Abstract

Herein, polypyrrole-based porous carbon (PPC) was prepared by ZnCl2 activation for toluene adsorption from paraffin liquid. The structure properties were adjusted by a dosage of activating agents and carbonization temperature. The result with a 3:1 mass ratio of ZnCl2/PPy at 600 °C showed the highest micropore area and percentage of micropore volume of 1105 m2/g and 86.26%, respectively. In addition, the PPC surface was rich in functional groups and obtained a high N-doped content from 7.00 to 8.82%. The toluene adsorption behavior onto the PPC was comprehensively investigated including isotherms, kinetics, and thermodynamics. The adsorption isotherm accorded with the Freundlich model well, and the kinetic model was fitted more closely to the pseudo-second-order chemisorption. The thermodynamic research uncovered that the adsorption was spontaneous and an endothermic process in essence. The ZnCl2 activation mechanism is discussed based on TG/TGA curves and pore structure analysis at last. The devised way of synthesized microporous carbon is green and simple, which is suited to mass production for the adsorption of toluene from paraffin liquid and reducing environmental pollution.
1. Introduction
Mineral oils are intricate admixtures of hydrocarbons consisting of saturated hydrocarbons (MOSH) and aromatic hydrocarbons (MOAH), mainly alkylated like toluene,1 which are known as paraffin liquid or white oils.2−4 Paraffin liquid (white oils) is widely required for variety of use in industries,5 cosmetics,4 and food or drugs products1,6,7 on the basis of different properties and the level of purity. Aromatic compounds are totally undesirable constituents of paraffin liquid due to deleterious effects on product performance and their mutagenicity or potential skin-carcinogenicity,6,8,9 which must meet very severe criteria in terms of residuary levels. However, these hazardous substances can easily be involved up to 30% and even to 50%, limiting wider application of paraffin liquid.10 Significantly, there is an urgent need to exploit an efficient, economical, and environmentally friendly method to remove aromatic hydrocarbons from paraffin liquid.
With respect to the way of the separation of aromatic hydrocarbons from paraffin liquid for now, there are two main methods, that is, a sulfonated method or catalytic hydrogenation.4,6,11−13 The first mentioned is a treatment with sulfuric acid (oleum) or sulfur trioxide, which has low efficiency and serious effects on the environment. The other method refines by hydrogen with a catalyst at high temperature and pressure to obtain a saturated hydrocarbon, which undertakes the high equipment cost and the risk of working in a flammable and explosive gas production environment.
Meanwhile, the adsorption method has been widely applied to the gas or liquid phase for the past several years14−17 and common adsorbents include molecular sieves,18 silica gel,19,20 Al2O3,21 metal–organic frameworks (MOFs),22,23 and porous carbon materials.24−26 Recently, porous carbon materials have drawn increasing attention on a multitude of applications because they endow high porosity and excellent mechanical/chemical stability and can be prepared relatively economically.27−30 Luna et al.31 evaluated the adsorption behavior of commercial activated carbons on polyaromatic hydrocarbons (PAHs) from heavy naphthenic oils (8 wt %), which reported adsorption capacities of more than 160 mg/g for PAHs. Multiwalled carbon nanotubes with KOH activation, removing toluene from aqueous solutions, were synthesized by Yu et al.32 Kinetic data was well matched to the pseudo-second-order model in their work. They also investigated the effects of different oxygen contents of porous carbons on toluene adsorption in another research,33 which demonstrated the evidence of maximum adsorption capacities first increasing then decreasing. The results indicated that the most outstanding group was that of a 3.2% oxygen content, and N-doped porous carbons were also proven to be beneficial for adsorption in both gas and liquid phases in previous research.27,34
In this study, toluene, as a typical pollution in paraffin liquid, was captured by an efficient adsorbent. Polypyrrole (PPy) is an environmentally benign polymer with high nitrogen content. ZnCl2 is cheap and often applied as an activation agent, which is explored little on the mechanism. As a result, the polypyrrole-based porous carbons (PPCs), synthesized by different doses of the ZnCl2 activation agent and the carbonization temperature, were prepared and characterized. Consequently, the adsorbent with three times of a mass activation agent to polypyrrole at 600 °C (PPC-3-600) was verified as the most suitable one for toluene adsorption. PPC-3-600 showed superior toluene adsorption of 2817.19 mg/g from paraffin liquid (C0 = 6 g/L) at 318 K with a high surface area and micropore area of 1258 and 1105 m2/g, respectively. Additionally, the adsorption activity including adsorption isotherms, adsorption kinetics, and thermodynamic research were evaluated as well as the ZnCl2 activation mechanism.
2. Results and Discussion
2.1. Characterization
2.1.1. SEM and TEM Analysis
Figure 1 and Figure S1 illustrate the structure and morphology of the products. The skeleton of samples is constructed with abundant sphere-like particles of 200–400 nm in diameter with regular shapes (Figure 1a). Closer inspection of the figures shows that the stacking of the balls produces plenty of gaps, which forms pore channel structures and increases the specific surface areas with ZnCl2 activation. On the contrary, it is obvious that spheres without activation compact each other closely, and limited gaps or a pore channel was produced (Figure S1b), fitting well to the following BET analysis. The crack-free surface suggests that the PPC is thermally and morphologically stable with ZnCl2 and the calcination activation process. Besides, the TEM images of the PPC also indicate a sphere-like morphology. The HR-TEM (Figure 1d) graphic uncovers a disordered or amorphous structure of the PPC, which may arise from the π–π interaction of partial polypyrrole chains being alike in that of aromatic groups.35 Also, it is obvious from the HR-TEM image that plenty of micropores are homogeneous on the PPC surface, which may derive from the pyrolysis of PPy within the calcination process.36
Figure 1.
SEM (a–c) and HRTEM (d) images of PPC-3-600.
