Abstract
The stability of the liquid water phase on Mars has been examined on the basis of fundamental thermodynamic principles. The analysis considers the atmospheric pressure and temperature conditions prevalent on Mars. Because of the very low atmospheric pressure on Mars, water cannot exist in the liquid form. However, salt dissolution can reduce the freezing point and elevate the boiling point of aqueous solutions. This is interesting in the light of the discovery of perchlorate, sulphate, sodium, potassium, and calcium ions over the Martian surface. The effect of different perchlorate salts on the freezing and boiling points of water while considering their saturation solubility under varying ionic conditions is key to this analysis. It is shown that under an average atmospheric pressure of 600 Pa, the saturated solution of sodium perchlorate (NaClO4) is stable in the liquid phase in the temperature range between 240 and 275 K. The triple point of water under this condition is shifted to 269 K with a saturation solubility of 14.4 mass % of the salt. However, a saturated solution of magnesium perchlorate (Mg(ClO4)2) renders this temperature range wider from 198 to 296 K, with the triple point being located at 269 K (salt saturation at 13.5 mass % salt). In case the water is contaminated with a mixture of these salts, an increased stability is predicted for liquid water down to 180 K and up to at least 298 K. This is caused by the increased ionic strength that enhances the freezing point depression and boiling point elevation of the solution. Thus, in the extreme and uneventful conditions of saturation by mixtures of salts, liquid water can be stable on Mars between 180 K and at least up to 298 K. Below this temperature, water exists as a glacier and above, as steam only.
Introduction
The presence of water on other planets, especially on Mars has always intrigued mankind as this could be central to understanding the hydrologic cycle and potential for the existence of life on Mars. Evidence for the presence of water on Mars was presented as recurring slope lineae, narrow streaks of low reflectance compared to the surrounding terrain, that grow incrementally in the down slope direction during warm seasons, a pattern consistent with transient flow of volatile species, from photos sent by the Mars Reconnaissance Orbiter.1 Also gullies, a geographical feature found in abundance on the Martian surface was proposed to be created by liquid water formed by melting of ice during warmer seasons on Mars.2
Brine flows (or seeps) have been proposed to explain the formation of these recurring slope lineae. Evidence for the presence of hydrated salts at various locations suggests that these might be the reason for recurring slope lineae. The hydrated salts are also consistent with spectral absorption features that were detected.3−5 The prominent ones among them are perchlorates of sodium, magnesium, calcium, and potassium, chlorates of sodium and potassium in addition to ferric and ferrous sulphates. These salts can lower the freezing point of water and lower its evaporation rate too.6−12 These were postulated as reasons for the increased stability of liquid water on the surface of present-day Mars.13
However, very recent photographic evidences from the compact reconnaissance imaging spectrometer for the Mars (CRISM) onboard of the Mars Reconnaissance Orbiter have suggested that the formation of gullies on Mars cannot be explained by theory of flowing water.14 Thus, the existence of liquid water on Mars itself is a matter of doubt or debate. In this backdrop, it looked appropriate to undertake a theoretical analysis of the stability of liquid water on Mars from a thermodynamic perspective. Thus, this paper attempts to find an answer to this question by the way of analyzing the thermodynamic stability of liquid water on Mars, considering the atmospheric conditions on Mars and the hygroscopic nature of various salts. There are a few research articles which have dealt with such matters individually before11−13,20 by looking into the freezing and evaporation of brine solutions of perchlorates, sulfates, and so forth, without incorporating the effects of lower atmospheric temperature and pressure on Mars. Here, we have attempted to model the overall phase stability of liquid water on Mars by considering binary solutions formed by the dissolution of perchlorates of sodium and magnesium under the atmospheric conditions prevalent on Mars. The modeling was carried out using Pitzer equations and the equations of Ge et al.16,17 for boiling point elevation and freezing point depression of highly ionic solutions. These models were solved numerically using a computer program. For modeling of binary solutions, we have borrowed the binary Pitzer parameters from FREZCHEM,10,21 whereas for modeling of ternary solutions, we have attempted to theoretically estimate ternary interaction parameters and cation–cation interaction parameters using numerical methods. The solubility data for the solutions were taken from the literature.10 This paper is an attempt toward this objective, using the latest theoretical models and their experimental validation, wherever possible.
