Abstract
Herpes simplex virus encephalitis (HSVE) usually presents as a monophasic disease. Symptomatic HSVE relapsing with seizures, encephalopathy, or involuntary movements associated with anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis have been recently reported. We report 2 cases of adult post-HSVE anti-NMDAR encephalitis from Portugal. Two female patients aged 50 years and 30 years were diagnosed with herpes simplex virus type 2 and type 1 encephalitis, respectively. After the initial improvement with specific treatment and despite virologic negativization, both patients suffered clinical, electroencephalographic, and imaging deterioration. The autoimmune encephalitis hypothesis was confirmed with the demonstration of anti-NMDAR antibodies in both cerebrospinal fluid and serum. Both responded to human immunoglobulin and methylprednisolone, with progressive gain of autonomy along the follow-up period. Thymectomy for thymic hyperplasia diagnosed during follow-up was performed in 1 patient. Although being rare, post-HSVE anti-NMDAR encephalitis should be considered in all cases of symptomatic recrudescence after HSVE, since adequate immune-modulating treatment improves the outcome. The role of thyme hyperplasia in autoimmune encephalitis pathogenesis needs better understanding.
Keywords: herpes virus simplex encephalitis, herpes simplex type 2, encephalitis relapse, autoimmune NMDA-R-receptor antibody encephalitis, thyme hyperplasia
Introduction
Herpes simplex virus encephalitis (HSVE) is one of the most common neurological infectious emergencies. With specific treatment, HSVE usually follows a monophasic course with progressive improvement.1,2 However, despite adequate treatment and virological negativization, after initial clinicoradiological improvement, neurological deterioration can occur in some patients.3,4 There is growing evidence that these relapsing post-HSVE manifestations are caused by secondary brain autoimmune disorder.4 The presence of immunoglobulin G antibodies against the GluN1 subunit of the N-methyl-d-aspartate receptor (NMDAR; anti-NMDAR antibodies) and the clinical improvement after treatment with immune modulators support the diagnosis of post-HSVE anti-NMDAR encephalitis as the cause of the relapsing symptoms.5 The literature on clinical presentation, treatment, and outcome of post-HSVE anti-NMDAR encephalitis in adults is very sparse. Herein, we report 2 cases of post-HSVE anti-NMDAR encephalitis from Portugal.