2.1.2. XRD and Raman Analysis
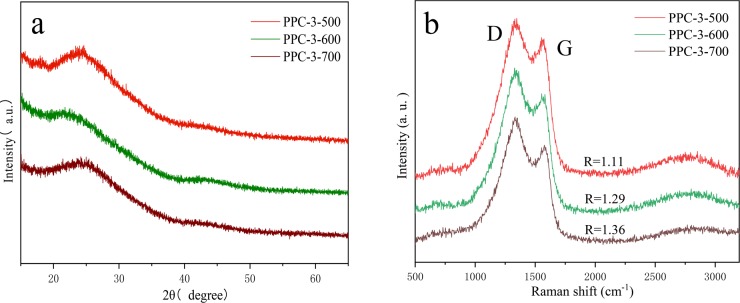
XRD in the wide-angle region (Figure 2a) was measured to further investigate the crystal structure of carbonization materials. The most relevant characteristic peaks at 2θ = 24.3 and 44° can be ascribed to the (002) and (101) planes of hexagonal carbon, respectively.37 The weak and broad intensity peaks indicate that the carbonization samples are predominantly amorphous and possess low degrees of graphitization, fitting well to the TEM observation, which is quite reasonable because N-doped carbon materials induce defect sites and destroy the carbon lattice, and graphene fragments and heterogeneous defects are generated on carbon materials after the activation process as a result.38,39 The Raman spectra can further demonstrate that two typical peaks locate at 1339 and 1565 cm–1 (Figure 2b) in accordance with the D band and G band, respectively. The D band reflects the vacancies and impurities of a hexagonal symmetry in the carbon structure, which is assigned to disordered sp2-hybidized carbon, fitting well with the next XPS. The G band indicates the graphitic structure relating to the 2E2g mode of a two-dimensional network structure. The extent of carbon-containing defects can be revealed by the intensity ratio of the D and G band from previous research (R = ID/IG).28 The R value of PPC-3-500/600/700 is 1.11, 1.29, and 1.36, respectively, which means more defect sites produced with the temperature increasing.
Figure 2.
(a) XRD patterns and (b) Raman spectrum of PPC-3-500, PPC-3-600, and PPC-3-700.
2.1.3. FTIR Analysis
A series of samples are depicted on an FTIR spectra in Figure 3. In detail, the bands at 1285 cm–1 is attributed to C–N stretching vibration of PPy.40 It is apparent that the sharp peak at 1626 cm–1 should be assigned to the fundamental vibrations of the PPy ring or C=O, which is consistent with the results in a previous report.41,42 Furthermore, the bands appearing at 3450 and 3720 cm–1 can be ascribed to N–H and O–H stretching vibrations, respectively,27 wherein a possible explanation for O–H might be adsorbed moisture or residual hydroxy groups of oxidizing reactions. These functional groups would be deeper analyzed and testified in following XPS. The results significantly suggest that all the samples contain abundant nitrogen and oxygen functional groups, which increase much the active sites in the surface43 and further improve the surface properties for adsorption. Moreover, the rising of carbonization temperature and increasing of the ZnCl2 ratio would not destroy these functional groups as a consequence of all the samples having similar peaks in the spectra. Taken together, strong evidence is found in that the samples have a rich surface chemical property for adsorption.
Figure 3.

FTIR spectra for PPC-3-500, PPC-0-600, PPC-3-600, and PPC-3-700.
2.1.4. Pore Structure Analysis
The effect of the ZnCl2 dosage and carbonization temperature on product structure can be explored via N2 ad-/desorption techniques. The isotherms (Figure 4a) exhibit a sharp N2 adsorption increment at a relative pressure of 0–0.1 and then feature a long adsorption plateau without a hysteresis loop ranging from 0.1 to 1.0, indicating the type I(a) shape of the Langmuir isotherm according to the IUPAC classification. These results suggest that the PPC series are porous materials with plenty of narrow micropores,44 which is also in accordance with TEM analysis. The NLDFT model and the micropore size distribution (HK method) are calculated and shown in Figure S2 and Figure 4b, respectively, which are in good agreement. Moreover, the pore structure depends on the ZnCl2 dosage terrifically. The samples without activation shows a poor SSA of 11 m2/g at 600 °C and a PPC-1-600 increase to 568 m2/g. PPC-3-600 concentrates on a small size of 0.3762 nm but possesses the highest micropore area of 1105 m2/g. Besides, the percentage of micropore volume can be up to 86.26% (Table 1). Similar outstanding properties of a 3:1 ratio with ZnCl2 activating porous carbons were also obtained in the other results.45,46 As the ratio increases to 4:1, the surface area, total pore volume, and percentage of micropore volume progressively decrease as opposed to the micropore size enlarging to 0.6046 nm, which might be ascribed to superfluous activation agents for pore formation resulting in pore coalescence.47
Figure 4.
(a) N2 adsorption/desorption isotherms at 77 K (the solid is adsorption, and the hollow is desorption) and (b) micropore size distribution.
Table 1. Textural Parameters of PPC.
