Abstract
The novel SARS coronavirus SARS-CoV-2 pandemic may be particularly deleterious to patients with underlying cardiovascular disease (CVD). The mechanism for SARS-CoV-2 infection is the requisite binding of the virus to the membrane-bound form of angiotensin-converting enzyme 2 (ACE2) and internalization of the complex by the host cell. Recognition that ACE2 is the coreceptor for the coronavirus has prompted new therapeutic approaches to block the enzyme or reduce its expression to prevent the cellular entry and SARS-CoV-2 infection in tissues that express ACE2 including lung, heart, kidney, brain, and gut. ACE2, however, is a key enzymatic component of the renin-angiotensin-aldosterone system (RAAS); ACE2 degrades ANG II, a peptide with multiple actions that promote CVD, and generates Ang-(1–7), which antagonizes the effects of ANG II. Moreover, experimental evidence suggests that RAAS blockade by ACE inhibitors, ANG II type 1 receptor antagonists, and mineralocorticoid antagonists, as well as statins, enhance ACE2 which, in part, contributes to the benefit of these regimens. In lieu of the fact that many older patients with hypertension or other CVDs are routinely treated with RAAS blockers and statins, new clinical concerns have developed regarding whether these patients are at greater risk for SARS-CoV-2 infection, whether RAAS and statin therapy should be discontinued, and the potential consequences of RAAS blockade to COVID-19-related pathologies such as acute and chronic respiratory disease. The current perspective critically examines the evidence for ACE2 regulation by RAAS blockade and statins, the cardiovascular benefits of ACE2, and whether ACE2 blockade is a viable approach to attenuate COVID-19.
Listen to this article’s corresponding podcast at: https://ajpheart.podbean.com/e/covid-19-ace2-and-the-cardiovascular-consequences/.
Keywords: ACE2, ANG II, COVID-19, renin-angiotensin system, SARS-CoV-2, statins
The rapid and progressive spread of the novel SARS coronavirus SARS-CoV-2 pandemic that causes coronavirus-induced disease (COVID-19) has profoundly affected the health of thousands of individuals, strained national health care systems, and significantly impacted global economic stability. The characteristics of SARS-CoV-2 that particularly distinguish this disease from influenza are a higher transmission rate combined with a greater risk of mortality from COVID-19 primarily due to acute respiratory distress syndrome (ARDS) (16). While the major cause of mortality from COVID-19, particularly in older adults and those with compromised immune systems, is respiratory failure, a number of patients exhibit cardiovascular-related pathologies including congestive heart failure (CHF) and brain medullary cardiorespiratory dysfunction (6, 16, 32, 33, 55, 60). The cardiovascular complications and the focus on ACE2 as the coreceptor for SARS-CoV-2, as well as the apparent confusion in the literature between the ACE and ACE2 components of the renin-angiotensin-aldosterone system (RAAS), has prompted the current perspective.
Viral infections are dependent on cellular entry of the virus that uses the cellular machinery of the host to replicate multiple viral copies which are subsequently shed by the host cell. Coronaviruses such as SARS-CoV-2 and SARS-CoV-1 are now known to use the host protein angiotensin-converting enzyme-2 (ACE2, EC 3.4.17.23) as a coreceptor to gain intracellular entry into the lungs and brain (17, 30, 52, 53, 62). ACE2 is a membrane-bound peptidase with the majority of the protein that comprises the NH2-terminal peptide domain including the catalytic site oriented extracellularly (3.4). ACE2 is expressed in essentially all tissues, with greatest activity in the ileum and kidney followed by adipose tissue, heart, brain stem, lung, vasculature, stomach, liver, and nasal and oral mucosa based on activity data in the mouse that generally parallel ACE2 mRNA levels in humans (13, 53, 62), although discrepancies between mRNA levels and ACE2 activity or protein expression are evident (10, 11, 47). ACE2 has access to peptides in the circulation (both maternal and fetal), renal tubular fluid, cerebrospinal fluid, interstitial fluid, and bronchial fluid. Consensus of evidence from various studies favors a primary role of ACE2 to efficiently degrade ANG II to Ang-(1–7). ACE2 is not an aminopeptidase as recently described by Zheng et al. (60) as its catalytic action that removes the COOH-terminal phenylalanine residue of ANG II characterizes ACE2 as a carboxypeptidase. This single catalytic event reduces ANG II, the major effector of the RAAS that promotes hypertension (HTN) in part by attenuating baroreceptor sensitivity (BRS) for control of heart rate and promoting vasoconstriction, sodium retention, oxidative stress, inflammation, and fibrosis, as well as increases the bioactive peptide Ang-(1–7) that opposes the ANG II-ANG II type 1 (AT1) receptor axis through its anti-inflammatory and antifibrotic actions, as well as enhancing BRS (Fig. 1). Thus, the ACE2 peptidase pathway constitutes a key inflexion point in the processing pathway of the RAAS. Consequently, the loss of ACE2 may shift the system to an overall higher ANG II and lower Ang-(1–7) tone (4, 5, 39). In contrast, ACE forms ANG II and degrades Ang-(1–7), which produces the opposite processing of ACE2 and promotes an increase in blood pressure, inflammation, and fibrosis (Fig. 1). ACE2 hydrolyzes other peptides including apelin and des-arginine bradykinin (des-Arg1-BK): apelin exhibits cardioprotective actions (48), while des-Arg1-BK promotes inflammation via stimulation of the B1 receptor (44). The extent that these peptides functionally contribute to the effects of altered ACE2 activity is not well established, although increased levels of des-Arg1-BK enhancing pulmonary inflammation would be deleterious (44).
