Abstract
Background
Spinal cerebrospinal fluid (CSF) leak can lead to intracranial hypotension and is an important differential diagnosis to consider in patients with sudden-onset chronic daily headaches. Pars interarticularis (PI) fracture is a potential rare cause of suspected spinal CSF leak.
Methods
This is a retrospective case series of 6 patients with suspected spinal CSF leak evaluated between January 2016 and September 2019. All patients received a magnetic resonance imaging (MRI) of the brain with and without gadolinium, MRI whole spine and full spine computed tomography (CT) myelogram. Targeted epidural patches with fibrin sealant were performed. Treatment response at return visit (3 months post-patch) was documented.
Results
Six patients (4 females, 2 males) were diagnosed with a suspected spinal CSF leak and PI fracture. Mean age at the time of headache onset was 39 years old, and a range from 32 to 50 years old. Mean time to targeted epidural patches with fibrin sealant was 4.5 years. All 6 patients had PI fractures identified on CT myelogram and received targeted epidural patches with fibrin sealant at the site of the PI fracture. All patients had significant improvement in their headache intensity.
Conclusion
Our study highlights: 1) the importance of PI fracture as a possible culprit of suspected spinal CSF leak in patients with intracranial hypotension; 2) the added benefit of CT imaging for detecting bony abnormalities such as fractures in patients with intracranial hypotension; and 3) the successful treatment of suspected spinal CSF leak when targeting the fracture site.
Keywords: Spinal cerebrospinal fluid leak, Spinal CSF leak, Intracranial hypotension, Pars interarticularis fracture
Background
Spinal cerebrospinal fluid (CSF) leak can lead to intracranial hypotension and is an important differential diagnosis to consider in patients with sudden onset chronic daily headaches. This entity can often be confused with other chronic headache disorders, such as new daily persistent headache and chronic migraine. The most common clinical manifestation of a spinal CSF leak is orthostatic headache (worse upright) [1]. However, there is a wide variation of clinical presentation in headache arising from a spinal CSF leak including non-positional headache [1–4], second half of the day headache [5], exertional headache [3, 6], cough headache [7], thunderclap headache [8–10], and paradoxical headache (in which symptoms are worse when supine) [11–15].
In addition to orthostatic headache, patients with spontaneous intracranial hypotension (SIH) can experience a broad clinical spectrum of symptoms, including cochleovestibular dysfunction [16, 17], cranial nerve palsies, visual blurring, upper limb symptoms, encephalopathy, autonomic dysfunction, and cognitive dysfunction [18–20]. This is presumptively due to sagging of the brain causing significant traction, distortion or compression of anchoring and supporting structures [21]. Several imaging modalities have been utilized to aid in the diagnosis of spinal CSF leak including magnetic resonance imaging (MRI) of the brain with and without gadolinium, MRI whole spine, radioisotope cisternography, magnetic resonance myelogram, computed tomography (CT) myelogram or digital subtraction myelogram, each with proposed individual benefits and drawbacks [22, 23].
Spinal CSF leak is commonly precipitated by traumatic events such as motor vehicle accidents, sports injuries, and medical procedures such as dural or epidural punctures [22]. However, only 30% of patients with SIH can identify an inciting event or trauma, with the majority of patients unable to trace the onset of symptoms to any unusual circumstances at all [24]. SIH may arise in an area of a pre-existing dural sac weakness secondary to connective tissue disorders or spinal conditions such as herniated disc or spondylotic spurs [22]. However, there is no available medical literature highlighting osseous fracture as a potential cause of suspected spinal CSF leak, including pars interarticularis (PI) fracture. PI fracture is a stress fracture involving the posterior vertebral arch. This injury occurs in the lower lumbar region, most often at L5 and S1 [25]. Discovery of a PI fracture is often an incidental imaging finding in an asymptomatic patient or in an adolescent athlete involved in sports requiring repetitive lumbar loading in extension and rotation [26]. In this setting, patient may present with acute or insidious onset lower back pain. We suspect PI fracture can rarely be a potential cause of suspected spinal CSF leak. In this retrospective case series, we identified PI fractures in patients with suspected spinal CSF leak and their clinical outcomes were reported.
