Abstract
The stereotyped features of brain structure, such as the distribution, morphology and connectivity of neuronal cell types across brain areas, are those most likely to explain the remarkable capacity of the brain to process information and govern behaviors. Recent advances in anatomical methods, including the simple but versatile isotropic fractionator and several whole-brain labeling, clearing and microscopy methods, have opened the door to an exciting new era in comparative brain anatomy, one that has the potential to transform our understanding of the brain structure-function relationship by representing the evolution of brain complexity in quantitative anatomical features shared across species and species- or clade-specific. Here we discuss these methods and their application to mapping brain cell count and cell type distributions—two particularly powerful neural correlates of vertebrate cognitive and behavioral capabilities.
Introduction
The ultimate goal of neuroscience is to understand the structure and function of the human brain. However, much of neuroscience research is done on nonhuman model species that are amenable to experiments and genetic manipulations, with an increasing focus on the laboratory mouse in addition to a few other traditional animal models, such as the rat, prairie vole, rhesus macaque, marmoset, and zebra finch. Here we make a case for the importance of comparative studies across a much broader range of mammalian and vertebrate species, applying modern but versatile anatomical methods to disentangle which neural features are shared across distantly related species and which are species-or clade-specific. We review how recently acquired estimates of neuron numbers advanced our understanding of the evolution of vertebrate intelligence and highlight the potential of newly developed microscopy and brain clearing and labeling techniques for quantitative assessment of neural cell type-based correlates of cognitive and behavioral capabilities. Finally, we postulate that while the primary focus of contemporary evolutionary neuroscience has been on the identification of homologies at various levels of nervous system organization [1–5], detailed analyses of both shared and divergent features of brain anatomy can bring new information on the evolution of brain complexity and information-processing capacity.
From brain size to neuron numbers
Brain size is the predominant surrogate measure of brain functional capacity in comparative and cognitive neuroscience. However, it is neurons and their connections that provide the substrate for cognition and behavior. The total number of neurons, the basic computational units of the brain, is therefore a much better approximation of the brain’s computational capacity than brain size alone.
Historically, unbiased stereological techniques such as the optical fractionator have been the methods of choice to determine cellular composition of specific brain regions [6]. However, applying stereology to major brain divisions or an entire brain is problematic, because it is highly laborious and requires independent sampling of structures that exhibit dramatically different cytoarchitectures and cell packing densities. To circumvent these technical limitations, a non-stereological technique – the isotropic fractionator (IF) – was developed [7,8]. This method involves mechanical dissociation of fixed brain tissue into a homogenous suspension of free cell nuclei, which are then counted, and immunocytochemically identified to estimate the proportion of non-neuronal (glial and endothelial) cells and neurons. In terms of repeatability and accuracy, the IF is comparable to stereology [9–11], but it is inexpensive and much less time consuming. The IF has been extensively used to assess the total numbers of neurons, glial cells and glia to neuron ratio in whole brains and dissected brain parts across diverse vertebrates and to determine how numbers of neurons and glial cells scale with brain size in various clades. So far, such data were gathered for 68 mammalian species, representing primates, rodents, carnivores, artiodactyls, insectivores, afrotherians and marsupials [12]**[13,14],[15]*. Recently, similar data were also acquired for 28 species of birds, representing mainly songbirds and parrots but also a few more basal avian groups [16]**, and for a single reptile [17] and fish species [18]**, and much broader comparative studies are in progress. While the current sampling is certainly not sufficient to reconstruct the big picture of mammalian (even less so vertebrate) brain evolution in quantitative terms, the utilization of the IF has already yielded several important insights.
Primates have relatively high cortical neuronal densities that almost do not decrease with body and brain size, in contrast to other mammalian groups, where neuronal numbers exhibit negative allometry with respect to brain size [21]. As a result, primate brains accommodate many more neurons than equivalently sized brains of non-primate mammals. Notably, the human brain, with its 86 billion neurons, is built with the same space-saving cellular scaling rules that apply to other primates and in this respect appears to be “merely” a scaled-up primate brain [22,23] Counterintuitively, the cerebellum houses a large majority (typically 70–90%) of all brain neurons in mammals, whereas the cerebral cortex or pallium that accounts for most of the mammalian brain volume house a minority (10–30%) of brain neurons [14]**,[24]. Remarkably, the human cerebral cortex contains a mere 19% of brain neurons but represents 82% of brain mass [22,23]. The African elephant is an more extreme case of this biased distribution, as its brain is about three times larger and contains three times more neurons (257 billion) than the human brain, however 97.5% of these neurons (251 billion) are located in the cerebellum [25].
