Significance Statement
Cytosine methylation plays a key role in determining cell fate and response to stimuli. Using mice with kidney-specific deletion of genes encoding de novo DNA methyltransferases Dnmt3a and Dnmt3b, the authors showed that these genes are responsible for methylation of gene regulatory regions that act as enhancers during kidney development but are then decommissioned in adult mice. Although the knock-out mice displayed no obvious kidney abnormalities at baseline, they showed resistance to induced AKI. The authors also discovered that human kidney disease risk loci were enriched on fetal regulatory regions (enhancers) that were decommissioned by Dnmt3a/3b and no longer active in the adult kidney. These findings suggest that adult kidney diseases could have a developmental origin and that genetic and epigenetic (such as Dnmt3a/3b) factors could converge on the same genetic regions resulting in kidney disease development.
Keywords: de novo methylation, kidney development, fetal enhancer, WGBS, Dnmt3a, Dnmt3b
Visual Abstract
Abstract
Background
Cytosine methylation is an epigenetic mark that dictates cell fate and response to stimuli. The timing and establishment of methylation logic during kidney development remains unknown. DNA methyltransferase 3a and 3b are the enzymes capable of establishing de novo methylation.
Methods
We generated mice with genetic deletion of Dnmt3a and Dnmt3b in nephron progenitor cells (Six2CreDnmt3a/3b) and kidney tubule cells (KspCreDnmt3a/3b). We characterized KspCreDnmt3a/3b mice at baseline and after injury. Unbiased omics profiling, such as whole genome bisulfite sequencing, reduced representation bisulfite sequencing and RNA sequencing were performed on whole-kidney samples and isolated renal tubule cells.
Results
KspCreDnmt3a/3b mice showed no obvious morphologic and functional alterations at baseline. Knockout animals exhibited increased resistance to cisplatin-induced kidney injury, but not to folic acid–induced fibrosis. Whole-genome bisulfite sequencing indicated that Dnmt3a and Dnmt3b play an important role in methylation of gene regulatory regions that act as fetal-specific enhancers in the developing kidney but are decommissioned in the mature kidney. Loss of Dnmt3a and Dnmt3b resulted in failure to silence developmental genes. We also found that fetal-enhancer regions methylated by Dnmt3a and Dnmt3b were enriched for kidney disease genetic risk loci. Methylation patterns of kidneys from patients with CKD showed defects similar to those in mice with Dnmt3a and Dnmt3b deletion.
Conclusions
Our results indicate a potential locus-specific convergence of genetic, epigenetic, and developmental elements in kidney disease development.
Cytosine methylation is erased and reestablished between generations. DNA methylation is removed from the zygote by the blastocyst stage and reinstated during embryonic development.1 De novo methyltransferases 3a (Dnmt3a) and 3b (Dnmt3b) play key roles in establishing new methylation patterns.2 Dnmt3a-deficient animals die several weeks after birth and Dnmt3b-deficient animals die in utero, indicating the essential roles of Dnmt3a- and Dnmt3b-mediated de novo methylation in development.
Cytosine methylation has several important functions. Most cytosines in the genome are methylated for efficient silencing of transposable elements.3 Transposable elements are the footprint of ancient integrated retroviruses, making up close to 50% of the genome.4 Recent studies indicated that Dnmt1 deletion in Six2-positive nephron progenitors resulted in a release of transposable-element silencing, endogenous retroviral expression, cytokine release, and a downstream severe kidney developmental defect.5
Cytosine methylation is believed to be a key regulator of gene expression.6 Gene regulatory regions, such as promoters and enhancers, contain cytosine-guanine (CpG)–rich regions (islands). In general, unmethylated promoters are permissive to transcription-factor binding and are associated with active gene expression. Methylated promoters exclude transcription factors, therefore they are associated with gene repression. Methylation of promoter and enhancer regions plays a key role in stabilizing linage decisions and restricting lineage fates. In addition to promoters, enhancers are critical for establishing cell type–specific gene regulation and gene expression. Enhancers are enriched for cell type–specific transcription factor binding sites to ensure cell-specific gene regulation. Cell type–specific genes often have multiple enhancers that loop around and join promoters to establish a cell type–specific gene expression pattern. Six2 is a critical transcription factor in kidney development. Six2-positive progenitors can undergo a symmetric and asymmetric division to renew or to commit and differentiate into specialized nephron epithelium segments.7,8 The role of cytosine methylation in this process is poorly understood.
Kidney disease is a complex gene environmental disease, affecting 800-million people worldwide. Genome-wide association analyses have been conducted to understand the heritability of kidney function, which uncovered close to 300 loci associated with disease risk.9–11 Each nucleotide variation only increases disease risk by a minuscule amount, however, they should explain close to 50% of disease risk in aggregate. It has been proposed that human disease-associated genetic variants are enriched on cell type–specific enhancer regions.12 Nucleotide-sequence changes at enhancer regions could alter transcription-factor binding, leading to quantitative differences in gene expression contributing to disease development. Upon analyzing kidney disease risk loci, we found that only 20%–30% of identified loci are located in regions annotated as enhancers in adult kidney samples. The underlying mechanism explaining the disease development that is associated with regions with no detectable regulatory function in the adult human kidney remains unknown. These regions might be specific for rare kidney cell types or a disease or developmental stage that is not captured by bulk analysis of adult human kidney tissue samples.
Environmental and nutritional alterations play equally important roles in kidney disease development.13–15 Intrauterine nutrient availability is known to be an important determinant of hypertension and kidney disease development, so called “prenatal programming.”16,17 Because epigenome-editing enzymes need substrates from the intermediate metabolism, it has been proposed that the epigenome might play a key role in prenatal programming. Nutrient availability, such as the presence of diabetes, remains the most significant risk factor for kidney disease development.18 Indeed, the effect of poor glycemic control on diabetic kidney disease development can be observed even decades after improved metabolic control.19,20 It also remains unclear how environmental and genetic factors interact and lead to kidney disease development.
To understand the role of de novo methylation in kidney cell differentiation, we generated mice with genetic deletion of Dnmt3a and Dnmt3b in nephron progenitor cells (NPCs) and tubule cells, using Six2Cre and KspCre (Six2CreDnmt3a/3b and KspCreDnmt3a/3b), respectively. Whole-genome bisulfite sequencing (WGBS) and reduced representation bisulfite sequencing (RRBS) identified significant changes in the methylome of kidney tubule cells. We showed that Dnmt3a and Dnmt3b play important roles in de novo methylation of fetal enhancers that were initially bound by Six2. The decline in Six2 expression during development was associated with a loss of H3K27ac and an increase in methylation. These fetal enhancers decommissioned by Dnmt3a and Dnmt3b were enriched for kidney disease risk loci. Diseased kidney samples showed a methylation pattern that was similar to the Dnmt3a/3b knockout animals. Overall, our data suggest that changes brought on by Dnmt3a/3b might be important for human kidney disease.
Methods
Animal Strains
Mice were raised and maintained in a barrier facility. Experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and were performed in accordance with the institutional guidelines. For folic acid–induced nephropathy mouse models, 8-week-old male mice were injected with folic acid (250 mg/kg, dissolved in 300 mM sodium bicarbonate) intraperitoneally and euthanized on day 7. For the cisplatin-induced injury model, 8-week-old male mice were injected with cisplatin (25 mg/kg) intraperitoneally and euthanized on day 3.
Real-Time RT-PCR
RNA was isolated from mouse kidney using Trizol (Invitrogen) and was reverse transcribed using the cDNA Archival Kit (Life Technologies). Real-time RT-PCR was performed using the SYBR Green Master Mix (Applied Biosystems). Primer pair sequences are shown in (Supplemental Table 1).
BUN and Creatinine Level
Serum creatinine was measured using Creatinine Enzymatic and Creatinine Standard (Pointe Scientific). Serum BUN was measured using Infinity Urea Liquid Stable Reagent (Thermo Scientific). Both measurements were performed according to the manufacturers’ instructions.
