to the editor: Emerging clinical reports from China, Italy, and the United States reveal that SARS-CoV-2 is associated with a prominent incidence of cardiovascular morbidities and complications (1, 36), including myocarditis, acute myocardial infarction, and worsening heart failure. These cardiovascular manifestations have been encountered in preceding epidemics of corona viruses, namely Severe Acute Respiratory Syndrome (SARS) and Middle-East Respiratory Syndrome (MERS), as well as during H1N1 influenza outbreaks (1, 36). Moreover, several preliminary reports indicate that a vast majority of patients with comorbidities are prone to SARS-CoV-2 complications (50–86%). Congestive heart failure (CHF), chronic kidney disease (CKD), diabetes, and pulmonary diseases are the principal-identified clinical conditions predisposing to SARS-CoV-2-induced morbidities and mortality (7). Although pneumonia, the principal alarming feature of SARS-CoV-2 infection, could by itself evoke cardiovascular complications as a result of hypoxemia, systemic inflammation and enhanced myocardial oxygen demand, a direct cardiovascular injury, likely develops, initiated by binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2), widely expressed in myocardial and vascular endothelial cells, leading to adverse cardiovascular consequences. The following short commentary outlines potential mechanisms by which elimination of ACE2 by this virus may lead to deleterious cardiovascular outcome.
ACE2 is a transcellular protein predominantly expressed in the heart, vasculature, kidney, lung, brain, intestine, and testis and is usually located at the apical side of cells attached to basal membrane (Fig. 1) (30). In the heart, ACE2 is widely expressed in all cardiac cell types, including endothelial cells, smooth muscle cells in the myocardial vasculature and in cardiac myocytes (2, 9). ACE2 has 400-fold affinity to angiotensin II (ANG II), as compared with the classic ACE, and it converts ANG II to angiotensin-(1–7) [Ang-(1–7)] (31). The latter short peptide exerts vasodilatory, natriuretic/diuretic, anti-inflammatory, and antifibrotic effects via Mas receptor (MasR). Noteworthy, both clinical (2, 3, 10, 13, 37) and experimental (2) heart failure is characterized by upregulation of cardiac ACE2 and enhanced Ang-(1–7) generation, which may represent a cardioprotective compensatory response aimed at reducing or preventing cardiac remodeling (28) (Fig. 1). In agreement with this notion, targeted overexpression of cardiac ACE2 by applying local injection of lenti-viral vector in Sprague-Dawley normotensive rats significantly attenuated cardiac hypertrophy and myocardial fibrosis induced by prolonged ANG II administration (15). Similarly, overexpression of ACE2 in cardiac tissues of spontaneously hypertensive rats decreased cardiac remodeling in as was evident by reduced left ventricular wall thickness and perivascular fibrosis (6), probably via reduction of collagen production (11). Collectively, these animal studies highlight a cardioprotective role for the ACE2–Ang-(1–7)–MasR axis.
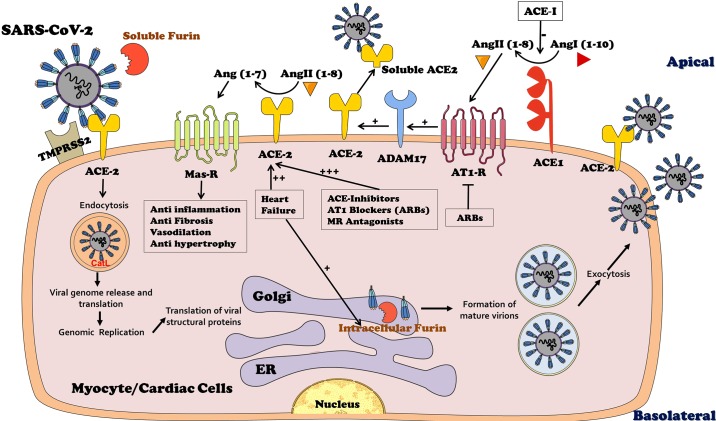
Fig. 1.