The cellular composition of avian brains markedly differs from that found in mammals [18]**. Brains of songbirds and parrots feature exceptionally high neuronal densities, surpassing even those of primates. Their brains thus contain about twice as many neurons as primate brains of the same mass. Neuronal densities in the pallium of songbirds and parrots exceed those observed in the primate pallium by a factor of 3–4. Hence, these birds have much higher proportions of brain neurons (33–55% in songbirds and 46–61% in parrots) located in the pallium, compared to primates and other mammals. Consequently, large-brained parrots and corvids have forebrain neuron counts equal to or greater than primates with much larger brains. The emu, the red junglefowl and the pigeon, all species representing more basal bird clades, share small telencephalic and dominant cerebellar neuronal fractions and generally lower neuronal densities. Therefore, their brains and particularly forebrains harbor much smaller absolute numbers of neurons than brains of equivalently sized songbirds or parrots and are closer to mammals in the distribution of neurons to different brain parts.
These findings have two important implications. First, the highest numbers of neurons in the pallial telencephalon have been found in those species that are well known for their remarkable cognitive abilities [26],[27]**,[28]**,[29],[30], namely in humans, followed by great apes, the African elephant, large monkeys, large parrots and corvids (Figure 1). Thus, number of pallial neurons seems to be a better correlate of cognitive abilities than number of all brain neurons or neurons in other brain parts [18]**,[31], even though abundant evidence also demonstrates the role of the cerebellum in various cognitive functions (for review, see [32,33]) and increase of its size is associated with multiple independent evolutionary occurrences of increased behavioral complexity in mammals [34],[35]**. Information processing capacity of elephant brain is lowered by a thin cortex, low neuron packing density and low axonal conduction velocity [36]*. By contrast, a short interneuronal distance, a corollary of the extremely high neuron packing densities, likely results in a high speed of information processing, which further enhances cognitive abilities of parrots and corvids. Thus, the large numbers of neurons concentrated in high densities in the telencephalon contributes to the remarkable intelligence of these birds, despite their small brains in absolute terms [18]**
Figure 1.
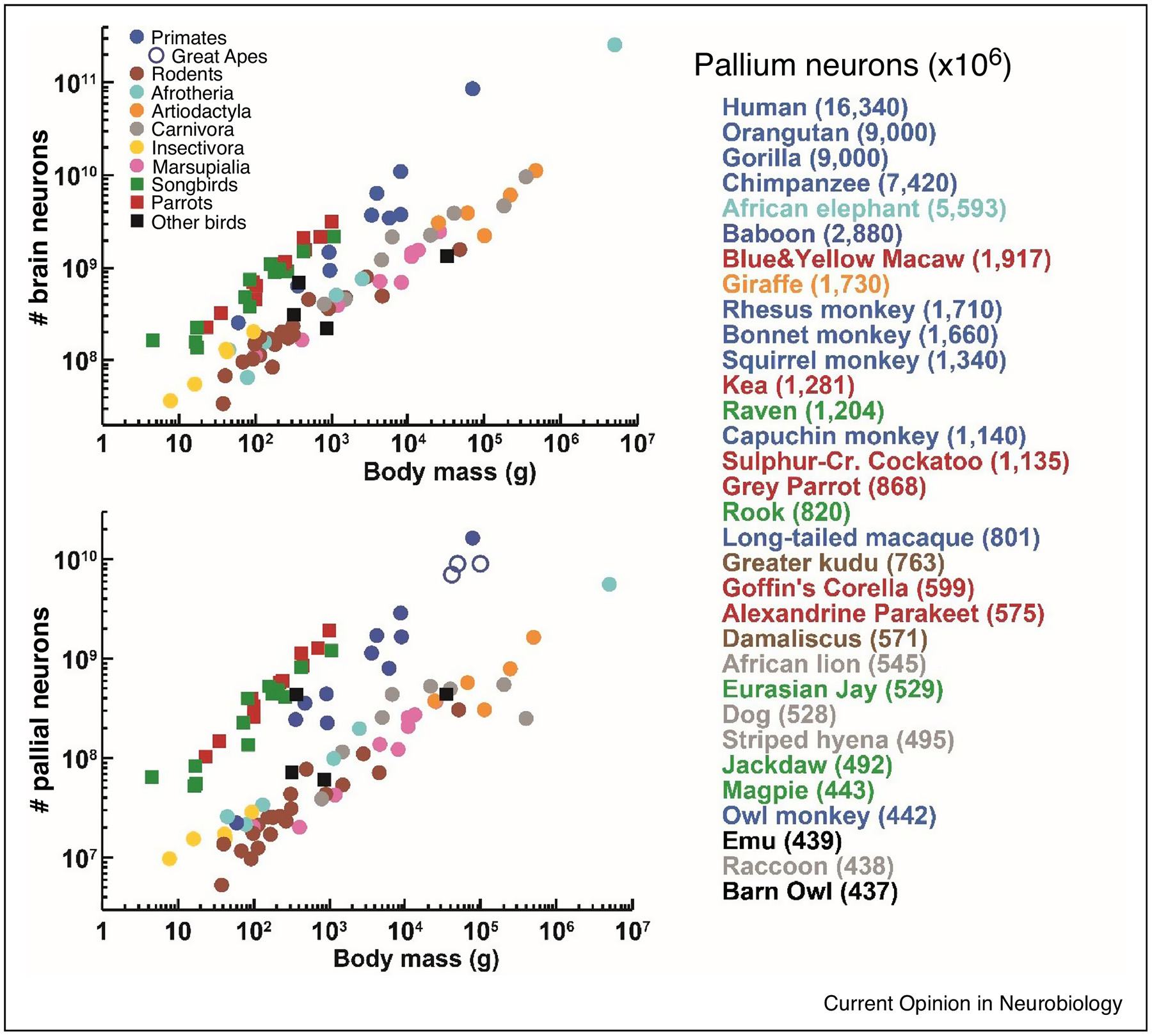
Neuron numbers in birds and mammals. (a) Total number of brain neurons plotted as a function of body size. (b) Total number of neurons located in the pallial telencephalon plotted as a function of body size. Each point represents the average value for one species. (c) Species having a high number of pallial neurons ranked in descending order. The species means are given in brackets. Data from published reports [14–18,40], pallial neurons estimates for the orangutan and gorilla from [31].