Staining
Kidneys were harvested from mice, rinsed in PBS, fixed in 10% formalin, and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin.
Isolation of CDH16+ Cells
Kidneys were harvested from control and KspCreDnmt3a/3b double knockout mice and minced using a razor blade. About 0.25 g tissue was digested in 1.17 ml RPMI plus 50 μl Enzyme D, 25 μl Enzyme R, and 6.75 μl Enzyme A from the Multi Tissue Dissociation Kit 1 (Miltenyi Biotec) and incubated for 10 minutes at 37°C. Kidney was then dissociated using 21-gauge and 26.5-gauge needles and incubated for 10 minutes at 37°C. The dissociation step was repeated twice. To neutralize the enzymes, 10% serum was added. Cells were then filtered through the 70-μm nylon mesh to isolate single cells and they were then centrifuged at 1000 rpm for 5 minutes. The pellet was treated with red blood cell lysis buffer and washed with PBS. The cell suspension was incubated with CDH16 antibody (Santa Cruz). CDH16-positive cells were magnetically isolated using anti-mouse IgG microbeads (Miltenyi Biotec) following the manufacturer’s instruction.
RRBS
Genomic DNA from whole kidney was isolated using the DNeasy Kit (Qiagen). Libraries were generated from the isolated DNA using the Premium Reduced Representation Bisulfite Sequencing Kit (Diagenode) following the manufacturer’s instruction. The 2100 Bioanalyzer (Agilent) and High Sensitivity DNA Kit (Agilent) was used for quality check. After trimming adapter and low-quality reads using Trim Galore version 0.5.0 (https://github.com/FelixKrueger/TrimGalore) with the option “–rrbs,” Bismark version 0.19.121 was applied to align reads to the mouse genome (mm10). MethylKit version 1.8.122 was used to quantify the methylation level of CpG sites covered by at least five reads and to calculate the methylation difference between Dnmt3a/3b and control samples. CpG sites with methylation difference >20% and q value <0.01 were analyzed in edmr version 0.6.4.123 to identify differentially methylation regions (DMRs) with at least three CpG sites and methylation difference >20%.
WGBS
Genomic DNA was isolated from Cdh16-positive cells using the MagAttract HMW DNA Kit (Qiagen) according to the manufacturer’s instruction. The concentration of DNA was measured using the Quant-iT PicoGreen dsDNA Assay kit (Life Technologies) following the manufacturer’s instruction. DNA quality was checked on agarose gel. After trimming adapter and low-quality reads by Trim Galore, Bismark was used for alignment to the mouse genome (mm10), deduplication, and quantification of methylation level for each CpG site. SMART version 2.2.824 was used to perform de novo genome segmentation with default thresholds. The fragments with at least five CpG sites and methylation difference >20% were identified as DMRs.
RNA Sequencing
Total RNA from whole kidneys were isolated using the RNeasy Mini Kit (Qiagen). RNA quantity and quality was analyzed on the 2100 Bioanalyzer (Agilent) using the RNA 6000 Pico Kit (Agilent). Sequencing reads were aligned to the mouse genome using STAR version 2.2.125 and gene expression was quantified using RSEM version 1.3.1.26 Comprehensive gene annotation (gencode.vM18.annotation.gtf) was obtained from GENCODE.27 Differentially expressed genes (DEGs) were identified using edgeR version 3.24.3,28 with the thresholds of false discovery rate <0.001 and log2 fold change >1. Functional enrichment of DEGs were performed using DAVID database version 6.829 and the modPhEA database.30
Functional Annotation of DMRs
We downloaded mouse kidney reference epigenomes, including WGBS and histone modifications (embryonic day 14.5 [E14.5], E15.5, postnatal day 0 [P0], and 8 weeks old) from the ENCODE website (Y. He, M. Harihran, D. U. Gorkin, D. E. Dickel, C. Luo, R. G. Castanon, et al., unpublished observations; D. U. Gorkin, I. Barozzi, Y. Zhang, A. Y. Lee, B. Li, Y. Zhao, et al., unpublished observations). To define regulatory elements, mouse kidney chromatin states (E14.5, E15.5, E16.5, and P0) were downloaded from http://enhancer.sdsc.edu/enhancer_export/ENCODE/chromHMM/replicated/. These chromatin states were estimated by ChromHMM (D. U. Gorkin, I. Barozzi, Y. Zhang, A. Y. Lee, B. Li, Y. Zhao, et al., unpublished observations) using a 15-state model. ChromHMM uses a combinatorial pattern of histone modifications (H3K4me1, H3K4me2, H3K4me3, H3K27ac, H3K27me3, H3K9ac, H3K9me3, and H3K36me3). The 15-state model was further simplified into four states including promoter, enhancer, transcription, and other. Chromatin states from multiple data sets were merged. BEDTools version 2.27.031 was used to intersect chromatin states and DMRs.
Adult kidney chromatin states from 8-week-old mouse were downloaded from https://github.com/gireeshkbogu/chromatin_states_chromHMM_mm9 and lifted over to mm10 using the LiftOver tool (https://genome.ucsc.edu/cgi-bin/hgLiftOver). These chromatin states were estimated by ChromHMM using the 15-state model using the combinatorial patterns of chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-seq) profiles (H3K4me1, H3K4me3, H3K36me3, H3K27me3, H3K27ac, CTCF, and RNA polymerase II) obtained from the mouse ENCODE project.32 Similarly, the 15-state model was simplified into four states such as promoter, enhancer, transcription, and other. Chromatin accessibility of DMRs in kidneys of 8-week-old male C57BL/6J mice were quantified by deepTools version 3.1.233 using bigwig files obtained from the Mouse sci-ATAC-seq Atlas.34 Transcription factor motif enrichment analysis of DMRs was performed using findMotifsGenome.pl of HOMER version 4.10.3.35 GREAT version 3.0.036 was used to predict functions of cis-regulatory regions.
Six2 Binding Sites in NPCs
Six2 binding sites (ChIP-seq peaks) in NPCs were downloaded from GUDMAP database (RID:Q-Y4CY).37 Six2 peaks identified in at least two of the three replicates were used for further analysis. To build a control set, shuffled regions, matching in size and number, were generated using BEDTools shuffle.
Topologically Associating, Domain-Constrained Map of Enhancer DMR-DEG Associations
We obtained a topologically associating domain-constrained map of enhancer-promoter associations from a reference (D. U. Gorkin, I. Barozzi, Y. Zhang, A. Y. Lee, B. Li, Y. Zhao, et al., unpublished observations). BEDTools was used for intersect analysis. The Spearman correlation test was performed to examine the relationship between enhancer DMR (eDMR) methylation and DEG expression.
Enrichment of Genome-Wide Association Study Single-Nucleotide Polymorphisms in Developmental DMRs and Dnmt3a and Dnmt3b Double Knockout DMRs
Genome-wide association study (GWAS) single-nucleotide polymorphisms (SNPs) were obtained from the GWAS catalog (gwas_catalog_v1.0-associations_e96_r2019–06–20.tsv).38 After filtering out SNPs for missing coordinates and significance (P<5×10−8), 79,744 SNPs were used for follow-up analysis. The University of California Santa Cruz (UCSC) Genome Browser LiftOver function was used to lift over the mouse coordinates into human coordinates,39 resulting in a final set of 36,045 GWAS SNPs. The hypergeometric test was used to determine the significance of disease trait and enrichment of DMRs.
SNPs showing a significant association with eGFR were downloaded from recent publications.9,40,41 The significant (P<5×10−8) SNPs from different studies were combined (26,637). The human SNP coordinates were converted to mouse genome (mm10) using LiftOver from the UCSC Genome Browser,39 resulting in a final set of 7923 eGFR SNPs.
Quantification and Statistical Analysis
Statistical analyses were performed using R or GraphPad Prism software (GraphPad Software, La Jolla, CA). Two-tailed t test or Wilcoxon signed rank sum test was used to compare two groups. One-way ANOVA was used to compare multiple groups. Spearman rank correlation was used to determine the correlation. When needed, multiple testing correction was performed using the false discovery rate.
