Abstract
Background:
Although risk-based selection of ever-smokers for lung-cancer screening could improve efficiency over US Preventive Services Task Force (USPSTF) guidelines, it preferentially selects older ever-smokers with lower life-expectancies due to comorbidities.
Objectives:
To compare selecting ever-smokers for screening based on gains in life-expectancy vs. based on lung cancer risk.
Design:
Cohort analyses and model-based projections.
Setting:
US population of ever-smokers aged 40-84 years.
Participants:
n=130,964 National Health Interview Survey participants, representing ~60 million US ever-smokers during 1997-2015.
Interventions:
Annual CT screening for 3 years vs. no screening.
Measurements:
Estimated lung-cancer deaths-averted and life-years gained after developing a mortality model.
Results:
Using the calibrated and validated mortality model in US ever-smokers aged 40-84 years and selecting equivalent 8.3 million ever-smokers to match USPSTF-guideline eligibility in 2013-2015, our analysis estimated that life-gained-based versus risk-based selection would increase the total life-expectancy from CT screening (633,400 versus 607,800 years) but prevent fewer lung-cancer deaths (52,600 versus 55,000) in the population. The 1.56 million individuals included in life-gained-based but not risk-based selection were younger (mean age=59 versus 75 years) with fewer comorbidities (mean=0.75 versus 3.7).
Limitations:
Estimates are model-based and presume the implementation of lung-cancer screening with short-term effectiveness similar to that from trials.
Conclusion:
Life-gained-based selection could maximize the benefits of lung-cancer screening in the US population by including ever-smokers who have not only high lung-cancer risk but also high life-expectancy.
Funding Source:
Intramural Research Program of the National Institute of Health/National Cancer Institute.
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
INTRODUCTION
Population-wide cancer-screening programs can reduce cancer-mortality by detecting disease at curable stages (1). However, cancer screening also has harms, such as anxiety, costs, unnecessary follow-up procedures, and overdiagnosis (2,3). Consequently, there is a growing recognition that screening programs need to selectively include individuals at highest cancer risk for whom the benefits of screening outweigh its harms (4-6). Such risk-based screening strategies (7,8) presume that individuals at highest risk also experience the greatest benefit, measured conventionally as prevented cancer death. However, preventing cancer death may not always translate to a gain in life-expectancy (life-gained), another measure of benefit. Indeed, the major risk factors that confer high-risk of cancer, such as older age, smoking duration and intensity, and intermediary comorbid conditions, also increase risk of mortality from other causes (9).
Lung cancer is an ideal example where individuals at highest risk may not always experience the greatest life-gained from screening, i.e., low-dose computed tomography (CT) which has been shown to be efficacious in two large randomized trials (10,11). Using risk-prediction models to select ever-smokers for CT screening is estimated to prevent more lung cancer deaths than the US Preventive Services Task Force (USPSTF) recommendations (12,13). However, preventing more lung cancer deaths may not always translate to substantial gains in life-expectancy. Such risk-based screening-selection would preferentially include older heavy-smokers with multiple co-morbidities who have reduced life-expectancy, and consequently, reduced life-gained from screening (14-18). Indeed, recent microsimulation analyses have shown that, while using risk-prediction models can further reduce lung cancer deaths, the life-years gained may be more modest (19) and that unless high risk patients also have sufficiently long life-expectancy, patients may not experience a net benefit from screening (20). Recognizing these issues, some health-care providers (e.g. the Veterans Administration) require at least 5 years of life-expectancy for high-risk individuals to enter screening (21) while other medical societies have declined to endorse risk-based lung-cancer screening altogether (22). These considerations underscore a much-needed alternative to risk-based screening strategies that also incorporate life-expectancy, and thereby, life-gained from screening.
To enable life-gained-based selection for lung cancer screening, we developed and validated an individualized prediction model for overall mortality/life-expectancy in US ever-smokers. We utilized this model to estimate individualized life-gained from an NLST-like CT screening program (10) in US ever-smokers aged 40-84 years, called the Life Years gained From Screening-CT (LYFS-CT) model. We investigated outcomes for selection of ever-smokers for CT screening in the US population using a life-gained-based versus a risk-based strategy.
METHODS
Overview
An individual’s life-expectancy in the absence of screening reflects their age, smoking intensity and duration, and comorbidities. For individuals undergoing NLST-like CT screening program (3 annual screens and 5-year follow-up), screening reduces the probability of death from lung cancer over the next 5 years by 20.4% as observed in the NLST for CT versus radiography (10). The life-gained from screening is the life-expectancy difference between the presence/absence of CT screening, i.e., the difference between the integrals of the survival curves from the current age to a maximum possible attainable age. Thus, deaths averted in an NLST-like CT screening program primarily accrue during the screening period and a short time post-screening (total: ~5 years), while gains in life-expectancy accrues over the first 5 years as well as beyond due to a greater probability of extended survival to older ages (additional details are provided in the eMethods).
Development and validation of overall mortality model for U.S. Ever-Smokers
We utilized data from the National Health Interview Surveys (NHIS) to develop and validate an individualized prediction model for overall mortality/life-expectancy in US ever-smokers aged 40-84 years in the absence of CT screening. The NHIS is an annual cross-sectional, multistage probability sample of approximately 87,500 individuals representing the noninstitutionalized civilian US population for that year (23). NHIS data collected through 2009 have been linked with the National Death Index, with follow-up through December 31, 2011 (24).