| samples | BET surface area (m2/g) | micropore area (m2/g) | HK most probable aperture (nm) | total pore volume (cm3/g) | micropore volume (cm3/g) | percentage of micropore volume (%) |
|---|---|---|---|---|---|---|
| PPC-3-500 | 548 | 484 | 0.6108 | 0.294 | 0.2377 | 80.85 |
| PPC-0-600 | 11 | 0.9954 | 0.041 | 0.0039 | ||
| PPC-1-600 | 569 | 497 | 0.3597 | 0.295 | 0.2485 | 84.24 |
| PPC-3-600 | 1258 | 1105 | 0.3762 | 0.629 | 0.5426 | 86.26 |
| PPC-4-600 | 1055 | 891 | 0.6046 | 0.536 | 0.4496 | 83.88 |
| PPC-3-700 | 1040 | 857 | 0.6039 | 0.540 | 0.4458 | 82.56 |
In terms of the carbonization temperature, it seems similar to the effect of ZnCl2 dosage. PPC-3-500 obtains a low SSA of 548 m2/g and total pore volume of 0.295 cm3/g owing to pyrolysis reactions just commencing and the insufficiency heat provided at this calcination temperature for volatilization and pore channel development. Nevertheless, the quantity of heat is more adequate with the temperature up to 600 °C so that the pyrolysis reactions are carried out completely and most volatile matter and gas are released gradually, thus causing the growth of more pores and getting a high SSA and total pore volume of 1258 m2/g and 0.629 cm3/g, respectively. However, the pore properties reduce again with a temperature increment to 700 °C, which may be on account of the sintering effect followed by shrinkage and realignment of the carbon as well as lessened pore volume.48
2.1.5. XPS Analysis
XPS investigation was performed on representative samples and carried out to obtain more detailed information of surface functional components, chemical composition, and atomic percentages. Carbon peaks (C 1s) at 284.8 eV, nitrogen peaks (N 1s) at 400.1 eV, and oxygen peaks (O 1s) at 532 eV for PPC-3-500, PPC-3-600, and PPC-3-700 °C can be seen in Figure 5a. The high-resolution C 1s photoelectron spectra (Figure 5b) include four common signals, which can be ascribed to the aromatic carbon/C–C, C–O/C–N, C=O, and O–C=O/C=N bonding.29,38,44 The lowest energy contribution at ∼284.7 eV is attributed to graphitic sites of the amorphous carbon, which is consistent with XRD, and the contribution at ∼285.5 eV is attributed as the sp2 C bonded to N in the aromatic structure. The peak at ∼286.3 eV is ascribed to the sp3-hybridized C, but the highest energy (∼288. 9 eV) is ascribed to the sp2-hybridized C in the aromatic ring attached to nitrogen functional groups.49,50 In addition, the carbon content increases with the temperature (Table 2). Figure 5c compares the N 1s intercorrelations among the three patterns of the PPC. According to their different environments, these signals can be deconvoluted into four component peaks located at ∼398.5, ∼399.7, ∼400.3, and ∼401.1 eV, which are attributed to pyridinic (N-6), amine/amide/imine, pyrrolic/pyridonic (N-5), and quaternary nitrogen (N-Q), respectively.51,52 What stands out in the figure is that the intensity of the N-5 and amine/amide/imine peak grows much weaker and its proportion is clearly less; nevertheless, the peaks of N-6 and N-Q rise a lot. The steadier N-6 and N-Q suggest the continuous development of pyridinic nitrogen, such as via transformation of pyridone and imine to pyridine.52 Although the amount of N atoms decreases (Table 2), the abundant N species (N-6 and N-Q) could supply enough active sites.39 The results of high-resolution O 1s spectra are depicted in Figure 5d, which clearly reveals the existence of several oxygen-based groups including carboxyl or carbonyl groups (C=O), epoxy, hydroxyl, or carboxyl groups (−O–/–OH), and carboxyl groups (−COOH).51,53 Interestingly, increasing the temperature to 700 °C leads to a loss of −O–/–OH and even disappearance of −COOH peaks while an apparent growth on C=O. An implication of this is the possibility that −COOH or −O–/–OH is converted to C=O with thermal treatment. Nevertheless, temperature causes a total O atomic reduction from 8.86 to 6.46% (Table 2).
Figure 5.
(a) XPS survey spectra, (b) C 1s, (c) N 1s, and (d) O 1s high-resolution photoelectron spectra of PPC-3-500, PPC-3-600, and PPC-3-700.
Table 2. Chemical Composition from XPS Data.
| samples | C (atomic %) | N (atomic %) | O (atomic %) |
|---|---|---|---|
| PPC-3-500 | 82.32 | 8.82 | 8.86 |
| PPC-3-600 | 84.41 | 7.02 | 8.57 |
| PPC-3-700 | 86.54 | 7.00 | 6.46 |
2.1.6. Analysis of Thermal Characteristic
TGA was conducted to evaluate the thermal stability of PPy with ZnCl2. It mirrors the evidence that ZnCl2 activation occurs in three main stages in Figure 6. The first small amount of region descent, accorded with the small peak in the DTG curve, is attributed to the evaporation of adsorbed moisture.54 Then, a slow weight loss with a broad temperature between 100 and 350 °C should correspond to the pyrolysis of organic matter and carboxylic or hydroxyl groups.33 Finally, a sharp drop with the temperature changing from 350 to 600 °C is assigned to the decomposition of carbon in the PPC. From this time onward, the basic structure of carbon is steadily formed and enters into aromatization.
Figure 6.

TG and DTG thermograms of PPy at a 3:1 impregnation ratio of ZnCl2.
2.2. Adsorption of Toluene and Activation Mechanism
2.2.1. Toluene Adsorption Performance
Figure 7 exhibits the adsorption performance of different PPC-A-B (A = 1, 2, 3, 4; B = 500, 600, 700 °C) materials for toluene from paraffin liquid solution. The adsorption capacity is significantly enhanced as the BET surface area and total pore volume increases (Table 1), which indicates that the adsorbability of the PPC could get a close correlation of the pore properties. Meanwhile, the micropore area takes the main part of all the samples so it is reasonable to speculate that the micropores play a crucial part in toluene adsorption, suiting the former results of researchers.31,33 The best performance for each temperature is 822.84, 2817.19, and 1855.03 mg/g with triple ZnCl2. Therefore, it is PPC-3-600 with the highest micropore area of 1105 m2/g and appropriate pore properties (micropore size and volume of 0.3762 nm and 0.5426 cm3/g, respectively) that displays the most outstanding performance for toluene adsorption, which is chosen for further investigation.
Figure 7.

Comparison of adsorption capacity on toluene with different PPCs (20 mg adsorbent, C0 = 6 g/L, 318 K).
2.2.2. Adsorption Isotherm
Various adsorption models have been used to match toluene adsorption on PPC-3-600. The Langmuir model assumes that the surface properties of the adsorbent were homogeneous and only monolayer adsorption could occur on the surface of the adsorbent. The isotherms can hardly fit with the Langmuir model, so its image is not shown here. The Freundlich isotherm model is non-linear and hypothesizes heterogeneous surfaces, limited sorption sites, and variable potential energy interactions.55 The adsorption isotherms of toluene at 298, 308, and 318 K are shown in Figure 8a following the linearized Freundlich model (Supporting Information, eq S1). The results of calculation parameters are shown in Table S1 with all regression coefficients (R2) more than 0.984. Freundlich supposed that the adsorption capacity increased with the adsorbate concentration.56 Notionally, an infinite amount of adsorption can be obtained.