Data Availability
All sequencing data (WGBS, RRBS, and RNA sequencing) have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) and are accessible through GEO accession number GSE134267.
Results
Deletion of de Novo Methyltransferases Dnmt3a and Dnmt3b in Renal Progenitors
To understand the role of de novo methyltransferases in kidney development and maturation, we generated double knockout transgenic mice by crossing the Dnmt3af/f mice and the Dnmt3bf/f mice with KspCre mice (KspCreDnmt3a/3b or KspCreDKO) (Figure 1A). KspCre mice express Cre recombinase under the control of the mouse cadherin 16. Chd16 or Ksp-cadherin is expressed from E11.5 in the developing kidney (Supplemental Figure 1A). Its expression increases as the kidney matures (Supplemental Figure 1B). Cell type–specific expression and open chromatin data indicated that it is expressed in distal tubules, collecting duct, loop of Henle, and proximal tubules in adult kidney (Supplemental Figure 1, C and D). KspCreDnmt3a/3b mice were born at the expected Mendelian ratio. The genetic deletion was confirmed by quantitative RT-PCR in kidneys of 3-day-old mice (Figure 1B). Transcript levels of Dnmt3a and Dnmt3b were lower in KspCreDnmt3a/3b mice (Figure 1B); however, as reported earlier, the expression of Dnmt3b was around the detection limit at birth (Figure 1B).
Figure 1.
Phenotypic characterization of KspCreDnmt3a/Dnmt3b mice. (A) Breeding scheme for generating KspCreDnmt3a/3b double knockout mice (KspCreDKO). (B) Transcript levels of Dnmt3a and Dnmt3b in kidneys of 3-day-old control and KspCreDnmt3a/3b mice. Data are represented as mean±SEM; P value was calculated by two-tailed t test. (C) Serum creatinine measurement in 3-week-old control and KspCreDnmt3a/3b mice. Data are represented as mean±SEM. (D) Representative images of haemotoxylin and eosin–stained kidney sections of control and KspCreDnmt3a/3b mice. Scale bar: upper 20µm; bottom: 10µm. (E) Relative mRNA levels of kidney segment markers Slc34a1, Slc12a1, Slc12a3, and Aqp2 in control and KspCreDnmt3a/3b mice on day 3, 10, 21 and 56. Data are represented as mean±SEM. *P<0.05, **P<0.01, ***P<0.001 by two-way ANOVA with post hoc Tukey test. (F) Serum BUN levels in control and KspCreDnmt3a/3b mice with or without cisplatin treatment. Data are represented as mean±SEM; P value was calculated by one-way ANOVA. (G) Relative mRNA level of AKI markers (Kim1 and Lcn2) and cytokines (Cd68 and Ccl2). Data are represented as mean±SEM; P value was calculated by one-way ANOVA. (H) Relative mRNA level of kidney segment markers such as Slc34a1, Slc12a1, and Slc12a3 in control and Six2CreDnmt3a/3b mice with or without cisplatin treatment. Data are represented as mean±SEM; P value was calculated by one-way ANOVA. Con, control; Veh, vehicle.
KspCreDnmt3a/3b mice showed no obvious renal phenotypic alterations at baseline. Serum creatinine level of KspCreDnmt3a/3b was comparable to littermate controls (Figure 1C). Kidney structural analysis showed no observable abnormalities (Figure 1D). To further understand the role of Dnmt3a and Dnmt3b in renal development, we quantified the expression of renal segment–specific markers (Figure 1E). Slc34a1 (proximal tubule), Slc12a1 (loop of Henle), Slc12a3 (distal tubule), and Aqp2 (collecting duct) were quantified on day 3, 10, 21, and 56. Gene expression levels showed minor alterations in the developing and maturing kidneys, however they were overall similar between adult KspCreDnmt3a/3b mice and littermate controls (Figure 1E).
Next, we analyzed whether KspCreDnmt3a/3b mice show alterations in response to injury. To model AKI, we treated control and KspCreDnmt3a/3b mice with cisplatin. Serum BUN level of cisplatin-treated mice was significantly increased, confirming the injury. Serum BUN level was lower in KspCreDnmt3a/3b mice when compared with cisplatin-treated controls (Figure 1F). Tubule injury markers such as kidney injury molecule 1 (Kim-1) and Lipocalin-2 (Lcn2) were lower in KspCreDnmt3a/3b mice compared with controls (Figure 1G). The macrophage marker Cd68 and cytokine Ccl2 were higher in cisplatin-treated animals but they were less prominent in KspCreDnmt3a/3b mice (Figure 1G). Kidney segment–specific marker genes, such as the proximal tubule marker Slc34a1 and loop of Henle marker Slc12a1 were decreased after cisplatin treatment and these changes were comparable in KspCreDnmt3a/3b mice (Figure 1H). We also analyzed the injury response in the folic acid–induced kidney fibrosis model. We did not observe significant differences between control and KspCreDnmt3a/3b mice in the folic acid–induced kidney fibrosis model (Supplemental Figure 2).
Because Chd16 expression is segment specific and expresses at later stages of development, we next genetically deleted Dnmt3a and Dnmt3b in nephron progenitors using the Six2Cre mice (Six2CreDnmt3a/3b or Six2CreDKO) (Supplemental Figure 3A). Six2 is expressed at E11.5, and it labels the self-renewing nephron progenitors that give rise to all nephron epithelia5 (Supplemental Figure 1, A, B, and D). Six2CreDnmt3a/3b mice were born at the expected Mendelian ratio. Kidney sections of 3-week-old Six2CreDnmt3a/3b mice showed no obvious structural abnormalities (Supplemental Figure 3B). Transcript levels of kidney segment markers (Slc34a1, Slc12a1, Slc12a3, and Aqp2) in 3-week-old Six2CreDnmt3a/3b mice were comparable to littermate controls (Supplemental Figure 3C). Taken together, Dnmt3a and Dnmt3b appeared dispensable in adult kidney tubule cells at baseline. However, they seem to confer some resistance to AKI, but not to kidney fibrosis.
Dnmt3a and Dnmt3b Play Important Roles in Establishing Methylation Patterns during Kidney Development
To examine the contribution of Dnmt3a and Dnmt3b to kidney cytosine methylation changes during development, we performed RRBS on whole-kidney samples obtained from 3-week-old control and KspCreDnmt3a/3b and Six2CreDnmt3a/3b mice (Figure 2A). On average, RRBS quantified methylation levels of approximately 2.4-million CpG sites representing approximately 10.8% of CpG sites in the mouse genome. Using stringent criteria (of q value <0.01 and methylation difference >20%), we identified 7184 DMRs in kidneys of Six2CreDnmt3a/3b mice and 1457 DMRs in kidneys of KspCreDnmt3a/3b mice (Figure 2B). As expected, most regions showed lower methylation levels in Dnmt3a/3b knockout mice (94.3% of the DMRs in Six2CreDnmt3a/3b and 71.3% in KspCreDnmt3a/3b mice) (Figure 2B), indicating that Dnmt3a and Dnmt3b play a key role in de novo methylation. Regions that failed to gain methylation in the Dnmt3ab/3b double knockout kidneys were mostly fetal kidney enhancers (Supplemental Figure 4).
Figure 2.
