Abstract
Background and Purpose:
Due to the potential for high mortality and neurologic complications of rheumatoid meningitis (RM), awaiting biopsy confirmation may delay vital treatment intervention. Our aim was to describe the clinical presentations of RM in our population and determine whether meningeal biopsy impacted diagnosis, treatment, and outcomes.
Methods:
A retrospective chart review was completed for patients at Mayo Clinic with a diagnosis of RM within the last 28 years. Those with identified alternative inflammatory, infectious, or neoplastic causes of pachymeningitis or leptomeningitis were excluded.
Results:
Fourteen patients meeting inclusion/exclusion criteria were identified. All patients were positive for rheumatoid factor or cyclic citrullinated peptide. All patients had magnetic resonance imaging abnormalities characterized by pachymeningeal and/or leptomeningeal enhancement. Of the 10 patients who underwent biopsy, nonspecific findings were seen in 74%. All patients except one were treated with corticosteroids with subsequent symptomatic improvement. Radiographic improvement or resolution was seen in 10 (83%) of 12. Patients improved with corticosteroid treatment, including those who were diagnosed with RM on clinical basis without undergoing a biopsy as well.
Conclusions:
This retrospective review displays the myriad of clinical presentations of RM. It also suggests that with appropriate exclusion of infectious, neoplastic, and other autoimmune etiologies, biopsy may not be necessary to initiate treatment.
Keywords: rheumatoid arthritis, rheumatoid meningitis, rheumatoid pachymeningitis, biopsy
Introduction
Rheumatoid arthritis (RA) is an inflammatory disease primarily affecting synovial joints. It has several well-known extra-articular manifestations including those within the central and peripheral nervous system. Potential neurological manifestations of RA include cervical radiculopathy secondary to atlantoaxial subluxation,1,2 compressive myelopathy, mononeuritis multiplex,3 and carpal tunnel syndrome. Vasculitis involving the central nervous system has also been described.4,5 Rheumatoid meningitis (RM) is a rare complication of RA manifesting as meningitis, resulting in focal neurologic deficits, stroke-like episodes,6 headache, seizures, and/or encephalopathy.4 Rheumatoid meningitis can affect the pachymeninges, leptomeninges, or both.2 Oftentimes, clinical symptoms can precede radiographic findings by months.7 The differential diagnosis of RM is broad, particularly in the setting of immunosuppression commonly used to treat patients with RA. Etiologies including infection, for example, Mycobacterium tuberculosis (MTB), endemic mycoses, syphilis, neurosarcoidosis, idiopathic hypertrophic pachymeningitis, immunoglobulin G4 (IgG4)-related hypertrophic meningitis, granulomatosis with polyangiitis, Sjögren syndrome, lymphoma, and meningeal metastases must be excluded.2 Although there are no established diagnostic criteria for RM, this condition has typically been substantiated through meningeal biopsy revealing a combination of rheumatoid nodules, nonspecific meningeal inflammation, or vasculitis.4 Rheumatoid meningitis should be considered in all patients with RA, especially those with long-standing disease, presenting with new neurologic symptoms localizing to the central nervous system.
We reviewed the clinical and diagnostic features of 14 patients with RM evaluated at our institution. The purpose of this study was to determine whether meningeal biopsy influenced the diagnosis, treatment, and outcomes in this RM cohort.
Methods
This was a retrospective chart review completed searching for patients from 1990 to present within the Mayo Clinic system with a diagnosis of RM. All data for the study were obtained from existing institutional review board—approved data within the Mayo Clinic system.
Key search words included “rheumatoid arthritis,” “pachymeningitis,” “leptomeningitis,” “chronic meningitis,” and “meningitis NOS.” A series of searches was conducted using both keywords and International Classification of Disease, Ninth Revision codes. Charts were then individually reviewed to verify diagnoses. Patients were classified as having RA only if a rheumatologist had made that diagnosis either prior to or at time of presentation with RM. Rheumatoid meningitis was diagnosed based on clinical judgment of both a rheumatologist and a neurologist along with exclusion of other infectious, neoplastic, and autoimmune etiologies. Specifically, RM was diagnosed if the following were reasonably excluded: sarcoidosis, granulomatosis with polyangiitis, idiopathic hypertrophic pachymeningitis, hematologic malignancy, solid organ malignancy, meningeal metastases, and infection (eg, MTB, fungal, syphilis). A myriad of additional infectious studies were selectively obtained depending on clinical presentation and immune state. Data regarding clinical presentation, RA disease features, laboratory findings, radiologic results including chest imaging, biopsy findings, treatment, and outcomes were extracted by detailed chart review. All diagnostic evaluations were not completed at the time of presentation with neurological symptoms as some patients were secondary referrals. Diagnostic findings from outside facility workups and our institution were included. In a small proportion of our patients, diagnostic testing was completed up to 4 years from neurological symptom onset.