Second, it has been clearly shown that absolute brain or brain region size is not a good indicator of number of neurons when comparison is made across distantly related species. For instance, the pallium of the African elephant is ~200 times larger but accommodates only ~3-times more neurons than the pallium of the blue-and-yellow macaw [18]**,[25]. Thus, absolute size of brain or brain region is a reliable proxy for its computational / functional capacity only when comparison is made within a group of animals following the same neuronal scaling rules.
The IF has been also used to map cellular and neuronal densities across cortex in the short-tailed opossum [37], mouse [38] and eight species of primates including human [39], [40]*, [41,42]. All these distantly related mammals share the same basic pattern: high neuronal densities in primary sensory areas and trend of decreasing neuronal densities along the caudal to rostral axis of the cortex. Typically, the highest neuron packing is observed in the primary visual cortex, the lowest in motor and premotor cortex. The described density patterns are more pronounced in large-brained primates [39],[40]*,[41], and this has been corroborated by a recent stereological study [43]. However, rather than a specialization of the primate brain, the pronounced differences in neuron packing densities across cortical sheet reflect the fact that neurons are morphologically more differentiated in large-brained mammals. Indeed, the described density pattern likely reflects different information processing in sensory and higher-order cortical areas: sensory neurons are smaller, have smaller dendritic arbors and are activated by relatively few inputs, whereas neurons serving integrative functions are larger, have larger dendritic arbors with more synaptic contacts driving their activation [40]*.
In contrast to this growing body of information on taxon-specific cellular scaling rules, almost nothing is known about within species variation in neuronal numbers and densities. A recent study has shown that selection for larger brains also leads to a matching increase in the number of neurons in the guppy Poecilia reticulata [20]**. Remarkably, a 12% difference in neuronal numbers arose within just five generations of artificial selection, suggesting that evolution of larger brains harboring an increased number of neurons can occur very rapidly. Low variation in neuron numbers and a weak correlation between brain mass and number of neurons has been observed in laboratory mice [44] and captive-bred Madagascar ground geckos Paroedura picta (Němec lab, unpublished data). Neuron number and density variation in wild populations remains unknown.
In summary, the introduction of the IF opened up a whole new line of research, as the quantification of the total numbers of brain neurons across broad taxonomic scales can be used to infer evolutionary histories and selective pressures leading to independent emergence of high brain processing capacity underlying the evolution of intelligence.
From neuron numbers to neural circuits
The simple IF method described above has enabled the use of neuron cell counts in place of brain size in comparative studies, demonstrating, for example, the cognitive relevance of neuronal numbers in the vertebrate pallial telencephalon. Yet the separation between neuronal and non-neuronal cells does not capture the astounding diversity of neuronal cell types known to neuroscientists since the first visualization of brain cytoarchitecture by Camillo Golgi and Santiago Ramón y Cajal more than 100 years ago. Thus in addition to the differences in neuron numbers, differences in neuronal cell type distributions and their ratios across anatomical regions, representing local and long-range cell type-specific neural circuits, are fundamental determinants of brain function. The study of cell type-based composition of the brain of different species is therefore the next frontier in comparative neuroscience.
