Abstract
Oxidative damage is one of the major contributors to retinal degenerative diseases such as age-related macular degeneration (AMD), while RPE mediated antioxidant defense plays an important role in preventing retinopathies. However, the regulatory mechanisms of antioxidant signaling in RPE cells are poorly understood. Here we show that transcription factor MITF regulates the antioxidant response in RPE cells, protecting the neural retina from oxidative damage. In the oxidative stress-induced retinal degeneration mouse model, retinal degeneration in Mitf+/- mice is significantly aggravated compared to WT mice. In contrast, overexpression of Mitf in Dct-Mitf transgenic mice and AAV mediated overexpression in RPE cells protect the neural retina against oxidative damage. Mechanistically, MITF both directly regulates the transcription of NRF2, a master regulator of antioxidant signaling, and promotes its nuclear translocation. Furthermore, specific overexpression of NRF2 in Mitf+/- RPE cells activates antioxidant signaling and partially protects the retina from oxidative damage. Taken together, our findings demonstrate the regulation of NRF2 by MITF in RPE cells and provide new insights into potential therapeutic approaches for prevention of oxidative damage diseases.
Keywords: Antioxidant, Retinal degeneration, NRF2, RPE, MITF
Graphical abstract
Highlights
-
•
MITF haploinsufficiency exacerbates oxidative stress-induced retinal degeneration.
-
•
Specific overexpression of MITF in RPE cells protects retinas from oxidative damage in vivo.
-
•
MITF directly regulates the transcription and nuclear translocation of NRF2.
-
•
Partial rescue of retinal oxidative damage in Mitf ±mice by gene transfer mediated RPE cell specific expression of NRF2.
1. Introduction
Oxidative stress is a common risk factor in normal aging and many neural degenerative diseases. The retina is composed of retinal pigment epithelial cells (RPE) and the neural retina, which is critical for visual function and particularly susceptible to oxidative damage due to its physiological structure and high metabolic activity [[1], [2], [3]]. Retinal oxidative damage has been identified as one of the major causative factors in retinal degenerative diseases, including age-related macular degeneration (AMD) [[4], [5], [6]]. Protecting the neural retina from oxidative damage is a potential therapeutic approach for retinal degenerative diseases and is an area of active investigation [7].
The RPE consists of a single layer of epithelial cells between the neural retina and the choroid, from which it is separated by Bruch's membrane. It constitutes the outer blood retinal barrier and plays vital roles in retinal physiology including light absorption, epithelial transport, anti-oxidative activity, ion buffering of the photoreceptors, secretion of growth factors, involvement in the visual cycle, and phagocytosis of shed photoreceptor outer segments among others [2,8]. RPE cells have evolved effective defense mechanisms against oxidative damage, which are instrumental in clearing reactive oxygen species (ROS) and counterbalancing the oxidative stress of the highly metabolically active retina [8]. Deficiency in RPE cell antioxidant ability is critically implicated in the pathogenesis of AMD, while increases in the antioxidant capability of RPE cells could protect the neural retina from oxidative damage [9].
NRF2 (nuclear factor erythroid 2-related factor 2, NFE2L2) and PGC1α (peroxisome proliferator-activated receptor-gamma coactivator 1 alpha, Ppargc1α) are two key regulators in maintaining the redox balance of cells through activating multiple genes that combat oxidation and free radicals [10,11]. Under basal conditions, NRF2 is anchored in the cytoplasm by KEAP1 (Kelch-like ECH-associated protein-1). When cells are attacked by ROS or electrophilic agents, NRF2 dissociates from KEAP1 and rapidly translocates into the nucleus where it forms a heterodimer with the MAF (small masculoaponeurotic fibrosarcoma) proteins, and then binds to ARE (antioxidant response element) consensus sequences in its target genes, leading to induction of the antioxidant response [12]. Currently, mounting evidence suggests that RPE redox homeostasis relies on the activation of NRF2. Nrf2-deficient mice exhibit age-related pathology characteristic of AMD, including RPE cell degeneration and visual hypofunction [[13], [14], [15], [16], [17], [18]], while AAV mediated Nrf2 expression promotes photoreceptor survival in mouse models of inherited retinal degeneration [19]. As a key regulator of the antioxidant pathway, NRF2 is tightly regulated by several mechanisms [20]. Most studies have been focused on post-transcriptional regulation, including nuclear translocation, stability, and transcriptional activity. p62 (also named SQSTM1, sequestosome 1) has been reported to promote the nuclear translocation of NRF2 through competitively binding with KEAP1 in the cytoplasm, which binds NRF2 confining it to the cytoplasm and facilitating its ubiquitination [21,22]. In RPE cells, X box-binding protein 1 (XBP1) was reported to regulate the translation of NRF2 [23]. However, studies of the mechanism of NRF2 regulation at the transcriptional level in RPE cells are limited.
RPE cells are precisely regulated by a variety of transcription factors and signaling pathways, both during development and after maturation [24]. Among them, MITF (Microphthalmia-associated transcription factor) is a crucial transcription factor that plays an irreplaceable role in RPE development and cellular functions [25]. In humans, MITF mutations were reported to be associated with Waardenburg Syndrome (WS), Tietz albinism deafness syndrome (TADS), Coloboma, Osteopetrosis, Microphthalmia, Macrocephaly, Albinism and deafness (COMMAD), nonsyndromic hearing loss, melanoma and renal carcinoma [[26], [27], [28], [29], [30], [31]]. Mitf-deficient mice exhibit pathological features of albinism, microphthalmia, retinal degeneration and deafness [[32], [33], [34], [35]], although the pathogenic mechanisms have only been partially elucidated.
MITF is highly expressed in RPE cells [36], and regulates various RPE cellular functions. It has been demonstrated that MITF regulates RPE development and differentiation, melanogenesis, cell migration, proliferation, growth factor secretion and visual cycle function [[37], [38], [39], [40], [41]]. We previously demonstrated that MITF regulates mitochondrial biogenesis through PGC1α in ARPE-19 cells [42]. However, the mechanisms through which MITF dysfunction induces retinal degeneration are still only partially understood. Unaddressed questions include whether MITF regulates the RPE antioxidant defense system in vivo and also whether RPE cell specific expression of MITF protects the neural retina from oxidative damage.
In order to address these questions, we used the sodium iodate (NaIO3)- induced retinal degeneration mouse model and showed that MITF haploinsufficiency exacerbates oxidative stress-induced retinal degeneration in Mitf+/- mice. Conversely, overexpression of MITF in RPE cells using Dct-Mitf transgenic mice or AAV-MITF mediated gene transfer protects the mouse neural retina against oxidative damage. Mechanistically, MITF protects against oxidative stress at least partially through regulating the expression and nuclear translocation of NRF2, a master regulator of antioxidant signaling pathways [43]. Moreover, regulation of NRF2 by MITF is similar to that seen in other cell types besides the RPE. Since oxidative damage is one of the key causative factors for numerous human diseases, and NRF2 is reported to be a master regulator of antioxidant signaling, the function of MITF in regulating NRF2 and its downstream antioxidant signaling might have therapeutic value for the prevention or treatment of retinal degeneration and other oxidative stress-mediated human diseases.
2. Results
2.1. MITF haploinsufficiency exacerbates oxidative damage-induced retinal degeneration
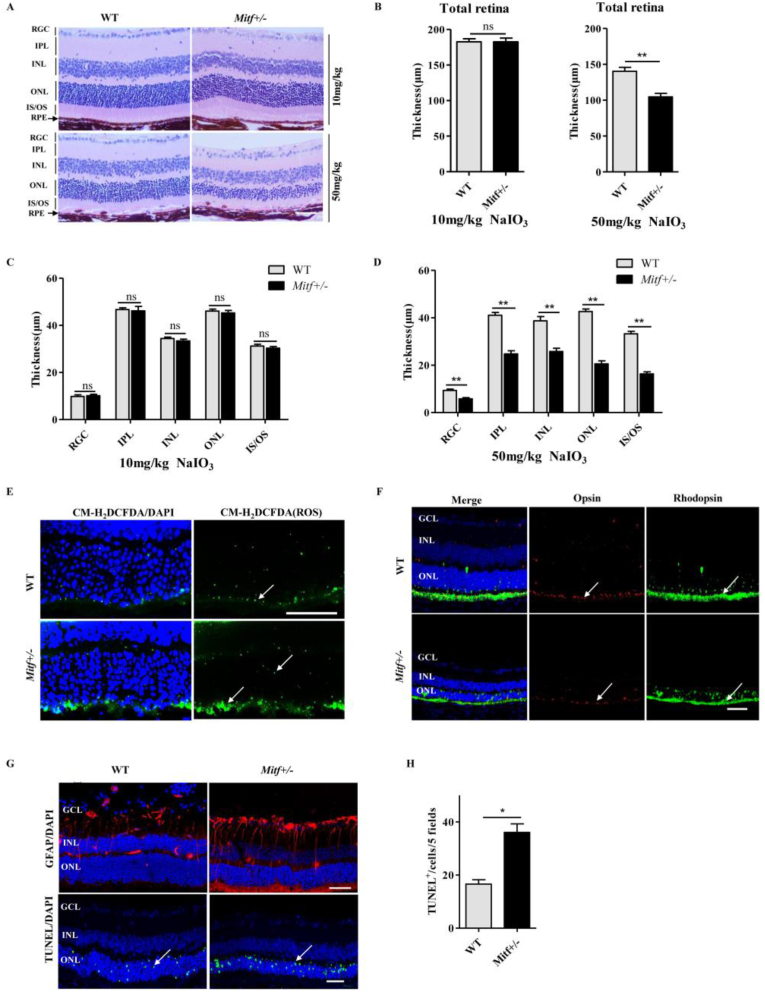
We have previously shown that Mitf−/− mice show serious retinal degeneration, and overexpression of MITF in ARPE-19 cells can increase resistance to oxidative stress [42], although it is unclear whether MITF regulates RPE antioxidant defense in vivo. As Mitf−/− mice lack mature RPE cells, it is difficult to use them for functional analysis of MITF action. To address the question of whether MITF regulates antioxidant signaling in RPE cells in vivo, we firstly used Mitf+/- mice, which have no visible defects in either the structure of the RPE and neural retina, or in the expression of Rhodopsin and Opsin (Fig. S1A-D), but do show decreased MITF protein levels. In order to determine whether MITF haploinsufficiency exacerbates retinal oxidative damage, 8-wk-old C57BL/6J (WT) and Mitf+/- mice were intraperitoneally injected with NaIO3, which is a stable oxidizing agent that targets primarily the RPE [44,45]. As shown in Fig. 1A–D, there is no significant difference in the structure of the RPE and neural retina between WT and Mitf+/- mice after injection of 10 mg/kg NaIO3. However, at a dose of 50 mg/kg NaIO3, the thickness of the Mitf+/- mouse retina was significantly reduced, including thickness of the retinal ganglion cells (RGC), inner nuclear layer (INL), outer nuclear layer (ONL), inner segment/outer segment (IS/OS), and the inner plexiform layer (IPL). Also, after injection of 50 mg/kg NaIO3, higher ROS production and lower expression levels of Rhodopsin and Opsin were detected in Mitf+/- mouse retinas (Fig. 1E and F). In addition, stronger TUNEL and GFAP positive signals were observed in the retinas of Mitf+/- than WT mice (Fig. 1G and H). Thus, the retinal degeneration was more severe in Mitf+/- than WT mice after NaIO3-induced retinal oxidative damage, which was also confirmed by results in the light/hyperoxia-induced retinal oxidative damage models (Fig. S2).
