Significance Statement
The genes and mechanisms underlying the association between diabetes or hypertension and CKD risk are unclear. The authors identified a recessive K572Q mutation in γ-adducin (Add3), which encodes a cytoskeletal protein (ADD3), in fawn-hooded hypertensive (FHH) rats—a mutation also reported in Milan normotensive (MNS) rats that develop renal disease. They demonstrated that FHH and Add3 knockout rats had impairments in the myogenic response of afferent arterioles and in renal blood flow autoregulation, which were rescued in Add3 transgenic rats. They confirmed the K572Q mutation’s role in altering the myogenic response in a genetic complementation study that involved crossing FHH and MNS rats. The work is the first to demonstrate that a mutation in ADD3 that causes renal vascular dysfunction also promotes susceptibility to kidney disease.
Keywords: ADD3, Genetics, Myogenic Response, chronic kidney disease, renal hemodynamics
Visual Abstract
Abstract
Background
The genes and mechanisms involved in the association between diabetes or hypertension and CKD risk are unclear. Previous studies have implicated a role for γ-adducin (ADD3), a cytoskeletal protein encoded by Add3.
Methods
We investigated renal vascular function in vitro and in vivo and the susceptibility to CKD in rats with wild-type or mutated Add3 and in genetically modified rats with overexpression or knockout of ADD3. We also studied glomeruli and primary renal vascular smooth muscle cells isolated from these rats.
Results
This study identified a K572Q mutation in ADD3 in fawn-hooded hypertensive (FHH) rats—a mutation previously reported in Milan normotensive (MNS) rats that also develop kidney disease. Using molecular dynamic simulations, we found that this mutation destabilizes a critical ADD3-ACTIN binding site. A reduction of ADD3 expression in membrane fractions prepared from the kidney and renal vascular smooth muscle cells of FHH rats was associated with the disruption of the F-actin cytoskeleton. Compared with renal vascular smooth muscle cells from Add3 transgenic rats, those from FHH rats had elevated membrane expression of BKα and BK channel current. FHH and Add3 knockout rats exhibited impairments in the myogenic response of afferent arterioles and in renal blood flow autoregulation, which were rescued in Add3 transgenic rats. We confirmed these findings in a genetic complementation study that involved crossing FHH and MNS rats that share the ADD3 mutation. Add3 transgenic rats showed attenuation of proteinuria, glomerular injury, and kidney fibrosis with aging and mineralocorticoid-induced hypertension.
Conclusions
This is the first report that a mutation in ADD3 that alters ACTIN binding causes renal vascular dysfunction and promotes the susceptibility to kidney disease.
Hypertension and diabetes are the leading risk factors for CKD, but the genes and mechanisms involved are not well understood. Genome-wide association studies (GWAS) have revealed multiple quantitative trait loci (QTL) that enhance the risk of diabetic and hypertension nephropathy.1–6 Knockout (KO) studies in mice and zebrafish have confirmed that some of the candidate genes can alter renal function.7 However, none of the human sequence variants have been shown to alter the expression or function of the candidate proteins and cause renal disease in a transgenic model.7,8
Genetic studies have also identified many regions of the genome that influence the susceptibility to renal disease in rodent models of hypertension and diabetes.9 Many candidate genes have been studied. However, only a polymorphism that prevents transcription of Rab38, which blocks the reuptake of filtered albumin,10 has been confirmed to produce proteinuria in fawn-hooded hypertensive (FHH) rats.
Previously, studies identified a QTL on chromosome 1 (Rf-1), which is associated with proteinuria and glomerulosclerosis in FHH rats.9,11–13 In subsequent studies, we demonstrated that the FHH rat exhibits impaired myogenic response and autoregulation of renal and cerebral blood.14,15 Transfer of a portion of the Rf-1 region, including γ-adducin (Add3), restored renal hemodynamics and attenuated proteinuria in an FHH.1BN congenic strain.14 We also identified sequence variants in Add3 in FHH rats14 and confirmed that knockdown of ADD3 expression impaired the myogenic response of renal and cerebral arteries.16
Genetic KO studies only establish that loss of a candidate gene has the potential to affect a phenotype. They provide no information as to whether a sequence variant alters function. Validation of a causal variant requires demonstration that expression of the wild-type (WT) protein restores function in a transgenic or congenic strain. Therefore, we created FHH.1BN congenic and Add3 knock-in transgenic rats on the FHH genetic background and Add3 KO rats on the FHH.1BN and Sprague Dawley genetic backgrounds to evaluate the role of the ADD3 mutation in altering renal hemodynamics and promoting CKD. We also performed a genetic complementation study in an F1 cross of FHH and Milan normotensive (MNS) rats that share the ADD3 mutation.
Methods
Animal Models
Experiments were conducted on male rats from colonies maintained at the University of Mississippi Medical Center (UMMC). The original FHH (FHH/EurMcwi), Milan hypertension (MHS), and MNS breeders were obtained from the Medical College of Wisconsin (MCW). The FHH.1BN congenic [FHH.1BN-(D1Rat09-D1Rat225)/Mcwi] rats were generated at the UMMC. The FHH.Add3K572 transgenic [FHHTg(CAG-Add3K572)Mcwi], FHH.1BN.Add3 KO, and Sprague Dawley.Add3 KO strains were generated at the MCW and characterized at the UMMC. The FHH × FHH.1BN and FHH × MNS F1 crosses were bred at the UMMC. All protocols were approved by the Institutional Animal Care and Use Committees at the UMMC and the MCW. Sequence variants in Add3 in different strains were aligned to Brown Norway (BN) reference genome and identified using the Genome Analysis Tool kit available at https://rgd.mcw.edu/rgdweb/report/gene/main.html?id=2043. The K572Q ADD3 mutation in our colonies was also validated by Sanger sequencing of PCR products using forward primer 5′-CATGTGCTGCAGGTCCGTTTATG-3′ and reverse primer 5′-CTGAGCAGAGCAGGTCCCTCTG-3′.
Generation of FHH.Add3K572 Transgenic Rats
A full-length rat WT Add3 cDNA obtained from an expression plasmid pCMV6-entry-Add3 purchased from Origene (Rockville, MD) was inserted in a sleeping beauty transposon vector. The expression of WT ADD3 in the transposon vector driven by a CAG promoter was first validated in a cell culture system, and then, the construct was injected into the pronucleus of oocytes collected from female FHH rats along with SB100 transposase mRNA as we previously reported.17 Transposon insertion sites were detected by ligation-mediated PCR.17 A single-transgene insertion was identified on chromosome 10, which is located >64 kbp away from the protein shisa-6 homolog precursor at its 5′ end and >360 kbp away from the phosphoinositide-interacting protein at the 3′ end. Confirmation of the insertion site was verified by genotyping each animal using a Tri-Primer PCR strategy as we reported previously.17 Heterozygous founders were intercrossed to derive a homozygous transgenic line that was used for all experiments.
Generation of KO Rats
Zinc-finger nuclease (ZFN) technology18,19 was used to KO Add3 in both the FHH.1BN congenic and Sprague Dawley strain backgrounds. A ZFN targeting the sequence ACCCGACTGAGGTGCtggagaAGAGAAATAAGATTCGGGA in exons 11 and 12 of the rat Add3 gene was designed and obtained from Sigma-Aldrich (St. Louis, MO). The ZFN mRNA was injected into the pronucleus of fertilized FHH.1BN and Sprague Dawley embryos and transferred to the oviduct of pseudopregnant females. Founders were identified using a Cel-1 assay.20 PCR genotyping of tail biopsies from the founders confirmed 68- and 14-bp deletions in FHH.1BN and Sprague Dawley genetic background, respectively, using forward primer 5′-GCCCCCATGAGTCACTACAC-3′ and reverse primer 5′-GCTACAGGAAGCATCTCCTGTG-3′. Founders with Add3 deletion were backcrossed to the parental strain to generate heterozygous F1 rats. Heterozygous F1 siblings were then intercrossed to derive a homozygous KO line used for all experiments.
