Women enrolled in HIV prevention trials in KwaZulu-Natal, South Africa between 2002 and 2012 have overlapping risk factors for pregnancy and HIV incidence. This emphasizes an urgent need for appropriate, targeted, individual-centred counseling for women participating in HIV prevention trials.
Keywords: HIV, HIV-prevention trials, pregnancy, risk-reduction counseling, STIs
Abstract
Background
Women enrolled in human immunodeficiency virus (HIV) prevention efficacy trials receive counseling on prevention of HIV, sexually transmitted infections (STIs), and pregnancy during every visit. Incident pregnancy has an impact on efficacy outcomes. Incidence rates of pregnancy and HIV/STIs among women who became pregnant and associated risk factors were assessed.
Methods
Data from 9165 women participating in HIV prevention trials in KwaZulu-Natal, South Africa from 2002–2012 were combined. Demographic and behavioral predictors of incidence pregnancy and incidence HIV and STIs were determined using Cox regression models.
Results
Overall pregnancy incidence was 9.6 per 100 person-year (py) (95% confidence interval [Cl], 9.1–10.3). Human immunodeficiency virus incidence among pregnant women was 5.93 per 100 py (95% Cl, 4.73–7.44). Incidence of STIs among pregnant women for Chlamydia trachomatis, Trichomonas vaginalis, Neisseria gonorrhoeae, and Treponema pallidum (syphilis) were 10.87, 7.42, 3.92, and 1.43 per 100 py, respectively. In the adjusted analyses, we observed overlapping risk factors for HIV acquisition during pregnancy, ie, young age, not married/not cohabitating, and low parity. The risk of pregnancy and HIV acquisition is more than 3 times higher among young women (<20 years of age).
Conclusions
We identified overlapping risk factors for pregnancy and HIV incidence, suggesting an urgent need for appropriate, targeted, individual-centred counseling for women participating in HIV prevention trials.
Approximately 270 000 people were newly infected with human immunodeficiency virus (HIV) in South Africa in 2016 [1]. The province of KwaZulu-Natal had the highest burden of HIV in 2015, with an estimated prevalence rate of 44.4% among antenatal attendees [2]. In the general population, the HIV prevalence and incidence in South Africa is high [3–5]. Risk factors for HIV acquisition during pregnancy, breastfeeding, and the postpartum period are reported to be attributed to biological (eg, elevated hormonal levels, immunological fluctuations, and changes in the vaginal microbiome) [6–11] and behavioral risks (eg, increased frequency of unprotected sexual intercourse and partner infidelity) [12–16]. In addition, structural risk factors for HIV acquisition include being young, unmarried, and not cohabiting with a stable or regular partner [17].
In addition to HIV, 4 curable sexually transmitted infections (STIs), namely, Chlamydia trachomatis, Neisseria gonorrhoeae, Treponema pallidum (syphilis), and Trichomonas vaginalis, continue to be endemic in South Africa [18–22]. In women, STIs have been associated with pelvic inflammatory disease, infertility, cervical cancer, ectopic pregnancy, miscarriage, as well as foetal and neonatal death [23, 24]. Sexually transmitted infections have been shown to enhance HIV acquisition and transmissibility with mechanisms including increased rate of HIV shedding in persons with concurrent infections [25–27]. A study conducted in Malawi by Taha et al [28] suggest that STIs such as N gonorrhoeae and T vaginalis were associated with increased risk of HIV acquisition during pregnancy and the postpartum period. The risk was 2-fold higher for T vaginalis and 4-fold higher for N gonorrhoeae.
Significant investments are made to test new biomedical interventions to prevent HIV among women in our setting, due to the high prevalence and incidence of HIV in South Africa [3–5]. Thus, we have been actively involved in several large-scale, multicenter HIV prevention clinical trials that investigate the efficacy of women-initiated biomedical interventions, namely, vaginal microbicides and pre-exposure prophylaxis, with limited success [29–36]. In general, these HIV prevention trials enroll woman aged between 18 and 45 years who are not intending to get pregnant but are at high risk of HIV acquisition. Given the limited data on incidence of pregnancy and concomitant incidence of HIV/STIs among pregnant women in a clinical trial setting, we used the opportunity to combine data from cohorts of women participating in HIV prevention trials, to better understand the rates of incident pregnancy as well as the rates of incident HIV/STIs in women who became pregnant. We hope the data will be useful for future clinical trial design and conduct as well as for local HIV/STI programmatic awareness in antenatal and family planning clinics.