Methodology
Clinical, paraclinical, and magnetic resonance information was collected from medical records. Cerebrospinal fluid (CSF) anti-NMDAR antibodies were documented using indirect immunofluorescence antibody test. Informed consent was obtained from both patients.
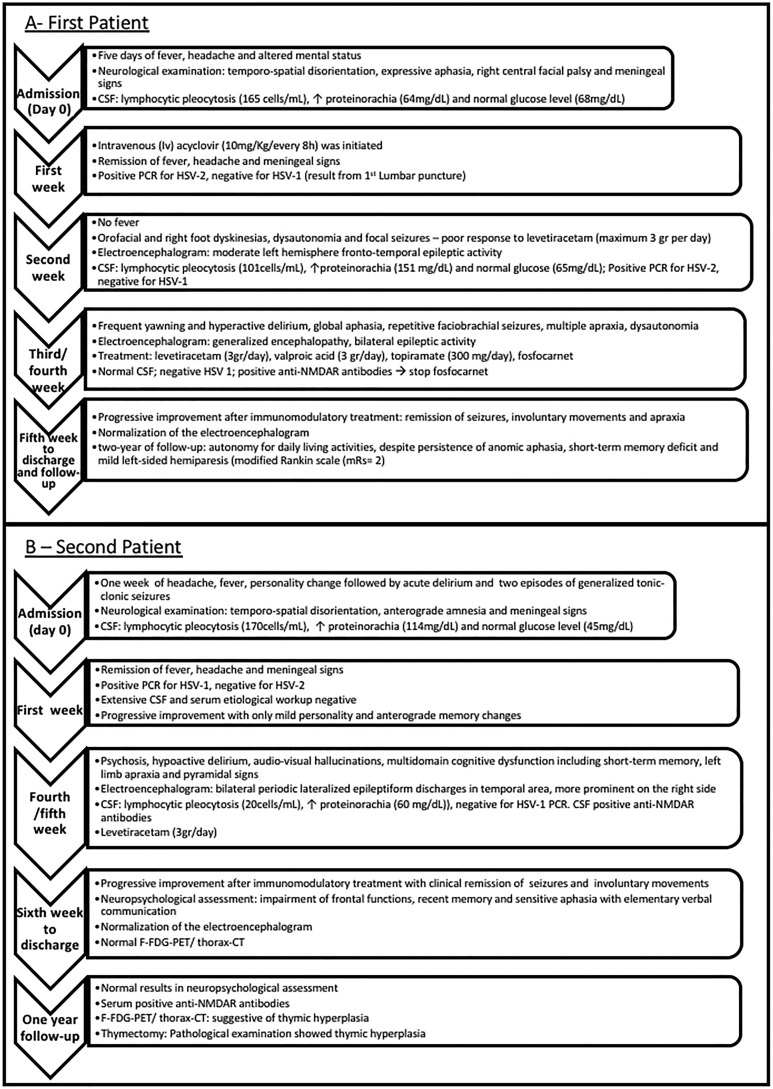
First Patient
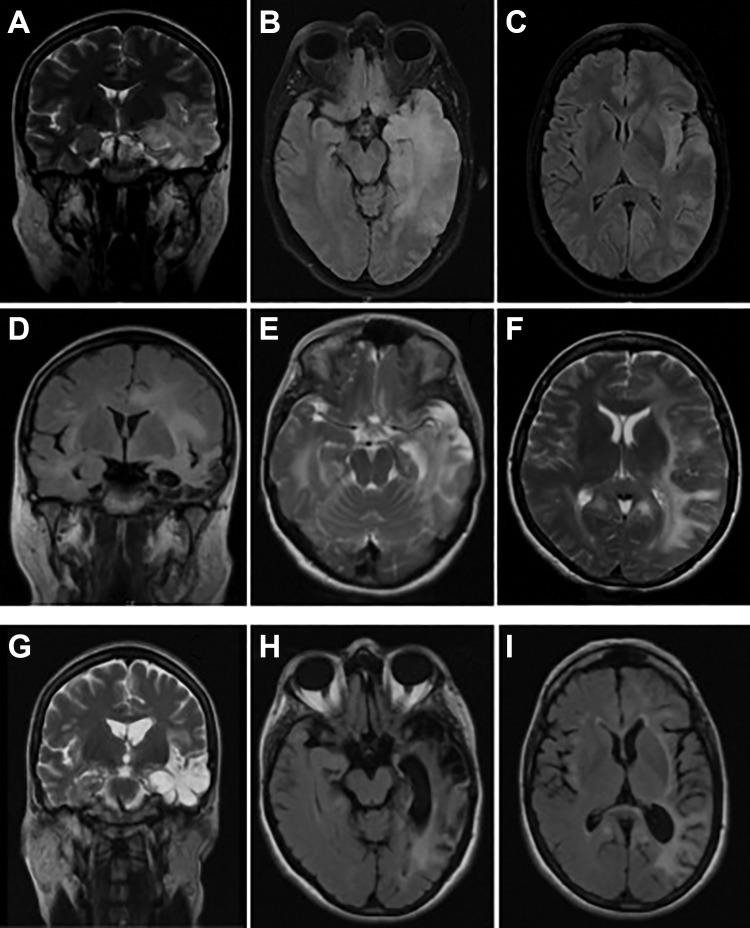
A previously healthy 50-year-old female was admitted because of an acute encephalitis (Figure 1A). The brain magnetic resonance imaging (MRI) revealed the presence of a left temporal lobe lesion compatible with acute HSVE (Figure 2A-C). Intravenous (IV) acyclovir (10 mg/kg every 8 hours) was started for presumed HSVE. Cerebrospinal fluid polymerase chain reaction (PCR) confirmed the presence of HSV-2 infection, and it was negative for HSV-1 (Figure 1A). After initial improvement, at the end of the second week after admission, her clinical condition got worse with orofacial and right foot dyskinesias, dysautonomia, and focal seizures with poor response to levetiracetam (maximum 3 g/d; Figure 1A). The CSF was still inflammatory and virological retesting sustained the diagnosis HSV-2 infection, and PCR was again negative for HSV-1. The temporal brain lesion was discreetly increased on the follow-up brain MRI (day 16). A presumable acyclovir-resistant HSV-2 infection was considered and foscarnet was started (day 21). Despite therapeutic optimization, progressive neurological worsening occurred with fluctuation in consciousness, transient tachycardia, global aphasia, orofacial and right foot dyskinesia, and right-sided faciobrachial dystonic seizures, which progressed to encephalopathy (Figure 1A). Focal seizures persisted despite antiepileptic treatment optimization (levetiracetam—3 g/d, valproic acid—3 g/d, topiramate—300 mg/d). The brain MRI disclosed extensive bilateral asymmetric white matter lesions (Figure 2D-F). At the fourth week, the CSF was normal, the PCR for HSV was negative, and foscarnet was stopped. The possibility of postinfectious immune complication was considered at the end of fourth week of hospitalization. The CSF antibodies associated with autoimmune encephalitis were requested and turned to be positive for the presence of anti-NMDAR antibodies (indirect immunofluorescence antibody test). Patient was started on human