–1350. 10.1016/j.fuel.2019.05.102. [DOI] [Google Scholar]
- Li Q. Z.; Lin B. Q.; Zhang J. K.; Shi J. J.; Dai H. M. Fractal characteristics of particle size distribution and its effects on the surface wetting performance of coal mine dusts. J. China Coal Soc. 2012, 37, 138–142. [Google Scholar]
- Yao Q.; Xu C.; Zhang Y.; Zhou G.; Zhang S.; Wang D. Micromechanism of coal dust wettability and its effect on the selection and development of dust suppressants. Process Saf. Environ. Prot. 2017, 111, 726–732. 10.1016/j.psep.2017.08.037. [DOI] [Google Scholar]
- Guo Q.; Ren W.; Shi J. Foam for coal dust suppression during underground coal mine tunneling. Tunnelling Underground Space Technol. 2019, 89, 170–178. 10.1016/j.tust.2019.04.009. [DOI] [Google Scholar]
- Zhou Q.; Qin B.; Wang F.; Wang H. Experimental investigation on the performance of a novel magnetized apparatus used to improve the dust suppression ability of surfactant-magnetized water. Powder Technol. 2019, 354, 149–157. 10.1016/j.powtec.2019.05.081. [DOI] [Google Scholar]
- Zhou G.; Ma Y.; Fan T.; Wang G. Preparation and characteristics of a multifunctional dust suppressant with agglomeration and wettability performance used in coal mine. Chem. Eng. Res. Des. 2018, 132, 729–742. 10.1016/j.cherd.2018.02.021. [DOI] [Google Scholar]
- Chen Y.; Xu G.; Huang J.; Liu X.; Zhao Z.; Eksteen J. Characterization of coal particles wettability in surfactant solution by using four laboratory static tests. Colloids Surf., A 2019, 567, 304–312. 10.1016/j.colsurfa.2019.01.068. [DOI] [Google Scholar]
- Meng J.; Yin F.; Li S.; Zhong R.; Sheng Z.; Nie B. Effect of different concentrations of surfactant on the wettability of coal by molecular dynamics simulation. Int. J. Min. Sci. Technol. 2019, 29, 577–584. 10.1016/j.ijmst.2019.06.010. [DOI] [Google Scholar]
- Han W. B.; Zhou G.; Zhang Q. T.; Pan H. W.; Liu D. Experimental study on modification of physicochemical characteristics of acidified coal by surfactants and ionic liquids. Fuel 2020, 266, 116966 10.1016/j.fuel.2019.116966. [DOI] [Google Scholar]
- Han W. B.; Zhou G.; Gao D. H.; Zhang Z. X.; Wei Z. Y.; Wang H. T.; Yang H. Q. Experimental analysis of the pore structure and fractal characteristics of different metamorphic coal based on mercury intrusion-nitrogen adsorption porosimetry. Powder Technol. 2020, 362, 386–398. 10.1016/j.powtec.2019.11.092. [DOI] [Google Scholar]
- Xu C.; Wang D.; Wang H.; Xin H.; Ma L.; Zhu X.; Zhang Y.; Wang Q. Effects of chemical properties of coal dust on its wettability. Powder Technol. 2017, 318, 33–39. 10.1016/j.powtec.2017.05.028. [DOI] [Google Scholar]
- Luo G. H.; Li B.; Ding Y. Y.; Zhang B. Study on influence of coal dust wettability by chemical composition and structure parameters. J. Dalian Jiaotong Univ. 2016, 37, 64–67. [Google Scholar]
- Hu F. Study on influence of coal composition upon hydrophilicity of coal dust. Min. Saf. Environ. Prot. 2014, 41, 19–22. [Google Scholar]
- Glanville J. O.; Haley L. H. Studies of coal dust wetting by surfactant solutions. Colloids Surf. 1982, 4, 209–212. 10.1016/0166-6622(82)80018-8. [DOI] [Google Scholar]