While the question of what defines a neuronal cell type is yet to be fully defined, there is a consensus on key descriptors that can be used to classify a cell population as a distinct cell type, including cellular morphology, electrical properties, and most recently molecular expression profile [45,46]. Historically, classic anatomical features have been used most widely as classification criteria for neurons identified in different parts of the brain, including the location and size of the soma, the appearance and size of dendritic trees, as well as the axonal projection and arborization patterns. Of course, the importance of the cell anatomy is not only as a classification criterion, as their morphology represents geometrical sampling of the synaptic input / output space and the assembly into local and long-range neural circuits [47]. Following on the anatomical classification, electrical properties, both passive and active, have been used to describe, name, and further classify neurons previously described on morphology and soma position alone [46]. Finally, the set of molecules expressed by neurons can be used for cell type classification, including protein markers such as neurotransmitters, neuropeptides, membrane transporters and ion channels [48], as well as new cell type-specific molecules that may be revealed by recent cell expression profiling methods, in particular single-cell RNA-seq (scRNA-seq) [49,50].
An essential criterion for the use of neuronal cell type classifications in comparative neuroscience is the selection of versatile cell type descriptors and the development of sampling methods that can be applied efficiently across different species. Clearly, some descriptors, such as detailed anatomical features, electrophysiological properties or scRNA-seq expression profiles, would be difficult to apply broadly due to the laborious nature and/or high cost of the respective methods. However, recent advances in anatomical methods developed initially for the mouse brain, including several automated light microscopy (LM) instruments, whole-brain clearing and tissue labeling methods, and computational methods for analysis of whole-brain datasets, can be translated to the study of brains of other species after some modifications. And while the laboratory mouse will continue to be a leading animal model for the study of brain structure and function due to its many experimental advantages (see below), it is imperative that in order to identify evolutionary meaningful brain organizing principles, the findings learned in the mouse must be tested and compared not only in other mammalian brains but also across selected vertebrates.
The laboratory mouse has undergone a remarkable transformation, from its main use as a genetic “knock-out” model into a broadly used animal model in systems neuroscience. This shift has been largely due to the development of mice expressing Cre (or Flp) recombinase as a marker “tag” from genes with neuron cell type-specific expression, which include major classes of inhibitory neurons, layer-specific cortical pyramidal neurons, neuromodulatory cell types and neuropeptide-defined hypothalamic neurons [46,51]. These reporter mice can be used for testing the function of cell type-specific circuits by optogenetic studies [52] and anatomical mapping of neuronal cell type distribution, morphology and connectivity [53]. It is the brainwide cell type distribution that we believe can be translated to other animal species and provide a powerful means of extending our knowledge of brain evolution to include cell type-defined neural circuit classification.
The first quantitative brainwide (qBrain) cell type mapping platform was based on an automated block-face microscopy called serial two-photon tomography (STPT) [54]**, combined with advanced computational methods for cell detection by machine learning algorithms and 3D reconstruction and registration of the imaged whole-brain datasets to the digital mouse brain atlas [55,56]. A proof-of-principle application of the qBrain platform to map the stereotyped distributions of three broad inhibitory cell types clearly demonstrated the power of unbiased cell type atlasing in uncovering novel principles of brain organization [57]** (Figure 2). First, a comparison of distribution of inhibitory neurons expressing somatostatin (SST), parvalbumin (PV) and vasoactive intestinal peptide (VIP) across the mouse brain isocortex revealed a novel and unexpected cortical organizing principle: sensory-motor areas were found to be dominated by output-modulating parvalbumin-positive interneurons, whereas association, including frontal, areas in contrast comprised large numbers of input-modulating somatostatin-positive interneurons [57]** (Figure 2). This shows that instead of a single canonical neural circuit [58], different cortical areas in the mouse brain comprise distinct cell type-based circuits that, based on computational modeling, are predicted to enable qualitatively distinct computations and functions [57]**, [49]. This finding strongly suggests that a similar pattern of “mixing and matching” of neuronal cell types to fit specific functions is present also in other species and may represent an efficient means for evolutionary changes underlying the emergence of high cognitive abilities.
Figure 2.

Cell type-based composition of the mouse brain. (a) Top views of STPT imaged mouse brains with computationally map whole-brain distributions of PV+ (2,959,233 ± 297,158), SST+ (2,268,932 ± 294,923) and VIP+ (442,265 ± 32,250) neurons (data are mean ± SD). (b) Cortical cell density mapping using a cortical flatmap (left). The heatmap displays of cell density per mm3 for the PV+ (middle), and SST+ (right) cell populations reveals lowdensity of PV+ and high density of SST+ in the medial frontal (blue arrow) and lateral association cortices (orange arrow) (adapted from [57]). (c) A schematic of the synaptic weight changes in cortical circuits with low PV / high SST (left) versus high PV / low SST (right) cell type distribution.
A second example of the power of cell type analysis over total cell counts concerns gender differences in brain anatomy representing neural substrates for sexually dimorphic behaviors. While the male mouse brain is larger than that of the female and some hypothalamic areas related to sexual behavior have statistically more neurons [60], the analysis of the three neuronal cell types revealed 8 regions with more SST+ or VIP+ neurons in the female brain and only one area linked to ejaculation with more SST+ neurons in the male brain [57]**. Since the SST+ and VIP+ neurons are mostly sparse cell types, this shows how cell type-based analysis can identify putative sex-dimorphic circuits that would be otherwise missed in total cell count comparisons.