Independent model-training and model-testing datasets were created using the NHIS surveys, years 1997-2009, by assigning surveys conducted in odd years (e.g. 1997, 1999, etc.) to model-training (to develop the model) and surveys conducted in even years (e.g. 1998, 2000, etc.) to model-testing (to test the developed model) (Table S1). This ensured an adequate time-span to capture calendar trends in overall mortality in each dataset. A Cox proportional hazards model was developed using age as the time-scale and overall mortality as the outcome in the training dataset (56,556 US ever-smokers aged 40-84 years representing 57,656,447 individuals). Details of variable selection and parameterization are presented in the eMethods of the Data Supplement. The overall mortality model was validated in the NHIS testing dataset (45,950 US ever-smokers aged 40-84 years representing 57,932,166 individuals). Validation was evaluated through calibration (the ratio of the number of model-predicted cases to the number of observed cases [estimated/observed] overall, by 5-year mortality risk, and within subgroups) and discrimination (the area under the receiver operating curve [AUC] statistic). Our validation evaluates model-prediction accuracy in the NHIS-targeted US population (reproducibility) (25,26); however, further external validations are needed to evaluate transportability of model-predictions to other populations/settings.
Estimation of individualized life-gained by CT screening: LYFS-CT model
In the absence of CT screening, the individualized overall mortality prediction includes the risk of lung cancer mortality and other causes of death. We also separately estimated the risk of lung cancer mortality over 5-years in the absence of CT screening using a previously validated lung cancer death risk assessment tool (LCDRAT) (8,13).
Because no data exist on the long-term mortality experience in the presence of CT screening, we applied to the overall mortality prediction the average treatment effect observed in the National Lung Screening Trial (NLST) -- a 20.4% reduction in LCDRAT-estimated lung cancer mortality risk for 5 years. This assumes: 1) the 20.4% reduction in the risk of lung cancer mortality from CT screening observed in the NLST applies to all US ever-smokers, including individuals outside the NLST-eligibility criteria (e.g. ages younger than 55 years and lower levels of smoking exposure). The homogeneity in lung cancer mortality rate ratios across risks within the NLST (27) lends support to this assumption; 2) the benefit of 3 rounds of CT screening on lung cancer mortality would only accrue over 5 years; and 3) CT screening does not affect non-lung cancer mortality (10).
To calculate life-gained from CT screening for each ever-smoker aged 40-84 years in the US population, we subtracted the estimated life-expectancy in the absence of screening from the counterfactual life-expectancy in the presence of 3 annual CT-screens (see eMethods).
Comparative performance of life-gained-based versus risk-based selection of US ever-smokers for CT screening
We projected outcomes of NLST-like CT lung cancer screening in a contemporary US cohort (NHIS 2013-2015, Table S1, 28,458 ever-smokers representing 60,712,710 individuals). We compared three strategies for the selection of ever-smokers for CT screening: 1) life-gained-based selection of ever-smokers aged 40-84 years; 2) risk-based selection of ever-smokers aged 40-84 years using the a lung cancer death risk model (LCDRAT); and 3) USPSTF guideline (ages 55-80, ≥30 pack-years, and ≤15 years since quit) (28). The USPSTF guideline selected ~8.3 million (13.6%) ever-smokers for CT screening, so for comparability, we utilized thresholds for life-gained (16.2 days) and risk (1.4% risk of lung cancer mortality over 5 years) that would select the same number of individuals as USPSTF guidelines.
Outcomes for comparison included measures of program-sensitivity (percent of screen-detectable lung cancer cases, lung cancer deaths, and life-years gained relative to screening all US ever-smokers aged 40-84 years), benefit (number of deaths averted and years of life-expectancy gained), harms (number of false-positive CT-screens), and efficiency (number needed to be screened [NNS] to prevent 1 lung cancer death, NNS per 10 life-years gained in the population screened, life-gained per screen-detected case/prevented death, and the ratio of false-positives per death prevented/10 life-years gained in the population screened).
Analyses were conducted in R using the survey package to account for the NHIS complex-sampling design (29,30).
Role of the funding source
The NIH provided salaries for research staff and had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
RESULTS
Model Description and Validation
Predictors of overall mortality included gender, race/ethnicity, calendar-eras (NHIS survey year), body-mass index (BMI), smoking (pack-years and years since quitting), and co-morbid medical conditions, such as diabetes, stroke and hypertension (Table 1). Overweight or obese BMI (greater than 25) was associated with lower mortality, after adjusting for a range of medical conditions (Table S2). Predictors of lung cancer death risk in LCDRAT (13) were age, gender, race/ethnicity, BMI, smoking (pack-years, duration, intensity, and quit-years), emphysema, and a family history of lung cancer (Table 1).
Table 1:
Models for lung cancer death (Katki et al., 2016) and of overall mortality.