Theory
Solubility of Salts
The atmospheric pressure on Mars varies from 30 to 1000 Pa with an average value of 600 Pa. The average surface temperature is −60 °C while the variation is from −140 °C at poles to 20 °C at noon near the equator.15,22
The Martian atmosphere contains mainly carbon dioxide (95.2%) and the other major component being nitrogen. The amount of water vapor present in the Martian atmosphere is about 1/1000th of that on earth. The liquid water on Mars is said to be contaminated with chlorides, sulphates, and perchlorates of Na, K, Mg, Ca, and so forth.23 Among these, perchlorates offer the highest ionic strength and are soluble enough in water to induce appreciable lowering of the freezing point and elevation of the boiling point. Sodium perchlorate has the maximum solubility in water at a given temperature as compared to perchlorates of magnesium and potassium. But even then, the magnesium perchlorate–water system is reported to show a lower eutectic temperature as compared to sodium perchlorate due to the higher ionic activity of magnesium perchlorate as compared to sodium perchlorate. The solubility of potassium perchlorate in water is generally poor (2.56 g of KClO4/100 g of water at 30 °C as compared to 222 g of NaClO4/100 g of water at 30 °C) (https://en.wikipedia.org/wiki/Solubility_table) and hence was not considered for this analysis. It does not improve even in the presence of sodium and magnesium perchlorates. Calcium perchlorate was not considered because under Martian conditions, as it is highly soluble, calcite and gypsum are more likely to precipitate out when compared to calcium perchlorate.11,24 Hence, the cases of binary solutions of sodium and magnesium perchlorate in water and the ternary solution of sodium perchlorate–magnesium perchlorate–water were only primarily considered for analysis of the phase behavior of the respective brine solutions on Mars. The solubility data taken from the literature are given in Table 1.10
Table 1. Saturation Solubility of Sodium Perchlorate and Magnesium Perchlorate in Water at Varying Temperatures10.
| sodium
perchlorate |
magnesium
perchlorate |
||
|---|---|---|---|
| temp. (K) | molality (m) | temp.(K) | molality (m) |
| 298.01 | 17.08 | 298.90 | 4.41 |
| 293.47 | 16.38 | 292.85 | 4.34 |
| 282.07 | 14.86 | 283.45 | 4.22 |
| 273.02 | 13.86 | 273.00 | 4.09 |
| 263.45 | 12.91 | 262.92 | 3.97 |
| 252.84 | 11.26 | 252.94 | 3.84 |
Here, individual cases of freezing point depression of water due to dissolution of sodium and magnesium perchlorates in water have been considered; the analysis considers the freezing point depression of a ternary system of NaClO4–Mg(ClO4)2–H2O too. The solubility data of sodium and magnesium perchlorate in water are given in Table 1.
Freezing Point of the Salt Solution under Martian Conditions
In fact, in Mars, the freezing point of salt solution is affected by two opposing phenomena. One is the depression in the freezing point of water due to salt dissolution and the other is an increase in its freezing point due to a lower atmospheric pressure prevalent on Mars, that is, as we apply lower pressure to the ice–water equilibrium, by Le Chatelier’s principle, the system will try to compensate by increasing the rate of the reaction of the side of the reaction where pressure is higher. It will do so by making itself fit into a higher volume, and because ice occupies a higher volume than water, the rate of conversion of water to ice increases, effectively leading to an increase in the freezing point of the ice–water system.
(The binary system refers to a solution of a single salt in water and the ternary system to a solution of two salts in water.)
Also, because the pressure coefficient of free energy change of the ice–water equilibrium is equal to the molar volume change which is negative for ice to water transition, an increase of pressure should cause a decrease in the melting point of ice and vice versa, that is, (dΔG/dP)T = ΔV (−ve) for ice-to-water transition.
Effect of the Salt Concentration and Ionic Strength on the Freezing Point of Water
Considering the cases of the saturated solutions of sodium perchlorate and magnesium perchlorate respectively in equilibrium with their solids,
| 1 |
| 2 |
Here, we have considered sodium perchlorate dihydrate and magnesium perchlorate hexahydrate because it is reported in earlier studies that these are the stable phases that precipitate out at the eutectic point of the solution.10
In case of perchlorates, the salt should be mostly in ionized form in solution.
| 3 |
where, aw is the activity of water.
Similarly, for the magnesium perchlorate hexa hydrate,
| 4 |
The detailed explanation of the Pitzer equation used in this work is given in the Supporting Information, S2.
The eutectic point was calculated by solving simultaneously the Pitzer equations and the solubility product, calculated from the fitted solubility curve (refer the Supporting Information part S1) from the experimental data given in Table 1. Here, the activities of ions, osmotic coefficients, and the activity of water obtained using Pitzer equations were simultaneously substituted in eq 5 till the solutions (output) converged to get the eutectic point. For calculating the freezing point depression (ΔTF), the equation15,16 was used
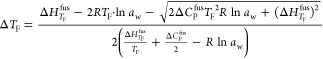 |
5 |
Here, TF is the normal freezing point of pure water (273.15 K); aw is the activity of water; ΔHTFfus is the enthalpy change of fusion of the pure solvent at TF, which is 6004.8 J/mol for water at 273 K; and ΔCPfus is the difference of heat capacities between the liquid and solid phases at TF, which is 38 J/mol/K for water.