METHODS
Study Population and Procedures
Data from 9165 consenting women enrolled in 5 phase II/III HIV prevention biomedical trials were combined. A detailed description of the study populations, the diagnostic tests used for HIV/STI testing, as well as pregnancy determination has been described elsewhere (see Supplementary Table S2) [29–31, 35, 36]. The main eligibility criteria were consistent across the trials and included the following: women aged 18 years or older; being sexually active; an HIV-negative result at screening and enrollment; a negative pregnancy test; no intention to become pregnant throughout the study; and willingness to provide written informed consent, follow study procedure, and reside in and around the study area for a minimum of 1 year. Women who were HIV positive at screening were referred to the local healthcare facilities for care and support. Women who seroconverted during the trial remained in the study and received ongoing counseling, and they were referred to local healthcare facilities for further care at the end of the study. At enrollment and at each follow-up visit, participants were tested for other curable STIs including C trachomatis, N gonorrhoeae, T pallidum (syphilis), and T vaginalis. Those who tested positive were treated before enrollment according to the study protocols, and treatment was provided according to the local South African guidelines. Women who were free of any STIs were enrolled in the trials. Women were also tested for HIV and pregnancy, received a pelvic examination, and were offered contraception free of charge at the site. Women were compensated with the amount of R150 ($11) for their time, travel, and refreshments. The protocol and informed consent forms were approved by the local ethics committee.
Risk Factors and Statistical Analysis
We considered a wide range of common sociodemographic and behavioral risk factors across trials for incident pregnancy and HIV including age at baseline (<20, 20–29, and 30+), marital/cohabitation status (yes/no), level of education (none, primary school, secondary school), contraceptive method (injectable contraception and other forms), parity (0, 1, 2+), and the number of sex partners in the last 3 months (1/2+). Time to pregnancy was calculated as the difference between the date of pregnancy and date of enrollment. Time of pregnancy was defined as the midpoint between the last negative and first positive pregnancy test result. Time to STI positivity was calculated as the time elapsed from enrollment to first positive STI diagnosis (defined as the midpoint between the last negative and first positive STI test result).
Kaplan-Meier curves were used to present the crude incidence rate of pregnancy, and the log rank test was used to test whether incidence differed between groups including age and parity. Cox proportional hazards regression was used to identify the predictors of incident pregnancy and HIV, respectively. Multivariate models were created using the variables with P < .1 in univariate analyses, and forward stepwise methods were used to finalize the multivariate models. Final multivariate models included only statistically significant factors with P < .05. Adjusted hazard ratios (aHRs) and their 95% confidence intervals (CIs) were presented from the multivariable analyses. All analysis was performed using Stata 14.0.
Estimating Population Attributable Risk Percentage
The population attributable risk percentage (PAR%) was used to quantify the impact of numerous factors on incidence of pregnancy, ie, proportion of pregnancy cases attributed to various risk factors after adjusting for potential confounders. In brief, PAR% and its 95% CIs were estimated for the proportion of pregnancies associated with the risk factor(s) of interest. This epidemiological measure was derived by combining the aHRs from the Cox regression models and the estimated prevalence of the risk factors listed in Table 1 [37].
Table 1.
HIV and STI Incidence in Women Who Became Pregnant During the Trials Compared With Those Who Remained Nonpregnant Throughout the Trials
| Disease | Pregnancy Status | Number of Infections | Incidence (per 100-Person Years) | 95% CI | P Value |
|---|---|---|---|---|---|
| Neisseria gonorrhoeae | Pregnant | 49 | 3.92 | 2.97–5.19 | .85 |
| Nonpregnant | 363 | 3.79 | 3.42–4.20 | ||
| Chlamydia trachomatis | Pregnant | 131 | 10.87 | 9.16–12.90 | .48 |
| Nonpregnant | 923 | 9.78 | 9.18–10.44 | ||
|
Treponema pallidum
(syphilis) |
Pregnant | 18 | 1.43 | 0.90–2.27 | .038 |
| Nonpregnant | 206 | 2.14 | 1.86–2.45 | ||
| Trichomonas vaginalis* | Pregnant | 49 | 7.42 | 5.61–9.82 | .19 |
| Nonpregnant | 362 | 6.85 | 6.18–7.59 | ||
| HIV | Pregnant | 75 | 5.93 | 4.73–7.44 | .14 |
| Nonpregnant | 683 | 7.05 | 6.54–7.60 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; STI, sexually transmitted infection.
