GEN-003 is a candidate therapeutic vaccine for herpes simplex virus type 2 (HSV-2). Immunization with GEN-003 antigen/MM2 adjuvant combinations of 60/50 µg and 60/75 µg produced significant reductions in HSV-2 viral shedding and lesion rates for up to 12 months.
Keywords: genital herpes, HSV-2, therapeutic vaccine
Abstract
Background
GEN-003 is a candidate therapeutic vaccine for genital herpes simplex virus type 2 (HSV-2). We compared virologic and clinical impact of varying GEN-003 doses.
Methods
Adults with symptomatic HSV-2 received placebo or GEN-003 (30 or 60 µg antigen with 25, 50, or 75 µg adjuvant). Viral shedding and lesion rates before vaccination were compared with those measured immediately after vaccination, then at weeks 29–33 and 53–57 after last dose.
Results
Compared with baseline shedding rates, the rate ratios for viral shedding immediately after treatment were as follows: 0.82 (95% confidence interval [CI], 0.49–1.36), 30 µg antigen/25 µg adjuvant (30/25) dose; 0.64 (95% CI, 0.45–0.92), 30/50 dose; 0.63 (95% CI, 0.37–1.10), 30/75 dose; 0.56 (95% CI, 0.36–0.88), 60/25 dose; 0.58 (95% CI, 0.38–0.89), 60/50 dose; 0.45 (95% CI, 0.16–0.79), 60/75 dose; and 0.98 (95% CI, 0.76–1.26), placebo. Lesion rate reductions by GEN-003 ranged from 31% to 69%, but lesion rates also decreased among placebo recipients (62%). Reductions in shedding and lesion rate were durable for 12 months for the 60 µg antigen plus 50 or 75 µg adjuvant groups. No serious adverse events occurred with vaccination.
Conclusions
The most efficacious vaccine combinations for GEN-003 were the 60 µg/50 µg and 60 µg/75 µg doses.
Herpes simplex virus type 2 (HSV-2) is a common sexually transmitted infection responsible for most cases of recurrent genital herpes [1]. Approximately 417 million people aged 15–49 years were living with HSV-2 infection worldwide in 2012 [2]. Infection may be characterized by recurrences of painful genital ulcers. It can also be asymptomatic [3, 4]. Current antiviral treatments provide clinical benefit but do not completely prevent genital herpes recurrences or subclinical viral shedding [5, 6]. When oral antiviral therapy is discontinued, viral shedding returns to pretreatment levels [5]. Prolonged antiviral treatment can be costly, and some patients are not willing to start, or are unlikely to comply with, daily suppressive therapy [7, 8].
GEN-003 is a novel therapeutic vaccine composed of a transmembrane deletion mutant of glycoprotein D (gD2ΔTMR), a large fragment of infected cell protein 4 (ICP4.2), and Matrix-M2 ([MM2] Novavax, Gaithersburg, MD), a saponin-derived adjuvant. In a prior clinical trial that enrolled subjects with genital HSV-2 infection, GEN-003 (30 µg of each antigen combined with 50 µg of MM2) reduced genital HSV-2 shedding and lesion rates and stimulated humoral and cellular responses [9, 10].
In this study, we report the results of a randomized Phase 2 trial comparing virologic and clinical effects of 6 antigen and adjuvant dose combinations. The primary objective was to compare the rate of viral shedding at baseline with the shedding rate immediately after completion of a 3-dose regimen. Secondary endpoints included the following: (1) an evaluation of the impact of GEN-003 on clinical disease measured by lesion rates, time to first recurrence, and proportion of subjects without recurrences at 6 and 12 months after treatment; (2) safety and tolerability; and (3) cellular and humoral immune responses to GEN-003 antigens.
METHODS
Study Subjects
Eligible subjects were aged 18–50 years with a diagnosis of genital HSV-2 infection for >1 year supported by one of the following: (1) Western blot for HSV-2 antibody; (2) type-specific polymerase chain reaction (PCR) or viral culture from genital skin or mucous membrane; or (3) a compatible clinical history with a positive result on the HSV-2 HerpeSelect 2 immunoglobulin G (IgG) enzyme-linked immunosorbent assay (index value >3.5) or HSV-2 IgG LIAISON assay. Other inclusion criteria included a history of 3–9 genital herpes recurrences in the 12 months preceding enrollment or, if currently on suppressive therapy, in the 12 months before starting such therapy.
Exclusion criteria included use of suppressive antiviral therapy within 7 days of the baseline viral shedding assessment, history of genital HSV-1 infection, history of any form of ocular HSV infection, HSV-related erythema multiforme, herpes meningitis, or herpes encephalitis. Immunocompromised persons and those seropositive for human immunodeficiency virus (HIV), with active hepatitis C virus or hepatitis B virus infection, or previously immunized with HSV-2 antigens were excluded. Use of effective contraception was required throughout the study.
Study Vaccine
Unblinded site pharmacists prepared GEN-003 vaccine by combining each antigen with MM2 diluted with Dulbecco’s phosphate-buffered saline to 0.5 mL. Dose groups were designated by the content of each antigen (µg) and adjuvant (µg) (30/25, 30/50, 30/75, 60/25, 60/50, or 60/75). Normal saline was used as placebo.
Study Procedures
This study (ClinicalTrials.gov identifier NCT02114060) was conducted at 17 centers in the United States between July 2014 and February 2016. Eligible subjects who provided informed consent were instructed to collect anogenital swab samples twice daily, using a standard procedure, and record the presence or absence of genital lesions daily for 28 days. Eligibility for randomization required subjects to collect at least 45 samples during their baseline swabbing period.
Subjects were randomized through a component of an electronic case report form to receive with equal probability placebo or 1 of the 6 GEN-003 antigen/adjuvant dose combinations. Subjects received 3 doses of their assigned treatment at 21 (±1) day intervals via intramuscular injection into the deltoid muscle of either arm. All study staff with direct subject contact were blinded to treatment assignment.
Swabbing procedures and recording of genital lesions were repeated immediately after the third dose of vaccine for 28 days (immediate postvaccination period) and then from weeks 29 to 33 and weeks 53 to 57 (referred to as 6 and 12 months after last dose of vaccine, respectively). For first recurrence of genital herpes, subjects were instructed to return to the clinic. The first date of the outbreak was recorded and a swab sample was collected.