Figure 8.
(a) Freundlich model and (b) D-R model of toluene adsorption onto the PPC-3-600.
To strengthen the understanding of the adsorption mechanism, the Dubinin–Radusckevich (D-R) model (Supporting Information, eqs S2–S4) was applied to estimate the nature of toluene adsorption (physical or chemical) in Figure 8b, which could describe adsorption on both homogeneous and heterogeneous surfaces.57 Generally, the adsorption energy (E) is greater than 8 kJ/mol, revealing chemical adsorption, and a value under 8 kJ/mol demonstrates physical adsorption.58 The E value of the toluene adsorption on PPC-3-600 is 615.98, 742.70, and 606.22 kJ/mol at 298, 308, and 313 K (Table S1), respectively, disclosing that the behavior covers chemical adsorption.
2.2.3. Adsorption Kinetics
Various adsorption kinetic models can address crucial information about the interaction mechanism of toluene on PPC-3-600. The contact time of toluene adsorption was studied at initial concentrations of 6, 7, and 8 g/L. As it can be seen in Figure 9, all the shapes of kinetics curves are similar and composed of a fast initial adsorption followed by a slow diffusion closing to a flat. Thus, the optimum balance time is 20, 24, and 30 h for initial toluene concentrations of 6, 7, and 8 g/L, respectively. The rapid adsorption process at the beginning can be traced to the access of abundant vacant sites for adsorption, and the gradual decline rate might be attributed to the pore diffusion resistance of toluene into PPC-3-600.58
Figure 9.
(a) Effect of contact time on the toluene adsorption to the PPC-3-600. (b) Linearly fitted curves of pseudo-first-order, (c) pseudo-second-order, and (d) intra-particle diffusion model for toluene adsorption on PPC-3-600 at 318 K. (C0 = 6, 7, 8 g/L)
Herein, pseudo-first-order, pseudo-second-order, and intraparticle diffusion models (Supporting Information, eq S5–S7) were applied to investigate the adsorption behavior. The applicability and kinetic parameters are presented in Figure 9b–d and Table S2. The R2 reveals that the pseudo-second-order (>0.991), an indication of a chemisorption mechanism,47 is more successful to fit the adsorption process than the pseudo-first-order model (>0.956). The intraparticle diffusion model elucidates that the capture of toluene by PPC-3-600 involved three steps. The first is ascribed as the diffusion of toluene to the PPC-3-600 surface, which is the rapidest process. The second linear step is the intraparticle diffusion, namely, the diffusion of toluene from the PPC-3-600 surface to the inside pores, and the last stage is deemed to diffusion into inner micropores wherein the particle diffusion shifts further slow down and reach final equilibrium.47,58
2.2.4. Thermodynamic Research
The adsorption thermodynamic parameters disclose deep information on internal energetic changes. As shown in Table S1, it can be sure that the maximum adsorption quantity increases with the temperature (298–318 K), which specifies an endothermic reaction of the adsorption process for toluene on PPC. The thermodynamic parameters including Gibbs free energy (ΔG0), enthalpy (ΔH0), and entropy (ΔS0) are determined by
| 1 |
| 2 |
where K0 is the thermodynamic constant. In Table S3, the plus value of ΔH0 certifies the adsorption to be an endothermic process. The plus ΔS0 value is derived from the enhancive randomness on the solid–liquid interphase on account of toluene adsorption. The minus ΔG0 elucidates the spontaneous process in nature.19 Furthermore, the greater absolute value of ΔG0 with temperature explains that the rising temperature can increase the spontaneity of adsorption.
2.2.5. Activation Mechanism
ZnCl2 is often used as a chemical activating agent.45,47,59,60 Although a chemical activation method is used widely to synthesis porous carbons, the mechanism is not very clear. First, ZnCl2 has a low melting point of 283–293 °C allowing better contact with the carbon surface above a 500 °C activation temperature. Meanwhile, the Zn2+ radius of 74 pm is less than that of common metal salt ions such as Na+ (102 pm), K+ (138 pm), and Ca2+ (100 pm), which can contribute to the PPC-A-B to obtain micropores from 0.3597 to 0.6108 nm. In addition, ZnCl2 is normally regarded as a dehydrant.45,61 On the one hand, it reacts with water to form hydroxy dichlorozincic acid (ZnCl2 + H2O → H[ZnCl2(OH)]) when the concentration of zinc chloride is high. The acid is corrosive and play an etching effect on PPy to clean the impurity and form a channel structure. On the other hand, ZnCl2 mixed with PPy might not volatilize as the temperature increases and, on the contrary, would cause some ZnCl2 or ZnO (ZnCl2 + −OH → ZnO + HCl) to remain in the pore structure, which can change the pyrolysis behavior of the carbon and obtain lower tar.59 At the same time, the yield of carbon is much higher as a consequence of ZnCl2 inducing plenty of H and O atoms in the carbon precursor to be removed as H2O rather than developing in hydrocarbons or oxygenated organic matter.60 It can be concluded from TG/DTG curves (Figure 6) and other characterizations that the PPy materials have been fully changed and optimized as well as surface properties with both ZnCl2 and temperature activation under 620 °C. Moreover, the rest of the cavities would offer additional porosity in the internal structure once ZnCl2/ZnO is removed by acid washing as shown in Figure 10. It can be true that the carbon framework aromatization with a more prominent pore structure was formed for PPC-A-600 compared to PPC-A-500. However, ZnCl2 is impeded as the activation reagent, and the pore properties diminish owing to the heat shrinkage as temperature rises up to 700 °C, which is a possible explanation for the pore structure of PPC-A-700.
Figure 10.
Diagram of activation mechanism of ZnCl2 for PPC preparation.