RESULTS
Incidence of Pregnancy
Of the 9165 women enrolled, 1034 (11.3%) women had at least one pregnancy during follow-up, with the overall pregnancy incidence rate of 9.6 per 100 person-years (py) (95% CI, 9.1–10.3).
Incidence of Human Immunodeficiency Virus and Sexually Transmitted Infections
Human immunodeficiency virus and STI incidence among pregnant and nonpregnant women are presented in Table 1. A total of 75 women who had at least one pregnancy during study follow-ups had incident HIV. The HIV incidence rate among women who became pregnant, expressed as number of pregnancies per 100 py, was 5.93, whereas the HIV incidence in nonpregnant women was 7.05 per 100 py. A total of 322 and 2537 cumulative STIs were recorded for pregnant and nonpregnant women, respectively. The incidence per 100 py among pregnant women of C trachomatis, T vaginalis, N gonorrhoeae, and T pallidum (syphilis) were 10.87, 7.42, 3.92, and 1.43, respectively. Likewise, the incidence of curable STIs among nonpregnant women during these trials were C trachomatis 9.78 per 100 py, followed by T vaginalis (6.85), N gonorrhoeae (3.79), and T pallidum (syphilis) (2.14). Sexually transmitted infection(s) incidence rates were not statistically significant by pregnancy status. Incidence of syphilis was slightly lower among pregnant women compared with nonpregnant women (1.43 vs 2.14, P = .04). Approximately one quarter of the women who acquired HIV during the trial were coinfected with at least 1 other STI (data not shown).
Predictors of Incidence Pregnancy and Human Immunodeficiency Virus
In the adjusted analyses, being younger (<20), not married/not cohabiting, using contraceptives other than injectable, and low parity (≤1 child) were all identified as independent predictors of pregnancy (Table 2). The risk of pregnancy was more than 3 times higher among young women (<20) compared with women aged 30 or above (P < .001). Likewise, relative to women aged 30 or above, women aged 20–29 had a 2.4 times higher risk of pregnancy. Unmarried women/not cohabitating were 30% more likely to have incident pregnancy compared with their married peers (P = .001). Women on oral contraceptives or not on any form of contraceptive were more likely to be at risk of incidence pregnancy (P < .001). A protective role of injectable contraceptives against pregnancy was also observed (P < .001). Women with no children were 2.4 times at higher risk of pregnancy relative to women with 2 or more children, respectively (P < 0.001).
Table 2.
Predictors of Pregnancy and HIV Seroconversion
| Pregnancy | HIV Seroconversion | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total (%) | Adjusted Hazard Ratio (95% Cl) |
P Value | PAR% | Adjusted Hazard Ratio (95% Cl) | P Value | PAR% |
| Age group | 51 (47, 54) | 50 (46, 54) | |||||
| <20 years | 10.40 | 3.23 (2.61–4.00) | <.001 | 3.20 (2.51–4.10) | <.001 | ||
| 20–29 | 55.47 | 2.41 (2.05–2.83) | <.001 | 2.36 (1.96–2.85) | <.001 | ||
| 30+ years | 33.80 | 1 (reference) | 1 (reference) | ||||
| Marital/cohabitation status | 45 (40, 51) | 75 (70, 78) | |||||
| Yes | 15.40 | 1 (reference) | 1 (reference) | ||||
| No | 84.60 | 1.34 (1.13–1.58) | .001 | 2.19 (1.68–2.84) | <.0001 | ||
| Education | 3 (1, 4) | ||||||
| None | 54.20 | 1 (reference) | 1 (reference) | ||||