- Yang J.; Wu X.; Gao J.; Li G. Surface characteristics and wetting mechanism of respirable coal dust. Min. Sci. Technol. 2010, 20, 365–371. 10.1016/S1674-5264(09)60209-X. [DOI] [Google Scholar]
- Zhou Q.; Qin B.; Wang J.; Wang H.; Fei W. Experimental investigation on the changes of the wettability and surface characteristics of coal dust with different fractal dimensions. Colloids Surf., A 2018, 551, 148–157. 10.1016/j.colsurfa.2018.05.005. [DOI] [Google Scholar]
- Xi X.; Jiang S.; Zhang W.; Wang K.; Shao H.; Wu Z. An experimental study on the effect of ionic liquids on the structure and wetting characteristics of coal. Fuel 2019, 244, 176–183. 10.1016/j.fuel.2019.01.183. [DOI] [Google Scholar]
- Tang H.; Zhao L.; Wei S.; Hu Y.; Han H. Surface characteristics and wettability enhancement of respirable sintering dust by nonionic surfactant. Colloids Surf., A 2016, 509, 323–333. 10.1016/j.colsurfa.2016.09.041. [DOI] [Google Scholar]
- Shutthanandan V.; Nandasiri M.; Zheng J.; Engelhard M. H.; Wu X.; Thevuthasan S.; Murugesan V. Applications of XPS in the characterization of Battery materials. J. Electron Spectrosc. Relat. Phenom. 2019, 231, 2–10. 10.1016/j.elspec.2018.05.005. [DOI] [Google Scholar]
- Hamm U. W.; Lazarescu V.; Kolb D. M. Adsorption of pyrazine on Au(111) and Ag(111) electrodes - An ex situ XPS study. J. Chem. Soc., Faraday Trans. 1996, 92, 3785–3790. 10.1039/ft9969203785. [DOI] [Google Scholar]
- Saheli G.; Liu W.; Lazik C.; Uritsky Y.; Bevan M.; Tang W.; Ma P.; Venkatasubramanian E.; Bobek S.; Kulshreshtha P.; Brundle C. R. Characterization of film materials in wafer processing technology development by XPS. J. Electron Spectrosc. Relat. Phenom. 2019, 231, 57–67. 10.1016/j.elspec.2018.03.007. [DOI] [Google Scholar]
- Kaveh N. S.; Wolf K. H.; Ashrafizadeh S. N.; Rudolph E. S. J. Effect of coal petrology and pressure on wetting properties of wet coal for CO2 and flue gas storage. Int. J. Greenhouse Gas Control 2012, 11, S91–S101. 10.1016/j.ijggc.2012.09.009. [DOI] [Google Scholar]
- Ni G. H.; Li Z.; Sun Q.; Li S.; Dong K. Effects of Bmim Cl ionic liquid with different concentrations on the functional groups and wettability of coal. Adv. Powder Technol. 2019, 30, 610–624. 10.1016/j.apt.2018.12.008. [DOI] [Google Scholar]
- Zhou L.; Yang S.; Hu B.; Yuan Z.; Wu H.; Yang L. Evaluating of the performance of a composite wetting dust suppressant on lignite dust. Powder Technol. 2018, 339, 882–893. 10.1016/j.powtec.2018.08.081. [DOI] [Google Scholar]
- Ward C. R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. 10.1016/j.coal.2016.07.014. [DOI] [Google Scholar]
- Sun Y.; Qi G.; Lei X.; Hui X.; Yi W. Extraction of Uranium in Bottom Ash Derived from High-Germanium Coals. Procedia Environ. Sci. 2016, 31, 589–597. 10.1016/j.proenv.2016.02.096. [DOI] [PubMed] [Google Scholar]
- Li H.; Zheng N.; Guo G.; Chen Y. Control measures for reduction of arsenic and cadmium contamination during underground coal gasification without shaft. J. Cleaner Prod. 2019, 219, 960–970. 10.1016/j.jclepro.2019.02.154. [DOI] [Google Scholar]
- Long J.; Zhang S.; Luo K. Discovery of anomalous molybdenum enrichment in lower Carboniferous coal and its availability and origin. J. Geochem. Explor. 2019, 200, 104–111. 10.1016/j.gexplo.2019.02.003. [DOI] [Google Scholar]
