Abstract
Mucus in the gastrointestinal (GI) tract is the primary point-of-interaction between humans and their gut microbiota. This not only intimates that mucus ensures protection against endogenous and exogenous opportunists but provision for the human microbiota to reside and flourish. With the emergence of living therapeutics, engineered microbes can deliver and produce increasingly complex medicine, and controlling the mucoadhesive properties of different microbial chassis can dictate dose-response in a patient. Here we present a redesigned, in vitro, plate-based assay to measure the mucus adhesion of various probiotics. Cell-mucus interactions were isolated by immobilizing mucus to the plate surface. Binding parameters were derived for each probiotic strain by measuring cell adhesion over a wide range of cell concentrations, providing dose-dependent adhesion metrics. Surface proteins and cell components known to influence mucoadhesion were then heterologously expressed or altered in Lactococcus lactis MG1363 and E. coli Nissle 1917 to control mucus-binding capacity, avidity, and cooperativity.
Keywords: Lactic acid bacteria, probiotics, microbiome, cell adhesion, mucus, mucoadhesion
As a new era of precision medicine and personalized therapeutics becomes increasingly mainstream, there too is heightened interest in our ability to leverage the human gut microbiota, a complex and rich community of approximately 1000 species of microbes living in the gastrointestinal (GI) tract. Although causality remains uncertain, recent evidence suggests a strong relationship between many disease states and a perturbed, dysbiotic, gut microbiota1,2. One strategy to restore microbial balance, and the basis of 282 clinical trials in the last decade3, is to introduce probiotics – microbes shown to confer health benefits. Probiotics have been shown to inhibit or exclude enteric pathogens4,5, modulate or improve mucosal barrier function6, reduce inflammatory responses7, ameliorate metabolic or toxin imbalances8, maintain intestinal pH, and contribute to neurohumoral signaling9. Furthermore, engineered probiotics as therapeutic delivery vehicles10-12 are being explored for pathogen eradication13, vaccines14, metabolic disorders, enzyme-replacement therapies15,16, cancer treatments17,18, and diagnostics19,20.
Somewhat counterintuitively, the stability of a healthy gut microbiota likely arises from its diversity and competitiveness rather than established cooperative metabolic networks21. Thus, an argument can be made that when developing probiotic therapeutics, one design focus should be to enhance factors conferring competitive opportunity (of course, while safeguarding against overgrowth). This dichotomy is further exemplified by the fact that lactic acid bacteria (LAB), which encompass the majority of common probiotics studied for disease amelioration, make up less than 1% of the human microbiota22. Furthermore, orally-administered probiotics are rarely detected past a week after ingestion, and persistence is highly strain- and patient-specific23, making dose-response relationships unreliable24. From an engineering perspective, directing a microbe toward a synthetic application inherently places a burden that conciliates competitive advantage compared to its natural counterpart in the body; essentially, current strategies cannot persist, or even survive, long enough to be effective.
The most prominent point-of-interaction between microbes and humans is at mucus membranes, revealing mucus adhesion as a prime target for controlling probiotic occupancy. Indeed, the cell-surface structures of commensal bacteria are hypothesized to have coevolved in the presence of mucus glycans, creating preferential mucoadhesive mechanisms25. Additionally, the extracellular polysaccharides (EPS) secreted for LAB biofilm formation can influence colonization through mucus associations26,27, while pathogenic biofilm formation is impeded by LAB extracellular polysaccharides and mucus itself28. Mucin has also been observed to increase surface-protease expression in LAB, suggesting the utilization of mucus as a nutrient source29. Despite these interactions and the mucus matrix of the GI tract providing a topography for microbes to find spatial niches, orally-administered natural probiotics and engineered microbial therapeutics do not currently provide any means of controlling biogeographical targeting, residence time, or an ability to colonize the gut. The ability to accurately measure and engineer mucus adhesion could address these limitations and provide a new avenue to increase efficacy of natural and engineered probiotic therapies.
Methodologies to quantify bacterial adhesion to mucus have been adapted from traditional cell adhesion assays and inherit similar advantages and disadvantages30. The general procedure behind these adhesion assays is to quantify the fraction of residual bacteria bound to mucus-functionalized surfaces after washes31. Quantifying residual bacteria can range from a simple cell count32 to surface plasmon resonance33 or atomic force microscopy34. The most common method to quantify mucus adhesion is to use a fluorescent indicator as a correlate for cell concentration. Surfaces are often modified by incubating with mucus or by culturing gut epithelial cell/organ tissue. However, recent studies showed that bacteria adhere to polystyrene, the support of mucus functionalization, as well or better than mucin- or cell culture-coated microplate wells35. Additionally, there are several examples of bacterial strains showing different adhesion characteristics depending on the mucus source36-38, and different mucus compositional profiles can be observed in co-culture models simply by differentiating the HT29 cell line with either methotrexate (MTX) or 5-fluorouracil (FU)39. The question arises then, whether these adhesion data are the result of the mucus composition affecting cell-cell associations, cell-mucus associations, or mucus-plastic associations. Measuring more holistic binding parameters when quantifying mucus adhesion would allow for better comparability across conditions.
In order to address these disconnects, we report an improved in vitro method for quantifying bacterial adhesion to mucus. Rather than relying on natural nonspecific interactions to coat a microplate with mucus, we covalently functionalized surfaces with mucus, providing robust and full coverage. Our assay is able to isolate the cell adhesion events associated directly with mucus. We then measure adhesion over a range of cell concentrations to develop strain-specific mucus-binding curves. By more clearly describing mucus adhesion using strain-specific quantifiers interpolated from a mucus binding curve, we can better compare various binding characteristics related to binding capacity, binding avidity, and cooperativity for common probiotic strains as well as common probiotic engineering strains. We also demonstrate how the expression of recombinant characterized and putative mucus-binding proteins, or deletion thereof, can be used to alter mucus-binding characteristics of probiotics and how these changes are reflected in the binding curves. Such altered mucus-binding has never been engineered previously. Overall, our methods and results provide a novel and robust approach to quantifying bacterial adhesion to mucus and advances design of microbial therapeutics with tunable pharmacological release factors.
RESULTS AND DISCUSSION
Silanization and EDC-coupling covalently binds mucus to the plate surface
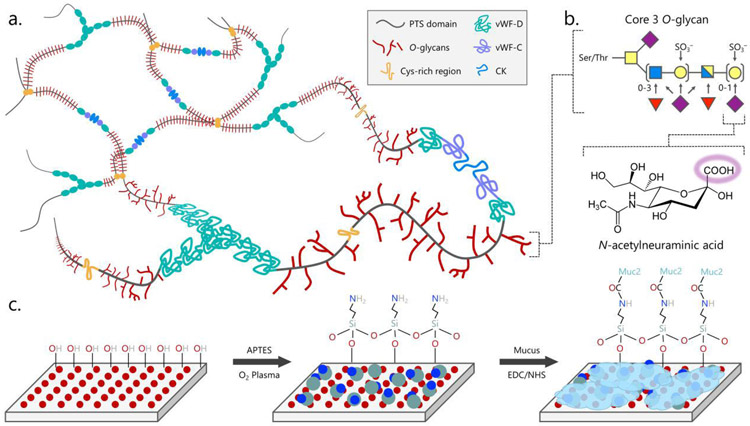
A comparison of traditional in vitro models used to study bacterial adhesion to the intestinal epithelium revealed that differences between bacterial strains binding to mucus are significantly confounded by their ability to strongly stick to the abiotic surface of the microplate35. To remedy this, we sought to covalently link mucus to the plate, providing full mucus coverage and removing cell-plate interactions. To perform this, the microplate surface was silanized with (3-aminopropyl)triethoxysilane (APTES) to provide a surface of available primary amine groups. The most abundant gel-forming mucin protein in the mammalian small intestine is Muc2, which is contains sialylated and sulfated terminal glycans that contain freely accessible carboxyl groups40,41. Using the primary amine on the plate and available carboxyl groups from the terminal sialic acids of Muc2, mucus was then crosslinked to the silanized microplate using a common N-ethyl-N’-(3-dimethyl-aminopropyl) carbodiimide and N-hydroxysuccinimide (EDC/NHS) reaction mixture (Figure 1). Porcine intestinal mucus (PIM) was used as an analog for human mucus because of the compositional and rheological similarities42,43. Though mammalian mucus glycobiology is diverse and species specific, core 3-derived O-glycans are relatively consistent (Figure 140 and are the major glycan structure of Muc2 in the mammalian intestine44.
Figure 1.
Schematic of mucus immobilization strategy
The structure of mucus presents an opportunity for covalent immobilization chemistry. Gel-forming mucins such as Muc2 form (a.) macromolecular sheets of trimers of dimers that crosslink or interact to form a mesh between von Willebrand Factor (vWF) domains and cysteine knots (CK). Each mucin monomer has a highly glycosylated proline-threonine/serine (PTS) domain with the (b.) highly sialylated core 3 O-glycan dominating in the intestines. Sialic acid, or N-acetylneuraminic acid (purple diamond), provides a freely available carboxylic acid that can be (c.) coupled to a primary amine presented on a glass or plastic surface using EDC/NHS crosslinking chemistry. Other common glycans include N-acetylglucosamine (blue square), N-acetylgalactosamine (yellow square), galactose (yellow circle), and fucose (red triangle).
