Abstract
Alzheimer disease (AD) is the most common cause of dementia in the elderly, and is characterized by extracellular deposition of β-amyloid and intracellular accumulation of hyperphosphorylated tau protein in the brain. These pathologic findings are identified postmortem. Various visual deficits in AD have been reported and there have been conflicting reports, through imaging and pathology studies, regarding the presence of changes in the globe that mirror Alzheimer changes in the brain. Moreover, both macular degeneration and glaucoma have been variously characterized as having AD-related features. We examined one or both eyes from 19 autopsy cases, 17 of which had varying degrees of AD-related changes, and 2 of which were age-matched controls. Three cases had glaucoma and 4 had macular degeneration. Immunohistochemistry for tau, β-amyloid, TDP-43, ubiquitin, and α-synuclein showed no evidence of inclusions, deposits or other protein accumulation in any case, in any part of the globe. This finding suggests that regardless of the severity of changes seen in the brain in AD, there are no similar changes in the globe.
Keywords: Alzheimer disease, Biomarkers, Ocular changes
INTRODUCTION
Alzheimer disease (AD) is the most common cause of dementia in the elderly, and is characterized by neuronal loss in the cerebral cortex, extracellular deposition of β-amyloid, and intracellular accumulation of hyperphosphorylated tau protein in the brain. The final diagnosis is made based on autopsy brain findings. Various visual deficits in AD have been reported and there have been conflicting reports, through imaging and pathology studies, regarding the presence of changes in the lens and retina that mirror Alzheimer changes in the brain. This study aims to evaluate the presence of these changes in the eye for possible use in diagnosis and disease monitoring.
The ocular globe contains a rich supply of nerve tissue. The retina is composed of multiple layers of neurons and axons connected by synapses, including the optic nerve fiber layer, ganglion cell layer, inner and outer plexiform layers, inner and outer nuclear layers, and a layer of rods and cones. The retina also contains glial cells, such as Müller glia, which support the neurons. In addition, structures such as the cornea, iris, and ciliary body are well innervated by sensory or autonomic nerve fibers. This extensive supply of neurons, glia, and nerves make evaluating eye structures for AD-related changes an attractive area for exploration.
The notion that AD changes occur in the eye has been present for decades, with initial postmortem studies showing an association of AD with moderate reduction in retinal ganglion cells, thinning of the retinal nerve-fiber layer, and axonal degeneration of the optic nerve (1, 2). More recent studies using optical coherence tomography have confirmed these findings, as well as shown functional defects in the retina (3–8) and retinal vasculature changes (9). In addition, patients with AD have an increased risk for age-related macular degeneration (10).
Numerous transgenic mouse models of AD have reported retinal findings of hyperphosphorylated tau and β-amyloid (11–15). Other studies on mouse models using spectrophotometric and fluorochrome plaque-labeling techniques have reported that the amyloid deposits in retina can precede cognitive decline (16), and can be identified through noninvasive imaging modalities (16–18).
Reports of deposits in humans have been varied. The first reports using light microscopy showed no neurofibrillary tangles or amyloid angiopathy in 4 (1) and 12 autopsy retinas from patients with AD (2). The latter study also confirmed these findings via evaluation of ultrastructural characteristics.