Results
Fourteen patients with RM were identified. The mean age at diagnosis was 66.6 years; 8 (57%) of 14 of patients were men, 6 (43%) of 14 women. The most common presenting symptoms were headache 7 (50%) of 14 and seizure 7 (50%) of 14. Hemiparesis occurred in 5 (36%) of 14. Other symptoms included hearing loss, gait instability, hemisensory loss, and altered mental status. Timing of RM in the RA disease course varied extensively. Rheumatoid meningitis presented in long-standing RA (>20 years) in 5 patients and at time of RA diagnosis in 3 patients. Of these 3, one patient presented with neurological symptoms preceding the joint symptoms by 12 months, while the second one never developed articular symptoms (likely as immunosuppressive therapy was initiated at neurological presentation). The third patient developed RM 6 months after the onset of joint symptoms. None of the newly diagnosed patients with RA had evidence of erosions or rheumatoid nodules. Rheumatoid arthritis was well controlled in all patients with established RA, except one. Joint imaging with plain radiography was done in 10 patients and showed erosions in 3 (30%). Subcutaneous nodules were seen in 4 (31%) of 13 patients, while lung nodules were seen in 6 (46%) of 13.
Diagnostic findings are detailed in Table 1. Rheumatoid factor (RF) was positive in 11 (79%) of 14, whereas anti–cyclic citrullinated peptide (CCP) antibody was positive in 10 (83%) of 12. All patients were positive for either RF or CCP, while 7 (50%) of 14 were positive for both. Erythrocyte sedimentation rate (ESR) was elevated in 7 (64%) of 11, and C-reactive protein (CRP) was elevated in 9 (75%) of 12. Importantly, both ESR and CRP were normal in 3 (27%) of 11 patients. Immunoglobulin G4 antibodies were tested in 4 patients and were negative. Cerebrospinal fluid (CSF) cell count was recorded in 13 of 14 patients and was elevated in 77% (range: 7-239 cells/µL) with a lymphocytic predominance in 80% of those patients. One patient had a neutrophilic predominance, whereas 2 had monocytic. Cerebrospinal fluid protein was elevated in 11 (79%) of 14 with a median elevation 87 mg/dL (range: 47-155 mg/dL). Cerebrospinal fluid glucose was normal in 12 (86%) of 14; the remaining patients had mildly decreased values. Cerebrospinal fluid cytology was unremarkable in 12 (86%) of 14 with the remainder showing inflammation; all were negative for malignancy. Chest imaging was negative for malignancy in all assessed 13 (93%) of 14.
Table 1.
Diagnostic Evaluation of Patient With RMa,b.