Dnmt3a and Dnmt3b play important roles in establishing methylation patterns during kidney development. (A) Schematic representation of RRBS on whole-kidney lysates of KspCreDnmt3a/3b, SixCreDnmt3a/3b and littermate controls. (B) Volcano plot showing methylation changes in (DMR or differentially methylated cytosines [DMCs]) in SixCreDnmt3a/3b (left), KspCreDnmt3a/3b (middle), and development (right). The x axis shows mean methylation differences and the y axis shows statistical significance (as −log10[q value]). (C) The overlap between DMRs identified in SixCreDnmt3a/3b, KspCreDnmt3a/3b and during kidney development (P0–P21). (D) Overlap of hypo-DMRs identified in KspCreDnmt3a/3b and SixCreDnmt3a/3b. (E) Methylation changes during mouse kidney development (E14.4, E15.5, E16.5, and P0), followed by methylations in KspCreDnmt3a/3b, SixCreDnmt3a/3b and littermate controls (left). Mean methylation levels from zero (blue) to one (red); mean methylation levels of DMRs in fetal, littermate controls, KspCreDnmt3a/3b and SixCreDnmt3a/3b kidneys (right). Mean methylation difference between control kidney and fetal, KspCreDnmt3a/3b and SixCreDnmt3a/3b kidney were compared by Wilcoxon signed rank sum test. #P=3.8×10−6, &P=0.84, *P value <2.2×10−16. DKO, double knockout.
Next we examined global methylation patterns at birth (P0) and at 3 weeks of age (P21) (Figure 2B), which is the time when nephron development ceases in mice. As reported earlier, there was a significant gain in CpG sites methylation (69.1%) during this period (P0–P21). This is consistent with the terminal differentiation of cells during this period. Developmental DMRs failed to gain methylation in Dnmt3a/3b knockout mice (chi-squared test P=1.05×10−33; Figure 2C). This effect was more pronounced in the Six2CreDnmt3a/3b animals compared with KspCreDnmt3a/3b mice. There was a limited overlap between hypo-DMRs observed in Six2CreDnmt3a/3b and KspCreDnmt3a/3b mice (Figure 2, D and E), indicating that the targets of Dnmt3a and Dnmt3b are spatially and temporally different. In summary, cytosine methylation was severely reduced in kidneys of mice lacking de novo methyltransferases such as Six2CreDnmt3a/3b and KspCreDnmt3a/3b.
Dnmt3a and Dnmt3b Are Necessary for de Novo Methylation of Fetal-Specific Enhancers
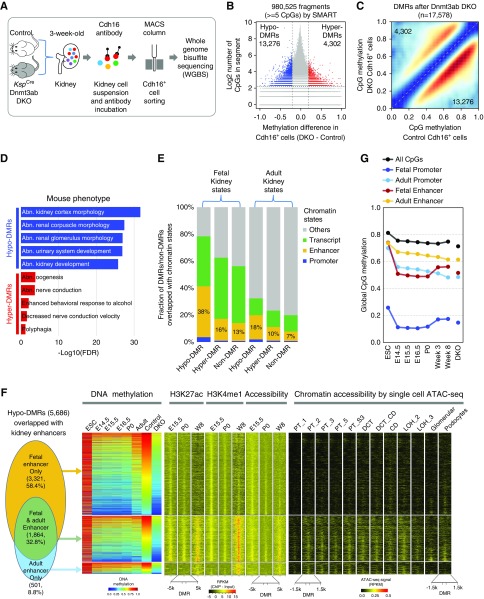
For accurate cell type–specific, base-resolution methylome analysis, we performed WGBS on sorted Cdh16 (Ksp-cadherin)-positive cells (Figure 3A). Cells were isolated from kidneys of 3-week-old KspCreDnmt3a/3b and control mice. The methylation patterns of Cdh16-positive cells showed strong concordance with whole-kidney methylation (Supplemental Figure 5A). To identify DMRs, we first segmented the genome into 980,525 fragments, each of them containing at least five CpG sites, as established in the SMART method (Supplemental Figure 5B).24 The differential methylation analysis identified 17,578 fragments (DMRs) showing at least 20% change in methylation between KspCreDnmt3a/3b mice and controls (Figure 3B, Supplemental Table 2). Consistently, >75% (13,276) of DMRs showed a lower methylation level (hypo-DMRs) in kidneys of KspCreDnmt3a/3b mice. Loci with intermediate (40%–80%) methylation level were mostly affected in the KspCreDnmt3a/3b mice (Figure 3C). Functional annotation of these DMRs indicated that demethylation events tended to be close to genes associated with morphogenesis and kidney development (Figure 3D).
Figure 3.
Base-resolution methylome analysis of isolated tubule cells in control and KspCreDnmt3a/3b mice. (A) Experimental design. (B) Volcano plot, x axis shows differentially methylated regions (20% change in at least five CpG of any fragment) and y axis shows the number of DMRs. (C) Methylation level of 17,578 DMRs in Cdh16+ cells in control (y axis) and KspCreDnmt3a/3b (x axis). Color (higher red) indicates the number of DMRs in that group. (D) Mouse phenotypes enriched by hypo-DMRs and hyper-DMRs in KspCreDnmt3a/3b based on GREAT for functional annotation. (E) The overlap between hypo-DMRs and hyper-DMRs in KspCreDnmt3a/3b mice and different chromatin states in fetal and adult kidneys. Chromatin states from fetal and adult kidneys were used to classify the genome location of each segment by WGBS. (F) Functional annotation of hypo-DMRs (5686, lost methylation in Dnmt3a/3b knockout mice). First panel showed the number and fraction of hypo-DMRs overlapped with fetal and/or adult enhancers. Second panel shows the methylation levels of hypo-DMRs during kidney development, control, and KspCreDnmt3a/3b. The data were ordered according to the methylation level in control kidneys. Third panel shows density of chromatin mark (associability, H3K27ac, and H3K4me1) in each hypo-DMR and its flanking regions (5 kb) in fetal kidney (E15.5, P0) and adult kidney (8 weeks old). The last panel shows the chromatin accessibility by single cell assay for transposase-accessible chromatin using sequencing single cell (ATAC-seq) in adult kidney (8-week-old) cell types. (G) Global CpG methylation levels in kidney enhancers and promoters at different stages of development and in Dnmt3a/3b knockout mice. Abn., abnormal; CD, collecting duct; DCT, distal tubule; DKO, double knockout; ESC, embryonic stem cell; FDR, false discovery rate; LOH, loop of Henle; Podo, podocyte; PT, proximal tubule; RPKM, reads per kilobase of transcript per million mapped reads; W8, week 8.
To define regions that are specifically altered by Dnmt3a- and Dnmt3b-mediated differential methylation, we mapped DMRs to functional regulatory elements. Kidney-specific functional regulatory elements such as enhancers and promoters were annotated by integrating multiple histone ChIP data obtained from fetal and adult mouse kidney samples.42 We found that Dnmt3a- and Dnmt3b-mediated DMRs were enriched on enhancers, but hardly ever observed on promoter regions (Figure 3E, Supplemental Figure 5C). When we compared fetal and adult kidneys, we found that loci with lower methylation (hypo-DMRs) in Dnmt3a/3b knockout mice were strongly enriched on fetal-enhancer regions (38% versus 13%, chi-squared test P<2.2×10−16), and to lesser degree on regions identified as transcribed regions in fetal kidneys (Figure 3E).
Next, we wanted to understand the fate of the fetal enhancers and the role of methylation. Upon analyzing cytosine methylation on a genome-wide scale during kidney development, we found that adult promoters and enhancers showed a mild, gradual decline in global methylation level, indicating their openness was determined earlier (Figure 3G). On the other hand, fetal enhancers and promoters gained significant methylation during the P0–P21 time period. Kidneys of Dnmt3a/3b knockout mice failed to gain methylation, consistent with their role as de novo methyltransferases (Figure 3G).
To further explore the function of Dnmt3a/3b in kidney development, we identified 3334 DMRs that showed methylation changes during development and also showed changes in Dnmt3a/3b knockout mice. Most (58.5%) of these shared DMRs underwent de novo methylation in development but failed to increase their methylation in Dnmt3a/3b knockout mice, and were enriched in fetal-enhancer regions (Supplemental Figures 5, C and D, and 6). When focused our analysis on the hypo-DMRs that overlapped with kidney enhancers, we found that 58% were fetal specific whereas only a small fraction (8.8%) of hypo-DMRs were annotated as enhancers only in the adult mouse kidney (Figure 3F, Supplemental Figure 5C). Hypo-DMRs gained methylation, losing enhancer marks and chromatin accessibility in the adult kidney (Figure 3F, Supplemental Figure 5, E and F). These results revealed Dnmt3a and Dnmt3b were necessary for de novo methylation and decommissioning of fetal-specific enhancers.