Fig. 1.
MITF haploinsufficiency exacerbates NaIO3-induced retinal degeneration. (A) HE staining of the WT and Mitf+/- retinal sections after 10 mg/kg or 50 mg/kg NaIO3 intraperitoneal injection for 4 days. (B–D) Retinal thickness analysis of the NaIO3 injection mice based on the results of (A), which showed no significant difference between the WT and Mitf+/- mice at the dose of 10 mg/kg NaIO3, while the thickness of the retinal layers in Mitf+/- mice were decreased compared to the WT mice at the dose of 50 mg/kg NaIO3, including the layers of RGC, IPL, INL, ONL and IS/OS. (E) Green fluorescent probe CM-H2DCFDA detected ROS production in Mitf+/- and WT mice retina after 50 mg/kg NaIO3 injection, which showed the ROS production increased in the retina of Mitf+/- mice (white arrow indicated). (F) Immunostaining for Rhodopsin and Opsin was carried out in Mitf+/- and WT mice after injection of 50 mg/kg NaIO3 (white arrow indicated). (G) GFAP and TUNEL staining in the mice retina after injection of 50 mg/kg NaIO3 (white arrow indicated). (H) Quantification of the TUNEL positive cells based on the results of (G). Scale bar: 50 μm. (n = 6; ns, no significant difference; **P < 0.01, *P < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.2. Overexpression of MITF protects the retina against oxidative damage in Dct-Mitf transgenic mice
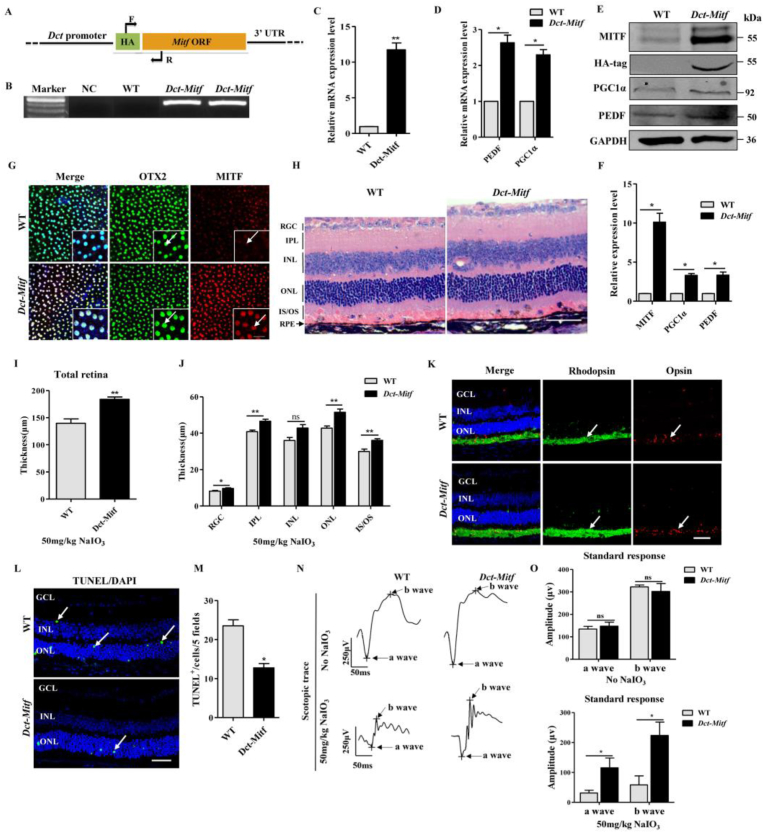
The above results demonstrated that MITF haploinsufficiency exacerbates oxidative stress-induced retinal degeneration. However, it was still unclear whether overexpression of MITF in RPE cells could protect the neural retina against oxidative damage. In order to address this question, we constructed Dct-HA-Mitf transgenic mice, which express a HA-MITF fusion protein with HA (hemagglutinin) as a tagged protein sequence (Fig. 2A). The HA-Mitf genotypic positive mice were used for MITF expression analysis and functional studies (Fig. 2B). Real-time PCR showed that the mRNA levels of Mitf (Fig. 2C) and its target genes Pedf (pigment epithelium-derived factor) and Pgc1α in Dct-Mitf RPE cell were up-regulated (Fig. 2D). Western blotting indicated the protein levels of MITF, HA-tag, PGC1α and PEDF were increased in Dct-Mitf RPE cells (Fig. 2E and F). Double immunostaining of OTX2 (orthodenticle homeobox 2) and MITF was performed on 8-wk-old WT and Dct-Mitf RPE flat-mounts, which confirmed that MITF expression is elevated in Dct-Mitf RPE cells (Fig. 2G). To determine whether overexpression of MITF in RPE cells affects the normal retinal structure, the retinal histological structure of 8-week-old WT and Dct-Mitf mice was analyzed under normal conditions, which showed no significant differences (Fig. S3A-D).
Fig. 2.
Overexpression of MITF protects the retina from oxidative damage in Dct-Mitf transgenic mice. (A) Schematic diagram of the Mitf overexpression transgenic mice, F and R are primers used for genotyping, and HA is the molecular tag. (B)Dct-Mitf transgenic mouse genotyping result. Wild type (WT); negative control (NC). (C, D) Real-time PCR showed the mRNA levels of Mitf (C) and Pedf and Pgc1α (D) were increased in the RPE cells of Dct-Mitf mice RPE cells. (E) Western blotting analysis of the protein levels of MITF, HA-tag, PGC1α and PEDF in the RPE cells of Dct-Mitf mice. (F) Quantification of western blotting bands based on the results of (E). (G) Double immunostaining for OTX2 and MITF was performed on WT or Dct-Mitf RPE flat-mount. Enlarged images in the white line boxes, which showed that MITF expression was upregulated in Dct-Mitf RPE (white arrow indicated). Scale bar: 20μm. (H–J) HE staining showed that the thickness of the retinal layers of Dct-Mitf mice was thicker than the WT after 4 days of 50 mg/kg NaIO3 injection, including the RGC, IPL, ONL and IS/OS layers. Scale bar: 50μm. (K) Immunostaining showed the expression levels of Rhodopsin and Opsin were higher in the Dct-Mitf retinas after injection of 50mg/kg NaIO3 (white arrow indicated). Scale bar: 50μm. (L) TUNEL staining showed that the apoptotic cell number was decreased in Dct-Mitf retina after injection 50mg/kg NaIO3 (white arrow indicated). Scale bar: 50μm. (M) Quantitative analysis of the TUNEL positive cell number based on the results of Figure L. (N, O) ERG scotopic traces showed higher amplitude of a-wave and b-wave in Dct-Mitf mice after injection 50mg/kg NaIO3. There was no significant difference in the group without NaIO3 injection. (n = 6; ns, no significant difference; **P < 0.01, *P < 0.05).
We then analyzed whether MITF overexpression could protect the neural retina against oxidative damage. 8-wk-old WT and Dct-Mitf mice were intraperitoneally injected with 50 mg/kg NaIO3. Four days after the NaIO3 injection, HE staining showed the Dct-Mitf mouse retina was thicker than that of WT mice, including the layers of RGC, IPL, ONL and IS/OS (Fig. 2H–J). Immunostaining showed higher Rhodopsin and Opsin levels and fewer TUNEL positive cells in the Dct-Mitf mouse retinas compared to those of WT mice (Fig. 2K-M). ERG results showed a higher a-wave and b-wave amplitude in Dct-Mitf than WT mice after the injection of 50mg/kg NaIO3, while there is no significant difference under normal conditions (Fig. 2N, O).
2.3. Gene transfer mediated RPE cell specific overexpression of MITF protects the mouse retina against oxidative damage
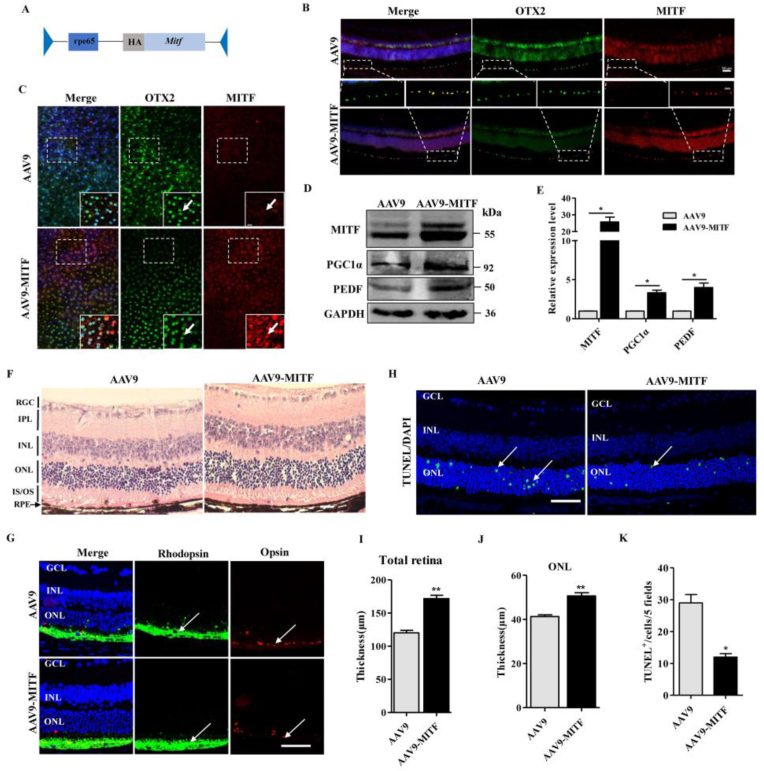
The above data indicated that the increasing of MITF in RPE cells could preserve the neural retina from oxidative damage. We further addressed the approach of protecting neural retina from oxidative damage through Mitf gene transfer. As MITF is specifically expressed in RPE cells, we used the RPE cell specific RPE65 (retinoid isomerohydrolase RPE65) promoter as previously described [46] and constructed an AAV9-p.RPE65-Mitf adeno-associated viral expression vector (hereafter referred to as AAV9-MITF) (Fig. 3A). Two weeks after the injection of AAV9 empty vector virus or AAV9-MITF into the subretinal space of 6-wk-old mice, MITF and OTX2 double immunostaining was performed on retinal sections or RPE flat-mounts. As the data in Fig. 3B and C shows, the MITF expression level was increased in the RPE after infection with the AAV9-MITF virus. Additionally, western blotting indicated that MITF, as well as its downstream target genes PGC1α and PEDF were up-regulated in mouse RPE after AAV9-MITF virus infection (Fig. 3D and E). Thus, AAV9-p.RPE65-Mitf gene transfer results in overexpression of MITF in the RPE and also activates expression of its downstream target genes. To determine whether AAV9-MITF gene transfer might alter normal retinal structure, two weeks after AAV9 injection, mouse retinas were analyzed by HE staining and Rhodopsin and Opsin expression. There were no significant differences between the AAV9 and AAV9-MITF injected mouse eyes (Fig. S4A-D).
