Abstract
Genus Galanthus (Amaryllidaceae) includes 19 species in Europe and the Middle East. The Flora of Bulgaria recognizes two species: G. nivalis L. and G. elwesii Hook. Galanthus elwesii is characterized by relatively high morphological variability, leading some authors to identify some populations as G. gracilis Celak. However, the occurrence of G. gracilis in the Bulgarian flora is disputed. The hypothesis was that populations previously identified as G. gracilis belong indeed to a separate species. Therefore, the objective of this study was to compare G. nivalis and G. elwesii with plants from populations previously identified as G. gracilis. Morphological, DNA, embryological and anatomical analyzes were conducted to meet the objective. The morphological characteristics and DNA dendrogram revealed that G. gracilis and G. elwesii were situated in the same cluster and had significant morphological similarity, whereas plants from populations identified as G. nivalis were dissimilar in morphology and situated in a separate cluster. The revealed features of the generative sphere showed similarities across the species. Scanning electron microscopy (SEM) analyses of the surface revealed that the anticillinal walls of G. elwesii and G. gracilis were straight, while those of G. nivalis were wavy. This research demonstrated that the plants of G. elwesii and those from populations identified as G. gracilis are morphologically, embryologically and genetically similar, thus refuting the hypothesis. This study did not provide sufficient evidence to support the claim of the existence of G. gracilis in the Bulgarian flora; the populations identified as G. gracilis in Bulgaria may be forms of G. elwesii.
Keywords: Ecology, Genetics, Plant biology, Biodiversity, Botany, Galanthus, Plant morphology, DNA, Plant embryology, Plant anatomy
Ecology; Genetics; Plant biology; Biodiversity; Botany; Galanthus; Plant morphology; DNA; Plant embryology; Plant anatomy
1. Introduction
Genus Galanthus (Amaryllidaceae) is homogeneous group and comprises about 19 species, plus a small number of subspecies, varieties and natural hybrids (Davis, 1999, 2001; Zubov and Davis, 2012; World Checklist of Selected Plant Families, 2018). Galanthus species are distributed in Europe, Asia Minor and Near East. Galanthus have economic value because of their ornamental potential and their use as landscape plants (Jovanović et al., 2018). In addition, Galanthus species contain alkaloids that have shown pharmacological activity (Zhong, 2005). Genus Galanthus has been the subject of a number of taxonomic revisions; however, there is no consensus with the enumeration of the species, subspecies, and varieties. The difficulty with separating (distinguishing) individual species in the Galanthus genus is due to several reasons: (1) the lack of clearly identifiable morphological characteristics; (2) the comparatively small number of simplified, morphological parts, which is characteristic of monocotyledonous plants; (3) most species of the genus have the same chromosomal number (2n = 2x = 24); and (4) the individual species of Galanthus easily hybridize with each other (Davis and Barnett, 1997; Zonneveld et al., 2003; Lledó et al., 2004). As a result, there are various taxonomic schemes of the Galanthus genus and lack of concensus among botanists, as reflected in the various monographs of Stern (1956), Artjushenko (1966, 1970), and Zeybek and Sauer (1995). Morphological features and anatomical characteristics of the leaves have been the basis of taxonomic patterns in the genus (Artjushenko, 1966, 1970; Davis and Barnett, 1997).
In recent years, DNA and Inter-Simple Sequence Repeat (ISSR) markers research have successfully been applied to resolve plant systematic problems (Anne, 2006). The ISSR markers proved to be a powerful tool for assessing genetic variation and elucidation of genetic relationships within and among species (Agarwal et al., 2008; Arif et al., 2010). Due to its multilocus assessment capability in a single reaction, their application bares sufficient potential of identifying (for the identification of) unique profiles for each genotype and can easily be applicable for different organisms (Lopes et al., 2014; Hilooğlu and Sözen, 2017).
Previously, genome size (Cx-value) was applied as a new criterion to investigate the relationships within the genus Galanthus (Zonneveld et al., 2003). Molecular phylogenetic studies have been conducted by Lledó et al. (2004), Moore et al. (1991), Meerow et al. (2006), Larsen et al. (2011). The cyto-embryological characteristics are more stable traits than others such as the morphological ones, and thus, it becomes possible to establish cyto-embryological similarity in species, and on this basis find associated relationships among species, genera, families, and orders of plants that are externally morphologically different (Poddubnaya-Arnoldi, 1976). The embryological characteristic, the peculiarities of structures and processes in the generative sphere, and the type of reproductive system are specific for each species, which helps with the determination of the species belonging. Therefore, in this investigation, an embryological study of populations of Bulgarian Galanthus species was conducted to provide additional data to clarify their taxonomic identity.
There is no consensus among botanists about the Galanthus species distributed in the Bulgarian flora. For example, various authors in different editions of the book Flora of Bulgaria, provided inconsistent information about Galanthus in Bulgaria:
1) Velenovsky (1891, 1898) – G. nivalis L., G. gracilis Celak (=G. bulgaricus Vel.)., G. maximus Vel.;
2) Stojanov and Stefanov (1924; 1933; 1948) – G. nivalis (var. gracilis Celak V., var. maximus Vel.);
3) Jordanov (1964) - G. nivalis L.;
4) Stojanov et al. (1966) - G. nivalis L. (var. nivalis., var. graecus (Orph.) Staj. et Stef.)., G. elwesii (formae maximus);
5) Kozucharov (1992) - G. nivalis L. and G. elwesii Hook.
6) Delipavlov et al. (2003) - G. nivalis L., G. elwesii Hook., G. gracilis Celak.
One of the main issues with the determination of the number of Galanthus species in the Bulgarian flora is the variability of the basic morphological features used by the taxonomic botanists. Within the same Galanthus species, there is diversity in the leaf and bulb sizes and forms (Jordanov 1964). Taxonomic problem exists with the distinction of G. elwesii Hook fil (senso lato, s.l.). and the similar taxon G. graecus Orph. and G. gracilis Celak. Taxonomic solutions for these species are made mainly on the basis of morphological features and variability of the populations under the influence of environmental conditions (Delipavlov et al., 2003). Overall, Galanthus' research in Bulgaria focuses on two aspects; (1) morphometric variability, and (2) phytochemical composition.
Sidjimova (2006, 2008) conducted a study on morphometrical variability of natural populations of G. elwesii (s.l.) at 29 different sites and found significant correlation between length and width of outer and inner perianth segments. Of the samples tested, 73% were homogeneous for morphological features, while 29.4% showed significant variability. The results confirm the clone-population structure of the species G. elwesii (Sidjimova, 2006). Тhe authros concluded that these clone-populations belong to one species with no differentiation on subspecies level (Sidjimova, 2006).
A phytochemical differentiation of populations, (including those populations that are the subject of this study) that separated G. nivalis and G. elwesii populations in Bulgaria were conducted by Sidjimova (2008) and Berkov et al. (2008, 2011). Berkov et al. (2011) studied the alkaloid patterns of two occasionally sympatric (same geographical area) G. nivalis and G. elwesii populations and found that the two were distinguished by the type of alkaloids. This provided chemotaxonomical support for the division of G. nivalis and G. elwesii into different taxons. Berkov et al. (2011) studied alkaloid diversity in 25 G elwesii and 7 G nivalis Bulgarian populations of the species and reported that G. elwesii and G. nivalis populations were separated.
However, there is still no definite answer to the question of does G. gracilis (syn. G. graecus) exist in the Bulgarian flora, and if yes, what are the features that describe it. The working hypothesis of this study was that G. elwesii plants and those from populations identified as G. gracilis are morphologically, embryologically and genetically disimilar. Therefore, the objective of this study was to compare G. nivalis and G. elwesii with plants from populations previously identified as G. gracilis. For this purpose, morphological, DNA, embryological, anatomical, and palynological studies were conducted.
2. Material and methods
2.1. Plant material
The species previously identified as Galanthus nivalis, G. elwesii, and G. gracilis were determined according to Delipavlov et al. (2003). Plant samples from six different populations were collected. Thirty specimens per population per species were collected. The localities of populations, coordinates, elevation and exposure are presented in Table 1.
Table 1.
Samples with the GPS coordinates of Genus Galanthus collected in Bulgaria.
| No. | Species | Locality |
|---|---|---|
| 1 | G. elwesii | Sredna gora: Bogdan hut, in the forest, 1495 m alt., 42°60′60.3″ N, 24°45′14.4″E, NW exposition, 21°. |
| 2 | G. elwesii | Central Rhodopes: near the road Belovo-Yundola, over Yadenitza river, 776 m alt., NE exposition, 42°14′42.0″ N; 23°96′03.1″E. |
| 3 | G. gracilis | Central Rhodopes: Bachkovo village, near the road from Monastery to children's camp, 485 m; NE exposition, 41°93′30.2″ N, 24°86′60.5″ E. |
| 4 | G. gracilis | Sofia region: Makocevo village, “Sinigerov Dol” locality, 650 m; NE exposition, 42°68′96.6″ N, 23°82′31.3″ E. |
| 5 | G. nivalis | South Black sea: Primorsko, near Ropotamo River, “Lavskata glava” locality 9,7 m alt., 42°30′80.0″ N, 27°72′35.3″ E. |
| 6 | G. nivalis | West Balkan Mt.: Belogradchik, “Venetza”, 575m. alt., 43°62′36.8″N, 22°67′67.1″E. |
The morphometrical, DNA, embryological, and anatomical analyses were performed on fresh plant tissue, and whole plant samples (including bulbs) that were collected during flowering. The Scanning Electron Microscopy (SEM) analyses were done on air-dried seeds and leaves.