*Final optimized conditions for full mucus coverage require 1% PIM, 10x higher than other conditions, and ELISA luminescence values increased proportionally.
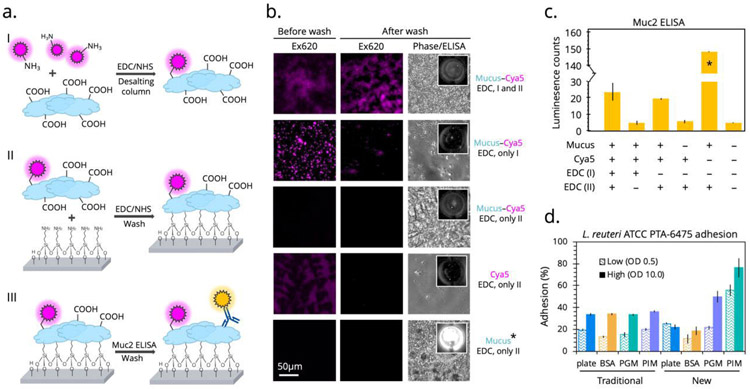
Previous reports indicate that it is possible to immobilize mucus using EDC-coupling, wherein mucus provides primary amine groups for the reaction33. Because our proposed reaction scheme requires mucin to donate carboxyl groups, we wanted to confirm that EDC-coupling was indeed possible with the plate surface and that the reaction was not hindered by a self-reaction involving amines within the mucins themselves. To accomplish this, we performed two EDC reactions in tandem. First, between fluorescent sulfo-cyanine5 amine (cya5) and PIM in which mucus provides carboxyl groups and a subsequent filtration removes any unbound fluorophore. Second, between PIM and the microplate in which vigorous wash steps can separate non-specifically bound mucus from that which is covalently attached to the plate. Fluorescence was observed before and after washing, and the presence of mucus was confirmed by a follow-up Muc2-specific enzyme-linked immunosorbent assay (ELISA) (Figure 2a). As shown in Figure 2b, when both EDC-coupling reactions are performed, not only is fluorescence observed initially, suggesting covalent linking to the mucus, but it remains after multiple washes, suggesting the mucus is stably linked to the microplate surface. Absence of the EDC reagent at either stage results in no fluorescence or plate binding, as expected. Similar results were observed from the subsequent Muc2-specific ELISA (Figure 2c). These results confirm the directionality of the EDC-coupling reaction and that mucus was covalently bound to the microtiter plate. Covalently bound mucus provided further opportunities for optimizing the cell adhesion assay, including automated wash steps and added shear stress during the incubation using orbital shaking.
Figure 2.
Validating the modified cell mucoadhesion assay and comparing to the traditional assay
(a.) Two EDC/NHS reactions were performed in tandem followed by a Muc2-specific ELISA to determine the success of the mucus immobilization. (b.) Fluorescence microscopy was then implemented to measure of the robustness and directionality of the coupling between mucus and the sulfo-cyanine5 fluorophore and between mucus and the plate surface. Each condition was imaged before and after vigorous washing and after the ELISA. (c.) Luminescence counts were quantified to determine mucus coverage, with the final optimized condition (1% PIM) measuring 10-fold higher than other conditions, as expected. (d.) With these final conditions, the traditional adhesion assay was compared to the newly optimized assay using the strong binder L. reuteri ATCC PTA-6475 as a metric for success on different protein surfaces. Percent adhesion was calculated from fluorescent output of cFDA-stained cells.
Modified microtiter plate assay reveals dependence of cell adhesion on mucus type
Bacterial strains isolated from mammalian intestines have been reported to preferentially bind mucus over other general proteins such as bovine serum albumin (BSA)38. Unfortunately, when using the traditional cell adhesion assay to test the mucoadhesive ability of Lactobacillus reuteri ATCC PTA-6475 at OD600 0.5, we were not able replicate preferential binding to mucus over BSA (Figure 2d). Only after implementing our newly modified covalent immobilization, did we observe significant differences between binding to polystyrene plate, BSA, porcine gastric mucus (PGM), and PIM by L. reuteri ATCC PTA-6475. Specifically, there was a larger percent of adhered L. reuteri ATCC PTA-6475 cells to PIM than the plate, BSA, and PGM (Figure 2d), potentially recapitulating this strain’s co-evolution with, and preferential binding to, intestinal niches.
It has been previously reported that mucus gel reconstituted from commercially available PGM does not accurately replicate the pH-dependent rheological characteristics (viscosity and elasticity) of native mucus45 and has an altered structure, likely from industrial processing46. Polymer rigidity, influenced by the negative charges of sulfates and terminal sialic acids, is an important determinant of the viscosity and elasticity of mucus47, which may explain the diminished cell adhesion capacity observed with the processed PGM (Figure 2d). Maintaining the glycan chemical identity of mucus is more important than macromolecular structural integrity when interrogating mucus adhesion. This is highlighted by the fact that a common mucoadhesion assay is to separate, purify, and freeze mucins to be later used in dot-blot assays in which no mucus gel is required48. As shown in Figure 2b, Muc2 antibody recognizes the covalently attached mucus indicating that important epitopes are preserved through our method processing.
We obtained similar preferential binding to covalently immobilized PIM at a high cell density (OD600 10.0) of L. reuteri ATCC PTA-6475 while the traditional assay demonstrated equivalent and non-specific maximum binding capacity of 35% under all conditions (Figure 2d). This was likely observed because the more vigorous washing required at higher cell loadings removes non-covalently attached proteins from the plastic surface. Additionally, while L. reuteri ATCC PTA-6475 did not appear to bind any better to PGM than BSA or the plate at the low cell density, the binding capacity significantly increased at the higher cell density, becoming higher than the 35% maximum threshold of the uncoated plate (Figure 2d). It can then be inferred that covalently immobilized mucus enables detection of specific cell-mucus interactions rather than non-specific cell-plastic binding events that dominate on non-covalently functionalized plates. A final important aspect of the new assay is a higher throughput and automated wash procedure, adding consistency between technician operability without sacrificing replicate variability.
Different probiotics display vastly different mucoadhesive characteristics
Cell mucoadhesion is typically reported as a percent or fractional residual bound cells at a single cell concentration, often at OD600 0.537,38,48-51. To assess binding characteristics in greater resolution, we measured adhesion of several probiotic strains and species across a wide range of cell concentrations (OD600 0.01 to 100). Adherence was calculated by staining the cells with the fluorescent indicator carboxyfluorescein diacetate (cFDA) and detecting the fluorescence at each concentration before and after washes. The dye only fluoresces when taken up by the cell and deacetylated by native esterases. In order to avoid non-specific surface binding that may affect cell mucoadhesion, we stained the cells at a much lower concentration than typically used52 (10 μM versus 100 μM). There is no observed cytotoxicity at this concentration53, a sign that membrane integrity is maintained.
While there are some previous reports measuring dose-dependent adhesion of probiotics, these were either performed using a Caco2 monolayer54, therein not directly measuring mucus adhesion, or performed at very low cell concentrations (OD600 < 0.1), likely due to the limited dynamic range of the traditional assay55. With our more robust immobilized mucus plate, we aimed to develop a more quantitative and descriptive mucoadhesive measurement by expanding the dose range, introducing shear stress in the form of orbital plate shaking, and fitting a sigmoidal curve to the adhesion data to make direct comparisons without the need to normalize between strains. With many cell-mucus interactions determined to be lectin-like recognition of mucus glycans by surface proteins56-59, a sigmoidal Hill-type shape60 was chosen with the hypothesis that cell-mucus binding mirrors the energetics of ligand-receptor binding.
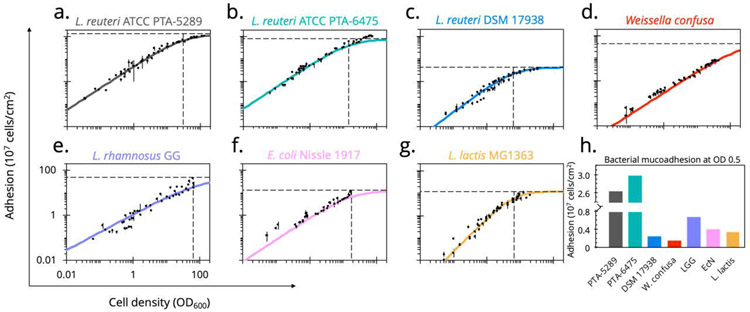
Using this method, distinct strain-specific differences between the mucoadhesive abilities of common probiotics become apparent (Figure 3). This was particularly interesting for L. reuteri human isolates. L. reuteri ATCC PTA-6475 and L. reuteri ATCC PTA-5289, which are nearly identical at the genome level61 clustered in the MLSA (multilocus sequence analysis) lineage II phylogenic group62. Notably, these strains also contain variations of cell and mucus-binding protein A (CmbA)49 and have been previously reported to have strong adhesion to mucus and intestinal epithelial cells (IEC)63. In contrast, L. reuteri DSM 17938 is from a different phylogenic branch (lineage VI) and has a much lower reported adherence to IECs48, which concurs with our observation that it attains a low maximum adherence capacity (Amax = 4.3×107 cells/cm2).
Figure 3.
