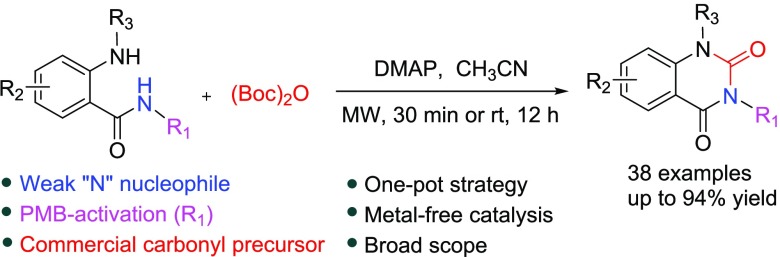
Abstract
The one-pot synthesis of quinazoline-2,4-diones was developed in the presence of 4-dimethylaminopyridine (DMAP) by metal-free catalysis. The commercially available (Boc)2O acted as a key precursor in the construction of the 2-position carbonyl of quinazolinediones. The p-methoxybenzyl (PMB)-activated heterocyclization could smoothly proceed at room temperature instead of the microwave condition. This strategy is compatible with a variety of substrates with different functional groups. Furthermore, this protocol was utilized to smoothly prepare Zenarestat with a total yield of 70%.
Introduction
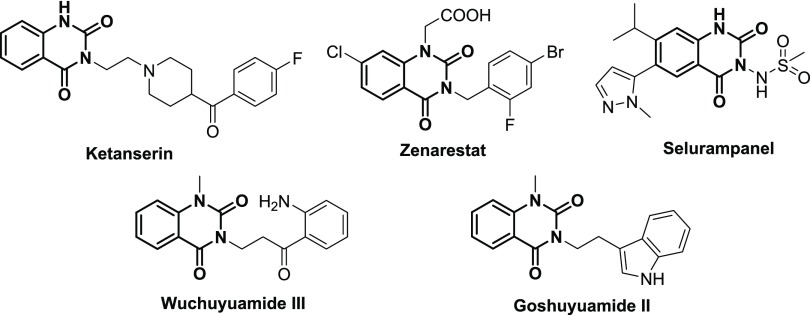
Quinazolinediones are an important class of nitrogen-containing heterocycles in medicinal chemistry with a broad range of bioactivities, such as antihypertensive,1,2 antidiabetic,3 antimicrobial,4,5 antimalarial,6,7 and anticancer activities.8−10 Structures shown in Figure 1 are representative examples of commercial drugs or biologically active compounds and natural products containing the quinazoline-2,4-dione core.11−15 Among them, Zenarestat is a therapeutic agent against diabetic neuropathy. Due to the importance of quinazoline-2,4-diones in medicinal chemistry, various synthetic methods have been reported to construct this core.16−31 The representative strategies for the construction of quinazoline-2,4-diones have been summarized in the introduction part of our previous article.32 The key precursor reagents such as urea, triphosgene, CO, CO2, dimethylformamide (DMF), 1,1′-carbonyldiimidazole (CDI), and KOCN are crucial to the introduction of the 2-position carbonyl group. However, there are limited methods available to construct quinazoline-2,4-diones with electron-withdrawing groups from 2-aminobenzamides.
Figure 1.
Representative examples of quinazoline-2,4-diones.
Di-tert-butyl dicarbonate [(Boc)2O] is widely used in organic synthesis to introduce the tert-butoxycarbonyl (Boc) protecting group into amines. In some cases, (Boc)2O is also used to form ureas in the presence of 4-dimethylaminopyridine (DMAP).33 DMAP, as a well-known superacylation catalyst, is used to address difficult acylations.34 The combined use of (Boc)2O and DMAP may be a good option to embed the carbonyl group in the quinazolinediones.
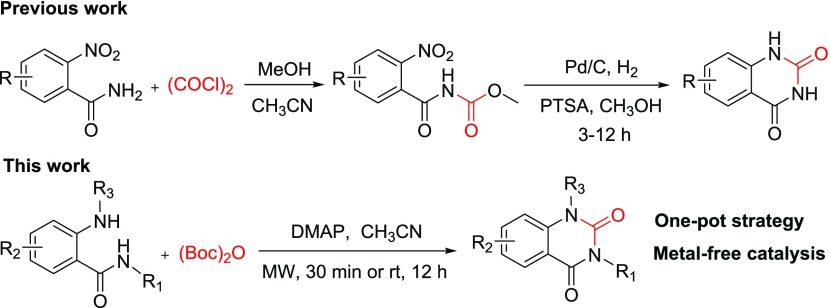
Recently, our group developed a p-toluenesulfonic acid (PTSA)-catalyzed hydrogenation condensation strategy to synthesize various quinazoline-2,4(1H,3H)-diones using oxalyl chloride as the key precursor reagent (Scheme 1). This two-step approach has several advantages, such as no further purification, mild reaction conditions, high yields, and low-toxicity reagents, but limited in substrate applicability.32 To further simplify the procedure and extend the substrate scope, we focus on the exploration of an appropriate 2-position carbonyl precursor to construct quinazoline-2,4-dione. Herein, we report on the development of the one-pot synthesis of quinazoline-2,4-diones from 2-aminobenzamides using (Boc)2O as the key precursor in the presence of DMAP (Scheme 1).
Scheme 1. Strategies for the Construction of Substituted Quinazoline-2,4-diones.
Results and Discussion
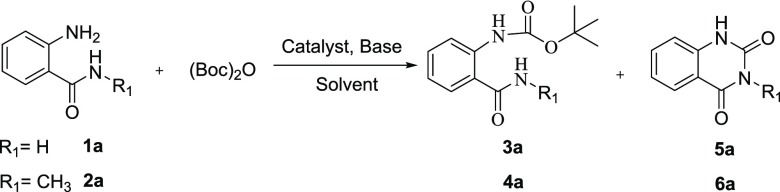
Initially, 2-aminobenzamide (1a) and 2-amino-N-methylbenzamide (2a) were chosen as the model substrates to explore the possible formation of quinazoline-2,4-diones using (Boc)2O as the carbonyl donor. The results are summarized in Table 1. The first reaction from substrate 1a was attempted at room temperature under the Boc protecting condition in the solvent of CH2Cl2, with Et3N as the base and DMAP as the catalyst.
Table 1. Optimization of Reaction Conditionsa.
| yield (%) |
||||||
|---|---|---|---|---|---|---|
| entry | R1 | catalyst | base | solvent | 3a/4a | 5a/6a |
| 1b | H | DMAP | Et3N | CH2Cl2 | 9 | 33 |
| 2b | H | Et3N | CH2Cl2 | 9 | 13 | |
| 3b | H | DMAP | CH2Cl2 | 79 | ||
| 4b | H | CH2Cl2 | 10 | 47 | ||
| 5b | H | TBD | CH2Cl2 | NRe | ||
| 6b | H | DBU | CH2Cl2 | NRe | ||
| 7b | H | DABCO | CH2Cl2 | 36 | ||
| 8b | H | DMAP | THF | 58 | ||
| 9b | H | DMAP | DMF | 61 | ||
| 10b | H | DMAP | CH3CN | 94 | ||
| 11b | CH3 | DMAP | CH3CN | 46 | 46 | |
| 12c | CH3 | DMAP | CH3CN | 21 | 59 | |
| 13d | CH3 | DMAP | CH3CN | 92 | ||
All reactions were conducted using 1a/2a (1 mmol, 1.0 equiv), (Boc)2O (1.5 mmol, 1.5 equiv), catalyst (0.1 mmol, 0.1 equiv), solvent (3 mL), isolated yield.
The reaction was run at room temperature for 12 h.
The reaction was run at reflux for 12 h.
The reaction was run under the microwave (MW) condition for 30 min.
No reaction.
Inspiringly, the desired product 5a was obtained in 33% yield, although the side product 3a was obtained in 9% yield (Table 1, entry 1). The result indicated that it is feasible to construct the quinazoline-2,4-dione scaffold using (Boc)2O from 1a. It was found that the reaction yield decreased without the catalyst of DMAP (Table 1, entry 2), while it showed significant improvement without the additional base Et3N with a yield of 79% (Table 1, entry 3). The heterocyclization could not proceed completely in the absence of DMAP after 12 h (Table 1, entry 4). The replacement of DMAP with 1,5,7-triazabicyclo(4.4.0)dec-5-ene (TBD), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and 1,4-diazabicyclo[2.2.2]octane (DABCO) failed to obtain any improvement (Table 1, entries 5–7). Prompted by this result, optimal solvents were examined to afford more satisfactory results. Changing CH2Cl2 to other organic solvents such as tetrahydrofuran (THF) and DMF led to lower yields in the range 58–61% (Table 1, entries 8, 9 vs entry 3). To our delight, the result showed that CH3CN was the optimal solvent toward the formation of 5a in 94% yield (Table 1, entry 10).
However, when we used the protocol of entry 10 to treat substrate 2a, the heterocyclization could not transform completely to 6a even under the reflux condition for 12 h (Table 1, entries 11 and 12). Fortunately, the yield of 6a increased to 92% under the microwave (MW) condition, and the reaction time for complete conversion was shortened to 30 min (Table 1, entry 13). Consequently, we decided to use entries 13 and 10 as the optimal condition to investigate the scope and application of the reaction separately.