- Bratskaya S. Y.; Volk A. S.; Ivanov V. V.; Ustinov A. Y.; Barinov N. N.; Avramenko V. A. A new approach to precious metals recovery from brown coals: Correlation of recovery efficacy with the mechanism of metal-humic interactions. Geochim. Cosmochim. Acta 2009, 73, 3301–3310. 10.1016/j.gca.2009.03.010. [DOI] [Google Scholar]
- Dai S. F.; Ren D. Y.; Ma S. M. The cause of endemic fluorosis in western Guizhou Province, Southwest China. Fuel 2004, 83, 2095–2098. 10.1016/j.fuel.2004.03.016. [DOI] [Google Scholar]
- Liu Y.; Luo K.; Li L.; Shahid M. Z. Fluoride and sulfur dioxide indoor pollution situation and control in coal-burning endemic area in Zhaotong, Yunnan, China. Atmos. Environ. 2013, 77, 725–737. 10.1016/j.atmosenv.2013.05.043. [DOI] [Google Scholar]
- Zheng B.; Ding Z.; Huang R.; Zhu J.; Yu X.; Wang A.; Zhou D.; Mao D.; Su H. Issues of health and disease relating to coal use in southwestern China. Int. J. Coal Geol. 1999, 40, 119–132. 10.1016/S0166-5162(98)00064-0. [DOI] [Google Scholar]
- Dai S.; Tian L.; Chou C.; Zhou Y.; Zhang M.; Zhao L.; Wang J.; Yang Z.; Cao H.; Ren D. Mineralogical and compositional characteristics of Late Permian coals from an area of high lung cancer rate in Xuan Wei, Yunnan, China: Occurrence and origin of quartz and chamosite. Int. J. Coal Geol. 2008, 76, 318–327. 10.1016/j.coal.2008.09.001. [DOI] [Google Scholar]
- Zhao F. H.Study on the Mechanism of Distributions and Occurrences of Hazardous Minor and Trace Elements in Coal and Leaching Experiments of Coal Combustion Residues. Ph.D. Thesis, China University of Mining and Technology, May 1997. [Google Scholar]
- Wang W. F.; Qin Y.; Song D. Y. The parameters for assessing the environmental and human health impacts of toxic elements in coal. Coal Mine Environ. Prot. 2002, 16, 8–14. [Google Scholar]
- Ren D. Y.; Zhao F. H.; Zhang J. Y.; Xu D. W. A preliminary study on genetic type of enrichment for hazardous minor and trace elements in coal. Earth Sci. Front. 1999, 6, 17–22. [Google Scholar]
- Tang X.; Zhang Y.. Trace Elements in Chinese Coal; 1st ed.; The Commercial Press: Beijing, 2004; pp 2–20. [Google Scholar]
- Wang W. F.; Qin Y.; Song D. Y. Modes of occurrence on hazardous trace elements in coal. Coal Geol. China 2003, 15, 10–13. [Google Scholar]
- Gürdal G. Abundances and modes of occurrence of trace elements in the Can coals (Miocene), Canakkale-Turkey. Int. J. Coal Geol. 2011, 87, 157–173. 10.1016/j.coal.2011.06.008. [DOI] [Google Scholar]
- Dai S. F.; Zeng R. S.; Sun Y. Z. Enrichment of arsenic, antimony, mercury, and thallium in a Late Permian anthracite from Xingren, Guizhou, Southwest China. Int. J. Coal Geol. 2006, 66, 217–226. 10.1016/j.coal.2005.09.001. [DOI] [Google Scholar]
- Dai S. F.; Ren D. Y.; Tang Y. G.; Yue M.; Hao L. M. Concentration and distribution of elements in Late Permian coals from western Guizhou Province, China. Int. J. Coal Geol. 2005, 61, 119–137. 10.1016/j.coal.2004.07.003. [DOI] [Google Scholar]
- Dai S. F.; Ren D. Y.; Tang Y. G.; Yue M.; Hao L. M.; et al. Mineralogy and geochemistry of boehmite-rich coals: New insights from the Haerwusu Surface Mine, Jungar Coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. 10.1016/j.coal.2008.01.001. [DOI] [Google Scholar]