| Factor | Coding | Overall Mortality | Lung cancer Death (LCDRAT) |
||
|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||
| 3 annual CT screens | Binary | — * | — | 0.796 † | (0.69-0.92) † |
| Age | Log term | — ‡ | — | 431.812 | (185.059,1007.578) |
| Gender (female) | Binary | 0.708 | (0.671,0.747) | 0.837 | (0.736,0.950) |
| Race | Categorical | ||||
| White, non-Hispanic | 1.000 | Reference | 1.000 | Reference | |
| Black, non-Hispanic | 1.294 | (1.195,1.402) | 1.482 | (1.176,1.869) | |
| Hispanic | 0.812 | (0.734,0.899) | 0.687 | (0.397,1.190) | |
| Asian or Other | 0.619 | (0.493,0.778) | 0.657 | (0.447,0.964) | |
| Education § | Trend | 0.930 | (0.915,0.944) | 0.908 | (0.873,0.944) |
| Calendar year assessed | Linear | 0.977 | (0.969,0.985) | — | — |
| BMI≤18.5 | Binary | — | — | 1.428 | (0.899,2.268) |
| BMI | Log term | — | — | 0.447 | (0.296,0.675) |
| Categorical | — | — | |||
| 20<−25 | 1.000 | Reference | |||
| ≤18.5 | 1.879 | (1.631,2.166) | |||
| 18.5<−20 | 1.370 | (1.219,1.539) | |||
| 25<−30 | 0.793 | (0.747,0.842) | |||
| 30<−35 | 0.815 | (0.755,0.880) | |||
| >35 | 0.980 | (0.894,1.074) | |||
| Pack years | Categorical | — | — | ||
| 0-<30 | 1.000 | Reference | |||
| 30-<40 | 1.743 | (1.354,2.244) | |||
| 40-<50 | 2.112 | (1.679,2.657) | |||
| 50+ | 2.446 | (1.861,3.214) | |||
| Sqrt term | 1.081 | (1.053,1.110) | |||
| Quit years | Log term ∥ | 0.839 | (0.818,0.860) | 0.686 | (0.640,0.735) |
| Years smoked | Log term | — | — | 1.395 | (1.099,1.771) |
| >1 pack/day | Binary | — | — | 1.273 | (1.054,1.539) |
| Cigs/day | Log term ∥ | 0.966 | (0.893,1.045) | — | — |
| Lung cancer family history ¶ | Trend ** | — | — | 1.525 | (1.300,1.789) |
| Use special equipment †† | Binary | 1.748 | (1.624,1.881) | — | — |
| Liver condition, past 1y | Binary | 1.650 | (1.416,1.923) | — | — |
| Emphysema | Binary | 1.615 | (1.491,1.749) | 1.741 | (1.450,2.090) |
| Diabetes | Binary | 1.518 | (1.422,1.620) | — | — |
| Weak/failing kidneys, past 1y | Binary | 1.475 | (1.288,1.689) | — | — |
| Prior cancer | Binary | 1.322 | (1.244,1.405) | — | — |
| Prior stroke | Binary | 1.319 | (1.212,1.436) | — | — |
| Prior heart attack | Binary | 1.292 | (1.187,1.412) | — | — |
| Coronary heart disease | Binary | 1.182 | (1.080,1.294) | — | — |
| Heart disease | Binary | 1.143 | (1.073,1.218) | — | — |
| Chronic bronchitis, past 1y | Binary | 1.130 | (1.037,1.232) | — | — |
| Hypertension | Binary | 1.118 | (1.066,1.173) | — | — |
| Angina pectoris | Binary | 0.912 | (0.829,1.004) | — | — |
Abbreviations: LCDRAT= Lung cancer Death Risk Assessment Tool; CT= Computed tomography; BMI= Body mass index; HR = Hazard Ratio; CI= Confidence interval; 1y = 1 year.
“—“ indicates that the specified risk factor/parameterization was not included as a covariate.
Represents the rate-ratio observed in the NLST over 5 years of follow-up.
Age is used as the time scale for the overall mortality model.
<12 grade=1, high-school graduate=2, post high-school but no college=3, some College=4, Bachelor’s degree=5, graduate school=6.
Natural logarithm of 1+quit-years
FDR = First-degree relatives (siblings, parents, children) with history of lung cancer.
Definition: No FDRs with lung cancer = 0, 1 FDR with lung cancer = 1, Two or more FDRs with lung cancer = 2.
Have health problems that require use of special equipment, such as a cane, a wheelchair, a special bed, or a special telephone.
The overall mortality model had good calibration and discrimination in the independent validation data set of US ever-smokers aged 40-84 years, overall (E/O=1.00, 95%CI=0.98-1.02; AUC=0.847; 95%CI=0.841-0.853), by USPSFT-eligibility, by 5-year mortality risk (E/O range=0.92-1.17), and within subgroups (NHIS year, age groups, gender, race, BMI, smoking categories, and number of comorbidities) (Table S3 and S4). The lung cancer death risk model (LCDRAT) had good calibration (E/O=0.94; 95%CI=0.84-1.05) and discrimination (AUC=0.78; 95%CI=0.76-0.80) in US ever-smokers (13).
Comparative performance of life-gained-based versus risk-based selection of US ever-smokers for CT screening
For screening the same number of ever-smokers as USPSTF criteria (8.3 million), Table 2 compares projected outcomes when selecting CT lung cancer screening eligibility based on individual life-gained (with a threshold of >16.2 days), individual lung cancer mortality risk (with a threshold of >1.4%) or USPSTF criteria. Compared to risk-based selection, life-gained-based selection increased life-expectancy outcomes gained from CT screening 1) overall (633,400 versus 607,800), 2) per screen-detected lung cancer case (1.45 versus 1.35), and 3) per lung cancer death-averted (12.1 versus 11.1). However, life-based selection led to fewer lung cancer deaths averted relative to risk-based (52,600 versus 55,000). Notably, both risk-based and life-gained-based selection would be superior to USPSTF recommendations by lung cancer deaths averted, life-years gained, but not by life years gained per lung cancer death averted or number of false positives.