Immunocytochemistry
Vascular smooth muscle cells (VSMCs) were isolated from pooled renal microvessels isolated from FHH and FHH.Add3 rats as we described previously.16,21 Briefly, renal microvessels were isolated using a sieving procedure and washed with ice-cold physiologic salt solution (PSS) containing (pH 7.4) 119 mM NaCl, 4.7 mM KCl, 1.6 mM CaCl2, 1.2 mM MgSO4, 18 mM NaHCO3, 1.2 mM NaH2PO4, 10 mM glucose, 0.03 mM EDTA, and 5 mM HEPES. The vessels were incubated in PSS supplemented with papain (22.5 U/ml) and dithiothreitol (2 mg/ml) at 37°C for 15 minutes with gentle rotation; then, they were pelleted and resuspended in PSS supplemented with collagenase (250 U/ml), trypsin inhibitor (10,000 U/ml), and elastase (2.4 U/ml) and incubated at 37°C with gentle rotation for another 15 minutes. After centrifugation, single cells were released into DMEM (Thermo Fisher Scientific, Waltham, MA) supplemented with 20% FBS and antibiotics and seeded in chamber slides that were precoated with Cell-Tak (Agilent, Santa Clara, CA). Immunocytochemistry was performed immediately on freshly isolated cells using primary antibodies against KCNMA1 (APC-107, 1:50; Alomone Labs, Jerusalem, Israel) and Adducin γ (sc-25733; Santa Cruz Biotechnology, Santa Cruz, CA) followed by incubation with Alexa Fluor–labeled secondary antibodies (Thermo Fisher Scientific). The slides were covered with an antifade mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Images were obtained using a Nikon Eclipse 55i microscope connected with a DS-FiL1 color camera (Nikon, Melville, NY). Isolated VSMCs were also cultured with 20% FBS DMEM on Cell-Tek–coated dishes. Early-passage (P2) VSMCs were incubated with the Adducin γ first antibody followed by Alexa Fluor–labeled secondary antibody or Alexa Fluor–labeled Phalloidin for F-actin (Thermo Fisher Scientific) staining. Cells were imaged using a Nikon C2 laser scanning confocal inverted microscope.
Western Blot
The renal cortex and freshly isolated primary VSMCs were homogenized in radioimmunoprecipitation assay buffer (Sigma-Aldrich) supplemented with protease and phosphatase inhibitors (Thermo Fisher Scientific). Membrane fractions were obtained by centrifugation at 11,000 × g for 15 minutes at 4°C. Aliquots of the renal cortical homogenates or kidney and VSMC membrane fractions (75 μg for the renal cortex and 35 μg for the kidney membrane and VSMC membranes) were electrophoresed on SDS-polyacrylamide gels, transferred to nitrocellulose membranes with a Trans-Blot Turbo Transfer System (Bio-Rad, Hercules, CA), and incubated with an anti-Add3 antibody (sc-365177; Santa Cruz Biotechnology) as previously described,16 and β-actin was used as a loading control.
Patch-Clamp Studies
VSMCs isolated from renal microvessels obtained from FHH, FHH.Add3, FHH.1BN congenic, and FHH.1BN Add3 KO rats were used for patch-clamp studies as we previously described.16 Whole-cell currents were recorded before and after blockade of the BK channel with iberiotoxin (IBTX; 10−7 M) using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). Clampfit software (version 10.0; Axon Instruments) was used for data acquisition and analysis. Differences in the size of the VSMCs were normalized by the expression of peak current (in picoamperes) as a current density (picoamperes per picrofarads).
Examination of BP and Proteinuria
Mean arterial pressure (MAP) detected by telemetry and proteinuria collected in metabolic cages was measured at 3-week intervals in FHH and FHH.Add3K572 transgenic rats from 12 to 22 weeks or weekly before and after induction of deoxycorticosterone (DOCA) salt hypertension for 3 weeks. Hypertension was induced in uninephrectomized rats by implantation of a 21-day release DOCA pellet (200 mg) with 1% sodium chloride drinking water. Urine protein concentrations were determined using the Bio-Rad Protein Assay buffer (Bio-Rad Laboratories, Hercules, CA).
The Myogenic Response of the Afferent Arteriole
Glomerulus-attached afferent arterioles (Af-arts) were microdissected and transferred to a temperature-controlled chamber. The Af-arts were cannulated using concentric pipettes, and the myogenic responses were determined by measuring the inner diameters of the Af-art in response to an elevation in perfusion pressures from 60 to 120 mm Hg using a digital CCD camera (Andor, Concord, MA) on an inverted microscope. The data were analyzed using NIS-Elements software (Nikon) as we previously reported.16,22
Autoregulation of Renal Blood Flow
Renal blood flow (RBF) autoregulation was compared in 9- to 12-week-old rats as previously described.23 Briefly, the rats were anesthetized with ketamine (30 mg/kg intramuscularly) and Inactin (50 mg/kg intraperitoneally). RBF was measured with a transonic flow probe (Transonic Systems Inc., Ithaca, NY) placed around the left renal artery. BP was measured via catheters implanted in the carotid and femoral arteries, and renal perfusion pressure (RPP) was adjusted with clamps placed on the abdominal aorta above and below the left renal artery. The left kidney was harvested for western blot or immunohistochemistry at the end of the experiment.
Glomerular Capillary Pressure
Glomerular capillary pressure (Pgc) was measured in 9- to 12-week-old FHH and FHH.1BN congenic rats as previously described.24 Briefly, rats were anesthetized with ketamine (30 mg/kg intramuscularly) and Inactin (50 mg/kg intraperitoneally). Catheters were placed in the femoral artery and jugular vein for the measurement of arterial pressure and intravenous infusion. Pgc was estimated by measuring stop-flow pressures in proximal tubules using a servo null micropressure device (model 900; World Precision Instruments, Sarasota, FL).
Glomerular Permeability to Albumin
These experiments were performed in 9- to 12-week-old Sprague Dawley, FHH, and FHH.Add3K572 transgenic rats. High molecular mass (500 kD) FITC-Dextran dissolved in saline was injected into anesthetized rats. Glomeruli were isolated, and permeability to albumin (Palb) was compared using a fluorescent dilution technique as previously reported.25 The reflection coefficient (σAlb) indicating the ratio of the change in fluorescence intensity after rapidly reducing albumin concentration in the bath (6%–4%) to the expected change (33%) in glomerular volume in response to the decrease in oncotic pressure was examined. The convective permeability to albumin (1 − σAlb) was compared as an index of the relative degree of glomerular injury between strains in populations of glomeruli.25,26
Assessment of Renal Injury
The kidneys were fixed in 10% buffered formalin solution, and paraffin sections (3 μm) were prepared and stained with Masson trichrome or hematoxylin-eosin. Thirty glomeruli per rat were evaluated for the degree of glomerular injury as previously described.27 The degree of glomerular and renal interstitial fibrosis was examined and calculated by the measuring percentage of blue collagen staining using the NIS-Elements D 4.6 software (Nikon). Areas that exhibited red fluorescence at the corticomedullary junction were also analyzed to access the formation of protein casts.
Paraffin sections were deparaffinized with xylene and rehydrated with ethanol as we previously described.27 The sections were then permeabilized followed by antigen retrieval and incubated with antibody to nephrin (1:50; Fitzgerald, Washington, DC) and Alexa Fluor–labeled secondary antibody. Endogenous fluorescence was quenched with 0.005% Evans blue. The slides were cover slipped using an antifade mounting medium with DAPI (Vector Laboratories), and the images were captured using a Nikon Eclipse 55i microscope equipped with a DS-Fil1 color camera (Nikon). Mean fluorescence intensities for nephrin staining per glomerulus were analyzed using the NIS-Elements D 4.6 software (Nikon) as we previously described.27
Statistical Analyses
All data are presented as mean values ±SEM. A two-way repeated measures ANOVA was used to compare the significance of differences in corresponding values between groups followed by a Holm–Sidak test. A P value <0.05 was considered to be significant.
Results
Identification of a p.Lys572Gln (K572Q) Mutation in ADD3 in the FHH and MNS Rats
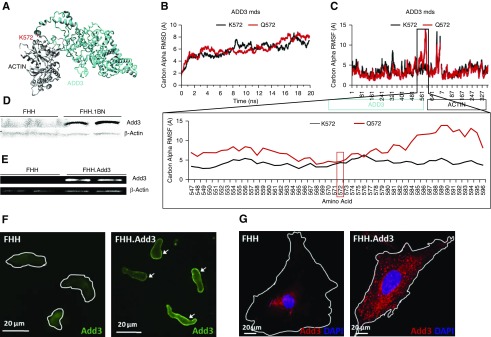
We identified a p.Lys572Gln (K572Q) mutation in ADD3 in FHH rats. The same mutation was previously reported by Tripodi et al.28 in MNS rats, which are also susceptible to kidney injury. The K572 allele in ADD3 was reported to be expressed in 13 normotensive strains, including Sprague Dawley, BN, and August Copenhagen Irish, and five hypertensive strains, including MHS and spontaneously hypertensive rats (SHRs).28 Amino acid 572 is located in exon 13 at a critical ADD3-ACTIN interaction site (Figure 1A). The ADD3 mutation destabilizes a region from amino acids 547–596 (Figure 1, B and C). These changes are predicted to alter the ability of ADD3 to regulate the actin cytoskeleton, which influences signal transduction and membrane trafficking.29 We confirmed that ADD3 expression was reduced in membrane fractions prepared from the kidney (Figure 1D) and renal VSMCs (Figure 1E) of FHH rats. ADD3 redistributed from the cell membrane to the cytoplasm of freshly isolated (Figure 1F) and primary cultured renal VSMCs (Figure 1G) in FHH versus FHH.Add3 rats.