Fig. 1.

Processing and functional scheme of the renin-angiotensin system. The protease renin converts the precursor angiotensinogen to angiotensin I (ANG I), which is subsequently converted to ANG II by dipeptidyl carboxypeptidase angiotensin-converting enzyme (ACE). ANG II binds to the ANG II type 1 receptor (AT1R) to stimulate inflammation, fibrosis, oxidative stress, and an increase in blood pressure. ANG II is metabolized to ANG III and ANG IV through various aminopeptidases (APs). ANG I and ANG II are converted to Ang-(1–7) via endopeptidases (NEP) and the monocarboxypeptidase ACE2, respectively. Ang-(1–7) binds to the Mas receptor (Mas-R) to exert anti-inflammatory and antifibrotic actions, stimulate the release of nitric oxide, and reduce blood pressure. Ang-(1–7) is metabolized to Ang-(1–5) by ACE. Major forming and degrading pathways are depicted by solid and dashed lines, respectively. SARS-CoV-2 binds to ACE2 to stimulate internalization of both the virus and peptidase that may remove ACE2 from this pathway.
As to the mechanism for the intracellular entry by SARS-CoV-2 and SARS-CoV, the viral coat expresses a protein termed SPIKE (S protein) that contains a receptor-binding region that binds to the extracellular domain of ACE2 with high affinity of 15 nM (50). Cleavage of the S protein along dibasic arginine sites by the host protease TMPRSS2 to generate the S1 and S2 subunits is a critical step for S2-induced membrane fusion and viral internalization by endocytosis with ACE2 in the pulmonary epithelium (17, 21). The S protein is a not a substrate for ACE2, nor does SARS-CoV-2 bind to ACE. Wrapp and colleagues (52) suggest the greater virulence of SARS-CoV-2 may reflect that the S1 protein exhibits markedly higher affinity for ACE2 as compared with that of SARS-CoV. ACE2 internalization by SARS-CoV-2 would potentially result in the loss of ACE2 at the cell surface and voids a key pathway for the cell to degrade ANG II and generate the CVD-protective Ang-(1–7). Indeed, an increase in the overall ratio of ANG II:Ang-(1–7) following ACE2 internalization may exacerbate the pulmonary tissue damage initially provoked by SARS-CoV-2. In turn, the reduction in ACE2 may contribute to chronic loss of pulmonary function and increased tissue fibrosis due to COVID-19.
The extent to which SARS-CoV-2 infects the heart or other cardiovascular tissues once it enters the circulation and potentially contributes to the myocarditis associated with COVID-19 is unknown (16, 18, 55). In fact, the impact of SARS-CoV-2 on the cardiovascular system apart from the lung is not established. Cardiovascular tissues or cells that express ACE2 are potentially at risk for SARS-CoV-2 infection; however, other factors including expression of the host proteases that prime the virus are required for infection as well (17, 21). In patients with underlying CVD, the loss of ACE2 by SARS-CoV-2-induced internalization would be predicted to exacerbate CVD acutely and perhaps long term (56). ACE2 is the primary route of ANG II metabolism and Ang-(1–7) generation in the heart, and the loss of this carboxypeptidase may compromise cardiac function apart from or in addition to viral infection (3, 12, 24, 38, 40, 43, 56). ACE2 is highly expressed in the tubular epithelium of the kidney, and the loss of the enzyme may contribute to altered sodium transport leading to an increase in blood volume and pressure, as well as both acute and chronic effects on kidney injury (4, 5, 10, 22, 24, 43, 50). On the basis of studies on SARS-CoV and recent reports of the presence of viral load in the brain stem with SARS-CoV-2, a similar transfer of virus to the brain by ACE2 may occur via internalization and transport by various cranial nerves (32, 35). Certainly, neuronal cell death as a result of viral infection would disrupt these vital functions (32). In addition, loss of ACE2 in brain cardiovascular centers short of neuronal death may impair proper autonomic nervous system regulation of blood pressure and potentially respiration (52). The loss of ACE2 in the brain stem may facilitate an increase in sympathetic drive, alterations in the baroreflex, and exacerbation of hypertension (1, 8, 54). Reduced expression of ACE2 in the vasculature may also promote endothelial dysfunction and inflammation and exacerbate existing atherosclerosis and diabetes (9, 34, 42, 45, 56, 59). A loss of pulmonary ACE2 may exacerbate hypertension, respiratory distress, and fibrosis post-viral infection (20, 30, 44). Cell surface diminution of ACE2 may contribute to widespread inflammation observed with COVID-19. We note that the ACE2 protein collectrin that facilitates amino acid transport and is expressed in multiple tissues (kidney, brain, vasculature, and pancreas) lacks the extracellular peptide domain of ACE2 and is not expected to directly bind and enable internalization of SARS-CoV-2 (7).
