Immunocompromised mice were infected with an influenza virus and treated with viral neuraminidase or polymerase inhibitors, or combinations thereof. Combination therapy increased survival times but did not prevent the emergence of variants resistant to neuraminidase inhibitors.
Keywords: influenza, combination therapy, neuraminidase inhibitor, polymerase inhibitor, drug resistance
Abstract
Background
Treatment of immunocompromised, influenza virus–infected patients with the viral neuraminidase inhibitor oseltamivir often leads to the emergence of drug-resistant variants. Combination therapy with compounds that target different steps in the viral life cycle may improve treatment outcomes and reduce the emergence of drug-resistant variants.
Methods
Here, we infected immunocompromised nude mice with an influenza A virus and treated them with neuraminidase (oseltamivir, laninamivir) or viral polymerase (favipiravir) inhibitors, or combinations thereof.
Results
Combination therapy for 28 days increased survival times compared with monotherapy, but the animals died after treatment was terminated. Mono- and combination therapies did not consistently reduce lung virus titers. Prolonged viral replication led to the emergence of neuraminidase inhibitor–resistant variants, although viruses remained sensitive to favipiravir. Overall, favipiravir provided greater benefit than neuraminidase inhibitors.
Conclusions
Collectively, our data demonstrate that combination therapy in immunocompromised hosts increases survival times, but does not suppress the emergence of neuraminidase inhibitor–resistant variants.
Treatment of influenza relies on antiviral compounds that target the viral neuraminidase (NA) or polymerase proteins. Currently, 4 compounds are marketed that block the enzymatic activity of NA (reviewed in [1, 2]). Oseltamivir phosphate (OS; administered orally twice daily for 5 days [3]) is the most frequently prescribed NA inhibitor. Zanamivir has to be inhaled twice daily, usually for 5 days (http://www.fda.gov/drugs/drugsafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm183783.htm). Peramivir is administered as a single intravenous dose [4]. Laninamivir octanoate (LAO) is a long-acting NA inhibitor that remains effective in animals and humans for several days [5–7]; accordingly, a single inhaled dose of this compound is sufficient to treat influenza virus infections. All 4 inhibitors structurally resemble the natural substrate of NA and act as inhibitors by occupying the catalytic site in NA. They differ in their side chains, which affects their bioavailability and the rate of emergence of NA proteins resistant to their antiviral effects [8, 9].
Oseltamivir can reduce the duration of symptoms [10, 11] and the severity of disease [12, 13]. However, treatment of immunocompromised individuals with oseltamivir often leads to the emergence of drug-resistant viruses [14–20], presumably because delayed virus clearance increases the opportunity for resistant variants to emerge. In immunocompromised hosts, infections with such oseltamivir-resistant influenza viruses can be fatal [14–16, 20–23]. Most oseltamivir-resistant viruses are attenuated but can become dominant in nature (reviewed in [24, 25]).
An inhibitor of the influenza viral polymerase complex (favipiravir [FA], formerly known as T-705) [26, 27]) is approved in Japan for restricted use against NA inhibitor–resistant pandemic influenza viruses [28], and has been tested in a phase 3 clinical trial in the United States (https://clinicaltrials.gov/ct2/show/NCT01728753). To date, FA-resistant influenza viruses have not been reported.
Combination therapy (ie, simultaneous treatment with ≥2 drugs) may increase antiviral efficacy and reduce the frequency of drug resistance compared with monotherapy. In particular, combinations of compounds that interfere with different steps in the viral life cycle may suppress viral replication and the emergence of drug-resistant variants more efficiently than monotherapies. Several studies have found beneficial effects of combination therapy compared with monotherapy [29, 30]. For example, a recent study demonstrated a beneficial effect of oseltamivir/favipiravir combination therapy vs both monotherapies in influenza virus–infected mice. However, this study did not assess the potential benefits of combination therapy in immunocompromised hosts.
Nude mice lack a thymus and cannot generate mature T cells and thus have been used as a model for immunodeficiency [31]. In nude mice infected with a human influenza virus, virus clearance was delayed and the survival rate was reduced compared with genetically matched control mice [32]. Here, we used nude mice to assess the therapeutic value of combination therapy compared with monotherapy in an immunocompromised host infected with influenza virus.
METHODS
Mouse Infections
Six-week-old female BALB/c (BALB/c CrSlc) and nude (BALB/c-nu/nu) mice were purchased from Japan SLC Inc (Shizuoka, Japan). To confirm prolonged virus replication in immunocompromised mice compared with immunocompetent animals, we assessed the virus titers in BALB/c and nude mice as follows: Animals were anesthetized with isoflurane and intranasally infected with 103 plaque-forming units (PFU) of mouse-adapted A/California/04/2009 (H1N1; MA-CA04). Changes in body weight and survival were monitored for 14 days. Three randomly selected mice per group were euthanized on days 3, 6, 9, and 11 postinfection and virus titers in the lungs were determined by using plaque assays in Madin-Darby canine kidney (MDCK) cells.
To compare the survival rates of influenza virus–infected mice treated with mono- or combination therapy, nude mice were anesthetized with isoflurane and intranasally infected with 104 PFU of MA-CA04 virus. One hour postinfection, groups of 5 mice were treated with OS (25 mg/kg), LAO (1.5 mg/kg), and FA (20 mg/kg or 30 mg/kg) alone or in combination. The compounds were administered orally (OS and FA) or intranasally (LAO) once daily (OS and FA) or once a week (LAO) for 5 or 28 days. Survival and clinical signs were monitored daily for 2 months. In a separate study, animals were infected and treated as described above. On days 3, 7, 14, 21, and 28 postinfection, 3 randomly selected mice per group were euthanized and virus titers in the lungs were determined as described above.
Additional details are described in the Supplementary Data.
RESULTS
Influenza Virus Infection in BALB/c Nude Mice Results in Delayed Virus Clearance
To establish that influenza virus clearance is delayed in BALB/c-nu/nu (nude) mice compared with wild-type BALB/c mice, we intranasally infected animals with 103 PFU of a mouse-adapted variant of A/California/04/2009 (H1N1) virus (MA-CA04) that causes detectable virulence in BALB/c mice [33]. MA-CA04 replicated efficiently in the lungs of infected BALB/c mice on days 3 and 6 postinfection, but the virus infection was cleared by days 9–11 postinfection (Table 1). In contrast, virus titers remained high in the lungs of nude mice on day 11 postinfection (Table 1), demonstrating delayed virus clearance in these animals.