Fig. 3.
AAV-mediated RPE cell specific overexpression of MITF could protect the mice retina against oxidative damage. (A) Schematic representation of AAV9-p.RPE65-Mitf construction. (B, C) Two weeks after the injection of AAV9 or AAV9-MITF into the subretinal space of 6-wk-old WT mice, double immunostaining of OTX2 and MITF was performed on retinal sections (B) or RPE flat-mount (C) as indicated. Enlarged images in the white line boxes, which showed that the MITF expression was upregulated in the RPE specifically after the infection of AAV9-MITF virus (white arrow indicated). Scale bar: 20μm. (D) Western blotting analysis of the protein levels of MITF, PGC1α and PEDF in RPE cells after the infection of AAV9-MITF virus. (E) Quantification of western blotting bands based on the result (D). (F, I and J) HE staining showed the total retinal thickness and ONL layer were thicker in the AAV9-MITF infected mice retina than the AAV9 infected eyes after 4 days of 50 mg/kg NaIO3 injection. Scale bar: 20μm. (G) Immunostaining of the Rhodopsin and Opsin in the AAV9 or AAV9-MITF virus-infected mouse retinas after injection of 50mg/kg NaIO3 (white arrow indicated). Scale bar: 50μm. (H) TUNEL staining showed the number of apoptotic cells was decreased in the AAV9-MITF virus-infected mouse retinas after injection of 50mg/kg NaIO3 (white arrow indicated). (K) Quantitative analysis of the TUNEL positive cell number based on the results of (H). Scale bar: 50 μm. (n = 6; ns, no significant difference; **P < 0.01, *P < 0.05).
In order to assess whether exogenously expression of Mitf by gene transfer could protect the retina against oxidative damage, the left eyes of 6-wk-old WT mice were injected with AAV9, and the right eyes with AAV9-MITF virus. Two weeks after injection of the virus the mice were intraperitoneally injected with 50 mg/kg NaIO3 and four days later the retinal structure was examined. Compared to the AAV9 injected retinas, the AAV9-MITF virus injected RPE cell layers were relatively intact, and the thickness of the ONL and the total retina were thicker as well (Fig. 3F, I, J). Consistently, higher expression levels of Rhodopsin and Opsin (Fig. 3G) and lower TUNEL-positive signals were detected in the retinas of AAV9-MITF mice (Fig. 3H, K). Thus, gene transfer mediated RPE cell specific overexpression of MITF can protect the neural retina from oxidative damage. However, these results didn't delineate the mechanism through which MITF regulates the antioxidant signaling pathways in RPE cells.
2.4. MITF directly regulates NRF2 expression through binding to its promoter
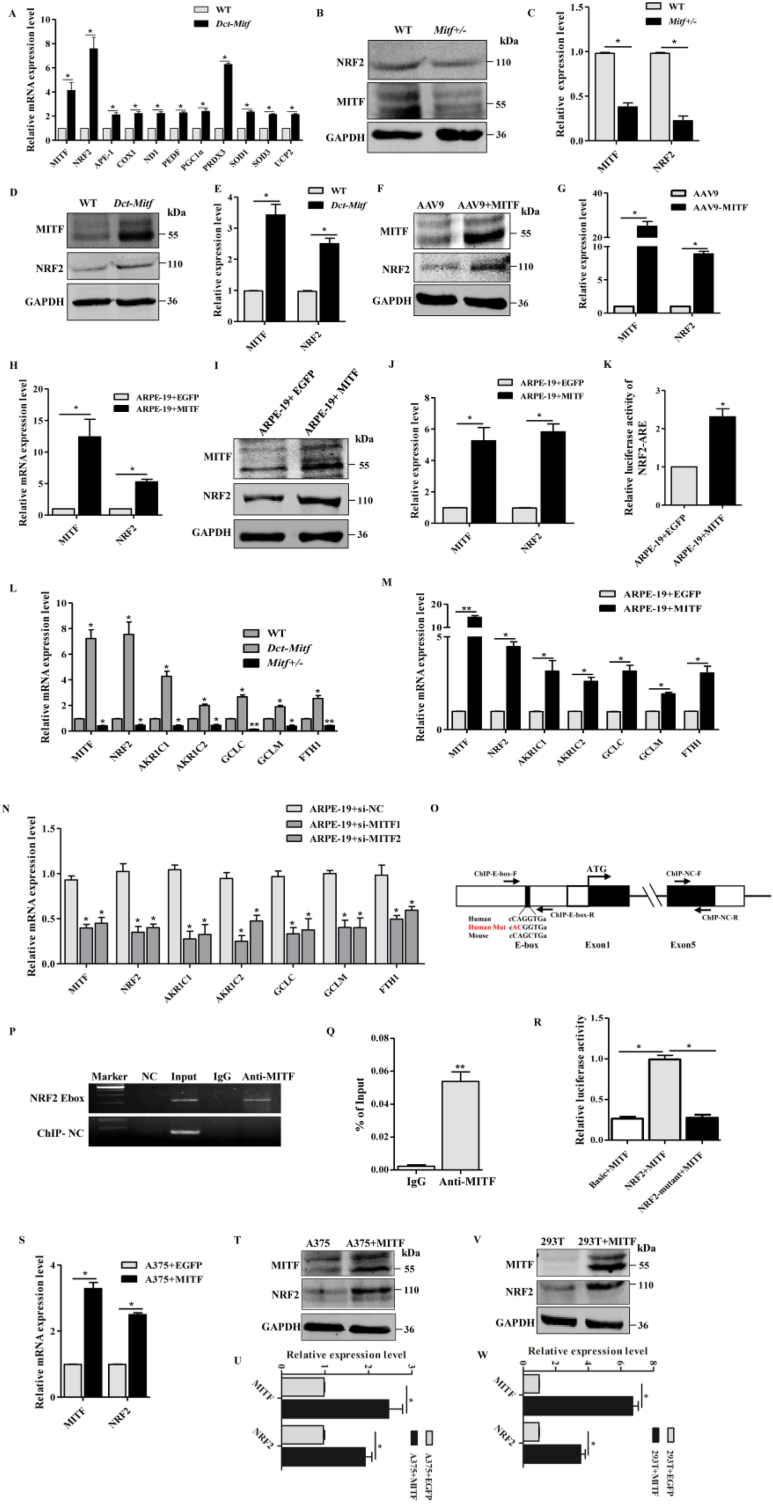
To address the molecular mechanisms through which MITF regulates antioxidant signaling, we subsequently explored antioxidant genes that might be regulated by MITF in RPE cells. Analysis of the expression of antioxidant genes by real-time PCR was carried out to determine which were upregulated in Dct-Mitf mice RPE cells relative to WT mice (Fig. 4A). Among them, Nrf2 has been reported to be a master regulator in the antioxidant pathway [47]. Nrf2 knockout mice respond to oxidative stress with changes that present as age related retinopathy [16], while pharmacological or microRNA potentiated Nrf2 activation enhanced the antioxidant ability of RPE cell and protected the neural retina from oxidative stress induced retinal degeneration [48,49]. In order to elucidate whether MITF regulates the NRF2 dependent antioxidant signaling pathway in RPE cells, NRF2 protein levels were measured in Mitf +/-and Dct-Mitf mouse RPE cells. As shown in Fig. 4B and C, NRF2 protein levels were decreased in Mitf ±mouse RPE cells compared to those of WT mice, and were upregulated in Dct-Mitf mice (Fig. 4D and E) and AAV9-MITF injected mice RPE cells (Fig. 4F and G). In addition, we also confirmed these results in MITF-overexpressing ARPE-19 cells in culture (ARPE-19 + MITF cells), in which increasing MITF levels promoted the expression of NRF2 at both the mRNA and protein levels (Fig. 4H–J).
Fig. 4.