More recent reports have evaluated for the presence of deposits with immunohistochemistry, starting with evaluation by Loffler et al of amyloid precursor protein (APP), β-amyloid, and non-phosphorylated tau in the retina of older patients and patients with retinitis pigmentosa (RP) and age-related macular degeneration (ARMD, 19). No age-related changes were identified in the staining pattern of tau. Retinal ganglion cells of older cases and the retinal pigment epithelium of eyes with RP and ARMD showed increased staining for APP. Patchy staining of β-amyloid was also identified within the sub-retinal pigment epithelium of older subjects. Of note, in this study, none of the 40 total cases were from patients with known AD. Another study examined eyes from 19 cases (ages 49 to 87 years), of which 2 had clinical dementia, and found no phospho-tau or β-amyloid deposits in any of the cases (20). This study also reported that older patients had increased frequency of ubiquitin and α-synuclein intracytoplasmic inclusions in the inner nuclear layer of the retina (20). Koronyo-Hamaoui et al reported β-amyloid deposits in postmortem eyes by immunohistochemistry on cryosections, in 8 cases of definitive AD and 5 cases of suspected early stage AD. Plaques were not detected in 5 age-matched controls (17). A study by Schön et al showed that the eyes of 6 AD patients were all negative for β-amyloid, and 5 of 6 had deposits of hyperphosphorylated tau (21). A study by Ho et al revealed no evidence of phospho-tau or β-amyloid deposits in any part of the eyes of 11 cases of autopsy-confirmed AD (22). Through a variety of techniques including immunohistochemistry and Western blot, 2 studies by the same group showed that ocular lenses from subjects with AD and Down syndrome contain deposits of β-amyloid (23, 24). However, some have raised concerns regarding performing and validating these findings, with Michael et al identifying no material diagnostic for amyloid (25, 26).
Many incongruous findings exist in the literature (Table 1). Antibodies not used in the autopsy diagnosis of AD were used, such as APP, and non-standard techniques were used, including staining performed on cryosections. Small cohorts and cohorts without established diagnoses of AD were examined. Some did not concurrently examine the brain, making the assessment of comparable deposits impossible. In this study, we evaluate a sizeable cohort, examining both the eyes and brain of each case for AD-related changes and other pathological findings, using the standard techniques used for autopsy diagnosis of AD.
TABLE 1.
Description of Cases, Tissue Processing, Methods, Specific Stains Used, and Results in Each Publication That Examined Human Eyes for AD Related Neuropathological Changes
| Citation | Cases description | Tissue processing | Method(s) | Specific stain | Results |
|---|---|---|---|---|---|
| Hinton et al (1) | 4 retinas from patients with AD (autopsy confirmed) | Dissected retina snap-frozen. Cryostat sections air dried, then fixed in 10% buffered formalin | Staining | H&E, Thioflavine S | Negative for NFTs1, neuritic plaques, and amyloid angiopathy |
| Blanks et al (2) | 12 retinas from patients with AD (autopsy confirmed) | Dissected retina snap-frozen. Cryostat sections air dried, then fixed in 10% buffered formalin | Staining | H&E, Thioflavine S | Negative for NFTs and amyloid angiopathy |
| Koronyo-Hamaoui et al (17) | 8 patients with definite AD, 5 patients with possible or probable AD, and 5 age-matched controls (autopsy confirmed) |
|
IHC2 | Positive for β-amyloid in AD patients’ retinas by IF and IP staining (negative in 5 age matched controls) | |
| Löffler et al (19) | Eyes fixed in 10% buffered formalin (except 2 controls were fixed in 1% glutaraldehyde-4% formaldehyde), paraffin embedded | IHC |
|
|
|
| Leger et al (20) |
|
Eyes formalin fixed, paraffin embedded | IHC |
|
|
| Schön et al (21) |
|
Eyes formalin (4%) fixed, paraffin embedded | IHC |
|
|
| Ho et al (22) | Eyes from 11 AD cases and 6 age-matched controls (autopsy confirmed) | Eyes fixed in buffered formalin, paraffin embedded | IHC | Anti-phospho-tau (clone AT8, Research Diagnostic Inc., Flanders, NJ)Anti-β-amyloid (clone 6F/3D, Dako, Carpinteria, CA) | Negative for phospho-tau and β-amyloid |
| Goldstein et al (23) | Lenses from 4 patients with AD, and 4 age-matched controls (autopsy confirmed) | IHC, EM, WB |
|
Lenses show focal increased positivity for β-amyloid by IHC and EM in AD cases, co-localized with cataracts | |
| Moncaster et al (24) | Lenses from 3 patients with AD, 4 patients with Down syndrome, and 3 controls (autopsy confirmed) |
|
IHC, EM, ELISA, WB, peptide sequencnig |
|
Lenses positive for β-amyloid by IHC and EM in Down syndrome patients |
| Michael et al (25) | Eyes from 21 patients with AD (17 with clinical dementia, 4 autopsy confirmed) and 15 age matched controls (7 with cataracts) | Lenses dissected from globes, fixed in 3.6% buffered formaldehyde, paraffin embedded | IHC, staining |
|
Lenses negative for β-amyloid by IHC and Congo red staining |
Lesions in the eye do not parallel typical neuropathological changes in the brain in AD.
