Abstract
Mucoid bacteria, predominately Pseudomonas aeruginosa, are commonly associated with decline in pulmonary function in children with cystic fibrosis (CF), and are thought to persist at least in part due to a greater propensity toward forming biofilms. We isolated a higher frequency of mucoid Streptococcus pneumoniae (Sp) expressing high levels of capsular polysaccharides from sputa from children with CF, compared to those without CF. We compared biofilm formation and maturation by mucoid and non-mucoid isolates of Sp collected from children with and without CF. Non-mucoid Sp serotype 19A and 19F isolates had significantly higher levels of biofilm initiation and adherence to CF epithelial cells than did serotype 3 isolates. However, strains expressing high levels of capsule had significantly greater biofilm maturation, as evidenced by increased density and thickness in static and continuous flow assays via confocal microscopy. Finally, using a serotype 3 Sp strain, we showed that highly encapsulated mucoid phase variants predominate during late adherence and better colonize CFTR–/– as compared to wild-type mice in respiratory infection studies. These findings indicate that overexpression of capsule can enhance the development of mature pneumococcal biofilms in vitro, and may contribute to pneumococcal colonization in CF lung disease.
Keywords: Streptococcus pneumoniae, cystic fibrosis, biofilm, mucoid, serotype, type 3 capsule
Mucoid Streptococcus pneumonia were collected more frequency from children with cystic fibrosis (CF) compared to children without CF, and enhanced the development of mature pneumococcal biofilms, thus may contribute to bacterial colonization in CF lung disease.
INTRODUCTION
Streptococcus pneumoniae (Sp) is a respiratory pathogen that is a common cause of morbidity and mortality worldwide, especially in infants less than 2 years of age, the elderly, and those with underlying medical conditions (Calbo and Garau 2010). Sp is commonly carried in the human upper respiratory tract. Sp can also cause a range of infections that include otitis media, sinusitis, mastoiditis, bacteremia, sepsis, bacterial meningitis and community acquired pneumonia. Although Sp is associated with a wide range of respiratory and systemic infections, little is known about the role of Sp in cystic fibrosis (CF) lung disease. Several studies have reported the presence of Sp in CF patient sputa (Burns et al.1998; del Campo et al.2005; Maeda et al.2011).
CF is a recessive genetic disorder caused by mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, resulting in abnormal regulation and function of the CFTR protein, which normally regulates the transport of chloride, sodium and water across epithelial cells (Cutting 2010). These changes can lead to the production of abnormally thick mucus that blocks clearance of the lungs and airways, thereby providing a favorable environment for opportunistic bacterial infection (Heeckeren et al.1997; Mathee et al.1999; Guss et al.2011).
Mucoid bacteria, especially Pseudomonas aeruginosa, are commonly observed in patients with CF disease and in the case of mucoid P. aeruginosa have been significantly correlated with deterioration of pulmonary function in CF (Gosselin et al.1995; Gosselin et al.1998; Laurans et al.2006; Emerson et al.2010; Martha et al.2010), increased resistance to phagocytosis and antibiotics, heightened inflammation of the airways (Saiman et al.1992; Martha et al.2010; Rau et al.2010) and in the formation of biofilms (Høiby, Ciofu and Bjarnsholt 2010; Lee et al.2011). Biofilms are multicellular microbial communities embedded in a complex matrix of extracellular proteins, polysaccharides and DNA, following adherence of bacteria to a surface (Costerton et al.1987). Mucoid P. aeruginosa have elevated levels of adherence in mature biofilms and maintain their ability to form well-differentiated biofilms in chronic lung infection (Lee et al.2011) and can persist in chronic CF lung disease (Jelsbak et al.2007; Hall-Stoodley and Stoodley 2009; Rau et al.2010). Sp biofilms have been demonstrated in vitro (Allegrucci et al.2006; Oggioni et al.2006; Allegrucci and Sauer 2007; Moscoso, García and López 2009) and in vivo (Reid et al.2009; Perez et al.2014; Wren et al.2014; Murrah et al.2015); however, the role of mucoid phenotype in Sp biofilm formation and in CF lung disease have not been described. In this study, using serotype-matched isolates (type 3, 19A and 19F) from CF and non-CF patient sputa, we compared initial and late attachment of mucoid and non-mucoid Sp to CFTR deficient primary lung epithelial cells, to polystyrene plates, to flow chamber glass slides and in CFTR–/– mouse lungs during the different stages of biofilm formation.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Clinical isolates for this study were selected from Sp isolated from routine clinical sputum cultures patients from the Clinical Microbiology Laboratory at Children's of Alabama in Birmingham under a protocol approved by the UAB Institutional Review Board. Pneumococcal isolates were identified by alpha-hemolysis and colony morphology on 5% sheep blood agar, Gram's stained smear, optochin disk sensitivity and bile solubility (Converse and Dillon 1977; Finland and Barnes 1977). A CF diagnosis was confirmed by (i) examination of medical records and (ii) enrollment in the Children's Hospital CF Center registry database as part of the Cystic Fibrosis Foundation Registry. Clinical isolates of Sp from CF sputa were compared to isotype-matched lower respiratory isolates from NCF patients, submitted to the Clinical Microbiology Laboratory as part of routine clinical care. Laboratory strain WU2 (serotype 3; Briles et al.1981) and its unencapsulated mutant JD908 (Dillard and Yother 1994) were used to characterize different stages of Sp biofilm formation (Table 1). Bacteria were cultured at 37°C in Todd-Hewitt broth containing 0.5% yeast extract (THY) and stored at −80°C in media containing 10% glycerol. New titered freezer cultures of Sp WU2 and JD908 were prepared from frozen stocks according to standard culture procedures. Sp were grown to an optical density of 0.4–0.5 at 600 nm, stored as aliquots into freezer vials and stored in THY containing 16% glycerol at −80°C until use.
Table 1.
Streptococcus pneumoniae strains used in this study.a
| Strain | Serotype | Colony description | Strain type |
|---|---|---|---|
| WU2 | 3 | Mucoid | Lab strain |
| JD908 | 3 | Non-mucoid | Lab strain |
| CHB 875 | 3 | Mucoid | CF |
| CHB 455 | 3 | Mucoid | CF |
| CHB 179 | 3 | Mucoid | Non-CF |
| CHB 1126 | 3 | Mucoid/Non-mucoid | CF |
| CHB 620 | 3 | Mucoid | CF |
| CHB 756 | 3 | Mucoid | CF |
| CHB 803 | 3 | Mucoid | CF |
| CHB 1058 | 19A | Non-mucoid | CF |
| CHB 692 | 19A | Non-mucoid | Non-CF |
| CHB 607 | 19A | Non-mucoid | CF |
| CHB 477 | 19F | Non-mucoid | CF |
| CHB 399 | 19F | Non-mucoid | CF |
| CHB 240 | 19F | Non-mucoid | Non-CF |
Capsular serotyping and capsule quantitation methods
Isolates were serotyped using the multiplex bead assay (Hoiby et al.1976; Yu et al.2005), which included Sp serotypes 1, 2, 3, 4, 5, 6A, 6B, 6C, 7F/A, 7B/7C/40/15A/F, 8, 9N, 9V, 10A/39, 11A/D/F, 11Aβ, 12F/B, 13, 14, 15B/(C), 16F, 17F/A, 18C, 19A, 19F, 20, 22F/A, 23A, 23B, 23F, 25F/A/38, 31, 33F/A, 34, 35B and 35F/47F. Isolates not reacting with any of the listed serotypes were considered nontypeable (NT) (Yu et al.2005). Sp bacterial DNA was analyzed using the PCF targeting the first gene of the capsule operon, cpsA to identify the 3, 19A and 19F-specific capsular genes. The reaction products were separated by agarose electrophoresis, and visualized under UV light. All of the Sp type 3 strains were observed to be mucoid based on their colony morphology (Dennis et al.2015). Bacteria were cultured at 37°C in Todd-Hewitt broth containing 0.5% yeast extract (THY) and stored at −80°C in 10% glycerol. Capsular polysaccharide for serotype 3 strains were quantified using the Stains-All assay for measuring acidic polysaccharides and a normal curve was constructed using Sp WU2 as the reference point as previously described (Magee and Yother 2001).