Table 2:
Projected outcomes from 3 strategies for selecting US ever-smokers for screening
| Life-gained-based * | Risk-based † | USPSTF ‡ | ||||
|---|---|---|---|---|---|---|
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
| Screen-eligible ever-smokers, millions | 8.3 | (7.9, 8.7) | 8.3 | (7.9, 8.7) | 8.3 | (7.9, 8.7) |
| % of all screen-detectable cases§ | 56.0% | (54.5%, 57.4%) | 57.3% | (55.9%, 58.8%) | 44.8% | (43.2%, 46.5%) |
| % of all Lung cancer deaths§ | 58.5% | (57.1%, 60.0%) | 61.3% | (59.9%, 62.6%) | 46.0% | (44.4%, 47.7%) |
| % of all Life-years gained§ | 47.9% | (46.6%, 49.2%) | 46.0% | (44.6%, 47.3%) | 40.7% | (39.4%, 42.1%) |
| Screen-detected cases after 5 y, thousands | 438 | (413, 463) | 449 | (424, 475) | 351 | (329, 373) |
| Lung cancer deaths averted after 5 y, thousands | 52.6 | (49.6, 55.5) | 55.0 | (52.0, 58.1) | 41.3 | (38.8, 43.9) |
| Life-years gained, thousands | 633 | (602, 665) | 608 | (577, 639) | 538 | (510, 567) |
| NNS per LCD averted | 158 | (152, 164) | 151 | (146, 156) | 201 | (191, 210) |
| NNS per 10 LYG | 131 | (128, 134) | 136 | (133.2, 140) | 154 | (149 159) |
| LYG per screen-detected case ∥ | 1.45 | (1.42, 1.48) | 1.35 | (1.33, 1.38) | 1.53 | (1.50, 1.57) |
| LYG per LCD averted ¶ | 12.1 | (11.8, 12.3) | 11.1 | (10.8, 11.3) | 13.0 | (12.7, 13.4) |
| Number of false positives, millions | 5.9 | (5.7, 6.2) | 6.0 | (5.7, 6.3) | 5.6 | (5.4, 5.9) |
| Number of false positives per LCD averted | 113 | (109, 117) | 109 | (105, 112) | 137 | (131, 142) |
| Number of false positives per 10 LYG | 93.7 | (92.0, 95.4) | 98.3 | (96.3, 100) | 105 | (102, 107) |
Abbreviations: USPSTF = U.S. Preventive Service Task Force; LCD = lung cancer death, LYG = life years gained, NNS = number needed to screen
Life-gained-based selection of 8.3 million – age 40-84, gain in life-expectancy from undergoing CT screening of at least 16.2 days
Risk-based selection of 8.3 million – age 40-84, 5-year lung cancer death risk of 1.4% or greater, as estimated by the Lung cancer Death Risk Assessment Tool (LCDRAT) (Katki et al., 2016)
USPSTF criteria – age 55-80, smoked in past 15 years, 30 pack-years
Percentage are calculated by comparing against the number of lung cancer incidence, lung cancer deaths, and life-gained that would occur if all U.S. ever-smokers, aged 40-84 years, were screened.
Life years gained per screen-detected cases = total life years gained in the screened population divided by the total number of screen-detected cases
Life years gained per lung cancer death averted = total life years gained in the screened population divided by the total number of averted lung cancer deaths.
Comparison of life-gained-based vs. risk-based selection of US ever-smokers for screening
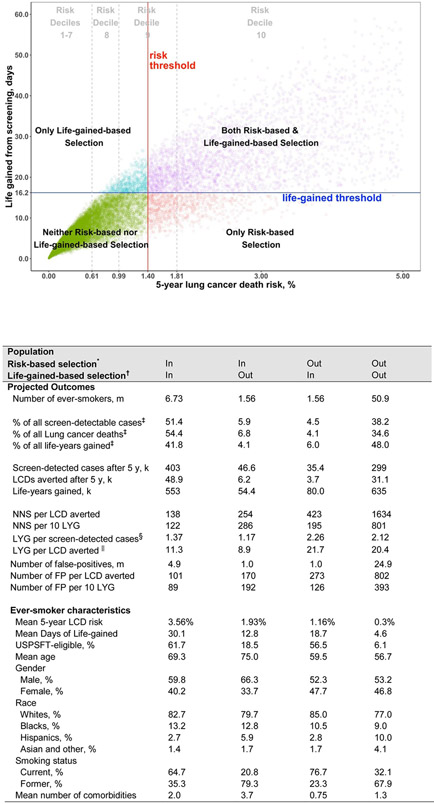
Figure 1 illustrates the selection of US ever-smokers aged 40-80 years by a life-gained-based vs. risk-based strategy. Most individuals with a high (at least 1.81% after 5-years) risk of lung cancer death (e.g. the highest LCDRAT risk decile among US ever-smokers, aged 40-84) would be screened by either life-gained-based (86%) or risk-based (100%) selection, compared with 56.4% by USPSTF criteria. For individuals with low (falling below 0.61% after 5-years) lung cancer death risks (e.g. the lowest 7 LCDRAT risk deciles), all were excluded by both life-gained-based and risk-based selection, compared with 3% selected by the USPSTF criteria (Figure 1, Table S5). Thus, choice of life-gained-based versus risk-based selection criteria primarily affects individuals at moderately high (falling between 0.61% and 1.81% after 5-years) risk of lung cancer death (e.g. risk deciles 8 and 9). The 1.56 million individuals eligible for screening in life-gained-based selection but not in risk-based selection (Figure 1 inset, Table S6) have lower 5-year risk of lung cancer death (mean: 1.16% versus 1.93%) but higher gain in life-expectancy per lung cancer death averted (mean: 21.7 versus 8.9 years). The life-gained-based selection strategy tend to be younger, women, African-American, current smokers, and to have fewer comorbid medical conditions (Figure 1 inset and Table S7).
Figure 1. Life-gained from screening and lung cancer death risk in US ever-smokers, age 40-84.
Estimates for contemporary US ever-smokers are based on the National Health Interview Survey (NHIS) 2013-2015. The plot shows 5-year lung cancer death risk up to 5%, but 721 NHIS subjects had risks of 5<−21%. 5-year lung cancer death risk and life-gained thresholds of 1.4% and 16.2 days, respectively, are the risk-based and life-gained-based thresholds that correspond to selecting the same number of ever-smokers (8.3 million) as selected by the US Preventive Services Task Force criteria. Projected outcomes and characteristics of the ever-smokers who are in both risk- and life-gained-based selections, in risk-based only, in life-gained-based only, or in neither are presented in detail in the Figure 1 inset Table. Risk deciles 1-10 of 5-year lung cancer death risks in NHIS are defined as <0.04%, 0.04%-<0.07%, 0.07%-<−0.11%, 0.11%-<0.18%, 0.18%-<0.27%, 0.27%-<0.41%, 0.41%-<0.61%, 0.61%-<0.99%, 0.99%-<1.81%, 1.81 or greater, respectively. The proportions selected by risk deciles by each of the selections are given in Table S5.