In contrast to ACE, endogenous circulating levels of soluble ACE2 are generally quite low to nondetectable and would not adequately sequester SARS-CoV-2 in the circulation to prevent viral dissemination (3). While a clinical trial on infusion of recombinant ACE2 was recently proposed and subsequently withdrawn (NCT04287686), the extent that soluble ACE2 would compete for SARS-CoV-2 binding to reduce viremia infection and alleviate tissue injury is unknown. This approach would likely have little impact on viral infection via the respiratory system or the gut, although an increase in circulating ACE2 to augment the Ang-(1–7):ANG II ratio may improve SARS-CoV-2-induced organ injury such as ARDS and attenuate subsequent viral infection of other tissues. However, ACE2 infusion may decrease circulating ANG II and increase Ang-(1–7) levels to the extent that blood pressure dysregulation leading to relative hypotension could occur in patients with COVID-19 in later stages of disease including septic or cardiogenic shock (28).
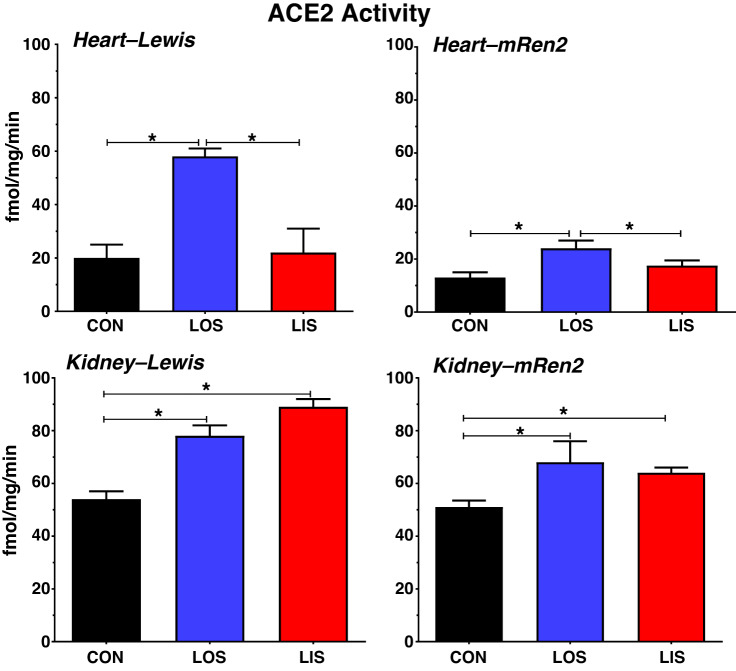
Experimental studies generally support the notion that RAAS blockade stimulates ACE2 expression and/or activity, although there appear to be differential responses to AT1 receptor antagonists (ARBs) versus ACE inhibitors (ACEIs), as well as tissue-dependent responses. In normotensive Lewis and hypertensive mRen2.Lewis male rats, we found that the ARB losartan increased ACE2 activity in the heart by two- to threefold as shown in Fig. 2 (11, 22); a similar increase in cardiac ACE2 activity (~2-fold) was reported for the ARB eprosartan in rats with CHF (27). The ACEI lisinopril, however, either failed to increase cardiac ACE2 activity (Lewis) or stimulated to a lesser extent than losartan (mRen2) (Fig. 2), despite similar reductions in blood pressure (11, 22) Plasma and cardiac tissue contents of Ang-(1–7) paralleled the increase in ACE2 activity following losartan treatment in the Lewis rats (11). In the kidney of both strains, losartan and lisinopril increased ACE2 activity (10, 22), although to a lesser degree compared with the heart (Fig. 2). Burchill et al. (2) found that the ACEI ramipril reduced cardiac ACE2 activity to the level of the control group in a rat model of acute kidney injury (AKI). Wang et al. (49) recently showed that various ARBs (olmesartan, losartan, valsartan, candesartan, telmisartan, and irbesartan) all increased ACE2 protein to a similar extent (~2-fold) in the hearts of aorta-constricted mice. In patients with chronic kidney disease (CKD), urinary ACE2 levels (an index of renal tubular expression) in those treated with ACEIs or ARBs were similar to the untreated group (36). Furthermore, Lely et al. (31) found no effect of ACEI treatment on ACE2 protein expression in renal biopsy samples from patients with various renal pathologies, as well as in recipients of kidney transplant. In contrast, only patients treated with ACEI exhibited an increase in intestinal ACE2 mRNA levels as compared with those on ARBs; however, ACE2 protein or activity were not assessed to validate the mRNA results (46).
