Summary
Ca2+ oscillations that depend on inositol-1,4,5-trisphosphate (IP3) have been ascribed to biphasic Ca2+ regulation of the IP3 receptor (IP3R) or feedback mechanisms controlling IP3 levels in different cell types. IP3 uncaging in hepatocytes elicits Ca2+ transients that are often localized at the subcellular level and increase in magnitude with stimulus strength. However, this does not reproduce the broad baseline-separated global Ca2+ oscillations elicited by vasopressin. Addition of hormone to cells activated by IP3 uncaging initiates a qualitative transition from high-frequency spatially disorganized Ca2+ transients, to low-frequency, oscillatory Ca2+ waves that propagate throughout the cell. A mathematical model with dual coupled oscillators that integrates Ca2+-induced Ca2+ release at the IP3R and mutual feedback mechanisms of cross-coupling between Ca2+ and IP3 reproduces this behavior. Thus, multiple Ca2+ oscillation modes can coexist in the same cell, and hormonal stimulation can switch from the simpler to the more complex to yield robust signaling.
Subject Areas: Cell Biology, Specialized Functions of Cells, Mathematical Biosciences
Graphical Abstract

Highlights
-
•
Ca2+ oscillations driven by IP3R (class 1) and PLC (class 2) occur in the same cell
-
•
IP3 uncaging elicits brief and often spatially localized class 1 Ca2+ oscillations
-
•
GPCRs elicit whole-cell Ca2+ oscillations and waves via a hybrid class 2 mechanism
-
•
Dual Ca2+ feedback on IP3R and PLC ensures a robust response to hormonal stimulation
Cell Biology; Specialized Functions of Cells; Mathematical Biosciences
Introduction
Oscillations of intracellular Ca2+ represents a fundamental cellular signaling mechanism regulating a multitude of process such as fertilization, metabolism, and secretion (Rooney et al., 1989, Bootman et al., 1997b, Berridge et al., 2000, Berridge, 2016). In non-excitable cells, oscillations of cytosolic Ca2+ ([Ca2+]c) are generated by activation of hormone receptors coupled to phospholipase C (PLC) that produce the second messenger inositol-1,4,5-trisphosphate (IP3), which activates IP3 receptor Ca2+ release channels (IP3R) in the endoplasmic reticulum (ER). The Ca2+ signaling toolkit of channels, pumps, and regulatory proteins is well defined (Berridge et al., 2000, Berridge, 2017), but the mechanisms driving cyclical changes in [Ca2+]c have yet to be resolved. In hepatocytes, several IP3-dependent hormones regulate metabolism and a number of other functions via baseline-separated [Ca2+]c oscillations. Hepatocytes exhibit frequency modulation, whereby the [Ca2+]c oscillation frequency is determined by agonist concentration, but the individual Ca2+ spikes have constant amplitude and rate of rise, and propagate as intracellular [Ca2+]c waves at a constant velocity, independent of agonist dose (Rooney et al., 1989, Thomas et al., 1991, Gaspers and Thomas, 2005, Bartlett et al., 2014). Moreover, these hormone-induced Ca2+ transients are an all-or-none phenomenon, whereby the Ca2+ waves propagate at full strength across the whole cell. In the intact liver, Ca2+ waves propagate from cell to cell through gap junctions, and hence across the entire liver lobules to regulate hepatic metabolism and glucose output (Gaspers et al., 2019). We propose that Ca2+-dependent activation of PLC provides a feedforward mechanism to elicit robust cell-wide Ca2+ increases throughout the cytoplasm, ensuring maximal activation of Ca2+-dependent processes. Alterations in Ca2+ homeostasis and signaling have been shown in diseases of the liver (Bartlett et al., 2017, Arruda and Hotamisligil, 2015), therefore elucidating the mechanisms that maintain Ca2+ oscillations is critical to understanding Ca2+ dysregulation in disease states such as fatty liver disease and diabetes.
Experimental and mathematical modeling studies have explored the [Ca2+]c oscillatory phenomenon without reaching a consensus in terms of mechanism. Two distinct hypotheses have been proposed: class 1 oscillations, which arise solely due to the biphasic effects Ca2+ on IP3R gating (Bezprozvanny et al., 1991, Thurley and Falcke, 2011, De Young and Keizer, 1992, Marchant and Parker, 2001) and rely only on a continuous elevation of IP3, and class 2 oscillations, which rely on Ca2+ feedback regulation of IP3 levels such that [Ca2+]c oscillations are coupled to, and dependent on, IP3 oscillations (Politi et al., 2006, Salazar et al., 2008, Dupont and Erneux, 1997). Currently, cells are classified into class 1 Ca2+-induced Ca2+ release (CICR) or class 2 IP3 dynamics-dependent Ca2+ oscillators. Experimental evidence from different cell types is used to support each of the two models, and thus the discrepant findings may reflect differences in the cellular complement and subcellular distributions of the Ca2+ signaling toolkit components.
Mathematical models based on biphasic regulation of the IP3R by Ca2+, effectively IP3-dependent CICR, yield Ca2+ oscillations with a high frequency and a narrow sensitivity to PLC activity and IP3 concentration (Bezprozvanny et al., 1991, De Young and Keizer, 1992, Sneyd et al., 2017, Thurley and Falcke, 2011). This is borne out by the nature of the [Ca2+]c signals observed in cell types where experimental evidence indicates that class 1 Ca2+ oscillations occur. Hepatocytes and many other secretory cells generate [Ca2+]c oscillations with long interspike intervals (ISIs) and kinetics that are independent of agonist dose, which cannot be readily reproduced by class 1 oscillatory models. The long periods at basal [Ca2+]c between Ca2+ transients at low hormone doses, together with a broad dynamic range of frequency modulation, suggests that these [Ca2+]c oscillations are controlled by other feedback loops such as those that regulate IP3 generation and metabolism (Meyer and Stryer, 1988, Politi et al., 2006, Gaspers et al., 2014, Kummer et al., 2000). In the hepatocyte modeling studies of Kummer et al. (Kummer et al., 2000), an autocatalytic activity of the Gα subunit of the G-protein drives dynamic PLC activation and hence IP3 oscillations, with Ca2+ causing negative feedback on PLC. However, these activities have not been reported experimentally, and although their model reproduces baseline spiking, it also leads to chaotic behavior that is not usually observed in our studies. In general, models that rely on Ca2+-dependent IP3 degradation or feedback inhibition of PLC activity do not fully reproduce the types of [Ca2+]c oscillations generated by G-protein-coupled receptor (GPCR)-linked hormones in hepatocytes (Gaspers et al., 2014, Dupont et al., 2003). By contrast, class 2 mathematical models incorporating positive Ca2+ feedback on IP3 formation can reproduce the [Ca2+]c oscillatory patterns observed in these cells (Gaspers et al., 2014, Meyer and Stryer, 1988, Politi et al., 2006). In most models involving positive Ca2+ feedback on IP3 formation, a component of Ca2+ activation of the IP3R is also generally required to reproduce the observed waveform of baseline-separated [Ca2+]c oscillations with a rapidly rising phase (Politi et al., 2006, Gaspers et al., 2014), making the relevant class 2 models a hybrid of class 2 and class 1 properties.
Our previous work has demonstrated that expression of a recombinant IP3 buffer in hepatocytes slows or completely eliminates (at high expression levels) hormone-induced [Ca2+]c oscillations and waves (Gaspers et al., 2014). These data, combined with mathematical modeling of the predicted effects of an IP3 buffer, argue that a periodic rapid rise in IP3 via Ca2+ feedforward activation of PLC is essential for generating [Ca2+]c oscillations in hepatocytes. Previously, we showed that PLC inhibition does not affect [Ca2+]c oscillations generated by IP3 uncaging in hepatocytes, indicating that agonist-dependent activation of PLC is a prerequisite for the feedforward regulation seen with hormonal stimulation (Bartlett et al., 2015). Furthermore, we identified both positive and negative feedback regulation of Ca2+ oscillations by protein kinase C (PKC), at least part of which is due to inhibition of GPCR-dependent PLC activation. It is possible that this could underlie the unique and distinctively reproducible Ca2+ spike shapes elicited by activation of different GPCRs in hepatocytes (Rooney et al., 1989).