Figure 1 inset Table: Projected outcomes and characteristics of US ever-smokers by life-gained-based and risk-based selections
Abbreviations: m = millions, k = thousands, LCD = Lung cancer death; LYG = life-years gained, y = year, FP = false positives, USPSTF = US Preventive Services Task Force
* Risk-based selection of 8.3 million – age 40-84, 5-year lung cancer death risk of 1.4% or greater, as estimated by the Lung cancer Death Risk Assessment Tool (LCDRAT) (Katki et al., 2016)
† Life-gained-based selection of 8.3 million – age 40-84, gain in life-expectancy from undergoing CT screening of at least 16.2 days
‡ Percentage are calculated by comparing against the number of lung cancer incidence, lung cancer deaths, and life-gained that would occur if all U.S. ever-smokers, aged 40-84 years, were screened.
§ Life years gained per screen-detected cases = total life years gained in the screened population divided by the total number of screen-detected cases
∥ Life years gained per lung cancer death averted = total life years gained in the screened population divided by the total number of averted lung cancer death.
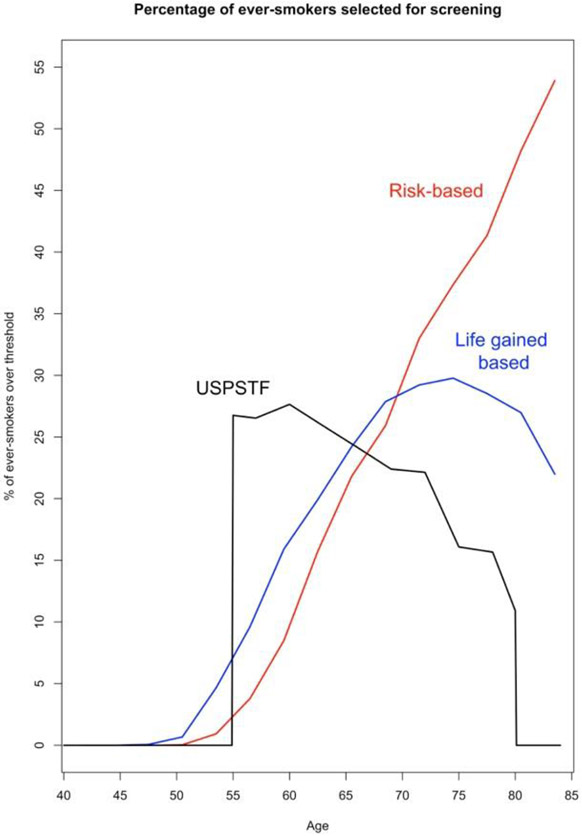
Figure 2 illustrates the preferential selection of younger individuals by a life-gained-based versus risk-based strategies. A life-gained-based versus a risk-based strategy selected more younger ever-smokers (7.7% versus 2.9% 50-59 year-olds and 22.9% versus 19.8% 60-69 year-olds), and substantially fewer older ever-smokers (28.1% versus 40.2% 70 year-olds or older). Notably, neither strategy selected many individuals younger than 50 years (only 0.1% of ever-smokers aged 47-49 by a life-gained-based strategy and none <47). USPSTF guidelines select by far the most people at age 55 (before they have accrued appreciable lung cancer risk) and the fewest after age 65 (thereby excluding many older ever-smokers with both high risk for lung cancer and high life-expectancy).
Figure 2: US ever-smokers selected for screening by age: life-gained-based, risk-based, and USPSTF.
USPSTF selection (8.3 million) – age 55-80, 30+ pack-years, current smoker or quit in past 15 years
Life-gained-based selection of 8.3 million – age 40-84, gain in life-expectancy from undergoing CT screening of at least 16.2 days
Risk-based selection of 8.3 million – age 40-84, 5-year lung cancer death risk of 1.4% or greater, as estimated by the Lung cancer Death Risk Assessment Tool (LCDRAT) (Katki et al., 2016)
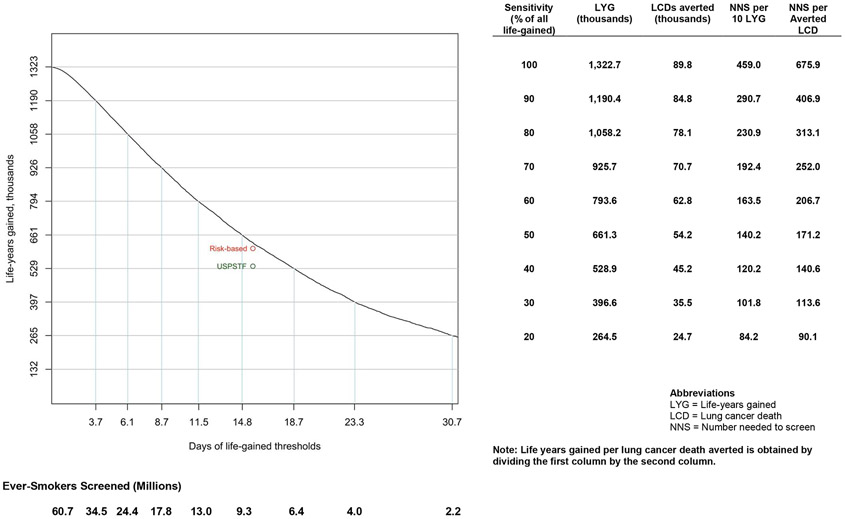
Figure 3 shows the number of gainable life-years, number of preventable lung cancer deaths, NNS per 10 life-years gained in the population screened, and NNS per prevented lung cancer death across the range of potential life-gained selection thresholds. A similar figure for risk-based selection thresholds is shown in Figure S2. These estimates allow comparisons of alternative selection strategies for CT screening, such as selection of ever-smokers to match the NNS per prevented death or NNS per 10 years of life-gained in the screened population. For example, a life-gained threshold of 12.4 days (Table S8a) would screen 11.9 million ever-smokers at the same NNS of 153.9 per 10 life-years gained as the USPSTF recommendations, provide an estimated gain of 773,800 life-years (58.5% of gainable life-years), and avert 61,600 lung cancer deaths (68.6% of preventable lung cancer deaths). By contrast, a risk-based threshold of 1.06% lung cancer death risk over 5-years would screen 11.1 million ever-smokers at the same NNS of 153.9 per 10 life-years gained, provide an estimated gain of 721,100 life-years (54.5% of gainable life-years), and avert 62,000 lung cancer deaths (69.1% of preventable lung cancer deaths). Similar comparisons in Table S8b presents life-gained-based and risk-based strategies matching the same NNS of 200.5 per prevented death as the USPSTF recommendations. In summary, compared to USPSTF, both life-gained-based and risk-based selection strategies could include more ever-smokers for screening, prevent more deaths, and gain more life-years while maintaining the same USPSTF-efficiency. For risk- and life-gained thresholds that screen similar numbers of ever-smokers, risk-based selection maximizes the number of averted lung cancer deaths while life-gained-based selection maximizes life-years gained in the population.
Figure 3: Projected outcomes from different life-gained-based thresholds in US ever-smokers aged 40 to 84 years.
CONCLUSIONS