NFT = neurofibrillary tangles,
IHC = immunohistochemistry,
IF = immunofluorescence,
IP = immunoperoxidase,
RP = retinitis pigmentosa,
ARMD = age-related macular degeneration,
APP = amyloid precursor protein,
RPE = retinal pigment epithelium,
EM = electron microscopy,
WB = Western blot.
MATERIALS AND METHODS
We examined one (4 cases) or both eyes (13 cases) from 17 autopsy cases of AD and both eyes from 2 age-matched controls chosen from autopsy records from Massachusetts General Hospital. Demographic data as well as concurrent ophthalmological changes were recorded. The brain from each case was examined for pathological abnormalities, with sections taken from the superior and inferior frontal cortex, motor sensory strip, superior and inferior parietal cortex, temporal lobe, cingulate gyrus, calcarine cortex, hippocampus, basal ganglia, amygdala, thalamus, brainstem, and cerebellum. Immunohistochemistry for tau was performed on superior frontal cortex, superior and inferior parietal cortex, calcarine cortex, hippocampus, amygdala, and cingulate gyrus. Immunohistochemistry for β-amyloid was performed on these sections and basal ganglia, midbrain and cerebellum. The sections taken and immunohistochemistry performed were based on standard protocols established to determine the presence of AD-related changes in the brain, recorded by Thal stage, Braak and Braak tangle stage, and CERAD age-related plaque score (27–32). These staging systems are based on the stereotypical spatiotemporal progression of β-amyloid positive plaques and phospho-tau positive neurofibrillary tangles (NFTs) in AD. Thal stage is based on a progression of amyloid deposition in 5 stages: Stage 1 or isocortical; Stage 2, with additional allocortical deposits (entorhinal cortex, hippocampal formation, amygdala, insular, and cingulated cortices); Stage 3, with added involvement of subcortical nuclei including striatum, basal forebrain cholinergic nuclei, thalamus and hypothalamus, and white matter; Stage 4, distinguished by the involvement of brainstem structures, including red nucleus, substantia nigra, reticular formation of the medulla oblongata, superior and inferior colliculi; and Stage 5, with additional amyloid deposits in the pons (reticular formation, raphe nuclei, locus ceruleus) and the molecular layer of the cerebellum (27). Braak tangle stage includes 6 stages of NFTs: the first NFTs appear in the transentorhinal region (stage I) and the entorhinal cortex, then in the CA1 region of the hippocampus (stage II). Next, NFTs appear in limbic structures such as the subiculum of the hippocampal formation (stage III) and the amygdala, thalamus, and claustrum (stage IV). NFTs subsequently extend to all isocortical areas (isocortical stage), with the associative areas being affected prior and more severely (stage V) than the primary sensory, motor, and visual areas (stage VI) (28). CERAD age-related plaque score is based on the density of neuritic plaques (reported as sparse, moderate and severe) in the most severely affected region of isocortex (frontal, temporal, or parietal), and the patient’s age at death (29). All neuropathological and ophthalmological evaluations were performed at the same institution.