3. Conclusions
The intention of the current work is to determine the adsorption properties and behavior of porous carbons based on polypyrrole for toluene from paraffin liquid. In a word, the PPC is prepared successfully and characterized using several methods. Comprehensive studies including isotherms, kinetic, thermodynamic studies, and activation mechanisms are conducted on its adsorption for toluene. The most prominent finding to appear on this work is that activation temperature and ZnCl2 dosage have a significant effect on adsorption capacity, that is, increasing first then reducing. The results indicate that PPC-3-600 obtains superior behavior for toluene adsorption of 2817.19 mg/g from paraffin liquid (C0 = 6 g/L) at 318 K. Simultaneously, the surface area is as high as 1258 m2/g, and one unanticipated finding is that the percentage of micropore volume of PPC-3-600 can attain 86.26%. In addition, the PPC owns rich functional groups in FRIR and XPS analysis, which may facilitate the adsorption. The toluene adsorption isotherm onto the PPC-3-600 is well fitted by Freundlich models, which indicate heterogeneous surfaces of adsorbents. The kinetics study suggests that the pseudo-second-order equation provides an outstanding match, indicating a chemisorption mechanism. Moreover, various thermodynamic parameters such as ΔG0, ΔH0, and ΔS0 are calculated, showing adsorption to be endothermic and spontaneous in the nature of the process. In conclusion, this work has stated toluene adsorption on PPC from paraffin liquid with an inexpensive and simple synthesized method to quantity production, peculiar use for sustainable base oils, and toluene pollutant adsorption.
4. Materials and Methods
4.1. Synthesis of Materials
All reaction reagents were obtained from Shanghai Sinopharm Chemical Reagent Co., Ltd. of China. The PPC synthesis consisted of two typical steps. First of all, polypyrrole (PPy) was synthesized by a modified oxidative method.27 Typically, 3.6 mL of pyrrole (chemical purity, CP) was added to 0.5 M FeCl3 (100 mL) solution with magnetic stirring at room temperature for 2 h to obtain PPy (Scheme 1). After oxidation, PPy was filtered and washed repeatedly to neutral. The filtered products were dried immediately with vacuum drying at 80 °C for 12 h. Second, the prepared PPy and activating agent ZnCl2 (analytical reagents, AR) was mixed with the mass ratio 1:x (x = 1, 2, 3, 4) and grinded until well blended. Then, the mixture was directly carbonized at the temperature of 500–700 °C for 3 h with a heat addition of 5 °C/min in the N2 tube furnace. After cooling off, the carbonization products were washed with hydrochloric acid (5 wt %) and deionized water to neutral pH and lastly dried, defining as PPC-A-B (A refers to the mass ratio of PPy to ZnCl2 of 1:1, 1:2, 1:3, and 1:4; B means the carbonization temperature of 500, 600, and 700 °C).
Scheme 1. Synthetic Procedures of PPy.
4.2. Characterizations
The surface topography and microstructure of the adsorbents were observed with scanning electron microscopy (SEM, Hitachi SU8010, Japan) and high-resolution transmission electron microscopy (TEM, FEI Tecnai G2 20 S-TWIN, U.S.A.). Fourier transform infrared spectroscopy (FTIR) spectra were measured on a Nicolet Nexus 470 spectrometer (U.S.A.) with a KBr pellet method. The X-ray photoelectron spectroscopy (XPS) was investigated with a Multilab 2000 spectrometer using an Al Kα excitation source. The XPS spectra were analyzed by XPS Peak 4.1 software based on a Shirley background type and fixed Lorentzian-Gaussian ratio of 80%. The crystal phase of products was conducted using X-ray diffraction (XRD) on a D8 Advance diffractometer (Germany). After degassing at 200 °C for 4 h, N2 ad-/desorption isotherms were measured with a JW-BK100C instrument at liquid N2 temperature (77 K). The specific surface areas (SSAs) were calculated by the Brunauer–Emmett–Teller (BET) model, while the pore size distribution was obtained from the Barrett–Joyner–Halenda (BJH), Horvath–Kawazoe (HK), and nonlocal density functional theory (NLDFT) models. Thermogravimetric analysis (TGA) and derivative thermogravity analysis (DTG) of samples were performed with a TG 209 F3 Tarsus (NETZSCH, Germany) from indoor temperature to 700 °C.
4.3. Adsorption Experiments
The static adsorption was conducted by dispersing 20.0 mg of PPC into 50 mL of toluene solution with initial concentrations (C0) of 6 g/L in paraffin liquid (CAS: 8012-95-1). Sample bottles were fixed in a shaker (SHZ-82A, Changzhou, China), operating at 318 K and 130 rpm for at least 24 h, and the blank group without the addition of PPC was carried out to prove that the decrease of toluene was not attributed to the adsorption on the glass bottle wall or evaporation loss but the adsorption of the PPC indeed. To investigate the adsorption isotherm and thermodynamics, 20.0 mg of adsorbents was added into 50 mL of toluene solution of 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 g/L, and the water bath temperature was set as 298, 308, and 318 K. For kinetic studies, initial toluene concentrations of 6, 7, and 8 g/L were performed at specific time intervals from 0.5 to 48 h.
After centrifugation to obtain the supernatant, the concentrations of toluene were detected by ultraviolet–visible (UV–vis) absorption spectra on UV-2600 (Shimadzu, Japan) with a 2,2,4-trimethylpentane (blank) baseline and Lambert beer’s law. The adsorption capacity of toluene (Qe, mg/g) was expressed as
| 3 |
where C0 and Ce represent the initial and equilibrium toluene concentrations (g/L); V (L) and m (g) are the solution volume and adsorbent mass.
All absorbance data was detected continuously for at least three times, and only the mean value was reported with an accuracy of ±5%.
Acknowledgments
We sincerely acknowledge the financial support of the Hubei Natural Science Foundation (no. BZY16022, China).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00461.