Subcellular Ca2+ blips and puffs are local events that can recruit other Ca2+ release sites to summate into global [Ca2+]c oscillations and propagate as saltatory [Ca2+]c waves (Bootman et al., 1997a, Bootman and Berridge, 1995). Intracellular uncaging of IP3 has been employed as a tool to mimic hormone-induced Ca2+ responses and interrogate the basic parameters of localized and global Ca2+ release events (Marchant et al., 1999, Marchant and Parker, 2001, Smith et al., 2009, Lock et al., 2017). The local Ca2+ signals generated by uncaging IP3 can provide information about the sensitivity of a given cell to IP3 and the speed and extent of CICR via the resident Ca2+ release channels. Moreover, CICR at the level of IP3R channels is sufficient to drive [Ca2+]c oscillations and Ca2+ wave propagation. However, IP3 uncaging does not recapitulate the [Ca2+]c responses to physiological hormonal stimuli in hepatocytes.
The current consensus is that cells exhibit either class 1 or class 2 Ca2+ oscillations and that these occur unambiguously (Sneyd et al., 2006, Sneyd et al., 2017). However, the present data obtained in hepatocytes suggest that these mechanisms are not mutually exclusive. Photorelease of IP3 alone elicits high-frequency [Ca2+]c oscillations with an amplitude, width, and rate of rise that increase with stimulus strength. These responses can be observed in local Ca2+ puff sites or globally across the cell, and importantly, they are not perturbed by the expression of an intracellular IP3 buffer. We conclude that these are CICR-dependent [Ca2+]c oscillations. However, there is a dramatic shift in the oscillatory pattern upon the addition of a hormone to cells responding to flash photolysis of caged IP3. The [Ca2+]c oscillations in the presence of vasopressin (VP) become much broader, with a prolonged ISI, a very regular frequency, and manifesting as Ca2+ waves that consistently propagate through the entire cell. A mathematical model incorporating both CICR at the IP3R and hormone-dependent Ca2+ feedback on IP3 generation faithfully reproduces both modes of [Ca2+]c oscillations and the ability to transition between them. These data show that class 1 and class 2 [Ca2+]c oscillations can coexist in the same cell and demonstrate that hormone-dependent [Ca2+]c oscillations require dual feedback loops that dynamically control Ca2+ release and IP3 levels during the oscillation cycle. Importantly, class 2 oscillations dominate over class 1 in the presence of hormone.
Results
Graded [Ca2+]c Responses to Flash Photolysis of Caged IP3
The genetically encoded Ca2+ indicators GCaMP3 and RGECO-1 were used to detect [Ca2+]c responses elicited by IP3 uncaging and activation of the PLC-coupled VP receptor (V1R). Expression of molecular Ca2+ indicators offers a number of advantages over chemical indicators such as fluo4. The apparent affinities for Ca2+ of GCaMP3 and RGECO1 (405 and 449 nM, respectively; Akerboom et al., 2013) are comparable with that of fluo4 (335 nM), but they have a higher quantum yield and are typically expressed at a lower effective concentration. They are also less diffusible and so are less likely to perturb local Ca2+ dynamics. It is also an advantage that they have an exclusive cytosolic location (unlike the chemical indicators, which also load into subcellular compartments). Empirically, we find that these genetically encoded Ca2+ indicators are well suited to the resolution of small and local [Ca2+]c signals. In the present study, we analyzed the local, global, and propagating properties of [Ca2+]c oscillations elicited by global IP3 uncaging and VP treatment, with a view to dissecting the mechanisms driving baseline-separated [Ca2+]c oscillations.
Hormonal regulation of metabolism in hepatocytes has been extensively characterized by our group, and we have shown that [Ca2+]c oscillation frequency, rather than amplitude, encodes signal strength (Bartlett et al., 2014, Gaspers and Thomas, 2005, Rooney et al., 1989, Thomas and Robb-Gaspers, 1996). Different GPCRs give rise to [Ca2+]c oscillations with distinct Ca2+ spike shapes, reflected primarily in the rate of decay of each Ca2+ spike, and this too is independent of agonist dose. By contrast, photorelease of caged IP3 in GCaMP3-expressing hepatocytes induced [Ca2+]c oscillations whose amplitude and duration increased in a dose-dependent manner (Figure 1A; see also Transparent Methods). Analysis of global (whole-cell) [Ca2+]c responses showed that as the number of UV pulses was increased, the [Ca2+]c peak height, peak width (measured as full width at half maximum; FWHM), rate of rise, and frequency increased, and there was a concomitant decrease in ISI (Figures 1B–1E). For each of these experiments, 5 nM VP was also added. VP-induced [Ca2+]c oscillations had amplitudes and rates of Ca2+ rise that were similar to the maximum response to IP3 uncaging, whereas the [Ca2+]c peak widths and ISI were much longer than was observed with IP3 uncaging.
Figure 1.
Effect of Incremental Flash Photolysis of Caged IP3 on [Ca2+]c Oscillation Properties
Isolated hepatocytes were transfected with GCaMP3, cultured overnight, and then loaded with caged IP3 (2 μM; 1 h).
(A) Representative trace showing cytosolic Ca2+ responses to photolysis of caged IP3 elicited by rapid trains of 1, 2, 3, and 4 UV pulses (arrows). After a recovery period, the same cells were stimulated with 5 nM vasopressin (VP).
(B–E) Summary data for the effect of increasing UV stimulation compared with VP for [Ca2+]c spike amplitude (B), peak width measured as full width at half maximum (FWHM) (C), rate of rise (D), and interspike interval (ISI) (E). Data are mean ± SEM of the second [Ca2+] transient for amplitude, width, and rate of rise, and 3 consecutive oscillations for ISI after UV pulse or 5 nM VP addition (11 cells from 6 independent experiments).
We have previously demonstrated that hormone-induced Ca2+ oscillations are inhibited by the PLC inhibitor U73122, whereas oscillations induced by photorelease of caged IP3 are insensitive to PLC inhibition (Bartlett et al., 2015). Thus, without GPCR activation the mechanisms required to drive regenerative IP3 increases are lacking. Furthermore, in contrast to the sustained hormone-induced [Ca2+]c oscillations, the responses elicited by caged IP3 run down, as shown by the decrease in Ca2+ transient amplitude (Figure 1A). Taken together, these data demonstrate that an increase in IP3 alone does not recapitulate the effect of GPCR activation.