Figure 1.
A p.Lys572Gln (K572Q) mutation in the ADD3 protein alters the actin binding site in the FHH rat. (A) Model of ADD3 (cyan) docked to ACTIN (gray) using HADDOCK web server followed by energy minimization indicates that the location of K572 (red) is at a critical ADD3-ACTIN binding site. (B) ADD3 protein models containing K572 or Q572 allele were run for 20 ns of molecular dynamics simulations (mds) using the AMBER03 force field to reach dynamic equilibrium. (C) The individual amino acid movement was assessed for both ADD3 and ACTIN, and a zoomed-in view of the 572 location demonstrates that the amino acid mutation p.Lys572Gln (K572Q) destabilizes the region from amino acids 547–596. (D) Representative western blot indicating the expression of an immunoreactive band at the apparent molecular mass of ADD3 (94 kD) in the membrane fraction of the kidney of FHH.1BN congenic but not in FHH rats; β-actin was used as a loading control. (E) ADD3 was detected in the membrane fraction of primary renal VSMCs isolated from FHH.Add3 transgenic rats that carry the reference K572 allele, but it was barely detectable in FHH rats; β-actin was used as a loading control. Distribution of ADD3 in the membrane of (F) freshly isolated and (G) cultured primary renal VSMCs of FHH rats is reduced relative to FHH.Add3 transgenic rats. White lines indicate the outline of the cells.
Validation of ADD3 Protein Expression in the Newly Generated Rat Models
The Add3 transgenic and KO rat models were created on the FHH, FHH.1BN, and Sprague Dawley genetic backgrounds. The FHH rat expresses the Q572 allele. The FHH.1BN congenic rat has a 2.4-Mbp region of chromosome 1, including Add3, from BN rats introgressed onto the FHH background. Both FHH.1BN congenic and Sprague Dawley rats carry the reference K572 allele of ADD3. Transgenic FHH-Tg(CAG-Add3K572) Mcwi rats (referred to as FHH.Add3) were created using a Sleeping Beauty transposon transgenic technique17 to overexpress the reference ADD3 allele (K572) in FHH rats (Supplemental Figure 1A). ZFN technology18,19 was used to KO the reference K572 allele of ADD3 in both the FHH.1BN congenic and Sprague Dawley strains that do not express ADD3 (Supplemental Figure 1, B and C). We refer to these strains as FHH.1BN Add3 KO and Sprague Dawley.Add3 KO, respectively.
Effects of ADD3-K572Q Variant on the F-Actin Cytoskeleton and IBTX-Sensitive BK Channel Current in VSMCs Isolated from FHH Rats
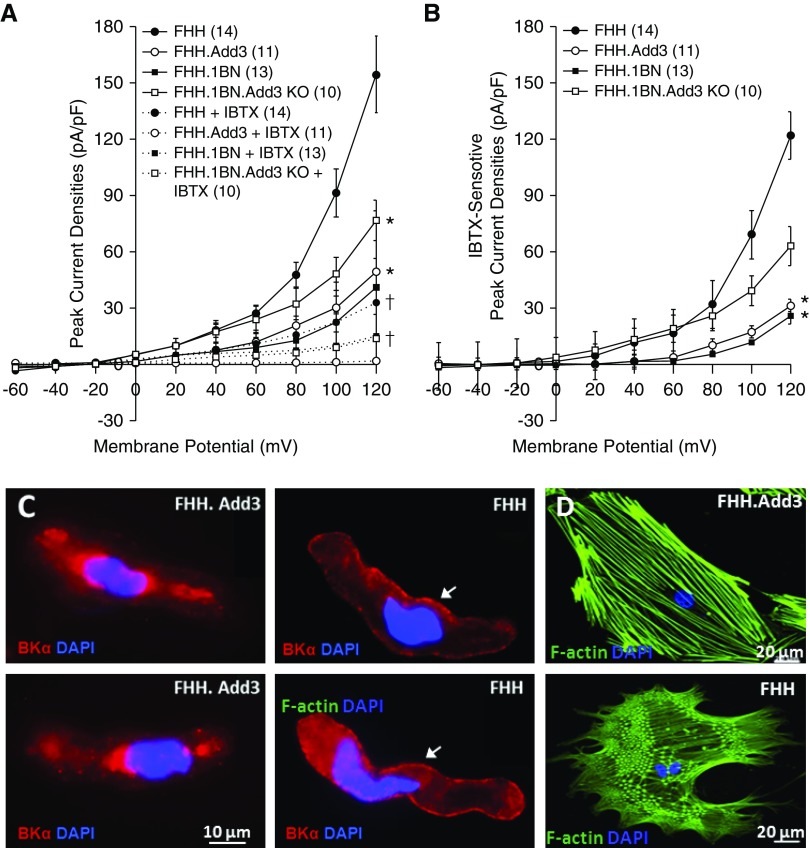
IBTX-sensitive BK peak current densities were higher in VSMCs of FHH and FHH.1BN Add3 KO rats than controls (Figure 2, A and B). The expression of pore-forming BK α-subunits was elevated in the plasma membrane of renal VSMCs of FHH versus FHH.Add3K572 rats (Figure 2C). The actin cytoskeleton was altered in primary cultured VSMCs isolated from FHH rats with the loss of F-actin stress filaments and the formation of a more branched F-actin network (Figure 2D).
Figure 2.
A p.Lys572Gln (K572Q) mutation in the ADD3 protein in the FHH rat is associated with elevated IBTX-sensitive potassium (BK) channel current and disruption of the F-actin cytoskeleton in VSMCs. (A and B) Comparison of IBTX-sensitive potassium channel current densities in VSMCs isolated from renal vasculature of FHH, congenic, Add3 transgenic, and KO rats. Numbers in parentheses indicate the number of animals studied. *Significant differences compared with the corresponding values in the WT strains; †significant differences before and after IBTX within the same. (C) Immunocytochemistry demonstrating elevated expression of BK α-subunits in the plasma membrane of renal VSMCs isolated from FHH compared with FHH.Add3 rats. (D) The actin cytoskeleton was disrupted in primary cultured VSMCs isolated from FHH compared with FHH.Add3 rats.