| Case | RF Titer (IU/mL) | CCP Titer | ESR (mm/1 hour) | CRP (mg/L) | CSF Cells (mm3) | CSF Differential (%) | CSF Protein (mg/dL) |
|---|---|---|---|---|---|---|---|
| 1 | 107 | 219.8 | 36 | 18.3 | 7.8 | 56 lymphocytes | 95 |
| 2 | >800 | 51.9 | 108 | 176 | 12 | 95 lymphocytes | 72 |
| 3 | 124 | >100 | NR | NR | 34 | 79 lymphocytes | 49 |
| 4 | 115 | >250 | 37 | 38 | 239 | 72 neutrophils | 39 |
| 5 | <15 | >250 | 49 | 10.3 | NR | NR | 118 |
| 6 | 108b | 25.6 | 20 | 5 | 7 | 47 monocytes | 61 |
| 7 | 280 | >250 | 35 | 4.2 | 30.6 | 97 lymphocytes | 31 |
| 8 | 63 | >250 | 2 | <3 | 2 | 73 lymphocytes | 47 |
| 9 | <15 | >250 | NR | 14.8 | 2 | 86 lymphocytes | 106 |
| 10 | <15 | >250 | 11 | <3 | 74 | 85 lymphocytes | 87 |
| 11 | 70 | NR | 15 | <3 | 72 | 92 lymphocytes | 155 |
| 12 | 100 | NR | 72 | 6.26 | 23 | 45 lymphocytes, 44 monocytes | 73 |
| 13 | 45b | <2b | NR | NR | 3 | 61 monocytes | 28 |
| 14 | 292 | 15.4b | 90 | 15 | 148 | 92 lymph | 103 |
Abbreviations: CCP, cycli citrullinated peptide; CRP, C-reactive protein; CSF, cerebrospinal fluid; Diff, differential; ESR, erythrocyte sedimentation rate; NR, not recorded; RF, rheumatoid factor; RM, rheumatoid meningitis.
aNormal values: RF < 15 IU/mL, CCP < 20, ESR 0 to 22 mm/1 hour, CRP < 8 mg/L, CSF cells 0 to 5 mm3, CSF protein 14 to 45 mg/dL.
bValues obtained during diagnostic period, up to 2-year variance from CSF values. Case 6: 2 years prior, NR at time of CSF. Case 13: 2 years after initial CSF. Case 14: 6 months later than RF and CSF.
Table 2 describes the imaging and biopsy characteristics of each case. All patients demonstrated enhancement of the pachymeninges, leptomeninges, or both, with 12 (86%) of 14 having a frontoparietal predominance (Table 2). Asymmetric involvement was appreciated in 11 (78.6%) of 14. In the 10 patients who underwent biopsy, 90% showed nonspecific inflammation or granulomatous necrosis. Rheumatoid nodules and vasculitic changes were not seen histologically in our series. No infectious organisms were identified. Biopsy was not pursued in 4 patients given symptomatic improvement with treatment. Diagnostic confidence of RM impacted this decision. There were no significant differences in regard to treatment or treatment response in patients with or without biopsy.
Table 2.
Enhancement Patterns and Pathology of Patients With RM.
| Case | Enhancement Pattern | Location | Pathology |
|---|---|---|---|
| 1 | Pachy/lepto | Primary R parietal | No inflammation or necrosis, abundant macrophages, no plasma cells |
| 2 | Pachy | Posterior fossa | NP |
| 3 | Pachy/lepto | Bifrontal, max L | Necrotizing granulomatous inflammation |
| 4 | Pachy/lepto | Bifrontal, max R | Nonspecific chronic inflammation, abundant macrophages, positive T and B lymphocytes |
| 5 | Pachy/lepto | Frontoparietal, R | NP |
| 6 | Pachy/lepto | Frontal, R | Mild chronic and necrotizing inflammation, positive lymphocytes, positive histiocytes |
| 7 | Pachy/lepto | Frontoparietal, R | Severe inflammation with necrotizing granulomas; numerous plasma cells |
| 8 | Pachy/lepto | Frontoparietal, R | Acute-chronic inflammation, extensive necrosis, multinucleated giant cells |
| 9 | Lepto | Frontoparietal, occipital | NP |
| 10 | Pachy | Frontal, R | Mixed chronic inflammation |
| 11 | Pachy/lepto | Bilateral, R>L | NP |
| 12 | Lepto | Frontoparietal, L | Necrotizing granulomatous meningitis |
| 13 | Pachy | Bifrontal dural mass | Laminar necrotizing inflammation, positive plasma cells, positive T and B lymphocytes, positive histiocytes |
| 14 | Lepto | Frontoparietal, L | Necrotizing granulomatous process, positive plasma cells |
Abbreviations: lepto, leptomeningeal; max, maximal; NP, not performed; pachy, pachymeningeal; RM, rheumatoid meningitis.