We propose a novel framework for the selection of US ever-smokers for CT lung cancer screening based on the individualized increase in life-expectancy from screening (a life-gained-based strategy). To enable implementation of this strategy, we developed and independently validated a mortality model to estimate individualized gain in life-expectancy for screening US ever-smokers ages 40-84 years (LYFS-CT model). We show that compared to risk-based selection, life-gained-based selection of ever-smokers maximizes the life-years gained from CT screening in a population. However, life-gained-based selection would result in fewer lung cancer deaths prevented. Thus, the selection of a life-gained threshold also needs to consider the number of deaths prevented, the balance of benefits and harms, program-efficiency and costs.
Life-gained-based selection represents a much-needed alternative to risk-based selection. Previous modeling work (19,20) suggested the importance of considering life-expectancy in risk-based screening. We have generalized this to a novel life-gained-based approach to screening with an individualized life-gained calculator that could be used clinically. Although both risk-based and life-gained strategies generally exclude the lowest risk individuals, a life-gained approach preferentially includes ever-smokers with not only high-enough pre-screening lung cancer risk to benefit from CT screening (i.e., averted-death), but also high post-screening benefit (i.e., life-expectancy). Due to the preferential inclusion of younger, current smokers, with fewer comorbid medical conditions, life-gained selection maximizes the benefits (increase in life-expectancy from screening) and might reduce the potential harms (e.g., major complications) (10,31). Indeed, individuals included for screening in life-gained but not risk-based selection have substantially higher gain in life-expectancy per prevented lung cancer death (21.7 versus 8.9 years) when compared to those included in risk-based selection alone. We caution that a risk-based approach with a minimum life-expectancy cannot preferentially include moderate-risk younger or healthier individuals with long life-expectancy. Thus risk-based screening with minimum life-expectancy is not equivalent to life-gained-based screening.
We refrain from proposing a specific life-gained threshold for the selection of US ever-smokers for CT screening owing to several reasons. First, a life-gained threshold needs to ensure that enough lung cancer deaths are prevented while maximizing the life-years gained in the population and maintaining screening efficiency. Balancing trade-offs between death-prevention/life-extension and screening efficiency/costs may be inherently a social rather than scientific question. Second, our estimates of life-gained from screening are based on an NLST-like screening program rather than a real-life setting of many annual rounds of screening for eligible ever-smokers. Likewise, our estimates of screening-positivity criteria utilized NLST definitions, which may not apply in the era of Lung-RADS or volumetrics. Third, our estimates do not account for individualized harms of screening (e.g., complications from follow-up procedures) (10,27). Lastly, our life-gained estimations do not incorporate quality-of life metrics (19,20,32,33). These considerations are beyond the scope of the current work. Nevertheless, we provide estimated outcomes across the range of life-gained thresholds, which could form the basis for the determination of screening thresholds.
We submit that a shift to life-gained selection could have broad implications for precision-cancer screening. Individualized life-gained explicitly quantifies into a single metric several implicit clinical considerations—disease-risk, life-expectancy, comorbidity/performance status, and the probability of benefits and harms of procedures/interventions (20,33). Life-gained-based selection could also unify the metrics for cancer screening across stakeholders—patients, clinicians, cost-effectiveness modelers (35,36), and guidelines committees (28,37).
Importantly, our proposed life-gained-based strategy could readily address two key issues facing population-wide cancer screening— ages at initiation/cessation of screening (38) and the identification of individuals for whom the benefits of screening outweigh its harms (14-17). In contrast to risk-based selection, a life-gained-based selection circumvents the need for ages at initiation/cessation of screening because it naturally excludes individuals without both high-enough disease-risk to benefit from screening and high-enough life-expectancy to gain life-years from screening, regardless of age. This selection strategy also explicitly identifies individuals with fewer co-morbidities, better performance status, and favorable benefit-harm ratios. Thus, under a life-gained-based framework, guidelines need to only specify a minimum gain in life-expectancy relative to harms to recommend individuals for screening.