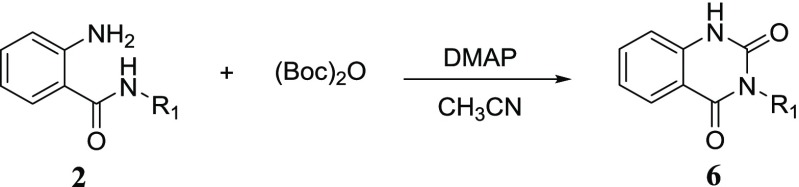
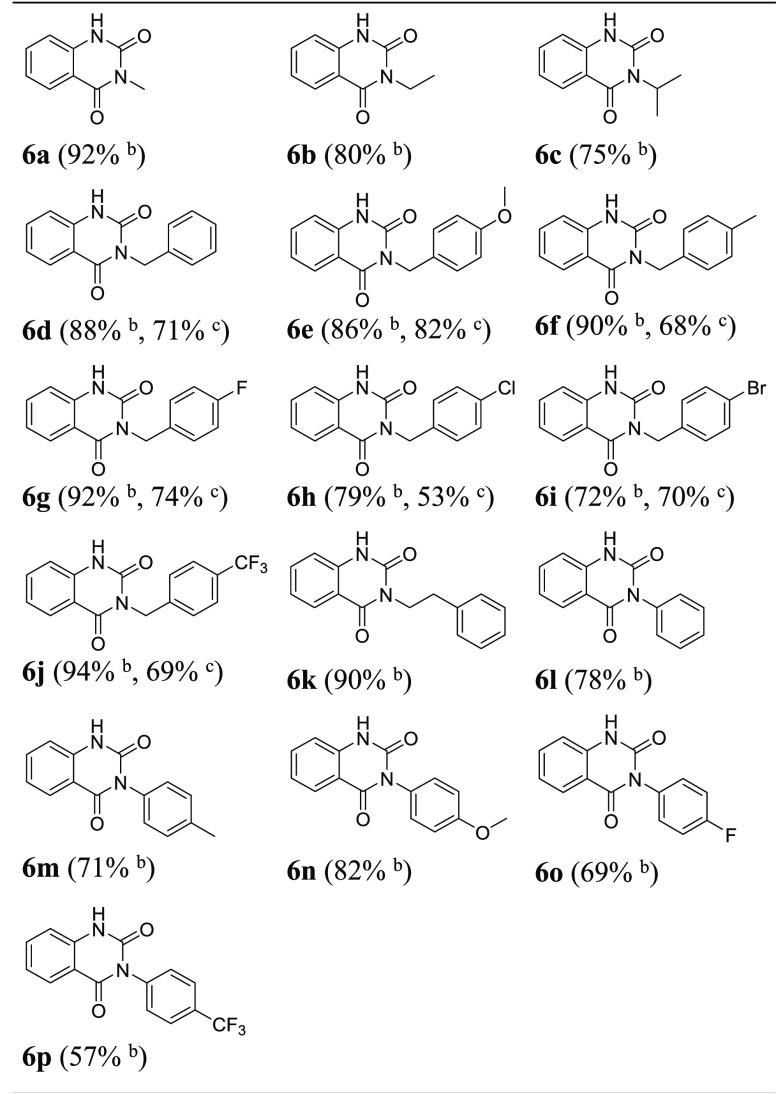
With the optimal conditions in hand, we then attempted to apply them to synthesize a series of 3-substituted quinazoline-2,4-diones. As shown in Table 2, the 2-amino-N-substituted benzamide substrates 2a–p smoothly underwent heterocyclization to afford 3-substituted quinazoline-2,4-diones 6a–p in good to excellent yields. We were pleased to find that the reaction was tolerant to alkyl groups (6a–c, 6k), benzyl groups (6d–j), and aryl groups (6l–p) at the 3-position of quinazoline-2,4-dione. When the R1 were electron-donating groups such as alkyl and benzyl, the target products were obtained in excellent yield up to 90%, especially for 6a, 6f, 6g, 6j, and 6k. It is noticed that substrates with aryl substituents gave moderate to good yields ranging from 57 to 82% under MW conditions (6l–p). Among them, the p-methoxyphenyl group had a strong positive effect on this transformation in 82% yield (6n). Based on the capability of the benzyl group to facilitate heterocyclization under MW conditions, we further investigated the outcome when the reaction was run at room temperature. We were pleased to see that the target N-benzyl substituted quinazolinediones were obtained in moderate to good yields ranging from 53 to 82% at room temperature (6d–j).
Table 2. Synthesis of 3-Substituted Quinazoline-2,4-diones 6a–pa.
All reactions were conducted using 2a–p (1 mmol, 1.0 equiv), (Boc)2O (1.5 mmol, 1.5 equiv), DMAP (0.1 mmol, 0.1 equiv), isolated yield.
The reaction was run in 3 mL of CH3CN under the MW condition for 30 min.
The reaction was run in 10 mL of CH3CN at room temperature for 12 h.
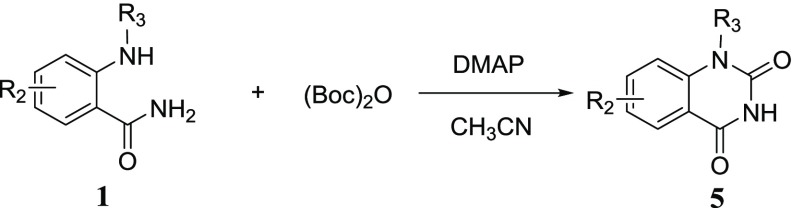
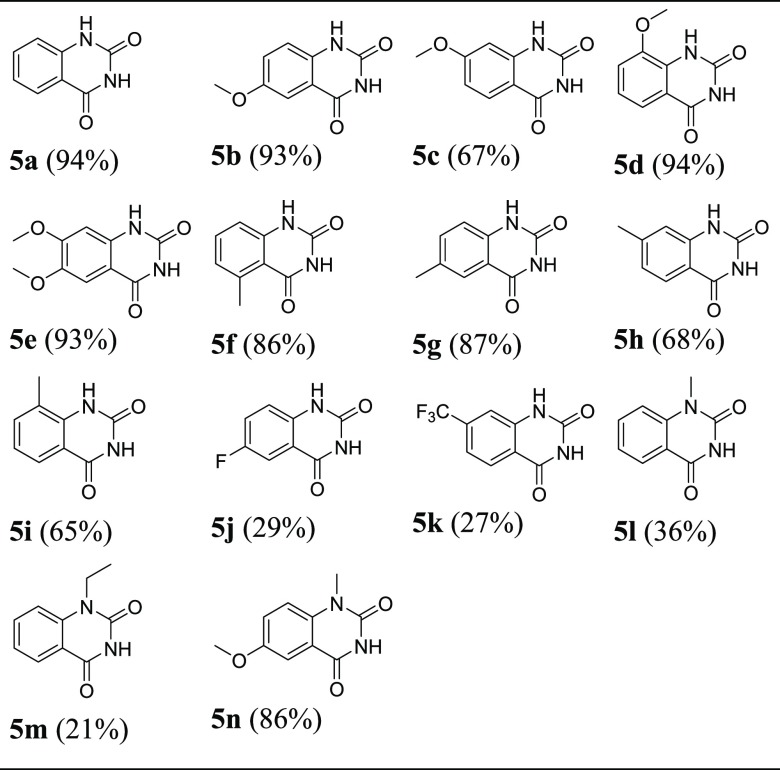
To investigate the substrate profile of this reaction, various substituted 2-aminobenzamide substrates with electron-donating and electron-withdrawing substituents on the phenyl ring were employed to react with (Boc)2O under the optimized conditions. It was indicated that the substrates containing electron-donating groups such as methoxyl and methyl generally gave higher yields (5b–i in Table 3), while the substrates containing an electron-deficient group like fluorine and trifluoromethyl substituents gave very low yields (5j, 5k in Table 3). Moreover, the para-substituted amino group with methoxyl or methyl, which improves the nucleophilicity of the amino group, gave higher yields (5b, 5e, 5g in Table 3). Additionally, the 1-substituted quinazoline-2,4-diones could also be constructed in the current reaction system but in very low yields (5l, 5m in Table 3). The possible reason may be the steric hindrance effect, as the compound 5m with the ethyl group gave a lower yield compared to 5l with the methyl group. It demonstrated that the electron-donating group on the phenyl ring was a crucial factor to facilitate the heterocyclization exemplified by 5n vs 5l with the yields of 86% and 36%, respectively.
Table 3. Synthesis of Quinazolin-2,4-diones Derivative 5a–na.
All reactions were conducted using 1a–n (1 mmol, 1.0 equiv), (Boc)2O (1.5 mmol, 1.5 equiv), DMAP (0.1 mmol, 0.1 equiv), CH3CN (3.0 mL), under the MW condition for 30 min, isolated yield.
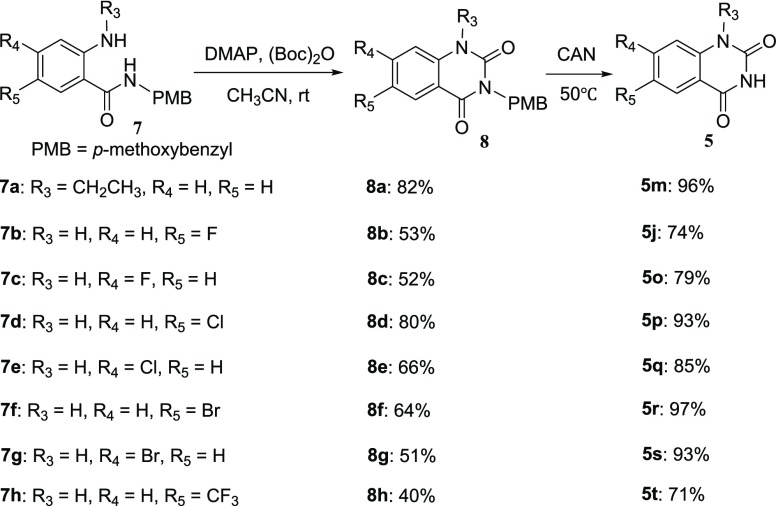
Subsequently, we focused on addressing how to improve the yield of substrates with various electron-deficient groups. Inspired by the benzyl group to activate the reaction based on the results in Table 2, a series of substrates containing the N-p-methoxybenzyl (PMB) (N-PMB) group with various electron-deficient substitutes at different positions were investigated. We were pleased to find that PMB-activated products 8 were obtained with obviously improved yields even at room temperature compared to the corresponding nonactivated substrates under MW conditions (8a/8b in Scheme 2 vs yields for 5m/5j in Table 3). Furthermore, the PMB-activated heterocyclization could smoothly occur at room temperature in moderate to good yields (8a–h in Scheme 2). The quinazoline-2,4-diones (5m, 5j, and 5o–t) were afforded through the N-PMB deprotection of 8 in the presence of ceric ammonium nitrate (CAN) in good to excellent yields. The results proved that this method is noteworthy for its utility in preparing quinazoline-2,4-diones with various functional groups at different positions in high efficiency.
Scheme 2. PMB-Activated Substituted Quinazoline-2,4-dione Formation.