Effect of Atmospheric Pressure on the Freezing Point
The effect of atmospheric pressure on the freezing points of these eutectic systems was calculated by applying basic thermodynamic principles to the following equilibrium, by applying the Clapeyron law
The free energy change in the system is
| 6 |
where, ΔV is the molar volume change, and ΔS is the the molar entropy change
| 7 |
When pure ice melts, the molar volume decreases while entropy increases. Therefore, the pressure coefficient of the melting point is negative.
The density of water is 997 kg/m3 and that of ice is 920 kg/m3
| 8 |
| 9 |
By applying the Clapeyron law, we get
| 10 |
We know, ΔH/ΔV = (6010 J/mol)/(1.51 × 10–6 m3/mol) = −3.98 × 109 Pa.
Assume that ΔH and ΔV are constant over a small temperature range, and integrating over dT and dP, we get
| 11 |
or
| 12 |
| 13 |
that is, ΔT/T = 2.5 × 10–5, T = melting point of the sodium perchlorate–water system.
We get dT/dP = −6 × 10–3 K·Pa–1.
This implies that the increase in the melting point of ice due to a drop in the atmospheric pressure can be to a maximum of 0.006 K only (assuming that the atmospheric pressure drops from 1 to zero bar). The variation in the melting point of water due to seasonal variations in the atmospheric pressure on Mars amounts to a maximum of 5.18 × 10–5 K only (considering the Martian atmospheric pressure variations from 30 to 1000 Pa). Hence, for all practical purposes, the effect of atmospheric pressure on the melting point of ice can be neglected.
Boiling Point Variation of Water under Martian Conditions
The boiling point of liquid water in Mars is also affected by two factors: Elevation in the boiling point due to dissolution of the salt and depression in the normal boiling point due to the reduced atmospheric pressure on Mars.
Boiling Point of Pure Liquid Water on Mars
Variations in the atmospheric pressure have a profound influence on the boiling point of any liquid. As the atmospheric pressure varies from place to place in Mars, it is interesting to examine the impact of this variation on the phase behavior of the salt–water system. The average temperature on Mars is about −60 °C, although it can vary from −140 °C near the poles during the winter to as much as +20 °C at mid-day near the equator. The surface atmospheric pressure varies from 30 to 1000 Pa with an average of 600 Pa. For all practical purposes, an average atmospheric pressure of 600 Pa is considered for calculating the boiling point of saturated salt solutions.
The boiling point of pure water at reduced pressure was calculated using the Clausius–Clapeyron equation, and then, the increase in the boiling point due to salt dissolution was accounted for.
Applying the Clausius–Clapeyron relation for pure water
| 14 |
P1 and P2 are 101,300 and 600 Pa, respectively, and T1 is 373.15 K; substituting
Boiling point of pure water (TB = T2) was obtained as 268.19 K (−4.96 °C) at 600 Pa, that is, −4.96 °C is the normal boiling point of pure water in Mars.
Elevation in the Boiling Point due to Salt Dissolution
For calculating the boiling point elevation due to salt dissolution, the following equation15,16 was used
 |
15 |
where, TB is the
normal boiling point of pure water on Mars (268.19 K); aw is the activity of water; 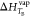 is the enthalpy change of vaporization
of pure water at TB, which is 40,660 J/mol;
and ΔCvap is the difference of heat
capacities between the vapor and liquid phases at TB, which is −43.05 J/mol/K for water.
is the enthalpy change of vaporization
of pure water at TB, which is 40,660 J/mol;
and ΔCvap is the difference of heat
capacities between the vapor and liquid phases at TB, which is −43.05 J/mol/K for water.
The activity of water was calculated using the Pitzer equation as detailed in Supporting Information, S2.
The boiling point elevation of a ternary mixture of sodium and magnesium perchlorate in water was also evaluated using the Van’t Hoff equation
| 16 |
where, i is the Van’t Hoff factor; i = 2 for sodium perchlorate, and i = 3 for magnesium perchlorate.
Kb is the ebullioscopic constant, Kb = 0.512 K·kg/molal for water, and m is the molality of the solution. This was done to have an indicative value of the elevation in the boiling point with increasing concentration.
Ternary System of Solution of Sodium Perchlorate and Magnesium Perchlorate in Water
The Pitzer parameters of binary solutions were taken from the literature.10 These values have been widely reported and have been proven to match the results from experiments. Ternary Pitzer parameters for perchlorates are not widely reported mainly because of the lack of experimental data for their theoretical estimation. Toner et al. in their work10 had determined them by generating experimental data and modeling using Pitzer equations. However, they had used these parameters for modeling of freezing of ternary solutions. Here, we have attempted to model both freezing and boiling of ternary solution of sodium and magnesium perchlorate in water. The cation–cation interaction parameter (θNa+,Mg2+) and the ternary interaction parameter (ψNa+,Mg2+,ClO4–) were obtained by numerically solving the experimental solubility data using the Pitzer equation.