Corticosteroids were the treatment of choice in all but one case, as shown in Table 3. Treatment varied between oral prednisone or dexamethasone and 3- to 5-day courses of intravenous methylprednisolone. Twelve (86%) of 14 patients received a corticosteroid taper, and 9 (64%) of 14 were placed on an immunosuppressive agent, including rituximab, azathioprine, etanercept, and methotrexate. The mean duration of prednisone treatment prior to tapering was known for 9 (75%) of 12 patients with a mean of 4.7 weeks (range: 2-12 weeks). All patients had symptomatic improvement following treatment. There was no difference in outcomes in patients treated with oral versus parenteral corticosteroids. Ten (83%) of the 12 patients who underwent repeat imaging showed complete or near complete resolution of abnormal enhancement with a mean imaging interval to improvement of 6.7 months (range: 2-24 months). The 2 patients without radiological improvement had persistent symptoms. One of these patients had not undergone a biopsy.
Table 3.
Treatment and Outcomes of Patients With RM.
| Case | Initial Treatment | Maintenance | Biopsy (Y/N) | Follow-Up MRI | Residual Symptoms |
|---|---|---|---|---|---|
| 1 | 5d IVMP | Prednisone taper | Y | NP | None |
| 2 | 3d IVMP | Prednisone taper | N | Resolution, 2 months | Hearing loss |
| 3 | 3d IVMP, ×2 | Prednisone taper, rituximab | Y | Improvement, 16 months | None |
| 4 | 3d IVMP | Prednisone taper | Y | Resolution, 4 months | None |
| 5 | 4d IVMP | Prednisone taper | N | Resolution, 2 months | None |
| 6 | 5d IVMP | Orednisone taper, rituximab | Y | Near complete resolution, 4 months | None |
| 7 | RIPE, low-dose prednisone | low-dose prednisone, rituximab | Y | Resolution, 2 years | None |
| 8 | Weekly IVMP | Prednisone taper, rituximab | Y | No change | HA |
| 9 | Prednisone 40 mg | Prednisone taper, rituximab | N | NP | Episodic hemiparesis |
| 10 | Dexamethasone | Dexamethasone taper | Y | Resolution, 3 months | NA |
| 11 | None | Azathioprine, etanercept | N | Improvement, 5 months | None |
| 12 | Prednisone 60 mg | Prednisone taper, methotrexate | Y | Resolution, 5 months | None |
| 13 | 5d IVMP | Prednisone taper, methotrexate | Y | No change | Infrequent seizures |
| 14 | Prednisone 60 mg | Prednisone taper, azathioprine | Y | Improvement, 5 months | None |
Abbreviations: IVMP, intravenous methylprednisolone; MRI, magnetic resonance imaging; NP, not performed; RIPE, rifampin, isoniazid, pyrazinamide, ethambutol; RM, rheumatoid meningitis.
Discussion
Rheumatoid meningitis is a rare entity that may occur at any time throughout the RA disease course or even as the first manifestation of the disease.8 Prompt identification and treatment results in excellent clinical outcomes. Although prior studies have reported high mortality rates,9 excellent outcomes can be achieved with prompt identification and treatment. This case series demonstrates that a detailed diagnostic evaluation of clinically suspected RM in a patient with RA should include appropriate blood work, CSF, and radiologic studies to establish the diagnosis. Evaluation necessitates exclusion of infectious causes of pachymeningitis including mycobacterial infection (eg, TB), fungal—namely endemic mycotic, and spirochetal (syphilis); noninfectious to include GPA (granulomatosis with polyangiitis), Sjögren disease, sarcoidosis, IgG4 disease, and neoplastic (lymphoma or leptomeningeal carcinomatosis). Proposed diagnostic studies and scenarios in which to consider biopsy are outlined in Table 4. The purpose of this testing is primarily to exclude other autoimmune, infectious, and neoplastic disorders. Meningeal or dural biopsy results in this RM cohort failed to show specific pathologic evidence of RM. Furthermore, upon retrospective review, there were no clear differences in treatment and outcomes in those who underwent biopsy relative to those who did not. These findings suggest that the role of meningeal biopsy is primarily to exclude alternative conditions particularly in atypical clinical scenarios or in situations of diagnostic uncertainty. Biopsy should not result in treatment delay. We suggest that meningeal biopsy should be strongly considered in patients with seronegative RA, in patients with new-onset RA presenting with suspected RM, or in patients with RM not responding promptly to corticosteroids. Biopsy should also be pursued in patients with suspicion of atypical infection, patients with systemic evidence of IgG4 disease, isolated dural involvement, suspicion of malignancy, and patients with overlapping disease features.