Continuous Uncaging of IP3
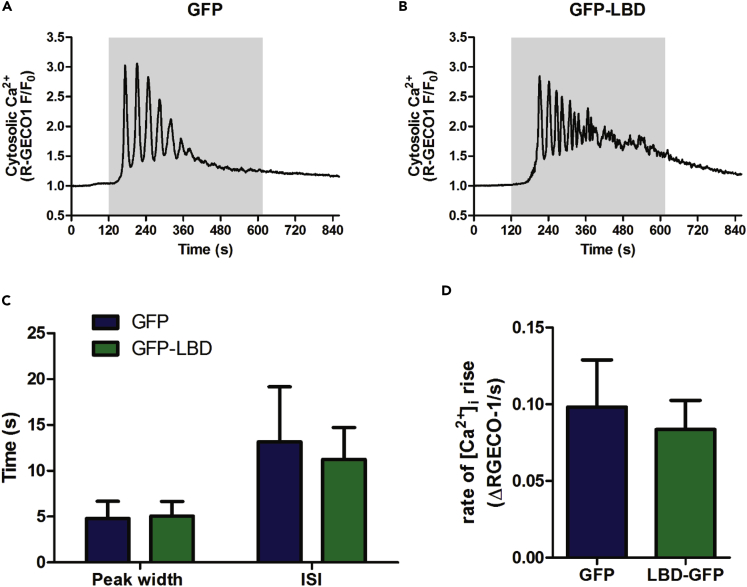
Caged IP3 can also be photolyzed slowly using a xeon light source and UV illumination over a prolonged period (as opposed to the discrete UV laser flashes reported in the previous section) to more closely mimic the continuous generation of IP3 during hormonal stimulation. In the majority of cells (72% ± 6%, mean ± SEM, 45 cells from 3 independent experiments), continuous IP3 uncaging resulted in a slow monophasic rise in [Ca2+]c throughout the cell, but [Ca2+]c oscillations were also observed in ~25% cells (Figures S1A and S1B). Similar to the observations with flash photolysis of caged IP3, these [Ca2+]c oscillations had a higher frequency and shorter spike duration than hormone-induced oscillations, whereas the rate of [Ca2+]c rise was the same (Figures S1C and S1D). We have previously shown that expression of the ligand-binding domain of rat IP3R type 1 (LBD) acts as an intracellular IP3 buffer, which slows hormone-induced [Ca2+]c oscillations and waves in primary hepatocytes (Gaspers et al., 2014). In that study we showed that LBD expression did not affect the rate of rise or amplitude of the slow monophasic [Ca2+]c response to continuous IP3 uncaging. As shown in Figure 2, LBD expression also had no effect on the peak width, ISI, or rate of rise of the oscillatory [Ca2+]c responses generated by slow uncaging of IP3. Thus, in the absence of hormone, when IP3 reaches the threshold for CICR-mediated [Ca2+]c oscillations the kinetic properties of these oscillations is unaffected by the presence of an IP3 buffer, as predicted for a class 1 model.
Figure 2.
Buffering of Intracellular IP3 Has No Effect on Ca2+ Oscillations Elicited by Slow Release of Caged IP3
(A–D) Hepatocytes cotransfected with RGECO1 and either GFP (A) or GFP-LBD (B) were loaded with caged IP3 (2 μM; 1 h). The gray area shows the duration of slow IP3 uncaging elicited by low-intensity UV illumination (50-ms exposures from xenon lamp at 2 Hz). Expression of GFP-LBD had no effect on the peak width (FWHM) or ISI (C) or the rate of Ca2+ rise (D). Data are mean ± SEM of 10 cells from 4 independent experiments.
Subcellular Organization of Ca2+ Signals Elicited by Global Flash Photolysis of Caged IP3
The preceding data describe only the integrated whole-cell [Ca2+]c events elicited by IP3 uncaging, but IP3 uncaging can also cause localized subcellular Ca2+ release. Local Ca2+ release responses termed Ca2+ puffs have been observed in Xenopus oocytes after photorelease of caged IP3 (Marchant and Parker, 2001), and similar localized Ca2+ release events have also been reported in mammalian cell lines (Smith et al., 2009, Thomas et al., 2000, Tovey et al., 2001). Local Ca2+ release events have not previously been reported in hepatocytes in response to photorelease of caged IP3 (Bartlett et al., 2015, Gaspers et al., 2014) or following hormone stimulation (Rooney et al., 1990, Thomas et al., 1996). In the present study, the use of a genetically encoded Ca2+ indicator that has less effect on Ca2+ diffusion and buffering than small molecule chemical indicator dyes has revealed spatial heterogeneity in the [Ca2+]c responses to submaximal levels of photoreleased IP3. As shown in Figure 3, low levels of IP3, particularly in the threshold range elicited by 1 or 2 UV pulses, caused localized [Ca2+]c transients at discreet subcellular locations in ~40% cells (23 of 59 cells, 6 independent experiments). In the other cells analyzed, where the initial IP3 uncaging presumably exceeded this threshold, oscillatory propagating Ca2+ waves or a single global [Ca2+]c response were observed (summarized in Figure 3E). Importantly, the data of Figure 3E show a progression from predominantly local events, to propagating oscillations, to global [Ca2+]c elevations as the UV flash density was increased.
Figure 3.
Spatial Properties of [Ca2+]c Responses Elicited by Flash Photolysis of caged IP3
Isolated hepatocytes were transfected with GCaMP3, cultured overnight, and then loaded with caged IP3 (2 μM; 1 h).
(A) Single video frames showing focal [Ca2+]c release events. Scale bar, 10 μM.
(B and C) Representative traces of changes in [Ca2+]c at the local puff site (red) and distal pole (blue) of two individual hepatocytes during IP3 uncaging.
(D) Interpuff interval (IPI, red) and interspike interval (ISI, blue) during the 3 UV pulse response from (C).
(E) Ordinal plot of the Ca2+ response pattern for cells with increasing UV pulse density (bursts of 1–4 flashes, as indicated). Response patterns of increasing strength are classified as follows: no response, local Ca2+ transients at a discrete puff site, partial propagation where Ca2+ waves only propagated across the cell intermittently, propagation where Ca2+ waves consistently propagated across the cell, and global for whole-cell Ca2+ responses that were not spatially resolved (summary of 59 cells from 6 independent experiments).
The localized Ca2+ release events observed with IP3 uncaging in hepatocytes (Figure 3A) were 3.3 ± 0.3 μm in diameter (mean ± SEM, n = 23 cells from 6 independent experiments), which is similar to the Ca2+ puffs reported in other cell types, including Xenopus oocytes (Marchant and Parker, 2001, Tovey et al., 2001, Thomas et al., 2000). We therefore refer to the localized hepatocyte Ca2+ transients in the present study as Ca2+ puffs, although they differ somewhat from most previous reports in having a longer duration. Another difference from previous reports of Ca2+ puffs elicited by IP3 is that they typically occurred at a single repeating location at the cell periphery in the primary cultured hepatocyte (87% of cells). Significantly, these “eager” Ca2+ release sites corresponded to the site of Ca2+ wave initiation by hormones in the same cell. This polarization may reflect the reported subcellular distribution of IP3 receptors in hepatocytes, with the more sensitive type 2 IP3R predominantly in the apical region where hormone-induced Ca2+ waves originate and type 1 IP3R uniformly distributed across the cell (Hernandez et al., 2007, Nagata et al., 2007). In cells with a diffuse distribution of IP3Rs, Ca2+ puffs occur in multiple locations, although there are still discrete eager sites (Smith et al., 2009, Tovey et al., 2001, Marchant and Parker, 2001). Thus, the functional polarization of the hepatocyte allows for a stable Ca2+ release site that presumably engages multiple IP3R clusters to form a discrete integrated Ca2+ puff site.
To characterize the local and global Ca2+ events in each cell, [Ca2+]c in the region of interest at the Ca2+ puff site and at the distal pole of the cell were analyzed. Figures 3B and 3C show example Ca2+ traces from two cells with the puff site and distal pole superimposed. In these studies the [Ca2+]c signals at the distal region reflect the propagation of Ca2+ waves from the puff site (Rooney et al., 1990, Gaspers et al., 2019, Gaspers and Thomas, 2005). We have determined the frequency of oscillations at each site from the interval between Ca2+ transients; by convention the term inter-puff interval (IPI) is used for events at the Ca2+ puff site and inter-spike interval (ISI) for events that propagate across the whole cell. Submaximal levels of IP3 uncaging frequently generated localized Ca2+ oscillations at the puff site that did not propagate across the whole cell, as shown in Figure 3B, 1 UV flash, and Figure 3C, 2 UV flashes. As the stimulus strength was increased (number of UV flashes), [Ca2+]c waves were observed to propagate across the cell, radiating from the puff site. However, the effect of IP3 uncaging on Ca2+ responses at the distal pole of the cell did not always mirror the Ca2+ oscillations at the puff site. In some cases only the first Ca2+ transient at the puff site propagated across the cell as a single Ca2+ wave (Figure 3B). When multiple Ca2+ transients at the puff site did propagate as Ca2+ waves they often declined in amplitude. Importantly, not all [Ca2+]c transients at the puff site were transmitted across the hepatocyte to the distal pole (Figure 3C). This failure to propagate manifests in the difference in IPI and ISI plotted in Figure 3D for the 3 UV uncaging response in Figure 3C (see also Figure 5E). The continuum of increasingly effective propagation with increasing UV flash density is summarized in Figure 3E.