Dnmt3a and Dnmt3b Are Required for Decommissioning of Fetal Enhancers Bound by Developmental Transcription Factors
Because DMRs were enriched on developmental-enhancer regions, we were interested to understand whether we could identify critical transcription factors associated with these sites. We used transcription factor motif analysis established by HOMER35 to analyze DMRs from the WGBS data set. Upon comparing regions that gained methylation during development, we found a measurable enrichment for homeobox transcription factors, including Six2 which is known to play a key role in kidney development (Figure 4A). Through examining DMRs identified in kidneys of KspCreDnmt3a/3b mice, we again found enrichment for homeobox transcription factors, such as Six2 binding sites (Figure 4A). The overlap between the developmental DMRs and Dnmt3a/3b knockout DMRs showed significant enrichment for kidney developmental transcription factor binding sites including Hoxc9, Six2, Six1, and Dlx3 (Figure 4A). Six2, a kidney developmental transcription factor, is required for nephron progenitor maintenance.7,43 These results indicate that a good portion of fetal enhancers that are decommissioned by Dnmt3a/3b are bound by Six2.
Figure 4.
KspCreDnmt3a/3b DMRs are enriched for developmental transcription factor binding. (A) Transcription factor motif enrichment (HOMER) of DMRs, during kidney development (P0–P21), in control versus KspCreDnmt3a/3b kidneys. Fragments that gained methylation during development but failed to gain methylation in Dnmt3a/3b knockout mice. (B) The degree of overlap between DMRs that are in enhancer regions and on Six2 binding sites in nephron progenitors (NPC) from ChIP-seq (cyan) compared with background (gray). (C) Methylation differences in control versus KspCreDnmt3a/3b kidneys (left) or adult versus fetal kidneys (right) that overlap with Six2 binding versus background shuffled region. (D) Scatter plot of methylation changes of Six2 binding sites in NPCs, during kidney development (x axis) and in control versus KspCreDnmt3a/3b (y axis). The red dots represent the fragments gaining methylation in adult kidneys, but not in KspCreDnmt3a/3b mice. (E) IGV genome browser view of the Pax2 region (chromosome 19: 44729363–44851852). DNA methylation changes in hypo-DMRs (lower methylation in KspCreDnmt3a/3b), note the methylation pattern in the developing mouse kidney (E15.5, P0), wild-type and Dnmt3a/3b knockout (DKO) mice, H3K27ac enhancer mark, and Six2 binding. Note the failure to increase in methylation of a fetal-enhancer region. (F) Global CpG methylation in NPC Six2 peaks and shuffled regions at different stages of kidney development. (G) Correlation between gene expression of Six2 and CpG methylation of segments overlapped with NPC Six2 peaks and shuffled regions. Wilcoxon signed rank sum test was carried out to calculate the significance of difference between NPC Six2 peaks and shuffled regions, and P values was provided for each comparison. ESC, embryonic stem cell.
Because motif analysis cannot distinguish among closely related transcription factors, we next specifically examined Six2 binding sites identified in NPCs by ChIP-seq.37 The Six2 peaks in NPCs were affected by methylation because they were enriched for fetal hypo-DMRs (Figure 4B) compared with a shuffled background. More than half (56%) of the fragments that overlapped with NPC Six2 peaks gained methylation in development but failed to be methylated in KspCreDnmt3a/3b mice (Figure 4, C and D, Supplemental Figure 7A). These fragments were localized to genes that are known to play roles in kidney development. For example, Six2 peaks in nephron progenitors overlapped with the distal enhancer of Pax2 which is a critical regulator of kidney development.44 This enhancer showed low methylation levels in the fetal kidney and its methylation level increased in the adult kidney (Figure 4E, Supplemental Figure 7A). In contrast to the wild-type mice, this enhancer failed to gain methylation in kidneys of the KspCreDnmt3a/3b mice and the regions remained similar to those in fetal kidneys. Furthermore, chromatin conformation capture contact matrices revealed interactions between this enhancer and the Pax2 locus (Supplemental Figure 7B). Integrative analysis revealed that Six2 binding sites had lower methylation levels in fetal kidneys and gained methylation after birth (Figure 4F), indicating an interaction between Six2 binding and methylation changes. For example, pioneering transcription factors not only play roles in opening closed chromatin sites during development, but their binding footprint can be observed even after the transcription factor is no longer expressed.45 To explore this hypothesis, we calculated the correlation of fragment methylation and Six2 expression during kidney development. The Six2-bound fragment methylation showed negative correlation with Six2 expression, which was particularly obvious for fetal enhancers (Figure 4G). For example, methylation of a locus on chromosome 7 was significantly negatively correlated (Spearman ρ=−0.97, P value=0.0002) with the expression of Six2 (Supplemental Figure 8). This region included Six2 binding sites that failed to gain methylation in Dnmt3a/3b knockout mice. These results indicated that DNA methylation, mediated by Dnmt3a and Dnmt3b, preferentially affected enhancer regions bound by Six2 during kidney development. The methylation of these sites correlated with Six2 expression.
Transcriptional Changes Observed in KspCreDnmt3a/3b Mice
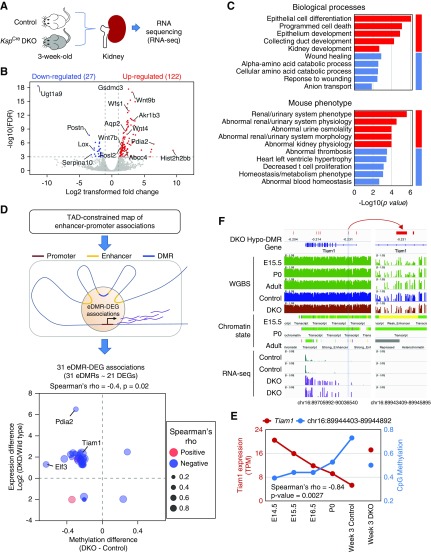
Next, we performed unbiased gene expression analysis by RNA sequencing of kidneys of control and KspCreDnmt3a/3b mice (Figure 5A). We identified 149 genes that passed the significance threshold for differential expression (DEGs). Consistent with the demethylation events, most (82%, 122/149) DEGs showed an increase in their expression in KspCreDnmt3a/3b mice, including several kidney developmental genes such as Wnt4 and Wnt9b (Figure 5B). Functional annotation indicated that DEGs were enriched for kidney development and epithelial differentiation functions (Figure 5C).
Figure 5.
Dnmt3a- and Dnmt3b-mediated methylation represses developmental genes in late development stage. (A) Schematics of the experiments. (B) Gene expression changes in KspCreDnmt3a/3b mice. Volcano plot; x axis shows fold-change difference, and y axis shows statistical difference (−log false discovery rate [−logFDR]). Red dots represent genes with higher expression in KspCreDnmt3a/3b, whereas blue dots represent genes with lower expression. (C) Function enrichment (biologic processes and mouse phenotype) analysis of differentially regulated genes. Red colors represent genes with increased expression, whereas blue represent decreased expression. (D) Enhancer and promoter associations obtained from topologically associating domain (TAD)–constrained maps. eDMRs represent enhancers that also showed differential methylation. The association (Spearman rank correlation coefficient) between eDMRs and associated genes and significance was calculated and showed. (E) Methylation (of eDMR) and expression correlation of Tiam1 locus in kidney development and in KspCreDnmt3a/3b mice. Transcripts per million (TPM) was used for quantification of RNA expression. Spearman rank correlation coefficient and significance was calculated and showed. (F) IGV genome browser view of the Tiam1 locus (chromosome 16: 89944403–89944892), including WGBS tracks during kidney development E15.5–P0, chromatin state and gene expression by RNA sequencing in adult and KspCreDnmt3a/3b mice. The right panel is a zoom-in region of the eDMR. DKD, diabetic kidney disease; DKO, double knockout.