Table 1.
Virus Titers in the Lungs of Wild-type and Nude BALB/c Mice Infected With Mouse-Adapted A/California/04/2009 Virusa
| Virus Titers, Mean Log10 PFU/g ± SD | |||||||
|---|---|---|---|---|---|---|---|
| BALB/c | BALB/c-nu/nu | ||||||
| Day 3 (n = 3) |
Day 6 (n = 3) |
Day 9 (n = 3) |
Day 11 (n = 3) |
Day 3 (n = 3) |
Day 6 (n = 3) |
Day 9 (n = 3) |
Day 11 (n = 3) |
| 7.6 ± 0.5 | 6.5 ± 0.2 | 1.7, <1.7, <1.7 | <1.7, <1.7, <1.7 | 8.0 ± 0.2 | 7.6 ± 0.3 | 7.5 ± 0.4 | 7.2 ± 0.2 |
The detection limit was 1.7 log10 PFU/g.
Abbreviations: PFU, plaque-forming units; SD, standard deviation.
aWild-type BALB/c and BALB/c-nu/nu mice were intranasally infected with 103 PFU of mouse-adapted A/California/04/2009 virus. Three mice from each group were euthanized on days 3, 6, 9, and 11 postinfection for virus titration in the lungs of infected animals. When virus was not detected from all 3 mice, individual titers were recorded.
Survival Times of Influenza Virus–Infected Nude Mice Treated With Antiviral Compounds
To compare the efficacy of mono- and combination therapy for influenza virus infection in immunocompromised hosts, we intranasally infected nude mice with 104 PFU of MA-CA04 virus. The higher dose compared to the pilot study was chosen to cause lethal infection. One hour after virus infection, groups of 5 mice each were treated with the viral NA inhibitors OS (the most frequently prescribed NA inhibitor) or LAO (which needs to be administered only once during a standard 5-day treatment), and/or with the viral polymerase inhibitor FA (Table 2). For FA, 2 different doses (20 mg/kg [FA20] and 30 mg/kg [FA30]) were tested. Compounds were administered daily (OS and FA) or once a week (LAO), all animals were treated for 5 days (the recommended treatment course in humans) or 28 days (Table 2); both arms of the study were carried out in the same experiment. Survival and clinical signs were monitored daily for up to 2 months. In a second experiment (described in detail in the following section), mice were infected and treated as described above and euthanized on days 3, 7, 14, 21, or 28 to determine lung virus titers. Mice that succumbed to virus infection were included in the calculation of survival times, whereas euthanized animals (ie, those used for virus titration) were censored (see Supplementary Data for details of our analysis).
Table 2.
Summary of Treatment Groups
| Group No. | Compound(s) | Concentration(s) | Treatment Regimen | Total No. |
|---|---|---|---|---|
| 5-day treatment | ||||
| 1 | Control | NA | PBS and methylcellulosea | 20b |
| 2 | OSc | 25 mg/kg | Once daily | 20 |
| 3 | LAOd | 1.5 mg/kg | Once | 20 |
| 4 | FA20e | 20 mg/kg | Once daily | 20 |
| 5 | FA30f | 30 mg/kg | Once daily | 20 |
| 6 | OS + FA20 | 25 mg/kg + 20 mg/kg | Once daily | 20 |
| 7 | LAO + FA20 | 1.5 mg/kg + 20 mg/kg | Once (LAO)/once daily (FA20) | 20 |
| 8 | OS + FA30 | 25 mg/kg + 30 mg/kg | Once daily | 20 |
| 9 | LAO + FA30 | 1.5 mg/kg + 30 mg/kg | Once (LAO)/once daily (FA30) | 22g |
| 28-day treatment | ||||
| 10 | Control | NA | PBS and methylcellulosea | 17h |
| 11 | OS | 25 mg/kg | Once daily | 17 |
| 12 | LAO | 1.5 mg/kg | Once per week | 17 |
| 13 | FA20 | 20 mg/kg | Once daily | 17 |
| 14 | FA30 | 30 mg/kg | Once daily | 17 |
| 15 | OS + FA20 | 25 mg/kg + 20 mg/kg | Once daily | 17 |
| 16 | LAO + FA20 | 1.5 mg/kg + 20 mg/kg | Once per week (LAO)/once daily (FA20) | 17 |
| 17 | OS + FA30 | 25 mg/kg + 30 mg/kg | Once daily | 17 |
| 18 | LAO + FA30 | 1.5 mg/kg + 30 mg/kg | Once per week (LAO)/once daily (FA30) | 17 |
Abbreviations: FA20, low-dose favipiravir (20 mg/kg); FA30, high-dose favipiravir (30 mg/kg); LAO, laninamivir octanoate; NA, not applicable; OS, oseltamivir phosphate; PBS, phosphate-buffered saline.
aPBS (intranasal control) was administered once per week; methylcellulose (oral control) was administered daily.
bFive mice for the survival study and 15 mice to assess lung virus titers (5 time points; 3 mice per time point).
cAdministered orally.
dAdministered intranasally.
eAdministered orally.
fAdministered orally.
gTwo extra mice were available, which were added to group 9.
hFive mice for the survival study and 12 mice to assess lung virus titers (4 time points; 3 mice per time point).
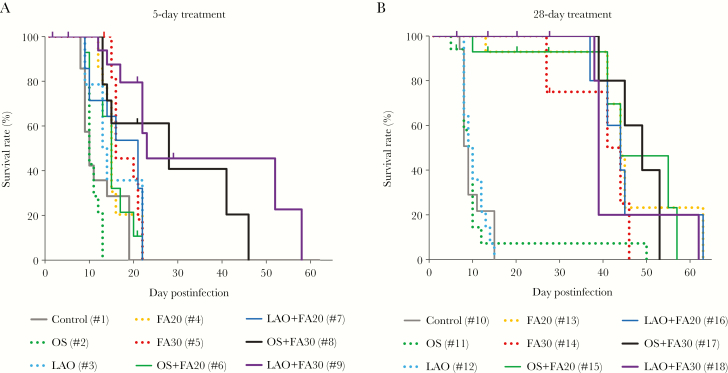
Monotherapy for 5 days did not increase survival times compared with untreated mice (Figures 1 and 2A; Supplementary Table 1A). In contrast, 5-day treatment with the higher dose of FA in combination with an NA inhibitor significantly increased survival times compared with the untreated control group. Thus, combination therapy may prolong survival compared with monotherapies (Figure 2A; Supplementary Table 1A). However, all mice died once treatment was terminated.