The initial step after the invasion of Severe Acute Respiratory Syndrome (SARS)-CoV-2 is binding to membranal angiotensin-converting enzyme 2 (ACE2) widely expressed in cardiac cells including endothelial cells, smooth muscle cells in the myocardial vasculature and in cardiac myocytes. ACE2 is responsible for the conversion of ANG II to Ang-(1–7) that exerts beneficial effects on the cardiac tissue such as vasodilation, antifibrosis, and anti-inflammation via Mas receptor (MasR). The binding of SARS-CoV-2 to ACE2 is preceded by furin-mediated exposure of the viral receptor binding protein (RBP) localized to S-glycoprotein (S1 domain of the viral spike). Furin is abundant in the heart both intracellulary and in the circulation as a free enzyme, making it a key factor in the uncovering of RBP and eventually in SARS-CoV-2 transmission. In addition, furin enhances the affinity of the virus to ACE2 by not only exposing the viral binding site on S1 domain but also revealing the effusion site on the S2 domain in the viral spike. Consequently, the virus undergoes endocytosis and massive replication accompanied by profound activation by cathespsin L (CatL) and the abundant intracellular furin. The activated intracellular SARS-CoV-2 undergoes exocytosis where it binds again to ACE2 elsewhere, thus creating a vicious feed-forward devastating cycle. Importantly, heart failure is characterized by enhanced expression of myocardial ACE2, which is further upregulated by ACE-I, angiotensin receptor blockers (ARBs), and mineralocorticoid-receptor (MR) antagonists, thus sensitizing ACE2 expressing target organs to SARS-CoV-2. ADAM metallopeptidase domain 17 (ADAM 17) is responsible for shading of ACE2, a process stimulated by ANG II type 1 receptors (AT1-R) and may explain why renin-angiotensin-aldosterone system inhibitors augment ACE2 expression. ER, endoplasmic reticulum.
Binding of the SARS viral spike glycoprotein to ACE2 triggers its internalization along with the virus (13). This might be of supreme importance for cardiomyocytes of patients with heart failure, characterized by intense upregulation of ACE2 (9, 10, 37) (Fig. 1). Possibly, intracellular translocation of SARS-CoV-2 coupled with ACE2 leads to its depletion in cell membranes. It is tempting to assume that consequent ACE2 elimination might participate in many features of acute corona virus infection. Among such clinical characteristics are decompensation of preexisting CHF, respiratory distress irrespective to left-ventricular backward failure (due to impaired pulmonary capillary endothelium and endothelial barrier function), acute kidney failure (reflecting altered renal microcirculation and hypoxic injury), and diarrhea (caused by injured gut microcirculation with hypoxic damage and injured mucosal barrier.
Indeed, corona virus has already been shown to induce myocardial inflammation and dysfunction accompanied with adverse cardiac outcomes in patients with SARS, assumedly due to downregulation/elimination of the myocardial ACE2 system (25). Support for this concept emerges from previous experimental reports demonstrating cardiac contractility defects in rats with reduced X chromosomal-derived ACE2 expression and heart failure with pulmonary congestion in ACE2-knockout mice (ACE2-KO) (4, 34). These undesired changes were prominent in males and progressed with age, coincidentally overlapping the observations that elderly and men are more susceptible to SARS-CoV-2-induced serious infection. Interestingly, the hearts of animals depleted of ACE2 exhibited similar changes that occur after coronary artery disease or bypass surgery in humans (5). Subsequent studies demonstrated extended infarct size, reduced contractility, altered ventricular remodeling, and increased mortality following myocardial infarction (MI) induced by ligation of the proximal LAD in mice with ACE2 deletion, as compared with their wild-type controls (20). Moreover, these mice showed enhanced oxidative stress and concomitant upregulation of proinflammatory cytokines, plausibly parallel to the observed hypersensitive immunological response reported in patients with SARS-CoV-2 infection.