| Primary | 41.30 | 0.94 (0.67–1.31) | .718 | 0.93 (0.81–1.07) | .296 | ||
| Secondary | 4.50 | 0.91 (0.65–1.27) | .591 | 1.01 (0.73–1.42) | .931 | ||
| Contraception | 58 (55, 60) | 20 (15, 23) | |||||
| Other | 24.3 | 1 (reference) | <.001 | 1 (reference) | |||
| Injectable | 51.20 | 0.24 (0.20–0.28) | <.001 | 1.36 (1.14–1.63) | <.001 | ||
| Pill | 9.89 | 1.42 (1.20–1.70) | <.001 | 0.71 (0.51–1.00) | .050 | ||
| No | 14.58 | 1.20 (1.01–1.42) | .035 | 1.17 (0.91–1.48) | .212 | ||
| Parity | 52 (48, 57) | 47 (42, 53) | |||||
| 0 | 12.40 | 2.41 (1.95–2.97) | <.001 | 1.86 (1.45–2.38) | <.001 | ||
| 1 | 43.10 | 1.08 (0.90,1.31) | .392 | 1.76 (1.48–2.10) | <.001 | ||
| 2+ | 44.50 | 1 (reference) | 1 (reference) | ||||
| Number of Sexual Partners | 5 (3, 10) | ||||||
| 1 | 1 (reference) | 1 (reference) | |||||
| 2+ | 13.44 | 1.27 (0.98–1.63) | .063 | 1.39 (1.08–1.78) | .010 | ||
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; PAR%, population attributable risk percentage.
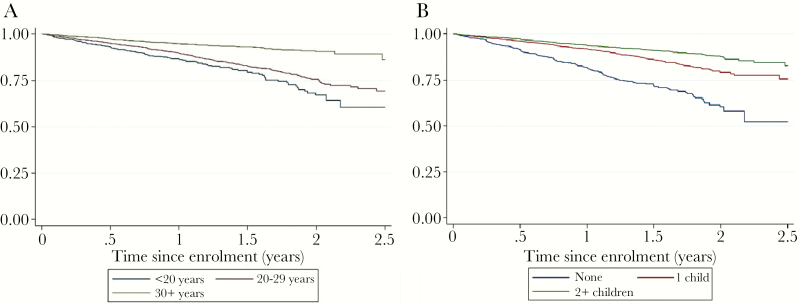
Kaplan-Meier curves of pregnancy incidence stratified by age Figure 1A and parity Figure 1B are presented. The data demonstrate that the probability of incident pregnancy significantly increases after the first year of enrollment. Pregnancy incidence was highest among women aged less than 25 (see Supplementary Table S1a) and women with 1 child or no children (see Supplementary Table S1b).
Figure 1.
Kaplan-Meier survival curves of pregnancy incidence stratified by (a) age and (b) parity.
We observed statistical significant predictors of HIV incidence during pregnancy including age, marital status, methods of contraception, parity, and number of sexual partners (Table 2). Women aged <20 years and 20–29 years were 3.2 and 2.4 times more likely to acquire HIV compared with women aged 30 or above, respectively. Unmarried women/not cohabitating had a 2.2 times higher risk of HIV seroconversion compared with married women/cohabitating (P < .001). Women who used progestin injectable contraception, depot-medroxyprogesterone acetate, were also at increased risk for HIV (aHR, 1.36; 95% CI, 1.14–1.63; P < .001). Furthermore, women with a lower parity showed an increased risk of HIV seroconversion (aHR, 1.86; 95% CI, 1.45, 2.38; P < .001). Women with multiple sexual partners were significantly at higher risk of HIV seroconversion (aHR, 1.39; 95% CI, 1.08–1.78; P = .01).
The population attributable risks (PAR%) and their 95% CIs are also presented in Table 2. In the adjusted analysis, approximately 50% of the incident pregnancies and HIV seroconversions were associated with younger women aged <30. Being unmarried/not cohabitating was observed as a PAR for incident pregnancies and HIV seroconversions (ie, 45% and 75%, respectively). Use of injectable contraception at baseline had profound effects on pregnancy and HIV incidence rates. Fifty-eight percent of the all incident pregnancies were associated with either not using contraceptives or using pills or other methods of contraception (excluding injectable contraception). On the other hand, use of injectable rather than other methodologies was associated with 20% of the HIV seroconversions. Women with 1 child or no children were likely to have 52% and 47% of the pregnancies and HIV seroconversions, respectively.