The collected samples were from populations previously described as G. elwesii and G. gracilis based on the morphological description of the species.
2.2. Morphometrical analysis
The morphometric characteristics were measured and characterized as follows: Bulb diameter (cm); Stem length (cm); Leaf length (cm); Leaf width (cm); Outer perianth segments length (cm); and Outer perianth segments width (cm).
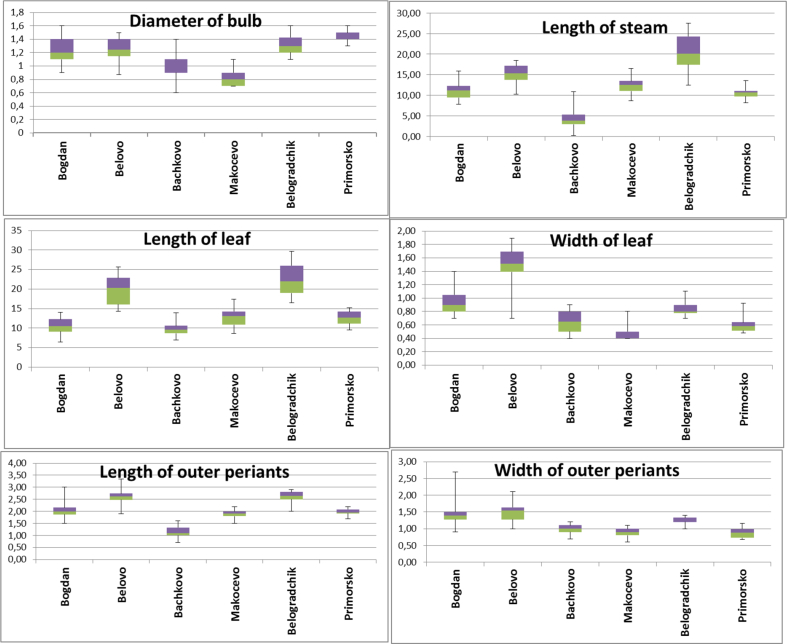
Thirty specimens per population per species were collected by random sampling. Аverage (X̅), minimum - maximum (min - max), and standart deviation (SD) of all characteristics were calculated by Microsoft Excel (2010) (Figure 1). All values were statistically significant (p-value ≤ 0.05). The measurements were taken with accuracy of 0.1 cm. Voucher specimens of the species were deposited at the Herbarium of the Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences (SOM) (Thiers, 2012).
Figure 1.
Box plots of measured characteristics (leaf length and width, bulb diameter, stem height, outher perianths length and width); G. elwesii –Bogdan, Belovo-Yundola; G. gracilis – Bachkovo, Makocevo; G. nivalis - Primorsko, Belogradchik.
2.3. DNA extraction
Genomic DNA was extracted from 200 mg of fresh leaf tissue using the innu PREP DNA Kit (Analytik Jena) (Zietkiewicz et al., 1994). Visualization of the isolated DNA was done after electrophoretic separation of the products in 1.5 % agarose gel by staining with ethidium bromide.
2.3.1. Inter-Simple Sequence Repeats (ISSR) analysis and polymerase chain reaction (PCR) amplification
ISSR analysis was performed by PCRs in a QB-96 Thermal Cycler (Quanta Biotech, London, UK). Sequences of ISSR primers used to perform PCRs are listed in Table 2. PCR reactions were performed in 25 μL reaction volumes, where for each reaction: My Taq HS red mix (Bioline) – 12.5 μL; primer – 1.5 μL; H2O – 15 μL; and 1 μl genomic DNA were used. Visualization of the PCR products was done after electrophoretic separation of the products on 2 % agarose gel cast in TBE buffer, stained with ethidium romide and photographed under UV light. DNA fragment size was determined by comparing with the 100 bp Rainbow extended DNA ladder (Bioron) and analyzed with Gel Analyzer 2010 software.
Table 2.
Sequences of ISSR primers used to perform PCRs, number of bends amplified, polymorphic bands, and annealing temperature.
| Primer Name | DNA sequence (5’ 3’) | Total number of bands | Number of polymorphic bands | Annealing temperature (0С) |
|---|---|---|---|---|
| ISSR1 | AG (8)CTG | 6 | 6 | 48.2 |
| ISSR2 | AG (8)YT | 12 | 12 | 51.4 |
| ISSR3 | GA (8)YC | 14 | 13 | 53.9 |
| ISSR4 | CA (8)RC | 10 | 10 | 53.9 |
| ISSR5 | CA (7)RG | 13 | 12 | 49.0 |
| ISSR6 | CA (8)AA | 12 | 11 | 50.0 |
2.3.2. DNA analysis
The results of the ISSR reactions across six accessions were processed in a binary system for band presence ‘‘1″ or absence ‘‘0″ for each primer. The reliable and intensive bands only were scored. The number of monomorphic and polymorphic amplification products generated by each primer of each marker system was determined. The binary data were used to estimate levels of polymorphism by dividing the polymorphic bands by the total number of bands scored. Molecular data gathered throughout the current study were used for calculating relative genetic distances and producing hierarchical clusters with the “SPSS for Windows” statistical package.
2.4. Embryological research
For the embryological study, flower buds and flowers at different developmental stages from the above cited population of G. nivalis, G. elwesii and G. gracilis were fixed in a mixture of FAA (formalin: glacial acetic acid: 70 % ethanol in ratio 5:5:90 parts). Then the samples were embedded in paraffin, cut into 9 to 15-μm sections with a rotary microtome Leica, and treated according to the classical paraffin methods (Sundara, 2000). The sections were stained with Heidenhain's haematoxylin and included in Enthelan in order to obtain permanent microscope slides. The slides were observed on the Olympus CX21 light microscope and the stage of development of the generative sphere was described.
2.5. Anatomical research in leaves
The materials for the study were collected from natural populations identified as G. nivalis, G. elwesii, G. gracilis according to Delipavlov et al. (2003). Leaf samples were taken during the flowering period in 2015–2016. The epidermis and the transverse section were studied from the middle part of the lamina. Semi-stable microscopic preparations were made. For light microscope (LM) examination an Amplival microscope was used. The measurements were made with a reed eyepiece-micrometer and pictures were taken with a digital light microscope Motic DMBA 210. The study included the number and location of stomata per mm2, and width and length of guard stomata cells in μm. The following indicators were measured in transverse section: height of epidermal cells in the upper (adaxial, ad) and lower (abaxial, ab) surface in μm, the thickness of mesophyll in μm, and length and width of the lysоgenous cavities in μm. The data were processed with the mathematical method of descriptive statistics, and the quantitative indicators included the arithmetic average (X), average error (err), and standard deviation (SD). For each indicator 50 measurements were made.
2.6. Scanning electron microscopy (SEM) analysis of leaves, seeds, and pollen grains
The scanning electron microscope (SEM) used in this investigation was an FEI Quanta 600 SEM at the Microscopy Facility at Oregon State University, United States. Sample prepration included placing small samples into a fixative, 1% paraformaldehyde and 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer with pH 7.4. The samples were soaked in fixative for 2 h, followed by two rinses in 0.1M Cacodyalte buffer, 15 min each, and dehydration in acetone (10%,30, 50, 70,90, 95, 100-100%), 10–15 min each, followed by critical point drying (two ‘bomb flushes’ at chamber pressure to 5 °C, fill chamber with CO2). The samples were left to vent for 5 min and then the preocedure was repeated. The dry samples were mounted onto an aluminum SEM stub with double stick carbon tape. Samples were sputter coated with a Cressington 108A sputter coater from Ted Pella with Au/Pd, 60/40 mix.
For leaf surfaces, the terminology and classification of Bаrthlott et al. (1998) were used.
For seed morphology description of species, the shape, as well as the structure of the spermoderm were determined. In this case, the terminology and classification described by Barthlott and Ehler (1977) were used. For pollen surface we used the terminology and classification described by Punt et al. (2007).
2.7. Light microscopy (LM) analysis of pollen
A light microscope (LM) analysis of pollen grains was conducted to elucidate the peculiarities of the main parameters of the pollen. Measurements were made with an eyepiece micrometer (16x) and an an Amplival microscope, and the images were taken with a light digital camera Motic DMBA-210. The light analysis of pollen included pollen size along the long axis (P; X̅ - average) and the short axis (E; X̅ - average) (Table 3.). The grouping of pollen grains, according to size, was made following the classification proposed by Halbritter et al. (2009).
Table 3.
Pollen size of the three Galanthus species measured on light microscope.
| Species | Long axis (P) μm |
Short axis (E) μm |
|---|---|---|
| X̅±SDerr. | X̅±err | |
| G. elwesii | 24.36 ± 0.56 | 18.27 ± 0.34 |
| G. gracilis | 24.63 ± 0.49 | 18.45 ± 0.32 |
| G. nivalis | 25.90 ± 0.63 | 18.18 ± 0.30 |
X̅ - average; err – average error.
3. Results and discussion
3.1. Values of morphometrical characteristics
Values of the measured indices are presented in Table 4 and Figure 1. The general variability of the Galanthus species in Bulgaria was compared based on the measurements of 6 morphological traits of 60 samples of G. nivalis (of two populations), G. elwesii (of two populations) and species defined as G. gracilis (from two populations).