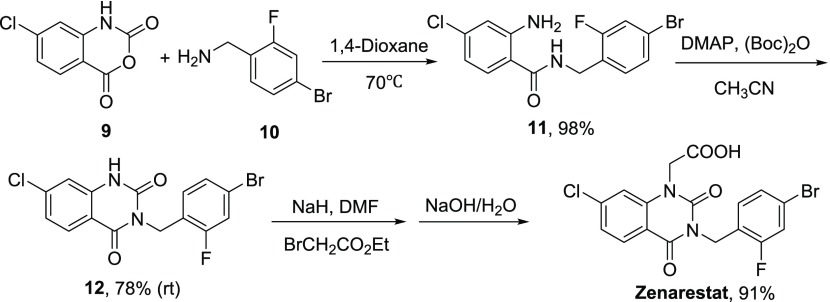
Zenarestat (Figure 1), an aldose reductase inhibitor, attracted our attention due to its particular structure containing the N-substituted benzyl group. To validate the methodology, we synthesized the Zenarestat by the optimized reaction condition. A convenient synthetic route is shown in Scheme 3. First, the substituted 2-aminobenzamide 11 was obtained from commercially available starting materials 9 and 10 in 98% yield. Then, the heterocyclization could transform from 11 to 12 at room temperature for 12 h in 78% yield without column chromatographical purification. Zenarestat was afforded from the key intermediate 12 through substitution and hydrolysis in good yield. Notably, this novel method not only displayed high efficiency with total yield of 70% but also avoided regioselective alkylation of quinazolinedione disclosed in the literature.35
Scheme 3. Synthetic Strategy of Zenarestat by the Optimized Reaction Condition.
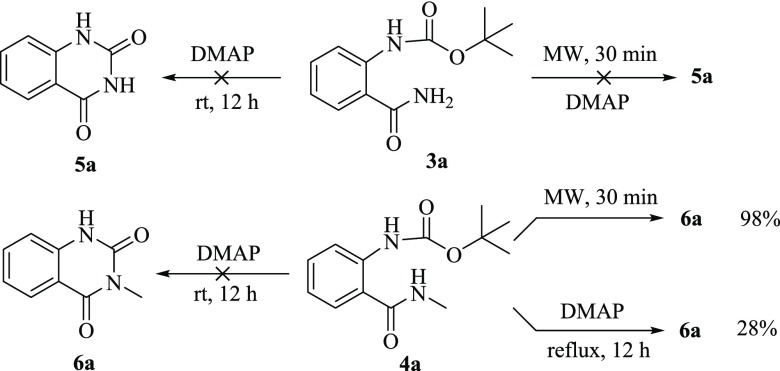
To elucidate the possible pathway of this heterocyclization, we first investigated the transformation of compounds 3a and 4a. However, compound 3a did not undergo cyclization to form 5a at room temperature or MW conditions in the presence of DMAP as speculated (Scheme 4). Meanwhile, compound 4a could smoothly convert to the corresponding quinazolinedione 6a in the absence of DMAP under the microwave condition for 30 min. It indicated that the alkyl-substituted amido group contributed to the cyclization.
Scheme 4. Exploration of the Possible Pathway.
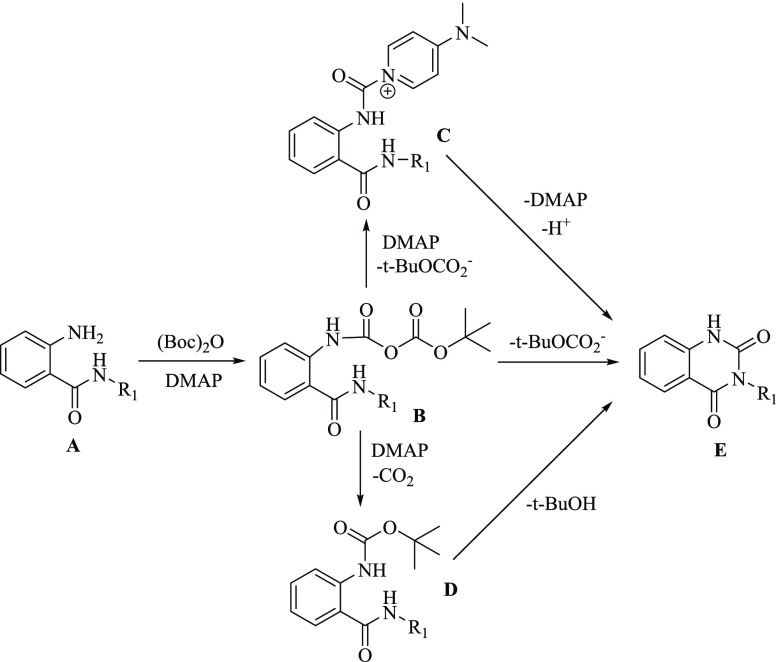
For the formation of the target product quinazoline-2,4-diones E, two reaction pathways are proposed according to the reactions of amines with (Boc)2O–DMAP via the unstable carbamic–carbonic anhydride intermediate B(34) (Scheme 5). One way is the intramolecular ammonolysis from B to E. The other way is the formation of the active intermediate C catalyzed by DMAP, followed by the intramolecular attack to afford E. Based on the above result in Scheme 4, the third pathway from D to E was suggested. Although the reaction could not occur from D to E at room temperature due to the weak nucleophilicity of benzamide and poor reactivity of bulky ester, it took place smoothly under the MW condition (R1 = CH3) to form E (Scheme 4). This could be the possible reason why substrates under the MW condition generally gave higher yields.
Scheme 5. Proposed Pathway for the Synthesis of Quinazolinediones with (Boc)2O–DMAP.
Conclusions
In summary, we have developed a novel metal-free catalysis approach to construct substituted quinazoline-2,4-diones, starting from 2-aminobenzamides and (Boc)2O, based on DMAP-catalyzed heterocyclization in good to excellent yields. In particular, the PMB-activated strategy could broaden the reaction scope and offer an additional route to the formation of quinazolinediones under mild conditions. The reaction is applicable to a wide range of substrates with various functional groups at different positions, and most desired products can be obtained without requiring column chromatographical purification. The high efficiency and simple manipulation render this one-pot reaction an attractive method for the synthesis of quinazolinediones.
Experimental Section
General
The reagents and solvents were obtained from commercial suppliers and used without further purification. 1H and 13C NMR spectra were recorded on a Varian 400 or 500 MHz NMR spectrometer with dimethyl sulfoxide (DMSO)-d6 as a solvent. Chemical shifts are referenced to the residual solvent peak and reported in parts per million (ppm) (δ scale), and all coupling constant (J) values are given in hertz. Electrospray ionization-high-resolution mass spectrometry (ESI-HRMS) data were measured on a Thermo Exactive Orbitrap plus spectrometer. Melting points were determined on a Yanaco MP-J3 microscope melting point apparatus. All microwave reactions were carried out in single-mode CEM Explorer SP 48. Substrates 1, 2, and 11 were prepared according to the reported procedures.36−40
General Procedure for the Formation of 2-Aminobenzamides 7a–h
To a stirred suspension of isatoic anhydride (1.0 equiv) in dioxane was added 4-methoxybenzylamine (1.5 equiv) at room temperature. The mixture was then warmed up to 100 °C and stirred for 2 h. The reaction mixture was evaporated in vacuo and then purified by column chromatography (petroleum ether (PE)/ethyl acetate (EA) = 100:80) to give the desired product.
General Procedure A for the Formation of Quinazoline-2,4-diones (5a–n, 6a–p)
A 10 mL sealed tube was charged with 2-aminobenzamide (1.0 mmol), (Boc)2O (1.5 mmol), and DMAP (0.1 mmol) in CH3CN (3 mL). The reaction mixture was heated at 150 °C in MW at 150 W, 10 psi for 30 min. After cooling to room temperature, the mixture was filtrated. The obtained solid was washed with 3 mL of CH3CN and dried to give the desired product.
General Procedure B for the Formation of Quinazoline-2,4-diones (6d–j, 8a–h, 12)
To a solution of 2-aminobenzamide (1.0 mmol) in CH3CN (10 mL) were added (Boc)2O (1.5 mmol) and DMAP (0.1 mmol). The reaction mixture was stirred at room temperature for 12 h and then filtrated. The obtained solid was washed with 3 mL of CH3CN and dried to give the desired product.
General Procedure C for Deprotection of PMB (5m, 5j, 5o–t)
To a solution of obtained 8 (1.0 equiv) in CH3CN was added ceric ammonium nitrate (CAN) (4.0 equiv). The reaction mixture was heated at 50 °C for 2–3 h. After cooling to room temperature, the mixture was filtrated. The obtained solid was washed with 3 mL of CH3CN and dried to give the desired product.
Quinazoline-2,4(1H,3H)-dione (5a)
A white solid (152 mg, 94% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.24 (brs, 2H), 7.92 (d, J = 8.0 Hz, 1H), 7.66 (t, J = 8.0 Hz, 1H), 7.22–7.19 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 163.0, 150.4, 141.0, 135.0, 127.1, 122.4, 115.4, 114.5; ESI-HRMS m/z calcd for C8H7N2O2 [M + H]+ 163.0502, found: 163.0499.
6-Methoxyquinazoline-2,4(1H,3H)-dione (5b)
A white solid (178 mg, 93% yield, general procedure A), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.25 (brs, 1H), 11.00 (brs, 1H), 7.33 (s, 1H), 7.28 (dd, J = 9.0, 2.5 Hz, 1H), 7.12 (d, J = 9.0 Hz, 1H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.8, 154.7, 150.1, 135.1, 123.9, 117.0, 114.9, 108.1, 55.6; ESI-HRMS m/z calcd for C9H9N2O3 [M + H]+ 193.0608, found: 193.0615.
7-Methoxyquinazoline-2,4(1H,3H)-dione (5c)
A white solid (129 mg, 67% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.10 (brs, 1H), 11.02 (brs, 1H), 7.80 (d, J = 8.8 Hz, 1H), 6.76 (dd, J = 8.8, 2.4 Hz, 1H), 6.62 (d, J = 2.4 Hz, 1H), 3.82 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 164.4, 162.4, 150.6, 142.9, 128.9, 110.6, 107.8, 98.4, 55.7; ESI-HRMS m/z calcd for C9H9N2O3 [M + H]+ 193.0608, found: 193.0613.