Equations S1–S4: linear of Freundlich and Dubinin–Radusckevich adsorption isotherm models, pseudo-first-order and pseudo-second-order adsorption kinetic models; Figure S1: SEM images of PPC-3-500, PPC-0-600, and PPC-3-700; Figure S2: pore size distribution of samples calculated with NLDFT models; Figure S3: N2 adsorption/desorption isotherms of PPC-0-600; and Tables S1–S3: parameters of adsorption isotherm, kinetics, and thermodynamics (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Moret S.; Scolaro M.; Barp L.; Purcaro G.; Sander M.; Conte L. S. Optimisation of pressurised liquid extraction (PLE) for rapid and efficient extraction of superficial and total mineral oil contamination from dry foods. Food Chem. 2014, 157, 470–475. 10.1016/j.foodchem.2014.02.071. [DOI] [PubMed] [Google Scholar]
- Urlaub J.; Norwig J.; Schollmayer C.; Holzgrabe U. 1H NMR analytical characterization of mineral oil hydrocarbons (PARAFFINS) for pharmaceutical use. J. Pharm. Biomed. Anal. 2019, 169, 41–48. 10.1016/j.jpba.2019.01.036. [DOI] [PubMed] [Google Scholar]
- Rawlings A. V.; Lombard K. J. A review on the extensive skin benefits of mineral oil. Int. J. Cosmet. Sci. 2012, 34, 511–518. 10.1111/j.1468-2494.2012.00752.x. [DOI] [PubMed] [Google Scholar]
- Petry T.; Bury D.; Fautz R.; Hauser M.; Huber B.; Markowetz A.; Mishra S.; Rettinger K.; Schuh W.; Teichert T. Review of data on the dermal penetration of mineral oils and waxes used in cosmetic applications. Toxicol. Lett. 2017, 280, 70–78. 10.1016/j.toxlet.2017.07.899. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Qiu S.; Liu L.; Xing W.; He L.; Hou Y.; Fang M.; Gui Z.; Song L.; Hu Y. Hierarchical hollow SiO2@TiO2 sphere structure for enhancing the lubrication and photo-catalytic degradation of liquid paraffin. Composites, Part B 2019, 167, 599–607. 10.1016/j.compositesb.2019.03.019. [DOI] [Google Scholar]
- Nash J. F.; Gettings S. D.; Diembeck W.; Chudowski M.; Kraus A. L. A Toxicological Review of Topical Exposure to White Mineral Oils. Food Chem. Toxicol. 1996, 34, 213–225. 10.1016/0278-6915(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Tennant D. R. The usage, occurrence and dietary intakes of white mineral oils and waxes in Europe. Food Chem. Toxicol. 2004, 42, 481–492. 10.1016/j.fct.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Biles R. W.; Mckee R. H.; Lewis S. C.; Scala R. A.; DePass L. R. Dermal carcinogenic activity of petroleum-derived middle distillate fuels. Toxicology 1988, 53, 301–314. 10.1016/0300-483X(88)90222-3. [DOI] [PubMed] [Google Scholar]
- Kimber I.; Carrillo J. C. Oral exposure to mineral oils: Is there an association with immune perturbation and autoimmunity?. Toxicology 2016, 344-346, 19–25. 10.1016/j.tox.2016.01.008. [DOI] [PubMed] [Google Scholar]
- García-Cicourel A. R.; Janssen H.-G. Direct analysis of aromatic hydrocarbons in purified mineral oils for foods and cosmetics applications using gas chromatography with vacuum ultraviolet detection. J. Chromatogr. A 2019, 1590, 113–120. 10.1016/j.chroma.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Joseph M.; Dibijak J.; Griffith I.. Production of Technical White Mineral Oil. US 3,629,096, 1967.
- Rausch M. K.Making a White Oil by Two Stage of Catalytic Hydrogenation. US 3,459,656 A, 1969.
- Nussbaum M. L.; Knaggs E A.. Process for Sulfonation. US 4,148,821, 1979.
- Anfar Z.; Amedlous A.; el Fakir A. A.; Ahsaine H. A.; Zbair M.; Lhanafi S.; el Haouti R.; Jada A.; el Alem N. Combined Methane Energy Recovery and Toxic Dye Removal by Porous Carbon Derived from Anaerobically Modified Digestate. ACS Omega 2019, 4, 9434–9445. 10.1021/acsomega.9b00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent P.; Belmabkhout Y.; Burd S. D.; Cairns A. J.; Luebke R.; Forrest K.; Pham T.; Ma S.; Space B.; Wojtas L.; Eddaoudi M.; Zaworotko M. J. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. 10.1038/nature11893. [DOI] [PubMed] [Google Scholar]
- Woellner M.; Hausdorf S.; Klein N.; Mueller P.; Smith M. W.; Kaskel S. Adsorption and Detection of Hazardous Trace Gases by Metal-Organic Frameworks. Adv. Mater. 2018, 30, 1704679. 10.1002/adma.201704679. [DOI] [PubMed] [Google Scholar]
- Li B.; Xiong H.; Xiao Y. Progress on Synthesis and Applications of Porous Carbon Materials. Int. J. Electrochem. Sci. 2020, 1363–1377. 10.20964/2020.02.04. [DOI] [Google Scholar]
- Ma S.; Sun D.; Yuan D.; Wang X. S.; Zhou H. C. Preparation and gas adsorption studies of three mesh-adjustable molecular sieves with a common structure. J. Am. Chem. Soc. 2009, 131, 6445–6451. 10.1021/ja808896f. [DOI] [PubMed] [Google Scholar]
- Chen J.; Qu R.; Zhang Y.; Sun C.; Wang C.; Ji C.; Yin P.; Chen H.; Niu Y. Preparation of silica gel supported amidoxime adsorbents for selective adsorption of Hg(II) from aqueous solution. Chem. Eng. J. 2012, 209, 235–244. 10.1016/j.cej.2012.08.030. [DOI] [Google Scholar]