Before this study, the highest dose of MM2 studied was 50 µg [10]. For this reason, randomization to the 75-µg MM2 dose was initially limited to 12 subjects in each GEN-003 antigen dose group. Safety data from 7 days after the first dose for these subjects were reviewed by an independent Data Safety Monitoring Board before enrollment of additional subjects.
Subjects in the placebo group were offered randomization to one of the active combination doses after completion of swabbing after the third vaccine dose. Thus, no comparison of shedding over time was possible for this group.
During the study, subjects were not permitted to take daily suppressive antiviral medication. In the event of a genital herpes recurrence, a 3-day course of valacyclovir could be prescribed at the discretion of the investigator, provided it occurred outside the swab collection periods.
Study Assessments
Safety and tolerability assessments included reactogenicity (daily recording by patients of specified local reactions [tenderness, pain, swelling, and erythema] and systemic events [myalgia, fatigue, nausea, vomiting, diarrhea, fatigue, and fever], performed for 7 days after each immunization). All other adverse events (AEs) were captured from the first immunization until 28 days after the last dose. Serious AEs (SAEs) and AEs of special interest (AESIs), the latter comprising new onset medical conditions of autoimmune origin, were recorded throughout the study. Adverse events were graded by severity using specified criteria [11]. Standard clinical laboratory evaluations were performed 7 days after each dose and at 28 days after the last dose.
Laboratory Measurement of Herpes Simplex Virus Type 2 Deoxyribonucleic Acid
Anogenital swabs were tested for HSV-2 deoxyribonucleic acid (DNA) using quantitative real-time PCR as previously described [12]. The limit of detection was 1 DNA copy per 20-µL reaction.
Statistical Methods
SAS version 9.3 software (SAS Institute, Cary, NC) was used for analysis of data. Efficacy analyses are reported for the intent-to-treat population, defined as all subjects randomized regardless of whether they received any treatment. The safety population included all subjects who received at least 1 dose of their assigned treatment. Subjects were analyzed according to the treatment they received. Sample size estimates were based on predicted changes in HSV-2 shedding rate from baseline. The HSV-2 shedding rate was calculated for each assessment period as the number of anogenital swabs with HSV-2 detected divided by the total number of swabs collected during that period, both for the individual subjects and the overall treatment groups. Similar computations were done for lesion rates. Viral shedding and lesion rates in each GEN-003 dose group were analyzed in a longitudinal Poisson mixed model with a random intercept, using a log link, to test for differences versus baseline (http://biostats.bepress.com/uwbiostat/paper410/). A ranking analysis of change from baseline for viral shedding rates to the immediate posttreatment period was performed among the 7 treatment groups. Ranking probabilities were calculated using observed estimates of the mean and standard deviation change from baseline in shedding rate for each group as parameters for assumed normal distributions. Data for each of the 7 distributions were then simulated, and the 7 groups were ranked for each simulation.
The proportion of subjects who were recurrence-free at 6 and 12 months was based on subject-reported recurrences and was compared across GEN-003 dose groups using a χ2 test. Time to the first subject-reported recurrence after the third dose was estimated by Kaplan-Meier analysis.
Study Oversight
The study was designed collaboratively by investigators at Genocea Biosciences and the University of Washington. The study protocol was approved by the institutional review board at each study center, and each subject provided written informed consent. The study was conducted in accordance with the International Council for Harmonisation Guideline for Good Clinical Practice and the ethical principles of the Declaration of Helsinki (2000).
RESULTS
Study Population
Of 457 subjects screened, 310 were randomized. Forty-five subjects were randomized to placebo and the 30/50 dose group and 44 to each of the other dose groups. All randomized subjects received at least 1 dose of GEN-003 or placebo. The most common reasons for screen failure were noncompliance with baseline swab collection, abnormal laboratory measurements, or seronegativity for HSV-2 (Supplementary Figure S1). Overall, 230 of the 265 (87%) subjects who were randomized to 1 of the 6 GEN-003 dose combinations completed the study through 12 months. Fifteen subjects (5%) discontinued study treatment. All 45 subjects in the placebo group completed the study until 28 days after the last dose.
Mean subject age was 36 years (range, 19–50). Most subjects were women (70%). Time from initial diagnosis of HSV-2 and number of reported annual recurrences were similar between groups (Table 1).
Table 1.
Baseline Demographics and Disease Characteristics
| Characteristic | Placebo N = 45 | GEN-003 (µg of antigens/µg of adjuvant) | |||||
|---|---|---|---|---|---|---|---|
| 30/25 N = 44 | 30/50 N = 45 | 30/75 N = 44 | 60/25 N = 44 | 60/50 N = 44 | 60/75 N = 44 | ||
| Mean age, year (range) | 35.5 (19–50) |
36.1 (21–50) |
36.2 (23–49) |
37.0 (19–50) |
35.9 (23–50) |
35.1 (21–49) |
37.6 (24–50) |
| Female sex, n (%) | 32 (71.1) | 36 (81.8) | 31 (68.9) | 32 (72.7) | 30 (68.2) | 26 (59.1) | 29 (65.9) |
| Race, n (%) | |||||||
| White | 26 (57.8) | 22 (50.0) | 26 (57.8) | 26 (59.1) | 31 (70.5) | 30 (68.2) | 28 (63.6) |
| African American | 16 (35.6) | 19 (43.2) | 16 (35.6) | 15 (34.1) | 12 (27.3) | 13 (29.5) | 13 (29.5) |
| Other | 3 (6.7) | 3 (6.8) | 3 (6.7) | 3 (6.8) | 1 (2.3) | 1 (2.3) | 3 (6.8) |
| Mean time from initial diagnosis of HSV-2 to randomization, years (range) | 8.0 (1–31) |
10.7 (1–30) |
9.0 (1–30) |
10.7 (1–33) |
11.2 (2–31) |
9.1 (1–30) |
11.7 (2–33) |
| Mean number of episodes in last 12-month period without suppression (range)a | 5.2 (3–9) |
5.1 (3–9) |
5.3 (39) |
5.4 (3–9) |
4.6 (3–8) |
5.5 (3–9) |
4.9 (3–9) |
| Ever on suppressive therapy before study entry, n (%) | 25 (55.6) |
28 (63.6) |
29 (64.4) |
28 (63.6) |
32 (72.7) |
29 (65.9) |
26 (59.1) |
| Current treatment with antiviral suppression therapy, n (%)b | 8 (17.8) |
10 (22.7) |
5 (11.1) |
10 (22.7) |
10 (22.7) |
12 (27.3) |
9 (20.5) |
| History of oral lesions or HSV-1 diagnoses, n (%) | 11 (24.4) |
8 (18.2) |
11 (24.4) |
13 (29.5) |
11 (25.0) |
8 (18.2) |
12 (27.3) |
Abbreviations: HSV-1, herpes simplex virus type 1.