- Dai S. F.; Ren D. Y.; Hou X. Q.; Shao L. Y. Geochemical and mineralogical anomalies of the late Permian coal in the Zhijin coalfield of southwest China and their volcanic origin. Int. J. Coal Geol. 2003, 55, 117–138. 10.1016/S0166-5162(03)00083-1. [DOI] [Google Scholar]
- Dai S. F.; Chou C. L.; Yue M.; Luo K. L.; Ren D. Y. Mineralogy and geochemistry of a Late Permian coal in the Dafang Coalfield, Guizhou, China: influence from siliceous and iron-rich calcic hydrothermal fluids. Int. J. Coal Geol. 2005, 61, 241–258. 10.1016/j.coal.2004.09.002. [DOI] [Google Scholar]
- Dai S. F.; Wang X. B.; Chen W. M.; Li D. H.; Chou C. L.; Zhou Y. P.; Zhu C. S.; Li H.; Zhu X. W.; Xing Y. W.; Zhang W. G.; Zou J. H. A high-pyrite semianthracite of Late Permian age in the Songzao Coalfield, southwestern China: Mineralogical and geochemical relations with underlying mafic tuffs. Int. J. Coal Geol. 2010, 83, 430–445. 10.1016/j.coal.2010.06.004. [DOI] [Google Scholar]
- Dai S. F.; Ren D. Y.; Zhou Y. P.; Chou C. L.; Wang X. B.; Zhao L.; Zhu X. W. Mineralogy and geochemistry of a superhigh-organic-sulfur coal, Yanshan Coalfield, Yunnan, China: Evidence for a volcanic ash component and influence by submarine exhalation. Chem. Geol. 2008, 255, 182–194. 10.1016/j.chemgeo.2008.06.030. [DOI] [Google Scholar]
- Dai S. F.; Zhang W. G.; Seredin V. V.; Ward C. R.; Hower J. C.; Song W. J.; Wang X. B.; Li X.; Zhao L. X.; Kang H.; Zheng L. C.; Wang P. P.; Zhou D. Factors controlling geochemical and mineralogical compositions of coals preserved within marine carbonate successions: A case study from the Heshan Coalfield, southern China. Int. J. Coal Geol. 2013, 109–110, 77–100. 10.1016/j.coal.2013.02.003. [DOI] [Google Scholar]
- Zhuang X. G.; Su S. C.; Xiao M. G.; Li J.; Alstuey A.; Querol X. Mineralogy and geochemistry of the Late Permian coals in the Huayingshan coal-bearing area, Sichuan Province, China. Int. J. Coal Geol. 2012, 94, 271–282. 10.1016/j.coal.2012.01.002. [DOI] [Google Scholar]
- Zhuang X.; Querol X.; Plana F.; Alastuey A.; Lopez-Soler A.; Wang H. Determination of elemental affinities by density fractionation of bulk coal samples from the Chongqing coal district, Southwestern China. Int. J. Coal Geol. 2003, 55, 103–115. 10.1016/S0166-5162(03)00081-8. [DOI] [Google Scholar]
- Dai S. F.; Ren D. Y.; Chou C. L.; Finkelman R. B.; Seredin V. V.; Zhou Y. P. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. 10.1016/j.coal.2011.02.003. [DOI] [Google Scholar]
- Yuan J.; Na C.; Qi L.; Xiong M.; Guo J.; Zheng H. Coal use for power generation in China. Resour., Conserv. Recycl. 2018, 129, 443–453. 10.1016/j.resconrec.2016.03.021. [DOI] [Google Scholar]
- Liu F.; Tao L.; Sajid M.; Li X. Optimization for China’s coal flow based on matching supply and demand sides. Resour., Conserv. Recycl. 2018, 129, 345–354. 10.1016/j.resconrec.2016.08.013. [DOI] [Google Scholar]