Methods
Six cases of suspected spinal CSF leak secondary to PI fracture were identified retrospectively. All patients were evaluated between January 2016 to September 2019 at the Stanford CSF leak headache clinic. All cases were presented at the neuroradiology/headache neurology CSF leak conference with headache specialists, anesthesiologists and neuroradiologists in attendance. All patients received an MRI of the brain with and without gadolinium, MRI whole spine and full spine CT myelogram. No bedside lumbar puncture was performed. These studies were interpreted by board-certified neuroradiologists. All targeted epidural patches with fibrin sealant were performed by a team member board-certified in Headache, Anesthesiology, and Pain Medicine (IC). Treatment response at return visit (3 months post-patch) was documented based on a pain intensity scale from 0 to 10, 10 being the most severe. This study received IRB approval (eProtocol 52,298, IRB 61, Registration 4947) from Stanford University.
Statistical analysis
This is a descriptive case series study only, so no statistical analysis was utilized.
Results
Six patients (4 females and 2 males) had suspected spinal CSF leak based on their clinical and/or radiological findings. All 6 patients complained of daily unremitting orthostatic headaches. Patient 3, 4, and 5 had migraine features (throbbing/pounding, moderate to severe intensity, worse with activity, photophobia, phonophobia, and nausea) with their headaches. Patient 1, 2, and 6 had severe occipital pressure pain with their headaches. They had a number of concomitant symptoms including blurry vision, tinnitus, ear fullness, altered taste, vertigo, neck pain, interscapular pain, autonomic dysfunction and/or cognitive dysfunction (Table 1). Mean age at the time of onset of headache was 39 years old, with a range from 32 to 50 years old. Mean time to targeted epidural patches with fibrin sealant was 4.5 years. All 6 patients had limited therapeutic response to pharmacological treatment listed in Table 1. Four patients had an MRI brain reported as normal and 2 patients had radiological features suggestive of intracranial hypotension (Patient 1 and 2 had pachymeningeal enhancement [Fig. 1a, b], patient 2 had subdural collections [Fig. 1c]). All 6 patients had PI fracture identified on CT myelogram. Patient 1 and 6 recalled an inciting event (after hiking and after a mechanical fall respectively) with onset of headache within 24 h. Patient 1 complained of lower back pain. Four patients had contrast nerve sleeves enhancement at multiple spinal levels and patient 1 had epidural fluid collection on CT myelogram. Opening pressure was not recorded in 5 patients and 1 patient (Patient 5) had an opening pressure measured at 18 cm H20 from the CT myelogram. The mean number of interventions before targeted treatment at the PI fracture site was 3.8. The clinical and radiological characteristics for the 6 documented patients are included in Table 2, while treatment method and response are documented in Table 3. All 6 patients had an improvement (Greater than 50% reduction) in their headache intensity at their return visit (3 months post-patch) documented based on a pain intensity scale from 0 to 10, 10 being the most severe after treatment targeted at the PI fracture site (Table 3). All 6 patients indicated a subjective improvement of their concomitant symptoms and reported a reduction in their analgesics use. Patient 1 and 4 developed suspected rebound intracranial hypertension 6 months after treatment with reverse postural headache (worse when supine), improved with diuretics and/or carbonic anhydrase inhibitors.
Table 1.
Patient Medical History
| Patient | Past Medical History | Concomitant Symptoms | Pharmacological Treatments |
|---|---|---|---|
| 1 | None | Neck stiffness, tinnitus, interscapular pain, lower back pain, autonomic dysfunction and anxiety | Ibuprofen |
| Oxycodone | |||
| 2 | None | Blurry vision, tinnitus, ear fullness, vertigo and cognitive dysfunction | Oxycodone |
| Ibuprofen | |||
| 3 | Ehlers-Danlos Syndrome | Tinnitus, vertigo, neck pain, autonomic and cognitive dysfunction | Sertraline |
| Migraine | Sumatriptan | ||
| Naproxen | |||
| 4 | Depression | Blurry vision, neck pain, tinnitus, ear fullness, altered taste, interscapular pain, autonomic and cognitive ysfunction | Amlodipine |
| Hypertension | Amitriptyline | ||
| Migraine | Naproxen | ||
| 5 | Prior C5/6 discectomy Depression | Blurry vision, neck pain, tinnitus, ear fullness, altered taste, interscapular pain, autonomic and cognitive dysfunction | Amitriptyline |
| Acetaminophen | |||
| 6 | Corneal transplant | Neck pain, tinnitus, ear fullness, difficulty swallowing and cognitive dysfunction | Acetaminophen |
Fig. 1.