Next, we tested whether enhancer methylation correlates with gene expression changes. To identify targets of enhancer DMR (eDMR), we obtained topologically associating, domain-constrained maps for accurate enhancer-promoter associations (D. U. Gorkin, I. Barozzi, Y. Zhang, A. Y. Lee, B. Li, Y. Zhao, et al., unpublished observations). We identified 31 associations between 31 eDMRs and 21 DEGs (eDMR~DEG). The methylation changes in these eDMRs were associated with changes in their target gene expression (Figure 5D, Supplemental Figure 9A, Supplemental Table 3). Most of the eDMR-DEG associations (84%) were direction consistent, such as lower methylation was associated with higher expression (Figure 5D). For example, an eDMR on the Tiam1 locus showed lower methylation in KspCreDnmt3a/3b mice and an increase in transcript expression of Tiam1 (Figure 5, E and F, Supplemental Figure 9B). Tiam1 was reported to play a role in Wnt signaling and epithelial-mesenchymal transition,46,47 and it is mostly silenced in adult kidney tubules. Overall, the effect of Dnmt3a- and Dnmt3b-mediated methylation changes on gene expression modulation was modest, and mostly affected genes involved in kidney development.
Dnmt3a- and Dnmt3b-Mediated Methylation Changes Are Enriched for Kidney Disease Genetic Risk Loci
GWASs have identified nucleotide variations that are enriched in patients with CKD. Previously, we showed that a good portion of such loci are enriched on enhancer regions in the adult kidney,10 however, more than half of GWAS loci remain unannotated. Here, we hypothesized that kidney disease risk loci might be specific to the developmental stage, i.e., might be active in the fetal tissue, explaining the lack of regulatory annotation in the adult human kidney. As we showed earlier, such fetal enhancers are specifically methylated and decommissioned by Dnmt3a/3b in adult kidney.
As a first step, we overlapped the entire human GWAS catalog and DMRs identified during development. We found variants associated with kidney function (Figure 6A) and other kidney-associated traits were specifically localized to kidney developmental DMRs. Next, we narrowed the DMRs only those were found to be methylated by Dnmt3a and Dnmt3b. These DMRs showed a strong enrichment for kidney function–associated traits (Figure 6A). Functional annotation revealed that 68% of DMRs that overlapped with kidney function–associated SNPs were fetal kidney–specific enhancers, which was significantly higher than expected by chance (chi-squared test P=1.08×10−12; Figure 6B, Supplemental Figure 10A, Supplemental Table 4). Cross-species comparison indicated these fetal kidney enhancers were conserved between mouse and human, both in sequence and in their methylation patterns (Supplemental Figure 10, B and C). Specifically, we integrated the different enhancer marks (H3K27ac and H3K4me1) in fetal (E15.5) and adult (week 8) kidneys, and identified 51 GWAS loci that overlapped with kidney enhancers (Supplemental Table 5). Most of these enhancers (59%) were positive for both enhancer marks (H3K27ac and H3K4me1) in the fetal stage, but only 28% of them remained positive for both marks in the adult kidney (chi-squared test P=0.0052) (Figure 6C, Supplemental Figure 10A). To confirm the GWAS catalog–based finding (which only reports the top associated SNPs), we combined a comprehensive list of 26,637 SNPs that were significantly associated with eGFR (eGFR-SNPs) in the most recent GWAS studies9,40,41 (Figure 6D). More than a half (51%) of DMRs overlapped with eGFR-SNPs were localized to fetal enhancers, and 49% of enhancers that overlapped with eGFR-SNPs were enriched for enhancer marks (H3K27ac and K3K4me1) in fetal kidneys (Figure 6, E and F). These results raise the possibility that genetic variants in fetal enhancers decommissioned by Dnmt3a and Dnmt3b contribute to human kidney disease development.
Figure 6.
Dnmt3a/3b-methylated regions harbor kidney disease risk loci. (A) Enrichment of human disease risk loci (obtained from GWAS catalog) in developmental and Dnmt3a/3b double knockout DMRs. Only the significantly enriched human kidney disease–related traits were shown, and the significance was represented by color from white to dark blue. (B) Genomic location of DMRs overlapping with kidney disease risk variants. Chromatin states from fetal and adult kidneys were used to classify the genome location of each DMR. (C) Histone-modification transition from fetal mouse kidney to adult mouse kidney in enhancers overlapping with kidney function associated traits. ChIP-seq peaks of H3K27ac and H3K4me1 were used to classify the transition pattern of each enhancer. (D) Integration of eGFR-associated SNPs (eGFR-SNPs) from recently published GWAS.9,40,41 The combined eGFR-SNPs after the lift over to mouse genome (mm10) were overlapped with WGBS segments. (E) Genomic location of DMRs overlapped with eGFR-SNPs. (F) Histone-modification transition from fetal mouse kidney to adult mouse kidney in enhancers overlapping with eGFR-SNPs. (G) DNA-methylation changes during mouse kidney development and human diabetic kidney disease (DKD). Spearman rank correlation coefficient and significance was calculated and showed. (H) Gene expression of Uncx and CpG methylation of eDMR overlapped with kidney function–associated SNPs during kidney development from E14.5 to 8 weeks after birth. sCR, serum creatinine; TPM, transcripts per million; eGFRcrea, estimated glomerular filtration rate based on serum creatinine levels.
To further understand the clinical significance of our findings, we analyzed the methylation of DMRs that overlapped with GWAS SNPs associated with kidney function–related traits in microdissected kidney tubule samples obtained from healthy subjects and patients with diabetic kidney disease.14 Methylation changes during kidney development were significantly negatively correlated with methylation changes observed in diabetic kidney disease (Figure 6, G and H), suggesting the methylation patterns established during development were either reversed in diabetic kidney disease or failed to establish during development.
For example, GWASs have revealed that nucleotide variants nearby UNCX were significantly associated with kidney functions (Supplemental Figure 10D). The methylation pattern of this locus in healthy human kidneys was similar to the mouse kidney, including a large area of lowly methylated region (Figure 7, A and B). Kidney function–associated GWAS variants were localized to a fetal enhancer which showed the active enhancer marks H3K27ac and H3k4me1 in fetal kidneys. Although the region remained minimally H3K4me1 positive in the adult kidney, this region was no longer positive for H3K27ac, indicating that it was not an active enhancer in adult kidneys. This region showed an increase in methylation level during development and failed to gain methylation in absence of Dnmt3a and Dnmt3b, indicating the key role of Dnmt3a and Dnmt3b in establishing the methylation of this GWAS region. Consistent with the notion that this region is a fetal-specific active enhancer, Uncx/UNCX was expressed in fetal but not in adult kidney (Figures 6H and 7A, Supplemental Figure 10E), indicating the important role of Dnmt3a and Dnmt3b in decommissioning fetal enhancers. Finally, when compared with healthy kidney samples, kidney tubules from patients with diabetic kidney disease showed strong similarities to kidneys of Dnmt3a/3b knockout mice, such as the loss of cytosine methylation of this region (Figure 7B). In addition to the UNCX/Uncx locus, we also examined the Hoxd/HOXD locus. Again, we found a similar pattern, such as conservation between the human and mouse locus, Dnmt3a/3b-mediated methylation, and decommissioning of fetal enhancers (Figure 7, C and D, Supplemental Figure 10F). In summary, our results indicate that Dnmt3a- and Dnmt3b-mediated methylation of fetal enhancers are enriched on kidney disease risk loci.
Figure 7.
Genetic and epigenetic features of UNCX and HOXD human kidney disease associated loci. (A) IGV genome browser of the mouse Uncx locus (chromosome 5 [chr5]: 139515562–139580379). The top panel shows GWAS and kidney function (eGFR)–associated variants followed by methylation patterns (WGBS) in the developing kidney and Dnmt3a/3b knockout kidney, Six2 binding, H3K27ac and H3K4me1 (enhancer marks), and gene expression by RNA sequencing (RNA-seq). (Note the failure of methylation of the enhancer region in absence of Dnmt3a/3b). (B) IGV genome browser view of the human UNCX locus (chr7: 1239604–1308516). Lift-over from the UCSC Genome Browser was used to identify the conserved region between human and mouse UNCX/Uncx locus. LocusZoom showing association between SNPs and kidney function. PhastCons conservation scores among 46 vertebrate species. Whole-genome bisulfite methylation patterns were shown in control and diabetic kidney disease (DKD) samples (note the lower methylation in DKD samples). (C and D) IGV genome browser view of the mouse Hoxd and human HOXD loci.