These values were verified by plotting the theoretically determined osmotic coefficient against the experimentally determined osmotic coefficient10 and was found to have a close match (a typical plot of Φ vs concentration is shown in Figure S3 in the Supporting Information S2). The temperature dependency of ψNa+,Mg2+,ClO4– and θNa+,Mg2+ was not determined because of the lack of solubility data at lower temperatures. However, the variation of Pitzer parameters on temperature is minimal for temperatures from 298.15 K to temperatures as low as 200 K.10 The variation of Pitzer parameters with temperatures above 298.15 K was not studied because of the lack of experimental data. The values of ψNa+,Mg2+,ClO4– and θNa+,Mg2+ were found to be −0.0274 and −0.0043, respectively.
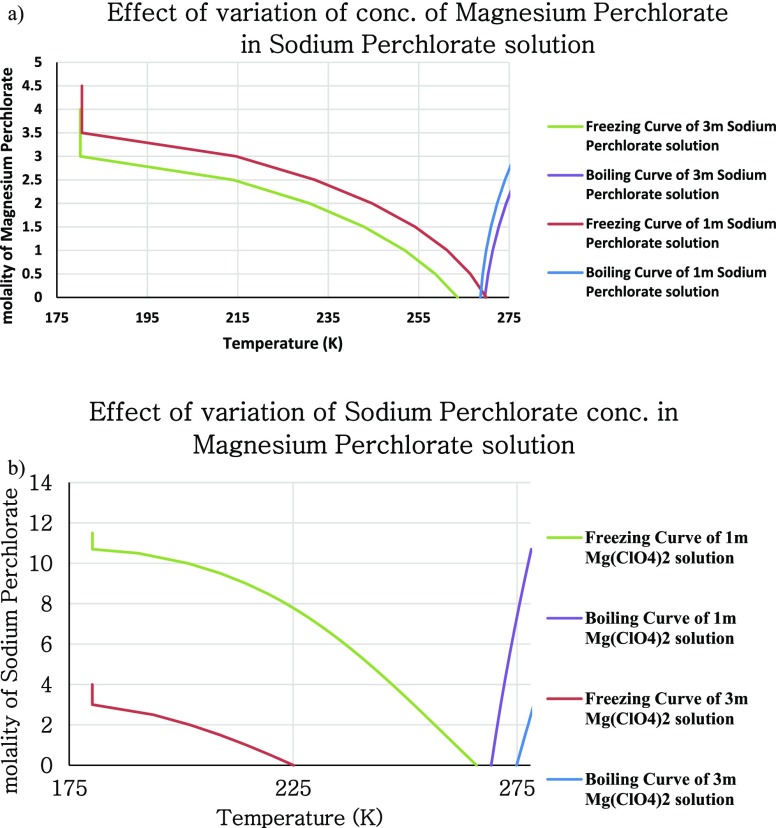
The freezing point depression for the ternary system for varying concentrations of sodium and magnesium perchlorates and the plots are shown in Figure 3.
Figure 3.
Phase diagrams depicting the freezing and boiling point profiles at (a) varying concentrations of sodium perchlorate in 1 and 3 m magnesium perchlorate solution and (b) varying concentrations of magnesium perchlorate in 1 and 3 m sodium perchlorate solutions.
The eqs 3–5 and 15 and Pitzer equations (i–xii, in the Supporting Information 2) were solved using a computer program.
Results
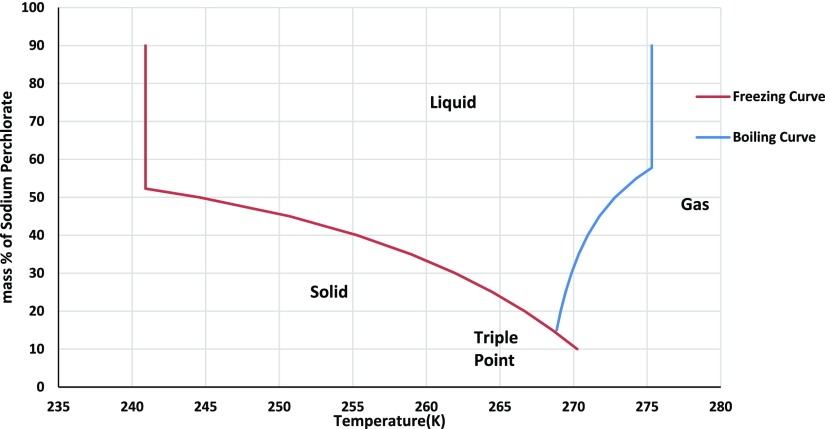
The predicted freezing points and boiling points of the water–sodium perchlorate liquid system under Martian conditions have been used to generate the phase diagram. The freezing point results are well in agreement with the freezing point data published earlier.10,11 As can be seen from Figure 1, above a threshold concentration of 14.4 mass % of sodium perchlorate in water, the liquid phase of water becomes stable across a very small temperature window (268.787–268.814 K) under the Martian atmospheric pressure conditions. This temperature range widens as the concentration of sodium perchlorate in water increases and reaches a maximum (from 240.9 to 275.32 K) at a concentration of 57.81% by mass of the salt. Although a temperature window of 268.787–268.814 K may seem to have no practical significance, it may be noted that this temperature and salt concentration may be seen as the tipping point for the formation of liquid water under Martian conditions. In the final temperature window of 240.9–275.32 K, the higher end point is 275.32 > 273.15 K because of the elevation in the boiling point due to dissolution of salts.