- Wurzbacher J. A.; Gebald C.; Steinfeld A. Separation of CO2 from air by temperature-vacuum swing adsorption using diamine-functionalized silica gel. Energy Environ. Sci. 2011, 4, 3584–3592. 10.1039/c1ee01681d. [DOI] [Google Scholar]
- Cai W.; Yu J.; Jaroniec M. Template-free synthesis of hierarchical spindle-like γ-Al2O3 materials and their adsorption affinity towards organic and inorganic pollutants in water. J. Mater. Chem. 2010, 20, 4587–4594. 10.1039/b924366f. [DOI] [Google Scholar]
- An J.; Rosi N. L. Tuning MOF CO2 adsorption properties via cation exchange. J. Am. Chem. Soc. 2010, 132, 5578–5579. 10.1021/ja1012992. [DOI] [PubMed] [Google Scholar]
- Xue D. X.; Belmabkhout Y.; Shekhah O.; Jiang H.; Adil K.; Cairns A. J.; Eddaoudi M. Tunable Rare Earth fcu-MOF Platform: Access to Adsorption Kinetics Driven Gas/Vapor Separations via Pore Size Contraction. J. Am. Chem. Soc. 2015, 137, 5034–5040. 10.1021/ja5131403. [DOI] [PubMed] [Google Scholar]
- Bakhtiari N.; Azizian S. Nanoporous Carbon Derived from MOF-5: A Superadsorbent for Copper Ions. ACS Omega 2018, 3, 16954–16959. 10.1021/acsomega.8b02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K.; Che R.; Cao Q.; Sun Z.; Yue Q.; Deng Y. Designed Fabrication and Characterization of Three-Dimensionally Ordered Arrays of Core–Shell Magnetic Mesoporous Carbon Microspheres. ACS Appl. Mater. Interfaces 2015, 7, 5312–5319. 10.1021/am508683p. [DOI] [PubMed] [Google Scholar]
- Meng F.; Song M.; Wei Y.; Wang Y. The contribution of oxygen-containing functional groups to the gas-phase adsorption of volatile organic compounds with different polarities onto lignin-derived activated carbon fibers. Environ. Sci. Pollut. Res. 2019, 26, 7195–7204. 10.1007/s11356-019-04190-6. [DOI] [PubMed] [Google Scholar]
- Sevilla M.; Valle-Vigón P.; Fuertes A. B. N-Doped Polypyrrole-Based Porous Carbons for CO2 Capture. Adv. Funct. Mater. 2011, 21, 2781–2787. 10.1002/adfm.201100291. [DOI] [Google Scholar]
- He X.; Male K. B.; Nesterenko P. N.; Brabazon D.; Paull B.; Luong J. H. T. Adsorption and desorption of methylene blue on porous carbon monoliths and nanocrystalline cellulose. ACS Appl. Mater. Interfaces 2013, 5, 8796–8804. 10.1021/am403222u. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Liu Y.; Zhou C.; Luo G.; Zhang S.; Chen J. A novel porous carbon derived from hydrothermal carbon for efficient adsorption of tetracycline. Carbon 2014, 77, 627–636. 10.1016/j.carbon.2014.05.067. [DOI] [Google Scholar]
- Mao N.; Huang L.; Shuai Q. Facile Synthesis of Porous Carbon for the Removal of Diclofenac Sodium from Water. ACS Omega 2019, 4, 15051–15060. 10.1021/acsomega.9b01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna F. M. T.; Filho A. N. O.; Araújo C. C. B.; Azevedo D. C. S.; Cavalcante C. L. Jr. Adsorption of Polycyclic Aromatic Hydrocarbons from Heavy Naphthenic Oil Using Commercial Activated Carbons. 1. Fluid-Particle Studies. Ind. Eng. Chem. Res. 2016, 55, 8176–8183. 10.1021/acs.iecr.6b01059. [DOI] [Google Scholar]
- Yu F.; Wu Y.; Li X.; Ma J. Kinetic and thermodynamic studies of toluene, ethylbenzene, and m-xylene adsorption from aqueous solutions onto KOH-activated multiwalled carbon nanotubes. J. Agric. Food Chem. 2012, 60, 12245–12253. 10.1021/jf304104z. [DOI] [PubMed] [Google Scholar]
- Yu F.; Ma J.; Wu Y. Adsorption of toluene, ethylbenzene and m-xylene on multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. J. Hazard. Mater. 2011, 192, 1370–1379. 10.1016/j.jhazmat.2011.06.048. [DOI] [PubMed] [Google Scholar]
- Sánchez-Sánchez Á.; Suárez-García F.; Martínez-Alonso A.; Tascón J. M. D. Synthesis, characterization and dye removal capacities of N-doped mesoporous carbons. J. Colloid Interface Sci. 2015, 450, 91–100. 10.1016/j.jcis.2015.02.073. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Zhang J.; Song W.; Liu Z. Controllable Synthesis of Conducting Polypyrrole Nanostructures. J. Phys. Chem. B 2006, 110, 1158–1165. 10.1021/jp054335k. [DOI] [PubMed] [Google Scholar]
- Qie L.; Chen W.-M.; Wang Z.-H.; Shao Q.-G.; Li X.; Yuan L.-X.; Hu X.-L.; Zhang W.-X.; Huang Y.-H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. 10.1002/adma.201104634. [DOI] [PubMed] [Google Scholar]
- Su F.; Poh C. K.; Chen J. S.; Xu G.; Wang D.; Li Q.; Lin J.; Lou X. W. Nitrogen-containing microporous carbon nanospheres with improved capacitive properties. Energy Environ. Sci. 2011, 4, 717–724. 10.1039/C0EE00277A. [DOI] [Google Scholar]
- Ariharan A.; Viswanathan B.; Nandhakumar V. Nitrogen-incorporated carbon nanotube derived from polystyrene and polypyrrole as hydrogen storage material. Int. J. Hydrogen Energy 2018, 43, 5077–5088. 10.1016/j.ijhydene.2018.01.110. [DOI] [Google Scholar]
- Chen L.-F.; Zhang X.-D.; Liang H.-W.; Kong M.; Guan Q.-F.; Chen P.; Wu Z.-Y.; Yu S.-H. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano 2012, 6, 7092–7102. 10.1021/nn302147s. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Yao B.; Hu B.; Huo K.; Chen W.; Zhou J. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci. 2013, 6, 470. 10.1039/c2ee23977a. [DOI] [Google Scholar]