The eyes and brain were removed at autopsy, fixed in formalin, and sections taken were paraffin embedded. For each globe, 2 cross sections were processed to include representative structures, including lens, optic nerve and retina. Five-μm-thick sections were stained with hematoxylin and eosin (H&E) and examined. Immunohistochemistry for tau (Dako, Carpinteria, CA; Ref: A0024), β-amyloid (Dako; clone 6F3D; Ref: M0872), ubiquitin (Thermo Scientific, Waltham, MA), and α-synuclein (Thermo Scientific; Ref: RB-9026-P) was subsequently performed on each eye available for each autopsy case. Immunohistochemistry for TDP-43 (Proteintech, Rosemont, IL; Ref: 10782-2-AP) was performed on 11 cases. Appropriate positive controls were used on each run, including known AD brain autopsy cases for tau and β-amyloid. Good antibody penetrance of retinal tissue was observed using GFAP immunohistochemistry (Dako), with positive staining of Müller cells ((33), Fig. 1). This served as a positive control for the staining methods.
FIGURE 1.
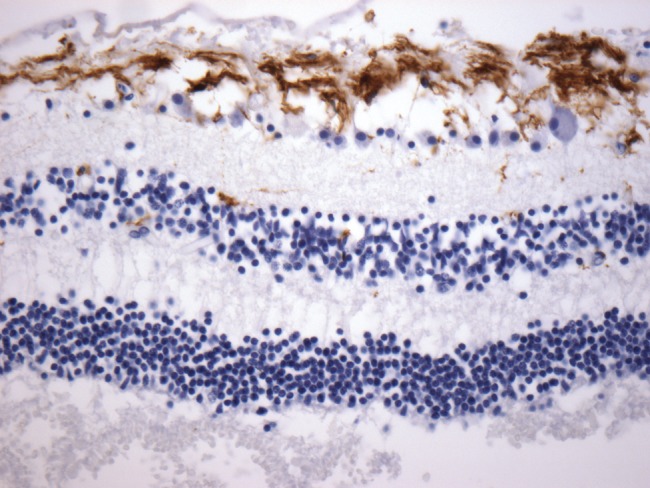
Sample immunohistochemical stains at 40x show good antibody penetrance for GFAP in retinal tissue, with positive staining of Müller glia.
RESULTS
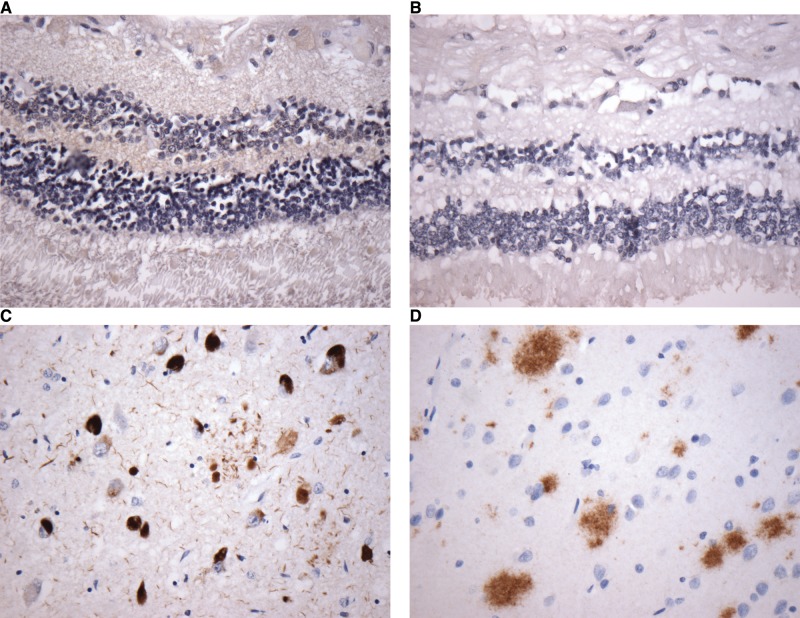
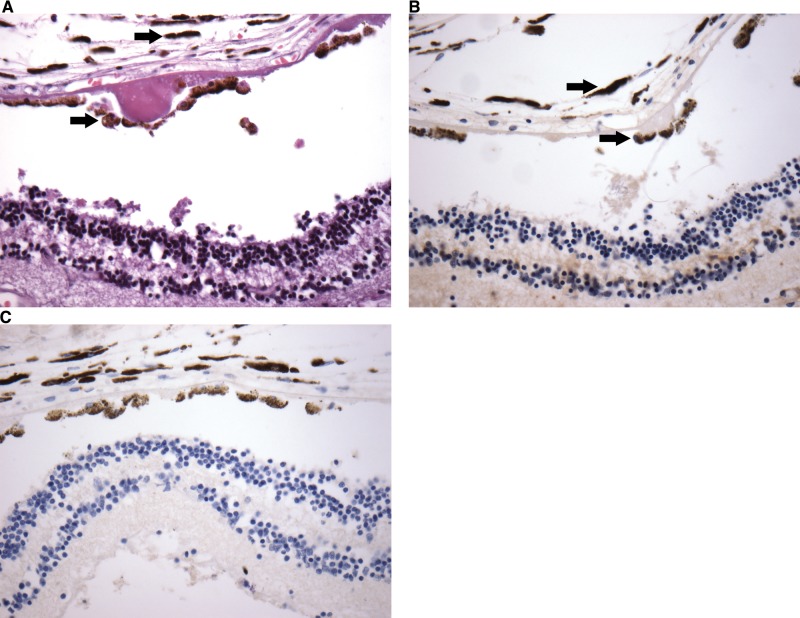
The mean age of the cohort was 77 years with 52% male patients. Seventeen cases had varying degrees of AD-related changes (Table 2). No known cases of familial AD were included in the series. The presence of AD-related changes, cerebral amyloid angiopathy, and other neuropathological and ophthalmic findings for each case are shown in Table 2. Two age-matched controls were included (Cases 18, 19). No definite inclusions were identified with immunohistochemistry for tau, β-amyloid, ubiquitin, TDP-43, or α-synuclein in any part of the eye, including retina (Fig. 2A, B), lens, and optic nerve. Immunohistochemistry was performed in the standard fashion on brain sections for diagnosis of AD, which included stains for tau and β-amyloid (Fig. 2C, D). Concurrent ophthalmological observations on