8-Methoxyquinazoline-2,4(1H,3H)-dione (5d)
A white solid (180 mg, 94% yield, general procedure A), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.28 (brs, 1H), 10.49 (brs, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.13 (t, J = 8.0 Hz, 1H), 3.88 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.8, 150.1, 146.3, 131.1, 122.3, 118.0, 115.5, 115.1, 56.3; ESI-HRMS m/z calcd for C9H9N2O3 [M + H]+ 193.0608, found: 193.0615.
6,7-Dimethoxyquinazoline-2,4(1H,3H)-dione (5e)
A white solid (207 mg, 93% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.10 (brs, 1H), 10.92 (brs, 1H), 7.26 (s, 1H), 6.68 (s, 1H), 3.83 (s, 3H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.5, 155.0, 150.5, 145.1, 136.6, 107.2, 106.3, 97.8, 55.9, 55.8; ESI-HRMS m/z calcd for C10H11N2O4 [M + H]+ 223.0713, found: 223.0708.
5-Methylquinazoline-2,4(1H,3H)-dione (5f)
A white solid (151 mg, 86% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.06 (brs, 1H), 11.01 (brs, 1H), 7.45 (t, J = 8.0 Hz, 1H), 7.02 (d, J = 8.0 Hz, 1H), 6.94 (d, J = 7.2 Hz, 1H), 2.65 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 163.7, 150.1, 142.2, 141.0, 133.8, 125.2, 113.5, 112.6, 22.2; ESI-HRMS m/z calcd for C9H9N2O2 [M + H]+ 177.0659, found: 177.0662.
6-Methylquinazoline-2,4(1H,3H)-dione (5g)
A white solid (153 mg, 87% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.20 (brs, 1H), 11.05 (brs, 1H), 7.69 (dd, J = 1.6, 0.8 Hz, 1H), 7.47–7.45 (m, 1H), 7.08 (d, J = 8.4 Hz, 1H), 2.32 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.9, 150.4, 138.8, 136.0, 131.6, 126.5, 115.3, 114.2, 20.3; ESI-HRMS m/z calcd for C9H9N2O2 [M + H]+ 177.0659, found: 177.0664.
7-Methylquinazoline-2,4(1H,3H)-dione (5h)
A white solid (120 mg, 68% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.12 (brs, 2H), 7.77 (s, 1H), 7.00–6.95 (m, 2H), 2.36 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.8, 150.5, 145.6, 141.0, 127.0, 123.7, 115.1, 112.1, 21.5; ESI-HRMS m/z calcd for C9H9N2O2 [M + H]+ 177.0659, found: 177.0659.
8-Methylquinazoline-2,4(1H,3H)-dione (5i)
A white solid (114 mg, 65% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.33 (brs, 1H), 10.39 (brs, 1H), 7.78–7.76 (m, 1H), 7.49–7.47 (m, 1H), 7.09 (t, J = 7.6 Hz, 1H), 2.34 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 163.0, 150.6, 139.3, 136.1, 124.8, 124.2, 122.1, 114.6, 17.3; ESI-HRMS m/z calcd for C9H9N2O2 [M + H]+ 177.0659, found: 177.0663.
6-Fluoroquinazoline-2,4(1H,3H)-dione (5j)
A white solid (52 mg, 29% yield, general procedure A; 71 mg, 74% yield, general procedure C), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.40 (brs, 1H), 11.19 (brs, 1H), 7.60–7.55 (m, 1H), 7.55–7.51 (m, 1H), 7.22–7.17 (m, 1H); 13C NMR (100 MHz, DMSO-d6) δ 162.1, 157.3 (d, JF,C = 238 Hz), 150.0, 137.5, 122.9 (d, JF,C = 25 Hz), 117.6, 115.4, 112.0 (d, JF,C = 24 Hz); ESI-HRMS m/z calcd for C8H6FN2O2 [M + H]+ 181.0408, found: 181.0404.
7-(Trifluoromethyl)quinazoline-2,4(1H,3H)-dione (5k)
A white solid (63 mg, 27% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.54 (brs, 1H), 11.40 (brs, 1H), 8.09 (d, J = 8.4 Hz, 1H), 7.49 (dd, J = 8.4, 1.6 Hz, 1H), 7.46 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 162.1, 150.2, 141.3, 134.2 (q, JF,C = 32 Hz), 128.8, 123.4 (q, JF,C = 271 Hz), 118.4, 117.5, 112.3; ESI-HRMS m/z calcd for C9H6F3N2O2 [M + H]+ 231.0371, found: 231.0376.
1-Methylquinazoline-2,4(1H,3H)-dione (5l)
A white solid (64 mg, 36% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.54 (brs, 1H), 8.0 (dd, J = 8.0, 1.6 Hz, 1H), 7.79–7.74 (m, 1H), 7.42 (d, J = 8.4 Hz, 1H), 7.30–7.26 (m, 1H), 3.45 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 161.9, 150.3, 141.7, 135.3, 127.4, 122.5, 115.6, 114.7, 29.5; ESI-HRMS m/z calcd for C9H9N2O2 [M + H]+ 177.0659, found: 177.0659.
1-Ethylquinazoline-2,4(1H,3H)-dione (5m)
A white solid (39 mg, 21% yield, general procedure A; 149 mg, 96% yield, general procedure C), mp 219–221 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.53 (brs, 1H), 8.02 (d, J = 6.5 Hz, 1H), 7.75 (d, J = 7.0 Hz, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.27 (t, J = 7.0 Hz, 1H), 4.08 (d, J = 6.5 Hz, 2H), 1.18 (t, J = 6.9 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 161.8, 149.8, 140.6, 135.4, 127.7, 122.4, 115.8, 114.5, 37.0, 12.4; ESI-HRMS m/z calcd for C10H11N2O2 [M + H]+ 191.0815, found: 191.0812.
6-Methoxy-1-methylquinazoline-2,4(1H,3H)-dione (5n)
A white solid (178 mg, 86% yield, general procedure A), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.51 (brs, 1H), 7.45 (s, 1H), 7.38 (s, 2H), 3.82 (s, 3H), 3.43 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 161.7, 154.7, 150.1, 135.9, 123.3, 116.5, 116.4, 109.1, 55.7, 29.6; ESI-HRMS m/z calcd for C10H11N2O3 [M + H]+ 207.0764, found: 207.0761.
7-Fluoroquinazoline-2,4(1H,3H)-dione (5o)
A white solid (75 mg, 79% yield, general procedure C), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.34 (brs, 1H), 11.24 (brs, 1H), 7.97–7.89 (m, 1H), 7.04–6.96 (m, 1H), 6.91–6.83 (m, 1H); 13C NMR (100 MHz, DMSO-d6) δ 165.8 (d, JF,C = 249 Hz), 162.0, 150.3, 142.9 (d, JF,C = 13 Hz), 130.2 (d, JF,C = 11 Hz), 111.3, 110.3 (d, JF,C = 23 Hz), 101.5 (d, JF,C = 26 Hz); ESI-HRMS m/z calcd for C8H6FN2O2 [M + H]+ 181.0408, found: 181.0406.
6-Chloroquinazoline-2,4(1H,3H)-dione (5p)
A white solid (145 mg, 93% yield, general procedure C), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.43 (brs, 1H), 11.27 (brs, 1H), 7.81 (t, J = 2.2 Hz, 1H), 7.70–7.64 (m, 1H), 7.18 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ 161.8, 150.0, 139.7, 134.8, 126.3, 125.9, 117.5, 115.8; ESI-HRMS m/z calcd for C8H6ClN2O2 [M + H]+ 197.0112, found: 197.0109.
7-Chloroquinazoline-2,4(1H,3H)-dione (5q)
A white solid (110 mg, 85% yield, general procedure C), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.39 (brs, 1H), 11.23 (brs, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.21 (d, J = 8.4 Hz, 1H), 7.17 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 162.1, 150.2, 141.9, 139.3, 129.0, 122.5, 114.7, 113.3; ESI-HRMS m/z calcd for C8H6ClN2O2 [M + H]+ 197.0112, found: 197.0113.
6-Bromoquinazoline-2,4(1H,3H)-dione (5r)
A white solid (149 mg, 97% yield, general procedure C), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.43 (brs, 1H), 11.26 (brs, 1H), 7.93 (s, 1H), 7.78 (d, J = 8.0 Hz, 1H), 7.11 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ 161.7, 150.0, 140.1, 137.5, 128.9, 117.8, 116.2, 113.8; ESI-HRMS m/z calcd for C8H6BrN2O2 [M + H]+ 240.9607, found: 240.9602.
7-Bromoquinazoline-2,4(1H,3H)-dione (5s)
A white solid (114 mg, 93% yield, general procedure C), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.39 (brs, 1H), 11.22 (brs, 1H), 7.79 (d, J = 8.0 Hz, 1H), 7.36–7.33 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.2, 150.1, 142.0, 129.0, 128.2, 125.3, 117.6, 113.6; ESI-HRMS m/z calcd for C8H6BrN2O2 [M + H]+ 240.9607, found: 240.9602.
6-(Trifluoromethyl)quinazoline-2,4(1H,3H)-dione (5t)
Following general procedure C, 5t was purified by column chromatography (CH2Cl2/CH3OH = 100:1) to afford a white solid (65 mg, 71% yield), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.55 (brs, 1H), 11.37 (brs, 1H), 8.07 (d, J = 8.0 Hz, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.44 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 162.0, 150.1, 141.2, 134.2 (q, JF,C = 32 Hz), 128.7, 123.4 (q, JF,C = 272 Hz), 118.3, 117.4, 112.2; ESI-HRMS m/z calcd for C9H6F3N2O2 [M + H]+ 231.0376, found: 231.0376.
3-Methylquinazoline-2,4(1H,3H)-dione (6a)
A white solid (162 mg, 92% yield, general procedure A), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.43 (brs, 1H), 7.93 (d, J = 7.0 Hz, 1H), 7.64 (d, J = 7.0 Hz, 1H), 7.19 (dd, J = 13.5, 7.5 Hz, 2H), 3.26 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.2, 150.4, 139.4, 134.9, 127.3, 122.5, 115.1, 113.8, 27.1; ESI-HRMS m/z calcd for C9H9N2O2 [M + H]+ 177.0659, found: 177.0659.