Figure 1.
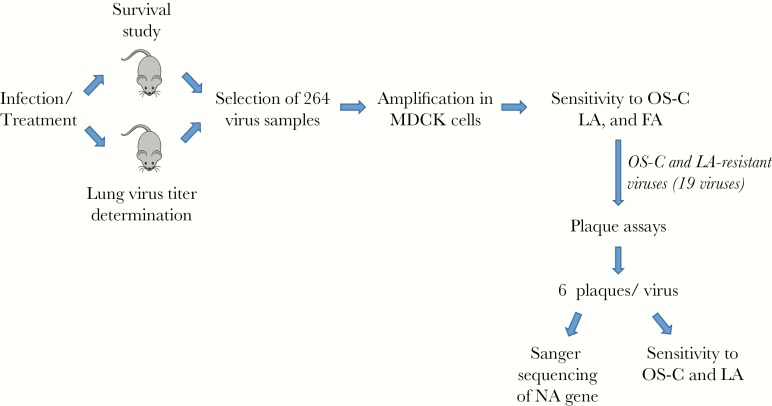
Schematic overview of the drug combination study. Nude mice were infected and treated as described in the text and summarized in Table 2. Survival was assessed (Figure 2; Supplementary Table 1) and lung virus titers were measured (Table 3; Supplementary Table 2). We then selected 264 virus samples (Supplementary Table 3) for further analysis, amplified them in Madin-Darby canine kidney (MDCK) cells, and determined their sensitivity to oseltamivir carboxylate (OS-C), laninamivir (LA), and favipiravir (FA) (Supplementary Table 4). For 19 viruses that were resistant to OS-C and LA (Table 4), we performed plaque assays, isolated 6 viral plaques each, determined the neuraminidase (NA) sequences by Sanger sequencing, and tested their sensitivity to OS-C, LA, and FA (Supplementary Table 5).
Figure 2.
Survival times of nude mice infected with MA-CA04 virus and treated with mono- or combination therapy. Groups of 17–22 mice were infected and treated as described in the text and summarized in Table 2. A, Five-day treatment with mono- or combination therapy. B, Twenty-eight–day treatment with mono- or combination therapy. Statistical analyses are shown in Supplementary Table 1. Abbreviations: FA, favipiravir; LAO, laninamivir octanoate; OS, oseltamivir phosphate.
Influenza virus clearance is delayed in immunocompromised individuals, requiring prolonged treatment with antiviral compounds. Treatment with NA inhibitors for 28 days did not significantly improve survival times compared with untreated nude mice (Figure 2B; Supplementary Table 1B). However, both doses of FA with or without an NA inhibitor increased survival times significantly compared with untreated mice. Likewise, combination treatments of FA and a neuraminidase inhibitor resulted in prolonged survival compared with OS or LAO monotherapy. Collectively, these data demonstrate that treatment with FA may be more beneficial than treatment with OS or LAO, and that combination therapies with NA and polymerase inhibitors may be more effective in preventing death than currently established monotherapy with NA inhibitors.
Prolonged treatment with OS alone did not significantly increase survival times compared with 5-day therapy (Figure 2A and 2B; Supplementary Table 1C). In contrast, 28-day monotherapy with LAO or FA at either dose significantly increased survival times compared with 5-day treatment. In addition, 28-day combination treatments resulted in prolonged survival compared with 5-day treatments, with the exception of the LAO + FA30 group (Supplementary Table 1C).
Together, these data indicate that (1) FA is more effective than OS or LAO in delaying death in influenza virus-infected nude mice; (2) some combination therapies are more beneficial than monotherapies; and (3) a 28-day FA or LAO treatment regimen prolongs survival relative to a 5-day regimen. Nonetheless, even combination therapy for 28 days did not prevent death after treatment was withdrawn.
Lung Virus Titers and Lung Pathology of Influenza Virus–Infected Nude Mice Treated With Antiviral Compounds
All infected mice succumbed to influenza virus infection after the termination of antiviral therapy, demonstrating that even prolonged combination therapy did not clear the infection. We, therefore, assessed the lung virus titers of infected animals after mono- or combination therapy for 5 or 28 days (Figure 1, Table 3; Supplementary Table 2). Animals were infected and treated as described previously. On days 3, 7, 14, 21, and 28 postinfection, 3 mice per group were euthanized and virus titers in the lungs were determined.
Table 3.