Adherence assay to primary lung epithelial cells
CF and normal human primary airway epithelial cells were obtained from the UAB Gregory Fleming James Cystic Fibrosis Research Center Airway Tissue Procurement Program and grown to confluent monolayers. Sp (5 × 104 CFU/ml) were added to the plates and incubated with epithelial cells in supplemented growth medium at 37°C for 4 h to measure initial adherence of Sp to epithelial cells. After incubation, media was removed and cells were washed gently with phosphate-buffered saline (PBS) pH 7.4 to eliminate unbound bacteria without disturbing adherent bacteria. Epithelial cells were detached by treatment with trypsin, and remnant bacteria in the wells were re-suspended by vigorous pipetting, serially diluted in PBS, and total number of viable adherent bacteria assessed by plate-count.
Crystal violet biofilm assay
Biofilm initiation was measured as adherence to a polystyrene microtiter dish, as quantified by optical density following staining with crystal violet as previously described (Southey-Pillig, Davies and Sauer 2005). To measure initial adherence (4 h) and late adherence (24 h), 5 × 102 CFU of Sp in THY were added to 96-well plates, and incubated at 37°C. Following incubation, media was removed, and plates were carefully washed once with deionized water. Biofilms were stained with 0.1% crystal violet for 15 min, washed three times with deionized water and allowed to dry for 15 min. Biofilms were solubilized in 100% ethanol and absorbance measured at 600 nm.
Adherence assay to polystyrene
Twenty-four well polystyrene plates were inoculated with 5 × 104 CFU of Sp in THY, then incubated at 37°C for 4 h to measure initial adherence and for 24 h to measure late adherence. Media was carefully replenished after 12 h. After incubation, media was removed from wells and bacteria were washed gently with THY to remove unattached cells. Remnant bacteria in the wells were re-suspended and dissociated from the plate by vigorous pipetting with 1 mL of THY, then removed and serially diluted in PBS pH 7.4 and the total number of viable adherent bacteria assessed by plate count as above. The unattached bacteria were also serially diluted in PBS and plated on blood agar to determine the numbers of viable unattached bacteria. Percent bacteria attached were calculated in comparison to the total number of attached and unattached bacteria for each sample.
Continuous-flow biofilm assays
Biofilm cultures were grown using a continuous-flow system as previously described (Hong et al.2007). Bacteria were cultured in Stovall Convertible Flow Cell continuous-flow chambers (Stovall Life Science, Inc., Greensboro, NC). After inoculation, THY media flow was arrested for 3 h, and the flow cell was inverted to enable adhesion of the bacteria to the flow cell coverslip. Medium flow was resumed at a constant rate using a peristaltic pump (Cole Parmer, Vernon Hills, IL) and visualized by confocal laser scanning microscopy using a Zeiss LSM 510 laser scanning microscope at 24, 48 and 72 h time-points. Biofilm structural measures were obtained using confocal microscopy analyzed using COMSTAT software (Heydorn et al.2000). To assess bacterial viability within biofilms, biofilms were stained with BacLight Live-Dead reagent (Molecular Probes) at 24, 48 and 72 h.
Mice and lung infections
CFTRm1Unc−/−(FABP-hCFTR) [fatty acid-binding protein-CFTR) homozygotes were obtained from the CF facility at UAB. Mice have their intestinal defect corrected by the presence of a functional human CFTR gene expressed from a rat intestinal FABP gene promoter. Cftrtm1UncTg (FABPhCFTR) mice were anesthetized lightly with Isoflurane. Following anesthesia, suspensions of 40 μl of lactated Ringer's solution containing 5 × 105–1 × 106 CFU bacteria were introduced into the nares of the mice to induce aspiration pneumonia. After 1, 3 or 5 days, the mice were sacrificed. The lungs of the sacrificed mice were lavaged through the trachea with 1ml of lactated Ringer's. The lungs were harvested and placed into 1 ml of lactated Ringer's solution in a stomacher bag, homogenized, serially diluted, and plated onto blood agar with gentamicin (4 μg/ml) in serial threefold dilutions. The blood was collected by retro-orbital bleeding, serial diluted and plated on blood agar with gentamicin (4 μg/ml) in serial threefold dilutions.
Statistical analysis
Statistical analysis was performed using Students’ t-test or one-way ANOVA to calculate P-values for each comparison using the GraphPad InStat software version 3.10 2009 for Windows.
RESULTS
Sp isolate characterization
During the study period, 119 Streptococcus pneumoniae isolates were identified from 98 different CF patients accounting for 35.25% (98/278) of the hospital's CF Center patient registry. During the same time period 40 Sp isolates were cultured from children without CF (NCF) patient sputa. The predominant serotypes in CF patient sputum were 19A, 3 and 19F in order of decreasing frequency of isolation and 19A, NT and among the strains from NCF patients, the most common serotypes were 19A and 19F (unpublished data).
Thirteen different CF and NCF clinical isolates and laboratory strain WU2 (Briles et al.1981) along with its unencapsulated mutant JD908 (Dillard and Yother 1994) are shown in Table 1. These Sp isolates of serotypes 3, 19A and 19F were selected based on their high prevalence in sputa from the CF patient population at Children's of Alabama in Birmingham, AL consistent with previous reports of pneumococcal serotypes from CF respiratory tract (Hoiby et al.1976) and were characterized for their ability to adhere and form initial and mature Sp biofilms in vitro. All clinical Sp 19A and 19F isolates used in this study, although encapsulated, were classified as relatively ‘non-mucoid’ in comparison to the highly mucoid type 3 isolates. All clinical serotype 3 isolates used were classified as mucoid. The colony morphology of the fresh type 3 strains from the CF patients was very different from what we have ever observed for type 3 isolates from non-CF patients. The type 3 isolates from CF patients had strong colony structure that made it hard to disrupt them with cotton swabs or wire loop and made it easy to push the intact colony gently over the surface of a blood agar plate. WU2 was classified as mucoid and the WU2 derivative JD908 (cps3S-WU2; Dillard and Yother 1994) produced no polysaccharide capsule (acapsular) and was classified as non-mucoid. We additionally measured the amount of capsule produced by each serotype 3 isolate using the Stains-All assay for measuring acidic polysaccharides (Table 2). Serotype 3 isolate CHB1126 when isolated from CF patient sputum, produced mucoid colonies from stock cultures. However during biofilm adherence assays, CHB1126 always produced some colony variants with a less-mucoid phenotype in addition to those with the highly mucoid phenotype.