3-Ethylquinazoline-2,4(1H,3H)-dione (6b)
A white solid (153 mg, 80% yield, general procedure A), mp 202–204 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.40 (brs, 1H), 7.93 (d, J = 6.5 Hz, 1H), 7.65 (s, 1H), 7.20–7.17 (m, 2H), 3.90–3.95 (m, 2H), 1.15 (t, J = 6.9 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 161.7, 150. 0, 139.4, 134.9, 127.4, 122.5, 115.1, 113.9, 35.1, 13.0; ESI-HRMS m/z calcd for C10H11N2O2 [M + H]+ 191.0815, found: 191.0813.
3-Isopropylquinazoline-2,4(1H,3H)-dione (6c)
A white solid (153 mg, 75% yield, general procedure A), mp 97–98 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.27 (brs, 1H), 7.92 (d, J = 8.0 Hz, 1H), 7.63 (t, J = 7.5 Hz, 1H), 7.20–7.14 (m, 2H), 5.17–5.12 (m, 1H), 1.44 (s, 3H), 1.43 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.3, 150.2, 139.5, 134.9, 127.5, 122.4, 114.9, 114.3, 44.4, 19.3; ESI-HRMS m/z calcd for C11H13N2O2 [M + H]+ 205.0972, found: 205.0970.
3-Benzylquinazoline-2,4(1H,3H)-dione (6d)
A white solid (223 mg, 88% yield, general procedure A; 179 mg, 71% yield, general procedure B), mp 230–231 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.53 (brs, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.70–7.65 (m, 1H), 7.32–7.30 (m, 4H), 7.25–7.19 (m, 3H), 5.09 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.1, 150.3, 139.5, 137.5, 135.3, 128.4, 127.6, 127.2, 122.7, 115.3, 113.8, 43.2; ESI-HRMS m/z calcd for C15H13N2O2 [M + H]+ 253.0972, found: 253.0978.
3-(4-Methoxybenzyl)quinazoline-2,4(1H,3H)-dione (6e)
A white solid (243 mg, 86% yield, general procedure A; 232 mg, 82% yield, general procedure B), mp 224–225 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.49 (brs, 1H), 7.94 (d, J = 7.5 Hz, 1H), 7.66 (t, J = 7.5 Hz, 1H), 7.28 (d, J = 8.5 Hz, 2H), 7.21 (dd, J = 12.5, 8.0 Hz, 2H), 6.86 (d, J = 8.5 Hz, 2H), 5.02 (s, 2H), 3.71 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.0, 158.5, 150.3, 139.5, 135.2, 129.5, 129.4, 127.5, 122.7, 115.3, 113.8, 55.1, 42.6; ESI-HRMS m/z calcd for C16H15N2O3 [M + H]+ 283.1077, found: 283.1086.
3-(4-Methylbenzyl)quinazoline-2,4(1H,3H)-dione (6f)
A white solid (240 mg, 90% yield, general procedure A; 182 mg, 68% yield, general procedure B), mp 240–242 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.51 (brs, 1H), 7.94 (dd, J = 8.8, 1.6 Hz, 1H), 7.69–7.65 (m, 1H), 7.23–7.19 (m, 4H), 7.11 (d, J = 8.0 Hz, 2H), 5.05 (s, 2H), 2.26 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.0, 150.3, 139.5, 136.3, 135.2, 134.5, 130.8, 128.9, 127.7, 127.5, 115.3, 113.8, 43.0, 20.7; ESI-HRMS m/z calcd for C16H15N2O2 [M + H]+ 267.1128, found: 267.1134.
3-(4-Fluorobenzyl)quinazoline-2,4(1H,3H)-dione (6g)
A white solid (248 mg, 92% yield, general procedure A; 200 mg, 74% yield, general procedure B), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.54 (brs, 1H), 7.95 (d, J = 7.5 Hz, 1H), 7.66 (t, J = 7.5 Hz, 1H), 7.40 (dd, J = 8.0, 6.0 Hz, 2H), 7.20 (t, J = 7.5 Hz, 2H), 7.14 (t, J = 9.0 Hz, 2H), 5.08 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.0, 161.4 (d, JF,C = 242 Hz), 150.3, 139.5, 135.3, 133.7, 129.9, 127.5, 122.7, 115.3, 115.2 (d, JF,C = 26 Hz), 113.8, 42.6; ESI-HRMS m/z calcd for C15H12FN2O2 [M + H]+ 271.0877, found: 271.0883.
3-(4-Chlorobenzyl)quinazoline-2,4(1H,3H)-dione (6h)
A white solid (226 mg, 79% yield, general procedure A; 151 mg, 53% yield, general procedure B), mp 245–247 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.55 (brs, 1H), 7.95 (dd, J = 8.0, 1.6 Hz, 1H), 7.70–7.66 (m, 1H), 7.39–7.33 (m, 4H), 7.28–7.15 (m, 2H), 5.07 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.0, 150.2, 139.5, 136.5, 135.3, 131.8, 129.6, 128.4, 127.5, 122.7, 115.3, 113.7, 42.7; ESI-HRMS m/z calcd for C15H12ClN2O2 [M + H]+ 287.0582, found: 287.0570.
3-(4-Bromobenzyl)quinazoline-2,4(1H,3H)-dione (6i)
A white solid (239 mg, 72% yield, general procedure A; 232 mg, 70% yield, general procedure B), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.56 (brs, 1H), 7.95 (dd, J = 8.8, 1.2 Hz, 1H), 7.70–7.66 (m, 1H), 7.52–7.49 (m, 2H), 7.31–7.27 (m, 2H), 7.22 (t, J = 8.0 Hz, 2H), 5.06 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.0, 150.2, 139.5, 136.9, 135.3, 131.3, 129.9, 127.5, 122.7, 120.3, 115.3, 113.7, 42.7; ESI-HRMS m/z calcd for C15H12BrN2O2 [M + H]+ 331.0077, found: 331.0071.
3-(4-(Trifluoromethyl)benzyl)quinazoline-2,4(1H,3H)-dione (6j)
A white solid (300 mg, 94% yield, general procedure A; 221 mg, 69% yield, general procedure B), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.60 (brs, 1H), 7.96 (d, J = 8.0 Hz, 1H), 7.71–7.66 (m, 3H), 7.54 (d, J = 8.0 Hz, 2H), 7.25–7.23 (m, 2H), 5.19 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.1, 150.3, 142.2, 139.6, 135.3, 128.2, 127.8 (q, JF,C = 32 Hz), 127.5, 125.3, 124.3 (q, JF,C = 270 Hz), 122.8, 115.4, 113.7, 43.0; ESI-HRMS m/z calcd for C16H12F3N2O2 [M + H]+ 321.0845, found: 321.0852.
3-Phenethylquinazoline-2,4(1H,3H)-dione (6k)
A white solid (239 mg, 90% yield, general procedure A), mp 219–221 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.44 (brs, 1H), 7.94 (dd, J = 8.0, 1.6 Hz, 1H), 7.68–7.64 (m, 1H), 7.32–7.29 (m, 2H), 7.25–7.19 (m, 5H), 4.13–4.09 (m, 2H), 2.90–2.86 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.8, 150.1, 139.5, 138.7, 135.0, 128.7, 128.5, 127.4, 126.4, 122.5, 115.2, 113.8, 41.3, 33.4; ESI-HRMS m/z calcd for C16H15N2O2 [M + H]+ 267.1128, found: 267.1121.
3-Phenylquinazoline-2,4(1H,3H)-dione (6l)
A white solid (185 mg, 78% yield, general procedure A), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.54 (brs, 1H), 7.94 (d, J = 7.5 Hz, 1H), 7.70 (t, J = 7.5 Hz, 1H), 7.48 (t, J = 7.5 Hz, 2H), 7.42 (t, J = 7.5 Hz, 1H), 7.32 (d, J = 7.0 Hz, 2H), 7.23 (t, J = 8.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.3, 150.3, 139.9, 135.8, 135.3, 129.2, 128.9, 128.2, 127.7, 122.6, 115.3, 114.4; ESI-HRMS m/z calcd for C14H11N2O2 [M + H]+ 239.0815, found: 239.0821.
3-(4-Methylphenyl)quinazoline-2,4(1H,3H)-dione (6m)
A white solid (178 mg, 71% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.51 (brs, 1H), 7.93 (dd, J = 8.4, 1.6 Hz, 1H), 7.71–7.67 (m, 1H), 7.29–7.26 (m, 2H), 7.24–7.21 (m, 2H), 7.20–7.16 (m, 2H), 2.37 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.3, 150.3, 139.9, 137.5, 135.2, 133.2, 129.4, 128.9, 127.7, 122.6, 115.3, 114.4, 20.8; ESI-HRMS m/z calcd for C15H13N2O2 [M + H]+ 253.0972, found: 253.0968.
3-(4-Methoxyphenyl)quinazoline-2,4(1H,3H)-dione (6n)
A white solid (219 mg, 82% yield, general procedure A), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.50 (brs, 1H), 7.93 (dd, J = 8.4, 1.6 Hz, 1H), 7.71–7.67 (m, 1H), 7.24–7.20 (m, 4H), 7.03–6.99 (m, 2H), 3.81 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 162.5, 158.9, 150.5, 139.9, 135.2, 130.1, 128.3, 127.7, 122.5, 115.2, 114.4, 114.1, 55.4; ESI-HRMS m/z calcd for C15H13N2O3 [M + H]+ 269.0921, found: 269.0919.
3-(4-Fluorophenyl)quinazoline-2,4(1H,3H)-dione (6o)
A white solid (177 mg, 69% yield, general procedure A), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.55 (brs, 1H), 7.94 (d, J = 8.0 Hz, 1H), 7.70 (t, J = 8.0 Hz, 1H), 7.40–7.37 (m, 2H), 7.31 (t, J = 9.0 Hz, 2H), 7.24–7.22 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.3, 161.6 (d, JF,C = 243 Hz), 150.3, 139.9, 135.3, 132.0, 131.3, 127.7, 122.6, 115.7 (d, JF,C = 22 Hz), 115.3, 114.4; ESI-HRMS m/z calcd for C14H10FN2O2 [M + H]+ 257.0721, found: 257.0715.