Dose-dependent mucus-binding curves for common probiotics
(a.-g.) Using our optimized plate assay, the number of cells adhered to immobilized mucus was measured over a large range of concentrations for common probiotics. Data points are reported as the mean values of quadruplicate wells across three independent experiments. Hill-type sigmoidal curves were fit, with curve parameters a metric for strain-specific mucoadhesive characteristics (Table 1). (h.) With the traditional assay typically performed at OD600 0.5, comparative analysis across strains was visualized at this density.
Escherichia coli Nissle 1917 (EcN) is an extensively studied probiotic sold over-the-counter as Mutaflor® to treat chronic inflammatory and infectious intestinal diseases and is used significantly in the development of microbial therapeutics16,64-69. Though there are conflicting reports whether EcN can persist in human intestines68,70, it has been shown to colonize the porcine intestinal tract71. Therefore, our results indicate a surprisingly poor ability to adhere to PIM, as determined by this assay (Amax 12.9×107 cells/cm2). Like L. reuteri DSM 17938, its binding plateaus to its maximum relatively easily, reaching half maximum adhesion at ODA50 17.7 (Table 1). It has been suggested that biofilm-like aggregation is necessary for EcN colonization72 and that mucoadhesion is influential in initial binding before other competitive mechanisms take over. Additionally, compared to pathogenic strains, commensal E. coli have more flexibility in their ability to perform metabolic shifts depending on the mono- and disaccharide carbon sources available from mucin glycans73. This suggests commensal E. coli, such as EcN, may utilize mucus by finding a nutritional niche rather than adhering as a persistence mechanism.
Table 1.
Summary of mucus-binding curve parameters for common probiotics
| Strain |
aAmax (×107 cells/cm2) |
bODA50 | n |
|---|---|---|---|
| L. reuteri ATCC PTA-6475 | 77.4 ± 3.1 | 14.3 ± 1.5 | 0.98 ± 0.05 |
| L. reuteri ATCC PTA-5289 | 133.5 ± 7.5 | 30.0 ± 4.1 | 0.97 ± 0.05 |
| L. reuteri DSM 17938 | 4.3 ± 0.3 | 6.2 ± 1.1 | 1.11 ± 0.11 |
| Weissella confusa | 44.7 ± 0.0 | a203.7 ± 0.1 | 0.95 ± 0.001 |
| L. rhamnosus GG | 44.5 ± 0.3 | a99.0 ± 0.8 | 0.80 ± 0.02 |
| E. coli Nissle 1917 | 12.9 ± 0.2 | 17.7 ± 0.2 | 0.98 ± 0.04 |
| L. lactis MG1363 | 6.8 ± 0.4 | 3.7 ± 0.4 | 1.52 ± 0.14 |
Parameters determined from sigmoidal curve fitting of experimental adhesion data, shown with 95% confidence intervals
Extrapolated value from curve fitting
Standard curves to correlate OD600 to CFU/mL are reported for each strain in Table S1.
Another commonly used clinical probiotic is Lactobacillus rhamnosus GG (ATCC 53103), which has been reported to have strong mucoadhesion primarily through SpaCBA pilus-mediated interactions74. Notably, both EcN and L. rhamnosus GG show moderate mucus-binding ability and use moonlighting proteins with the secondary function of mucus adhesion but have quite different binding characteristics. While the probiotic nature of Weissella confusa is in question75, it was observed to have a similar binding capacity (Amax 45 ×107 cells/cm2) to L. rhamnosus GG, which corroborates a report of these strains binding strongly to HT29-MTX, a mucus-producing differentiated epithelial cell line76.
Finally, Lactococcus lactis is undoubtedly the most well-studied LAB and the basis of centuries of food engineering. Importantly, L. lactis MG1363 showed poor binding ability to PIM compared to other probiotic strains (Figure 3g). Despite similar curve parameters to L. reuteri DSM 17938, L. lactis MG1363 has a higher sigmoidal coefficient, creating a tighter window of concentrations in which mucus binding is influential compared to other strains.
Expression or removal of surface proteins can be used to alter adhesion characteristics
There are several surface proteins characterized on LAB that either moonlight or directly mediate mucoadhesion49. A database search revealed a number of known and putative mucus-associated proteins found on the L. reuteri strains being evaluated. Constitutively expressing three of these proteins in L. lactis MG1363 highly, moderately, and minimally altered the stain mucoadhesion characteristics.
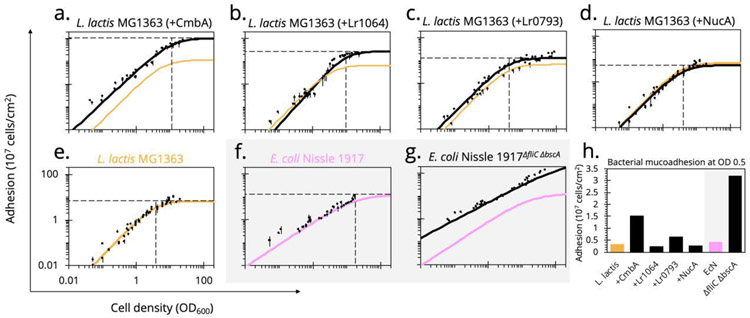
Cell and mucus-binding protein (CmbA) has been reported to significantly impact the ability of L. reuteri ATCC PTA-6475 to bind to Caco-2 cells and PIM25. The protein also contains a 288 base pair tandem repeat region, a characteristic found in surface proteins associated with mucus binding in various LAB77. When expressing CmbA on the surface of L. lactis MG1363, the mucus-binding ability of the strain drastically improved, creating a binding curve with similar parameters to that of L. reuteri ATCC PTA-6475 (Figure 4a and Table 2).
Figure 4.
Altered dose-dependent mucoadhesion by heterologous protein expression
Known and putative mucus-associating surface proteins from L. reuteri were heterologously expressed in L. lactis MG1363. The (e.) wild-type L. lactis MG1363 binding curve (orange) was (a.-c.) compared to the engineered counterpart (black) as well as the L. reuteri strain from which the protein was sourced (green or blue). (d.) As a display control, an inactivated NucAE41Q did not alter mucus binding compared to the wild-type strain. Alternatively, the flagellum of EcN, considered a known mucus-binding factor, and cellulose synthase, a contributor to biofilm formation, were (g.) knocked out but surprisingly improved mucoadhesion compared to the (f.) wild-type (pink). (h.) With the traditional assay typically performed at OD600 0.5, comparative analysis across strains was visualized at this density.
Table 2.
Summary of mucus-binding curve parameters when altering mucoadhesive protein expression
| Strain |
aAmax (× 107 cells/cm2) |
ODA50 | n |
|---|---|---|---|
| L. lactis MG1363 | 6.8 ± 0.4 | 3.7 ± 0.4 | 1.52 ± 0.14 |
| + NucAE41Q | 5.5 ± 0.4 | 4.0 ± 0.5 | 1.5 ± 0.13 |
| + CmbA | 104.3 ± 4.6 | 11.5 ± 0.8 | 1.37 ± 0.12 |
| + Lr1064 | 27.8 ± 1.7 | 9.4 ± 0.9 | 1.64 ± 0.12 |
| + Lr0793 | 13.0 ± 0.5 | 4.1 ± 0.5 | 1.48 ± 0.22 |
| E. coli Nissle 1917 | 12.9 ± 0.2 | 17.7 ± 0.2 | 0.98 ± 0.04 |
| ΔfliC,ΔbscA | 368.3 ± 5.5 | a230.5 ± 3.6 | 0.78 ± 0.02 |
Parameters determined from sigmoidal curve fitting of experimental adhesion data, shown with 95% confidence intervals
Extrapolated value from curve fitting
Despite the observed poor mucus-binding ability of L. reuteri DSM 17938, the cell surface of L. reuteri SD2112, the genetically equivalent parental strain, has been characterized with multiple identified putative mucus binding proteins, including Lr0793 and Lr106478,79. The protein Lr0793 is an ATP-binding cassette (ABC)-transporter component of an amino acid transporter, homologous to collagen-binding protein (CnBP) and mucus adhesion promoting protein A (MapA) with 97% sequence identity. These homologs have been described as binding to collagen type I80, gastric mucus81, and Caco-2 cells77. However, expressing Lr0793 on the surface of L. lactis MG1363 only slightly improved maximum adherence capacity to PIM compared to wild-type while ODA50 remained unchanged (Figure 4c and Table 2). Similarly, Lr1064 is an ABC-transporter component of a metal ion transporter and member of the LraI family82. These proteins have been reported to be adhesins of oral streptococci77. When expressed on L. lactis MG1363, Lr1064 increased the maximum adherence capacity (27.8×107 cells/cm2) while keeping the ODA50 similar to wild-type, thus moderately improving mucoadhesion (Figure 4b and Table 2). Additionally, when testing the binding ability of L. lactis MG1363 expressing Lr1064 on BSA rather than PIM, there was no observed improved adhesion, reinforcing the observed differences are in fact mucus-specific cell adhesion caused from surface protein expression (Figure S1a). This further emphasizes that our processing does not alter mucin glycans necessary for cell adhesion. These studies demonstrate the binding of recombinant L. lactis recapitulates that of the strain from which the mucus binding proteins were sourced.
Expression of each mucus-associated protein was confirmed by Western Blot (Figure S2), and surface display was confirmed by flow cytometry (Figure S3) using anti-His-tag antibodies. The pSIP expression vectors have previously shown successful secretion of the reporter nuclease A (NucA) in LAB83. We displayed an inactive mutant (NucAE41Q) on L. lactis, as a display control, and confirmed that this strain demonstrated no improvement in mucus binding (Figure 4d), again reiterating that the L. reuteri proteins enhance binding in a mucus-specific manner.