Although it was not the focus of the current study, we determined high rates of adverse pregnancy outcomes, which may potentially be attributed to very high rates of STIs and HIV among these women. In brief, of 1034 pregnancies, 92 (9%) resulted in elective abortion. Among the remaining pregnancies (ie, 942), 131 (15%) adverse events were observed. The vast majority of these events were identified as miscarriage 92 (70%), 21 (16%) of them were premature but live birth, whereas 18 death/still births were observed (14%).
DISCUSSION
This large study of combined data from several cohorts provides insight and raises concern that despite the counseling and care provided to all participants in clinical trials, the risk of pregnancy, STIs, and HIV remained high. Our data suggests the need for more individualized intensive counseling based on women’s needs at different time points of their participation in the trial. Although we need to recruit and retain women at risk of HIV acquisition in these trials, we also need to minimize risk factors that lead to attrition or loss of “on product” time that can compromise trial efficacy endpoints. Given that these trials are HIV endpoint driven, women who become pregnant in these trials are required to stop study product until after the end of pregnancy and or breastfeeding period; therefore, they minimize their contribution to efficacy endpoints. Our study underscores the fact that the very group that we are targeting for HIV prevention (young women) are the ones that are likely to become pregnant and go off study product and are likely to acquire HIV during pregnancy. Human immunodeficiency virus incidence among young women aged between 18 and 25 years in South Africa is one of the highest in the world [3, 5, 38]. Furthermore, we report on the incidence of unwanted pregnancies (9% elective abortion) in the same age group which is of concern. Overall HIV incidence during pregnancy of 5.93 per 100 py was similar to the pooled HIV incidence during pregnancy/postpartum reported in a meta-analysis of 19 cohorts (4.7 per 100 py, 95% Cl = 3.3, 6.1 per 100 py) [39]. The incidence of HIV during pregnancy in our study was slightly higher than that reported by Chetty et al [40] in a survey conducted among women in rural KwaZulu-Natal (5.93 vs 4.5 per 100 py).
We identified overlapping risk factors for HIV seroconversion and incidence of pregnancy such as young age, marital/cohabitating status, and parity. Thus, we suggest that behavioral and structural drivers do indeed play a significant role in both pregnancy and HIV acquisition. In agreement with Chetty et al [40], we show that young women were more likely to acquire both HIV and pregnancy. In our study, young adolescent women under the age of 20 years had a 3.2 times higher risk of pregnancy compared with women 30 years and older. The same cohort was also 3.2 times more likely to acquire HIV, a trend consistent with that reported in another study conducted in KwaZulu-Natal [41]. Comparatively, women who did not become pregnant had an HIV incidence rate of 7.05 per 100 py, suggesting that during pregnancy, sexual intercourse may be decreased, hence the risk is slightly lower or, alternately, those women who did not become pregnant were likely to be on a reliable contraception but had a higher incidence of STIs due to risky sexual behavior. However, we do not know whether the women who did not become pregnant were intending to actively become pregnant or had no desire to have a pregnancy.
The rapid increase in the probability of pregnancy after 1 year of follow-up draws attention to the waning effect of HIV and pregnancy prevention counseling and suggests that more intensive, targeted, and tailored counseling is warranted after the first year of enrollment. In this regard, we are currently developing a combined risk assessment tool for HIV and pregnancy prevention for clinical trial participants so that individualized and focused counseling strategies may be implemented. We previously reported on an HIV risk assessment tool [42], which is currently being implemented in our HIV prevention vaccine trials. Although these risk assessment tools for appropriate and targeted counseling are indeed useful in a clinical trial research setting, we believe that they would be excellent for use in general infectious disease and reproductive health clinics in our setting.