How can the cell type-based analysis of brain structure be applied to other animal species? While the above described experiments rely on genetically engineered Cre-expressing mice for convenient labeling of the selected cell types, recently developed whole mouse brain immunolabeling and brain clearing methods can be directly applied to other animal brains for cell type detections, with the caveat that there needs to be an antibody recognizing the cell type-specific protein marker in the selected species. There are several methods for whole-brain immunolabeling and clearing, including CLARITY [61], iDISCO+ [62]*, vDISCO [63] and CUBIC-X [64] that, while developed initially for the mouse brain, can be applied to other tissues even larger than the mouse brain, as demonstrated for example on a whole-mouse body preparation [63]. Alternatively, traditional anatomical methods based on brain tissue sections can also be largely automated and standardized, as recently demonstrated for the marmoset brain [65]** and zebra finch brain [66]*, offering further flexibility with respect to the brain size that can be analyzed by this approach. Finally, while the use of immunolabeling is clearly a limitation for applying these methods to a broad range of species, we envision that this could be overcome by advances in whole-brain fluorescence in situ hybridization (FISH) mRNA detection, as was recently described for the iDISCO protocol [67], perhaps even with single-molecule FISH methods, such as MERFISH [68]*, seqFISH [69], or STARmap [70], to further enhance the power of identifying cell types with more complex transcriptome signatures in selected homologous brain areas in different species.
Conclusion
We propose that a broad utilization of the IF in synergy with a more selective use of the newly available methods for quantitative whole-brain atlasing of cell type distributions can revolutionize comparative and cognitive neuroscience. The IF is a highly versatile tool that can be employed to estimate neuronal numbers across hundreds of species, generating an unprecedented wealth of comparative data on the evolution of brain complexity in vertebrates, ultimately identifying independent evolutionary drives in neuron-based information-processing capacity. This will also allow a rational selection of model species that deserve further scrutiny of cell type-based composition of the brain. While the methods for neuronal cell type atlasing need further improvements, especially in versatility and costs, to be more broadly applicable, the possibility to map and compare cell-type-specific brain circuits subserving sensory–motor, emotional and cognitive functions in representative vertebrate species will dramatically enhance our ability to assess how evolutionary diversification or convergence in brain anatomy contributes to the broad behavioral repertoires seen across vertebrates.
Highlights.
Maps of cell count and cell type distributions reveal evolution of brain complexity.
Number and distribution of neurons and glial cells is species-and clade-specific.
Number of neurons in pallial telencephalon correlates with cognitive abilities.
Isotropic fractionator can be used to estimate cell numbers in hundreds of species.
Whole-brain immunolabeling and FISH are tools for cell type distribution mapping.
Acknowledgements
We thank Kristina Kverková for reading of the manuscript and discussions and Martin Kocourek for his assistance with preparation of Figure 1. This study was supported by Czech Science Foundation (18-15020S to PN and by NIH (U01 MH105971 and U01 MH114824 to PO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References:
- 1.Briscoe SD, Ragsdale CW: Evolution of the Chordate Telencephalon. Curr Biol 2019, 29:R647–R662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liebeskind BJ, Hillis DM, Zakon HH, Hofmann HA: Complex Homology and the Evolution of Nervous Systems. Trends Ecol Evol 2016, 31:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Duran JM, Pang K, Borve A, Le HS, Furu A, Cannon JT, Jondelius U, Hejnol A: Convergent evolution of bilaterian nerve cords. Nature 2018, 553:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tosches MA, Laurent G: Evolution of neuronal identity in the cerebral cortex. Curr Opin Neurobiol 2019, 56:199–208. [DOI] [PubMed] [Google Scholar]
- 5.Striedter GF, Northcutt RG: Brains Through Time: A Natural History of Vertebrates: Oxford University Press; 2019. [Google Scholar]
- 6.West MJ: Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci 1999, 22:51–61. [DOI] [PubMed] [Google Scholar]
- 7.Herculano-Houzel S, Lent R: Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci 2005, 25:2518–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young NA, Flaherty DK, Airey DC, Varlan P, Aworunse F, Kaas JH, Collins CE: Use of flow cytometry for high-throughput cell population estimates in brain tissue. Front Neuroanat 2012, 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herculano-Houzel S, von Bartheld CS, Miller DJ, Kaas JH: How to count cells: the advantages and disadvantages of the isotropic fractionator compared with stereology. Cell Tissue Res 2015, 360:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahney J, von Bartheld CS: Validation of the isotropic fractionator: comparison with unbiased stereology and DNA extraction for quantification of glial cells. J Neurosci Methods 2014,222:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller DJ, Balaram P, Young NA, Kaas JH: Three counting methods agree on cell and neuron number in chimpanzee primary visual cortex. Front Neuroanat 2014, 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngwenya A, Nahirney J, Brinkman B, Williams L, Iwaniuk AN: Comparison of estimates of neuronal number obtained using the isotropic fractionator method and unbiased stereology in day old chicks (Gallus domesticus). J Neurosci Methods 2017, 287:39–46. [DOI] [PubMed] [Google Scholar]
- 13.Neves K, Guimarães DM, Rayêe D, Valério-Gomes B, Iack PM, Lent R, Mota B: The reliability of the isotropic fractionator method for counting total cells and neurons. J Neurosci Methods 2019,326:108392. [DOI] [PubMed] [Google Scholar]
- 14.Herculano-Houzel S, Catania K, Manger PR, Kaas JH: Mammalian Brains Are Made of These: A Dataset of the Numbers and Densities of Neuronal and Nonneuronal Cells in the Brain of Glires, Primates, Scandentia, Eulipotyphlans, Afrotherians and Artiodactyls, and Their Relationship with Body Mass. Brain BehavEvol 2015, 86:145–163. [DOI] [PubMed] [Google Scholar]; **This paper provides the numbers and cell densities of neurons and nonneuronal cells for 40 species representing five mammalian clades.