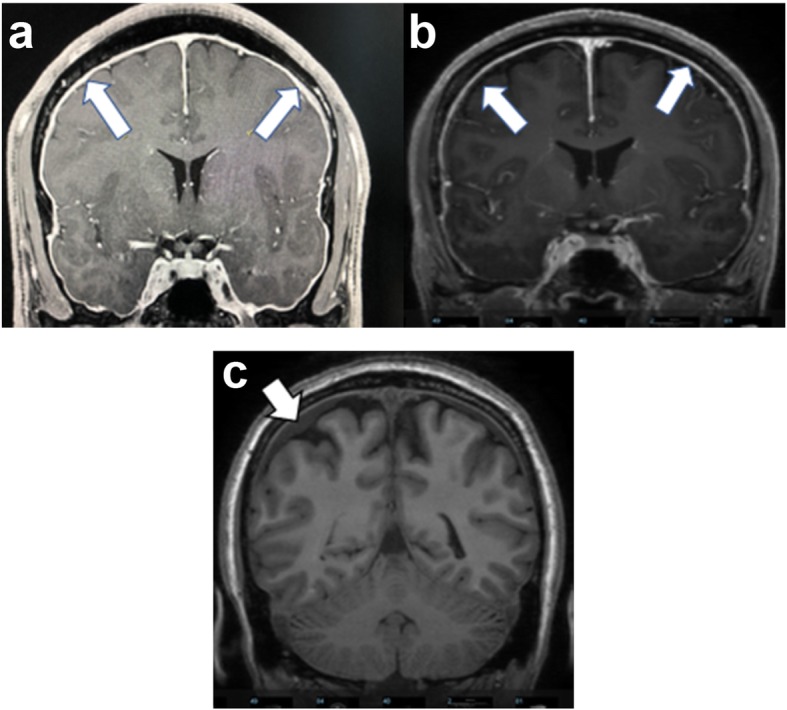
a Patient 1 b Patient 2: Magnetic Resonance Imaging with gadolinium (Coronal T1 Spoiled Recalled Gradient-Echo) demonstrated diffuse pachymeningeal enhancement (arrows), a feature of intracranial hypotension. c: Patient 2: Magnetic Resonance Imaging (Coronal T1) demonstrated subdural collections (arrow), a feature of intracranial hypotension
Table 2.
Patient Characteristics
| Patient # (Age Gender) | Age of onset | Inciting event | Orthostatic headache (Worse upright) | MRI head (with and without gadolinium) | MRI spine | CT myelogram | Previous treatmenta (# of patches) |
|---|---|---|---|---|---|---|---|
| 1 (35 M) | 32 | Post-hiking | Yes | Diffuse pachymeningeal enhancement | Central disc protrusion at T7/8, T11/12 | Bilateral L5 PI # | 4 |
| Diffuse epidural fluid collection in the whole spine | |||||||
| Epidural fluid collection extending from L1-L5 | |||||||
| 2 (52F) | 50 | None | Yes | Diffuse pachymeningeal enhancement | Severe grade 3 L4/5 spondylolisthesis | Bilateral L4 PI # | 3 |
| L4/5 anterolisthesis | |||||||
| Subdural collections | Mildly prominent perineural spaces at bilateral C7-T1, right T7/8, T8/9, bilateral T9/10 | ||||||
| Small left S2 perineural cyst | |||||||
| 3 (38F) | 34 | None | Yes | Normal | Mild degenerative changes in the C and L spine | Bilateral L5 PI # | 4 |
| Multi-disc degeneration from C3-C6 | |||||||
| Small amount of contrast seen in multiple perineural sleeves throughout the spine | |||||||
| 4 (49F) | 39 | None | Yes | Normal | C3/4, C4/5, C6/7 disc bulges | Bilateral L3 PI # | 2 |
| Grade 1 L3/4 anterolisthesis | |||||||
| L3/4 anterolisthesis | Nerve root sleeve opacification at the left C6/7, C7/T1, L5/S1 and right T12/L1 | ||||||
| S1 Tarlov cyst | |||||||
| 5 (38F) | 34 | None | Yes | Normal | Prior discectomy at C5/6 | S1 PI # (right) | 3 |
| Bilateral nerve root sleeves at C5/6, C6/7, C7/T1, T7/8, T9/10, T10/11 and all L levels | |||||||
| 6 (49 M) | 45 | Fall | Yes | Normal | Normal | Bilateral L5 PI # | 7 |
| Calcified osteophyte on left L5/S1 facet join |
C Cervical T Thoracic L Lumbar S Sacral PI #: Pars Interarticularis fracture
aAll patients received conservative treatment plus a combination of the following: blind epidural blood patches, targeted blood patches and/or fibrin sealant
Table 3.