Figure 1.
Phase diagram of sodium perchlorate−water system on mars.
Below a floor value of 240.9 K, water always exists as glacier irrespective of salt solubility and atmospheric pressure. Similarly, above 275.3 K, water exists only as vapor on Mars.
At 240.9 K, the salt concentration of sodium perchlorate is 52.28% by mass. The maximum boiling point for the sodium perchlorate–water mixture was calculated to be 275.32 K and occurred at a concentration of 57.81% by mass of sodium perchlorate. This means that under saturated sodium perchlorate salt conditions, liquid water is stable between 240.9 and 275.32 K, given the triple point for the system appearing at 269 K.
Similar to the sodium perchlorate–water system, the phase diagram of the magnesium Perchlorate–water mixture was also predicted. Teutectic (freezing) for magnesium perchlorate was calculated to be 193.4 K at a salt concentration of 45.86 mass % (refer Figure 2). The maximum boiling point for the magnesium perchlorate–water mixture was calculated to be 296.63 K that occurred at a concentration of 58.36% by mass of magnesium perchlorate. The new triple point is 269 K with a salt saturation solubility of 13.5% by mass. The eutectic point of 193.4 K for ice–Mg(ClO4)2 solution predicted in this paper differs from the values reported elsewhere,10,11 which report the eutectic temperature of the magnesium perchlorate–water mixture in the range of 205–216 K. The disagreement is due to the fact that the computations carried out in this paper used eq 5 for the calculation of the freezing point instead of the methodology used in the FREZCHEM model, which is the sequential method of solving. Several experimental works also report the eutectic point of the magnesium perchlorate system to be between 205 and 212 K. However, here, while freezing a highly ionic solution, it can be observed that once the excess salt starts precipitating out, the liquid solution is no more having a uniform concentration, and the effects of convective heat transfer begins to play a role due to density differences. This can lead to pockets of solutions, such as bubbles in a foam, having sizes ranging from millimetres to micrometres in dimensions, which may be the last to freeze. Because in experiments, we can measure only the temperature of maximum 2–3 locations; chances are there that such pockets may be missed. These pockets are formed inside the ice and may not be visible to naked eyes. Additionally, it may also be noted that the experimental rapid estimation of concentration of perchlorates in solution via titration is also highly prone to error.19
Figure 2.
Phase diagram of the magnesium perchlorate–water system on Mars.
In both of these graphs (Figures 1 and 2), it can be seen that the change in the freezing point/boiling point due to the increased concentration of the salt is not linear. This is due to the nonlinear increase in the activity of the ions with a linear increase in the mass percentage of salt dissolution, which is attributed to the highly ionic nature of perchlorates. This is also seen from eqs 5 and 15 used for calculating the freezing point depression and boiling point elevations of the solution, where we can see that ΔT is not a linear function of aw. Beyond certain concentrations, further salt dissolution is not possible, and that is why both graphs have a portion of “x = constant” at the end [linear part].
The eutectic point as determined experimentally in this work is nearly 198 K and is closer to the prediction in this work.
From the phase diagrams of sodium and magnesium perchlorate, it is evident that the rate of change of the freezing and boiling point with the concentration of salt is higher for magnesium perchlorate as compared to sodium perchlorate. This is certainly attributed to the higher ionic strength of Mg(ClO4)2 and its corresponding impact on the activity of water as compared with NaClO4.18
More than 96.5% of water on earth is a part of oceans which contain multiple salts in varying concentrations. Assuming a similar situation on Mars, we can expect the liquid water there to be contaminated with diverse types of salts dissolved to different extents. Here, we have considered the case of a ternary solution of sodium–magnesium perchlorate in water so as to understand the impact of dissolution of multiple salts on the phase stability of water.