aDuring year before initiation of suppressive therapy for those on suppressive therapy.
bTreatment with antiviral suppression therapy at the time of screening (all subjects discontinued antiviral therapy at least 7 days before the baseline swab collection period).
Effect of GEN-003 Antigen and Adjuvant Dose Combinations on Herpes Simplex Virus Type 2 Shedding Rate
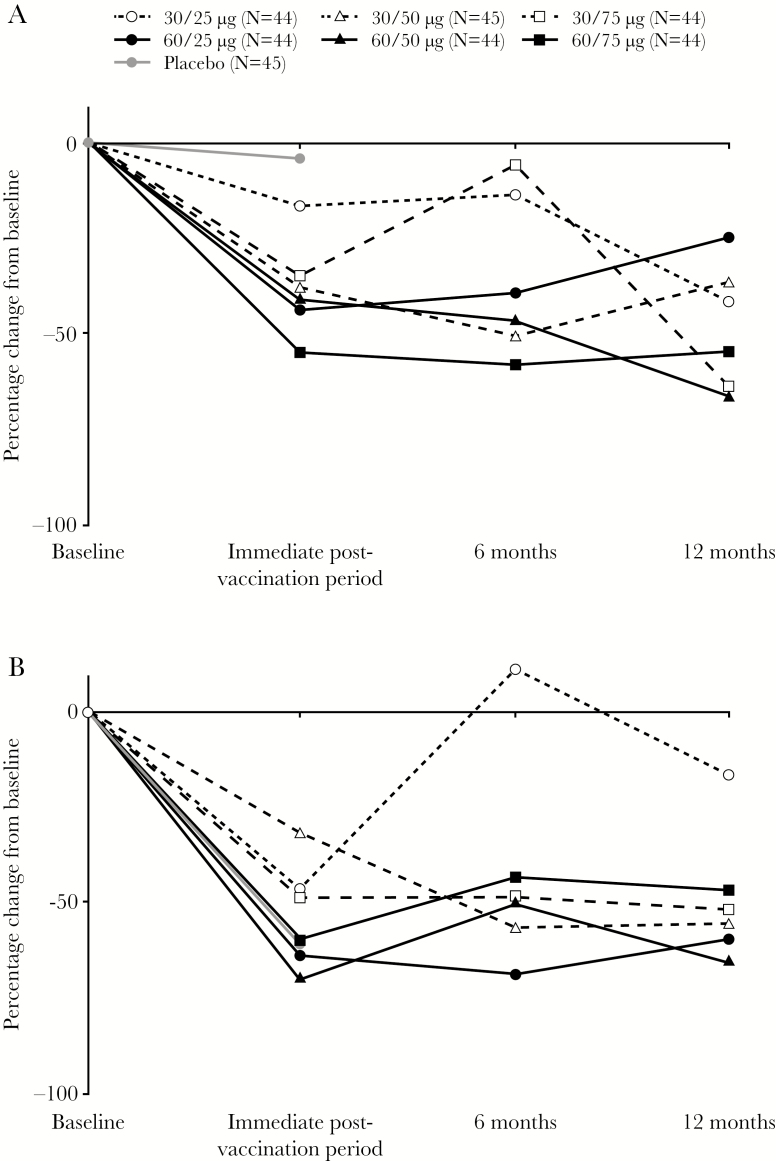
During each 28-day collection period, a mean of at least 95% of the anogenital swab samples were collected for each treatment group, except during the immediate postvaccination collection period for the 60/25 group (92%). Overall, HSV-2 was detected in 19% (3225 of 16814) of swabs collected at baseline. Baseline viral shedding rate was 23.2% for placebo and ranged from 13.6% to 27.1% for the 6 active dose combinations. During the 28 days immediately after the last dose, shedding was unchanged for the placebo group (22.2%) and ranged from 8.5% to 16.0% for the active dose combinations. Rate ratios for viral shedding immediately after treatment by treatment group were as follows: 0.82 (95% confidence interval [CI], 0.49–1.36), 30/25 dose; 0.64 (95% CI, 0.45–0.92), 30/50 dose; 0.63 (95% CI, 0.37–1.10), 30/75 dose; 0.56 (95% CI, 0.36–0.88), 60/25 dose; 0.58 (95% CI, 0.38–0.89), 60/50 dose; 0.45 (95% CI, 0.16–0.79), 60/75 dose; and 0.98 (95% CI, 0.76–1.26), placebo (Table 2; Figure 1A). Twelve months after the last dose, statistically significant reductions in rate ratios for viral shedding were as follows: 0.34 (95% CI, 0.19–0.61; P = .0003), 30/75 dose; 0.38 (95% CI, 0.25–0.57; P < .0001), 60/50 dose; and 0.43 (95% CI, 0.23–0.82; P = .01), 60/75 dose.
Table 2.