3-(4-(Trifluoromethyl)phenyl)quinazoline-2,4(1H,3H)-dione (6p)
A white solid (175 mg, 57% yield, general procedure A), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.64 (brs, 1H), 7.95 (d, J = 7.5 Hz, 1H), 7.88 (d, J = 8.0 Hz, 2H), 7.72 (t, J = 7.5 Hz, 1H), 7.62 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 7.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.2, 150.0, 140.0, 139.7, 135.4, 130.4, 128.8 (d, JF,C = 32 Hz), 127.7, 126.0, 124.6 (d, J = 271 Hz), 122.7, 115.4, 114.4; ESI-HRMS m/z calcd for C15H10F3N2O2 [M + H]+ 307.0689, found: 307.0682.
2-(Ethylamino)-N-(4-methoxybenzyl)benzamide (7a)
A white solid (1.00 g, 84% yield), mp 90–92 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.79 (brs, 1H), 7.74 (brs, 1H), 7.59 (d, J = 7.5 Hz, 1H), 7.27 (d, J = 7.5 Hz, 1H), 7.23 (d, J = 8.5 Hz, 2H), 6.88 (d, J = 8.0 Hz, 2H), 6.65 (d, J = 8.5 Hz, 1H), 6.54 (t, J = 7.5 Hz, 1H), 4.35 (d, J = 5.5 Hz, 2H), 3.72 (s, 3H), 3.13–3.08 (m, 2H), 1.18 (t, J = 7.0 Hz, 3H); ESI-HRMS m/z calcd for C17H21N2O2 [M + H]+ 285.1598, found: 285.1590.
2-Amino-5-fluoro-N-(4-methoxybenzyl)benzamide (7b)
A white solid (1.20 g, 88% yield), mp 114–116 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.77 (t, J = 8.0 Hz, 1H), 7.38 (dd, J = 10.4, 3.2 Hz, 1H), 7.23 (d, J = 8.8 Hz, 2H), 7.07–7.02 (m, 1H), 6.88 (d, J = 8.8 Hz, 2H), 6.70 (dd, J = 9.2, 5.2 Hz, 1H), 6.32 (brs, 2H), 4.34 (d, J = 6.0 Hz, 2H), 3.72 (s, 3H); ESI-HRMS m/z calcd for C15H16FN2O2 [M + H]+ 275.1190, found: 275.1185.
2-Amino-4-fluoro-N-(4-methoxybenzyl)benzamide (7c)
A white solid (1.13 g, 82% yield), mp 110–112 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.71 (t, J = 6.0 Hz, 1H), 7.59 (dd, J = 8.8, 6.8 Hz, 1H), 7.23 (d, J = 8.4 Hz, 2H), 6.88 (d, J = 8.8 Hz, 2H), 6.76 (brs, 2H), 6.47 (dd, J = 12.0, 2.8 Hz, 1H), 6.33–6.29 (m, 1H), 4.34 (d, J = 6.0 Hz, 2H), 3.72 (s, 3H); ESI-HRMS m/z calcd for C15H16FN2O2 [M + H]+ 275.1190, found: 275.1186.
2-Amino-5-chloro-N-(4-methoxybenzyl)benzamide (7d)
A white solid (1.23 g, 85% yield), mp 142–144 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.83 (t, J = 6.0 Hz, 1H), 7.58 (d, J = 2.4 Hz, 1H), 7.23 (d, J = 8.8 Hz, 2H), 7.16 (dd, J = 8.8, 2.4 Hz, 1H), 6.90–6.87 (m, 2H), 6.72 (d, J = 8.8 Hz, 1H), 6.57 (brs, 2H), 4.33 (d, J = 5.6 Hz, 2H), 3.72 (s, 3H); ESI-HRMS m/z calcd for C15H16ClN2O2 [M + H]+ 291.0895, found: 291.0890.
2-Amino-4-chloro-N-(4-methoxybenzyl)benzamide (7e)
A white solid (0.95 g, 82% yield), mp 122–124 °C. 1H NMR (500 MHz, CDCl3) δ 7.26 (d, J = 7.5 Hz, 2H), 7.21 (d, J = 8.0 Hz, 1H), 6.89 (d, J = 8.5 Hz, 2H), 6.68 (s, 1H), 6.57 (d, J = 7.5 Hz, 1H), 6.19 (brs, 1H), 5.73 (brs, 2H), 4.51 (d, J = 5.0 Hz, 2H), 3.80 (s, 3H); ESI-HRMS m/z calcd for C15H16ClN2O2 [M + H]+ 291.0895, found: 291.0891.
2-Amino-5-bromo-N-(4-methoxybenzyl)benzamide (7f)
A white solid (1.48 g, 88% yield), mp 153–155 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.84 (t, J = 6.0 Hz, 1H), 7.69 (d, J = 2.4 Hz, 1H), 7.26 (dd, J = 9.2, 2.8 Hz, 1H), 7.23 (d, J = 8.4 Hz, 2H), 6.89 (d, J = 8.8 Hz, 2H), 6.67 (d, J = 8.8 Hz, 1H), 6.59 (brs, 2H), 4.33 (d, J = 5.6 Hz, 2H), 3.72 (s, 3H); ESI-HRMS m/z calcd for C15H16BrN2O2 [M + H]+ 335.0390, found: 335.0381.
2-Amino-4-bromo-N-(4-methoxybenzyl)benzamide (7g)
A white solid (1.16 g, 87% yield), mp 141–143 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.78 (t, J = 6.0 Hz, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.23 (d, J = 8.8 Hz, 2H), 6.92 (d, J = 2.0 Hz, 1H), 6.88 (d, J = 8.4 Hz, 2H), 6.75–6.54 (m, 3H), 4.33 (d, J = 5.6 Hz, 2H), 3.72 (s, 3H); ESI-HRMS m/z calcd for C15H16BrN2O2 [M + H]+ 335.0390, found: 335.0382.
2-Amino-N-(4-methoxybenzyl)-5-(trifluoromethyl)benzamide (7h)
A white solid (370 mg, 85% yield), mp 175–177 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.93 (t, J = 6.0 Hz, 1H), 7.69 (d, J = 8.4 Hz, 1H), 7.24 (d, J = 8.8 Hz, 2H), 7.05 (d, J = 1.6 Hz, 1H), 6.89 (d, J = 8.4 Hz, 2H), 6.79 (dd, J = 8.4, 2.0 Hz, 1H), 6.74 (brs, 2H), 4.37 (d, J = 5.6 Hz, 2H), 3.73 (s, 3H); ESI-HRMS m/z calcd for C16H16F3N2O2 [M + H]+ 325.1158, found: 325.1157.
1-Ethyl-3-(4-methoxybenzyl)quinazoline-2,4(1H,3H)-dione (8a)
Following general procedure B, 8a was purified by column chromatography (CH2Cl2/CH3OH = 100:1) to afford a white solid (253 mg, 82% yield), mp 120–121 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.08 (dd, J = 8.0, 1.6 Hz, 1H), 7.80–7.76 (m, 1H), 7.51 (d, J = 8.4 Hz, 1H), 7.32–7.28 (m, 3H), 6.89–6.85 (m, 2H), 5.07 (s, 2H), 4.15 (q, J = 7.2 Hz, 2H), 3.71 (s, 3H), 1.21 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 158.5, 150.0, 139.4, 135.6, 129.4, 129.3, 128.2, 122.8, 115.0, 114.5, 113.8, 55.1, 43.7, 38.4, 12.5; ESI-HRMS m/z calcd for C18H19N2O3 [M + H]+ 311.1390, found: 311.1382.
6-Fluoro-3-(4-methoxybenzyl)quinazoline-2,4(1H,3H)-dione (8b)
A white solid (160 mg, 53% yield, general procedure B), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.56 (brs, 1H), 7.64 (dd, J = 8.6, 3.0 Hz, 1H), 7.57 (td, J = 8.7, 3.0 Hz, 1H), 7.28 (d, J = 8.4 Hz, 2H), 7.23 (dd, J = 8.8, 4.2 Hz, 1H), 6.86 (d, J = 8.4 Hz, 2H), 5.00 (s, 2H), 3.71 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 161.2, 158.5, 157.4 (d, JF,C = 239 Hz), 149.9, 136.1, 129.3, 129.2, 123.1 (d, JF,C = 24 Hz), 117.5, 114.7, 113.7, 112.3 (d, JF,C = 23 Hz), 55.0, 42.7; ESI-HRMS m/z calcd for C16H14FN2O3 [M + H]+ 301.0983, found: 301.0979.
7-Fluoro-3-(4-methoxybenzyl)quinazoline-2,4(1H,3H)-dione (8c)
A white solid (157 mg, 52% yield, general procedure B), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.61 (brs, 1H), 7.99 (dd, J = 8.8, 6.2 Hz, 1H), 7.28 (d, J = 8.7 Hz, 2H), 7.05 (td, J = 8.7, 2.5 Hz, 1H), 6.91 (dd, J = 9.8, 2.5 Hz, 1H), 6.85 (d, J = 8.7 Hz, 2H), 4.99 (s, 2H), 3.70 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.8 (d, JF,C = 250 Hz), 161.1, 158.4, 150.1, 141.4, 130.8, 129.3, 129.2, 113.7, 110.8, 110.7 (d, JF,C = 23 Hz), 101.3 (d, JF,C = 23 Hz), 55.0, 42.6; ESI-HRMS m/z calcd for C16H14FN2O3 [M + H]+ 301.0983, found: 301.0978.
6-Chloro-3-(4-methoxybenzyl)quinazoline-2,4(1H,3H)-dione (8d)
A white solid (252 mg, 80% yield, general procedure B), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.63 (brs, 1H), 7.86 (d, J = 2.5 Hz, 1H), 7.69 (dd, J = 8.7, 2.5 Hz, 1H), 7.28 (d, J = 8.8 Hz, 2H), 7.20 (d, J = 8.7 Hz, 1H), 6.85 (d, J = 8.7 Hz, 2H), 4.99 (s, 2H), 3.70 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 160.9, 158.5, 149.9, 138.3, 134.9, 129.3, 129.1, 126.5, 126.3, 117.4, 115.1, 113.7, 55.0, 42.8; ESI-HRMS m/z calcd for C16H14ClN2O3 [M + H]+ 317.0687, found: 317.0682.