Lung Virus Titers of MA-CA04–Infected Nude Mice Treated With the Indicated Neuraminidase and/or Polymerase Inhibitorsa
| Group | Treatmentb | Virus Titer, Mean Log10 PFU ± SD/g | ||||
|---|---|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| 5-day treatment | ||||||
| 1 | Control | 7.7 ± 0.3 | 7.1 ± 0.2 | 6.7 ± 0.3 | NAc | NAc |
| 2 | OS | 8.0 ± 1.0 | 6.9 ± 0.4 | 6.4 ± 1.0 | NAc | NAc |
| 3 | LAO | 7.9 ± 0.1 | 7.7 ± 0.5 | 6.8 ± 0.3 | NAc | NAc |
| 4 | FA20 | 6.6 ± 1.4 | 6.9 ± 0.3 | 7.3 ± 0.3 | 4.8, 7.1, NAc | NAc |
| 5 | FA30 | 5.6 ± 0.4d | 7.0 ± 0.2 | 7.1 ± 0.5 | 6.8 ± 0.1 | NAc |
| 6 | OS + FA20 | 5.0 ± 0.4d,e | 5.3 ± 1.5d | 6.6 ± 0.3 | 6.7, NAc | NAc |
| 7 | LAO + FA20 | 6.9 ± 0.4e | 4.2 ± 0.4d,e | 7.2 ± 0.3 | 6.8 ± 0.8 | NAc |
| 8 | OS + FA30 | 7.0 ± 0.3f | 4.5 ± 0.2d,e | 6.6 ± 0.3 | 6.6 ± 0.4 | 7.4, NA, NAc |
| 9 | LAO + FA30 | 7.1 ± 0.3f | 6.2 ± 0.3e | 7.0 ± 0.1 | 7.0 ± 0.4 | 7.3, 7.2, NAc |
| 28-day treatment | ||||||
| 10 | Control | 7.7 ± 0.3 | 7.4 ± 0.2 | 6.6, 7.2, NAc | NAc | NAc |
| 11 | OS | 8.0 ± 1.0 | 6.7 ± 0.7 | NAc | NAc | NAc |
| 12 | LAO | 7.9 ± 0.1 | 7.6 ± 0.5 | 6.1, NA, NAc | NAc | NAc |
| 13 | FA20 | 6.6 ± 1.4 | 5.2 ± 1.0d | 6.2 ± 0.2 | 6.2 ± 0.2 | 5.9 ± 0.8 |
| 14 | FA30 | 5.6 ± 0.4d | 6.1 ± 0.6 | 6.3 ± 0.1 | 5.8 ± 0.5g | 6.3 ± 0.2 |
| 15 | OS + FA20 | 5.0 ± 0.4d,e | 5.4 ± 0.7 | 6.2 ± 0.4 | 6.6 ± 0.6 | 6.1 ± 0.9 |
| 16 | LAO + FA20 | 6.9 ± 0.4 | 6.3 ± 0.3e,h | 3.4 ± 0.4d,e,g | 6.0 ± 0.4 | 6.1 ± 0.8 |
| 17 | OS + FA30 | 7.0 ± 0.3f | 6.2 ± 0.2h | 4.7 ± 1.7d,e,g | 5.8 ± 0.3 | 6.3 ± 0.1 |
| 18 | LAO + FA30 | 7.1 ± 0.3f | 6.3 ± 0.1e | 5.7 ± 0.5g | 6.6 ± 0.9 | 6.2 ± 0.2g |
Abbreviations: FA20, low-dose favipiravir (20 mg/kg); FA30, high-dose favipiravir (30 mg/kg); LAO, laninamivir octanoate; OS, oseltamivir phosphate; NA, not applicable; PFU, plaque-forming units; SD, standard deviation.
aMice were intranasally infected with 104 PFU of virus.
Three mice from each group were euthanized on days 3, 7, 14, 21, and 28 postinfection.
bTreatment details are shown in Table 2.
cAnimal(s) succumbed to infection before the day of sampling.
dVirus titers were significantly lower than in untreated animals;
eVirus titers in animals treated with combination therapy were significantly lower than in those treated with monotherapy.
fVirus titers after combination therapy were higher than those after FA30 monotherapy.
gVirus titers in the 28-day treatment group were significantly lower than those in the respective 5-day treatment group.
hVirus titers after the 28-day treatment were higher than after the 5-day treatment.
At several time points, animals treated with mono- or combination therapy for 5 or 28 days had significantly lower lung virus titers than untreated animals, but most reductions were moderate and not consistent, that is, the titers were reduced at 1 or 2 time points, but rebounded at later time points (Table 3; Supplementary Table 2A and 2B). None of the drug treatments suppressed virus replication completely.
The comparison of mono- and combination therapy revealed reduced lung virus titers for several combination therapies (Table 3; Supplementary Table 2 C and 2D); however, most differences were not significant. Two combination therapies (OS + FA30 and LAO + FA30) resulted in significantly higher lung virus titers on day 3 compared with FA30 monotherapy.
A 28-day treatment regimen was more beneficial in reducing virus titers than a 5-day treatment regimen for several groups including FA20 (day 7), FA30 (days 7, 14, and 21), LAO + FA20 (day 14), OS + FA30 (day 14), and LAO + FA30 (days 14 and 28) (Table 3; Supplementary Table 2E), demonstrating that prolonged FA treatment with or without an NA inhibitor was efficacious. In contrast, lung virus titers of mice in 2 groups (LAO + FA20 on day 7, and OS + FA30 on day 7) were relatively low when the drugs were administered for 5 days, but significantly higher when the drugs were administered for 28 days (Table 3).
Direct comparison of monotherapies revealed that FA reduced lung virus titers compared with neuraminidase inhibitors on some, but not all days (Supplementary Table 2F and 2G).
Collectively, in most groups, mono- or combination therapy did not significantly reduce lung virus titers, consistent with our finding that all mice succumbed to their infection after treatment was withdrawn. However, as observed in our survival study (see Figure 2), treated animals survived longer than untreated controls.
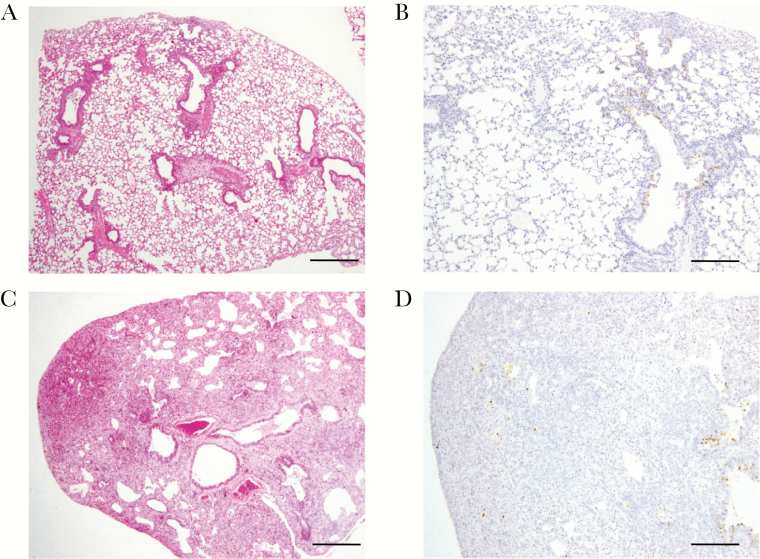
Pathological evaluation on day 28 postinfection showed that prolonged combination treatment with NA and polymerase inhibitors reduced inflammation in the lungs of these animals compared with those treated for 5 days (Figure 3). In mice treated with LAO + FA30 for 28 days, there was limited inflammation around the bronchi near the central airway on day 28 postinfection (Figure 3A and 3B). By contrast, mice treated with LAO + FA30 for 5 days showed widespread inflammation on day 28 postinfection (Figure 3C and 3D). Consistent with the survival data, these results demonstrate the benefit of combination therapy vs monotherapy.