MITF directly regulates the expression of NRF2 through binding to its promoter region. (A) Relative mRNA expression levels of Mitf and a subset of antioxidant genes were carried out in the WT or Dct-Mitf mice RPE cells by real-time PCR. Gapdh was used as endogenous control, and the value of each gene in the WT group was normalized as 1. (B, C) Western blotting analysis of MITF and NRF2 in WT or Mitf+/- mice RPE cells. (D, E) Western blotting analysis of MITF and NRF2 in WT or Dct-Mitf mice RPE cells. (F, G) Western blotting analysis of MITF and NRF2 in the RPE cells of AAV9-MITF or AAV9 virus-infected mice. (H) Real-time PCR analyzed the MITF and NRF2 mRNA levels in ARPE-19 + MITF cell. (I, J) Western blotting analysis the protein level of MITF and NRF2 in ARPE-19 + MITF cells. (K) The luciferase activity of ARE was measured in ARPE-19 cells as indicated, when the endogenous MITF was knocked-down with specific siRNA. (L) Real-time PCR showed that relative mRNA expression levels of Mitf, Nrf2 and its downstream antioxidant target gene were upregulated in Dct-Mitf mice RPE cell and decreased in Mitf+/- mice RPE cell. (M) Real-time PCR showed that overexpression of MITF upregulated the levels of NRF2 and its antioxidant target genes, (N) but knockdown of MITF reduced their transcription levels in ARPE-19 cells. (O) Schematic diagram of potential binding sites motif (E-box) for MITF in the human NRF2 gene promoter region, which is conserved between human and mouse. Red font marks the mutant E-box. (P) ChIP-PCR and enrichment analysis (Q) were performed in ARPE-19 + MITF cells, which showed that MITF can bind to the NRF2 proximal promoter. (R) Luciferase reporter assay was carried out in 293T cells, which showed that MITF signficantly stimulated the transcription of the wild type but not the E-box mutant (Red font in O) NRF2 promoter. (S) Real-time PCR showed that overexpression of MITF in A375 cells upregulated the expression of NRF2. (T, U) Western blotting analysis the protein level of NRF2 in MITF overexpression A375 cells. (V, W) Western blotting analysis the protein level of NRF2 in MITF overexpression 293T cells. (n = 4, **P < 0.01, *P < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
These results indicate that MITF promotes NRF2 expression in RPE cells, but not whether MITF actives NRF2 dependent antioxidant signaling. NRF2 controls both the basal and oxidative stress-inducible expression of antioxidant genes that share in a common ARE consensus sequences [50]. Here, we first showed that pARE-luc (a reporter gene plasmid) elevated the transcriptional activity of the cis-acting element ARE in ARPE-19 + MITF cells (Fig. 4K), and confirmed similar results in 293T cells (Fig. S5). Moreover, we selected known downstream antioxidant genes of NRF2 and tested their expression levels. Real-time PCR showed that the relative mRNA expression levels of Mitf, Nrf2 and its known downstream antioxidant genes were increased in Dct-Mitf and decreased in Mitf+/- mice RPE cells (Fig. 4L). In addition, overexpression of MITF upregulated the levels of NRF2 and its target genes (Fig. 4M), while interference of MITF reduced their transcription levels in ARPE-19 cells (Fig. 4N). Taken together, these data suggested that MITF not only promotes the expression of NRF2, but also its physiologic functions in regulating antioxidant signaling.
The above data demonstrated that MITF regulates the expression of NRF2 in RPE cells, but did not elucidate the mechanism through which MITF regulates NRF2. To address this question, we analyzed the promoter region of the human NRF2 gene and identified a conserved E-box binding site for MITF within 7102 bp upstream of the transcription start site (NM_001145413, Fig. 4O), consistent with earlier ChIP-seq results in HA-MITF overexpressing melanoma cell line using HA antibody [51]. In fact, ARPE-19 + MITF cell ChIP-PCR assays indicated that amplimers containing NRF2 E-box detected positive signals in both Input and anti-MITF lanes, while the ChIP-NC primers detected a positive band only in the Input lane, but not in any immunoprecipitated sample (Fig. 4P). Quantitation by real-time PCR, showed a significant enrichment of anti-MITF ChIP compared to IgG (Fig. 4Q). Additionally, MITF showed strong transcriptional activity with the NRF2 wild type but not the E-box mutation promoter in dual luciferase reporter assays (Fig. 4R). Taken together, these results demonstrated that MITF directly binds to the NRF2 promoter and actives its transcription.
In order to examine whether MITF regulates the expression of NRF2 in general, we further analyzed their expressional regulation in other cell types. As shown in Fig. 4S-U, overexpression of MITF can up-regulate NRF2 mRNA and protein levels significantly in the melanoma cell line A375. In addition, overexpression of MITF in 293T cells, which do not express endogenous MITF, also promotes the expression of NRF2 in these cells (Fig. 4V, W). These results suggest that MITF might have a general role in regulation of NRF2 expression in antioxidant signaling.
2.5. MITF promotes NRF2 nuclear translocation predominantly via up-regulating p62
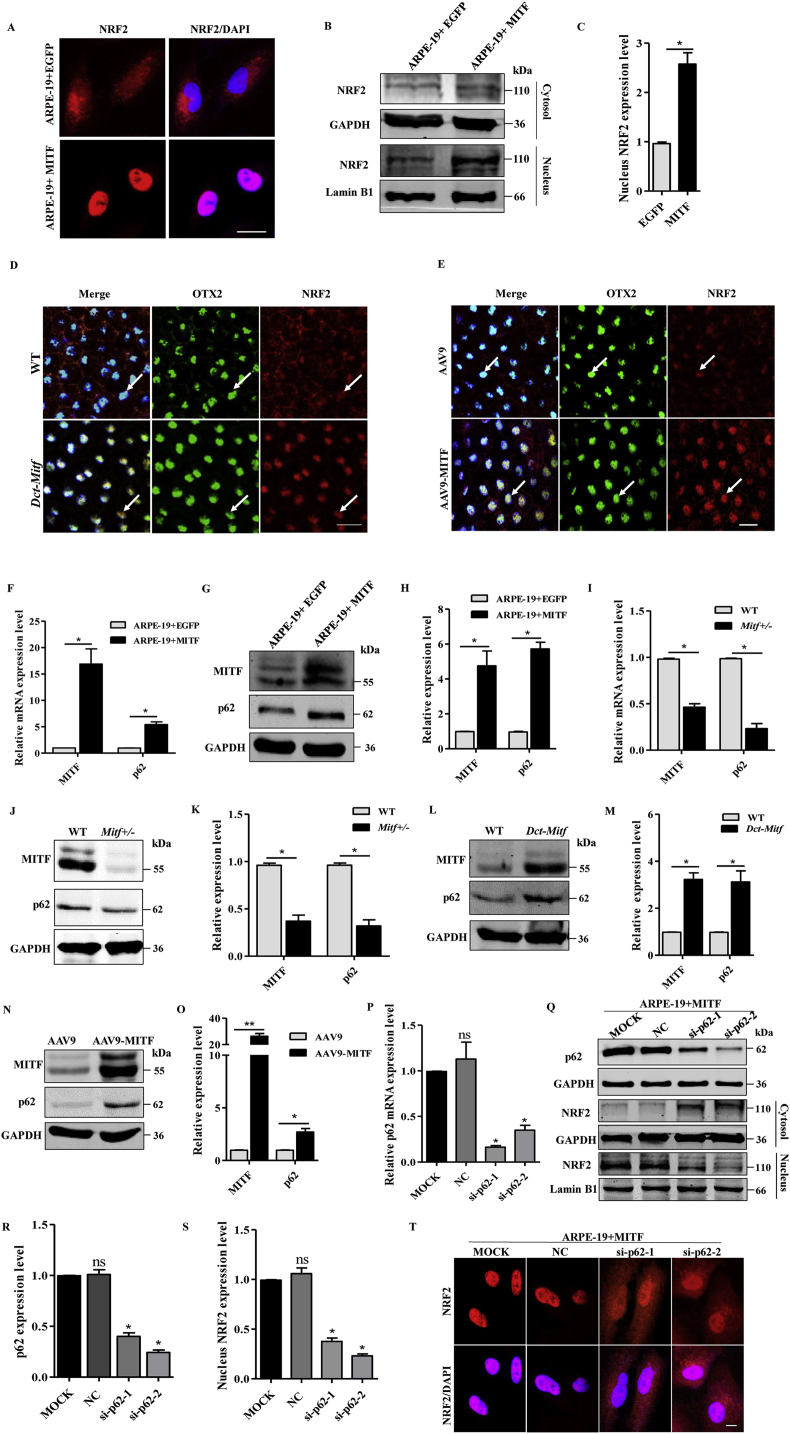
In addition to MITF regulation of NRF2 expression, it remained possible that MITF altered the activity of NRF2 post-transcriptionally. This was examined by comparing NRF2 immunofluorescence in ARPE-19 + MITF to control ARPE-19 cells. As seen in Fig. 5A, NRF2 positive signals are observed in both the cytoplasmic and nuclear compartments in ARPE-19 + EGFP cells, whereas it was predominantly nuclear in ARPE-19 + MITF cells, suggesting that MITF might regulate the nuclear translocation of NRF2 in RPE cells. Confirming this observation, western blotting of NRF2 isolated from the nuclear and cytoplasmic compartments showed that overexpression of MITF significantly increased the nuclear level of NRF2 in ARPE-19 cells (Fig. 5B and C). Moreover, nuclear levels of NRF2 were also increased in Dct-Mitf mice RPE cells compared to those of WT mice (Fig. 5D, Fig. S6), and treatment with AAV9-MITF also elevated nuclear levels of NRF2 compared to the control group (Fig. 5E). These results clearly indicate that MITF not only increases the transcription of NRF2, but also promotes its nuclear translocation, although they did not delineate the mechanism.
Fig. 5.
MITF promotes NRF2 nuclear translocation predominantly via up-regulating p62 expression. (A) Immunostaining of NRF2 in ARPE-19 + EGFP or ARPE-19 + MITF cells as indicated. Scale bar: 20μm. (B, C) Western blotting analysis of the nuclear and cytoplasmic proteins using specific antibodies as indicated. (D) Double immunostaining of OTX2 and NRF2 was performed on WT or Dct-Mitf mice RPE flat-mount. Enlarged images in the white line boxes, which indicated that NRF2 nuclear localization was increased in Dct-Mitf RPE cells (white arrow indicated). Scale bar: 20μm. (E) Immunostaining result showed that NRF2 nuclear translocation was increased in the AAV9-MITF infected mice RPE cells (white arrow indicated). Scale bar: 20μm. (F) Real-time PCR showed that MITF upregulated the mRNA level of p62 in ARPE-19 cells. (G) Western blotting analysis of the p62 protein level in ARPE-19 cell. (H) Quantification of western blot bands based on the result of (G). (I) Relative mRNA levels of Mitf and p62 were carried out by real-time PCR in WT or Mitf ±mice RPE cells, which were decreased in Mitf+/- mice RPE cells. (J) Western blotting analysis of MITF and p62 in WT or Mitf+/- mice RPE cells. (K) Quantification of western blotting bands based on the result of (J). (L) Western blotting analysis of MITF and p62 in WT or Dct-Mitf mice RPE cells. (M) Quantification of western blot bands based on the result of (L). (N) Western blotting analysis of MITF and p62 in WT mice RPE cells after injection of AAV or AAV-MITF virus. (O) Quantification of western blotting bands based on the result of (N). (P)p62 knockdown efficiency was carried out by real-time PCR in ARPE-19 + MITF cells. (Q, R and S) Protein levels of p62 and NRF2 were analyzed by western blotting, which showed that knockdown of p62 in ARPE-19 + MITF cells reduces the nuclear translocation of NRF2, but increases cytoplasmic NRF2 level. (T) Immunostaining of NRF2 in ARPE-19 + MITF cells, which showed that after knockdown of p62 in ARPE-19 + MITF cell, the nuclear translocation of NRF2 was partial reversed. Scale bar: 20μm.Representative images and quantitative data (n = 4, **P < 0.01, *P < 0.05).