Table 4.
Values of the measured morphometrical characters of the three Galanthus species from different populations.
| Population/species | Indicators |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bulb diameter cm |
Stem length cm |
Leaf length cm |
Leaf width cm |
Outer periants lenght cm |
Outer periants width cm |
|||||||
| X̅±SD | min-max | X̅±SD | min-max | X̅±SD | min-max | X̅±SD | min-max | X̅±SD | min-max | X̅±SD | min-max | |
| Bogdan, G. elwesii | 1.2 ± 0.2 | 0.9–1.6 | 11.2 ± 2.3 | 7.8–17 | 10.8 ± 2.4 | 6.5–16 | 1.1 ± 0.2 | 0.7–1.5 | 2.0 ± 0.4 | 1.5–3 | 1.5 ± 0.4 | 0.9–2.8 |
| Belovo, G. elwesii | 1.3 ± 0.2 | 0.8–1.8 | 15.9 ± 3.2 | 10.3–27 | 20.6 ± 4.0 | 14.3–30 | 1.0 ± 0.2 | 0.7–1.4 | 2.2 ± 0.3 | 1.5–3.0 | 1.4 ± 0.3 | 0.9–2.0 |
| Bachkovo, G. gracilis | 0.9 ± 0.2 | 0.5–1.4 | 11.1 ± 2.7 | 10.3–18.4 | 9.9 ± 1.6 | 7.0–14.4 | 0.7 ± 0.2 | 0.4–1.1 | 1.7 ± 0.3 | 0.7–2.3 | 1.0 ± 0.2 | 0.7–1.8 |
| Макоcevo,G. gracilis | 0.8 ± 0.1 | 0.7–1.1 | 9.9 ± 2.0 | 6.2–14 | 9.9 ± 2.3 | 5.6–14.4 | 0.5 ± 0.1 | 0.4–0.8 | 1.9 ± 0.2 | 1.5–2.2 | 0.9 ± 0.1 | 0.6–1.1 |
| Belogradchik, G. nivalis | 1.1 ± 0.2 | 0.9–1.5 | 20.7 ± 3.7 | 12.5–27.5 | 22.7 ± 3.8 | 16.4–29.6 | 0.9 ± 0.1 | 0.7–1.2 | 2.3 ± 0.2 | 1.8–2.7 | 1.2 ± 0.1 | 0.9–1.4 |
| Primorsko, G. nivalis | 1.3 ± 0.2 | 0.8–1.6 | 14.6 ± 2.8 | 7.4–21.0 | 14.3 ± 3.0 | 6.1–21.1 | 0.6 ± 0.1 | 0.5–1.0 | 2.0 ± 0.1 | 1.7–2.2 | 0.8 ± 0.2 | 0.5–1.0 |
X̅-average; SD-standart deviation; min-max – minimum-maximum.
The measurements of the bulb diameter revealed that plants identified as G. gracilis had the smallest bulb size (0.8–0.9 cm) out of the three species. The results from this study on bulb diameter are within the minimum sizes specified by Davis (1999, 2001) for the Galanthus genus. Plants belonging to G. elwesii and G. nivalis had similar bulb size (1.1–1.3 cm) and these values exceeded the ones reported by Davis (2001). Also, plants identified as G. gracilis had the smallest stem and leaf lengths compared with the other two species (Table 4). The leaf lenght of G. gracilis ranged from 5.6 cm (min) to 14.4 cm (max). These results were relatively close to those obtained for a population of G. elwesii at Bogdan. However, the stem length in one population of G. gracilis (Bachkovo) and G. elwesii (Bogdan) were the same (11.1 and 11.2 cm, respectively). Similarly, plants from a population of G. elwesii (Belovo 15.9 cm) and a population of G. nivalis (Primorsko 14.6 cm) had equal values for stem lenght. The longest stems were measured in G. nivalis plants from the Belogradchik population (20.7 cm). Leaf lenght varied from 10.8 to 20.6 cm in G. elwesii and from 14.3 to 22.7 cm in G. nivalis. The widest leaves were measured in G. elwesii plants (1.0–1.1 cm); leaf width in the rest of the samples ranged from 0.5 to 0.9 cm.
The most noticeable difference in measured indices between G. elwesii and G. gracilis was the length of outer periants. The average value of the outer perianth lenght for G. nivalis was 2.0–2.3 cm, for G. elwesii it was 2.0–2.2 cm, and for G. gracilis it was 1.7–1.9 cm respectively. The outer perianth lenght of G. nivalis were closer to that of G. elwesii. In this study, the length of external periants was greater than those reported previously for this genus (Webb, 1980).
For the width of outer periants, mean values were 1.4–1.5 cm for G. elwesii and 0.9–1.0 cm for G. gracilis. Overall, the results from this study showed significant morphological variation, both between species, and also between the populations within a species. However, the results on the morphological characteristics did not define a clear trend that would provide us a basis for making taxonomic decisions.
3.2. ISSR analysis
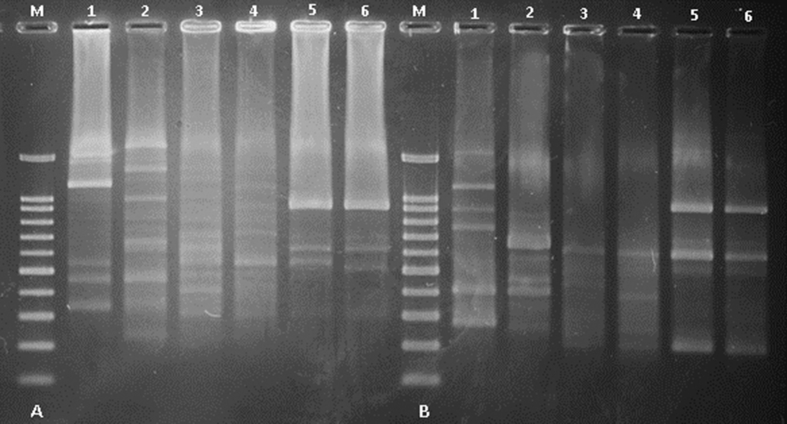
Initial screening with pre-selected ISSR markers was aimed at verifying the capacity of the selected marker system to reveal sufficient polymorphism both within and between the two species. The use of different ISSR primers led to revealing different numbers of polymorphic bands among the samples of genus Galanthus. As exemplified in Figure 2 by the products of PCR with primer ISSR1 and ISSR3, a number of polymorphic bands between the different samples were observed. The ISSR primer, presenting monomorphic bands was excluded from the analysis, making this primer unsuitable for the purpose of our study. Generally, a greater number of polymorphic bands are desirable and a prerequisite for more accurate detection of genetic diversity among specimens. Based on the 4 polymorph ISSR markers, a total of 45 polymorphic bands were obtained. We have clearly distinguished fragments ranging in size from 300 to 2200 base pairs. The average number of bands generated with the polymorphic primer was 6 and a maximum of 12 for primer C12.
Figure 2.
(The figure as uncropped original image is also provided as Supplemental Figure 1). ISSR patterns of Galanthus species: 1 - G elwesii 1 (Belovo); 2 - G. elwesii 2 (Bogdan); 3 - G. gracilis 1 (Bachkovo); 4 - G. gracilis 2 (Makotsevo); 5 - G. nivalis 1 (Primorsko); and 6 - G. nivalis 1 (Belogradchik).generated with primers 3 (A) and 1(B). Line M – 100bp Rainbow extended DNA ladder.
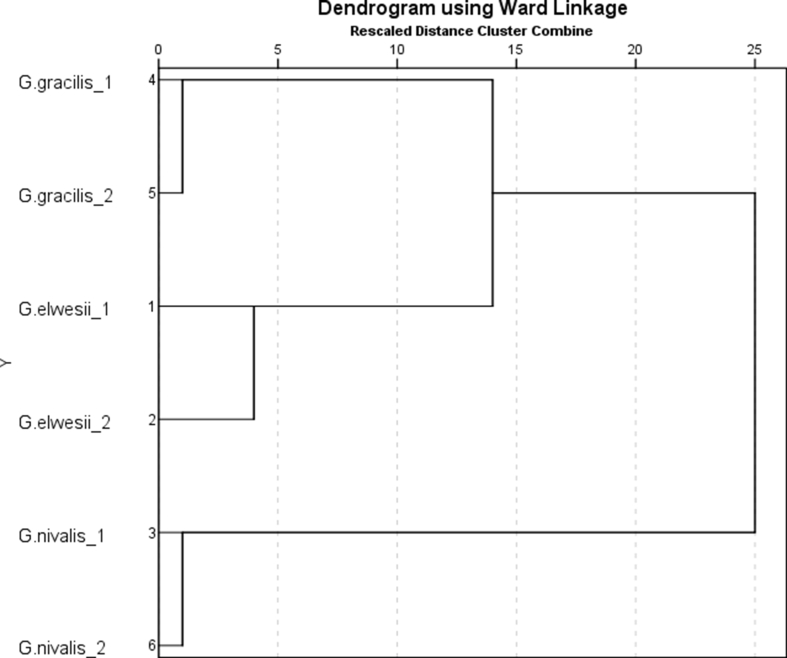
As a result of the screening of several primers and optimizing the PCR conditions, six of the ISSR primers were identified to produce informative polymorphisms in these plant samples. Clearly detectable amplified fragments ranged from 300 to 2100 bp in size. The average number of clear bands generated per polymorphic primer was 6, with a maximum of 14 for primer ISSR3. In total 64 (91%) polymorphic fragments were produced from 70 loci amplified. The molecular data collected were used to calculate relative genetic distances and to create hierarchical clusters (Figure 3). After processing the results of the ISSR analysis, an SPSS statistical processing program was implemented and a dendrogram was constructed presenting the phylogenetic relationships between the different species. Six ISSR primers were used to get information on the presence of genetic polymorphisms. Cluster analysis based on grouped Galanthus species into two main clusters revealed the difference between the samples. The first cluster combined G. elwesii 1 and 2 also G.gracilis 1 and 2, which correspond to their family affiliation. These results agree with those previously reported by Zonneveld et al. (2003), in which DNA analyses confirmed the close relationships in some species pairs, including those between G. nivalis and G. reginae-olgae, between G. krasnovii and G. platyphyllus, and between G. gracilis and G. elwesii. The results from this study indicated that G. elwesii and G. gracilis are more closely related, compared to G. nivalis, which was situated in a separate cluster. To further accurately identify the genetic diversity among the two species, additional molecular analysis is envisaged, using a large set of ISSR primers exhibiting high levels of polymorphism in combination with the taxonomical data.