As there are more than one salt, different eutectic concentrations are available, and the lowest freezing point predicted for the same is 180.17 K. Some of the cases of these ternary mixtures are shown in Figure 3 (calculations shown are as per eqs 3–5 and 15 and, i–xii in the Supporting Information S2).
Here, plots of different concentrations of the ternary mixture have been made such that the concentration of one of the salts is kept constant while the other one is varied. As can be seen from the above-mentioned phase diagrams, the presence of magnesium perchlorate along with sodium perchlorate further widens the temperature window for existence of liquid water on Mars, that is, the freezing point is lowered down to 180 K. As a general trend, the impact of sodium perchlorate concentration on depression in the freezing point and elevation in the boiling point seems to be quite linear, whereas that of magnesium perchlorate shows a second-order dependency. In other words, the depression in the freezing point and elevation in the boiling point for unit change in concentration of magnesium perchlorate is more pronounced unlike for sodium perchlorate. This is obviously due to an increased ionic strength of Mg(ClO4)2 vis-à-vis sodium perchlorate.
The concentration of the highest boiling mixture has not been shown because the equation for the boiling point elevation and Pitzer equations fail to converge together at higher concentrations. This is mainly because the Pitzer parameters were modeled for temperatures from 298 K and below only. Because the boiling point of the ternary mixture was above 298 K, the model did not yield any meaningful result. Experimental determination of boiling point elevation of the ternary mixture could not be carried out because of the hazardous nature of the perchlorate salts. An effort was made to compute the boiling point of the ternary mixture using the Van’t Hoff equation.25 The data generated are given in Table 2. The boiling points of perchlorate solutions whether single or in mixture of salts computed by Van’t Hoff or Pitzer models normally deviate from the experimental results mainly at higher concentrations. This is attributed to the impact of the ionic strength of perchlorates especially at higher salt concentrations.
Table 2. Boiling Points of Varying Concentrations of the Ternary Mixture; Using the Van’t Hoff Equation.
| molality of sodium perchlorate | molality of magnesium perchlorate | boiling point of ternary mixture, K |
|---|---|---|
| 1 | 3 | 279.24 |
| 3 | 2 | 281.80 |
| 5 | 1 | 284.36 |
| 4 | 3 | 286.92 |
| 10 | 1 | 297.16 |
From the above-mentioned results, it becomes clear that the dissolution of perchlorate salts in water stabilizes the liquid phase of water to some extent under Martian conditions. However, this warrant meeting certain conditions that are unlikely on Mars. For example, water should meet beds of salts or salt mixtures that are present in quantities to get the water saturated. This analysis has considered the Martian atmospheric pressure to be 600 Pa. In some areas, this pressure may be lower, which will further destabilize the formation of liquid water at a given temperature.
Discussion and Conclusion
Based on thermodynamic analyses, it is evident that pure water cannot exist as liquid under Martian atmospheric conditions. Mars as we know is abundant in salts especially perchlorates and sulphates. Some of these salts are highly hygroscopic in nature which can lead to stabilization of highly concentrated liquid solutions. The dissolution of these salts in water opens up small windows of temperature over which the solution exists as a liquid. These windows increase with increasing concentration of the salt in the solution. For example, the normal freezing point and boiling point of liquid water on Mars are 273 and 268 K, respectively. These points being shifted from the triple point of the water–water vapor–ice system this would imply that ice will sublime directly to water vapor at this pressure. On addition of a small amount of sodium or magnesium perchlorate, the freezing point drops below 273 K, and boiling point gets raised above 268 K. Finally, at some concentration, the theoretical freezing point of the solution becomes less than the boiling point, and the solution exists as liquid over Mars in that small temperature window. For example, at a minimum concentration of 14.4% by mass of sodium perchlorate, the solution exists as liquid in a temperature window between 268.787 and 268.814 K. As the concentration of salt increases, this window widens till the limiting concentration, above which no more salt gets dissolved into the solution. This concentration is 57.81% by mass for sodium perchlorate, and the largest temperature window over which a saturated sodium perchlorate solution can exist as liquid is 240.9–275.32 K.
The largest temperature window over which a saturated magnesium perchlorate solution can exist as liquid is between 193 and 296 K. Here again, as in the case of sodium perchlorate solution, the concentration of 13.5% by mass of salt and the temperature window of 268.770 and 268.816 K may be viewed as the tipping point above which the liquid water phase is stable under Martian conditions. The higher ionic strength of Mg2+ as compared to Na+ at the same solubility makes the system more sensitive to magnesium perchlorate than sodium perchlorate.
When multiple perchlorate salts are simultaneously present, the freezing point of such a mixture goes as low as 180 K. This can be attributed to the combined effect of the multiple salts leading to higher ionic strengths. All the above-mentioned analyses results were calculated assuming the average Martian atmospheric pressure of 600 Pa. In certain areas where pressure may be lower, such as in high altitudes, the chances of finding liquid water may be further reduced. On the other hand, in areas where pressure might be higher as in craters, the chances of discovering liquid water will be more.