Fig. 2.
Influence of angiotensin II (ANG II) type 1 receptor or angiotensin-converting enzyme (ACE) blockade on ACE2 activity in the heart and kidney of normotensive Lewis and hypertensive mRen2.Lewis rats. Chronic blockade with losartan (LOS) increased cardiac ACE2 activity by 3-fold in normotensive Lewis and 2-fold hypertensive mRen2.Lewis (mRen2) male rats. Lisinopril (LIS) treatment had little or no effect on cardiac ACE2 activity in these strains. Chronic LOS or LIS treatment increased renal ACE2 activity in the Lewis (1.3- and 1.7-fold, respectively) and mRen2 (1.3- and 1.2-fold, respectively). ACE2 activity is the amount of Ang-(1–7) converted from ANG II in the plasma membrane fraction (fmol Ang-(1–7)·mg protein−1·min−1) sensitive to the ACE2 inhibitor MLN4760 (3). Data are means ± SE; n = 6–8. *P < 0.05 (10, 11, 22). CON, control.
In the brain stem of older rats, losartan treatment increased ACE2 mRNA levels twofold; ACE2 was the primary peptidase to generate Ang-(1–7) in this brain region (8, 14). Chronic exercise may be another important stimulus of ACE2 in the brain and the periphery (37). In the rostral ventrolateral medulla (RVLM), an exercise regimen markedly increased ACE2 protein as compared with both the control and CHF experimental groups (26). However, this raises the potential issue that while exercise is clearly associated with improved cardiovascular outcomes in chronic situations, exercise may contribute to a greater risk of SARS-CoV-2 infection. Keidar et al. (25) reported that the mineralocorticoid antagonist spironolactone increased ACE2 activity fourfold in monocyte-derived macrophages from patients with CHF; however, spironolactone failed to increase cardiac ACE2 significantly in experimental CHF (27). Apart from RAAS blockade, experimental studies reveal that statins also augment the ACE2 expression. Tikoo et al. (45) reported an increase in ACE2 protein in both heart and kidney (~2-fold) of atorvastatin-treated atherosclerotic rabbits that was associated with epigenetic modifications of the ACE2 gene. Fluvastatin treatment significantly enhanced the effects of insulin to augment cardiac ACE2 protein expression in diabetic rats (41). To our knowledge, the influence of ARB or ACEI treatments combined with statins on ACE2 expression has not been established. Finally, the peroxisome proliferator-activated receptor-γ (PPAR-γ) may influence ACE-2 expression as well. The PPAR-γ agonist rosiglitazone increased ACE2 protein levels twofold in the aorta of hypertensive rats following aortic coarctation (39). Oudit and colleagues (61) found that telmisartan, a partial PPAR-γ agonist, also increased ACE2 protein expression in aorta which was associated with greater PPAR-γ content in the spontaneously hypertensive rat. The extent that ARBs with PPAR-γ agonistic actions such as telmisartan and irbesartan exhibit a greater effect on ACE2 expression in different tissues is unknown, although Wang et al. (49) found no difference in the increase in cardiac ACE2 among six different ARBs that included both telmisartan and irebesartan.
The influence of RAAS blockade on pulmonary ACE2 has not been evaluated thoroughly, but ACEI and ARB treatment may improve outcomes in patients with ARDS (27). In experimental studies, Yuan et al. (57) reported reduced ACE2 protein in the lungs of rats subjected to chronic smoking and that losartan treatment was beneficial but failed to increase ACE2 in either the control or the smoking-exposed groups. However, there are inconsistencies in the stated conclusions of this study that are not supported by the data, as well as the extremely high ANG II content reported in the lung tissue (>10 µg/mg or 10 nmol/mg protein) whereby ANG II would comprise 1% of the total protein content in lung (57). In a model of LPS-induced ARDS, losartan improved pulmonary function and inflammation (51). Losartan treatment was associated with higher ACE2 activity in bronchoalveolar lavage fluid (BALF) compared with that of the ARB-treated controls; losartan reduced ACE2 activity by 50% in the ventilated control group (49). Changes in ANG II and Ang-(1–7) BALF content evaluated by HPLC-mass spectroscopy paralleled alterations in ACE2 activity (51). We are not aware of studies in animals or humans that have examined the effects of ACEI on pulmonary ACE2, and the discrepancy between ARBs and ACEIs to augment ACE2 activity clearly requires further evaluation. The effect of ARBs or ACEIs on the expression of the SPIKE proteases on the host cell that facilitate binding and entry of SARS-CoV-2 is also unknown. ARBs substantially increase the circulating levels of ANG II arising from the disinhibition of kidney renin release, and whether the higher ANG II levels compete for SARS-CoV-2 binding to ACE2 is unclear. In collaborative studies with the McCray laboratory, we reported that the SPIKE protein from SARS-CoV did not attenuate hydrolysis of ANG II to Ang-(1–7) by soluble ACE2, thus it may be unlikely that ANG II or other peptide substrates would directly interfere with SAR-CoV-2 binding and internalization (23).