H&E included cataracts (2 cases), age-related macular degeneration (3 cases), open angle glaucoma (4 cases), and optic nerve atrophy (1 case) (Table 2; Fig. 3A). No cerebral amyloid angiopathy was identified within the retinal vasculature of any case. No immunohistochemical staining for phospho-tau, β-amyloid, ubiquitin, TDP-43, or α-synuclein was identified in these ophthalmic pathologies (Fig. 3B, C).
TABLE 2.
Degrees of AD-Related Changes, and Additional Brain and Eye Pathology
| Case | Thal | Braak and Braak stage | CERAD | NIA-AlzAssn score | CAA-Vonsattel grade | Other brain pathology | Eye pathology |
|---|---|---|---|---|---|---|---|
| 1 | 4 | VI | Frequent | A3B3C3 | 0 | Open angle glaucoma | |
| 2 | 4-5 | VI | Frequent | A3B3C3 | Focal 1 | Remnant of cataract | |
| 3 | 4-5 | V-VI | Frequent | A3B3C3 | 0 | Microinfarcts | Open angle glaucoma |
| 4 | 5 | V-VI | Frequent | A3B3C3 | Focal 1 | Microinfarcts | Macular degeneration |
| 5 | 5 | VI | Frequent | A3B3C3 | Focal 1 | ||
| 6 | 5 | VI | Frequent | A3B3C3 | Diffuse 3 | Hippocampal sclerosis with TDP-43 inclusions | |
| 7 | 5 | VI | Frequent | A3B3C3 | 0 | Open angle glaucoma | |
| 8 | 4 | V | Moderate | A3B3C2 | 2-3 | Cupped optic n. with atrophy consistent with glaucoma, soft drusen | |
| 9 | 3 | VI | Frequent | A2B3C3 | 1 | Cupped optic n., soft drusen | |
| 10 | 4 | V | Moderate | A3B3C2 | 1 | Focal chorioretinal scar | |
| 11 | 3 | VI | Moderate | A2B3C2 | 0 | ||
| 12 | 3 | VI | Moderate | A2B3C2 | Diffuse 4 | ||
| 13 | 3 | VI | Moderate | A2B3C2 | Focal 1 | Diffuse Lewy body disease | Early macular degeneration |
| 14 | 3 | II | Moderate | A2B1C2 | 0 | Lewy body disease, remote microinfarcts | Macular degeneration |
| 15 | 3 | III-IV | Sparse | A2B2C1 | Diffuse 3 | ||
| 16 | 4 | II | Moderate | A3B1C2 | 0 | ||
| 17 | 3 | II | Moderate | A2B1C2 | 0 | Cataract remnant, retinal tear | |
| 18 | 0 | IV | 0 | A0B2C0 | 0 | Fibrous metaplasia of RPE1, band keratopathy, scar | |
| 19 | 0 | II | 0 | A0B1C0 | 0 | Multiple system atrophy, cerebellar type | Optic n. atrophy |
RPE = retinal pigment epithelium.