Table 2.
Serotype 3 strains used in this study: capsule production relative to WU2.
| Strain | Capsule quantification (%) | Colony description | Strain type |
|---|---|---|---|
| WU2 | 100 | Mucoid | Lab strain |
| JD908 | 0 | Non-mucoid | WU2 derivative |
| CHB 875 | 49.80 | Mucoid | CF |
| CHB 455 | 58.86 | Mucoid | CF |
| CHB 179 | 63.39 | Mucoid | Non-CF |
| CHB 1126 | 121.99 | Mucoid/Non-mucoid | CF |
| CHB 620 | 143.51 | Mucoid | CF |
| CHB 756 | 428.45 | Mucoid | CF |
| CHB 803 | 434.03 | Mucoid | CF |
Sp static adherence to primary lung epithelial cells
We examined mucoid and non-mucoid pneumococcal adherence to CF and NCF primary lung epithelial cells to assess the effect of epithelial CFTR deficiency on initial biofilm attachment. Assays measuring Sp attachment to both CF and NCF cells indicated that after a 4-h incubation, CFTR deficiency did not have a significant effect on Sp adherence. (Fig. 1). No strain showed a significant difference between ability to adhere to CF compared to NCF cells after 4 h, with the exception of CHB1058 (Sp type 19A, non-mucoid), which adhered slightly better to NCF cells than to CF cells (P ≤ 0.05). These results indicated that CFTR deficiency may not have a biologically important effect on the initial adherence of Sp to lung epithelia in static culture.
Figure 1.
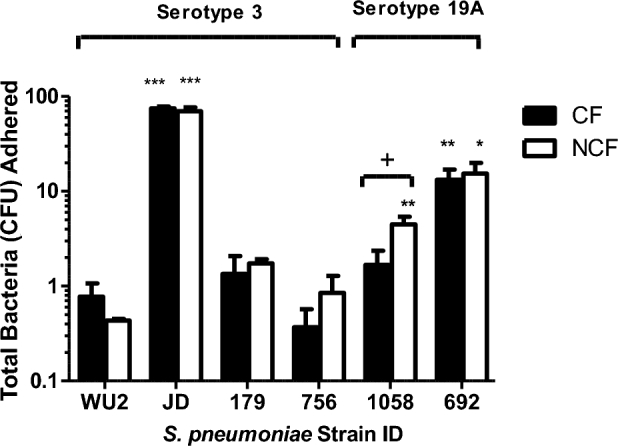
Adherence of S. pneumoniae to primary lung epithelial cells. Early attachment of individual mucoid and non-mucoid isolates of S. pneumoniae was measured to CF primary epithelial cells (black bars) and NCF primary epithelial cells (white bars) to assess initial bacterial adherence to biological surfaces. Percent bacteria attached to epithelial cells in 6-well plates were calculated in comparison to the total number of attached bacteria plus unattached bacteria that were recovered in media wash for each sample after 4 h incubations by dividing attached CFUs by total CFUs. All experiments were performed in duplicate and repeated independently at least once. Bars represent the group means + SEM of samples from all assays. Statistical analyses were performed using a two-tailed Student's t-test. Comparisons are either strain vs. WU2 (A single asterisk denotes P ≤ 0.05, double asterisks denotes P ≤ 0.01, triple asterisks denotes P ≤ 0.001) or adherence to CF vs. NCF cells. (A single plus sign denotes P ≤ 0.05).
We observed, however, that isolates with a non-mucoid phenotype were more adherent to both CF and NCF epithelial cell types in 4-h adherence assays than isolates with mucoid phenotype. Adherence for each strain was compared statistically to that of type 3 reference strain WU2. Among the two type 19A strains, (both non-mucoid), CHB692 adhered significantly better to both CF and NCF epithelial cells than WU2 (P ≤ 0.01) and CHB1058 adhered significantly better to NCF epithelial cells than Sp WU2 (P ≤ 0.05). Strain JD908 (non-mucoid, acapsular mutant of WU2) adhered highly significantly better than Sp WU2 (P ≤ 0.05). In contrast to the non-mucoid strains, both mucoid type 3 clinical strains (Sp CHB756 and CHB179) showed no significant differences in 4-h adherence to CF or NCF cells from that of WU2. These result suggests that the high serotype 3 capsule production and the mucoid phenotype may hinder initial adherence to epithelial cells.
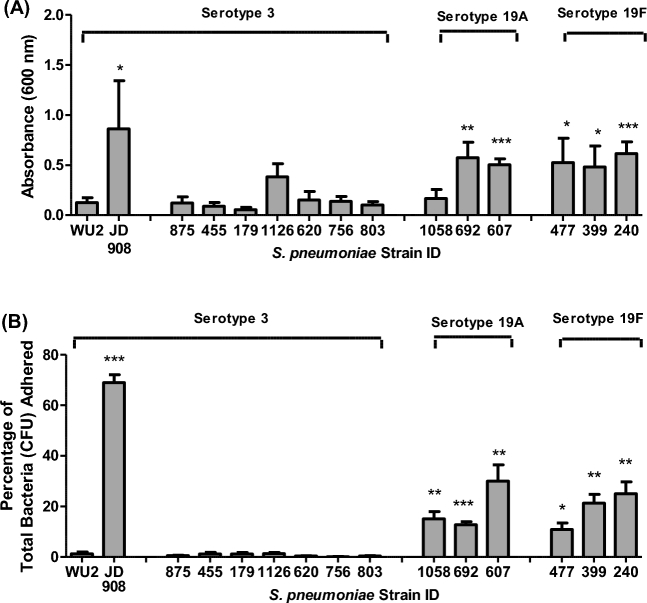
Sp 4-hour static biofilms on polystyrene
Static biofilm assays were employed to evaluate biofilm initiation (4 h) to 96-well polystyrene plates using crystal violet assays (Fig. 2A). All strains were compared directly to WU2. Fig. 2A illustrates that when crystal violet staining was used to measure initial attachment, five of six group 19 strains (all non-mucoid) had significantly greater adherent biomass compared to the reference strain WU2 (P ≤ 0.05). No clinical mucoid type 3 strains had significantly greater attached biomass than WU2. The only strain on a type 3 genetic background with significantly greater staining in the crystal violet assay than WU2 was JD908, the acapsular non-mucoid WU2 variant (P ≤ 0.05). No statistically significant differences were observed between clinical strains collected from CF patients compared to those collected from NCF patients within each serotype.
Figure 2.
Initial adherence of S. pneumoniae serotypes 3, 19A and 19F to polystyrene wells. Early attachment of serotype 3, 19A and 19F S. pneumoniae isolates to polystyrene wells was measured by (A) staining adhered cells with crystal violet to measure differences in biofilm biomass after 4 h incubations or by (B) quantifying the CFUs that remained attached to 24-well polystyrene plates and the CFUs that were recovered in THY wash after 4 h incubations and calculating the percentage adhered by dividing attached CFUs by total CFUs. Each sample used in both assays was tested in duplicate wells and all assays were repeated at least once. Bars represent the group means + SEM of samples from all assays. Statistical analysis was performed using a two-tailed Student's t-test. Comparisons are vs. WU2. (A single asterisk denotes P ≤ 0.05, double asterisks denote P ≤ 0.01 and triple asterisks denote P ≤ 0.001).