Figure 3. .
Histopathological findings in mice infected with mouse-adapted CA04 virus and treated with laninamivir octanoate (LAO) + high-dose favipiravir (FA30) for 5 or 28 days. Lung histopathology in mice infected with mouse-adapted CA04 and treated with LAO and FA30 for 28 days (A and B) or for 5 days (C and D). Shown are hematoxylin and eosin staining (A and C) and immunostaining against influenza virus nucleoprotein (NP) antigen (B and D) on day 28 postinfection. Scale bars: 500 µm (A and C); 200 µm (B and D).
Emergence of Drug Resistance
Treatment of influenza virus–infected, immunocompromised patients with NA inhibitors frequently results in the emergence of drug-resistant variants [14, 17–21, 23, 34]. To assess the emergence of drug resistance in animals treated with NA and/or polymerase inhibitors, we selected 264 lung samples from mice of all groups (Figure 1), with an emphasis on late time points (Supplementary Table 3). All samples except number 84 (which we were unable to amplify) were tested for resistance to oseltamivir carboxylate (OS-C, the active form of OS), laninamivir (LA; the active form of LAO), and FA (Supplementary Table 4).
To date, resistance to FA has not been reported. Hence, we lacked a positive control to establish an 50% inhibitory concentration (IC50) value that would indicate resistance to FA. We detected very minor increases in IC50 values in several samples obtained from FA-treated mice but do not know the significance of this finding.
In accordance with the World Health Organization guidelines, NAs that displayed reduced inhibition (10- to 100-fold and 5- to 50-fold increases in susceptibility to NA inhibitors for influenza A and B viruses, respectively) are shaded in light gray in Supplementary Table 4, whereas NAs that displayed highly reduced inhibition (>100-fold and >50-fold increases in susceptibility to NA inhibitors for influenza A and B viruses, respectively) are shaded in dark gray.
The 5-day treatment regimen resulted in only 1 sample (number 61, group 3) with reduced inhibition in the presence of OS-C (obtained from an LAO-treated mouse). Treatment for 28 days with NA inhibitors resulted in more frequent emergence of drug resistance, in particular at late time points of sampling (Supplementary Table 4). Drug resistance typically emerged in mice treated with combination therapy, most likely because the combination treatment prolonged survival. Treatment of influenza virus–infected mice with OS resulted in viruses with increased IC50 values to this inhibitor, but not to LA; by contrast, LAO treatment of influenza virus–infected mice resulted in viruses with increased IC50 values to this inhibitor and/or OS-C. We detected 19 samples with increased IC50 values to both OS-C and LA (Table 4; and shown in boldface type in Supplementary Table 4), all of which were isolated from animals treated with LAO + FA. Thus, prolonged treatment of immunocompromised mice with combination therapy does not suppress viral replication sufficiently to prevent the emergence of mutants with resistance to NA inhibitors.
Table 4.
Viral Samples With Reduced Sensitivity to Oseltamivir Carboxylate and Laninamivir, Sorted by Day of Sampling
| Sample No. | Group No.a | Treatmenta | Day of Sampling | IC50, nM | |
|---|---|---|---|---|---|
| OS-C | LA | ||||
| 111 | 16 | LAO + FA20 | 14 | 83 | 12 |
| 181 | 16 | LAO + FA20 | 21 | 178 | 27 |
| 182 | 16 | LAO + FA20 | 21 | 94 | 13 |
| 187 | 18 | LAO + FA30 | 21 | 85 | 32 |
| 188 | 18 | LAO + FA30 | 21 | 141 | 37 |
| 189 | 18 | LAO + FA30 | 21 | 189 | 25 |
| 220 | 16 | LAO + FA20 | 28 | 200 | 24 |
| 221 | 16 | LAO + FA20 | 28 | 222 | 14 |
| 222 | 16 | LAO + FA20 | 28 | 216 | 27 |
| 226 | 18 | LAO + FA30 | 28 | 82 | 45 |
| 227 | 18 | LAO + FA30 | 28 | 215 | 21 |
| 228 | 18 | LAO + FA30 | 28 | 107 | 35 |
| 234 | 16 | LAO + FA20 | 37 | 60 | 44 |
| 235 | 18 | LAO + FA30 | 38 | 250 | 29 |
| 238 | 18 | LAO + FA30 | 39 | 109 | 21 |
| 243 | 16 | LAO + FA20 | 41 | 241 | 31 |
| 248 | 16 | LAO + FA20 | 44 | 166 | 21 |
| 250 | 16 | LAO + FA20 | 45 | 37 | 13 |
| 264 | 16 | LAO + FA20 | 63 | 86 | 22 |
Abbreviations: FA20, low-dose favipiravir (20 mg/kg); FA30, high-dose favipiravir (30 mg/kg); IC50, 50% inhibitory concentration; LA, laninamivir; LAO, laninamivir octanoate; OS-C, oseltamivir carboxylate.
aDetails of treatment groups and treatments are shown in Table 2.
Assessment of Mutations That Confer Drug Resistance to Neuraminidase Inhibitors
For virus samples with resistance to both NA inhibitors, we performed plaque assays in MDCK cells, picked 6 viral plaques each, amplified them in MDCK cells, and tested them for resistance to OS-C and LA (Figure 1; Supplementary Table 5). We also sequenced the NA segments of these viruses (Supplementary Table 5). For several lung virus samples, all 6 viral plaques encoded the same mutation in NA. However, most lung virus samples contained virus populations that encoded combinations of wild-type and/or mutant NA proteins.
The most frequently detected mutation was R152K (Supplementary Table 5), which confers oseltamivir resistance to influenza B viruses [35, 36] but is typically not associated with influenza A virus resistance to NA inhibitors. Here, we detected it after prolonged combination therapy with LAO + FA, resulting in moderate-to-high resistance to OS-C and LA. Several viruses encoded an E119G mutation, which resulted in moderate-to-high resistance to LA, but not to OS-C. Mutations at this position have previously been shown to confer resistance to NA inhibitors [17, 21, 37]. In our study, the E119G mutation was also detected together with a P120S mutation, resulting in high resistance to LA; this combination of mutations has not been reported previously. Moreover, we isolated a variant with E119D/N378D mutations in NA that conferred high resistance to OS-C and LA with IC50 values of 822 nM and 428 nM, respectively. Clonal analysis of viral samples revealed coexistence of the R152K and E119G or E119D mutations in viral populations; however, we did not detect viral clones with mutations at both positions.