Figure 3.
Dendrogram based on polymophisms revealed with six ISSR primes for samples G elwesii 1 (Belovo); G. elwesii 2 (Bogdan); G. gracilis 1 (Bachkovo); G. gracilis 2 (Makotsevo); and G. nivalis 1 (Primorsko); and G. nivalis 1 (Belogradchik).
3.3. Leaf anatomy study
To solve the taxonomic issues in the Galanthus genus, it is necessary to combine morphological examinations of the species with the histological structure of the leaf plate (Davis and Barnett, 1997). The peculiarities of the epidermal complex and the structure of the mesophyll are taxonomic characteristics that have been used in a number of taxonomic studies of the genus (Stern, 1956; Artjushenko, 1966, 1970; Davis and Barnett, 1997; Zeybek and Sauer, 1995).
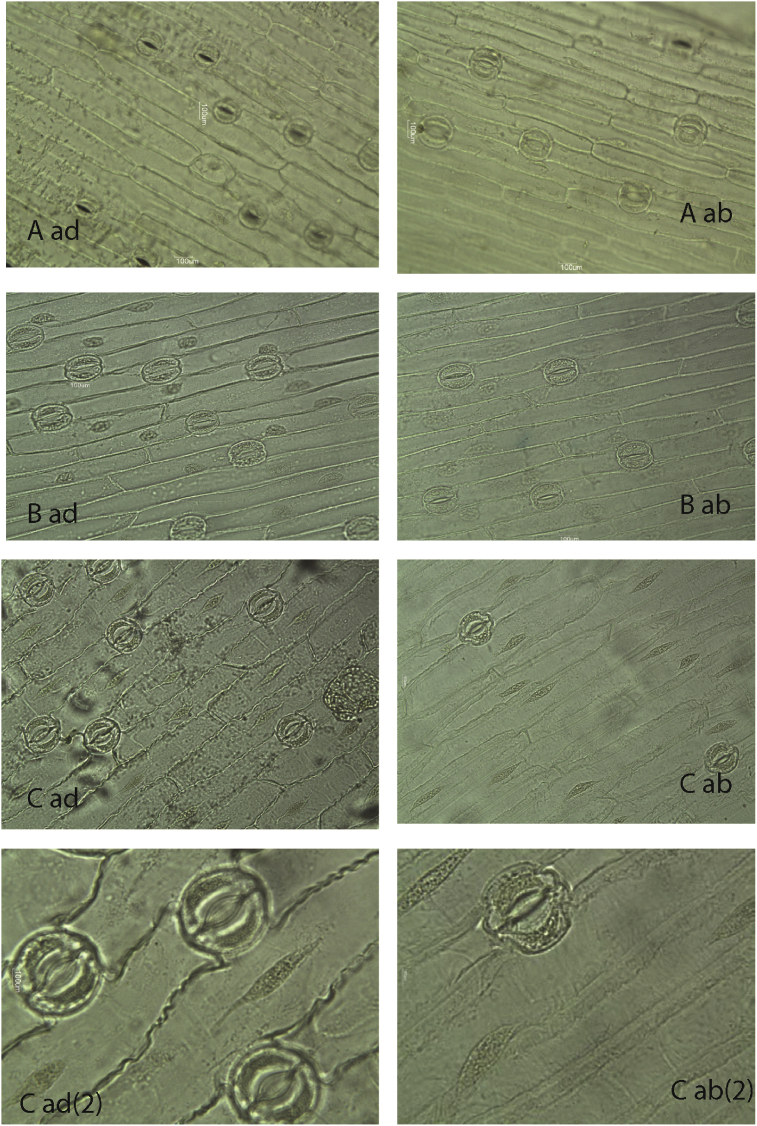
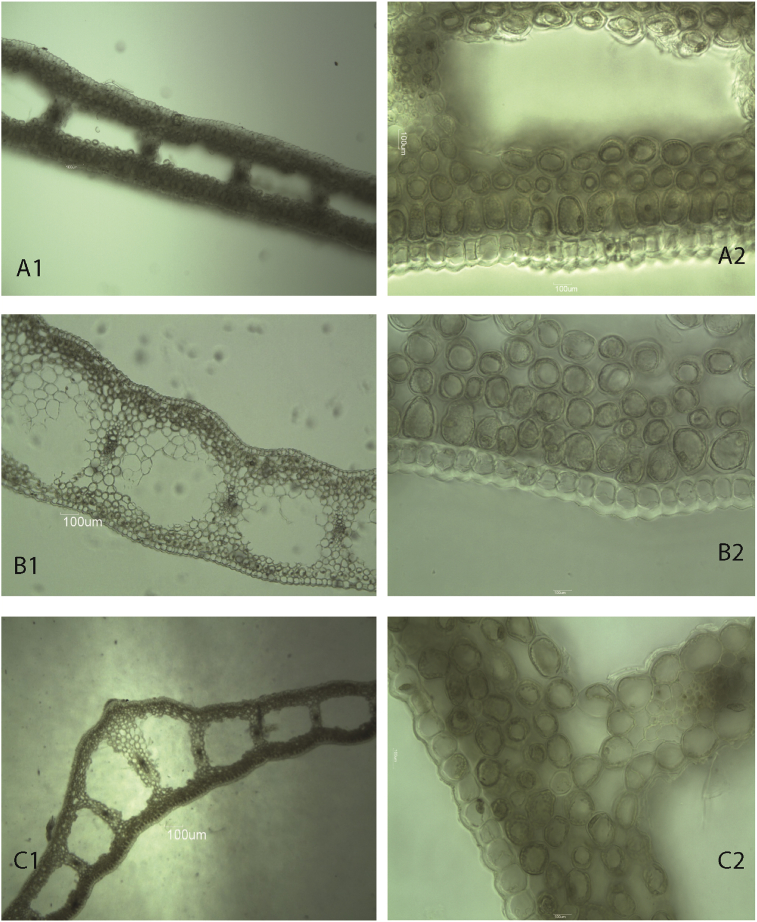
The results from this study showed that the main epidermal cells were elongated and varied in size and form (short, strongly elongated and transient type) (Figure 4A,B,C). Most specific were the main epidermal cells in G. nivalis; the lateral walls of the cells on the upper (adaxial) epidermis were waved and the lateral walls of the cells on the lower epidermis were significantly thickened (Figure 4C).
Figure 4.
General view of epidermis cells, stomata of G. gracilis (A - ad, ab surface, LM 10 × 10); G. elwesii (B - ad, ab surface, LM 10 × 10); G. nivalis (C - ad, ab surface, LM10 × 10, LM 10 × 40).
The side walls of the epidermal cells of G. elwesii and the plants that were identified as G. gracilis were straight. Variations in the slope of the transverse cell walls of the main epidermal cells was observed in all three species. The transverse walls were positioned in one of the following ways: tapered edges at acute angles (defining a triangular tapered end of the cell), straight transverse walls (defining a rectangular shape of the cells), and rounded edges (defining a shape of rounded oval cells). The cells of the plants identified as G. gracilis (Figure 4A) were characterized by the most constant shape (rounded oval). In this study, spherical thickening was observed on the major epidermal cells in all three species, but these were more pronounced on the lower (abaxial) surface of G. nivalis.
The results from this study indicated that in G. nivalis, the lateral walls of the cells on the abbaxial side were strongly thickened, which was not observed in the other two species (Figure 4C). Apparently, this is the first report on such thickening; no relevant information was found in the literature about these peculiarities of epidermal cells.
The stomata were found on both the upper and lower leaf surfaces, which determines the leaves as amphistomatic. The stomata numbers were greater on the abaxial surface, ranging from 57.5 to 67.5 per mm2 (Table 5). The guard cells had a bean-like shape. Generally, the guard cell shape is closely related to the physiological state of the cell (Willmer and Fricker, 1996). The stomata type of the species was of an anomocytic (irregular celled) type.
Table 5.