7-Chloro-3-(4-methoxybenzyl)quinazoline-2,4(1H,3H)-dione (8e)
A white solid (210 mg, 66% yield, general procedure B), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.61 (brs, 1H), 7.93 (d, J = 8.5 Hz, 1H), 7.33–7.22 (m, 3H), 7.20 (s, 1H), 6.86 (d, J = 8.1 Hz, 2H), 4.99 (s, 2H), 3.71 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 161.2, 158.5, 150.0, 140.4, 139.4, 129.5, 129.3, 129.1, 122.8, 114.5, 113.7, 112.7, 55.0, 42.7; ESI-HRMS m/z calcd for C16H14ClN2O3 [M + H]+ 317.0687, found: 317.0686.
6-Bromo-3-(4-methoxybenzyl)quinazoline-2,4(1H,3H)-dione (8f)
A white solid (230 mg, 64% yield, general procedure B), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.63 (brs, 1H), 8.00 (d, J = 2.4 Hz, 1H), 7.82 (dd, J = 8.7, 2.4 Hz, 1H), 7.28 (d, J = 8.2 Hz, 2H), 7.14 (d, J = 8.7 Hz, 1H), 6.85 (d, J = 8.2 Hz, 2H), 5.00 (s, 2H), 3.71 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 160.8, 158.5, 149.9, 138.6, 137.6, 129.3, 129.1, 117.7, 115.5, 114.0, 113.7, 55.0, 42.8; ESI-HRMS m/z calcd for C16H14BrN2O3 [M + H]+ 361.0182, found: 361.0189.
7-Bromo-3-(4-methoxybenzyl)quinazoline-2,4(1H,3H)-dione (8g)
A white solid (185 mg, 51% yield, general procedure B), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.58 (brs, 1H), 7.85 (d, J = 8.4 Hz, 1H), 7.37 (d, J = 8.4 Hz, 1H), 7.35 (d, J = 1.7 Hz, 1H), 7.27 (d, J = 8.7 Hz, 2H), 6.85 (d, J = 8.7 Hz, 2H), 4.99 (s, 2H), 3.70 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 161.3, 158.4, 150.0, 140.5, 129.5, 129.3, 129.1, 128.4, 125.6, 117.5, 113.7, 113.0, 55.0, 42.7; ESI-HRMS m/z calcd for C16H14BrN2O3 [M + H]+ 361.0182, found: 361.0180.
6-(Trifluoromethyl)-3-(4-methoxybenzyl)quinazoline-2,4(1H,3H)-dione (8h)
Following general procedure B, 8h was purified by column chromatography (CH2Cl2/CH3OH = 100:1) to afford a white solid (140 mg, 40% yield), mp > 250 °C. 1H NMR (500 MHz, DMSO-d6) δ 11.76 (brs, 1H), 8.14 (d, J = 8.2 Hz, 1H), 7.52 (d, J = 8.2 Hz, 1H), 7.48 (s, 1H), 7.29 (d, J = 8.2 Hz, 2H), 6.86 (d, J = 8.5 Hz, 2H), 5.02 (s, 2H), 3.71 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 158.5, 150.0, 139.7, 134.2 (q, JF,C = 32 Hz), 129.3, 129.2, 129.0, 123.3 (q, JF,C = 272 Hz), 118.5, 116.7, 113.7, 112.1, 55.0, 42.8; ESI-HRMS m/z calcd for C17H14F3N2O3 [M + H]+ 351.0951, found: 351.0949.
3-(4-Bromo-2-fluorobenzyl)-7-chloroquinazoline-2,4(1H,3H)-dione (12)
A white solid (300 mg, 78% yield, general procedure B), mp > 250 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.70 (brs, 1H), 7.93 (d, J = 8.4 Hz, 1H), 7.53 (dd, J = 10.0, 2.0 Hz, 1H), 7.33 (dd, J = 8.0, 1.6 Hz, 1H), 7.26 (dd, J = 8.4, 1.6 Hz, 1H), 7.22–7.21 (m, 1H), 7.18 (d, J = 8.0 Hz, 1H), 5.07 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.3, 159.9 (q, JF,C = 249 Hz), 150.0, 140.7, 139.6, 130.4, 129.6, 127.6, 123.7, 122.9, 120.3, 118.6, 114.7, 112.7, 37.2; ESI-HRMS m/z calcd for C15H10BrClFN2O2 [M + H]+ 382.9593, found: 382.9578.
General Procedures for the Preparation of Zenarestat
To a suspension of 12 (0.30 g, 0.78 mmol) in dry DMF (4 mL) was added NaH (0.031 g, 60% dispersion in mineral oil, 0.78 mmol) in batches cooled with an ice bath. The reaction mixture was stirred at 0 °C for 4 h, followed by the addition of a solution of ethyl bromoacetate (0.19 g, 1.17 mmol) in dry DMF (4.0 mL) dropwise. The reaction mixture was stirred at room temperature for an additional 6 h. The solvent was removed under reduced pressure, and to the residue was added aqueous sodium hydroxide solution (0.049 g in 12 mL of H2O). The reaction mixture was heated to reflux and stirred for 1 h until the completion of the reaction. The hot solution was filtered to remove insoluble particulate. After cooling to room temperature, the filtrate was acidified with 1 N HCl to pH 3–4. The suspension was cooled with an ice bath for 1 h and then filtrated. The obtained solid was washed with 5 mL of aqueous methanol (1:1) and dried to afford the Zenarestat (313 mg, 91%, two-step) as a white solid. mp 225–226 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.33 (brs, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.65 (s, 1H), 7.54 (d, J = 9.5 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.15 (t, J = 8.0 Hz, 1H), 5.14 (s, 2H), 4.90 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 169.4, 160.4, 159.9 (d, JF,C = 249 Hz), 150.4, 141.2, 140.6, 130.4, 130.1, 127.7, 123.6, 123.4, 120.6, 118.8 (d, JF,C = 25 Hz), 114.8, 113.5, 45.3, 38.2; ESI-HRMS m/z calcd for C17H12BrClFN2O4 [M + H]+ 440.9648, found: 440.9666.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grant no. 81703362 and the CAMS Innovation Fund for Medical Sciences under Grant CAMS-2016-I2M-1-010.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01104.
Copies of 1H NMR and 13C NMR spectra for quinazoline-2,4-diones (5, 6, 8, and 12), 2-aminobenzamide (7), and Zenarestat (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Khan I.; Ibrar A.; Abbas N.; Saeed A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthetic approaches and multifarious applications. Eur. J. Med. Chem. 2014, 76, 193–244. 10.1016/j.ejmech.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Ismail M. A. H.; Barker S.; Abou El Ella D. A.; Abouzid K. A. M.; Toubar R. A.; Todd M. H. Design and synthesis of new tetrazolyl- and carboxy-biphenylylmethyl-quinazolin-4-one derivatives as angiotensin II AT1 receptor antagonists. J. Med. Chem. 2006, 49, 1526–1535. 10.1021/jm050232e. [DOI] [PubMed] [Google Scholar]
- Crespo I.; Giménez-Dejoz J.; Porté S.; Cousido-Siah A.; Mitschler A.; Podjarny A.; Pratsinis H.; Kletsas D.; Parés X.; Ruiz F. X.; Metwally K.; Farrés J. Design, synthesis, structure-activity relationships and X-ray structural studies of novel 1-oxopyrimido[4,5-c]quinoline-2-acetic acid derivatives as selective and potent inhibitors of human aldose reductase. Eur. J. Med. Chem. 2018, 152, 160–174. 10.1016/j.ejmech.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Castellani B.; Diamanti E.; Pizzirani D.; Tardia P.; Maccesi M.; Realini N.; Magotti P.; Garau G.; Bakkum T.; Rivara S.; Mor M.; Piomelli D. Synthesis and characterization of the first inhibitor of N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD). Chem. Commun. 2017, 53, 12814–12817. 10.1039/C7CC07582K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z.-Y.; Hong W. D.; Cui X.-P.; Gao H.-C.; Wu P.-P.; Chen Y.-S.; Shen D.; Yang Y.; Zhang B.-J.; Taylor M. J.; Ward S. A.; O’Neill P. M.; Zhao S.-Q.; Zhang K. Synthesis and structure-activity relationship of N4-benzylamine-N2-isopropyl-quinazoline-2,4-diamines derivatives as potential antibacterial agents. RSC Adv. 2017, 7, 52227–52237. 10.1039/C7RA10352B. [DOI] [Google Scholar]
- Bouchut A.; Rotili D.; Pierrot C.; Valente S.; Lafitte S.; Schultz J.; Hoglund U.; Mazzone R.; Lucidi A.; Fabrizi G.; Pechalrieu D.; Arimondo P. B.; Skinner-Adams T. S.; Chua M. J.; Andrews K. T.; Mai A.; Khalife J. Identification of novel quinazoline derivatives as potent antiplasmodial agents. Eur. J. Med. Chem. 2019, 161, 277–291. 10.1016/j.ejmech.2018.10.041. [DOI] [PubMed] [Google Scholar]
- Rojas-Aguirre Y.; Hernández-Luis F.; Mendoza-Martínez C.; Sotomayor C. P.; Aguilar L. F.; Villena F.; Castillo I.; Hernández D. J.; Suwalsky M. Effects of an antimalarial quinazoline derivative on human erythrocytes and on cell membrane molecular models. Biochim. Biophys. Acta, Biomembr. 2012, 1818, 738–746. 10.1016/j.bbamem.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Kuang Y.; Sechi M.; Nurra S.; Ljungman M.; Neamati N. Design and synthesis of novel reactive oxygen species inducers for the treatment of pancreatic ductal adenocarcinoma. J. Med. Chem. 2018, 61, 1576–1594. 10.1021/acs.jmedchem.7b01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.; Ji M.; Yao H.-P.; Cao R.; Zhao H.-L.; Wang X.-Y.; Chen X.-G.; Xu B.-L. Discovery of quinazoline-2,4(1H,3H)-dione derivatives as novel PARP-1/2 inhibitors: design, synthesis and their antitumor activity. Org. Biomol. Chem. 2018, 16, 3189–3202. 10.1039/C8OB00286J. [DOI] [PubMed] [Google Scholar]