Finally, our knowledge of the cardiovascular consequences of SARS-CoV-2 infection in patients at this early point is quite limited. Liu et al. (33) recently reported that the circulating levels of ANG II were significantly higher in patients with COVID-19 than those of healthy controls that would be consistent with lower ACE2 activity. Moreover, plasma ANG II content significantly correlated with both the viral load in BALF and pulmonary function in the SARS-CoV-2 cohort (33). Thus, as suggested by Liu and colleagues, whether a direct ANG II ACE2 interaction occurs or changes in pulmonary or cardiac function in these patients alters RAAS expression cannot be ascertained. However, this clinical study comprised only 12 patients, and circulating ACE2 or ACE levels were not determined. Moreover, an increase in circulating ANG II may reflect changes in a number of RAAS components as opposed to solely a reduction in ACE2 activity (Fig. 1). Plasma ANG II levels in the study of Liu et al. (33) ranged from 100 pM in healthy controls to 500 pM in the SARS-CoV-2 cohort [100–500 pg/ml] using an ELISA-based method in which the patient plasma was directly assayed; these values are 5–10-fold higher than expected ANG II levels in plasma but are in an acceptable range particularly if the patients were ventilated (3). In contrast, their previous study using a different ELISA reported plasma ANG II values in control subjects of 5 nM [5,000 pg/ml] that increased up to 20 nM [20,000 pg/ml] in patients with the influenza A H7N9N1 virus that far exceeds accepted plasma ANG II concentrations (19). This latter study underscores that appropriate methods to accurately quantify ANG II, ACE2, and other RAAS component are vital to establish the role of the RAAS in patients with COVID-19. Biochemical approaches to assess the peptide and protein components of the RAAS in plasma and tissues, as well as the expected endogenous peptide values, were recently reviewed by Chappell (3). The study of Liu et al. (33) also failed to include blood pressure data for each patient which could substantiate the higher circulating levels of ANG II. Patients with more severe COVID-19 are reported to have hypokalemia and higher blood pressure as compared with those with milder COVID-19 that would support a role for a stimulated ANG II-AT1 receptor axis (6). Current clinical data on the ACE2-Ang-(1–7) pathway, however, are quite limited relative to the experimental data, especially in regard to the effects of ACEI and ARB. Furthermore, the existing evidence, though novel and insightful, often comes from smaller cross-sectional observational studies with incomplete RAAS measurements that cannot fully account for potential sources of bias and confounding.
There are essentially no clinical data on how ACEIs or ARBs may impact the ACE2-Ang-(1–7) pathway in lung, heart or brain. Thus, additional data are urgently needed on the effects of ACEI and ARB on human pulmonary disease and RAAS expression, particularly the response of the ACE2-Ang-(1–7)-Mas receptor axis. Ang-(1–7) itself may potentially serve as a novel therapeutic to treat COVID-19. In both LPS- and acid-induced ARDS with high-stretch ventilation, Ang-(1–7) infusion improved oxygenation, reduced the acute inflammatory response, and reduced subsequent tissue fibrosis (51, 58). Clinical trials are in development to test the effects of the ARB losartan in patients with COVID-19 (NCT04311177 and NCT04312009). Upregulation of the ACE2-Ang-(1–7) pathway of the RAAS is well known to counter-regulate the proinflammatory and profibrotic effects of the ACE-ANG II-AT1 receptor axis in experimental models of HTN and CVD (4, 5, 24, 40). Indeed, experimental studies demonstrate that the ACE2-Ang-(1–7) pathway mediates some of the beneficial effects of ACEI and ARB in these diseases including improved regulation of autonomic control of blood pressure (3, 5, 40, 56), though data in humans remains limited. Experimental evidence to date strongly suggests that ANG II may promote acute lung injury and ARDS induced by coronaviruses, including SARS-CoV, SARS-CoV-2/COVID-19, and possibly in MERS-CoV (20, 30, 51).
We emphasize that further investigation into these potential mechanisms is urgently required, now given the complex interplay of the RAAS and novel coronaviruses such as SARS-CoV-2. Particularly relevant information includes the status of the RAAS at baseline, during and after the infection and during progression of COVID-19 and following recovery. Comprehensive assessments of the full complement of RAAS components across this timeline particularly in concert with medical management of the patient during different phases of the disease are required to establish whether the ACE-ANG II-AT1 receptor versus ACE2-Ang-(1–7)-Mas receptor pathways are beneficial or detrimental at a given point in time. This situation is not surprising since the RAAS has both an immediate role in blood pressure and fluid balance regulation and a longer-term impact on chronic oxidative stress, inflammation and fibrosis. Clinical trials are required for a greater understanding of the impact of treatment with ACEIs and ARBs (as well as statins and aldosterone blockers) medications, known to be highly effective for mitigating the impact of hypertension and diabetes on the heart, kidney and vasculature, on infection rates and severity of disease, to directly target our understanding of proposed mechanisms of injury. Moreover, precise, rigorous, and appropriate methods are required to correctly characterize the RAAS phenotype, whether in patients with COVID-19 or in animals in experimental studies, particularly with respect to ACE2 activity or protein expression that may provide insight into the role of ACE2 in the progression of SARs-CoV-2 infection and, importantly, whether blockade of the peptidase is an appropriate step, at least acutely and targeted to the pulmonary system rather than systemically (56). Thus, a shift to studies of the multifaceted roles of the RAAS in the setting of infectious disease is warranted, which should not occur in isolation from the other well-known roles of the system in cardiovascular homeostasis, particularly given the recent focus on the RAAS and the balance of ANG II and Ang-(1–7) in critical care medicine such as in septic shock (15, 28).