Measured indices of the leaves of the three Galanthus species in μm.
| Indicators: | X̅ ± err. | SD. | ||
|---|---|---|---|---|
| Number of stomata per mm2 | G. nivalis | Upper surface (ad) | 40.4 ± 1.5 | 8.4 |
| G. elwesii | 47.9 ± 1.8 | 9.8 | ||
| G. gracilis | 63.3 ± 1.9 | 10.8 | ||
| G. nivalis | Lower surface (ab) | 57.5 ± 2.4 | 13.3 | |
| G. elwesii | 63.3 ± 2.8 | 15.3 | ||
| G. gracilis | 67.5 ± 2.3 | 12.9 | ||
| Stomata length, μm | G. nivalis | Upper surface (ad) | 39.8 ± 0.3 | 1.7 |
| G. elwesii | 47.5 ± 0.4 | 2.2 | ||
| G. gracilis | 31.7 ± 0.2 | 1.1 | ||
| G. nivalis | Lower surface (ab) | 42.8 ± 0.4 | 2.6 | |
| G. elwesii | 43.5 ± 0.4 | 2.4 | ||
| G. gracilis | 35.2 ± 0.2 | 1.3 | ||
| Somata width, μm | G. nivalis | Upper surface (ad) | 36.0 ± 0.6 | 3.3 |
| G. elwesii | 34.0 ± 0.3 | 1.9 | ||
| G. gracilis | 32.5 ± 0.2 | 1.6 | ||
| G. nivalis | Lower surface (ab) | 32.8 ± 0.4 | 1.8 | |
| G. elwesii | 35.3 ± 0.3 | 1.7 | ||
| G. gracilis | 32.2 ± 0.3 | 1.7 | ||
| Leaf thickness, μm | G. nivalis | 290.2 ± 3.0 | 16.5 | |
| G. elwesii | 315.6 ± 1.6 | 9.1 | ||
| G. gracilis | 136.9 ± 1.9 | 10.5 | ||
| Lysigenous cavities width, μm | G. nivalis | 140.6 ± 2.5 | 14.1 | |
| G. elwesii | 189.4 ± 2.0 | 11.3 | ||
| G. gracilis | 33.6 ± 1.1 | 6.3 | ||
| Lysigenous cavities length, μm | G. nivalis | 228.0 ± 1.6 | 9.2 | |
| G. elwesii | 289.7 ± 2.73 | 14.9 | ||
| G. gracilis | 112.0 ± 2.6 | 14.55 | ||
X̅-average; SD-standart deviation.
The transverse sections prepared in this study showed that the leaf thickness of G. elwesii (316 μm) was the greatest while that of G. gracilis (137 μm) was the thinnest. The leaves were characterized by the following histological structure: a single layer epidermis, a mesophyll made up of oval parenchymal cells, closed collateral vascular bundles, and lysigenous cavities between them (Figure 5A1,B1,C1). A similar histological structure of the leaves was established in G. elwesii and G. gracilis (Figure 5A2,B2). The first row of mesophyll cells below the epidermal layer in both species was made of of elongated cylindrical cells (palisades) that pass into oval cells towards the end of the leaf. In G. nivalis, the mesophyll was made of cells with similar forms (Figure 5C2). This feature of mesophyllic cells is a trait that can be used to distinguish the two species (G. elwesii and G. nivalis). Previously, the shape and size of lysigenous cavities in the leaf plate have been used as basis for some taxonomic decisions for the genus Galanthus (Artjushenko, 1970). From the measurements conducted in this study, the largest (189 μm width and 289 μm length) lysigenous cavities were found in G. elwesii and the smallest ones in G. gracilis (33 μm wide and 112 μm long) (Table 5). However, the results from this study demonstrated that the shape and size of lysigenous cavities may vary significantly within one leaf in all three species, since there were lysigenous cavities in the process of formation. Therefore, lysigenous cavity form and shape should not be used for taxonomic decisions.
Figure 5.
Transverse section general view, lysigenous cavities of G. gracilis (A1, A2); G. elwesii (B1, B2); G. nivalis (C1, C2) LM10 × 10, LM 10 × 40.
3.4. Scanning electron microscopy (SEM) analysis of leaf surfaces
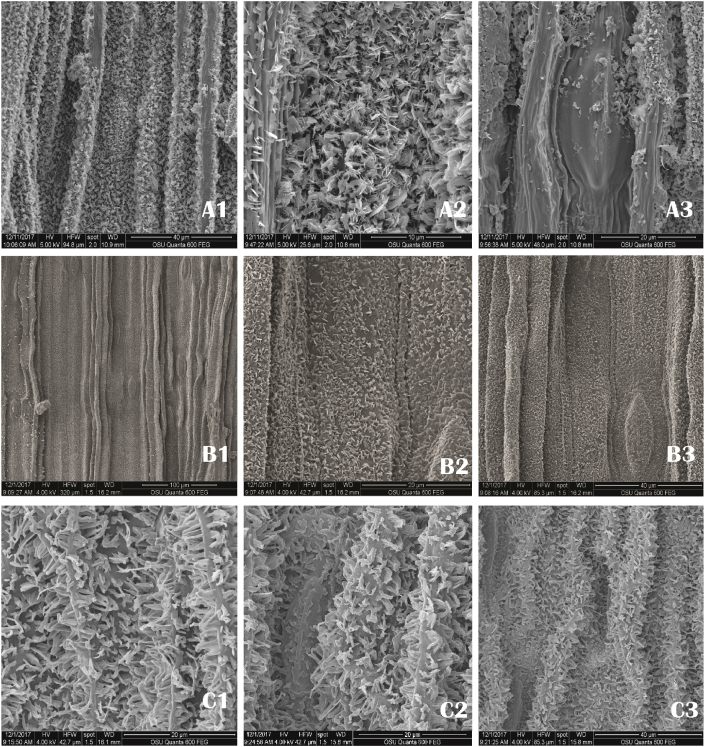
Epicuticular waxes that cover the epidermis of some plants are characterized by a considerable diversity in their ultrastructure and chemical composition (Bаrthlott et al., 1998). Leaf wax, manifest as the leaf color, is a character of taxonomic significance in Galanthus (Davis and Barnett, 1997). The SEM analysis in this study showed that the leaf surface of the three species was wavy, with multiple folds. Pericilinal walls had many stratiations, clearly expressed in G. elwesii and G. nivalis. The results from this study showed that epicuticular waxes were clearly distinguishable and different between G. elwesii and G. nivalis (Figure 6 B2, C2). Polygonal rodlet wax structures were observed in G. nivalis. The stomata in G. nivalis were submerged/surrounded by waxes longitudinally aggregated that formed layers (Figure 6 C3). In G. nivalis, in addition to the observed polygonal rodlets, there were also single plates of waxes. In G. elwesii and G. gracilis, the predominant were the plate waxes, curled, among which were also single waxes with stick-like form. The orientation of waxes and crystalloloids is of taxonomic importance when describing the leaf surface (Bаrthlott et al., 1998). In this study, parallel stacked rodlets/plates were found in all three species (Figure6 A2,B2,C2).
Figure 6.
Scanning Electron Microscopy (SEM) analysis of leaves surfaces of G. gracilis (A1, A2, A3); G. elwesii (B1, B2, B3); G. nivalis (C1, C2, C3).
3.5. Scanning electron microscopy (SEM) analyses of the pollen
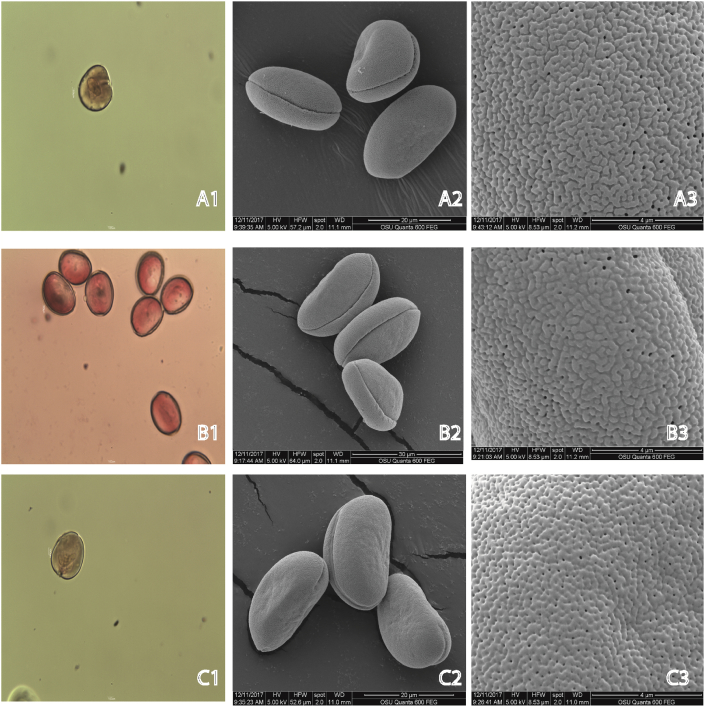
The peculiarities of the pollen grains in combination with other traits of the plant species are used in plant systematics (Halbritter et al., 2009). Overall, in this study, the pollen grains of G. elwesii and G. gracilis can be defined as small (10–24.6 μm), while the pollen grains of G. nivalis were slightly larger (medium 25.9 μm), if the classification of Halbritter et al. (2009) is followed (Table 3). Overall the family is characterized by a strong variation in the size of the pollen grains (Erdtman, 1952). In all three species, the pollen was oval-shaped (prolate), ovate-extended in its distal part (prolate shaped) (Figure 7A1,B1,C1). Pores were located over the entire pollen grain surface (Figure 7 A3,B3,C3). The pollen grains in all three species were single-apperture, with a longitudinal position of the apperture (Figure 7 A1,B2,C2). The exine was reticulate, micro-rugulate. In G. nivalis, the aperture membrane of the colpus (an elongated aperture) was smooth (Figure 7C2). The pollen grains form and surface found in this study is characteristic for most members of the family Amaryllidaceae (Dönmez and Işik, 2008; Erdtman, 1952; Moore et al., 1991).