Static biofilm attachment was also determined by quantifying adherent colony-forming units (CFUs) to polystyrene by % CFU bound (Fig. 2B). After 4 h incubations (Fig. 2B), all group 19 strains showed significantly greater percentages of adherent bacteria than WU2 (P ≤ 0.05) and JD908 was again the only strain with a type 3 genetic background that showed significantly greater adherence than WU2 (P ≤ 0.001). No clinical mucoid type 3 isolates had percentages of adherent bacteria that were significantly higher than WU2. No differences were observed between clinical strains collected from CF patients compared to those collected from NCF patients within the type 3 or group 19 serotypes using the adherence assay. These results further indicated that serotypes 19A and 19F clinical isolates, which were all scored as non-mucoid phenotypes, adhered better than mucoid type 3 clinical isolates during the initial adherence stages of static biofilm formation.
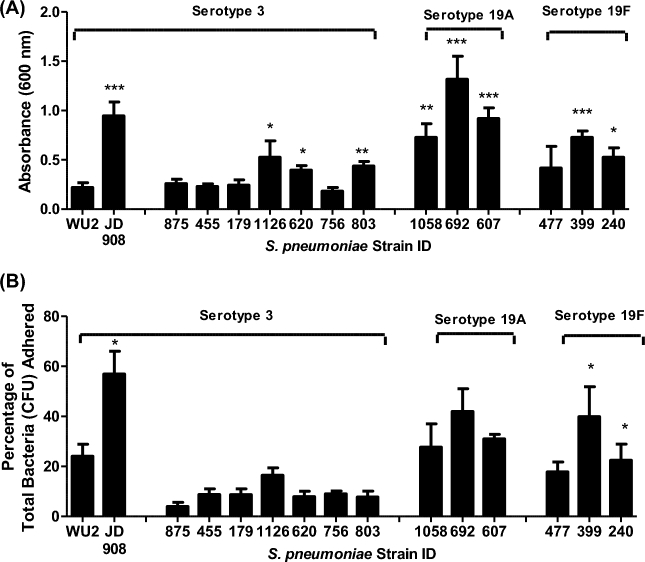
Sp 24-hour static biofilms on polystyrene
Crystal violet and CFU adherence assays were employed to assess late stage static adherence (24-h) to polystyrene plates (Fig. 3). Using crystal violet staining (Fig. 3A), five of six group 19 strains and JD908 had significantly greater adherent biomass than WU2 (P ≤ 0.05) at 24 h, which was the same trend observed after 4 h. However, at 24 h three of seven mucoid type 3 strains had significantly greater crystal violet staining than did WU2. This was in contrast to the 4-h data (Fig. 2) where no clinical type 3 strain had greater biomass than WU2. Using CFU adherence assays (Fig. 3B), only two of six group 19 strains and JD908 had a significantly greater percentage of adherent bacteria than WU2 (P ≤ 0.05) at 24 h, whereas all six group 19 strains had a greater biomass than WU2 at 4 h. However, for the percentage of CFU bound to the polystyrene at 24 h, none of the type 3 isolates higher binding than did than WU2 (Fig. 3B), which mimicked the trend observed at 4 h (Fig. 2).
Figure 3.
Adherence of S. pneumoniae serotypes 3, 19A and 19F to polystyrene wells. Late attachment of serotype 3 and serotypes 19A and 19F S. pneumoniae isolates was measured by (A) staining adhered cells with crystal violet to measure differences in biofilm biomass after 4 h incubations or by (B) quantifying the CFUs that remained attached to 24-well polystyrene plates and the CFUs that were recovered in THY wash after 4 h incubations. Percentage of total bacteria adhered was determined by dividing attached CFUs by total CFUs. Each sample used in both assays was tested in duplicate wells and all assays were repeated at least once. Bars represent the group means + SEM of samples from all assays. Statistical analysis was performed using a two-tailed Student's t-test. Comparisons are vs. WU2. (A single asterisk denotes P ≤ 0.05, double asterisks denote P ≤ 0.01 and triple asterisks denote P ≤ 0.001).
Adherence data were further analyzed for each strain by directly comparing crystal violet staining at 4 h and 24 h to determine whether biomass increased from the early to late stage of biofilm development (Table 3). Using crystal violet staining, 71% (five of seven) of clinical mucoid type 3 isolates showed significant increases in biomass from 4 to 24 h (P ≤ 0.05). This trend was evident in only 50% (three of six) of non-mucoid clinical isolates. Using the CFU adherence assay, 86% (six of seven) of clinical mucoid type 3 isolates had significant increases in attachment capability (P ≤ 0.05) whereas only 17% (one of six) of non-mucoid isolates had a significant increase in the percentage of adherent bacteria. The results of both assays suggest that from 4 to 24 h, mucoid strains show a significantly greater increase in adherence from the early to late stage of biofilm formation. Thus, non-mucoid phenotype appears to be advantageous early in pneumococcal biofilm formation, and mucoid phenotype shows greater potential for further biofilm maturation with longer incubation time.
Table 3.
Streptococcus pneumoniae serotypes 3, 19A and 19F 4 h vs. 24 h adherence.
| Strain | Serotype | Colony description | Crystal violet assay (4 h/24 h) | CFU adherence assay (4 h/24 h) |
|---|---|---|---|---|
| WU2 | 3 | Mucoid | 0.13/0.22 | 1.2/18.8** |
| JD908 | 3 | Non-mucoid | 0.61/0.95 | 68.9/47.9 |
| CHB 875 | 3 | Mucoid | 0.12/0.26 | 0.5/4.9 |
| CHB 455 | 3 | Mucoid | 0.09/0.28* | 1.2/4.9* |
| CHB 179 | 3 | Mucoid | 0.06/0.25** | 1.3/7.6* |
| CHB 1126 | 3 | Mucoid/Non-mucoid | 0.32/0.53* | 1.3/20.9** |
| CHB 620 | 3 | Mucoid | 0.15/0.4* | 0.3/6.3* |
| CHB 756 | 3 | Mucoid | 0.14/0.18 | 0.1/7.6*** |
| CHB 803 | 3 | Mucoid | 0.1/0.44*** | 0.4/10.2* |
| CHB 1058 | 19A | Non-mucoid | 0.17/0.73** | 15.1/15.6 |
| CHB 692 | 19A | Non-mucoid | 0.57/1.31* | 12.7/35.4* |
| CHB 607 | 19A | Non-mucoid | 0.5/0.92* | 30/28.4 |
| CHB 477 | 19F | Non-mucoid | 0.52/4.16 | 10.8/22.1 |
| CHB 399 | 19F | Non-mucoid | 0.48/0.73 | 21/31.9 |
| CHB 240 | 19F | Non-mucoid | 0.62/0.53 | 25/23.3 |
aThe calculations in this table are based on the results shown in figures 2 and 3. Statistical significance between 4 hr and 24 hr results are indicated by *, ≤0.05; **, ≤0.01; and ***, ≤0.001. Where no statistical significance is indicated, the 4- and 24-hour data are not statistically different.