DISCUSSION
Here, we tested combinations of influenza virus NA and polymerase inhibitors in immunocompromised mice and detected prolonged survival after combination therapy compared with monotherapy; however, lung virus titers were not reduced consistently and all animals succumbed to their infection after the antiviral treatment was terminated. The combination of NA and polymerase inhibitors was chosen because these compounds target different viral proteins and differ in their mode of action.
Oseltamivir treatment of influenza virus–infected, immunocompromised patients often leads to the emergence of variants resistant to this compound. In an effort to avoid this, oseltamivir treatment may be combined with additional NA inhibitors and/or other antiviral compounds such as adamantanes or ribavirin (reviewed in [29]). In clinical settings, antiviral compounds are typically administered sequentially (rather than in combination) and proper controls are not available. In a small clinical study of immunocompromised patients (n = 7), combination therapy with oseltamivir, amantadine, and ribavirin reduced virus titers compared with those of a patient who received monotherapy; drug-resistant variants were detected in the patient treated with oseltamivir monotherapy, but not in the patients who received combination therapy [38].
In a recent study in immunocompetent mice, Marathe et al [39] tested oseltamivir and favipiravir mono- and combination therapies as treatment for infection with a highly pathogenic influenza virus of the H5N1 subtype; drug treatment was initiated 2–5 days after infection and continued for 5 days. Compared with untreated controls, mice treated with OS, FA, or OS/FA showed improved virus clearance, reduced lung virus titers, and/or increased survival rates. Monotherapy with FA was more beneficial than OS monotherapy. Combination therapy completely protected the mice from lethal H5N1 virus infection, whereas the monotherapies did not. In our study in immunocompromised mice, relatively high virus titers were detected in mouse lungs throughout the treatment period, but combination therapy increased the survival times in several treatment groups. Consistent with the study by Marathe et al [39], we also found that treatment with FA was more beneficial than treatment with OS. Collectively, these studies in immunocompetent [39] and immunocompromised (our data) mice demonstrate that treatment with a polymerase inhibitor may be more beneficial than treatment with OS, and that OS/FA combination therapy may improve the outcome of influenza virus infections compared with monotherapy.
Drug combination therapy may suppress the emergence of drug-resistant variants, but comprehensive studies are lacking to prove or disprove this concept. Marathe et al [39] did not detect NA mutations that conferred resistance to OS, perhaps because the samples were collected within 8 days of infection of the mice. In contrast, we isolated a number of viral samples with increased IC50 values to OS-C and/or LA, particularly at the late stage of infection. Thus, prolonged virus replication at relatively high titers facilitated the emergence of mutants resistant to NA inhibitors. The emergence of drug-resistant variants did not correlate with virus titers or the time of death.
We isolated a number of viruses with resistance to both OS-C and LA that typically encoded the R152K mutation, which is associated with OS-resistant influenza B viruses [35, 36, 40–42]. However, the R152K mutation has been detected in an A/Puerto Rico/8/34 (H1N1) virus isolated from mice treated with OS [43]; this mutation increased the IC50 values to OS-C and LA by 17- and 4.5-fold, respectively, compared with the wild-type virus [43]. Here, we frequently detected this mutation after treatment with LAO, resulting in low-to-moderate resistance to LA and OS-C (Supplementary Table 5).
Several studies have demonstrated that mutations at position 119 of N1 NAs confer resistance to NA inhibitors. Samson et al [37] isolated human H1N1 viruses with the NA-E119A or NA-E119K/G147E mutations after virus passages in cultured cells in the presence of LA. Tamura et al [17] reported the emergence of the H275Y and E119G/D mutations in an immunocompromised child treated with OS and zanamivir. Treatment of an influenza virus–infected transplant recipient with OS, followed by zanamvir, resulted in the emergence of a human H1N1 virus with the H275Y mutation (acquired after OS treatment) and the E119D mutation (acquired after treatment with zanamivir) [21]. We detected the E119D mutation in combination with the N378D mutation; this double-mutant showed resistance to both OS-C and LA (Supplementary Table 5). Similarly, L’Huillier et al [21] reported that the E119D mutation conferred moderate-to-high resistance to OS, LA, zanamivir, and peramivir.
We detected the E119G mutation individually and in combination with the P120S or N378D mutation (Supplementary Table 5). The E119G mutation conferred resistance to LA, but not to OS-C, consistent with other studies that showed that NA-E119G conferred resistance to LA, zanamivir, and peramivir, but not to OS-C [37, 44, 45]. Two viruses possessing both the NA-E119G and P120S mutations displayed very high IC50 values of 275 nM and 520 nM, respectively, to LA, suggesting that the P120S mutation may increase the inhibitory effect of the E119G mutation. The amino acid at position 120 of NA has not been linked to increased resistance to NA inhibitors; however, its location next to residue 119 suggests a direct or indirect effect on the interaction with NA inhibitors.
Collectively, these findings demonstrate that the E119D mutation in NA confers broad resistance to NA inhibitors, whereas the E119G mutation confers resistance to LA, zanamivir, and peramivir, but not to OS-C. A human H1N1 virus with the E119G mutation was attenuated in mice and ferrets [46], suggesting that such a variant may not become dominant in nature.
We did not detect viruses with substantially increased resistance to FA, consistent with other studies [39, 47]. The action of this compound, which mimics purine nucleotides [48, 49], may make the emergence of resistant variants highly unlikely, although a chikungunya virus with resistance to FA has been reported [50].
In summary, combination therapy with NA and polymerase inhibitors increased the survival times of influenza virus–infected immunocompromised mice. However, the treatments tested here did not result in virus clearance; rather, virus replication continued at relatively high levels throughout the treatment period, providing an opportunity for the emergence of NA inhibitor–resistant variants. Novel antiviral approaches are needed to eradicate influenza virus infections from immunocompromised hosts.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. K., M. Y., and Y. K. conceived the study and designed the experiments. M. K., T. J. S. L., S. Y., M. I., N. N., and H. H. performed the experiments and statistical analyses. M. K., G. N., and Y. K. analyzed the data and wrote the manuscript.