Figure 7.
Light microsopy (LM 10 × 10) and Scanning Electron Microscopy (SEM) analyses in pollen., G. gracilis (A1, A2, A3); G. elwesii (B1, B2, B3); G. nivalis (C1, C2, C3).
3.6. Scanning electron microscopy (SEM) analyses of seeds
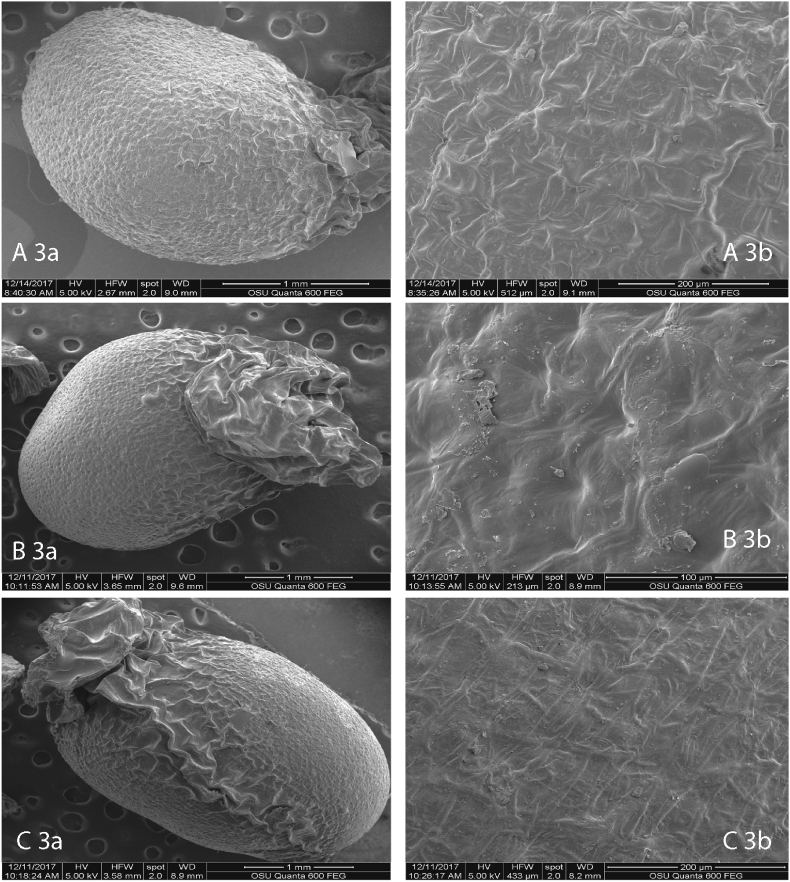
The seed form is more or less genetically determined (Werker, 1997), however, species in the genus may often display phenotypic or genotypic variability (Chkhaidze et al., 2014). In this study, the seed pattern ranged from oval-shaped (G. nivalis, and G. elwesii) to egg-shaped (G. gracilis) (Figure 8 A3a,b; B3a,b; C3a,b). We observed that the seed spermoderm grows more pronounced at one end of the seed and forms a hood (seed with elaiosome). From a systematic point of view, particularly important features of the spermoderm are the shape of the cells, the edge, and the appearance of the anticlinal and periclinal walls (Takhtajan 1991). The surface of the seeds of the three species in this study ranged from Tabular type (G. nivalis) to a slightly Concave type (G. elwesii and G. gracilis) (Figure 8 A3b; B3b; C3b). The anticlinal and pericillinal walls of the three species in this study had a rounded edge with insignificant striation, more pronounced in G. elwesii and in G. gracilis. It should be noted that the surface of the seed spermoderm in the area near the elaiosome had more pronounced concavities (Concave type). Similar seed form and surface have been observed in G. cilicicus and G. peshmenii (Yüzbaşioğlu et al., 2013).
Figure 8.
SEM analysis of seeds; G. gracilis (A3a,b); B 3a, b – G. elwesii (B3a,b); G. nivalis (C3a,b).
3.7. Embryological study
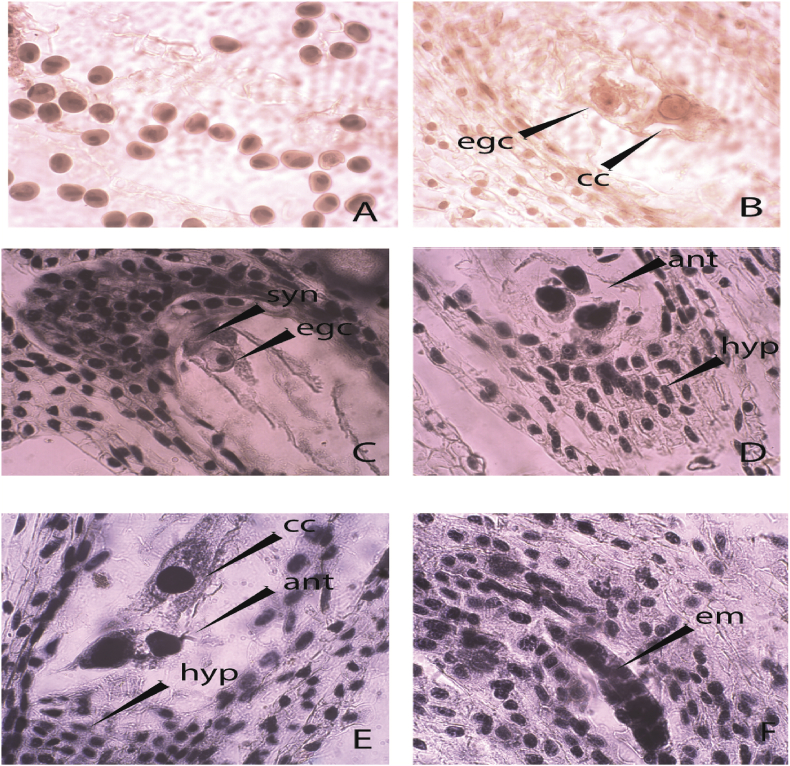
In this study, the features of the generative sphere showed similarities between the studied species, more clearly expressed between G. elwesii and G. gracilis, and namely:
Male generative sphere: The anthers were tetrasporangiate. The anther wall developed after the Monocotyledonous-type according to Davis’ classification (Davis, 1966). It consisted of four layers: epidermis, fibrous endothecium, one ephemeral middle layer, and glandular tapetum. The sporogenous tissue was multilayered. The microsporogenesis passed successively and as a result, tetrahedral microspore tetrads formed predominantly. At the time of shedding, the mature pollen grains were two-celled (Figure 9A).
Figure 9.
Pecularities of the generative sphere in the studied Galantus species: A) mature two-celled pollen grains in G. nivallis; B) mature anatropous ovule with egg cell and cenral cell in the ES cavity of G. nivallis; C) mature anatropous ovule with egg cell and synergids in the embry sac (ES) cavity of G. elwesii; D) and of G. gracilis E) and G. elwesii F) asterad type embryo in the ES cavity of G. gracilis.(x400); egc – egg cell; cc – central cell; syn – synergids; ant – antipodals; hyp – hypostase; em – embryo.
Female generative sphere: The Gynoecium was syncarpous, inferior. The ovary was two-locular. The ovule was anatropous, bitegmic and crassinucellate (Figure 9B,C). Embryo-sac development followed Polygonum-type. Synergids were hooked and with filiform apparatus (Figure 9C). The antipodal apparatus consisted of 3 cells that did not proliferate (Figure 9D). Polar nuclei fused prior to fertilization and formed the central cell that was located near the antipodals (Figure 9E) or the egg cell (in G. nivalis) (Figure 9 B). At the chalazal end of the embryo sac a hypostase was present (Figure 9D,E). The Endosperm formation passes a nuclear stage; the embryogenesis follows an asterad-type of development (Figure 9F).
4. Concluding remarks
4.1. Values of morphometrical characters
Generally, the morphological characteristic data showed that the mean values of the measured indices in G. elwesii were greater than those of G. gracilis. The least variable were the leaf width and length, and the width of the outer periants. The analysis of the variability of the morphometric characteristics revealed a lack of discretion in the variability. This does not allow us to define clear feature that may help to distinguish and separate G. elwesii from G. gracilis.
The results from study partially agree with those from a previous morphometric study of G. elwesii in Bulgaria (Sidjimova, 2006). Similar contradictory results have been found for some populations in Ukraine (Budnikov, 2011) and Serbia (Jovanović et al., 2018). The morphometric characteristics of G. elwesii and G. gracilis are not sufficiently reliable to make taxonomic decisions, because both species have the same morphological features and the same color properties (Jovanović et al., 2012). In each of the populations, individual plants may fully fit the morphometric description of both G. elwesii and G. gracilis.