intravenous immunoglobulin (IVIg; 23 g/d) and methylprednisolone (MP; 1 g/d) pulses for 5 days followed by oral prednisolone and human immunoglobulin sessions along 8 months. With immune-modulating treatment, marked clinical and radiological (Figure 2G-I) improvement occurred. After 2-year follow-up, despite the persistence of discrete anomic aphasia, short-term memory deficit, and mild left-sided hemiparesis (modified Rankin Scale [mRS] = 2), she regained autonomy for daily living activities. The malignancy workup yielded negative results.
Figure 1.
Timeline of clinical evolution and treatment of the patient 1 (A) and patient 2 (B).
Figure 2.
Brain magnetic resonance imaging (MRI) evolution of the first patient. Initial MRI—coronal T2 imaging (A) and axial fluid-attenuated inversion recovery (FLAIR) imaging (B-C) showing hyperintensity in the left temporal lobe involving the hippocampus and hyperintensity in left temporal and insular lobes, respectively; post-herpes simplex virus encephalitis (HSVE) anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis—coronal FLAIR imaging (D) showing extensive left temporal and insular lobes hyperintensity with dilatation of temporal horn and choroid fissure; axial T2 imaging (E-F) with bilateral hyperintensity in temporal and insular lobes, with predominance in the left side with parietal and frontal extension; follow-up imaging (after immunotherapy and approximately 10 weeks after initiation of acyclovir)—coronal T2 imaging (G) showing left temporal region of increase T2 signal with cystic degeneration and ex-vacuo dilatation of temporal horn; axial FLAIR imaging (H-I) with left temporoparietal gliosis.
Second Patient
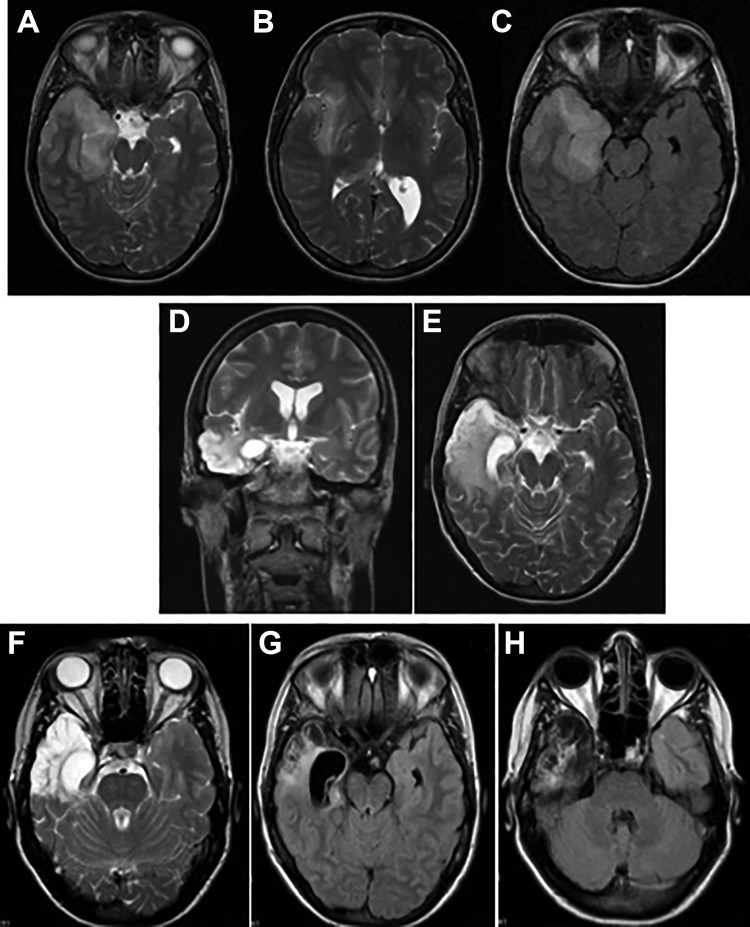
A 33-year-old woman with unremarkable past history was also diagnosed with acute encephalitis (Figure 1B). The brain MRI revealed a right temporal lobe lesion suggestive of acute encephalitis (Figure 3A-C). Herpes simplex virus 1 PCR became positive in CSF (Figure 1B), and IV acyclovir was initiated (10 mg/kg every 8 hours). A progressive improvement was noticed on the first week of treatment. On the fourth week, her condition started to deteriorate (Figure 1B), with mild personality change which progressed rapidly to hypoactive delirium, audiovisual hallucinations, severe short-term memory, language, executive–visual–spatial functions impairment, left limb apraxia, and pyramidal signs. No dysautonomic or movement disorders features were perceived.