GRANTS
This work was supported by National Institutes of Health Grants HL-146818, HL-05952, HD-084227, and HL-56973; American Heart Association Grants AHA-151521 and AHA-18TPA34170522; and Cardiovascular Sciences Center Grant CVSC-830114 and by the Hypertension and Vascular Research Center and the Farley Hudson Foundation.
DISCLOSURES
No conflicts of interest are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.C. conceived and designed research; M.C.C. performed experiments; M.C.C. analyzed data; M.C.C. interpreted results of experiments; M.C.C. prepared figures; A.M.S., D.D., and M.C.C. drafted manuscript; A.M.S., D.D., and M.C.C. edited and revised manuscript; A.M.S., D.D., and M.C.C. approved final version of manuscript.
REFERENCES
- 1.Alenina N, Bader M. ACE2 in brain physiology and pathophysiology: Evidence from transgenic animal models. Neurochem Res 44: 1323–1329, 2019. doi: 10.1007/s11064-018-2679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burchill L, Velkoska E, Dean RG, Lew RA, Smith AI, Levidiotis V, Burrell LM. Acute kidney injury in the rat causes cardiac remodelling and increases angiotensin-converting enzyme 2 expression. Exp Physiol 93: 622–630, 2008. doi: 10.1113/expphysiol.2007.040386. [DOI] [PubMed] [Google Scholar]
- 3.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol 310: H137–H152, 2016. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the Angiotensin converting enzyme 2-Angiotensin (1-7)-MAS receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne) 4: 201–215, 2014. doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappell MC. Emerging evidence for a functional ACE2-angiotensin-(1–7)-Mas receptor axis: more than regulation of blood pressure? Hypertension 50: 596–603, 2007. doi: 10.1161/HYPERTENSIONAHA.106.076216. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Li X, Song Q, Hu C, Su F, Dai J. Hypokalemia and clinical implications in patients with Coronavirus Disease 2019 (COVID-19). [Preprint]. MedRxiv, 2020. doi: 10.1101/2020.02.27.20028530. [DOI] [PMC free article] [PubMed]
- 7.Danilczyk U, Sarao R, Remy C, Benabbas C, Stange G, Richter A, Arya S, Pospisilik JA, Singer D, Camargo SM, Makrides V, Ramadan T, Verrey F, Wagner CA, Penninger JM. Essential role for collectrin in renal amino acid transport. Nature 444: 1088–1091, 2006. doi: 10.1038/nature05475. [DOI] [PubMed] [Google Scholar]
- 8.Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Tallant EA, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol 93: 694–700, 2008. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong B, Zhang C, Feng JB, Zhao YX, Li SY, Yang YP, Dong QL, Deng BP, Zhu L, Yu QT, Liu CX, Liu B, Pan CM, Song HD, Zhang MX, Zhang Y. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol 28: 1270–1276, 2008. doi: 10.1161/ATVBAHA.108.164715. [DOI] [PubMed] [Google Scholar]
- 10.Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann Tallant E, Smith RD, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int 68: 2189–2196, 2005. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–2610, 2005. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 12.Garabelli PJ, Modrall JG, Penninger JM, Ferrario CM, Chappell MC. Distinct roles for angiotensin-converting enzyme 2 and carboxypeptidase A in the processing of angiotensins within the murine heart. Exp Physiol 93: 613–621, 2008. doi: 10.1113/expphysiol.2007.040246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides 26: 1270–1277, 2005. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliam-Davis S, Gallagher PE, Payne VS, Kasper SO, Tommasi EN, Westwood BM, Robbins ME, Chappell MC, Diz DI. Long-term systemic angiotensin II type 1 receptor blockade regulates mRNA expression of dorsomedial medulla renin-angiotensin system components. Physiol Genomics 43: 829–835, 2011. doi: 10.1152/physiolgenomics.00167.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleeson PJ, Crippa IA, Mongkolpun W, Cavicchi FZ, Van Meerhaeghe T, Brimioulle S, Taccone FS, Vincent JL, Creteur J. Renin as a marker of tissue perfusion and prognosis in critically ill patients. Crit Care Med 47: 152–158, 2019. doi: 10.1097/CCM.0000000000003544. [DOI] [PubMed] [Google Scholar]