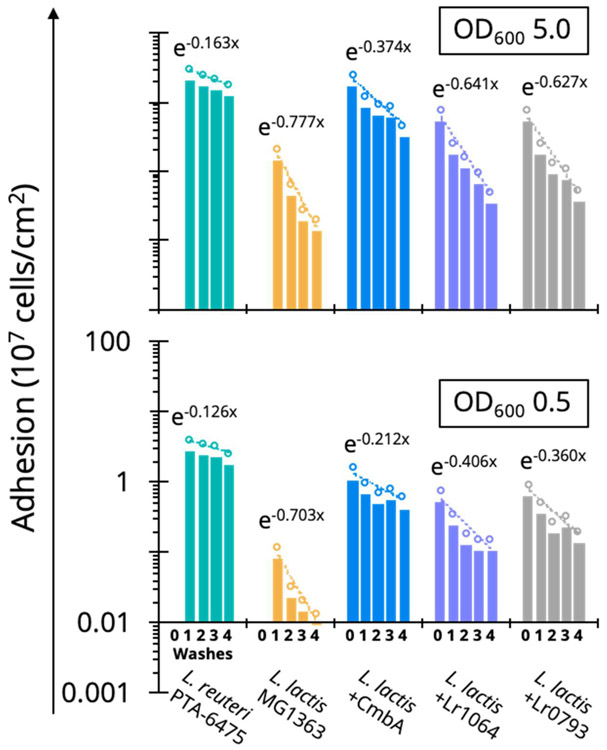
In consideration of altering mucus adhesion to increase probiotic residence time in the gut, the engineered L. lactis MG1363 strains were more resilient against washout on the plate than the wild-type counterpart. By measuring fluorescence over successive washes, strong mucus binders, such as L. reuteri ATCC PTA-6475, not only adhere at a higher initial density, but they remain bound over multiple applications of shear stress (Figure 5). This resilience is also recapitulated to different degrees in the engineered L. lactis MG1363 strains, indicated by a less negative slope over the washing steps (i.e. fewer cells removed compared to wild-type L. lactis MG1363). The improvement is more prominent at a low cell density of OD600 0.5 compared to OD600 5.0 (Figure 5), which may be a result of saturating the mucus binding sites available on the plate at higher cell densities. Lactic acid bacteria are some of the few species that can survive in the harsh conditions of the stomach and small intestine, resulting in low microbial biomass (102-105 cells/mL) in this section of the GI tract84. Our results suggest residence time of delivered probiotics or microbial biotherapeutics can be increased in the small intestines without necessarily requiring competitive binding or displacement. We have performed a preliminary competition assay, testing the ability of a strong mucus binder to displace a poor mucus binder, and again only observe competition only at very high cell densities (Figure S4).
Figure 5.
Mucoadhesive resilience against washout for engineered LAB
By observing the remaining fluorescent cells adhered to the plate over successive washes, a washout factor can be calculated from the trend (the exponential slope). The less negative the slope, the fewer cells are washed away each round, suggesting that not only can strong mucus binders (L. reuteri ATCC PTA-6475) adhere at higher initial density, but they are more resilient in the presence of shear stress. Resilience can be engineered by expressing mucus-adhesion factors on the surface of a poor mucus binder (L. lactis MG1363). Resilience against washout is also more apparent at a low cell density (OD600 0.5) when mucus binding sites are not saturated on the microtiter plate.
While mucus adhesion influences residence time and biogeography, the primary selective pressure in the gut is likely centered around metabolism. For example, in a mouse microbiota with low diversity (associated with dysbiosis), EcN was observed to shift from gluconate to N-acetylglucosamine metabolism as a stress response85. Additionally, commensal E. coli are more flexible and sequential in their carbon utilization while pathogenic E. coli consume multiple carbon sources simultaneously in a slash and burn approach, highlighting the different strategies between scavenger and infection systems in the gut73.
The major mucus adhesin on EcN is considered to be the flagellum, formed with the structural subunit FliC86. Additionally, EcN expresses a cellulose synthase (BscA) necessary for biofilm formation and adhesion to epithelial cells87. We sought to assess the role of FliC and BcsA in EcN mucus binding. Somewhat unexpectedly, and counter to previous reports, deleting both genes (EcNΔfliC,ΔbscA) increase the mucoadhesive ability of EcN (Figure 4g), shown by a curve shift up and to the left. This relationship is reversed, however, when the adhesion of each strain was subsequently tested on polystyrene alone (Figure S1b). Recently, the E. coli flagellum was shown to adhere more tightly to hydrophobic abiotic surfaces than hydrophilic substrates and may in fact impede other surface adhesins as a tradeoff for mobility88. This behavior is consistent with our results in Figure 4g, suggesting the adhesion of EcN to the hydrophilic mucus surface was improved by removing the flagellum, and it is possible that previous measurements of EcN mucoadhesion were either confounded by the cells binding other available abiotic surfaces like plastic. These results emphasize the importance of covalently immobilizing mucus and measuring global binding parameters across a wide range of cell concentrations. Beyond the initial observation that more EcNΔfliC,ΔbscA cells remain bound than EcN, the binding parameters show a stark difference in curve shape between the wild-type and knockout strains, perhaps suggesting different mechanisms of adhesion (Table 2). This is further emphasized when observing binding on polystyrene alone and with the observation that the removal of the flagellum improves binding to PIM and BSA indiscriminately (Figure S1). Despite a 46% increase in adhesion at OD600 0.5, EcNΔfliC,ΔbscA retains a similar curve shape to EcN because nonspecific interactions dominate the adhesion of both cell types to polystyrene (Figure S1c). Curve shapes are altered when protein-protein interactions (mucus-specific or otherwise) at surfaces dominate (Figure 4h).
METHODS
Microbial strains, plasmids, and growth conditions
All lactobacilli and Weissella confusa were cultured in de Man-Rogosa-Sharpe (MRS) medium (RPI Corp, Mt. Prospect, IL) at 37 °C in static microaerobic conditions. Lactococcus lactis subsp. cremoris MG1363 was cultured in GM17 medium (M17 broth (BD Difco, Franklin Lakes, NJ), 0.5 % (w/v) glucose) at 30 °C in static microaerobic conditions. E. coli strains were cultured in lysogeny broth (LB) (VWR International, Randor, PA) at 37 °C with rotary shaking at 250 rpm. All media was solidified using 1.5 % (w/v) agar (Teknova Inc, Hollister, CA). E. coli TG1ΔendA was used as a host for the construction of the pSIP-based expression vectors listed in Table S2 and cultured as above only supplemented with erythromycin (200 μg/mL) (RPI Corp). When L. lactis subsp. cremoris MG1363 was used as hosts for these vectors, the strains were cultured as above only supplemented with erythromycin (5 μg/mL).
All cloning was performed in E. coli TG1ΔendA. Beginning with the plasmid pSIP411, the inducible promoter PsppQ89 was replaced with the constitutive synthetic promoter P1190 using the surrounding sites for restriction endonucleases BglII and NcoI. The genomic DNA of L. reuteri ATCC PTA-6475 and L. reuteri DSM 17938 were used as templates to amplify the known and putative mucus-associating factors in this study using colony PCR. The pSIP411-P11 backbone, lr1064, and lr0793 were digested with NcoI and XbaI while cmbA was digested with NdeI and XbaI. A site mutation was introduced into nucA using overlap PCR and the inactive nucAE41Q was inserted into a surface-display pSIP411-P11 variant (previously unpublished) by digestion with XhoI and XbaI. This created a fusion construct of secretion signal peptide lp305091, the N-terminal LysM anchoring motif lp301491, and nucAE41Q. All cloning enzymes were purchased from New England Biolabs (Ipswich, MA).
Transformation conditions
L. lactis MG1363 transformations require GSGM17 medium (GM17, 0.5 M sucrose, 2.5% (w/v) glycine). Electrocompetent cells were prepared by first growing in 5 mL GM17 overnight from a frozen stock optimized for GSGM17 growth. The resulting overnight was subcultured 1:9 in 10 mL GSGM17 and grown overnight. This final overnight culture was subcultured 1:7 in 40 mL GSGM17 and grown 6-7 h (OD600 0.17). Cells were centrifuged at 3000 × g at 4 °C for 25 min and washed once in 40 mL ice-cold EP buffer (0.5 M sucrose, 10 % (w/v) glycerol). The cells were then suspended in 20 mL ice-cold EP buffer and incubated for 15 min on ice. Finally, cells were suspended in 400 μL ice-cold EP buffer and stored at −80 °C in 50 μL aliquots. To transform, 500 ng DNA, always diluted in dH2O to a final volume of 5 μL, was added to 50 μL electrocompetent cells and incubated on ice for 2 min. This mix was transferred to a pre-chilled 2 mm electroporation cuvette and electroporation was performed (2.0 kV, 25 μF, 200 Ω) using a Gene Pulser Xcell (Bio-Rad Laboratories, Hercules, CA) electroporation system. Cells were recovered by immediately adding 950 μL pre-warmed GM17 supplemented with recovery salts (20 mM MgCl2, 2 mM CaCl2) and incubating statically at 30 °C for 1.5 h. Cells were plated on selective GM17 with erythromycin (5 μg/mL) and grown for at least 24 h at 30 °C.
