Case Presentation
A 52-year-old right-hand dominant man with prior history of unprovoked deep vein thrombosis and pulmonary embolism, substance use, and 1 year of recurrent seizures and progressive magnetic resonance imaging (MRI) abnormalities presented to a tertiary care hospital with breakthrough seizures and worsening encephalopathy.
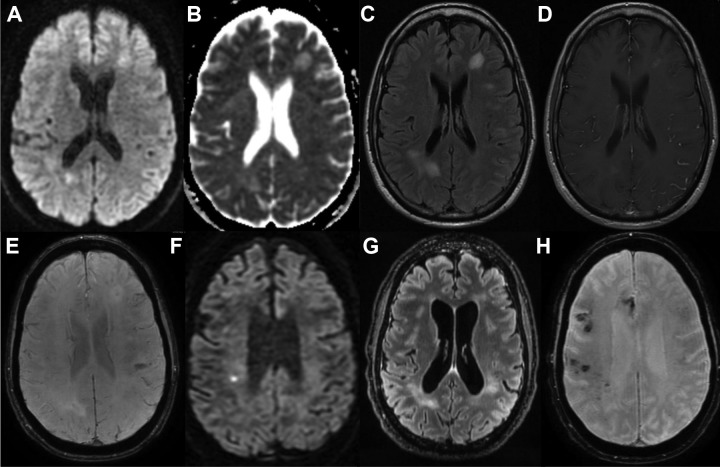
In February 2017, approximately 1 year prior to presentation, the patient developed spells during which he spoke nonsensically, lost consciousness, and awoke with aphasia for several hours. After his third episode in April 2017, the patient presented to a local hospital where brain MRI showed enhancing white matter lesions and few areas of increased susceptibility (Figure 1A-D). Cerebrospinal fluid (CSF) showed 1 white blood cell (WBC), elevated protein (94), normal glucose (44), normal immunoglobulin G (IgG) index, and matched oligoclonal bands in serum and CSF. An electroencephalogram (EEG) showed mild asymmetric slowing of the left frontal lobe. He was started on levetiracetam for suspected seizures and treated for presumed acute disseminated encephalomyelitis with intravenous (IV) methylprednisolone for 3 days with improvement in symptoms.
Figure 1.
Progressive white matter lesions and microhemorrhages. A-D, Axial MRI sequences at patient’s initial 4/2017 presentation including diffusion weighted imaging (DWI) (A), apparent diffusion coefficient (B), T2 fluid attenuated inversion recovery (FLAIR) (C), and T1 postcontrast (D). E, MRI SWAN from 5/2017. F-H, MRI sequences at patient’s latest 2/2018 presentation including axial DWI (F), T2 FLAIR (G), SWAN (H). MRI indicates magnetic resonance imaging; SWAN, susceptibility weighted angiography.
In March 2017, patient had another episode of “incoherent speech” and presented to a local hospital where MRI demonstrated worsening abnormalities, including a large lesion in the left frontal lobe concerning for left middle cerebral artery infarct. Digital subtraction angiography (DSA) showed incidental left ophthalmic artery aneurysm and left posterior communication artery infundibulum. He was treated with another course of high-dose methylprednisolone for 3 days with some improvement in speech before transfer to a tertiary care center for further workup.
A neurovascular workup including vessel imaging, cardiac monitoring, and transesophageal echocardiogram was unrevealing. Hemoglobin A1c was 5.6% and low-density lipoprotein was 103. Urinary toxicological screen was negative. Additional serum tests demonstrated leukocytosis (19 500/mm3), normocytic anemia (hematocrit, 35.7%), normal coagulation studies, and hypercoagulable testing including for antiphospholipid antibody syndrome was unrevealing. A rheumatological panel showed mildly elevated proteinase-3 antibody but unremarkable complement levels, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), antinuclear antibodies, double-stranded DNA, rheumatoid factor, anti-Smith/ribonucleoprotein, and anti-Sjögren's-syndromerelated antigen A, anti-Sjögren's-syndrome-related antigen B (SSA/SSB). Infectious testing for Lyme, syphilis, human immunodeficiency virus, and hepatitis B and C viruses was unremarkable, although a Quantiferon Gold test was positive in the setting of prior bacillus Calmette-Guerin vaccination. Other unremarkable workup included serum protein electrophoresis, immunoglobulin subclasses, cryoglobulins, vitamin B12, and thyroid function tests. MRI brain showed multiple patchy enhancing T2 hyperintense lesions in subcortical white matter including a large lesion in left frontal lobe, with multiple microhemorrhages on susceptibility weighted angiography sequences (Figure 1E). Positron emission tomography (PET) was unremarkable. Repeat EEG showed mild disorganization of the background, focal slowing of the left hemisphere, and multifocal epileptiform discharges of the left posterior quadrant.