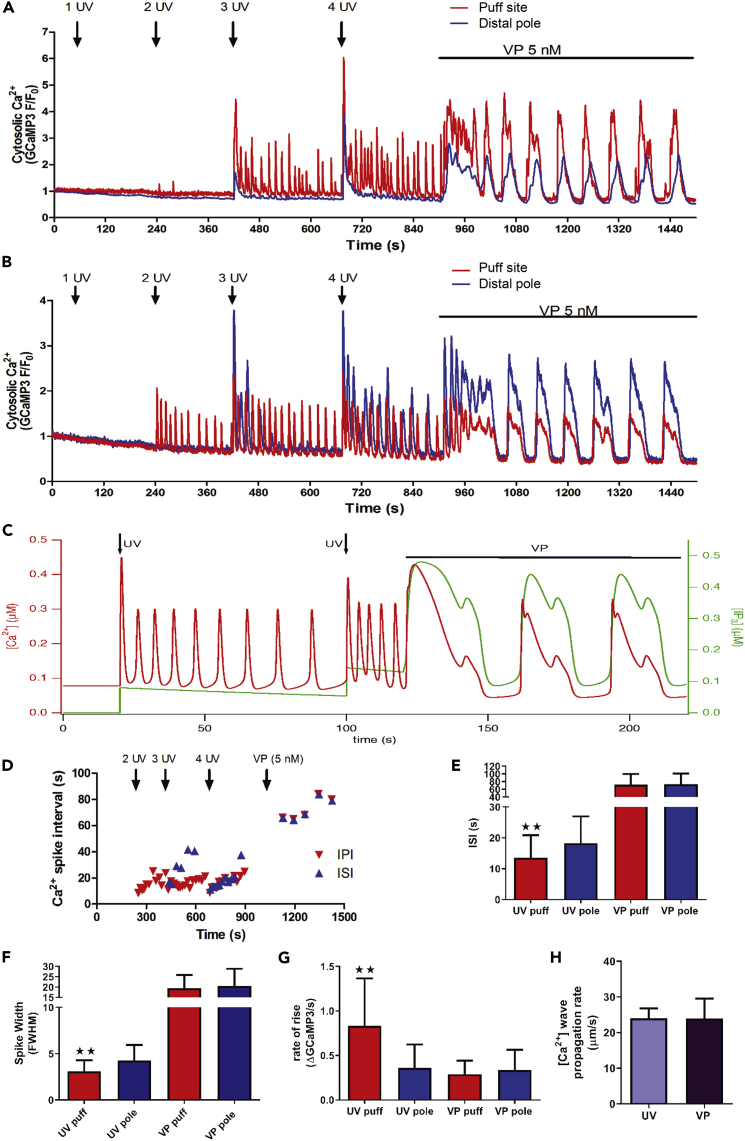
Figure 5.
Fast [Ca2+]c Oscillations Elicited by IP3 Uncaging Transition to Broad Baseline-Separate [Ca2+]c Oscillations in the Presence of Hormone
Isolated hepatocytes were transfected with GCaMP3, cultured overnight, and then loaded with caged IP3 (2 μM; 1 h). Rapid trains of 1, 2, 3, or 4 UV pulses were applied as indicated (arrows) followed by the addition of VP (5 nM).
(A and B) Representative traces showing cytosolic [Ca2+]c oscillations at puff site (red) and distal pole (blue) of two cells in response to IP3 uncaging and VP (see also Videos S1 and S2).
(C) Mathematical model combining class 1 and class 2 oscillation mechanisms predicts the observed behavior in this paradigm (red line [Ca2+]c, green line [IP3]).
(D) IPI and ISI of cell shown in (B) in response to UV pulses and VP (transition period between UV and VP responses not shown).
(E–G) Comparison of ISI (E), spike width (FWHM) (F), and rate of rise (G) at the puff site and distal pole of hepatocytes.
(H) Comparison of [Ca2+]c wave propagation rate elicited by UV and VP. Data are mean ± SEM of 10 cells from 5 independent experiments. ∗∗p < 0.01, paired Student's t test.
In contrast to IP3 uncaging, stimulation with a PLC-linked hormone such as VP leads to Ca2+ oscillations that consistently propagate across the entire cell throughout the active dose range, with the characteristic long-duration [Ca2+]c spikes described earlier (Figures 1 and S2; Gaspers and Thomas, 2005, Bartlett et al., 2014, Rooney et al., 1990). Moreover, low concentrations of hormone cause baseline-separated broad [Ca2+]c oscillations with long 1- to 5-min ISIs. We attempted to mimic this behavior by eliciting periodic IP3 uncaging with a similar frequency using single UV flash events (Figure S3). However, this did not reproduce the typical whole-cell baseline-separated Ca2+ oscillations observed with hormone. Instead multiple local Ca2+ transients were observed with the initial UV flashes. Although these did propagate to the distal pole as more IP3 was released into the hepatocyte, the [Ca2+]c spikes elicited by periodic uncaging never took on the broad profile of those observed with VP.
Effect of IP3 Uncaging during Hormone-Induced [Ca2+]c Oscillations
Photolysis of caged IP3 during a train of hormone-induced [Ca2+]c oscillations has been explored as an approach to determine whether receptor-mediated [Ca2+]c oscillations are dependent on oscillating or steady-state IP3 levels (Sneyd et al., 2006, Sneyd et al., 2017). Mathematical modeling predicts that class 1 oscillating cells show an increase in frequency following a pulse of IP3, and this has been demonstrated experimentally (Sneyd et al., 2006). For class 2 oscillating cells it has been predicted that an increase in [IP3] will result in a delay before Ca2+ oscillations recommence, because the IP3 level needs to fall before a subsequent Ca2+ spike can be initiated (Sneyd et al., 2006).
In hepatocytes, photolysis of caged IP3 during a train of VP-induced [Ca2+]c oscillations either slightly reduced the Ca2+ oscillation frequency (Figure 4A, 22/32 cells) or in some cases led to a temporary arrest of oscillations, as predicted from class 2 models (Figure 4B, 7/32 cells). In cells where the global hormone-induced [Ca2+]c oscillations were arrested, small local [Ca2+]c puffs were often observed during the interruption in the whole-cell [Ca2+]c oscillations. The decrease in oscillation frequency observed in the majority of cells is shown in Figure 4C, with mean pre- and post-photolysis values of 0.76 ± 0.07 min−1 and 0.59 ± 0.09 min−1, respectively (mean ± SEM for 32 hepatocytes from 4 independent experiments; 5 min pre- and post-UV; p < 0.001 by paired Student's t test). When the same UV pulse protocol was applied to VP-stimulated hepatocytes not loaded with caged IP3, the pre- and post-flash values were 0.84 ± 0.07 min−1 and 0.77 ± 0.09 min−1, respectively (15 cells from 2 independent experiments; p = 0.15). The decrease in frequency following IP3 uncaging was associated with an increase in Ca2+ transient width from 17.1 ± 1.1 s to 19.8 ± 1.6 s FWHM (Figure 4D; mean ± SEM for 26 hepatocytes from 4 independent experiments; p < 0.01 by paired Student's t test). As shown in Figure 4E, stronger IP3 uncaging (3 UV laser pluses) during a train of VP-induced [Ca2+]c oscillations caused a more pronounced prolongation of the [Ca2+]c spikes, but did not increase the oscillation frequency as would be expected for a class 1 mechanism. Presumably under the conditions of Figure 4E the uncaged IP3 extends the time required for IP3 levels to decay and hence delays Ca2+ refilling of the ER. Taken together, the data of Figure 4 are consistent with the hypothesis that hormones elicit predominantly class 2 [Ca2+]c oscillations that depend on fluctuations of IP3 in hepatocytes, because an increase in [IP3] did not translate to an increase in oscillation frequency.