Effects of ADD3-K572Q Variant on the Myogenic Response of the Af-art and Autoregulation of RBF
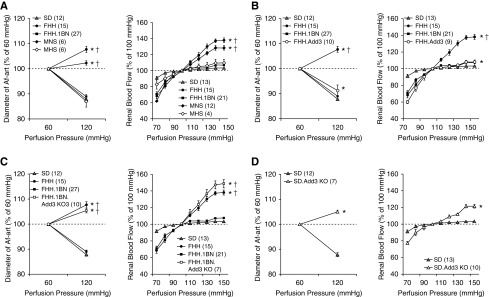
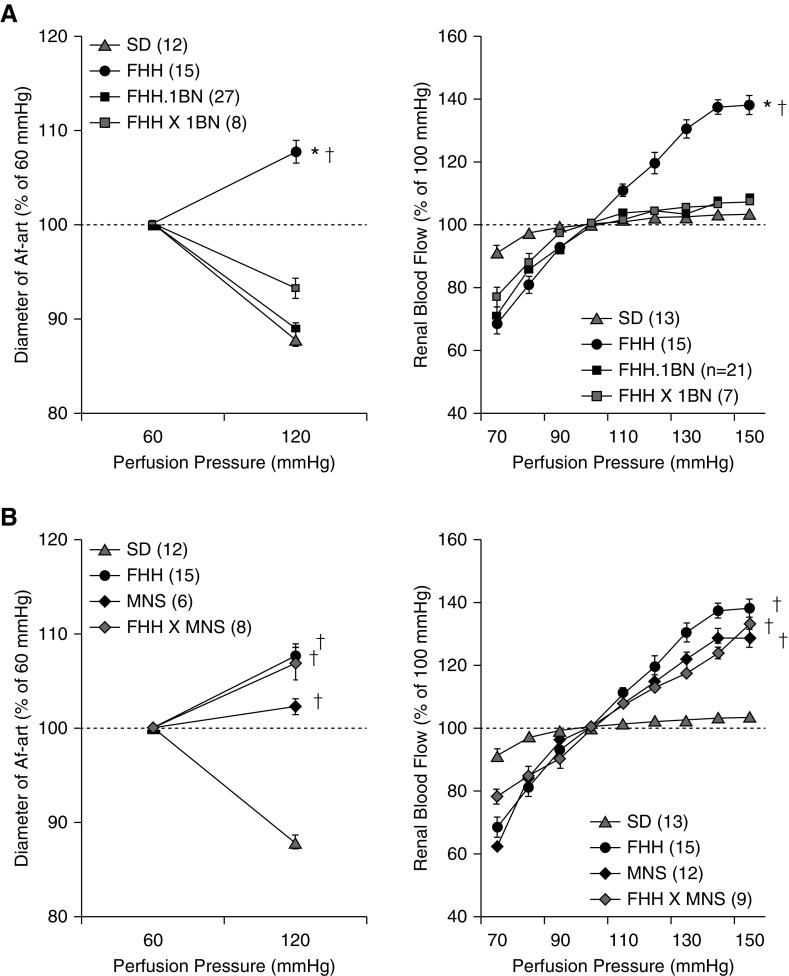
The diameter of the Af-art decreased by 12.1%±0.8% in Sprague Dawley rats (carrying K572 allele) when perfusion pressure was increased, and RBF only increased by 3.5%±0.9% when RPP was elevated from 100 to 150 mm Hg (Figure 3A). Both FHH.1BN congenic and MHS rats displayed a normal myogenic response and autoregulation. However, the Af-art failed to constrict in FHH and MNS rats, and RBF increased by 38.0%±3.0% when RPP was increased. The renal vascular impairment was rescued in FHH.1BN congenic and FHH.Add3K572 transgenic rats (Figure 3B). KO of Add3 abolished the myogenic response and RBF autoregulation in FHH.1BN congenic (Figure 3C) and Sprague Dawley rats (Figure 3D).
Figure 3.
The ADD3-K572Q variant impairs the myogenic response of the Af-art and autoregulation of RBF. (A) The diameter of the renal Af-art in rats carrying K572 allele, including Sprague Dawley, FHH.1BN congenic, and MHS rats, decreased when perfusion pressure increased from 60 to 120 mm Hg (left panel). RBF was well autoregulated when RPP elevated from 100 to 150 mm Hg in these strains (right panel). In contrast, Af-art did not constrict following elevations in pressure in FHH and MNS rats carrying Q572 allele, and RBF was poorly autoregulated when RPP was elevated from 100 to 150 mm Hg. (B) Expression of WT ADD3 protein in FHH.1BN congenic and FHH.Add3 transgenic rats rescued the Af-art myogenic response (left panel) and RBF autoregulation (right panel). (C) KO of Add3 in the FHH.1BN genetic background abolished the Af-art myogenic response (left panel) and RBF autoregulation (right panel). (D) KO of Add3 in Sprague Dawley rats also abolished the myogenic response of the Af-art (left panel) and RBF autoregulation (right panel). Numbers in parentheses indicate the number of animals studied. *Significant difference compared with the corresponding value in the WT strains; †significant difference from the corresponding value in Sprague Dawley rats.
Genetic Complementation Studies
The Af-art constricted in an F1 cross of FHH × FHH.1BN rats when perfusion pressure was increased but dilated in FHH × MNS rats (Figure 4). RBF increased by 7.3%±1.7% in FHH × FHH.1BN versus 33.1%±4.4% in FHH × MNS rats when RPP was elevated from 100 to 150 mm Hg. The recessive inhibitory effect of Q572 ADD3 was confirmed in cerebral arteries isolated heterozygous or homozygous Sprague Dawley.Add3 KO rats (Supplemental Figure 2). These results demonstrate that one copy of the reference Add3 K572 allele is sufficient to maintain the myogenic response and provides strong genetic evidence that the mutant ADD3 underlies renal vascular dysfunction in FHH and MNS rats.
Figure 4.
A genetic complementation study confirms that a shared ADD3 mutation causes renal vascular dysfunction in FHH and MNS rats. (A) One copy of ADD3 WT K572 allele in an F1 cross of FHH and FHH.1BN congenic (FHH × 1BN) rats restored the Af-art myogenic response (left panel) and RBF autoregulation (right panel) relative to FHH rats. (B) Two copies of ADD3 mutant Q572 allele in an F1 cross of FHH and MNS rat (FHH × MNS) abolished the Af-art myogenic response (left panel) and RBF autoregulation (right panel). Numbers in parentheses indicate the number of animals studied. *Significant difference compared with the corresponding value in the WT strains; †significant difference from the corresponding value in Sprague Dawley rats.
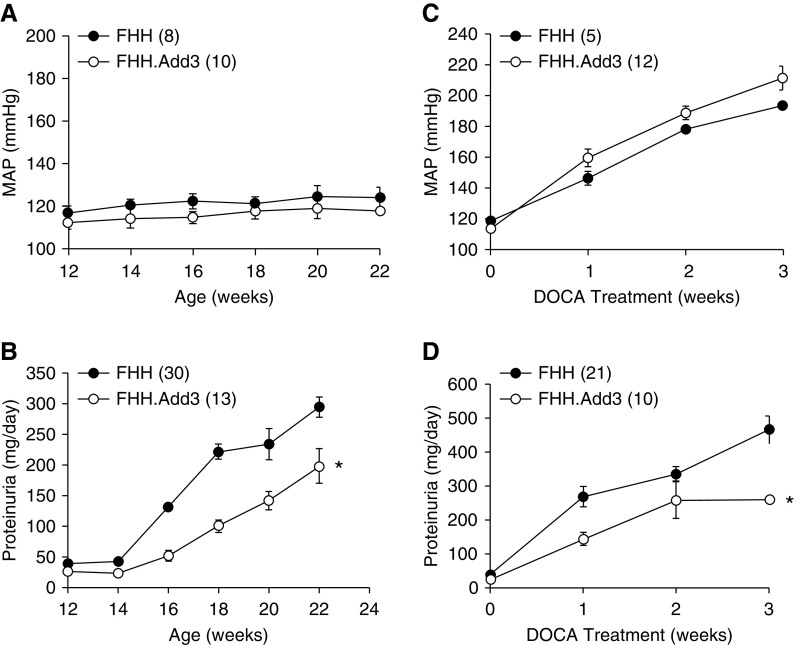
Effects of ADD3-K572Q Variant on MAP and Renal Injury
The consequence of the ADD3 mutation in FHH rats on the development of CKD with aging and hypertension was evaluated. MAP was similar in FHH and FHH.Add3K572 transgenic rats as they aged from 12 to 22 weeks (Figure 5A) and after induction of DOCA/salt hypertension (Figure 5C). Proteinuria increased from 37.6±2.2 to 294.0±16.0 mg/d in 12- versus 22-week-old FHH rats but rose to a lesser extent in age-matched FHH.Add3K572 rats (Figure 5B). Similarly, protein excretion was lower in FHH.Add3K572 than FHH rats after the induction of DOCA/salt hypertension (Figure 5D).
Figure 5.
The ADD3-K572Q variant increases mean arterial pressure (MAP) and urine protein excretion with age and after induction of hypertension in FHH rats. (A) MAP was similar in 12- to 22-week-old FHH and FHH.Add3 transgenic rats. (B) Proteinuria was significantly lower in 12- to 22-week-old FHH.Add3 transgenic compared with FHH rats. (C) MAP rose to the same extent in FHH.Add3 transgenic versus FHH rats after induction of DOCA/salt hypertension. (D) Urine protein excretion was lower in hypertensive FHH.Add3 transgenic versus FHH rats. Numbers in parentheses indicate the number of animals studied. *Significant difference compared with the corresponding value in FHH rats.