Discussion
Here, via integrating mouse genetic studies and genome-wide methylome and expression profiling, we elucidated the role of Dnmt3a and Dnmt3b in renal tubule epithelium in development, maturation, adult, and diseased mouse kidneys. Using base-resolution temporal profiling, we described dynamic changes of DNA methylation during kidney development. Globally, we observed the largest decline in global methylation level between embryonic stem cells and renal progenitors, whereas the greatest increase in methylation was observed during postnatal maturation (P0–P21). In mice, during the first 3 weeks, new nephrons are formed, epithelial cells proliferate, and the kidney enlarges drastically.48 Regions that act as enhancers in the adult kidney, show very small and gradual changes in their methylation level during development. Changes in methylation, on the other hand, are associated with alterations in transcript expression that again occur in the postnatal stage. These results indicate that the epigenetic state of these regions is likely established early during kidney development and their postnatal expression is mostly transcriptionally controlled.
Our results indicated that kidney-specific fetal enhancers underwent important changes during postnatal kidney development. We observed a substantial increase in enhancer methylation after birth (P0–P21). Histone-modification data indicated that the change in methylation (at birth) was associated with a loss of H3K27ac, a histone mark that defines active enhancers. Dnmt3a and Dnmt3b play a critical role in methylation of these fetal enhancers and we identified thousands of enhancer regions whose methylation was not established in absence of Dnmt3a and Dnmt3b. These enhancers show intermediate methylation levels at the fetal stage. Although their methylation increases to the adult stage, they do not seem to gain full methylation in the normal adult mouse kidney. Previously, these loci have also been called “vestigial enhancers.”49 Here we showed that Dnmt3a and Dnmt3b played key roles in methylation of these fetal enhancers. Fetal enhancers were the most significantly enriched group among the DMRs.
Six2 expression shows a strong correlation with the openness and methylation of fetal enhancers. Fetal enhancers are enriched for Six2 binding. Furthermore, the increase in methylation of Six2-bound regions is strongly correlated with the decrease in Six2 expression. Dnmt3a and Dnmt3b play key roles in methylation of Six2-bound fetal-enhancer regions. It seems that Six2-bound enhancers do not achieve full methylation in the adult kidney, indicating that Six2 might act as a pioneering factor, which will need to be tested in future experiments.
Although we observed a failure of full silencing of developmental genes in the Dnmt3a and Dnmt3b knockout mice, it was highly unexpected to observe minimal phenotypic changes at baseline and after injury in these animals. This is a key contrast to the observed methylation changes and to the key role Dnmt3a and Dnmt3b in other progenitor compartments. Dnmt3a is essential for hematopoietic stem cell (HSC) differentiation because Dnmt3a-null HSCs show a marked decline in differentiation capacity over serial transplantation, resulting in accumulation of undifferentiated HSCs in the bone marrow.50 Moreover, mutations in DNMT3A are prevalent in myeloid malignancies51–53 and lymphoid leukemias,54 consistent with its important function in hematopoiesis. The role of Dnmt3a and Dnmt3b seems to be more pronounced in rapidly proliferating stem cells such as HSCs, where methylation loss over time is associated with leukemia development. However, it is worth noting that not all animals develop malignancy and even those that develop disease will do so relatively later in life. These results indicate that cells exhibit significant plasticity in their enhancer methylation level. In addition, it seems that enhancer methylation is not critical for cell-fate stabilization. This notion will need further experimental evidence. Furthermore, although Dnmt3a/3b knockout mice did not show alterations in a CKD model, it showed increased resistance to AKI, which requires rapid proliferation and cell differentiation.
Here we show that developmental enhancers, whose de novo methylation is specifically mediated by Dnmt3a and Dnmt3b, are enriched for kidney disease genetic risk loci. These regions are annotated as active enhancers in the fetal kidney, many bound by Six2, a critical kidney developmental transcription factor. However, these regions are no longer annotated as active enhancers in the adult stage. Fetal enhancer methylation level increases during development and Dnmt3a and Dnmt3b are responsible for the methylation of these regions. These genetic variants have not been functionally annotated in the past because these regions are no longer active enhancers in adult kidneys. Furthermore, methylation of these regions shows strong correlation with gene expression. However, because these regions are methylated in the adult kidney, the target gene expression is limited to the fetal stage. It will be important to study how these regions contribute to kidney disease development.
Here we propose a locus-specific convergence of genetic and epigenetic factors in kidney disease development. The interplay of sequence and post-translational variations could explain how genetic and environmental factors could contribute to common disease development.55 Furthermore, because environmental and nutrient availability are critical in establishing the epigenome, it is possible that Dnmt3a- and Dnmt3b-mediated methylation changes play roles in kidney disease development. We found striking similarities when we compared methylation of the UNCX and HOXD9 regions in healthy human and mouse kidneys. However, methylation of diseased human kidney samples was more similar to Dntm3a and Dnmt3b knockout kidneys, raising the interesting possibility that human kidney disease–specific epigenetic changes are already established during fetal development. This hypothesis was raised by Barker et al.56 in the past, however it has never been conclusively proven that these changes are mediated by epigenetic factors.
In summary, we established the critical role of Dnmt3a and Dnmt3b in mouse kidney development. Dnmt3a and Dnmt3b play critical roles in de novo methylation and decommissioning of fetal-enhancer regions. Interestingly, most of their effect in mice is observed in the postnatal period when the most significant change in methylation occurs and is associated with the decline in Six2 expression. Fetal enhancers methylated by Dnmt3a and Dnmt3b appear to harbor key kidney disease risk loci, potentially indicating their key roles in kidney disease development and the locus-specific convergence of genetic and epigenetic factors.
Disclosures
The Susztak laboratory is supported by Bayer, Boehringer Ingelheim, Celgene, Gilead, GSK, Lilly, Merck, ONO Pharma, and Regeneron for work that is not related to this manuscript.
Funding
Work in the Susztak laboratory is supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK076077, R01 DK087635, and DP3 DK108220. Dr. Park is supported by American Diabetes Association training grant #1-17-PDF-036.
Supplementary Material
Acknowledgments
This study was conceived of and led by Dr. Susztak, Dr. Liu, and Dr. Guan. Dr. Guan performed all animal and cell experiments. Dr. Liu performed all computational analysis. Ms. Ma, Dr. Li, Dr. Park, and Dr. Sheng helped with animal care and data analysis. Dr. Liu, Dr. Guan, and Dr. Susztak wrote the mauscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019080797/-/DCSupplemental.
Supplemental Figure 1. The spatial and temporal expression of Six2 and Cdh16 (Ksp) in the mouse kidney.
Supplemental Figure 2. Effect of Dnmt3a/3b deletion in FA-induced kidney fibrosis model.
Supplemental Figure 3. Phenotypic characterization of Six2CreDnmt3a/3b mice.
Supplemental Figure 4. Genomic location of DMRs observed in Dnmt3a/3b double knock-out.
Supplemental Figure 5. Cell-type specific base resolution methylation changes in KspCreDnmt3a/3b mice.
Supplemental Figure 6. IGV genome browser of Hypo-DMR regions.
Supplemental Figure 7. Dnmt3a/3b mediated methylation changes overlap with fetal enhancers and Six2-binding.
Supplemental Figure 8. Correlation between methylation changes and Six2 expression.
Supplemental Figure 9. Methylation and gene expression correlation of topologically defined enhancer DMRs and their target genes.
Supplemental Figure 10. Species conservation of kidney enhancers and their epigenetic changes in development and kidney disease.