A brain biopsy was recommended but the patient and family declined. He was discharged on aspirin and atorvastatin, and transitioned from levetiracetam to valproate. Repeat CSF studies 2 weeks after steroids demonstrated 1 WBC, slightly elevated protein (69 mg/dL), no oligoclonal bands, and normal IgG index (0.6). Surveillance MRIs after discharge were concerning for progressive disease including a new infarct of right superior cerebellum, worsening supratentorial and infratentorial fluid attenuated inversion recovery hyperintensity, microhemorrhage, and global parenchymal atrophy, though the patient remained clinically stable.
In December 2017, the patient had a prolonged seizure for which he was intubated and treated at a local hospital. Lacosamide was added to valproate, and high-dose prednisone was restarted with a long taper. During outpatient follow-up in January 2018, he was seizure-free and eager to resume part-time employment. Examination at the time demonstrated mild nonfluent aphasia, binocular diplopia on left superior temporal gaze, and a partial left homonymous hemianopsia. Due to ongoing diagnostic uncertainty, he was instructed to taper off steroids with plan for brain biopsy and repeat imaging.
During steroid reduction, the patient began experiencing behavior change marked by perseverative thoughts and actions, increased anxiety, and paranoia, before being readmitted in February 2018 after a cluster of seizures, at which point the authors first encountered the patient.
Initial Evaluation
In addition to the history elicited above, further evaluation revealed family history significant for stroke and pulmonary embolism. He endorsed a 15-year smoking history with substance use in last 18 months including alcohol, cocaine, and possible methamphetamine. The patient was married with 2 children, and he had continued to maintain a high-functioning career until shortly after the onset of his present illness. Medications included prednisone 10 mg twice daily, lacosamide, valproate, aspirin, and atorvastatin.
On mental status examination, he was alert with fluent spontaneous speech that was partially comprehensible. He answered limited simple questions and followed one-step commands with repeated prompting. He was unable to name objects or repeat. Dysconjugate exotropic gaze was apparent when he looked to the left. He wiggled fingers and toes less briskly on the right. He perseverated on performing finger-nose-finger without dysmetria.
Interval laboratory testing since prior hospitalization demonstrated mild thrombocytopenia, elevated CRP 40.7 (reference, <6.3 mg/L), and elevated lactate dehydrogenase (LDH) 234 (reference, 102-199 U/L). Repeat antiproteinase 3 was negative. The remainder of the patient’s laboratory testing was not significantly changed; MRI brain showed new foci of acute ischemia marked by reduced diffusion within the supratentorial white matter (Figure 1F), with interval progression of T2 hyperintensities (Figure 1G) and peripheral microhemorrhages (Figure 1H).