Figure 3.
Brain magnetic resonance imaging (MRI) evolution of the second patient. Initial MRI—axial T2 imaging (A-B) and axial fluid-attenuated inversion recovery (FLAIR) imaging (C) showing hyperintensity in right temporoinsular area; post-herpes simplex virus encephalitis (HSVE) anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis—coronal (D) and axial (E) T2 imaging with hyperintensity evolving temporomesial area; follow-up imaging (after immunotherapy and approximately 24 weeks after initiation of acyclovir)—axial T2 imaging (F) and axial FLAIR imaging (G-H) showing hyperintensity with atrophy in the right temporoinsular area, and atrophy and gliosis in the right temporal area and hippocampus, respectively.
The follow-up brain MRI showed similar lesions with severe right hippocampus atrophy (Figure 3D and E). Intravenous acyclovir (10 mg/kg every 8 hours) was restarted for presumable recurrent HSV encephalitis, but the neurological worsening persisted. A postinfectious immune complication was considered, and CSF antibodies associated with autoimmune encephalitis were requested and turned to be positive for the presence of anti-NMDAR antibodies (indirect immunofluorescence antibody test). IVIg (25 g/d) and MP (1 g/d) pulses were administered for 5 days followed by oral prednisolone. Gradual clinical and radiological improvement were noticed, although neuropsychological assessment revealed severe impairment of frontal functions, recent memory, and sensitive aphasia with elementary verbal communication. The malignancy workup, including positron emission tomography (PET) scan, yielded negative results. After 1-year follow-up, a progressive clinical improvement was perceivable with normal results in neuropsychological assessment and no abnormalities on the physical examination (mRS = 0). Magnetic resonance imaging (Figure 3F-H) showed a severe right temporal atrophy. Anti-N-methyl-d-aspartate receptor antibodies were still positive in serum. At this time, the positron emission tomography with fluorine-18-fluorodeoxyglucose (F-FDG-PET) and thorax computed tomography were repeated and they were suggestive of thymic hyperplasia. She underwent thymectomy, and pathological examination showed thymic hyperplasia but no signs of teratoma. No relapses were noticed in 1-year follow-up postthymectomy.