As noted by Rønsted et al. (2013) the plants of G. elwesii distributed on the Balkan Peninsula, compared to those in Turkey, are significantly smaller in size. Therefore, they proposed a review and revision of the taxonomic status of G. elwesii distributed in the Balkans.
G. elwesii and G. gracilis have a green mark on the inner perianths and therefore they are easily distinguishable from G. nivalis that do not possess this feature.
4.2. Leaf anatomy study
The anatomical characteristics of the leaf have been used for making taxonomic decisions in the genus Galanthus (Artjushenko, 1966, 1970; Davis and Barnett, 1997). The main taxonomic markers used by various investigators were features of the mesophyll, type of major epidermal cells, and shape and size of lysigenous cavities. This study used these basic markers to compare the plants from the three species. In this study the anatomical analyses demonstrated that the leaves of G. elwesii and those identified as G. gracilis had a similar histological structure. This uniformity in the leaf structure gave Davis and Barnett (1997) the basis for grouping the two species into one group.
Unlike some previous reports (Artjushenko, 1965, 1966, 1970), this study found variation in the shape of the main epidermal cells. The shape of epidermal cells depends on the slope of the transverse walls. Such variation of epidermal cells was also observed by Davis and Barnett (1997). The observed variability in the form of major epidermal cells suggests that this trait should not be used for making systematic botany decisions in the Galanthus genus.
Usually the characteristics of the stomata complex is considered to have high taxonomic value (Cutler et al., 2007; Willmer and Fricker, 1996). In this study, there were no significant differences between the stomata characteristics of plants identified as three separate species. The observed variation in the stomata density and sizes is directly related to specific environmental conditions of the populations natural habitats (Davis and Barnett, 1997).
The mesophyll of both G. elwesii and G. gracilis was composed of two types of parenchyma cells (palisades, elongated and oval spongy mesophyll), while in G. nivalis, the mesophyll comprised one type of parenchymal cells only (oval spongy mesophyll). A characteristic feature of Galanthus leaf structure is the mesophyllic voids noted in the literature as air cavities. These gaps were identified as lysigenous cavities in this study, because observations revealed cells at various stages of lysing their cell walls (cell wall debris or semi-destroyed cells). The lysigenous cavities in the leaves were also used for taxonomic identification of species by other researchers (Artjushenko, 1965, 1970). However, this study found variation in lisigenous cavities size and shape. Davis and Barnett (1997) reported that in G. gracilis the lysigenous cavities were significantly larger than those in other species of the genus (to large 160–200 × 520-430 μm). In our study, this was not confirmed. However, we think that lysigenous cavities can not be considered a reliable trait and used as a systematic mark; our observations found that the lysigenous cavities in the different parts of the leaves vary in both size and degree of differentiation.
Leaf waxes, manifested as leaf colors, are a trait of taxonomic significance for the genus Galanthus (Davis and Barnett, 1997). No comparative SEM analysis of morphological peculiarities of the Galanthus species was found in the accessible literature. In this study, the epicucular waxes of G. nivalis and G. elwesii were clearly different. In G. elwesii and G. gracilis, the curled waxes plates were predominant. Also in this study, the SEM analysis of the pollen grains and seeds showed similar characteristics of the three species.
4.3. Embryological study
In this study, the specificities in the reproductive biology and sexual structure of the three Galanthus species were found to be common to the genus, and do not allow for differentiation of the species. The proper flow of processes in the generative sphere leading to the formation of male and female gametophytes provide the species with high reproductive potential. The analysis suggested that the formation of a large amount of mature fertile pollen, the absence of fetal deflection as degenerations of the generative structures were a condition for good reproduction of the species. The lack of apomixis, on the one hand, characterizes them as strictly sexual species, but on the other hand it is a prerequisite for their predicted low plasticity, and hence probably poor adaptation to changes in the environment. The observations showed that the three types of embryonic structures were similar. There is no data in the literature on the embryology of these species.
4.4. ISSR analysis and conclusion
Due to the limited number of molecular studies focused on European gene pool investigation, it is necessary to perform plant material identification via the application of molecular marker systems. As this study demonstrated, G. nivalis, G. elwesii, and G. gracilis have high morphological variation of individual vegetative parts. In order to cover this diversity, it is necessary to conduct a genetic study of all Galanthus populations in Bulgaria. ISSRs could identify a significant number of polymorphisms in the plant species. These characteristics would be important for the practical applications of a DNA marker system in plant taxonomy.
The following general conclusions can be drawn from the results of the study comparing G. nivalis and G. elwesii and the plants identified as G. gracilis: (1) There is morphological variation, both between and within the species. Within a species population, different transitions (ranges) in the leaf and stem size, and bulb diameter were established. (2) The anatomical structure of the leaves in G. elwesii and G. gracilis was similar, whereas that of G. nivalis was different. (3) The embryological characteristics were the same for G. elwesii, G. nivalis, and the plants identified as G. gracilis. (4) From the SEM pollen and seed analysis, it became clear that in G. elwesii and the plants designated as G. gracilis had the same surface characteristics including epicuticular waxes. Overall, this study demonstrated that the studied populations defined as G. gracilis are most probably a form of G. elwesii. Therefore, this study refutes the hypothesis and the claim of previous reports that populations defined as G. gracilis belong to a separate species (G. gracilis, and not to G. elwesii). On the other hand, G. elwesii and G. nivalis had clearly identifiable (morphological, anatomical, DNA, and SEM) characteristics as separate species.
Declarations
Author contribution statement
Ivanka Semerdjieva: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Boryana Sidjimova, Elina Yankova-Tsvetkova, Milena Kostova, Valtcho D. Zheljazkov: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data, reviewed and edited the paper.
Funding statement
Most of the research was supported by the Scientific Research Centre of the Agricultural University in Plovdiv, Bulgaria: Project №12–15 awarded to Dr. Semerdjieva and colleagues. The SEM analsyes were conducted at Oregon State University (OSU), USA. The SEM anlyses and the article publishing charge were covered by Dr. Jeliazkov (Zheljazkov) at OSU, USA.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Teresa Sawyer from the Microscopy Facility at Oregon State University (OSU). We gratefully acknowledge funding from OSU for the scanning electron microscopy analyses.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Agarwal M., Shrivastava N., Padh H. Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep. 2008;27(4):617–631. doi: 10.1007/s00299-008-0507-z. https://link.springer.com/article/10.1007/s00299-008-0507-z [DOI] [PubMed] [Google Scholar]
- Anne C. Choosing the right molecular genetic markers for studying biodiversity: from molecular evolution to practical aspects. Genetica. 2006;127(1-3):101–120. doi: 10.1007/s10709-005-2485-1. [DOI] [PubMed] [Google Scholar]
- Arif I., Bakir A., Khan M., Al Farhan N., Al Homaidan H., Bahkali A., Al Sadoon A., Shobrak M. A brief review of molecular techniques to assess plant diversity. Int. J. Mol. Sci. 2010;11:2079–2096. doi: 10.3390/ijms11052079. https://www.mdpi.com/1422-0067/11/5/2079/htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artjushenko Z. A contribution to the taxonomy of the genus Galanthus. Botulinum J. 1965;50(10):1430–1447. [in Russian] [Google Scholar]
- Artjushenko Z. Vol. 51. 1966. Critical Review of the Genus Galanthus L. Botanicals Reports, Moscow; pp. 1437–1451. [in Russian] [Google Scholar]
- Artjushenko Z. Nauka; Leningrad: 1970. Амариллисоϑые in the SSSR. Morphology, Systematics and Used. [in Russian] [Google Scholar]
- Barthlott W., Ehler N. . F. Steiner Verlag; Stuttgart, 105 S: 1977. Raster-Elektronenmikroskopie der Epidermis-Oberflächen von Spermatophyten. Trop. subtrop. Pflanzenwelt 19, Akad. Wiss. Lit. Mainz. [Google Scholar]