Clinical Discussion: Differential Diagnosis
Discussant: Megan Richie, MD
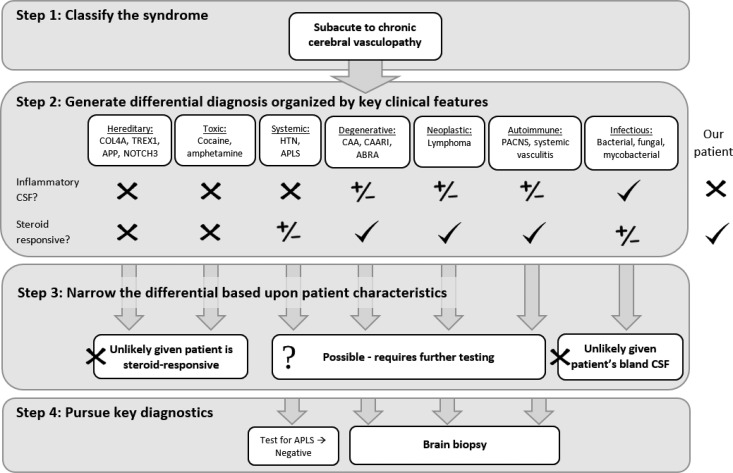
This patient presents with a waxing and waning chronic leukoencephalopathy with associated microhemorrhage and infarction. The combination of these features is suggestive of a vasculopathy, including autoimmune, degenerative, systemic, neoplastic, toxic, hereditary, and infectious causes (Figure 2). Given the pattern of infarcts, top autoimmune contenders include primary angiitis of the central nervous system versus a secondary small- or medium-vessel systemic vasculitis such as polyarteritis nodosa, ANCA-associated vasculitis, or Behςet’s syndrome. Cerebral amyloid angiopathy (CAA) is a degenerative condition in which progressive amyloid deposition causes vasculopathy and subsequent accumulation of microhemorrhages; CAA may also have associated inflammation (CAA-related inflammation or CAARI) or vasculitis (amyloid-β related angiitis or ABRA1). Systemic conditions that lead to vasculopathy include chronic hypertension and antiphospholipid antibody syndrome (APLS).
Figure 2.
Approach to the differential diagnosis. Steps 1 and 2 outline a broad approach to subacute to chronic cerebral vasculopathy, while step 3 narrows the differential diagnosis based upon the individual characteristics of this patient and step 4 illustrates the key diagnostic steps necessary to explore the remaining possibilities.
Vasculopathy associated with neoplasm is typically due to primary angioinvasion, such as can occur in lymphoma or breast cancer. Intravascular lymphoma (IVL) is a rare subtype of lymphoma that can present with a variety of symptoms due to occlusion of capillaries and postcapillary venules, involving widespread systemic circulation including the central nervous system (CNS) or isolated to skin (“cutaneous variant”).2 In Western countries, the most common presenting symptoms apart from skin lesions are related to CNS disease with rapidly progressive neurologic signs3,4; MRI brain may show small vessel ischemic strokes and microhemorrhages.5 Serum testing typically demonstrates hematologic abnormalities including elevated LDH (80%), anemia (65%), thrombocytopenia (29%), leukopenia (24%), and elevated CRP or ESR (43%).2
Numerous toxic causes could produce this syndrome, including recreational substances such as cocaine or methamphetamine, or iatrogenic causes such as radiation therapy. Hereditary vasculopathies that may lead to microhemorrhage and/or ischemia include mutations in COL4A, TREX1, amyloid precursor protein, or NOTCH3. Finally, over a year of symptoms without fulminant progression would be unusual for infectious causes, but could occur in fungal, mycobacterial, or atypical bacterial infections such as Treponema pallidum or Tropheryma whipplei.
Several clinical features of this case are helpful in narrowing this differential diagnosis. First, the CSF is not inflammatory, significantly lowering the likelihood of infection. In contrast, autoimmune processes such as vasculitides remain on the differential, as many of these patients do not have inflammatory CSF profiles.6 A second critical element in this case is the steroid responsivity, which makes toxic and hereditary causes much less likely. Noninflammatory CAA is likewise unlikely, both due to the patient’s steroid responsivity and infarcts, although CAARI and ABRA remain on the differential. Hypertensive vasculopathy would also not respond to steroids while APLS potentially would. Finally, most neoplasms would not respond to steroids so exquisitely with the primary exception of lymphoma. This leaves a final differential diagnosis of primary or secondary vasculitis, CAARI, ABRA, APLS, or IVL.