Figure 4.
Effect of IP3 Uncaging on [Ca2+]c Responses Elicited by Vasopressin
(A–D) Isolated hepatocytes were transfected with GCaMP3, cultured overnight and then loaded with caged IP3 (2 μM; 1h). Hepatocytes were stimulated with vasopressin (VP) and then IP3 uncaging was achieved by a brief (1-s) pulse of 340-nm light from the microscope fluorescence illuminator Xeon lamp. Representative traces showing (A) a slight slowing of Ca2+ oscillation frequency or (B) the temporary arrest of oscillations. Summary data showing a decrease in oscillation frequency (C) and an increase in peak width (D) after the IP3 uncaging event (data are mean ± SEM from ≥26 individual hepatocytes from 4 independent experiments, ∗∗p < 0.01, ∗∗∗p < 0.001 paired Student's t test).
(E) Representative trace of hepatocyte stimulated with VP and then IP3 uncaging with 3 UV flashes from UV laser.
Addition of hormone during IP3-induced [Ca2+]coscillations causes a qualitative shift in Ca2+ oscillation properties
Our previous work (Bartlett et al., 2015) and data presented in the current study, demonstrate that release of IP3 alone is incapable of reproducing oscillatory [Ca2+]c transients with the same properties as those observed with hormone stimulation. To further test the hypothesis that baseline-separated Ca2+ oscillations depend on cross-coupling of IP3 and Ca2+ we reversed the paradigm of Figure 4 and examined the effect of adding VP during a train of oscillations induced by IP3 uncaging. Flash photolysis events were incrementally increased, and then VP was added while cells were still responding to the uncaged IP3. The [Ca2+]c signals in puff sites and at the distal pole of the cell were then compared between uncaging and hormone-induced responses. Strikingly, the application of VP resulted in a dramatic shift in oscillatory behavior from high-frequency, small-width oscillations, only some of which propagated across the whole cell (as summarized in Figure 3E), to lower-frequency broad [Ca2+]c oscillations that always engaged the entire cell (Figures 5A and 5B; see also Videos S1 and S2). The IPI and ISI for the cell shown in Figure 5B are plotted in Figure 5D. These data show reliable and complete propagation of the hormone-induced [Ca2+]c oscillations throughout the cell, whereas the higher-frequency [Ca2+]c transients with IP3 uncaging were irregular and inconsistent in engaging the distal pole of the cell. Videos S1 and S2 show this cell (in center) and other cells that exhibit similar behavior. Video S1 shows the changes in [Ca2+]c in response to UV flash events and then subsequent VP addition; increases in GCaMP3 fluorescence intensity reflect increased Ca2+. Note that the UV flashes are global across the whole file, but appear as white dots on the images due to the position of the spinning disk at the moment of the flash. Video S2 is a differential image series of the same data; the red intensity is proportional to the rate of GCaMP3 fluorescence increase overlaid on the gray scale image. This video is included to better resolve the spatially localized [Ca2+]c changes, as well as the wave front during Ca2+ wave propagation. It should be noted that only the rising phase (not sustained) of each Ca2+ transient is observed in the red differential image (Video S2), so this does not show the full duration of Ca2+ transients (seen in Video S1).
GCaMP3 fluorescence images were collected at 10 Hz with a spinning disk confocal microscope (excitation, 488 nm; emission 510-nm-long band-pass filter). UV flash events are annotated at the top left and cover the entire field (appearing as white dots on images due to the position of the spinning disk). Time of vasopressin addition is also annotated on the top left. Traces of cells from this video are shown in Figure 5; video starts at 200 s
Data shown in Video S1 were processed using a differential algorithm to identify sites of Ca2+ increase. Red intensity is proportional to the rate of rise of GCaMP3 fluorescence and is overlaid on the initial gray scale image to identify the location and relative basal fluorescence intensity of GCaMP3-expressing cells. Note that only the rising phase of each Ca2+ transient is visualized in the red differential image, so this does not show the full duration of Ca2+ transients (which can be seen in Video S1).
This switch in [Ca2+]c oscillation properties between IP3 uncaging and activation of a PLC-linked GPCR such as VP can be recapitulated in a mathematical model that combines class 1 and class 2 oscillation mechanisms (see Transparent Methods). Specifically, the simulation presented in Figure 5C incorporates positive and negative feedback at the IP3R using parameters similar to those described previously (Sneyd et al., 2006), which is sufficient to generate [Ca2+]c oscillations in the presence of incremental sustained elevations of IP3 (green line). A stepped increase in [IP3] increases the oscillation frequency with no requirement for dynamic changes of IP3. Introduction of VP, which activates PLC and allows positive feedback of Ca2+ on IP3 formation, yields much broader [Ca2+]c oscillations with sustained periods of baseline [Ca2+]c, similar to those observed in Figures 5A and 5B. The model even recapitulates the secondary Ca2+ peaks often seen during the falling phase of [Ca2+]c in the experimental data in response to VP (this is also seen in cells that have not experienced prior IP3 uncaging events, Figure S2). Importantly, the qualitatively different oscillation patterns shown in Figure 5C are obtained with a single model that allows both intrinsic IP3R regulation by Ca2+ and Ca2+ regulation of IP3 levels in the presence of hormone.
At subcellular resolution, the broad hormone-dependent [Ca2+]c oscillations in hepatocytes typically propagated starting at the same puff site that was activated during IP3 uncaging. Following addition of VP, a single broad [Ca2+]c response with complicated oscillatory behavior was often observed during the transition to baseline-separated [Ca2+]c oscillations (Figures 5A and 5B). [Ca2+]c transients generated by uncaging IP3 had a higher frequency at the puff site (Figure 5E), a narrower spike width (Figure 5F), and a faster rate of [Ca2+]c rise (Figure 5G) compared with the distal pole of the cell. Hormone-induced Ca2+ oscillations had a much greater width (FWHM, Figure 5F), with greater ISI (Figures 5D and 5E), compared with responses generated solely by uncaging IP3. Unlike the responses generated by IP3 uncaging, no differences in spike width, ISI, or rate of rise between the puff site and distal regions of the cell were observed with hormone-dependent [Ca2+]c oscillations. These data show a clear switch in [Ca2+]c oscillation properties between IP3 uncaging and stimulation via a PLC-linked GPCR in hepatocytes. Our modeling studies reveal that the ability of hormones to generate all-or-none oscillatory propagating Ca2+ waves can be ascribed to the Ca2+-dependent stimulation of PLC, which only occurs with GPCR activation. This positive feedback of Ca2+ on PLC ensures that cellular IP3 levels consistently reach a level that is sufficient to engage the whole cell during each Ca2+ oscillation in the presence of hormone. By contrast, in the absence of hormone there is no stimulation of PLC activity when IP3 is uncaged, resulting in local responses at the “eager” Ca2+ release sites and fast narrow [Ca2+]c transients that do not reliably propagate throughout the cell.