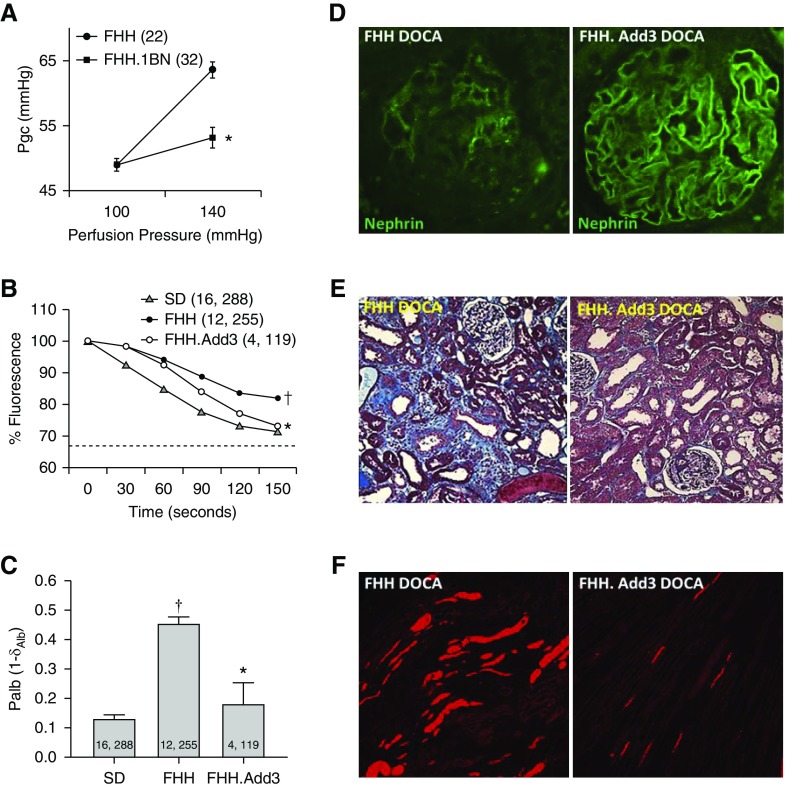
Effects of ADD3-K572Q Variant on Pgc, Palb, and Renal Injury after Induction of Hypertension
Pgc increased by 15 mm Hg in FHH rats when RPP was increased versus only 4 mm Hg in the age-matched FHH.1BN congenic strain (Figure 6A). Using a fluorescence dilution technique, we confirmed that Palb was elevated in FHH rats as previously reported.25 Overexpression of ADD3K572 rescued the elevated Palb in FHH.Add3K572 transgenic rats (Figure 6, B and C). Hypertensive FHH.Add3K572 rats exhibited greater glomerular nephrin expression (Figure 6D, Supplemental Figure 3A), reduced glomerular injury, fibrosis (Figure 6E, Supplemental Figure 3, B and C), and less protein cast formation (Figure 6F, Supplemental Figure 3D) than hypertensive FHH rats.
Figure 6.
The ADD3-K572Q variant alters Pgc, Palb, and glomerular function in FHH rats after induction of hypertension. (A) Pgc estimated from measurements of stop-flow pressure in the early proximal tubules increased to a lesser extent from 49.0±1.0 to 53.1±1.6 mm Hg in 3-month FHH.1BN rats when RPP was increased from 100 to 140 mm Hg versus from 48.8±0.9 to 63.6±1.2 mm Hg in age-matched FHH rats. Numbers in parentheses indicate the number of glomeruli studied. (B and C) Fluorescence intensity changes (percentages) in glomeruli isolated from Sprague Dawley, FHH, and FHH.Add3 transgenic rats in response to a change in albumin concentration in the bath albumin concentration from 6% to 4% are presented. Palb is calculated as 1 − σAlb in glomeruli isolated from Sprague Dawley, FHH, and FHH.Add3 transgenic rats; σAlb is the convective albumin reflection coefficient. Numbers in parentheses or bars indicate the number of animals and glomeruli studied. *Significant difference from the corresponding values in WT strains; †significant difference from the IBTX values in Sprague Dawley rats. (D) Glomerular nephrin expression is lower in FHH versus FHH.Add3 transgenic rats after 3 weeks of DOCA/salt hypertension. Quantitation is presented in Supplemental Figure 3A. (E) Glomerular injury and renal interstitial fibrosis were reduced FHH.Add3 transgenic versus FHH rats after 3 weeks of DOCA/salt hypertension. Quantitation is presented in Supplemental Figure 3, B and C. (F) Tubular protein cast formation (red stain) on hematoxylin-eosin–stained sections in hypertensive FHH.Add3 transgenic versus FHH rats. Quantitation is presented in Supplemental Figure 3D.
Discussion
Patients who are diabetic or hypertensive are at higher risk for the development of CKD.30 There is a significant genetic component because less than half of these individuals ever develop renal disease.31,32 GWAS have identified >100 regions of the genome that influence the susceptibility to CKD.33 However, identification of the specific variants involved has proven to be difficult due to the heterogeneous patient populations, differences in disease progression, and uncontrolled environmental factors.31
Our group has been studying CKD susceptibility using inbred FHH rats, which reduces genetic heterogeneity and allows for control of environmental influences.12,13,31,34 This strain is characterized by proteinuria, glomerulosclerosis, renal interstitial fibrosis, and mild hypertension as they age.11,35–41 FHH rats are also more susceptible to hypertension-induced renal injury than control August Copenhagen Irish and BN strains.39–41 We previously reported that FHH rats do not autoregulate RBF due to impaired myogenic response in renal arterioles.14,16,42 Five QTLs (Rf-1 to Rf-5) for proteinuria have been identified in this strain.31 Two of these loci (Rf-1 and Rf-2) are on rat chromosome 1.40 We subsequently identified a mutation in the Rab38 gene located in the Rf-2 region that contributes to proteinuria in FHH rats by attenuating tubular reuptake of filtered protein.10,40 More recently, a variant in the Rab38 has been linked to proteinuria in patients who are diabetic.4 However, introgression of the WT Rab38 gene in congenic or transgenic strains had no effect on the renal vascular function,10,40 suggesting other genes in the Rf-1 QTL must alter renal hemodynamics and contribute to CKD in FHH rats.
We reported that substitution of a portion of the Rf-1 region, including Add3, attenuated proteinuria and rescued the impaired myogenic response and autoregulation of renal and cerebral blood flow14,15 in an FHH.1BN congenic strain. We established Add3 as a candidate gene by showing that knockdown of its expression impaired the myogenic response of renal and cerebral arterioles ex vivo.16 We confirmed that FHH and MNS rats, which are both susceptible to renal injury,42–44 share the mutant Q572 locus in ADD3, whereas the reference K572 allele is expressed in 5 hypertensive strains and 13 of 18 normotensive strains sequenced in a previous study.28
Adducin is a cytoskeletal protein composed of heterodimers of α- (ADD1) and β-subunits (ADD2) or α- and γ-subunits (ADD3).29 ADD3 is expressed in most tissues, including blood vessels, podocytes, and distal tubules in the kidney.16,29,45 These tetrameric proteins have similar structures, including a globular head, a neck region important for dimerization, and a positively charged C terminus essential for dimerization and the association of adducin with actin, spectrin, and the plasma membrane.29,46 The tails of the ADD subunits are critical to its ability to cap F-actin and prevent excess polymerization.29,47,48 Phosphorylation of this region by protein kinases C and A dissociates ADD and spectrin from the membrane and disrupts actin capping and the cytoskeleton.46
Our results indicate that the K572Q mutation in ADD3 destabilizes the region of amino acids 547–596 at a critical ADD3-ACTIN interaction site. This region also contains three serine phosphorylation sites that regulate the function of this protein.49 The mutation is predicted to alter the configuration of ADD3, disassociate it from the membrane, and diminish its ability to regulate the actin cytoskeleton in VSMCs, which influences signal transduction and membrane trafficking.29 This prediction was confirmed by our finding of reduced ADD3 expression in membrane fractions prepared from the kidney and primary renal VSMCs of FHH rats. Consistent with our previous reports,16,50 we also found that ADD3 redistributed to a perinuclear location in freshly isolated and cultured primary VSMCs from FHH rats in association with the loss of F-actin stress filaments and the formation of a more branched F-actin network.
However, confirmation that the K572Q mutation is causal requires demonstration that the expression of the WT protein restores function. In this regard, the myogenic response of the Af-art and RBF autoregulation was impaired in FHH and MNS rats carrying the Q572 mutant allele and Add3 KO rats on both Sprague Dawley and FHH.1BN genetic backgrounds. The response was intact in Sprague Dawley and MHS rats, and it was rescued in FHH.1BN and FHH.Add3K572 rats that express WT ADD3.