Isolation of primary porcine intestinal mucus
The intestines of 8-10-week-old pigs were obtained from a local supplier (Research 87 Inc, Boylston, MA). The small intestine was sectioned into 60 cm segments, squeezed of chyme, and placed in ice-cold phosphate buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4). Each section was then splayed longitudinally, washed by dipping into a tray of ice-cold PBS, and moved to a second tray of ice-cold PBS. Pinning one end of an intestinal segment, a silicone spatula was used to scrape the mucus layer, placing the mucus in a beaker of ice-cold PBS containing a cOmplete protease inhibitor cocktail tablet (Roche, Basel, Switzerland). The crude PIM was centrifuged at 11000 × g at 4 °C for 10 min, and the supernatant was centrifuged again at 26000 × g at 4 °C for 15 min using an Optima L-90K (Beckman Coulter, Brea, CA) ultracentrifuge with a 70-Ti fixed angle rotor (Beckman Coulter). This clarified PIM supernatant was then lyophilized and stored at 4 °C.
Plate surface functionalization
The wells of 96-well clear polystyrene U-bottom (Greiner Bio-One, Kremsmunster, Austria) microtiter plates were activated by oxygen plasma etching using a PE-200 (Plasma Etch, Inc. Carson City, NV) industrial benchtop plasma processing system with a R301 RF Generator (Seren IPS, Inc., Vineland, NJ) power supply (105 W, 1 min plasma time, 50 psi vacuum pressure, 20 cc/min O2 flow). Immediately, 200 μL of APTES (5% v/v in ethanol) was added to each well, and the plate was sealed with parafilm and incubated at room temperature for 15 min. The plate was then washed twice with ethanol and dried with an air stream, always minimizing exposure to humidity or water. A 1% PIM solution was prepared in PBS. Separately, a 10x solution of EDC (Thermo Fisher Scientific, Waltham, MA) was made in PBS. The EDC was added to the 1% PIM to a final concentration of 5 mM and placed on a rocker for 5 min. During this time, NHS (TCI Chemicals, Portland, OR) was weighed out and added to the 1% PIM to a final concentration of 10 mM, again using a rocker to mix for 5 min. Finally, 200 μL of the PIM/EDC/NHS was added to each well of the APTES functionalized plate. The plate was then sealed with parafilm and incubated at room temperature for 2 h before being left at 4 °C overnight.
Fluorescent labelling of bacteria
All strains were grown to early stationary phase in 200 mL of their respective growth media (approximately 10 h for LAB strains). To promote flagellum expression E. coli was grown for 24 h at 30 °C at 85 rpm. Cells were then centrifuged at 3000 × g at 4 °C for 10 min and washed three times with 50 mL PBS. Cells were then diluted in PBS to OD600 1.0 and labelled by the addition of carboxyfluorescein diacetate (cFDA) (Thermo Fisher Scientific) to a final concentration of 10 μM by incubating at 37 °C for 40 min with rotary shaking at 85 rpm. Again, carboxyfluorescein (cF)-labelled cells were washed three times as above and suspended in 10 mL PBS.
Mucus adhesion assay
Stained cells were diluted using PBS from the 10 mL stock to OD600 values approximately evenly spaced across three orders of magnitude. The mucus solution left after plate functionalization was removed from the microtiter plate by aspiration. Two hundred microliters of each dilution were plated in four technical replicates. A separate 225 μL of each OD600 dilution was placed in microfuge tubes, and both the plate and tubes were then incubated at 37 °C for 4 h with orbital shaking at 500 rpm. Using an AquaMax4000 (Molecular Devices, San Jose, CA) plate washer with a cell wash head, the plate was washed three times with PBS (center aspiration 50 μL/well/s; wall dispense 7.2 mL/plate/s; probe height 3 mm) ending with a final aspiration step. The adhered cells were lysed by adding 200 μL lysis buffer (1% (w/v) SDS, 0.1 M NaOH) and incubating at 37 °C for 1 h with rotary shaking at 750 rpm. As a control, each strain was incubated in lysis buffer for 1 h and then plated to check for survival. Even at extremely dense cell concentrations (OD600 10.0), no growth was observed, confirming full cell lysis (data not shown; plates showed no colonies after 48hr). Additionally, the 225 μL cell dilutions were centrifuged at 5000 × g for 5 min and suspended in lysis buffer as fluorescence controls, to relate bulk fluorescence to cell density. All lysates were transferred to a 96-well F-bottom black FluoTrac (Greiner Bio-One) microtiter plate. The plate was passed over with a flame to remove bubbles and fluorescence was measured (Ex/485 nm, Em/520 nm) using a SpectraMax M3 (Molecular Devices) plate reader. Blank wells containing only lysis buffer were used for background subtraction.
The traditional mucus adhesion assay was adopted from MacKenzie, et al38, Briefly, 200 μL of 0.1% PIM in PBS was added to a 96-well F-bottom black FluoTrac (Greiner Bio-One) microtiter plate and incubated overnight at 4 °C. The plate was then washed three times with PBS, by vacuum aspirating and adding PBS with a multichannel electronic pipette at the slowest dispense speed. The plate was incubated for 1 h at room temperature with Pierce Protein-Free Blocking Buffer (Thermo Fisher Scientific) and again washed with PBS three times. The cF-labelled cells were added at OD600 0.5 and incubated for 4 h at 37 °C under static conditions. After another three washes with PBS, remaining cells were lysed with lysis buffer. Fluorescence was measured as above.
Adhesion curve fitting and statistical analysis
Plate data was background corrected by subtracting the relative fluorescence units (RFU) of blank wells containing only lysis buffer. The means of experimental replicates were used for further data analysis. By plotting the standards as RFU vs OD600, the slope of a linear trend (C1) was used to associate RFU per unit OD600. This relationship, along with the volume-to-surface-area relationship (V/A) derived from a microtiter plate schematic (Figure S5a) and the CFU/mL per OD600 (C2), can be used to convert values to adhered cells per unit area (Ay):
Values for C1 and C2 are summarized in Table S1. A typical sigmoidal (Hill-type) curve was adapted to model fit cell adhesion:
Here, Ay is the adhered cells per unit area at cell concentration y, Amax is the maximum possible adhered cells per unit area, OD is the OD600 for cell concentration y, ODA50 is the OD600 required for half the maximum number of cells to adhere to the well, and n is the sigmoid factor. The curve parameters were obtained by using the nonlinear curve fitting function in MATLAB (fitnlm) with robust bi-square least squares regression analysis, which applies a weighting factor to discount outliers. Plots of cells per unit area versus OD600 were created to visualize the adhesion differences between strains.
CONCLUSIONS
Our improved mucus adhesion assay isolates mucus-specific interactions by immobilizing a confluent layer of mucus to a plate surface. This removes previously observed variability caused through non-specific adhesion mechanisms to the polystyrene while retaining the benefits of a minimalistic adhesion assay. The assay is further improved by implementing automated wash steps for consistency, and, measuring adhesion over a wide range of cell concentrations facilitates the interpolation of a mucus-binding curve. Curve parameters offer a more global metric for strain-specific mucoadhesion without the need to normalize to a reference strain. We observe clear dose-dependent differences in the binding ability of a number of probiotic strains with the curve parameters extending these relationships beyond the traditional measurement of percent adhesion at one cell density. Importantly, known and putative mucus-associated factors were expressed on the surface of a poor mucus binder, L. lactis MG1363, and mucoadhesion was significantly altered, reinstating the mucus-specific interactions of these cell-surface elements. Furthermore, a new understanding of EcN mucoadhesion was observed with the polar flagellum, originally considered a major mucoadhesin, actually impeding mucus adhesion and more general hydrophilic surface adhesion. This emphasizes the advantage of our updated assay over a traditional approach in which nonspecific interactions with the abiotic plate surface likely confounded mucoadhesion measurements. These results offer promise in the delivery and control of drug-release in the emerging field of living microbial therapeutics.
Supplementary Material
Figure S1. Adhesion of L. lactis MG1363 and E. coli Nissle 1917 to different substrates
Figure S2. Western blots to confirm recombinant expression of mucus-associated factors
Figure S3. Flow-cytometry confirms surface display of recombinant mucus-associated factors
Figure S4. Competitive binding of L. reuteri and engineered L. Lactis
Figure S5. Mucus characterization after EDC reaction and schematic of Greiner U-bottom plate geometry
Table S1. Linear relationships between RFU and OD600 and OD600 and CFU/mL for each strain
Table S2. List of strains, plasmids, and primers (XLSX)
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Huan-Hsuan Hsu and Prof. Xiaocheng Jiang for their advisement and resources when developing the surface immobilization procedure. A special thanks to Josef R. Bober for his advisement with surface-display in LAB.
FUNDING SOURCES
This work was financially supported by grant numbers R03HD090444 and DP2HD091798 of the National Institutes of Health and a Tufts Collaborates! grant from Tufts University.