Viral Shedding and Lesion Rates
| Time point | Placebo N = 45 | GEN-003 (µg of antigens/µg of adjuvant) | ||||||
|---|---|---|---|---|---|---|---|---|
| 30/25 N = 44 | 30/50 N = 45 | 30/75 N = 44 | 60/25 N = 44 | 60/50 N = 44 | 60/75 N = 44 | |||
| Viral Shedding Rate | ||||||||
| Baseline | N | 44 | 44 | 45 | 44 | 44 | 44 | 44 |
| Proportion (%)a | 555/2396 (23.2) | 325/2387 (13.6) | 431/2465 (17.5) | 349/2406 (14.5) | 465/2386 (19.5) | 648/2387 (27.1) | 452/2387 (18.9) | |
| Immediate postvaccination period | N | 45 | 43 | 40 | 43 | 44 | 42 | 41 |
| Proportion (%)a | 535/2411 (22.2) | 259/2297 (11.3) | 230/2136 (10.8) | 218/2337 (9.3) | 247/2254 (11.0) | 362/2263 (16.0) | 186/2180 (8.5) | |
| Rate ratio (95% CI)b | 0.98 (0.76–1.26) |
0.82 (0.49–1.36) |
0.64 (0.45–0.92) |
0.63 (0.37–1.10) |
0.56 (0.36–0.88) |
0.58 (0.38–0.89) |
0.45 (0.16–0.79) |
|
| Within-group P valuec | .88 | .44 | .02 | .11 | .01 | .01 | .01 | |
| Month 6 | N | NA | 38 | 38 | 41 | 40 | 38 | 39 |
| Proportion (%)a | NA | 241/2039 (11.8) | 179/2067 (8.7) | 308/2246 (13.7) | 251/2124 (11.8) | 297/2045 (14.5) | 165/2091 (7.9) | |
| Rate ratio (95% CI)b | NA | 0.92 (0.66–1.29) |
0.54 (0.29–1.01) |
0.94 (0.61–1.45) |
0.61 (0.43–0.87) |
0.55 (0.40–0.77) |
0.41 (0.26–0.63) |
|
| Within-group P valuec | NA | .62 | .05 | .78 | .01 | .0004 | <.0001 | |
| Month 12 | N | NA | 36 | 36 | 39 | 38 | 37 | 38 |
| Proportion (%)a | NA | 154/1944 (7.9) | 217/1952 (11.1) | 112/2145 (5.2) | 303/2056 (14.7) | 186/2021 (9.2) | 176/2041 (8.6) | |
| Rate ratio (95% CI)b | NA | 0.64 (0.35–1.17) |
0.69 (0.37–1.31) |
0.34 (0.19–0.61) |
0.79 (0.50–1.25) |
0.38 (0.25–0.57) |
0.43 (0.23–0.82) |
|
| Within-group P valuec | NA | .15 | .25 | .0003 | .32 | <.0001 | .01 | |
| Viral Lesion Rate | ||||||||
| Baseline | N | 44 | 44 | 45 | 44 | 44 | 44 | 44 |
| Proportion (%) | 207/1271 (16.3) | 117/1245 (9.4) | 115/1276 (9.0) | 178/1234 (14.4) | 187/1236 (15.1) | 156/1236 (12.6) | 155/1233 (12.6) | |
| Immediate postvaccination period | N | 45 | 43 | 40 | 43 | 44 | 42 | 41 |
| Proportion (%)d | 77/1239 (6.2) | 59/1191 (5.0) | 71/1143 (6.2) | 89/1208 (7.4) | 66/1206 (5.5) | 46/1182 (3.9) | 58/1151 (5.0) | |
| Rate ratio (95% CI)e | 0.38 (0.23–0.63) |
0.55 (0.34–0.91) |
0.67 (0.37–1.23) |
0.50 (0.26–0.95) |
0.36 (0.17–0.73) |
0.30 (0.16–0.59) |
0.41 (0.20–0.87) |
|
| Within-group P valuec | .0002 | .02 | .19 | .03 | .005 | .0005 | .02 | |
| Month 6 | N | NA | 39 | 38 | 41 | 40 | 38 | 39 |
| Proportion (%)d | NA | 115/1094 (10.5) | 41/1053 (3.9) | 86/1148 (7.5) | 54/1135 (4.8) | 67/1059 (6.3) | 78/1085 (7.2) | |
| Rate ratio (95% CI)e | NA | 1.22 (0.82–1.82) |
0.43 (0.19–0.99) |
0.51 (0.28–0.93) |
0.32 (0.18–0.58) |
0.51 (0.30–0.85) |
0.57 (0.34–0.95) |
|
| Within-group P valuec | NA | .32 | .05 | .03 | .0001 | .01 | .03 | |
| Month 12 | N | NA | 36 | 36 | 39 | 38 | 37 | 38 |
| Proportion (%)d | NA | 79/1000 (7.9) | 40/1002 (4.0) | 76/1089 (7.0) | 66/1068 (6.2) | 46/1043 (4.4) | 71/1060 (6.7) | |
| Rate ratio (95% CI)e | NA | 0.95 (0.54–1.66) |
0.42 (0.18–0.96) |
0.46 (0.23–0.92) |
0.42 (0.21–0.83) |
0.35 (0.18–0.71) |
0.53 (0.31–0.89) |
|
| Within-group P valuec | NA | .85 | .04 | .03 | .01 | .003 | .02 | |
Abbreviations: CI, confidence interval; HSV-1, herpes simplex virus type 1; NA, not applicable.
aData are number of swab specimens testing positive for HSV/total number of swab specimens tested (%).
bShedding rate ratios were calculated as the proportion of swab specimens testing positive for HSV/proportion testing positive at baseline.
cPercentage ratios and P values were based on a Poisson mixed model with treatment group, visit, and treatment group by visit interaction (fixed effects) and subject (random effect). Statistically significant P values are in bold.
dData are number of swabbing days on which a lesion was detected/total number of swabbing days (%).
eLesion rate ratios were calculated as the proportion of swabbing days on which a lesion was detected/proportion of swabbing days at baseline.
Figure 1.
Percentage change in viral shedding rate (A) and lesion rate (B) from baseline.