- Han F.; Li D.; Li W.-C.; Lei C.; Sun Q.; Lu A.-H. Nanoengineered Polypyrrole-Coated Fe2O3@C Multifunctional Composites with an Improved Cycle Stability as Lithium-Ion Anodes. Adv. Funct. Mater. 2013, 23, 1692–1700. 10.1002/adfm.201202254. [DOI] [Google Scholar]
- Li Y.; Li D.; Rao Y.; Zhao X.; Wu M. Superior CO2, CH4, and H2 uptakes over ultrahigh-surface-area carbon spheres prepared from sustainable biomass-derived char by CO2 activation. Carbon 2016, 105, 454–462. 10.1016/j.carbon.2016.04.036. [DOI] [Google Scholar]
- Guo D.; Shibuya R.; Akiba C.; Saji S.; Kondo T.; Nakamura J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. 10.1126/science.aad0832. [DOI] [PubMed] [Google Scholar]
- Chandra V.; Yu S. U.; Kim S. H.; Yoon Y. S.; Kim D. Y.; Kwon A. H.; Meyyappan M.; Kim K. S. Highly selective CO2 capture on N-doped carbon produced by chemical activation of polypyrrole functionalized graphene sheets. Chem. Commun. 2012, 48, 735–737. 10.1039/C1CC15599G. [DOI] [PubMed] [Google Scholar]
- Sun L.; Tian C.; Li M.; Meng X.; Wang L.; Wang R.; Yin J.; Fu H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. 10.1039/C3TA10897J. [DOI] [Google Scholar]
- Yorgun S.; Vural N.; Demiral H. Preparation of high-surface area activated carbons from Paulownia wood by ZnCl2 activation. Microporous Mesoporous Mater. 2009, 122, 189–194. 10.1016/j.micromeso.2009.02.032. [DOI] [Google Scholar]
- Chang B.; Guan D.; Tian Y.; Yang Z.; Dong X. Convenient synthesis of porous carbon nanospheres with tunable pore structure and excellent adsorption capacity. J. Hazard. Mater. 2013, 262, 256–264. 10.1016/j.jhazmat.2013.08.054. [DOI] [PubMed] [Google Scholar]
- Mohanty K.; Jha M.; Meikap B. C.; Biswas M. N. Preparation and Characterization of Activated Carbons from Terminalia Arjuna Nut with Zinc Chloride Activation for the Removal of Phenol from Wastewater. Ind. Eng. Chem. Res. 2005, 44, 4128–4138. 10.1021/ie050162+. [DOI] [Google Scholar]
- Vinu A.; Ariga K.; Mori T.; Nakanishi T.; Hishita S.; Golberg D.; Bando Y. Preparation and Characterization of Well-Ordered Hexagonal Mesoporous Carbon Nitride. Adv. Mater. 2005, 17, 1648–1652. 10.1002/adma.200401643. [DOI] [Google Scholar]
- Kang K. Y.; Lee B. I.; Lee J. S. Hydrogen adsorption on nitrogen-doped carbon xerogels. Carbon 2009, 47, 1171–1180. 10.1016/j.carbon.2009.01.001. [DOI] [Google Scholar]
- Shi K.; Ren M.; Zhitomirsky I. Activated Carbon-Coated Carbon Nanotubes for Energy Storage in Supercapacitors and Capacitive Water Purification. ACS Sustainable Chem. Eng. 2014, 2, 1289–1298. 10.1021/sc500118r. [DOI] [Google Scholar]
- Wang X.; Liu C.-G.; Neff D.; Fulvio P. F.; Mayes R. T.; Zhamu A.; Fang Q.; Chen G.; Meyer H. M.; Jang B. Z.; Dai S. Nitrogen-enriched ordered mesoporous carbons through direct pyrolysis in ammonia with enhanced capacitive performance. J. Mater. Chem. A 2013, 1, 7920–7926. 10.1039/c3ta11342f. [DOI] [Google Scholar]
- Xu H.; Jia W.; Ren S.; Wang J. Magnetically responsive multi-wall carbon nanotubes as recyclable demulsifier for oil removal from crude oil-in-water emulsion with different pH levels. Carbon 2019, 145, 229–239. 10.1016/j.carbon.2019.01.024. [DOI] [Google Scholar]
- Qian Q.; Machida M.; Tatsumoto H. Preparation of activated carbons from cattle-manure compost by zinc chloride activation. Bioresour. Technol. 2007, 98, 353–360. 10.1016/j.biortech.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Yuan M.; Tong S.; Zhao S.; Jia C. Q. Adsorption of polycyclic aromatic hydrocarbons from water using petroleum coke-derived porous carbon. J. Hazard. Mater. 2010, 181, 1115–1120. 10.1016/j.jhazmat.2010.05.130. [DOI] [PubMed] [Google Scholar]
- Freundlich H. Adsorption in solution. J. Phys. Chem. 1906, 57, 1361–1368. [Google Scholar]
- Saleh T. A.; Sarı A.; Tuzen M. Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem. Eng. J. 2017, 307, 230–238. 10.1016/j.cej.2016.08.070. [DOI] [Google Scholar]
- Duan Q.; Li X.; Wu Z.; Alsaedi A.; Hayat T.; Chen C.; Li J. Adsorption of 17β-estradiol from aqueous solutions by a novel hierarchically nitrogen-doped porous carbon. J. Colloid Interface Sci. 2019, 533, 700–708. 10.1016/j.jcis.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Srinivasan M. P.; Ni Y. Novel activation process for preparing highly microporous and mesoporous activated carbons. Carbon 2001, 39, 877–886. 10.1016/S0008-6223(00)00198-6. [DOI] [Google Scholar]
- Yue Z.; Mangun C. L.; Economy J. Preparation of fibrous porous materials by chemical activation 1. ZnCl2 activation of polymer-coated fibers. Carbon 2002, 40, 1181–1191. 10.1016/S0008-6223(01)00268-8. [DOI] [Google Scholar]
- He X.; Ling P.; Qiu J.; Yu M.; Zhang X.; Yu C.; Zheng M. Efficient preparation of biomass-based mesoporous carbons for supercapacitors with both high energy density and high power density. J. Power Sources 2013, 240, 109–113. 10.1016/j.jpowsour.2013.03.174. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.