Table 4.
Proposed Workup for Suspected RM.
| Serum | Cerebrospinal Fluid |
|---|---|
|
|
|
|
|
|
| |
| |
| |
|
|
| |
When to consider biopsy:
| |
Abbreviations: ACE, angiotensin-converting enzyme; ANA, anti-nuclear antibody; ANCA, anti-nuclear cytoplasmic antibody; CRP, C-reactive protein; ENA, extractable nuclear antigen; ESR, erythrocyte sedimentation rate; IgG, immunoglobulin G; MTB, Mycobacterium tuberculosis; RA, rheumatoid arthritis; RM, rheumatoid meningitis; RPR, rapid plasma reagin.
A diagnosis of RM requires careful assessment. Patients require a rheumatologist verified diagnosis of RA. Proper exclusion of other etiologies must be pursued in serum and CSF studies. Due to similarities in neurological clinical presentation and radiographic findings, IgG4-related disease must be included in the differential diagnosis for patients with suspected RM.10 Four patients within our study were tested for IgG4 antibodies and all were normal. Three of these patients had a biopsy performed but only one was specifically stained for IgG4 and was negative. Lack of familiarity with this disease entity likely resulted in few patients being specifically tested for IgG4 antibodies. Current diagnostic criteria for IgG4 disease require typical histopathologic features on biopsy including dense lymphoplasmacytic infiltrate, storiform fibrosis, and obliterative phlebitis.11 Presence of at least 2 of these histopathological features gives greater diagnostic certainty and should be accompanied by immunohistochemical staining showing elevated IgG4 levels or an elevated IgG:IgG4 ratio.12 Importantly, presence of giant cells, granulomas, and neutrophilic infiltrates argues against IgG4 disease unless a concomitant disease exists.12,13 Other inflammatory conditions such as RA and granulomatosis with polyangiitis can cause elevated IgG4 in tissue but lack the typical histopathological features of IgG4 disease. Immunoglobulin G4 meningitis typically only involves the dura, and headaches and cranial neuropathies are the most common presenting symptoms14; the latter were not seen in our patients. To make matters more complicated, IgG4 leptomeningitis has been reported in patients with RA15,16 although it is not entirely clear that RA did not play a causative role.17 A careful clinical evaluation to look for more common manifestations of IgG4, for example, salivary gland, lacrimal gland, orbital, retroperitoneal involvement, and so on, should be pursued, as almost half have systemic involvement.14 Lack of dural involvement argues against the presence of IgG4 disease. In patients with systemic disease involvement, it is imperative that IgG4 disease be excluded.
Rheumatoid arthritis disease duration, high disease activity, and presence of nodules or erosions were not predictive of development of RM. On the contrary, almost all 10 (91%) of 11 had well-controlled disease and the majority had nonerosive disease. This would suggest that RM occurs almost independent of underlying RA disease activity as has been reported previously.4 More interestingly, RM can be the presenting feature of RA, which can make the diagnosis even more challenging.
In a review of 24 previously reported autopsy cases of RM in the literature, Kato et al describes clinical features of RM.9 Similar to our case series, male and female genders seem to be equally affected, unlike RA itself which is 3 times more common in women.18 The duration of RA can also range from newly diagnosed to long-standing disease. No patients were seronegative for RA. Interestingly, Kato et al describes a majority of cases that resulted in death. Only 8 of 24 patients reported improvement.9 This may be in part due to lack of familiarity with the disease process and less aggressive immunosuppressant intervention at that time. In this study, 10 patients received no treatment, which likely led to higher mortality rates. It is also unclear from this study how intense the therapies were in terms of corticosteroid dosing. The high mortality rates are suspected to be slightly skewed due to primary use of autopsy data. Interestingly, however, the majority of our patients improved both clinically and radiographically with therapy, stressing the need for aggressive intervention. In the review by Kato et al, only 12 (50%) of 24 pathology cases demonstrated rheumatoid nodules, with the majority (67%) revealing meningeal inflammation. In review of recent case reports, rheumatoid nodules were not required for diagnosis.10,7 The article by Kato et al was an autopsy study, whereas a biopsy is limited by sampling size and may miss pathological findings.