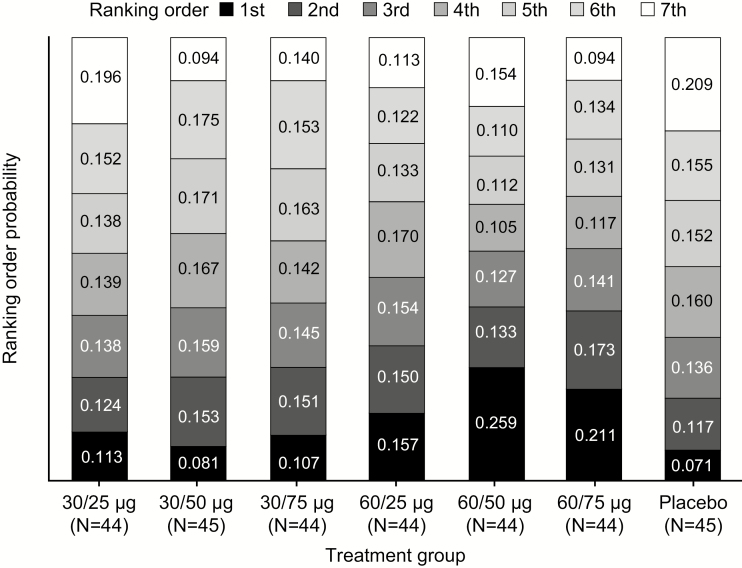
Ranking analysis of the change from baseline in viral shedding rates during the immediate postvaccination collection period showed that the 60/50 and 60/75 dosing combinations were most likely to lead to the greatest reduction in shedding rate (Figure 2). The probability of ranking first was 0.259, 60/50 dose; 0.211, 60/75 dose; 0.157, 60/25 dose; 0.113, 30/25 dose; 0.081, 30/50 dose; and 0.071, placebo.
Figure 2.
Ranking analysis of GEN-003 doses based on change in viral shedding rate from baseline to the immediate, 28-day postvaccination period. Ranking order probability was based on simulated data from assumed normal distributions of change from baseline in shedding rates for each treatment group.
Effect of GEN-003 Antigen and Adjuvant Dose Combinations on Lesion Rate
Self-reported lesions were recorded on 13% (1115 of 8731) of days during the baseline period. Baseline lesion rate was 16.3% for placebo and ranged from 9.0% to 15.1% for the active doses. During the 28 days immediately after the last dose, lesion rates were lower than baseline for all treatment groups, including placebo (6.2%), and ranged from 3.9% to 7.4% among the active treatment groups. Rate ratios for lesion rates were 0.55 (95% CI, 0.34–0.91), 30/25 dose; 0.67 (95% CI, 0.37–1.23), 30/50 dose; 0.50 (95% CI, 0.26–0.95), 30/75 dose; 0.36 (95% CI, 0.17–0.73), 60/25 dose; 0.30 (95% CI, 0.16–0.59), 60/50 dose; 0.41 (95% CI, 0.20–0.87), 60/75 dose; and 0.38 (95% CI, 0.23–0.63), placebo (Table 2; Figure 1B). Twelve months after the last dose, the rate ratios for lesion rates were as follows: 0.95 (95% CI, 0.54–1.66; P = .85), 30/25 dose; 0.42 (95% CI, 0.18–0.96; P = .04), 30/50 dose; 0.46 (95% CI, 0.23–0.92; P = .03), 30/75 dose; 0.42 (95% CI, 0.21–0.83; P = .01), 60/25 dose; 0.35 (95% CI, 0.18–0.71; P = .003), 60/50 dose; and 0.53 (95% CI, 0.31–0.89; P = .02), 60/75 dose.
Effect of GEN-003 Antigen and Adjuvant Dose Combinations on Recurrences
Six months after the last vaccine dose, 30%–46% of GEN-003-treated subjects were recurrence-free and 16%–32% were recurrence-free at 12 months (Table 3). The proportion of recurrence-free subjects did not differ significantly across GEN-003 doses at 6 or 12 months. The median time (days) to first recurrence was 101 (95% CI, 62–179), 30/25 dose; 64 (95% CI, 39–105), 30/50 dose; 124 (95% CI, 49–191), 30/75 dose; not estimable for the 60/25 dose; 98 (95% CI, 55–208), 60/50 dose; and 149 (95% CI, 80–198), 60/75 dose.
Table 3.
Proportion of Recurrence-Free Subjects at 6 and 12 Months
| Time point | Status | GEN-003 (µg of antigens/µg of adjuvant) | P Valuea | |||||
|---|---|---|---|---|---|---|---|---|
| 30/25 N = 44 | 30/50 N = 45 | 30/75 N = 44 | 60/25 N = 44 | 60/50 N = 44 | 60/75 N = 44 | |||
| 6 months, n (%) | Yes | 13 (29.5) | 14 (31.1) | 17 (38.6) | 20 (45.5) | 16 (36.4) | 13 (29.5) | .69 |
| No | 27 (61.4) | 23 (51.1) | 24 (54.5) | 21 (47.7) | 23 (52.3) | 26 (59.1) | ||
| Unknown | 4 (9.1) | 8 (17.8) | 3 (6.8) | 3 (6.8) | 5 (11.4) | 5 (11.4) | ||
| 12 months, n (%) | Yes | 8 (18.2) | 11 (24.4) | 7 (15.9) | 14 (31.8) | 13 (29.5) | 9 (20.5) | .43 |
| No | 31 (70.5) | 27 (60.0) | 33 (75.0) | 27 (61.4) | 26 (59.1) | 30 (68.2) | ||
| Unknown | 5 (11.4) | 7 (15.6) | 4 (9.1) | 3 (6.8) | 5 (11.4) | 5 (11.4) | ||
aAcross treatment-group χ2 test of homogeneity of proportions (excluding unknown counts).