Discussion
Ca2+ oscillation and waves generated by the PLC-linked GPCRs are a major class of Ca2+ signals that regulate a host of intracellular processes. In hepatocytes, hormone-induced [Ca2+]c oscillations regulate cellular metabolism, bile secretion, gene expression, and glucose homeostasis (Amaya and Nathanson, 2013, Bartlett et al., 2014). The hepatocyte was one of the first cell types to be shown to signal through Ca2+ oscillations, and these cells remain one of the best examples of frequency-modulated signaling, with a broad frequency response range and long-period baseline-separated [Ca2+]c transients (Bartlett et al., 2014, Hajnoczky et al., 1995, Rooney et al., 1989, Woods et al., 1986, Woods et al., 1987). The mechanisms that give rise to [Ca2+]c oscillations have been the topic of much study and discussion, which has been complicated by the existence of two distinct paradigms that have been evidenced in different cellular systems: class 1 oscillations that are essentially intrinsic to the IP3R and rely on positive and negative feedback effects of Ca2+ on the Ca2+ release channel (Sneyd et al., 2006, Sneyd et al., 2017, Thurley and Falcke, 2011) and class 2 oscillations in which Ca2+ feedback acts at the level of IP3 formation and/or breakdown (Dupont and Erneux, 1997, Gaspers et al., 2014, Harootunian et al., 1991, Politi et al., 2006, Sneyd et al., 2006). It has generally been considered that the main determinants dictating the utilization of these mechanisms lie in the cellular complement of Ca2+ toolkit components, and that the higher-frequency-intrinsic IP3R oscillator will tend to dominate in the presence of sufficiently elevated IP3.
In the present study, we demonstrate the coexistence of both types of oscillatory Ca2+ signaling in primary rat hepatocytes and show that in contrast to the IP3R-level class 1 oscillation mechanism dominating, class 2 mechanisms become important when an agonist is added to activate the GPCR-dependent PLC and elicit IP3 formation. Moreover, Ca2+ oscillations elicited by uncaging IP3 and those generated by the hormone VP are qualitatively different and generated by distinct mechanisms, but, nevertheless, share components of the same Ca2+ toolkit. Both mechanisms can be recapitulated in a single mathematical model without changing any model parameters, but simply allowing for an agonist-dependent formation of IP3 in a Ca2+-regulated manner. The simplicity of this divergent behavior in a convergent model makes it clear that the same elements of the Ca2+ signaling toolkit give rise to the full range of Ca2+ signaling in hepatocytes. Thus, at least in the hepatocyte, the class 1 and class 2 oscillation mechanisms are not mutually exclusive, but integrate to give rise to the complex patterns of [Ca2+]c signals seen in these cells. In a hybrid class 2 model, Ca2+ and IP3 oscillations are co-dependent, and in effect cross-coupled through mutual feedback regulation. Positive Ca2+ feedback on PLC ensures that sufficient IP3 is produced for a global cell-wide [Ca2+]c response and drives Ca2+ release, whereas positive Ca2+ feedback on the IP3R ensures the rapid rising phase of the Ca2+ transients. In the context of the "Ca2+ toolkit" described by Berridge and others (Berridge et al., 2000, Bootman and Berridge, 1995, Bootman et al., 1997b), the key determinant of this type of Ca2+ oscillation is the involvement of Gαq-linked GPCRs that engage the Ca2+-sensitive PLCβ enzymes. By contrast, photorelease of caged IP3 initiates solely class 1 Ca2+ oscillations, and whereas this is a non-physiological stimulus, other physiological stimuli that act through IP3 but do not activate a Ca2+-sensitive PLC could still elicit [Ca2+]c oscillations through predominantly class 1 mechanisms in hepatocytes, for example, growth factors that signal through PLCγ (Baffy et al., 1992) and agonists that enhance IP3R activity through, for example, cAMP-dependent phosphorylation (Joseph and Ryan, 1993, Bartlett et al., 2014).
An important characteristic of the [Ca2+]c oscillations elicited by PLCβ-linked GPCRs is their robustness, both in the temporal domain and in their spatial organization. In the presence of hormones such as VP, Ca2+ transients occur with a regular period and relatively little variation of ISI, whereas the Ca2+ transients occurring in response to IP3 uncaging are much more stochastic. This implies a larger contribution from deterministic components under the conditions of hormone stimulation (Thurley et al., 2014), which likely reside in the metabolic steps of IP3 formation and breakdown. Presumably it is the linked cascade of metabolic steps and Ca2+ feedback on these that gives rise to the other notable difference between [Ca2+]c oscillations induced by IP3 uncaging and hormone treatment, which is the much greater duration of the Ca2+ transients elicited by hormone. These broader [Ca2+]c transient kinetics are known to be independent of agonist dose, but do show characteristically distinct shapes with different agonists (Bartlett et al., 2014, Green et al., 1993, Rooney et al., 1989).
A potential explanation for the agonist-specific [Ca2+]c spike shapes is differential modulation of the generation and/or metabolism of IP3 by distinct GPCRs. An additional component of the Gq-linked PLC pathway is the generation of diacylglycerol and activation of PKC, concurrent with the production of IP3. Many components of the Ca2+ signaling toolkit are substrates for PKC isoforms, and the activity of these kinases could contribute to oscillatory IP3 production (Nash et al., 2001, Woodring and Garrison, 1997, Bartlett et al., 2015). GPCRs can be phosphorylated by PKC (Nash et al., 2001), which decreases their coupling to G proteins. Consistent with this, we and others have shown that PKC inhibition enhances hormone-induced Ca2+ signaling, whereas acute PKC activation with phorbol ester decreases Ca2+ oscillation frequency (Bartlett et al., 2015, Sanchez-Bueno et al., 1990). PKC phosphorylation may also modify the activity of IP3Rs (Matter et al., 1993, Arguin et al., 2007), and thus has the potential to affect the onset or duration of class 1 oscillations. Indeed, we have shown that acute phorbol ester treatment increases the frequency of Ca2+ oscillations induced by uncaging IP3 (Bartlett et al., 2015). However, it is important to note that a PKC feedback mechanism to regulate Ca2+ or IP3 metabolism was not included in the modeling data presented here. Thus, although PKC activity may account for agonist-dependent differences in Ca2+ transient dynamics, the only factor required for the switch between class 1 and class 2 oscillations in our model is allowing the Ca2+ feedforward activation of PLC (Ca2+ and IP3 cross-coupling).
In addition to the longer duration of the Ca2+ transients in the presence of hormones, it is also noteworthy that hormone-induced [Ca2+]c oscillations sustain much longer periods of baseline [Ca2+]c between Ca2+ transients when compared with IP3 uncaging. During these periods there is no overt stochastic Ca2+ spiking like that seen with IP3 uncaging. Indeed, hormone-mediated Ca2+ signaling not only yields more robust low-frequency oscillations but also appears to suppress stochastic behavior between Ca2+ transients. Nevertheless, both IP3 uncaging and agonist stimulation are associated with dose-dependent increases in [Ca2+]c oscillation frequency (present work and Bartlett et al., 2014, Bartlett et al., 2015, Gaspers et al., 2014).