Treatment Response
| Patient | Treatment method (epidural patch with fibrin sealant, paramedian approach) a | Treatment response | |
|---|---|---|---|
| Pre-treatmentb | Post-treatmentb (3 months post-patch) | ||
| 1a) | Bilateral L4/L5, right L5/S1 | Upright: 3 | Upright: 0 |
| Supine: 2 | Supine: 0 | ||
| 2 | Bilateral L4/L5 | Upright: 8 | Upright: 4 |
| Supine: 2 | Supine: 1 | ||
| 3 | Bilateral L4/L5, left L5/S1 | Upright: 8 | Upright: 2 |
| Supine: 0 | Supine: 0 | ||
| 4b) | Bilateral L3/4 | Upright: 8 | Upright: 4 |
| Supine: 4 | Supine: 2 | ||
| 5 | Right L4/L5, right L5/L6 | Upright: 7 | Upright: 3 |
| Supine: 0 | Supine: 0 | ||
| 6 | Bilateral L5/S1 | Upright: 4 | Upright: 1 |
| Supine: 3 | Supine: 0 | ||
a3 ml of fibrin sealant to each site
bPain intensity scale: 0–10, 10 being the most severe
a) At 6 months – suspected rebound intracranial hypertension managed with acetazolamide 250 mg twice daily
b) At 6 months – suspected rebound intracranial hypertension managed with amiloride 10 mg and furosemide 60 mg daily
Case illustration (Patient 1)
A 32-year-old male developed a severe positional headache following a hiking trip. The headache location was occipital in nature. He also complained of tinnitus, interscapular pain, lower back pain, autonomic dysfunction and anxiety shortly after the onset of his orthostatic headache. Ibuprofen and oxycodone provided no relief. His past medical history was unremarkable. Neurological examination was unremarkable. MRI of the brain with and without gadolinium demonstrated diffuse pachymeningeal enhancement (Fig. 1a). Full spine CT myelogram revealed a ventral cervicothoracic epidural fluid collection extending from the mid cervical spine to approximately T8 (Fig. 2). He underwent 4 patches over a span of 2 years: 2 non-directed lumbar epidural blood patches, bilateral transforaminal T6–7 and right transforaminal T10–11 epidural blood patches with fibrin sealant (3 ml each site), left paramedian C7-T1 and right T2-T3 paramedian interlaminar blood patches with catheters threaded under fluoroscopic guidance to the ventral epidural space (10 ml and 5 ml respectively). The 2 non-directed lumbar epidural blood patches provided transient and mild relief, but the subsequent 2 targeted patches provided no relief. Finally, the bilateral L5 PI fracture site (Fig. 3) was targeted with bilateral paramedian L4–5 and right paramedian L5–S1 epidural patches with fibrin sealant (3 ml each site). In contrast with his previous patches, he noted resolution of his orthostatic headache with pain intensity dropped from 3 to 0 upright, on a scale of 0 to 10, 10 being the most severe at his return visit (3 months post-patch). He discontinued his ibuprofen and oxycodone intake completely. He later returned to his full-time employment with minimal concomitant symptoms.
Fig. 2.