The results of our genetic complementation study indicated that the myogenic response of the Af-art and autoregulation of RBF was impaired in an F1 cross of FHH and MNS rats that share the K572Q mutation in ADD3 but normalized in a cross of FHH and FHH.1BN rats with one copy of the WT ADD3 allele. In addition, the impaired myogenic response in Sprague Dawley.Add3 KO rats was restored when they were crossed with Sprague Dawley rats carrying the WT K572 ADD3. These results indicate that the shared mutation in ADD3 plays a causal role in renal vascular dysfunction in FHH and MNS rats.
An activating mutation in ADD1, but not Add3, has been linked to the development of hypertension,51–57 stroke,58 and cardiovascular dysfunction59–61 in human genetic association studies and the development of hypertension in MHS rats.52,62 Previous studies focused on the role of a G460W mutation in ADD1 in promoting hypertension by altering actin polymerization and enhancing sodium transport in the kidney.52,54,63–66 However, there are now a few reports that mutations in ADD3 are also linked to human disease. In this regard, a rare variant in ADD3 found in three families was associated with cognitive dysfunction and nephrotic syndrome in one of these families.67 A G367D mutation in ADD3 that impaired dimerization of ADD3 with ADD1, actin capping, and disrupted the cytoskeleton in fibroblasts, was associated with cerebral palsy in related families.68 Finally, SNPs near the Add3 gene have been linked to biliary atresia.69
The mechanism by which loss of adducin function impairs the myogenic response remains to be determined. We reported that the impaired myogenic response is associated with an elevation in BK channel activity.14,15,70 The myogenic response could be restored by blocking the BK channel with IBTX. In this study, the expression of BK channels in the membrane and channel activity was elevated in VSMCs isolated from FHH rats. The elevated BK channel activity was normalized by the expression of WT-ADD3 in FHH.1BN congenic and FHH.Add3 transgenic strains. Elevated BK channel activity was also seen in ADD3 KO rats. Increased BK channel activity could be secondary to disruption of the actin cytoskeleton that increases its trafficking into the membrane or reduces recycling of the channel into endosomes. Alternatively, changes in the actin cytoskeleton could affect the actin-myosin contractile mechanism, the membrane expression of other ion channels, or signaling mechanisms.29,48,50
Hyperfiltration and increased Pgc are thought to contribute to podocyte injury in salt-sensitive hypertensive and diabetic models.71,72 In this study, we confirmed that Pgc and Palb were elevated in FHH rats. Renal injury was attenuated in FHH.Add3K572 rats with reduced protein excretion, less glomerular injury, and increased nephrin expression. Renal interstitial fibrosis and protein cast formation were reduced in hypertensive FHH.Add3K572 rats. Moreover, the development of proteinuria and CKD with aging was attenuated in FHH.Add3K572 relative to FHH rats. These results support the view that the Q572 mutation in ADD3 contributes to the development of kidney disease, at least in FHH and MNS rats.
A remaining question is whether renal hemodynamics and the susceptibility to proteinuria are altered in MNS, Wistar Kyoto (WKY), Buffalo, and Lewis rats that also express Q572 ADD3.28 The myogenic response of the Af-Art73–75 and the autoregulation of RBF76 were impaired in WKY versus SHR and MNS versus MHS rats.44 SHR and MHS rats are less susceptible to CKD than their control strains.9,77–79 Buffalo rats develop focal glomerular sclerosis as they age, but renal hemodynamics have not been studied.9 We have reported that the myogenic response of the Af-Art in Lewis rats was blunted to the same extent as in WKY relative to SHR,75 but their susceptibility to hypertension or diabetic-induced nephropathy versus other strains has not been evaluated. These findings suggest that the myogenic response would be reduced in other strains expressing Q572 ADD3. However, the development of proteinuria in response to hypertension or diabetes may be blunted relative to FHH rats because this strain has variants in other genes (Rab38 and Sorcs1) that impair the reuptake of filtered protein.10,40,80
The contribution of mutations in ADD3 to the development of CKD in humans is uncertain. A region of human chromosome 10 near ADD3 was associated with diabetic and nondiabetic CKD in black sib-pairs.81 This finding was replicated in a more extensive study of diabetic sib-pairs82 and in a longitudinal study of whites in a Utah pedigree.83 However, the QTLs in these studies were broad (40 cM). The most recent GWAS meta-analysis for CKD suggests that ADD3 is 5–6 Mbp from the nearest loci.33 However, our examination of the Genome Aggregation Database (https://macarthurlab.org/2019/10/16/gnomad-v3-0/) revealed there are 492 nonsynonymous ADD3 variants, of which 227 are damaging. A K571H mutation in human ADD3, analogous to the K572Q in the FHH rat, is the most prevalent variant. There are five other variants nearby that alter charged amino acids in the actin binding site and many others that modify phosphorylation sites in the tail of ADD3 that control its interactions with actin, spectrin, and membrane phospholipids. However, all of these mutations are rare variants (frequency <0.1%) that are challenging to link to CKD, even in very large GWAS. In addition, multiple isoforms of ADD3 have been identified, one of which deletes amino acids 576–607 within the critical actin binding region.84 Thus, we believe that additional genetic studies are needed that pool results from diabetic and hypertensive subjects with damaging genotypes to discern if these rare variants in ADD3 are associated with the CKD in more susceptible populations. Nevertheless, these results indicate that variants in ADD3 that alter the actin cytoskeleton and impair renal hemodynamics have the potential to increase the susceptibility to diabetic and nondiabetic CKD in individual patients and families with one of these rare mutations.
Numerous QTLs and candidate genes for CKD have been identified in animal and GWAS.9,33 However, no variants have been identified that alter the myogenic response of renal arterioles and autoregulation of RBF. This study used novel ADD3 KO and transgenic rats and a genetic complementation approach in FHH and MNS rats to confirm that a loss-of-function mutation in ADD3 that alters ACTIN binding causes renal vascular dysfunction and promotes kidney disease in MNS and FHH rats.
Disclosures
Dr. Geurts reports grants from the National Institutes of Health during the conduct of the study. Dr. Prokop reports grants from National Institutes of Health during the conduct of the study. Dr. Roman reports grants from National Institutes of Health during the conduct of the study. All remaining authors have nothing to disclose.
Funding
This study was supported in part by National Institutes of Health grants AG050049 (to Dr. Fan), AG057842 (to Dr. Fan), P20GM104357 (to Dr. Roman and Dr. Fan), DK104184 (to Dr. Roman) and HL138685 (to Dr. Roman) and American Heart Association grant 16GRNT31200036 (to Dr. Fan).
Supplementary Material
Acknowledgments
We thank Dr. Allen Cowley Jr. (Medical College of Wisconsin) for providing us with MNS and MHS rats.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Pulling the Hood off Genetic Susceptibility to Hypertensive Renal Disease,” on pages 667–668.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019080784/-/DCSupplemental.
Supplemental Figure 1. Comparison of the expression of ADD3 protein in kidney of Sprague Dawley, FHH, FHH.Add3 transgenic, and KO rats.
Supplemental Figure 2. The vascular effects of the K572Q ADD3 variant are autosomal recessive.
Supplemental Figure 3. Comparison of glomerular nephrin staining, glomerular injury scores, percentage of renal interstitial fibrosis, and protein casts in hypertensive FHH and FHH.Add3 transgenic rats.