FIGURE 2.
Sample immunohistochemical stains at 40x show retina with no evidence of phospho-tau- (A) or β-amyloid (B)-positive inclusions. Brain sections of hippocampus from the same case show prominent tau (C) and β-amyloid (D) accumulation.
FIGURE 3.
Sample macular degeneration H&E (A) with no evidence phospho-tau- (B) or β-amyloid (C)-positive inclusions. Numerous melanin granules (arrows) are seen in association with retinal pigment epithelium and choroid, and are not to be confused with positive staining by immunohistochemistry. All stains at magnification: 40x.
DISCUSSION
AD is the most common cause of dementia in the elderly. A particular focus has been placed on early diagnosis to initiate treatment as early as possible. The eye is a particularly appealing site given it contains an abundant supply of nervous tissue. This includes retina, which holds multiple interconnected layers of neurons and transmits to the optic nerve, and well-innervated structures such as the cornea and the iris. The globe is directly connected to the brain and is accessed more easily than the brain for potential diagnostic tests. In addition to early diagnosis, ocular tests could possibly monitor AD progression or response to treatment.
In our study, immunohistochemistry for tau, β-amyloid, TDP-43, ubiquitin, and α-synuclein showed no evidence of inclusions, deposits or other protein accumulation in any case, in any part of the globe. Immunohistochemistry standardization is indispensable for reliable and reproducible results. We used identical protocols and positive controls for each globe and brain case. Each specimen, following removal at autopsy, was fully formalin fixed for a minimum of 2 weeks. Identical tissue processing was performed, including paraffin embedding and slide-drying time and temperature. The same antibodies, antibody dilution, antigen retrieval, incubation times, and automated platforms were used, to ensure consistent results. Tau deposition and β-amyloid plaques were present in the brain of these cases, and showed strongly positive immunostaining. These findings suggest that regardless of the severity of changes seen in the brain in AD, there are no similar changes in the globe. AD cannot be readily diagnosed or monitored via ophthalmological examination using methods with similar sensitivity to standard immunohistochemical techniques.
These findings are in agreement with some studies and in disagreement with others (Table 1). Multiple studies, including those by Hinton et al, Loffler et al, and Leger et al failed to identify any inclusions, with the latter 2 confirming their findings with immunohistochemistry (1, 19, 20). Schön et al and Ho et al found similar findings with immunohistochemistry, with the exception of hyperphosphorylated but not fibrillar tau found in the retina from AD patients by Schön et al (21, 22). Koronyo-Hamaoui et al identified amyloid plaques in early and confirmed AD through immunofluoresecent analysis of frozen material, which may be a more sensitive diagnostic test (17). In addition, they examined retinal whole mounts, which includes more tissue than cross sectional evaluation used here.