- 15.Jardim-Messeder D, Lambert K, Noctor S, Pestana FM, de Castro Leal ME, Bertelsen MF, Alagaili AN, Mohammad OB, Manger PR, Herculano-Houzel S: Dogs have the most neurons, though not the largest brain: trade-off between body mass and number of neurons in the cerebral cortex of large carnivoran species. Front Neuroanat 2017, 11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dos Santos SE, Porfirio J, da Cunha FB, Manger PR, Tavares W, Pessoa L, Raghanti MA, Sherwood CC, Herculano-Houzel S: Cellular Scaling Rules for the Brains of Marsupials: Not as “Primitive” as Expected. Brain Behav Evol 2017, 89:48–63. [DOI] [PubMed] [Google Scholar]
- 17.Kverková K, Bělíková T, Olkowicz S, Pavelková Z, O’Riain MJ, Šumbera R, Burda H, Bennett NC, Němec P: Sociality does not drive the evolution of large brains in eusocial African mole-rats. Sci Rep 2018, 8:9203. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The first paper to use neuronal numbers as a proxy for brain processing capacity in testing an evolutionary hypothesis. The results of the study in African mole-rats, which range from solitary to eusocial, suggest that social living per se does not select strongly for brain enlargement unless coupled with Machiavellian interactions affecting individual fitness.
- 18.Olkowicz S, Kocourek M, Lučan RK, Porteš M, Fitch WT, Herculano-Houzel S, Němec P: Birds have primate-like numbers of neurons in the forebrain. Proc Natl Acad Sci USA 2016, 113:7255–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This paper provides data on cellular composition of the brains of 28 avian species and solves a long standing enigma of cognitive biology: How do birds achieve remarkable cognitive prowess with small brains? Brains of songbirds and parrots contain very large numbers of neurons concentrated in high densities in the telencephalon. Thus, large-brained parrots and corvids have forebrain neuron counts equal to or greater than primates with much larger brains.
- 19.Ngwenya A, Patzke N, Manger PR, Herculano-Houzel S: Continued growth of the central nervous system without mandatory addition of neurons in the Nile crocodile (Crocodylus niloticus). Brain Behav Evol 2016, 87:19–38. [DOI] [PubMed] [Google Scholar]
- 20.Marhounová L, Kotrschal A, Kverková K, Kolm N, Němec P: Artificial selection on brain size leads to matching changes in overall number of neurons. Evolution 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study provides the first direct experimental evidence that selection for brain mass leads to matching changes in number of neurons and shows that brain size evolution is intimately linked to the evolution of neuron numbers and cognition.
- 21.Herculano-Houzel S, Collins CE, Wong P, Kaas JH: Cellular scaling rules for primate brains. Proc Natl Acad Sci USA 2007, 104:3562–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Filho WJ, Lent R, Herculano-Houzel S: Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. JComp Neurol 2009, 513:532–541. [DOI] [PubMed] [Google Scholar]
- 23.Herculano-Houzel S: The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci USA 2012, 109:10661–10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herculano-Houzel S: Coordinated scaling of cortical and cerebellar numbers of neurons. Front Neuroanat 2010, 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herculano-Houzel S, Avelino-de-Souza K, Neves K, Porfirio J, Messeder D, Mattos Feijo L, Maldonado J, Manger PR: The elephant brain in numbers. Front Neuroanat 2014, 8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deaner RO, Isler K, Burkart J, Van Schaik C: Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav Evol 2007, 70:115–124. [DOI] [PubMed] [Google Scholar]
- 27.MacLean EL, Hare B, Nunn CL, Addessi E, Amici F, Anderson RC, Aureli F, Baker JM, Bania AE, Barnard AM: The evolution of self-control. Proc Natl Acad Sci USA 2014, 111:E2140–E2148. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Utilizing a standardized methodology, a large consortium of authors compared cognitive performance of 567 individual representing 36 species of birds and mammals in two problem solving tasks measuring self-control and found that absolute brain size is the best predictor of performance across species.