References
- 1.Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, et al.: Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 39: 457–466, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Okano M, Bell DW, Haber DA, Li E: DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99: 247–257, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Gifford WD, Pfaff SL, Macfarlan TS: Transposable elements as genetic regulatory substrates in early development. Trends Cell Biol 23: 218–226, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al.; International Human Genome Sequencing Consortium: Initial sequencing and analysis of the human genome [published correction appears in Nature 412: 565, 2001]. Nature 409: 860–921, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Li SY, Park J, Guan Y, Chung K, Shrestha R, Palmer MB, et al.: DNMT1 in Six2 progenitor cells is essential for transposable element silencing and kidney development. J Am Soc Nephrol 30: 594–609, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckerman P, Ko YA, Susztak K: Epigenetics: A new way to look at kidney diseases. Nephrol Dial Transplant 29: 1821–1827, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al.: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanner N, Vornweg J, Combes A, Wilson S, Plappert J, Rafflenbeul G, et al.: DNA methyltransferase 1 controls nephron progenitor cell renewal and differentiation. J Am Soc Nephrol 30: 63–78, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wuttke M, Li Y, Li M, Sieber KB, Feitosa MF, Gorski M, et al.; Lifelines Cohort Study; V. A. Million Veteran Program: A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 51: 957–972, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu C, Huang S, Park J, Park Y, Ko YA, Seasock MJ, et al.: Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat Med 24: 1721–1731, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko YA, Yi H, Qiu C, Huang S, Park J, Ledo N, et al.: Genetic-variation-driven gene-expression changes highlight genes with important functions for kidney disease. Am J Hum Genet 100: 940–953, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, et al.: Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518: 337–343, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gluck C, Qiu C, Han SY, Palmer M, Park J, Ko YA, et al.: Kidney cytosine methylation changes improve renal function decline estimation in patients with diabetic kidney disease. Nat Commun 10: 2461, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Guan Y, Sheng X, Gluck C, Seasock MJ, Hakimi AA, et al.: Functional methylome analysis of human diabetic kidney disease. JCI Insight 4: e128886, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G, Chen H, Ren S, Xia M, Zhu J, Liu Y, et al.: Aberrant DNA methylation of mTOR pathway genes promotes inflammatory activation of immune cells in diabetic kidney disease. Kidney Int 96: 409–420, 2019. [DOI] [PubMed] [Google Scholar]
- 16.Vehaskari VM, Aviles DH, Manning J: Prenatal programming of adult hypertension in the rat. Kidney Int 59: 238–245, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Hoppe CC, Evans RG, Moritz KM, Cullen-McEwen LA, Fitzgerald SM, Dowling J, et al.: Combined prenatal and postnatal protein restriction influences adult kidney structure, function, and arterial pressure. Am J Physiol Regul Integr Comp Physiol 292: R462–R469, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Keating ST, van Diepen JA, Riksen NP, El-Osta A: Epigenetics in diabetic nephropathy, immunity and metabolism. Diabetologia 61: 6–20, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato M, Natarajan R: Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol 15: 327–345, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Susztak K: Understanding the epigenetic syntax for the genetic alphabet in the kidney. J Am Soc Nephrol 25: 10–17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krueger F, Andrews SR: Bismark: A flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27: 1571–1572, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, et al.: methylKit: A comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol 13: R87, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Garrett-Bakelman FE, Akalin A, Zumbo P, Levine R, To BL, et al.: An optimized algorithm for detecting and annotating regional differential methylation. BMC Bioinformatics 14[Suppl 5]: S10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Liu X, Zhang S, Lv J, Li S, Shang S, et al.: Systematic identification and annotation of human methylation marks based on bisulfite sequencing methylomes reveals distinct roles of cell type-specific hypomethylation in the regulation of cell identity genes. Nucleic Acids Res 44: 75–94, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al.: STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Dewey CN: RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12: 323, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, et al.: GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res 47: D766–D773, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson MD, McCarthy DJ, Smyth GK: edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Sherman BT, Lempicki RA: Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Weng MP, Liao BY: modPhEA: Model organism Phenotype Enrichment Analysis of eukaryotic gene sets. Bioinformatics 33: 3505–3507, 2017. [DOI] [PubMed] [Google Scholar]
- 31.Quinlan AR, Hall IM: BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bogu GK, Vizán P, Stanton LW, Beato M, Di Croce L, Marti-Renom MA: Chromatin and RNA maps reveal regulatory long noncoding RNAs in mouse. Mol Cell Biol 36: 809–819, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramírez F, Dündar F, Diehl S, Grüning BA, Manke T: deepTools: A flexible platform for exploring deep-sequencing data. Nucleic Acids Res 42: W187–W191, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cusanovich DA, Hill AJ, Aghamirzaie D, Daza RM, Pliner HA, Berletch JB, et al. : A single-cell atlas of in vivo mammalian chromatin accessibility. Cell 174: 1309–1324.e18, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al.: Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, et al.: GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 28: 495–501, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien LL, Guo Q, Lee Y, Tran T, Benazet JD, Whitney PH, et al.: Differential regulation of mouse and human nephron progenitors by the six family of transcriptional regulators. Development 143: 595–608, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al.: The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res 47: D1005–D1012, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al.: The human genome browser at UCSC. Genome Res 12: 996–1006, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris AP, Le TH, Wu H, Akbarov A, van der Most PJ, Hemani G, et al.: Trans-ethnic kidney function association study reveals putative causal genes and effects on kidney-specific disease aetiologies. Nat Commun 10: 29, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellwege JN, Velez Edwards DR, Giri A, Qiu C, Park J, Torstenson ES, et al.: Mapping eGFR loci to the renal transcriptome and phenome in the VA Million Veteran Program. Nat Commun 10: 3842, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ernst J, Kellis M: Chromatin-state discovery and genome annotation with ChromHMM. Nat Protoc 12: 2478–2492, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, et al.: Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25: 5214–5228, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouchard M, Souabni A, Mandler M, Neubüser A, Busslinger M: Nephric lineage specification by Pax2 and Pax8. Genes Dev 16: 2958–2970, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayran A, Khetchoumian K, Hariri F, Pastinen T, Gauthier Y, Balsalobre A, et al.: Pioneer factor Pax7 deploys a stable enhancer repertoire for specification of cell fate. Nat Genet 50: 259–269, 2018. [DOI] [PubMed] [Google Scholar]
- 46.Heuberger J, Birchmeier W: Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2: a002915, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edeling M, Ragi G, Huang S, Pavenstädt H, Susztak K: Developmental signalling pathways in renal fibrosis: The roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol 12: 426–439, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Ettou S, Khalid M, Taglienti M, Jain D, Jung YL, et al.: EED, a member of the polycomb group, is required for nephron differentiation and the maintenance of nephron progenitor cells. Development 145: dev157149, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hon GC, Rajagopal N, Shen Y, McCleary DF, Yue F, Dang MD, et al.: Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. Nat Genet 45: 1198–1206, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, et al.: Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet 44: 23–31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al.: DNMT3A mutations in acute myeloid leukemia. N Engl J Med 363: 2424–2433, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walter MJ, Ding L, Shen D, Shao J, Grillot M, McLellan M, et al.: Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia 25: 1153–1158, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, et al.: Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet 43: 309–315, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Grossmann V, Haferlach C, Weissmann S, Roller A, Schindela S, Poetzinger F, et al.: The molecular profile of adult T-cell acute lymphoblastic leukemia: Mutations in RUNX1 and DNMT3A are associated with poor prognosis in T-ALL. Genes Chromosomes Cancer 52: 410–422, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Chamberlain AA, Lin M, Lister RL, Maslov AA, Wang Y, Suzuki M, et al.: DNA methylation is developmentally regulated for genes essential for cardiogenesis. J Am Heart Assoc 3: e000976, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM: Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 36: 62–67, 1993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data (WGBS, RRBS, and RNA sequencing) have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) and are accessible through GEO accession number GSE134267.