We do not underestimate the complexities surrounding the implementation of a life-gained-based screening strategy. Uptake of lung cancer screening has been very low in the United States (~2% of USPSTF-eligible ever-smokers were screened in 2016) (39). This low-uptake is perhaps partly related to the time-involved (40) and the difficulty in collecting accurate smoking information (41), a requirement for USPSTF recommendations as well as risk-based lung cancer screening strategies. A shift to life-gained-based screening selection would no doubt compound problems related to the time, effort, feasibility, and reliability of the collection of information on demographics, anthropometrics, risk-behaviors, as well as multiple health conditions, many of which may not be readily available in health records. Likewise, ethical issues for not offering/continuing screening for otherwise high-risk individuals based on life-expectancy, while common to all screening strategies (e.g. age-based and risk-based), would also need to be considered. Professional societies and guidelines committees recommend discontinuation of screening in older individuals and/or those with low life-expectancy (38,42). However, patients may be reluctant to hear about life-expectancy or forego screening based on life-expectancy (43,44). Recent research suggests that elderly individuals are more likely to forego screening when faced with shared decision-making and positive messaging that conveys that the screening test is unlikely to help them live longer (43,44).
Medicare and Medicaid guidelines require a counseling and shared decision-making visit prior to undergoing lung cancer screening (45). Our individualized calculators could be utilized for such doctor—patient communication, tailored to a specific patient. For example, a clinician could use our tools to communicate patient-specific risk of lung cancer death in the absence of CT screening, probability of lung cancer detection/false-positive result on screening, probability of death averted by screening, and the life-gained from undergoing screening. The individualized life-gained from CT screening model (LYFS-CT) is available in our lcrisks and lcmodels R packages and in an excel spreadsheet on our website (46,47).
Our observations have public-health implications. As lung cancer screening moves towards risk-based selection strategies, screening programs would increasingly include older individuals with multiple co-morbidities, which might inadvertently limit the population-level benefit of lung cancer screening. Instead, our life-gained framework could aid in the development of lung cancer screening guidelines such that benefits are maximized, harms are minimized, and the number of prevented deaths, effectiveness, and efficiency are maintained at high-levels.
Supplementary Material
ACKNOWLEDGEMENT
Funding/Support: This study was supported by the Intramural Research Program of the US National Institutes of Health (NIH)/National Cancer Institute.
Footnotes
Conflict of Interest Disclosures: Dr. Berg reports personal fees from Medial EarlySign, LLC, and GRAIL. The Lung Cancer Death Risk Assessment Tool (LCDRAT) was previously proposed by co-authors of this manuscript. No other authors reported disclosures.
Role of the Funder/Sponsor: The NIH had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Ethics Committee Approval: The National Institutes of Health Office of Human Subjects Research deemed this study exempt from institutional review board review.
Reproducible Research Statement:
Study protocol: Not available. Data set and Statistical code: Available from Dr. Cheung (e-mail, li.cheung@nih.gov).
REFERENCES
- 1.Wilson JMG, Jungner G. Principles and practices of screening for disease. Geneva: WHO, 1968. [Google Scholar]
- 2.Patz EF Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174(2):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shieh Y, Eklund M, Sawaya GF, Black WC, Kramer BS, Esserman LJ. Population-based screening for cancer: hope and hype. Nat Rev Clin Oncol. 2016;13(9):550–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach PB. Raising the bar for the U.S. Preventive Services Task Force [Editorial]. Ann Intern Med. 2014;160:365–6. [DOI] [PubMed] [Google Scholar]
- 5.Wood DE, Kazerooni EA, Baum SL, Eapen GA, Ettinger DS, Hou L, et al. Lung cancer screening, version 3.2018, NCCN clinical practice guidelines in oncology. JNCCN. 2018;16:412–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draft Research Plan: Lung Cancer: Screening. U.S. Preventive Services Task Force, May 2018. (https://www.uspreventiveservicestaskforce.org/Page/Document/draft-research-plan/lungcancer-screening1). Accessed April 18, 2019.
- 7.Ten Haaf K, Jeon J, Tammemägi MC, Han SS, Kong CY, Plevritis SK, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med. 2017;14:e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katki HA, Kovalchik SA, Petito LC, Cheung LC, Jacobs E, Jemal A, et al. Implications of nine risk prediction models for selecting ever-smokers for computed tomography lung cancer screening. Ann Intern Med. 2018;169(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Ann Intern Med. 2012;157:571–3. [DOI] [PubMed] [Google Scholar]
- 10.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. ; National Lung Screening Trial Research Team. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Koning HJ, van der Aalst C, Ten Haaf K, Oudkerk M. Effects of volume CT lung cancer screening: mortality results of the NELSON randomized-controlled population based trial. 2018 World Conference on Lung Cancer Abstract PL02.05. Presented September 25, 2018 in Toronto, CA. [Google Scholar]
- 12.Tammemägi MC. Application of risk prediction models to lung cancer screening: a review. J Thorac Imaging. 2015;30:88–100. [DOI] [PubMed] [Google Scholar]
- 13.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315:2300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould MK. Clinical practice. Lung cancer screening with low-dose computed tomography. N Engl J Med. 2014;371:1813–20 [DOI] [PubMed] [Google Scholar]
- 15.Schneider D, Arenberg A. Competing mortality in cancer screening: a teachable moment. JAMA Intern Med. 2015;175(6):896–897. [DOI] [PubMed] [Google Scholar]
- 16.Howard DH, Richards TB, Bach PB, Kegler MC, Berg CJ. Comorbidities, smoking status, and life expectancy among individuals eligible for lung cancer screening. Cancer. 2015;121(24):4341–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera MP, Tanner NT, Silvestri G, Detterbeck FC, Tammemägi MC, Young RP, et al. Incorporating coexisting chronic illness into decisions about patient selection for lung cancer screening. An official American Thoracic Society research statement. Am J Respir Crit Care Med. 2018:198(2):e3–e13. [DOI] [PubMed] [Google Scholar]
- 18.Tanner NT, Dai L, Bade BC, Gebregziabher M, Silvestri G. Assessing the generalizability of the national lung screening trial: comparison of patients with stage 1 disease. Am J Respir Crit Care Med. 2017:196(5):602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar V, Cohen JT, van Klaveren D, Soeteman DI, Wong JB, Neumann PJ, et al. Risk-targeted lung cancer screening: a cost-effectiveness analysis. Ann Intern Med. 2018;168(3):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caverly TJ, Cao P, Hayward RA, Meza R. Identifying patients for whom lung cancer screening is preference-sensitive: a microsimulation study. Ann Intern Med. 2018;169(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lung cancer screening saves lives. Veterans Health Administration, March 2018. (https://www.va.gov/health/newsfeatures/2018/march/ldct-screening-enhances-cancer-care-for-veterans.asp). Accessed April 18, 2019.