Acknowledgments. We thank Yosuke Furuta for helpful advice, and Susan Watson for editing the manuscript.
Financial support. This work was supported by Strategic Basic Research Programs from the Japan Science and Technology Agency; Leading Advanced Projects for Medical Innovation from the Japan Agency for Medical Research and Development (AMED); Grants-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan (grant numbers 16H06429, 16K21723, and 16H06434); the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from the MEXT and AMED; by JSPS KAKENHI (grant number 25460562); and the Center for Research on Influenza Pathogenesis funded by the National Institute for Allergy and Infectious Diseases (contract number HHSN272201400008C).
Potential conflicts of interest. Y. K. is a co-founder of FluGen; has received grant support from Chugai Pharmaceuticals, Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc, Denka Seiken Co. Ltd, and Ttsumura and Co; and has received royalties from MedImmune. Y. K. holds the following patents: Recombinant influenza viruses for vaccines and gene therapy (US Patent No.: US 8715940 B2); Viruses comprising mutant ion channel protein (US Patent No. 6872395); Mutant cells with altered sialic acid (US Patent No. 7176021); Filovirus vectors and noninfectious filovirus-based particles (US Patent No. 7211378); Signal for packaging of influenza virus vectors (US Patent No. 7585657 B2); Viruses encoding mutant membrane protein (US Patent No. 7588769 B2); Recombinant influenza vectors with a pol-II promoter and ribozymes for vaccines and gene therapy (US Patent No. 7723094); Recombinant influenza vectors with tandem transcription units (US Patent No. 7968101 B2); Adenoviral vectors for influenza virus production (US Patent No. 8043856); Viruses comprising mutant ion channel protein (US Patent No. 8057806); Cell-based systems for producing influenza vaccines (US Patent No. 8163523); Influenza B viruses with reduced sensitivity to neuraminidase inhibitor (US Patent No. 8465960); High titer recombinant influenza viruses for vaccine and gene therapy (US Patent No. 8475806 B2); Influenza A virus with attenuating mutations in NS2 protein (US Patent No. 8507247); and Neuraminidase-deficient live influenza vaccines (US Patent No. 8597661). G. N. is a co-founder of FluGen and has received royalties from MedImmune. She holds the following patents: Recombinant influenza viruses for vaccines and gene therapy (US Patent Number: 8715940 B2); Filovirus vectors and noninfectious filovirus-based particles (US Patent No. 7211378); and Recombinant influenza vectors with tandem transcription units (US Patent No. 7968101B2). M. Y. holds the following patents: Laninamivir octanoate for influenza therapy (JP Patent No. 3209946); Laninamivir and laninamivir octanoate for H5N1 influenza therapy (JP Patent No. 5286251 and 6,012,076); and Laninamivir octanoate and other related compounds for influenza prophylaxis (JP Patent No. 4205314 and No. 4827197). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Li TC, Chan MC, Lee N. Clinical implications of antiviral resistance in influenza. Viruses 2015; 7:4929–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McKimm-Breschkin JL, Fry AM. Meeting report: 4th ISIRV antiviral group conference: novel antiviral therapies for influenza and other respiratory viruses. Antiviral Res 2016; 129:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schirmer P, Holodniy M. Oseltamivir for treatment and prophylaxis of influenza infection. Expert Opin Drug Saf 2009; 8:357–71. [DOI] [PubMed] [Google Scholar]
- 4. Alame MM, Massaad E, Zaraket H. Peramivir: a novel intravenous neuraminidase inhibitor for treatment of acute influenza infections. Front Microbiol 2016; 7:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiso M, Kubo S, Ozawa M et al. Efficacy of the new neuraminidase inhibitor CS-8958 against H5N1 influenza viruses. PLoS Pathog 2010; 6:e1000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamashita M. Laninamivir and its prodrug, CS-8958: long-acting neuraminidase inhibitors for the treatment of influenza. Antivir Chem Chemother 2010; 21:71–84. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–75. [DOI] [PubMed] [Google Scholar]
- 8. Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–85. [DOI] [PubMed] [Google Scholar]
- 9. Laborda P, Wang SY, Voglmeir J. Influenza neuraminidase inhibitors: synthetic approaches, derivatives and biological activity. Molecules 2016; 21 pii:E1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015; 385:1729–37. [DOI] [PubMed] [Google Scholar]
- 11. Muthuri SG, Venkatesan S, Myles PR et al. PRIDE Consortium Investigators Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014; 2:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar D, Michaels MG, Morris MI et al. American Society of Transplantation H1N1 Collaborative Study Group Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis 2010; 10:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oboho IK, Reed C, Gargiullo P et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–15. [DOI] [PubMed] [Google Scholar]
- 14. Renaud C, Boudreault AA, Kuypers J et al. H275Y mutant pandemic (H1N1) 2009 virus in immunocompromised patients. Emerg Infect Dis 2011; 17:653–60; quiz 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gooskens J, Jonges M, Claas EC, Meijer A, Kroes AC. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. J Infect Dis 2009; 199:1435–41. [DOI] [PubMed] [Google Scholar]
- 16. Hurt AC, Leang SK, Tiedemann K et al. Progressive emergence of an oseltamivir-resistant A(H3N2) virus over two courses of oseltamivir treatment in an immunocompromised paediatric patient. Influenza Other Respir Viruses 2013; 7:904–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamura D, DeBiasi RL, Okomo-Adhiambo M et al. Emergence of multidrug-resistant influenza A(H1N1)pdm09 virus variants in an immunocompromised child treated with oseltamivir and zanamivir. J Infect Dis 2015; 212:1209–13. [DOI] [PubMed] [Google Scholar]
- 18. Rogers MB, Song T, Sebra R et al. Intrahost dynamics of antiviral resistance in influenza A virus reflect complex patterns of segment linkage, reassortment, and natural selection. MBio 2015; 6. doi:10.1128/mBio.02464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore C, Galiano M, Lackenby A et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Vries E, Stelma FF, Boucher CA. Emergence of a multidrug-resistant pandemic influenza A (H1N1) virus. N Engl J Med 2010; 363:1381–2. [DOI] [PubMed] [Google Scholar]
- 21. L’Huillier AG, Abed Y, Petty TJ et al. E119D neuraminidase mutation conferring pan-resistance to neuraminidase inhibitors in an A(H1N1)pdm09 isolate from a stem-cell transplant recipient. J Infect Dis 2015; 212:1726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamura D, DeBiasi RL, Okomo-Adhiambo M et al. Emergence of multidrug-resistant influenza A(H1N1)pdm09 virus variants in an immunocompromised child treated with oseltamivir and zanamivir. J Infect Dis 2015; 212:1209–13. [DOI] [PubMed] [Google Scholar]
- 23. Tramontana AR, George B, Hurt AC et al. Oseltamivir resistance in adult oncology and hematology patients infected with pandemic (H1N1) 2009 virus, Australia. Emerg Infect Dis 2010; 16:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe DM, Howley PM, Cohen JI. et al. eds. Fields Virology. 6th ed Vol 1 Philadelphia, PA: Lippincott Williams & Wilkins, 2013:1186–243. [Google Scholar]
- 25. Hurt AC. The epidemiology and spread of drug resistant human influenza viruses. Curr Opin Virol 2014; 8:22–9. [DOI] [PubMed] [Google Scholar]
- 26. Furuta Y, Takahashi K, Fukuda Y et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother 2002; 46:977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Furuta Y, Takahashi K, Kuno-Maekawa M et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother 2005; 49:981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toyama Chemical Co. Ltd. The new drug application approval of Avigan tablet 200 mg in Japan for the anti-influenza virus drug https://www.toyama-chemical.co.jp/eng/news/news140324e.html. Accessed 24 March 2014.