References
- 1.Palmer ND, Ng MC, Hicks PJ, Mudgal P, Langefeld CD, Freedman BI, et al.: Evaluation of candidate nephropathy susceptibility genes in a genome-wide association study of African American diabetic kidney disease. PLoS One 9: e88273, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandholm N, Salem RM, McKnight AJ, Brennan EP, Forsblom C, Isakova T, et al.; DCCT/EDIC Research Group: New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet 8: e1002921, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyengar SK, Sedor JR, Freedman BI, Kao WH, Kretzler M, Keller BJ, et al.; Family Investigation of Nephropathy and Diabetes (FIND): Genome-wide association and trans-ethnic meta-analysis for advanced diabetic kidney disease: Family Investigation of Nephropathy and Diabetes (FIND). PLoS Genet 11: e1005352, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pattaro C, Teumer A, Gorski M, Chu AY, Li M, Mijatovic V, et al.ICBP Consortium; AGEN Consortium; CARDIOGRAM; CHARGe-Heart Failure Group; ; ECHOGen Consortium: Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun 7: 10023, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pattaro C, Köttgen A, Teumer A, Garnaas M, Böger CA, Fuchsberger C, et al.CARDIoGRAM Consortium; ICBP Consortium; CARe Consortium; ; Wellcome Trust Case Control Consortium 2 (WTCCC2): Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet 8: e1002584, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CT, Garnaas MK, Tin A, Kottgen A, Franceschini N, Peralta CA, et al.; CKDGen Consortium: Genetic association for renal traits among participants of African ancestry reveals new loci for renal function. PLoS Genet 7: e1002264, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKnight AJ, Duffy S, Maxwell AP: Genetics of diabetic nephropathy: A long road of discovery. Curr Diab Rep 15: 41, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Cañadas-Garre M, Anderson K, Cappa R, Skelly R, Smyth LJ, McKnight AJ, et al.: Genetic susceptibility to chronic kidney disease - some more pieces for the heritability puzzle. Front Genet 10: 453, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz A, Kreutz R: Mapping genetic determinants of kidney damage in rat models. Hypertens Res 35: 675–694, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Rangel-Filho A, Lazar J, Moreno C, Geurts A, Jacob HJ: Rab38 modulates proteinuria in model of hypertension-associated renal disease. J Am Soc Nephrol 24: 283–292, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López B, Ryan RP, Moreno C, Sarkis A, Lazar J, Provoost AP, et al.: Identification of a QTL on chromosome 1 for impaired autoregulation of RBF in fawn-hooded hypertensive rats. Am J Physiol Renal Physiol 290: F1213–F1221, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ: Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 12: 44–51, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Brown DM, Van Dokkum RP, Korte MR, McLauglin MG, Shiozawa M, Jacob HJ, et al.: Genetic control of susceptibility for renal damage in hypertensive fawn-hooded rats. Ren Fail 20: 407–411, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Burke M, Pabbidi M, Fan F, Ge Y, Liu R, Williams JM, et al.: Genetic basis of the impaired renal myogenic response in FHH rats. Am J Physiol Renal Physiol 304: F565–F577, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pabbidi MR, Juncos J, Juncos L, Renic M, Tullos HJ, Lazar J, et al.: Identification of a region of rat chromosome 1 that impairs the myogenic response and autoregulation of cerebral blood flow in fawn-hooded hypertensive rats. Am J Physiol Heart Circ Physiol 304: H311–H317, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan F, Pabbidi MR, Ge Y, Li L, Wang S, Mims PN, et al.: Knockdown of Add3 impairs the myogenic response of renal afferent arterioles and middle cerebral arteries. Am J Physiol Renal Physiol 312: F971–F981, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan F, Geurts AM, Murphy SR, Pabbidi MR, Jacob HJ, Roman RJ: Impaired myogenic response and autoregulation of cerebral blood flow is rescued in CYP4A1 transgenic Dahl salt-sensitive rat. Am J Physiol Regul Integr Comp Physiol 308: R379–R390, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, et al.: Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325: 433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan F, Geurts AM, Pabbidi MR, Smith SV, Harder DR, Jacob H, et al.: Zinc-finger nuclease knockout of dual-specificity protein phosphatase-5 enhances the myogenic response and autoregulation of cerebral blood flow in FHH.1BN rats. PLoS One 9: e112878, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JC, Holmes MC, Wang J, Guschin DY, Lee YL, Rupniewski I, et al.: An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol 25: 778–785, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Fan F, Sun CW, Maier KG, Williams JM, Pabbidi MR, Didion SP, et al.: 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PLoS One 8: e82482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge Y, Murphy SR, Fan F, Williams JM, Falck JR, Liu R, et al.: Role of 20-HETE in the impaired myogenic and TGF responses of the Af-Art of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 307: F509–F515, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roman RJ, Cowley AW Jr.: Characterization of a new model for the study of pressure-natriuresis in the rat. Am J Physiol 248: F190–F198, 1985. [DOI] [PubMed] [Google Scholar]
- 24.Williams JM, Fan F, Murphy S, Schreck C, Lazar J, Jacob HJ, et al.: Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 302: R1209–R1218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan F, Chen CC, Zhang J, Schreck CM, Roman EA, Williams JM, et al.: Fluorescence dilution technique for measurement of albumin reflection coefficient in isolated glomeruli. Am J Physiol Renal Physiol 309: F1049–F1059, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savin VJ, Sharma R, Lovell HB, Welling DJ: Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol 3: 1260–1269, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, He X, Murphy SR, Zhang H, Wang S, Ge Y, et al.: Knockout of dual-specificity protein phosphatase 5 protects against hypertension-induced renal injury. J Pharmacol Exp Ther 370: 206–217, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripodi G, Szpirer C, Reina C, Szpirer J, Bianchi G: Polymorphism of γ-adducin gene in genetic hypertension and mapping of the gene to rat chromosome 1q55. Biochem Biophys Res Commun 237: 685–689, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka Y, Li X, Bennett V: Adducin: Structure, function and regulation. Cell Mol Life Sci 57: 884–895, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams EN, Mathis KW: Buffering chronic kidney disease with sodium bicarbonate. Clin Sci (Lond) 132: 1999–2001, 2018. [DOI] [PubMed] [Google Scholar]
- 31.O’Meara CC, Lutz MM, Sarkis AB, Xu H, Kothinti RK, Hoffman M, et al.: A 4.1-Mb congenic region of Rf-4 contributes to glomerular permeability. J Am Soc Nephrol 23: 825–833, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alicic RZ, Rooney MT, Tuttle KR: Diabetic kidney disease: Challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wuttke M, Li Y, Li M, Sieber KB, Feitosa MF, Gorski M, et al.Lifelines Cohort Study; ; V. A. Million Veteran Program: A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 51: 957–972, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiozawa M, Provoost AP, van Dokkum RP, Majewski RR, Jacob HJ: Evidence of gene-gene interactions in the genetic susceptibility to renal impairment after unilateral nephrectomy. J Am Soc Nephrol 11: 2068–2078, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Kuijpers MH, Gruys E: Spontaneous hypertension and hypertensive renal disease in the fawn-hooded rat. Br J Exp Pathol 65: 181–190, 1984. [PMC free article] [PubMed] [Google Scholar]
- 36.Kuijpers MH, de Jong W: Spontaneous hypertension in the fawn-hooded rat: A cardiovascular disease model. J Hypertens Suppl 4: S41–S44, 1986. [PubMed] [Google Scholar]
- 37.Gilboa N, Rudofsky U, Magro A: Urinary and renal kallikrein in hypertensive fawn-hooded (FH/Wjd) rats. Lab Invest 50: 72–78, 1984. [PubMed] [Google Scholar]
- 38.Kuijpers MH, de Jong W: Relationship between blood pressure level, renal histopathological lesions and plasma renin activity in fawn-hooded rats. Br J Exp Pathol 68: 179–187, 1987. [PMC free article] [PubMed] [Google Scholar]
- 39.Provoost AP, Shiozawa M, Van Dokkum RP, Jacob HJ: Transfer of the Rf-1 region from FHH onto the ACI background increases susceptibility to renal impairment. Physiol Genomics 8: 123–129, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, et al.: RF-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the fawn-hooded hypertensive rat. J Am Soc Nephrol 16: 852–856, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Mattson DL, Kunert MP, Roman RJ, Jacob HJ, Cowley AW Jr.: Substitution of chromosome 1 ameliorates L-NAME hypertension and renal disease in the fawn-hooded hypertensive rat. Am J Physiol Renal Physiol 288: F1015–F1022, 2005. [DOI] [PubMed] [Google Scholar]