- 22.Mazzone PJ, Silvestri GA, Patel S, Kanne JP, Kinsinger LS, Wiener RS, et al. Screening for Lung Cancer: CHEST Guideline and Expert Panel Report. Chest 2018; 153(4): 954–85. [DOI] [PubMed] [Google Scholar]
- 23.National Health Interview Survey. Centers for Disease Control and Prevention (CDC), April 2019. (http://www.cdc.gov/nchs/nhis). Accessed April 18, 2019.
- 24.National Center for Health Statistics Data Linkage: 2011 Public-Use Linked Mortality Files. Centers for Disease Control and Prevention (CDC), February 2019. (https://www.cdc.gov/nchs/data-linkage/mortality-public.htm). Accessed April 18, 2019.
- 25.Justice JC, Covingsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515–24. [DOI] [PubMed] [Google Scholar]
- 26.Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovalchik SA, Tammemägi M, Berg CD, Caporaso NE, Riley TL, Korch M, et al. Targeting of low-dose CT screening according to the risk of lung cancer death. N Engl J Med. 2013;369:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyer VA, U. S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 29.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2012. [Google Scholar]
- 30.Lumley T Analysis of complex survey samples. J Stat Softw. 2004;9(1): 1–19. [Google Scholar]
- 31.Black WC. Importance of individualized decision making for lung cancer screening. Radiology. 2018;289(1): 225–226. [DOI] [PubMed] [Google Scholar]
- 32.Cressman S, Peacock SJ, Tammemägi MC, Evans WK, Leighl NB, Goffin JR, et al. The cost-effectiveness of high-risk lung cancer screening and drivers of program efficiency. J Thorac Oncol. 2017;12(8):1210–1222. [DOI] [PubMed] [Google Scholar]
- 33.Heale W. Individualised and personalised QALYs in exceptional treatment decisions. J Med Ethics 2016; 42(10): 665–71. [DOI] [PubMed] [Google Scholar]
- 34.Keating NL, Pace LE. Breast cancer screening in 2018: time for shared decision making. JAMA. 2018; 319(17):1814–1815. [DOI] [PubMed] [Google Scholar]
- 35.de Koning HJ, Meza R, Plevritis SK, ten Haaf K, Munshi VN, Jeon J, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(5):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandelblatt JS, Stout NK, Schechter CB, van der Broek, Miglioretti DL, Krapcho M, et al. Collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med. 2016. February 16;164(4):215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siu AL; U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296. [DOI] [PubMed] [Google Scholar]
- 38.American Cancer Society guidelines for the early detection of cancer. American Cancer Society, May 2018. (https://www.cancer.org/healthy/find-cancer-early/cancer-screening-guidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer.html). Accessed April 18, 2019.
- 39.Pham D, Bhandari S, Oechsli M, et al. Lung cancer screening rates: data from the lung cancer screening. J Clin Oncol. 2018;36 no. 15_suppl 6504–6504. [Google Scholar]
- 40.Brenner AT, Malo TL, Margolis M, Lafata JE, James S, Vu MB, et al. Evaluating Shared Decision Making for Lung Cancer Screening. JAMA Intern Med. 2018;178(10):1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polubriaginof F, Salmasian H, Albert DA, Vawdrey DK. Challenges with collecting smoking status in electronic health records. AMIA Annu Symp Proc. 2017;2017:1392–1400. [PMC free article] [PubMed] [Google Scholar]
- 42.Workgroup AGSCW. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950–960. [DOI] [PubMed] [Google Scholar]
- 43.Torke AM, Schwartz PH, Holtz LR, Montz K, Sachs GA. Older adults and forgoing cancer screening: I think it would be strange. JAMA Intern Med. 2013;173(7):526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenborn NL, Lee K, Pollack CE, Armacost K, Dy SM, Bridges JFP, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177(8):1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Syrek Jensen T, Chin J, Ashby L, Hermansen J, Hutter JD. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). Centers for Medicare and Medicaid Services, 2015. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274 (accessed April 17, 2019). [Google Scholar]
- 46.Cheung LC, Katki HA. lcrisks: Lung cancer risk models for screening. https://dceg.cancer.gov/tools/risk-assessment/lcrisks (accessed April 19, 2019).
- 47.Cheung LC, Katki HA. lcmodels: R package for predictions from published lung cancer models. https://dceg.cancer.gov/tools/risk-assessment/lcmodels (accessed April 19, 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.