It has been reported that p62 can promote nuclear translocation of NRF2 through competitively binding KEAP1 [21,22]. Our previous work demonstrated that MITF promotes the expression of PGC1α, which can target p62 [42,52]. To determine the role of p62 in MITF induced NRF2 nuclear translocation, we initially examined p62 expression levels in RPE cells in vivo and ex vivo. As seen in Fig. 5F–H, MITF up-regulates p62 expression in ARPE-19 cells at both the mRNA and protein levels. p62 Expression is decreased in Mitf+/- RPE cells (Fig. 5I–K) but is increased in both Dct-Mitf (Fig. 5L, M) and AAV9-MITF injected mice RPE cells (Fig. 5N, O). These results show that MITF positively regulates the expression of p62 in RPE cells both in vivo and ex vivo, but do not show whether MITF acts through p62 to promote the NRF2 nuclear translocation directly. This question was addressed by seeing whether knockdown of p62 could inhibit NRF2 nuclear translocation in ARPE-19 + MITF cells. After 48 h of efficient knockdown of p62 in ARPE-19 cells (Fig. 5P–R), western blotting showed a significant reduction in the nuclear translocation of NRF2, while its cytoplasmic level was increased (Fig. 5Q, S), which was confirmed by immunofluorescent localization of NRF2 in ARPE-19 + MITF cells with or without knockdown of p62 (Fig. 5T).
2.6. Partial rescue of retinal oxidative damage in Mitf+/- mice by gene transfer mediated RPE cell specific overexpression of NRF2
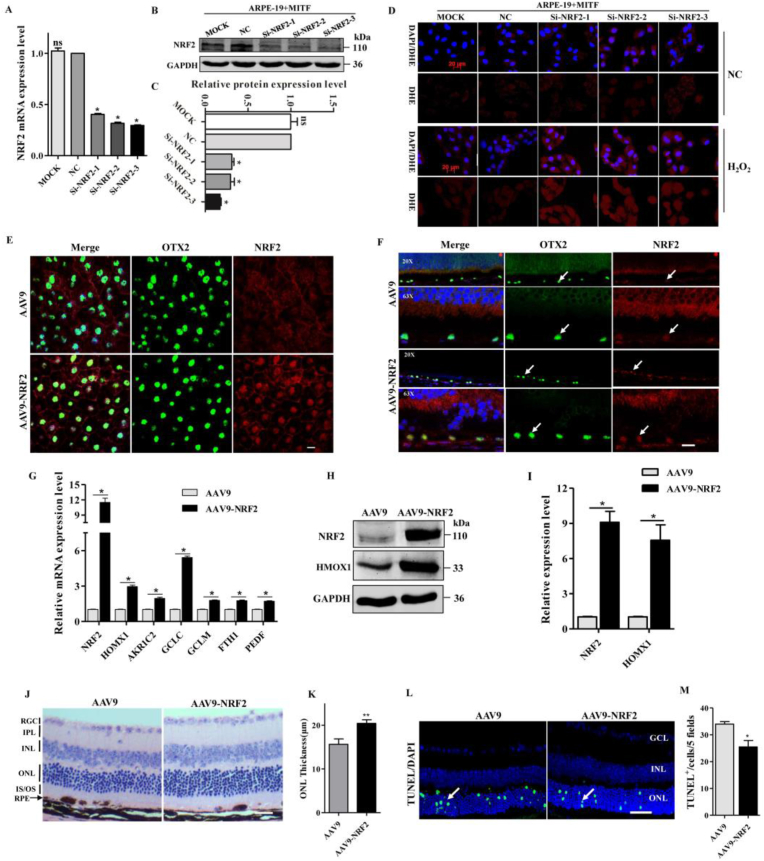
The above results demonstrated that MITF directly regulates the expression of NRF2 and promotes its nuclear translocation, but it remained unclear whether NRF2 mediates MITF function in antioxidant responses in RPE cells. To address this question, we knocked-down NRF2 in ARPE-19 + MITF cells using a specific siRNA (Fig. 6A–C), which didn't affect the expression of MITF, but down-regulated the levels of the NRF2 downstream target genes (Fig. S7). Under normal conditions, there is only a minimal difference in the ROS level between the si-NC and si-NRF2 transfected ARPE-19 + MITF cells, but exposure of the cells to 0.65 mmol/l H2O2 resulted in a marked increase in ROS levels in NRF2 knockdown but not the si-NC transfected ARPE-19 + MITF cells (Fig. 6D). We also evaluated whether gene transfer-mediated expression of Nrf2 in Mitf+/- RPE cells could protect the retina from oxidative damage. Mouse Nrf2 (NM_010902) was inserted into the AAV9-p.RPE65-Nrf2 vector (AAV9-NRF2) and injected into the subretinal space of mice. Two weeks later, immunostaining showed that NRF2 was specifically overexpressed in the RPE cells of AAV9-NRF2 injected mice (Fig. 6E and F). Quantitation of both mRNA (Fig. 6G) and protein levels (Fig. 6H and I) of NRF2 target genes confirmed the activation of NRF2-dependent antioxidant signaling in mouse RPE cells after gene transfer mediated high expression of NRF2 in vivo.
Fig. 6.
Gene transfer mediated RPE cell specific overexpression of NRF2 could partial rescue retinal oxidative damage in Mitf ±mice. (A)NRF2 knockdown efficiency was carried out by real-time PCR in ARPE-19 + MITF cells as indicated. (B) NRF2 protein was analyzed by western blotting in ARPE-19 + MITF cell after transfected with si-NRF2. (C) Quantification of western blotting bands based on the result of (B). (D) Immunostaining of DHE (red signal) in ARPE-19 + MITF cells, which showed that after knockdown of NRF2, the intracellular ROS production increased with 4h H2O2 treatment. Scale bar: 20 μm (n = 3). (E, F) Two weeks after the injection of AAV9 or AAV9-NRF2, double immunostaining of OTX2 and NRF2 was performed on RPE flat-mount (E) or retinal sections (F) as indicated, which showed that the NRF2 expression was upregulated in the RPE specifically after the infection of AAV9-NRF2 virus (white arrow indicated), scale bar: 10μm. (G) Real-time PCR showed that relative mRNA expression levels of Nrf2 and its downstream target genes were upregulated in the AAV9-NRF2 injected mice RPE cell. (H) Western blotting analysis of the protein levels of NRF2 and HMOX1 in mice RPE cells after the infection of AAV9-NRF2 virus. (I) Quantification of western blotting bands based on the result of (H). (J, K) HE staining showed that the thickness of ONL layer was thicker in the AAV9-NRF2 infected mice retina than the AAV9 infected eyes after 4 days of 50 mg/kg NaIO3 injection, scale bar: 50μm. (L) TUNEL staining showed that the number of apoptotic cells was decreased in the AAV9-NRF2 virus-infected mouse retinas after injection 50mg/kg NaIO3 (white arrow indicated), scale bar: 50μm. (M) Quantitative analysis of the TUNEL positive cell number based on the results of (L). (n = 6; ns, no significant difference; **P < 0.01, *P < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Two weeks after injection of AAV9 into the left subretinal space and AAV9-NRF2 into the right of 6-wk-old Mitf+/- mice eyes, the mice were intraperitoneally injected with 50 mg/kg NaIO3 and four days later, the retinal structure and TUNEL staining were examined. Compared to the AAV9 injected retina, the AAV9-NRF2 injected Mitf+/- mice retina have a thicker ONL layer and a lower percentage of TUNEL positive cells (Fig. 6J-M). These results suggest that while overexpression of NRF2 in RPE cells of Mitf+/- mice could not completely compensate for the MITF haploinsufficiency, it could partially rescue the neural retina from oxidative damage.
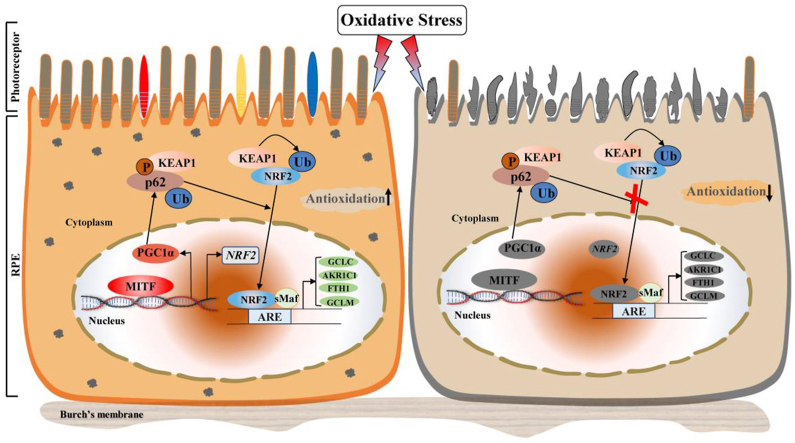
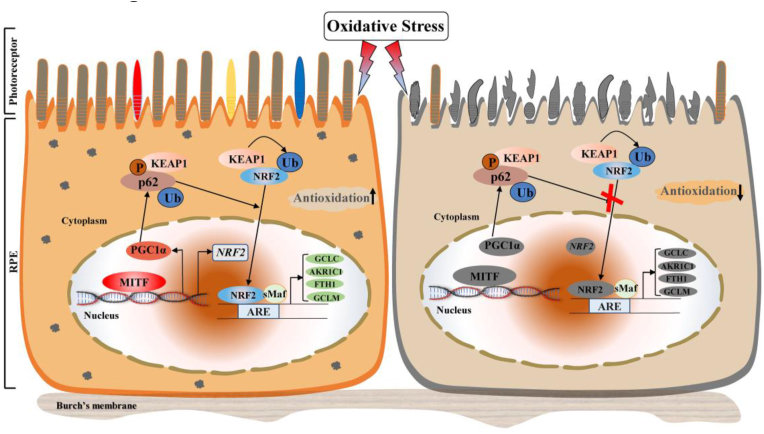
Taken as a whole, our data demonstrate that MITF directly regulates transcription of NRF2 directly by binding to the promoter region and also promotes its nuclear translocation partially through upregulating p62. This then activates antioxidant pathway signaling in RPE cells and protects the neural retina from oxidative stress-induced degeneration (Fig. 7).
Fig. 7.
Graphical summary of MITF action as a potent antioxidant in RPE cells through regulating NRF2-dependant antioxidant signaling to protect the neural retina against oxidative stress-induced degeneration. MITF directly binds to the NRF2 promoter to stimulate its transcription. Meanwhile, MITF up-regulates p62 to promote the nuclear translocation of NRF2, which actives the antioxidant signaling in RPE cells and protects the neural retina against oxidative damage (left). MITF dysfunction will impair the NRF2-dependent antioxidant signaling and exacerbates retinal degeneration under oxidative stress (right).