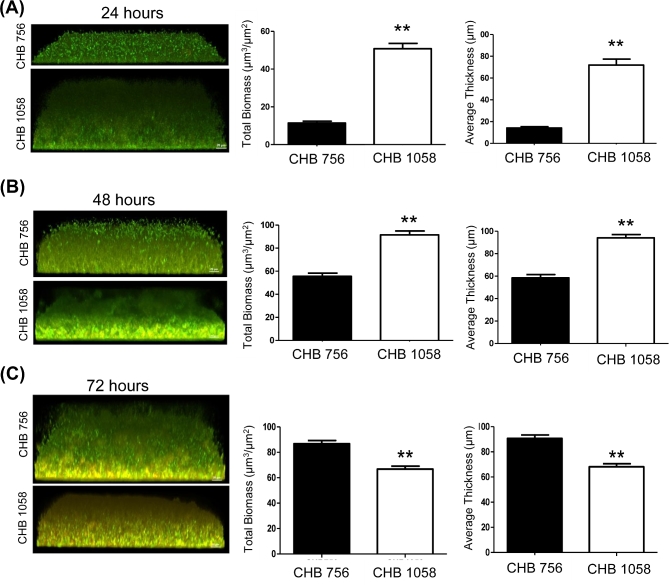
Sp biofilms in continuous media flow cells
Longer term biofilm formation for a clinical mucoid serotype 3 (CHB756) and a non-mucoid 19A (CHB1058) strain was assessed using flow-cell chambers with continuous media flow as previously described (Hong et al.2007). Confocal images with Live/Dead BacLight staining were taken to examine cell structure, stability, and viability during mature stages of biofilm formation. CHB756 and CHB1058, each isolated from CF patient sputa, were examined and compared after 24, 48 and 72 h of biofilm growth under flow cell conditions. As shown in Fig. 4, at 24 and 48 h, biofilms formed by CHB1058 (type 19A) were significantly denser than those formed by CHB756 (type 3; P ≤ 0.01), similar to observations in static assays. Interestingly, by 72 h, biofilms formed by mucoid CHB756 had a significantly greater average thickness and overall biomass than those formed by non-mucoid CHB1058 (P ≤ 0.01), consistent with the finding that mucoid phenotype shows greater potential for further biofilm maturation with longer incubation time.
Figure 4.
Mature biofilm formation of serotypes 3 and serotype 19A S. pneumoniae isolates. Mature biofilms of mucoid type 3 (CHB756) and non-mucoid type 19A (CHB1058) S. pneumoniae were evaluated by confocal microscopy using a continuous media flow biofilm chambers. Biofilm formation was evaluated at 24 h (A), 48 h (B) and 72 h (C) to compare biofilm viability and density. The green is staining is viable cells, the red is staining dead cells and yellow indicates co-localization of the two. Integrated Intensity of red and green fluorescent staining was measured by COMSTAT software.
Role of type 3 colony morphology variants of CHB1126 during Sp biofilm formation
During the adherence assays, we observed the consistent emergence of non-mucoid colony variants (N-MCVs) from CHB1126, a serotype 3 mucoid isolate initially collected from CF patient sputum. We prepared stocks of a single N-MCV and a single mucoid colony variant (MCV) recovered after growth on blood agar. The mucoid variants similar to the other clinical type 3 isolates collected from CF sputa were large, glossy, and hard disrupt with cotton swabs or wire loop on blood agar plates where as non-mucoid variants were flat and easy to manipulate. Non-mucoid and MCVs are shown in Fig. 5A. These non-mucoid and mucoid variants were grown to an optical density of 0.4–0.5 at 600 nm in THY, stored at −80°C until use and designated as 1126-NM and 1126-M, respectively. Single slice confocal images with Live/Dead BacLight staining were taken of 1126-M, wild-type (WT) and NM after 72 h of biofilm formation using flow cell chambers (Fig. 5B). After 72 h of biofilm development, confocal images confirm that a WT (mixed) phenotype was more advantageous than a non-mucoid or mucoid phenotype. Quantitative analysis by Metamorph software indicated that total cell integrated intensity of 1126-WT was greater than that formed by 1126-NM and 1126-M (Fig. 5C). These data suggest that both mucoid and non-MCVs may contribute to biofilm maturation.
Figure 5.
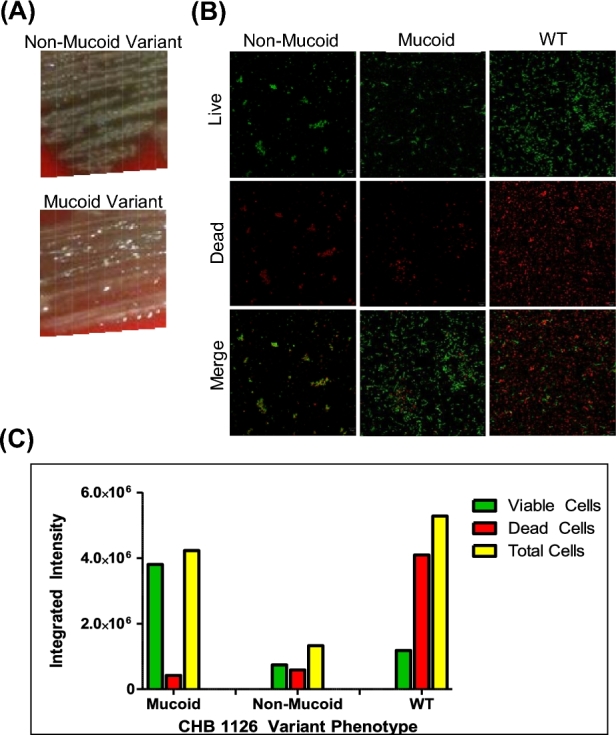
Biofilm formation by serotype 3 S. pneumoniae isolate CHB1126. Confocal microscopy with BacLight staining was employed after 72 h to compare biofilm viability and robustness of mucoid, non-mucoid and WT/mixed variants of serotype 3 CHB 1126 Sp. Morphology of non-mucoid and mucoid variants of CHB 1126 on blood agar (A). The green is staining is viable cells, the red is staining dead cells and yellow indicates co-localization of the two (B). Integrated intensity of red and green fluorescent staining was measured by Metamorph software (C).
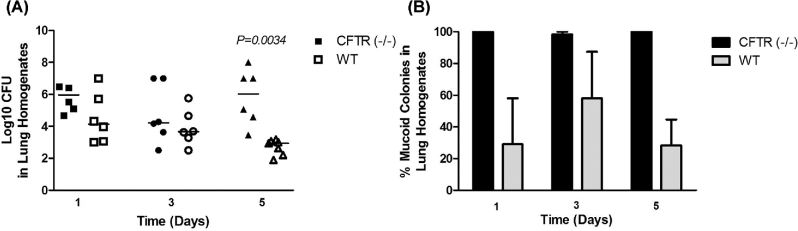
Role of type 3 colony variants during lung disease
To determine whether mucoid type 3 variants have a colonization advantage in CF lung disease, we infected congenic CFTR–/– and WT C57BL mice with 1126-WT, and measured the ability of mice to clear lung bacteria. Additionally, we quantified the percentage of mucoid and non-MCVs that emerged in homogenized lungs on day 1, 3 and 5 post-infection. No bacteria were detected in the blood of infected mice on days 1, 3 and 5 post-infection in any experiments (not shown). There was no significant difference in the number of CFUs recovered from CFTR–/– and WT mice lung on days 1 and 3 post-infection, but on day 5 post-infection, we recovered significantly more bacteria from CFTR–/– mice lungs than WT-mice lungs (Fig. 6A), and importantly, nearly 100% of CHB1126 colony variants recovered from CFTR–/– mice on day 1, 3 and 5 were highly mucoid (Fig. 6B). Thus, we conclude from these findings that the mucoid serotype 3 variants were selected for in vivo and may have a selective advantage over non-MCVs in congenic CFTR–/– mouse lungs compared with wild-type mice lungs after 5 days of pneumococcal infection.