Being unmarried contributed to 45% and 75% of incidence pregnancy and HIV, respectively. Cultural fertility expectations have been suggested to influence the pregnancy incidence in young women [43]. In our setting, due to high levels of poverty and unemployment, many men are unable to pay “lobola” (bride price); this may contribute to the high prevalence of unmarried women in the community as well as nonmarital fertility. Fertility among South African women has declined over time to an average of 3.5 children per women, in part due to postponement of childbearing and access to contraception [43]. Although there are reports that fertility is stabilizing in older women, in younger women, pregnancy incidence is reported to be approximately 65 per 1000 aged 15–19 years, with a staggering HIV incidence of 17.2 per 100 py in the same age group [44]. Our findings confirm this report, and we agree with Tanser et al [38] in that young South African women have an 80% lifetime risk of HIV. Parity was a significant risk factor for pregnancy and HIV incidence. Those women who had no children were at higher risk of both pregnancy and HIV acquisition. This finding was contradictory to the dose-response relationship between gravidity and HIV observed by Chetty et al [40], where the number of prior pregnancies increased risk of HIV. In a Ugandan study, 1% of pregnant women reported having multiple partners in the previous year, whereas over one third of male partners of pregnant women disclosed other sexual partners [12].
We observed high rates of incident pregnancy despite access to contraception at research site and assessing women’s contraception use at each study visit through counseling. These findings underscore the need to provide more in-depth counseling on pregnancy prevention and developing a rapport with the participants, allowing for more open and honest dialogue on the women’s reproductive health needs.
Our data showed that approximately 60% of all pregnancies would be avoided if women were using injectable contraception. Although using injectable progestin contraception as a family planning method would potentially prevent the majority of the pregnancies, it was associated with 20% of HIV acquisitions.
The observed high incidence of C trachomatis and T vaginalis has also been previously demonstrated in the general population [28], with limited data on STI among pregnant women. Our findings are of grave concern because untreated chlamydial and trichomonas infections are associated with adverse pregnancy and neonatal outcomes including miscarriage, preterm birth, and low birth weight [23, 24, 45]. Furthermore, maternal chlamydial infections may result in neonatal conjunctivitis and pneumonia [46]. Currently in South Africa, programs consider HIV prevention, but STI prevention counseling is frequently neglected. Reproductive health programs need to give serious consideration to the prevalence and incidence of STI and their sequelae among both pregnant and non-pregnant women.
Human immunodeficiency virus prevention trials target women at risk of HIV acquisition, and the inclusion criteria include women not being pregnant nor intending to be pregnant during the course of the trial. However, the very population that we target may not benefit from new technology being tested due to high pregnancy rates, which may have an impact on efficacy outcomes. It seems that the desire for a child does indeed increase after the first year of trial participation. In future trial design and implementation, researchers should consider intensifying pregnancy/HIV/STI prevention counseling and ensure adherence to contraception after the first year of study participation, especially among women who are nulliparous, unmarried, under the age of 20 years, and have incident STIs. Depending on the product being tested, if there are sufficient preclinical and clinical data on the safety of the drug during pregnancy, consenting women with incident pregnancy should be allowed to continue on the product with careful monitoring of pregnancy outcomes. These data would be useful when introducing an efficacious product in the general population.
Limitations
A limitation to the study is that our findings are based on women in communities who volunteered to participate in clinical trials and who may consider themselves at risk of HIV acquisition. We cannot generalize these findings beyond this group. However, of note is that our findings do not differ widely from those in general population surveys. Although all STIs were treated, there was no information on partner treatment or resistance testing conducted during these trials. A recent Kenyan study demonstrated that partner notification and STI treatment was effective, accepted, and feasible among pregnant/postpartum women and their partners in reducing recurrent STIs in pregnancy [47].