- Radić S.; Medunic G.; Kuharic Z.; Roje V.; Maldini K.; Vujcic V.; Krivohlavek A. The effect of hazardous pollutants from coal combustion activity: Phytotoxicity assessment of aqueous soil extracts. Chemosphere 2018, 199, 191–200. 10.1016/j.chemosphere.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Man C. K.; Gibbins J. R. Factors affecting coal particle ignition under oxyfuel combustion atmospheres. Fuel 2011, 90, 294–304. 10.1016/j.fuel.2010.09.006. [DOI] [Google Scholar]
- Yu H.; Wang C.; Pang L.; Cui Y.; Chen D. Inhibiting effect of coal fly ash on minimum ignition temperature of coal dust clouds. J. Loss Prev. Process Ind. 2019, 61, 24–29. 10.1016/j.jlp.2019.05.018. [DOI] [Google Scholar]
- Moroń W.; Ferens W.; Czajka K. M. Explosion of different ranks coal dust in oxy-fuel atmosphere. Fuel Process. Technol. 2016, 148, 388–394. 10.1016/j.fuproc.2016.03.007. [DOI] [Google Scholar]
- Liu S. H.; Cheng Y. F.; Meng X. R.; Ma H. H.; Song S. X.; Liu W. J.; Shen Z. W. Influence of particle size polydispersity on coal dust explosibility. J. Loss Prev. Process Ind. 2018, 56, 444–450. 10.1016/j.jlp.2018.10.005. [DOI] [Google Scholar]
- Man C. K.; Harris M. L. Participation of large particles in coal dust explosions. J. Loss Prev. Process Ind. 2014, 27, 49–54. 10.1016/j.jlp.2013.11.004. [DOI] [Google Scholar]
- Cao W.; Huang L.; Zhang J.; Xu S.; Qiu S.; Pan F. Research on Characteristic Parameters of Coal-dust Explosion. Procedia Eng. 2012, 45, 442–447. 10.1016/j.proeng.2012.08.183. [DOI] [Google Scholar]
- Zlochower I. A.; Sapko M. J.; Perera I. E.; Brown C. B.; Harris M. L.; Rayyan N. S. Influence of specific surface area on coal dust explosibility using the 20-L chamber. J. Loss Prev. Process Ind. 2018, 54, 103–109. 10.1016/j.jlp.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.; Liu Z.; Zhao E.; Lin S.; Qiu L.; Qian J.; Liu H.; Xia S. Comparison of behavior and microscopic characteristics of first and secondary explosions of coal dust. J. Loss Prev. Process Ind. 2017, 49, 382–394. 10.1016/j.jlp.2017.08.005. [DOI] [Google Scholar]
- Harris M. L.; Sapko M. J.; Zlochower I. A.; Perera I. E.; Weiss E. S. Particle size and surface area effects on explosibility using a 20-L chamber. J. Loss Prev. Process Ind. 2015, 37, 33–38. 10.1016/j.jlp.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.; Wei W.; Huang W.; Du B.; Liu L.; Zhu J. Experimental investigations on the roles of moisture in coal dust explosion. J. Taiwan Inst. Chem. Eng. 2014, 45, 2325–2333. 10.1016/j.jtice.2014.05.022. [DOI] [Google Scholar]
- Thomas G. O. On the Conditions Required for Explosion Mitigation by Water Sprays. Process Saf. Environ. Prot. 2000, 78, 339–354. 10.1205/095758200530862. [DOI] [Google Scholar]
- Xu H.; Li Y.; Zhu P.; Wang X.; Zhang H. Experimental study on the mitigation via an ultra-fine water mist of methane/coal dust mixture explosions in the presence of obstacles. J. Loss Prev. Process Ind. 2013, 26, 815–820. 10.1016/j.jlp.2013.02.014. [DOI] [Google Scholar]
- Li Q.; Wang K.; Zheng Y.; Ruan M.; Mei X.; Lin B. Experimental research of particle size and size dispersity on the explosibility characteristics of coal dust. Powder Technol. 2016, 292, 290–297. 10.1016/j.powtec.2016.01.035. [DOI] [Google Scholar]