- 28.Kabadayi C, Taylor LA, von Bayern AM, Osvath M: Ravens, New Caledonian crows and jackdaws parallel great apes in motor self-regulation despite smaller brains. R Soc Open Sci 2016,3:160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Güntürkün O, Bugnyar T: Cognition without cortex. Trends Cogn Sci 2016, 20:291–303. [DOI] [PubMed] [Google Scholar]
- 30.Lambert ML, Jacobs I, Osvath M, von Bayern AM: Birds of a feather? Parrot and corvid cognition compared. Behaviour 2019, 156: 505–594.. [Google Scholar]
- 31.Herculano-Houzel S: Numbers of neurons as biological correlates of cognitive capability. Curr Opin Behav Sci 2017, 16:1–7. [Google Scholar]
- 32.Sokolov AA, Miall RC, Ivry RB: The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci 2017, 21:313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barton RA: Embodied cognitive evolution and the cerebellum. Philos Trans R Soc Lond B Biol Sci 2012, 367:2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barton RA, Venditti C: Rapid Evolution of the Cerebellum in Humans and Other Great Apes. Curr Biol 2017, 27:1249–1250. [DOI] [PubMed] [Google Scholar]
- 35.Smaers JB, Turner AH, Gomez-Robles A, Sherwood CC: A cerebellar substrate for cognition evolved multiple times independently in mammals. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dicke U, Roth G: Neuronal factors determining high intelligence. Philos Trans R Soc Lond B Biol Sci 2016, 371:20150180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seelke AM, Dooley JC, Krubitzer LA: The cellular composition of the marsupial neocortex. J Comp Neurol 2014, 522:2286–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herculano-Houzel S, Watson C, Paxinos G: Distribution of neurons in functional areas of the mouse cerebral cortex reveals quantitatively different cortical zones. Front Neuroanat 2013, 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH:Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci USA 2010, 107:15927–15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins CE, Turner EC, Sawyer EK, Reed JL, Young NA, Flaherty DK, Kaas JH: Cortical cell and neuron density estimates in one chimpanzee hemisphere. Proc Natl Acad Sci USA 2016, 113:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner EC, Young NA, Reed JL, Collins CE, Flaherty DK, Gabi M, Kaas JH: Distributions of Cells and Neurons across the Cortical Sheet in Old World Macaques. Brain Behav Evol 2016, 88:1–13. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro PFM, Ventura-Antunes L, Gabi M, Mota B, Grinberg LT, Farfel JM, Ferretti-Rebustini REL, Leite REP, Filho WJ, Herculano-Houzel S: The human cerebral cortex is neither one nor many: neuronal distribution reveals two quantitatively different zones in the gray matter, three in the white matter, and explains local variations in cortical folding. Front Neuroanat 2013, 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charvet CJ, Cahalane DJ, Finlay BL: Systematic, Cross-Cortex Variation in Neuron Numbers in Rodents and Primates. Cereb Cortex 2013, 25:147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herculano-Houzel S, Messeder DJ, Fonseca-Azevedo K, Pantoja NA: When larger brains do not have more neurons: increased numbers of cells are compensated by decreased average cell size across mouse individuals. Front Neuroanat 2015, 9:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ecker JR, Geschwind DH, Kriegstein AR, Ngai J, Osten P, Polioudakis D, Regev A, Sestan N, Wickersham IR, Zeng H: The BRAIN Initiative Cell Census Consortium: Lessons Learned toward Generating a Comprehensive Brain Cell Atlas. Neuron 2017, 96:542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng H, Sanes JR: Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci 2017, 18:530. [DOI] [PubMed] [Google Scholar]
- 47.Ascoli GA, Wheeler DW: In search of a periodic table of the neurons: Axonal-dendritic circuitry as the organizing principle. BioEssays 2016, 38:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.L Rees C, M White C, A Ascoli G: Neurochemical markers in the mammalian brain: structure, roles in synaptic communication, and pharmacological relevance. Current medicinal chemistry 2017,24:3077–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro E, Biezuner T, Linnarsson S: Single-cell sequencing-based technologies will revolutionize whole-organism science. Nature Reviews Genetics 2013, 14:618. [DOI] [PubMed] [Google Scholar]
- 50.Yuste R, Hawrylycz M, Aalling N, Arendt D, Armananzas R, Ascoli G, Bielza C, Bokharaie V, Bergmann T, Bystron I et al. : A community-based transcriptomics classification and nomenclature of neocortical cell types [Internet]. arXiv.org 2019, 1909.0 3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang ZJ, Zeng H: Genetic approaches to neural circuits in the mouse. Annu Rev Neurosci 2013,36:183–215. [DOI] [PubMed] [Google Scholar]