CONCLUSIONS
Given the need to recruit and retain women at risk for HIV, in biomedical HIV prevention trials, a better understanding of the population we target is essential because it assesses the needs and risks for both pregnancy and HIV prevention and provides appropriate individualized education and counseling. Human immunodeficiency virus prevention trials are costly and require high rates of accrual and retention together with high-quality data to measure the desired efficacy outcomes. The high rates of incident pregnancy and incidence of HIV/STIs in the trial population warrants a change in our counseling approach to address reproductive health and HIV prevention needs of women during the course of their trial participation with a more focused and targeted approach when the risk is the highest.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all the teams who conducted the original studies as well as the sponsors including the Bill & Melinda Gates Foundation (BMGF), University of California, San Francisco, (grant number 21082 to MIRA study), UK Department for International Development (DFID), British Medical Research Council (G0100137 to MDP301 study), National Institute for Allergy and Infectious Diseases (NIAID; U01AI048008 to HPTN035 study), National Institute for Allergy and Infectious Diseases (NIAID; U01AI069422 to MTN003 study), and Population Council, US Agency for International Development (USAID), Bill & Melinda Gates Foundation (BMGF; CB04.106G-7 to Population council Carraguard trial), as well as the research participants and communities who contributed to this study through participation in clinical trials. We give special thanks to Jill Hanass-Hancock for her input in reviewing this manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Joint United Nations Program on HIV/AIDS (UNAIDS). Global AIDS Update. Geneva, Switzerland: 2016. [Google Scholar]
- 2. South African National Department of Health (DOH). National Antenatal Sentinel HIV & Syphilis Survey Report. 2015. Available at: https://www.health-e.org.za/wp-content/uploads/2016/03/Dept-Health-HIV-High-Res-7102015.pdf. Accessed 01 September 2017. [Google Scholar]
- 3. Karim QA, Kharsany AB, Frohlich JA, et al. . Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. Int J Epidemiol 2011; 40:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shisana O, Simbayi RT, Zuma LC, et al. . South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town: HSRC Press,2014. [Google Scholar]
- 5. Ramjee G, Wand H, Whitaker C, et al. . HIV incidence among non-pregnant women living in selected rural, semi-rural and urban areas in KwaZulu-Natal, South Africa. AIDS Behav 2012; 16:2062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheffield JS, Wendel GD Jr, McIntire DD, Norgard MV. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci 2009; 16:20–31. [DOI] [PubMed] [Google Scholar]
- 7. Morrison C, Fichorova RN, Mauck C, et al. . Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr 2014; 66:109–17. [DOI] [PubMed] [Google Scholar]
- 8. Masson L, Passmore JA, Liebenberg LJ, et al. . Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015; 61:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kinuthia J, Drake AL, Matemo D, et al. . HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS 2015; 29:2025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MacIntyre DA, Chandiramani M, Lee YS, et al. . The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep 2015; 5:8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taha TE, Hoover DR, Dallabetta GA, et al. . Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 1998; 12:1699–706. [DOI] [PubMed] [Google Scholar]
- 12. Gray RH, Li X, Kigozi G, et al. . Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet 2005; 366:1182–8. [DOI] [PubMed] [Google Scholar]
- 13. Jones HE, Browne FA, Myers BJ, et al. . Pregnant and nonpregnant women in Cape Town, South Africa: drug use, sexual behavior, and the need for comprehensive services. Int J Pediatr 2011; 2011:353410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peltzer K, Mlambo G. Sexual HIV risk behaviour and associated factors among pregnant women in Mpumalanga, South Africa. BMC Pregnancy Childbirth 2013; 13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Businge CB, Longo-Mbenza B, Mathews V. Risk factors for incident HIV infection among antenatal mothers in rural Eastern Cape, South Africa. Glob Health Action 2016; 9:29060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keating MA, Hamela G, Miller WC, Moses A, Hoffman IF, Hosseinipour MC. High HIV incidence and sexual behavior change among pregnant women in Lilongwe, Malawi: implications for the risk of HIV acquisition. PLoS One 2012; 7:e39109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramjee G, Moonsamy S, Abbai NS, Wand H. Individual and population level impact of key HIV risk factors on HIV incidence rates in Durban, South Africa. PLoS One 2016; 11:e0153969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naidoo S, Wand H, Abbai NS, Ramjee G. High prevalence and incidence of sexually transmitted infections among women living in Kwazulu-Natal, South Africa. AIDS Res Ther 2014; 11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kapiga S, Kelly C, Weiss S, et al. . Risk factors for incidence of sexually transmitted infections among women in South Africa, Tanzania, and Zambia: results from HPTN 055 study. Sex Transm Dis 2009; 36:199–206. [DOI] [PubMed] [Google Scholar]