Figure 6.
The effect of mucoidy on serotype 3 colonization and residence in CF mouse lungs. CFTR–/– mice (black dots) and WT-mice (white dots) were infected with 1 × 105 CFU of mucoid serotype 3 isolate CHB1126 (n = 6; A). The percentage of mucoid colonies and non-MCVs was calculated from lung homogenates (B). The horizontal lines indicate the median for each group. CFTR–/– mice and WT-mice were directly compared for each strain using the two-tailed T test. Experiments were repeated at least twice. P-values ≥ 0.05 are not shown.
DISCUSSION
In CF lung disease, biofilms are increasingly recognized as an important component of bacterial persistence, subsequent inflammation and decline of patient health (Brogden, Guthmiller and Taylor 2005; Lee et al.2011). It is thus essential to gain a better understanding of how bacteria utilize phenotypic and molecular adaptation to persist in host environments, and how bacterial modifications impact disease progression. Three distinct stages of pneumococcal biofilm formation have been described, including initial attachment, cluster formation and biofilm maturation (Allegrucci and Sauer 2007). Mucoid phenotype and capsular regulation are thought to play a role in bacterial adherence and in biofilm formation for some bacterial species, most notably Pseudomonas aeruginosa, yet the role of mucoidy and capsule in the development of pneumococcal biofilms and the potential impact on CF lung disease is not known. In this report we describe a direct analysis of the relative ability of mucoid and non-mucoid Sp isolates collected from CF patient sputum to adhere to biotic and abiotic surfaces during the various stages of biofilm formation in vitro and in vivo using assays of Sp static adherence to polystyrene plates and primary CF lung epithelial cells and studies of mature phases of biofilm formation using flow cell assays and a mouse model of CF lung disease.
We first assessed mucoid and non-mucoid Sp attachment to primary CF and NCF epithelial cells. Initial epithelial adherence is an important step for bacterial colonization in many infections (Selinger and Reed 1979), and the association of bacteria with epithelial tissue may play an important role in biofilm formation and pathogenesis (Oggioni et al.2006; Allegrucci and Sauer 2007; Moscoso, García and López 2009). Thus, an understanding of Sp attachment to CF lung epithelia may provide insight into how biofilm forming Sp behave in the CF lung. During initial (4-h) pneumococcal adherence to human bronchial epithelial cells, non-encapsulated pneumococci have been reported to adhere in higher numbers than encapsulated strains (Adamou et al.1998). Furthermore, the presence of polysaccharide capsule has been shown to significantly reduce the ability of Sp to adhere to and penetrate A549 lung epithelial cells (Talbot, Paton and Paton 1996; Hammerschmidt et al.2005). In our analysis of Sp adherence to primary epithelial cells, we found that non-encapsulated type 3 and non-mucoid group 19 strains adhere in significantly higher numbers than mucoid type 3 strains to both CF and Non-CF cell types.
In our examination of Sp adherence to polystyrene plates, we show that non-mucoid serotypes 19A and 19F and a non-mucoid type 3 strain also bind polystyrene plates in greater numbers than mucoid serotype 3 strains at the 4-h time point. These results were consistent whether adherence was quantitated by crystal violet staining or and a CFU adherence assay. The variations that were observed between the two measures of adherence were probably due to the fact that crystal violet staining could detect dead adherent bacteria, whereas CFU quantification could only detect viable bacteria. We further report that after 24 h of incubation, although non-mucoid strains remained better able to adhere, the mucoid strains have more significant increases in capacity for biofilm adherence as compared to the 4-h studies. These findings indicate that non-mucoid group 19 strains are more successful at early attachment and cell cluster formation, yet mucoid strains show greater capacity for improvement later in biofilm development. During our assessment of mature biofilm formation using flow cell chambers, we demonstrated that after 3 days of biofilm formation, a mucoid phenotype may be advantageous for development of high biomass and biofilm stability than a non-mucoid phenotype, and that serotype 3 strains may be able to achieve greater biofilm density than group 19 strains. Allegrucci and Sauer (2007) also demonstrated that 3-day-old biofilms formed by large mucoid type 3 pneumococci obtained from pediatric nasal washes have greater biomass and average thickness than biofilms formed by small mucoid type 3 pneumococci (Allegrucci and Sauer 2007).
Whereas the P. aeruginosa mucoid phenotype is linked to alginate production (Martin et al. 1993a,b; Hay et al.2009), pneumococcal mucoidy is correlated with high production of polysaccharide capsule (Martínez and Melero 2000; Magee and Yother 2001). Pseudomonas aeruginosa alginate overproduction has been demonstrated to impair early biofilm attachment, yet is more advantagteous for microcolony formation as part of biofiom formation (Hentzer et al.2001; Hay et al.2009). In this connection, our findings suggest that low capsule production is favored for initial Sp adherence. Low capsule has been suggested to be advantageous for initial adherence due to high hydrophobicity that increases bacterial aggregation (Allegrucci and Sauer 2007). Efficient initial attachment by non-mucoid strains with host cells may also be due to greater exposure of surface molecules that aid in adhesion (Magee and Yother 2001; Hammerschmidt et al.2005). Adhesins present on the Sp surface normally shielded by the capsule are exposed in non-mucoid and acapsular strains, and as a result are more readily available for binding to host cell surfaces (Adamou et al.1998). However, pneumococcal capsule is the major mechanism of clearance and renders the organism resistant to opsonophagocytosis (Hammerschmidt et al.2005), and even small amounts of capsule can retard clearance of pneumococci in mucus (Nelson et al.2007). Thus, depending on the degree of local inflammation low levels of mucoidy may decrease the ability of bacteria to survive over time in vivo due to decreased ability to persist in harsh environments such as CF lungs. Capsule may allow for better immune evasion and survival in the lung, leading to chronic infection. Expression of capsule seems to be varied at different stages of invasive infection, and capsular regulation likely allows better ability to adapt to various ecological niches in the human host. Two pneumococcal colony phase variants that play a role in establishing adherence have been previously described (Kim and Weiser 1998; Waite, Struthers and Dowson 2001). The transparent phase has less capsular polysaccharide, which exposes adhesive molecules and allows more efficient adherence to epithelial cells. Whereas the opaque form produces two to six times more capsular polysaccharide than the transparent form (Kim and Weiser 1998) and is more virulent and common in systemic infections (Calbo and Garau 2010). The ability of pneumococci to switch between a transparent form that facilitates initial adherence and carriage, and an opaque form that poorly adheres but is better adapted to develop better in vivo biofilms, and evade the host immune response during inflammation or invasive infection suggests that these phenotypic variants are adapted for different stages of disease and that polysaccharide capsule plays an essential role during adherence as well as during disease progression. Recently, the underlying mechanism for phase variation was shown to consists of genetic rearrangements resulting in six different bacterial subpopulations with distinct methylation patterns and gene expression profiles. Furthermore, in vivo selection for variants was demonstrated to have a direct effect on virulence (Manso et al.2014).