- 29. Dunning J, Baillie JK, Cao B, Hayden FG; International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Antiviral combinations for severe influenza. Lancet Infect Dis 2014; 14:1259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim WY, Young Suh G, Huh JW et al. Korean Society of Critical Care Medicine H1N1 Collaborative Triple-combination antiviral drug for pandemic H1N1 influenza virus infection in critically ill patients on mechanical ventilation. Antimicrob Agents Chemother 2011; 55:5703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou J, Yang XQ, Fu Z et al. Increased pathogenesis and inflammation of airways from respiratory syncytial virus infection in T cell deficient nude mice. Med Microbiol Immunol 2008; 197:345–51. [DOI] [PubMed] [Google Scholar]
- 32. Wells MA, Albrecht P, Ennis FA. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol 1981; 126:1036–41. [PubMed] [Google Scholar]
- 33. Sakabe S, Ozawa M, Takano R, Iwastuki-Horimoto K, Kawaoka Y. Mutations in PA, NP, and HA of a pandemic (H1N1) 2009 influenza virus contribute to its adaptation to mice. Virus Res 2011; 158:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruiz-Carrascoso G, Casas I, Pozo F, González-Vincent M, Pérez-Breña P. Prolonged shedding of amantadine- and oseltamivir-resistant influenza A(H3N2) virus with dual mutations in an immunocompromised infant. Antivir Ther 2010; 15:1059–63. [DOI] [PubMed] [Google Scholar]
- 35. Gubareva LV, Matrosovich MN, Brenner MK, Bethell RC, Webster RG. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J Infect Dis 1998; 178:1257–62. [DOI] [PubMed] [Google Scholar]
- 36. Jackson D, Barclay W, Zürcher T. Characterization of recombinant influenza B viruses with key neuraminidase inhibitor resistance mutations. J Antimicrob Chemother 2005; 55:162–9. [DOI] [PubMed] [Google Scholar]
- 37. Samson M, Abed Y, Desrochers FM et al. Characterization of drug-resistant influenza virus A(H1N1) and A(H3N2) variants selected in vitro with laninamivir. Antimicrob Agents Chemother 2014; 58:5220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seo S, Englund JA, Nguyen JT et al. Combination therapy with amantadine, oseltamivir and ribavirin for influenza A infection: safety and pharmacokinetics. Antivir Ther 2013; 18:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marathe BM, Wong SS, Vogel P et al. Combinations of oseltamivir and T-705 extend the treatment window for highly pathogenic influenza A(H5N1) virus infection in mice. Sci Rep 2016; 6:26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gubareva LV, Webster RG, Hayden FG. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob Agents Chemother 2001; 45:3403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wetherall NT, Trivedi T, Zeller J et al. Evaluation of neuraminidase enzyme assays using different substrates to measure susceptibility of influenza virus clinical isolates to neuraminidase inhibitors: report of the neuraminidase inhibitor susceptibility network. J Clin Microbiol 2003; 41:742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McKimm-Breschkin J, Trivedi T, Hampson A et al. Neuraminidase sequence analysis and susceptibilities of influenza virus clinical isolates to zanamivir and oseltamivir. Antimicrob Agents Chemother 2003; 47:2264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kubo S, Tokumitsu A, Tomozawa T, Kakuta M, Yamashita M. High and continuous exposure of laninamivir, an anti-influenza drug, may work suppressively to generate low-susceptibility mutants in animals. J Infect Chemother 2012; 18:69–74. [DOI] [PubMed] [Google Scholar]
- 44. Baek YH, Song MS, Lee EY et al. Profiling and characterization of influenza virus N1 strains potentially resistant to multiple neuraminidase inhibitors. J Virol 2015; 89:287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pizzorno A, Bouhy X, Abed Y, Boivin G. Generation and characterization of recombinant pandemic influenza A(H1N1) viruses resistant to neuraminidase inhibitors. J Infect Dis 2011; 203:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pizzorno A, Abed Y, Rheaume C, Bouhy X, Boivin G. Evaluation of recombinant 2009 pandemic influenza A (H1N1) viruses harboring zanamivir resistance mutations in mice and ferrets. Antimicrob Agents Chemother 2013; 57:1784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baranovich T, Wong SS, Armstrong J et al. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol 2013; 87:3741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sangawa H, Komeno T, Nishikawa H et al. Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase. Antimicrob Agents Chemother 2013; 57:5202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jin Z, Tucker K, Lin X et al. Biochemical evaluation of the inhibition properties of favipiravir and 2’-C-methyl-cytidine triphosphates against human and mouse norovirus RNA polymerases. Antimicrob Agents Chemother 2015; 59:7504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Delang L, Segura Guerrero N, Tas A et al. Mutations in the chikungunya virus non-structural proteins cause resistance to favipiravir (T-705), a broad-spectrum antiviral. J Antimicrob Chemother 2014; 69:2770–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.