Safety
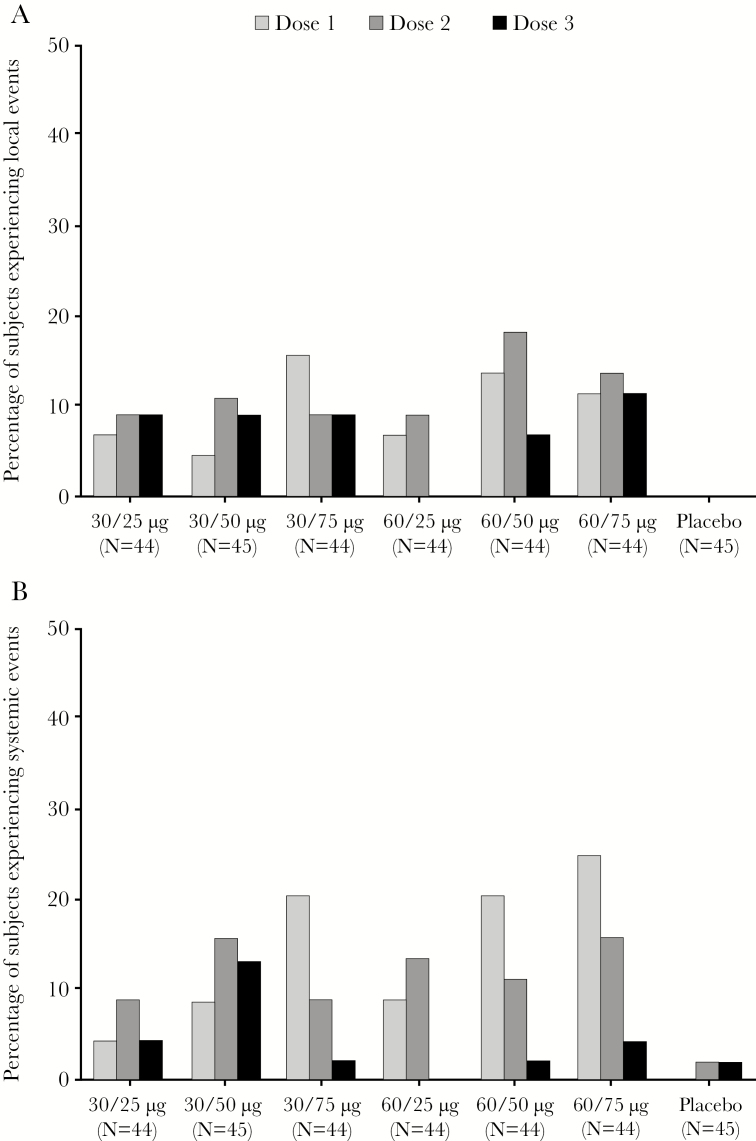
Most subjects treated with GEN-003 experienced reactogenicity events (ie, solicited AEs) within 7 days of any dose (≥98% in each GEN-003 group versus 62% of placebo recipients) (Table 4). Grade 3 reactogenicity occurred in 20%–43% and 4% of GEN-003 and placebo recipients, respectively. No grade 4 reactogenicity was reported. An effect of adjuvant dose on the incidence of grade 3 systemic events was evident after the first dose of the vaccination series (Figure 3). No other consistent effects of antigen or adjuvant dose on the incidence or severity of local reactions or systemic events were observed. There was no increase in the incidence of grade 3 local reactions with repeated doses, whereas grade 3 systemic events tended to decrease in frequency with subsequent doses (Figure 3).
Table 4.
Number (%) of Subjects With Reactogenicity Events, Adverse Events, or Other Safety Events
| GEN-003 (µg of antigens/µg of adjuvant) | |||||||
|---|---|---|---|---|---|---|---|
| Event, n (%)a | Placebo N = 45 | 30/25 N = 44 | 30/50 N = 45 | 30/75 N = 44 | 60/25 N = 44 | 60/50 N = 44 | 60/75 N = 44 |
| Reactogenicityb | |||||||
| Any | 28 (62.2) | 44 (100.0) | 44 (97.8) | 44 (100.0) | 44 (100.0) | 43 (97.7) | 43 (97.7) |
| Grade 1 (Mild) | 10 (22.2) | 7 (15.9) | 7 (15.6) | 4 (9.1) | 3 (6.8) | 6 (13.6) | 4 (9.1) |
| Grade 2 (Moderate) | 16 (35.6) | 26 (59.1) | 28 (62.2) | 27 (61.4) | 31 (70.5) | 19 (43.2) | 20 (45.5) |
| Grade 3 (Severe) | 2 (4.4) | 11 (25.0) | 9 (20.0) | 13 (29.5) | 10 (22.7) | 18 (40.9) | 19 (43.2) |
| Grade 4 (Life-threatening) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any local reaction | 14 (31.1) | 43 (97.7) | 44 (97.8) | 43 (97.7) | 42 (95.5) | 43 (97.7) | 42 (95.5) |
| Any systemic event | 26 (57.8) | 41 (93.2) | 42 (93.3) | 41 (93.2) | 40 (90.9) | 42 (95.5) | 41 (93.2) |
| AEs | |||||||
| Any | 24 (53.3) | 22 (50.0) | 22 (48.9) | 28 (63.6) | 26 (59.1) | 23 (52.3) | 18 (40.9) |
| Grade 1 (Mild) | 18 (40.0) | 16 (36.4) | 13 (28.9) | 18 (40.9) | 17 (38.6) | 20 (45.5) | 13 (29.5) |
| Grade 2 (Moderate) | 7 (15.6) | 9 (20.5) | 12 (26.7) | 12 (27.3) | 12 (27.3) | 12 (27.3) | 10 (22.7) |
| Grade 3 (Severe) | 3 (6.7) | 0 | 6 (13.3) | 4 (9.1) | 1 (2.3) | 4 (9.1) | 2 (4.5) |
| Grade 4 (Life-threatening) | 0 | 1 (2.3) | 2 (4.4) | 1 (2.3) | 0 | 1 (2.3) | 1 (2.3) |
| SAEs | 0 | 1 (2.3) | 2 (4.4) | 1 (2.3) | 2 (4.5) | 1 (2.3) | 1 (2.3) |
| Treatment-related AEsc | 5 (11.1) | 8 (18.2) | 10 (22.2) | 13 (29.5) | 9 (20.5) | 13 (29.5) | 11 (25.0) |
| Treatment-related SAEsc | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Discontinuation due to reactogenicity | 0 | 0 | 1 (2.2) | 1 (2.3) | 0 | 0 | 1 (2.3) |
| Discontinuation due to AEs | 0 | 0 | 1 (2.2) | 0 | 2 (4.5) | 1 (2.3) | 1 (2.3) |
Abbreviations: AE, adverse event; SAE, serious adverse event.
aSubjects are counted once in each category in which they have data.
bReactogenicity events occurring within 7 days after any dose of GEN-003 or placebo.
cTreatment-related AEs/SAEs are AEs/SAEs with relationship to investigational product assessed by the investigator as likely.