Here, we demonstrated that a pneumococci strain isolated from CF patient sputum could undergo reversible phase variation during biofilm formation, similar to that previously reported (Allegrucci and Sauer 2007). In all biofilm assays, a clinical serotype 3 isolate CHB1126 occasionally produced non-mucoid phase variants during attachment, thus selective pressures during biofilm formation appeared to influence the appearance of non-MCVs from a mucoid strain. It is likely that some mucoid isolates may be capable of down regulating capsule expression to allow for initial attachment to lung epithelia in significant numbers. Allegrucci and Sauer (2007) described the emergence of colony morphology variants in biofilms formed by type 3 pneumococci (Allegrucci et al.2006). Phenotypic variants have also been described to emerge during mouse nasal colonization (Briles et al.2005). Although phenotypic conversions can sometimes be random (Dybvig 1993), selective pressures and environmental factors that influence biofilm formation may have implications on capsule production, which must be carefully regulated in order to enable the pathogen to colonize and subsequently survive within the host. The specific mechanisms and environmental conditions that influence pneumococcal capsular polysaccharide expression are not well defined, yet it has been shown that mutations in serotype 3-specific genes of the type 3 capsule loci, cps3DSUM can alter capsule production and mucoid phenotype (Dillard and Yother 1994; Magee and Yother 2001). In addition, capsule production was recently shown to be negatively and directly regulated by a GntR family regulator, CpsR, which was demonstrated to have important implications for both pneumococcal colonization and invasive disease (Wu et al.2016).
It is important to note that in vitro assays do not account for environmental stressors that typically occur in human lungs such as opsonophagocytosis, and acapsular and non-mucoid strains may not display the same levels of adherence or biofilm success during infection in vivo. Our study demonstrated that mucoid pneumococcal colony phase variants may also have a selective advantage in CF lung disease. Using an aspiration pneumonia disease model, we previously demonstrated more severe pneumococcal lung disease in in CFTR–/– mice compared to WT-mice infected with type 3 Sp strains isolated from CF patients (Dennis et al.2015), suggesting that mucoid capsular serotype 3 Sp could exploit the CF defect of the CFTR–/– mice. Lack of CFTR in transgenic CF mice has similarly been shown to lead to increased levels of P. aeruginosa in the lung, which are associated with hypersusceptibility of CF mice to mucoid P. aeruginosa infection (Gosselin et al.1998; Williams, Dehnbostel and Blackwell 2010). Here, our results support the association of a highly mucoid Sp with poor prognosis in CFTR–/– mice. Previous work showed no difference in pro-inflammatory cytokine secretion or recruitment of neutrophils to sites of infection between WT and CFTR–/– mice infected with mucoid pneumococci (Dennis et al.2015), thus high susceptibility to infection in CF lungs has been attributed in part to bacterial adaptation within the CF airways, specifically to the transition of bacteria to mucoid phenotypes (Martin et al.1993) and biofilm formation (Lee et al.2011). Our observations that mucoid type 3 Sp isolates appear more frequently in the lungs of CF patients than in normal individuals is consistent with previous reports (Hoiby et al.1976), and that CFTR–/– mice are more susceptible to highly mucoid Sp than WT-mice, both support the hypothesis that mucoid pneumococci may have an advantage in the environment of the CF lung that may lead to chronic infection.
Additional in vivo studies need to be conducted to further assess the implications of mucoidy, serotype and polysaccharide capsule on the development of pneumococcal biofilms in the CF lung. Further studies also need to explore the effectiveness of antibiotic therapy for organisms within biofilms during CF lung disease. Current antibiotic therapy acts predominantly on planktonic cells and may alleviate the acute symptoms of the lung infection, but may not be as effective at eliminating bacteria residing within biofilm communities. Continued investigation of biofilm morphology can help to better explain biofilm-related pathogenesis and show promise towards the development of new therapies to battle infections associated with mucoid bacteria in CF lung disease.
Acknowledgements
We are grateful to Yvette Hale who was instrumental in the animal studies and helped to create a laboratory environment that was conducive to this research.
FUNDING
This work was supported in part by the UAB Cystic Fibrosis Research Center (P30 DK072482) and by grant R01AI118805.
Conflict of interest. None declared.
REFERENCES
- Adamou JE, Wizemann TM, Barren P et al. Adherence of Streptococcuspneumoniae to human bronchial epithelial cells (BEAS-2B). Infect Immun 1998;66:820–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegrucci M, Sauer K. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J Bacteriol 2007;189:2030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegrucci M, Hu FZ, Shen K et al. Phenotypic characterization of Streptococcuspneumoniae biofilm development. J Bacteriol 2006;188:2325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles DE, Novak L, Hotomi M et al. Nasal colonization with Streptococcuspneumoniae includes subpopulations of surface and invasive pneumococci. Infect Immun 2005;73:6945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles DE, Nahm M, Schroer K et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcuspneumoniae. J Exp Med 1981;153:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden KA, Guthmiller JM, Taylor CE. Human polymicrobial infections. Lancet North Am Ed 2005;365:253–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JL, Emerson J, Stapp JR et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 1998;27:158–63. [DOI] [PubMed] [Google Scholar]
- Calbo E, Garau J. Of mice and men: innate immunity in pneumococcal pneumonia. Int J Antimicrob Agents 2010;35:107–13. [DOI] [PubMed] [Google Scholar]
- Converse GM, Dillon HC. Epidemiological studies of Streptococcuspneumoniae in infants: methods of isolating pneumococci. J Clin Microbiol 1977;5:293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Cheng KJ, Geesey GG et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol 1987;41:435–64. [DOI] [PubMed] [Google Scholar]
- Cutting GR. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci 2010;1214:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo R, Morosini MI, de la Pedrosa EG et al. Population structure, antimicrobial resistance, and mutation frequencies of Streptococcuspneumoniae isolates from cystic fibrosis patients. J Clin Microbiol 2005;43:2207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EA, Coats MT, Griffin SE et al. The effects of CFTR and mucoid phenotype on susceptibility and innate immune responses in a mouse model of pneumococcal lung disease. PLoS One 2015;10:e0140335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard JP, Yother J. Genetic and molecular characterization of capsular polysaccharide biosynthesis in Streptococcuspneumoniae type 3. Mol Microbiol 1994;12:959–72. [DOI] [PubMed] [Google Scholar]
- Dybvig K. DNA rearrangements and phenotypic switching in prokaryotes. Mol Microbiol 1993;10:465–71. [DOI] [PubMed] [Google Scholar]
- Emerson J, McNamara S, Buccat AM et al. Changes in cystic fibrosis sputum microbiology in the United States between 1995 and 2008. Pediatr Pulmonol 2010;45:363–70. [DOI] [PubMed] [Google Scholar]