3. Discussion
The RPE is critical for maintaining homeostasis of the neural retina through numerous biological functions, including its antioxidant properties [2,8]. RPE abnormalities are associated with several retinopathies, while defects in the antioxidant ability of the RPE generally are related to AMD [4]. One approach to treat RPE deficiency induced degenerative retinal disease is to replace deficient cells with healthy and fully functional RPE cells. Another potential approach is to elevate the endogenous neuroprotective functions of the RPE, including supplying neuroprotective factors and enhancing the antioxidant defense system. Here, we show that the transcription factor MITF acts as a potent antioxidant inducer to protect the neural retina from oxidative stress-induced degeneration by regulating NRF2 dependent antioxidant signaling in RPE cells. Haploinsufficiency of MITF in RPE cells exacerbates oxidative stress induced retinal degeneration, while overexpression of MITF in RPE cells does not impair the retinal physiological structure, but can enhance its antioxidant ability and protect the neural retina from oxidative damage in both Mitf transgenic and AAV9-p.RPE65-Mitf mediated gene transfer mice.
NRF2 and PGC1α are two transcription factors important in regulating cellular redox balance. PGC1α was reported to regulate the expression of NRF2 [11,53], while the PGC1α promoter also contains ARE consensus sequences for the binding of NRF2 [54], but, it is unclear whether a positive feed-back regulatory loop between NRF2 and PGC1α exists in RPE cells. Moreover, mice deficient in Pgc1α are susceptible to light-induced retinal damage [55] and conditional knockout of Pgc1α in adult mice resulted in RPE dysfunction and severe photoreceptor degeneration [56], while Nrf2 knockout mice exhibit age-related retinopathies [16]. Nrf2/Pgc1α double knockout mice exhibit significant age-dependent RPE degeneration, accumulation of oxidative stress markers and damaged mitochondria [13]. These results suggest that PGC1α and NRF2 might share overlapping functions and possible cross-talk in antioxidant pathways.
NRF2 is a master regulator of antioxidant pathway signaling. Its activation regulators or co-factors have been well studied, including p62 [57]. However, the transcriptional regulation of NRF2 is still largely uncharacterized. In RPE cells, XBP1 positively regulates NRF2 at the protein but not mRNA level [23] and ID2 (inhibitor of DNA binding 2) was also reported to regulate expression of NRF2, especially under conditions of oxidative stress [58]. Here we demonstrate that MITF directly regulates the transcription of NRF2 by binding to the promoter region and activating its transcription in RPE and other cells. Interestingly, we have previously demonstrated that MITF directly regulates the expression of PGC1α in ARPE-19 cells [42], which suggests MITF might play a key role in antioxidant signaling through regulating both PGC1α and NRF2 simultaneously.
p62 has been demonstrated to regulate nuclear translocation of NRF2, and our data indicate that MITF promotes the nuclear translocation of NRF2 in RPE cells partial through p62. However, the mechanism through which MITF regulate p62 is still unclear. Our previous work has demonstrated that MITF directly regulates the expression of PGC1α in RPE cells [42], which was reported to activate expression of p62 [52]. Thus, one possible mechanism through which MITF might promote the expression of p62 is through regulation of PGC1α. The precise regulatory network among MITF, NRF2 and PGC1α is not only intrinsically interesting, but is also important for understanding the regulatory mechanisms of antioxidant signaling.
Although our data suggests that MITF regulates the antioxidant pathway through NRF2 and protects the neural retina from oxidative damage, it also regulates other target genes in RPE cells, including PGC1α and PEDF, which also have neuroprotective and/or antioxidant functions [42,59]. In fact, our data show that overexpression of NRF2 in Mitf ±RPE cells only partially rescues the retina from NaIO3-induced oxidative degeneration. It is possible that MITF also regulates other target genes in different functional pathways, and that combination of NRF2 with other MITF downstream factors, such as PEDF and PGC1α might lead to a more complete rescue.
Retinal degenerative diseases lead to visual impairment, and blindness in severe cases. The RPE plays important roles in the pathological mechanisms of these diseases, while RPE cell dysfunction can lead to various types of retinopathies including RP (retinitis pigmentosa) and AMD, which still lack effective treatment. Preventing vision loss in patients suffering from retinal degenerations is a major challenge. Currently, hundreds of genes have been implicated in retinal degenerations (RetNet:https://sph.uth.edu/retnet/disease. htm), even though the genes responsible for approximately 40% of retinal degeneration cases are currently unknown [60]. This indicates that the total number of genetic mutations capable of causing retinal degenerations is huge. To address each genetic deficit individually would be a difficult and expensive approach. Although the genes and mutations causing retinal degeneration vary, many retinopathies share common characteristics, including oxidative stress, protein misfolding, abnormalities of the visual cycle, and photoreceptor damage [61]. The RPE plays a critical role in maintaining retinal structure through providing trophic factors, antioxidant activity, maintaining ionic balance, phagocytosis of shed photoreceptor outer segments and involvement in the visual cycle. MITF previously has been reported to regulate the expression of trophic factors, visual cycle enzymes and antioxidants in RPE cells [39,41,42,59]. It is reasonable to hypothesize that increasing the level of MITF in RPE cells might enhance multiple cellular functions to protect against retinal degeneration. In fact, a transgenic approach of ectopic expression of MITF in Mitf deficient mouse RPE cells can partially rescue the microphthalmia and RPE pigmentation seen in this model [62]. Our current data show that high expression of MITF can protect the retina from oxidative damage, but, whether MITF could prevent or slow the retinal degeneration in inherited retinal degeneration diseases is an important and interesting question which will need to be investigated in suitable animal models.
Besides RPE cells, MITF and NRF2 are also widely expressed in other cell types, suggesting that they might have a role in antioxidant responses in general. Interestingly, MITF also positively regulates the expression of NRF2 in melanoma and 293T cells, suggesting that regulation of NRF2 by MITF is a general phenomenon and suggested that MITF might play roles in regulating antioxidant signaling pathways through NRF2 in other non-ocular oxidative diseases. Consistent with this hypothesis, MITF has been reported to participate in antioxidant regulation in melanocyte and melanoma cell lines, and was associated with melanocyte destruction in vitiligo [63,64]. Beside RPE and melanocytes, MITF mutations were also reported to be associated with nonsyndromic hearing loss in patients [31] and Mitf mutant mice also show cardiac hypertrophy and reduced nephron number [65,66], while NRF2 and oxidative stress have also been implicated in cardiomyopathies and kidney diseases [43,67,68]. This suggests that MITF might also protect other tissues from oxidative damage in extraocular disease models.
In summary, this study shows that MITF plays a key role in antioxidant signaling by regulating the antioxidant master regulator, NRF2. Increasing MITF levels in RPE cells can prevent the neural retina from oxidative stress induced degeneration. As oxidative damage contributes to numerous human diseases, MITF with its potent ability to regulate NRF2 and antioxidant signaling, might have therapeutic value for the prevention or treatment of retinal oxidative damage and oxidative stress diseases in general.
4. Material and methods
4.1. Animals
Mitf mi-vga9 mutant mice [33], here designated Mitf−/− mice were from NIH Dr. Arnheiter. The HA-Mitf plasmid and Dct promoter were from NIH Dr. Heinz Arnheiter and University of Maryland School of Medicine Dr. Thomas J. Hornyak separately. The construction of Dct-HA-Mitf transgenic mouse was performed in Nanjing Biomedical Research Institute of Nanjing University, China. The wild type mice were purchased from the Charles River Laboratories, China. All the mice were kept on the C57BL/6J background and rd8 mutation of Crb1 gene was excluded. Experimental mice were bred in the SPF facility of Wenzhou Medical University, and the animal care and experimental procedures were performed in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Statement on the Use of Animals in Ophthalmic and Vision Research and were approved by the Wenzhou Medical University Animal Care and Use Committee (wydw2019-0353).
4.2. NaIO3 induced retinal degeneration
NaIO3 induced retinal degeneration animal model was previously reported to be used for studying retinal oxidative damage [45,69]. NaIO3 (BBI Life Sciences) was dissolved in sterile normal saline at a stock concentration of 1% (w/v), and was given via intraperitoneal injection (10mg/kg or 50mg/kg). The control group was the mice injected with the same volume of normal saline. After 4 days, the eyeballs were taken for the follow-up experiments.
4.3. Electroretinography (ERG)
Methods for mice ERG were as described previously [70]. A RETIport ERG system (Roland Consult, Germany) was used to record the dark-adapted ERGs and Photopic ERGs of mice. The a-wave and b-wave amplitudes in the ERG responses were analyzed.
4.4. Immunostaining
For immunostaining, mice eyes were fixed in 4% paraformaldehyde (PFA) for 2 h, dehydrated in 30% sucrose, and embedded in OCT compounds and snap frozen using liquid nitrogen. Sections (10μm) were collected on a cryostat, and fixed in 4% PFA for 10 min. After rinsing, the sections were blocked with 3% BSA for 1 h at room temperature. The samples were incubated with specific primary antibodies overnight at 4 °C. Staining was indicated by appropriate secondary antibodies. The information of the antibodies used is shown in supplementary Table S1. Each stain was performed on a glass slide of at least 6 animals under each condition, sex in half. Immunostaining results were observed and photographed on a Zeiss con-focal microscope.
4.5. ROS fluorescence assay
ROS levels were determined using the GENMED kit (Genmed Scientifics Inc., Shanghai, China) according to the manufacturer's instructions. Briefly, prepare 10μm thick unfixed retinal frozen sections, adds 200μl of preheated GENMED staining solution incubate for 30 min in a light-protected moistened incubator at 37 °C. Immediate observation under a fluorescence microscope. The method for determining ROS in ARPE-19 + MITF cell was described in our previous work [42].
4.6. Histological analysis and TUNEL staining
Histological analysis of the mice retina was carried out by HE staining. The methods for retinal image acquisition and thickness analysis and comparison were as described previously [71]. Briefly, under a 100X magnification, the Zen analysis software system was used to measure the thickness of the ventral and dorsal retinas of the mice, respectively, from the optic nerve head to the peripheral retina at a distance between 100μm and 1200μm, the average was taken. At least 6 eyeballs were measured in each group. TUNEL staining was performed in the cryopreserved tissue sections using a TUNEL Kit (Roche), according to the instructions. Then the Images were obtained on a Zeiss confocal microscope.