- Richter S.; Gioffreda B. Synthesis, molecular modelling and biological evaluation of 4-amino-2(1H)-quinazolinone and 2,4(1H,3H)-quinazolidone derivatives as antitumor agents. Arch. Pharm. 2011, 344, 810–820. 10.1002/ardp.201000312. [DOI] [PubMed] [Google Scholar]
- Fakhraian H.; Heydary M. Reinvestigation of the synthesis of ketanserin (5) and its hydrochloride salt (5.HCl) via 3-(2-chloroethyl)-2,4-(1H,3H)-quinazolinedione (2) or dihydro-5H-oxazole(2,3-b)quinazolin-5-one (1). J. Heterocycl. Chem. 2014, 51, 151–156. 10.1002/jhet.1897. [DOI] [Google Scholar]
- Lee Y. S.; Chen Z.; Kador P. F. Molecular modeling studies of the binding modes of aldose reductase inhibitors at the active site of human aldose reductase. Bioorg. Med. Chem. 1998, 6, 1811–1819. 10.1016/S0968-0896(98)00139-4. [DOI] [PubMed] [Google Scholar]
- Shiro T.; Fukaya T.; Tobe M. The chemistry and biological activity of heterocycle-fused quinolinone derivatives: A review. Eur. J. Med. Chem. 2015, 97, 397–408. 10.1016/j.ejmech.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Su X.-L.; Xu S.; Shan Y.; Yin M.; Chen Y.; Feng X.; Wang Q.-Z. Three new quinazolines from Evodia rutaecarpa and their biological activity. Fitoterapia 2018, 127, 186–192. 10.1016/j.fitote.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Jin H.-Z.; Du J.-L.; Zhang W.-D.; Chen H.-S.; Lee J.-H.; Lee J.-J. A novel alkaloid from the fruits of Evodia officinalis. J. Asian Nat. Prod. Res. 2007, 9, 685–688. 10.1080/10286020601103296. [DOI] [PubMed] [Google Scholar]
- Mathew T.; Papp A. A.; Paknia F.; Fustero S.; Prakash G. K. S. Benzodiazines: recent synthetic advances. Chem. Soc. Rev. 2017, 46, 3060–3094. 10.1039/C7CS00082K. [DOI] [PubMed] [Google Scholar]
- Reddy M. M.; Sivaramakrishna A. Remarkably flexible quinazolinones-synthesis and biological applications. J. Heterocycl. Chem. 2019, 942–954. 10.1002/jhet.3844. [DOI] [Google Scholar]
- Hulla M.; Chamam S. M. A.; Laurenczy G.; Das S.; Dyson P. J. Delineating the mechanism of ionic liquids in the synthesis of quinazoline-2,4(1H,3H)-dione from 2-aminobenzonitrile and CO2. Angew. Chem., Int. Ed. 2017, 56, 10559–10563. 10.1002/anie.201705438. [DOI] [PubMed] [Google Scholar]
- Li J.-R.; Chen X.; Shi D.-X.; Ma S.-L.; Li Q.; Zhang Q.; Tang J.-H. A new and facile synthesis of quinazoline-2,4(1H,3H)-diones. Org. Lett. 2009, 11, 1193–1196. 10.1021/ol900093h. [DOI] [PubMed] [Google Scholar]
- Willis M. C.; Snell R. H.; Fletcher A. J.; Woodward R. L. Tandem palladium-catalyzed urea arylation-intramolecular ester amidation: Regioselective synthesis of 3-alkylated 2,4-quinazolinediones. Org. Lett. 2006, 8, 5089–5091. 10.1021/ol062009x. [DOI] [PubMed] [Google Scholar]
- Liu F.-S.; Ping R.; Zhao P.-H.; Gu Y.-Q.; Gao J.; Liu M.-S. Succinimide-based ionic liquids: An efficient and versatile platform for transformation of CO2 into quinazoline-2,4(1H,3H)-diones under mild and solvent-Free conditions. ACS Sustainable Chem. Eng. 2019, 7, 13517–13522. 10.1021/acssuschemeng.9b03154. [DOI] [Google Scholar]
- Zhu A.-L.; Tang M.-J.; Lv Q.-Z.; Li L.-J.; Bai S.-K.; Li Q.-Q.; Feng W.-L.; Li Q.-X.; Wang J.-J. Fixation of CO2 in structurally diverse quinazoline-2,4(1H,3H)-diones under ambient conditions. J. CO2 Util. 2019, 34, 500–506. 10.1016/j.jcou.2019.07.038. [DOI] [Google Scholar]
- Xiao Y.-Q.; Kong X.-Q.; Xu Z.-C.; Cao C.-S.; Pang G.-S.; Shi Y.-H. Efficient synthesis of quinazoline-2,4(1H,3H)-diones from CO2 catalyzed by N-heterocyclic carbene at atmospheric pressure. RSC Adv. 2015, 5, 5032–5037. 10.1039/C4RA13752C. [DOI] [Google Scholar]
- Sharafi-Kolkeshvandi M.; Nikpour F. A facile and convenient approach for the one-pot synthesis of 2,4(1H,3H)-quinazolinediones. Chin. Chem. Lett. 2012, 23, 431–433. 10.1016/j.cclet.2012.01.027. [DOI] [Google Scholar]
- Li X.-Q. The simple synthesis of quinazoline-2,4-dione derivatives using Boc strategy. Chin. Chem. Lett. 2009, 20, 1201–1203. 10.1016/j.cclet.2009.05.001. [DOI] [Google Scholar]
- Chen S.-Q.; Wang Z.; Hu J.-Y.; Guo Y.-F.; Deng T.-L. Efficient transformation of CO2 into quinazoline2,4(1H,3H)-diones at room temperature catalyzed by a ZnI2/NEt3 system. New J. Chem. 2019, 43, 16164–16168. 10.1039/C9NJ04302K. [DOI] [Google Scholar]
- Mizuno T.; Ishino Y. Highly effcient synthesis of 1H-quinazoline-2,4-diones using carbon dioxide in the presence of catalytic amount of DBU. Tetrahedron 2002, 58, 3155–3158. 10.1016/S0040-4020(02)00279-X. [DOI] [Google Scholar]
- Koay N.; Campeau L.-C. Efficient preparation of 3-substituted quinazolinediones directly from anthranilic acids and isocyanates. J. Heterocycl. Chem. 2011, 48, 473–478. 10.1002/jhet.551. [DOI] [Google Scholar]
- Mohammadi A. A. One-pot syntheses of some new 2,4(1H,3H)-quinazolinedione derivatives in the absence of catalyst. J. Heterocycl. Chem. 2017, 54, 2075–2078. 10.1002/jhet.2778. [DOI] [Google Scholar]
- Miyata T.; Mizuno T.; Nagahama Y.; Nishiguchi I.; Hirashima T.; Sonoda N. Facile synthesis of 2,4-Dioxo-1,2,3,4-tetrahydroquinazlines by sulfur-assisted carbonylation with carbon monoxide. Heteroat. Chem. 1991, 2, 473–475. 10.1002/hc.520020409. [DOI] [Google Scholar]
- Wang S.-L.; Yang K.; Yao C.-S.; Wang X.-S. Green synthesis of quinazolinone derivatives catalyzed by iodine in ionic liquid. Synth. Commun. 2012, 42, 341–349. 10.1080/00397911.2010.524340. [DOI] [Google Scholar]
- Wang P.-X.; Wang Y-N.; Lin Z.-Y.; Li G.; Huang H.-H. Facile and efficient synthesis of quinazoline-2,4(1H,3H)-diones through sequential hydrogenation condensation. Synth. Commun. 2018, 48, 1183–1189. 10.1080/00397911.2018.1439173. [DOI] [Google Scholar]
- Wang M.-L.; Han J.-L.; Si X.-J.; Hu Y.-M.; Zhu J.-D.; Sun X. Effective approach to ureas through organocatalyzed one-pot process. Tetrahedron Lett. 2018, 59, 1614–1618. 10.1016/j.tetlet.2017.11.030. [DOI] [Google Scholar]
- Basel Y.; Hassner A. Di-tert-butyl dicarbonate and 4-(dimethylamino)pyridine revisited. Their reactions with amines and alcohols. J. Org. Chem. 2000, 65, 6368–6380. 10.1021/jo000257f. [DOI] [PubMed] [Google Scholar]
- Goto S.; Tsuboi H.; Kanoda M.; Mukai K.; Kagara K. The process development of a novel aldose reductase inhibitor, FK366. Part 1. Improvement of discovery process and new syntheses of 1-substituted quinazolinediones. Org. Process Res. Dev. 2003, 7, 700–706. 10.1021/op0340661. [DOI] [Google Scholar]
- Hour M. J.; Yang J. S.; Chen T. L.; Chen K. T.; Kuo S. C.; Chung J. G.; Lu C. C.; Chen C. Y.; Chuang Y. H. The synthesized novel fluorinated compound (LJJ-10) induces death receptor- and mitochondria-dependent apoptotic cell death in the human osteogenic sarcoma U-2 OS cells. Eur. J. Med. Chem. 2011, 46, 2709–2721. 10.1016/j.ejmech.2011.03.059. [DOI] [PubMed] [Google Scholar]
- Oh B. K.; Ko E. B.; Han J. W.; Oh C. H. Synthesis of N-heteropolycyclic compounds including quinazolinone skeleton using Friedel–Crafts alkylation. Synth. Commun. 2015, 45, 758–766. 10.1080/00397911.2014.987353. [DOI] [Google Scholar]
- Gavin J. T.; Annor-Gyamfi J. K.; Bunce R. A. Quinazolin-4(3H)-ones and 5,6-dihydropyrimidin-4(3H)-ones from β-aminoamides and orthoesters. Molecules 2018, 23, 2925 10.3390/molecules23112925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Zu W.; Zhang J.-L.; Xu L. Chemoselective N-arylation of aminobenzamides via copper catalysed Chan-Evans-Lam reactions. Org. Biomol. Chem. 2017, 15, 9288–9292. 10.1039/C7OB02491F. [DOI] [PubMed] [Google Scholar]
- Bonne D.; Dekhane M.; Zhu J. Exploiting the dual reactivity of o-isocyanobenzamide: Three-component synthesis of 4-imino-4H-3,1-benzoxazines. Org. Lett. 2005, 7, 5285–5288. 10.1021/ol052239w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.