ABBREVIATIONS
- ABC
ATP-binding cassette
- APTES
(3-aminopropyl)triethoxysilane
- BSA
bovine serum albumin
- BscA
cellulose synthase catalytic subunit A
- cFDA
carboxyfluorescein diacetate
- CmbA
cell and mucus binding protein A
- CnBP
collagen binding protein
- EcN
E. coli Nissle 1917
- EDC
N-ethyl-N’-(3-dimethyl-aminopropyl) carbodiimide
- ELISA
enzyme-linked immunosorbent assay
- EPS
extracellular polysaccharide
- FU
5-fluorouracil
- GI
gastrointestinal
- IEC
intestinal epithelial cell
- LAB
lactic acid bacteria
- MapA
mucus adhesion promoting protein A
- MTX
methotrexate
- NHS
N-hydroxysuccinimide
- NucA
nuclease A
- PGM
porcine gastric mucus
- PIM
porcine intestinal mucus
- PTS
proline-threonine/serine
- RFU
relative fluorescence unit
Footnotes
CONFLICTS OF INTEREST
None.
ASSOCIATED CONTENT
The following files are available free of charge.
SUPPORTING INFORMATION
Additional methods and accompanying figures to confirm mucus adhesion, the expression of mucus-associated proteins, and the specificity of the subsequent mucus binding experiments. A table listing all primers, strains, and plasmids.
• Methods for fluorescence microscopy, ELISA, and Western blot analysis, flow cytometry, and gene knockouts in E, coli Nissle 1917
REFERENCES
- (1).Shreiner AB; Kao JY; Young VB The Gut Microbiome in Health and in Disease. Curr Opin Gastroenterol 2015, 31 (1), 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Cho I; Blaser MJ The Human Microbiome: At the Interface of Health and Disease. Nat Rev Genet 2012, 13 (4), 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).US National Library of Medicine. Search: Probiotic; Lactic Acid Bacteria; Intestine | Exclude Unknown | Completed | 01/012018 - 12/31/2018 https://www.clinicaltrials.gov.
- (4).Ollech JE; Shen NT; Crawford C. v.; Ringel Y Use of Probiotics in Prevention and Treatment of Patients with Clostridium Difficile Infection. Best Pract Res, Clin Gastroenterol 2016, 30 (1), 111–118. [DOI] [PubMed] [Google Scholar]
- (5).Shafaghi A; Pourkazemi A; Khosravani M; Fakhrie Asl S; Amir Maafi A; Atrkar Roshan Z; Abaspour Rahimabad J The Effect of Probiotic Plus Prebiotic Supplementation on the Tolerance and Efficacy of Helicobacter Pylori Eradication Quadruple Therapy: A Randomized Prospective Double Blind Controlled Trial. Middle East J Dig Dis 2016, 8 (3), 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mennigen R; Nolte K; Rijcken E; Utech M; Loeffler B; Senninger N; Bruewer M Probiotic Mixture VSL#3 Protects the Epithelial Barrier by Maintaining Tight Junction Protein Expression and Preventing Apoptosis in a Murine Model of Colitis. Am J Physiol-Gastr Liver Physiol 2009, 296 (5), G1140–G1149. [DOI] [PubMed] [Google Scholar]
- (7).Gionchetti P; Rizzello F; Helwig U; Venturi A; Lammers KM; Brigidi P; Vitali B; Poggioli G; Miglioli M; Campieri M Prophylaxis of Pouchitis Onset with Probiotic Therapy: A Double-Blind, Placebo-Controlled Trial. Eur J Gastroenterol Hepatol 2003, 15 (7), 835. [DOI] [PubMed] [Google Scholar]
- (8).Krishnan S; Ding Y; Saedi N; Choi M; Sridharan G. v.; Sherr DH; Yarmush ML; Alaniz RC; Jayaraman A; Lee K Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep 2018, 23 (4), 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Hyland N; Stanton C The Gut-Brain Axis: Dietary, Probiotic, and Prebiotic Interventions on the Microbiota; London, UK: Academic Press is an imprint of Elsevier, 2016. [Google Scholar]
- (10).Bober JR; Beisel CL; Nair NU Synthetic Biology Approaches to Engineer Probiotics and Members of the Human Microbiota for Biomedical Applications. 2018, No. March, 277–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Waller MC; Bober JR; Nair NU; Beisel CL Toward a Genetic Tool Development Pipeline for Host-Associated Bacteria. Curr Opin Microbiol 2017, 38, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Mays ZJ; Nair NU Synthetic Biology in Probiotic Lactic Acid Bacteria: At the Frontier of Living Therapeutics. Curr Op Biotechnol 2018, 53, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hwang IY; Koh E; Wong A; March JC; Bentley WE; Lee YS; Chang MW Engineered Probiotic Escherichia Coli Can Eliminate and Prevent Pseudomonas Aeruginosa Gut Infection in Animal Models. Nat Commun 2017, 8, 15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Rosales-Mendoza S; Angulo C; Meza B Food-Grade Organisms as Vaccine Biofactories and Oral Delivery Vehicles. Trends Biotechnol 2016, 34 (2), 124–136. [DOI] [PubMed] [Google Scholar]
- (15).Isabella VM; Ha BN; Castillo MJ; Lubkowicz DJ; Rowe SE; Millet YA; Anderson CL; Li N; Fisher AB; West KA; et al. Development of a Synthetic Live Bacterial Therapeutic for the Human Metabolic Disease Phenylketonuria. Nat Biotechnol 2018, 36 (August), 857–867. [DOI] [PubMed] [Google Scholar]
- (16).West KA; Perreault M; Kurtz CB; Wagner DA; Charbonneau MR; Dagon Y; Degar AJ; Kotula JW; Millet YA; Brennan AM; et al. An Engineered E. Coli Nissle Improves Hyperammonemia and Survival in Mice and Shows Dose-Dependent Exposure in Healthy Humans. Sci Transl Med 2019, 11 (475), eaau7975. [DOI] [PubMed] [Google Scholar]
- (17).Chowdhury S; Castro S; Coker C; Hinchliffe TE; Arpaia N; Danino T Programmable Bacteria Induce Durable Tumor Regression and Systemic Antitumor Immunity. Nat Med 2019, 25 (July), 1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhang Y; Zhang Y; Xia L; Zhang X; Ding X; Yan F; Wu F Escherichia Coli Nissle 1917 Targets and Restrains Mouse B16 Melanoma and 4T1 Breast Tumors through Expression of Azurin Protein. Appl Environ Microbiol 2012, 78 (21), 7603–7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Danino T; Prindle A; Kwong GA; Skalak M; Li H; Allen K; Hasty J; Bhatia SN Programmable Probiotics for Detection of Cancer in Urine. Sci Transl Med 2015, 7 (289), 289ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Riglar DT; Giessen TW; Baym M; Kerns SJ; Niederhuber MJ; Bronson RT; Kotula JW; Gerber GK; Way JC; Silver PA Engineered Bacteria Can Function in the Mammalian Gut Long-Term as Live Diagnostics of Inflammation. Nature Biotechnology 2017, 35 (7), 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Coyte KZ; Schluter J; Foster KR The Ecology of the Microbiome: Networks, Competition, and Stability. Sci 2015, 350 (6261), 663–666. [DOI] [PubMed] [Google Scholar]
- (22).Douillard FP; de Vos WM Functional Genomics of Lactic Acid Bacteria: From Food to Health. Microb Cell Fact 2014, 13 (Suppl 1), S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Derrien M; van Hylckama Vlieg JET Fate, Activity, and Impact of Ingested Bacteria within the Human Gut Microbiota. Trends Microbiol 2015, 23 (6), 354–366. [DOI] [PubMed] [Google Scholar]
- (24).Ouwehand AC A Review of Dose-Responses of Probiotics in Human Studies. Benefic Microbes 2017, 8 (2), 143–151. [DOI] [PubMed] [Google Scholar]
- (25).Boekhorst J; Helmer Q; Kleerebezem M; Siezen RJ Comparative Analysis of Proteins with a Mucus-Binding Domain Found Exclusively in Lactic Acid Bacteria. Microbiol 2006, 152 (1), 273–280. [DOI] [PubMed] [Google Scholar]
- (26).Lebeer S; Verhoeven TLA; Francius G; Schoofs G; Lambrichts I; Dufrêne Y; Vanderleyden J; de Keersmaecker SCJ Identification of a Gene Cluster for the Biosynthesis of a Long, Galactose-Rich Exopolysaccharide in Lactobacillus Rhamnosus GG and Functional Analysis of the Priming Glycosyltransferase. Appl Environ Microbiol 2009, 75 (11), 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Salas-Jara M; Ilabaca A; Vega M; García A Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms 2016, 4 (4), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Caldara M; Friedlander RS; Kavanaugh NL; Aizenberg J; Foster KR; Ribbeck K Mucin Biopolymers Prevent Bacterial Aggregation by Retaining Cells in the Free-Swimming State. Curr Biol 2012, 22 (24), 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wickström C; Chávez de Paz L; Davies JR; Svensäter G Surface-Associated MUC5B Mucins Promote Protease Activity in Lactobacillus Fermentum Biofilms. BMC Oral Health 2013, 13 (43), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Mackie AR; Goycoolea FM; Menchicchi B; Caramella CM; Saporito F; Lee S; Stephansen K; Chronakis IS; Hiorth M; Adamczak M; et al. Innovative Methods and Applications in Mucoadhesion Research. Macromol Biosci 2017, 17 (8), 1600534. [DOI] [PubMed] [Google Scholar]