Furthermore, preliminary alarming data from SARS-CoV-2-infected patients suggested that those treated with renin-angiotensin-aldosterone (RAAS) inhibitors such as angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin-receptor blockers (ARBs) experienced severe symptoms with a higher mortality rate as compared with nonuser counterparts (7, 26). Noteworthy, cardiac ACE2 expression is markedly enhanced in response to RAAS blockade by ACEi (24), ARB (8, 18, 19), and even by mineralocorticoid receptor (MR) antagonist (19, 21) (Fig. 1). Conceivably, this is translated into increased vulnerability of patients with RAAS blockade during SARS-CoV-2 infection. Furthermore, several studies have demonstrated that binding of ANG II to its AT1 receptors in target organs, including the heart, activates ADAM 17, a sheddase affecting ACE2 (22) (Fig. 1). Conclusively, blocking ANG II synthesis or its binding to AT1 receptors by RAAS inhibitors likely leads to the upregulation of ACE2 and eventually hypersensitizing the heart to SARS-CoV-2 infection. Enhancement of myocardial invasion by the virus due to enhanced ACE2 likely plays an important role.
An additional player that may contribute to the vulnerability of patients with heart failure to SARS-CoV-2 is furin, also termed paired basic amino acid-cleaving enzyme (PACE). Furin is essential for permeating viral functionality as it cleaves viral envelope trimeric transmembrane glycoprotein (S) (12, 14, 29). This S-glycoprotein, vital for the entry of the virus into the cell, contains two functional domains: an ACE2-binding domain (also called receptor-binding domain (RBD) and a second domain essential for fusion of the viral and cell membranes (23, 33, 35). Furin activity exposes the binding and fusion domains essential for the entry of the virus into the cell (32). Furin presents mainly intracellularly and to a lesser extent in the circulation (16) (Fig. 1), where it converts ventricular proBNP to active BNP, an important physiological process in heart failure subjects. Patients with heart failure are specifically characterized by upregulation of cardiac furin, providing an additional potential explanation for their vulnerability Covid-19 infection (17) (Fig. 1). Moreover, furin is detected in circulating T cells that are activated during infections (27). This may form a feed-forward loop of furin-facilitated coronavirus replication that may be responsible for hypersensitive immunological response (cytokine storm) in some patients, leading to fulminant myocarditis, devastating lung injury, and lethal multiorgan failure.
Collectively, evidently ACE2 exerts beneficial effects on cardiac function under normal conditions and particularly in the presence of heart failure. Moreover, some of the cardioprotective effects of ACE inhibitors, ARBs, and MR blockers are mediated by their positive impact on ACE2 abundance in cardiac tissues. Nevertheless, in patients infected with SARS-CoV-2, ACE2 may transform to a Trojan horse. Its binding with ACE2 neutralizes the advantageous cardiac effects of this enzyme, especially in patients with heart failure. The susceptibility of these subjects to life-threatening SARS-CoV-2 infection could be attributed to the simultaneous upregulation of both ACE2 and furin in the diseased myocardium and to the wide use of RAAS inhibitors in this population (Fig. 1). Therefore, temporary blockade of the viral binding site on ACE2 or furin by immunological or pharmacological means in patients infected with SARS-CoV-2 may compose new therapeutic strategies in combating this unprecedented formidable viral threat.
GRANTS
Z. Abassi acknowledges research support from the Israel Science Foundation Grant 544/18.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.A.A. and E.E.K. prepared figure; Z.A.A., S.A., E.E.K., and S.N.H. drafted manuscript; Z.A.A., S.A., E.E.K., and S.N.H. edited and revised manuscript; Z.A.A., S.A., E.E.K., and S.N.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Safa Kinaneh for technical assistance.