- 42.van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ: Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol 276: R855–R863, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Pugliese G, Pricci F, Barsotti P, Iacobini C, Ricci C, Oddi G, et al.: Development of diabetic nephropathy in the Milan normotensive strain, but not in the Milan hypertensive strain: Possible permissive role of hemodynamics. Kidney Int 67: 1440–1452, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Ge Y, Fan F, Didion SP, Roman RJ: Impaired myogenic response of the afferent arteriole contributes to the increased susceptibility to renal disease in Milan normotensive rats. Physiol Rep 5: e13089, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He X, Wang S, Guo Y, Gao W, Roman RJ, Fan F: Down regulation of gamma-adducin diminishes glomerular function and promotes hypertension related chronic kidney disease. Hypertension 74[Suppl 1]: A130, 2019 [Google Scholar]
- 46.Matsuoka Y, Li X, Bennett V: Adducin is an in vivo substrate for protein kinase C: Phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol 142: 485–497, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi R, Bennett V: Mapping the domain structure of human erythrocyte adducin. J Biol Chem 265: 13130–13136, 1990. [PubMed] [Google Scholar]
- 48.Joshi R, Gilligan DM, Otto E, McLaughlin T, Bennett V: Primary structure and domain organization of human alpha and beta adducin. J Cell Biol 115: 665–675, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lundby A, Secher A, Lage K, Nordsborg NB, Dmytriyev A, Lundby C, et al.: Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat Commun 3: 876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, Travis O, He X, Fan F, Roman RJ: Down-regulation of gamma-Adducin disrupts the actin cytoskeleton in FHH rats and may contribute to the development of hypertension-induced renal injury. FASEB J 32: 721.10, 2018 [Google Scholar]
- 51.Manunta P, Bianchi G: Pharmacogenomics and pharmacogenetics of hypertension: Update and perspectives--the adducin paradigm. J Am Soc Nephrol 17[Suppl 2]: S30–S35, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Bianchi G, Ferrari P, Staessen JA: Adducin polymorphism: Detection and impact on hypertension and related disorders. Hypertension 45: 331–340, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Lanzani C, Citterio L, Jankaricova M, Sciarrone MT, Barlassina C, Fattori S, et al.: Role of the adducin family genes in human essential hypertension. J Hypertens 23: 543–549, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Staessen JA, Bianchi G: Adducin and hypertension. Pharmacogenomics 6: 665–669, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Cwynar M, Staessen JA, Tichá M, Nawrot T, Citterio L, Kuznetsova T, et al.; European Project On Genes in Hypertension (EPOGH) Investigators: Epistatic interaction between alpha- and gamma-adducin influences peripheral and central pulse pressures in white Europeans. J Hypertens 23: 961–969, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Bianchi G, Tripodi G, Casari G, Salardi S, Barber BR, Garcia R, et al.: Two point mutations within the adducin genes are involved in blood pressure variation. Proc Natl Acad Sci U S A 91: 3999–4003, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bianchi G, Manunta P, Glorioso N: Clinical impact of adducin polymorphism. J Hypertens 27: 1325–1327, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Zafarmand MH, van der Schouw YT, Grobbee DE, de Leeuw PW, Bots ML: Alpha-adducin Gly460Trp variant increases the risk of stroke in hypertensive Dutch women. Hypertension 51: 1665–1670, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Gerhard T, Gong Y, Beitelshees AL, Mao X, Lobmeyer MT, Cooper-DeHoff RM, et al.; INVEST Investigators: Alpha-adducin polymorphism associated with increased risk of adverse cardiovascular outcomes: Results from GENEtic Substudy of the INternational VErapamil SR-trandolapril STudy (INVEST-GENES). Am Heart J 156: 397–404, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuznetsova T, Citterio L, Herbots L, Carpini SD, Thijs L, Casamassima N, et al.: Effects of genetic variation in adducin on left ventricular diastolic function as assessed by tissue Doppler imaging in a Flemish population. J Hypertens 26: 1229–1236, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Zagato L, Kuznetsova T, Tripodi G, Zerbini G, Richart T, et al.: Angiotensin-converting enzyme I/D and alpha-adducin Gly460Trp polymorphisms: From angiotensin-converting enzyme activity to cardiovascular outcome. Hypertension 49: 1291–1297, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Zagato L, Modica R, Florio M, Torielli L, Bihoreau MT, Bianchi G, et al.: Genetic mapping of blood pressure quantitative trait loci in Milan hypertensive rats. Hypertension 36: 734–739, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Tripodi G, Valtorta F, Torielli L, Chieregatti E, Salardi S, Trusolino L, et al.: Hypertension-associated point mutations in the adducin alpha and beta subunits affect actin cytoskeleton and ion transport. J Clin Invest 97: 2815–2822, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang JG, Staessen JA, Tizzoni L, Brand E, Birkenhäger WH, Fagard R, et al.: Renal function in relation to three candidate genes. Am J Kidney Dis 38: 1158–1168, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Manunta P, Citterio L, Lanzani C, Ferrandi M: Adducin polymorphisms and the treatment of hypertension. Pharmacogenomics 8: 465–472, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Efendiev R, Krmar RT, Ogimoto G, Zwiller J, Tripodi G, Katz AI, et al.: Hypertension-linked mutation in the adducin alpha-subunit leads to higher AP2-mu2 phosphorylation and impaired Na+,K+-ATPase trafficking in response to GPCR signals and intracellular sodium. Circ Res 95: 1100–1108, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Gonçalves S, Patat J, Guida MC, Lachaussée N, Arrondel C, Helmstädter M, et al.: A homozygous KAT2B variant modulates the clinical phenotype of ADD3 deficiency in humans and flies. PLoS Genet 14: e1007386, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kruer MC, Jepperson T, Dutta S, Steiner RD, Cottenie E, Sanford L, et al.: Mutations in γ adducin are associated with inherited cerebral palsy. Ann Neurol 74: 805–814, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng G, Tang CS, Wong EH, Cheng WW, So MT, Miao X, et al.: Common genetic variants regulating ADD3 gene expression alter biliary atresia risk. J Hepatol 59: 1285–1291, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Pabbidi MR, Mazur O, Fan F, Farley JM, Gebremedhinm D, Harder DR, et al. : Enhanced large conductance K+ channel activity contributes to the impaired myogenic response in the cerebral vasculature of Fawn Hooded Hypertensive rats. Am J Physiol Heart Circ Physiol 306: H989–H1000, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burke M, Pabbidi MR, Farley J, Roman RJ: Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12: 845–858, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R: Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren Y, D’Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA: Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 298: H1769–H1775, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ito S, Juncos LA, Carretero OA: Pressure-induced constriction of the afferent arteriole of spontaneously hypertensive rats. Hypertension 19[Suppl]: II164–II167, 1992. [DOI] [PubMed] [Google Scholar]
- 75.Imig JD, Falck JR, Gebremedhin D, Harder DR, Roman RJ: Elevated renovascular tone in young spontaneously hypertensive rats. Role of cytochrome P-450. Hypertension 22: 357–364, 1993. [DOI] [PubMed] [Google Scholar]
- 76.Iversen BM, Sekse I, Ofstad J: Resetting of renal blood flow autoregulation in spontaneously hypertensive rats. Am J Physiol 252: F480–F486, 1987. [DOI] [PubMed] [Google Scholar]
- 77.Floege J, Hackmann B, Kliem V, Kriz W, Alpers CE, Johnson RJ, et al.: Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: A podocyte disease. Kidney Int 51: 230–243, 1997. [DOI] [PubMed] [Google Scholar]
- 78.Stella P, Cusi D, Duzzi L, Bianchi G: Relations between hypertension and glomerulosclerosis in first-generation hybrid rats of the Milan strains. Child Nephrol Urol 11: 6–9, 1991. [PubMed] [Google Scholar]
- 79.Brandis A, Bianchi G, Reale E, Helmchen U, Kühn K: Age-dependent glomerulosclerosis and proteinuria occurring in rats of the Milan normotensive strain and not in rats of the Milan hypertensive strain. Lab Invest 55: 234–243, 1986. [PubMed] [Google Scholar]
- 80.Lazar J, O’Meara CC, Sarkis AB, Prisco SZ, Xu H, Fox CS, et al.: SORCS1 contributes to the development of renal disease in rats and humans. Physiol Genomics 45: 720–728, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freedman BI, Rich SS, Yu H, Roh BH, Bowden DW: Linkage heterogeneity of end-stage renal disease on human chromosome 10. Kidney Int 62: 770–774, 2002. [DOI] [PubMed] [Google Scholar]
- 82.Iyengar SK, Fox KA, Schachere M, Manzoor F, Slaughter ME, Covic AM, et al.: Linkage analysis of candidate loci for end-stage renal disease due to diabetic nephropathy. J Am Soc Nephrol 14[Suppl 2]: S195–S201, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Hunt SC, Hasstedt SJ, Coon H, Camp NJ, Cawthon RM, Wu LL, et al.: Linkage of creatinine clearance to chromosome 10 in Utah pedigrees replicates a locus for end-stage renal disease in humans and renal failure in the fawn-hooded rat. Kidney Int 62: 1143–1148, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Katagiri T, Ozaki K, Fujiwara T, Shimizu F, Kawai A, Okuno S, et al.: Cloning, expression and chromosome mapping of adducin-like 70 (ADDL), a human cDNA highly homologous to human erythrocyte adducin. Cytogenet Cell Genet 74: 90–95, 1996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.