Other methods for early diagnosis of AD via ocular biomarkers have been suggested, such as β-site APP-cleaving enzyme 1 (BACE1 [34]) and retinal vascular changes. In APP/PS-1 transgenic mice, changes in BACE1 expression level occur earlier in the retina than in the brain and occur before behavioral deficits, but these observations may reflect transgene promoter effects (34). Several groups have examined retinal blood vessels. Frost and colleagues compared brain amyloid plaque burden to multiple retinal vasculature parameters obtained through retinal photography and were able to predict healthy individuals with high plaque burden based on 2 of these parameters (venular branching asymmetry factor and arteriolar length-to-diameter ratio) (35). Williams et al showed that subjects with lower venular fractal dimension and lower arteriolar tortuosity were more likely to have AD (9).
The increased risk of ARMD in AD is discussed in the literature (10), although the pathophysiology of this connection is not understood. It has been suggested that tau or β-amyloid deposits may play a role (10, 36). Three cases in our group of patients had both AD and ARMD. None of these globes had phospho-tau or β-amyloid deposits in the eye, with specific focus on the retinal pigment epithelium, choriocapillaris, and drusen. In addition, reports have shown an increased incidence of primary open angle glaucoma among AD patients, and some patients with glaucoma have shown an increase in phospho-tau in specific parts of the retina (36). In our 4 cases of concurrent AD and open angle glaucoma, no phospho-tau deposits within the retina were identified. This suggests that deposits of these proteins do not play a prominent role in the pathogenesis of ARMD and glaucoma in patients with AD. Finally, there have been mixed reports of β-amyloid deposits in the lenses from patients with cataracts and AD (23, 25). The observations, made indirectly with an optical imaging approach, suggest amyloid deposits in the lens, but no amyloid deposits were observed in the lens of the patients examined in this series.
In conclusion, upon examination of both brain and eyes from AD subjects using the standard techniques used at Massachusetts General Hospital, no similar AD changes are found in the globe. This is in disagreement with some prior studies, and could be explained by differences in technique and sensitivity. In addition, no deposits were found in patients with both AD and other ophthalmic pathologies, indicating that deposits similar to those in the brain are not part of the pathophysiology of these diseases.
ACKNOWLEDGMENTS
We thank Dr. E. Tessa Hedley-Whyte for her contribution to the excellent characterization of the brain cases used in this study. We also acknowledge the constructive comments of the reviewers, which have significantly improved the final manuscript.
REFERENCES
- 1. Hinton DR, Sadun AA, Blanks JC, et al. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med 1986;315:485–7 [DOI] [PubMed] [Google Scholar]
- 2. Blanks JC, Hinton DR, Sadun AA, et al. Retinal ganglion cell degeneration in Alzheimer's disease. Brain Res 1989;501:364–72 [DOI] [PubMed] [Google Scholar]
- 3. Kesler A, Vakhapova V, Korczyn AD, et al. Retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Clin Neurol Neurosurg 2011;113:523–6 [DOI] [PubMed] [Google Scholar]
- 4. Ascaso FJ, Cruz N, Modrego PJ, et al. Retinal alterations in mild cognitive impairment and Alzheimer's disease: An optical coherence tomography study. J Neurol 2014;261:1522–30 [DOI] [PubMed] [Google Scholar]
- 5. Cheung CY, Ong YT, Hilal S, et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 2015;45:45–56 [DOI] [PubMed] [Google Scholar]
- 6. Coppola G, Di Renzo A, Ziccardi L, et al. Optical Coherence tomography in alzheimer's disease: A meta-analysis. PLoS One 2015;10:e0134750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pillai JA, Bermel R, Bonner-Jackson A, et al. Retinal nerve fiber layer thinning in Alzheimer's disease: A case-control study in comparison to normal aging, Parkinson's disease, and non-Alzheimer's dementia. Am J Alzheimers Dis Other Demen 2016;31:430–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reed BT, Behar-Cohen F, Krantic S.. Seeing early signs of Alzheimer's Disease through the lens of the eye. Curr Alzheimer Res 2017;14:6–17 [DOI] [PubMed] [Google Scholar]
- 9. Williams MA, McGowan AJ, Cardwell CR, et al. Retinal microvascular network attenuation in Alzheimer's disease. Alzheimers Dement (Amst) 2015;1:229–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frost S, Guymer R, Aung KZ, et al. Alzheimer’s disease and the early signs of age-related macular degeneration. Curr Alzheimer Res 2016;13:1259–66 [DOI] [PubMed] [Google Scholar]