- 16.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of 183 coronavirus disease 2019 in China. N Engl J Med. 2020. February 28 [Epub ahead of print]. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann M, Kleine-Wever H, Kruger N, Muller M, Drotsten C, Pholhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry in target cells. Cell 181: 1–10, 2020. doi: 10.1016/j.cell.2020.02.05.32243785 [DOI] [Google Scholar]
- 18.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang F, Guo J, Zou Z, Liu J, Cao B, Zhang S, Li H, Wang W, Sheng M, Liu S, Pan J, Bao C, Zeng M, Xiao H, Qian G, Hu X, Chen Y, Chen Y, Zhao Y, Liu Q, Zhou H, Zhu J, Gao H, Yang S, Liu X, Zheng S, Yang J, Diao H, Cao H, Wu Y, Zhao M, Tan S, Guo D, Zhao X, Ye Y, Wu W, Xu Y, Penninger JM, Li D, Gao GF, Jiang C, Li L. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat Commun 5: 3595–3602, 2014. doi: 10.1038/ncomms4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol 93: e01815–e01818, 2019. doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from the transgenic Ren-2 rat. Am J Physiol Heart Circ Physiol 291: H2166–H2172, 2006. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 23.Jia HP, Look DC, Tan P, Shi L, Hickey M, Gakhar L, Chappell MC, Wohlford-Lenane C, McCray PB Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol 297: L84–L96, 2009. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol 11: 413–426, 2014. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keidar S, Gamliel-Lazarovich A, Kaplan M, Pavlotzky E, Hamoud S, Hayek T, Karry R, Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res 97: 946–953, 2005. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 26.Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol (1985) 108: 923–932, 2010. doi: 10.1152/japplphysiol.00840.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karram T, Abbasi A, Keidar S, Golomb E, Hochberg I, Winarver J, Hoffman A, Abassi Z. Effects of spironolactone and eprotosartan on cardiac remodeling and ACE isoforms in rats with experimental heart failure. Am J Physiol Heart Circ Physiol 289: H1351–H1358, 2005. doi: 10.1152/ajpheart.01186.2004. [DOI] [PubMed] [Google Scholar]
- 28.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, Busse LW, Altaweel L, Albertson TE, Mackey C, McCurdy MT, Boldt DW, Chock S, Young PJ, Krell K, Wunderink RG, Ostermann M, Murugan R, Gong MN, Panwar R, Hästbacka J, Favory R, Venkatesh B, Thompson BT, Bellomo R, Jensen J, Kroll S, Chawla LS, Tidmarsh GF, Deane AM; ATHOS-3 Investigators . Angiotensin II for the treatment of vasodilatory shock. N Engl J Med 377: 419–430, 2017. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Choi SM, Lee J, Park YS, Lee CH, Yim JJ, Yoo CG, Kim YW, Han SK, Lee SM. Effect of renin-angiotensin system blockage in patients with acute respiratory distress syndrome: a retrospective case control study. Korean J Crit Care Med 32: 154–163, 2017. doi: 10.4266/kjccm.2016.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol 204: 587–593, 2004. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- 32.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVCID-19 patients. J Med Virol 2020. February 27 [Epub ahead of print]. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63: 364–374, 2020. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, Levitt KS, Oudit GY, Al-Omran M, Stewart DJ, Slutsky AS, Peterson MD, Backx PH, Penninger JM, Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol 295: H1377–H1384, 2008. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 35.Mao L, Wang M, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Li Y, Jin H, Bo H. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. [Preprint.] MedRExiv, 2020. 10.1101/2020.02.22.20026500.