The most common CSF profile in our patients was a nonspecific lymphocytic pleocytosis with an elevated protein. Interestingly, 2 patients had a monocytic predominance and 1 neutrophilic, indicating that patients with RM can present with a variety of CSF profiles including a large range of CSF WBCs. It is important to note that both CSF cell count and protein can also be normal in RM, as was the case in one patient who had a delay to diagnosis but ultimately achieved remission after treatment. There have been prior suggestions that elevated interleukin-6 (IL-6) or RF in the CSF may support the diagnosis,19-21 but this is not always feasible due to laboratory and cost restrictions. Only 2 of our patients had RF evaluated in the CSF; results were unremarkable in both cases. Interleukin-6 was not evaluated in any patient. In an effort to simplify the workup for this rare disease process, it is unlikely that CSF RF and IL-6 be necessary components of the investigation.
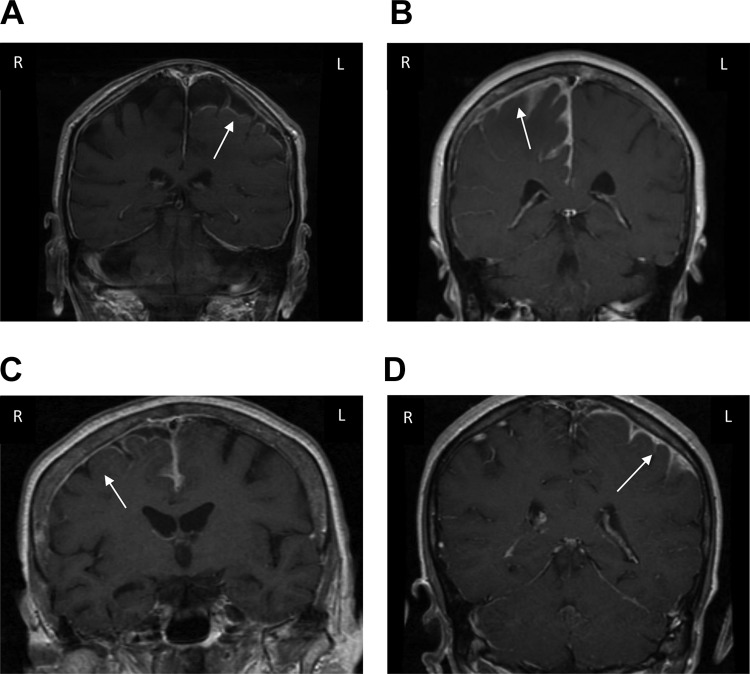
Interestingly, 12 (86%) of 14 patients had a frontoparietal predominance to the abnormal enhancement observed on brain magnetic resonance imaging (MRI; Figure 1). The true significance of this remains unclear. In general, it is well known that RM should be in the differential diagnosis for pachymeningeal or leptomeningeal enhancement.22 Etiologies such as tuberculosis and certain fungal infections (eg, coccidioidomycosis, cryptococcus) have a predilection for the basilar cisterns. Neurosarcoidosis also typically affects the basal leptomeninges.23 That said, these remain important diagnostic considerations in cases of pachymeningitis even in the absence of basilar involvement. A predominant location of MRI abnormalities has not previously been described though it is unclear if a specific location would be more likely to be RM versus other infectious, inflammatory, or neoplastic etiologies. Asymmetric involvement of RM was seen in 11 (78.6%) of 14 patients. Asymmetric radiographic findings have been described10 but remain unique for RM. Although this is unlikely specific, asymmetric findings should not exclude the possibility of RM.
Figure 1.
Brain magnetic resonance imaging, coronal T1-weighted contrast-enhanced sequences. A, Abnormal enhancement left posterior frontal and parietal leptomeningeal region with diffuse pachymeningeal enhancement. B, Diffuse pachymeningeal and leptomeningeal right and posterior frontal and anterior parietal enhancement with mild edema. C, Abnormal leptomeningeal enhancement over the superior portion of the right hemisphere with extension to the left frontal and parietal leptomeninges. D, Enhanced meningeal and dural thickening in the left hemisphere, maximal posterior Sylvian fissure. Subtle enhancement right hemisphere.