It is to be remarked that these calculations are applicable for ideal saturated solution conditions only, which are unlikely thus diminishing the probability of the stable liquid water phase on Mars except on special occasion of water encountering beds of mixtures of salts and experience higher atmospheric pressures. Still the kinetics of phase changes are not favorable as a large amount of enthalpy change associates with such phase changes, that is, the high latent heat of fusion for water (6004.8 J/mol) and high latent heat of vaporization of water (40,660 J/mol) of water is going to prevent any rapid phase changes because in reality, there is no mechanism of sudden heating or sudden cooling existing in the nature. This must be the reason for the Martian terrain fooling the cameras on board Mars orbiters from time to time, as also reported by NASA.14
Acknowledgments
Authors are grateful to Prof K.L. Sebastian, Indian Institute of Science, Bangalore, India, for fruitful discussions. They appreciate the help rendered by K Sunitha in generating the freezing point data while Dr K.S. Santhosh Kumar, VSSC, is thanked for editorial help.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00444.
Solubility curves of sodium and magnesium perchlorates, description of the Pitzer model, and the codes used for predicting the results (PDF)
Author Present Address
§ Department of Polymer Science and Rubber Technology, Cochin University of Science and Technology, Cochin 682 022, Kerala, India.
Author Contributions
The idea was conceived by both the authors, and the manuscript was written through contributions of both the authors. Both authors have given approval to the final version of the manuscript.
No funding sources.
The authors declare no competing financial interest.
Supplementary Material
References
- Ojha L.; Wilhelm M. B.; Murchie S. L.; McEwen A. S.; Wray J. J.; Hanley J.; Massé M.; Chojnacki M. Spectral evidence for hydrated salts in recurring slope lineae on Mars. Nat. Geosci. 2015, 8, 829–832. 10.1038/ngeo2546. [DOI] [Google Scholar]
- Mellon M. T.; Phillips R. J. Recent gullies on Mars and the source of liquid water. J. Geophys. Res. 2001, 106, 23165–23179. 10.1029/2000JE001424. [DOI] [Google Scholar]
- Hecht M. H.; Kounaves S. P.; Quinn R. C.; West S. J.; Young S. M. M.; Ming D. W.; Catling D. C.; Clark B. C.; Boynton W. V.; Hoffman J.; DeFlores L. P.; Gospodinova K.; Kapit J.; Smith P. H. Detection of perchlorate and the soluble chemistry of Martian soil at the Phoenix Lander site. Science 2009, 325, 64–67. 10.1126/science.1172466. [DOI] [PubMed] [Google Scholar]
- Glavin D. P.; Freissinet C.; Miller K. E.; Eigenbrode J. L.; Brunner A. E.; Buch A.; Sutter B.; Archer P. D. Jr.; Atreya S. K.; Brinckerhoff W. B.; Cabane M.; Coll P.; Conrad P. G.; Coscia D.; Dworkin J. P.; Franz H. B.; Grotzinger J. P.; Leshin L. A.; Martin M. G.; McKAy C.; Ming D. W.; Navarro-González R.; Pavlov A.; Steele A.; Summons R. E.; Szopa C.; Teinturier S.; Mahaffy P. R. Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. J. Geophys. Res. 2013, 118, 1955–1973. 10.1002/jgre.20144. [DOI] [Google Scholar]
- Ehlmann B. L.; Edwards C. S. Mineralogy of the Martian surface. Annu. Rev. Earth Planet. Sci. 2014, 42, 291–315. 10.1146/annurev-earth-060313-055024. [DOI] [Google Scholar]
- Pestova O. N.; Myund L. A.; Khripun M. K.; Prigaro A. V. Polythermal study of the systems M(ClO4)2-H2O (M2CDMg2C, Ca2C, Sr2C, Ba2C). Russ. J. Appl. Chem. 2005, 78, 409–413. 10.1007/s11167-005-0306-z. [DOI] [Google Scholar]
- Chevrier V.; Hanley J.; Altheide T. Stability of perchlorate hydrates and their liquid solutions at the Phoenix landing site, Mars. Geophys. Res. Lett. 2009, 36, L10202. 10.1029/2009GL037497. [DOI] [Google Scholar]
- Hanley J.; Chevrier V. F.; Berget D. J.; Adams R. D. Chlorate salts and solutions on Mars. Geophys. Res. Lett. 2012, 39, L08201. 10.1029/2012GL051239. [DOI] [Google Scholar]