The greater robustness of Ca2+ signaling with hormones when compared with direct photorelease of IP3 is also apparent at the spatial level. The [Ca2+]c oscillations elicited by global uncaging of IP3 are characterized by local events, which may be sustained in only a small subcellular region, or propagate to other parts of the cell. Even within the same cell, and during a single uncaging event, there can be a mixture of local Ca2+ transients, partial propagation, and global Ca2+ waves. When they do occur, these global Ca2+ signals resulting from IP3 uncaging vary in their kinetic properties in different parts of the cell, tending to decrease in rates of Ca2+ rise and increase in duration from the initial puff site to the distal pole of the cell. By contrast, the [Ca2+]c oscillations induced by hormone treatment always propagate throughout the cell as a full Ca2+ wave, and do so with kinetic properties that are sustained across the cell. Significantly, the hormone-induced [Ca2+]c oscillations and waves typically originate from the same “eager” Ca2+ puff sites that are seen with IP3 uncaging. These data suggest that the subcellular distribution of IP3 receptor populations plays a key role in determining the initiation of Ca2+ signaling in response to hormones in hepatocytes. However, the combination of the class 1 IP3R CICR mechanism with the class 2 positive feedforward of Ca2+ on PLC to generate sufficient IP3 yields a more robust signal that ensures that Ca2+ release is not spatially restricted in the presence of hormone and gives rise to the properties of propagating intracellular Ca2+ waves in hepatocytes. Thus, the combination of two types of interacting Ca2+ oscillation mechanisms give rise to a uniform intracellular Ca2+ signal with fixed kinetics and relatively low frequency and limits stochastic behavior between Ca2+ transients.
Limitations of the Study
In this study we challenge the dogma that different cell types elicit Ca2+ oscillations via class 1 or class 2 mechanisms and clearly demonstrate that both phenomena can be observed in primary rat hepatocytes. It is likely that other cell types, particularly those with functional polarization such as epithelial cells, may also generate Ca2+ oscillations via a hybrid class 2 mechanism, but examining different cell types was beyond the scope of the present study. A further test of our proposed mechanism would be to demonstrate unequivocally that IP3 levels oscillate with hormone and not with photorelease of caged IP3. However, it was not possible to make single-cell IP3 measurements during IP3 uncaging and cytosolic Ca2+ recording. We and others have shown previously that hormone-induced oscillations in IP3 occur concurrently with Ca2+ oscillations using Förster resonance energy transfer (FRET)-based IP3 sensors (Gaspers et al., 2014, Tanimura et al., 2009). However, combining flash photolysis of caged IP3 with detection of IP3 with FRET probes is technically challenging, because the UV excitation used for uncaging can quench the FRET fluorophore, and the required blue light excitation may elicit uncontrolled IP3 uncaging. As an alternative approach we utilized an IP3 buffer to perturb IP3 dynamics and demonstrated that fast Ca2+ oscillations still occur when IP3 is photoreleased in the presence of IP3 buffering (Figure 2), whereas hormone-induced Ca2+ oscillations are supressed by the expression of the IP3 buffer (Gaspers et al., 2014).
Acknowledgments
GCaMP3 was a gift from Loren Looger (Addgene plasmid #22692). R-GECO-1 was a gift from Robert Campbell (Addgene plasmid #32444). GCaMP3 and R-GECO-1 were acquired with an MTA between Addgene and Rutgers-NJMS. This work was supported by the Thomas P. Infusion Endowed Chair and NIH R01DK078019 (to A.P.T), NIH R01DE019245-11 (to J.S.), and the University of Auckland Doctoral Scholarship (I.C.).
Author Contributions
P.J.B., A.P.T, and J.S. designed the research. P.J.B. and I.C. performed the research. P.J.B. and I.C. analyzed the data. P.J.B. and A.P.T. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: May 22, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101062.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Supplemental Information
References
- Akerboom J., Carreras Calderón N., Tian L., Wabnig S., Prigge M., Tolö J., Gordus A., Orger M.B., Severi K.E., Macklin J.J. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 2013;6:2. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya M.J., Nathanson M.H. John Wiley & Sons, Inc; 2013. Calcium Signaling in the Liver. Comprehensive Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguin G., Regimbald-Dumas Y., Fregeau M.-O., Caron A.Z., Guillemette G. Protein kinase C phosphorylates the inositol 1,4,5-trisphosphate receptor type 2 and decreases the mobilization of Ca2+in pancreatoma AR4-2J cells. J. Endocrinol. 2007;192:659–668. doi: 10.1677/JOE-06-0179. [DOI] [PubMed] [Google Scholar]
- Arruda A.P., Hotamisligil G.S. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015;22:381–397. doi: 10.1016/j.cmet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffy G., Yang L., Michalopoulos G.K., Williamson J.R. Hepatocyte growth factor induces calcium mobilization and inositol phosphate production in rat hepatocytes. J. Cell Physiol. 1992;153:332–339. doi: 10.1002/jcp.1041530213. [DOI] [PubMed] [Google Scholar]
- Bartlett P.J., Antony A.N., Agarwal A., Hilly M., Prince V.L., Combettes L., Hoek J.B., Gaspers L.D. Chronic alcohol feeding potentiates hormone-induced calcium signalling in hepatocytes. J. Physiol. 2017;595:3143–3164. doi: 10.1113/JP273891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett P.J., Gaspers L.D., Pierobon N., Thomas A.P. Calcium-dependent regulation of glucose homeostasis in the liver. Cell Calcium. 2014;55:306–316. doi: 10.1016/j.ceca.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Bartlett P.J., Metzger W., Gaspers L.D., Thomas A.P. Differential regulation of multiple steps in inositol 1,4,5- trisphosphate signaling by protein kinase C shapes hormone-stimulated Ca2+ oscillations. J. Biol. Chem. 2015;290:18519–18533. doi: 10.1074/jbc.M115.657767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 2016;96:1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Calcium signalling in health and disease. Biochem. Biophys. Res. Commun. 2017;485:5. doi: 10.1016/j.bbrc.2017.01.098. [DOI] [PubMed] [Google Scholar]
- Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny L., Watras J., Ehrlich B.E. Bell-shaped calcium-response curves of lns(l,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bootman M., Niggli E., Berridge M., Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J. Physiol. 1997;499(Pt 2):307–314. doi: 10.1113/jphysiol.1997.sp021928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M.D., Berridge M.J. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Berridge M.J., Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/s0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- De Young G.W., Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc. Natl. Acad. Sci. U S A. 1992;89:9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G., Erneux C. Simulations of the effects of inositol 1,4,5-trisphosphate 3-kinase and 5-phosphatase activities on Ca2+ oscillations. Cell Calcium. 1997;22:321–331. doi: 10.1016/s0143-4160(97)90017-8. [DOI] [PubMed] [Google Scholar]
- Dupont G., Houart G., de Koninck P. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium. 2003;34:485–497. doi: 10.1016/s0143-4160(03)00152-0. [DOI] [PubMed] [Google Scholar]
- Gaspers L.D., Bartlett P.J., Politi A., Burnett P., Metzger W., Johnston J., Joseph S.K., Hofer T., Thomas A.P. Hormone-induced calcium oscillations depend on cross-coupling with inositol 1,4,5-trisphosphate oscillations. Cell Rep. 2014;9:1209–1218. doi: 10.1016/j.celrep.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspers L.D., Pierobon N., Thomas A.P. Intercellular calcium waves integrate hormonal control of glucose output in the intact liver. J. Physiol. 2019;597:2867–2885. doi: 10.1113/JP277650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspers L.D., Thomas A.P. Calcium signaling in liver. Cell Calcium. 2005;38:329–342. doi: 10.1016/j.ceca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Green A.K., Dixon C.J., Mclennan A.G., Cobbold P.H., Fisher M.J. Adenine dinucleotide-mediated cytosolic free Ca2+ oscillations in single hepatocytes. FEBS Lett. 1993;322:197–200. doi: 10.1016/0014-5793(93)81567-j. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G., Robb-Gaspers L.D., Seitz M.B., Thomas A.P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Harootunian A.T., Kao J.P., Paranjape S., Adams S.R., Potter B.V., Tsien R.Y. Cytosolic Ca2+ oscillations in REF52 fibroblasts: Ca(2+)-stimulated IP3 production or voltage-dependent Ca2+ channels as key positive feedback elements. Cell Calcium. 1991;12:153–164. doi: 10.1016/0143-4160(91)90017-9. [DOI] [PubMed] [Google Scholar]