Corticosteroids remain the treatment of choice for initial and long-term treatment. In our study, improvement was based on clinical symptoms and radiographic improvement. Four of our patients were managed with corticosteroids alone. There were no differences in outcomes in patients receiving oral steroids versus intravenous. In contrast to other older studies in which cyclophosphamide was frequently used,24,25 rituximab was used in 4 of our cases with good results (likely due to greater awareness and availability). Other agents included methotrexate and azathioprine. In only one case, etanercept was used. There is a report of adalimumab possibly inducing RM.26 Patients 2, 6, and 14 were currently or previously treated with adalimumab, all with symptomatic and radiographic improvement with treatment. Although it is unclear whether adalimumab has a direct role in the development of RM, exposure to this medication should increase clinical suspicion.
Limitations to this study include the retrospective design, small sample size, and that the diagnosis of some RM cases was based solely on expert clinical judgment. It is likely that cases were missed due to the rarity of this disease process and thus not diagnosed with RM. Also, suspect cases of meningitis of unknown etiology may at times truly represent RM, but patients are often lost to follow-up before identification of RA. Pathologic findings as demonstrated by our review are often nonspecific and should not result in reluctance to diagnose cases as RM. It is also possible that the process of RM is entirely independent of underlying RA and/or may represent a secondary autoimmune process, as may be suggested by the lack of association with underlying disease activity. On the other hand, the fact that it responds well to immunosuppressive treatment and in autopsy studies of RM, rheumatoid nodules have been identified lends credence to the likely association between the 2. Potential cases of IgG4 could have been missed as no cases in our review prior to 2016 tested specifically for IgG4 antibodies. This likely reflects the limited awareness and availability of testing for IgG4 disease, particularly in earlier cases. However, overall clinical picture of our patients, corresponding laboratory results and strong articular component argues against a primary IgG4 disease process in our patients.
Conclusions
This case series reviews serologic, CSF, radiologic, and meningeal biopsy findings in 14 patients diagnosed with RM at our institution. Based upon this review, we found that (1) meningeal biopsy had limited utility in providing definitive pathologic confirmation of RM in our patients, (2) a nonspecific meningeal biopsy did not appear to influence treatment or outcome relative to those who did not undergo biopsy, and (3) neuroradiologic features showed a frontoparietal predominance of gadolinium enhancement. Assuming that other causes of pachymeningitis have been excluded, we posit that biopsy should not delay treatment in clinically suspected cases as RM is highly responsive to immunosuppressive therapy.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Angela M. Parsons, DO, MS  https://orcid.org/0000-0002-0506-894X
https://orcid.org/0000-0002-0506-894X
References
- 1. Weissman BN, Aliabadi P, Weinfeld MS, Thomas WH, Sosman JL. Prognostic features of atlantoaxial subluxation in rheumatoid arthritis patients. Radiology. 1982;144(4):745–751. [DOI] [PubMed] [Google Scholar]
- 2. DeQuattro K, Imboden J. Neurologic manifestations of rheumatoid arthritis. Rheum Dis Clin North Am. 2017;43(4):561–571. [DOI] [PubMed] [Google Scholar]
- 3. Bougea A, Anagnostou E, Konstantinos G, George P, Triantafyllou N, Kararizou E. A systematic review of peripheral and central nervous system involvement of rheumatoid arthritis, systemic lupus erythematosus, primary Sjögren’s syndrome, and associated immunological profiles. Int J Chronic Dis. 2015;2015:910352 Erratum in: Int J Chronic Dis. 2016;2016: 9854813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bathon JM, Moreland LW, DiBartolomeo AG. Inflammatory central nervous system involvement in rheumatoid arthritis. Semin Arthritis Rheum. 1989;18(4):258–266. [DOI] [PubMed] [Google Scholar]