Discussion
Cross-reactivity induced by the immune response to the HSV infection exposing central nervous system antigens otherwise normally unexposed is the most reasonable mechanism explaining the occurrence of post-HSVE anti-NMDAR encephalitis.6,7 Although more frequently described after HSV1 encephalitis, post-HSVE autoimmune encephalitis following HSV2 infection also occurs.3 It remains to clarify why anti-NMDAR encephalitis does not occur after other herpes virus infections, such as human herpesvirus 6, Varicella-zoster virus, and Epstein-Barr virus.8 In the reported cases, post-HSVE NMDA-R encephalitis symptoms started between 28 and 90 days after onset of HSVE, with patients at different phases of antiviral treatment. This suggests a relation between the amount of antigen released as a result of the HSV-induced neuronal damage with the overactivation of immune system. In that sense, delayed treatment with acyclovir may potentiate the development of post-HSVE NMDA-R encephalitis.9,10 As in similar cases, behavioral–psychiatric alterations, cognitive dysfunction, headache, and abnormal movements were the main clinical manifestations, and the virus was not detectable in CSF at the time the complication occurred. As in our cases, the diagnosis of post-HSVE NMDA-R encephalitis was not initially considered in the majority of published cases, raising a strong possibility of underdiagnoses of this treatable condition. Patients with post-HSVE NMDA-R encephalitis respond to high-dose steroids and IVIg or plasma exchange, particularly if promptly started.9 Second-line therapies, such as rituximab and cyclophosphamide, are indicated for refractory post-HSVE NMDA-R encephalitis.11 For some authors, the occurrence of post-HSVE NMDA-R encephalitis raises the discussion regarding the relevance of corticotherapy in HSVE treatment to prevent autoimmunity.6 Small studies have shown that corticosteroids are safe, do not interfere with the virological resolution, and can improve the prognosis of HSVE.12 Since prompt immunotherapy is crucial for a good outcome, it is important to consider early the diagnosis of autoimmune encephalitis in all patients with relapsing symptoms after HSVE. Thymic tumors, mostly thymoma, have been associated with autoimmune encephalitis such as CASPR2, GABA-A and B, AMPA-R, GAD65, and ANNA-1, but not with NMDA-R encephalitis.13 In one of the patients, thymic hyperplasia was diagnosed and treated after clinical improvement. This fact limits our conviction regarding the possible contributive association with the development of post-HSVE NMDA-R encephalitis. However, the chronic persistence of NMDA-R antibodies raises an interesting hypothesis that thymus can be involved in autoimmune encephalitis pathogenesis. In this particular case, thymectomy was considered because thymic pathology and its role in eventually future relapsing symptoms were unknown.
In conclusion, our cases are illustrative of the need for increasing awareness of this entity in order to reduce delay in diagnosis. In addition, we discuss possible findings that may contribute to better understand the immune-mediated mechanisms that can explain the occurrence of post-HSVE NMDA-R encephalitis.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ana André  https://orcid.org/0000-0003-1921-4095
https://orcid.org/0000-0003-1921-4095
References
- 1. McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997;63(3):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics. 2016;13(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sköldenberg B, Aurelius E, Hjalmarsson A, et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol. 2006;253(2):163–170. [DOI] [PubMed] [Google Scholar]
- 4. Armangue T, Moris G, Cantarín-Extremera V, et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology. 2015;85(20):1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prüss H, Finke C, Höltje M, et al. N-methyl-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72(6):902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galli J, Clardy SL, Piquet AL. NMDAR encephalitis following herpes simplex virus encephalitis. Curr Infect Dis Rep. 2017;19(1):1. [DOI] [PubMed] [Google Scholar]
- 7. Linnoila J, Rosenfeld M, Dalmau J. Neuronal surface antibody-mediated autoimmune encephalitis. Semin Neurol. 2014;34(4):458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schabitz WR, Rogalewski A, Hagemeister C, Bien CG. VZV brainstem encephalitis triggers NMDA receptor immunoreaction. Neurology. 2014;83(24):2309–2311. [DOI] [PubMed] [Google Scholar]
- 9. Schein F, Gagneux-Brunon A, Antoine JC, et al. Anti-N-methyl-d-aspartate receptor encephalitis after Herpes simplex virus-associated encephalitis: an emerging disease with diagnosis and therapeutic challenges. Infection. 2017;45(4):545–549. [DOI] [PubMed] [Google Scholar]
- 10. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramos-Estebanez C, Lizarraga KJ, Merenda A. A systematic review on the role of adjunctive corticosteroids in herpes simplex virus encephalitis: is timing critical for safety and efficacy? Antivir Ther. 2013;19(2):133–139. [DOI] [PubMed] [Google Scholar]
- 13. Nwabuobi LA, Pellinen JC, Wisniewski TM. Thymoma-associated panencephalitis: a newly emerging paraneoplastic neurologic syndrome. Neuroimmunol Neuroinflamm. 2017;4(6):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]