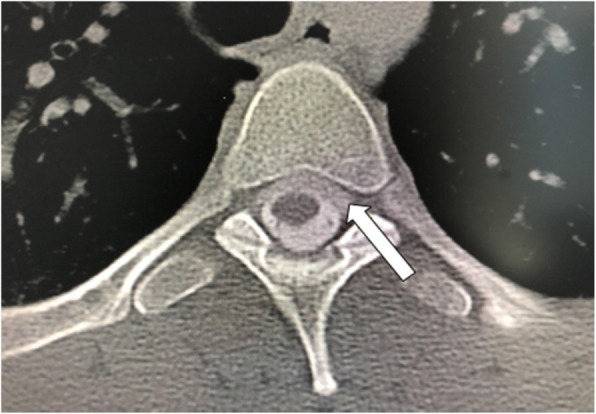
Computed Tomography Myelogram (axial view) demonstrated a ventral cervicothoracic epidural CSF collection (arrow) extending from the mid cervical spine to mid thoracic level (Patient 1)
Fig. 3.
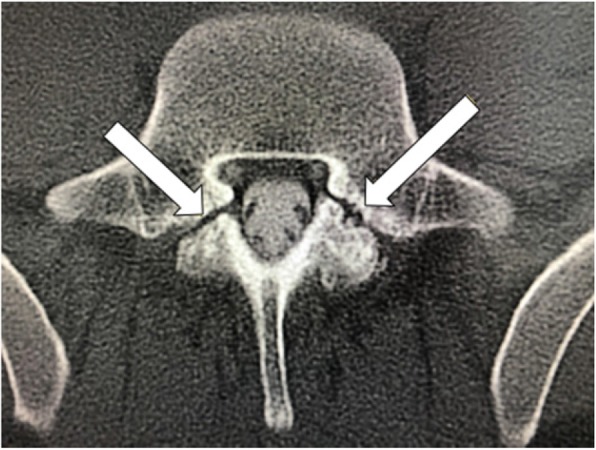
Computed Tomography Myelogram (axial view) demonstrated bilateral L5 pars interarticularis fractures (arrows) (Patient 1)
Discussion
All 6 patients suffered from chronic daily headaches and were diagnosed with suspected spinal CSF leak based on their clinical and/or radiological findings. Spinal CSF leak may resolve spontaneously or through conservative measures such as bed rest, oral hydration, generous caffeine intake and use of an abdominal binder [22, 23]; however, this was not seen in our patients. They did not respond to pharmacological treatment, conservative measures and failed multiple interventions including non-directed epidural blood patches and epidural patches targeted at levels outside of the PI fracture site, such as at the level of the contrast enhanced nerve sleeves and extradural collection. This highlights that in many cases of spinal CSF leak, it is imperative to identify the site of spinal CSF leak for targeted treatment with epidural patches to maximize response rate [23]. These cases highlight the difficulty in successfully identifying a target site.
The exact location of spinal CSF leak is often unknown. MRI brain may suggest signs of intracranial hypotension (subdural collections, pachymeningeal enhancement, venous engorgement, pituitary hyperemia and brain sagging), but these have no localizing value [23]. Spinal imaging such as MRI or CT myelogram can confirm a leak based on contrast in the extradural space or nerve sleeves, but may be falsely localizing [27]. Our patients had targeted low volume (3 ml each site) epidural patches with fibrin sealant at the site of the PI fractures with significant treatment response (Greater than 50% reduction in pain intensity) at their 3 month return visit, which suggests the PI fractures may be the culprit for their suspected spinal CSF leak. Previous reports have demonstrated intracranial hypotension or pseudomeningocele as potential complications from other orthopedic injuries including spondylolisthesis and vertebroplasty [28, 29]. PI fractures were detected from the CT myelogram and were missed on the MRI spine in 5 out of the 6 patients. Our cases illustrate an added advantage of CT myelogram in the workup of suspected spinal CSF leak for osseous pathology [30].
Patient 1 demonstrated a transient and mild relief from his non-targeted lumbar epidural patches, which suggests a possibility that prior treatments were coincidentally injected at or near the site of PI fracture since the lumbar region is often a standard location for non-directed epidural blood patches. Limited information was available from chart review on the other patients’ prior treatment response.