Figure 3.
Percentage of subjects experiencing local (A) and systemic (B) grade 3 reactogenicity events.
The 2 most common local reactions associated with GEN-003 were pain and tenderness at the injection site. The 2 most common systemic reactions were fatigue and myalgias. We compared pooled local reactogenicity events and pooled systemic reactogenicity events in the vaccine groups to placebo. Local and systemic reactogenicity events were significantly more common in all GEN-003 vaccine groups compared with placebo (Supplementary Table S1).
The number of subjects who experienced unsolicited AEs was similar across GEN-003 dose groups (41%–64%) and the placebo group (53%) (Table 4). The most common AEs were headache, upper respiratory tract infection, and chills (Supplementary Table S2). Grade 3 or 4 AEs were uncommon and were also noted in the placebo group (Supplementary Table S3). Eight subjects experienced 10 SAEs (bipolar disorder [2 episodes in 1 subject], cholecystitis, diverticulitis, femur fracture, myocardial infarction, overdose, pyelonephritis, viral infection, and postlumbar puncture syndrome [in 1 subject each]). No SAEs were considered treatment-related. No deaths or AESIs were reported. Eight subjects (3%) who received GEN-003 and no placebo recipients discontinued dosing due to reactogenicity (n = 3) or AEs (n = 5 [rash, upper respiratory infection, headache, and confusional state, and circumoral edema in 1 subject each; muscle spasms, constipation, and pruritis in 1 subject]). No differences in laboratory results were observed among treatment groups or with repeated doses.
Immunology
The IgG antibody titers to both GEN-003 antigens increased among all doses by at least 0.8 log. Responses to the 30/25 dose tended to be slightly lower than responses to other doses at all timepoints. The gD2ΔTMR IgG responses were higher than ICP4.2 responses at baseline and at all posttreatment responses. The CD4+ IFN-γ+ T-cell responses peaked at 8 days after the first dose and gradually declined thereafter. Responses were generally GEN-003 dose-related with CD4+ IFN-γ+ frequencies of 0.049% for the 30/25 dose, peaking at 0.141% for the 60/50 dose for ICP4.2. The CD4+ IFN-γ+ T-cell responses to gD2ΔTMR were higher, with 0.092% for the 30/25 dose, peaking at 0.179% for the 60/50 dose. The frequencies of CD4+ IFN-γ+ T cells for the 60/75 dose were lower than the other 60 µg antigen-containing doses at 0.073% for ICP4.2 and 0.128% for gD2ΔTMR (L.K.M., data not shown; manuscript in preparation).
DISCUSSION
GEN-003 reduced viral shedding for up to 12 months after completion of a 3-dose series, with the 60/50 and 60/75 doses representing the most promising combinations for further evaluation based on viral shedding and overall safety assessment. We selected viral shedding as the primary parameter for comparison because it is an objective measure of antiviral effect and underlies the key elements of genital HSV infection: recurrences and transmission to an uninfected sexual partner. Lesion rates were also most effectively reduced at the 60/50 and 60/75 doses at 6 and 12 months. Of note, placebo patients were offered vaccine after the initial period. Therefore, patients who were not unblinded at that time were known to have received the active vaccine, although both the participants and the investigators remained blinded to the dose.
The magnitude of the reductions in viral shedding rates seen in this study are consistent with the findings of a previous Phase 1/2 study, in which the 30/50 dose of GEN-003 decreased viral shedding in the 28 days after immunization by approximately 50%. In that study, although the durability of this response appeared to be limited, few subjects provided shedding rate data at 12 months after the last dose, making effect estimates at that time-point uncertain [10]. In the current study, doses containing 60 µg of each antigen generally provided greater durability of effect than doses of 30 µg of antigen. The efficacy of the 50 and 75 µg doses of adjuvant appeared similar, whereas the reactogenicity of the higher adjuvant dose was greater.
The clinical outcomes of GEN-003 were lesion rates, the proportions of subjects who remained recurrence-free at 6 and 12 months, and the time to the first recurrence of genital herpes. The variability of lesion rates, as seen in the brief reduction (first period only) in lesions seen in the lowest dose group as well as the placebo group, reinforces our decision to use shedding as the main outcome. The observed reduction in lesion rates for the placebo group immediately after dosing remains unexplained. Possible causes include expectations of benefit given (1) the 6 in 7 chances of randomization to an active dose or (2) the impact of chance in quantifying an episodic phenomenon. Despite the apparent reduction in lesion rate, there was no concurrent reduction in viral shedding in the placebo group. This phenomenon was not observed in the placebo group in the Phase 1/2 study, with similar lesion rates before (7.2%) and after (9.1%) vaccination [10]. In addition, although there were apparent differences in viral shedding and lesion rates among the GEN-003 dose groups through month 12, there were no observable differences across other clinical assessments. The proportion of subjects free from recurrences at 6 and 12 months was not a differentiating factor across the active doses, probably a consequence of small sample size. Nevertheless, in studies of antiviral treatments, the proportion of subjects recurrence-free at 12 months who received placebo was 6% and 4% [13, 14], although differences in subject populations may limit the ability to compare across products. The analysis of time to first recurrence across the dose groups also failed to identify differences, likely also the result of small sample size.
Solicited local and systemic events were more frequent in all GEN-003 groups compared with the placebo group. Despite substantial reports of tenderness, fatigue, and myalgia among GEN-003 recipients, few persons withdrew from the study. No grade 4 reactogenicity events, treatment-related SAEs, AESIs, or deaths occurred during the study. These observations indicate that GEN-003 displays an acceptable reactogenicity and safety profile for a therapeutic vaccine.