- Finland M, Barnes MW. Changes in occurrence of capsular serotypes of Streptococcuspneumoniae at Boston City Hospital during selected years between 1935 and 1974. J Clin Microbiol 1977;5:154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D, DeSanctis J, Boulé M et al. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonasaeruginosa. Infect Immun 1995;63:3272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D, Stevenson MM, Cowley EA et al. Impaired ability of Cftr knockout mice to control lung infection with Pseudomonasaeruginosa. Am J Respir Crit Care Med 1998;157:1253–62. [DOI] [PubMed] [Google Scholar]
- Guss AM, Roeselers G, Newton IL et al. Phylogenetic and metabolic diversity of bacteria associated with cystic fibrosis. ISME J 2011;5:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol 2009;11:1034–43. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt S, Wolff S, Hocke A et al. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun 2005;73:4653–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ID, Gatland K, Campisano A et al. Impact of alginate overproduction on attachment and biofilm architecture of a supermucoid Pseudomonasaeruginosa strain. Appl Environ Microb 2009;75:6022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeckeren A, Walenga R, Konstan MW et al. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonasaeruginosa. J Clin Invest 1997;100:2810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M, Teitzel GM, Balzer GJ et al. Alginate overproduction affects Pseudomonasaeruginosa biofilm structure and function. J Bacteriol 2001;183:5395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000;146:2395–407. [DOI] [PubMed] [Google Scholar]
- Hoiby N, Hoff GE, Jensen K et al. Serological types of Diplococcuspneumoniae isolated from the respiratory tract of children with cystic fibrosis and children with other diseases. Scand J Respir Dis 1976;57:37–40. [PubMed] [Google Scholar]
- Hong W, Pang B, West-Barnette S et al. Phosphorylcholine expression by nontypeable Haemophilusinfluenzae correlates with maturation of biofilm communities in vitro and in vivo. J Bacteriol 2007;189:8300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby N, Ciofu O, Bjarnsholt T. Pseudomonasaeruginosa biofilms in cystic fibrosis. Future Microbiology 2010;5:1663–74. [DOI] [PubMed] [Google Scholar]
- Jelsbak L, Johansen HK, Frost AL et al. Molecular epidemiology and dynamics of Pseudomonasaeruginosa populations in lungs of cystic fibrosis patients. Infect Immun 2007;75:2214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcuspneumoniae. J Infect Dis 1998;177: 368–77. [DOI] [PubMed] [Google Scholar]
- Laurans M, Arion A, Fines-Guyon M et al. [Pseudomonasaeruginosa and cystic fibrosis: first colonization to chronic infection]. Arch Pediatr 2006;13:S22–29. [PubMed] [Google Scholar]
- Lee B, Schjerling CK, Kirkby N et al. Mucoid Pseudomonasaeruginosa isolates maintain the biofilm formation capacity and the gene expression profiles during the chronic lung infection of CF patients. APMIS 2011;119:263–74. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Elborn JS, Parkins MD et al. Population structure and characterization of viridans group streptococci (VGS) including Streptococcuspneumoniae isolated from adult patients with cystic fibrosis (CF). J Cyst Fibros 2011;10:133–9. [DOI] [PubMed] [Google Scholar]
- Magee AD, Yother J. Requirement for capsule in colonization by Streptococcuspneumoniae. Infect Immun 2001;69:3755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manso AS, Chai MH, Atack JM et al. A random six-phase switch regulates pneumococcal virulence via global epigenetic changes. Nat Commun 2014;5:5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martha B, Croisier D, Fanton A et al. Factors associated with mucoid transition of Pseudomonasaeruginosa in cystic fibrosis patients. Clin Microbiol Infect 2010;16:617–23. [DOI] [PubMed] [Google Scholar]
- Martin D, Schurr M, Mudd M et al. Differentiation of Pseudomonasaeruginosa into the alginate-producing form: inactivation of mucB causes conversion to mucoidy. Mol Microbiol 1993a;9:497–506. [DOI] [PubMed] [Google Scholar]
- Martin DW, Schurr MJ, Mudd MH et al. Mechanism of conversion to mucoidy in Pseudomonasaeruginosa infecting cystic fibrosis patients. Proc Natl Acad Sci 1993b;90:8377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I, Melero J. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J Gen Virol 2000;81:2715–22. [DOI] [PubMed] [Google Scholar]
- Mathee K, Ciofu O, Sternberg C et al. Mucoid conversion of Pseudomonasaeruginos by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 1999;145:1349–57. [DOI] [PubMed] [Google Scholar]
- Moscoso M, García E, López R. Pneumococcal biofilms. Int Microbiol 2009;12:77–85. [PubMed] [Google Scholar]
- Murrah KA, Pang B, Richardson S et al. Nonencapsulated Streptococcuspneumoniae causes otitis media during single-species infection and during polymicrobial infection with nontypeable Haemophilusinfluenzae. Pathogens and Disease2015;73: DOI: 10.1093/femspd/ftu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AL, Roche AM, Gould JM et al. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun 2007;75:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oggioni MR, Trappetti C, Kadioglu A et al. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol Microbiol 2006;61:1196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez AC, Pang B, King LB et al. Residence of Streptococcuspneumoniae and Moraxellacatarrhalis within polymicrobial biofilm promotes antibiotic resistance and bacterial persistence in vivo. Pathogens Disease 2014;70:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau MH, Hansen SK, Johansen HK et al. Early adaptive developments of Pseudomonasaeruginosa after the transition from life in the environment to persistent colonization in the airways of human cystic fibrosis hosts. Environ Microbiol 2010;12:1643–58. [DOI] [PubMed] [Google Scholar]
- Reid SD, Hong W, Dew KE et al. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis 2009;199:786–94. [DOI] [PubMed] [Google Scholar]
- Saiman L, Cacalano G, Gruenert D et al. Comparison of adherence of Pseudomonasaeruginosa to respiratory epithelial cells from cystic fibrosis patients and healthy subjects. Infect Immun 1992;60:2808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger DS, Reed WP. Pneumococcal adherence to human epithelial cells. Infect Immun 1979;23:545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southey-Pillig CJ, Davies DG, Sauer K. Characterization of temporal protein production in Pseudomonasaeruginosa biofilms. J Bacteriol 2005;187:8114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot UM, Paton AW, Paton JC. Uptake of Streptococcuspneumoniae by respiratory epithelial cells. Infect Immun 1996;64:3772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite RD, Struthers JK, Dowson CG. Spontaneous sequence duplication within an open reading frame of the pneumococcal type 3 capsule locus causes high-frequency phase variation. Mol Microbiol 2001;42:1223–32. [DOI] [PubMed] [Google Scholar]
- Williams BJ, Dehnbostel J, Blackwell TS. Pseudomonasaeruginosa: host defence in lung diseases. Respirology 2010;15:1037–56. [DOI] [PubMed] [Google Scholar]
- Wren JT, Blevins LK, Pang B et al. Influenza A virus alters pneumococcal nasal colonization and middle ear infection independently of phase variation. Infect Immun 2014;82:4802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Xu H, Zheng Y et al. CpsR, a GntR family regulator, transcriptionally regulates capsular polysaccharide biosynthesis and governs bacterial virulence in Streptococcuspneumoniae. Sci Rep 2016;6:29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Lin J, Benjamin WH et al. Rapid multiplex assay for serotyping pneumococci with monoclonal and polyclonal antibodies. J Clin Microbiol 2005;43:156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]