Clinical Discussion: Diagnostic Workup
Discussants: Megan Richie, MD, and Christopher McGraw, MD, PhD
In this patient, APLS testing is already unremarkable, and CSF, DSA, and extensive MRI have not yielded a specific diagnosis. In the workup of vasculopathy, these tests can be supportive but are not usually confirmatory. For example, the MRI findings of IVL are heterogeneous and nonspecific and include infarcts, focal enhancement, and white matter hyperintensity.5 Additionally, while DSA can be helpful in the workup of a vasculopathy, it has low sensitivity in small vessel processes and does not distinguish between inflammatory or noninflammatory causes.3 Finally, while CSF testing may be helpful to rule-in an inflammatory process, CSF may only be modestly abnormal in primary CNS vasculitis, and normal results should not be used to exclude the diagnosis in the setting of abnormal imaging or other clinically compatible features.6
In this patient, further imaging with an amyloid PET scan to investigate amyloid-related conditions could be considered, but ultimately tissue would be required to differentiate between CAA, CAARI, and ABRA. Vasculitis and IVL likewise are diagnosed by biopsy. However, while brain tissue may offer a diagnosis, when possible biopsy of more easily accessible sites should be prioritized.7 Peripheral blood smear may very occasionally demonstrate IVL.2 Random skin biopsy has been demonstrated to be helpful to diagnose IVL by several authors8,9 and should be pursued even in the absence of identifiable lesions if IVL is in the differential. Our patient had a skin biopsy of an ulcerated wound on his left upper extremity that was unrevealing. Since systemic imaging with CT and PET did not identify an alternative target, brain biopsy is the next logical step.
Ideally, brain biopsy should be obtained prior to the start of steroids, especially when lymphoma is on the differential, as steroids may quickly and dramatically reduce the yield of the biopsy due to an antineoplastic effect.10 Recommended targets would be an area of enhancement ideally with associated reduced diffusion in a noneloquent area of brain, obtaining 1 cm3 sample of grey matter, white matter, and leptomeninges. This patient’s steroids were rapidly weaned and a brain biopsy subsequently obtained.
Brain Biopsy
Discussants: Andrew W. Bollen, MD, DVM, and Sean P. Ferris, MD, PhD
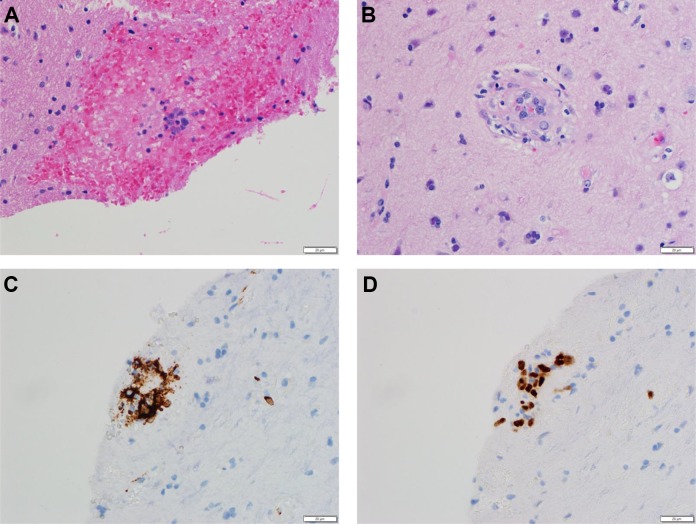
The brain biopsy specimen showed multiple small vessels associated with microhemorrhage and rare vessels containing enlarged, atypical lymphocytes with irregular nuclear borders and prominent nucleoli (Figure 3A and B). Level sections demonstrated multiple additional vessels with enlarged, atypical lymphocytes, which were positive for the B-cell markers CD20 (Figure 3C) and PAX5 (Figure 3D), and negative for CD3 (not shown). These histologic and immunohistochemical findings are diagnostic of intravascular large B-cell lymphoma.11 Intravascular large B-cell lymphoma tends to involve small and intermediate-sized blood vessels and can be a patchy process.2 A single negative biopsy may not reliably exclude the diagnosis, particularly if the initial biopsy was performed after recent steroid use, so in patients where there is high clinical suspicion for IVL, a second biopsy may be necessary.
Figure 3.
Brain biopsy. Hematoxylin and eosin stained sections at ×400 magnification demonstrate enlarged, atypical lymphocytes associated with microhemorrhage (A) or within small vessels (B). Immunohistochemically stained sections at ×400 magnification show the atypical lymphocytes are positive for the B-cell markers CD20 (C) and PAX5 (D).