- 20. Mlisana K, Naicker N, Werner L, et al. . Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis 2012; 206:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abbai NS, Wand H, Ramjee G. Sexually transmitted infections in women participating in a biomedical intervention trial in Durban: prevalence, coinfections, and risk factors. J Sex Transm Dis 2013; 2013:358402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feldblum PJ, Lie CC, Weaver MA, et al. . Baseline factors associated with incident HIV and STI in four microbicide trials. Sex Transm Dis 2010; 37:594–601. [PubMed] [Google Scholar]
- 23. World Health Organization (WHO). Report on global sexually transmitted infection surveillance 2013. 2014. Availabe at: http://apps.who.int/iris/bitstream/10665/112922/1/9789241507400_eng.pdf. Accessed 4 June 2017. [Google Scholar]
- 24. World Health Organization (WHO). Draft global health sector strategies: sexually transmitted infections, 2016–2021. 2015. Availabe at: http://apps.who.int/gb/ebwha/pdf_files/EB138/B138_31-en.pdf. Accessed 4 June 2017. [Google Scholar]
- 25. Zhang X, Wang C, Hengwei W, et al. . Risk factors of HIV infection and prevalence of co-infections among men who have sex with men in Beijing, China. AIDS 2007; 21(Suppl 8):S53–7. [DOI] [PubMed] [Google Scholar]
- 26. Patterson TL, Semple SJ, Staines H, et al. . Prevalence and correlates of HIV infection among female sex workers in 2 Mexico-US border cities. J Infect Dis 2008; 197:728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagot N, Ouédraogo A, Foulongne V, et al. . Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med 2007; 356:790–9. [DOI] [PubMed] [Google Scholar]
- 28. Taha TE, Gray RH. Genital tract infections and perinatal transmission of HIV. Ann N Y Acad Sci 2000; 918:84–98. [DOI] [PubMed] [Google Scholar]
- 29. Padian NS, van der Straten A, Ramjee G, et al. . Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet 2007; 370:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCormack S, Ramjee G, Kamali A, et al. . PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet 2010; 376:1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skoler-Karpoff S, Ramjee G, Ahmed K, et al. . Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 372:1977–87. [DOI] [PubMed] [Google Scholar]
- 32. Thigpen MC, Kebaabetswe PM, Paxton LA, et al. . Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 33. Van Damme L, Corneli A, Ahmed K, et al. . Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Microbicides Trials Network (MTN). MTN statement on decision to discontinue use of Tenofovir gel in VOICE, a major HIV prevention study in women. 2011. Available at: https://mtnstopshiv.org/news/mtn-statement-decision-discontinue-use-tenofovir-gel-voice-major-hiv-prevention-study-women. Accessed 20 April 2017. [Google Scholar]
- 35. Abdool Karim SS, Richardson BA, Ramjee G, et al. . Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS 2011; 25:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marrazzo JM, Ramjee G, Richardson BA, et al. . Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wand H, Ramjee G. Assessing and evaluating the combined impact of behavioural and biological risk factors for HIV seroconversion in a cohort of South African women. AIDS Care 2012; 24:1155–62. [DOI] [PubMed] [Google Scholar]
- 38. Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339:966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014; 11:e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chetty T, Vandormael A, Thorne C, Coutsoudis A. Incident HIV during pregnancy and early postpartum period: a population-based cohort study in a rural area in KwaZulu-Natal, South Africa. BMC Pregnancy Childbirth 2017; 17:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naicker N, Kharsany AB, Werner L, et al. . Risk factors for HIV acquisition in high risk women in a generalised epidemic setting. AIDS Behav 2015; 19:1305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wand H, Reddy T, Naidoo S, et al. . A simple risk prediction algorithm for HIV transmission: results from HIV prevention trials in KwaZulu Natal, South Africa (2002–2012). AIDS Behav 2018; 22:325–36. [DOI] [PubMed] [Google Scholar]
- 43. Moultrie TA, McGrath N. Teenage fertility rates falling in South Africa. S Afr Med J 2007; 97:442–3. [PubMed] [Google Scholar]
- 44. Darroch JE, Singh S. Trends in contraceptive need and use in developing countries in 2003, 2008, and 2012: an analysis of national surveys. Lancet 2013; 381:1756–62. [DOI] [PubMed] [Google Scholar]
- 45. Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev 2004; 17:794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clin Infect Dis 2011; 53(Suppl 3):S99–102. [DOI] [PubMed] [Google Scholar]
- 47. Unger JA, Matemo D, Pintye J, et al. . Patient-delivered partner treatment for chlamydia, gonorrhea, and trichomonas infection among pregnant and postpartum women in Kenya. Sex Transm Dis 2015; 42:637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.