- Berkov S., Bastida J., Sidjimova B., Viladomat F., Codina C. Alkaloid diversity in Galanthus elwesii and Galanthus nivalis. Chem. Biodivers. 2011;8(1):115–130. doi: 10.1002/cbdv.200900380. [DOI] [PubMed] [Google Scholar]
- Berkov S., Bastida J., Sidjimova B., Viladomat F., Codina C. Phytochemical differentiation of Galanthus nivalis and Galanthus elwesii (Amaryllidaceae): a case study. Biochem. Syst. Ecol. 2008;36(8):638–645. [Google Scholar]
- Bаrthlott W., Neinhuis C., Cutler D., Ditsch F., Meusel I., Theisen I., Wilhelmi H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998;126:237–260. [Google Scholar]
- Budnikov G. Morphological variation of specimens and populations of Galanthus nivalis L. in western regions of Ukraine. Thaiszia – J. Bot., Košice. 2011;21:95–109. ISSN 1210-0420. [Google Scholar]
- Chkhaidze N., Goginashvili N., Zurabishvili M., Manvelidze Z. Some morphological and anatomical descriptions of seed in Galanthus woronowii losinsk. From Western Georgia. Mod. Phytomorphol. 2014;6:59–66. [Google Scholar]
- Cutler D., Botha T., Stevenson D. Blackwell Publishing; 2007. Plant Anatomy. An Applied Approach. [Google Scholar]
- Davis A.P. The genus Galanthus – snowdrops in the wild. In: Bishop M., Davis A.P., Grimshaw J.A., editors. Monograph of Cultivated Galanthus. The Griffin Press; Maidenhead: 2001. pp. 9–63. [Google Scholar]
- Davis G. John Wiley & Sons, Ltd; New York, London, Sydney: 1966. Systematic Embryology of the Angiosperms. [Google Scholar]
- Davis A.P. Timber Press; Portland: 1999. The Genus Galanthus. A Botanical Magazine Monograph. published in association with the Royal Botanic Gardens, Kew. [Google Scholar]
- Davis A., Barnett J. The leaf anatomy of the genus Galanthus L. Arnaryllidaceae J. St.-Hil.). Bot. J. Linn. Soc. 1997;123:333–352. [Google Scholar]
- Delipavlov D., Chesmedziev I., Popova M., Terzijski D., Kovachev I. Genus Galanthus. In: Delipavlov D., editor. Determinant of Plant in Bulgaria. Agricultural University; Plovdiv: 2003. pp. 452–453. [in Bulgarian] [Google Scholar]
- Dönmez E.A., Işik I. Pollen morphology of Turkish Amaryllidaceae, ixioliriaceae and iridaceae. Grana. 2008;47(1):15–38. [Google Scholar]
- Erdtman G. Almqvist & Wiksell; Stockholm: 1952. Pollen Morphology and Plant Taxonomy. Angiosperms (An Introduction to Palynology. I) pp. 37–41. [Google Scholar]
- Halbritter H., Ulrich S., Grímsson F., Weber M., Zetter R., Hesse M., Buchner R., Svojtka M., Frosch-Radivo M. Springer Science & Business Media; 2009. Pollen Terminology: an Illustrated Handbook. (eBook) [Google Scholar]
- Hilooğlu M., Sözen E. Population genetic structure of endemic Verbascum alyssifolium in Erzincan region of Turkey. Fresenius Environ. Bull. 2017;26(2a):1756–1764. [Google Scholar]
- Jordanov D. Genus Galanthus L. In: Jordanov D., Izdatelstvo na B.A.N., editors. Vol. 2. 1964. pp. 318–319. (Flora of the Republic of Bulgaria). Sofia. [in Bulgarian] [Google Scholar]
- Jovanović F., Obratov-Petković D., Zlatković B. Vrsta Galanthus gracilis Celak. (Amaryllidaceae) u flori serbije. Bull. Forresti. 2012;106:101–112. [in Serbian] [Google Scholar]
- Jovanović F., Obratov-Petković D., Bjedov I., Živanović I., Braunović S., Cirković-Mitrović T., Tomovic G. Morphological variability of snowdrops in the central part of the balkan Peninsula. Hortscience. 2018;53(8):1119–1124. [Google Scholar]
- Kozucharov S. Field Guide to the Vascular Plants in Bulgaria. Nauka & Izkustvo; Sofia: 1992. [in Bulgarian] [Google Scholar]
- Larsen M.M., Adsersen A., Davis A.P., Lledó M.D., Jäger A.K., Rønsted N. Using a phylogenetic approach to selection of target plants in drug discovery of acetylcholinesterase inhibiting alkaloids in Amaryllidaceae tribe Galantheae. Biochem. Syst. Ecol. 2011;38(5):1026–1034. [Google Scholar]
- Lledó M., David A., Crespo M., Chase M., Fay M. Phylogenetic analysis of Leucojum and Galanthus (Amaryllidaceae) based on plastid matK and nuclear ribosomal spacer (ITS) DNA sequences and morphology. Plant Syst. Evol. 2004;246(3-4):223–243. [Google Scholar]
- Lopes M.S., Mendonça D., Bettencourt S.X., Borba A.R., Melo C., Baptista C., Machado, da Cȃmara A. Genetic diversity of an Azorean endemic and endangered plant species inferred from inter-simple sequence repeat markers. AoB Plants. 2014;6:plu034. doi: 10.1093/aobpla/plu034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerow A., Francisco-Ortega J., Kuhn D., Schnell R. Phylogenetic relationships and biogeography within the eurasian clade of Amaryllidaceae based on plastid and nrDNA ITS sequences: lineage sorting in a reticulate area? Syst. Bot. 2006;31(1):42–60. [Google Scholar]
- Moore P.D., Webb J.A., Collinson M.E. Blackwell Sci. Publ; London: 1991. Pollen Analysis. [Google Scholar]
- Poddubnaya-Arnoldi V.A. Nauka; Moskva: 1976. Cytoembryology of the Angiosperm. [in Russian] [Google Scholar]
- Punt W., Hoen P., Blackmore S., Nilsson S., Le Thomas A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007;143(1-2):1–81. [Google Scholar]
- Rønsted N., Zubov D., Bruun-Lund S., Davis A.P. Snowdrops falling slowly in to place: an improved phylogeny for Galanthus (Amaryllidaceae) Mol. Phylogenetics Evol. 2013;69(1):205–217. doi: 10.1016/j.ympev.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Sidjimova B. Morphometrical variability in Bulgarian Galanthus elwesii (Amaryllidaceae) In: Ivanova D., editor. Plant, Fungal and Habitat Diversity Investigation and Conservation. Proceedings of IV Balkan Botanical Congres, 20-26 June 2006. Institute of Botany, Sofia; Sofia: 2006. pp. 205–210. [Google Scholar]
- Sidjimova B. Department of Plant and Fungal Diversity; BAS, Sofia: 2008. Biological and Phytochemical Study of Species of Genus Galanthus L. In Bulgaria. PhD thesis. [in Bulgarian] [Google Scholar]
- Stern F.C. The Royal Horticural Society, Vincent Square, London; London: 1956. Snowdrops and Snowflakes. [Google Scholar]
- Stojanov N., Stefanov B. 1924. Flora in Bulgaria I. Sofia. [in Bulgarian] [Google Scholar]
- Stojanov N., Stefanov B. 1933. Flora in Bulgaria II. Sofia. [in Bulgarian] [Google Scholar]
- Stojanov N., Stefanov B. 1948. Flora in Bulgaria III. Sofia. [in Bulgarian] [Google Scholar]
- Stojanov N., Stefanov B., Kitanov B. 1966. Flora in Bulgaria IV (1). Sofia. [in Bulgarian] [Google Scholar]
- Sundara R.S. Anmol Publ. PVT LTD; New Delhi: 2000. Practical Manual of Plant Anatomy and Embryology. [Google Scholar]
- Takhtajan A. Columbia Univ. Press; New York: 1991. Evolutionary Trends in Flowering Plants. [Google Scholar]
- Thiers B. 2012. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff.http://sweetgum.nybg.org/ih/ New York Botanical Garden's. [continuously updated]. Virtual Herbarium. 622. [Google Scholar]
- Velenovsky J. 1891. Flora Bulgarica. Sofia. [in Bulgarian] [Google Scholar]
- Velenovsky J. Suplementum I; Sofia: 1898. Flora Bulgarica. [in Bulgarian] [Google Scholar]
- Webb D.A. Genus Galanthus. In: Tutin T.G., Heywood V.H., Burges N.A., Moore D.M., Valentine D.H., Walters S.M., Webb D.A., editors. V. Cambridge Univ. Press; Cambridge, UK: 1980. pp. 77–78. (Flora Europaea). [Google Scholar]
- Werker E. Gebruder Borntraeger; Berlin, Germany: 1997. Seed Anatomy; p. 424. [Google Scholar]
- Willmer C., Fricker M. Springer-Science business Media, B.V; 1996. Stomata. [Google Scholar]
- World Cheklist of Selected Plant Families . Kew Garden; 2018. http://apps.kew.org/wcsp/qsearch.do;jsessionid=56A58C50E62834507137259ECD D7B0E0 [Google Scholar]
- Yüzbaşioğlu I., Aykur C., Çinbilgel I., Gőktürk R.S., Deniz I.G., Bozkurt M. Some notes on Galanthus cilicicus and Galanthus peshmenii (Amaryllidaceae) Biol. Divers. Conserv. 2013;6(1):153–160. ISSN 1308-8084 Online; ISSN 1308-5301. [Google Scholar]
- Zeybek N., Sauer E. Ege Üniversitesi Basimevi; Izmir: 1995. Türkiye kardelenleri (Galanthus L.) I beitrage zur türkischen schneeglöckhen (Galanthus L.) I/ [Google Scholar]
- Zhong J. Amaryllidaceae and sceletium alkaloids. Nat. Prod. Rep. 2005;22:111–126. doi: 10.1039/b316106b. [DOI] [PubMed] [Google Scholar]
- Zietkiewicz Е., Rafalski А., Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-Anchored polymerase chain reaction amplification. Genomics. 1994;20(2):176–183. doi: 10.1006/geno.1994.1151. https://reader.elsevier.com/reader/sd/pii/S0888754384711529?token=252457083E56E6CA8811E5B5D7DED104F0E53C0436F1756DACF63082E087ED33351AC37ED50306770E0F818E3051C05B [DOI] [PubMed] [Google Scholar]
- Zonneveld B., Grimshaw J., Davis A. The systematic value of nuclear DNA content in Galanthus. Plant Syst. Evol. 2003;241(1-2):89–102. https://link.springer.com/article/10.1007/s00606-003-0016-z [Google Scholar]
- Zubov D., Davis A. Galanthus panjutinii sp. nov.: a new name for an invalidly published species of Galanthus (Amaryllidaceae) from the northern Colchis area of Western Transcaucasia. Phytotaxa. 2012;50:55–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.