4.7. Cell culture and transfection
The ARPE-19 cell line was purchased from ATCC and cultured in F12/DMEM (Gibco) supplemented with 10% FBS and 1% penicillin/streptomycin at 5% CO2, 37 °C. A375 and 293T cells were cultured in DMEM growth medium with 10% FBS and 1% penicillin/streptomycin at 5% CO2, 37 °C. MITF-overexpressing ARPE-19 cells (ARPE-19 + MITF) were produced and described in our previous work [39]. The siRNAs (400nM/60mm dish) were transfected into the cell using lipo2000 (Mirus Corp), and plasmid DNA was transfected into the cell using PolyJet™ reagent (Signagen) at the indicated concentrations according to the manufacturer's instructions. siRNA sequences were designed and synthesized by Gene Pharma Co., Ltd (Shanghai) as follows: si-NC:5′ UUCUCCGAACGUGUCACGUTT; si-NRF2-1: 5′ CGCUCAGUUACAACUAGAUTT; si-NRF2-2: 5′ CCCGUUUGUAGAUGACAAUTT; si-NRF2-3: 5′ CCAGUUGACAGUGAACUCATT; si-MITF-1: GCUCAUGGACUUUCCCUUAdTdT; si-MITF-2: GUAUGCAGAUGGAUGAUGUdTdT; si-p62-1: 5′ GGAACAGAUGGAGUCGGAUTT; si-p62-2: 5′ CCUACGUGAAGGAUGACAUTT.
4.8. Western blotting and quantitative real-time PCR
The methods for western blotting and quantitative real-time PCR were described previously [39,42,72]. GAPDH was used as the endogenous control in quantitative real-time PCR experiments, the relative value of each gene was normalized to GAPDH. Primers used in quantitative real-time PCR are listed in Table S2. A full list of antibodies is shown in Table S1, which were recognized by the fluorescein-conjugated secondary antibodies (LI-COR) at room temperature for 1 h in the dark. The protein bands were scanned using the odyssey CLx system (LI-COR) and quantitative densitometry of the bands was performed using ImageJ software. All experiments were performed a minimum of 4 times.
4.9. Chromatin immunoprecipitation
ChIP analysis was carried out in ARPE-19 + MITF cells as described in our previous work [72] using a Magna ChIP™ A/G Chromatin Immunoprecipitation Kit (Millipore) following the supplier's suggested protocol, and antibodies of anti-Human IgG (Abcam) and anti-MITF (D5) (Neo Markers, Fremont CA). Primers used for ChIP-PCR were as follows: NRF2-ChIP-F: gatatgattcatgttccacctgg; NRF2-ChIP-R: cccctgagatccttccttca; NRF2-ChIP–NC–F: ctgtaagtcctggtcatcgga; NRF2-ChIP–NC–R: tgaagtcaacaacagggaggt. MITF ChIP enrichment analysis was carried out by real-time PCR and normalized with the Input group.
4.10. Luciferase reporter assay
The 641bp human NRF2 promoter was amplified using the primers of NRF2-Report-F: atacgcgtggctggtctcaaactcctgggct and R: atctcgaggagaatgacaggaaggtggtggg, which was cloned into the PGL3-basic vector (Promega) between XhoI and MluI restriction sites. Luciferase activity was carried out as described previously [42,72].
4.11. AAV9 vector construction and virus injection
AAV9-p.RPE65-MCS-SV40-PolyA vector was acquired from the Shanghai Genechem Co., LTD, China. Mouse HA-Mitf (NM_008601) was from NIH Dr. Arnheiter, which was constructed into the AAV9 vector using the restriction enzymes NcoI/HindIII. Mouse Nrf2 (NM_010902) was constructed into the AAV9 vector using the restriction enzymes EcoRI/HindIII. Approximately 0.5μl of AAV9, AAV9-MITF or AAV-NRF2 virus supernatant (1012–1013 genome copies/ml) was injected into the subretinal space of the mice, using a pulled angled glass pipette under direct observation aided by a dissecting microscope under dim light.
4.12. Statistical analysis
Each experiment was repeated a minimum of four times and all quantitative results were presented as mean ± standard deviation (SD). Each mouse experiment was carried out using at least 6 mice, half of each sex. All statistical analyses were carried out by a non-parametric Mann-Whitney U test. P < 0.05 was considered as a significant difference.
Author contributions
Conceived and designed the experiments: X Ma, L Hou. Performed the experiments: S Han, J Hua, X Hu, G Zheng, S Jian, X Ma. Analyzed the data: S Han, J Hua, J Yang, X Ma, L Hou. Contributed reagents/materials/analysis tools: X Ma, L Hou, J Chen, J Qu, J Wang, H Li. Wrote the manuscript: S Han, JF Hejtmancik, X Ma, L Hou. Revised the manuscript: S Han, JF Hejtmancik, X Ma, L Hou.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
We would like to thank NIH Dr. Heinz Arnheiter and University of Maryland School of Medicine Dr. Thomas J. Hornyak for providing Mitf mi-vga9 mutant mice, plasmids and reagents. This work is supported by the National Natural Science Foundation of China (81870664, 81770946, 81570892, 81600748 and 81800838), the Research Grant of Wenzhou Medical University Eye Hospital (KYQD151211, YNZD201605). This study was also supported by the Project of State Key Laboratory of Ophthalmology, Optometry and Visual Science, Wenzhou Medical University (437201804G).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101537.
Contributor Information
Jia Qu, Email: jqu@wz.zj.cn.
Xiaoyin Ma, Email: xyma2015@wmu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Hyttinen J.M.T., Blasiak J., Niittykoski M., Kinnunen K., Kauppinen A., Salminen A. DNA damage response and autophagy in the degeneration of retinal pigment epithelial cells-Implications for age-related macular degeneration (AMD) Ageing Res. Rev. 2017;36:64–77. doi: 10.1016/j.arr.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 3.Yu D.Y., Cringle S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005;80:745–751. doi: 10.1016/j.exer.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Khandhadia S., Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expet Rev. Mol. Med. 2010;12:e34. doi: 10.1017/S146239941000164X. [DOI] [PubMed] [Google Scholar]
- 5.Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 6.Blasiak J., Pawlowska E., Szczepanska J., Kaarniranta K. Interplay between autophagy and the ubiquitin-proteasome system and its role in the pathogenesis of age-related macular degeneration. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias M.F., Joo K., Kemp J.A., Fialho S.L., da Silva Cunha A., Jr., Woo S.J. Molecular genetics and emerging therapies for retinitis pigmentosa: basic research and clinical perspectives. Prog. Retin. Eye Res. 2018;63:107–131. doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Simo R., Villarroel M., Corraliza L., Hernandez C., Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. J. Biomed. Biotechnol. 2010;2010 doi: 10.1155/2010/190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswal M.R., Han P., Zhu P., Wang Z., Li H., Ildefonso C.J. Timing of antioxidant gene therapy: implications for treating dry AMD. Invest. Ophthalmol. Vis. Sci. 2017;58:1237–1245. doi: 10.1167/iovs.16-21272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaramillo M.C., Zhang D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldelli S., Aquilano K., Ciriolo M.R. Punctum on two different transcription factors regulated by PGC-1alpha: nuclear factor erythroid-derived 2-like 2 and nuclear respiratory factor 2. Biochim. Biophys. Acta. 2013;1830:4137–4146. doi: 10.1016/j.bbagen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 13.Felszeghy S., Viiri J., Paterno J.J., Hyttinen J.M.T., Koskela A., Chen M. Loss of NRF-2 and PGC-1alpha genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2019;20:1–12. doi: 10.1016/j.redox.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Ramirez M., Hernandez C., Ruiz-Meana M., Villarroel M., Corraliza L., Garcia-Dorado D. Erythropoietin protects retinal pigment epithelial cells against the increase of permeability induced by diabetic conditions: essential role of JAK2/PI3K signaling. Cell. Signal. 2011;23:1596–1602. doi: 10.1016/j.cellsig.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Liao R., Yan F., Zeng Z., Wang H., Qiu K., Xu J. Insulin-like growth factor-1 activates PI3K/Akt signalling to protect human retinal pigment epithelial cells from amiodarone-induced oxidative injury. Br. J. Pharmacol. 2018;175:125–139. doi: 10.1111/bph.14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Z., Chen Y., Wang J., Sternberg P., Freeman M.L., Grossniklaus H.E. Age-related retinopathy in NRF2-deficient mice. PloS One. 2011;6 doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta S., Cano M., Ebrahimi K., Wang L., Handa J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachdeva M.M., Cano M., Handa J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong W., MacColl Garfinkel A.E., Li Y., Benowitz L.I., Cepko C.L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Invest. 2015;125:1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paladino S., Conte A., Caggiano R., Pierantoni G.M., Faraonio R. Nrf2 pathway in age-related neurological disorders: insights into MicroRNAs. Cell. Physiol. Biochem. 2018;47:1951–1976. doi: 10.1159/000491465. [DOI] [PubMed] [Google Scholar]
- 21.Ichimura Y., Waguri S., Sou Y.S., Kageyama S., Hasegawa J., Ishimura R. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 23.Chen C., Zhong Y., Wang J.J., Yu Q., Plafker K., Plafker S. Regulation of Nrf2 by X box-binding protein 1 in retinal pigment epithelium. Front. Genet. 2018;9:658. doi: 10.3389/fgene.2018.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharti K., Nguyen M.T., Skuntz S., Bertuzzi S., Arnheiter H. The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigm. Cell Res. 2006;19:380–394. doi: 10.1111/j.1600-0749.2006.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X., Li H., Chen Y., Yang J., Chen H., Arnheiter H. The transcription factor MITF in RPE function and dysfunction. Prog. Retin. Eye Res. 2019 doi: 10.1016/j.preteyeres.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Bertolotto C., Lesueur F., Giuliano S., Strub T., de Lichy M., Bille K. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature. 2011;480:94–98. doi: 10.1038/nature10539. [DOI] [PubMed] [Google Scholar]
- 27.George A., Zand D.J., Hufnagel R.B., Sharma R., Sergeev Y.V., Legare J.M. Biallelic mutations in MITF cause coloboma, Osteopetrosis, microphthalmia, macrocephaly, albinism, and deafness. Am. J. Hum. Genet. 2016;99:1388–1394. doi: 10.1016/j.ajhg.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tassabehji M., Newton V.E., Liu X.Z., Brady A., Donnai D., Krajewska-Walasek M. The mutational spectrum in Waardenburg syndrome. Hum. Mol. Genet. 1995;4:2131–2137. doi: 10.1093/hmg/4.11.2131. [DOI] [PubMed] [Google Scholar]