The factorial design facilitating comparisons across different antigen and adjuvant doses, measured by change from baseline analyses, is a key strength of this study, and the consistency seen across dose combinations and with the Phase 1/2 study reinforces the robustness of the results. Another strength was the confirmation of viral shedding and lesion rate evaluations as indicators of biologic activity and potential clinical benefit in the development of new therapeutic agents for genital herpes [15]. This study has some limitations. The placebo group was only followed for 28 days after the third dose, preventing comparison of the clinical and shedding efficacy of the vaccine with placebo beyond this time point. However, comparison with the placebo group was not vital to the primary objective of the study to differentiate among the doses but was included as a comparator for reactogenicity. Despite these limitations, we believe that GEN-003 shows promise as a therapeutic vaccine for HSV-2. In preparation for Phase 3 trials, studies are under way to determine the performance of a new formulation of GEN-003 modified to allow for an increase in the scale of production. Because lesion rate will be an important outcome for Phase 3 trials, work is under way to create better tools to more accurately collect genital herpes recurrence data. Full results of immunogenicity studies in this population will be reported separately, which is an important corollary to the clinical outcomes reported here.
Currently available therapeutic options for recurrent HSV-2 infections include (1) short-term treatment beginning with the first signs of recurrence or (2) chronic daily suppression with nucleoside analogues, valacyclovir, acyclovir, or famciclovir [3]. However, chronic daily therapy does not completely suppress outbreaks [16], has cost implications [7], and requires daily adherence. With a high global prevalence [2], an established link between HSV-2 and subsequent HIV acquisition [17], the often overlooked psychological effects of genital HSV infection [18], and the inability of antiviral oral treatments to completely eliminate viral shedding, there is a clear need for new therapeutic options. An effective therapeutic vaccine is an attractive option due to its novel mechanism of action and potential for improved adherence.
CONCLUSIONS
In conclusion, GEN-003 immunization resulted in significant reductions in viral shedding and lesion rates for up to 12 months. The GEN-003 antigen/MM2 adjuvant combinations of 60/50 µg and 60/75 µg produced the most consistent and durable virologic and clinical effects.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: IDWeek 2015, October 7–11, 2015, San Diego, CA; ASM Microbe 2016, June 16–20, 2016, Boston, MA.
Acknowledgments. We thank the participating study volunteers, clinicians, nurses, and laboratory technicians who made this trial possible. Medical writing support was provided by Drs. Joanna Chapman and Rick Flemming (Aspire Scientific Ltd., Bollington, UK).
Financial support. This study was funded by Genocea Biosciences Inc. (Cambridge, MA).
Potential conflicts of interest. N. V. W. has been a consultant for Genocea Biosciences for herpes simplex virus vaccines and has served as the principal investigator on clinical trials related to herpes vaccines with Vical and Genocea Biosciences. K. F. receives research funding from Genocea Biosciences and Vical. P. A. L. receives research funding from Genocea Biosciences and Vical and has served as a consultant for Genocea Biosciences, Vical, and Abbott Diagnostics. D. I. B. receives funding from Genocea Biosciences for preclinical studies of vaccines and has been a consultant to Genocea Biosciences, Vical, GlaxoSmithKline, and Merck for herpes virus vaccines. T. W. has served as the principal investigator on clinical trials related to herpes vaccines with Vical, Genocea Biosciences, GlaxoSmithKline, and Merck. R. M. N. has served as the principal investigator on clinical trials related to herpes vaccines with Vical and GlaxoSmithKline. R. B. has received funding from Genocea Biosciences. J. K. has received research funding from Genocea Biosciences and Vical. S. T. has received research funding from Genocea Biosciences for clinical studies on herpes simplex virus vaccines. W. K. has served as the principal investigator on clinical trials related to herpes for both Genocea and Vical and received funding for participation in those trials. G. L. worked as Medical Director for Tekton Research and thus worked as a principal investigator on Genocea herpes vaccine trials. A. M. is owner of IND 2 Results. B. Z. and S. T. were employees of Genocea Biosciences at the time of the study. S. H. is an employee and stock owner of Genocea Biosciences. A. W. is a consultant for Aicuris, Amgen, and GlaxoSmithKline and has received travel reimbursement from Admedus and Viblok. She has received funds for sponsored projects for Genocea Biosciences and Vical. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gnann JW Jr, Whitley RJ. Genital herpes. N Engl J Med 2016; 375:1906. [DOI] [PubMed] [Google Scholar]
- 2. Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015; 10:e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Workowski KA, Bolan GA; Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 4. Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med 1994; 121:847–54. [DOI] [PubMed] [Google Scholar]
- 5. Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis 2004; 190:1374–81. [DOI] [PubMed] [Google Scholar]
- 6. Johnston C, Saracino M, Kuntz S, et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet 2012; 379:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonnar PE. Suppressive valacyclovir therapy to reduce genital herpes transmission: good public health policy?McGill J Med 2009; 12:39–46. [PMC free article] [PubMed] [Google Scholar]
- 8. Romanowski B, Zdanowicz YM, Owens ST. In search of optimal genital herpes management and standard of care (INSIGHTS): doctors’ and patients’ perceptions of genital herpes. Sex Transm Infect 2008; 84:51–6. [DOI] [PubMed] [Google Scholar]
- 9. Flechtner JB, Long D, Larson S, et al. Immune responses elicited by the GEN-003 candidate HSV-2 therapeutic vaccine in a randomized controlled dose-ranging phase 1/2a trial. Vaccine 2016; 34:5314–20. [DOI] [PubMed] [Google Scholar]
- 10. Bernstein DI, Wald A, Warren T, et al. Therapeutic vaccine for genital herpes simplex virus-2 infection: findings from a randomized trial. J Infect Dis 2017; 215:856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Food and Drug Administration. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials 2007. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf. Accessed 12 November 2017.
- 12. Skoberne M, Cardin R, Lee A, et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs. J Virol 2013; 87:3930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. FAMVIR (famciclovir). Prescribing Information 2011. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020363s037lbl.pdf. Accessed 12 November 2017.
- 14. VALTREX (valacyclovir hydrochloride). Prescribing Information 2008. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020487s014lbl.pdf. Accessed 12 November 2017.
- 15. Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest 2011; 121:4600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med 2008; 168:1137–44. [DOI] [PubMed] [Google Scholar]
- 17. Schiffer JT, Gottlieb SL. Biologic interactions between HSV-2 and HIV-1 and possible implications for HSV vaccine development. Vaccine 2017. pii: S0264-410X(17)31273-2. doi: 10.1016/j.vaccine.2017.09.044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merin A, Pachankis JE. The psychological impact of genital herpes stigma. J Health Psychol 2011; 16:80–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.