- Li Q.; Tao Q.; Yuan C.; Zheng Y.; Zhang G.; Liu J. Investigation on the structure evolution of pre and post explosion of coal dust using X-ray diffraction. Int. J. Heat Mass Transfer 2018, 120, 1162–1172. 10.1016/j.ijheatmasstransfer.2017.12.137. [DOI] [Google Scholar]
- Cao W.; Gao W.; Liang J.; Xu S.; Pan F. Flame-propagation behavior and a dynamic model for the thermal-radiation effects in coal-dust explosions. J. Loss Prev. Process Ind. 2014, 29, 65–71. 10.1016/j.jlp.2014.02.002. [DOI] [Google Scholar]
- Cao W.; Qin Q.; Cao W.; Lan Y.; Chen T.; Xu S.; Cao X. Experimental and numerical studies on the explosion severities of coal dust/air mixtures in a 20-L spherical vessel. Powder Technol. 2017, 310, 17–23. 10.1016/j.powtec.2017.01.019. [DOI] [Google Scholar]
- Mittal M. Limiting oxygen concentration for coal dusts for explosion hazard analysis and safety. J. Loss Prev. Process Ind. 2013, 26, 1106–1112. 10.1016/j.jlp.2013.04.012. [DOI] [Google Scholar]
- Li Y.; Xu H.; Wang X. Experimental Study on the Influence of Initial Pressure on Explosion of Methane-coal Dust Mixtures. Procedia Eng. 2013, 62, 980–984. 10.1016/j.proeng.2013.08.151. [DOI] [Google Scholar]
- Song C. X. Experimental study on coal dust explosion characteristics under impact of coal elemental composition. Coal Technol. 2015, 34, 189–191. [Google Scholar]
- Li Q.; Yuan C.; Tao Q.; Zheng Y.; Zhao Y. Experimental analysis on post-explosion residues for evaluating coal dust explosion severity and flame propagation behaviors. Fuel 2018, 215, 417–428. 10.1016/j.fuel.2017.11.093. [DOI] [Google Scholar]
- Orumwense F. O. Estimation of the wettability of coal from contact angles using coagulants and flocculants. Fuel 1998, 77, 1107–1111. 10.1016/S0016-2361(97)00223-8. [DOI] [Google Scholar]
- Kozłowski M. XPS study of reductively and non-reductively modified coals. Fuel 2004, 83, 259–265. 10.1016/j.fuel.2003.08.004. [DOI] [Google Scholar]
- Raymundo-Piñero E.; Cazorla-Amorós D.; Linares-Solano A.; Find J.; Wild U.; Schlögl R. Structural characterization of N-containing activated carbon fibers prepared from a low softening point petroleum pitch and a melamine resin. Carbon 2002, 40, 597–608. 10.1016/S0008-6223(01)00155-5. [DOI] [Google Scholar]
- Kapteijn F.; Moulijn J. A.; Matzner S.; Boehm H. P. The development of nitrogen functionality in model chars during gasification in CO2 and O2. Carbon 1999, 37, 1143–1150. 10.1016/S0008-6223(98)00312-1. [DOI] [Google Scholar]
- Perry D. L.; Grint A. Application of XPS to coal characterization. Fuel 1983, 62, 1024–1033. 10.1016/0016-2361(83)90135-7. [DOI] [Google Scholar]
- Mahoney S. A.; Rufford T. E.; Johnson D.; Dmyterko A. S. K.; Rodrigues S.; Esterle J.; Rudolph V.; Steel K. M. The effect of rank, lithotype and roughness on contact angle measurements in coal cleats. Int. J. Coal Geol. 2017, 179, 302–315. 10.1016/j.coal.2017.07.001. [DOI] [Google Scholar]
- Liu H.; Li Y.; Wang W.; Chen P. A theoretical study of liquid bridge force between natural gas hydrate particles. Nat. Gas Ind. 2013, 33, 109–113. [Google Scholar]
- Su X. B.; Wang Q.; Song J. X.; Chen P. H.; Yao S.; Hong J. T.; Zhou F. D. Experimental study of water blocking damage on coal. J. Pet. Sci. Eng. 2017, 156, 654–661. 10.1016/j.petrol.2017.06.048. [DOI] [Google Scholar]