Patients 1 and 4 developed suspected rebound intracranial hypertension 6 months post-patch based on a change in their headache characteristics (headache worse lying supine). This information was relayed to our center from their primary providers. This illustrates a potential risk of spinal CSF leak treatment long term. Our patients had a prolonged clinical course before the PI fracture was identified as the suspected leak site. It is possible that contributed to an increased risk of rebound intracranial hypertension [31].
As for the PI fracture, the treatment is usually nonsurgical (rest and bracing). Surgery (laminectomy or posterior lumbar fusion) may be required if symptoms persist or remain bothersome [25]. Patient 1 complained of lower back pain, which could be a direct symptom of PI fracture, however his subjective improvement after the patch argued against the PI fracture being the cause and lower back pain is not an uncommon symptom in SIH [20].
Limitations
Some limitations were seen with this study: Two out of the 6 patients (Patient 1 and 2) met the diagnostic criteria of “headache attributed to low CSF pressure” as per the International Classification of Headache Disorders Third Edition [32]. The other 4 patients had suspected CSF leak based on supportive clinical and radiological findings, such as orthostatic headaches, enhancing nerve sleeves and/or favorable response to treatment. All cases were presented at the neuroradiology/headache neurology CSF leak conference and the diagnosis of suspected CSF leak was agreed upon a panel of headache specialists, anesthesiologists and neuroradiologists. Opening pressure was not measured in 5 patients and patient 5 had a normal opening pressure recorded [33]. Certainly, a detection of CSF pressure < 6 cm H2O supports the diagnosis of CSF leak, however, it is not uncommon to have a normal opening pressure with CSF leak, especially in chronic CSF leak patients. A normal opening pressure does not rule out a CSF leak [23]. All patients reported subjective improvement with their concomitant symptoms at their return visit, but not quantified. Long term follow-up (beyond 3 months) data are not available since our center is a tertiary referral center and our patients returned back to their primary and referring providers for subsequent follow-up. This is a retrospective case series limited by lack of control subjects and small population size. It is also subject to selection and information bias.
Conclusion
The diagnosis and management of spinal CSF leak causing SIH remains a challenge, especially in cases where the site of suspected CSF leak is difficult to detect. There are limitations to each imaging modality and often a combination is required to confirm and localize a CSF leak. Our study highlights: 1) the importance of PI fracture as a possible culprit of suspected spinal CSF leak in patients with intracranial hypotension; 2) the added benefit of CT imaging for detecting bony abnormalities such as fractures in patients with intracranial hypotension; and 3) the successful treatment of suspected spinal CSF leak when targeting the fracture site. If no clinical improvement is observed after adequate conservative measures (bed rest, oral hydration, generous caffeine intake and use of an abdominal binder) or non-targeted epidural patches in the context of a non-localizing spinal CSF leak, further testing may be warranted to explore an osseous pathology, such as occult fractures. No conclusions can be drawn regarding the frequency with which PI fractures are complicated by CSF leaks. Future research in a larger cohort of patients is required to explore the likelihood of detecting PI fracture in patients with non-localizing spinal CSF leak and to identify any risk factors associated with both PI fracture and spinal CSF leak. Long term follow-up data are required to explore the effectiveness and potential complication (i.e rebound intracranial hypertension) from targeted patches to the fracture site.
Acknowledgements
Not Applicable.
Abbreviations
- CSF
Cerebrospinal Fluid
- CT
Computed Tomography
- MRI
Magnetic Resonance Imaging
- PI
Pars Interarticularis
- SIH
Spontaneous Intracranial Hypotension
Authors’ contributions
TLHC wrote the manuscript. RC and NH reviewed and edited the manuscript. SH and BL obtained and interpreted the images. IC examined, performed the targeted blood patches and reviewed the manuscript. All authors read and approved the final version of the manuscript.
Funding
No funding was received for this study.
Availability of data and materials
The material analyzed during the current study are available from the corresponding author on reasonable request. Permission was obtained to access the patient data and the data was de-identified upon data collection.
Ethics approval and consent to participate
This study received ethics approval from the Institutional Review Board at Stanford University – Panel on Medical Human Subjects. eProtocol#: 52298, IRB 61, Registration 4947.