REFERENCES
- 1.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020. March 19 [Epub ahead of print]. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 26: 369–375, 2005. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- 3.Clarke NE, Turner AJ. Angiotensin-converting enzyme 2: the first decade. Int J Hypertens 2012: 307315, 2012. doi: 10.1155/2012/307315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 5.Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res 98: 463–471, 2006. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 6.Díez-Freire C, Vázquez J, Correa de Adjounian MF, Ferrari MF, Yuan L, Silver X, Torres R, Raizada MK. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics 27: 12–19, 2006. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- 7.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med pii: S2213-2600(20)30116-8, 2020. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–2610, 2005. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 295: H2373–H2379, 2008. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goulter AB, Goddard MJ, Allen JC, Clark KL. ACE2 gene expression is up-regulated in the human failing heart. BMC Med 2: 19, 2004. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobe JL, Der Sarkissian S, Stewart JM, Meszaros JG, Raizada MK, Katovich MJ. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci (Lond) 113: 357–364, 2007. doi: 10.1042/CS20070160. [DOI] [PubMed] [Google Scholar]
- 12.Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk HD, Garten W. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 360: 358–361, 1992. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- 13.Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD, Timens W, Turner AJ, Navis G, van Goor H. The emerging role of ACE2 in physiology and disease. J Pathol 212: 1–11, 2007. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosaka M, Nagahama M, Kim WS, Watanabe T, Hatsuzawa K, Ikemizu J, Murakami K, Nakayama K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem 266: 12127–12130, 1991. [PubMed] [Google Scholar]
- 15.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol 90: 783–790, 2005. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 16.Ichiki T, Burnett JC Jr. Post-transcriptional modification of pro-BNP in heart failure: is glycosylation and circulating furin key for cardiovascular homeostasis? Eur Heart J 35: 3001–3003, 2014. doi: 10.1093/eurheartj/ehu381. [DOI] [PubMed] [Google Scholar]
- 17.Ichiki T, Huntley BK, Burnett JC Jr. BNP molecular forms and processing by the cardiac serine protease corin. Adv Clin Chem 61: 1–31, 2013. doi: 10.1016/B978-0-12-407680-8.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 43: 970–976, 2004. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 19.Karram T, Abbasi A, Keidar S, Golomb E, Hochberg I, Winaver J, Hoffman A, Abassi Z. Effects of spironolactone and eprosartan on cardiac remodeling and angiotensin-converting enzyme isoforms in rats with experimental heart failure. Am J Physiol Heart Circ Physiol 289: H1351–H1358, 2005. doi: 10.1152/ajpheart.01186.2004. [DOI] [PubMed] [Google Scholar]
- 20.Kassiri Z, Zhong J, Guo D, Basu R, Wang X, Liu PP, Scholey JW, Penninger JM, Oudit GY. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail 2: 446–455, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- 21.Keidar S, Gamliel-Lazarovich A, Kaplan M, Pavlotzky E, Hamoud S, Hayek T, Karry R, Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res 97: 946–953, 2005. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 22.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280: 30113–30119, 2005. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ocaranza MP, Godoy I, Jalil JE, Varas M, Collantes P, Pinto M, Roman M, Ramirez C, Copaja M, Diaz-Araya G, Castro P, Lavandero S. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension 48: 572–578, 2006. doi: 10.1161/01.HYP.0000237862.94083.45. [DOI] [PubMed] [Google Scholar]
- 25.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 39: 618–625, 2009. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perico L, Benigni A, Remuzzi G. Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. Nephron 23: 1–9, 2020. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, Andersson J, Shevach EM, Quezado M, Bouladoux N, Roebroek A, Belkaid Y, Creemers J, O’Shea JJ. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature 455: 246–250, 2008. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos RA, Ferreira AJ, Simões E Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol 93: 519–527, 2008. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 29.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol 3: 753–766, 2002. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 31.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 32.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell pii: S0092-8674(20)30262-2, 2020. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94: e00127-20, 2020. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M, Tatara Y, Shiota A, Sugano S, Takeda S, Rakugi H, Ogihara T. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 47: 718–726, 2006. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W.. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. [Preprint]. bioRxiv. 2020. doi: 10.1101/2020.01.26.919985. [DOI] [PMC free article] [PubMed]
- 36.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol, 2020. March 5 [Epub ahead of print]. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation 108: 1707–1712, 2003. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]