- 36.Mizuiri S, Aoki T, Hemmi H, Arita M, Sakai K, Aikawa A. Urinary angiotensin-converting enzyme 2 in patients with CKD. Nephrology (Carlton) 16: 567–572, 2011. doi: 10.1111/j.1440-1797.2011.01467.x. [DOI] [PubMed] [Google Scholar]
- 37.Nunes-Silva A, Rocha GC, Magalhaes DM, Vaz LN, Salviano de Faria MH, Simoes E Silva AC. Physical exercise and ACE2-angiotensin-(1–7)-mas receptor Axis of the renin angiotensin system. Protein Pept Lett 24: 809–816, 2017. doi: 10.2174/0929866524666170728151401. [DOI] [PubMed] [Google Scholar]
- 38.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res 118: 1313–1326, 2016. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sánchez-Aguilar M, Ibarra-Lara L, Del Valle-Mondragón L, Rubio-Ruiz ME, Aguilar-Navarro AG, Zamorano-Carrillo A, Ramírez-Ortega MD, Pastelín-Hernández G, Sánchez-Mendoza A. Rosiglitazone, a ligand to PPAR, improves blood pressure and vascular function through Renin-Angiotensin System regulation. PPAR Res 2019: 1371758, 2019. doi: 10.1155/2019/1371758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos RAS, Oudit GY, Verano-Braga T, Canta G, Steckelings UM, Bader M. The renin-angiotensin system: going beyond the classical paradigms. Am J Physiol Heart Circ Physiol 316: H958–H970, 2019. doi: 10.1152/ajpheart.00723.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin YH, Min JJ, Lee JH, Kim EH, Kim GE, Kim MH, Lee JJ, Ahn HJ. The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Heart Vessels 32: 618–627, 2017. doi: 10.1007/s00380-016-0936-5. [DOI] [PubMed] [Google Scholar]
- 42.Sahara M, Ikutomi M, Morita T, Minami Y, Nakajima T, Hirata Y, Nagai R, Sata M. Deletion of angiotensin-converting enzyme 2 promotes the development of atherosclerosis and arterial neointima formation. Cardiovasc Res 101: 236–246, 2014. doi: 10.1093/cvr/cvt245. [DOI] [PubMed] [Google Scholar]
- 43.Simões E Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 107: 154–162, 2016. doi: 10.1016/j.phrs.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, McCray PB Jr, Chappell M, Hackam DJ, Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol 314: L17–L31, 2018. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tikoo K, Patel G, Kumar S, Karpe PA, Sanghavi M, Malek V, Srinivasan K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol 93: 343–351, 2015. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Vuille-dit-Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J, Hamie QM, Meier CF, Hunziker S, Forraas-Kaufmann Z, Kuyumcu S, Fox M, Schwizer W, Fried M, Lindenmeyer M, Götze O, Verrey F. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 47: 693–705, 2015. doi: 10.1007/s00726-014-1889-6. [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Lai FM, Lai KB, Chow KM, Kwan CH, Li KT, Szeto CC. Discrepancy between intrarenal messenger RNA and protein expression of ACE and ACE2 in human diabetic nephropathy. Am J Nephrol 29: 524–531, 2009. doi: 10.1159/000185629. [DOI] [PubMed] [Google Scholar]
- 48.Wang W, McKinnie SM, Farhan M, Paul M, McDonald T, McLean B, Llorens-Cortes C, Hazra S, Murray AG, Vederas JC, Oudit GY. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects in the Cardiovascular System. Hypertension 68: 365–377, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06892. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Ye Y, Gong H, Wu J, Yuan J, Wang S, Yin P, Ding Z, Kang L, Jiang Q, Zhang W, Li Y, Ge J, Zou Y. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1-7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J Mol Cell Cardiol 97: 180–190, 2016. doi: 10.1016/j.yjmcc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Williams VR, Scholey JW. Angiotensin-converting enzyme 2 and renal disease. Curr Opin Nephrol Hypertens 27: 35–41, 2018. doi: 10.1097/MNH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 51.Wösten-van Asperen RM, Lutter R, Specht PA, Moll GN, van Woensel JB, van der Loos CM, van Goor H, Kamilic J, Florquin S, Bos AP. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol 225: 618–627, 2011. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 52.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 12: 8–15, 2020. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1-7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol 300: R804–R817, 2011. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis 9712: 30136–3, 2020. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yousif MHM, Dhaunsi GS, Makki BM, Qabazard BA, Akhtar S, Benter IF. Characterization of Angiotensin-(1-7) effects on the cardiovascular system in an experimental model of type-1 diabetes. Pharmacol Res 66: 269–275, 2012. doi: 10.1016/j.phrs.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Yuan YM, Luo L, Guo Z, Yang M, Ye RS, Luo C. Activation of renin-angiotensin-aldosterone system (RAAS) in the lung of smoking-induced pulmonary arterial hypertension (PAH) rats. J Renin Angiotensin Aldosterone Syst 16: 249–253, 2015. doi: 10.1177/1470320315576256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zambelli V, Bellani G, Borsa R, Pozzi F, Grassi A, Scanziani M, Castiglioni V, Masson S, Decio A, Laffey JG, Latini R, Pesenti A. Angiotensin-(1-7) improves oxygenation, while reducing cellular infiltrate and fibrosis in experimental Acute Respiratory Distress Syndrome. Intensive Care Med Exp 3: 44, 2015. doi: 10.1186/s40635-015-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C, Zhao YX, Zhang YH, Zhu L, Deng BP, Zhou ZL, Li SY, Lu XT, Song LL, Lei XM, Tang WB, Wang N, Pan CM, Song HD, Liu CX, Dong B, Zhang Y, Cao Y. Angiotensin-converting enzyme 2 attenuates atherosclerotic lesions by targeting vascular cells. Proc Natl Acad Sci USA 107: 15886–15891, 2010. doi: 10.1073/pnas.1001253107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020. March 5 [Epub ahead of print]. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong JC, Ye JY, Jin HY, Yu X, Yu HM, Zhu DL, Gao PJ, Huang DY, Shuster M, Loibner H, Guo JM, Yu XY, Xiao BX, Gong ZH, Penninger JM, Oudit GY. Telmisartan attenuates aortic hypertrophy in hypertensive rats by the modulation of ACE2 and profilin-1 expression. Regul Pept 166: 90–97, 2011. doi: 10.1016/j.regpep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med, 2020. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]