Consent for publication
Written consent for publication was obtained from each patient.
Competing interests
The authors have no conflicts of interest (financial or non-financial) to disclose relevant to this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mea E, Chiapparini L, Savoiardo M, Franzini A, Bussone G, Leone M. Clinical features and outcomes in spontaneous intracranial hypotension: a survey of 90 consecutive patients. Neurol Sci. 2009;30(Suppl 1):S11–S13. doi: 10.1007/s10072-009-0060-8. [DOI] [PubMed] [Google Scholar]
- 2.Ferrante E, Savino A. Nonpostural headache by spontaneous intracranial hypotension. Headache. 2003;43(2):127–129. doi: 10.1046/j.1526-4610.2003.03030.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang S-J, Fuh J-L. Exertional but not postural headache resulting from spontaneous intracranial hypotension. Acta Neurol Taiwanica. 2005;14(1):36–37. [PubMed] [Google Scholar]
- 4.Schievink WI, Smith KA. Nonpositional headache caused by spontaneous intracranial hypotension. Neurology. 1998;51(6):1768–1769. doi: 10.1212/wnl.51.6.1768. [DOI] [PubMed] [Google Scholar]
- 5.Leep Hunderfund AN, Mokri B. Second-half-of-the-day headache as a manifestation of spontaneous CSF leak. J Neurol. 2012;259(2):306–310. doi: 10.1007/s00415-011-6181-z. [DOI] [PubMed] [Google Scholar]
- 6.Mokri B. Spontaneous CSF leaks mimicking benign exertional headaches. Cephalalgia. 2002;22(10):780–783. doi: 10.1046/j.1468-2982.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- 7.Evans RW, Boes CJ. Spontaneous low cerebrospinal fluid pressure syndrome can mimic primary cough headache. Headache. 2005;45(4):374–377. doi: 10.1111/j.1526-4610.2005.05076.x. [DOI] [PubMed] [Google Scholar]
- 8.Schievink WI, Wijdicks EF, Meyer FB, Sonntag VK. Spontaneous intracranial hypotension mimicking aneurysmal subarachnoid hemorrhage. Neurosurgery. 2001;48(3):513–516. doi: 10.1097/00006123-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Famularo G, Minisola G, Gigli R. Thunderclap headache and spontaneous intracranial hypotension. Headache. 2005;45(4):392–393. doi: 10.1111/j.1526-4610.2005.05082_1.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrante E, Savino A. Thunderclap headache caused by spontaneous intracranial hypotension. Neurol Sci. 2005;26(Suppl 2):s155–s157. doi: 10.1007/s10072-005-0433-6. [DOI] [PubMed] [Google Scholar]
- 11.Schievink WI, Chu RM, Maya MM, Johnson JP, Cohen HCM. Spinal manifestations of spontaneous intracranial hypotension. J Neurosurg Spine. 2013;18(1):96–101. doi: 10.3171/2012.10.SPINE12469. [DOI] [PubMed] [Google Scholar]
- 12.Mokri B, Aksamit AJ, Atkinson JLD. Paradoxical postural headaches in cerebrospinal fluid leaks. Cephalalgia. 2004;24(10):883–887. doi: 10.1111/j.1468-2982.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 13.Lupo I, Salemi G, Fierro B, Brighina F, Daniele O, Caronia A, et al. Headache in cerebrospinal fluid volume depletion syndrome: a case report. Funct Neurol. 2006;21(1):43–46. [PubMed] [Google Scholar]
- 14.Liu H, Kaye A, Comarda N, Li M. Paradoxical postural cerebrospinal fluid leak–induced headache: report of two cases. J Clin Anesth. 2008;20(5):383–385. doi: 10.1016/j.jclinane.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Kalarickal PL, Rosinia F. A case of paradoxical presentation of postural postdural puncture headache. J Clin Anesth. 2012;24(3):255–256. doi: 10.1016/j.jclinane.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Isildak H, Albayram S, Isildak H. Spontaneous intracranial hypotension syndrome accompanied by bilateral hearing loss and venous engorgement in the internal acoustic canal and positional change of audiography. J Craniofac Surg. 2010;21(1):165–167. doi: 10.1097/SCS.0b013e3181c50e11. [DOI] [PubMed] [Google Scholar]