- Hernandez E., Leite M.F., Guerra M.T., Kruglov E.A., Bruna-Romero O., Rodrigues M.A., Gomes D.A., Giordano F.J., Dranoff J.A., Nathanson M.H. The spatial distribution of inositol 1,4,5-trisphosphate receptor isoforms shapes Ca2+ waves. J. Biol. Chem. 2007;282:10057–10067. doi: 10.1074/jbc.M700746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.K., Ryan S.V. Phosphorylation of the inositol trisphosphate receptor in isolated rat hepatocytes. J. Biol. Chem. 1993;268:23059–23065. [PubMed] [Google Scholar]
- Kummer U., Olsen L.F., Dixon C.J., Green A.K., Bornberg-Bauer E., Baier G. Switching from simple to complex oscillations in calcium signaling. Biophysical J. 2000;79:1188–1195. doi: 10.1016/S0006-3495(00)76373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J.T., Smith I.F., Parker I. Comparison of Ca2+ puffs evoked by extracellular agonists and photoreleased IP3. Cell Calcium. 2017;63:43–47. doi: 10.1016/j.ceca.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant J., Callamaras N., Parker I. Initiation of IP(3)-mediated Ca(2+) waves in Xenopus oocytes. EMBO J. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant J.S., Parker I. Role of elementary Ca(2+) puffs in generating repetitive Ca(2+) oscillations. EMBO J. 2001;20:65–76. doi: 10.1093/emboj/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter N., Ritz M.F., Freyermuth S., Rogue P., Malviya A.N. Stimulation of nuclear protein kinase C leads to phosphorylation of nuclear inositol 1,4,5-trisphosphate receptor and accelerated calcium release by inositol 1,4,5-trisphosphate from isolated rat liver nuclei. J. Biol. Chem. 1993;268:732–736. [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc. Natl. Acad. Sci. U S A. 1988;85:5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata J., Guerra M.T., Shugrue C.A., Gomes D.A., Nagata N., Nathanson M.H. Lipid rafts establish calcium waves in hepatocytes. Gastroenterology. 2007;133:256–267. doi: 10.1053/j.gastro.2007.03.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M.S., Young K.W., Challiss R.A., Nahorski S.R. Intracellular signalling. Receptor-specific messenger oscillations. Nature. 2001;413:381–382. doi: 10.1038/35096643. [DOI] [PubMed] [Google Scholar]
- Politi A., Gaspers L.D., Thomas A.P., Hofer T. Models of IP3 and Ca2+ oscillations: frequency encoding and identification of underlying feedbacks. Biophys. J. 2006;90:3120–3133. doi: 10.1529/biophysj.105.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T.A., Sass E.J., Thomas A.P. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J. Biol. Chem. 1989;264:17131–17141. [PubMed] [Google Scholar]
- Rooney T.A., Sass E.J., Thomas A.P. Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J. Biol. Chem. 1990;265:10792–10796. [PubMed] [Google Scholar]
- Salazar C., Politi A.Z., Hofer T. Decoding of calcium oscillations by phosphorylation cycles: analytic results. Biophys. J. 2008;94:1203–1215. doi: 10.1529/biophysj.107.113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Bueno A., Dixon C.J., Woods N.M., Cuthbertson K.S., Cobbold P.H. Inhibitors of protein kinase C prolong the falling phase of each free-calcium transient in a hormone-stimulated hepatocyte. Biochem. J. 1990;268:627–632. doi: 10.1042/bj2680627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I.F., Wiltgen S.M., Parker I. Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 2009;45:65–76. doi: 10.1016/j.ceca.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneyd J., Han J.M., Wang L., Chen J., Yang X., Tanimura A., Sanderson M.J., Kirk V., Yule D.I. On the dynamical structure of calcium oscillations. Proc. Natl. Acad. Sci. U S A. 2017;114:1456–1461. doi: 10.1073/pnas.1614613114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneyd J., Tsaneva-Atanasova K., Reznikov V., Bai Y., Sanderson M.J., Yule D.I. A method for determining the dependence of calcium oscillations on inositol trisphosphate oscillations. Proc. Natl. Acad. Sci. U S A. 2006;103:1675–1680. doi: 10.1073/pnas.0506135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A., Morita T., Nezu A., Shitara A., Hashimoto N., Tojyo Y. Use of fluorescence resonance energy transfer-based biosensors for the quantitative analysis of inositol 1,4,5-trisphosphate dynamics in calcium oscillations. J. Biol. Chem. 2009;284:8910–8917. doi: 10.1074/jbc.M805865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.P., Bird G.S., Hajnoczky G., Robb-Gaspers L.D., Putney J.W., JR. Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- Thomas A.P., Renard D.C., Rooney T.A. Spatial and temporal organization of calcium signalling in hepatocytes. Cell Calcium. 1991;12:111–126. doi: 10.1016/0143-4160(91)90013-5. [DOI] [PubMed] [Google Scholar]
- Thomas A.P., Robb-Gaspers L.D. Subcellular and multicellular organization of calcium signaling in liver. In: Bailey G.W., Corbett J.M., Dimlich R.V.M., Michael J.R., Zaluzec N.J., editors. Microscopy and Microanalysis 1996. San Francisco Press, Inc; 1996. pp. 736–737. [Google Scholar]
- Thomas D., Lipp P., Tovey S.C., Berridge M.J., LI W., Tsien R.Y., Bootman M.D. Microscopic properties of elementary Ca2+ release sites in non-excitable cells. Curr. Biol. 2000;10:8–15. doi: 10.1016/s0960-9822(99)00258-4. [DOI] [PubMed] [Google Scholar]
- Thurley K., Falcke M. Derivation of Ca2+ signals from puff properties reveals that pathway function is robust against cell variability but sensitive for control. Proc. Natl. Acad. Sci. U S A. 2011;108:427–432. doi: 10.1073/pnas.1008435108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurley K., Tovey S.C., Moenke G., Prince V.L., Meena A., Thomas A.P., Skupin A., Taylor C.W., Falcke M. Reliable encoding of stimulus intensities within random sequences of intracellular Ca2+ spikes. Sci. Signal. 2014;7:ra59. doi: 10.1126/scisignal.2005237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey S.C., de Smet P., Lipp P., Thomas D., Young K.W., Missiaen L., de Smedt H., Parys J.B., Berridge M.J., Thuring J. Calcium puffs are generic InsP3;-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J. Cell Sci. 2001;114:3979. doi: 10.1242/jcs.114.22.3979. [DOI] [PubMed] [Google Scholar]
- Woodring P.J., Garrison J.C. Expression, purification, and regulation of two isoforms of the inositol 1,4,5-trisphosphate 3-kinase. J. Biol. Chem. 1997;272:30447–30454. doi: 10.1074/jbc.272.48.30447. [DOI] [PubMed] [Google Scholar]
- Woods N.M., Cuthbertson K.S., Cobbold P.H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986;319:600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- Woods N.M., Cuthbertson K.S.R., Cobbold P.H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987;8:79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GCaMP3 fluorescence images were collected at 10 Hz with a spinning disk confocal microscope (excitation, 488 nm; emission 510-nm-long band-pass filter). UV flash events are annotated at the top left and cover the entire field (appearing as white dots on images due to the position of the spinning disk). Time of vasopressin addition is also annotated on the top left. Traces of cells from this video are shown in Figure 5; video starts at 200 s
Data shown in Video S1 were processed using a differential algorithm to identify sites of Ca2+ increase. Red intensity is proportional to the rate of rise of GCaMP3 fluorescence and is overlaid on the initial gray scale image to identify the location and relative basal fluorescence intensity of GCaMP3-expressing cells. Note that only the rising phase of each Ca2+ transient is visualized in the red differential image, so this does not show the full duration of Ca2+ transients (which can be seen in Video S1).