- 52.Fenno L, Yizhar O, Deisseroth K: The development and application of optogenetics. Annu Rev Neurosci 2011, 34:389–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo L, Callaway EM, Svoboda K: Genetic Dissection of Neural Circuits: A Decade of Progress. Neuron 2018, 98:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ragan T, Kadiri LR, Venkataraju KU, Bahlmann K, Sutin J, Taranda J, Arganda-Carreras I, Kim Y, Seung HS, Osten P: Serial two-photon tomography for automated ex vivo mouse brain imaging. Nat Methods 2012, 9:255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This paper introduces STPT as the first automated method for high-resolution imaging of fluorescently labeled mouse brains
- 55.Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL, Hawrylycz M, Dang C: Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res 2013, 41:D996–D1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, Arganda-Carreras I, Ng L,Hawrylycz MJ, Rockland KS, et al. : Mapping social behavior-induced brain activation at cellular resolution in the mouse. Cell Rep 2015, 10:292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim Y, Yang GR, Pradhan K, Venkataraju KU, Bota M, Garcia Del Molino LC, Fitzgerald G, Ram K, He M,Levine JM, et al. : Brain-wide Maps Reveal Stereotyped Cell-Type-Based Cortical Architecture and Subcortical Sexual Dimorphism. Cell 2017, 171:456–469 e422. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study provides the first whole-brain analysis of neuronal cell type distribution in the mouse brain, demonstrating the power of comprehensive unbiased anatomical analyses in discovering unexpected anatomical organizing principles, such as novel cortical hierarchy and sex-dimorphic cell type distributions.
- 58.Douglas RJ, Martin KA: Mapping the matrix: the ways of neocortex. Neuron 2007, 56:226–238. [DOI] [PubMed] [Google Scholar]
- 59.Wang X-J: A microcircuit model of prefrontal functions: ying and yang of reverberatory neurodynamics in cognition. The frontal lobes 2006:92–127. [Google Scholar]
- 60.Yang T, Shah NM: Molecular and neural control of sexually dimorphic social behaviors. Curr Opin Neurobiol 2016, 38:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung K, Deisseroth K: CLARITY for mapping the nervous system. Nat Methods 2013, 10:508–513. [DOI] [PubMed] [Google Scholar]
- 62.Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou Y, et al. : Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell 2016, 165:1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper describes an optimized procedure for whole-brain immunostaining and clearing that represents a versatile method for labeling of brains from different species.
- 63.Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Foerstera B, Zhao S, Bhatia HS, Parra-Damas A, Mrowka L, Theodorou D: Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Edited by: Nature Publishing Group; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murakami TC, Mano T, Saikawa S, Horiguchi SA, Shigeta D, Baba K, Sekiya H, Shimizu Y, Tanaka KF, Kiyonari H: A three-dimensional single-cell-resolution whole-brain atlas using CUBIC-X expansion microscopy and tissue clearing. Nature Neurosci 2018, 21:625. [DOI] [PubMed] [Google Scholar]
- 65.Lin MK, Takahashi YS, Huo B-X, Hanada M, Nagashima J, Hata J, Tolpygo AS, Ram K, Lee BC, Miller MI:A high-throughput neurohistological pipeline for brain-wide mesoscale connectivity mapping of the common marmoset. Elife 2019, 8:e40042. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Powerful application of an automated traditional histological pipeline to generate a digital atlas and connectivity map of the common marmoset brain.
- 66.Karten HJ, Brzozowska-Prechtl A, Lovell PV, Tang DD, Mello CV, Wang H, Mitra PP: Digital atlas of the zebra finch (Taeniopygia guttata) brain: A high-resolution photo atlas. J Comp Neurol 2013,521:3702–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Application of an automated traditional histological pipeline to generating a digital atlas of the zebra finch brain.
- 67.Guo W, Liu X, Hu Q, Huang F, Li N, Zhang Q, Li Y, Xiong F, Luo Q, Zeng S: Whole-mount in situ hybridization of mouse brain to precisely locate mRNAs via fluorescence tomography. Journal of biophotonics 2019, 12:e201800249. [DOI] [PubMed] [Google Scholar]
- 68.Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, Rubinstein ND, Hao J,Regev A, Dulac C: Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 2018, 362:eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Powerful application of MERFISH to the analysis of cell types in the mouse hypothalamus.
- 69.Shah S, Lubeck E, Zhou W, Cai L: seqFISH accurately detects transcripts in single cells and reveals robust spatial organization in the hippocampus. Neuron 2017, 94:752–758. e751. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, Evans K, Liu C, Ramakrishnan C,Liu J: Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 2018, 361:eaat5691. [DOI] [PMC free article] [PubMed] [Google Scholar]