Management
Discussants: Christopher McGraw, MD, PhD, and Sara LaHue, MD
There are no randomized trials investigating the optimal treatment of IVL, therefore patient management tends to be individualized, and new chemotherapeutic regimens are continuing to be tested (recently reviewed by Nizamutdinov et al7). Commonly, patients are treated with a regimen of rituximab and anthracycline-based chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone) known as R-CHOP12,13 in combination with more CNS-penetrant therapy (eg, intrathecal methotrexate, cytarabine, and prednisolone)14,15 or high-dose methotrexate. However, relapse is common, particularly in patients with CNS disease.2
Our patient received high-dose IV solumedrol followed by R-CHOP and high-dose methotrexate. Despite this therapy, there was clinical concern for recurrence in the setting of progressive worsening of encephalopathy, although concurrent lung, urine, and skin infections also likely contributed. The decision was made not to pursue further cancer-directed workup or therapy. He lived at home with support from his wife. His nonfluent aphasia and seizure control were stable. He required a wheelchair for mobility. At last follow-up 4 months later, he continued to live at home despite continued decline due to fatigue in the setting of recurrent urinary tract infections. Patient and family were pursuing comfort care.
Footnotes
Authors’ Note: Christopher M. McGraw, MD, PhD, Sara C. LaHue, MD, contributed equally to the work.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Christopher M. McGraw, MD, PhD  https://orcid.org/0000-0002-5460-4550
https://orcid.org/0000-0002-5460-4550
Keywords: progressive cerebrovascular disease, central nervous system diseases, seizures, epilepsy
References
- 1. Moussaddy A, Levy A, Strbian D, Sundararajan S, Berthelet F, Lanthier S. Inflammatory cerebral amyloid angiopathy, amyloid-β–related angiitis, and primary angiitis of the central nervous system: similarities and differences. Stroke. 2015;46(9):e210–213. [DOI] [PubMed] [Google Scholar]
- 2. Ponzoni M., Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132(15):1561–1567. [DOI] [PubMed] [Google Scholar]
- 3. Chapin JE, Davis LE, Kornfeld M, Mandler RN. Neurologic manifestations of intravascular lymphomatosis. Acta Neurol Scand. 2009;91(6):494–499. [DOI] [PubMed] [Google Scholar]
- 4. Brunet V, Marouan S, Routy JP, et al. Retrospective study of intravascular large B-cell lymphoma cases diagnosed in Quebec. Medicine (Baltimore). 2017;96(5):e5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams RL, Meltzer CC, Smirniotopoulos JG, Fukui MB, Inman M. Cerebral MR imaging in intravascular lymphomatosis. AJNR Am J Neuroradiol. 1998;19(3):427–431. [PMC free article] [PubMed] [Google Scholar]
- 6. Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol. 2009;66(6):704–709. [DOI] [PubMed] [Google Scholar]
- 7. Nizamutdinov D, Patel NP, Huang JH, Fonkem E. Intravascular lymphoma in the CNS: options for treatment. Curr Treat Options Neurol. 2017;19(10):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gill S, Melosky B, Haley L, ChanYan C. Use of random skin biopsy to diagnose intravascular lymphoma presenting as fever of unknown origin. Am J Med. 2003;114(1):56–58. [DOI] [PubMed] [Google Scholar]
- 9. Sitthinamsuwan P, Chinthammitr Y, Pattanaprichakul P, Sukpanichnant S. Random skin biopsy in the diagnosis of intravascular lymphoma. J Cutan Pathol. 2017;44(9):729–733. [DOI] [PubMed] [Google Scholar]
- 10. Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2017. [Google Scholar]
- 12. Sawada T, Omuro Y, Kobayashi T, et al. Long-term complete remission in a patient with intravascular large B-cell lymphoma with central nervous system involvement. OncoTargets Ther. 2014;7:2133–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwamuro M, Kimura K, Kondo E, Otsuka F. Hyperintense lesion in the pons in intravascular lymphoma. Intern Med. 2015;54(18):2421–2422. [DOI] [PubMed] [Google Scholar]
- 14. Kikuchi J, Kaneko Y, Kasahara H, et al. Methotrexate-associated intravascular large b-cell lymphoma in a patient with rheumatoid arthritis. Intern Med. 2016;55(12):1661–1665. [DOI] [PubMed] [Google Scholar]
- 15. Sekiguchi Y, Shimada A, Imai H, et al. Intravascular large B-cell lymphoma with pontine involvement successfully treated with R-CHOP therapy and intrathecal administration: a case report and review of literature. Int J Clin Exp Pathol. 2014;7(6):3363–3369. [PMC free article] [PubMed] [Google Scholar]