- 17.Srimanee D, Pasutharnchat N, Phanthumchinda K. Bilateral subdural hematomas and hearing disturbances caused by spontaneous intracranial hypotension. J Med Assoc Thail. 2009;92(11):1538–1543. [PubMed] [Google Scholar]
- 18.Amemiya S, Takahashi K, Mima T, Yoshioka N, Miki S, Ohtomo K. Reversible alterations of the neuronal activity in spontaneous intracranial hypotension. Cephalalgia. 2016;36(2):162–171. doi: 10.1177/0333102415585085. [DOI] [PubMed] [Google Scholar]
- 19.Nakano M, Umehara F. Liquorrhea with multiple subdural hematomas causing reversible prospagnosia. Rinsho Shinkeigaku. 2012;52(2):96–101. doi: 10.5692/clinicalneurol.52.96. [DOI] [PubMed] [Google Scholar]
- 20.Capizzano AA, Lai L, Kim J, Rizzo M, Gray L, Smoot MK, et al. Atypical presentations of intracranial hypotension: comparison with classic Spontaneous intracranial hypotension. AJNR Am J Neuroradiol. 2016;37(7):1256–1261. doi: 10.3174/ajnr.A4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokri B. Spontaneous low pressure, low CSF volume headaches: spontaneous CSF leaks. Headache. 2013;53(7):1034–1053. doi: 10.1111/head.12149. [DOI] [PubMed] [Google Scholar]
- 22.Mokri B, Spontaneous CSF. Leaks: low CSF volume syndromes. Neurol Clin. 2014;32(2):397–422. doi: 10.1016/j.ncl.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286–2296. doi: 10.1001/jama.295.19.2286. [DOI] [PubMed] [Google Scholar]
- 24.Schievink WI, Louy C. Precipitating factors of spontaneous spinal CSF leaks and intracranial hypotension. Neurology. 2007;69(7):700–702. doi: 10.1212/01.wnl.0000267324.68013.8e. [DOI] [PubMed] [Google Scholar]
- 25.Mansfield JT, Wroten M. StatPearls. Treasure Island (FL): StatPearls Publishing; 2019. Pars Interarticularis Defect. [PubMed] [Google Scholar]
- 26.Cavalier R, Herman MJ, Cheung EV, Pizzutillo PD. Spondylolysis and spondylolisthesis in children and adolescents: I. diagnosis, natural history, and nonsurgical management. J Am Acad Orthop Surg. 2006;14(7):417–424. doi: 10.5435/00124635-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Schievink WI, Maya MM, Chu RM, Moser FG. False localizing sign of cervico-thoracic CSF leak in spontaneous intracranial hypotension. Neurology. 2015;84(24):2445–2448. doi: 10.1212/WNL.0000000000001697. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y-W, Tseng P-T, Tsui H-W, Hsu S-P, Kuo H-C. Intracranial hypotension as a rare complication of Vertebroplasty: a case report. Acta Neurol Taiwanica. 2015;24(3):97–101. [PubMed] [Google Scholar]
- 29.Novais G, Ratilal B, Pappamikail L, Branco P, Reis N. Spontaneous pseudomeningocele associated with lumbar spondylolisthesis: a case report and review of the literature. Surg Neurol Int. 2017;8:221. doi: 10.4103/sni.sni_179_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mokri B. Spontaneous Intracranial Hypotension. Continuum. 2015;21(4 Headache):1086–1108. doi: 10.1212/CON.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 31.Schievink WI, Maya MM, Jean-Pierre S, Moser FG, Nuño M, Pressman BD. Rebound high-pressure headache after treatment of spontaneous intracranial hypotension: MRV study. Neurol Clin Pract. 2019;9(2):93–100. doi: 10.1212/CPJ.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 33.Whiteley W, Al-Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67(9):1690–1691. doi: 10.1212/01.wnl.0000242704.60275.e9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The material analyzed during the current study are available from the corresponding author on reasonable request. Permission was obtained to access the patient data and the data was de-identified upon data collection.