- Altheide T.; Chevrier V.; Nicholson C.; Denson J. Experimental investigation of the stability and evaporation of sulfate and chloride brines on Mars. Earth Planet. Sci. Lett. 2009, 282, 69–78. 10.1016/j.epsl.2009.03.002. [DOI] [Google Scholar]
- Toner J. D.; Catling D. C.; Light B. A revised Pitzer model for low temperature soluble salt assemblages at Pheonix site, Mars. Geochim. Cosmochim. Acta 2015, 166, 327–343. 10.1016/j.gca.2015.06.011. [DOI] [Google Scholar]
- Toner J. D.; Catling D. C.; Light B. Modelling salt precipitation from brines on Mars: Evaporation versus freezing origins of soil salts. Icarus 2015, 250, 451–461. 10.1016/j.icarus.2014.12.013. [DOI] [Google Scholar]
- Marion G. M.; Catling D. C.; Zahnle K. J.; Claire M. W. Modelling aqueous perchlorate chemistries with applications to Mars. Icarus 2010, 207, 675–685. 10.1016/j.icarus.2009.12.003. [DOI] [Google Scholar]
- Martín-Torres F. J.; Zorzano M.-P.; Valentín-Serrano P.; Harri A.-M.; Genzer M.; Kemppinen O.; Rivera-Valentin E. G.; Jun I.; Wray J.; Bo Madsen M.; Goetz W.; McEwen A. S.; Hardgrove C.; Renno N.; Chevrier V. F.; Mischna M.; Navarro-González R.; Martínez-Frías J.; Conrad P.; McConnochie T.; Cockell C.; Berger G.; Vasavada A. R.; Sumner D.; Vaniman D. Transient liquid water and water activity at Gale crater on Mars. Nature 2015, 8, 357–361. 10.1038/ngeo2412. [DOI] [Google Scholar]
- Núñez J. I.; Barnouin O. S.; Murchie S. L.; Seelos F. P.; McGovern J. A.; Seelos K. D.; Buczkowski D. L. New Insights into gully formation on Mars: Constraints from composition as seen by MRO/CRISM. Geophys. Res. Lett. 2016, 43, 8893. 10.1002/2016GL068956. [DOI] [Google Scholar]
- Eydelman A.Temperature on the Surface of Mars, The Physics Factbook. 2001, https://hypertextbook.com/facts/2001/AlbertEydelman.shtml (accesssed January 12, 2016).
- Ge X.; Wang X. Calculations of Freezing Point Depression, Boiling Point Elevation, Vapor Pressure and Enthalpies of Vaporization of Electrolyte Solutions by a Modified Three-Characteristic Parameter Correlation Model. J. Solution Chem. 2009, 38, 1097–1117. 10.1007/s10953-009-9433-0. [DOI] [Google Scholar]
- Ge X.; Wang X.; Zhang M.; Seetharaman S. Correlation and Prediction of Activity and Osmotic Coefficients of Aqueous Electrolytes at 298.15 K by the Modified TCPC Model. J. Chem. Eng. Data 2007, 52, 538–547. 10.1021/je060451k. [DOI] [Google Scholar]
- Willey J. D. The Effect of Ionic Strength on the Solubility of an Electrolyte. J. Chem. Educ. 2004, 81, 1644. 10.1021/ed081p1644. [DOI] [Google Scholar]
- Rao B. K. S.; Laddha G. S. Rapid estimation of chlorate in presence of perchlorate. Fresenius’ Z. für Anal. Chem. 1959, 167, 410–415. 10.1007/bf00458633. [DOI] [Google Scholar]
- Marion G. M., Catlin D. C., Claire M., Zahnle K. J.. Modelling aqueous perchlorate chemistries with applications to mars. 40th Lunar and Planetary Science Conference, 2009; p P1959.
- Marion M. G., Grant S. A.. FREZCHEM: A Chemical-Thermodynamic Model for Aqueous solutions at Subzero temperatures. Special Report 94-18, 1994.
- Mars Facts, NASA, Retrieved June 20, 2013.
- Nikolakakos G.; Whiteway J. A. Laboratory Investigation of Perchlorate deliquescence at the surface of Mars with a Raman Scattering Lidar. Geophys. Res. Lett. 2015, 42, 7899–7906. 10.1002/2015GL065434. [DOI] [Google Scholar]
- Nuding D. L.; Rivera-Valentin E. G.; Davis R. D.; Gough R. V.; Chevrier V. F.; Tolbert M. A. Deliquescence and efflorescence of Calcium Perchlorate: An investigation of stable aqueous solution relevant to Mars. Icarus 2014, 243, 420–428. 10.1016/j.icarus.2014.08.036. [DOI] [Google Scholar]
- Van’t Hoff J. H. Die Rolle des osmotischen Druckes in der Analogie zwischen Lösungen und Gasen. Z. Phys. Chem. 1887, 1, 481–493. 10.1515/zpch-1887-0151. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.