- (31).Khalili AA; Ahmad MR A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int J Mol Sci 2015, 16 (8), 18149–18184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Miyoshi Y; Okada S; Uchimura T; Satoh E A Mucus Adhesion Promoting Protein, MapA, Mediates the Adhesion of Lactobacillus Reuteri to Caco-2 Human Intestinal Epithelial Cells. Biosci, Biotechnol, Biochem 2006, 70 (7), 1622–1628. [DOI] [PubMed] [Google Scholar]
- (33).Uchida H; Fujitani K; Kawai Y; Kitzawa H; Horii A; Shiiba K; Saito K; Saito T A New Assay Using Surface Plasmon Resonance (SPR) to Determine Binding of the Lactobacillus Acidophilus Group to Human Colonic Mucin. Biosci, Biotechnol, Biochem 2004, 68 (5), 1004–1010. [DOI] [PubMed] [Google Scholar]
- (34).Gunning AP; Kavanaugh D; Thursby E; Etzold S; Mackenzie DA; Juge N Use of Atomic Force Microscopy to Study the Multi-Modular Interaction of Bacterial Adhesins to Mucins. Int J Mol Sci 2016, 17 (11), 1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Laparra JM; Sanz Y Comparison of in Vitro Models to Study Bacterial Adhesion to the Intestinal Epithelium. Lett Appl Microbiol 2009, 49 (6), 695–701. [DOI] [PubMed] [Google Scholar]
- (36).Le DTL; Tran TL; Duviau MP; Meyrand M; Guérardel Y; Castelain M; Loubière P; Chapot-Chartier MP; Dague E; Mercier-Bonin M Unraveling the Role of Surface Mucus-Binding Protein and Pili in Muco-Adhesion of Lactococcus Lactis. PLoS One 2013, 8 (11), e79850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Li XJ; Yue LY; Guan XF; Qiao SY The Adhesion of Putative Probiotic Lactobacilli to Cultured Epithelial Cells and Porcine Intestinal Mucus. J Appl Microbiol 2008, 104 (4), 1082–1091. [DOI] [PubMed] [Google Scholar]
- (38).MacKenzie DA; Jeffers F; Parker ML; Vibert-Vallet A; Bongaerts RJ; Roos S; Walter J; Juge N Strain-Specific Diversity of Mucus-Binding Proteins in the Adhesion and Aggregation Properties of Lactobacillus Reuteri. Microbiol 2010, 156 (11), 3368–3378. [DOI] [PubMed] [Google Scholar]
- (39).Leteurtre E; Gouyer V; Rousseau K; Moreau O; Barbat A; Swallow D; Huet G; Lesuffleur T Differential Mucin Expression in Colon Carcinoma HT-29 Clones with Variable Resistance to 5-Fluorouracil and Methotrexate. Biol Cell 2004, 96 (2), 145–151. [DOI] [PubMed] [Google Scholar]
- (40).Larsson JMH; Karlsson H; Sjövall H; Hansson GC A Complex, but Uniform O-Glycosylation of the Human MUC2 Mucin from Colonic Biopsies Analyzed by NanoLC/MSn. Glycobiol 2009, 19 (7), 756–766. [DOI] [PubMed] [Google Scholar]
- (41).Koropatkin NM; Cameron EA; Martens EC How Glycan Metabolism Shapes the Human Gut Microbiota. Nat Rev Microbiol 2012, 10 (5), 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Lai SK; Wang YY; Wirtz D; Hanes J Micro- and Macrorheology of Mucus. Adv Drug Del Rev 2009, 61 (2), 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Yakubov GE; Papagiannopoulos A; Rat E; Easton RL; Waigh TA Molecular Structure and Rheological Properties of Short-Side-Chain Heavily Glycosylated Porcine Stomach Mucin. Biomacromol 2007, 8 (11), 3467–3477. [DOI] [PubMed] [Google Scholar]
- (44).Bergstrom KSB; Xia L Mucin-Type O-Glycans and Their Roles in Intestinal Homeostasis. Glycobiology 2013, 23 (9), 1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Celli JP; Turner BS; Afdhal NH; Ewoldt RH; McKinley GH; Bansil R; Erramilli S; Erramilli S Rheology of Gastric Mucin Exhibits a PH-Dependent Sol-Gel Transition. Biomacromol 2007, 8 (5), 1580–1586. [DOI] [PubMed] [Google Scholar]
- (46).Wagner CE; Wheeler KM; Ribbeck K Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu Rev Cell Dev Biol 2018, 34 (1), 189–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Zanin M; Baviskar P; Webster R; Webby R The Interaction between Respiratory Pathogens and Mucus. Cell Host Microbe 2016, 19 (2), 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Jonsson H; Ström E; Roos S Addition of Mucin to the Growth Medium Triggers Mucus-Binding Activity in Different Strains of Lactobacillus Reuteri in Vitro. FEMS Microbiol Lett 2001, 204 (1), 19–22. [DOI] [PubMed] [Google Scholar]
- (49).Jensen H; Roos S; Jonsson H; Rud I; Grimmer S; van Pijkeren JP; Britton RA; Axelsson L Role of Lactobacillus Reuteri Cell and Mucus Binding Protein A (CmbA) in Adhesion to Intestinal Epithelial Cells and Mucus in Vitro. Microbiol UK 2014, 160 (PART 4), 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Roos S; Jonsson H A High-Molecular-Mass Cell-Surface Protein from Lactobacillus Reuteri 1063 Adheres to Mucus Components. Microbiol 2002, 148 (2), 433–442. [DOI] [PubMed] [Google Scholar]
- (51).Etzold S; MacKenzie DA; Jeffers F; Walshaw J; Roos S; Hemmings AM; Juge N Structural and Molecular Insights into Novel Surface Exposed Mucus Adhesins from Lactobacillus Reuteri Human Strains. Mol Microbiol 2014, 92 (3), 543–556. [DOI] [PubMed] [Google Scholar]
- (52).Celebioglu HU; Olesen SV; Prehn K; Lahtinen SJ; Brix S; Abou Hachem M; Svensson B Mucin- and Carbohydrate-Stimulated Adhesion and Subproteome Changes of the Probiotic Bacterium Lactobacillus Acidophilus NCFM. Journal of Proteomics 2017, 163, 102–110. [DOI] [PubMed] [Google Scholar]
- (53).Wang XQ; Duan XM; Liu LH; Fang YQ; Tan Y Carboxyfluorescein Diacetate Succinimidyl Ester Fluorescent Dye for Cell Labeling. Acta Biochim Biophys Sinica 2005, 37 (6), 379–385. [DOI] [PubMed] [Google Scholar]
- (54).Lee YK; Lim CY; Teng WL; Ouwehand AC; Tuomola EM; Salminen S Quantitative Approach in the Study of Adhesion of Lactic Acid Bacteria to Intestinal Cells and Their Competition with Enterobacteria. Appl Environ Microbiol 2000, 66 (9), 3692–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Mukherjee S; Ramesh A Bacteriocin-Producing Strains of Lactobacillus Plantarum Inhibit Adhesion of Staphylococcus Aureus to Extracellular Matrix: Quantitative Insight and Implications in Antibacterial Therapy. J Med Microbiol 2015, 64 (12), 1514–1526. [DOI] [PubMed] [Google Scholar]
- (56).Valeriano VD; Bagon BB; Balolong MP; Kang DK Carbohydrate-Binding Specificities of Potential Probiotic Lactobacillus Strains in Porcine Jejunal (IPEC-J2) Cells and Porcine Mucin. J Microbiol 2016, 54 (7), 510–519. [DOI] [PubMed] [Google Scholar]
- (57).Latousakis D; Nepravishta R; Rejzek M; Wegmann U; le Gall G; Kavanaugh D; Colquhoun IJ; Frese S; MacKenzie DA; Walter J; et al. Serine-Rich Repeat Protein Adhesins from Lactobacillus Reuteri Display Strain Specific Glycosylation Profiles. Glycobiol 2019, 29 (1), 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Tallon R; Arias S; Bressollier P; Urdaci MC Strain- and Matrix-Dependent Adhesion of Lactobacillus Plantarum Is Mediated by Proteinaceous Bacterial Compounds. J Appl Microbiol 2007, 102 (2), 442–451. [DOI] [PubMed] [Google Scholar]
- (59).Petrova MI; Lievens E; Verhoeven TLA; MacKlaim JM; Gloor G; Schols D; Vanderleyden J; Reid G; Lebeer S The Lectin-like Protein 1 in Lactobacillus Rhamnosus GR-1 Mediates Tissue-Specific Adherence to Vaginal Epithelium and Inhibits Urogenital Pathogens. Sci Rep 2016, 6 (October), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Hill A v. The Possible Effects of Aggregation of Haemoglobin on Its Dissociation Curves. J Physiol London 1910, 40, IV–VII. [Google Scholar]
- (61).Walter J; Britton RA; Roos S Host-Microbial Symbiosis in the Vertebrate Gastrointestinal Tract and the Lactobacillus Reuteri Paradigm. Proc Natl Acad Sci USA 2011, 108 Suppl, 4645–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Oh PL; Benson AK; Peterson DA; Patil PB; Moriyama EN; Roos S; Walter J Diversification of the Gut Symbiont Lactobacillus Reuteri as a Result of Host-Driven Evolution. ISME J 2010, 4 (3), 377–387. [DOI] [PubMed] [Google Scholar]