- 5. Kurne A, Karabudak R, Karadag O, et al. An unusual central nervous system involvement in rheumatoid arthritis: combination of pachymeningitis and cerebral vasculitis. Rheumatol Int. 2009;29(11):1349–1353. [DOI] [PubMed] [Google Scholar]
- 6. Bourgeois P, Rivest J, Bocti C. Rheumatoid meningitis presenting with stroke-like episodes. Neurology. 2014;82(17):1564–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexander S, Cicco M, Pohl U, Cifelli A. Rheumatoid disease: an unusual cause of relapsing meningoencephalitis. BMJ Case Rep. 2018. doi:10.1136/bcr-2017-222587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lattanzi S, Cagnetti C, Di Bella P, Scarpelli M, Silvestrini M, Provinciali L. Leptomeningeal inflammation in rheumatoid arthritis. Neurol Neuroimmunol Neuroinflamm. 2014;1(4):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kato T, Hoshi K, Sekijima Y, et al. Rheumatoid meningitis: an autopsy report and review of the literature. Clin Rheumatol. 2003;22(4):475–480. [DOI] [PubMed] [Google Scholar]
- 10. Choi S, Ho Park Y, Kim J, Han J, Choe G, Kim S. Pearls & Oy-sters: asymmetric meningeal involvement is a common feature of rheumatoid meningitis. Neurology. 2017;88(12):e108–e110. [DOI] [PubMed] [Google Scholar]
- 11. Wallace ZS, Carruthers MN, Khosroshahi A, et al. IgG4-related disease and hypertrophic pachymeningitis. Medicine (Baltimore). 2013;92(4):206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deshpande V, Chan Z, Yi E, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–1192. [DOI] [PubMed] [Google Scholar]
- 13. Stone J, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366(6):539–551. [DOI] [PubMed] [Google Scholar]
- 14. Lu L, Della-Torre E, Stone J, Clark S. IgG4-related hypertrophic pachymeningitis: clinical features, diagnostic criteria, and treatment. JAMA Neurol. 2014;71(6):785–793. [DOI] [PubMed] [Google Scholar]
- 15. Mehta S., Switzer J, Biddinger P, Rojiani A. IgG4-related leptomeningitis: a reversible cause of rapidly progressive cognitive decline. Neurology. 2014;82(6):540–542. [DOI] [PubMed] [Google Scholar]
- 16. Lindstrom K, Cousar J, Lopes M. IgG4-related meningeal disease: clinic-pathological features and proposal for diagnostic criteria. Acta Neuropathol. 2010;120(6):765–776. [DOI] [PubMed] [Google Scholar]
- 17. Baptista B, Casian A, Gunawardena H, DCruz D, Rice C. Neurological manifestations of IgG4-related disease. Curr Treat Options Neurol. 2017;19(4):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scott D, Wolfe F, Huizinga T. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. [DOI] [PubMed] [Google Scholar]
- 19. Jones SE, Belsley NA, McLoud TC, Mullins ME. Rheumatoid meningitis: radiologic and pathologic correlation. AJR Am J Roentgenol. 2006;186(4):1181–1183. [DOI] [PubMed] [Google Scholar]
- 20. Markenson JA, McDougal JS, Tsairis P, Lockshin MD, Christian CL. Rheumatoid meningitis: a localized immune process. Ann Intern Med. 1979;90(5):786–789. [DOI] [PubMed] [Google Scholar]
- 21. Matsushima M, Yaguchi H, Niino M, et al. MRI and pathological findings of rheumatoid meningitis. J Clin Neurosci. 2010;17(1):129–132. [DOI] [PubMed] [Google Scholar]
- 22. Smirniotopoulous J, Murphy F, Rushing E, Schroeder J. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27(2):525–551. [DOI] [PubMed] [Google Scholar]
- 23. Lacomis D. Neurosarcoidosis. Curr Neuropharmacol. 2011;9(3):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chou RC, Henson JW, Tian D, Hedley-Whyte ET, Reginato AM. Successful treatment of rheumatoid meningitis with cyclophosphamide but not infliximab. Ann Rheum Dis. 2006;65(8):1114–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yucel AE, Kart H, Aydin P, et al. Pachymeningitis and optic neuritis in rheumatoid arthritis: successful treatment with cyclophosphamide. Clin Rheumatol. 2001;20(2):136–139. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed M, Luggen M, Herman J, et al. Hypertrophic pachymeningitis in rheumatoid arthritis after adalimumab administration. J Rheumatol. 2006;33(11):2344–2346. [PubMed] [Google Scholar]