- 11. Liu B, Rasool S, Yang Z, et al. Amyloid-peptide vaccinations reduce β-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer's transgenic mice. Am J Pathol 2009;175:2099–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ning A, Cui J, To E, et al. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci 2008;49:5136–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perez SE, Lumayag S, Kovacs B, et al. Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer's disease. Invest Ophthalmol Vis Sci 2009;50:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan Z, Ge J.. Amyloid-beta, the retina, and mouse models of Alzheimer disease. Am J Pathol 2010;176:2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao H, Chang R, Che H, et al. Hyperphosphorylation of tau protein by calpain regulation in retina of Alzheimer's disease transgenic mouse. Neurosci Lett 2013;551:12–6 [DOI] [PubMed] [Google Scholar]
- 16. More SS, Vince R.. Hyperspectral imaging signatures detect amyloidopathy in Alzheimer's mouse retina well before onset of cognitive decline. ACS Chem Neurosci 2015;6:306–15 [DOI] [PubMed] [Google Scholar]
- 17. Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer's patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 2011;54 Suppl: 1:S204–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heaton GR, Davis BM, Turner LA, et al. Ocular biomarkers of Alzheimer's disease. Cent Nerv Syst Agents Med Chem 2015;15:117–25 [DOI] [PubMed] [Google Scholar]
- 19. Löffler KU, Edward DP, Tso MO.. Immunoreactivity against tau, amyloid precursor protein, and beta-amyloid in the human retina. Invest Ophthalmol Vis Sci 1995;36:24–31 [PubMed] [Google Scholar]
- 20. Leger F, Fernagut PO, Canron MH, et al. Protein aggregation in the aging retina. J Neuropathol Exp Neurol 2011;70:63–8 [DOI] [PubMed] [Google Scholar]
- 21. Schön C, Hoffmann NA, Ochs SM, et al. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One 2012;7:e53547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ho CY, Troncoso JC, Knox D, et al. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer's and Parkinson's disease patients. Brain Pathol 2014;24:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldstein LE, Muffat JA, Cherny RA, et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer's disease. Lancet 2003;361:1258–65 [DOI] [PubMed] [Google Scholar]
- 24. Moncaster JA, Pineda R, Moir RD, et al. Alzheimer's disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS One 2010;5:e10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michael R, Rosandić J, Montenegro GA, et al. Absence of β-amyloid in cortical cataracts of donors with and without Alzheimer's disease. Exp Eye Res 2013;106:5–13 [DOI] [PubMed] [Google Scholar]
- 26. Tian T, Zhang B, Jia Y, et al. Promise and challenge: the lens model as a biomarker for early diagnosis of Alzheimer's disease. Dis Markers 2014;2014:826503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thal DR, Rüb U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800 [DOI] [PubMed] [Google Scholar]
- 28. Braak H, Braak E.. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 29. Mirra SS, Heyman A, et al. The Consortium to establish a registry for Alzheimer disease (CERAD). Part II Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–86 [DOI] [PubMed] [Google Scholar]
- 30. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hyman BT, Phelps CH, Beach TG, et al. National institute on aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson PT, Alafuzoff Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol 2012;71:362–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bringmann A, Pannicke T, Grosche J, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res 2006;25:397–424 [DOI] [PubMed] [Google Scholar]
- 34. Li L, Luo J, Chen D, et al. BACE1 in the retina: A sensitive biomarker for monitoring early pathological changes in Alzheimer's disease. Neural Regen Res 2016;11:447–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frost S, Kanagasingam Y, Sohrabi H, et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer's disease. Transl Psychiatry 2013;2:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ho WL, Leung Y, Tsang AW, et al. Review: Tauopathy in the retina and optic nerve: Does it shadow pathological changes in the brain? Mol Vis 2012;18:2700–10 [PMC free article] [PubMed] [Google Scholar]