- (63).Jensen H; Grimmer S; Naterstad K; Axelsson L In Vitro Testing of Commercial and Potential Probiotic Lactic Acid Bacteria. Int J Food Microbiol 2012, 153 (1-2), 216–222. [DOI] [PubMed] [Google Scholar]
- (64).Lasaro MA; Salinger N; Zhang J; Wang Y; Zhong Z; Goulian M; Zhu J F1C Fimbriae Play an Important Role in Biofilm Formation and Intestinal Colonization by the Escherichia Coli Commensal Strain Nissle 1917. Appl Environ Microbiol 2009, 75 (1), 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Maltby R; Leatham-Jensen MP; Gibson T; Cohen PS; Conway T Nutritional Basis for Colonization Resistance by Human Commensal Escherichia Coli Strains HS and Nissle 1917 against E. Coli O157:H7 in the Mouse Intestine. PLoS One 2013, 8 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Ou B; Yang Y; Tham WL; Chen L; Guo J; Zhu G Genetic Engineering of Probiotic Escherichia Coli Nissle 1917 for Clinical Application. Appl Microbiol Biotechnol 2016, 100 (20), 8693–8699. [DOI] [PubMed] [Google Scholar]
- (67).Scaldaferri F; Gerardi V; Mangiola F; Lopetuso LR; Pizzoferrato M; Petito V; Papa A; Stojanovic J; Poscia A; Cammarota G; et al. Role and Mechanisms of Action of Escherichia Coli Nissle 1917 in the Maintenance of Remission in Ulcerative Colitis Patients: An Update. World J Gastroenterol 2016, 22 (24), 5505–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Hancock V; Dahl M; Klemm P Probiotic Escherichia Coli Strain Nissle 1917 Outcompetes Intestinal Pathogens during Biofilm Formation. J Med Microbiol 2010, 59 (4), 392–399. [DOI] [PubMed] [Google Scholar]
- (69).Joeres-Nguyen-Xuan TH; Boehm SK; Joeres L; Schulze J; Kruis W Survival of the Probiotic Escherichia Coli Nissle 1917 (EcN) in the Gastrointestinal Tract given in Combination with Oral Mesalamine to Healthy Volunteers. Inflamm Bowel Dis 2010, 16 (2), 256–262. [DOI] [PubMed] [Google Scholar]
- (70).Anderson GG; Palermo JJ; Schilling JD; Roth R; Heuser J; Hultgren SJ Intracellular Bacterial Biofilm-like Pods in Urinary Tract Infections. Sci 2003, 301 (5629), 105–107. [DOI] [PubMed] [Google Scholar]
- (71).Barth S; Duncker S; Hempe J; Breves G; Baljer G; Bauerfeind R Escherichia Coli Nissle 1917 for Probiotic Use in Piglets: Evidence for Intestinal Colonization. J Appl Microbiol 2009, 107 (5), 1697–1710. [DOI] [PubMed] [Google Scholar]
- (72).Kankainen M; Paulin L; Tynkkynen S; von Ossowski I; Reunanen J; Partanen P; Satokari R; Vesterlund S; Hendrickx AP; Lebeer S; et al. Comparative Genomic Analysis of Lactobacillus Rhamnosus GG Reveals Pili Containing a Human-Mucus Binding Protein. Proc Natl Acad Sci USA 2009, 106 (40), 17193–17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Fabich AJ; Jones SA; Chowdhury FZ; Cernosek A; Anderson A; Smalley D; McHargue JW; Hightower GA; Smith JT; Autieri SM; et al. Comparison of Carbon Nutrition for Pathogenic and Commensal Escherichia Coli Strains in the Mouse Intestine. Infect Immun 2008, 76 (3), 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Nishiyama K; Sugiyama M; Mukai T Adhesion Properties of Lactic Acid Bacteria on Intestinal Mucin. Microorganisms 2016, 4 (34), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Fairfax MR; Lephart PR; Salimnia H Weissella Confusa: Problems with Identification of an Opportunistic Pathogen That Has Been Found in Fermented Foods and Proposed as a Probiotic. Front Microbiol 2014, 5 (JUN), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Ayeni FA; Sánchez B; Adeniyi BA; de los Reyes-Gavilán CG; Margolles A; Ruas-Madiedo P Evaluation of the Functional Potential of Weissella and Lactobacillus Isolates Obtained from Nigerian Traditional Fermented Foods and Cow’s Intestine. Int J Food Microbiol 2011, 147 (2), 97–104. [DOI] [PubMed] [Google Scholar]
- (77).Båth K; Roos S; Wall T; Jonsson H The Cell Surface of Lactobacillus Reuteri ATCC 55730 Highlighted by Identification of 126 Extracellular Proteins from the Genome Sequence. FEMS Microbiol Lett 2005, 253 (1), 75–82. [DOI] [PubMed] [Google Scholar]
- (78).Aleljung P; Shen W; Rozalska B; Hellman U; Ljungh Å; Wadström T Purification of Collagen-Binding Proteins of Lactobacillus Reuteri NCIB 11951. Curr Microbiol 1994, 28 (4), 231–236. [DOI] [PubMed] [Google Scholar]
- (79).Roos S; Aleljung P; Robert N; Lee B; Wadström T; Lindberg M; Jonsson H A Collagen Binding Protein from Lactobacillus Reuteri Is Part of an ABC Transporter System? FEMS Microbiol Lett 1996, 144 (1), 33–38. [DOI] [PubMed] [Google Scholar]
- (80).Rojas M; Ascencio F; Conway PL Purification and Characterization of a Surface Protein from Lactobacillus Fermentum 104R That Binds to Porcine Small Intestinal Mucus and Gastric Mucin. Appl Environ Microbiol 2002, 68 (5), 2330–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Saraiva TDL; Morais K; Pereira VB; de Azevedo M; Rocha CS; Prosperi CC; Gomes-Santos AC; Bermudez-Humaran L; Faria AMC; Blottiere HM; et al. Milk Fermented with a 15-Lipoxygenase-1-Producing Lactococcus Lactis Alleviates Symptoms of Colitis in a Murine Model. Curr Pharma Biotechnol 2015, 16 (5), 424–429. [DOI] [PubMed] [Google Scholar]
- (82).Jenkinson HF Cell Surface Protein Receptors in Oral Streptococci. FEMS Microbiol Lett 1994, 121 (2), 133–140. [DOI] [PubMed] [Google Scholar]
- (83).Troge A; Scheppach W; Schroeder BO; Rund SA; Heuner K; Wehkamp J; Stange EF; Oelschlaeger TA More than a Marine Propeller - the Flagellum of the Probiotic Escherichia Coli Strain Nissle 1917 Is the Major Adhesin Mediating Binding to Human Mucus. Int J Med Microbiol 2012, 302 (7–8), 304–314. [DOI] [PubMed] [Google Scholar]
- (84).Walter J; Ley R The Human Gut Microbiome: Ecology and Recent Evolutionary Changes. Annu Rev Microbiol 2011, 65 (1), 411–429. [DOI] [PubMed] [Google Scholar]
- (85).Crook N; Ferreiro A; Gasparrini AJ; Pesesky MW; Gibson MK; Wang B; Sun X; Condiotte Z; Dobrowolski S; Peterson D; et al. Adaptive Strategies of the Candidate Probiotic E. Coli Nissle in the Mammalian Gut. Cell Host Microbe 2019, 25 (4), 499–512.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Monteiro C; Saxena I; Wang X; Kader A; Bokranz W; Simm R; Nobles D; Chromek M; Brauner A; Brown RM; et al. Characterization of Cellulose Production in Escherichia Coli Nissle 1917 and Its Biological Consequences. Environ Microbiol 2009, 11 (5), 1105–1116. [DOI] [PubMed] [Google Scholar]
- (87).Friedlander RS; Vogel N; Aizenberg J Role of Flagella in Adhesion of Escherichia Coli to Abiotic Surfaces. Langmuir 2015, 31 (22). [DOI] [PubMed] [Google Scholar]
- (88).Karlskås IL; Maudal K; Axelsson L; Rud I; Eijsink VGH; Mathiesen G Heterologous Protein Secretion in Lactobacilli with Modified PSIP Vectors. PLoS One 2014, 9 (3), e91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Rud I; Jensen PR; Naterstad K; Axelsson L A Synthetic Promoter Library for Constitutive Gene Expression in Lactobacillus Plantarum. Microbiol 2006, 152 (4), 1011–1019. [DOI] [PubMed] [Google Scholar]
- (90).Mathiesen G; Sveen A; Brurberg MB; Fredriksen L; Axelsson L; Eijsink VGH Genome-Wide Analysis of Signal Peptide Functionality in Lactobacillus Plantarum WCFS1. BMC Genomics 2009, 10 (1), 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Fredriksen L; Kleiveland CR; Olsen Hult LT; Lea T; Nygaard CS; Eijsink VGH; Mathiesen G Surface Display of N-Terminally Anchored Invasin by Lactobacillus Plantarum Activates NF-KB in Monocytes. Appl Environ Microbiol 2012, 78 (16), 5864–5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Adhesion of L. lactis MG1363 and E. coli Nissle 1917 to different substrates
Figure S2. Western blots to confirm recombinant expression of mucus-associated factors
Figure S3. Flow-cytometry confirms surface display of recombinant mucus-associated factors
Figure S4. Competitive binding of L. reuteri and engineered L. Lactis
Figure S5. Mucus characterization after EDC reaction and schematic of Greiner U-bottom plate geometry
Table S1. Linear relationships between RFU and OD600 and OD600 and CFU/mL for each strain
Table S2. List of strains, plasmids, and primers (XLSX)