- 29.Tassabehji M., Newton V.E., Read A.P. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat. Genet. 1994;8:251–255. doi: 10.1038/ng1194-251. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama S., Woods S.L., Boyle G.M., Aoude L.G., MacGregor S., Zismann V. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;480:99–103. doi: 10.1038/nature10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z., Chen Q.D., Zhao L.P., Ma J., Zhang T.S., Pang J.X. A novel variant in MITF in a child from Yunnan-Guizhou Plateau with autosomal dominant inheritance of nonsyndromic hearing loss: a case report. Mol. Med. Rep. 2018;17:6054–6058. doi: 10.3892/mmr.2018.8627. [DOI] [PubMed] [Google Scholar]
- 32.Steingrimsson E., Arnheiter H., Hallsson J.H., Lamoreux M.L., Copeland N.G., Jenkins N.A. Interallelic complementation at the mouse Mitf locus. Genetics. 2003;163:267–276. doi: 10.1093/genetics/163.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodgkinson C.A., Moore K.J., Nakayama A., Steingrimsson E., Copeland N.G., Jenkins N.A. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- 34.Smith L.E., Wesolowski E., McLellan A., Kostyk S.K., D'Amato R., Sullivan R. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 35.Garcia-Llorca A., Aspelund S.G., Ogmundsdottir M.H., Steingrimsson E., Eysteinsson T. The microphthalmia-associated transcription factor (Mitf) gene and its role in regulating eye function. Sci. Rep. 2019;9:15386. doi: 10.1038/s41598-019-51819-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakayama A., Nguyen M.T., Chen C.C., Opdecamp K., Hodgkinson C.A., Arnheiter H. Mutations in microphthalmia, the mouse homolog of the human deafness gene MITF, affect neuroepithelial and neural crest-derived melanocytes differently. Mech. Dev. 1998;70:155–166. doi: 10.1016/s0925-4773(97)00188-3. [DOI] [PubMed] [Google Scholar]
- 37.Adijanto J., Castorino J.J., Wang Z.X., Maminishkis A., Grunwald G.B., Philp N.J. Microphthalmia-associated transcription factor (MITF) promotes differentiation of human retinal pigment epithelium (RPE) by regulating microRNAs-204/211 expression. J. Biol. Chem. 2012;287:20491–20503. doi: 10.1074/jbc.M112.354761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bharti K., Gasper M., Ou J., Brucato M., Clore-Gronenborn K., Pickel J. A regulatory loop involving PAX6, MITF, and WNT signaling controls retinal pigment epithelium development. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma X., Pan L., Jin X., Dai X., Li H., Wen B. Microphthalmia-associated transcription factor acts through PEDF to regulate RPE cell migration. Exp. Cell Res. 2012;318:251–261. doi: 10.1016/j.yexcr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Smith S.B., Defoe D.M. Autoradiographic and biochemical assessment of rod outer segment renewal in the vitiligo (C57BL/6-mivit/mivit) mouse model of retinal degeneration. Exp. Eye Res. 1995;60:91–96. doi: 10.1016/s0014-4835(05)80086-7. [DOI] [PubMed] [Google Scholar]
- 41.Wen B., Li S., Li H., Chen Y., Ma X., Wang J. Microphthalmia-associated transcription factor regulates the visual cycle genes Rlbp1 and Rdh5 in the retinal pigment epithelium. Sci. Rep. 2016;6:21208. doi: 10.1038/srep21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua J., Chen H., Chen Y., Zheng G., Li F., Qu J. MITF acts as an anti-oxidant transcription factor to regulate mitochondrial biogenesis and redox signaling in retinal pigment epithelial cells. Exp. Eye Res. 2018;170:138–147. doi: 10.1016/j.exer.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Schmidlin C.J., Dodson M.B., Madhavan L., Zhang D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019;134:702–707. doi: 10.1016/j.freeradbiomed.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enzmann V., Row B.W., Yamauchi Y., Kheirandish L., Gozal D., Kaplan H.J. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp. Eye Res. 2006;82:441–448. doi: 10.1016/j.exer.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Gong L., Liu F., Xiong Z., Qi R., Luo Z., Gong X. Heterochromatin protects retinal pigment epithelium cells from oxidative damage by silencing p53 target genes. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E3987–E3995. doi: 10.1073/pnas.1715237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Meur G., Lebranchu P., Billaud F., Adjali O., Schmitt S., Bezieau S. Safety and long-term efficacy of AAV4 gene therapy in patients with RPE65 leber congenital amaurosis. Mol. Ther. 2018;26:256–268. doi: 10.1016/j.ymthe.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klettner A. Oxidative stress induced cellular signaling in RPE cells. Front. Biosci. 2012;4:392–411. doi: 10.2741/s275. [DOI] [PubMed] [Google Scholar]
- 48.Saito Y., Kuse Y., Inoue Y., Nakamura S., Hara H., Shimazawa M. Transient acceleration of autophagic degradation by pharmacological Nrf2 activation is important for retinal pigment epithelium cell survival. Redox Biol. 2018;19:354–363. doi: 10.1016/j.redox.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jadeja R.N., Jones M.A., Abdelrahman A.A., Powell F.L., Thounaojam M.C., Gutsaeva D. Inhibiting microRNA-144 potentiates Nrf2-dependent antioxidant signaling in RPE and protects against oxidative stress-induced outer retinal degeneration. Redox Biol. 2019;28 doi: 10.1016/j.redox.2019.101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Canc. Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 51.Strub T., Giuliano S., Ye T., Bonet C., Keime C., Kobi D. Essential role of microphthalmia transcription factor for DNA replication, mitosis and genomic stability in melanoma. Oncogene. 2011;30:2319–2332. doi: 10.1038/onc.2010.612. [DOI] [PubMed] [Google Scholar]
- 52.Salazar G., Cullen A., Huang J., Zhao Y., Serino A., Hilenski L. SQSTM1/p62 and PPARGC1A/PGC-1alpha at the interface of autophagy and vascular senescence. Autophagy. 2019:1–19. doi: 10.1080/15548627.2019.1659612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gleyzer N., Vercauteren K., Scarpulla R.C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.St-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jager S. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 55.Egger A., Samardzija M., Sothilingam V., Tanimoto N., Lange C., Salatino S. PGC-1alpha determines light damage susceptibility of the murine retina. PloS One. 2012;7 doi: 10.1371/journal.pone.0031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosales M.A.B., Shu D.Y., Iacovelli J., Saint-Geniez M. Loss of PGC-1alpha in RPE induces mesenchymal transition and promotes retinal degeneration. Life Sci Alliance. 2019;2 doi: 10.26508/lsa.201800212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tebay L.E., Robertson H., Durant S.T., Vitale S.R., Penning T.M., Dinkova-Kostova A.T. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015;88:108–146. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan Y., Huang Z., Long C., Ning J., Zhang H., Kuang X. ID2 protects retinal pigment epithelium cells from oxidative damage through p-ERK1/2/ID2/NRF2. Arch. Biochem. Biophys. 2018;650:1–13. doi: 10.1016/j.abb.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y., Yang J., Geng H., Li L., Li J., Cheng B. Photoreceptor degeneration in microphthalmia (Mitf) mice: partial rescue by pigment epithelium-derived factor. Dis Model Mech. 2019;12 doi: 10.1242/dmm.035642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L., Chen Y., Jiao X., Jin C., Jiang D., Tanwar M. Homozygosity mapping and genetic analysis of autosomal recessive retinal dystrophies in 144 consanguineous Pakistani families. Invest. Ophthalmol. Vis. Sci. 2017;58:2218–2238. doi: 10.1167/iovs.17-21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lobanova E.S., Finkelstein S., Li J., Travis A.M., Hao Y., Klingeborn M. Increased proteasomal activity supports photoreceptor survival in inherited retinal degeneration. Nat. Commun. 2018;9:1738. doi: 10.1038/s41467-018-04117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michael H.T., Graff-Cherry C., Chin S., Rauck C., Habtemichael A.D., Bunda P. Partial rescue of ocular pigment cells and structure by inducible ectopic expression of mitf-M in MITF-deficient mice. Invest. Ophthalmol. Vis. Sci. 2018;59:6067–6073. doi: 10.1167/iovs.18-25186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu F., Fu Y., Meyskens F.L., Jr. MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/Ref-1. J. Invest. Dermatol. 2009;129:422–431. doi: 10.1038/jid.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi Q., Zhang W., Guo S., Jian Z., Li S., Li K. Oxidative stress-induced overexpression of miR-25: the mechanism underlying the degeneration of melanocytes in vitiligo. Cell Death Differ. 2016;23:496–508. doi: 10.1038/cdd.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tshori S., Gilon D., Beeri R., Nechushtan H., Kaluzhny D., Pikarsky E. Transcription factor MITF regulates cardiac growth and hypertrophy. J. Clin. Invest. 2006;116:2673–2681. doi: 10.1172/JCI27643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phelep A., Laouari D., Bharti K., Burtin M., Tammaccaro S., Garbay S. MITF - a controls branching morphogenesis and nephron endowment. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1007093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ge Z.D., Lian Q., Mao X., Xia Z. Current status and challenges of NRF2 as a potential therapeutic target for diabetic cardiomyopathy. Int. Heart J. 2019;60:512–520. doi: 10.1536/ihj.18-476. [DOI] [PubMed] [Google Scholar]
- 68.Yamawaki K., Kanda H., Shimazaki R. Nrf2 activator for the treatment of kidney diseases. Toxicol. Appl. Pharmacol. 2018;360:30–37. doi: 10.1016/j.taap.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 69.Yang Y., Ng T.K., Ye C., Yip Y.W., Law K., Chan S.O. Assessing sodium iodate-induced outer retinal changes in rats using confocal scanning laser ophthalmoscopy and optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2014;55:1696–1705. doi: 10.1167/iovs.13-12477. [DOI] [PubMed] [Google Scholar]
- 70.Kong S., Du X., Peng C., Wu Y., Li H., Jin X. Dlic1 deficiency impairs ciliogenesis of photoreceptors by destabilizing dynein. Cell Res. 2013;23:835–850. doi: 10.1038/cr.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arjunan P., Lin X., Tang Z., Du Y., Kumar A., Liu L. VEGF-B is a potent antioxidant. Proc. Natl. Acad. Sci. U. S. A. 2018;115:10351–10356. doi: 10.1073/pnas.1801379115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma X., Hua J., Zheng G., Li F., Rao C., Li H. Regulation of cell proliferation in the retinal pigment epithelium: differential regulation of the death-associated protein like-1 DAPL1 by alternative MITF splice forms. Pigment Cell Melanoma Res. 2018;31:411–422. doi: 10.1111/pcmr.12676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.